- 1Department of General Surgery, the First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 2Jiangsu Key Lab of Cancer Biomarkers, Prevention and Treatment, Jiangsu Collaborative Innovation Center for Cancer Personalized Medical University, Nanjing, China
- 3Department of General Surgery, Jiangyin People’s Hospital Affiliated to Nantong University, Wuxi, China
Background: Recently, the use of immunochemotherapy in the treatment of advanced gastric cancer (GC) has been increasing and programmed cell death protein 1 (PD-1) inhibitors combined with chemotherapy has become the first-line treatment for advanced GC. However, few studies with small sample sizes have examined this treatment regimen to assess its effectiveness and safety in the neoadjuvant treatment phase of resectable local advanced GC.
Materials and methods: Herein, we systematically searched PubMed, Cochrane CENTRAL, and Web of Science for clinical trials on neoadjuvant immunochemotherapy (nICT) in advanced GC. The primary outcomes were effectiveness [evaluated by major pathological response (MPR) and pathological complete response (pCR)] and safety [assessed by grade 3–4 treatment-related adverse events (TRAEs) and postoperative complications]. A meta-analysis of non-comparative binary results was performed to aggregate the primary outcomes. Direct comparative analysis was used to compare pooled results of neoadjuvant chemotherapy (nCT) with nICT. The outcomes emerged as risk ratios (RR).
Results: Five articles with 206 patients were included, and all of them were from the Chinese population. The pooled pCR and MPR rates were 26.5% (95% CI: 21.3%–33.3%) and 49.0% (95% CI: 42.3%–55.9%), while grade 3–4 TRAEs and post-operative complication rates were 20.0% (95% CI: 9.1%–39.8%) and 30.1% (95% CI: 23.1%–37.9%), respectively. Direct comparison showed that with the exception of grade 3–4 TRAEs and postoperative complications, all outcomes including pCR, MPR, and R0 resection rate favoured nICT to nCT.
Conclusion: nICT is a promising strategy for use as an advisable neoadjuvant treatment for patients with advanced GC in Chinese population. However, more phase III randomized controlled trials (RCTs) will be required to further consolidate the efficacy and safety of this regimen.
Introduction
In China, gastric cancer (GC) is the fourth most common cancer and third leading cause of cancer-related deaths (1). It is estimated that if gastric cancer risk cannot be effectively controlled, the global burden of gastric cancer is predicted to increase to 1.8 million new cases and 1.3 million deaths by 2040 (2).To date, great progress has been made in understanding the pathogenesis of GC, and surgery remains the backbone of curative treatment (3). Although D2 radical surgery is beneficial, the 5-year survival rate of patients with GC remains below 50% (4). To improve the prognosis of patients with advanced GC, several clinical studies have confirmed that neoadjuvant therapy in locally advanced GC can downstage the tumour, increase the R0 resection rate, and reduce the risk of postoperative recurrence, thereby improving patient outcomes compared with surgery alone (5, 6). Moreover, with ongoing developments in medicine, immunotherapy has started gaining approval in clinical settings, thus changing the landscape of tumour treatment with satisfactory results being observed in the treatment of melanoma and non-small cell lung cancer (7, 8). Immunotherapy has also shown promising results in the treatment of GC. For instance, the Checkmate 649 and Orient-16 studies confirmed that chemotherapy combined with PD-1 inhibitors has significant improvement in overall survival (OS) (HR 0·71;98·4% CI 0·59–0·86; p<0·0001 and HR 0.660; 95% CI 0.505–0.864; P=0.0023, respectively) and progression-free survival (PFS) (HR 0·68; 98% CI 0·56–0·81; p<0·0001 and HR 0.628; 95% CI 0.489–0.805; P=0.0002, respectively) versus chemotherapy alone in patients with a programmed cell death 1 ligand 1(PD-L1) combined positive score (CPS)>5 (9, 10). KEYNOTE-012 and -059 trials confirmed the efficacy of immune checkpoint inhibitors (ICIs) in patients with metastatic GC (11, 12). KEYNOTE-012 reported that overall response was achieved in 8 (22%) of 36 patients while 17 (53%) of 32 patients developed tumour lesion regression. KEYNOTE-059 presented an objective response rate of 15.5% (95% CI 10.1%–22.4%; 23 of 148 patients) in patients with PD-L1-positive tumours. Consequently, immunotherapy is now generally accepted globally as the first-line treatment for advanced GC. However, whether immunotherapy has benefit in the early stages of GC treatment, such as in the neoadjuvant phase, is a current research focus. Furthermore, immunotherapy combined with chemotherapy has been used clinically in GC as neoadjuvant therapy (e.g., in the NCT04354662, NCT04119622, and NCT04694183 trials), while large-scale clinical trials are yet be conducted to assess its efficacy and safety. Hence, this systematic review and meta-analysis of eligible data was performed to assess the efficacy and safety of neoadjuvant immunochemotherapy (nICT) by pathological complete response (pCR), major pathological response (MPR), R0 surgical resection (clinical and complete microscopic resection of the tumour) rate, grade 3–4 treatment-related adverse events (TRAEs), and postoperative complications, in an attempt to provide a more reliable basis for exploring novel therapeutic strategies for GC.
Materials and methods
Data sources and search strategy
In current study we followed the Preferred Reporting Items for Systematic Reviews and Meta analyses (PRISMA) and Reporting of Surrogate Endpoint Evaluation using Meta analyses (ReSEEM) guidelines (13, 14). We systematically searched PubMed, Medline, Web of Science and Cochrane Library electronic databases to 1 February 2023 for all clinical trials that tested nICT in advanced GC. The detailed search strategy and inclusion criteria are exhibited in online supplemental materials.
Data extraction
The following variables were extracted from all the included clinical trials, if available: pCR, MPR, R0 surgical resection rate; grade 3–4 TRAEs and incidence of postoperative complications. Other details such as the immune checkpoint inhibitor (ICI) regimen and sample size are also shown in the information sheet.
Statistical analysis
Data from the individual included studies were entered into a spread sheet for further analysis. Review Manage (RevMan) software version 5.4 was used to perform the statistical analysis. A meta-analysis of the non-comparative binary results was performed based on the most of the involved studies, which were one-arm clinical trials. For evaluating neoadjuvant therapy effectiveness and safety, the aggregated odds ratio (OR) and 95% confidence interval (CI) were transformed into occurrence rates (synthesis of detailed data in the supplementary information). P< 0.05 for Q test or I2 > 50% for I2 test was deemed to indicate significant heterogeneity in the literature, random effects model was adopted; otherwise, a fixed effects model was used (15, 16). The level of significance for all results was set at P < 0.05. Funnel plots were performed to evaluate possible publication bias (online Supplemental Figure 2).
Risk of bias assessment
Since studies on neoadjuvant immunochemotherapy were mostly non-randomized single-arm clinical trial without comparison groups. Methodological Index for Nonrandomized Studies was used to assess the risk of bias in eligible studies (17).
Results
Eligible studies
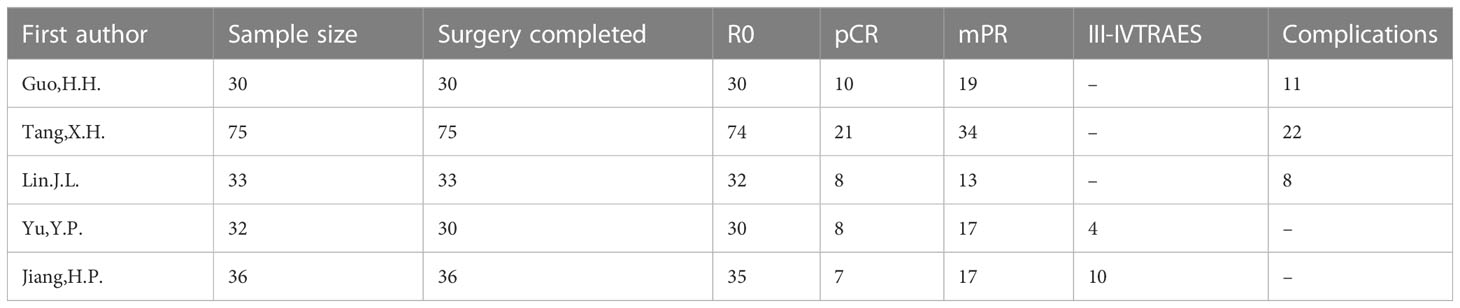
Five studies (18–22), with a total of 206 enrolled patients were included (Supplemental Figure 1). Details of the incorporated studies are shown in Table 1 and Supplemental Table 1. The literature quality of the included studies is summarised in Supplemental Table 2.
Evaluation of effectiveness outcomes
To assess the efficacy of nICT, both the pCR and MPR rates were used. In included studies, the pCR rates ranged from 19.4%–33.3%. The pooled pCR rate was 26.5% (95% CI: 21.8%–33.3%) (Figure 1A). In addition, the MPR rates ranged from 39.4%–63.4%, with an aggregated MPR rate of 49.0% (95% CI: 42.3%–55.9%) (Figure 1B).
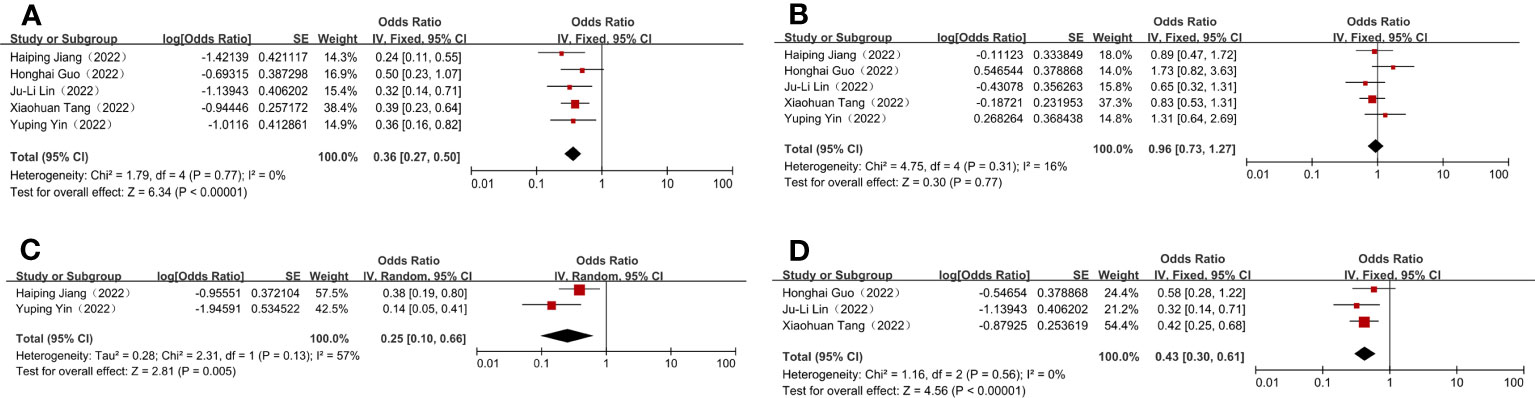
Figure 1 Efficacy and safety evaluation of neoadjuvant immunotherapy plus chemotherapy for locally advanced and resectable gastric cancer. (A) Pathological complete response (pCR); (B) Major pathological response (MPR); (C) Grade 3–4 treatment-related adverse events (TRAEs); (D) Surgical complication.
Evaluation of safety outcomes
The incidence of grade 3–4 TRAEs was recorded as a measure of the safety of nICT, in line with the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCICTCAE16; version 4.0). The combined incidence of grade 3–4 TRAEs was 20.0% (95% CI: 9.1%–39.8%) (Figure 1C). Three studies reported the precise number of postoperative complications, with the incidences ranging from 24.2%–36.7%, and having a combined incidence of 30.1% (95% CI: 23.1%–37.9%) (Figure 1D). Additionally, the R0 resection rates were 100% in most studies; therefore, it was complex to normalise and analyse the extracted data. The incidence of these outcomes is shown in Table 2.
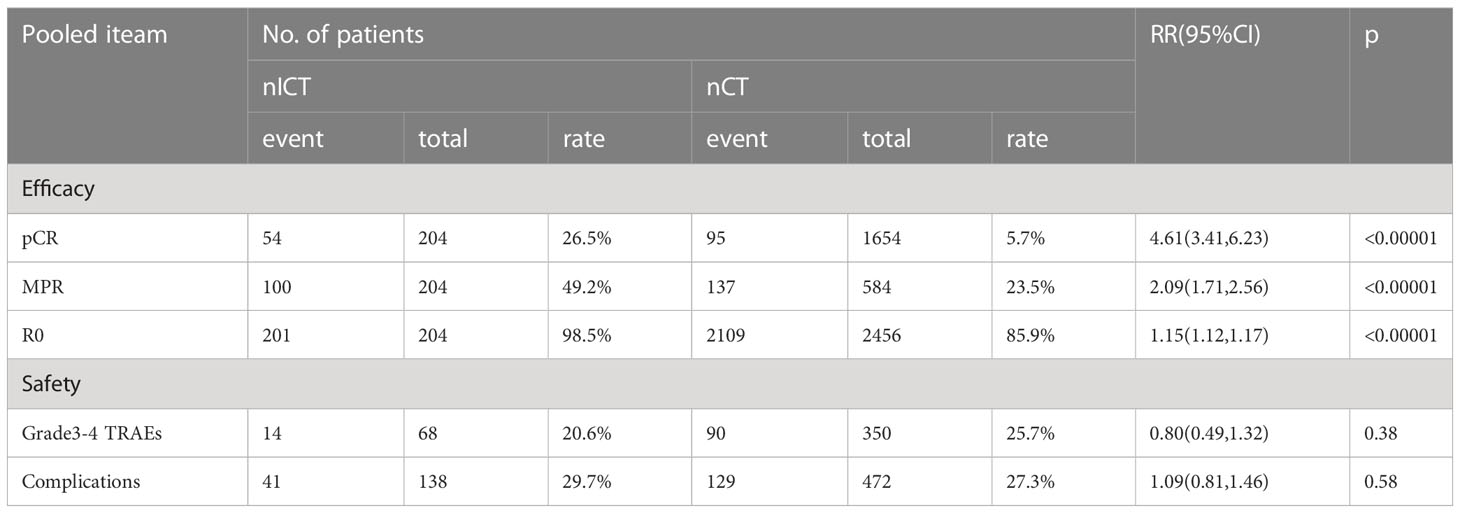
Table 2 The pairwise comparisons efficacy and safety for neoadjuvant immunotherapy combined with chemotherapy (nICT) and neoadjuvant chemotherapy(nCT).
Direct comparative analysis of neoadjuvant treatments
As most phase II clinical trials were nICT trials in GC, the majority of them were one-arm studies. Owing to this, a network meta-analysis could not be used to compare the efficacy and safety of nICT with that of reported nCT. Thus, based on the existing nCT-included studies (Supplemental Table 3), pairwise comparisons were conducted on the incidences of pCR, MPR, R0 resection, as well as grade 3–4 TRAEs and surgical complications associated with nICT and nCT to identify which neoadjuvant therapy regimen was more conducive for patients with GC. The combined outcomes suggested that there were significant differences between nICT and nCT in terms of pCR, MPR, and R0 resection rate, respectively, [(RR = 4.61; 95% CI: 3.41–6.23; p < 0.01), (RR =2.09; 95% CI: 1.71–2.56; p < 0.01), (RR =1.15; 95% CI: 1.12–1.17; p < 0.01)] (Table 2). Nevertheless, based on pooled outcomes, grade 3–4 TRAEs and surgical complications did not differ significantly between nICT and nCT, respectively, [(RR = 0.80; 95% CI: 0.49–1.32; p >0.05), (RR =1.09; 95% CI: 0.81–1.46; p >0.05)] (Table 2). Taken together, the above results demonstrate that nICT and nCT had comparable rates of grade 3–4 TRAEs and surgical complications, while nICT had higher rates of pCR, MPR, and R0 resection. Therefore, nICT has the potential to be a recommended neoadjuvant treatment for patients with GC.
Discussion
In the last several years, there has been rapid development of immunotherapy for patients with GC. In 2016, the KEYNOTE-012 trial was the first to demonstrate the potential of GC immunotherapy and lay the foundation for future clinical applications or studies of immunotherapy for GC (12). As third-line therapy, the ATTRACTION-2 study reported superior anti-tumour activity of nivolumab in patients with advanced GC/esophagogastric junction cancer (EGJC) previously treated with chemotherapy (23, 24). In addition, this therapeutic regimen significantly prolonged the survival of patients. KEYNOTE-061 investigated the efficacy of pembrolizumab versus paclitaxel as second-line treatment in patients with PD-1 positive GC/EGJC; nonetheless, the results showed no significant OS improvement with pembrolizumab over paclitaxel (25). Interestingly, a 2020 retrospective study reported by the American Society of Clinical Oncology (ASCO) demonstrated that pembrolizumab significantly prolonged OS and PFS in patients with a high tumour mutation burden (TMB-H) (TMB≥10 mut/MB) in the KEYNOTE-061 cohort (26). Moreover, some studies have found that pembrolizumab could benefit patients with advanced GC with microsatellite instability-high (MSI-H) cancer cells (27, 28). These findings suggest that identifying biomarkers to accurately screen the population with GC that is suitable for immunotherapy is a significant direction in the current research of this disease. The KEYNOTE-062 study was the first multicentre randomised controlled phase 3 clinical trial to evaluate the first-line treatment efficacy of pembrolizumab in patients with GC/EGJC. However, compared to chemotherapy alone, the combination of pembrolizumab and chemotherapy did not result in superior OS and PFS (29). Encouragingly, CheckMate-649, ATTRACTION-04, KEYNOTE-659, and ORIENT-16 reported that first-line treatment with PD-1 inhibitors in combination with chemotherapy could benefit patients with advanced GC (9, 10, 30, 31).
The above reports have prompted several researchers to apply immunotherapy in the neoadjuvant treatment strategy of GC. Furthermore, the curative effect of nICT was preliminarily demonstrated and immunochemotherapy showed great potential (18–22, 32). However, to date, the efficiency and safety of nICT in locally advanced GC have not yet been systematically assessed. Simultaneously, a large number of randomised controlled trials evaluating the clinical efficacy and safety of nICT in GC are lacking. Therefore, this study conducted a quantitative summary of reported studies to provide initial evidence and guidance for use in clinical decision-making during the neoadjuvant treatment of GC. To the best of our knowledge, the present meta-analysis of clinical trials on nICT for resectable advanced GC is the first in its field.
In 1982, Frei et al. first proposed the concept of neoadjuvant chemotherapy(nCT), which refers to systemic chemotherapy given before local treatment (surgery or radiotherapy) of malignant tumors, also known as initial chemotherapy, to show that it is different from postoperative adjuvant chemotherapy (33). Its main purpose is to reduce the volume of tumor lesions in patients or eliminate metastatic cancer cells in advance, which helps to improve the state before surgery and create favorable conditions for subsequent surgery (34). Immunotherapy mainly includes programmed death receptor 1/programmed cell death ligand 1 (PD-1/PD-L1) and cytotoxic T lymphocyte associated antigen-4 (CTLA-4) inhibitors (35, 36). In recent years, immunotherapy has been gradually applied in the treatment of tumors and it has shown unprecedented efficacy in several tumors (7, 8). This has prompted people to combine traditional neoadjuvant chemotherapy with immunotherapy to treat some advanced tumors to form a new neoadjuvant treatment: neoadjuvant immunochemotherapy(nICT) (37).
In this study, the combined rates of pCR, MPR, and R0 for nICT were 26.5%, 49.0%, and 98.5%, respectively, demonstrating the favourable outcome of this therapy in patients with GC. Regarding nCT, pCR, MPR, and R0 rates were 5.7%, 23.5%, and 85.9%, respectively. These outcomes indicated that nICT was superior to nCT, with statistically significant differences being observed (P<0.00001 for all) (Table 2). The incidence of grade 3–4 TRAEs and post-operative complications was 20.6% and 29.7% in nICT, and 25.7% and 27.3% in nCT, respectively, with no statistical differences observed (P=0.38, P=0.58, respectively) (Table 2). Fortunately, only a few fatal postoperative complications were reported in the included studies, and only one patient died as a result of hemophagocytic syndrome and renal insufficiency (22). In addition, a study of neoadjuvant nivolumab and ipilimumab for resectable GC reported pCR and MPR rates of 58.6% and 72.4%, respectively, indicating that patients obtained a better pathological response, making it easier to achieve a satisfactory prognosis (32). There may be two reasons for the high pCR and MPR rates observed in this aforementioned study. First, this clinical trial used a combination of PD-1 and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) inhibitors to treat patients with advanced GC. In 2018, the CheckMate-032 study showed that nivolumab alone or nivolumab combined with ipilimumab had high anti-tumour activity and prolonged OS in patients with metastatic esophagogastric cancer (38). Second, the study included patients with deficient mismatch repair/microsatellite instability-high (dMMR)/MSI-H cancer cells, indicating that these patients may have had better responsiveness to immunotherapy (39). Besides, a study on the efficacy of neoadjuvant nivolumab monotherapy for resectable GC showed that the pCR and MPR rates were 3.23% and 16.1%, which were lower than those associated with nICT (40). Further, a previous study demonstrated that nCT enhances the expression of multiple checkpoint molecules and the infiltration of CD4+ and CD8+ immune cells in GC, and the molecular change levels of checkpoints are positively correlated with each other (41). Therefore, ICIs combined with chemotherapy may be more effective than ICIs alone in neoadjuvant treatment of advanced GC. In summary, the above outcomes showed the acceptable efficacy and safety of neoadjuvant immunochemotherapy. Furthermore, it is believed that clinical studies, such as ATTRACTION-05 (42) and KEYNOTE-585 (43) trials, which are currently underway, will provide more evidence on the clinical application of nICT.
There are several limitations to this study. First, in light of the fact that some studies have not reached their endpoints, some survival indicators (such as PFS and OS) could not be investigated. Second, although an extensive literature search was performed, a small number of studies have been included, with inadequate sample sizes and most of them being single-arm studies. Our study also has the following limitations:(I) We conducted a direct pairwise comparison between the nICT and nCT groups and could not fully consider the baseline characteristics between the two sets. (II) The lack of randomised controlled trials (RCTs) may have led to instability and deviations in the study findings. (III) Subgroup analysis of different PD-1 inhibitors was not conducted to evaluate the best immunochemotherapy regimen for clinical application. Furthermore, the patients in this study were all from the Chinese population. The above findings are limited to evaluating the efficacy of neoadjuvant immunochemotherapy for advanced GC in China, which may be difficult to generalize to the whole population. At the same time, we also look forward to more clinical trials of neoadjuvant immunochemotherapy in the treatment of advanced gastric cancer at home and abroad in the future, so as to evaluate its efficacy and safety more comprehensively.
In conclusion, this systematic review and meta-analysis of five non-randomised clinical studies indicated promising effectiveness and safety of nICT in patients with resectable advanced GC in China, providing preliminary clinical evidence for the widespread use of this therapeutic strategy. The results of these studies provide confidence for future research, and RCTs with long-term follow-up are needed to comprehensively evaluate the merits of the nICT for patients with resectable gastric cancer, providing larger sample sizes and complete data to validate the findings of this study.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
HX, TL, GS: methodology, data curation, software, writing-original draft. WW, ZH, JX, YQ, HL: conceptualization, investigation, roles/writing—original draft, writing—review and editing. HG, LW, DZ: resources, supervision,validation. LY, FL, ZX: funding acquisition, supervision, writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported grants from National Natural Science Foundation of China (grant number: 82072708); Youth Program of National Natural Science Foundation of China (grant number: 81902461).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1193614/full#supplementary-material
Abbreviations
GC, gastric cancer; PD-1, programmed cell death protein 1; nICT, neoadjuvant immunochemotherapy; MPR, major pathological response; pCR, pathological complete response; TRAEs, treatment-related adverse events; nCT, neoadjuvant chemotherapy; RR, risk ratios; CI, confidence interval; OS, overall survival; PFS, progression-free survival;PD-L1, programmed cell death 1 ligand 1; CPS, combined positive score; ICIs, immune checkpoint inhibitors; EGJC, esophagogastric junction cancer; TMB-H, high tumour mutation burden; MSI-H, microsatellite instability-high; CTLA-4, cytotoxic T lymphocyte-associated antigen-4; dMMR/MSI-H, mismatch repair deficient/microsatellite instability-high; RCTs, randomised controlled trials.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Morgan E, Arnold M, Camargo MC, Gini A, Kunzmann AT, Matsuda T, et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020-40: a population-based modelling study. Eclinicalmedicine (2022) 47:101404. doi: 10.1016/j.eclinm.2022.101404
3. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet (2020) 396:635–48. doi: 10.1016/S0140-6736(20)31288-5
4. Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol (2005) 23:7114–24. doi: 10.1200/JCO.2005.14.621
5. Japanese Gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer (2021) 24:1–21. doi: 10.1007/s10120-020-01042-y
6. Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, et al. The Chinese society of clinical oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond) (2021) 41:747–95. doi: 10.1002/cac2.12193
7. Zimmer L, Livingstone E, Hassel JC, Fluck M, Eigentler T, Loquai C, et al. Adjuvant nivolumab plus ipilimumab or nivolumab monotherapy versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet (2020) 395:1558–68. doi: 10.1016/S0140-6736(20)30417-7
8. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-Small-Cell lung cancer. N Engl J Med (2018) 378:2078–92. doi: 10.1056/NEJMoa1801005
9. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet (2021) 398:27–40. doi: 10.1016/S0140-6736(21)00797-2
10. Xu J, Jiang H, Pan Y, Gu K, Cang S, Han L, et al. Sintilimab plus chemotherapy (chemo) versus chemo as first-line treatment for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma (ORIENT-16): first results of a randomized, double-blind, phase III study. Ann Oncol (2021) 32:S1331. doi: 10.1016/j.annonc.2021.08.2133
11. Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol (2018) 4:e180013. doi: 10.1001/jamaoncol.2018.0013
12. Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol (2016) 17:717–26. doi: 10.1016/S1470-2045(16)00175-3
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj (2021) 372:n71. doi: 10.1136/bmj.n71
14. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. Bmj (2017) 358:j4008. doi: 10.1136/bmj.j4008
15. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
16. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst (1959) 22:719–48.
17. Malgie J, Schoones JW, Pijls BG. Decreased mortality in coronavirus disease 2019 patients treated with tocilizumab: a rapid systematic review and meta-analysis of observational studies. Clin Infect Dis (2021) 72:e742–49. doi: 10.1093/cid/ciaa1445
18. Jiang H, Yu X, Li N, Kong M, Ma Z, Zhou D, et al. Efficacy and safety of neoadjuvant sintilimab, oxaliplatin and capecitabine in patients with locally advanced, resectable gastric or gastroesophageal junction adenocarcinoma: early results of a phase 2 study. J Immunother Cancer (2022) 10:e003635. doi: 10.1136/jitc-2021-003635
19. Lin JL, Lin JX, Lin JP, Zheng CH, Li P, Xie JW, et al. Safety and efficacy of camrelizumab in combination with nab-paclitaxel plus s-1 for the treatment of gastric cancer with serosal invasion. Front Immunol (2021) 12:783243. doi: 10.3389/fimmu.2021.783243
20. Tang X, Li M, Wu X, Guo T, Zhang L, Tang L, et al. Neoadjuvant PD-1 blockade plus chemotherapy induces a high pathological complete response rate and anti-tumor immune subsets in clinical stage III gastric cancer. Oncoimmunology (2022) 11:2135819. doi: 10.1080/2162402X.2022.2135819
21. Guo H, Ding P, Sun C, Yang P, Tian Y, Liu Y, et al. Efficacy and safety of sintilimab plus XELOX as a neoadjuvant regimen in patients with locally advanced gastric cancer: a single-arm, open-label, phase II trial. Front Oncol (2022) 12:927781. doi: 10.3389/fonc.2022.927781
22. Yin Y, Lin Y, Yang M, Lv J, Liu J, Wu K, et al. Neoadjuvant tislelizumab and tegafur/gimeracil/octeracil (S-1) plus oxaliplatin in patients with locally advanced gastric or gastroesophageal junction cancer: early results of a phase 2, single-arm trial. Front Oncol (2022) 12:959295. doi: 10.3389/fonc.2022.959295
23. Chen LT, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, et al. A phase 3 study of nivolumab in previously treated advanced gastric or gastroesophageal junction cancer (ATTRACTION-2): 2-year update data. Gastric Cancer (2020) 23:510–19. doi: 10.1007/s10120-019-01034-7
24. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (2017) 390:2461–71. doi: 10.1016/S0140-6736(17)31827-5
25. Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu MH, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet (2018) 392:123–33. doi: 10.1016/S0140-6736(18)31257-1
26. Shitara K, Ozguroglu M, Bang YJ, Di Bartolomeo M, Mandal M, Ryu MH, et al. The association of tissue tumor mutational burden (tTMB) using the foundation medicine genomic platform with efficacy of pembrolizumab versus paclitaxel in patients (pts) with gastric cancer (GC) from KEYNOTE-061. J Clin Oncol (2020) 38:4537. doi: 10.1200/JCO.2020.38.15_suppl.4537
27. Chao J, Fuchs CS, Shitara K, Tabernero J, Muro K, Van Cutsem E, et al. Assessment of pembrolizumab therapy for the treatment of microsatellite instability-high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 clinical trials. JAMA Oncol (2021) 7:895–902. doi: 10.1001/jamaoncol.2021.0275
28. Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite Instability/Mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol (2020) 38:1–10. doi: 10.1200/JCO.19.02105
29. Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol (2020) 6:1571–80. doi: 10.1001/jamaoncol.2020.3370
30. Boku N, Ryu MH, Kato K, Chung HC, Minashi K, Lee KW, et al. Safety and efficacy of nivolumab in combination with s-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol (2019) 30:250–58. doi: 10.1093/annonc/mdy540
31. Kawazoe A, Yamaguchi K, Yasui H, Negoro Y, Azuma M, Amagai K, et al. Safety and efficacy of pembrolizumab in combination with s-1 plus oxaliplatin as a first-line treatment in patients with advanced gastric/gastroesophageal junction cancer: cohort 1 data from the KEYNOTE-659 phase IIb study. Eur J Cancer (2020) 129:97–106. doi: 10.1016/j.ejca.2020.02.002
32. André T, Tougeron D, Piessen G, de la Fouchardière C, Louvet C, Adenis A, et al. Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in localized deficient mismatch Repair/Microsatellite instability-high gastric or esophagogastric junction adenocarcinoma: the GERCOR NEONIPIGA phase II study. J Clin Oncol (2022) 41:255-265. doi: 10.1200/JCO.22.00686
33. Frei ER. Clinical cancer research: an embattled species. Cancer (1982) 50:1979–92. doi: 10.1002/1097-0142(19821115)50:10<1979::aid-cncr2820501002>3.0.co;2-d
34. King TA, Morrow M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat Rev Clin Oncol (2015) 12:335–43. doi: 10.1038/nrclinonc.2015.63
36. Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy. Blood (2018) 131:58–67. doi: 10.1182/blood-2017-06-741033
37. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med (2022) 386:1973–85. doi: 10.1056/NEJMoa2202170
38. Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol (2018) 36:2836–44. doi: 10.1200/JCO.2017.76.6212
39. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science (2017) 357:409–13. doi: 10.1126/science.aan6733
40. Hasegawa H, Shitara K, Takiguchi S, Takiguchi N, Ito S, Kochi M, et al. A multicenter, open-label, single-arm phase I trial of neoadjuvant nivolumab monotherapy for resectable gastric cancer. Gastric Cancer (2022) 25:619–28. doi: 10.1007/s10120-022-01286-w
41. Yu Y, Ma X, Zhang Y, Zhang Y, Ying J, Zhang W, et al. Changes in expression of multiple checkpoint molecules and infiltration of tumor immune cells after neoadjuvant chemotherapy in gastric cancer. J Cancer (2019) 10:2754–63. doi: 10.7150/jca.31755
42. Terashima M, Kim YW, Yeh TS, Chung HC, Chen JS, Boku N, et al. ATTRACTION-05 (ONO-4538-38/BMS CA209844): a randomized, multicenter, double-blind, placebo-controlled phase 3 study of nivolumab (Nivo) in combination with adjuvant chemotherapy in pStage III gastric and esophagogastric junction (G/EGJ) cancer. Ann Oncol (2017) 28:778TiP. doi: 10.1093/annonc/mdx369.160
43. Bang YJ, Van Cutsem E, Fuchs CS, Ohtsu A, Tabernero J, Ilson DH, et al. KEYNOTE-585: phase 3 study of chemotherapy (chemo) plus pembrolizumab (pembro) vs chemo plus placebo as neoadjuvant/adjuvant treatment for patients (pts) with gastric or gastroesophageal junction (G/GEJ) cancer. J Clin Oncol (2018) 36:TPS4136. doi: 10.1200/JCO.2018.36.15_suppl.TPS4136
Keywords: resectable advanced gastric cancer, neoadjuvant immunochemotherapy, perioperative immunotherapy, neoadjuant chemotherapy, meta – analysis
Citation: Xu H, Li T, Shao G, Wang W, He Z, Xu J, Qian Y, Liu H, Ge H, Wang L, Zhang D, Yang L, Li F and Xu Z (2023) Evaluation of neoadjuvant immunotherapy plus chemotherapy in Chinese surgically resectable gastric cancer: a pilot study by meta-analysis. Front. Immunol. 14:1193614. doi: 10.3389/fimmu.2023.1193614
Received: 25 March 2023; Accepted: 12 June 2023;
Published: 23 June 2023.
Edited by:
Huashan Shi, Sichuan University, ChinaReviewed by:
Dongbo Jiang, Air Force Medical University, ChinaZheng Chen, University of Miami, United States
Jin Zhou, The First Affiliated Hospital of Soochow University, China
Ye Zhou, Fudan University, China
Haoran Qian, Zhejiang University, China
Copyright © 2023 Xu, Li, Shao, Wang, He, Xu, Qian, Liu, Ge, Wang, Zhang, Yang, Li and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zekuan Xu, eHV6ZWt1YW5AbmptdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Hao Xu
Hao Xu Tengyun Li
Tengyun Li Guoyi Shao3†
Guoyi Shao3† Zhongyuan He
Zhongyuan He Jianghao Xu
Jianghao Xu Yawei Qian
Yawei Qian Hongda Liu
Hongda Liu Li Yang
Li Yang Zekuan Xu
Zekuan Xu