- 1Department of Urology, Hokkaido University Hospital, Sapporo, Japan
- 2Division of Renal Surgery and Transplantation, Jichi Medical University, Shimotsuke, Tochigi, Japan
- 3Data Science Center, Promotion Unit, Institute of Health Science Innovation for Medical Care, Hokkaido University Hospital, Sapporo, Hokkaido, Japan
- 4Department of surgical pathology, Hokkaido University Hospital, Sapporo, Hokkaido, Japan
- 5Departments of Kidney Transplant Surgery, Sapporo City General Hospital, Sapporo, Hokkaido, Japan
Donor-specific antibodies (DSAs) are the main cause of graft loss over time. The direct pathway of alloantigen recognition is important in the pathogenesis of acute rejection. Recent studies have suggested that the direct pathway also contributes to the pathogenesis of chronic injury. Nevertheless, there are no reports on T-cell alloantigen response via the direct pathway in kidney recipients with DSAs. We analyzed the T-cell alloantigen response via the direct pathway in kidney recipients with DSAs (DSA+) or without DSAs (DSA−). A mixed lymphocyte reaction assay was implemented to assess the direct pathway response. DSA+ patients showed significantly higher CD8+ and CD4+ T cell responses to donor cells than DSA− patients. Furthermore, proliferating CD4+ T cells showed a marked increase in Th1 and Th17 responses in DSA+ patients than in DSA− patients. In a comparison between anti-donor and third-party responses, the anti-donor CD8+ and CD4+ T cell response was significantly lower than the anti-third-party response. In contrast, the donor-specific hyporesponsiveness was absent in DSA+ patients. Our study demonstrated that DSA+ recipients have a greater potential for developing immune responses against the donor tissues via the direct alloantigen recognition pathway. These data contribute to an understanding of DSAs pathogenicity during kidney transplantation.
Introduction
The development of immunosuppressants has reduced acute rejection and improved short-term outcomes after kidney transplantation. Donor-specific antibodies (DSAs) are associated with antibody-mediated rejection (AMR), which leads to poor outcomes. In particular, chronic active antibody-mediated rejection (CAAMR) induced by DSAs is the main cause of graft loss in the long term (1, 2). However, the exact role of DSAs in transplant immunity remains poorly understood.
T cells play a key role in the alloimmune response. Alloreactive T cells recognize HLA-mismatched tissue via two different pathways. The direct pathway is important in the pathogenesis of acute rejection in the early post-transplantation period (3). The mixed lymphocyte reaction (MLR) assay, which is a method to measure T-cell alloreactivity via the direct pathway has been used as a reliable monitoring system to predict acute rejection (4). In contrast, the indirect pathway of alloantigen recognition plays a pivotal role in late graft failure (5, 6). In addition, the activation of CD4+ T cells via the indirect pathway is critical for the generation of DSAs (7, 8). Therefore, several studies have focused on the indirect pathway in patients with chronic injury of AMR (9–12). However, recent studies have suggested that the direct pathway also contributes to the pathogenesis of chronic injury (13, 14). Nonetheless, to our knowledge, no study has evaluated the direct pathway of DSAs in patients with kidney transplants.
In this study, our aim was to investigate T-cell alloreactivity via the direct pathway in patients with DSAs undergoing kidney transplantation. We analyzed the T-cell alloantigen response via the direct pathway in kidney transplant recipients whose graft status was evaluated using graft biopsy.
Materials and methods
Patients and samples
In total, 96 patients who underwent a human leukocyte antigens (HLA) incompatible kidney transplantation between 1999 and 2020 were enrolled in the study. The inclusion criteria were functional longstanding living-donor kidney transplants for at least a year after transplantation. Among these recipients, 68 patients without donor-specific antibodies showed stable renal function (DSA− group). Twenty-eight patients were positive for donor-specific antibodies (DSA+ group). Patients were typically maintained on a triple immunosuppressive therapy, including calcineurin inhibitors (tacrolimus or cyclosporine), mycophenolate mofetil, and methylprednisolone. Some patients were subjected to steroid withdrawal and/or everolimus addition based on clinical assessment.
Isolated peripheral blood mononuclear cells (PBMCs) were collected in heparinized tubes from kidney transplant recipients, donors and third parties. Third party cells were obtained from patients in our laboratory who had previously been HLA typed. They were HLA mismatched with each recipient.
The study was approved by the Institutional Review Board of Hokkaido University Hospital (protocol 016-0389) and Sapporo City General Hospital (protocol R1-059-627). Written informed consent was obtained from all patients.
All blood samples from kidney transplant recipients were collected at the time of kidney allograft biopsy.
Assessment of DSA
Luminex single antigen beads assays (One Lambda, Canoga Park, CA, USA) were performed each year after kidney transplantation to identify donor-specific HLA class I and II antibodies. We defined DSA positivity as a mean fluorescence intensity greater than 1000.
Biopsy assessment
Protocol biopsies were performed each year after kidney transplantation, if possible. Some patients underwent kidney allograft biopsies owing to the development of de novo DSAs. All biopsy specimens were evaluated using light microscopy and C4d immunofluorescence staining.
Flow cytometry
Isolated PBMCs were analyzed by cell-surface staining using fluorescent antibodies against CD3 (SP34-2 and SK7), CD4 (L200), CD8 (SK1), CD19 (SJ25C1), CD24 (ML5), CD25 (M-A251), CD27 (M-T271), CD28 (CD28.2), CD38 (HIT2), CD45RO (UCHL1), CD56 (B159), CD57 (NK-1), CD69 (FN50), CD138 (MI15), PD1 (NAT105), HLA-DR (G46-6) (BD Pharmingen, San Diego, CA, USA), and CCR7 (G043H7) (BioLegend, San Diego, CA, USA). To assess the intracellular protein expression of FOXP3, INF-γ, IL-4, and IL-17A, the cells were permeabilized using the Foxp3/Transcription Factor Staining Buffer Set (eBioscience, San Diego, CA, USA) and stained with fluorescent antibodies against FOXP3 (PCH101, eBioscience), IFN- γ (B27, BD Pharmingen), IL-4 (8D4-8, BD Pharmingen), and IL-17A (eBio64DEC17, eBioscience). The cells were analyzed on the FACSverse (BD Biosciences, San Diego, CA, USA), and FlowJo software (BD Biosciences) was used for data analysis.
MLR assay
Responder T cells from recipients were purified by negative selection with an EasySep Human T Cell Isolation Kit (STEMCELL Technologies, Vancouver, BC, Canada). The isolated T cells were labeled with 3 μM carboxyfluorescein diacetate succimidyl ester (CFSE) (Invitrogen, Waltham, MA, USA) at 37°C for 5 min in a 5% CO2 incubator. Labeling of the T cells was terminated by adding cold phosphate-buffered saline containing 2% fetal bovine serum (Biological Industries, Cromwell, CT, USA). Stimulator PBMCs from donor or third parties were irradiated at 30 Gy. Responder T cells were cultured in 96-well U-bottom plates with the stimulator PBMCs at a 1:1 ratio. After 5 days, the cells were harvested and stained with antibodies, and the diluted CFSE signal was analyzed using flow cytometry. For intracellular cytokine staining, Leukocyte Activation Cocktail (BD Pharmingen) was added to the samples per the manufacturer’s protocol, followed by an additional incubation for 4 h.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 8 (GraphPad Software, Inc.; San Diego, CA, USA). Categorical variables were compared using the chi-square test, and Student’s t-test was used for continuous variables. To analyze the number of B cells and B-cell subsets, log transformations were used to reduce skewness. Results with P <0.05 were considered significant.
Results
Clinical characteristics of the patients
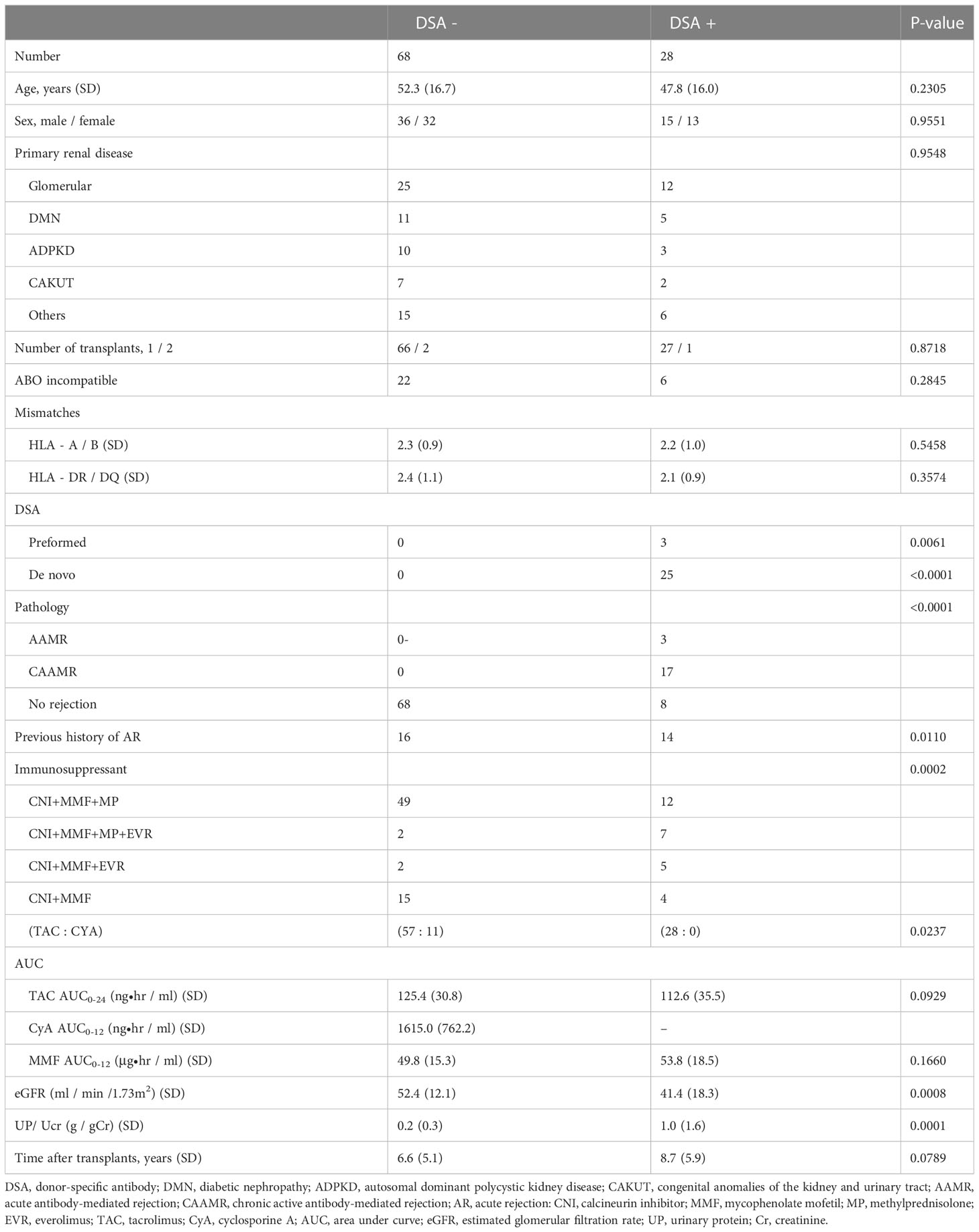
The patient characteristics of the two groups are summarized in Table 1. Age, sex, and the number of ABO-incompatible or HLA mismatches were not significantly different between the groups. All patients received calcineurin inhibitors and mycophenolate mofetil as maintenance immunosuppressants. The majority of DSA+ patients were treated with three or more immunosuppressants. An analysis of immunosuppressant blood levels showed no difference in the area under the curve for the use of tacrolimus and mycophenolate mofetil. The estimated glomerular filtration rate was significantly lower in the DSA+ group than in the DSA− group, which was indicative of graft damage induced by the DSAs or related immune responses. Additionally, total urinary protein/creatinine level was significantly higher in the DSA+ group than in the DSA- group. The time after transplantation was not significantly different between the groups. Among the patients in the DSA+ group, 3 had pre-formed DSAs and 25 developed de novo DSAs. None of the patients in the DSA− group showed incidents of rejection, whereas three DSA+ patients developed acute antibody-mediated rejection (AAMR), 17 DSA+ patients developed CAAMR, and 8 DSA+ patients had not yet shown indications of rejection of their kidney grafts.
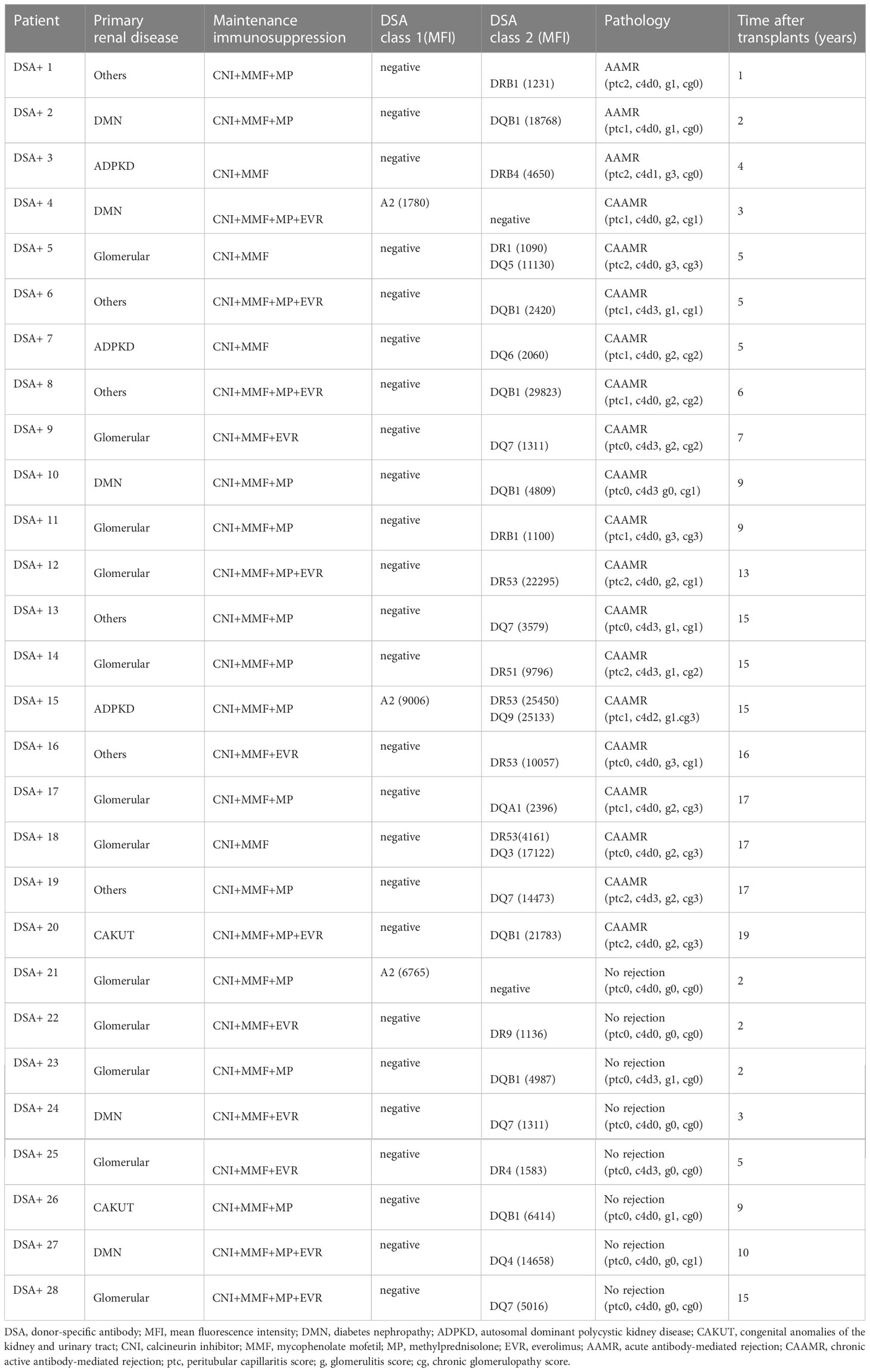
Immunological characteristics of DSA+ patients are summarized in Table 2. One patient developed both anti-HLA class I and II DSAs, two had anti-HLA class I DSAs, and the remaining patients had anti-HLA class II DSAs. The sum of the mean fluorescence intensity of DSA was 10260 ± 2374 (mean ± SEM). Biopsies from AAMR and CAAMR patients showed microvascular inflammation (glomerulitis and/or peritubular capillaritis score ≥2), while only CAAMR patients showed transplant glomerulopathy (chronic glomerulopathy score >0). In addition, one of three AAMR patients, seven of 17 CAAMR patients, and two of eight patients without rejection had C4d positive in in peritubular capillaries based on immunofluorescence.
Immunophenotypes of peripheral lymphocytes
The composition of peripheral blood lymphocytes was characterized in DSA− and DSA+ patients. CD3, CD19, and CD56 expression levels were monitored using flow cytometry, and no significant differences in the number of T (CD3+), B (CD19+), NK (CD3-CD56+), and NKT (CD3+CD56+) cells in the peripheral blood between the groups was observed (Figure 1). Next, the T and B cell subsets were further analyzed. The CD8+ or CD4+ T cell subsets were divided into naïve (CCR7+CD45RO−, TN), central memory (CCR7+CD45RO+, TCM), effector memory (CCR7−CD45RO+, TEM), and highly differentiated effector T cells without CD45RO expression that re-expressed CD45RA (CCR7−CD45RO−, TEMRA) (Supplemental Figure 1). We also assessed several more subsets of CD8+ or CD4+ T cells, such as activation (CD69+, and HLA-DR+), exhaustion (PD1+CD57-), and senescence (CD57+CD28-) (Supplemental Figure 1). B cell subsets were determined as follows: transitional B cells (CD19+CD24hiCD38hi), naïve B cells (CD19+CD24loCD38lo), memory B cells (CD19+CD27+), and plasma cells (CD38+CD138+) (Supplemental Figure 1). However, there were no significant differences in the number of T and B cell subsets between the groups (Figure 2).
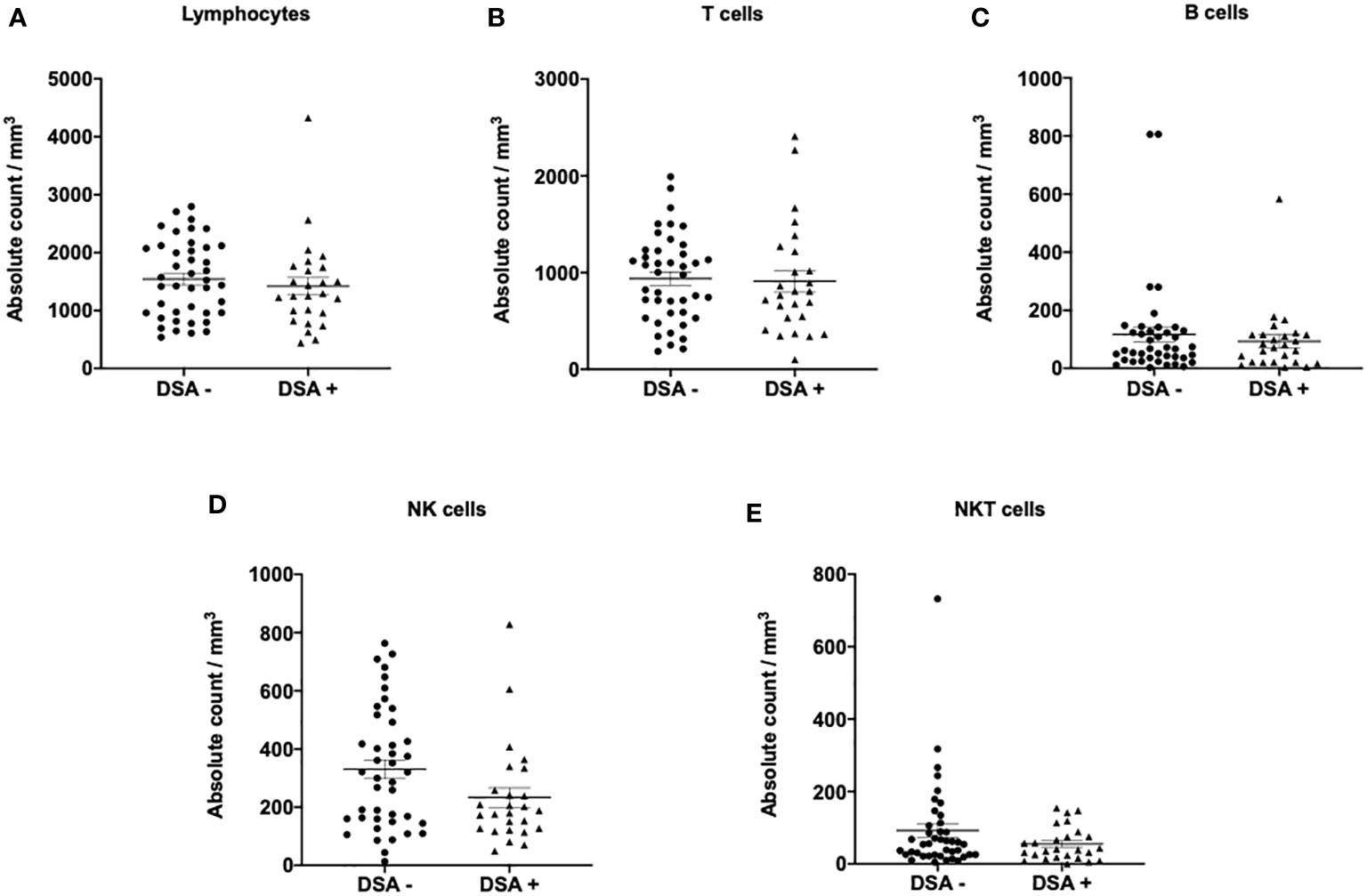
Figure 1 Lymphocyte subsets in patients without donor-specific antibodies (DSA -) and with DSA (DSA+). The number of (A) lymphocytes, (B) T cells (CD3+), (C) B cells (CD19+), (D) NK cells (CD3-CD56+), and (E) NKT cells (CD3+CD56+). No significant differences in the number of lymphocytes, T, B, NK, and NKT cells were observed in the peripheral blood between DSA- and DSA+ patients. Data are shown as mean ± SEM. An unpaired t-test was performed.
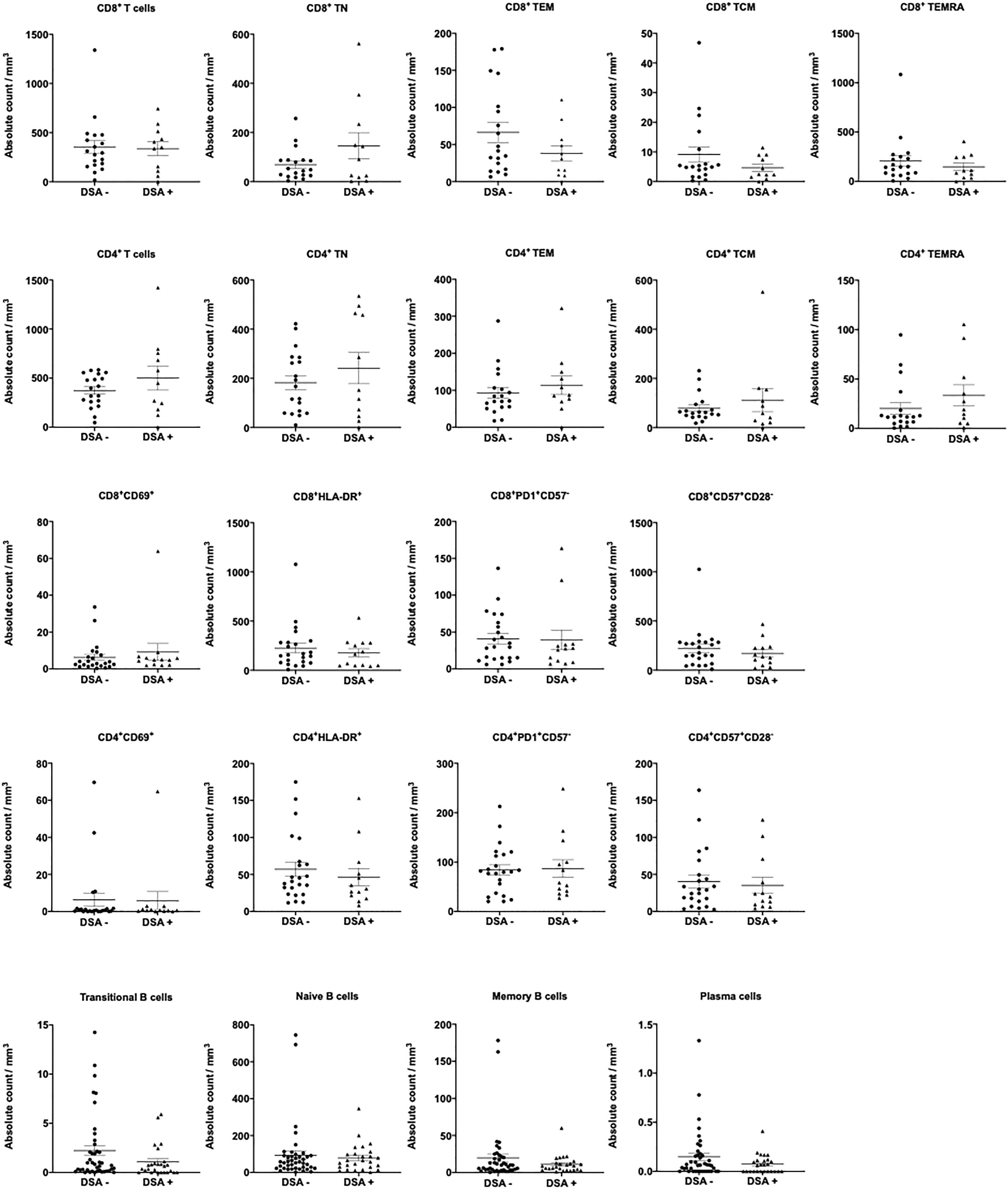
Figure 2 T and B cell subsets in patients without donor-specific antibodies (DSA-) and with DSA (DSA+). The number of T and B cell subsets was analyzed in the peripheral blood between DSA - and DSA + patients. There were no significant differences in T and B cell subsets between DSA- and DSA+ patients. Data are shown as the mean ± SEM. An unpaired t-test was performed.
Anti-donor CD8+ and CD4+ T-cell proliferation via the direct pathway in DSA+ patients
In a preliminary experiment, we evaluated the T-cell response against donor antigens via the direct pathway in 15 DSA− and 5 DSA+ patients. As previously reported in patients with acute rejection, PBMCs from the transplant recipients were labeled with CFSE and cultured with irradiated donor PBMCs for 5 days, after which the cultured cells were stained for CD4 and CD8 and analyzed using flow cytometry. No differences were observed in the anti-donor CD8+ and CD4+ T cell responses between the groups (Supplemental Figure 2). Unlike that in patients with acute cellular rejection, no marked T cell proliferation was observed in DSA+ patients.
To improve the accuracy of T-cell alloreactivity via the direct pathway, we used isolated T cells as responders, and this excluded the T cell response via the indirect pathway. Anti-donor CD8+ T-cell responses were found to be significantly higher in DSA+ patients than in DSA− patients (Figures 3A, B). Similarly, anti-donor CD4+ T-cell responses were significantly higher in DSA+ patients than in DSA− patients (Figures 3C, D).
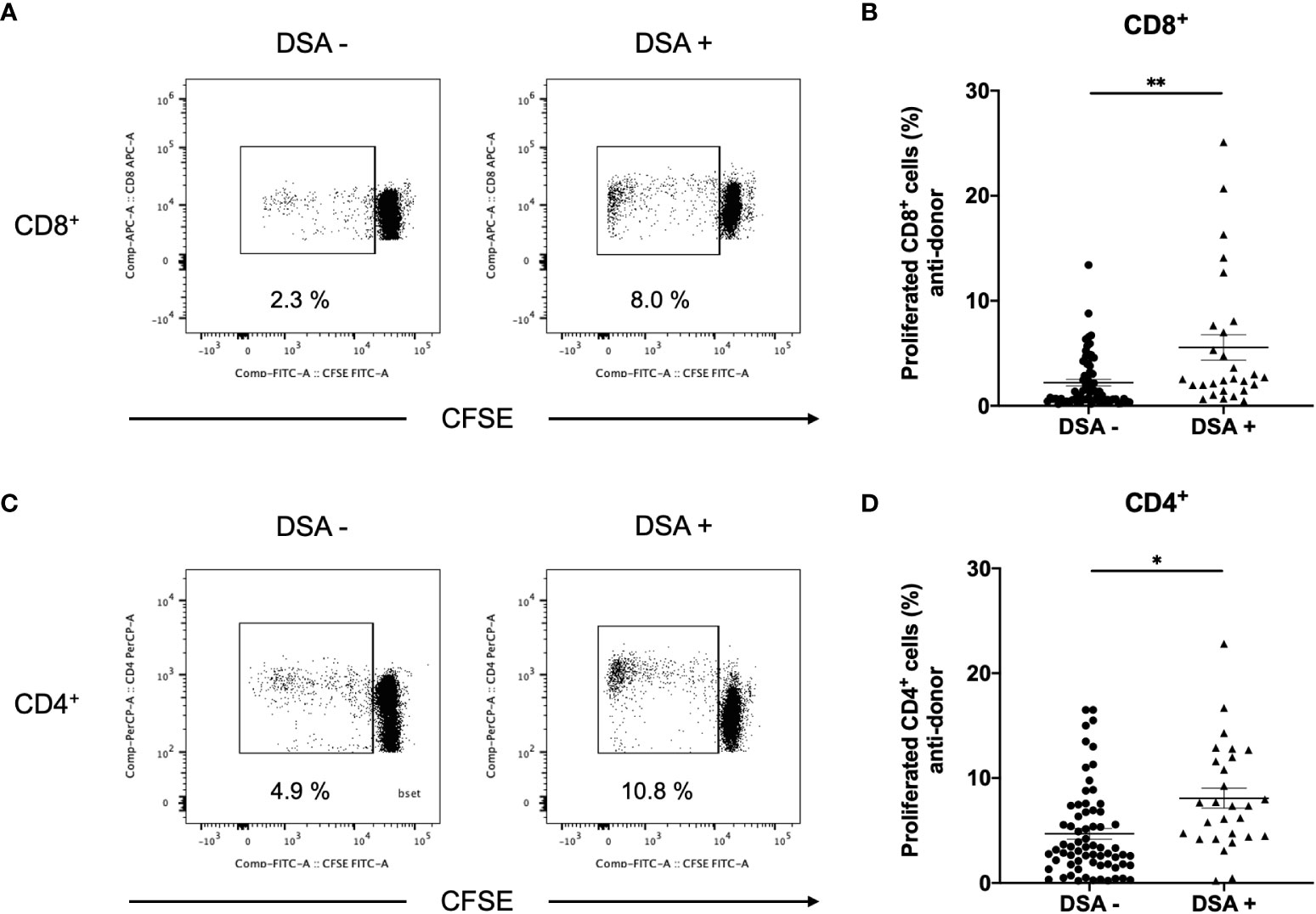
Figure 3 Aq1Anti-donor CD8+ and CD4+ T cell response in patients without donor-specific antibodies (DSA-) and with DSA (DSA+). The CD3+ cells isolated from DSA- and DSA+ patients were labeled with CFSE and were cultured with irradiated donor peripheral blood mononuclear cells for 5 days. The cultured cells were stained for CD4 and CD8. Representative flow cytometric data (A: CD8, C: CD4) and cell proliferation rate (B: CD8, D: CD4) are shown. Anti-CD8+ and CD4+ T-cell hyper-responses were observed in DSA+ patients, and they were significantly higher than those observed in DSA- patients, Data are presented as mean ± SEM. An unpaired t-test was performed. *p<0.01, **p<0.001.
Anti-donor Th1 and Th17 proliferation via the direct pathway in DSA+ patients
To identify CD4+ T cell subsets that showed expansion, responder T cells from DSA− and DSA+ patients were stained for INF- γ, IL-4, IL-17, and FOXP3 (Figure 4A). Although no differences were observed in the peripheral blood number of Th1 (CD4+INF-γ+) and Th17 (CD4+IL-17+) between DSA- and DSA+ patients (Supplemental Figure 3), proliferating CD4+ T cells showed a marked increase in the Th1 (CD4+INF- γ+) and Th17 (CD4+IL-17+) response in DSA+ patients compared with that in the DSA− patients (Figures 4B, D). In contrast, there were no differences in donor reactive Th2 (CD4+IL4+) or Treg (CD4+FOXP3+) cells between the groups (Figures 4C, E). These results suggest that DSAs are associated with the response of the classical proinflammatory Th1 and Th17 cells.

Figure 4 Anti-donor CD4+ T cell subset response in patients without donor-specific antibodies negative (DSA-) and with DSA (DSA+). T cells isolated from DSA- and DSA+ patients were labeled with CFSE and were cultured with irradiated donor peripheral blood mononuclear cells for 5 days. The cultured cells were stained for CD4, IFN-γ, IL-4, IL-17 and FOXP3. Representative flow cytometric data (A) and cell proliferation rate (B: CD4+IFN- γ +, C: CD4+IL-4+, D: CD4+IL-17+, E: CD4+FOXP3+) are shown. Proliferating CD4+ cells showed a marked increase in Th1 (CD4+IFN- γ+) and Th17 (CD4+IL-17+) response in DSA+ compared with DSA- those in patients (B, D). However, there were no differences in donor-reactive Th2 (CD4+IL-4+) or Treg (CD4+FOXP3+) cells between the groups (C, E). Data are presented as the mean ± SEM. An unpaired t-test was performed. *p<0.01, **p<0.0001.
We also explored the relationship between DSAs and the direct pathway response in rejection-free patients. Eight of the 28 patients with DSAs showed no transplant rejection (DSA+ without rejection). The anti-donor CD4+ T cell response was comparable between DSA+ patients without rejection and DSA− patients (Figure 5A). However, Th1 and Th17 responses were significantly higher in DSA+ patients without rejection than in DSA− patients (Figures 5B, C).
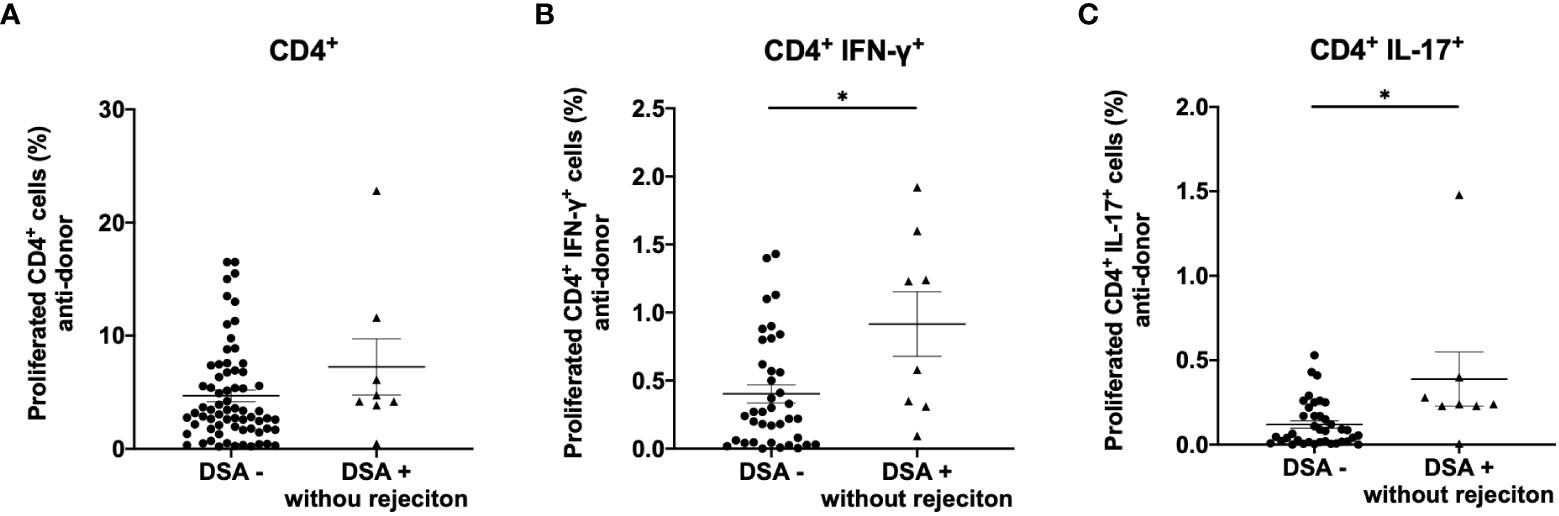
Figure 5 Analysis of anti-donor CD4+ T-cell response in patients with donor-specific antibodies without rejection (DSA+ without rejection). Anti-donor CD4+ T cell response was comparable between DSA+ without rejection and DSA- patients (A). However, a significantly higher proportion of the proliferating CD4+ T cells showed a Th1 (CD4+IFN- γ+) and Th17 (CD4+IL-17+) phenotype in the DSA+ patients without rejection than in DSA- patients (B, C). Data are shown as the mean ± SEM. An unpaired t-test was performed. *p<0.01.
Comparison between the anti-donor and third-party responses
To compare anti-donor and third-party responses, T cells isolated from DSA− and DSA+ patients were stimulated with third-party PBMCs. We hypothesized that immunosuppressant treatments can decrease the lymphocyte response to both donor and the third-party in kidney transplant patients. Interestingly, the anti-donor CD8+ and CD4+ T cell responses were significantly lower than the anti-third-party response in DSA− patients (Figures 6A, B, D, E). These results indicate that donor-specific hyporesponsiveness is observed in CD8+ and CD4+ T cells from DSA− patients and persists for 10 years after transplantation (Figures 6C, F).
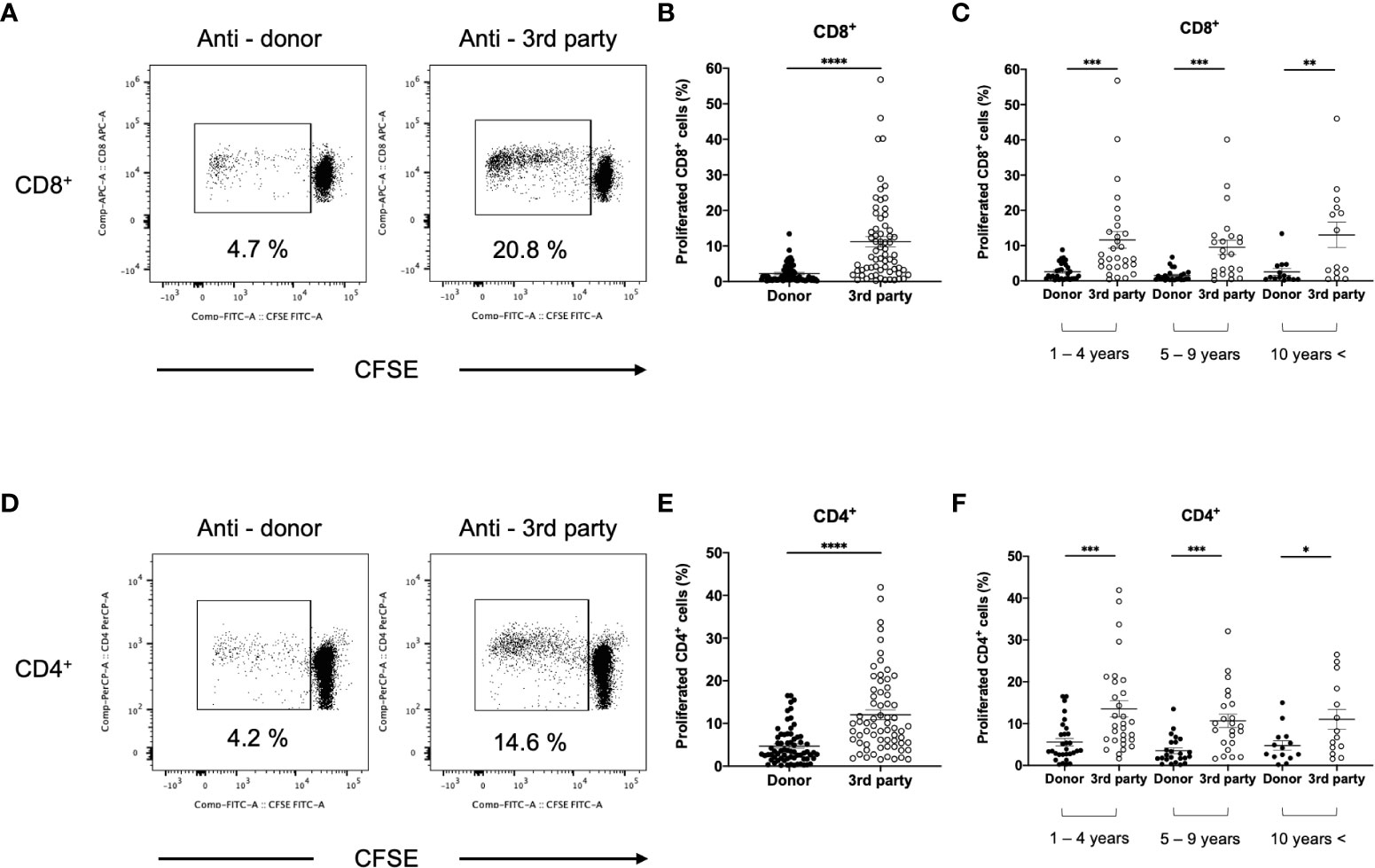
Figure 6 Comparison between anti-donor and third-party response in patients without donor-specific antibodies (DSA-). The isolated T cells from DSA- patients were labeled with CFSE and were cultured with irradiated donor or third-party peripheral blood mononuclear cells for 5 days. The cultured cells were stained for CD8 and CD4. Representative flow cytometric data (A: CD8, D: CD4) and proliferated cells rate (B: CD8, E: CD4) are shown. The response at 1-4, 5-9, and more than 10 (10 years <) years after transplantation is shown (C: CD8, F: CD4). Anti-donor CD8+ and CD4+ T-cell responses were significantly lower than the anti-third-party response (B, E). This donor-specific T-cell hyporesponsiveness persisted for 10 years after transplantation (C, F). Data are presented as the mean ± SEM. A paired t-test was performed. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
In DSA+ patients, the anti-donor CD8+ T-cell response was significantly lower than the third-party response (Figures 7A, B). In contrast, CD4+ T cell proliferation was similar in the donor and third-party (Figures 7C, D), indicating that donor-specific hyporesponsiveness of CD4+ T cells was absent in DSA+ patients.
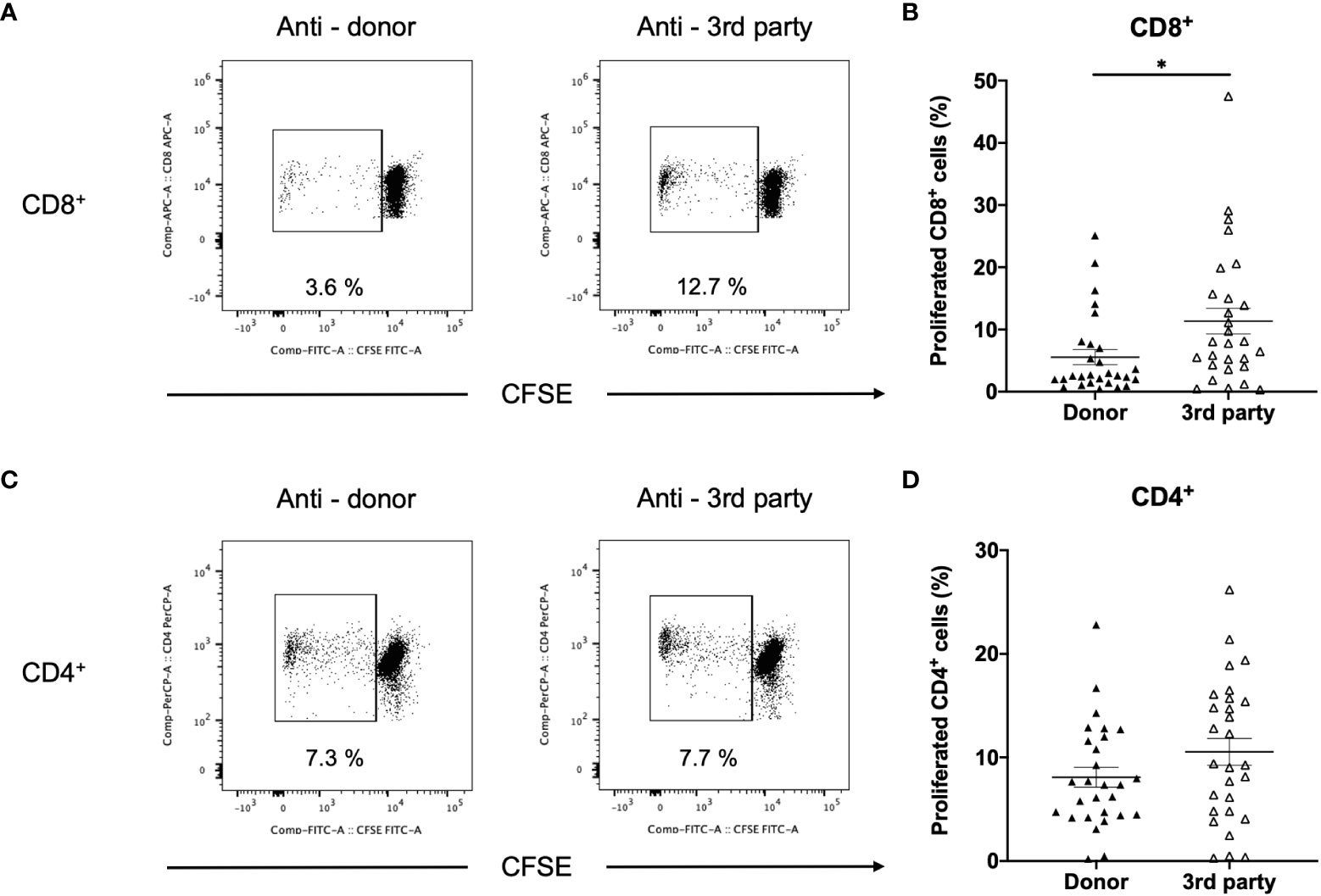
Figure 7 Comparison between anti-donor and third-party responses in patients with donor-specific antibodies (DSA+). T cells isolated from (DSA+) patients were labeled with CFSE and were cultured with irradiated donor or third-party peripheral blood mononuclear cells for 5 days. The cultured cells were stained for CD8 and CD4. Representative flow cytometric data (A: CD8, C: CD4) and proliferated cell rate (B: CD8, D: CD4) are shown. Anti-donor CD8+ T-cell response was significantly lower than the third-party response (B). However, CD4+ T-cell proliferation was comparable in terms of donor and third-party responses (D). Data are presented as mean ± SEM. A paired t-test was performed. *p<0.05.
Discussion
DSAs are a major risk factor of CAAMR and graft loss (1, 2). The importance of DSAs in kidney transplantation has been recognized for over 50 years (15). However, little is known about how DSAs affect the kidney allograft via direct pathway. Therefore, we explored the direct-pathway T-cell responses in 96 kidney transplant patients. Patients with DSAs displayed higher CD8+ and CD4+ responses to donors than those without DSAs. Moreover, activated CD4+ T cells showed a marked increase in Th1 and Th17 responses in patients with DSAs. Furthermore, no rejection patients with DSAs showed increased anti-donor Th1 and Th17 responses. We further demonstrated that donor-specific hyporesponsiveness of CD8+ and CD4+ T cells was observed in patients without DSAs, but the hyporesponsiveness of CD4+ T cells was absent in patients with DSAs.
T-cell allorecognition is still considered critical for short- and long-term outcomes of kidney transplantation. Alloreactive T cells recognize HLA-mismatched tissue via direct and indirect pathways. In the direct pathway, T cells recognize non-self MHC molecules present on the surface of donor cells. The direct pathway was regarded as short-lived and responsible for acute rejection in the early transplant period (3). The MLR assay is a method for measuring T cell alloreactivity via the direct pathway and has been widely used as a reliable monitoring system for acute T cell-mediated rejection in clinical transplantation (4, 16–18). We first performed the CFSE/MLR assay using PBMCs as responders and found that this assay could not distinguish between DSA+ and DSA− patients. Therefore, we used isolated T cells as responders, which exclude the T cell response via the indirect pathway (19). We identified that anti-donor CD8+ and CD4+ T-cell responses in DSA+ patients were significantly higher than those in DSA− patients. Therefore, the use of isolated T cells from transplant patients as responders revealed the activation of T cells via the direct pathway of DSAs. It is noteworthy that our study is the first to evaluate T-cell alloreactivity via the direct pathway of DSAs in clinical kidney transplant recipients.
Next, we focused on the differences in the CD4+ T cell phenotype between DSA+ and DSA− patients. Our study revealed a marked increase in Th1 and Th17 responses in DSA+ patients. The data suggest that Th1 and Th17 responses were activated in kidney transplant recipients with DSAs. Several studies have reported an association between CAAMR and Th1 and Th17 responses. Homs et al. reported that INF- γ and the transcription factor T-bet (both functionally defined markers of Th1 CD4 T cells) are more strongly expressed in the allografts of patients with CAAMR than in normal control patients and those without CAAMR (20). Several studies also showed an increased prevalence of the Th17-cell phenotype in kidney transplant recipients with CAAMR. Furthermore, Th17 immunity contributes to chronic allograft rejection in patients with lung and heart transplants (21–23). In our study, we performed a comparative analysis of different subsets of CD4+ T cells by staining responder T cells for INF-γ, IL-4, IL-17, and FOXP3. We observed a marked increase in Th1 and Th17 responses in DSA+ patients. A previous study reported that the decreased frequency of regulatory T cells in peripheral blood was associated with CAAMR in kidney recipients (22, 23); however, no differences in Treg expansion between DSA− and DSA+ recipients were observed.
We also found significantly higher Th1 and Th17 expansion via the direct pathway in rejection-free patients with DSAs. A recent study showed that transitional B cell frequencies were higher in rejection-free patients with de novo DSAs than in those without de novo DSAs (24). DSAs are associated with CAAMR, which is the main cause of graft loss in the long term. DSAs are considered a trigger for CAAMR. Therefore, rejection-free patients with DSAs might eventually develop CAAMR. This result indicates that monitoring Th1 and Th17 responses could identify patients with the potential for developing CAAMR. The identification of transplant patients with the potential for CAMMR permits therapeutic interventions that can be administered before histological changes occur in the graft tissue. Early interventions (plasmapheresis, pulse steroid, intravenous immunoglobulin, and rituximab) improve clinical outcomes in subclinical antibody-mediated rejection (25–27). Thus, prediction of the CAAMR incidence might prevent or delay CAAMR development. However, the number of DSA+ patients without rejection was small, and it has not been confirmed that they will develop CAAMR in the future. We must conduct long-term studies for confirmation.
Donor-specific hyporesponsiveness in CD4+ and CD8+ T cell responses was observed in DSA− patients during immunosuppressant therapy. A similar response has been reported in kidney recipients with induced immune tolerance from combined kidney and bone marrow transplantation (28). It has been suggested that the mechanism of donor-specific hyporesponsiveness and tolerance induces the clonal deletion of donor-reactive T cells (29). However, this phenomenon is controversial in solid organ recipients who receive immunosuppressive therapy. A recent study showed that donor-specific hyporesponsiveness occurs in both liver-kidney and solitary liver transplant patients, but this response was not observed in kidney recipients (30). Additionally, a few studies reported this hyporesponsiveness in kidney recipients with a stable renal function; however, recent studies were inconclusive because of the small number of patients and short follow-up periods (16, 31). This study is the first to clarify this phenomenon in kidney graft recipients using an adequate number of patients. In addition, we discovered that the donor-specific hyporesponsive state persist in the long term after transplantation. Meanwhile, we found that donor-specific hyporesponsiveness of CD4+ T cells was absent in DSA+ patients. Poggio et al. reported that ratios of donor/third-party enzyme-linked immunosorbent spot responses increased in chronic allograft nephropathy (CAN) (32). Additionally, CAN resulted in the development of DSAs to a greater degree than that in the control group. As the production of DSAs requires interactions between B cells and CD4+ T cells, we could detect the activation of donor-specific CD4+ T cell responses.
The strength of our study is that we analyzed a large number of patients during a long-term follow-up period. Furthermore, the overall status of the transplanted kidney was accurately evaluated in all recipients using graft biopsy. Nonetheless, there are some limitations to this study. First, we conducted a cross-sectional study and did not serially analyze the immune status of the recipients. Therefore, longitudinal and prospective studies that include monitoring patients before transplantation are ongoing. Second, our study did not include an independent validation cohort. Third, donor blood samples are required for our MLR assay. Thus, when we serially perform the MLR assay, we must collect blood samples from the donor several times. Currently, we are developing a new technique to increase the number of donor cells through incubation. If this technique is established successfully, then the donor blood will only need to be collected once for use at different time points. Fourth, we evaluated the direct allo-response in vitro assay using antigen-presenting cells (APCs) from donors in this study. However, the in vivo setting, viable APCs of donor origin are lacking after 1 year of transplantation. Thus, the more accurate in vitro correlate of the long-term transplant patient’s status is considered not the direct pathway but rather a pathway through recipient APCs cross-dressed with donor MHC molecules, termed the semi-direct pathway. Recent rodent studies indicated that the presentation of intact donor MHC molecules by donor APCs would lead to results similar to those with presentation of the same intact allogeneic MHC molecules by recipient APCs. Therefore, our MLR assay is certainly a rational and useful methods to assess T-cell alloresponse in clinical settings (33, 34).
In conclusion, our study demonstrated that DSA+ recipients have a greater potential of developing immune responses against the donor tissues via the direct alloantigen recognition pathway. These data contribute to an understanding of DSA pathogenicity during kidney transplantation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Hokkaido University Hospital (protocol 016-0389) and Sapporo City General Hospital (protocol R1-059-627). The patients/participants provided their written informed consent to participate in this study.
Author contributions
NI performed the experiments, analyzed the data, and wrote the manuscript. KH designed and supervised the experiments, analyzed the data, and wrote the manuscript. YTan performed the experiments. TT, SM, YTak, HHi, HS, TH, and HHa acquired the data. YI contributed to statistical design. TO performed the pathological analysis. DI and NS supervised the experiments. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, grant number 18K09156.
Acknowledgments
We would like to thank Editage (https://www.editage.jp/) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1164794/full#supplementary-material
Abbreviations
APCs, antigen presenting cells; AMR, antibody-mediated rejection; AAMR, acute antibody-mediated rejection; CAAMR, chronic active antibody-mediated rejection; CFSE, carboxyfluorescein diacetate succimidyl ester; DSA, donor-specific antibody; ESRD, end-stage renal disease; HLA, human leukocyte antigen; MLR, mixed lymphocyte reaction; PBMC, peripheral blood mononuclear cell.
References
1. El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, et al. Identifying specific causes of kidney allograft loss. Am J Transplant (2009) 9(3):527–35. doi: 10.1111/j.1600-6143.2008.02519.x
2. Sellares J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. (2012) 12(2):388–99. doi: 10.1111/j.1600-6143.2011.03840.x
3. van Besouw NM, Zuijderwijk JM, Vaessen LM, Balk AH, Maat AP, van der Meide PH, et al. The direct and indirect allogeneic presentation pathway during acute rejection after human cardiac transplantation. Clin Exp Immunol (2005) 141(3):534–40. doi: 10.1111/j.1365-2249.2005.02871.x
4. Tanaka Y, Ohdan H, Onoe T, Mitsuta H, Tashiro H, Itamoto T, et al. Low incidence of acute rejection after living-donor liver transplantation: immunologic analyses by mixed lymphocyte reaction using a carboxyfluorescein diacetate succinimidyl ester labeling technique. Transplantation (2005) 79(9):1262–7. doi: 10.1097/01.tp.0000161667.99145.20
5. Baker RJ, Hernandez-Fuentes MP, Brookes PA, Chaudhry AN, Lechler R. Comparison of the direct and indirect pathways of allorecognition in chronic allograft failure. Transplant Proc (2001) 33(1-2):449. doi: 10.1016/s0041-1345(00)02088-1
6. Sayegh MH. Carpenter CB role of indirect allorecognition in allograft rejection. Int Rev Immunol (1996) 13(3):221–9. doi: 10.3109/08830189609061749
7. Pettigrew GJ, Lovegrove E, Bradley JA, Maclean J. Bolton EM indirect T cell allorecognition and alloantibody-mediated rejection of MHC class I-disparate heart grafts. J Immunol (1998) 161(3):1292–8. doi: 10.4049/jimmunol.161.3.1292
8. Conlon TM, Saeb-Parsy K, Cole JL, Motallebzadeh R, Qureshi MS, Rehakova S, et al. Germinal center alloantibody responses are mediated exclusively by indirect-pathway CD4 T follicular helper cells. J Immunol (2012) 188(6):2643–52. doi: 10.4049/jimmunol.1102830
9. Baker RJ, Hernandez-Fuentes MP, Brookes PA, Chaudhry AN, Cook HT, Lechler RI. Loss of direct and maintenance of indirect alloresponses in renal allograft recipients: implications for the pathogenesis of chronic allograft nephropathy. J Immunol (2001) 167(12):7199–206. doi: 10.4049/jimmunol.167.12.7199
10. Hornick PI, Mason PD, Baker RJ, Hernandez-Fuentes M, Frasca L, Lombardi G, et al. Significant frequencies of T cells with indirect anti-donor specificity in heart graft recipients with chronic rejection. Circulation (2000) 101(20):2405–10. doi: 10.1161/01.cir.101.20.2405
11. Shiu KY, McLaughlin L, Rebollo-Mesa I, Zhao J, Burton H, Douthwaite H, et al. Graft dysfunction in chronic antibody-mediated rejection correlates with b-cell-dependent indirect antidonor alloresponses and autocrine regulation of interferon-γ production by Th1 cells. Kidney Int (2017) 91(2):477–92. doi: 10.1016/j.kint.2016.10.009
12. Shiu KY, McLaughlin L, Rebollo-Mesa I, Zhao J, Semik V, Cook HT, et al. B-lymphocytes support and regulate indirect T-cell alloreactivity in individual patients with chronic antibody-mediated rejection. Kidney Int (2015) 88(3):560–8. doi: 10.1038/ki.2015.100
13. Bestard O, Nickel P, Cruzado JM, Schoenemann C, Boenisch O, Sefrin A, et al. Circulating alloreactive T cells correlate with graft function in longstanding renal transplant recipients. J Am Soc Nephrol. (2008) 19(7):1419–29. doi: 10.1681/asn.2007050539
14. Herrera OB, Golshayan D, Tibbott R, Salcido Ochoa F, James MJ, Marelli-Berg FM, et al. A novel pathway of alloantigen presentation by dendritic cells. J Immunol (2004) 173(8):4828–37. doi: 10.4049/jimmunol.173.8.4828
15. Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med (1969) 280(14):735–9. doi: 10.1056/nejm196904032801401
16. Ghobrial II, Morris AG, Booth LJ. Clinical significance of In vitro donor-specific hyporesponsiveness in renal allograft recipients as demonstrated by the MLR. Transpl Int (1994) 7(6):420–7. doi: 10.1007/BF00346036
17. Ashokkumar C, Talukdar A, Sun Q, Higgs BW, Janosky J, Wilson P, et al. Allospecific CD154+ T cells associate with rejection risk after pediatric liver transplantation. Am J Transplant. (2009) 9(1):179–91. doi: 10.1111/j.1600-6143.2008.02459.x
18. van de Berg PJ, Yong SL, Koch SD, Lardy N, van Donselaar-van der Pant KA, Florquin S, et al. Characteristics of alloreactive T cells measured before renal transplantation. Clin Exp Immunol (2012) 168(2):241–50. doi: 10.1111/j.1365-2249.2011.04551.x
19. Hotta K, Aoyama A, Oura T, Yamada Y, Tonsho M, Huh KH, et al. Induced regulatory T cells in allograft tolerance via transient mixed chimerism. JCI Insight (2016) 1(10). doi: 10.1172/jci.insight.86419
20. Homs S, Mansour H, Desvaux D, Diet C, Hazan M, Buchler M, et al. Predominant Th1 and cytotoxic phenotype in biopsies from renal transplant recipients with transplant glomerulopathy. Am J transplant (2009) 9(5):1230–6. doi: 10.1111/j.1600-6143.2009.02596.x
21. Chung BH, Kim KW, Kim BM, Doh KC, Cho ML, Yang CW. Increase of Th17 cell phenotype in kidney transplant recipients with chronic allograft dysfunction. PloS One (2015) 10(12):e0145258. doi: 10.1371/journal.pone.0145258
22. Tsaur I, Gasser M, Aviles B, Lutz J, Lutz L, Grimm M, et al. Donor antigen-specific regulatory T-cell function affects outcome in kidney transplant recipients. Kidney Int (2011) 79(9):1005–12. doi: 10.1038/ki.2010.533
23. Ma L, Zhang H, Hu K, Lv G, Fu Y, Ayana DA, et al. The imbalance between tregs, Th17 cells and inflammatory cytokines among renal transplant recipients. BMC Immunol (2015) 16:56. doi: 10.1186/s12865-015-0118-8
24. Shabir S, Girdlestone J, Briggs D, Kaul B, Smith H, Daga S, et al. Transitional b lymphocytes are associated with protection from kidney allograft rejection: a prospective study. Am J Transplant. (2015) 15(5):1384–91. doi: 10.1111/ajt.13122
25. Rush D, Nickerson P, Gough J, McKenna R, Grimm P, Cheang M, et al. Beneficial effects of treatment of early subclinical rejection: a randomized study. J Am Soc Nephrol. (1998) 9(11):2129–34. doi: 10.1681/ASN.V9112129
26. Orandi BJ, Chow EH, Hsu A, Gupta N, Van Arendonk KJ, Garonzik-Wang JM, et al. Quantifying renal allograft loss following early antibody-mediated rejection. Am J Transplant. (2015) 15(2):489–98. doi: 10.1111/ajt.12982
27. Parajuli S, Joachim E, Alagusundaramoorthy S, Blazel J, Aziz F, Garg N, et al. Subclinical antibody-mediated rejection after kidney transplantation: treatment outcomes. Transplantation (2019) 103(8):1722–9. doi: 10.1097/TP.0000000000002566
28. Kawai T, Sachs DH, Sprangers B, Spitzer TR, Saidman SL, Zorn E, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant. (2014) 14(7):1599–611. doi: 10.1111/ajt.12731
29. Gorochov G, Larsen M, Parizot C, Brisson H, Nienen M, Kuchenbecker L, et al. Comment on “Tracking donor-reactive T cells: evidence for clonal deletion in tolerant kidney transplant patients”. Sci Transl Med (2015) 7(297):297le1. doi: 10.1126/scitranslmed.aab1994
30. Taner T, Gustafson MP, Hansen MJ, Park WD, Bornschlegl S, Dietz AB, et al. Donor-specific hypo-responsiveness occurs in simultaneous liver-kidney transplant recipients after the first year. Kidney Int (2018) 93(6):1465–74. doi: 10.1016/j.kint.2018.01.022
31. Ng WF, Hernandez-Fuentes M, Baker R, Chaudhry A, Lechler RI. Reversibility with interleukin-2 suggests that T cell anergy contributes to donor-specific hyporesponsiveness in renal transplant patients. J Am Soc Nephrol. (2002) 13(12):2983–9. doi: 10.1097/01.asn.0000042163.73539.d4
32. Poggio ED, Clemente M, Riley J, Roddy M, Greenspan NS, Dejelo C, et al. Alloreactivity in renal transplant recipients with and without chronic allograft nephropathy. J Am Soc Nephrol. (2004) 15(7):1952–60. doi: 10.1097/01.asn.0000129980.83334.79
33. Prunevieille A, Babiker-Mohamed MH, Aslami C, Gonzalez-Nolasco B, Mooney N, Benichou G. T Cell antigenicity and immunogenicity of allogeneic exosomes. Am J Transplant. (2021) 21(7):2583–9. doi: 10.1111/ajt.16591
Keywords: donor-specific antibody, direct alloantigen recognition pathway, immune monitoring, mixed lymphocyte reaction, kidney transplantation
Citation: Iwahara N, Hotta K, Iwami D, Tanabe T, Tanaka Y, Ito YM, Otsuka T, Murai S, Takada Y, Higuchi H, Sasaki H, Hirose T, Harada H and Shinohara N (2023) Analysis of T-cell alloantigen response via a direct pathway in kidney transplant recipients with donor-specific antibodies. Front. Immunol. 14:1164794. doi: 10.3389/fimmu.2023.1164794
Received: 13 February 2023; Accepted: 19 April 2023;
Published: 03 May 2023.
Edited by:
Jean Kwun, Duke University, United StatesReviewed by:
Min Hu, The University of Sydney, AustraliaWilliam James Burlingham, University of Wisconsin-Madison, United States
Copyright © 2023 Iwahara, Hotta, Iwami, Tanabe, Tanaka, Ito, Otsuka, Murai, Takada, Higuchi, Sasaki, Hirose, Harada and Shinohara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kiyohiko Hotta, aG90dGExMTI1QG1hYy5jb20=
 Naoya Iwahara1
Naoya Iwahara1 Kiyohiko Hotta
Kiyohiko Hotta Daiki Iwami
Daiki Iwami Tatsu Tanabe
Tatsu Tanabe Yoichi M. Ito
Yoichi M. Ito Takuya Otsuka
Takuya Otsuka Haruka Higuchi
Haruka Higuchi Hajime Sasaki
Hajime Sasaki Hiroshi Harada
Hiroshi Harada