- 1Blood Diseases Institute, Xuzhou Medical University, Xuzhou, Jiangsu, China
- 2Department of Hematology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China
- 3Key Laboratory of Bone Marrow Stem Cell, Xuzhou, Jiangsu, China
- 4Cancer Institute, Xuzhou Medical University, Xuzhou, Jiangsu, China
- 5Jiangsu Center for the Collaboration and Innovation of Cancer Biotherapy, Cancer Institute, Xuzhou, Medical University, Xuzhou, Jiangsu, China
- 6Center of Clinical Oncology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China
Introduction: Chimeric antigen receptor (CAR) T cell therapy has achieved unprecedented efficacy recently. However, the factors related to responses and durable remission are elusive. This study was to investigate the impact of pre-lymphodepletion (pre-LD) absolute lymphocyte count (ALC) on CAR T cell therapy outcomes.
Methods: We conducted a retrospective study of 84 patients with relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL) who underwent CAR T cell treatment at the Affiliated Hospital of Xuzhou Medical University between March 1,2016 and December 31, 2021. The enrolled patients were divided into high group and low group according to the optimal cutoff value of pre-LD ALC. The Kaplan-Meier analyses was used to calculate survival curves. The Cox proportional hazards model was used for univariate and multivariate analysis to assess the prognostic factors.
Results: The ROC showed that the optimal cutoff value of pre-LD ALC was 1.05 x 109/L. The overall response (defined as partial response or complete response) rate was significantly higher in patients with a high pre-LD ALC (75% versus 52.08%; P=0.032). Patients with a low pre-LD ALC had significantly inferior overall survival (OS) and progression-free survival (PFS) compared with those having a high pre-LD ALC (median OS, 9.6 months versus 45.17 months [P=0.008]; median PFS, 4.07 months versus 45.17 months [P= 0.030]). Meanwhile, low pre-LD ALC is an independent risk factor for PFS and OS.
Discussion: The data suggested that pre-LD ALC may serve as a helpful indicator to predict the outcomes of CAR T cell therapy in patients with R/R DLBCL.
Introduction
With a prevalence of about 24% non-Hodgkin’s lymphomas (NHL), diffuse large B-cell lymphoma (DLBCL) is a common lymphoma group whose prevalence increases with age (1). DLBCL has achieved a high cure rate in combination therapy with Rituximab-based treatment. However, there are approximately 10%-15% of patients no response, and 20%-30% of patients relapse (2–4). CAR T cell therapy has achieved significant efficacy in clinical trials targeting R/R B-NHL (5). At present, the two FDA (Food and Drug Administration)-approved anti-CD19 CAR products axicabtagene ciloleucel (6) and tisagenle cleucel (7), have achieved 52%-82% overall response rates (ORR) and 40%-54% complete remission rate (CRR) in DLBCL.
Studies have confirmed that the higher peak of blood CAR T cells expansion in vivo is a crucial indicator of therapeutic efficacy and associated with the persistence of responses. The persistence and function of CAR T cells are also correlated with the immunophenotype of T cell subsets (8, 9). Jacobson et al. (10) found that high c-reactive protein (CRP) levels on day 0 after CAR T cells infusion and larger tumor volume (>5cm) were associated with poor prognosis. However, no association was found between International Prognostic index (IPI), double or triple hit lymphoma, number of previous treatment lines, bridging therapy, cytokine release syndrome (CRS) or neurotoxicity, use of tocilizumab or steroid and efficacy in this study related to axicabtagene ciloleucel. Regrettably, the existing predictors are too less and not sufficiently convenient and economical. This current compels us to find more convenient indicators to identify which patients can most benefit from CAR T cell treatment.
The immune status is closely related to the outcome and prognosis of the disease. ALC, which represents the immune status of patients, is a prognostic factor of a variety of hematologic malignancies (11–13). Previous findings have demonstrated that lymphopenia is a poor prognostic indicator for solid malignancies (14) as well as hematological malignancies such as lymphoma (15) and acute myeloid leukemia (AML) (16). A meta-analysis of six studies involving 1206 patients with DLBCL confirmed the adverse effect of low ALC in patients who received CHOP combined with or without rituximab (17). Yet, whether pre-lymphodepletion (pre-LD) ALC could be a predictor in R/R DLBCL patients receiving CAR T cell therapy remains unclear. In this study, we assessed the impact of pre-LD ALC on the prognosis of 84 patients with R/R DLBCL treated with CAR T cells.
Methods
Patient selection
This is a retrospective study. 84 patients with R/R DLBCL were enrolled in the Affiliated Hospital of Xuzhou Medical University between March 1,2016 and December 31, 2021 (ChiCTR 2100049709). All enrolled patients met the 2016 World Health Organization (WHO) diagnostic criteria of the lymphoid neoplasms (18). Patients who were pregnant, nursing, or likely to become pregnant and those with mental or psychological illnesses, severe allergies, a history of severe allergies were excluded. Clinical data involved age, gender, Ann Arbor stage, IPI, previous therapies, complete blood count and lactate dehydrogenase (LDH) before lymphocyte depletion. The clinical trial has been approved by the Institutional Review Committee of the Affiliated Hospital of Xuzhou Medical University in accordance with the provisions of the Helsinki Declaration. All enrolled patients or their family members signed an informed consent form prior to the study.
CAR T cell manufacture and infusion
Peripheral blood mononuclear cells were collected by COMTEC hemocytometer (Fresenius Kabi [China] Investment Co, LTD.) for anti-CD19, anti-CD20 and anti-CD22 CAR T cells generation. Before CAR T cells infusion, 82 patients received lymphodepletion chemotherapy consisting of fludarabine (3 daily doses of 30mg/m2 on days -5 to -3) and cyclophosphamide (1 daily dose of 750 mg/m2 on day -5). Anti-CD19 CAR T cells alone or in combined with anti-CD22 or anti-CD20 CAR T cells were infused into the enrolled patients on day 0.
Evaluation criteria for efficacy and adverse reactions
All patients were evaluated according to the 2014 Lugano efficacy evaluation criteria (19). Responses were assessed as complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD). The overall response rate (ORR) was defined as ORR=CR+PR rates. Blood pressure, respiration, heart rate and other vital signs were detected during cell infusion. The main adverse reactions were CRS, which graded and managed according to the recommendations of Lee et al. (20).
Followed-up
The follow-up included an outpatient follow-up, a telephone follow-up and access to hospital records. The follow-up period was until December 31, 2021. PFS was defined as the time from CAR T cell infusion to disease progression, relapse or death. OS was determined as the time from CAR T cell infusion to death from any cause, loss of visit or final follow-up.
Statistical analysis
All data were analyzed using SPSS (version 25.0, SPSS Inc. Chicago, IL, USA). Categorical variables were analyzed using Pearson chi-square test, which is expressed as percentage. Two independent samples T test was used for two normally distributed continuous independent variables expressed as mean ± standard deviation while Mann-Whitney rank sum test was used for that not normally distributed variables expressed as the median and quartile. Kaplan-Meier analyses were used to describe survival curve. Cox proportional hazards model was used for univariate and multivariate analysis of PFS and OS. All tests were 2-sided, and a P value <.05 indicated a significant difference.
Results
Patient characteristics
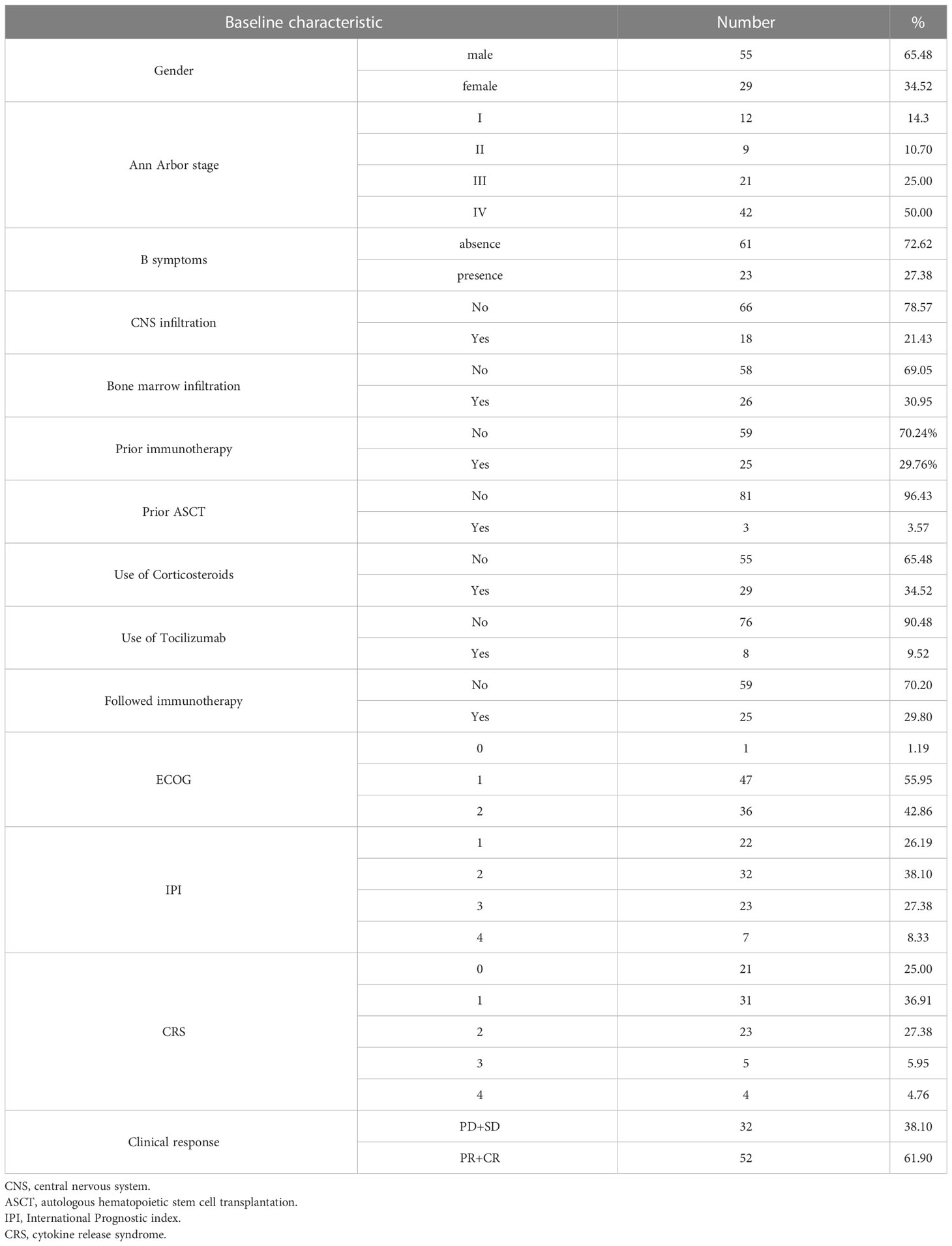
A total of 84 patients with R/R DLBCL were enrolled, and the basic characteristics are shown in Table 1. Fifty-five patients were male and twenty-nine patients were female. The median age was 51 years (range, 21 to 72 years) and 50% patients were over 60 years old. The median time from diagnosis to enrollment was 15.27 months. Three patients had undergone previous autologous hematopoietic stem cell transplantation (ASCT) and twenty-five had previous immunotherapy. There were fifty patients with elevated LDH. Sixty-three patients (75%) were in stage III or IV status and twenty-three patients (27.38%) had B symptoms according to the Ann Arbor clinical stage. Thirty-six patients (42.86%) had ECOG score of 2, and 30 patients (35.71%) had IPI score in medium-high risk to high-risk group. Thirty-one patients received anti-CD19 and anti-CD22 CAR T cell infusion; seventeen patients received anti-CD19 and anti-CD20 CAR T cell infusion; and thirty-six patients received anti-CD19 CAR T cell infusion alone.
Factors associated with pre-LD ALC
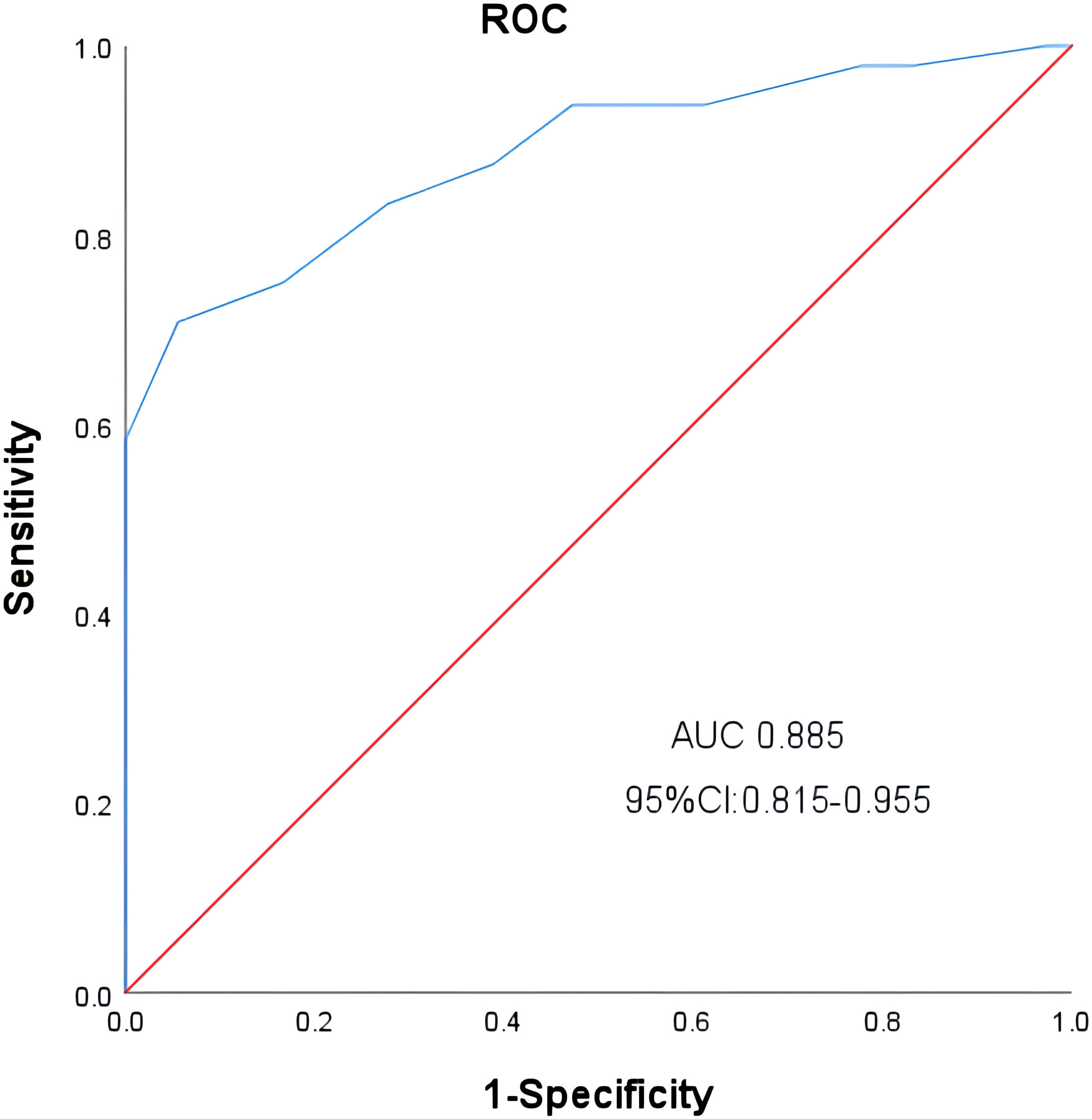
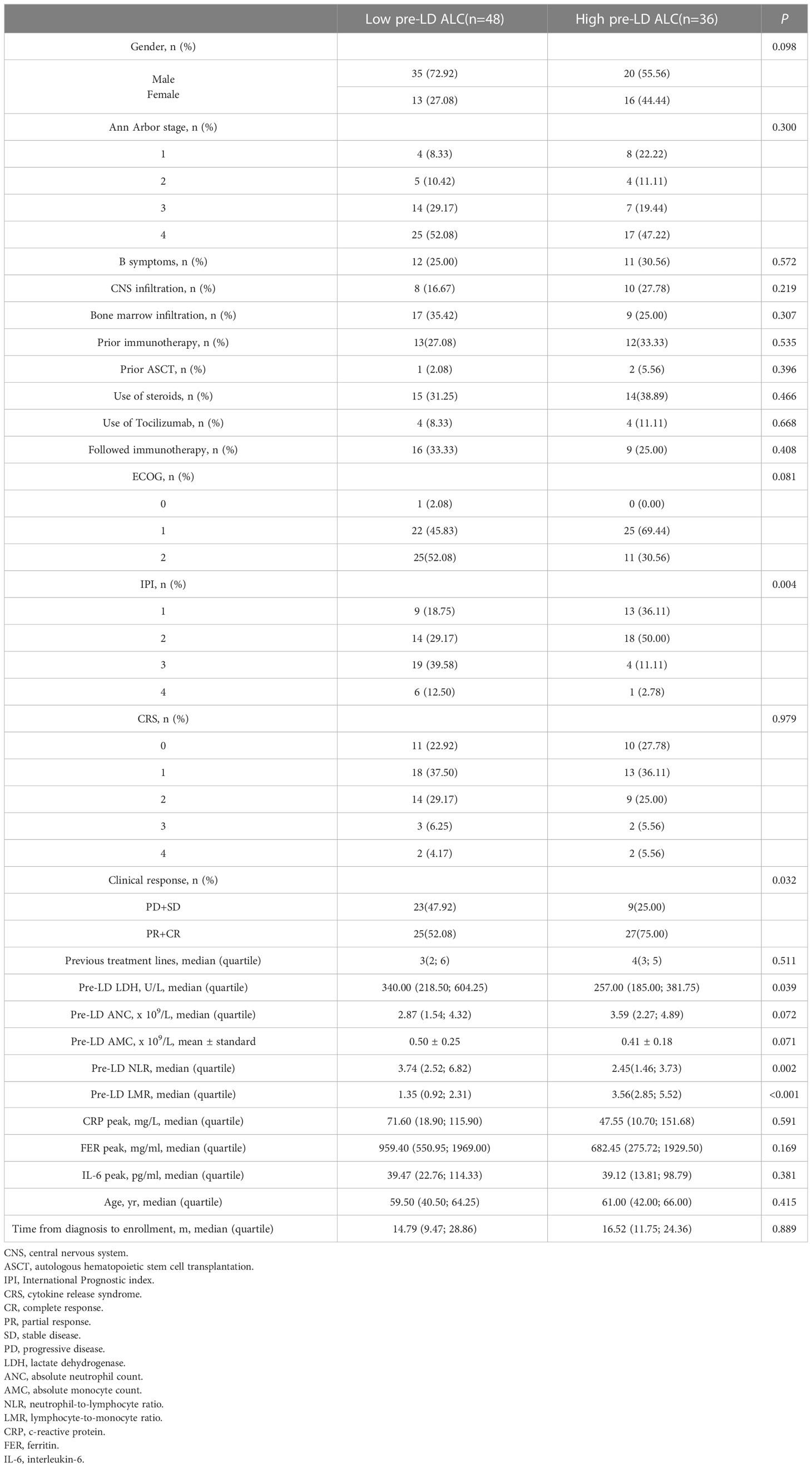
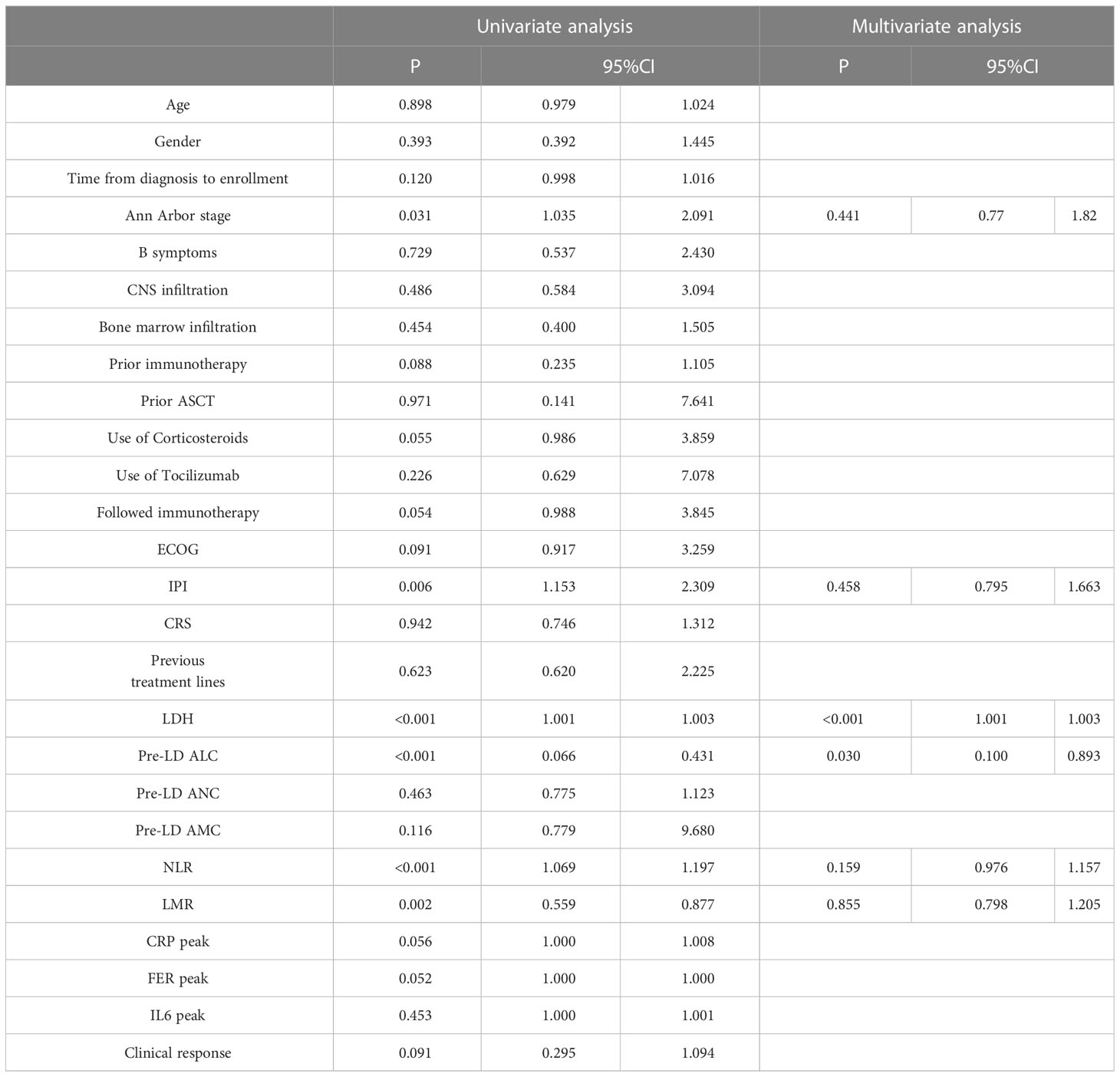
We obtained the optimal cutoff value of pre-LD ALC by receiver operating characteristic curve. The cutoff value was 1.05 x 109/L (sensitivity, 0.708; specificity, 0.944; area under the curve, 0.885; 95% confidence interval [CI], 0.815-0.955) (Figure 1). According to the optimal cut-off value of ALC, 48 patients were included in the low ALC group (<1.05 x 109/L), and 36 patients were included in the high ALC group (≥1.05 x 109/L). Compared to the high pre-LD ALC group, the low group had more patients with higher IPI score, worse clinical response, higher LDH and neutrophil-to-lymphocyte ratio (NLR), lower lymphocyte-to-monocyte ratio (LMR). There were statistically significant differences on IPI (p = 0.004), clinical response (p = 0.032), LDH (p = 0.039), NLR (p = 0.002), LMR (p<0.001). On the contrary, the two groups’ age, gender, ECOG, Ann Arbor stage, B symptoms, use of corticosteroids or tocilizumab etc. had no significant difference (Table 2). Particularly worth mentioning is that we did not find the relationship between pre-LD ALC and CRS (Table 2).
Survival analysis based on pre-LD ALC
In low pre-LD ALC group, the median PFS was 4.07 and the median OS was 9.6; In high pre-LD ALC group, the median PFS was 45.17 and the median OS was also 45.17. The results showed that OS (P< 0.001) and PFS (P< 0.001) in the low group were significantly shorter than those in the high group (P< 0.001) (Figures 2A, B) according to Kaplan-Meier survival curve.
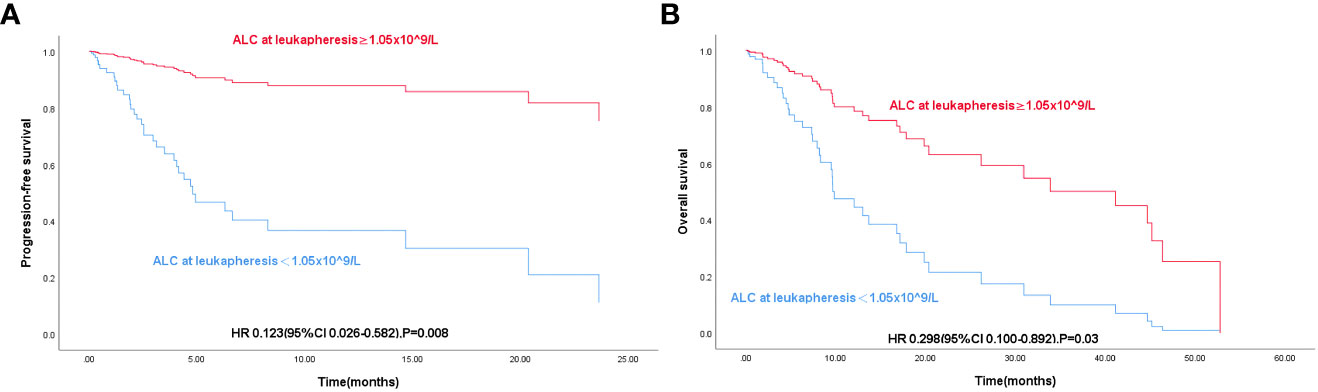
Figure 2 (A) K-M curve of progression-free survival in 84 patients with R/R DLBCL according to Pre-LD ALC grouping. (B) K-M curve of overall survival in 84 patients with R/R DLBCL according to Pre-LD ALC grouping.
Univariate and multivariate analysis of PFS and OS
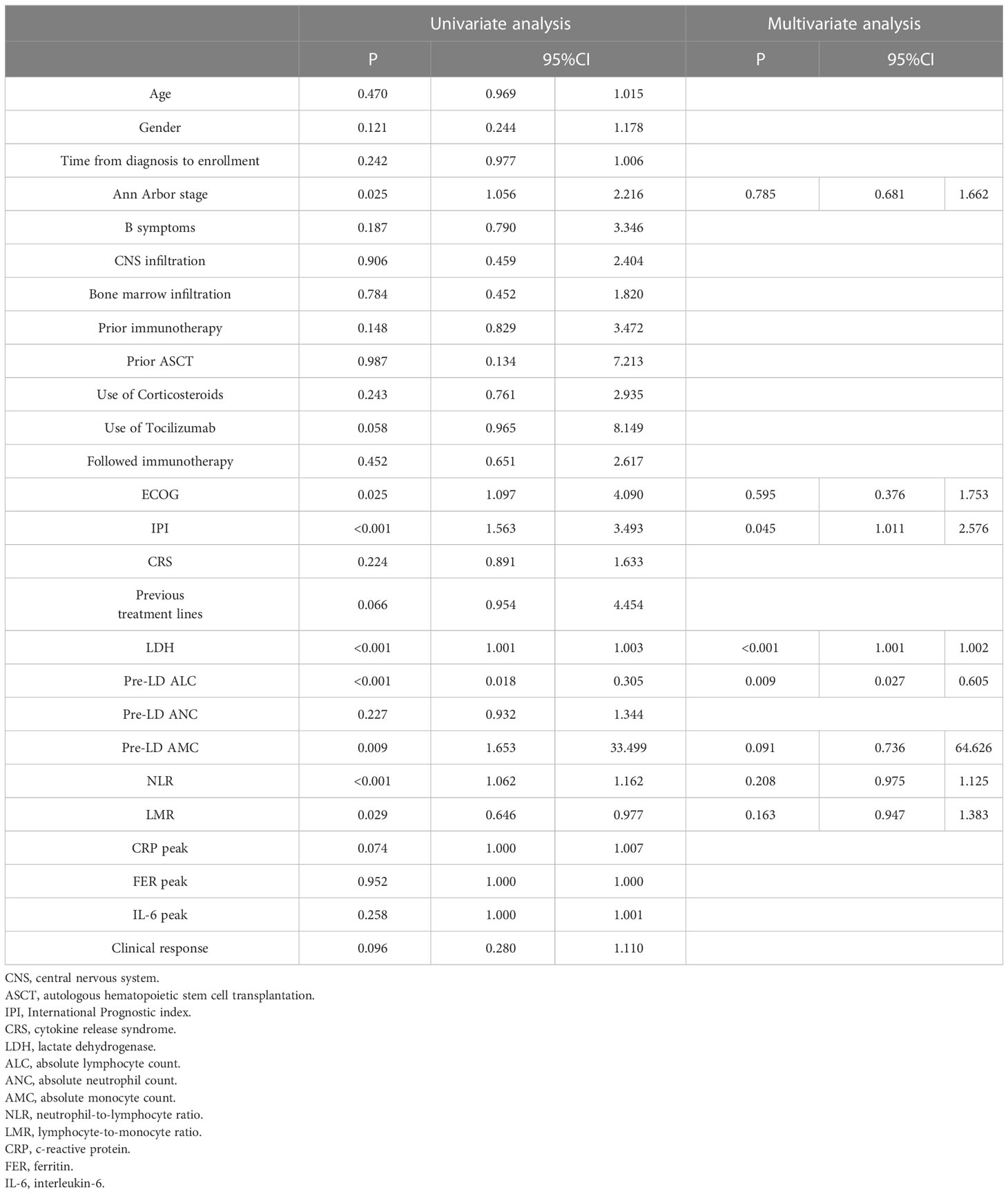
By cox univariate regression analysis, we found that Ann Arbor stage, ECOG score and IPI score, increased LDH, ALC < 1.05 x 109/L, increased NLR and decreased LMR were risk factors affecting OS and PFS in DLBCL patients (P < 0.05) (Tables 3, 4). In addition, PFS was also affected by increased monocyte (AMC) (P < 0.05) (Table 3). The significant variables in univariate regression analysis were included in the multivariate analysis model, and the results showed that elevated LDH (HR = 1.002, 95%CI: 1.001-1.003, P < 0.001) and ALC < 1.05 x 109/L (HR = 0.299, 95%CI: 0.100-0.893, P = 0.030) were an independent risk factor for OS in patients with DLBCL (Table 4). IPI score (HR = 1.614, 95%CI: 1.011-2.576, P = 0.045), elevated LDH (HR = 1.002, 95%CI: 1.001-1.002, P< 0.001) and ALC < 1.05 x 109/L (HR = 0.129, 95%CI: 0.027-0. 605, P = 0.009) were independent risk factors for PFS in patients with DLBCL (Table 3).
Discussion
DLBCL is a hematological malignancy with high heterogeneity. IPI, as the scoring system based on clinical and pathological features, is widely used to evaluate the prognosis of patients with DLBCL. However, it cannot really reflect the heterogeneity of cell morphology, immunophenotype and molecular biology in DLBCL patients, and the prognosis varies dramatically in patients with the same IPI score. Currently, finding new prognostic indicators with high accuracy and specificity is a hot spot in clinical diagnosis and treatment of DLBCL (21, 22).
It has been shown that the tumor microenvironment (TEM), which is composed of immune cells, stromal cells, and blood vessels, is crucial to the pathogenesis and clinical course of DLBCL. The tumor microenvironment can effectively identify tumors, and provide protection for tumor cells, promoting their growth and survival (23, 24). Also, it can help cancer cells escape host immune surveillance due to its interaction with lymphoma cells (25). Lymphocyte, an immune cell in TEM, has been shown to influence lymphoma patient survival. It plays a significant role in the immune surveillance in tumor patients and its depletion has been proved to be a key marker of host immunodeficiency (26). Studies (27–29) have shown that ALC can be used as an independent prognostic factor in leukemia, breast cancer, multiple myeloma, as well as other malignant tumors.
In this study, 57.0% of 84 patients with R/R DLBCL showed a decrease in ALC before lymphodepletion, possibly due to the toxic lytic effect of cytokines secreted by lymphoma cells on lymphocytes. We observed an ORR of 61.9%, which is similar to the known findings (7). High pre-LD ALC was associated with higher ORR and longer survival. Previous studies (30, 31)have shown that decreased ALC and B symptoms have been reported as poor prognostic indicators for NHL, which are independent of IPI and treatment regimen. We found PFS (P < 0.001) and OS (P < 0.001) in the high pre-LD ALC group were significantly longer than those in the low pre-LD ALC group, suggesting that pre-LD ALC is closely related to the prognosis of DLBCL patients and patients with low pre-LD ALC had a poor prognosis. In order to further explore the independent prognostic value of ALC, we then performed COX regression analysis. In univariate survival analysis, low LMR and pre-LD ALC < 1.05 x 109/L were risk factors for OS and PFS. But low LMR lost its prognostic value in multivariate analysis. Only pre-LD ALC < 1.05 x 109/L was an independent risk factor for OS and PFS in patients with DLBCL treated with CAR T cell. The reason may be that the immune response during chemotherapy is relatively weak in those with low lymphocytes, and the killing effect on tumor cells is small. NLR and LMR, as inflammatory biomarkers, represent the balance between the host immune system and TEM, and their changes will affect the normal immune response, immune prevention and other important functions (32, 33). Nevertheless, the synergistic effect of lymphocyte, monocyte and neutrophil may affect the predictive ability of LMR and NLR, which explains why their prognostic value were lost in the multifactorial analysis. In addition, we found that there were no statistically significant differences in Ann Arbor stage, age, B symptoms, ECOG, etc. between the two groups divided by pre-LD ALC of 1.05 x 109/L. The reason may be that lymphocyte immunoregulation is impaired, thus affecting the immune suppression and immune killing of lymphoma cells.
Since its formal introduction in 1993, IPI has been widely used to evaluate risk stratification of DLBCL for nearly 30 years. According to five indicators including age, LDH, ECOG score, Ann Arbor stage, and number of extra nodal involvement, patients are divided into four risk groups: low-risk, intermediate-low risk, intermediate-high risk, and high-risk. The 5-year OS rates were 84.5%, 70.1%, 53.1% and 64.2%, respectively (34). However, in an analysis of the prognosis of patients in the R-CHOP regimen group, Ngo (35)concluded that there was no significant difference in the 5-year OS rates between the low-risk groups and the intermediate-low risk groups, and between the intermediate-high risk groups and the high-risk groups, suggesting that in the era of rituximab, IPI was not sufficient to distinguish DLBCL with different prognosis. Likewise, IPI was not sufficient to predict OS independently in our study. As for malignant neoplastic diseases, malignant proliferation of tumor cells requires a large amount of energy consumption causing an increased glycolytic response, which in turn causes an increase LDH synthesis in patients (36). Excessive LDH has long been shown to be associated with poor prognosis in DLBCL, and similarly, elevated LDH was an independent risk factor for OS and PFS in our research. Whether ALC can be combined with IPI to form a new prognostic scoring system also requires further research and external validation.
The most common adverse effects of CAR T cell therapy are CRS and neurotoxic reactions. CRS are usually associated with cytokine levels, tumor burden and number of CAR T cell infusion and expansions. We have not found a correlation between Pre LD ALC and CRS. Previous studies have reported that the incidence of CRS in R/R B-NHL treated with CAR T cell ranged from 37% to 93% (37). Also, the occurrence of CRS can indicate the response to CAR T cell therapy to some extent. Patients without CRS often have the worse efficacy (38). In this study, 75% patients occurred CRS, mainly grade 1, which may be related to the fact that we reduced the tumor burden of patients as far as possible before infusion, such as FC regimen pretreatment and controlling the number of cell infusion. Corticosteroids attenuate toxicity by inhibiting CAR T cell proliferation and inflammatory cytokine production, but their effect on CAR T cell expansion and durable response remains unknown (39). In ZUMA-1 study (6), tocilizumab and corticosteroids were used primarily for grade ≥3 CRS and neurotoxic reactions, and the use of them did not seem to influence ORR or sustained response. In our univariate and multivariate survival analyses, no effect of tocilizumab or corticosteroid use on survival was found (P > 0.05). Nonetheless, the dose and duration of corticosteroid and tocilizumab after CAR T cell therapy remain clinically unanswered challenge.
This study has some limitations, such as single-center study, small sample size, short follow-up time. Therefore, studies with more samples are needed to confirm the potential prognostic value of pre-LD ALC in CAR T cell therapy. At the same time, external verification may improve our confidence in drawing solid conclusions.
In conclusion, our findings indicate that low pre-LD ALC is associated with inferior outcomes in R/R DLBCL patients treated with CAR T cells. Pre-LD ALC can serve as an independent prognostic indicator for these patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the ethics committee of the Affiliated Hospital of Xuzhou Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YYL, HZ, YL, and ZL proposed the project. ZL, KX, and JZ supervised the clinical study. YW, YS, HC, ZY, JC, WS, FZ, DL, and HS were responsible for patient recruitment. YYL and HZ collected and analyzed data, wrote the manuscript. YL, YS, YW, HC, ZY, JZ, KX, and ZL helped revise the manuscript. All authors discussed the data and the analytic methods and contributed to the final draft. YYL, HZ, and YL contributed equally to this work and should be considered co-first authors. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81770223).
Acknowledgments
We thank study participants and their families and staff of the Department of Hematology, the Affiliated Hospital of Xuzhou Medical University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1155216/full#supplementary-material
References
1. Liu Y, Barta SK. Diffuse large b-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol (2019) 94(5):604–16. doi: 10.1002/ajh.25460
2. Chaganti S, Illidge T, Barrington S, Mckay P, Linton K, Cwynarski K, et al. Guidelines for the management of diffuse large b-cell lymphoma. Br J Haematol (2016) 174(1):43–56. doi: 10.1111/bjh.14136
3. Coiffier B, Sarkozy C. Diffuse large b-cell lymphoma: r-CHOP failure-what to do? hematology. Am Soc Hematol Educ Program (2016) 2016(1):366–78. doi: 10.1182/asheducation-2016.1.366
4. Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR, et al. 2016 US Lymphoid malignancy statistics by world health organization subtypes. CA: Cancer J Clin (2016) 66(6):443–59. doi: 10.3322/caac.21357
5. Chavez JC, Locke FL. CAR T cell therapy for b-cell lymphomas. Best Pract Res Clin Haematol (2018) 31(2):135–46. doi: 10.1016/j.beha.2018.04.001
6. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory Large b-cell lymphoma. New Engl J Med (2017) 377(26):2531–44. doi: 10.1056/NEJMoa1707447
7. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse Large b-cell lymphoma. New Engl J Med (2019) 380(1):45–56. doi: 10.1056/NEJMoa1804980
8. Cappell KM, Sherry RM, Yang JC, Goff SL, Vanasse DA, McIntyre L, et al. Long-term follow-up of anti-CD19 chimeric antigen receptor T-cell therapy. J Clin Oncol (2020) 38(32):3805–15. doi: 10.1200/JCO.20.01467
9. Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult b cell ALL patients. J Clin Invest (2016) 126(6):2123–38. doi: 10.1172/JCI85309
10. Jacobson CA, Hunter BD, Redd R, Rodig SJ, Chen PH, Wright K, et al. Axicabtagene ciloleucel in the non-trial setting: outcomes and correlates of response, resistance, and toxicity. J Clin Oncol (2020) 38(27):3095–106. doi: 10.1200/JCO.19.02103
11. Zhaoyun L, Rong F. Predictive role of immune profiling for survival of multiple myeloma patients. Front Immunol (2021) 12:663748. doi: 10.3389/fimmu.2021.663748
12. Silzle T, Blum S, Schuler E, Kaivers J, Rudelius M, Hildebrandt B, et al. Lymphopenia at diagnosis is highly prevalent in myelodysplastic syndromes and has an independent negative prognostic value in IPSS-r-low-risk patients. Blood Cancer J (2019) 9(8):63. doi: 10.1038/s41408-019-0223-7
13. Laddaga FE, Ingravallo G, Mestice A, Tamma R, Maiorano E, Ribatti D, et al. Correlation between circulating blood and microenvironment T lymphocytes in diffuse large b-cell lymphomas. J Clin Pathol (2022) 75(7):493–7. doi: 10.1136/jclinpath-2020-207048
14. Yin T, Wang P, Yu J, Teng F. Treatment-related lymphopenia impairs the treatment response of anti-PD-1 therapy in esophageal squamous cell carcinoma. Int Immunopharmacol (2022) 106:108623. doi: 10.1016/j.intimp.2022.108623
15. Mohsen A, Taalab M, Abousamra N, Mabed M. Prognostic significance of absolute lymphocyte count, absolute monocyte count, and absolute lymphocyte count to absolute monocyte count ratio in follicular non-Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk (2020) 20(9):e606–15. doi: 10.1016/j.clml.2020.03.007
16. Le Jeune C, Bertoli S, Elhamri M, Vergez F, Borel C, Huguet F, et al. Initial absolute lymphocyte count as a prognostic factor for outcome in acute myeloid leukemia. Leuk Lymphoma (2014) 55(4):855–62. doi: 10.3109/10428194.2013.813504
17. Feng J, Wang Z, Guo X, Chen Y, Cheng Y, Tang Y, et al. Prognostic significance of absolute lymphocyte count at diagnosis of diffuse large b-cell lymphoma: a meta-analysis. Int J Hematol (2012) 95(2):143–8. doi: 10.1007/s12185-011-0993-6
18. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the world health organization classification of lymphoid neoplasms. Blood (2016) 127(20):2375–90. doi: 10.1182/blood-2016-01-643569
19. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the lugano classification. J Clin Oncol (2014) 32(27):3059–68. doi: 10.1200/JCO.2013.54.8800
20. Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood (2014) 124(2):188–95. doi: 10.1182/blood-2014-05-552729
21. Dave SS, Wright G, Tan B, Rosenwald A, Gascoyne RD, Chan WC, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. New Engl J Med (2004) 351(21):2159–69. doi: 10.1056/NEJMoa041869
22. Lin B, Chen C, Qian Y, Feng J. Prognostic role of peripheral blood lymphocyte/monocyte ratio at diagnosis in diffuse large b-cell lymphoma: a meta-analysis. Leuk Lymphoma (2015) 56(9):2563–8. doi: 10.3109/10428194.2015.1014367
23. Tamma R, Ingravallo G, Gaudio F, Annese T, Albano F, Ruggieri S, et al. Stat3, tumor microenvironment, and microvessel density in diffuse large b cell lymphomas. Leuk Lymphoma (2020) 61:567–74. doi: 10.1080/10428194.2019.1678154
24. Scott DW, Gascoyne RD. The tumour microenvironment in b cell lymphomas. Nat Rev Cancer (2014) 14:517–34. doi: 10.1038/nrc3774
25. Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol (2013) 14:1014–22. doi: 10.1038/ni.2703
26. Porrata LF, Ristow K, Habermann TM, Witzig TE, Inwards DJ, Markovic SN, et al. Absolute lymphocyte count at the time of first relapse predicts survival in patients with diffuse large b-cell lymphoma. Am J Hematol (2009) 84(2):93–7. doi: 10.1002/ajh.21337
27. Ege H, Gertz MA, Markovic SN, Lacy MQ, Dispenzieri A, Hayman SR, et al. Prediction of survival using absolute lymphocyte count for newly diagnosed patients with multiple myeloma: a retrospective study. Br J Haematol (2008) 141(6):792–8. doi: 10.1111/j.1365-2141.2008.07123.x
28. De Angulo G, Yuen C, Palla SL, Anderson PM, Zweidler-McKay PA. Absolute lymphocyte count is a novel prognostic indicator in ALL and AML: implications for risk stratification and future studies. Cancer (2008) 112(2):407–15. doi: 10.1002/cncr.23168
29. Ray-Coquard I, Cropet C, Sebban C, Sebban C, Le Cesne A, Judson I, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res (2009) 69(13):5383–91. doi: 10.1158/0008-5472.CAN-08-3845
30. Siddiqui M, Ristow K, Markovic SN, Witzig TE, Habermann TM, Colgan JP, et al. Absolute lymphocyte count predicts overall survival in follicular lymphomas. Br J Haematol (2006) 134(6):596–601. doi: 10.1111/j.1365-2141.2006.06232.x
31. Kim DH, Baek JH, Chae YS, Kim YK, Kim HJ, Park YH, et al. Absolute lymphocyte counts predicts response to chemotherapy and survival in diffuse large b-cell lymphoma. Leukemia (2007) 21(10):2227–30. doi: 10.1038/sj.leu.2404780
32. Sun HL, Pan YQ, He BS, Nie ZL, Lin K, Peng HX, et al. Prognostic performance of lymphocyte-to-monocyte ratio in diffuse large b-cell lymphoma: an updated meta-analysis of eleven reports. OncoTargets Ther (2016) 9:3017–23. doi: 10.2147/OTT.S96910
33. Porrata LF, Ristow K, Habermann T, Inwards DJ, Micallef IN, Markovic SN. Predicting survival for diffuse large b-cell lymphoma patients using baseline neutrophil/lymphocyte ratio. Am J Hematol (2010) 85:896–9. doi: 10.1002/ajh.21849
34. A predictive model for aggressive non-hodgkin’s lymphoma. N Engl J Med (1993) 329(14):987–94. doi: 10.1056/NEJM199309303291402
35. Ngo L, Hee SW, Lim LC, Tao M, Quek R, Yap SP, et al. Prognostic factors in patients with diffuse large b cell lymphoma: before and after the introduction of rituximab. Leuk Lymphoma (2008) 49(3):462–9. doi: 10.1080/10428190701809156
36. Romero GS, Moren MM, Prado GH, Sánchez-García FJ. Lactate contribution to the tumor microenvironment: mechanisms, effects on immune cells and therapeutic relevance. Front Immunol (2016) 7:52. doi: 10.3389/fimmu.2016.00052
37. Nair R, Neelapu SS. The promise of CAR T-cell therapy in aggressive b-cell lymphoma. best practice & research. Clin Haematol (2018) 31(3):293–8. doi: 10.1016/j.beha.2018.07.011
38. Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Trans Med (2015) 7(303):303ra139. doi: 10.1126/scitranslmed.aac5415
Keywords: chimeric antigen receptor T cell, diffuse large B-cell lymphoma, prelymphodepletion absolute, prognostic value, survival time
Citation: Lu Y, Zhu H, Liu Y, Wang Y, Sun Y, Cheng H, Yan Z, Cao J, Sang W, Zhu F, Li D, Sun H, Zheng J, Xu K and Li Z (2023) Prognostic value of prelymphodepletion absolute lymphocyte counts in relapsed/refractory diffuse large B-cell lymphoma patients treated with chimeric antigen receptor T cells. Front. Immunol. 14:1155216. doi: 10.3389/fimmu.2023.1155216
Received: 31 January 2023; Accepted: 17 April 2023;
Published: 02 May 2023.
Edited by:
Noah Isakov, Ben-Gurion University of the Negev, IsraelReviewed by:
Hua Wang, Sun Yat-sen University Cancer Center (SYSUCC), ChinaZhiming Li, Sun Yat-sen University Cancer Center (SYSUCC), China
Copyright © 2023 Lu, Zhu, Liu, Wang, Sun, Cheng, Yan, Cao, Sang, Zhu, Li, Sun, Zheng, Xu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junnian Zheng, am56aGVuZ0B4emhtdS5lZHUuY24=; Kailin Xu, bGlobWRAMTYzLmNvbQ==; Zhenyu Li, bGl6aGVueXVtZEAxNjMuY29t
†These authors have contributed equally to this work
 Yanyan Lu
Yanyan Lu Hong Zhu1,2,3†
Hong Zhu1,2,3†