- 1Department of Microbiology, Immunology and Transplantation, Allergy and Clinical Immunology Research Group, KU Leuven, Leuven, Belgium
- 2Department of Microbiology, Immunology and Transplantation, Laboratory of Adaptive Immunology, KU Leuven, Leuven, Belgium
- 3Department of Pharmaceutical and Pharmacological Sciences, Laboratory of Molecular Virology and Gene Therapy, KU Leuven, Leuven, Belgium
- 4Department of Microbiology, Immunology and Transplantation, Laboratory of Inborn Errors of Immunity, KU Leuven, Leuven, Belgium
- 5Department of Imaging and Pathology, Translational MRI, KU Leuven, Leuven, Belgium
- 6Department of Microbiology, Immunology and Transplantation, Experimental Laboratory Immunology, KU Leuven, Leuven, Belgium
- 7Laboratory of Lymphocyte Signaling and Development, Babraham Institute, Cambridge, United Kingdom
- 8Department of Pediatrics, University Hospitals Leuven, Leuven, Belgium
- 9Department of Cardiovascular Diseases, University Hospitals Leuven, Leuven, Belgium
Autosomal dominant Signal transducer and activator of transcription 1 (STAT1) gain-of-function (GOF) mutations result in an inborn error of immunity characterized by chronic mucocutaneous candidiasis, recurrent viral and bacterial infections, and diverse autoimmune manifestations. Current treatment consists of chronic antifungal therapy, antibiotics for concomitant infections, and immunosuppressive therapy in case of autoimmune diseases. More recently, treatment with Janus kinases 1 and 2 (JAK1/2) inhibitors have shown promising yet variable results. We describe a STAT1 GOF patient with an incidental finding of elevated cardiac troponins, leading to a diagnosis of a longstanding, slowly progressive idiopathic myocarditis, attributed to STAT1 GOF. Treatment with a JAK-inhibitor (baricitinib) mitigated cardiac inflammation on MRI but was unable to alter fibrosis, possibly due to the diagnostic and therapeutic delay, which finally led to fatal arrhythmia. Our case illustrates that myocarditis could be part of the heterogeneous disease spectrum of STAT1 GOF. Given the insidious presentation in our case, a low threshold for cardiac evaluation in STAT1 GOF patients seems warranted.
Introduction
Signal transducer and activator of transcription 1 (STAT1) gain-of-function (GOF) is an inborn error of immunity, affecting more than 400 patients worldwide. Autosomal dominant gain-of-function mutations lead to hyperexpression and hyperactivation of STAT1, which is a major transcription factor playing a pivotal role in regulating a range of biological processes upon cytokine stimulation. It is the most common genetic cause of inherited chronic mucocutaneous candidiasis (CMC), but patients also suffer from recurrent infections and a broad range of autoimmune manifestations (1). Current treatment consists of chronic antifungal therapy, antibiotics for concomitant infections, and immunosuppressive therapy in case of autoimmune diseases. More recently, treatment with Janus kinases 1 and 2 (JAK1/2) inhibitors have shown promising yet variable results (2–5). We describe a STAT1 GOF patient with an incidental finding of elevated cardiac troponins, leading to a diagnosis of a longstanding, slowly progressive idiopathic myocarditis, attributed to STAT1 GOF. Treatment with a JAK-inhibitor (baricitinib) mitigated cardiac inflammation on magnetic resonance imaging (MRI) but was unable to alter fibrosis, possibly due to the diagnostic and therapeutic delay, which finally led to fatal arrhythmia.
Case description
We report a case of a 55-year-old man with a de novo congenital STAT1 gain-of-function mutation (c963A>T, p.R321S), as previously reported and validated (Figure 1A) (1, 6–8). The patient presented with chronic mucocutaneous candidiasis (CMC) (Figure 1B), complicated by esophageal stenosis, respiratory infections, and bronchiectasis. He received continuous oral antimycotics since childhood, resulting in transient resistance to ketoconazole, fluconazole and itraconazole (Supplementary E1). Genetic and molecular diagnosis (Figure 1C) of STAT1 GOF was established at an age of 48 years.
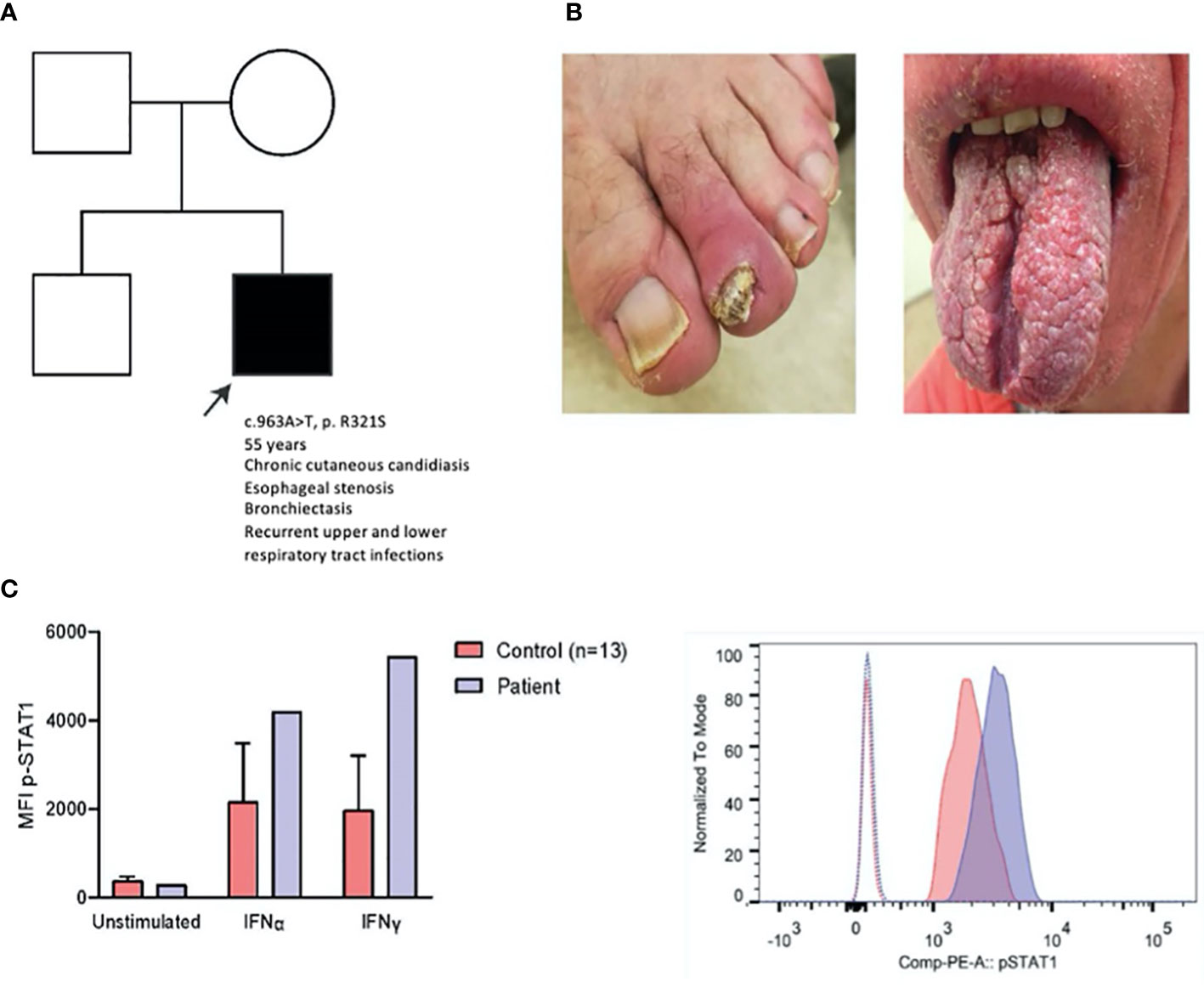
Figure 1 Clinical, genetic and laboratory findings in a patient with STAT1 GOF. (A) Patient pedigree. c.963A>T was demonstrated using Sanger sequencing (data not shown), (B) Clinical pictures of onychomycosis (left) and impact of CMC on the tongue (right), (C) p-STAT1 MFI of patient and age-matched controls (n=13) on CD14+ monocytes unstimulated and after IFNα (2000 IU/ml) and IFNγ (2000 IU/ml) stimulation for 15 minutes (left); representative histogram of p-STAT1 on CD14+ monocytes in patient and healthy control (right) after IFN-γ stimulation (15 min). MFI: mean fluorescence index.
The patient displayed stable respiratory dysfunction. Pulmonary evaluation (Supplementary E2) revealed a progressive obstructive pattern and mild diffusion defect. Chest computed tomography (CT) showed bilateral bronchiectasis. Remarkably, high-sensitivity cardiac troponins were elevated on a routine blood sampling at the age of 49, during a hospital admission for infectious enteritis (0.038 mcg/L, reference ≤ 0.013, Supplementary E2). No prior measurements were available, yet elevated troponins persisted. The patient did not report thoracic pain or other symptoms suggestive of ischemic heart disease or congestive heart failure. Blood pressure and jugular venous pressure were normal. No peripheral edema was noted. Electrocardiogram (Figure 2A) showed a pattern suggestive of right ventricular hypertrophy, biatrial enlargement and biphasic T waves in lateral leads V5-6. Transthoracic echocardiogram (TTE) showed biventricular concentric hypertrophy and mild diastolic dysfunction with increased left ventricular pressures. Cardiac MRI confirmed these findings, with increased native T1 and T2 values and enhancement of the subepicardial myocardium in the middle and apical zone of the left ventricle on late gadolinium enhancement (LGE), suggesting diffuse myocarditis (Figure 2B). Holter monitoring, performed in the context of two (pre)syncopal events, showed frequent ventricular extrasystoles and rare runs of non-sustained ventricular tachycardia (VT). Ischemic etiology was deemed unlikely based on CT imaging showing absence of coronary stenotic lesions. There were no clinical or radiological arguments for (chronic) pulmonary embolism as a cause of elevated right ventricular pressure. Also, arrhythmogenic cardiomyopathy was considered unlikely (9, 10). Whole exome sequencing for congenital cardiomyopathy was not performed (11, 12).
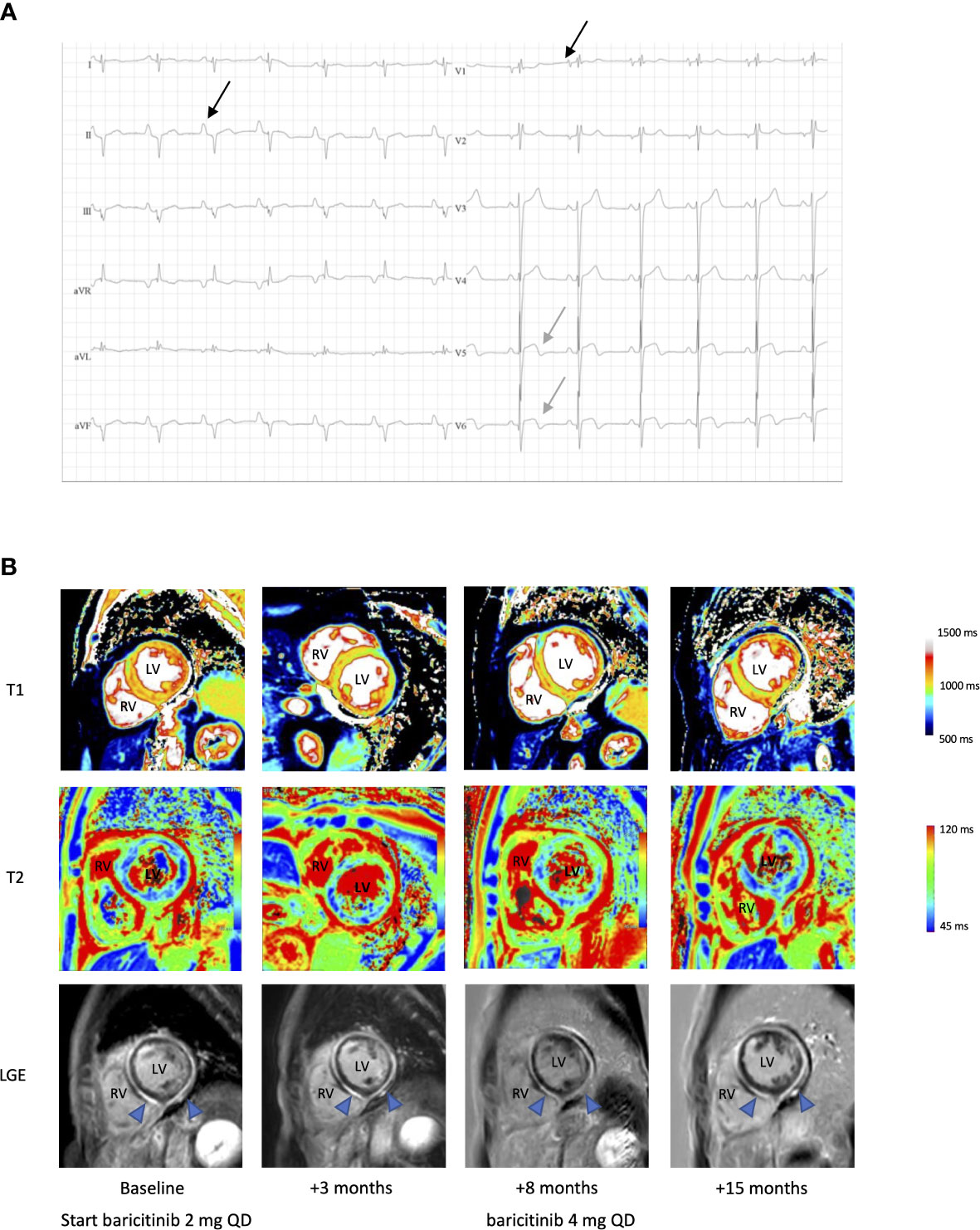
Figure 2 Electrocardiographic and radiological findings in a patient with STAT1 GOF and myocarditis. (A) ECG, showing bi-atrial enlargement (black arrow, biphasic P wave in V1 and enlarged P wave in II), extreme rightward QRS axis deviation and biphasic T waves in V5-6 (grey arrow). (B) Cardiac native T1 map, T2 map and late gadolinium enhancement (LGE) MR images pre- and post-baricitinib. LV, left ventricle; RV, right ventricle. Blue arrow in LGE showing diffuse enhanced subepicardial and myocardial enhancement compatible with active myocarditis, partial regression after 8 months of treatment, with further reduction at 15 months after a dose increase.
There were no arguments for invasive fungal infection based on repeated serum blood cultures, beta-D-glucan and aspergillus antigen measurements (Supplementary E2). PCR analysis on blood for common cardiotropic virus, including cytomegalovirus, Epstein-Barr virus, Parvovirus B19 and human herpesvirus-6 and –8 was negative rendering chronic infectious myocarditis improbable. Serology or PCR for other viral origins (enteroviral, adenoviral, human herpesvirus-7 or through microbial cell-free DNA analysis) was not accomplished. Negative autoimmune serology (Supplementary E2), together with the absence of other clinical or biochemical criteria, argued against myocarditis in the context of well-defined autoimmune diseases, although STAT1 GOF is associated with an increased likelihood for autoimmunity (1). Finally, drug-induced or hypersensitivity myocarditis was deemed improbable. Therefore, the tentative diagnosis of idiopathic myocarditis with unknown onset, presumably immune-mediated in the context of STAT1 GOF was made. No endomyocardial biopsy (EMB) was performed, considering the inherent risk of the procedure and the low probability of chronic viral induced myocarditis (13). Cardiac transplantation or an implantable cardioverter-defibrillator was refused after a multidisciplinary consult. During follow-up, left ventricular function decreased to a nadir left ventricular ejection fraction (LVEF) of 41% on TTE and 30% on MRI (Supplementary E2), with persistently active myocarditis on cardiac MRI. Conventional treatment for heart failure with reduced ejection fraction was initiated, including angiotensin converting enzyme inhibition (perindopril 5 mg QD), diuretic treatment (bumetanide 1 mg QD) and a beta blocker (carvedilol 6.25 mg BID). Initially no clinical deterioration was observed except for one episode of cardiac decompensation triggered by a diverticulitis. Because of active myocarditis with profound effects on systolic function, baricitinib, a JAK1/2 inhibitor, was started at a daily dose of 2 mg. After 3 and 8 months of treatment, partial regression of myocarditis was noted on MRI (Figure 2B). The patient reported stable exertional dyspnea and improvement of CMC was noted within 3 months of treatment initiation. During this period, there were no major infectious events or need for additional antibiotic treatment. After 8 months, baricitinib was increased to 4 mg daily, with further reduction of cardiac inflammation on MRI at 15 months. However, myocardial fibrosis already occurred, probably due to longstanding inflammation and resulted in persistent biventricular systolic failure. Unfortunately, shortly thereafter, the patient died from a cardiac arrest precipitated by a witnessed VT. Obduction was not performed.
Discussion
We report a STAT1 GOF patient with an incidental finding of elevated cardiac troponins due to myocarditis. The presenting symptom was a stable respiratory dysfunction which was initially attributed to recurrent respiratory tract infections and mild abnormalities on pulmonary function tests. Sporadic myocarditis has been associated with inborn errors of immunity (IEI) either in the context of infectious susceptibility or as an autoimmune manifestation (14, 15). Additionally, iatrogenic causes and direct molecular effects of the inborn error could potentially link myocarditis with IEI. However, to our knowledge, myocarditis in the context of STAT1 GOF has not been previously reported. Here, autoimmune myocarditis, as part of the spectrum of autoimmune manifestations in STAT1 GOF, or a myocarditis directly related to STAT1 GOF, was inferred.
Diagnosis was established based on cardiac MRI. This is the preferred diagnostic tool in stable patients with myocarditis as gadolinium contrast enhanced cardiac MRI can identify myocardial edema and fibrosis with high sensitivity (16). ECG findings such as sinus tachycardia with non-specific ST segment and T wave abnormalities can be present but their sensitivity is low (47%) (17). Inverted T waves in the lateral leads were present in our case, prompting a cardiac evaluation.
Treatment of myocarditis is supportive and the use of antiviral or immunomodulatory drugs including intravenous immunoglobulins for viral-induced or autoimmune myocarditis remains debated (16, 18). Interestingly, the use of JAK-inhibitors has shown a beneficial effect in selected cases of immune-mediated myocarditis associated with immune-checkpoint inhibitors (19, 20) and chronic graft versus host disease outside the context of STAT1 GOF (21). As reported in other cases of STAT1 GOF, ruxolitinib and baricitinib, both acting as JAK1/2 inhibitor, show promising results for improving both CMC as well as autoimmune manifestations (2–5). Importantly, not all patients are responsive and in some patients re-initiation after treatment interruption showed reduced efficacy. Moreover, an increased susceptibility to viral infections has been reported in these cases. Although the in vitro STAT1 phosphorylation index after stimulation with IFN-alpha and IFN-gamma initially normalized in our patient, considerable variation was observed during follow-up, despite good adherence (Supplementary E3). These in vitro findings might not serve as an ideal marker for treatment responses as mentioned earlier (4). Until further prospective multicenter data is available to guide the clinical use of this treatment, a careful risk-benefit analysis should be made on a case-by-case basis to evaluate the applicability of JAK inhibition. In this patient, baricitinib was started because of progressing active myocarditis, with increasing risk of end stage heart failure and sudden cardiac death. Treatment did not ameliorate clinical symptoms but led to a regression of active myocarditis on MRI, although biventricular failure persisted due to myocardial fibrosis, leading to fatal arrhythmia. Our patient had limited initial symptoms. Therefore, a low threshold for cardiac evaluation seems warranted in STAT1 GOF patients, especially when symptomatic.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics committee of University Hospitals Leuven (s58466). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
RS and LV were involved in the clinical care of the patient. XB and GF were responsible for the laboratory analysis. JB analyzed the MRI images. FS and RS planned the case report and initiated the first draft. All authors contributed to the article and approved the submitted version.
Funding
FS (11B5520N), SG (1S23017N), CH (1S07023N) are fellows of the Fonds Wetenschappelijk Onderzoek - Vlaanderen National Fund for Scientific Research (FWO). RS and IM are FWO senior clinical investigator fellow (1805518N and 1805523N, 1805821N). XB, AL, SH-B, IM, and RS are supported by the VIB Grand Challenge program (Translational science initiative on PID, GC01-C01). RS is supported by the FWO project financing (G054022N). IM and RS are members of the European Reference Network for Rare Immunodeficiency, Autoinflammatory and Autoimmune Diseases (Project ID No 739543).
Acknowledgments
We would like to thank Prof. Jan Ceuppens for the clinical care of this patient.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1095595/full#supplementary-material
References
1. Toubiana J, Okada S, Hiller J, Oleastro M, Gomez ML, Becerra JCA, et al. Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood (2016) 127(25):3154–64. doi: 10.1182/blood-2015-11-679902
2. Higgins E, Al Shehri T, McAleer MA, Conlon N, Feighery C, Lilic D, et al. Use of ruxolitinib to successfully treat chronic mucocutaneous candidiasis caused by gain-of-function signal transducer and activator of transcription 1 (STAT1) mutation. J Allergy Clin Immunol (2015) 135(2):551–553.e3. doi: 10.1016/j.jaci.2014.12.1867
3. Forbes LR, Vogel TP, Cooper MA, Castro-Wagner J, Schussler E, Weinacht KG, et al. Jakinibs for the treatment of immune dysregulation in patients with gain-of-function signal transducer and activator of transcription 1 (STAT1) or STAT3 mutations. J Allergy Clin Immunol (2018) 142(5):1665–9. doi: 10.1016/j.jaci.2018.07.020
4. Deyà-Martínez A, Rivière JG, Roxo-Junior P, Ramakers J, Bloomfield M, Guisado Hernandez P, et al. Impact of JAK inhibitors in pediatric patients with STAT1 gain of function (GOF) mutations–10 children and review of the literature. J Clin Immunol (2022) 42(5):1071. doi: 10.1007/s10875-022-01257-x
5. Meesilpavikkai K, Dik WA, Schrijver B, Nagtzaam NMA, Posthumus-van Sluijs SJ, van Hagen PM, et al. Baricitinib treatment in a patient with a gain-of-function mutation in signal transducer and activator of transcription 1 (STAT1). J Allergy Clin Immunol (2018) 142(1):328–330.e2. doi: 10.1016/j.jaci.2018.02.045
6. Okada S, Asano T, Moriya K, Boisson-Dupuis S, Kobayashi M, Casanova JL, et al. Human STAT1 gain-of-function heterozygous mutations: Chronic mucocutaneous candidiasis and type I interferonopathy. J Clin Immunol (2020) 40(8):1065–81. doi: 10.1007/s10875-020-00847-x
7. Giovannozzi S, Lemmens V, Hendrix J, Gijsbers R, Schrijvers R. Live cell imaging demonstrates multiple routes toward a STAT1 gain-of-Function phenotype. Front Immunol (2020) 11:1114. doi: 10.3389/fimmu.2020.01114
8. Giovannozzi S, Demeulemeester J, Schrijvers R, Gijsbers R. Transcriptional profiling of STAT1 gain-of-Function reveals common and mutation-specific fingerprints. Front Immunol (2021) 12:632997. doi: 10.3389/fimmu.2021.632997
9. Aquaro GD, de Luca A, Cappelletto C, Raimondi F, Bianco F, Botto N, et al. Prognostic value of magnetic resonance phenotype in patients with arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol (2020) 75(22):2753–65. doi: 10.1016/j.jacc.2020.04.023
10. Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCC, et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm (2019) 16(11):e301–72. doi: 10.1016/j.hrthm.2019.05.007
11. Ammirati E, Raimondi F, Piriou N, Sardo Infirri L, Mohiddin SA, Mazzanti A, et al. Acute myocarditis associated with desmosomal gene variants. JACC. Heart Failure (2022) 10(10):714–27. doi: 10.1016/j.jchf.2022.06.013
12. Bourfiss M, van Vugt M, Alasiri AI, Ruijsink B, Setten J, van Schmidt AF, et al. Prevalence and disease expression of pathogenic and likely pathogenic variants associated with inherited cardiomyopathies in the general population. Circulation Genomic Precis Med (2022) 15(6):e003704. doi: 10.1161/CIRCGEN.122.003704
13. Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: A scientific statement from the American heart association, the American college of cardiology, and the European society of cardiology endorsed by the heart failure society of America and the heart failure association of the European society of cardiology. Eur Heart J (2007) 28(24):3076–93. doi: 10.1093/eurheartj/ehm456
14. Gorbea C, Makar KA, Pauschinger M, Pratt G, Bersola JLF, Varela JD, et al. A role for toll-like receptor 3 variants in host susceptibility to enteroviral myocarditis and dilated cardiomyopathy. J Biol Chem (2010) 285(30):23208–23. doi: 10.1074/jbc.M109.047464
15. Laufs H, Nigrovic PA, Schneider LC, Oettgen H, del Nido P, Moskowitz IPG, et al. Giant cell myocarditis in a 12-Year-Old girl with common variable immunodeficiency. Mayo Clinic Proc (2002) 77(1):92–6. doi: 10.4065/77.1.92
16. Caforio ALP, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European society of cardiology working group on myocardial and pericardial diseases. Eur Heart J (2013) 34(33):2636–48. doi: 10.1093/eurheartj/eht210
18. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC). developed with the special contribution. Eur J Heart Failure (2016) 18(8):891–975. doi: 10.1002/ejhf.592
19. Liu Y, Jiang L. Tofacitinib for treatment in immune-mediated myocarditis: The first reported cases. J Oncol Pharm Pract (2020) 27(3):739–46.
20. Nguyen LS, Bretagne M, Arrondeau J, Zahr N, Ederhy S, Abbar B, et al. Reversal of immune-checkpoint inhibitor fulminant myocarditis using personalized-dose-adjusted abatacept and ruxolitinib: Proof of concept. J Immunother Cancer (2022) 10(4):e004699. doi: 10.1136/jitc-2022-004699
Keywords: primary immunodeficiencies, STAT1 GOF, myocarditis, infectious susceptibility, chronic mucocutaneous candidiasis
Citation: Staels F, Roosens W, Giovannozzi S, Moens L, Bogaert J, Iglesias-Herrero C, Gijsbers R, Bossuyt X, Frans G, Liston A, Humblet-Baron S, Meyts I, Van Aelst L and Schrijvers R (2023) Case report: Myocarditis in congenital STAT1 gain-of function. Front. Immunol. 14:1095595. doi: 10.3389/fimmu.2023.1095595
Received: 11 November 2022; Accepted: 07 March 2023;
Published: 20 March 2023.
Edited by:
Jacinta Bustamante, Université Paris Cité, FranceReviewed by:
Troy R. Torgerson, Allen Institute for Immunology, United StatesOlaf Neth, Institute of Biomedicine of Seville (CSIC), Spain
Copyright © 2023 Staels, Roosens, Giovannozzi, Moens, Bogaert, Iglesias-Herrero, Gijsbers, Bossuyt, Frans, Liston, Humblet-Baron, Meyts, Van Aelst and Schrijvers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rik Schrijvers, cmlrLnNjaHJpanZlcnNAdXpsZXV2ZW4uYmU=
 Frederik Staels
Frederik Staels Willem Roosens
Willem Roosens Simone Giovannozzi
Simone Giovannozzi Leen Moens
Leen Moens Jan Bogaert
Jan Bogaert Cecilia Iglesias-Herrero1,3
Cecilia Iglesias-Herrero1,3 Rik Gijsbers
Rik Gijsbers Xavier Bossuyt
Xavier Bossuyt Glynis Frans
Glynis Frans Adrian Liston
Adrian Liston Stephanie Humblet-Baron
Stephanie Humblet-Baron Isabelle Meyts
Isabelle Meyts Lucas Van Aelst
Lucas Van Aelst Rik Schrijvers
Rik Schrijvers