- Department of Immunology, University of Toronto, Toronto, ON, Canada
The IL-10/IL-10 receptor (IL-10R) axis plays an important role in attenuating neuroinflammation in animal models of Multiple Sclerosis (MS) and increased IL-10 has been associated with a positive response to MS disease modifying therapy. Because environmental factors play an important role in MS susceptibility and disease course, identification of environmental factors that impact the IL-10/IL-10R axis has therapeutic potential. In this review, we provide historical and updated perspectives of how IL-10R signaling impacts neuroinflammation, discuss environmental factors and intestinal microbes with known impacts on the IL-10/IL-10R axis, and provide a hypothetical model for how B cells, via their production of IL-10, may be important in conveying environmental “information” to the inflamed central nervous system.
1 The IL-10/IL-10R axis
In the context of disease, IL-10 and its cognate receptor IL-10R have been implicated in mitigating autoreactive T cell responses. One such context is multiple sclerosis (MS), a chronic, inflammatory disease of the central nervous system (CNS) that affects over 2 million people worldwide (1). The disease exhibits heterogeneous clinical presentation and is characterized by the infiltration of lymphocytes into the brain and spinal cord, resulting in demyelination and axonal loss (2). The animal model of MS, Experimental Autoimmune Encephalomyelitis (EAE), has been pivotal to our understanding of how such autoreactive T cells are primed, infiltrate the CNS and set up an inflammatory milieu that promotes demyelinating lesions (3). Early studies showed that myelin-specific Th2 cells could inhibit EAE via their production of Th2-associated cytokines (4–6). Subsequent work using IL-10 knockout and transgenic overexpression revealed that IL-10 is a key regulatory cytokine required to regulate EAE (7–9). However, the use of therapeutic IL-10 administration has yielded inconsistent outcomes in both EAE and MS (10–13). To contextualize these data, it is important to understand the underlying mechanism of IL-10 mediated anti-inflammatory processes and environmental factors that can modulate levels or activity of IL-10.
1.1 Historical significance of the IL-10/IL-10R axis
In 1989, Fiorentino and colleagues discovered a cytokine produced by Th2 cells acting directly on Th1 cells to inhibit their function in vitro (14). At the time, they named the secreted factor “cytokine synthesis inhibitory factor (CSIF)”, but it is now widely known as interleukin-10 (IL-10) (15, 16). Since this discovery, many innate immune cells (macrophages, monocytes, dendritic cells (DCs), and neutrophils) and adaptive immune cells (CD4+/CD8+ T cells and B cells) have been identified as producers of IL-10 (17). Early evidence supported the concept that IL-10 has an inhibitory effect on T effector cells via direct and indirect mechanisms (16, 18, 19). For example, IL-10 was shown to prevent T cell proliferation and cytokine production in an indirect manner by hampering the maturation and T cell stimulation capabilities of DCs (20–22), or by downregulating MHC class II expression on monocytes (23–26). On the other hand, IL-10 was also found to act directly on CD4+ T cells by inducing their anergy (27), suppressing the expansion of pathogenic Th17 cells (28, 29) and promoting the regulatory activity of CD4+ Foxp3+ regulatory T cells (Tregs) (30, 31) and CD4+ T regulatory type 1 (TR1) cells (32).
1.2 IL-10 producing cells
While T cells and myeloid cells collectively constitute a major cellular source of IL-10 (16, 33, 34), B cells also restrict inflammation via IL-10 in the context of neuroinflammation (as well as other autoimmune settings). Early work by Fillatreau and Anderton found that mice with B cell specific IL-10 deficiency fail to recover from EAE, and restoring this population with an adoptive transfer of IL-10+ B cells leads to disease recovery (35). Further studies have shown that regulatory B cell populations including Bregs, plasma cells (PCs) and plasmablasts can all limit the severity of EAE in an IL-10 dependent manner (36–39). The underlying regulatory mechanisms of B cell derived IL-10 are still being explored but it has been shown using human peripheral blood mononuclear cells (PBMCs) that plasmablast-derived IL-10 can hinder the ability of DCs to generate autoreactive T cells (39). Alternatively, IL-10+ Bregs in a murine model of arthritis have been shown to contribute to the induction of FoxP3+ Tregs and suppression of Th1/Th17 cells in vivo (37). Related human studies have found that MS patient B cells exhibit deficient IL-10 production following ex vivo stimulation (40). Following anti-CD20 induced MS remission, B cells that reconstitute the periphery regain their ability to produce IL-10 (41). Thus, B cell derived IL-10 plays a key role in regulating autoimmune inflammation and may contribute to the mechanism of action of anti-CD20 therapy in MS.
1.3 Downstream signaling through IL-10/IL-10R
Il-10 signals through the IL-10 receptor (IL-10R), a hetero-tetramer consisting of two alpha and two beta subunits (15). While the IL-10Rβ subunit can bind to other members of the IL-10 super family including IL-22 and IL-26 (34), the IL-10Rα subunit is specific to IL-10 (15). IL-10Rα is expressed at a basal level on most hematopoietic cells. However, certain immune populations have higher expression levels of IL-10Rα, especially upon immune activation (15). For instance, antigen-presenting cells (APCs) and other myeloid cells such as microglia have been shown to express high levels of IL-10Rα from development onwards (15, 42, 43). Conversely, naive CD4+ T-cells have low levels of steady state IL-10Rα expression that increases upon TCR stimulation both on multiple T-cells subsets in vivo and in vitro (28, 29).
Upon IL-10 binding, a cascade of intracellular signaling events occurs (Figure 1). This leads to the activation of tyrosine kinases Jak1 and Tyk2, which then reciprocally phosphorylate tyrosine residues of the IL-10Rα (15, 44). Receptor phosphorylation leads to the recruitment and phosphorylation of signal transducer and activator of transcription 3 (STAT3), which is then activated and subsequently translocates to the nucleus (45). In the nucleus STAT3 binds to STAT3-binding elements and activates the transcription of target genes, one of which is Suppressor Of Cytokine Signaling 3 (SOCS3). SOCS3 inhibits the transcription of pro-inflammatory cytokines such as IL-6 and TNFα. SOCS3 also inhibits IL-10 transcription, resulting in a negative feedback loop downstream of IL-10R signaling (15). Other STAT3 target genes include Bcl3, a known inhibitor of the NF-κβ pathway that can suppress the production of pro-inflammatory cytokines (46), and Ddit4, an mTOR inhibitor that has been shown to decrease the inflammatory activities of macrophages (47).
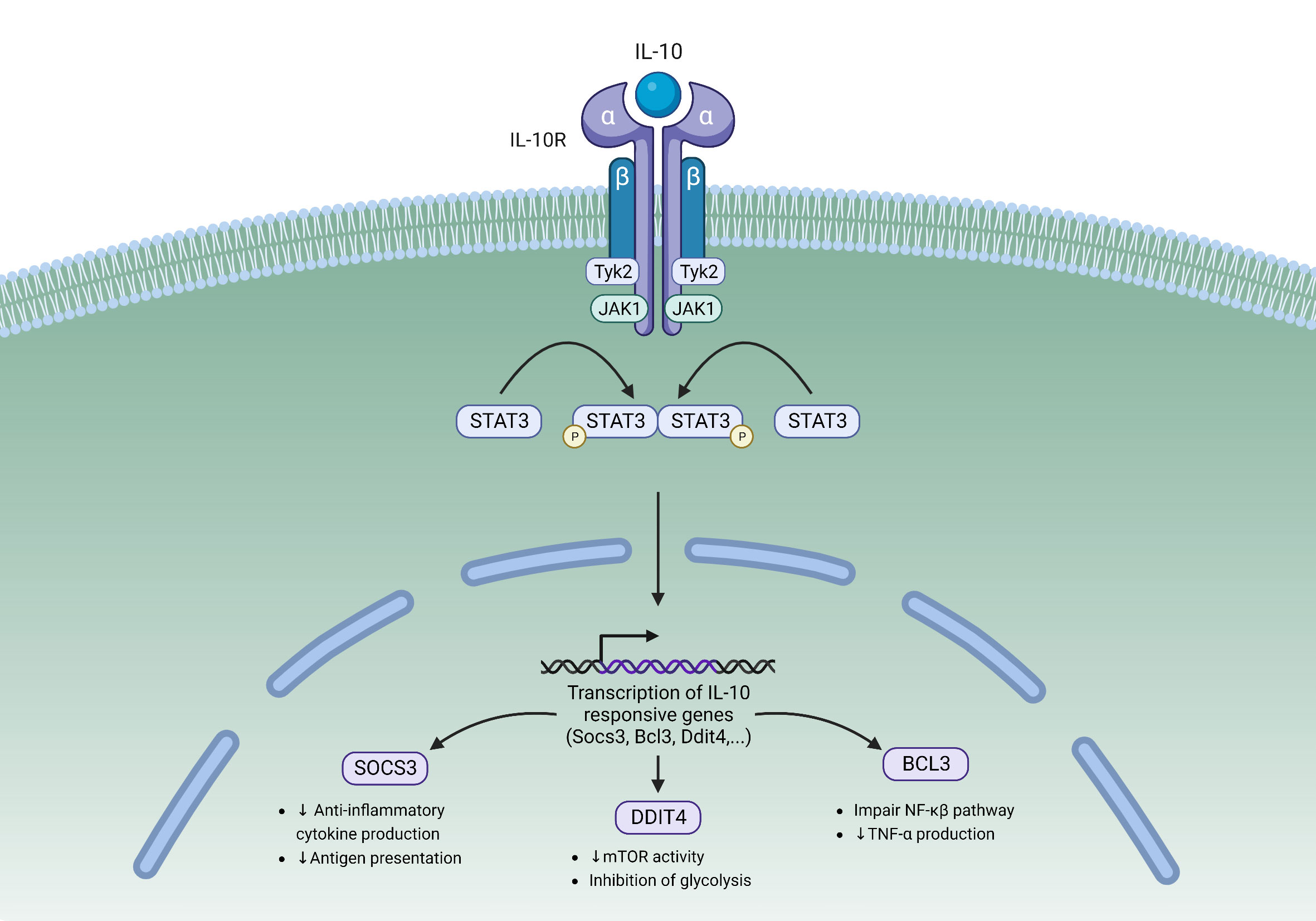
Figure 1 The IL-10/IL-10R Signaling pathway. IL-10 binding to its heterodimeric receptor leads to the phosphorylation of STAT3 by JAK1 and Tyk2. Upon phosphorylation, STAT3 translocates to the nucleus where it binds to STAT3-binding elements and activates the transcription of target genes. STAT3 is responsible for activating the transcription of several IL-10 responsive genes including SOCS3, Bcl3, and Ddit4. Tyk2, Tyrosine kinase 2; JAK1, Janus kinase 1; STAT3, Signal transducer and activator of transcription 3; SOCS3, Suppressor of cytokine signaling 3; DDIT4, DNA damage-inducible transcript 4.
1.4 Importance of IL-10R signaling during homeostasis – a focus on the gut
Much of the current understanding of the impact of IL-10R signaling has been elucidated in the context of gastrointestinal diseases such as inflammatory bowel disease (IBD), where the constant interaction between immune cells and the gut microbiota demands strict regulation within the local intestinal milieu. In humans, mutations to the IL10RA and IL10RB genes have both been strongly associated with infant colitis associated with defects in downregulation of proinflammatory cytokine secretion by monocytes (48–50). Others have also found that impaired IL-10R signaling in adult IBD patients is associated with increased T cell polarization towards a Th17 lineage, decreased IL-10-induced STAT3 phosphorylation, and increased pro-inflammatory cytokine expression in monocytes following in vitro stimulation (51–53).
In mice, deletion of IL-10Rβ results in spontaneous colitis (54) and deletion of IL-10Rα specifically in macrophages increases susceptibility to chemically induced colitis (55). In addition, IL-10R signaling in Foxp3+ Tregs is critical for suppressing pathogenic Th17 cells and IL-10R signaling in Th17 cells directly suppresses their expansion (29, 32). Beyond T cells, IL-10Rα deletion in monocytes/macrophages leads to an increase in IL-17 and IL-6 proinflammatory cytokine levels in serum, the production of nitric oxide (NO) and reactive oxygen species (ROS) by lamina propria macrophages, and an overall proinflammatory gene expression signature in intestinal macrophages (56, 57). Similarly, anti-IL-10Rα antibody blockade increases the expression of pro-inflammatory and STAT1-inducible genes such as Cxcl9 and Cxcl11 in colonic macrophages (58). Elimination of IL-10R in CD11c+ cells, including DCs, is associated with an amplified immune response to bacterial and fungal pathogens as well as allergens in the skin (55, 59, 60).
Taken together, IL-10R signaling has clear immunoregulatory roles in restraining inflammation in the gut – an environment that is constantly exposed to microbial antigens.
2 IL-10/IL-10R axis in MS and EAE: some paradoxes
The IL-10/IL-10R axis has been implicated as a key mechanism for constraining inflammation during MS/EAE. Several EAE studies have found that both global and cell specific IL-10 knockout leads to worsened disease, yet therapeutic administration of IL-10 has had mixed results in EAE and MS (10–13). In this section we explore the pro- and anti-inflammatory effects of IL-10 during MS/EAE and the contexts that separate potentially helpful versus harmful impacts of this cytokine.
2.1 Anti-inflammatory effects of IL-10/IL-10R signaling in EAE and MS
In EAE, IL-10 deficiency leads to increased disease incidence and severity (8), and mice with APCs that over-express IL-10 driven by a class II MHC promoter are strongly resistant to the development of EAE (7, 8). Moreover, several studies have found that serum IL-10 levels in MS patients are decreased prior to and during disease relapses, but are increased during remission (61–67). Profiling of CCR6+ myelin-reactive CD4+ T cells from MS patients also found that these cells had decreased IL-10 production in comparison to healthy control T cells (68). Furthermore, treatment with first-line disease-modifying therapies DMTs such as glatiramer acetate (GA) and interferon-beta (IFNβ) is associated with increased IL-10 production by PBMCs isolated from EAE mice and MS patients (61, 62, 69, 70). Of note, treatment with fingolimod, GA, and IFNβ also increases the proportion of IL-10 producing B cells in MS patients (71–73).
Several IL-10 producing cells have been implicated in the regulation of MS and EAE including regulatory T cells (Tregs) and B cells (Bregs) (30, 35, 38). A higher frequency of IL-10 producing Tregs in the CNS during EAE has been shown to correlate with disease recovery and depletion of these cells leads to an exacerbation of disease (74). Moreover, during EAE the loss of Breg derived IL-10 prevents disease recovery and leads to a Th1-dominant response (35). A link between IL-10 producing B cells and Foxp3+ Tregs has also been established during EAE. B cell deficient mice were shown to have lower levels of both IL-10 and Foxp3 expression in the spinal cord during EAE, suggesting that B cells may play a role in promoting Foxp3+ Treg accumulation in the CNS through an IL-10 dependent manner (75). Furthermore, plasma cells- the terminally-differentiated B cell typically associated with antibody production, have also been shown to contribute to protection against EAE through the production of IL-10 (38, 39).
Despite the clear role for IL-10 in limiting the severity of EAE (7, 8), and its association with reduced white matter lesions and an improved Expanded Disability Status Scale (EDSS) score in MS (63, 76), relatively less is known about the impact of its cognate receptor IL-10Rα in regulating neuroinflammation. CD4+ T cells derived from the blood of MS patients are relatively hyporesponsive to the immunosuppressive function of IL-10 in vitro compared to healthy controls, and this hyporesponsiveness is associated with impaired STAT3 phosphorylation (77), suggesting defects in IL-10Rα signaling. In addition, one allele of the IL-10Rα S138G polymorphism, which encodes for a loss-of-function allele for IL-10-induced STAT1 and STAT3 activation (78) is associated with MS disease susceptibility and severity in Tunisians (79), and two mutant alleles confers an increased risk for MS specifically in men who are normally less susceptible than women in developing relapsing-remitting MS (79). The same polymorphism has been linked to a higher risk of ulcerative colitis (80) and systemic lupus erythematosus (81, 82).
2.2 Pro-inflammatory effects of IL-10/IL-10R signaling in EAE and MS
While the bulk of research indicates that IL-10/IL-10R signaling contributes to the dampening of EAE/MS, some studies suggest otherwise. For example, IL-10 mRNA levels in serum are increased in MS patients in comparison to healthy controls (83–85), and in PBMCs IL10 mRNA levels are increased 2 weeks post-MSrelapse but subsequently return back to baseline after 4 weeks (64). However, it is unclear whether these increased levels of IL-10 mRNA/protein are involved in promoting pro-inflammatory conditions or represent a counter-regulatory mechanism that is triggered by neuroinflammation.
In two separate studies examining MOG35-55 EAE, IL-10rα deletion specifically in T cells reduced disease severity (86, 87). Liu et al. found that T cell specific IL10Ra deletion led to increased proportions of Tregs during the early phase of disease and an overall decrease in T cell accumulation during the disease course in the CNS and secondary lymphoid tissue (86). Using competitive bone marrow chimeras, T effector cells expressing IL10Ra exhibited a survival advantage over Il10Ra-deficient T effectors cells (86). In addition, Yogev et al. found that although CD4+ T cells are a relatively minor source of IL-10, T cell-derived IL-10 worsens EAE by acting on Th1 cells (and Th17 cells to a lesser extent) to promote their survival and proliferation in the CNS (87).
These results may explain why investigations into the therapeutic delivery of IL-10 have yielded mixed findings (10–13). As different immune populations produce IL-10 at different time points during MS/EAE, therapeutic efficacy could be dictated by the dose, delivery method, and timing.
3 Environmental factors that influence IL-10/IL-10R during MS/EAE
A person’s sex, age, diet, exercise, prior infections, geographic location, antibiotic use, exposure to pollution and early life factors (breastfeeding, mode of delivery) can all influence the composition of one’s microbiome (88). As such, the microbiome is a window into environmental exposures and accordingly has been studied for its potential role as a risk factor for MS incidence and/or severity (89, 90). However, there are also other environmental factors that can exert a direct impact on the immune system and by extension potentially on MS pathogenesis, independent of the microbiome. For example EBV infection and Vitamin D have been shown to act directly on immune cells in vitro, altering their functionality. In this section, we review how environmental factors can impact the IL-10/IL-10R axis in MS and EAE via the microbiome (section 3.1-3.2) or potentially independent of the microbiome (section 3.3-3.4)
3.1 The intestinal microbiome
The intestinal microbiome has a profound impact on host immunity even at distal sites such as the CNS (90, 91). Several human studies have revealed differences in the composition of the microbiome comparing patients with MS and healthy controls, the most common alterations being Akkermansia, Acinetobacter, and Parabacteriodes taxa (92–95). Shifts in microbiome composition in MS patients have also been associated with changes in immunomodulatory metabolites (96). However, causal associations between microbiome alterations in disease susceptibility or severity are difficult to establish in the real world. To address this, EAE models involving colonization of germ free or antibiotic treated mice via fecal microbial transplant (FMT) can be used to gain fundamental understanding into causality (94, 95).
Evidence that host commensal microbial communities influence IL-10 levels and subsequently CNS autoimmunity was first derived from antibiotic treatment studies. In these studies, oral administration of an antibiotic cocktail protected mice against the onset and severity of EAE. This phenomenon was associated with significantly increased levels of IL-10 secretion from cells isolated from secondary lymphoid tissue, specifically, IL-10 producing Foxp3+ Tregs (97, 98). Subsequently, Bacteriodes fragilis, a commensal bacteria that produces polysaccharide A (PSA), was found to be responsible for protection against EAE by triggering the activation of IL-10+ Tregs through the Toll-like receptor 2 pathway (99–102). Indeed, mice treated with oral PSA that were lacking IL-10 had similar clinical disease as wild-type mice, indicating that PSA and Treg mediated protection against disease requires IL-10 (99). Other intestinal commensal microbes have been implicated for their disease altering properties in EAE. For example, colonization with Prevotella histicola reduces EAE severity and is associated with increased IL-10 production by DCs (103). Cekanaviciute and colleagues identified a reduction in the bacterial genera Parabacteroides distasonis in MS patients and showed that P. distasonis exposure increases the differentiation of IL-10+ Tregs from healthy donor PBMCs in vitro. Moreover, in vivo monoclonization of germ free (GF) mice with P. distasonis significantly increased the amount of IL-10+ CD4+ T cells in the spleen and mesenteric lymph nodes (94). In two distinct models of EAE, Berer et al. found that GF mice colonized with fecal material from MS-affected twins exhibited increased incidence and severity of disease compared to mice colonized with fecal material from non-MS twins. The relative protection afforded by the non-MS twin FMT was abrogated by administration of an anti-IL-10 neutralizing antibody, indicating that the FMT influenced CNS autoimmunity in an IL-10 dependent manner. Moreover, the mice given the MS FMT had a marked absence of IL-10+ Treg induction in the mesenteric lymph nodes (95).
Several studies have indicated that both the prophylactic and therapeutic administration of probiotics can reduce the severity of MOG35-55 and PLP139-151 EAE (104–106). Probiotic treatment was shown to suppress Th17 cell differentiation, promote the expansion of IL-10 producing T cells in the mesenteric lymph nodes and the CNS, and increase systemic IL-10 levels in serum (104). Two separate MS patient studies have also shown that administration of probiotics can increase the relative frequency of IL-10+ Tregs and levels of IL-10 in serum from the blood (107, 108). Furthermore, administration of a probiotic containing Lactobacillus, Bifidobacterium and Streptococcus was found to increase the gene expression of IL-10RA on monocytes derived from MS patient PBMCs (108).
3.2 Diet
The human diet plays a key role in influencing the gut microbiome, thus identifying dietary factors that lie upstream of the microbiome provides insight into potential therapeutic interventions for MS patients. A link between diet and autoimmune neuroinflammation has been demonstrated. For example, a cellulose rich diet which promotes the accumulation of Lactobacillaceae in the intestine, alleviates EAE in conjunction with an increase in IL-10+ CD4+ T cells (109). The amino acid tryptophan, which is obtained through our diet, can induce regulatory IL-10 producing T cells both in vitro and in vivo during EAE (110, 111).
Short-chain fatty acids (SCFAs) including acetate, butyrate, and proprionate are produced by the colon during the bacterial fermentation of dietary fibers. Progressive MS patients have been shown to have lower levels of SCFAs in the blood. Of note, oral SCFA administration to mice increases the number of IL-10+ T cells in the CNS during EAE (112). Furthermore, SCFA treated glial cells induce the production of IL-10 by T cells in vitro (112). In the context of EAE, administration of the SCFA propionate exands CNS-resident Tregs in an IL-10R dependent mechanism that further leads to an increase in IL-10 production by Tregs (113). In MS, increases in Enterobacteriaceae have been shown to be accompanied by reduced SCFA levels, and these alterations were more pronounced in patients with a higher burden of disease (114).
Other dietary modulations have been shown to improve MS and EAE, although a direct link to IL10R signaling was not investigated. In a small cohort of MS patients, a high-vegetable/low-protein diet increased the abundance of fecal Lachnospiraceae that correlated with a decrease in IL-17+ CD4+ T cells and an increase in IL-10+CD14+ monocytes in the blood as well as a reduction in relapse rate compared to “western diet” MS patients (115). Administration of a nutritional supplementation of non-fermentable fiber in early adult life, which promotes increases in Helicobacter, Enterococcus, Desulfovibrio, Parabacteroides, Pseudoflavonifractor and Osillibacter and the production of cecal long chain fatty acids was shown to reduce the incidence of spontaneous EAE. Authors did not specifically report on IL-10 production but did observe an increase in T cell-derived IL-4 and IL-5 (116). Moreover, an isoflavone diet has also been shown to protect against EAE, and the isoflavone-free diet promoted a microbiome that was more reminiscent of an MS microbiome (117). Dietary guar gum has also been shown to attenuate EAE, notably independent of SCFA, and these beneficial effects are primarily due to reduced T cell priming and migration to the CNS (118). Lastly, intermittent fasting in the context of EAE increased the abundance of Lactobacillaceae, Bacteroidaceae, and Prevotellaceae in conjunction with a reduction in intestinal IL-17-producing T cells and improved EAE outcomes (119). In these dietary modulations, it will be of interest to examine their impact on IL-10 production and IL-10R signaling.
3.3 Vitamin D
During the cooler months, latitude is the strongest determinant for the amount of vitamin D produced by UVB radiation, thus the amount of vitamin D absorbed by skin drastically decreases as latitude increases (120). A meta-analysis published in 2011 found a significant increase in MS prevalence at higher latitudes (121). Similarly, individuals with a genetic predisposition to vitamin D deficiency are at higher risk of developing MS (122, 123). The expression of the MS risk gene HLA-DRB1*1501 is regulated by a vitamin D responsive promoter (124), and the level of serum 1,25(OH)2D3 in MS patients is inversely correlated with disease progression (125, 126), new CNS lesion formation (127), and risk of relapse (128, 129).
There are direct impacts of Vitamin D on the IL-10/IL-10R axis in the context of MS and EAE. Vitamin D supplementation trials in MS patient cohorts have shown that high dose vitamin D elevates the proportion of IL-10 producing CD4+ T cells (130), increases levels of cell proliferation (131), and leads to a global increase in IL-10 levels in the serum of relapsing remitting MS patients (132–134). However, the effect of 1,25(OH)2D3 on T cells may be indirectly linked to its influence on other immune cell subsets as since 1,25(OH)2D3 was found to dampen the differentiation and maturation of APCs resulting in increased production of IL-10 concomitant with a reduced generation of alloreactive T cells (135, 136). Although less studied in the context of CNS autoimmunity, 1,25(OH)2D3 also influences B cells and has been linked to enhanced IL-10 production by activated human B cells (137).
In the context of EAE, continual administration of 1,25(OH)2D3 inhibits clinical disease in both prophylactic and therapeutic modalities (138–141). Similar to in vitro experiments, vitamin D3 supplementation led to an increased production of IL-10 by spleen and lymph node CD4+ T cells and a skew towards Treg and Th2 phenotypes (140). Furthermore, adoptive transfer of DCs cultured with 1,25(OH)2D3 into EAE mice dampened disease severity, inhibited the infiltration of Th1/Th17 immune cells into the CNS while increasing the representation of IL-10+ CD4+ T cells (142). A causal relationship between vitamin D and the IL-10/IL-10R axis has been demonstrated: Specifically, unlike IL-10 sufficient littermates, vitamin D supplementation protects neither IL-10 nor IL-10Rb deficient mice from developing severe EAE. Since reciprocal bone marrow chimera experiments revealed that IL-10 derived from both the radiosensitive and radioresistant cell compartments was necessary for protection against EAE, the precise IL-10 producing cell type in this study remains unidentified, and the nature of the IL-10 receiving cell type was not determined (141). Further research into IL-10 sensing cells following vitamin D supplementation will be an important next step in elucidating its benefits for MS patients.
In summary, Vitamin D has a direct impact on the IL-10/IL-10R axis in MS and EAE. Vitamin D may also have an indirect impact on the IL-10/IL-10R axis via the microbiome (143), which in turn can impact neuroinflammation, however this is not well-studied.
3.4 EBV
Epstein-Barr Virus (EBV) is a common human gammaherpesvirus that persists in more than 90% of the population worldwide (144). Recently, a longitudinal analysis provided strong causal evidence that EBV infection is a necessary co-factor for the development of MS (145). Previous and ongoing research has led to the development of several hypotheses on how EBV confers a greater risk of MS susceptibility including molecular mimicry and the generation of pro-encephalitogenic B cells (146). Interestingly, EBV encodes a viral homolog of IL-10 (vIL-10) (also known as BCRF1), which has approximately 80% structural similarity to its human equivalent (147). However, vIL-10 acts as a selective agonist that binds with lower affinity to the IL-10R (148). Despite binding to the IL-10R, vIL-10 does not influence DC functioning to the same extent as endogenous IL-10 – it is a poor inducer of STAT3 phosphorylation and is less effective at dampening the production of pro-inflammatory cytokines following LPS treatment (149). In agreement, Jog et al. found that vIL-10 binding to IL-10R interfered with hIL-10 induced STAT3 phosphorylation, thus indirectly inhibiting the ability for IL-10 to induce anti-inflammatory cytokine production (150). Although the existing literature on vIL-10 and MS is limited, we do know that vIL-10 protects EBV infected B cells against detection and elimination by dampening the secretion of antiviral cytokines and by preventing NK cell mediated killing (151). This may allow for pro-inflammatory EBV+ B cells to persist and contribute to CNS autoimmunity. Exploring how vIL-10 influences B cell populations during MS may allow us to further understand how EBV influences MS disease.
4 B cells as a bridge between the environment and IL-10R signaling in MS/EAE
Since the first description of IL-10+ B cells, a heterogenous collection of Bregs have been described in various disease contexts, and the specific signals controlling their development- several which can be influenced by the environment, are now of significant interest (152). For example, in rheumatoid arthritis products from the gut microbiota drive the production of IL-1β and IL-6 which in turn promotes the differentiation of IL-10+ Bregs (153). Butyrate supplementation, a metabolite produced by the microbiota, promotes an increased frequency of IL-10+ Bregs in rheumatoid arthritis patients, and mice lacking IL-10 producing B cells do not experience the same disease suppression following butyrate treatment (154). Other studies have shown similar expansions of IL-10+ B cells in response to other microbiota-derived metabolites including acetate and pentanoate (155, 156), indicating the role of microbial communities in shaping IL-10 levels by modulating the B cell population.
Following B cell receptor engagement, B cells develop into plasma cells whose “day job” is to produce antibodies to protect the host against re-infection. However, plasma cells can also provide important regulatory functions, even at distal locations, through production of anti-inflammatory molecules such as IL-10. In the context of the CNS, complementary mouse and human studies have verified that gut-derived IgA+ plasma cells can migrate to the brain meninges at homeostasis (157). Studies in MS (158) and EAE (38) also detected microbiota-reactive IgA+ plasma cells originating from the gut in the inflamed CNS, and adoptive transfer of IgA+ plasma cells isolated from the small intestine can reduce EAE severity in an IL-10 dependent manner.
Of note, deletion of IL-10 production specifically in plasma cells results in exacerbated EAE, and adoptive transfer of IL-10 competent plasma cells into IL-10-/- EAE mice is sufficient to attenuate disease (38). This means that plasma cell derived IL-10 is both necessary and sufficient to dampen EAE, although it is highly likely that other IL-10 producing cells amplify these initial regulatory steps. With these data in mind, we propose a model whereby environmental factors operate through the gut microbiota promote IL-10+ B cell populations with the capacity to directly or indirectly regulate CNS autoimmunity during MS and EAE (Figure 2). It is likely that the recipients of IL-10 in this model are IL-10Rα expressing regulatory immune cell populations such as microglia, DCs, and regulatory T cell subsets. In support of this, there is evidence that IL-10+ Bregs are important for the differentiation of Tregs during EAE (75, 159, 160).
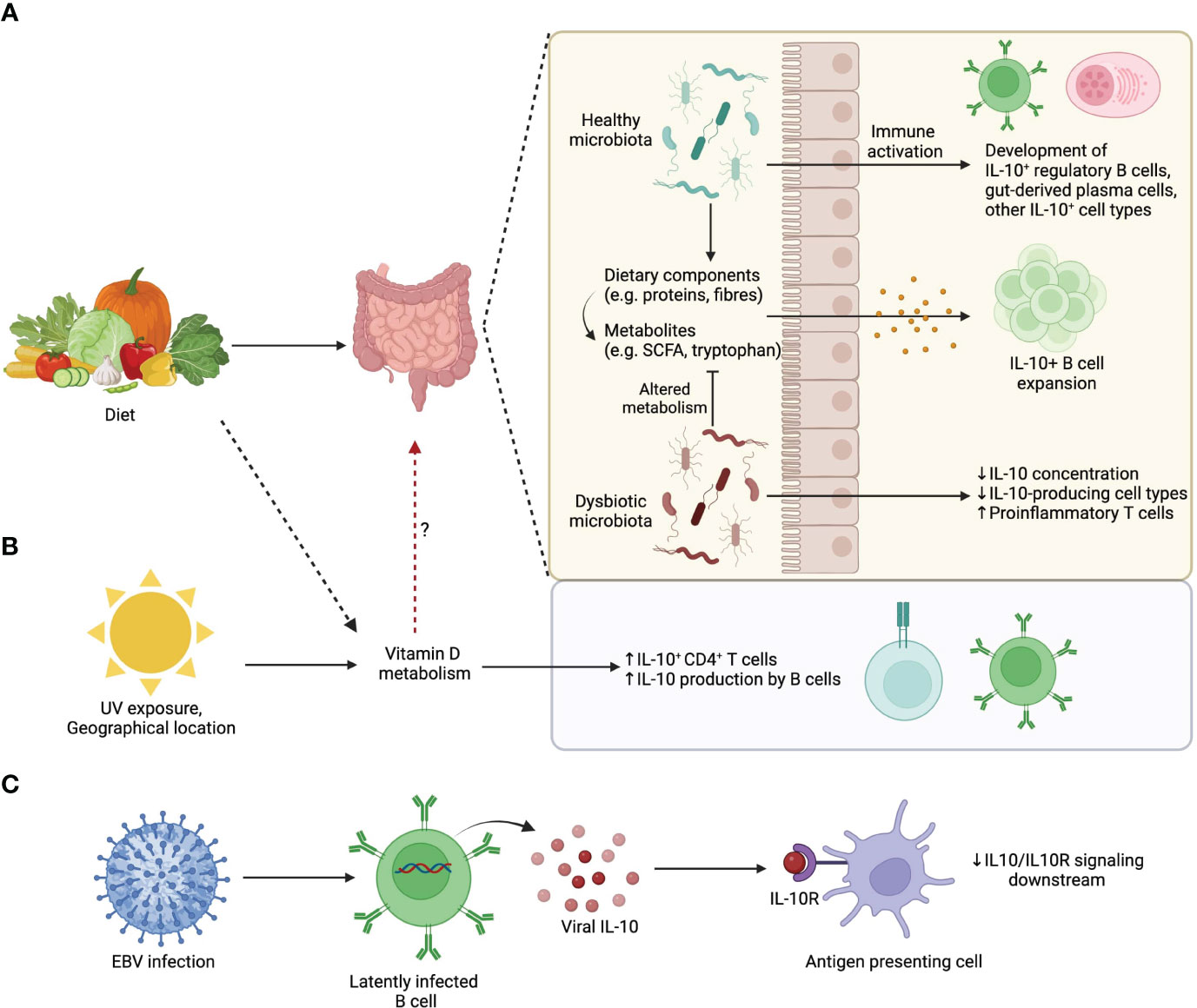
Figure 2 Environmental influences on IL-10-producing B cells. (A) Dietary patterns influence gut microbiota composition. A healthy microbiota and/or its associated diet-derived metabolites can promote the development of IL-10 producing commensal-reactive B cells and IgA+ plasma cells. (B) UV exposure from sunlight, which is influenced by geographical location, changes Vitamin D availability that upon metabolism promotes IL-10 production by lymphocytes. (C) EBV latently infected B cells produce viral homologs of IL-10 that compete with endogenous IL-10 for IL10R binding. Viral IL-10 homologues are less efficient than endogenous IL-10 at triggering downstream signaling.
5 Conclusions
While it has been shown that lower IL-10 levels have a negative impact on MS/EAE, and that environmental exposures impact IL-10 levels, our understanding of what IL-10R expressing cell types(s) respond to IL-10 to alter neuroinflammation, and the environmental factors that impact IL-10R signals, is less comprehensive. In this vein, it is critical to not only understand the specific environmental contexts that influence IL-10 production, but also what cell types receive IL-10. The conflicting evidence between IL-10 knockout studies and cell specific IL-10R knockout studies in EAE indicate that there is more to be understood about IL-10R signaling during CNS autoimmunity. Exploring how these IL-10R-expressing cell types respond to environmental stimuli reframes the focus from the IL-10 producing cell type(s) to the cell-specific downstream effects of IL-10R signaling, and how environmental factors impacts these signals. Furthermore, we propose that B cells are the critical link between environmental stimuli and IL-10R signaling during MS/EAE. Identifying environmental factors that modulate the IL-10/IL-10R axis has the potential to provide new insights into therapeutic intervention for MS patients.
Author contributions
EB wrote the article with editorial oversight from AW and JG. All authors contributed to the article and approved the submitted version.
Funding
The authors acknowledge funding from the MS Society of Canada for an operating grant (EGID#920164) to JG and an EndMS Doctoral award (EGID#3490) to AW.
Acknowledgments
We would like to thank the MS Society of Canada for their ongoing support of our research program.
Conflict of interest
JG is receiving reagents from Novartis and Roche to study the role of B cells in MS.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wallin MT, Culpepper WJ, Nichols E, Bhutta ZA, Gebrehiwot TT, Hay SI, et al. Global, regional, and national burden of multiple sclerosis 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol (2019) 18(3):269–85. doi: 10.1016/S1474-4422(18)30443-5
2. Compston A, Coles A. Multiple sclerosis. Lancet (2008) 372(9648):1502–17. doi: 10.1016/S0140-6736(08)61620-7
3. Steinman L. Assessment of animal models for MS and demyelinating disease in the design of rational therapy. Neuron (1999) 24(3):511–4. doi: 10.1016/S0896-6273(00)81107-1
4. Kuchroo VK, Prabhu Das M, Brown JA, Ranger AM, Zamvil SS, Sobel RA, et al. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: Application to autoimmune disease therapy. Cell (1995) 80(5):707–18. doi: 10.1016/0092-8674(95)90349-6
5. Cua DJ, Hinton DR, Stohlman SA. Self-antigen-induced Th2 responses in experimental allergic encephalomyelitis (EAE)-resistant mice. Th2-mediated suppression of autoimmune disease. J Immunol (1995) 155(8):4052–9.
6. Kuchroo VK, Anderson AC, Waldner H, Munder M, Bettelli E, Nicholson LB. T cell response in Experimental Autoimmune Encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire. Annu Rev Immunol (2003) 20:101–23. doi: 10.1146/annurev.immunol.20.081701.141316
7. Cua DJ, Groux H, Hinton DR, Stohlman SA, Coffman RL. Transgenic interleukin 10 prevents induction of experimental autoimmune encephalomyelitis. J Exp Med (1999) 189(6):1005. doi: 10.1084/jem.189.6.1005
8. Cohen SJ, Cohen IR, Nussbaum G. IL-10 mediates resistance to adoptive transfer experimental autoimmune encephalomyelitis in MyD88 –/– mice. J Immunol (2010) 184(1):212–21. doi: 10.4049/jimmunol.0900296
9. Bettelli E, Prabhu Das M, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J Immunol (1998) 161(7):3299–306. doi: 10.4049/jimmunol.161.7.3299
10. Cua DJ, Hutchins B, LaFace DM, Stohlman SA, Coffman RL. Central nervous system expression of IL-10 inhibits autoimmune encephalomyelitis. J Immunol (2001) 166(1):602–8. doi: 10.4049/jimmunol.166.1.602
11. Cannella B, Gao YL, Brosnan C, Raine CS. IL-10 fails to abrogate experimental autoimmune encephalomyelitis. J Neurosci Res (1996) 45(6):735–46. doi: 10.1002/(SICI)1097-4547(19960915)45:6<735::AID-JNR10>3.0.CO;2-V
12. Croxford JL, Feldmann M, Chernajovsky Y, Baker D. Different therapeutic outcomes in experimental allergic encephalomyelitis dependant upon the mode of delivery of IL-10: A comparison of the effects of protein, adenoviral or retroviral IL-10 delivery into the central nervous system. J Immunol (2001) 166(6):4124–30. doi: 10.4049/jimmunol.166.6.4124
13. Kwilasz AJ, Grace PM, Serbedzija P, Maier SF, Watkins LR. The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology (2015) 96(Pt A):55. doi: 10.1016/j.neuropharm.2014.10.020
14. Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med (1989) 170(6):2081–95. doi: 10.1084/jem.170.6.2081
15. Moore KW, De Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol (2003) 19:683–765. doi: 10.1146/annurev.immunol.19.1.683
16. Saraiva M, Vieira P, O’Garra A. Cytokines Focus: Biology and therapeutic potential of interleukin-10. J Exp Med (2020) 217(1):e20190418. doi: 10.1084/jem.20190418
17. Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol (2010) 10(3):170–81. doi: 10.1038/nri2711
18. Williams LM, Ricchetti G, Sarma U, Smallie T, Foxwell BMJ. Interleukin-10 suppression of myeloid cell activation — a continuing puzzle. Immunology (2004) 113(3):281. doi: 10.1111/j.1365-2567.2004.01988.x
19. Mittal SK, Roche PA. Suppression of antigen presentation by IL-10. Curr Opin Immunol (2015) 34:22. doi: 10.1016/j.coi.2014.12.009
20. Macatonia SE, Doherty TM, Knight SC, O’Garra A. Differential effect of IL-10 on dendritic cell-induced T cell proliferation and IFN-gamma production. J Immunol (1993) 150(9):3755–65. doi: 10.4049/jimmunol.150.9.3755
21. De Smedt T, Van Mechelen M, De Becker G, Urbain J, Leo O, Moser M. Effect of interleukin-10 on dendritic cell maturation and function. Eur J Immunol (1997) 27(5):1229–35. doi: 10.1002/eji.1830270526
22. Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol (1997) 159(10):4772–80. doi: 10.4049/jimmunol.159.10.4772
23. Waal Malefyt R, Haanen J, Spits H, Koncarolo MG, Te Velde A, Figdor C, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med (1991) 174(4):915–24. doi: 10.1084/jem.174.4.915
24. de Waal Malefyt R, Yssel H, de Vries JE. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol (1993) 150(11):4754–65. doi: 10.4049/jimmunol.150.11.4754
25. Ding L, Shevach EM. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J Immunol (1992) 148(10):3133–9. doi: 10.4049/jimmunol.148.10.3133
26. Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol (1991) 146(10):3444–51. doi: 10.4049/jimmunol.146.10.3444
27. Groux H, Bigler M, De Vries JE, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med (1996) 184(1):19–29. doi: 10.1084/jem.184.1.19
28. Kamanaka M, Huber S, Zenewicz LA, Gagliani N, Rathinam C, O’Connor W, et al. Memory/effector (CD45RBlo) CD4 T cells are controlled directly by IL-10 and cause IL-22–dependent intestinal pathology. J Exp Med (2011) 208(5):1027. doi: 10.1084/jem.20102149
29. Huber S, Gagliani N, Esplugues E, O’Connor W, Huber FJ, Chaudhry A, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3– and Foxp3+ regulatory CD4+ T cells in an interleukin-10 dependent manner. Immunity (2011) 34(4):554. doi: 10.1016/j.immuni.2011.01.020
30. Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity (2011) 34(4):566–78. doi: 10.1016/j.immuni.2011.03.018
31. Turovskaya O, Kim G, Cheroutre H, Kronenberg M, Madan R. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol (2009) 10(11):1178–84. doi: 10.1038/ni.1791
32. Brockmann L, Gagliani N, Steglich B, Giannou AD, Kempski J, Pelczar P, et al. IL-10 receptor signaling is essential for TR1 cell function in vivo. J Immunol (2017) 198(3):1130–41. doi: 10.4049/jimmunol.1601045
33. Boonstra A, Rajsbaum R, Holman M, Marques R, Asselin-Paturel C, Pereira JP, et al. Macrophages and myeloid dendritic cells, but not plasmacytoid dendritic cells, produce IL-10 in response to MyD88- and TRIF-dependent TLR signals, and TLR-independent signals. J Immunol (2006) 177(11):7551–8. doi: 10.4049/jimmunol.177.11.7551
34. Ouyang W, O’Garra A. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity (2019) 50(4):871–91. doi: 10.1016/j.immuni.2019.03.020
35. Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol (2002) 3(10):944–50. doi: 10.1038/ni833
36. Radomir L, Kramer MP, Perpinial M, Schottlender N, Rabani S, David K, et al. The survival and function of IL-10-producing regulatory B cells are negatively controlled by SLAMF5. Nat Commun (2021) 12(1):1–14. doi: 10.1038/s41467-021-22230-z
37. Carter NA, Vasconcellos R, Rosser EC, Tulone C, Muñoz-Suano A, Kamanaka M, et al. Mice lacking endogenous IL-10–producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol (2011) 186(10):5569–79. doi: 10.4049/jimmunol.1100284
38. Rojas OL, Pröbstel AK, Porfilio EA, Wang AA, Charabati M, Sun T, et al. Recirculating intestinal IgA-producing cells regulate neuroinflammation via IL-10. Cell (2019) 176(3):610–24. doi: 10.1016/j.cell.2018.11.035
39. Matsumoto M, Baba A, Yokota T, Nishikawa H, Ohkawa Y, Kayama H, et al. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity (2014) 41(6):1040–51. doi: 10.1016/j.immuni.2014.10.016
40. Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol (2007) 178(10):6092–9. doi: 10.4049/jimmunol.178.10.6092
41. Li R, Rezk A, Miyazaki Y, Hilgenberg E, Touil H, Shen P, et al. Proinflammatory GM-CSF-producing B cells in multiple sclerosis and B cell depletion therapy. Sci Transl Med (2015) 7(310):310ra166–310ra166. doi: 10.1126/scitranslmed.aab4176
42. Mass E, Ballesteros I, Farlik M, Halbritter F, Günther P, Crozet L, et al. Specification of tissue-resident macrophages during organogenesis. Science (2016) 353(6304):aaf4238. doi: 10.1126/science.aaf4238
43. Matcovitch-Natan O, Winter DR, Giladi A, Aguilar SV, Spinrad A, Sarrazin S, et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science (2016) 353(6301):aad8670. doi: 10.1126/science.aad8670
44. Finbloom DS, Winestock KD. IL-10 induces the tyrosine phosphorylation of tyk2 and Jak1 and the differential assembly of STAT1 alpha and STAT3 complexes in human T cells and monocytes. J Immunol (1995) 155(3):1079–90. doi: 10.4049/jimmunol.155.3.1079
45. Weber-Nordtt RM, Riley JK, Greenlund AC, Moore KW, Darnell JE, Schreiber RD. Stat3 recruitment by two distinct ligand-induced, tyrosine- phosphorylated docking sites in the interleukin-10 receptor intracellular domain. J Biol Chem (1996) 271(44):27954–61. doi: 10.1074/jbc.271.44.27954
46. Kuwata H, Watanabe Y, Miyoshi H, Yamamoto M, Kaisho T, Takeda K, et al. IL-10-inducible Bcl-3 negatively regulates LPS-induced TNF-α production in macrophages. Blood (2003) 102(12):4123–9. doi: 10.1182/blood-2003-04-1228
47. Ip WKE, Hoshi N, Shouval DS, Snapper S, Medzhitov R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Sci (80- ) (2017) 356(6337):513–9. doi: 10.1126/science.aal3535
48. Glocker E-O, Kotlarz D, Boztug K, Gertz EM, Schäffer AA, Noyan F, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med (2009) 361(21):2033. doi: 10.1056/NEJMoa0907206
49. Kotlarz D, Beier R, Murugan D, Diestelhorst J, Jensen O, Boztug K, et al. Loss of interleukin-10 signaling and infantile inflammatory bowel disease: Implications for diagnosis and therapy. Gastroenterology (2012) 143(2):347–55. doi: 10.1053/j.gastro.2012.04.045
50. Zhu L, Shi T, Zhong C, Wang Y, Chang M, Liu X. IL-10 and IL-10 receptor mutations in very early onset inflammatory bowel disease. Gastroenterol Res (2017) 10(2):65. doi: 10.14740/gr740w
51. Nunberg MY, Werner L, Kopylov U, Haberman Y, Lahad A, Weiss B, et al. Impaired IL-10 receptor-mediated suppression in monocyte from patients with crohn disease. J Pediatr Gastroenterol Nutr (2018) 66(5):779–84. doi: 10.1097/MPG.0000000000001795
52. Shouval DS, Biswas A, Goettel JA, McCann K, Conaway E, Redhu NS, et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity (2014) 40(5):706–19. doi: 10.1016/j.immuni.2014.03.011
53. Shouval DS, Konnikova L, Griffith AE, Wall SM, Biswas A, Werner L, et al. Enhanced TH17 responses in patients with IL10 receptor deficiency and infantile-onset IBD. Inflammation Bowel Dis (2017) 23(11):1950–61. doi: 10.1097/MIB.0000000000001270
54. Spencer SD, Di Marco F, Hooley J, Pitts-Meek S, Bauer M, Ryan AM, et al. The orphan receptor CRF2-4 is an essential subunit of the interleukin 10 receptor. J Exp Med (1998) 187(4):571. doi: 10.1084/jem.187.4.571
55. Pils MC, Pisano F, Fasnacht N, Heinrich JM, Groebe L, Schippers A, et al. Monocytes/macrophages and/or neutrophils are the target of IL-10 in the LPS endotoxemia model. Eur J Immunol (2010) 40(2):443–8. doi: 10.1002/eji.200939592
56. Li B, Alli R, Vogel P, Geiger TL. IL-10 modulates DSS-induced colitis through a macrophage–ROS–NO axis. Mucosal Immunol (2014) 7(4):869–78. doi: 10.1038/mi.2013.103
57. Zigmond E, Bernshtein B, Friedlander G, Walker CR, Yona S, Kim KW, et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity (2014) 40(5):720–33. doi: 10.1016/j.immuni.2014.03.012
58. Patik I, Redhu NS, Eran A, Bao B, Nandy A, Tang Y, et al. The IL-10 receptor inhibits cell extrinsic signals necessary for STAT1-dependent macrophage accumulation during colitis. Mucosal Immunol (2023) 16(3):233–49. doi: 10.1016/j.mucimm.2023.02.006
59. Girard-Madoux MJH, Kel JM, Reizis B, Clausen BE. IL-10 controls dendritic cell–induced T-cell reactivation in the skin to limit contact hypersensitivity. J Allergy Clin Immunol (2012) 129(1):143–150.e10. doi: 10.1016/j.jaci.2011.08.032
60. Teitz-Tennenbaum S, Viglianti SP, Roussey JA, Levitz SM, Olszewski MA, Osterholzer JJ. Autocrine IL-10 signaling promotes dendritic cell type-2 activation and persistence of murine cryptococcal lung infection. J Immunol (2018) 201(7):2004–15. doi: 10.4049/jimmunol.1800070
61. Özenci V, Kouwenhoven M, Huang YM, Xiao BG, Kivisäkk P, Fredrikson S, et al. Multiple sclerosis: levels of interleukin-10-secreting blood mononuclear cells are low in untreated patients but augmented during interferon-beta-1b treatment. Scand J Immunol (1999) 49(5):554–61. doi: 10.1046/j.1365-3083.1999.00546.x
62. Özenci V, Kouwenhoven M, Huang YM, Kivisäkk P, Link H. Multiple sclerosis is associated with an imbalance between tumour necrosis factor-alpha (TNF-α)- and IL-10-secreting blood cells that is corrected by interferon-beta (IFN-β) treatment. Clin Exp Immunol (2001) 120(1):147–53. doi: 10.1046/j.1365-2249.2000.01175.x
63. Waubant E, Gee L, Bacchetti P, Sloan R, Cotleur A, Rudick R, et al. Relationship between serum levels of IL-10, MRI activity and interferon beta-1a therapy in patients with relapsing remitting MS. J Neuroimmunol (2001) 112(1–2):139–45. doi: 10.1016/S0165-5728(00)00355-6
64. van Boxel-Dezaire AH, Hoff SC, van Oosten BW, Verweij CL, Dräger AM, Adèr HJ, et al. Decreased interleukin-10 and increased interleukin-12p40 mRNA are associated with disease activity and characterize different disease stages in multiple sclerosis. Ann Neurol (1999) 45(6):695–703. doi: 10.1002/1531-8249(199906)45:6<695::AID-ANA3>3.0.CO;2-R
65. Romme Christensen J, Börnsen L, Hesse D, Krakauer M, Sørensen PS, Søndergaard HB, et al. Cellular sources of dysregulated cytokines in relapsing-remitting multiple sclerosis. J Neuroinflamm (2012) 9(1):1–12. doi: 10.1186/1742-2094-9-215
66. Feigin VL, Krishnamurthi RV, Theadom AM, Abajobir AA, Mishra SR, Ahmed MB, et al. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol (2017) 16(11):877–97. doi: 10.1016/S1474-4422(17)30299-5
67. Fissolo N, Pappolla A, Rio J, Villar LM, Perez-Hoyos S, Sanchez A, et al. Serum levels of CXCL13 are associated with teriflunomide response in patients with multiple sclerosis. Neurol Neuroimmunol Neuroinflamm (2023) 10(1):e200050. doi: 10.1212/NXI.0000000000200050
68. Cao Y, Goods BA, Raddassi K, Nepom GT, Kwok WW, Love JC, et al. Distinct inflammatory profiles of myelin-reactive T cells from patients with multiple sclerosis. Sci Transl Med (2015) 7(287):287ra74. doi: 10.1126/scitranslmed.aaa8038
69. Aharoni R, Teitelbaum D, Leitner O, Meshorer A, Sela M, Arnon R. Specific Th2 cells accumulate in the central nervous system of mice protected against experimental autoimmune encephalomyelitis by copolymer 1. Proc Natl Acad Sci (2000) 97(21):11472–7. doi: 10.1073/pnas.97.21.11472
70. Miller A, Shapiro S, Gershtein R, Kinarty A, Rawashdeh H, Honigman S, et al. Treatment of multiple sclerosis with Copolymer-1 (Copaxone®): Implicating mechanisms of Th1 to Th2/Th3 immune-deviation. J Neuroimmunol (1998) 92(1–2):113–21. doi: 10.1016/S0165-5728(98)00191-X
71. Blumenfeld S, Staun-Ram E, Miller A. Fingolimod therapy modulates circulating B cell composition, increases B regulatory subsets and production of IL-10 and TGFβ in patients with Multiple Sclerosis. J Autoimmun (2016) 70:40–51. doi: 10.1016/j.jaut.2016.03.012
72. Ireland SJ, Guzman AA, O’Brien DE, Hughes S, Greenberg B, Flores A, et al. The effect of glatiramer acetate therapy on functional properties of B cells from patients with relapsing-remitting multiple sclerosis. JAMA Neurol (2014) 11):1421–8. doi: 10.1001/jamaneurol.2014.1472
73. Schubert RD, Hu Y, Kumar G, Szeto S, Abraham P, Winderl J, et al. IFN-β Treatment requires B cells for efficacy in neuroautoimmunity. J Immunol (2015) 194(5):2110–6. doi: 10.4049/jimmunol.1402029
74. McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ Regulatory cells within the central nervous system. J Immunol (2005) 175(5):3025–32. doi: 10.4049/jimmunol.175.5.3025
75. Mann MK, Maresz K, Shriver LP, Tan Y, Dittel BN. B cell regulation of CD4+CD25+ T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J Immunol (2007) 178(6):3447–56. doi: 10.4049/jimmunol.178.6.3447
76. Petereit HF, Pukrop R, Fazekas F, Bamborschke SU, Röpele S, Kölmel HW, et al. Low interleukin-10 production is associated with higher disability and MRI lesion load in secondary progressive multiple sclerosis. J Neurol Sci (2003) 206(2):209–14. doi: 10.1016/S0022-510X(02)00420-3
77. Martinez-Forero I, Garcia-Munoz R, Martinez-Pasamar S, Inoges S, de Cerio ALD, Palacios R, et al. IL-10 suppressor activity and ex vivo Tr1 cell function are impaired in multiple sclerosis. Eur J Immunol (2008) 38(2):576–86. doi: 10.1002/eji.200737271
78. Gasche C, Grundtner P, Zwirn P, Reinisch W, Shaw SH, Zdanov A, et al. Novel variants of the IL-10 receptor 1 affect inhibition of monocyte TNF-α Production. J Immunol (2003) 170(11):5578–82. doi: 10.4049/jimmunol.170.11.5578
79. Ben Fredj N, Aissi M, Ben Selma W, Mahmoud I, Nefzi F, Frih-Ayed M, et al. Association of the IL-10 receptor A536G (S138G) loss-of-function variant with multiple sclerosis in Tunisian patients. APMIS (2017) 125(5):444–51. doi: 10.1111/apm.12659
80. Galatola M, Miele E, Strisciuglio C, Paparo L, Rega D, Delrio P, et al. Synergistic effect of interleukin-10-receptor variants in a case of early-onset ulcerative colitis. World J Gastroenterol (2013) 19(46):8659. doi: 10.3748/wjg.v19.i46.8659
81. Peng H, Wang W, Zhou M, Li R, Pan HF, Ye DQ. Role of interleukin-10 and interleukin-10 receptor in systemic lupus erythematosus. Clin Rheumatol (2013) 32(9):1255–66. doi: 10.1007/s10067-013-2294-3
82. Peng H, Liu CY, Zhou M, Wen PF, Zhang M, Qiu LJ, et al. IL-10RB rs2834167 (A/G) polymorphism is associated with the susceptibility to systemic lupus erythematosus: Evidence from a study in Chinese han population. Inflammation (2013) 36(6):1218–24. doi: 10.1007/s10753-013-9658-3
83. Rodríguez-Sáinz M del C, Sánchez-Ramón S, Andrés C, Rodríguez-Mahou M, Muñoz-Fernández MA. Th1/Th2 cytokine balance and nitric oxide in cerebrospinal fluid and serum from patients with multiple sclerosis. Eur Cytokine Netw (2002) 13(1):110–4.
84. Martins TB, Rose JW, Jaskowski TD, Wilson AR, Husebye D, Seraj HS, et al. Analysis of proinflammatory and anti-inflammatory cytokine serum concentrations in patients with multiple sclerosis by using a multiplexed immunoassay. Am J Clin Pathol (2011) 136(5):696–704. doi: 10.1309/AJCP7UBK8IBVMVNR
85. Krakauer M, Sorensen P, Khademi M, Olsson T, Sellebjerg F. Increased IL-10 mRNA and IL-23 mRNA expression in multiple sclerosis: interferon-β treatment increases IL-10 mRNA expression while reducing IL-23 mRNA expression. Mult Scler J (2008) 14(5):622–30. doi: 10.1177/1352458507087136
86. Liu X, Alli R, Steeves M, Nguyen P, Vogel P, Geiger TL. The T cell response to IL-10 alters cellular dynamics and paradoxically promotes central nervous system autoimmunity. J Immunol (2012) 189(2):669–78. doi: 10.4049/jimmunol.1200607
87. Yogev N, Bedke T, Kobayashi Y, Brockmann L, Lukas D, Regen T, et al. CD4+ T-cell-derived IL-10 promotes CNS inflammation in mice by sustaining effector T cell survival. Cell Rep (2022) 38(13):110565. doi: 10.1016/j.celrep.2022.110565
88. Hasan N, Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ (2019) 7(8):e7502. doi: 10.7717/peerj.7502
89. Haase S, Wilck N, Haghikia A, Gold R, Mueller DN, Linker RA. The role of the gut microbiota and microbial metabolites in neuroinflammation. Eur J Immunol (2020) 50(12):1863–70. doi: 10.1002/eji.201847807
90. Pu A, Lee DSW, Isho B, Naouar I, Gommerman JL. The impact of IgA and the microbiota on CNS disease. Front Immunol (2021) 12:3782. doi: 10.3389/fimmu.2021.742173
91. Correale J, Hohlfeld R, Baranzini SE. The role of the gut microbiota in multiple sclerosis. Nat Rev Neurol (2022) 18(9):544–58. doi: 10.1038/s41582-022-00697-8
92. Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Soldan MMP, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep (2016) 6:28484. doi: 10.1038/srep28484
93. Jangi S, Gandhi R, Cox LM, Li N, Von Glehn F, Yan R, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun (2016) 7(1):1–11. doi: 10.1038/ncomms12015
94. Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci U S A (2017) 114(40):10713–8. doi: 10.1073/pnas.1711235114
95. Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A (2017) 114(40):10719–24. doi: 10.1073/pnas.1711233114
96. Zhou X, Baumann R, Gao X, Mendoza M, Singh S, Katz Sand I, et al. Gut microbiome of multiple sclerosis patients and paired household healthy controls reveal associations with disease risk and course. Cell (2022) 185(19):3467–3486.e16. doi: 10.1016/j.cell.2022.08.021
97. Yokote H, Miyake S, Croxford JL, Oki S, Mizusawa H, Yamamura T. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am J Pathol (2008) 173(6):1714. doi: 10.2353/ajpath.2008.080622
98. Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol (2009) 183(10):6041–50. doi: 10.4049/jimmunol.0900747
99. Ochoa-Repáraz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, et al. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol (2010) 3(5):487–95. doi: 10.1038/mi.2010.29
100. Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Begum-Haque S, Dasgupta S, et al. Central nervous system demyelinating disease protection by the human commensal bacteroides fragilis depends on polysaccharide A expression. J Immunol (2010) 185(7):4101–8. doi: 10.4049/jimmunol.1001443
101. Wang Y, Telesford KM, Ochoa-Repáraz J, Haque-Begum S, Christy M, Kasper EJ, et al. An intestinal commensal symbiosis factor controls neuroinflammation via TLR2-mediated CD39 signaling. Nat Commun (2014) 5:4432. doi: 10.1038/ncomms5432
102. Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A (2010) 107(27):12204–9. doi: 10.1073/pnas.0909122107
103. Mangalam A, Shahi SK, Luckey D, Karau M, Marietta E, Luo N, et al. Human gut-derived commensal bacteria suppress CNS inflammatory and demyelinating disease. Cell Rep (2017) 20(6):1269–77. doi: 10.1016/j.celrep.2017.07.031
104. Lavasani S, Dzhambazov B, Nouri M, Fåk F, Buske S, Molin G, et al. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PloS One (2010) 5(2):e9009. doi: 10.1371/journal.pone.0009009
105. Kwon HK, Kim GC, Kim Y, Hwang W, Jash A, Sahoo A, et al. Amelioration of experimental autoimmune encephalomyelitis by probiotic mixture is mediated by a shift in T helper cell immune response. Clin Immunol (2013) 146(3):217–27. doi: 10.1016/j.clim.2013.01.001
106. Kobayashi T, Suzuki T, Kaji R, Serata M, Nagata T, Ando M, et al. Probiotic upregulation of peripheral IL-17 responses does not exacerbate neurological symptoms in experimental autoimmune encephalomyelitis mouse models. Immunopharmacol Immunotoxicol (2012) 34(3):423–33. doi: 10.3109/08923973.2010.617755
107. Salami M, Kouchaki E, Asemi Z, Tamtaji OR. How probiotic bacteria influence the motor and mental behaviors as well as immunological and oxidative biomarkers in multiple sclerosis? A double blind clinical trial. J Funct Foods (2019) 52:8–13. doi: 10.1016/j.jff.2018.10.023
108. Tankou SK, Regev K, Healy BC, Cox LM, Tjon E, Kivisakk P, et al. Investigation of probiotics in multiple sclerosis. Mult Scler (2018) 24(1):58–63. doi: 10.1177/1352458517737390
109. Lu XY, Han B, Deng X, Deng SY, Zhang YY, Shen PX, et al. Pomegranate peel extract ameliorates the severity of experimental autoimmune encephalomyelitis via modulation of gut microbiota. Gut Microbes (2020) 12(1):1–14. doi: 10.1080/19490976.2020.1857515
110. Platten M, Ho PP, Youssef S, Fontoura P, Garren H, Hur EM, et al. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science (2005) 310(5749):850–5. doi: 10.1126/science.1117634
111. Yan Y, Zhang G-X, Gran B, Fallarino F, Yu S, Li H, et al. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J Immunol (2010) 185(10):5953–61. doi: 10.4049/jimmunol.1001628
112. Park J, Wang Q, Wu Q, Mao-Draayer Y, Kim CH. Bidirectional regulatory potentials of short-chain fatty acids and their G-protein-coupled receptors in autoimmune neuroinflammation. Sci Rep (2019) 9(1):1–13. doi: 10.1038/s41598-019-45311-y
113. Haase S, Mäurer J, Duscha A, Lee DH, Balogh A, Gold R, et al. Propionic acid rescues high-fat diet enhanced immunopathology in autoimmunity via effects on Th17 responses. Front Immunol (2021) 12:701626. doi: 10.3389/fimmu.2021.701626
114. Moles L, Delgado S, Gorostidi-Aicua M, Sepúlveda L, Alberro A, Iparraguirre L, et al. Microbial dysbiosis and lack of SCFA production in a Spanish cohort of patients with multiple sclerosis. Front Immunol (2022) 13:960761. doi: 10.3389/fimmu.2022.960761
115. Saresella M, Mendozzi L, Rossi V, Mazzali F, Piancone F, LaRosa F, et al. Immunological and clinical effect of diet modulation of the gut microbiome in multiple sclerosis patients: A pilot study. Front Immunol (2017) 8(OCT):289480. doi: 10.3389/fimmu.2017.01391
116. Berer K, Martínez I, Walker A, Kunkel B, Schmitt-Kopplin P, Walter J, et al. Dietary non-fermentable fiber prevents autoimmune neurological disease by changing gut metabolic and immune status. Sci Rep (2018) 8(1):1–12. doi: 10.1038/s41598-018-28839-3
117. Jensen SN, Cady NM, Shahi SK, Peterson SR, Gupta A, Gibson-Corley KN, et al. Isoflavone diet ameliorates experimental autoimmune encephalomyelitis through modulation of gut bacteria depleted in patients with multiple sclerosis. Sci Adv (2021) 7(28):eabd4595. doi: 10.1126/sciadv.abd4595
118. Fettig NM, Robinson HG, Allanach JR, Davis KM, Simister RL, Wang EJ, et al. Inhibition of Th1 activation and differentiation by dietary guar gum ameliorates experimental autoimmune encephalomyelitis. Cell Rep (2022) 40(11):111328. doi: 10.1016/j.celrep.2022.111328
119. Cignarella F, Cantoni C, Ghezzi L, Salter A, Dorsett Y, Chen L, et al. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab (2018) 27(6):1222–35. doi: 10.1016/j.cmet.2018.05.006
120. Kimlin MG, Olds WJ, Moore MR. Location and vitamin D synthesis: is the hypothesis validated by geophysical data? J Photochem Photobiol B Biol (2007) 86(3):234–9. doi: 10.1016/j.jphotobiol.2006.10.004
121. Simpson S, Blizzard L, Otahal P, van der Mei I, Taylor B. Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol Neurosurg Psychiatry (2011) 82(10):1132–41. doi: 10.1136/jnnp.2011.240432
122. Mokry LE, Ross S, Ahmad OS, Forgetta V, Smith GD, Leong A, et al. Vitamin D and risk of multiple sclerosis: A mendelian randomization study. PloS Med (2015) 12(8):e1001866. doi: 10.1371/journal.pmed.1001866
123. Rhead B, Bäärnhielm M, Gianfrancesco M, Mok A, Shao X, Quach H, et al. Mendelian randomization shows a causal effect of low vitamin D on multiple sclerosis risk. Neurol Genet (2016) 2(5):97. doi: 10.1212/NXG.0000000000000097
124. Ramagopalan SV, Maugeri NJ, Handunnetthi L, Lincoln MR, Orton SM, Dyment DA, et al. Expression of the multiple sclerosis-associated MHC class II allele HLA-DRB1*1501 is regulated by vitamin D. PloS Genet (2009) 5(2):e1000369. doi: 10.1371/journal.pgen.1000369
125. Smolders J, Menheere P, Kessels A, Damoiseaux J, Hupperts R. Association of vitamin D metabolite levels with relapse rate and disability in multiple sclerosis. Mult Scler (2008) 14(9):1220–4. doi: 10.1177/1352458508094399
126. Ascherio A, Munger KL, White R, Köchert K, Simon KC, Polman CH, et al. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol (2014) 71(3):306–14. doi: 10.1001/jamaneurol.2013.5993
127. Mowry EM, Waubant E, McCulloch CE, Okuda DT, Evangelista AA, Lincoln RR, et al. Vitamin D status predicts new brain magnetic resonance imaging activity in multiple sclerosis. Ann Neurol (2012) 72(2):234–40. doi: 10.1002/ana.23591
128. Runia TF, Hop WCJ, De Rijke YB, Buljevac D, Hintzen RQ. Lower serum vitamin D levels are associated with a higher relapse risk in multiple sclerosis. Neurology (2012) 79(3):261–6. doi: 10.1212/WNL.0b013e31825fdec7
129. Simpson S, Taylor B, Blizzard L, Ponsonby AL, Pittas F, Tremlett H, et al. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol (2010) 68(2):193–203. doi: 10.1002/ana.22043
130. Smolders J, Peelen E, Thewissen M, Tervaert JWC, Menheere P, Hupperts R, et al. Safety and T cell modulating effects of high dose vitamin D3 supplementation in multiple sclerosis. PloS One (2010) 5(12):e15235. doi: 10.1371/journal.pone.0015235
131. Mosayebi G, Ghazavi A, Ghasami K, Jand Y, Kokhaei P. Therapeutic effect of vitamin D3 in multiple sclerosis patients. Immunol Invest (2011) 40(6):627–39. doi: 10.3109/08820139.2011.573041
132. Hashemi R, Morshedi M, Jafarabadi MA, Altafi D, Hosseini-Asl SS, Rafie-Arefhosseini S. Anti-inflammatory effects of dietary vitamin D3 in patients with multiple sclerosis. Neurol Genet (2018) 4(6):278. doi: 10.1212/NXG.0000000000000278
133. Farsani ZS, Behmanesh M, Sahraian MA. Interleukin-10 but not transforming growth factor-β1 gene expression is up-regulated by vitamin D treatment in multiple sclerosis patients. J Neurol Sci (2015) 350(1–2):18–23. doi: 10.1016/j.jns.2015.01.030
134. Ashtari F, Toghianifar N, Zarkesh-Esfahani SH, Mansourian M. Short-term effect of high-dose vitamin D on the level of interleukin 10 in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled clinical trial. Neuroimmunomodulation (2015) 22(6):400–4. doi: 10.1159/000439278
135. Penna G, Adorini L. 1α,25-Dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol (2000) 164(5):2405–11. doi: 10.4049/jimmunol.164.5.2405
136. Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol (2000) 164(9):4443–51. doi: 10.4049/jimmunol.164.9.4443
137. Heine G, Niesner U, Chang HD, Steinmeyer A, Zügel U, Zuberbier T, et al. 1,25-dihydroxyvitamin D3 promotes IL-10 production in human B cells. Eur J Immunol (2008) 38(8):2210–8. doi: 10.1002/eji.200838216
138. Cantorna MT, Hayes CE, Deluca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci U S A (1996) 93(15):7861. doi: 10.1073/pnas.93.15.7861
139. Lemire JM, Clay Archer D. 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest (1991) 87(3):1103–7. doi: 10.1172/JCI115072
140. Haghmorad D, Yazdanpanah E, Jadid Tavaf M, Zargarani S, Soltanmohammadi A, Mahmoudi MB, et al. Prevention and treatment of experimental autoimmune encephalomyelitis induced mice with 1, 25-dihydroxyvitamin D3. Neurol Res (2019) 41(10):943–57. doi: 10.1080/01616412.2019.1650218
141. Spach KM, Nashold FE, Dittel BN, Hayes CE. IL-10 signaling is essential for 1,25-Dihydroxyvitamin D3-mediated inhibition of experimental autoimmune encephalomyelitis. J Immunol (2006) 177(9):6030–7. doi: 10.4049/jimmunol.177.9.6030
142. Xie Z, Chen J, Zheng C, Wu J, Cheng Y, Zhu S, et al. 1,25-dihydroxyvitamin D3-induced dendritic cells suppress experimental autoimmune encephalomyelitis by increasing proportions of the regulatory lymphocytes and reducing T helper type 1 and type 17 cells. Immunology (2017) 152(3):414–24. doi: 10.1111/imm.12776
143. Bellerba F, Muzio V, Gnagnarella P, Facciotti F, Chiocca S, Bossi P, et al. The association between Vitamin D and gut microbiota: A systematic review of human studies. Nutrients (2021) 13(10):3378. doi: 10.3390/nu13103378
144. Womack J, Jimenez M. Common questions about infectious mononucleosis. Am Fam Physician (2015) 91(6):372–6.
145. Bjornevik K, Cortese M, Healy BC, Kuhle J, Mina MJ, Leng Y, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Sci (80- ) (2022) 375(6578):296–301. doi: 10.1126/science.abj8222
146. Soldan SS, Lieberman PM. Epstein–Barr virus and multiple sclerosis. Nat Rev Microbiol (2022) 21(1):51–64. doi: 10.1038/s41579-022-00770-5
147. Hsu DH, De Waal Malefyt R, Fiorentino DF, Dang MN, Vieira P, Devries J, et al. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Sci (80- ) (1990) 250(4982):830–2. doi: 10.1126/science.2173142
148. Liu Y, de Waal Malefyt R, Briere F, Parham C, Bridon JM, Banchereau J, et al. The EBV IL-10 homologue is a selective agonist with impaired binding to the IL-10 receptor. J Immunol (1997) 158(2):604–13. doi: 10.4049/jimmunol.158.2.604
149. Raftery MJ, Wieland D, Gronewald S, Kraus AA, Giese T, Schönrich G. Shaping phenotype, function, and survival of dendritic cells by cytomegalovirus-encoded IL-10. J Immunol (2004) 173(5):3383–91. doi: 10.4049/jimmunol.173.5.3383
150. Jog NR, Chakravarty EF, Guthridge JM, James JA. Epstein Barr virus interleukin 10 suppresses anti-inflammatory phenotype in human monocytes. Front Immunol (2018) 9(OCT):2198. doi: 10.3389/fimmu.2018.02198
151. Jochum S, Moosmann A, Lang S, Hammerschmidt W, Zeidler R. The EBV immunoevasins vIL-10 and BNLF2a protect newly infected B cells from immune recognition and elimination. PloS Pathog (2012) 8(5):e1002704. doi: 10.1371/journal.ppat.1002704
152. Rosser EC, Mauri C. The emerging field of regulatory B cell immunometabolism. Cell Metab (2021) 33(6):1088–97. doi: 10.1016/j.cmet.2021.05.008
153. Rosser EC, Oleinika K, Tonon S, Doyle R, Bosma A, Carter NA, et al. Regulatory B cells are induced by gut microbiota–driven interleukin-1β and interleukin-6 production. Nat Med (2014) 20(11):1334–9. doi: 10.1038/nm.3680
154. Rosser EC, Piper CJM, Matei DE, Blair PA, Rendeiro AF, Orford M, et al. Microbiota-derived metabolites suppress arthritis by amplifying aryl-hydrocarbon receptor activation in regulatory B cells. Cell Metab (2020) 31(4):837–851.e10. doi: 10.1016/j.cmet.2020.03.003
155. Daïen CI, Tan J, Audo R, Mielle J, Quek LE, Krycer JR, et al. Gut-derived acetate promotes B10 cells with antiinflammatory effects. JCI Insight (2021) 6(7):e144156. doi: 10.1172/jci.insight.144156
156. Luu M, Pautz S, Kohl V, Singh R, Romero R, Lucas S, et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat Commun (2019) 10(1):1–12. doi: 10.1038/s41467-019-08711-2
157. Fitzpatrick Z, Frazer G, Ferro A, Clare S, Bouladoux N, Ferdinand J, et al. Gut-educated IgA plasma cells defend the meningeal venous sinuses. Nat (2020) 587(7834):472–6. doi: 10.1038/s41586-020-2886-4
158. Pröbstel AK, Zhou X, Baumann R, Wischnewski S, Kutza M, Rojas OL, et al. Gut microbiota-specific IgA+ B cells traffic to the CNS in active multiple sclerosis. Sci Immunol (2020) 5(53):eabc7191. doi: 10.1126/sciimmunol.abc7191
159. Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest (2008) 118(10):3420–30. doi: 10.1172/JCI36030
160. Jin G, Hamaguchi Y, Matsushita T, Hasegawa M, Le Huu D, Ishiura N, et al. B-cell linker protein expression contributes to controlling allergic and autoimmune diseases by mediating IL-10 production in regulatory B cells. J Allergy Clin Immunol (2013) 131(6):1674–1682.e9. doi: 10.1016/j.jaci.2013.01.044
Keywords: multiple sclerosis, central nervous system, experimental autoimmune encephalomyelitis, IL-10, IL-10R, environmental factors, B cells
Citation: Bugbee E, Wang AA and Gommerman JL (2023) Under the influence: environmental factors as modulators of neuroinflammation through the IL-10/IL-10R axis. Front. Immunol. 14:1188750. doi: 10.3389/fimmu.2023.1188750
Received: 17 March 2023; Accepted: 12 July 2023;
Published: 03 August 2023.
Edited by:
Javier Ochoa-Repáraz, Boise State University, United StatesReviewed by:
Ashutosh K. Mangalam, The University of Iowa, United StatesCopyright © 2023 Bugbee, Wang and Gommerman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jennifer L. Gommerman, amVuLmdvbW1lcm1hbkB1dG9yb250by5jYQ==
 Eryn Bugbee
Eryn Bugbee Angela A. Wang
Angela A. Wang Jennifer L. Gommerman
Jennifer L. Gommerman