- 1Department of Infectious Diseases, Zhongnan Hospital of Wuhan University, Wuhan, China
- 2Wuhan Research Center for Infectious Diseases and Cancer, Chinese Academy of Medical Sciences, Wuhan, China
- 3Wuchang District Center for Disease Control and Prevention, Wuhan, China
- 4Department of Nosocomial Infection Management, Zhongnan Hospital of Wuhan University, Wuhan, China
- 5Department of Pathology, Zhongnan Hospital of Wuhan University, Wuhan, China
- 6Department of Pathology, School of Basic Medical Sciences, Wuhan University, Wuhan, China
- 7Institute for Healthcare Artificial Intelligence, Guangdong No.2 Provincial People's Hospital, Guangzhou, China
- 8The University of North Carolina at Chapel Hill Project-China, Guangzhou, China
- 9Hubei Engineering Center for Infectious Disease Prevention, Control and Treatment, Wuhan, China
Background: Currently, data on long-term immune responses to a homogenous booster dose of the inactivated COVID-19 vaccine are still limited among people living with HIV (PLWH).
Methods: A prospective cohort study with a 13-month follow-up was conducted in China between March 2021 and August 2022 to evaluate the dynamics of SARS-CoV-2 specific humoral and cellular immunity against three doses of the inactivated COVID-19 vaccine from before the first dose until 6 months after the booster dose vaccination among PLWH in comparison to healthy controls (HC).
Results: 43 PLWH on antiretroviral therapy (ART) and 23 HC were enrolled. Compared with HC, the neutralizing antibodies (nAbs) levels among PLWH were significantly lower on days 14, 30, 60, 90, and 120 after the booster dose vaccination. Among PLWH, the nAbs titers on days 14, 30, and 60 after the booster dose were significantly higher than the peak of the second dose. However, on day 180 after the booster dose, the nAbs titers were similar to the peak of the second dose vaccination. Compared with HC, the frequencies of IFN-γ-secreting and TNF-α-secreting CD4+ and CD8+ T cells among PLWH were lower on days 14 and 180 after the booster dose vaccination. Among PLWH, increased T cell immunity was induced by the booster dose of the vaccine and kept stable on day 180 after the booster dose vaccination.
Conclusion: Although a homogenous booster dose following two doses of the inactivated COVID-19 vaccine among PLWH could elicit higher nAb titers, reduce antibody decay, and maintain T cell responses even 6 months after vaccination, the overall immunogenicity of the booster dose was found to be lower among PLWH than among healthy controls. Further strategies are needed to improve immunogenicity to the inactivated COVID-19 vaccine among PLWH.
Introduction
A high risk of COVID-19 mortality has been observed among people living with HIV infection (PLWH). Large-scale population-based retrospective cohort studies in South Africa and England reported that COVID-19 mortality increased 2~3-fold more in PLWH than in those without HIV infection (1, 2). Strengthening the prevention and control of SARS-CoV-2 infection in PWLH is crucial for global COVID-19 epidemic control.
COVID-19 vaccination is the primary strategy for preventing SARS-CoV-2 infection. The efficacy of two doses of COVID-19 inactivated vaccine for preventing symptomatic infections, hospitalizations, and deaths among healthy individuals has been verified by randomized clinical trials (3–5). In PLWH, persisting immunopathology may lead to impaired responses to vaccine despite virologic suppression (6). Our previous study indicated lower antibody responses against two doses of COVID-19 inactivated vaccine among PLWH than among healthy individuals (7, 8), and both our previous research and other studies observed decay in humoral immunity within 6 months after two doses of the inactivated or mRNA COVID-19 vaccination among people with immunosuppression, which may increase the risk of SARS-CoV-2 infection (9–11). Therefore, the booster vaccine is strongly proposed and expected to benefit PLWH.
Consistent with the results of previous studies on the booster the inactivated or mRNA COVID-19 vaccination in the general population (12, 13), we found that the level of neutralizing antibodies (nAbs) increased markedly and rapidly in PLWH on day 14 after a homogenous booster dose vaccination (14). However, data on the persistence of humoral and cellular immune responses following a homogenous booster dose of the inactivated COVID-19 vaccination among PLWH are still scarce. Here, we extend our previous research (8, 14) by conducting a prospective cohort study to investigate the longitudinal dynamics of the humoral and cellular responses of three doses of the inactivated COVID-19 vaccine among PLWH on stable antiretroviral therapy (ART) compared to healthy people.
Methods
Study participants
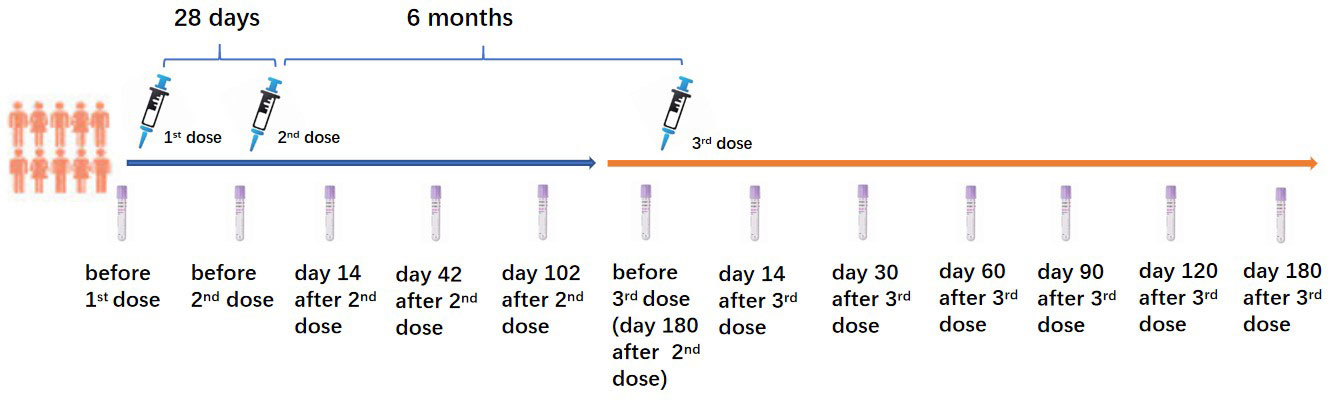
This prospective cohort study was performed in Wuhan, Hubei Province, China, between March 2021 and August 2022. The 43 PLWH and 23 healthy controls (HC) who voluntarily participated in this study and received a homogenous booster dose of the inactivated COVID-19 vaccination were recruited from our previous study (8). All PLWH received stable ART for at least one year. PLWH and HC who had a history of SARS-CoV-2 infection confirmed by SARS-CoV-2 IgG, IgM tests, and a SARS-CoV-2 nucleic acid test before the first dose of the inactivated COVID-19 vaccination were excluded. All participants received two primary doses of the inactivated COVID-19 vaccine (Sinopharm, Wuhan Institute of Biological Products Co. Ltd) with an interval of 28 days. The homogenous booster dose of the vaccine (Sinopharm, Wuhan Institute of Biological Products Co. Ltd) was administered 6 months after the second dose vaccination. CD4+ T lymphocyte count (CD4 count) was tested in all PLWH before the first dose vaccination. The China National HIV/AIDS Comprehensive Response Information Management System (CRIMS) was used to acquire the clinical and laboratory data of PLWH. Peripheral blood of PLWH and HC were collected for the immunogenicity analyses at the following time points (as shown in Figure 1): 3 days before the first dose (D0B1); 3 days before the second dose (D0B2), on days 14 (D14A2), 42 (D42A2), 102 (D102A2) after the second dose; 3 days before the booster dose (D0B3), on days 14 (D14A3), 30 (D30A3), 60 (D60A3), 90 (D90A3), 120 (D120A3), and 180 (D180A3) after the booster dose vaccination.
SARS-CoV-2 specific antibody responses examination
SARS-CoV-2 specific antibody responses of three doses of the inactivated vaccine were evaluated at all of the previously stated time points. The assessment of SARS-CoV-2 spike protein receptor-binding domain (RBD)-specific neutralizing antibodies (nAbs) was performed with in-house SARS-CoV-2 nAbs assay kits (Livzon, China). Semi-quantitative SARS-CoV-2 recombinant nucleocapsid (N) and RBD antigen-specific IgM and IgG antibodies were assessed with in-house-developed ELISA kits (Livzon, China). Seroconversion of nAbs was defined as the transition from baseline seronegative to seropositive or a 4-fold increase in antibody titers in seropositive participants at baseline (7).
Isolation of PBMCs
Peripheral blood mononuclear cells (PBMCs) were isolated using the Ficoll-Paque method. Cell pellets were treated with 5 ml RBC lysis buffer (Sigma-Aldrich) for 10 min followed by washing once with 5% FBS-PBS. PBMCs were counted and cryopreserved with fetal calf serum (FCS) containing 10% dimethyl sulfoxide (DMSO) in liquid nitrogen.
Intracellular cytokine staining and flow cytometry
SARS-CoV-2 specific CD4+ T and CD8+ T cell immunity against the inactivated COVID-19 vaccine were evaluated by intracellular cytokine staining (ICS) combined flow cytometry among PLWH and HC on day 14 after the second dose, before the booster dose, and on days 14, 30, and 180 after the booster dose vaccination. 1×106 PBMCs were incubated overnight with SARS-CoV-2 Spike peptide pool (1mg/ml) containing 23 peptides from the wild-type SARS-CoV-2 virus in a 200μL final volume medium in round-bottom 96-well plates at 37°C and 5% CO2. Brefeldin A (10μg/ml) (GolgiPlug, BD Biosciences) was added in the last 5 hours of incubation. Surface marker staining was performed with anti-CD3-PerCP-cy5.5 (clone UCHT1; Biolegend), anti-CD8-APC-Cy7 (clone SK1; Biolegend), and anti-CD4-PE-Cy7 (clone RPA-T4; Biolegend) for 30 min at 4°C in the dark. Fixation and permeabilization solution (Cytofix/Cytoperm; BD Biosciences) was added for 45 min at 4°C in the dark. Intracellular cytokines staining with anti-IFN-γ-PE (clone 4S.B3; Biolegend) and anti-TNF-α-APC (clone MAb11; Biolegend) was then performed according to the manufacturer’s instructions. The stained cells were fixed with 2% paraformaldehyde and then analyzed by flow cytometry. Data were analyzed with FlowJo software version 10, and the gating strategy is shown in Supplementary Figure 1.
Statistical analysis
SPSS 21.0 and GraphPad Prism 5.0 were used for data statistics and plotting. Variables were denoted as median (interquartile range, IQR) or proportion (%). The Kolmogorov-Smirnov test was used to analyze whether the measurement data conformed to the normal distribution. The group t-test was used to compare the continuous variables that conformed to the normal distribution, and the Mann-Whitney U test was used to compare continuous variables that weren’t normally distributed. The chi-square test or Fisher’s exact test was used to compare the categorical variables between the groups. p<0.05 was considered significant.
Results
Participant characteristics
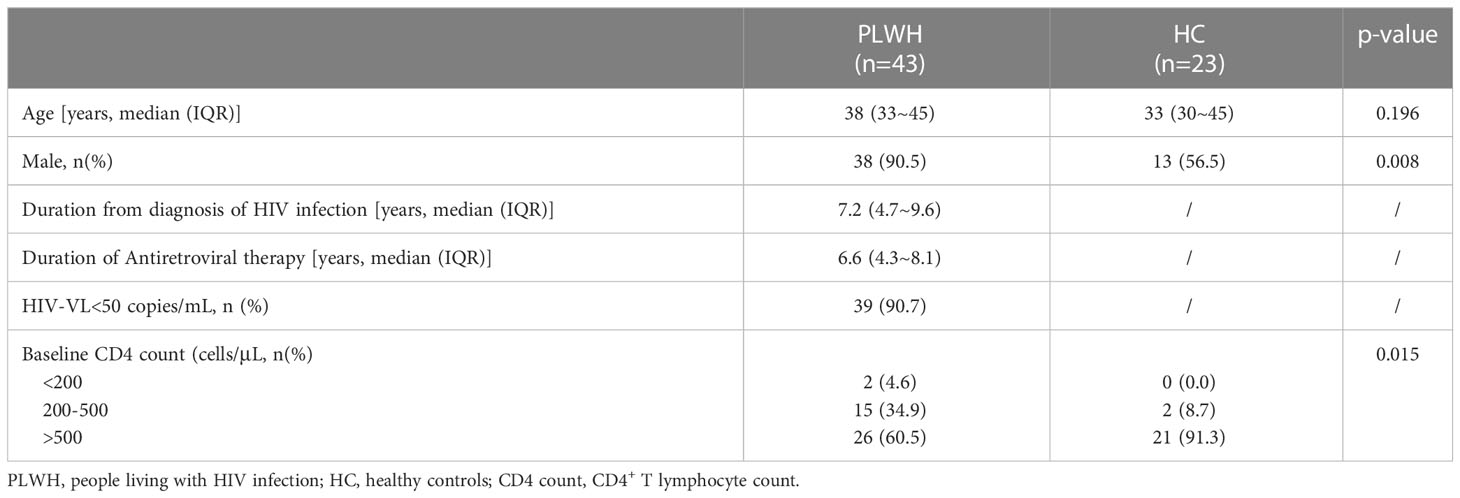
The median age of PLWH and HC was 38 (IQR 33~45) and 33 (30~45) years old without significant difference between the two groups. The proportion of male participants in PLWH was 90.5%, significantly higher than that in HC (56.5%, p=0.008). A total of 39 (90.7%) PLWH had an HIV viral load under 50 copies/mL and reached virological suppression. The CD4 count had significant difference between PWLH and HC (p=0.015). The proportion of CD4 count over 500 cells/μl in HC (91.3%) was higher than that in PLWH (60.5%). The characteristics of 43 PLWH and 23 HC enrolled are shown in Table 1.
Dynamics of nAbs against the inactivated COVID-19 vaccine
The dynamics of nAbs against three doses of the inactivated COVID-19 vaccine among PLWH and HC were evaluated. As shown in Figure 2A, in comparison to HC, the geometric mean titer (GMT) of nAbs in PLWH was significantly lower on day 14 after the second dose (p=0.001) and on days 14 (p=0.008), 30 (p=0.030), 60 (p=0.013), 90 (p=0.001), and 120 (p=0.047) after the booster dose vaccination. Furthermore, the nAbs of the second dose and the booster dose in PLWH peaked on day 42 and day 30 after vaccination, respectively, which were four weeks and two weeks behind the nAb peak appeared on day 14 after the second dose and the booster dose vaccination in HC.

Figure 2 (A) The geometric mean titer (GMT) of SARS-CoV-2 specific neutralizing antibodies (nAbs) among PLWH and HC at multiple time points. (B) The seroconversion rate of SARS-CoV-2 specific nAbs among PLWH and HC at multiple time points. (C) The GMT of SARS-CoV-2 specific IgM among PLWH and HC at multiple time points. (D) The GMT of SARS-CoV-2 specific IgG among PLWH and HC at multiple time points. D0B1: Day 0 before the first dose; D0B2: Day 0 before the second dose; D14A2: Day 14 after the second dose; D42A2: Day 42 after the second dose; D102A2: Day 102 after the second dose; D0B3: Day 0 before the booster dose; D14A3: Day 14 after the booster dose; D30A3: Day 30 after the booster dose; D60A3: Day 60 after the booster dose; D90A3: Day 90 after the booster dose; D120A3: Day 120 after the booster dose; D180A3: Day 180 after the booster dose.
Among PLWH, the nAbs titers on days 14 (p<0.001), 30 (p<0.001), and 60 (p=0.004) after the booster dose vaccination were significantly higher than the peak of nAbs titers of the second dose vaccination. Among HC, the nAbs titers on days 14 (p<0.001), 30 (p<0.001), 60 (p=0.002), and 90 (p=0.002) after the booster dose vaccination were significantly higher than the peak of nAbs titers of the second dose vaccination. In both PLWH and HC, the nAbs titers on day 180 after the booster dose were significantly higher than before the booster dose (i.e., on day 180 after the second dose, all p<0.001).
As shown in Figure 2B, the seroconversion rate of nAbs among HC on day 14 after the second dose (p=0.003) and on days 90 (p=0.003) and 120 (p=0.040) after the booster dose vaccination was significantly higher than that among PLWH. Among PLWH, the highest seroconversion rate of nAbs following the second and booster dose vaccination occurred on days 42 and 30 after vaccination, respectively. The latter was significantly higher than the former (80.6% vs. 43.9%, p=0.001). Among HC, the highest seroconversion rate of nAbs following the second and booster dose vaccination occurred on days 42 and 90 after vaccination, respectively, and there was no significant difference between them (90.9% vs. 68.2%, p=0.066).
Dynamics of IgM and IgG against the inactivated COVID-19 vaccine
The GMT of SARS-CoV-2 specific IgM on day 14 after the second dose (p=0.007) and on days 14 (p=0.015), 60 (p=0.004), 90 (p=0.008), 120 (p=0.006), and 180 (p=0.013) after the booster dose vaccination among PLWH was significantly lower than that among HC (shown in Figure 2C). The peak of IgM titers of the booster dose was less than that of the second dose in both PWLH and HC. However, there were no statistically significant differences.
The GMT of SARS-CoV-2 specific IgG on days 14 (p=0.004), 30 (p=0.015), 60 (p=0.018), and 90 (p=0.002) after the booster dose vaccination among PLWH was significantly lower than that among HC (shown in Figure 2D). The peak of IgG titers of the booster dose was significantly higher than that of the second dose in both PWLH and HC (all p<0.001).
T cell immune responses against the inactivated COVID-19 vaccine
For the comparison of PLWH and HC, as shown in Figure 3, the frequencies of IFN-γ+CD4+, IFN-γ+CD8+, TNF-α+CD4+, and TNF-α+CD8+ T cells among HC were significantly higher than that among PLWH on day 14 after the booster dose vaccination (p=0.004; p=0.005; p=0.024; p=0.005). The frequency of IFN-γ+CD8+ T cells in HC was significantly higher than in PLWH on day 180 after the booster dose vaccination (p=0.018).
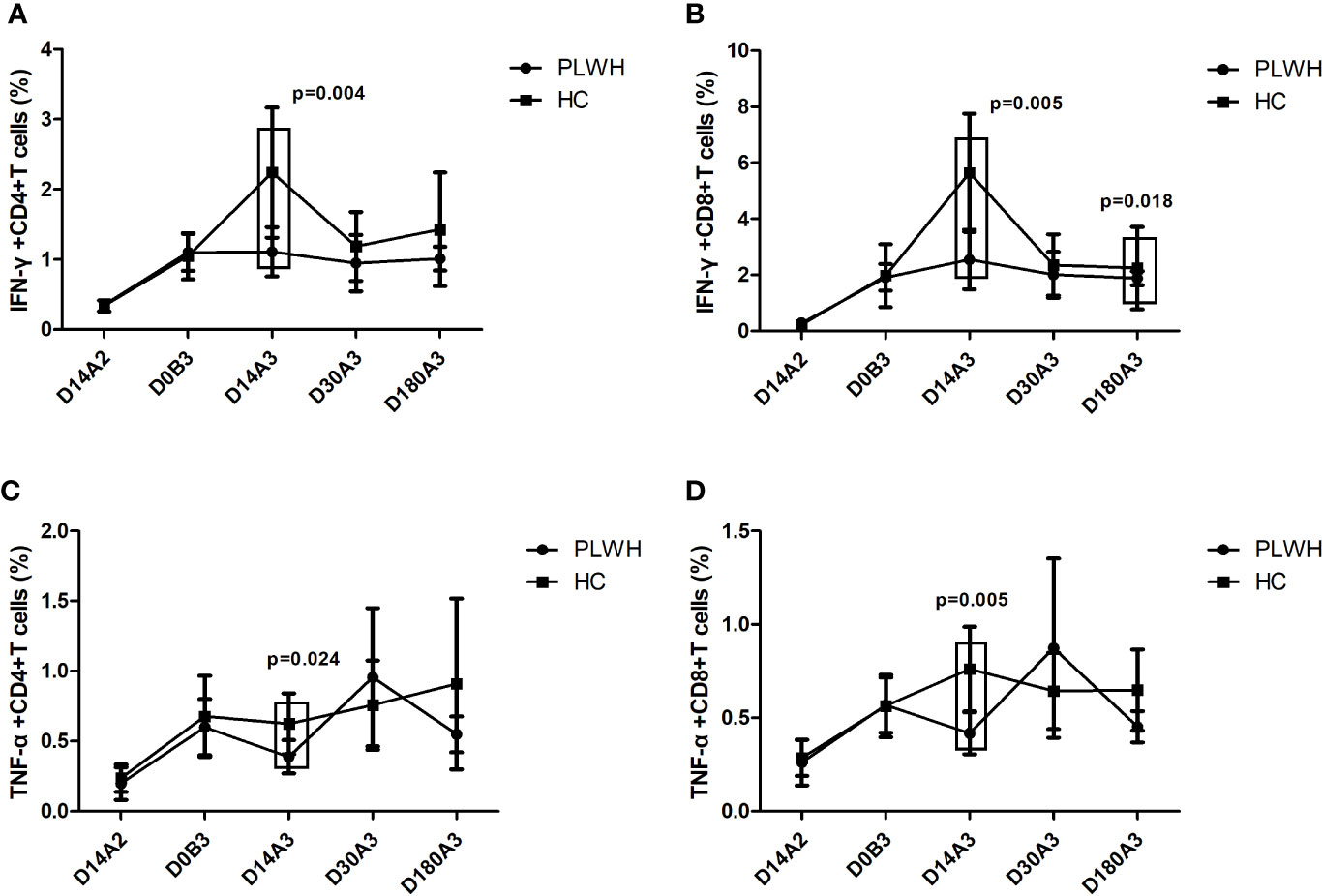
Figure 3 (A) The frequency of IFN-γ+CD4+T cells among PLWH and HC at multiple time points. (B) The frequency of IFN-γ+CD8+T cells among PLWH and HC at multiple time points. (C) The frequency of TNF-α+CD4+T cells among PLWH and HC at multiple time points. (D) The frequency of TNF-α+CD8+T cells among PLWH and HC at multiple time points. D14A2: Day 14 after the second dose; D0B3: Day 0 before the booster dose; D14A3: Day 14 after the booster dose; D30A3: Day 30 after the booster dose; D180A3: Day 180 after the booster dose.
As shown in Figure 4, among PLWH, the frequencies of IFN-γ+CD4+, IFN-γ+CD8+, TNF-α+CD4+, and TNF-α+CD8+ T cells on day 180 after the second dose (all p<0.001) and on days 14 (p<0.001; p<0.001; p<0.001; p=0.013), 30 (all p<0.001), and 180 (all p<0.001) after the booster dose were significantly higher than those on day 14 after the second dose vaccination. Furthermore, the frequencies of IFN-γ-secreting and TNF-α-secreting CD4+ and CD8+ T cells on day 180 after the booster dose were similar to those on day 180 after the second dose vaccination. Among HC, the frequencies of IFN-γ+CD4+, IFN-γ+CD8+, TNF-α+CD4+, and TNF-α+ CD8+ T cells before the booster dose (p<0.001; p<0.001; p=0.001; p=0.005) and on days 14 (all p<0.001), 30 (p<0.001; p<0.001; p=0.001; p<0.001) and 180 (p<0.001; p<0.001; p=0.003; p=0.001) after the booster dose were significantly higher than those on day 14 after the second dose vaccination.
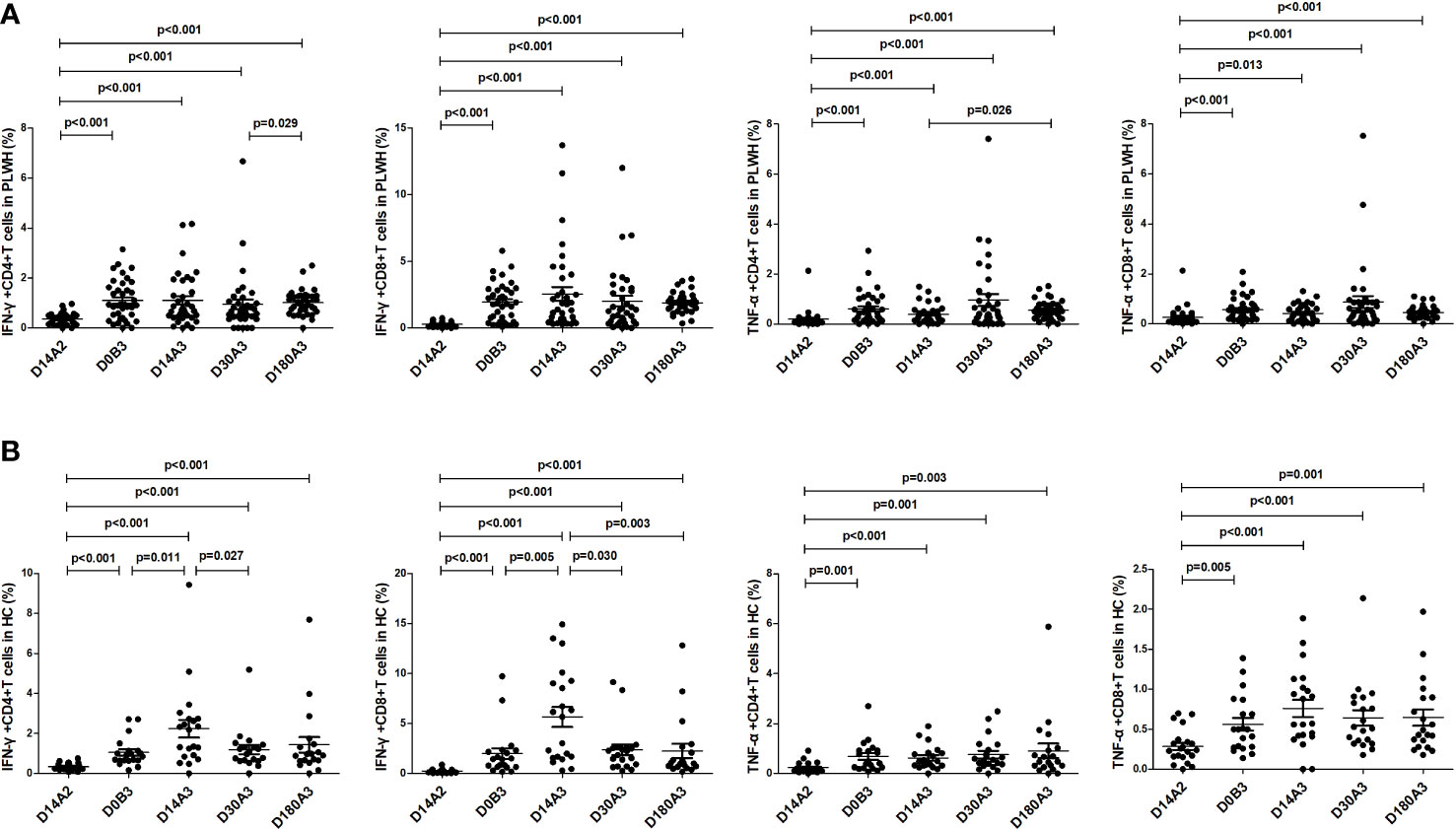
Figure 4 (A) The frequencies of IFN-γ-secreting and TNF-α-secreting CD4+ and CD8+ T cells in PLWH at multiple time points. (B) The frequencies of IFN-γ-secreting and TNF-α-secreting CD4+ and CD8+ T cells in HC at multiple time points. D14A2: Day 14 after the second dose; D0B3: Day 0 before the booster dose; D14A3: Day 14 after the booster dose; D30A3: Day 30 after the booster dose; D180A3: Day 180 after the booster dose.
Discussion
Monitoring the immune responses of PLWH to the booster dose of the COVID-19 vaccine is essential for planning future COVID-19 measures. Our previous study investigated the humoral immune responses to two doses of the inactivated COVID-19 vaccine within 6 months after vaccination among PLWH (8). However, little is known about the longitudinal humoral and T cell immunity against the booster dose of the inactivated COVID-19 vaccine among PLWH. Hence, we extended the existing literature by reporting the longitudinal dynamics of humoral and cellular responses within 6 months after the booster dose of the inactivated COVID-19 vaccination among PLWH. We found that among PLWH, although a homologous booster dose of the inactivated vaccine could dramatically increase the nAbs level and maintain stable T cell responses, immunogenicity was still significantly lower than among HC at multiple time points.
In our present study compared with two primary doses of the inactivated vaccine, the booster dose of the inactivated vaccine could induce a rapid and stronger nAbs level and seroconversion rate and increase T cell immunity among PLWH in the early period. These results align with the previous study’s result, which showed that the third dose of mRNA vaccine could elicit quick, robust antibody responses among PLWH (15). In healthy individuals, a homologous booster dose of the inactivated vaccine has been reported to rapidly boost the nAb level 3~5-fold compared to the second dose (16, 17). A non-randomized trial conducted on healthcare workers found that SARS-CoV-2 spike-specific memory B and T cells were still detectable 5 months after the second dose of the inactivated vaccination, which may form the basis of a rapid recall response to the booster dose of the vaccine (18). As observed in healthy individuals, a rapid and robust recall of immune responses following the booster dose of the inactivated vaccine may also exist in PLWH.
Our study reveals that the booster dose vaccination among PLWH reduced antibody decay maintained a higher nAb titer and sustained T cell responses even 6 months after vaccination. The limited literature on this supports our findings, as the previous study indicated that in healthy individuals who received three doses of the inactivated or mRNA COVID-19 vaccine, nAb titers decayed much slower within 6 months after booster dose administration than after primary two-dose regimens and still maintained a higher level 6 months after the booster dose vaccination (19, 20). It is well known that immune memory leads to the maintenance of immunity. The booster doses of both the inactivated and mRNA vaccine were reported to elicit extensive expansion of memory pools, especially robust SARS-CoV-2-specific memory B cells, which may contribute to the prolonged duration of humoral and cellular immunity (18, 21). Taken together, our study confirms the efficacy of the booster dose of the inactivated vaccine among PLWH for not only dramatically enhancing but also prolonging protective immunity against SARS-CoV-2 infection.
Lower immunogenicity against the booster dose of the vaccine was found at multiple time points among PLWH than among healthy individuals. These findings are consistent with immunogenicity toward the first and second doses of the COVID-19 vaccine. For example, previous studies reported that the humoral and cellular immune responses against two doses of the inactivated COVID-19 vaccine were lower, and the antibody responses were more delayed among PLWH than among healthy individuals (7, 22, 23). Our study supplements the comparison of the humoral and cellular immune responses against the booster dose vaccination between PLWH and HC, suggesting that the immunogenicity of the booster dose of the vaccine among PLWH is still lower than among healthy individuals. Lower immunogenicity in PLWH compared with HC has also been reported in other vaccine, such as the inactivated influenza and pneumococcal conjugate (24, 25). Previous studies indicated that PLWH with a CD4 count of less than 200 cells/μl had significantly weakened immune responses to COVID-19 vaccine compared with healthy individuals (26, 27). In our study, 95.4% of PLWH had a CD4 count over 200 cells/μl, most even had a CD4 count over 500 cells/μl. However, we still observed decreased immunogenicity to vaccine among PLWH compared to among HC. Chronic immune activation and immune dysregulation induced by HIV infection lead to immune damage of B cells and T cells, which may be related to lower immunogenicity against vaccine among PLWH (28, 29).
Our study has several limitations. First, the sample sizes of the PLWH and HC enrolled are relatively small; however, they are comparable to or larger than those of previously published studies (30, 31). Furthermore, significantly lower immunogenicity against the booster dose of the vaccine among PLWH than among HC was observed despite the limited sample size. Second, the time points for the humoral immunity assessment were not completely consistent between the second and third doses of the vaccine, but we demonstrated the antibody response differences between PLWH and HC using the same follow-up time points. Third, in vitro assays were not conducted to further investigate the efficacy of three doses of the inactivated vaccine against the variants. More studies are needed to assess the protection of the inactivated vaccine against the SARS-CoV-2 variants of concern among PLWH.
Conclusions
Among PLWH, the booster dose of the inactivated vaccine could induce stronger nAbs levels, sustain T cell immunity, and reduce antibody decay. However, the antibody and T cell responses against the booster dose of the inactivated vaccine were lower among PLWH than among healthy controls. Our study supports the efficacy of a booster dose of the inactivated vaccine in PLWH. However, additional strategies are needed to improve the immune response to the inactivated COVID-19 vaccine in PLWH.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Research and Ethics Committee of Zhongnan Hospital, Wuhan University, P. R. China (2020079K-1). The patients/participants provided their written informed consent to participate in this study.
Author contributions
KL and WT conceived and designed this investigation. YT, SZ, FM, and MW collected the original data. YT, SZ, FM, SW, and WG analyzed the data. YT and KL contributed to the writing of the paper. All authors read and approved the final manuscript.
Funding
This work was supported by the Medical Science and Technology Innovation Platform Support Project of Zhongnan Hospital, Wuhan University (PTXM2020008), Science and Technology Innovation Cultivation Fund of Zhongnan Hospital, Wuhan University (cxpy2017043), the Medical Science Advancement Program (Basic Medical Sciences) of Wuhan University (TFJC2018004), and Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2020-PT320-004).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1174379/full#supplementary-material
Supplementary Figure 1 | The gating strategy of the analysis of IFN-γ-secreting and TNF-α-secreting CD4+ and CD8+ T cells frequency in PLWH and HC.
References
1. Bhaskaran K, Rentsch CT, MacKenna B, Schultze A, Mehrkar A, Bates CJ, et al. HIV Infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV (2021) 8(1):e24–32. doi: 10.1016/S2352-3018(20)30305-2
2. Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases, South Africa. Risk factors for coronavirus disease 2019 (COVID-19) death in a population cohort study from the Western cape province, south Africa. Clin Infect Dis (2021) 73(7):e2005–15. doi: 10.1093/cid/ciaa1198
3. Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet (2021) 398(10296):213–22. doi: 10.1016/S0140-6736(21)01429-X
4. Fadlyana E, Rusmil K, Tarigan R, Rahmadi AR, Prodjosoewojo S, Sofiatin Y, et al. A phase III, observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of SARS-CoV-2 inactivated vaccine in healthy adults aged 18-59 years: an interim analysis in Indonesia. Vaccine (2021) 39(44):6520–8. doi: 10.1016/j.vaccine.2021.09.052
5. Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA (2021) 326(1):35–45. doi: 10.1001/jama.2021.8565
6. El CF, El SH. Vaccination in the adult patient infected with HIV: a review of vaccine efficacy and immunogenicity. Am J Med (2019) 132(4):437–46. doi: 10.1016/j.amjmed.2018.12.011
7. Zou S, Wu M, Ming F, Wu S, Guo W, Marley G, et al. Immune response and safety to inactivated COVID-19 vaccine: a comparison between people living with HIV and HIV-naive individuals. AIDS Res Ther (2022) 19(1):33. doi: 10.1186/s12981-022-00459-y
8. Zou S, Guo W, Wu S, Ming F, Tan Y, Wu M, et al. Six-month humoral immune response to inactivated COVID-19 vaccine among people living with HIV. Front Immunol (2022) 13:988304. doi: 10.3389/fimmu.2022.988304
9. Brisotto G, Muraro E, Montico M, Corso C, Evangelista C, Casarotto M, et al. IgG antibodies against SARS-CoV-2 decay but persist 4 months after vaccination in a cohort of healthcare workers. Clin Chim Acta (2021) 523:476–82. doi: 10.1016/j.cca.2021.10.035
10. Liao Y, Zhang Y, Zhao H, Pu J, Zhao Z, Li D, et al. Intensified antibody response elicited by boost suggests immune memory in individuals administered two doses of SARS-CoV-2 inactivated vaccine. Emerg Microbes Infect (2021) 10(1):1112–1115. doi: 10.1080/22221751.2021
11. Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning immune humoral response to BNT162b2 covid-19 vaccine over 6 months. N Engl J Med (2021) 385(24):e84. doi: 10.1056/NEJMoa2114583
12. Yue L, Zhou J, Zhou Y, Yang X, Xie T, Yang M, et al. Antibody response elicited by a third boost dose of inactivated SARS-CoV-2 vaccine can neutralize SARS-CoV-2 variants of concern. Emerg Microbes Infect (2021) 10(1):2125–2127. doi: 10.1080/22221751.2021.1996210
13. Falsey AR, Frenck RW Jr, Walsh EE, Kitchin N, Absalon J, Gurtman A, et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med (2021) 385(17):1627–9. doi: 10.1056/NEJMc2113468
14. Tan Y, Zou S, Ming F, Zhang Z, Xing Z, Wu S, et al. Early efficacy and safety of the booster dose inactivated COVID-19 vaccine among people living with HIV. J Acquir Immune Defic Syndr (2022) 90(3):e1–3. doi: 10.1097/QAI.0000000000002953
15. Lapointe HR, Mwimanzi F, Cheung PK, Sang Y, Yaseen F, Umviligihozo G, et al. People with HIV receiving suppressive antiretroviral therapy show typical antibody durability after dual COVID-19 vaccination, and strong third dose responses. J Infect Dis (2023) 227(7):838–49. doi: 10.1093/infdis/jiac229
16. Liang XM, Xu QY, Jia ZJ, Wu MJ, Liu YY, Lin LR, et al. A third dose of an inactivated vaccine dramatically increased the levels and decay times of anti-SARS-CoV-2 antibodies, but disappointingly declined again: a prospective, longitudinal, cohort study at 18 serial time points over 368 days. Front Immunol (2022) 13:876037. doi: 10.3389/fimmu.2022.876037
17. Zeng G, Wu Q, Pan H, Li M, Yang J, Wang L, et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect Dis (2022) 22(4):483–95. doi: 10.1016/S1473-3099(21)00681-2
18. Liu Y, Zeng Q, Deng C, Li M, Li L, Liu D, et al. Robust induction of b cell and T cell responses by a third dose of inactivated SARS-CoV-2 vaccine. Cell Discovery (2022) 8(1):10. doi: 10.1038/s41421-022-00373-7
19. Xin Q, Wu Q, Chen X, Han B, Chu K, Song Y, et al. Six-month follow-up of a booster dose of CoronaVac in two single-centre phase 2 clinical trials[J]. Nat Commun (2022) 13(1):3100. doi: 10.1038/s41467-022-30864-w
20. Ntanasis-Stathopoulos I, Karalis V, Sklirou AD, Gavriatopoulou M, Alexopoulos H, Malandrakis P, et al. Third dose of the BNT162b2 vaccine results in sustained high levels of neutralizing antibodies against SARS-CoV-2 at 6 months following vaccination in healthy individuals. Hemasphere (2022) 6(7):e747. doi: 10.1097/HS9.0000000000000747
21. Mise-Omata S, Ikeda M, Takeshita M, Uwamino Y, Wakui M, Arai T, et al. Memory b cells and memory T cells induced by SARS-CoV-2 booster vaccination or infection show different dynamics and responsiveness to the omicron variant. J Immunol (2022) 209(11):2104–13. doi: 10.4049/jimmunol.2200525
22. Huang X, Yan Y, Su B, Xiao D, Yu M, Jin X, et al. Comparing immune responses to inactivated vaccines against SARS-CoV-2 between people living with HIV and HIV-negative individuals: a cross-sectional study in China. Viruses (2022) 14(2):277. doi: 10.3390/v14020277
23. Ao L, Lu T, Cao Y, Chen Z, Wang Y, Li Z, et al. Safety and immunogenicity of inactivated SARS-CoV-2 vaccines in people living with HIV. Emerg Microbes Infect (2022) 11(1):1126–34. doi: 10.1080/22221751.2022.2059401
24. Madhi SA, Maskew M, Koen A, Kuwanda L, Besselaar TG, Naidoo D, et al. Trivalent inactivated influenza vaccine in African adults infected with human immunodeficient virus: double blind, randomized clinical trial of efficacy, immunogenicity, and safety. Clin Infect Dis (2011) 52(1):128–37. doi: 10.1093/cid/ciq004
25. Garrido HMG, Schnyder JL, Tanck MWT, Vollaard A, Spijker R, Grobusch MP, et al. Immunogenicity of pneumococcal vaccination in HIV infected individuals: a systematic review and meta-analysis. EClinicalMedicine (2020) 29-30:100576. doi: 10.1016/j.eclinm.2020.100576
26. Antinori A, Cicalini S, Meschi S, Bordoni V, Lorenzini P, Vergori A, et al. Humoral and cellular immune response elicited by mRNA vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in people living with human immunodeficiency virus receiving antiretroviral therapy based on current CD4 T-lymphocyte count. Clin Infect Dis (2022) 75(1):e552–63. doi: 10.1093/cid/ciac238
27. Benet S, Blanch-Lombarte O, Ainsua-Enrich E, Pedreño-Lopez N, Muñoz-Basagoiti J, Raïch-Regué D, et al. Limited humoral and specific T-cell responses after SARS-CoV-2 vaccination in PLWH with poor immune reconstitution. J Infect Dis (2022) 226(11):1913–23. doi: 10.1093/infdis/jiac406
28. Malaspina A, Moir S, Kottilil S, Hallahan CW, Ehler LA, Liu S, et al. Deleterious effect of HIV-1 plasma viremia on b cell costimulatory function. J Immunol (2003) 170(12):5965–72. doi: 10.4049/jimmunol
29. Okoye AA, Picker LJ. CD4(+) T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol Rev (2013) 254(1):54–64. doi: 10.1111/imr.12066
30. Feng Y, Zhang Y, He Z, Huang H, Tian X, Wang G, et al. Immunogenicity of an inactivated SARS-CoV-2 vaccine in people living with HIV-1: a non-randomized cohort study. EClinicalMedicine (2022) 43:101226. doi: 10.1016/j.eclinm.2021.101226
Keywords: HIV, COVID-19, inactivated vaccine, booster dose, antibody response, T cell immunity
Citation: Tan Y, Zou S, Ming F, Wu S, Guo W, Wu M, Tang W and Liang K (2023) A tale of two conditions: when people living with HIV meet three doses of inactivated COVID-19 vaccines. Front. Immunol. 14:1174379. doi: 10.3389/fimmu.2023.1174379
Received: 26 February 2023; Accepted: 31 May 2023;
Published: 19 June 2023.
Edited by:
Teodor Doru Brumeanu, Uniformed Services University of the Health Sciences, United StatesReviewed by:
Elizabeth De Gaspari, Adolfo Lutz Institute, BrazilMiguel de Mulder Rougvie, Cornell University, United States
Copyright © 2023 Tan, Zou, Ming, Wu, Guo, Wu, Tang and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiming Tang, V2VpbWluZ190YW5nQG1lZC51bmMuZWR1; Ke Liang, a2VsaWFuZ0B3aHUuZWR1LmNu
†These authors have contributed equally to this work
 Yuting Tan
Yuting Tan Shi Zou1,2†
Shi Zou1,2† Fangzhao Ming
Fangzhao Ming Songjie Wu
Songjie Wu Wei Guo
Wei Guo Weiming Tang
Weiming Tang Ke Liang
Ke Liang