- 1Blood Diseases Institute, Xuzhou Medical University, Xuzhou, Jiangsu, China
- 2Department of Hematology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China
- 3Jiangsu Key Laboratory of Bone Marrow Stem Cells, Xuzhou, Jiangsu, China
- 4Cancer Institute, Xuzhou Medical University, Xuzhou, Jiangsu, China
Background: Chimeric antigen receptor - T (CAR-T) cell therapy has shown remarkable efficacy in patients with relapsed/refractory multiple myeloma (R/R MM). However, a subset of patients still experienced progression or relapse, and the predictors of prognosis are little known. We analyzed the inflammatory markers before CAR-T cell infusion, to clarify their correlation with survival and toxicity.
Methods: This study involved 109 R/R MM patients who received CAR-T therapy between June 2017 and July 2021. Inflammatory markers, including ferritin, c-reactive protein (CRP), and interleukin-6 (IL-6) before CAR-T cell infusion were detected and then categorized by quartiles. Adverse events and clinical outcomes were compared between patients with upper quartile of inflammatory markers and patients with lower three quartiles of inflammatory markers. An inflammatory prognostic index (InPI) based on these three inflammatory markers was developed in this study. Patients were divided into 3 groups according to the InPI score, progression-free survival (PFS) and overall survival (OS) were compared among the groups. In addition, we explored the correlation between cytokine release syndrome (CRS) and pre-infusion inflammatory markers.
Results: We found that the pre-infusion high ferritin (hazard ratio [HR], 3.382; 95% confidence interval [CI], 1.667 to 6.863; P = .0007), high CRP (HR, 2.043; 95% CI, 1.019 to 4.097; P = .044), and high IL-6 (HR, 3.298; 95% CI, 1.598 to 6.808; P = .0013) were significantly associated with inferior OS. The formula of the InPI score was based on the HR value of these 3 variables. Three risk groups were formed: (good, 0 to 0.5 point; intermediate, 1 to 1.5 points; poor, 2 to 2.5 points). Median OS for patients with good, intermediate, and poor InPI was not reached, 24 months, and 4 months, respectively, and median PFS was 19.1 months, 12.3 months, and 2.9 months, respectively. In the cox proportional hazards model, poor InPI remained an independent prognostic factor for PFS and OS. Pre-infusion ferritin was negatively associated with CAR T-cell expansion normalized to baseline tumor burden. Spearman correlation analysis showed that pre-infusion ferritin and IL-6 levels positively correlated with the grade of CRS (P = .0369 and P = .0117, respectively). The incidence of severe CRS was higher in patients with high IL-6 compared with patients with low IL-6 (26% vs. 9%, P = .0405). Pre-infusion ferritin, CRP and IL-6 were positively correlated with each peak values within the first month after infusion.
Conclusions: Our results suggest that patients with elevated inflammation markers before CAR-T cell infusion are more likely to have poor prognosis.
Introduction
Chimeric antigen receptor - T (CAR – T) cells, which could recognize and kill tumor cells through major histocompatibility complex (MHC)-unrestricted pattern, is very promising in the era of immunotherapy (1). Multiple clinical trials have demonstrated unprecedented response rates of anti-BCMA CAR-T therapy in relapsed/refractory multiple myeloma (R/R MM) patients, regardless of previous treatment, ISS stage and cytogenetic risk (2). However, there was significant discrepancy in respect to long-term outcomes, and some patients experienced early progression or relapse (3, 4). Efforts have been made to boost and prolong the efficacy, including in vitro enriching memory phenotype T cells through culturing CAR-T cells with PI3K inhibitors (5), combination of γ-secretase inhibitor to increase the BCMA expression on the surface of MM cells (6) and “armed” CAR-T cells to transform an immune-suppressive signal into an immune-stimulatory signal (7).
Although the improvement of CAR-T cells and the exploration of new targets are undoubtedly critical, the identification of prognostic markers is also needed to help us distinguishing patients with poor prognosis for early intervention. It is generally believed that inflammation is critical for the oncogenesis and progression of tumor (8). A peripheral pro-inflammatory status has been reported to be related with worse outcomes in tumor patients treated with immune checkpoint inhibitors (9–11). Inflammatory markers, such as ferritin, c-reactive protein (CRP) and interleukin-6 (IL-6) have been widely proved to be related with cytokine release syndrome (CRS) during CAR-T cell therapy (12–15). The occurrence of severe CRS is associated with high early mortality (16), and the CRS-related complications such as delayed hematopoietic recovery, coagulopathy and cardiac disorders will also dispose patients to poor outcomes (17–19). To date, there are limited data regarding the correlation of circulating inflammatory markers and the prognosis of CAR-T therapy in the setting of MM. Herein, we conducted a retrospective study to clarify their correlation in a relatively large cohort of 109 R/R MM patients.
Patients and methods
Study population
This retrospective study included 109 patients with R/R MM treated with anti-BCMA CAR-T cells alone (Chinese Clinical Trial Registry, ChiCTR-1900026219) or combined with anti-CD19 CAR-T cells (ChiCTR-OIC-17011272) at the Affiliated Hospital of Xuzhou Medical University between June 2017 and July 2021. This study was approved by the Ethics Committee of Affiliated Hospital of Xuzhou Medical University and was conducted in accordance with the Declaration of Helsinki. The detailed inclusion and exclusion criteria could refer to previous studies (20, 21). Lymphodepletion conditioning chemotherapy was carried out in all patients, the regimen was fludarabine (30 mg/m2/d, days -5 to -3) and cyclophosphamide (750 mg/m2/d, day -5).
Data collection and therapeutic evaluation
Disease characteristics of patients were collected at enrollment, including age, gender, MM type, prior treatment, cytogenetic abnormalities. Laboratory data was obtained by retrieving electronic medical records. Baseline lactate dehydrogenase, albumin and beta-2 microglobulin data were defined as the latest data within 15 days prior to lymphodepletion. Baseline values of ferritin, CRP, and IL-6 were collected within 3 days before the CAR-T cell infusion, peak values were collected during the first month after infusion. CAR-T cell counts in peripheral blood were measured by flow cytometry at day 7, day 14, day 21, and day 28 post infusion. Efficacy was assessed according to the International Myeloma Working Group criteria (22). The severity of cytokine release syndrome (CRS) was evaluated according to the ASTCT consensus (23).
Statistical analysis
The deadline of follow-up for this study was August 31, 2022. OS was defined as the time from CAR T-cell infusion to death of any cause. Progression-free survival (PFS) was calculated from infusion to disease progression or death. Duration of response (DOR) was defined as the time from first partial response (PR) to progression or death. Quartile analysis was used to define the patients with high ferritin, high CRP and high IL-6, i.e., the upper quartile defined as high value, the lower three quartiles defined as low value. The difference between categorical variables was analyzed by Fisher’s exact test. The correlation between continuous variables was calculated by Spearman’s rank-order test. The log-rank test was used to compare the survival difference between groups. Factors with a P value <.2 or with clinical significance were included in the multivariate cox proportional hazards model. Two-sided P value <.05 was considered statistically significant. Statistical analysis was performed using SPSS 19.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
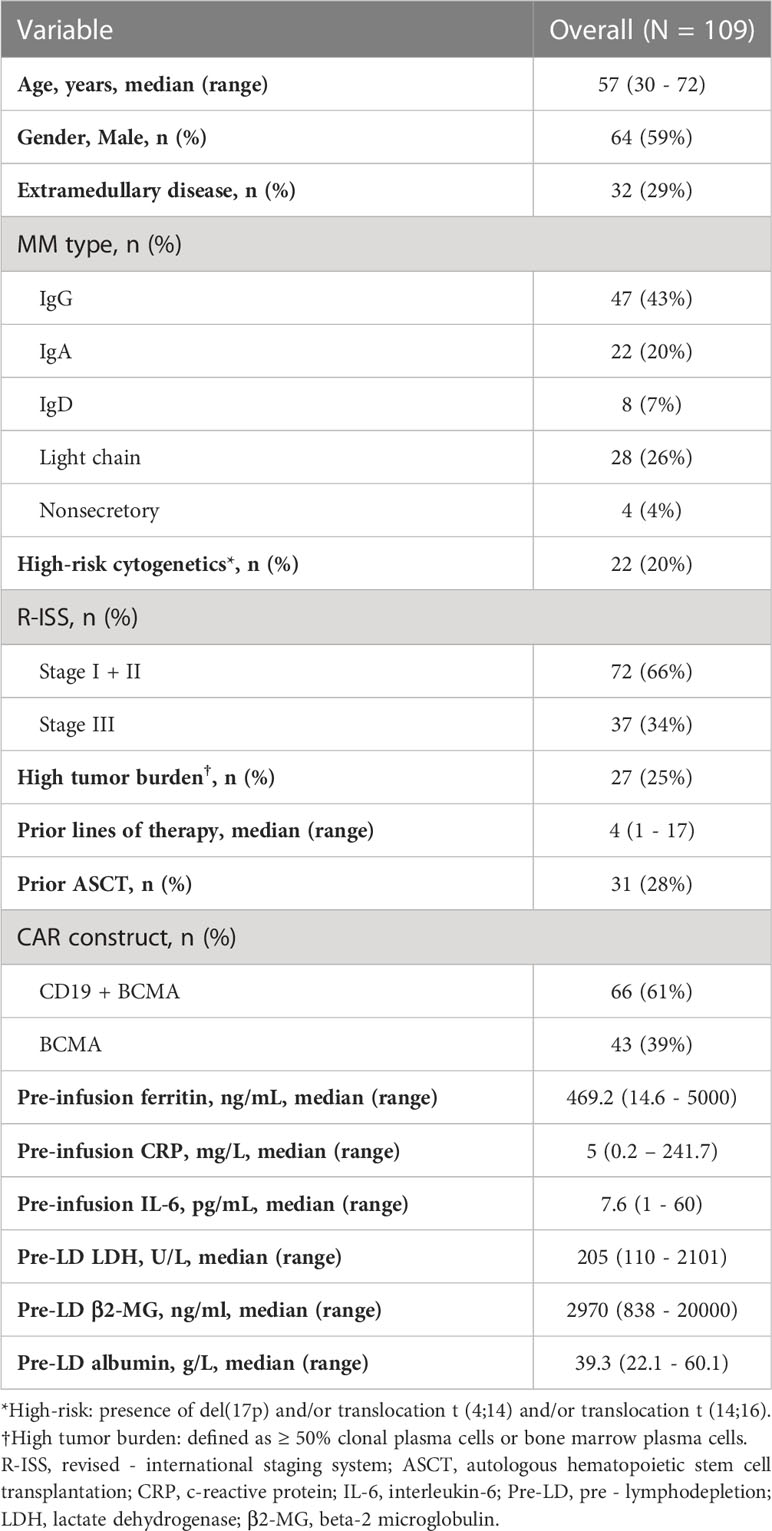
Baseline characteristics of 109 patients with R/R MM treated with CAR-T cells are summarized in Table 1. The median age was 57 years (range, 30 to 70 years). 59% of the patients were male. At enrollment, 32 (29%) of the patients had extramedullary disease (EMD), 22 (20%) had high-risk cytogenetics aberrations, 37 (34%) had revised international staging system (R-ISS) stage III diseases. Patients had a median of 4 lines of prior therapy. A total of 28% patients received prior autologous hematopoietic stem cell transplantation. The pre-infusion median ferritin was 469.2ng/mL (interquartile range [IQR], 251.8 – 882.3 ng/mL), and 62 (57%) patients had ferritin above the upper limit of normal (ULN). Median CRP was 5mg/L (IQR, 1.9 – 20.3 mg/L), and above the ULN in 53 (49%) patients. Median IL-6 was 7.6pg/mL (IQR, 3 – 14.1 pg/mL), and above the ULN in 55 (50%) patients.
Correlation between pre-infusion inflammatory markers and patient characteristics
Quartiles method was used to classify patients with high ferritin (> 882.3 ng/mL), high CRP (> 20.3 mg/L) and high IL-6 (> 14.1 pg/mL), i.e., the upper quartile was defined as high value. We then analyzed the correlation between these inflammatory markers and patients’ clinical and biological indicators. Both age, gender, high-risk cytogenetic, prior treatment and disease stage had no correlation with high-level pre-infusion inflammatory markers (Table S1). Interestingly, a significant higher proportion of patients with light chain myeloma had high ferritin (46% vs. 17%, P = .0021), high CRP (39% vs. 20%, P = .039), and high IL-6 (39% vs. 20%, P = .039) compared with those with non-light chain myeloma (Table S1). Patients with high ferritin had a higher tumor burden (median plasma cells in bone marrow, 38% vs 11%, P = .003) than those with low ferritin, also, there was a weak but significant association between ferritin levels and tumor burden (Spearman r = 0.2543, P = .0076) (Table S1 and Figure S1). However, we found no correlation between pre-infusion CRP and IL-6 with tumor burden.
Relationship between pre-infusion inflammatory markers and treatment response
The overall response rate was 85% (93/109) within 3 months after the infusion of CAR-T cells. Seventy-nine patients achieved a very good partial response (VGPR) or better response, and these patients had lower pre-infusion ferritin and IL-6, but not statistically significant (Figure S2). Except for high ferritin tended to be associated with decreased VGPR or better response rate (59% vs. 77%, P = .087), we found no significant association between other inflammatory markers and response rates (Table S2).
We further evaluated if there was a relationship between pre-infusion inflammatory markers and in vivo CAR T-cell expansion. However, Pre-infusion ferritin, CRP, and IL-6 were not associated directly with in vivo CAR T-cell expansion at indicated time points (days 7, 14, 21, and 28 post infusion) (Figure S3). Interestingly, ferritin, but not CRP and IL-6, was significantly (P <.05) but modestly (Spearman r < -0.3) associated with lower CAR T-cell expansion normalized to baseline tumor burden at days 7, 14, and 21 post infusion (Figures 1, S4).
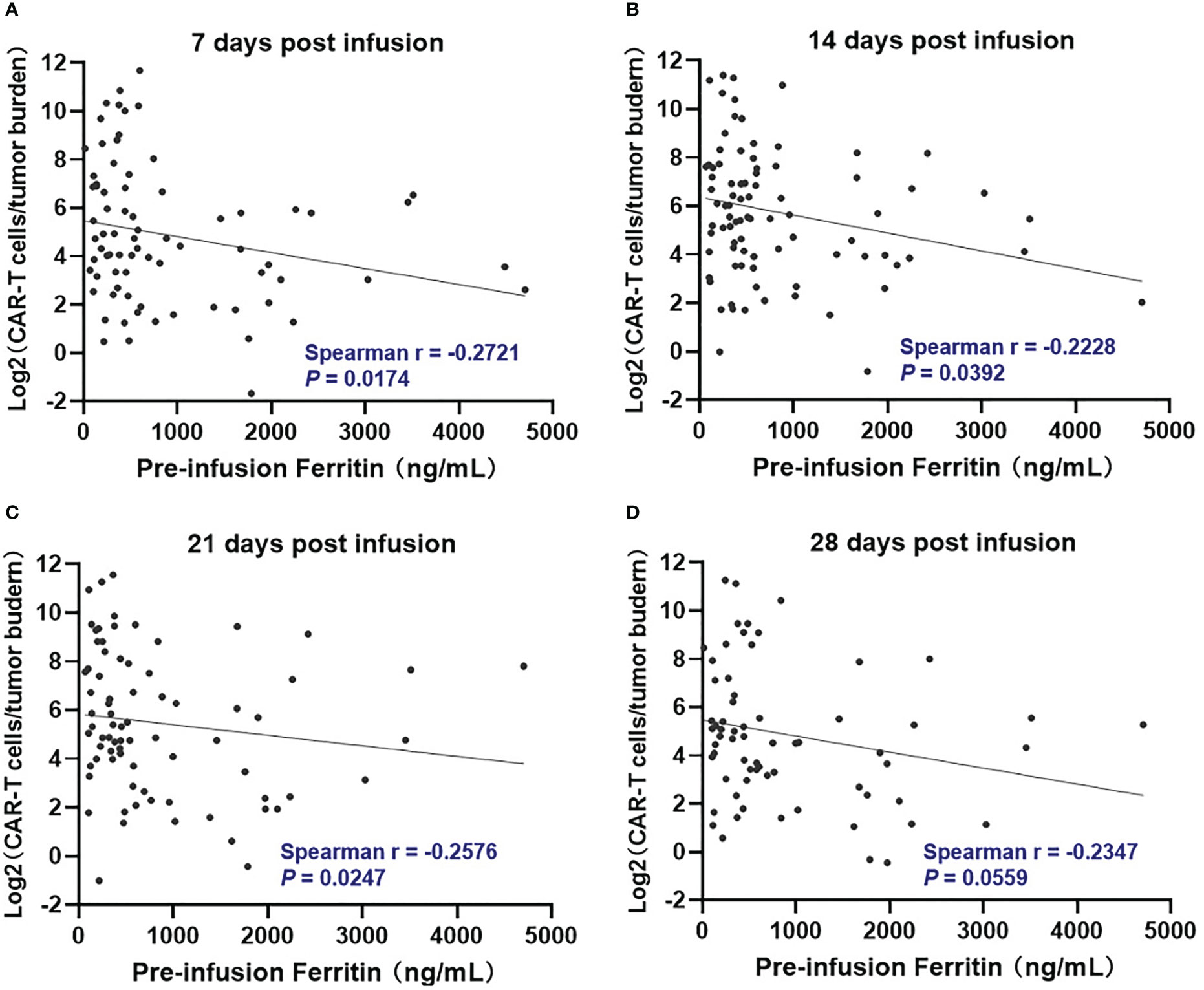
Figure 1 Correlation between pre-infusion ferritin and in vivo CAR T-cell expansion normalized to tumor burden during the first month following CAR-T cell infusion. At day 7, 14, and 21 post infusion (A–C), CAR-T cell expansion normalized to baseline tumor burden was negatively correlated with ferritin, and the correlation did not remain at day 28 (D). Tumor burden was defined as percentage of plasma cells in bone marrow. Spearman r value was calculated using the Spearman's correlation test.
High inflammatory markers were associated with decreased PFS and OS
The patients with high ferritin had significantly poorer OS and PFS compared with those with low ferritin (median OS: 14 months vs. 50.2 months, HR 3.382, P = .0007; median PFS: 5.7 months vs. 19 months, HR 2.611, P = .0015) (Figures 2A, 3A). High IL-6 also had similar adverse effects on OS and PFS (median OS: 14 months vs. not reached (NR), HR 3.298, P = .0013; median PFS: 8.4 months vs. 17.8 months, HR 2.026, P = .018) (Figures 2C, 3C). Patients with high CRP had inferior OS than patients with low CRP (median of 15.4 months vs. 36.5 months, HR 2.043, P = .044), but the difference in PFS was not significant (median of 10.3 months vs. 16.5 months, HR 1.261, P = .4142) (Figures 2B, 3B).
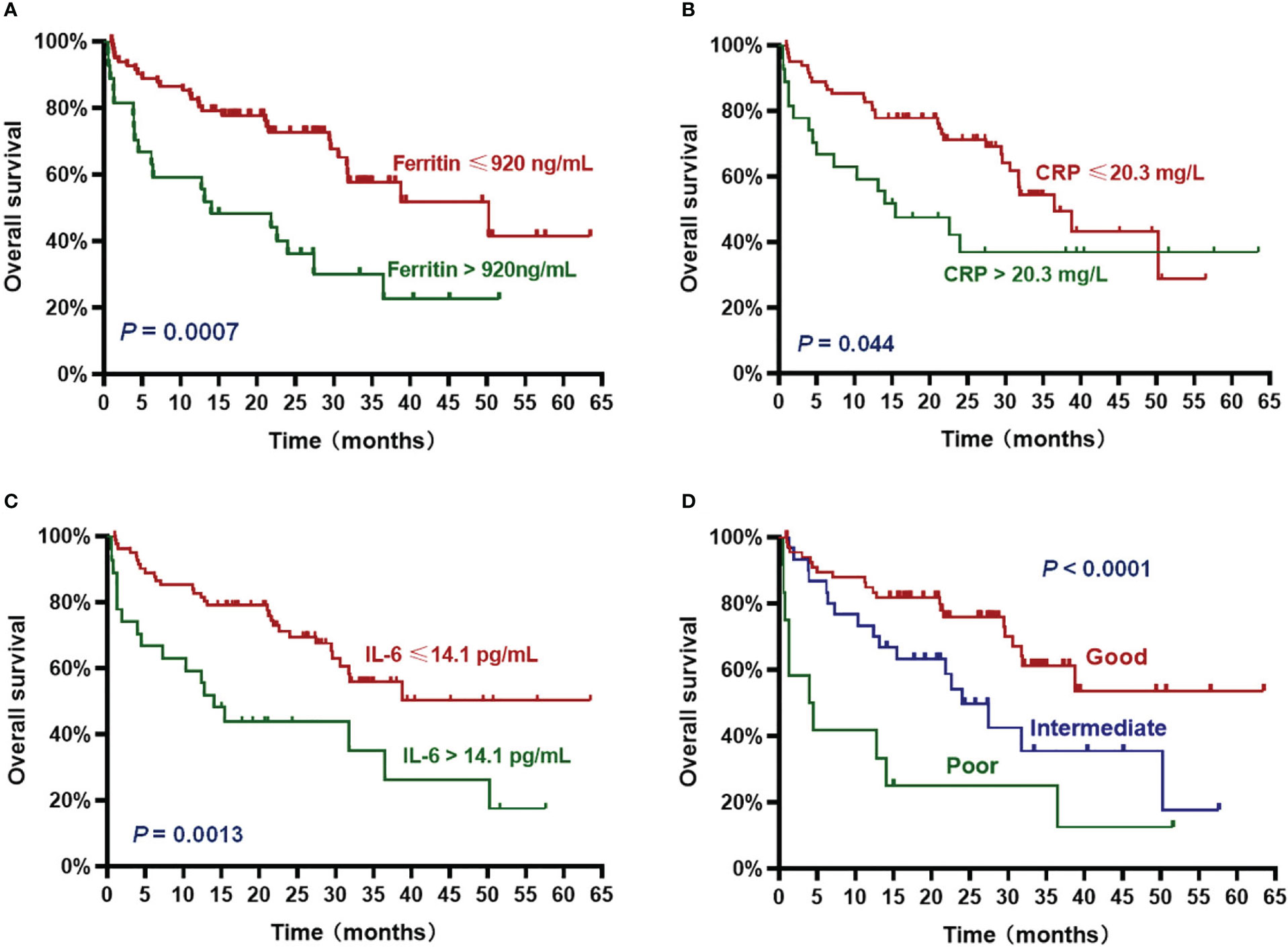
Figure 2 Overall survival (OS) according to inflammatory markers and InPI index. The patients with ferritin > 920 ng/mL (A), CRP > 20.3 mg/L (B), IL-6 > 14.1 pg/mL (C) and intermediate to poor InPI (D) had inferior OS. Survival curves were drawn according to the Kaplan-Meier method. The log-rank test was used to compare the difference in survival probability between two groups.
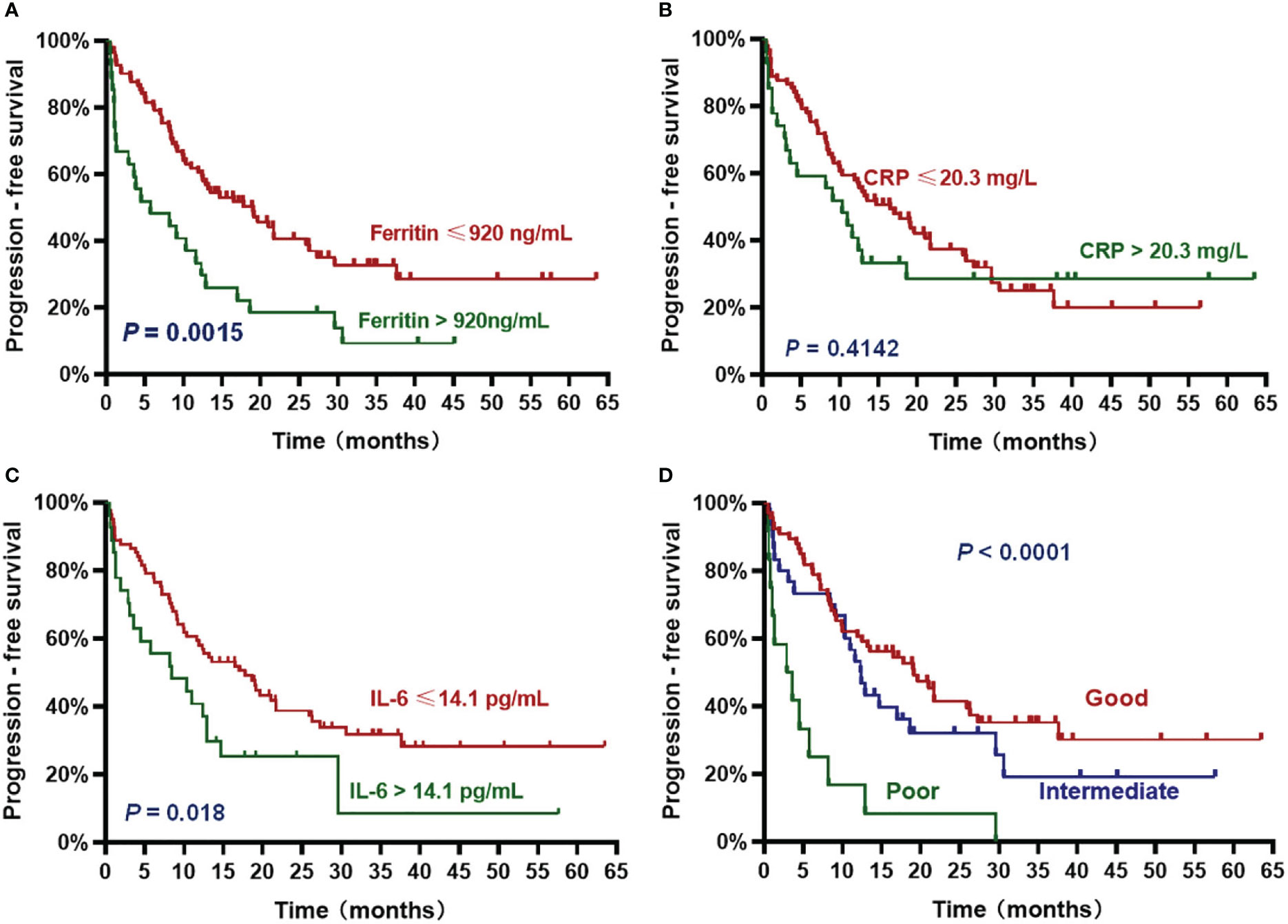
Figure 3 Progression-free survival (PFS) according to inflammatory markers and InPI index. The patients with ferritin > 920 ng/mL (A), IL-6 > 14.1 pg/mL (C) and intermediate to poor InPI (D) had inferior PFS. No significant association was found between CRP and PFS (B). Survival curves were drawn according to the Kaplan-Meier method. The log-rank test was used to compare the difference in survival probability between two groups.
Based on the HR values of ferritin, CRP and IL-6, inflammatory prognostic index (InPI) was developed, 0.5 point was assigned to high CRP, and 1 point was each assigned to high ferritin and high IL-6. According to the InPI score, patients were divided into 3 risk categories: good, 0 to 0.5 point; intermediate, 1 to 1.5 points; poor, 2 to 2.5 points. 67 (61%) of the patients had good InPI, 30 (28%) had intermediate InPI, and 12 (11%) had poor InPI. The median OS for patients with good, intermediate, and poor InPI was NR, 24 months (95% CI, 17.3 months to 30.7 months), and 4 months (95% CI, 0 to 9.4 months), respectively, and median PFS was 19.1 months (95% CI, 12.2 months to 26.0 months), 12.3 months (95% CI, 9.8 months to 14.9 months), and 2.9 months (95% CI, 0 to 6.8 months), respectively (both P <.0001) (Figures 2D, 3D).
To further determine whether InPI index was an independent prognostic factor for PFS and OS, we introduced potential influence covariates, including age, gender, EMD, number of therapy lines, type of MM, high-risk cytogenetic, R-ISS stage, tumor burden and InPI into cox proportional hazard model. The results showed that high InPI score still had independent adverse influence on OS (P = .009) and PFS (P = .01) even after adjusting for tumor burden (Table 2). In addition, high tumor burden was independent risk factor for PFS (HR: 2.512, 95% CI: 1.408 – 4.48, P = .002) and OS (HR: 2.249, 95% CI: 1.091 – 4.637, P = .028). EMD was independent risk factor for OS (HR: 2.077, 95% CI: 1.015 – 4.251, P = .046).
High inflammatory markers were associated with decreased DOR
Pre-infusion increases in ferritin and IL-6, but not CRP, were significantly associated with decreased DOR (HR 2.269, 95% CI 1.152 to 4.469, P = .0179, for high ferritin; HR 2.224, 95% CI 1.130 to 4.377, P = .0207, for high IL-6) (Figures S5A–C). The median DOR for patients with good, intermediate, and poor InPI was 21.1 months (95% CI, 14.1 months to 28.1 months), 13.8 months (95% CI, 6.4 months to 21.2 months), and 4 months (95% CI, 1.2 months to 6.8 months), respectively (Figure S5D).
The correlation between pre-infusion inflammatory markers and CRS
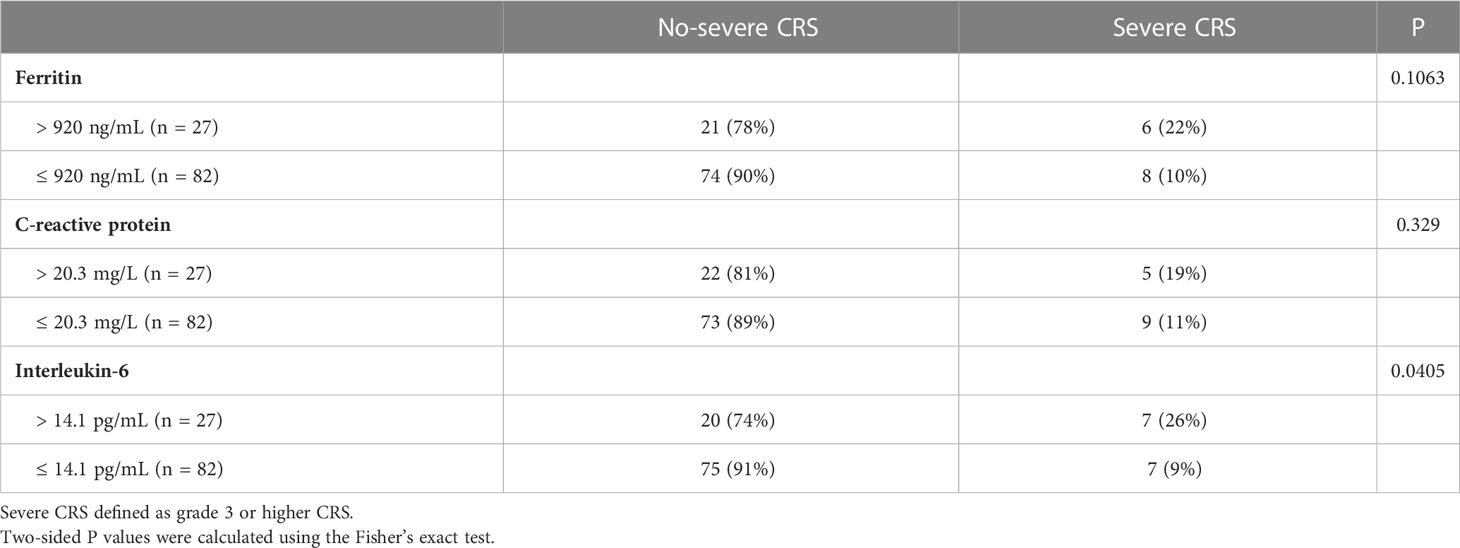
Of all patients, 91% experienced CRS. Grade 3 or higher CRS, defined as severe CRS, occurred in 14 (13%) patients. Median time to onset of CRS was 7d (0 – 28d), median duration of CRS was 4d (1d - 25d). Patients with lower pre-infusion concentration of ferritin and IL-6 were more likely to develop non-severe CRS than severe CRS (Table 3). When considering the severity of CRS as continuous variable, the levels of pre-infusion serum IL-6 (Spearman r = 0.241, P = .0117) and ferritin (Spearman r = 0.2, P = .0369) were positively correlated with the grade of CRS, no correlation was found between CRP concentration and CRS grade (Figures 4A–C). In addition, there was no correlation between inflammatory markers and the onset time of CRS or duration of CRS (Figures 4D–I). Furthermore, we found that the levels of pre-infusion ferritin, CRP and IL-6 were positively correlated with the post-infusion peak values of each of these markers (Spearman r = 0.49, P <.0001; Spearman r = 0.428, P <.0001; Spearman r = 0.352, P = .0002; respectively) (Figures 5A, E, I). Pre-infusion ferritin correlated with the peak values of CRP and IL-6 (Figures 5B, C), but the peak ferritin did not correlate with pre-infusion CRPand IL-6 (Figures 5D, G). There were positive correlations between IL-6 and CRP, regardless of baseline and peak values (Figures 5F, H). The InPI score also had positive correlation with the post-infusion peak levels of ferritin, CRP and IL-6 (Figures 5J–L).
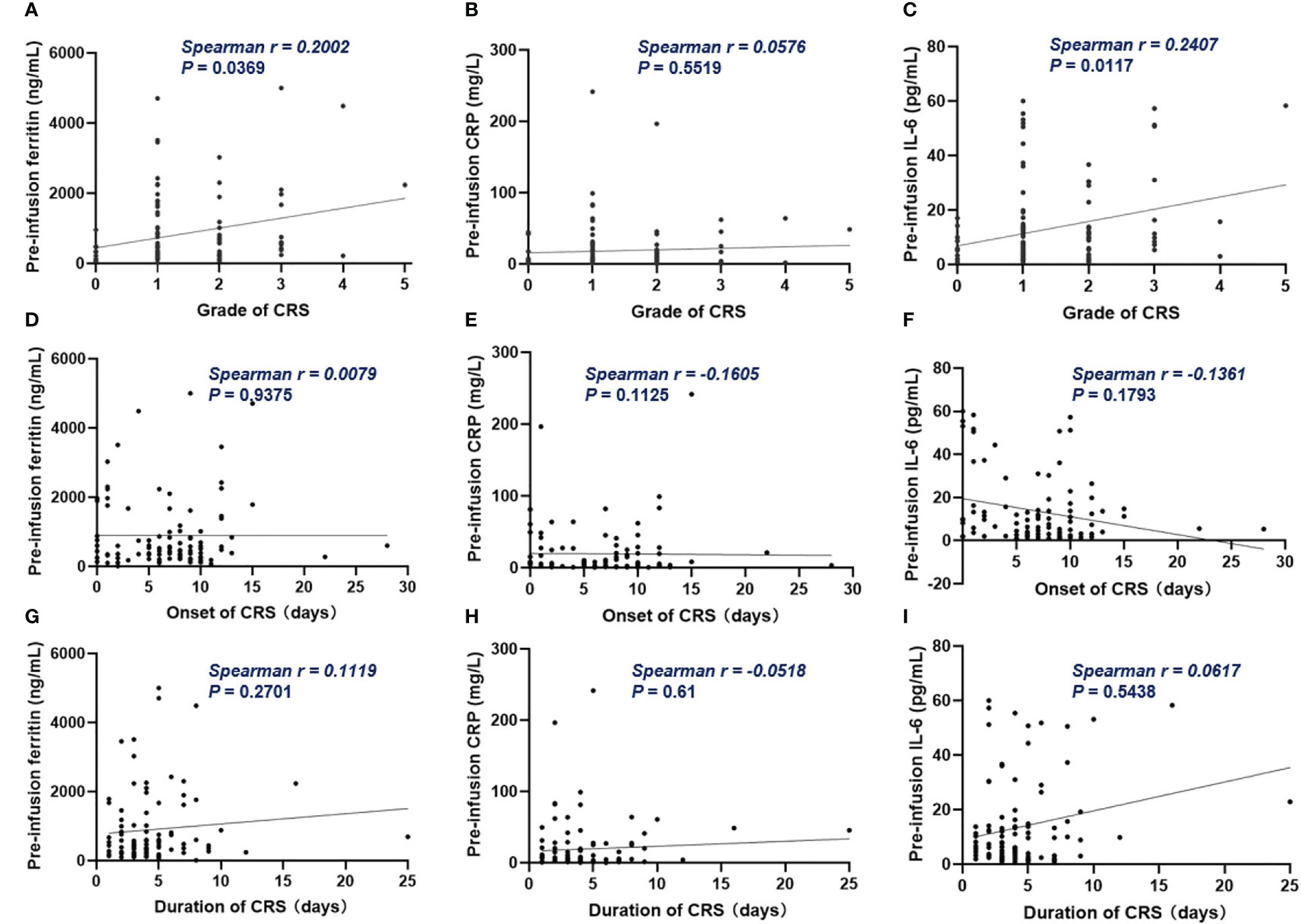
Figure 4 The correlation between inflammatory markers and CRS. (A, C) Pre-infusion ferritin and IL-6 correlated with the grade of CRS. (B) Pre-infusion CRP had no correlation with the grade of CRS. (D–I) The pre-infusion ferritin, CRP and IL-6 had no correlation with the onset and the duration of CRS. Spearman r value was calculated using the Spearman’s correlation test.
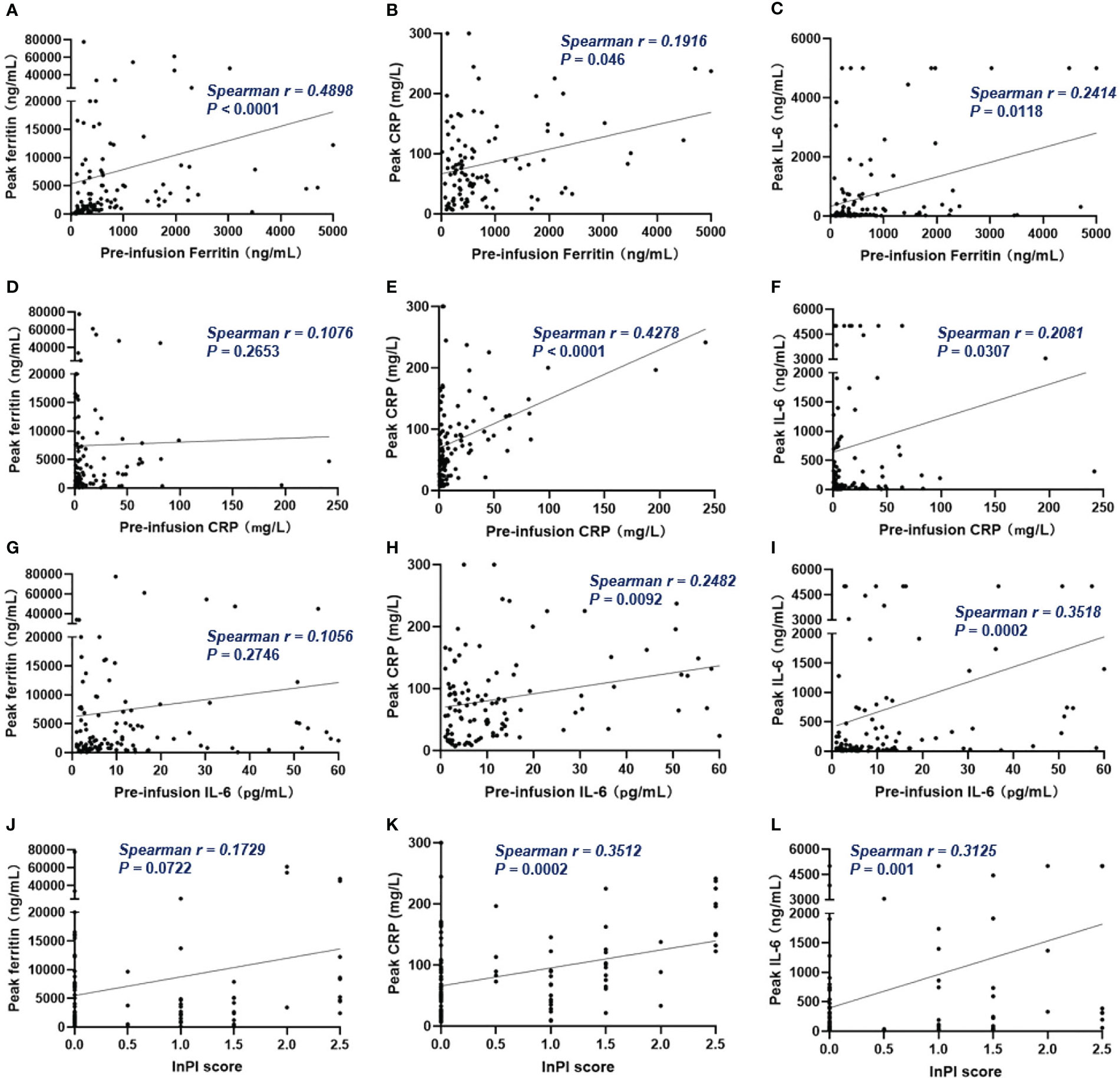
Figure 5 The correlation between pre-infusion inflammatory markers, InPI score and the post-infusion peak inflammatory markers. (A–C) Pre-infusion levels of ferritin correlated with the peak values of ferritin, CRP and IL-6. (D, G) Pre-infusion CRP and IL-6 had no correlation with peak ferritin. (E, F) Pre-infusion CRP correlated with peak CRP and IL-6. (H, I) Pre-infusion IL-6 correlated with peak CRP and IL-6. (J–L) InPI score positively correlated with peak ferritin, CRP, and IL-6. Spearman r value was calculated using the Spearman’s correlation test.
Discussion
Due to the high financial cost and the potentially life-threatening toxicities, it is of particular importance to early identify patients who will not benefit or less benefit from CAR-T cell therapy. Except for EMD, there are still no validated biomarkers for predicting prognosis in R/R MM patients following CAR-T therapy.
A cohort of 17 patients treated with LCAR-B38M revealed that EMD and anti-CAR T antibody constituted risk factors for early recurrence and progression (24). Que and colleagues found that the patients who had more than 6 lines of prior therapy had shorter PFS and OS, but when incorporating EMD into multivariate analysis, > 6 lines of therapy lost its predictive value, only EMD being an independently significant prognostic factor (25). Recently, zhang et al. reported that EMD, light chain type, high-risk cytogenetics, and > 3 prior therapeutic lines were independent risk factors of PFS, ECOG score of 2 and light chain type were independent risk factors of OS (26). Our results also showed that EMD was an independent risk factor that associated with OS. Besides, light chain type MM was identified as one of the risk factors for inferior OS in the univariate analysis, a marginal significant correlation remained in the multivariate analysis, though the exact mechanism is not clear. We think it deserve further study to verify and make clear of these findings.
An in-depth analysis from ZUMA-1 study in large B-cell lymphoma demonstrated that the levels of ferritin and IL-6 before CAR-T cell infusion had negative correlation with durable remission rates (27). One real world study of Axicabtagene Ciloleucel in patients with R/R B-cell non-Hodgkin lymphoma suggested that low CRP levels at baseline was associated with better response (28). However, the effect of inflammation on the prognosis of CAR-T therapy has not been reported in myeloma patients. Hence, we conducted a post-hoc analysis to elucidate their correlation. Our study proved that both pre-infusion high ferritin, high IL-6, and high CRP were risk factors for long-term survival, and patients with high ferritin and high IL-6 had shorter duration of remission. Further, we established a scoring system based on these three inflammatory indicators, defined as InPI index in this context. The InPI index helped us distinguished three groups of patients with different prognosis, the patients with a score of 2 to 2.5 points had the worst survival. Since high inflammation might partly be a proxy for disease burden or aggressive disease, we therefore brought variables that pertain to disease burden into multivariate analysis to determine the independent effect of InPI index. The results showed that after account for high tumor burden and EMD, poor InPI remained an independent predictor for durable remission and survival.
Inflammation has been extensively studied in the malignant progression of tumors, either by acting on cancerous cells or by acting on anti-tumor immunity (29, 30). The pro-inflammatory cytokine IL-6 might impair anti-tumor immunity through multi-aspect. It could restrict the differentiation of Th1 cells and decrease the production of interferon-γ, resulting in less mounting of CD8+ cytotoxic T cells in anti-tumor response (31). Besides, IL-6 could promote the differentiation of Th2 and Th17 cells, thereby tilt anti-tumor immune response to an immunosuppressive response (32, 33). Acute phase proteins such as CRP, fibrinogen and ferritin could be rapidly synthesized in liver after the stimulation of IL-6 (34). Our study also showed that high-level ferritin at baseline might adversely influence in vivo CAR-T amplification. These and our results led to a hypothesis that inflammation might be one of the most relevant factors for disease progression and long-term survival in CAR-T therapy, partially by affecting the activation and expansion of effector T cells.
Despite the superior efficacy of CAR-T therapy, its benefits might be offset by the serious adverse effects after infusion. CRS and immune effector cell-associated neurotoxicity syndrome (ICANS) are common adverse effects which are associated with endothelial activation injury and cytokine release. Due to the low incidence of ICANS in our center, we did not further discuss it. According to our recently published data, the occurrence of severe CRS was associated with poorer survival (15). Therefore, it is necessary to identify patients who will develop severe CRS preemptively. In this study, we found that the patients with high ferritin and IL-6 before CAR-T infusion had increased rates of severe CRS, and these inflammatory markers’ baseline levels positively correlated with the peak values after infusion. Peak levels of inflammatory markers had been reported to be correlated with the occurrence and the severity of CRS (35–38). Hay and colleagues reported that the pre-existing endothelial activation before conditioning and CAR-T cell infusion might increase the risk of severe CRS in patients receiving anti-CD19 CAR-T treatment (39). Researchers from University of Pennsylvania found that blood vessel endothelial cells are a key source of IL-6 during CRS (40). Together, these findings indicated that elevated pre-infusion inflammation might increase the risk of developing severe CRS. Although the small sample size of severe CRS in our cohort may limit the statistical power to detect the effect of pre-infusion inflammation on CRS, these might be important markers of concern. As the current predictive power of peak inflammatory markers usually occurs after severe symptoms have already appeared.
To our knowledge, this is the first study to illustrate the importance of pre-infusion inflammation on prognosis in R/R MM patients receiving CAR-T cell therapy. However, there are some limitations to our study. It is a single-center retrospective study. We had limited data to interpret the effect of inflammation at the time of apheresis on prognosis. In addition, because our findings were from a retrospective study, we did not take special interventions to ameliorate inflammation before CAR-T cell infusion. We also appreciate that there will be prospective studies to validate our findings by using commercial CAR-T products.
Conclusion
In conclusion, pre-infusion inflammation markers were useful predictors of durable remission and long-term survival, and might be risk factors for the subsequent development of severe CRS. Our data suggest that treating patients’ pre-infusion inflammation earlier in their course may improve durability of response to CAR-T cell therapy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics committee of the Affiliated Hospital of Xuzhou Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
ZL, KX and CC designed the study. YW, WC, JC, ZY, HC, FZ, WS, QW, CC and DL were responsible for patient enrollment. YL, XJ, LN, CW, JM and JJ collected the data. MS was responsible for the generation of CAR-T cells. YL and LN performed statistical analysis. YL wrote the manuscript. ZL, JQ, CC and KX revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by funding from the National Natural Science Foundation of China (grant 81770223, 82171762 and 82270232), Research and practice innovation program for Postgraduates of Jiangsu Province (KYCX22_2940).
Acknowledgments
We would like to thank all the patients and their families who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1169071/full#supplementary-material
References
1. Benmebarek MR, Karches CH, Cadilha BL, Lesch S, Endres S, Kobold S, et al. Killing mechanisms of chimeric antigen receptor (CAR) T cells. Int J Mol Sci (2019) 20(6). doi: 10.3390/ijms20061283
2. Parikh RH, Lonial S. Chimeric antigen receptor T-cell therapy in multiple myeloma: a comprehensive review of current data and implications for clinical practice. CA Cancer J Clin (2023). doi: 10.3322/caac.21771
3. Munshi NC, Anderson LJ, Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med (2021) 384(8):705–16. doi: 10.1056/NEJMoa2024850
4. Martin T, Usmani SZ, Berdeja JG, Agha M, Cohen AD, Hari P, et al. Ciltacabtagene autoleucel, an anti-b-cell maturation antigen chimeric antigen receptor T-cell therapy, for Relapsed/Refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol (2022) 41(6):1265-74. doi: 10.1200/JCO.22.00842
5. Raje NS, Shah N, Jagannath S, Kaufman JL, Siegel DS, Munshi NC, et al. Updated clinical and correlative results from the phase I CRB-402 study of the BCMA-targeted CAR T cell therapy bb21217 in patients with relapsed and refractory multiple myeloma. Blood (2021) 138(Supplement 1):548. doi: 10.1182/blood-2021-146518
6. Cowan AJ, Pont M, Dukesather B, TurtleMBBS CJ, Till BG, Nagengast AM, et al. Efficacy and safety of fully human bcma CAR T cells in combination with a gamma secretase inhibitor to increase bcma surface expression in patients with relapsed or refractory multiple myeloma. Blood (2019) 134(Supplement 1):204. doi: 10.1182/blood-2019-129405
7. Liu H, Lei W, Zhang C, Yang C, Wei J, Guo Q, et al. CD19-specific CAR T cells that express a PD-1/CD28 chimeric switch-receptor are effective in patients with PD-L1-positive b-cell lymphoma. Clin Cancer Res (2021) 27(2):473–84. doi: 10.1158/1078-0432.CCR-20-1457
8. Coussens LM, Werb Z. Inflammation and cancer. Nature (2002) 420(6917):860–7. doi: 10.1038/nature01322
9. Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol (2018) 4(3):351–7. doi: 10.1001/jamaoncol.2017.4771
10. Laino AS, Woods D, Vassallo M, Qian X, Tang H, Wind-Rotolo M, et al. Serum interleukin-6 and c-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J Immunother Cancer (2020) 8(1). doi: 10.1136/jitc-2020-000842
11. Keegan A, Ricciuti B, Garden P, Cohen L, Nishihara R, Adeni A, et al. Plasma IL-6 changes correlate to PD-1 inhibitor responses in NSCLC. J Immunother Cancer (2020) 8(2):e678. doi: 10.1136/jitc-2020-000678
12. Greenbaum U, Strati P, Saliba RM, Torres J, Rondon G, Nieto Y, et al. CRP and ferritin in addition to the EASIX score predict CAR-t-related toxicity. Blood Adv (2021) 5(14):2799–806. doi: 10.1182/bloodadvances.2021004575
13. Tedesco VT, Mohan C. Biomarkers for predicting cytokine release syndrome following CD19-targeted CAR T cell therapy. J Immunol (2021) 206(7):1561–8. doi: 10.4049/jimmunol.2001249
14. Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov (2016) 6(6):664–79. doi: 10.1158/2159-8290.CD-16-0040
15. Wang X, Zhao L, Wang J, Yao Y, Wang J, Ji S, et al. Correlation of cytokine release syndrome with prognosis after chimeric antigen receptor T cell therapy: analysis of 54 patients with relapsed or refractory multiple myeloma. Front Immunol (2022) 13:814548. doi: 10.3389/fimmu.2022.814548
16. Hernani R, Benzaquen A, Solano C. Toxicities following CAR-T therapy for hematological malignancies. Cancer Treat Rev (2022) 111:102479. doi: 10.1016/j.ctrv.2022.102479
17. Qi K, Yan Z, Cheng H, Chen W, Wang Y, Wang X, et al. An analysis of cardiac disorders associated with chimeric antigen receptor T cell therapy in 126 patients: a single-centre retrospective study. Front Oncol (2021) 11:691064. doi: 10.3389/fonc.2021.691064
18. Li H, Zhao L, Sun Z, Yao Y, Li L, Wang J, et al. Prolonged hematological toxicity in patients receiving BCMA/CD19 CAR-t-cell therapy for relapsed or refractory multiple myeloma. Front Immunol (2022) 13:1019548. doi: 10.3389/fimmu.2022.1019548
19. Jiang H, Liu L, Guo T, Wu Y, Ai L, Deng J, et al. Improving the safety of CAR-T cell therapy by controlling CRS-related coagulopathy. Ann Hematol (2019) 98(7):1721–32. doi: 10.1007/s00277-019-03685-z
20. Yan Z, Cao J, Cheng H, Qiao J, Zhang H, Wang Y, et al. A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: a single-arm, phase 2 trial. Lancet Haematol (2019) 6(10):e521–9. doi: 10.1016/S2352-3026(19)30115-2
21. Wang Y, Li C, Xia J, Li P, Cao J, Pan B, et al. Humoral immune reconstitution after anti-BCMA CAR T-cell therapy in relapsed/refractory multiple myeloma. Blood Adv (2021) 5(23):5290–9. doi: 10.1182/bloodadvances.2021004603
22. Palumbo A, Rajkumar SV, San MJ, Larocca A, Niesvizky R, Morgan G, et al. International myeloma working group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol (2014) 32(6):587–600. doi: 10.1200/JCO.2013.48.7934
23. Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant (2019) 25(4):625–38. doi: 10.1016/j.bbmt.2018.12.758
24. Xu J, Chen LJ, Yang SS, Sun Y, Wu W, Liu YF, et al. Exploratory trial of a biepitopic CAR T-targeting b cell maturation antigen in relapsed/refractory multiple myeloma. Proc Natl Acad Sci U S A (2019) 116(19):9543–51. doi: 10.1073/pnas.1819745116
25. Que Y, Xu M, Xu Y, Almeida V, Zhu L, Wang Z, et al. Anti-BCMA CAR-T cell therapy in Relapsed/Refractory multiple myeloma patients with extramedullary disease: a single center analysis of two clinical trials. Front Immunol (2021) 12:755866. doi: 10.3389/fimmu.2021.755866
26. Zhang M, Zhou L, Zhao H, Zhang Y, Wei G, Hong R, et al. Risk factors associated with durable progression-free survival in patients with relapsed or refractory multiple myeloma treated with anti-BCMA CAR T-cell therapy. Clin Cancer Res (2021) 27(23):6384–92. doi: 10.1158/1078-0432.CCR-21-2031
27. Locke FL, Rossi JM, Neelapu SS, Jacobson CA, Miklos DB, Ghobadi A, et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large b-cell lymphoma. Blood Adv (2020) 4(19):4898–911. doi: 10.1182/bloodadvances.2020002394
28. Jacobson CA, Hunter B, Armand P, Kamihara Y, Ritz J, Rodig SJ, et al. Axicabtagene ciloleucel in the real world: outcomes and predictors of response, resistance and toxicity. Blood (2018) 132(Supplement 1):92. doi: 10.1182/blood-2018-99-117199
29. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity (2019) 51(1):27–41. doi: 10.1016/j.immuni.2019.06.025
30. Shalapour S, Karin M. Pas de deux: control of anti-tumor immunity by cancer-associated inflammation. Immunity (2019) 51(1):15–26. doi: 10.1016/j.immuni.2019.06.021
31. Diehl S, Anguita J, Hoffmeyer A, Zapton T, Ihle JN, Fikrig E, et al. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity (2000) 13(6):805–15. doi: 10.1016/S1074-7613(00)00078-9
32. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature (2006) 441(7090):235–8. doi: 10.1038/nature04753
33. De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med (2011) 208(3):469–78. doi: 10.1084/jem.20101876
34. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol (2014) 6(10):a16295. doi: 10.1101/cshperspect.a016295
35. Yan Z, Zhang H, Cao J, Zhang C, Liu H, Huang H, et al. Characteristics and risk factors of cytokine release syndrome in chimeric antigen receptor T cell treatment. Front Immunol (2021) 12:611366. doi: 10.3389/fimmu.2021.611366
36. Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult b cell ALL patients. J Clin Invest (2016) 126(6):2123–38. doi: 10.1172/JCI85309
37. Wang Z, Han W. Biomarkers of cytokine release syndrome and neurotoxicity related to CAR-T cell therapy. biomark Res (2018) 6:4. doi: 10.1186/s40364-018-0116-0
38. Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med (2018) 24(6):739–48. doi: 10.1038/s41591-018-0036-4
39. Hay KA, Hanafi LA, Li D, Gust J, Liles WC, Wurfel MM, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood (2017) 130(21):2295–306. doi: 10.1182/blood-2017-06-793141
Keywords: chimeric antigen receptor T cell, relapsed/refractory, multiple myeloma, prognostic predictor, inflammation
Citation: Liu Y, Jie X, Nian L, Wang Y, Wang C, Ma J, Jiang J, Wu Q, Qiao J, Chen W, Cao J, Yan Z, Shi M, Cheng H, Zhu F, Sang W, Li D, Chen C, Xu K and Li Z (2023) A combination of pre-infusion serum ferritin, CRP and IL-6 predicts outcome in relapsed/refractory multiple myeloma patients treated with CAR-T cells. Front. Immunol. 14:1169071. doi: 10.3389/fimmu.2023.1169071
Received: 18 February 2023; Accepted: 03 April 2023;
Published: 19 April 2023.
Edited by:
Esra Akbay, University of Texas Southwestern Medical Center, United StatesReviewed by:
Said Dermime, National Center for Cancer Care and Research, QatarSimrit Parmar, University of Texas MD Anderson Cancer Center, United States
Copyright © 2023 Liu, Jie, Nian, Wang, Wang, Ma, Jiang, Wu, Qiao, Chen, Cao, Yan, Shi, Cheng, Zhu, Sang, Li, Chen, Xu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenyu Li, bGl6aGVueXVtZEAxNjMuY29t; Kailin Xu, bGlobWRAMTYzLmNvbQ==; Chong Chen, Y2NoZW5AeHpobXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Yang Liu
Yang Liu Xingxing Jie1,2,3†
Xingxing Jie1,2,3† Ying Wang
Ying Wang Qingyun Wu
Qingyun Wu Jianlin Qiao
Jianlin Qiao Wei Chen
Wei Chen Jiang Cao
Jiang Cao Zhiling Yan
Zhiling Yan Hai Cheng
Hai Cheng Feng Zhu
Feng Zhu Wei Sang
Wei Sang Chong Chen
Chong Chen Kailin Xu
Kailin Xu Zhenyu Li
Zhenyu Li