- 1The Second Clinical Medical College, Guangzhou University of Chinese Medicine, Guangzhou, China
- 2Guangzhou University of Chinese Medicine, Guangzhou, China
- 3The Second Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangdong Provincial Hospital of Chinese Medicine), Guangzhou, China
- 4Guangzhou Key Laboratory of Traditional Chinese Medicine for Prevention and Treatment of Emerging Infectious Diseases, Guangzhou, China
Background: Anti-type 2 inflammation therapy has been proposed as a treatment strategy for eosinophil-associated chronic airway disorders that could reduce exacerbations and improve lung function. We performed a meta-analysis of randomized controlled trials to assess the effectiveness of type 2 monoclonal antibodies (anti-T2s) for eosinophil-associated chronic airway disorders.
Methods: PubMed, Embase, Web of Science, and Cochrane Library were searched from their inception to 21 August 2022. Randomized clinical trials evaluating the effectiveness of anti-T2s versus placebo in the treatment of chronic airway diseases were selected. The outcomes were exacerbation rate and change in pre-bronchodilator forced expiratory volume in 1 s (FEV1) from baseline. The Cochrane Risk of Bias Assessment Tool 1.0 was used to evaluate the risk of bias, and the random-effects or fixed-effect model were used to pool the data.
Results: Thirty-eight articles concerning forty-one randomized clinical trials with 17,115 patients were included. Compared with placebo, anti-T2s therapy yielded a significant reduction in exacerbation rate in COPD and asthma (Rate Ratio (RR)=0.89, 95%CI, 0.83-0.95, I2 = 29.4%; RR= 0.59, 95%CI, 0.52-0.68, I2 = 83.9%, respectively) and improvement in FEV1 in asthma (Standard Mean Difference (SMD)=0.09, 95%CI, 0.08-0.11, I2 = 42.6%). Anti-T2s therapy had no effect on FEV1 improvement in COPD (SMD=0.05, 95%CI, -0.01-0.10, I2 = 69.8%).
Conclusion: Despite inconsistent findings across trials, anti-T2s had a positive overall impact on patients’ exacerbation rate in asthma and COPD and FEV1 in asthma. Anti-T2s may be effective in treating chronic airway illnesses related to eosinophils.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022362280.
1 Introduction
Chronic airway diseases pose a serious public health risk, causing 3.91 million deaths in 2017, accounting for 7% of all death worldwide, which is mainly attributable to chronic obstructive pulmonary disease (COPD) and asthma (1).
Elevated blood eosinophils, sputum eosinophils, or exhaled breath nitric oxide fraction (FeNO) are common manifestations of eosinophilic airway inflammation, which are associated with increased risk of patient complications, recurrent acute exacerbations, pneumonia, prolonged hospitalization, and increased morbidity and mortality (2–9). Patients with persistent eosinophilic airway inflammation may benefit from inhaled glucocorticosteroids (ICS) (10–14). Nevertheless, long-term ICS therapy may result in several unfavorable adverse events, such as osteoporosis, diabetes, cataracts, and higher infection risk (15, 16). Additionally, ICS is not always well-tolerated by patients.
Several monoclonal antibodies targeting particular inflammatory pathways have been created to address the complications mentioned above. Pathogenic factor-induced cellular release of cytokines, including interleukin (IL)-4, IL-5, IL-9, IL-13, IL-25, IL-33, immunoglobulin E (IgE) and thymic stromal lymphopoietin (TSLP) are closely related to eosinophilic airway inflammation (17, 18). Except for blocking the downstream targets, activation of toll-like receptor 9 (TLR9) has been shown to balance the T helper (Th) 1/Th2 axis (19). Type 2 monoclonal antibodies (anti-T2s) are effective in decreasing FeNO and eosinophil levels (20–24). However, results from previous research, which investigated the effectiveness of anti-T2s in reducing exacerbation rate and improving lung function, have been controversial. Therefore, we performed a meta-analysis of randomized controlled trials (RCTs) to examine the efficacy of anti-T2s for chronic eosinophilic airway diseases, investigating the possibility of endotype-guided strategy in the management of chronic airway disorders.
2 Methods
2.1 Protocol
The study protocol was registered at the International Prospective Register of Systematic Reviews (number CRD42022362280).
2.2 Data sources and search strategies
PubMed, Embase, Web of Science, and Cochrane Library were searched from their inception to 21 August 2022. We used the following search strategy to find all studies evaluating anti-T2s, including IL-5, IL-4, IL-9, IL-13, IL-25, IL-33, IgE, TSLP, and TLR9 for patients with eosinophil-associated COPD and asthma: (mepolizumab OR reslizumab OR benralizumab OR tralokinumab OR lebrikizumab OR dupilumab OR anti-interleukin OR MEDI-528 OR GSK679586 OR omalizumab OR tezepelumab OR AZD1419 OR CYT003 OR itepekimab OR XKH001) AND (asthma OR chronic obstructive diseases). The detailed search strategy is shown in Supplementary Table 1. Languages had no restrictions. The pertinent review articles and their citations were also checked.
2.3 Study selection
Endnote X9 software was adopted to manage the eligible studies during the literature screening and automatically remove duplicate documents. The following particular inclusion criteria were met (1): Participants: individuals (6 years of age or older) with asthma or COPD who met one or more criteria for eosinophilic inflammation at study enrolment or within the previous year. (2) Interventions: with anti-IL-5, anti-IL-4, anti-IL-13, anti-IL-9, anti-IL-25, anti-IL-33, anti-TSLP, anti-IgE or TLR9 agonist therapy at any dose. (3) Randomized placebo-controlled trials. (4) Reporting the following outcomes: exacerbation rate and change in pre-bronchodilator forced expiratory volume in 1 s (FEV1) from baseline.
Excluded criteria were as follows: (1) Studies did not involve eosinophilic endotype. (2) Interventions were not related to type 2 inflammation. (3) Studies did not assess the exacerbation rate or FEV1. (4) Not RCTs or literature types were reviews, letters, second analysis, or conferences.
The source data, together with the rate ratio (RR) or mean difference (MD), are given or can be computed from the data. All references were independently reviewed by two authors (YW and MH) following the selection criteria. Any disagreements were resolved through conversation or by a third author (JL).
2.4 Data extraction and quality assessment
The preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement was followed (25) (see Supplementary Table 5). Two authors (YW and MH) independently retrieved data from eligible studies using Excel 2019 in a standardized data extraction form in a blinded manner based on the authors, publication year, research design, patient characteristics (age, gender, etc.), the type of anti-T2s used, the dosage, the length of the therapy, the definitions of the outcomes, the exacerbation rate, and the change in FEV1. A third author (JL) was consulted to settle disagreements. Furthermore, we evaluated the risk of bias using Cochrane Risk of Bias Assessment Tool 1.0, which included sufficient sequence generation, allocation concealment, blinding of participants and staff, inadequate outcome data, selective reporting, and additional bias (26). Two senior researchers (RY and YL) evaluated the reliability of the evidence using the GRADE-profiler software (V.3.6, The GRADE Working Group, 2010), items including the risk of bias, inconsistency, indirectness, imprecision, and publication bias, and the evidence was assessed as 4 levels: high quality, moderate quality, low quality, and very low quality.
2.5 Statistical analysis
We conducted a series of meta-analysis to compare the efficacy of anti-T2s with a placebo. For dichotomous data (exacerbation rate), intervention effects were reported using RR and 95% confidence intervals (CI), whereas standard mean differences (SMD) and 95%CI were used for continuous data (FEV1). Following the Cochrane Handbook, we aggregated two or three intervention groups into a single intervention group when research demonstrates more than two arms (26). The chi-squared test and the I2 statistic were used to measure heterogeneity. Significant heterogeneity is indicated by an I2 value of more than 50% (27). When there was significant heterogeneity, a random effect model was applied, and meta-regression analyses were performed to investigate the possible origins of heterogeneity. Planned considerations included ages, risk of bias, and demographics (exacerbation history, type 2 inflammatory criteria, and so forth). In comparisons involving at least 10 trials, publication bias was examined using a funnel plot and Eggers’ test (28, 29). The influence of publication bias was estimated using the trim-and-fill method (30). Sensitivity analyses were carried out to assess the robustness of the overall effect sizes by removing one study at a time. Review Manager (V.5.4.1, The Cochrane Collaboration, 2020) and Stata (V.15.1) were used for all statistical analyses. A two-sided P value of 0.05 was considered statistically significant.
3 Results
3.1 Search results
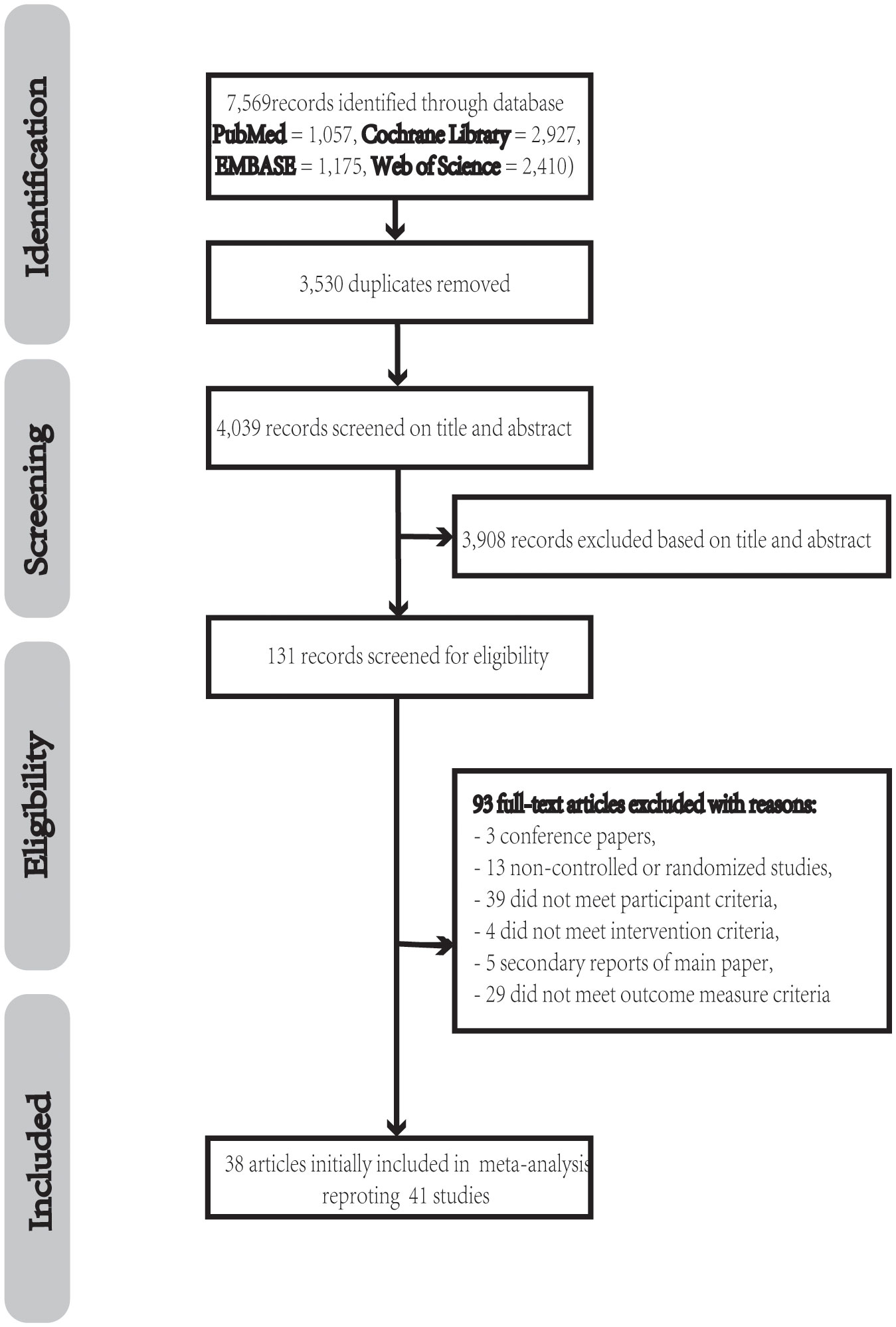
A total of 7569 potentially pertinent articles were found. 3530 duplicate records from among all the potential studies were eliminated, leaving 4039 papers for screening. We found and obtained 131 papers in full text for review after examining the titles and abstracts. Ninety-three of these publications were excluded due to the following reasons: improper population (n = 39) (31–69), incorrect intervention (n = 4) (70–73), improper outcomes (n =29) (74–102), non-RCT (n = 13) (103–115), conferences (n = 3) (116–118), and second analysis (n = 5) (119–123). In the end, this meta-analysis included 38 articles with 41 studies (Figure 1).
3.2 Description of included trials
Thirty-eight articles, covering forty-one trials with 17,115 individuals, were included (Supplementary Table 2). The number of subjects in the studies ranges from 61 to 1545. Eleven of these studies employed benralizumab (124–133), three reslizumab (23, 134, 135), two dupilumab (21, 136), one lebrikizumab (137), eight mepolizumab (22, 138–143), seven omalizumab (111, 144–149), two tezepelumab (150, 151), one astegolimab (152), one itepekimab (153), one AZD1419 (154), one quilizumab (155), one CYT003 (156) and two tralokinumab (157). The duration of the treatment ranges from 12 to 56 weeks, and the follow-up was 12 to 84 weeks. Six trials administered the monoclonal antibody by intravenous infusion (IV), thirty-three studies by subcutaneous (SC), one by inhalation, and one study comprised both IV and SC arms. Thirty-four studies included patients with asthma, whereas seven researches included those with COPD.
All included patients with COPD who had an exacerbation history. In thirty-four studies with asthma patients, seven studies included severe asthma, five studies included moderate to severe asthma, one study included mild to moderate asthma, twelve studies included refractory, uncontrolled, or persistent asthma, and the remaining studies did not specify asthma severity; patients in eight studies required medium to high dose ICS plus long-acting β2-agonists (LABA), two studies required at least medium ICS, one study required 6-month maintenance treatment with systemic glucocorticoids, and one study required not receiving ICS; seventeen studies required exacerbation history.
The definition of ‘type 2 inflammation’ varied among studies. Four studies were defined by FeNO levels, three studies were defined by a sputum eosinophil count, twenty-two studies were defined by baseline blood eosinophil counts, one study was defined by eosinophil counts in blood or sputum, one study was defined by baseline blood eosinophil counts or IgE levels, five by IgE levels, and one study defines by combinatorial biomarkers, including FeNO levels, eosinophil counts in blood or sputum, whereas four studies did not specify the criteria.
3.3 Efficacy outcomes
In chronic airway illnesses associated with eosinophils, we contrasted anti-T2s with a placebo. The primary outcome was the exacerbation rate. A COPD or asthma exacerbation was defined as a clinical worsening for at least three days, a temporary increase in the ICS background dosage, the need for systemic corticosteroid treatment, the consumption of antibiotics, hospitalization, or mortality resulting from an airway disease. The secondary endpoint was the change in FEV1 from baseline measured by spirometry. Since the number of studies on asthma is much higher than studies on COPD, to eliminate the influence, the population was divided into the asthma group and COPD group for meta-analysis, respectively.
3.3.1 Exacerbation rate in COPD
There were seven studies included to analyse anti-T2s’ efficacy in reducing the exacerbation rate of COPD. As a result, anti-T2s considerably reduced the exacerbation rate in COPD when compared to placebo (RR=0.89, 95%CI, 0.83-0.95, I2 = 29.4%, Figure 2A).
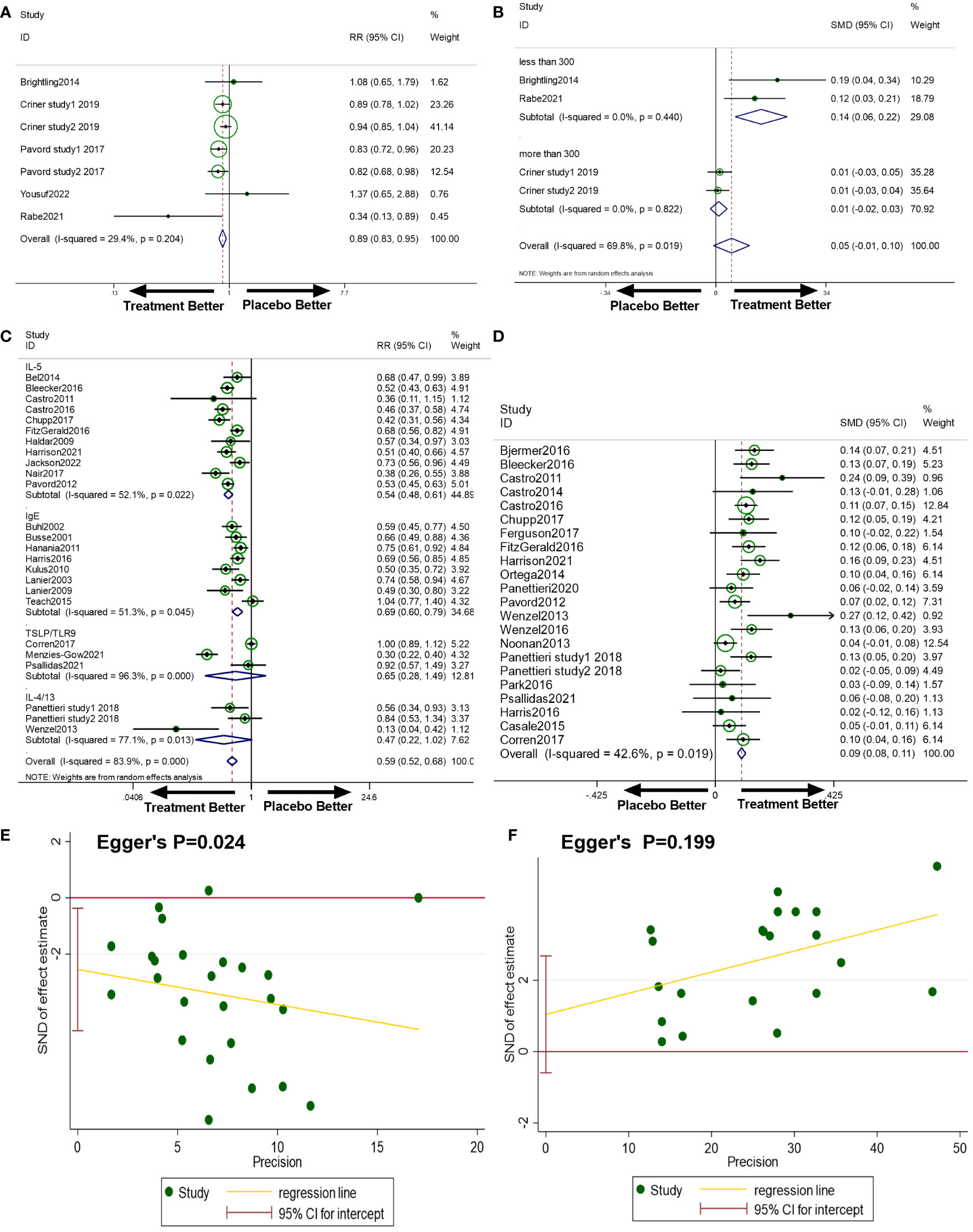
Figure 2 (A) The effect of anti-T2s versus placebo on exacerbation rate in COPD. Fixed-effect model. (B) The effect of anti-T2s versus placebo on FEV1 change from baseline in COPD. Random-effect model. (C) The effect of anti-T2s versus placebo on exacerbation rate in asthma. Random-effect model. (D) The effect of anti-T2s versus placebo on FEV1 change from baseline in asthma. Fixed-effect model. (E) Egger’s test of exacerbation rate in asthma in the meta-analysis. (F) Egger’s test of FEV1 in asthma in the meta-analysis. CI, confidence interval; SMD, standard mean difference; RR, rate ratio.
3.3.2 FEV1 in COPD
There were four studies included to analyse anti-T2s’ efficacy in improving FEV1 in COPD. As a result, anti-T2s improved pre-bronchodilator FEV1 in patients with COPD, whereas the difference was not statistically significant (SMD=0.05, 95%CI, -0.01-0.10, I2 = 69.8%, Figure 2B). Considering the between-study heterogeneity, a subgroup analysis based on the sample size of studies was applied. In the subgroup analysis, studies with a sample size of less than 300 subjects exhibited anti-T2s’ efficacy in improving FEV1 (SMD=0.14, 95%CI, 0.06-0.22, I2 = 0%), while studies with sample size of more than 300 patients showed no effect on FEV1 improvement (SMD=0.05, 95%CI, -0.01-0.10, I2 = 0%).
3.3.3 Exacerbation rate in asthma
There were twenty-five studies included. Anti-T2s considerably reduced the exacerbation rate in asthma when compared to placebo (RR=0.59, 95%CI, 0.52-0.68, I2 = 83.9%, Figure 2C). Publication bias on Egger’s test was present in this analysis (P=0.024, Figure 2E). But further investigation using the trim-and-fill test showed that this publishing bias did not affect the estimations (ie, no trimming was done because the data was unchanged).
Anti-IL-5 treatment was associated with a decreased incidence of asthma exacerbation compared to placebo in the subgroup analysis for different targets (RR=0.54, 95%CI, 0.48-0.61, I2 = 52.1%). Similarly, anti-IgE therapy achieved a reduction in exacerbation of asthma (RR=0.69, 95%CI, 0.60-0.79, I2 = 51.3%). The exacerbation rate was found decreasing with anti-IL-4/13, anti-TSLP, or TLR9 agonist medication when compared to placebo, although the difference was not statistically significant (RR=0.47, 95%CI, 0.22-1.02, I2 = 77.1%; RR=0.65, 95%CI, 0.28-1.49, I2 = 96.3%). Since the heterogeneity was partially decreased in subgroup analysis, different targets did not completely account for the between-study heterogeneity.
Univariable meta-regression using a random-effects model was performed and the results revealed that the criteria of ‘type 2 inflammation’, history of exacerbation, age, sample size, risk bias, severity, atopy, and different targets were not significantly associated with heterogeneity related to the exacerbation rate in asthma (Supplementary Table 3).
3.3.4 FEV1 in asthma
Data on pre-bronchodilator FEV1 were reported from twenty-two trials, of which seventeen reported a change in FEV1 from baseline, four reported a change in FEV1% from baseline, and one reported both.
Anti-T2s was associated with a substantial improvement in FEV1 change from baseline in a pooled analysis of twenty-two trials (SMD=0.09, 95%CI, 0.08-0.11, P<0.001, Figure 2D) with acceptable heterogeneity (I2 = 42.6%, P=0.019). No publication bias existed (Egger’s P=0.199, Figure 2F).
3.4 Risk of bias
A total of 25 researches (61.0%) adequately explained the randomization process. In 16 researches (39.0%), the random allocation was acknowledged, while in 25 studies (61.0%), allocation concealment was unclear. Research blinding was used in all of the investigations. 11 studies (26.8%) were at low risk for the outcome assessment’s blinding. There was a low risk to the integrity of the outcome data in 23 trials (56.1%). There was a low risk of selection bias for 37 (90.2%). The data from each study was insufficient to determine if the risk of other biases was low or high (Figures 3A, B). In 32 studies included to assess the exacerbation rate, 20 studies in total (62.5%) provided a comprehensive explanation of the randomization procedure. In 11 investigations (34.4%), the random allocation was confirmed, while allocation concealment was uncertain in 21 studies (65.6%). In each study, research blinding was performed. 7 studies (21.9%) had a low risk of blinding in outcome assessment. In 19 trials (59.4%), there was a low risk in the integrity of the outcome data. For 28 studies (87.5%), the risk of selection bias was low. In 26 studies included to assess FEV1, 18 studies (69.2%) in total provided a thorough explanation of the randomization procedure. In 12 investigations (46.2%), the random allocation was acknowledged, and allocation concealment was ambiguous in 14 studies (53.8%). In each study, research blinding was applied. 9 studies (34.6%) had a low risk of blinding outcome assessment. In 11 trials (42.3%), there was a low risk of the integrity of the outcome data. 25 studies (96.2%) had a low risk of selective bias.
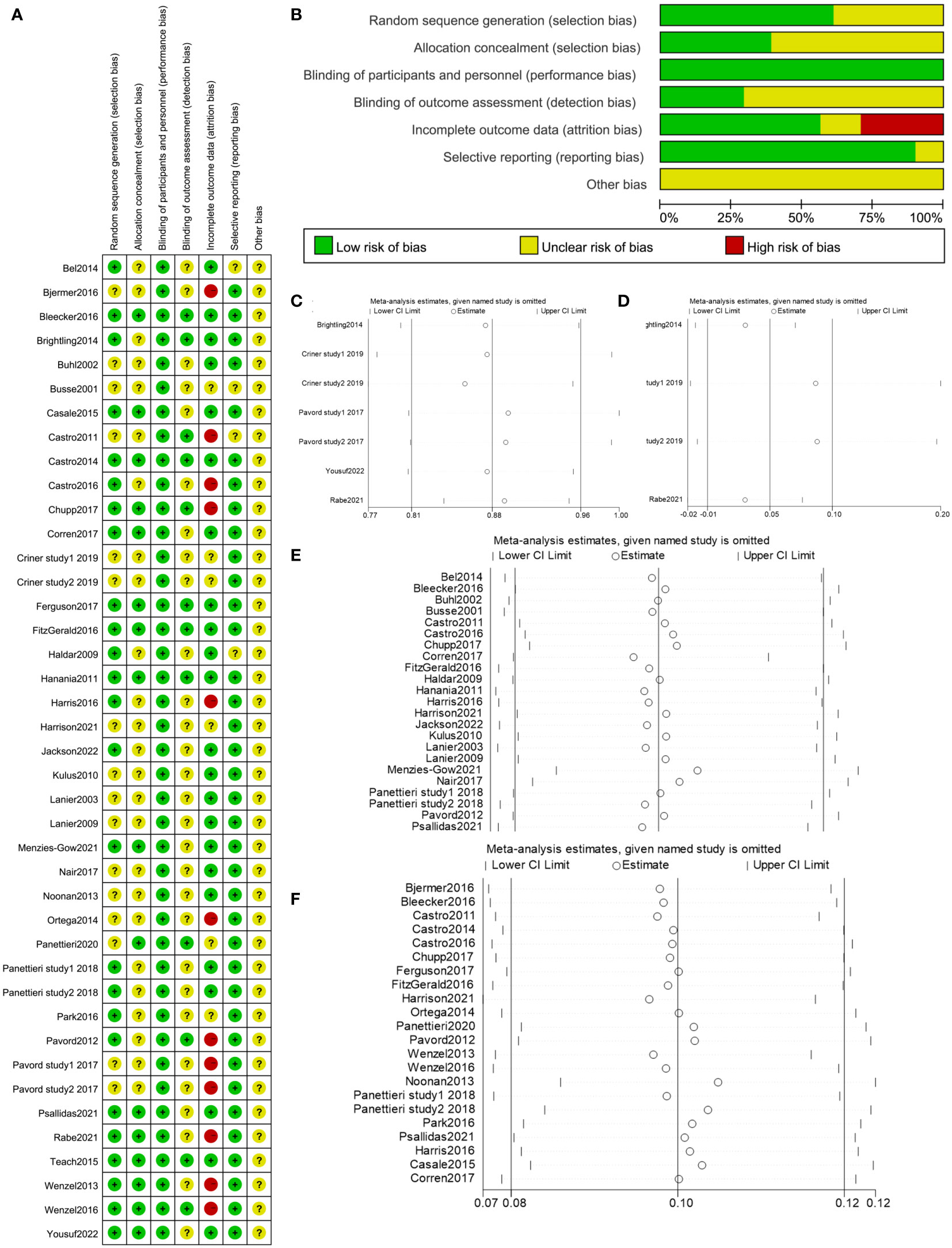
Figure 3 (A, B) Risk of bias summary of included studies. (C) Sensitivity analysis of exacerbation rate in COPD in the meta-analysis. (D) Sensitivity analysis of change in FEV1 from baseline in COPD in the meta-analysis. (E) Sensitivity analysis of exacerbation rate in asthma in the meta-analysis. (F) Sensitivity analysis of change in FEV1 from baseline in asthma in the meta-analysis.
3.5 Sensitivity analysis
By removing one study at a time, sensitivity analyses were utilized to examine the impact of each study on the combined results. The outcome demonstrated that there had been no appreciable changes to the results’ stability (Figures 3C–F), which supported the validity and dependability of our analysis.
3.6 Certainty of the evidence
Because of the considerable heterogeneity and inconsistent findings across trials, the evidence received a low-quality level in exacerbation rate in asthma and change in FEV1 from baseline in COPD, and a moderate-quality level in exacerbation rate in COPD and change in FEV1 from baseline in asthma (Supplementary table 4).
4 Discussion
This meta-analysis included 41 RCTs from 38 articles with 17,115 participants and investigated the effect of anti-T2s in patients with chronic eosinophilic airway diseases on exacerbation rate and FEV1. Our results showed that anti-T2s significantly reduces exacerbation rate in COPD and asthma, and improve FEV1 in asthma when compared to placebo, indicating that type 2 chronic airway disease patients can benefit from endotype-guided therapy as a treatment option.
Exacerbations are far more likely to cause morbidity and mortality (158, 159). One of the main objectives of chronic airway illness management is to reduce the exacerbation rate (22, 127). According to earlier investigations, increased blood and sputum eosinophilic counts are independent risk factors for exacerbations (160–162). Anti-T2s reduce the FeNO, eosinophil cationic protein, and eosinophil levels in airway inflammation (21–24), indicating that airway eosinophilia is a novel target, thus anti-T2s may be a potential approach to chronic eosinophilic airway disorders treatment. Our meta-analysis, which revealed a marked decline in the exacerbation rate in both COPD and asthma, validated the claim. Patients with COPD receiving anti-T2s medication in contrast to placebo experienced a reduced exacerbation. Although individuals with asthma receiving anti-T2s therapy had decreased exacerbations than those receiving a placebo, the heterogeneity was statistically significant. A subgroup analysis was conducted according to different targets, which revealed inconsistent results among subgroups. Anti-IL-5 and anti-IgE therapy both achieved a reduction in asthma exacerbation with acceptable heterogeneity, while anti-IL-4/13, anti-TSLP, and TLR9 agonist therapy had a decreased trend of exacerbations than placebo, and the difference was not statistically significant. The following factors may account for the inconsistent results among subgroups: (1) The inclusion criteria for each study within the current meta-analysis varied, which may have led to significant heterogeneity among study populations in terms of exacerbation risk, eosinophil count, and disease severity; (2) Different therapy regimens were varied; (3) Anti-IL-4/13 treatment has shown a less consistently positive impact on the exacerbation rate, as the previous study reported (163). Tralokinumab, an anti-IL-13 agent, did not affect the exacerbation rate in the study of Panettieri et al, but another cohort in the same article showed a statistically significant reduction in exacerbations (157). This may be an indication of the ambiguous impact of blocking the IL4/13 pathway on reducing the exacerbation rate in asthma (4). Anti-TSLP and TLR9 agonist therapy had fewer studies to evidence their efficacy, and the existing studies varied in ages of participants, disease severity, and so forth, resulting in obvious heterogeneity. However, the overall beneficial impact of anti-T2s in reducing asthma exacerbations is consistent across the meta-analysis, despite some lingering confounding factors.
The crucial identifying feature of chronic airway illnesses in the clinic and pathology is airflow limitation. The lung function test continues to be the gold standard in diagnosis and a crucial indicator of management, which is typically measured by the change in FEV1 (164). The results on FEV1 of asthma and COPD are inconsistent. Even though FEV1 in COPD patients improved, the difference was not statistically significant, which was consistent with previous studies (165–167). Overall, FEV1 considerably improved in asthma patients receiving anti-T2s. FEV1 alone may not be the optimum assessment for the management of chronic airway illnesses. The bias in results may be caused by variations in race, medication dosage, or even trial participants’ status and severity. The conflicting results between asthma and COPD could be attributed to the varying baseline FeNO, blood, or sputum eosinophilia thresholds. In addition, it was observed that former smokers achieved more pronounced benefits than current smokers in a prespecified subgroup analysis, which might be explained by a broad pro-inflammatory effect of cigarette smoke, indicating that smoking status had an impact on the effectiveness of anti-T2s in treating COPD (50). Further research should be done to determine the COPD-specific threshold of type 2 inflammation and explore the effect of anti-T2s in COPD patients with different smoking status to address these deficiencies.
According to the Cochrane handbook, ambiguous allocation concealment might exaggerate the estimated effect in subjective outcomes, while the bias in objective outcomes is not confirmed (26). In our study, the outcomes, including exacerbation rate and FEV1, are tending to be objective, and the impact of ambiguous allocation concealment remains unclear. Meanwhile, the GRADE system was applied to evaluate the reliability of our results, which had considered the bias judgments.
5 Limitations
Some potential restrictions must be taken into account. Firstly, it is difficult to determine the influence of the severity and initial therapy of included patients on the outcomes of the investigations. Secondly, a few of the research was conducted on a limited scale, which would limit their ability to investigate the true outcome. Thirdly, we failed to investigate the potential impact of disease severity, gender, and body mass index on outcomes given the limited data available. Fourthly, due to the finite number of specifically aimed at IL-4/13 pathway targeting, we were unable to further compare the effects of anti-IL-4 and anti-IL-4/13 treatment in subgroup analysis for exacerbation rate. Additionally, RCTs related to the anti-IL-9 agent were not included due to not meeting the inclusion criteria, RCTs related to anti-IL-25 therapy were in progress (NCT05128409), and RCTs of anti-T2s on COPD were under publication (NCT03615040, NCT03930732, NCT04456673). Finally, although using various intervention dosages and administration techniques, as recommended by the Cochrane handbook, we combined two or three intervention groups into a single arm, making it difficult to establish the ideal dosage. We should also be aware of the fact that different studies used various definitions of ‘type 2 inflammation’, and because no study included data on specific patients, we were unable to further examine the correlation between baseline levels of eosinophils or FeNO and treatment outcomes.
6 Conclusions
The current meta-analysis concluded that anti-T2s could considerably lessen exacerbations of chronic airway disorders. Therefore, anti-T2s may be effective in treating chronic airway illnesses associated with eosinophils. The findings highlight the effectiveness of endotype-guided treatment in chronic eosinophilic airway inflammation illnesses regardless of various background therapies and ‘type 2 inflammation’ criteria.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
YW, JL, and JC conceived this meta-analysis. YW, MH, and JZ extracted the data and wrote the manuscript. YLu did statistical analyses and checked them. KG helps the methods. RY and YLi evaluated the reliability of the evidence. YLu, RY, YLi, JL, and JC revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Guangzhou Key Laboratory of Combined Traditional Chinese and Western Medicine for Prevention and Treatment of Emerging and Sudden Infectious Diseases (202201020382); Basic and Applied Basic Research of Guangzhou City-University Joint Funding Project (202201020475, 2023A03J0227); Guangdong Provincial Bureau of Chinese Medicine (20225020, 20222071); the Fund of Guangzhou University of Chinese Medicine (2021YJZX012, 2022YBA06); the Guangdong Provincial Hospital of Chinese Medicine Fund (YN2019ML11 and YN2019QJ13); Research Fund for Zhaoyang Talents of Guangdong Provincial Hospital of Chinese Medicine (ZY2022KY10, ZY2022YL04).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1089710/full#supplementary-material
References
1. Li X, Cao X, Guo M, Xie M, Liu X. Trends and risk factors of mortality and disability adjusted life years for chronic respiratory diseases from 1990 to 2017: systematic analysis for the global burden of disease study 2017. Bmj (2020) 368:m234. doi: 10.1136/bmj.m234
2. Casciano J, Krishnan J, Small MB, Li C, Dotiwala Z, Martin BC. Progression to uncontrolled severe asthma: A novel risk equation. J Manag Care Spec Pharm (2017) 23(1):44–50. doi: 10.18553/jmcp.2017.23.1.44
3. Couillard S, Larivée P, Courteau J, Vanasse A. Eosinophils in COPD exacerbations are associated with increased readmissions. Chest (2017) 151(2):366–73. doi: 10.1016/j.chest.2016.10.003
4. David B, Bafadhel M, Koenderman L, De Soyza A. Eosinophilic inflammation in COPD: from an inflammatory marker to a treatable trait. Thorax (2021) 76(2):188–95. doi: 10.1136/thoraxjnl-2020-215167
5. Dotiwala Z, Casciano J, Davis JR, Fox K, Gopalan G, Rastogi S, et al. Effect of clinically significant thresholds of eosinophil elevation on health care resource use in asthma. Ann Allergy Asthma Immunol (2020) 125(2):182–9. doi: 10.1016/j.anai.2020.04.024
6. Gürsel G, Türktas H, Gökçora N, Tekin IO. Comparison of sputum and serum eosinophil cationic protein (ECP) levels in nonatopic asthma and chronic obstructive pulmonary disease. J Asthma (1997) 34(4):313–9. doi: 10.3109/02770909709067221
7. Casciano J, Krishnan J, Dotiwala Z, Li C, Sun SX. Clinical and economic burden of elevated blood eosinophils in patients with and without uncontrolled asthma. J Manag Care Spec Pharm (2017) 23(1):85–91. doi: 10.18553/jmcp.2017.23.1.85
8. Yun JH, Lamb A, Chase R, Singh D, Parker MM, Saferali A, et al. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol (2018) 141(6):2037–47.e10. doi: 10.1016/j.jaci.2018.04.010
9. Price DB, Rigazio A, Campbell JD, Bleecker ER, Corrigan CJ, Thomas M, et al. Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study. Lancet Respir Med (2015) 3(11):849–58. doi: 10.1016/S2213-2600(15)00367-7
10. Bafadhel M, Peterson S, De Blas MA, Calverley PM, Rennard SI, Richter K, et al. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med (2018) 6(2):117–26. doi: 10.1016/S2213-2600(18)30006-7
11. Lehtimäki L, Csonka P, Mäkinen E, Isojärvi J, Hovi SL, Ahovuo-Saloranta A. Predictive value of exhaled nitric oxide in the management of asthma: a systematic review. Eur Respir J (2016) 48(3):706–14. doi: 10.1183/13993003.00699-2016
12. Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med (2011) 184(5):602–15. doi: 10.1164/rccm.9120-11ST
13. Singh D, Kolsum U, Brightling CE, Locantore N, Agusti A, Tal-Singer R. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J (2014) 44(6):1697–700. doi: 10.1183/09031936.00162414
14. Siva R, Green RH, Brightling CE, Shelley M, Hargadon B, McKenna S, et al. Eosinophilic airway inflammation and exacerbations of COPD: A randomised controlled trial. Eur Respir J (2007) 29(5):906–13. doi: 10.1183/09031936.00146306
15. Saeed MI, Eklöf J, Achir I, Sivapalan P, Meteran H, Løkke A, et al. Use of inhaled corticosteroids and the risk of developing type 2 diabetes in patients with chronic obstructive pulmonary disease. Diabetes Obes Metab (2020) 22(8):1348–56. doi: 10.1111/dom.14040
16. Lodise TP, Li J, Gandhi HN, O'Brien G, Sethi S. Intraclass difference in pneumonia risk with fluticasone and budesonide in COPD: A systematic review of evidence from direct-comparison studies. Int J Chron Obstruct Pulmon Dis (2020) 15:2889–900. doi: 10.2147/COPD.S269637
17. Cowan DC, Cowan JO, Palmay R, Williamson A, Taylor DR. Effects of steroid therapy on inflammatory cell subtypes in asthma. Thorax (2010) 65(5):384–90. doi: 10.1136/thx.2009.126722
18. Wang W, Li Y, Lv Z, Chen Y, Li Y, Huang K, et al. Bronchial allergen challenge of patients with atopic asthma triggers an alarmin (IL-33, TSLP, and IL-25) response in the airways epithelium and submucosa. J Immunol (2018) 201(8):2221–31. doi: 10.4049/jimmunol.1800709
19. Campbell JD, Kell SA, Kozy HM, Lum JA, Sweetwood R, Chu M, et al. A limited CpG-containing oligodeoxynucleotide therapy regimen induces sustained suppression of allergic airway inflammation in mice. Thorax (2014) 69(6):565–73. doi: 10.1136/thoraxjnl-2013-204605
20. Yalcin AD, Celik B, Yalcin AN. Omalizumab (anti-IgE) therapy in the asthma-COPD overlap syndrome (ACOS) and its effects on circulating cytokine levels. Immunopharmacol Immunotoxicol (2016) 38(3):253–6. doi: 10.3109/08923973.2016.1173057
21. Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med (2013) 368(26):2455–66. doi: 10.1056/NEJMoa1304048
22. Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med (2009) 360(10):973–84. doi: 10.1056/NEJMoa0808991
23. Castro M, Mathur S, Hargreave F, Boulet LP, Xie F, Young J, et al. Reslizumab for poorly controlled, eosinophilic asthma a randomized, placebo-controlled study. Am J Respir Crit Care Med (2011) 184(10):1125–32. doi: 10.1164/rccm.201103-0396OC
24. Yalcin AD, Uzun R. Anti-IgE significantly changes circulating interleukin-25, vitamin-d and interleukin-33 levels in patients with allergic asthma. Curr Pharm Des (2019) 25(35):3784–95. doi: 10.2174/1381612825666190930095725
25. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol (Engl Ed) (2021) 74(9):790–9. doi: 10.1016/j.recesp.2021.06.016
26. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane Database systematic Rev (Online) (2022).
27. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
28. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
29. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Bmj (2011) 343:d4002. doi: 10.1136/bmj.d4002
30. Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics (2000) 56(2):455–63. doi: 10.1111/j.0006-341X.2000.00455.x
31. Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-Severe uncontrolled asthma. N Engl J Med (2018) 378(26):2486–96. doi: 10.1056/NEJMoa1804092
32. Bacharier LB, Maspero JF, Katelaris CH, Fiocchi AG, Gagnon R, de Mir I, et al. Dupilumab in children with uncontrolled moderate-to-Severe asthma. N Engl J Med (2021) 385(24):2230–40. doi: 10.1056/NEJMoa2106567
33. De Boever EH, Ashman C, Cahn AP, Locantore NW, Overend P, Pouliquen IJ, et al. Efficacy and safety of an anti-IL-13 mAb in patients with severe asthma: a randomized trial. J Allergy Clin Immunol (2014) 133(4):989–96. doi: 10.1016/j.jaci.2014.01.002
34. Brightling CE, Chanez P, Leigh R, O'Byrne PM, Korn S, She DW, et al. Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med (2015) 3(9):692–701. doi: 10.1016/S2213-2600(15)00197-6
35. Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med (2011) 365(12):1088–98. doi: 10.1056/NEJMoa1106469
36. Busse W, Brusselle G, Korn S, Kuna P, Magnan A, Cohen D, et al. Oral corticosteroid (OCS)-sparing effect of tralokinumab in severe, uncontrolled asthma: the TROPOS study. Eur Respir J (2018) 52(suppl 62):PA602. doi: 10.1183/13993003.congress-2018.PA602
37. Corren J, Weinstein S, Janka L, Zangrilli J, Garin M. Phase 3 study of reslizumab in patients with poorly controlled asthma: Effects across a broad range of eosinophil counts. Chest (2016) 150(4):799–810. doi: 10.1016/j.chest.2016.03.018
38. Corren J, Busse W, Meltzer EO, Mansfield L, Bensch G, Fahrenholz J, et al. A randomized, controlled, phase 2 study of AMG 317, an IL-4Rα antagonist, in patients with asthma. Am J Respir Crit Care Med (2010) 181(8):788–96. doi: 10.1164/rccm.200909-1448OC
39. Flood-Page P, Swenson C, Faiferman I, Matthews J, Williams M, Brannick L, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med (2007) 176(11):1062–71. doi: 10.1164/rccm.200701-085OC
40. Gauvreau GM, Boulet LP, Cockcroft DW, Fitzgerald JM, Carlsten C, Davis BE, et al. Effects of interleukin-13 blockade on allergen-induced airway responses in mild atopic asthma. Am J Respir Crit Care Med (2011) 183(8):1007–14. doi: 10.1164/rccm.201008-1210OC
41. Menzies-Gow A, Flood-Page P, Sehmi R, Burman J, Hamid Q, Robinson DS, et al. Anti-IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J Allergy Clin Immunol (2003) 111(4):714–9. doi: 10.1067/mai.2003.1382
42. Kips JC, O'Connor BJ, Langley SJ, Woodcock A, Kerstjens HAM, Postma DS, et al. Effect of SCH55700, a humanized anti-human interleukin-5 antibody, in severe persistent asthma: A pilot study. Am J Respir Crit Care Med (2003) 167(12):1655–9. doi: 10.1164/rccm.200206-525OC
43. Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet (london england) (2000) 356(9248):2144–8. doi: 10.1016/S0140-6736(00)03496-6
44. Korenblat P, Kerwin E, Leshchenko I, Yen K, Holweg CTJ, Anzures-Cabrera J, et al. Efficacy and safety of lebrikizumab in adult patients with mild-to-moderate asthma not receiving inhaled corticosteroids. Respir Med (2018) 134:143–9. doi: 10.1016/j.rmed.2017.12.006
45. Hanania NA, Korenblat P, Chapman KR, Bateman ED, Kopecky P, Paggiaro P, et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med (2016) 4(10):781–96. doi: 10.1016/S2213-2600(16)30265-X
46. Hodsman P, Ashman C, Cahn A, De Boever E, Locantore N, Serone A, et al. A phase 1, randomized, placebo-controlled, dose-escalation study of an anti-IL-13 monoclonal antibody in healthy subjects and mild asthmatics. Br J Clin Pharmacol (2013) 75(1):118–28. doi: 10.1111/j.1365-2125.2012.04334.x
47. Piper E, Brightling C, Niven R, Oh C, Faggioni R, Poon K, et al. A phase II placebo-controlled study of tralokinumab in moderate-to-severe asthma. Eur Respir J (2013) 41(2):330–8. doi: 10.1183/09031936.00223411
48. Nowak RM, Parker JM, Silverman RA, Rowe BH, Smithline H, Khan F. A randomized trial of benralizumab, an antiinterleukin 5 receptor alpha monoclonal antibody, after acute asthma. Am J Emerg Med (2015) 33(1):14–20. doi: 10.1016/j.ajem.2014.09.036
49. Oh CK, Leigh R, McLaurin KK, Kim K, Hultquist M, Molfino NA. A randomized, controlled trial to evaluate the effect of an anti-interleukin-9 monoclonal antibody in adults with uncontrolled asthma. Respir Res (2013) 14(1):93. doi: 10.1186/1465-9921-14-93
50. Rabe KF, Celli BR, Wechsler ME, Abdulai RM, Luo X, Boomsma MM, et al. Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: a genetic association study and randomised, double-blind, phase 2a trial. Lancet Respir Med (2021) 9(11):1288–98. doi: 10.1016/S2213-2600(21)00167-3
51. Oh C, Parker J, Geba G, Molfino N. Safety profile and clinical activity of multiple subcutaneous (SC) doses of MEDI-528, a humanized anti-interleukin-9 monoclonal antibody, in subjects with asthma Vol. [377]. Barcelona, Spain: European respiratory society annual congress (2010).
52. Sabogal Piñeros YS, Bal SM, van de Pol MA, Dierdorp BS, Dekker T, Dijkhuis A, et al. Anti-IL-5 in mild asthma alters rhinovirus-induced macrophage, b-cell, and neutrophil responses (MATERIAL). a placebo-controlled, double-blind study. Am J Respir Crit Care Med (2019) 199(4):508–17. doi: 10.1164/rccm.201803-0461OC
53. Russell RJ, Chachi L, FitzGerald JM, Backer V, Olivenstein R, Titlestad IL, et al. Effect of tralokinumab, an interleukin-13 neutralising monoclonal antibody, on eosinophilic airway inflammation in uncontrolled moderate-to-severe asthma (MESOS): a multicentre, double-blind, randomised, placebo-controlled phase 2 trial. Lancet Respir Med (2018) 6(7):499–510. doi: 10.1016/S2213-2600(18)30201-7
54. Scheerens H, Arron JR, Zheng Y, Putnam WS, Erickson RW, Choy DF, et al. The effects of lebrikizumab in patients with mild asthma following whole lung allergen challenge. Clin Exp Allergy (2014) 44(1):38–46. doi: 10.1111/cea.12220
55. Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med (2018) 378(26):2475–85. doi: 10.1056/NEJMoa1804093
56. Wechsler ME, Ruddy MK, Pavord ID, Israel E, Rabe KF, Ford LB, et al. Efficacy and safety of itepekimab in patients with moderate-to-Severe asthma. N Engl J Med (2021) 385(18):1656–68. doi: 10.1056/NEJMoa2024257
57. Szefler SJ, Roberts G, Rubin AS, Zielen S, Kuna P, Alpan O, et al. Efficacy, safety, and tolerability of lebrikizumab in adolescent patients with uncontrolled asthma (ACOUSTICS). Clin Trans Allergy (2022) 12(7):e12176. doi: 10.1002/clt2.12176
58. Corren J, Garcia Gil E, Griffiths JM, Parnes JR, van der Merwe R, Salapa K, et al. Tezepelumab improves patient-reported outcomes in patients with severe, uncontrolled asthma in PATHWAY. Ann Allergy Asthma Immunol (2021) 126(2):187–93. doi: 10.1016/j.anai.2020.10.008
59. Corren J, Lemanske RF Jr., Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med (2011) 365(12):1088–98. doi: 10.1056/NEJMoa1106469
60. Corren J, Tuyet-Hang P, Gil EG, Salapa K, Ren P, Parnes JR, et al. Baseline type 2 biomarker levels and response to tezepelumab in severe asthma. Allergy (2022) 77(6):1786–96. doi: 10.1111/all.15197
61. De Boever EH, Ashman C, Cahn AP, Locantore NW, Overend P, Pouliquen IJ, et al. Efficacy and safety of an anti-IL-13 mAb in patients with severe asthma: a randomized trial. J Allergy Clin Immunol (2014) 133(4):989–96. doi: 10.1016/j.jaci.2014.01.002
62. Diver S, Khalfaoui L, Emson C. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial (vol 9, pg 355, 2021). Lancet Respir Med (2021) 9(11):E106–E. doi: 10.1016/S2213-2600(21)00226-5
63. Garcia G, Magnan A, Chiron R, Girodet PO, Le Gros V,MH. A proof-of-concept randomized-controlled trial of omalizumab in patients with severe difficult to control nonatopic asthma. Eur Respir J (2012) 40(Suppl 56):856s [4692]. doi: 10.1164/ajrccm-conference.2012.185.1_MeetingAbstracts.A6764
64. Hendeles L, Khan YR, Shuster JJ, Chesrown SE, Abu-Hasan M. Omalizumab therapy for asthma patients with poor adherence to inhaled corticosteroid therapy. Ann Allergy Asthma Immunol (2015) 114(1):58–62.e2. doi: 10.1016/j.anai.2014.10.012
65. Ishizuka T, Menzies-Gow A, Okada H, Fukushima Y, Hayashi N, Colice G, et al. Efficacy and safety of tezepelumab in patients recruited in Japan who participated in the phase 3 NAVIGATOR study. Allergol Int (2022) 72(1):82–88. doi: 10.1016/j.alit.2022.07.004
66. Pillai P, Chan YC, Wu SY, Ohm-Laursen L, Thomas C, Durham SR, et al. Omalizumab reduces bronchial mucosal IgE and improves lung function in non-atopic asthma. Eur Respir J (2016) 48(6):1593–601. doi: 10.1183/13993003.01501-2015
67. Russell RJ, Chachi L, FitzGerald JM, Backer V, Olivenstein R, Titlestad IL, et al. Effect of tralokinumab, an interleukin-13 neutralising monoclonal antibody, on eosinophilic airway inflammation in uncontrolled moderate-to-severe asthma (MESOS): a multicentre, double-blind, randomised, placebo-controlled phase 2 trial. Lancet Respir Med (2018) 6(7):499–510. doi: 10.1016/S2213-2600(18)30201-7
68. Sverrild A, Hansen S, Hvidtfeldt M, Clausson C-M, Cozzolino O, Cerps S, et al. The effect of tezepelumab on airway hyperresponsiveness to mannitol in asthma (UPSTREAM). Eur Respir J (2022) 59(1):2101296. doi: 10.1183/13993003.01296-2021
69. Wechsler ME, Menzies-Gow A, Brightling CE. Evaluation of the oral corticosteroid-sparing effect of tezepelumab in adults with oral corticosteroid-dependent asthma (SOURCE): a randomised, placebo-controlled, phase 3 study (vol 10, pg 650, 2022). Lancet Respir Med (2022) 10(5):E73–E. doi: 10.1016/S2213-2600(21)00537-3
70. Polkey MI, Praestgaard J, Berwick A, Franssen FME, Singh D, Steiner MC, et al. Activin type II receptor blockade for treatment of muscle depletion in chronic obstructive pulmonary disease. A Randomized Trial Am J Respir Crit Care Med (2019) 199(3):313–20. doi: 10.1164/rccm.201802-0286OC
71. Yang F, Busby J, Heaney LG, Pavord ID, Brightling CE, Borg K, et al. Corticosteroid responsiveness following mepolizumab in severe eosinophilic asthma - a randomised, placebo-controlled crossover trial [MAPLE]. J Allergy Clin Immunol Pract (2022) 10(11):2925–34.e12. doi: 10.1016/j.jaip.2022.06.050
72. Eich A, Urban V, Jutel M, Vlcek J, Shim JJ, Trofimov VI, et al. A randomized, placebo-controlled phase 2 trial of CNTO 6785 in chronic obstructive pulmonary disease. Copd-Journal Chronic Obstructive Pulmonary Disease (2017) 14(5):476–83. doi: 10.1080/15412555.2017.1335697
73. Rubin AS, Souza-Machado A, Andradre-Lima M, Ferreira F, Honda A, Matozo TM. Effect of omalizumab as add-on therapy on asthma-related quality of life in severe allergic asthma: a Brazilian study (QUALITX). J Asthma (2012) 49(3):288–93. doi: 10.3109/02770903.2012.660297
74. Eck S, Castro M, Sinibaldi D, White W, Folliot K, Gossage D. Benralizumab effect on blood basophil counts in adults with uncontrolled asthma. Eur Respir J (2014) 44(Suppl 58):P297.
75. Laviolette M, Gossage DL, Gauvreau G, Leigh R, Olivenstein R, Katial R, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol (2013) 132(5):1086–+. doi: 10.1016/j.jaci.2013.05.020
76. Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med (2009) 360(10):985–93. doi: 10.1056/NEJMoa0805435
77. Busse WW, Brusselle GG, Korn S, Kuna P, Magnan A, Cohen D, et al. Tralokinumab did not demonstrate oral corticosteroid-sparing effects in severe asthma. Eur Respir J (2019) 53(2):1800948. doi: 10.1183/13993003.00948-2018
78. Pouliquen IJ, Kornmann O, Barton SV, Price JA, Ortega HG. Characterization of the relationship between dose and blood eosinophil response following subcutaneous administration of mepolizumab. Int J Clin Pharmacol Ther (2015) 53(12):1015–27. doi: 10.5414/CP202446
79. Swanson BN, Teper A, Hamilton JD, Zhang B, Staudinger H, Tian N. Dupilumab suppresses fractional exhaled nitric oxide (FeNO) and biomarkers of type 2 inflammation in adult patients with persistent uncontrolled asthma despite use of medium-to-high dose inhaled corticosteroids plus long-acting beta-agonists (ICS/LABAS). J Allergy Clin Immunol (2016) 137(2 SUPPL. 1):AB190. doi: 10.1016/j.jaci.2015.12.752
80. Apsangikar P, Ghadge P, Naik M, Nair S. Randomized comparative clinical study of first global omalizumab biosimilar with innovator product in moderate to severe persistent asthma. J Assoc Physicians India (2020) 68(12):61–5.
81. Ayres JG, Higgins B, Chilvers ER, Ayre G, Blogg M, Fox H. Efficacy and tolerability of anti-immunoglobulin e therapy with omalizumab in patients with poorly controlled (moderate-to-severe) allergic asthma. Allergy (2004) 59(7):701–8. doi: 10.1111/j.1398-9995.2004.00533.x
82. Borish LC, Nelson HS, Corren J, Bensch G, Busse WW, Whitmore JB, et al. Efficacy of soluble IL-4 receptor for the treatment of adults with asthma. J Allergy Clin Immunol (2001) 107(6):963–70. doi: 10.1067/mai.2001.115624
83. Bousquet J, Siergiejko Z, Swiebocka E, Humbert M, Rabe KF, Smith N, et al. Persistency of response to omalizumab therapy in severe allergic (IgE-mediated) asthma. Allergy (2011) 66(5):671–8. doi: 10.1111/j.1398-9995.2010.02522.x
84. Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest (2004) 125(4):1378–86. doi: 10.1378/chest.125.4.1378
85. Brodlie M, McKean MC, Moss S, Spencer DA. The oral corticosteroid-sparing effect of omalizumab in children with severe asthma. Arch Dis Child (2012) 97(7):604–9. doi: 10.1136/archdischild-2011-301570
86. Buhl R, Hanf G, Solèr M, Bensch G, Wolfe J, Everhard F, et al. The anti-IgE antibody omalizumab improves asthma-related quality of life in patients with allergic asthma. Eur Respir J (2002) 20(5):1088–94. doi: 10.1183/09031936.02.00016502
87. Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med (2011) 364(11):1005–15. doi: 10.1056/NEJMoa1009705
88. Chanez P, Contin-Bordes C, Garcia G, Verkindre C, Didier A, De Blay F, et al. Omalizumab-induced decrease of fc epsilon RI expression in patients with severe allergic asthma. Respir Med (2010) 104(11):1608–17. doi: 10.1016/j.rmed.2010.07.011
89. Djukanović R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, et al. Effects of treatment with anti-immunoglobulin e antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med (2004) 170(6):583–93. doi: 10.1164/rccm.200312-1651OC
90. Fahy JV, Cockcroft DW, Boulet LP, Wong HH, Deschesnes F, Davis EE, et al. Effect of aerosolized anti-IgE (E25) on airway responses to inhaled allergen in asthmatic subjects. Am J Respir Crit Care Med (1999) 160(3):1023–7. doi: 10.1164/ajrccm.160.3.9810012
91. Fahy JV, Fleming HE, Wong HH, Liu JT, Su JQ, Reimann J, et al. The effect of an anti-IgE monoclonal antibody on the early- and late- phase responses to allergen inhalation in asthmatic subjects. Am J Respir Crit Care Med (1997) 155(6):1828–34. doi: 10.1164/ajrccm.155.6.9196082
92. Gauvreau GM, O'Byrne PM, Boulet L-P, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med (2014) 370(22):2102–10. doi: 10.1056/NEJMoa1402895
93. Holgate ST, Chuchalin AG, Hébert J, Lötvall J, Persson GB, Chung KF, et al. Efficacy and safety of a recombinant anti-immunoglobulin e antibody (omalizumab) in severe allergic asthma. Clin Exp Allergy (2004) 34(4):632–8. doi: 10.1111/j.1365-2222.2004.1916.x
94. Li J, Kang J, Wang C, Yang J, Wang L, Kottakis I. Omalizumab improves quality of life and asthma control in chinese patients with moderate to severe asthma: a randomized phase III study. Allergy Asthma Immunol Res (2016) 8(4):319–28. doi: 10.4168/aair.2016.8.4.319
95. Massanari M, Nelson H, Casale T, Busse W, Kianifard F, Geba GP, et al. Effect of pretreatment with omalizumab on the tolerability of specific immunotherapy in allergic asthma. J Allergy Clin Immunol (2010) 125(2):383–9. doi: 10.1016/j.jaci.2009.11.022
96. Milgrom H, Berger W, Nayak A, Gupta N, Pollard S, McAlary M, et al. Treatment of childhood asthma with anti-immunoglobulin e antibody (omalizumab). Pediatrics (2001) 108(2):E36. doi: 10.1542/peds.108.2.e36
97. Noga O, Hanf G, Kunkel G. Immunological and clinical changes in allergic asthmatics following treatment with omalizumab. Int Arch Allergy Immunol (2003) 131(1):46–52. doi: 10.1159/000070434
98. Ohta K, Miyamoto T, Amagasaki T, Yamamoto M. Efficacy and safety of omalizumab in an Asian population with moderate-to-severe persistent asthma. Respirology (2009) 14(8):1156–65. doi: 10.1111/j.1440-1843.2009.01633.x
99. Scheerens H, Arron JR, Zheng Y, Putnam WS, Erickson RW, Choy DF, et al. The effects of lebrikizumab in patients with mild asthma following whole lung allergen challenge. Clin Exp Allergy (2014) 44(1):38–46. doi: 10.1111/cea.12220
100. Soler M, Matz J, Townley R, Buhl R, O'Brien J, Fox H, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J (2001) 18(2):254–61. doi: 10.1183/09031936.01.00092101
101. Tajiri T, Matsumoto H, Gon Y, Ito R, Hashimoto S, Izuhara K, et al. Utility of serum periostin and free IgE levels in evaluating responsiveness to omalizumab in patients with severe asthma. Allergy (2016) 71(10):1472–9. doi: 10.1111/all.12922
102. Zielen S, Lieb A, de la Motte S, Wagner F, de Monchy J, Fuhr R, et al. Omalizumab protects against allergen-induced bronchoconstriction in allergic (Immunoglobulin e-mediated) asthma. Int Arch Allergy Immunol (2013) 160(1):102–10. doi: 10.1159/000339243
103. Chipps BE, Hirsch I, Trudo F, Alacqua M, Zangrilli JG. Benralizumab efficacy for patients with fixed airflow obstruction and severe, uncontrolled eosinophilic asthma. Ann Allergy Asthma Immunol (2020) 124(1):79–86. doi: 10.1016/j.anai.2019.10.006
104. Busse WW, Bleecker ER, FitzGerald JM, Ferguson GT, Barker P, Brooks L, et al. Benralizumab for adolescent patients with severe, eosinophilic asthma: safety and efficacy after 3 years of treatment. J Allergy Clin Immunol (2021) 148(1):266–71.e2. doi: 10.1016/j.jaci.2021.02.009
105. Papi A, Corren J, Castro M, Domingo C, Rogers L, Chapman KR, et al. Dupilumab reduced impact of severe exacerbations on lung function in patients with moderate-to-severe type 2 asthma. Allergy (2022) 78(1):233–43. doi: 10.1183/13993003.congress-2021.OA2836
106. Busse WW, Maspero JF, Rabe KF, Papi A, Wenzel SE, Ford LB, et al. Liberty asthma QUEST: Phase 3 randomized, double-blind, placebo-controlled, parallel-group study to evaluate dupilumab Efficacy/Safety in patients with uncontrolled, moderate-to-Severe asthma. Adv Ther (2018) 35(5):737–48. doi: 10.1007/s12325-018-0702-4
107. Lugogo N, Domingo C, Chanez P, Leigh R, Gilson MJ, Price RG, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: A multi-center, open-label, phase IIIb study. Clin Ther (2016) 38(9):2058–70. doi: 10.1016/j.clinthera.2016.07.010
108. Austin CD, Edick MG, Ferrando RE, Solon M, Baca M, Mesh K, et al. A randomized, placebo-controlled trial evaluating effects of lebrikizumab on airway eosinophilic inflammation and remodeling in uncontrolled asthma (CLAVIER). Clin Exp Allergy (2020) 50(12):1342–51. doi: 10.1111/cea.13731
109. Baena-Cagnani CE, Teijeiro A, Canonica GW. Four-year follow-up in children with moderate/severe uncontrolled asthma after withdrawal of a 1-year omalizumab treatment. Curr Opin Allergy Clin Immunol (2015) 15(3):267–71. doi: 10.1097/ACI.0000000000000161
110. Corren J, Castro M, O'Riordan T, Hanania NA, Pavord ID, Quirce S, et al. Dupilumab efficacy in patients with uncontrolled, moderate-to-Severe allergic asthma. J Allergy Clin Immunol Pract (2020) 8(2):516–26. doi: 10.1016/j.jaip.2019.08.050
111. Hoshino M, Ohtawa J. Effects of adding omalizumab, an anti-immunoglobulin e antibody, on airway wall thickening in asthma. Respiration (2012) 83(6):520–8. doi: 10.1159/000334701
112. Humbert M, Berger W, Rapatz G, Turk F. Add-on omalizumab improves day-to-day symptoms in inadequately controlled severe persistent allergic asthma. Allergy: Eur J Allergy Clin Immunol (2008) 63(5):592–6. doi: 10.1111/j.1398-9995.2008.01654.x
113. Menzies-Gow A, Ponnarambil S, Downie J, Bowen K, Hellqvist Å, Colice G. DESTINATION: a phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the long-term safety and tolerability of tezepelumab in adults and adolescents with severe, uncontrolled asthma. Respir Res (2020) 21(1):279. doi: 10.1186/s12931-020-01541-7
114. Mukherjee M, Kjarsgaard M, Radford K, Huang C, Leigh R, Dorscheid DR, et al. Omalizumab in patients with severe asthma and persistent sputum eosinophilia. Allergy Asthma Clin Immunol (2019) 15:21. doi: 10.1186/s13223-019-0337-2
115. Rabe KF, FitzGerald JM, Bateman ED, Castro M, Pavord ID, Maspero JF, et al. Dupilumab is effective in patients with moderate-to-Severe uncontrolled GINA-defined type 2 asthma irrespective of an allergic asthma phenotype. J Allergy Clin Immunol In Pract (2022) 10(11):2916–24.e4. doi: 10.1016/j.jaip.2022.06.036
116. Swanson BN, Wang L, Ming J, Hamilton JD, Teper A, Dicioccio T. Exhaled nitric oxide (FENO) and t-helper 2 cell biomarkers: can they predict treatment response to dupilumab, an il-4ra antibody, in an eosinophilic asthma population? J Allergy Clin Immunol (2014) 133(2 Suppl):AB85. doi: 10.1016/j.jaci.2013.12.321
117. Ortega H, Liu M, Pavord I, Brusselle G, FitzGerald JM, Chetta A. Reduction in exacerbations with mepolizumab in severe eosinophilic asthma: MENSA study. Eur Respir J (2014) 44(Suppl 58):2906.
118. O'Byrne P, Boulet LP, Gauvreau G, Leon F, Sari S, White B. A single dose of medi-528, a monoclonal antibody against interleukin-9, is well tolerated in mild and moderate asthmatics in the phase ii trial mi-cp-138. Chest (2007) 132(4):478. doi: 10.1378/chest.132.4_MeetingAbstracts.478
119. Tohda Y, Nakamura Y, Fujisawa T, Ebisawa M, Arima K, Miyata M, et al. Dupilumab efficacy and safety in Japanese patients with uncontrolled, moderate-to-severe asthma in the phase 3 LIBERTY ASTHMA QUEST study. Allergology Int (2020) 69(4):578–87. doi: 10.1016/j.alit.2020.04.002
120. Maspero JF, Katelaris CH, Busse WW, Castro M, Corren J, Chipps BE, et al. Dupilumab efficacy in uncontrolled, moderate-to-Severe asthma with self-reported chronic rhinosinusitis. J Allergy Clin Immunology-in Pract (2020) 8(2):527–+. doi: 10.1016/j.jaip.2019.07.016
121. Bourdin A, Papi AA, Corren J, Virchow JC, Rice MS, Deniz Y, et al. Dupilumab is effective in type 2-high asthma patients receiving high-dose inhaled corticosteroids at baseline. Allergy: Eur J Allergy Clin Immunol (2021) 76(1):269–80. doi: 10.1111/all.14611
122. Busse WW, Maspero JF, Lu Y, Corren J, Hanania NA, Chipps BE, et al. Efficacy of dupilumab on clinical outcomes in patients with asthma and perennial allergic rhinitis. Ann Allergy Asthma Immunol (2020) 125(5):565–76.e1. doi: 10.1016/j.anai.2020.05.026
123. Robinson DS. Mepolizumab for severe eosinophilic asthma. Expert Rev Respir Med (2013) 7(1):13–7. doi: 10.1586/ers.12.84
124. Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta 2-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet (2016) 388(10056):2115–27. doi: 10.1016/S0140-6736(16)31324-1
125. Brightling CE, Bleecker ER, Panettieri RA, Bafadhel M, She DW, Ward CK, et al. Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: A randomised, double-blind, placebo-controlled, phase 2a study. Lancet Respir Med (2014) 2(11):891–901. doi: 10.1016/S2213-2600(14)70187-0
126. Ferguson GT, FitzGerald JM, Bleecker ER, Laviolette M, Bernstein D, LaForce C, et al. Benralizumab for patients with mild to moderate, persistent asthma (BISE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med (2017) 5(7):568–76. doi: 10.1016/S2213-2600(17)30190-X
127. Criner GJ, Celli BR, Brightling CE, Agusti A, Papi A, Singh D, et al. Benralizumab for the prevention of COPD exacerbations. N Engl J Med (2019) 381(11):1023–34. doi: 10.1056/NEJMoa1905248
128. FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor a monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet (2016) 388(10056):2128–41. doi: 10.1016/S0140-6736(16)31322-8
129. Castro M, Wenzel SE, Bleecker ER, Pizzichini E, Kuna P, Busse WW, et al. Benralizumab, an anti-interleukin 5 receptor alpha monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med (2014) 2(11):879–90. doi: 10.1016/S2213-2600(14)70201-2
130. Harrison TW, Chanez P, Menzella F, Canonica GW, Louis R, Cosio BG, et al. Onset of effect and impact on health-related quality of life, exacerbation rate, lung function, and nasal polyposis symptoms for patients with severe eosinophilic asthma treated with benralizumab (ANDHI): a randomised, controlled, phase 3b trial. Lancet Respir Med (2021) 9(3):260–74. doi: 10.1016/S2213-2600(20)30414-8
131. Panettieri RA, Welte T, Shenoy KV, Korn S, Jandl M, Kerwin EM, et al. Onset of effect, changes in airflow obstruction and lung volume, and health-related quality of life improvements with benralizumab for patients with severe eosinophilic asthma: Phase IIIb randomized, controlled trial (SOLANA) (vol 13, pg 115, 2020). J Asthma Allergy (2020) 13:135–. doi: 10.1016/j.jaci.2018.04.010
132. Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med (2017) 376(25):2448–58. doi: 10.1056/NEJMoa1703501
133. Park HS, Kim MK, Imai N, Nakanishi T, Adachi M, Ohta K, et al. A phase 2a study of benralizumab for patients with eosinophilic asthma in south Korea and Japan. Int Arch Allergy Immunol (2016) 169(3):135–45. doi: 10.2147/JAA.S240044
134. Castro M, Zangrilli J, Wechsler ME. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials (vol 3, pg 355, 2015). Lancet Respir Med (2016) 4(10):E50–E. doi: 10.1016/S2213-2600(15)00042-9
135. Bjermer L, Lemiere C, Maspero J, Weiss S, Zangrilli J, Germinaro M. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: A randomized phase 3 study. Chest (2016) 150(4):789–98. doi: 10.1016/j.chest.2016.03.032
136. Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang BZ, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta(2) agonist: A randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet (2016) 388(10039):31–44. doi: 10.1016/S0140-6736(16)30307-5
137. Noonan M, Korenblat P, Mosesova S, Scheerens H, Arron JR, Zheng Y, et al. Dose-ranging study of lebrikizumab in asthmatic patients not receiving inhaled steroids. J Allergy Clin Immunol (2013) 132(3):567–+. doi: 10.1016/j.jaci.2013.03.051
138. Chupp GL, Bradford ES, Albers FC, Bratton DJ, Wang-Jairaj J, Nelsen LM, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): A randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med (2017) 5(5):390–400. doi: 10.1016/S2213-2600(17)30125-X
139. Pavord ID, Chanez P, Criner GJ, Kerstjens HAM, Korn S, Lugogo N, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med (2017) 377(17):1613–29. doi: 10.1056/NEJMoa1708208
140. Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet (2012) 380(9842):651–9. doi: 10.1016/S0140-6736(12)60988-X
141. Jackson DJ, Bacharier LB, Gergen PJ, Gagalis L, Calatroni A, Wellford S, et al. Mepolizumab for urban children with exacerbation-prone eosinophilic asthma in the USA (MUPPITS-2): a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet (2022) 400(10351):502–11. doi: 10.1016/S0140-6736(22)01198-9
142. Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med (2014) 371(13):1198–207. doi: 10.1056/NEJMoa1403290
143. Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med (2014) 371(13):1189–97. doi: 10.1056/NEJMoa1403291
144. Buhl R, Soler M, Matz J, Townley R, O'Brien J, Noga O, et al. Omalizumab provides long-term control in patients with moderate-to-severe allergic asthma. Eur Respir J (2002) 20(1):73–8. doi: 10.1183/09031936.02.00278102
145. Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Della Cioppa G, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol (2001) 108(2):184–90. doi: 10.1067/mai.2001.117880
146. Hanania NA, Alpan O, Hamilos DL, Condemi JJ, Reyes-Rivera I, Zhu J, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med (2011) 154(9):573–82. doi: 10.7326/0003-4819-154-9-201105030-00002
147. Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol (2009) 124(6):1210–6. doi: 10.1016/j.jaci.2009.09.021
148. Lanier BQ, Corren J, Lumry W, Liu J, Fowler-Taylor A, Gupta N. Omalizumab is effective in the long-term control of severe allergic asthma. Ann Allergy Asthma Immunol (2003) 91(2):154–9. doi: 10.1016/S1081-1206(10)62170-9
149. Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ Jr., Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol (2015) 136(6):1476–85. doi: 10.1016/j.jaci.2015.09.008
150. Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med (2017) 377(10):936–46. doi: 10.1056/NEJMoa1704064
151. Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med (2021) 384(19):1800–9. doi: 10.1056/NEJMoa2034975
152. Yousuf AJ, Mohammed S, Carr L, Yavari Ramsheh M, Micieli C, Mistry V, et al. Astegolimab, an anti-ST2, in chronic obstructive pulmonary disease (COPD-ST2OP): a phase 2a, placebo-controlled trial. Lancet Respir Med (2022) 10(5):469–77. doi: 10.1016/S2213-2600(21)00556-7
153. Rabe KF, Celli BR, Wechsler ME, Abdulai RM, Luo X, Boomsma MM, et al. Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: a genetic association study and randomised, double-blind, phase 2a trial. Lancet Respir Med (2021) 9(11):1288–98. doi: 10.1016/S2213-2600(21)00167-3
154. Psallidas I, Backer V, Kuna P, Palmér R, Necander S, Aurell M, et al. A phase 2a, double-blind, placebo-controlled randomized trial of inhaled TLR9 agonist AZD1419 in asthma. Am J Respir Crit Care Med (2021) 203(3):296–306. doi: 10.1164/rccm.202001-0133OC
155. Harris JM, Maciuca R, Bradley MS, Cabanski CR, Scheerens H, Lim J, et al. A randomized trial of the efficacy and safety of quilizumab in adults with inadequately controlled allergic asthma. Respir Res (2016) 17:29. doi: 10.1186/s12931-016-0347-2
156. Casale TB, Cole J, Beck E, Vogelmeier CF, Willers J, Lassen C, et al. CYT003, a TLR9 agonist, in persistent allergic asthma - a randomized placebo-controlled phase 2b study. Allergy (2015) 70(9):1160–8. doi: 10.1111/all.12663
157. Panettieri RA, Sjobring U, Peterffy A, Wessman P, Bowen K, Piper E, et al. Tralokinumab for severe, uncontrolled asthma (STRATOS 1 and STRATOS 2): Two randomised, double-blind, placebo-controlled, phase 3 clinical trials. Lancet Respir Med (2018) 6(7):511–25. doi: 10.1016/S2213-2600(18)30184-X
158. Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA dissemination committee report. Allergy (2004) 59(5):469–78. doi: 10.1111/j.1398-9995.2004.00526.x
159. Brusselle G. Why doesn't reducing exacerbations decrease COPD mortality? Lancet Respir Med (2014) 2(9):681–3. doi: 10.1016/S2213-2600(14)70163-8
160. Bafadhel M, McKenna S, Terry S, Mistry V, Reid C, Haldar P, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med (2011) 184(6):662–71. doi: 10.1164/rccm.201104-0597OC
161. Zeiger RS, Schatz M, Li Q, Chen W, Tran T. Elevated blood eosinophil level is a risk factor for exacerbations in adult persistent asthma. J Allergy Clin Immunol Pract (2014) 2(6):741–50. doi: 10.1016/j.jaip.2014.06.005
162. Malinovschi A, Fonseca JA, Jacinto T, Alving K, Janson C. Exhaled nitric oxide levels and blood eosinophil counts independently associate with wheeze and asthma events in national health and nutrition examination survey subjects. J Allergy Clin Immunol (2013) 132(4):821–7.e1-5. doi: 10.1016/j.jaci.2013.06.007
163. Iftikhar IH, Schimmel M, Bender W, Swenson C, Amrol D. Comparative efficacy of anti IL-4, IL-5 and IL-13 drugs for treatment of eosinophilic asthma: A network meta-analysis. Lung (2018) 196(5):517–30. doi: 10.1007/s00408-018-0151-5
164. Reddel HK, Bateman ED, Becker A, Boulet LP, Cruz AA, Drazen JM, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J (2015) 46(3):622–39. doi: 10.1183/13993003.00853-2015
165. Lan SH, Lai CC, Chang SP, Hsu CC, Chen CH, Wang YH, et al. Efficacy and safety of anti-interleukin-5 therapy in patients with chronic obstructive pulmonary disease: A meta-analysis of randomized, controlled trials. J Microbiol Immunol Infect (2022) 55(1):26–35. doi: 10.1016/j.jmii.2020.11.001
166. Wang FP, Xiong XF, Liu T, Li SY, Cheng DY, Mao H. Anti-interleukin 5 therapy for eosinophilic asthma: A meta-analysis of randomized clinical trials. Clin Rev Allergy Immunol (2018) 54(2):318–30. doi: 10.1007/s12016-016-8588-x
Keywords: eosinophil-associated chronic airway diseases, efficacy, randomized controlled trials, meta-analysis, type 2 monoclonal antibodies
Citation: Wu Y, Huang M, Zhong J, Lu Y, Gan K, Yang R, Liu Y, Li J and Chen J (2023) The clinical efficacy of type 2 monoclonal antibodies in eosinophil-associated chronic airway diseases: a meta-analysis. Front. Immunol. 14:1089710. doi: 10.3389/fimmu.2023.1089710
Received: 04 November 2022; Accepted: 22 March 2023;
Published: 11 April 2023.
Edited by:
Elias J. Lolis, Yale University, United StatesReviewed by:
Xianwei Ye, Guizhou Provincial People’s Hospital, ChinaArzu Didem Yalcin, Academia Sinica, Taiwan
Mona Al-Ahmad, Kuwait University, Kuwait
Copyright © 2023 Wu, Huang, Zhong, Lu, Gan, Yang, Liu, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiankun Chen, Y2hlbmppYW5rdW5kb2N0b3JAMTI2LmNvbQ==; Jiqiang Li, bGlqaXFpYW5naml6aGVuQDE2My5jb20=; Yuntao Liu, aWFtbGl1eXVudGFvQDE2My5jb20=; Rongyuan Yang, eWFuZ3Jvbmd5dWFuQDE2My5jb20=; Yue Lu, Z3p5bHV5dWVAMTI2LmNvbQ==
 Yuan Wu
Yuan Wu Mengfen Huang
Mengfen Huang Jinyao Zhong1
Jinyao Zhong1 Yue Lu
Yue Lu Yuntao Liu
Yuntao Liu Jiankun Chen
Jiankun Chen