- 1Department of Medical Science, School of Medicine, Walailak University, Nakhon Si Thammarat, Thailand
- 2Research Center in Tropical Pathobiology, Walailak University, Nakhon Si Thammarat, Thailand
- 3Excellence Center of Community Health Promotion, Walailak University, Nakhon Si Thammarat, Thailand
- 4Department of Medical Clinical Science, School of Medicine, Walailak University, Nakhon Si Thammarat, Thailand
- 5BDMS Health Research Center, Bangkok Dusit Medical Service PLC, Bangkok, Thailand
- 6Walailak University Hospital, Walailak University, Nakhon Si Thammarat, Thailand
- 7Faculty of Health and Sports Science, Thaksin University, Paphayom, Phatthalung, Thailand
Background: There is a bidirectional relationship between obesity and depression. We investigated whether the coexistence of obesity and depression increases the risk of having severe depression and a high suicide risk in adolescents with major depressive disorder (MDD). Additionally, we explored the potential mechanisms linking the coexistence of obesity and depression to worse outcomes in these patients.
Methods: The odds of high suicide risk and severe depression were compared among MDD patients based on different body mass index (BMI) groups. Complete blood count (CBC) parameters, inflammatory ratios (neutrophil–lymphocyte ratio [NLR], monocyte-lymphocyte ratio [MLR], and platelet-lymphocyte ratio [PLR]), and cytokine levels (IFN-γ, IL-1β, IL-6, IL-8, MCP-1, TNF-α, and TGF-β1) were evaluated across BMI groups. Additionally, Pearson correlation coefficients (r) were assessed to understand the relationships between the 8Q and 9Q scores, CBC parameters, inflammatory ratios, cytokine levels, and BMI.
Results: A total of 135 antidepressant-naive adolescents with MDD were included. Overweight and obese MDD patients had higher odds of having high suicide risk and severe depression than lean individuals. Furthermore, they exhibited significantly higher white blood cell (WBC), and neutrophil counts. The NLR tended to be higher in obese MDD patients than in leans. Overweight and obese MDD patients had elevated levels of interleukin (IL)-1β and IL-6 compared to lean individuals, while TGF-β1 levels appeared to decline as body weight increased. BMI showed weak positive correlations with 8Q score, WBC count, neutrophil count, monocyte count, platelet count, neutrophil percentage, and NLR, and a weak negative correlation with lymphocyte percentage. The 8Q score displayed weak positive correlations with BMI, neutrophil percentage, monocyte percentages, NLR, and MLR, and a weak negative correlation with lymphocyte percentage.
Conclusion: The findings suggest that coexistence of overweight or obesity with depression heightened inflammatory responses, leading to worse outcomes and increased suicide risk in adolescents MDD patients.
1 Introduction
Depression is a common mental health disorders, affecting approximately 5% of adults worldwide. It is the second leading cause of death among people aged 15–29 (1). This condition is characterized as a chronic low-grade systemic inflammatory disease (2–4). Evidence from recent studies supports the notion that inflammatory processes play a crucial role in the pathophysiology of depression (3, 5–9). Various factors seem to be related to systemic inflammation and an increased risk of developing depression. These factors include stress, obesity, smoking, lack of physical activity, sleep disturbances, unhealthy diet, immune system dysregulation, cytokine imbalances, hormonal factors, increased gut permeability, and medication and medical conditions (10).
Obesity poses a significant health concern. In 2016, there were 1.9 billion overweight adults globally, of which 650 million were classified as obese. It is estimated that the number of individuals suffering from obesity worldwide will reach one billion by the year 2030 (10). Obesity and overweight are associated with an increased risk of various health conditions and diseases. These conditions can range from cardiovascular diseases to metabolic disorders (4, 11–16). These include cardiovascular diseases, type 2 diabetes, dyslipidemia, hypertension, certain cancers, sleep apnea and respiratory disorders, musculoskeletal conditions like osteoarthritis, susceptibility to infectious diseases, and even mental health disorders such as depression, and related conditions (10–16). If this trend continues, obesity-related conditions will likely become more prevalent. According to the global burden of disease, over 4 million people die each year as a result of being overweight or obese. Recently, studies have shown that obesity affects immune function and increases the risk of severe illness from coronavirus disease of 2019 (COVID-19) infection (17, 18).
White or visceral adipose tissue is the main depot for storing fat and serves as the largest endocrine organ that secretes adipokines, growth factors, cytokines, and chemokines into the bloodstream that affect endocrine and immune functions (2, 13, 15, 16, 18–23). Weight gain, obesity, chronic overnutrition, and aging lead to alterations in the white adipose tissue phenotype, characterized by the appearance of inflamed, dysfunctional, along with an increased infiltration of immune cells such as macrophages, T cells, and eosinophils (13). This inflammatory milieu leads to the release of proinflammatory cytokines and chemokines like interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and monocyte chemoattractant protein-1 (MCP-1) (8, 13, 15, 20, 22, 23). Such alterations trigger uncontrolled inflammatory responses, resulting in systemic low-grade inflammation. Consequently, chronic low-grade systemic inflammation of adipose tissue contributes to insulin resistance, a hallmark of obesity-related metabolic stress and the development of metabolic diseases (2, 4, 11–16). Previous studies have highlighted the link between obesity or overweight conditions and uncontrolled inflammatory responses or immune dysfunctions (2, 4, 11–16, 18–21).
Three systematic reviews and meta-analyses have illustrated the bi-directional relationship between obesity and depression (24–26). Firstly, Luppino et al. (2010) (24) conducted a systematic review and meta-analysis of 15 longitudinal studies. They found that overweight or obesity increased the risk of depression among adults (aged 20 years or older) but not among younger individuals (age < 20 years). Moreover, depression was found to increase the odds of developing obesity. In a subsequent systematic review and meta-analysis of 19 studies, Mannan et al. (2016) (25) confirmed this bi-directional link between obesity and depression. Their findings suggest that the direction of depression leading to obesity is stronger than the reverse direction. Furthermore, women in their reproductive years (18-49 years) are more susceptible to developing depression, compared with women more than 49 years old. A recent systematic review and meta-analysis study by Blasco et al. (2020) (26), covering 18 studies (9 cross-sectional, 6 longitudinal, and 3 clinical trials), also confirmed the bidirectional relationship between depression and obesity. It highlighted that obesity increases the risk of depression, particularly in women with recurrent depressive disorder, while depression, in turn, becomes a risk factor for obesity. Additionally, when obesity and depression coexist, it often results in poorer illness outcomes. Specifically, MDD patients with the highest BMI category reported more medical and psychiatric comorbidities. However, the precise mechanisms explaining how obesity leads to its complications, and those that explain how the coexistence of obesity and depression impacts the poor prognosis of depression remain unknown.
Neutrophils, monocytes, lymphocytes, and platelets serve as sources of substances involved in regulating inflammation, which are relevant to the pathophysiology of depression and contribute to depressive symptoms (3, 6–8). Neutrophil–lymphocyte ratio (NLR), monocyte-lymphocyte ratio (MLR), and platelet-lymphocyte ratio (PLR) are considered biomarkers of systemic inflammation. Recently, inflammatory ratios have been spotlighted as potential simple biomarkers for depression. Notably, a higher NLR has been linked to greater depression severity and has been proposed as a biomarker of suicidal risk in individuals with depression (27–30). Similarly, higher MLR, PLR, and platelet count are associated with more severe depression and an elevated risk of suicide (31–33). An increased MLR has been linked to both depression and suicidal severity in depressed patients (31, 34). Furthermore, a higher MLR has been suggested to be a more predictive biomarker for suicide attempts in adolescents with MDD than either NLR or PLR (31). Furthermore, MLR appears to be more responsive to selective serotonin reuptake inhibitor (SSRI) treatment compared to NLR or PLR (32). Additionally, higher baseline platelet counts have been associated with non-response to SSRI treatment in adolescents with MDD (32).
This study aimed to investigate whether the coexistence of obesity and depression increases the risk of having severe depression and a high suicide risk in adolescents with MDD. Additionally, we explored the potential mechanisms that link the comorbidity of obesity and depression to the poor prognosis in these adolescents, focusing on changes in complete blood count (CBC) parameters, inflammatory ratios (NLR, MLR, and PLR), and cytokine levels due to obesity. To achieve this, the odds of having high suicide risk and severe depression in patients with MDD were compared among different BMI groups. Furthermore, we compared CBC parameters, inflammatory ratios, and levels of proinflammatory cytokines (interferon (IFN)-γ, IL-1β, IL-6, IL-8, MCP-1, and TNF-α) and anti-inflammatory cytokine (transforming growth factor-beta 1 [TGF-β1]) between the different BMI groups. Lastly, we investigated the Pearson correlation coefficients (r) between the 8Q and 9Q scores, CBC parameters, and inflammatory ratios with BMI.
2 Materials and methods
2.1 Study design and participants
This study employed a cross-sectional design, involving university students aged 18–24 years who were visiting the psychiatry clinic, the outpatient department, of Walailak University Hospital in Nakhon Si Thammarat, southern Thailand. This study was conducted between November 2020 and March 2021. It was approved by The Institutional Review Board of Walailak University (WU-EC-MD-1-111-66), and all participants provided written informed consent. Each participant was diagnosed with MDD and had not been previously exposed to any antidepressant treatment. All adolescents diagnosed with MDD who were naïve to antidepressants and met the inclusion criteria were invited to participate in the study.
2.2 Diagnosis of major depressive disorder
A licensed psychiatrist made the diagnosis based on the criteria of the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) (31, 32).
2.3 Inclusion and exclusion criteria
All participants underwent comprehensive evaluation that included a routine physical examination, an assessment of physical health comorbidities, screening for COVID-19 infection using antigen test kit, an evaluation of other mental illnesses, examination of additional mental health data, and a review of their medical histories. A complete blood count (CBC) with differential analysis was performed on each participant. Additional laboratory tests and investigations were conducted if there were any concerns regarding physical health issues (31, 32).
2.3.1 Inclusion criteria
All patients diagnosed with MDD, aged 18–24 years, who were antidepressant-naive and visiting the outpatient unit of the university hospital, were included in the study when they provided informed consent and did not meet the specific exclusion criteria.
2.3.2 Exclusion criteria
The participants were excluded from the study if they met any of the following conditions: 1) physical comorbidities, except for being overweight or obese; 2) presence of fever, infectious diseases, or recent infections; 3) COVID-19 infection; 4) chronic physical illnesses or inflammatory diseases; 5) use of anti-inflammatory or immunosuppressive drugs; 6) other mood disorders or mental illnesses; 7) immune system and hematological system disorders; 8) pregnancy or having been pregnant within the past six months; and 9) usage of substances and alcohol or smoking more than 10 cigarettes per day (31, 32).
2.4 Severity of depression, suicidal behavior, and suicidality severity
The Patient Health Questionnaire-9 (PHQ-9 or 9Q) scale, a depression rating scale, was employed to evaluate the severity of depression. The scores of 5, 10, 15, and 20 on the PHQ-9 (9Q) scale correspond to mild, moderate, moderately severe, and severe depression, respectively. The assessment of suicidal behavior and suicidality severity were conducted using the 8Q scale, a Thai version of a suicidality module of the Mini International Neuropsychiatric Interview (M.I.N.I)5.0.0 (35). Based on the 8Q scale, suicidality was classified into four levels: no suicide risk (score = 0), low suicide risk (score = 1–8), moderate suicide risk (score = 9–16), and high suicide risk (score ≥ 17). Furthermore, participants were divided into three classification based on their suicidal behaviors:1) those without suicidal ideation (NSI), 2) those with suicidal ideation (SI), and 3) those who had attempted suicide (SA) (31, 32).
2.5 BMI measurement
Body mass index (BMI) was calculated using the formula of weight in kilograms divided by the height in meters squared (kg/m2). The BMI classification were as follow: A BMI of less than 24.9 kg/m2 was considered as lean, a BMI ranging from 25.0 to 29.9 kg/m2 was considered as overweight, and a BMI of 30 kg/m2 or higher was obese.
2.6 Laboratory study
Prior to beginning MDD-specific treatment, a 10-mL blood sample was collected from each participant. The complete blood count (CBC) with differential analysis required 2–3 mL. The remaining 7–8 mL were allowed to clot and subsequently utilized for the measurement of cytokines through the enzyme-linked immunosorbent assay (ELISA) technique.
2.6.1 Complete blood count test and inflammatory ratios
A routine CBC with differential analysis was conducted using an automated analyzer at the Central Laboratory of Walailak University Hospital. The inflammatory ratios, including NLR, PLR, and MLR, were calculated from the CBC parameters.
2.6.2 Cytokine measurement by ELISA
Sera were extracted from clotted blood and stored at -80°C until ready for analysis. The cytokines, including IFN-γ, IL-1β, IL-6, IL-8, MCP-1, TNF-α, and TGF-β1, were analyzed from serum samples using commercial ELISA kits as per the manufacturer’s instructions. The ELISA kits for measuring IFN-γ, IL-1β, IL-6, IL-8, and MCP-1 were purchased from BD Biosciences, while those for IL-1β, TNF-α, and TGF-β1 were purchased from R & D Systems. All samples were measured in duplicate (36).
2.7 Statistic analysis
Statistical analyses were performed with SPSS software (version 17.0, Chicago, IL, USA), except for the calculation of odds ratio, which was computed using an online calculator (37). To compare the groups, the chi-square test and analysis of variance (ANOVA) were used. The results are presented as mean ± SEM, SD, or odds ratios. The linear relationship between two continuous variables was conveyed through the Pearson correlation coefficient (r). A p-value less than 0.05 was considered statistically significant.
3 Results
The characteristics of the study participants are detailed in Supplementary Table S1. This study included 135 antidepressant-naïve adolescents diagnosed with MDD. Among them, 72.6% (98 participants) were lean, 14.1% (19 participants) were overweight, and 13.3% (18 participants) were obese MDD participants. Furthermore, 69.6% of the participants were female, and 91.1% were experiencing their first depressive episode. No significant differences in age, state of depressive episode status, and smoking status were observed between groups (Supplementary Table S1).
3.1 Association between 8Q and 9Q scores, suicide risk, depressive severity, suicidal behavior, and BMI in patients with MDD
The 8Q and 9Q scores, suicide risk, depressive severity, and suicidal behavior in patients with MDD with different BMI groups are shown in Supplementary Table S2. Overweight and obese patients with MDD were more likely to have higher 8Q and 9Q scores, a higher suicide risk, severe depression, and attempted suicide than lean patients with MDD, but this was not statistically significant (Supplementary Table S2).
Table 1 shows the impact of being overweight or obese on the odds of having high suicide risk and severe depression in patients with MDD. Overweight and obese individuals had higher odds of having high suicide risk (odds ratios: overweight, 3.02; 95% CI, 0.98–9.34, p -value of 0.028; obese, 2.51; 95% CI, 0.77–8.23, p -value of 0.063; overweight or obese, 2.77; 95% CI, 1.11–6.91, p -value of 0.015) and severe depression compared to lean individuals (odds ratios: overweight, 3.11; 95% CI, 1.12–8.60, p -value of 0.014; obese, 1.73; 95% CI, 0.58–5.13, p-value of 0.163; overweight or obese, 2.36; 95% CI, 1.05–5.29, p = 0.019).

Table 1 Influence of being overweight or obese on odds of having high suicide risk and severe depression in patients with MDD.
3.2 The complete blood count parameters and inflammatory ratios in different BMI groups in patients with MDD
Table 2 summarizes the CBC parameters and inflammatory ratios in patients with MDD in different BMI groups. One-way ANOVA revealed significant differences in red blood cell (RBC) count and hematocrit (p = 0.024 and 0.044, respectively). WBC and neutrophil counts increased significantly with increasing body weight in patients with MDD (p = < 0.0001 and < 0.0001, respectively). The lymphocyte percentage decreased with increasing body weight in patients with MDD, but this was not statistically different. Platelet count and NLR increased with increasing body weight in patients with MDD, but this was not statistically significant. Hemoglobin and monocyte counts were higher in overweight and obese patients with MDD compared to lean patients with MDD, but the difference was not statistically significant (p = 0.062 and 0.066, respectively).
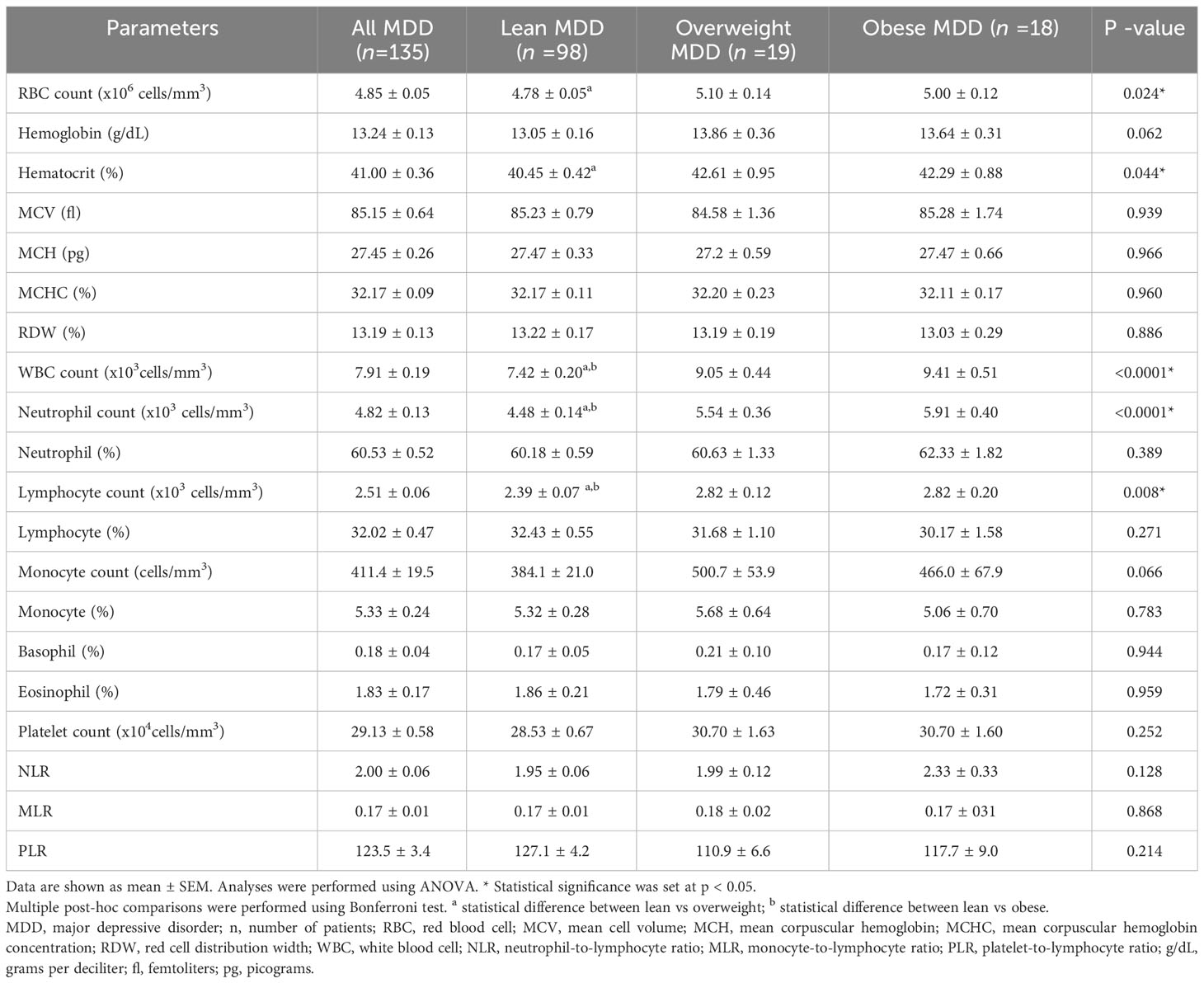
Table 2 The complete blood count parameters and inflammatory ratios in patients with MDD with different BMI groups.
Post-hoc comparisons were performed using the Bonferroni test. In overweight MDD patients, the RBC, hematocrit, WBC, and neutrophil counts were elevated compared to lean MDD patients. While hemoglobin and monocyte count showed an upward trend in overweight MDD, the differences were not statistically significant. Obese patients with MDD had higher WBC and neutrophil counts than their lean counterparts. Moreover, lymphocyte counts and NLR showed an increasing trend in obese patients compared to lean MDD patients. Comparing overweight and obese patients with MDD, no significant differences in CBC parameters or inflammatory ratios were observed.
3.3 Linear relationship between BMI, 8Q score, 9Q score, complete blood parameters, and inflammatory ratios in MDD patients
The p -value and Pearson correlation coefficients (r) of the linear relationship between two continuous variables are summarized in Table 3. BMI had a weak positive correlation with the 8Q score, RBC count, hemoglobin, hematocrit, WBC count, neutrophil count, neutrophil percentage, monocyte count, plate count, and NLR (with correlation coefficients of 0.12, 0.22, 0.20, 0.22, 0.35, 0.34, 0.13, 0.20, 0.10, and 0.18, respectively), and a weak negative correlation with lymphocyte count, lymphocyte percentage, and PLR (with correlation coefficients of -0.20, -0.18, and -0.10, respectively). The 8Q score had a moderate correlation with the 9Q score (with a correlation coefficient of 0.63 and a p -value of < 0.0001). The 8Q score had a weak positive correlation with BMI, red cell distribution width (RDW), neutrophil count, neutrophil percentage, monocyte count, monocyte percentage, NLR, and MLR (with correlation coefficients of 0.12, 0.013, 0.13, 0.18, 0.15, 0.11, 0.15, and 0.20, respectively), and a weak negative correlation with lymphocyte percentage (with a correlation coefficient of -0.26 and a p -value of 0.019). The 9Q score had a weak correlation with lymphocyte count, monocyte percentage, eosinophil percentage, platelet count, and MLR (correlation coefficients of 0.10, 0.12, -0.10, -0.16, and 0.16, respectively).
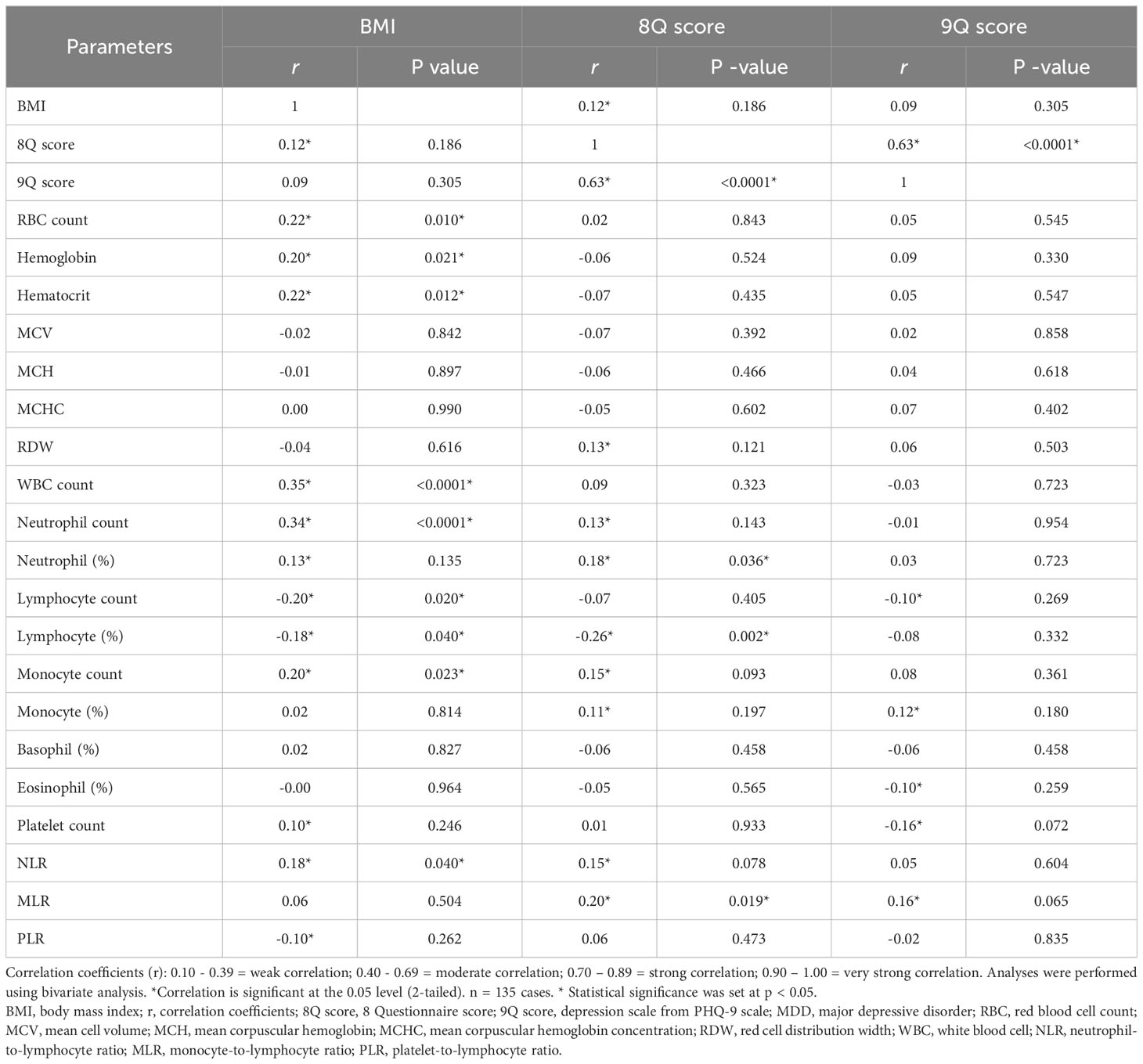
Table 3 The relation between BMI, 8Q score, 9Q score, CBC parameters and inflammatory ratios in patients with MDD.
3.4 Levels of serum inflammatory cytokines in different BMI groups in patients with MDD
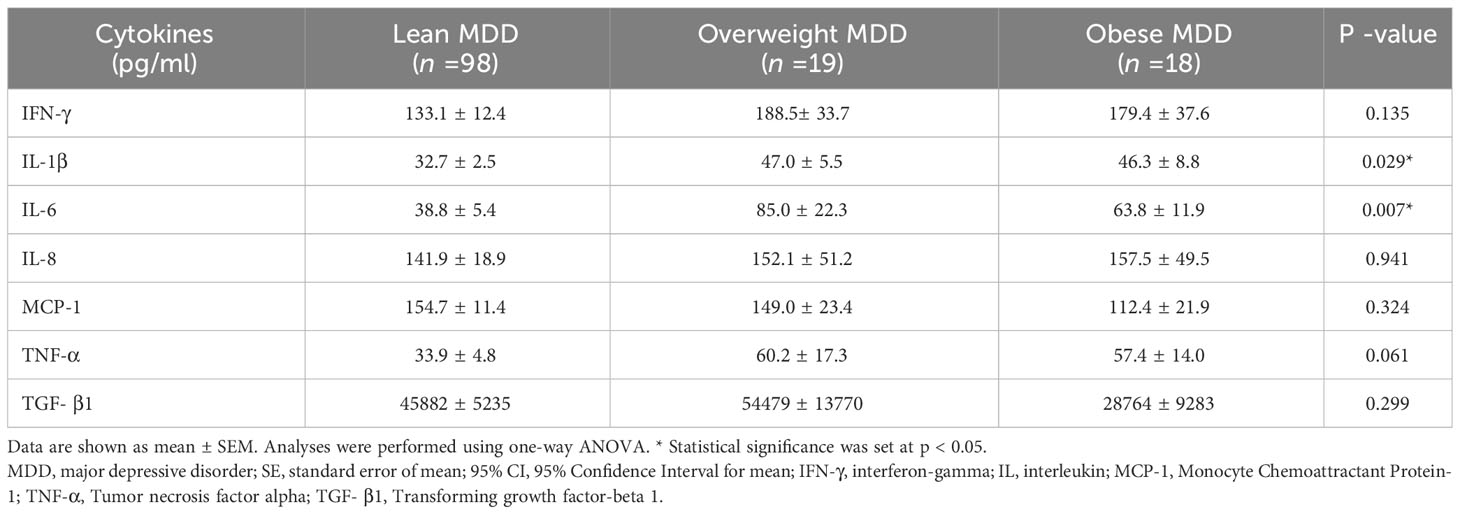
Table 4 summarizes levels of serum cytokines in patients with MDD with different BMI groups. Significant differences were observed in the levels of IL-1β and IL-6 among the BMI groups (p = 0.029 and 0.007, respectively). Levels of IFN-γ and TNF-α appeared to be higher in obese or overweight MDD patients compared to lean individuals, though the difference was not statistically significant (p = 0.135 and 0.061, respectively). TGF-β1 levels showed a decreasing trend with increasing body weight in patients with MDD, though this was not significant (p = 0.299).
Table 5 details the p-value of serum IL-1β and IL-6 levels in patients with MDD from various BMI groups as determined by post-hoc pairwise comparisons. Overweight patients with MDD exhibited significantly elevated levels of IL-1β and IL-6 compared to their lean counterparts (p = 0.036 and 0.003, respectively). Additional, obese patients with MDD had significantly higher IL-1β levels than lean patients with MDD (p = 0.005).
4 Discussion
Depression and obesity are now recognized as a state of chronic low-grade systemic inflammatory conditions. However, the impact of the coexistence of obesity and depression on the prognosis of depression and its precise mechanisms remain unclear. The present study aimed to determine whether coexist of overweight or obesity and depression increased the risk of having severe depression and a high risk of suicide. Additionally, this study investigated whether CBC parameters, inflammatory ratios, and proinflammatory cytokine production are influenced by the coexistence of obesity and depression in adolescents.
Comparing the odds of having high suicide risk and severe depression among different BMI groups of MDD patients, we found that overweight and obese MDD patients had higher odds of experiencing high suicide risk and severe depression compared to lean individuals. Our result is consistent with a previous study by Blasco et al. (2020) (26), who reported that the comorbidity of depression and obesity is a risk factor for poor prognosis.
Under lean conditions, adipose tissue is enriched in immune cells of type 2 immunity (predominantly anti-inflammatory/immune regulatory primed immune cells, such as M2-type macrophages, regulatory T cells (Treg), T-helper (Th)2, and type 2 innate lymphoid cells (ILC2). In obesity, the adipose immune system is dysregulated and replaced by inflammatory cells (for example, M1 macrophages, Th1, Th17, and CD8+ T cells) (13, 38) that secret proinflammatory cytokines such as IL-1β, IL-6, IL-17, and IFNγ (19– 21). Together, these changes lead to an elevated inflammatory state in obese individuals, with a predominance of neutrophils and hallmark increases in Th1 and Th17 cells (13, 19–21). This raises the possibility that the coexistence of two systemic inflammatory conditions (obesity and depression) will amplify the inflammatory responses and lead to worse outcomes and increased suicide risk in adolescents MDD patients.
To investigate the potential mechanisms that link the comorbidity of obesity and depression to the poor prognosis in these adolescents, we compared CBC parameters, inflammatory ratios, and levels of proinflammatory cytokines (IFN-γ, IL-1β, IL-6, IL-8, MCP-1, and TNF-α) and anti-inflammatory cytokine [TGF-β1]) between the different BMI groups. Here, we reported that WBC and neutrophil counts increased significantly with increasing body weight in patients with MDD. The lymphocyte percentage decreased with increasing body weight in patients with MDD, but this was not statistically different. Platelet count and NLR increased with increasing body weight in patients with MDD, but this was not statistically significant. When compared to lean patients with MDD, overweight patients with MDD had significantly higher RBC, WBC, neutrophil, and lymphocyte counts. The 8Q score, hemoglobin, hematocrit, and monocyte count tended to be higher in overweight MDD compared to lean individuals, but these were not statistically different. Obese patients with MDD had significantly higher WBC and neutrophil counts. The NLR tended to be higher in obese patients than in lean patients with MDD. In addition, the Pearson correlation coefficients (r) between the 8Q and 9Q scores, CBC parameters, and inflammatory ratios with BMI were analyzed. We reported that BMI had a weak positive correlation with the 8Q score, hemoglobin, hematocrit, RBC, WBC, neutrophil, monocyte, and plate counts, neutrophil percentage, and NLR, and a weak negative correlation with lymphocyte count, lymphocyte percentage, and PLR. The 8Q score had a weak positive correlation with BMI, neutrophil, and monocyte counts, neutrophil, and monocyte percentages, NLR, and MLR and a weak negative correlation with lymphocyte percentage.
There is emerging evidence supporting the association of immune system and pathophysiology of depression, with cytokines and chemokines standing front and center in this connection. Elevated levels of proinflammatory cytokines, including IL-1, IL-6, and TNF-α, have been consistently found in the blood of individuals experiencing MDD (5, 15, 22, 23), suggesting a heightened state of inflammation in these patients. Systemic inflammation can induce neuroinflammation by several mechanisms, including the break down of the blood-brain barrier, activation of glial cells associated with systemic immune activation, and effects on autonomic nerves via the organ-brain axis (39). Neuroinflammation, driven by these cytokines, can influence neurochemical imbalances, neurotransmitter function, and neuroendocrine activity and lead to neuron degeneration. This contributes to mood changes and the depressive symptoms seen in depression. Furthermore, these cytokines can disrupt the blood-brain barrier, promote oxidative stress, and stimulate the production of more inflammatory agents within the brain, creating a vicious cycle that may sustain or exacerbate depressive symptoms (39). In addition, a systematic review and meta-analysis, including 18 studies found that levels of IL-1β and IL-6 were significantly increased in blood and postmortem brain samples of patients with suicidality compared with both patients without suicidality and healthy control subjects (40). Contrary to proinflammatory cytokines, anti-inflammatory cytokines like IL-10 and TGF-β act to suppress inflammatory responses (38, 41). A decreased level of these cytokines may lead to unchecked inflammation, which is a consistent finding in many individuals with depression. As such, a balance between pro and anti-inflammatory cytokines is essential for mental well-being. Our results on cytokine levels in different BMI groups revealed that overweight and obese MDD patients had higher IL-1β and IL-6 levels than their lean individuals.Levels of IFN-γ and TNF-α also appeared to be higher in both overweight and obese patients when compared to lean MDD patients, while TGF-β1 levels appeared to decline as body weight increased. Collectively, our findings provide evidence that the coexistence of two low-grade systemic inflammatory conditions (obesity and depression) amplifies the inflammatory responses, consequently, leading to worse outcomes and increased suicide risk in adolescent MDD patients.
5 Conclusion
In adolescent MDD patients, the coexistence of overweight or obesity alongside depression amplifies the inflammatory status, which may contribute to a heightened severity of depression and a high suicide risk. In these patients, enhanced inflammation may be indicated by changes in CBC parameters including elevated levels of WBC, neutrophil, monocyte, and platelet counts, along with reduced lymphocyte percentages. This is further supported by increased inflammatory ratios (NLR and MLR), as well as elevated levels of the proinflammatory cytokines, specifically IL-1β and IL-6.
6 Limitations and strengths
This study has a limitation in that it was cross-sectional; thus, we cannot determine whether increasing total WBC, inflammatory immune cells (neutrophils, monocytes, platelets) lowered lymphocyte levels, and the expression of severe depressive symptoms in MDD followed or preceded obesity. Additionally, it is important to acknowledge that the physiological interactions between obesity, depression, and other environmental and endogenous factors are complex. Further studies on immune cell-adipocyte crosstalk, interactions of obese adipose tissues with high suicide risk and severe depressive symptoms, and mechanisms that link obese adipose tissue to poor prognosis of depression are required. The strengths of this study include the analysis of cytokines along with CBC with differentials and inflammatory ratios (NLR, MLR, and PLR) to figure out why adolescents with MDD who are overweight or obese are associated with higher suicide risk and severe depressive symptoms than lean individuals. Furthermore, we controlled for age, sex, and education level, as well as confounding factors or comorbidities that may affect inflammatory changes, such as other mood disorders, psychiatric disorders, immune system and hematological system disorders, infectious or inflammatory diseases, chronic physical illnesses, and other factors such as the use of alcohol, tobacco, antidepressants, and drugs that may affect inflammatory changes. Therefore, these variables did not affect the conclusions.
7 Implications
Our findings may have several implications. First, it raises the possibility that weight loss or reduced body weight could be a simple approach to lowering the risk of severe depressive symptoms and high suicide risk in MDD patients. Second, it is important to recognize that the impact of being overweight can be as profound as obesity itself in escalating the risk of severe depression and suicide in adolescents with MDD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Institutional Review Board of Walailak University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
PN, PP, and PK contributed to the conception, design of the study, and funding acquisition. PN, PP, and HJ collected data and performed the statistical analysis. PN wrote the first draft of the manuscript. PN, PP, PK, and KN wrote sections and revised the manuscript. All authors contributed to the manuscript revision, read, and approved the submitted version.
Funding
This study received funding from Walailak University, Thailand [grant number WU66233] and the BDMS Health Research Center, Bangkok Dusit Medical Services PLC, Thailand [grant number BMC-2023042701]. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
We would like to thank the staff from the Central Laboratory of Walailak University Hospital for their assistance with blood sample collection.
Conflict of interest
PK is employed by the Bangkok Dusit Medical Services PLC, Thailand.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1197775/full#supplementary-material
Abbreviations
IFN-γ, interferon-gamma; MCP-1, Monocyte Chemoattractant Protein-1; TNF-α, Tumor necrosis factor alpha; TGF- β1, Transforming growth factor-beta 1.
References
1. World Health Organization (WHO). Depression (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/depression (Accessed 4 December 2022).
2. Sun S, Ji Y, Kersten S, Qi L. Mechanisms of inflammatory responses in obese adipose tissue. Annu Rev Nutr (2012) 32(1):261–86. doi: 10.1146/annurev-nutr-071811-150623
3. Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Curr Top Behav Neurosci (2013) 14:135–51. doi: 10.1007/7854_2012_211
4. Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol (2016) 7:30. doi: 10.3389/fendo.2016.00030
5. Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci (2008) 9(1):46–56. doi: 10.1038/nrn2297
6. Felger JC. Imaging the role of inflammation in mood and anxiety-related disorders. Curr Neuropharmacol (2018) 16(5):533–58. doi: 10.2174/1570159X15666171123201142
7. Osimo EF, Pillinger T, Rodriguez IM, Khandaker GM, Pariante CM, Howes OD. Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav Immun (2020) 87:901–9. doi: 10.1016/j.bbi.2020.02.010
8. García-García ML, Tovilla-Zárate CA, Villar-Sot M, Juárez-Rojop IE, González-Castro TB, Genis-Mendoza AD, et al. Fluoxetine modulates the pro-inflammatory process of IL-6, IL-1β and TNF-α levels in individuals with depression: a systematic review and meta-analysis. Psychiatry Res (2022) 307:114317. doi: 10.1016/j.psychres.2021.114317
9. Berk M, Williams LJ, Jacka FN, O’Neil A, Pasco JA, Moylan S, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med (2013) 11(1):200. doi: 10.1186/1741-7015-11-200
10. World Health Organization (WHO). Obesity (2022). Available at: https://www.who.int/health-topics/obesity#tab=tab_1 (Accessed 4 December 2022).
11. León-Pedroza JI, González-Tapia LA, del Olmo-Gil E, Castellanos-Rodríguez D, Escobedo G, González-Chávez A. Low-grade systemic inflammation and the development of metabolic diseases: From the molecular evidence to the clinical practice. Cirugía y Cirujanos (English Edition) (2015) 83(6):543–51. doi: 10.1016/j.circen.2015.11.008
12. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature (2017) 542:177–85. doi: 10.1038/nature21363
13. Chung KJ, Nati M, Chavakis T, Chatzigeorgiou A. Innate immune cells in the adipose tissue. Rev Endocr Metab Disord (2018) 19(4):283–92. doi: 10.1007/s11154-018-9451-6
14. Radwan H, Ballout RA, Hasan H, Lessan N, Karavetian M, Rizk R. The epidemiology and economic burden of obesity and related cardiometabolic disorders in the United Arab Emirates: A systematic review and qualitative synthesis. J Obes (2018) 2018:1–23. doi: 10.1155/2018/2185942
15. Chait A, den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovascr Med (2020) 7:22. doi: 10.3389/fcvm.2020.00022
16. Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol (2021) 320(3):, C375–C391. doi: 10.1152/ajpcell.00379.2020
17. Obesity, Race/Ethnicity, and COVID-19, 2022. Centers for disease control and prevention (CDC). Available at: https://www.cdc.gov/obesity/data/obesity-and-covid-19.html#Increasing (Accessed 4 December 2022).
18. De Frel DL, Atsma DE, Pijl H, Seidell JC, Leenen PJM, Dik WA, et al. The impact of obesity and lifestyle on the immune system and susceptibility to infections such as COVID-19. Front Nutr (2020) 7:597600. doi: 10.3389/fnut.2020.597600
19. Kane H, Lynch L. Innate immune control of adipose tissue homeostasis. Trends Immunol (2019) 40:857–72. doi: 10.1016/j.it.2019.07.006
20. Liu R, Nikolajczyk BS. Tissue immune cells fuel obesity-associated inflammation in adipose tissue and beyond. Front Immunol (2019) 10:2. doi: 10.3389/fimmu.2019.01587
21. Schmidt V, Hogan AE, Fallon PG, Schwartz C. Obesity-mediated immune modulation: one step forward, (Th)2 steps back. Front Immunol (2022). doi: 10.3389/fimmu.2022.932893
22. Köhler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand (2017) 135(5):373–87. doi: 10.1111/acps.12698
23. Colasanto M, Madigan S, Korczak DJ. Depression and inflammation among children and adolescents: A meta-analysis. J Affect Disord (2020) 277:940–8. doi: 10.1016/j.jad.2020.09.025
24. Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BWJH, et al. Overweight, obesity, and depression. Arch Gen Psychiatry (2010) 67(3):220. doi: 10.1001/archgenpsychiatry.2010.2
25. Mannan M, Mamun A, Doi S, Clavarino A. Is there a bi-directional relationship between depression and obesity among adult men and women? Systematic review and bias-adjusted meta analysis. Asian J Psychiatr (2016) 21:51–66. doi: 10.1016/j.ajp.2015.12.008
26. Blasco BV, García-Jiménez J, Bodoano I, Gutiérrez-Rojas L. Obesity and depression: its prevalence and influence as a prognostic factor: A systematic review. Psychiatry Investig (2020) 17(8):715–24. doi: 10.30773/pi.2020.0099
27. Ekinci O, Ekinci A. The connections among suicidal behavior, lipid profile and low-grade inflammation in patients with major depressive disorder: a specific relationship with the neutrophil-to-lymphocyte ratio. Nord J Psychiatr (2017) 71(8):574–80. doi: 10.1080/08039488.2017.1363285
28. Kayhan F, Gündüz S, Ersoy SA, Kandeger A, Annagür BB. Relationships of neutrophil–lymphocyte and platelet–lymphocyte ratios with the severity of major depression. Psychiatr Res (2017) 247:332–5. doi: 10.1016/j.psychres.2016.11.016
29. Velasco A, Rodríguez-Revuelta J, Oli´e E, Abad I, Fernandez-Pel´aez, ´ A, Cazals A, et al. Neutrophil-to-lymphocyte ratio: a potential new peripheral biomarker of suicidal behavior. Eur Psychiatr (2020) 63(1):e14. doi: 10.1192/j.eurpsy.2019.20
30. Amitai M, Kaffman S, Kroizer E, Lebow M, Magen I, Benaroya-Milshtein N, et al. Neutrophil to-lymphocyte and platelet-to-lymphocyte ratios as biomarkers for suicidal behavior in children and adolescents with depression or anxiety treated with selective serotonin reuptake inhibitors. Brain Behav Immun (2022) 104:31–8. doi: 10.1016/j.bbi.2022.04.018
31. Puangsri P, Ninla-aesong P. Potential usefulness of complete blood count parameters and inflammatory ratios as simple biomarkers of depression and suicide risk in drug-naive, adolescents with major depressive disorder. Psychiatry Res (2021) 305:114216. doi: 10.1016/j.psychres.2021.114216
32. Puangsri P, Jinanarong V, Ninla-aesong P. Impact of antidepressant treatment on complete blood count parameters and inflammatory ratios in adolescents with major depressive disorder. J Psychiatr Res (2023) 157:26–35. doi: 10.1016/j.jpsychires.2022.11.017
33. Zheng Q, Liu J, Ji Y, Chen X, Liu B. Elevated levels of monocyte-lymphocyte ratio and platelet-lymphocyte ratio in adolescents with non-suicidal self-injury. BMC Psychiatry (2022) 22:618. doi: 10.1186/s12888-022-04260-z
34. Cheng Y, Wang Y, Wang X, Jiang Z, Zhu L, Fang S. Neutrophil-to lymphocyte ratio, platelet-to-lymphocyte ratio, and monocyte-to-lymphocyte ratio in depression: an updated systematic review and meta-analysis. Front Psychiatr (2022) 13:893097. doi: 10.3389/fpsyt.2022.893097
35. Department of mental health, Ministry of public health, Thailand. 2Q, 8Q, 9Q scale (2022). Available at: https://www.dmh.go.th/test/download/files/2Q%209Q%208Q%20(1).pdf (Accessed 2 December 2022).
36. Ninla-aesong P, Mitarnun W, Noipha K. Proinflammatory cytokines and chemokines as biomarkers of persistent arthralgia and severe disease after chikungunya virus infection: A 5-year follow-up study in Southern Thailand. Viral Immunol (2019) 32(10):442–52. doi: 10.1089/vim.2019.0064
37. Georgiev GZ. GIGA calculator (2022). Available at: https://www.gigacalculator.com/calculators/odds-ratio-calculator.php (Accessed 02 December 2022).
38. Touch S, Clément K, André S. T cell populations and functions are altered in human obesity and type 2 diabetes. Curr Diabetes Rep (2017) 17:81. doi: 10.1007/s11892-017-0900-5
39. Sun Y, Koyama Y, Shimada S. Inflammation from peripheral organs to the brain: how does systemic inflammation cause neuroinflammation? Front Aging Neurosci (2022) 14:903455. doi: 10.3389/fnagi.2022.903455
40. Black C, Miller BJ. Meta-analysis of cytokines and chemokines in suicidality: distinguishing suicidal versus nonsuicidal patients. Biol Psychiatry (2015) 78(1):28–37. doi: 10.1016/j.biopsych.2014.10.014
Keywords: depression, inflammation, inflammatory markers, overweight, obese, IL-1b, IL-6, suicide
Citation: Ninla-aesong P, Puangsri P, Kietdumrongwong P, Jongkrijak H and Noipha K (2023) Being overweight and obese increases suicide risk, the severity of depression, and the inflammatory response in adolescents with major depressive disorders. Front. Immunol. 14:1197775. doi: 10.3389/fimmu.2023.1197775
Received: 31 March 2023; Accepted: 13 October 2023;
Published: 01 November 2023.
Edited by:
Shuming Pan, Shanghai Jiao Tong University, ChinaReviewed by:
Angelos Halaris, Loyola University Chicago, United StatesSylvie Vancassel, INRA Centre Bordeaux-Aquitaine, France
Copyright © 2023 Ninla-aesong, Puangsri, Kietdumrongwong, Jongkrijak and Noipha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Putrada Ninla-aesong, cHV0cmFkYS5uaUBtYWlsLnd1LmFjLnRo
 Putrada Ninla-aesong
Putrada Ninla-aesong Pavarud Puangsri4
Pavarud Puangsri4