- 1Department of Ophthalmology, Renmin Hospital of Wuhan University, Wuhan, China
- 2Department of Gastroenterology, Renmin Hospital of Wuhan University, Wuhan, China
Background: Autoimmunity and inflammation are the main characteristics of rheumatic diseases and have both been found to be related to glaucoma. However, it remains unclear whether rheumatic diseases increase the risk of glaucoma. Here, we performed a Mendelian randomization (MR) analysis to investigate the causal effects of six common rheumatic diseases on glaucoma.
Methods: Six rheumatic diseases were included: ankylosing spondylitis (AS), rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Sicca syndrome/Sjögren’s sydrome (SS), dermatomyositis (DM), and gout. Glaucoma included primary open-angle glaucoma (POAG) and primary angle-closure glaucoma (PACG). Genetic variants associated with these rheumatic diseases and glaucoma were extracted from the genome-wide association studies and FinnGen8 database, respectively. First, a two-sample MR was used to investigate the potential causal association. Then, a multivariable MR was conducted to further verify the results. Inverse-variance weighted MR analysis was used as the main method, together with several sensitivity analyses.
Results: Two-sample MR suggests that AS is related to a higher risk of both POAG [odds ratio (OR): 1.28, 95% confidence interval (CI) 1.13–1.44; p = 1.1 × 10−4] and PACG (OR: 1.55, 95% CI: 1.09–2.09, p = 1.4 × 10−2). Multivariable MR shows a similar trend of the effect of AS on POAG (OR: 1.52, 95% CI: 1.22–1.90, p = 1.9 × 10−4) and PACG (OR: 2.05, 95% CI: 1.06–3.95, p = 3.2 × 10−2). No significant association was observed between the other five rheumatic diseases and glaucoma.
Conclusions: AS is related to an increased risk of POAG and PACG. We stress the importance of glaucoma screening for AS patients.
1 Introduction
Rheumatic diseases, including over 100 disorders, are a spectrum of autoimmune and/or inflammatory diseases that may damage the joints, muscles, bones, and organs (1). Rheumatic diseases are the most common cause of disability among US adults, surpassing heart diseases, diabetes, and cancers (2). As diseases with multi-organ involvement, rheumatic diseases can affect the brain, heart, lung, kidney, etc. (3–6). However, as “the window to the soul”, the eye has attracted relatively less attention in the field of rheumatology.
Glaucoma is a heterogeneous group of ocular diseases featured by degeneration of the optic nerve and retinal ganglion cell loss with multifactorial pathogenic causes (7). Currently, glaucoma is the leading global cause of irreversible blindness, with an estimated prevalence of 76.0 million cases worldwide (8). In 2020 alone, glaucoma has caused 3.6 million cases of blindness (9). Traditionally, increased eye pressure (intraocular pressure) has been viewed as a major risk factor for glaucoma development and progression (10). However, increased intraocular pressure is seen in approximately 70% of patients only, and some patients still exhibit disease progression despite the intraocular pressure being controlled within normal ranges, both of which indicate that there are other risk factors for glaucoma (11, 12). Taken together, it is crucial to explore the unidentified risk factors for glaucoma, which will facilitate early detection and timely treatment so as to reduce glaucoma-related blindness.
Notably, both autoimmunity and inflammation, the main characteristics of rheumatic diseases, have been found to be related to glaucoma (11, 13–15). This inspired us to speculate whether there is an association between rheumatic diseases and glaucoma.
Mendelian randomization (MR) is an established approach for making causal inferences in epidemiology (16). MR uses genetic variants identified in the genome-wide association studies (GWAS) as instrumental variables (IVs) (17). In MR, these IVs are used to assess the causal effects of defined exposures on a phenotype. At birth, according to Mendel’s second law, an individual is naturally assigned to either carry some IVs or not (18). Those who have inherited such IVs are steadily affected by IV-related exposures. When these individuals grow up, they either show a phenotype of interest (e.g., a disease) or not. Since these IVs are fixed at conception and typically not subjected to confounders, the differences in the studied phenotype are attributed to the differences in the exposure (18). For instance, if some IVs are strongly associated with depression (the exposure) and also associated with a higher risk of breast cancer (the phenotype), then it is likely that depression is causally associated with the risk of breast cancer (19).
In this study, we included six common rheumatic diseases, namely, ankylosing spondylitis (AS), rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Sicca syndrome/Sjögren’s syndrome (SS), dermatomyositis (DM), and gout. Glaucoma was divided into two categories: primary open-angle glaucoma (POAG) and primary angle-closure glaucoma (PACG). We first conducted a two-sample MR to evaluate the potential causality between the six rheumatic diseases and the two types of glaucoma. Then, the results were further verified by multivariable MR (MVMR) to provide a robust conclusion.
2 Materials and methods
An overview of the study design is shown in Figure 1. No additional ethical approval is needed due to the use of the publicly available GWAS and FinnGen data.
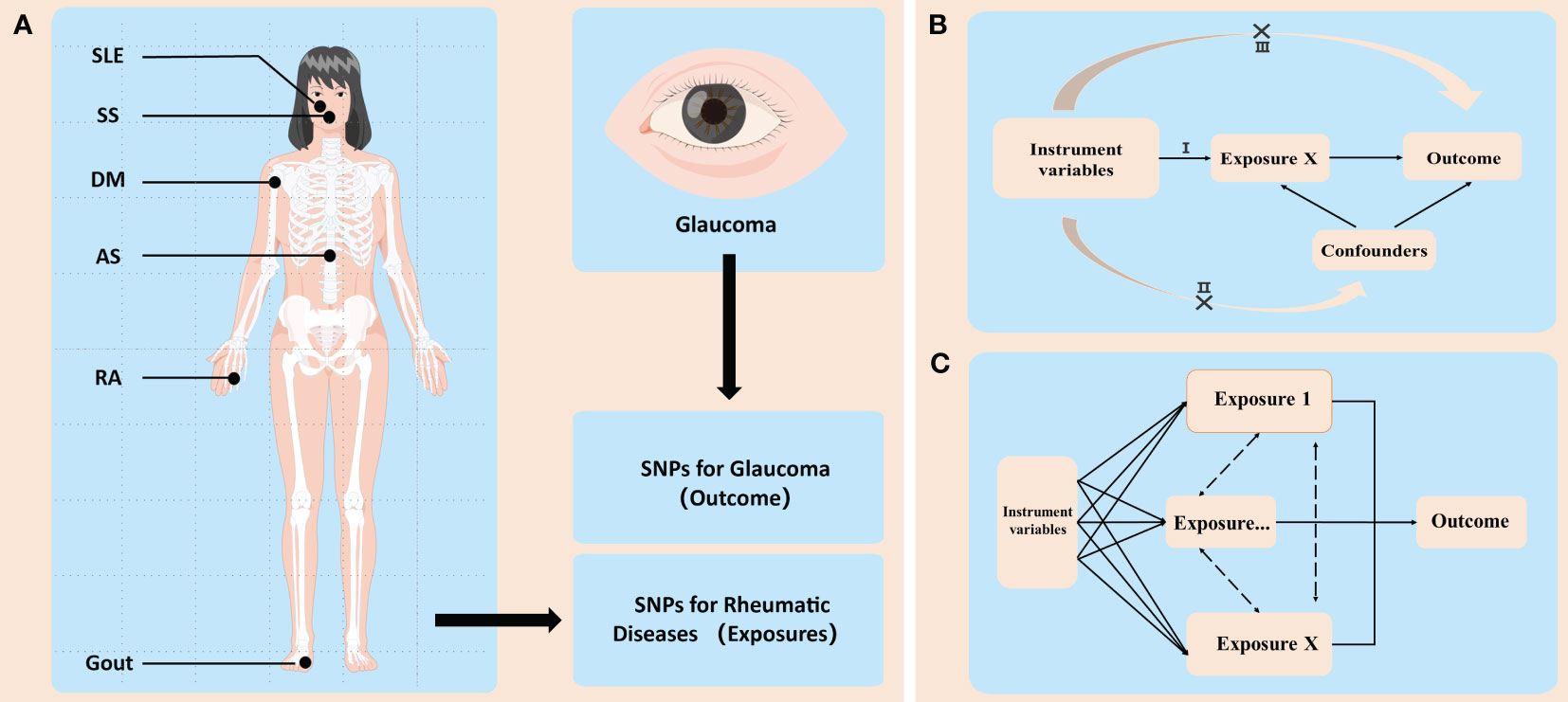
Figure 1 An overview of the study design. (A) GWAS (genome-wide association study) identified sequence variations, namely, single-nucleotide polymorphisms (SNPs), that exist throughout the human genome, and then screened disease-related SNPs from them. In this study, SNPs related with six common rheumatic diseases and two types of glaucoma were used. These rheumatic diseases included ankylosing spondylitis (AS), rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Sicca syndrome (SS), dermatomyositis (DM), and gout. Glaucoma is divided into two categories: primary open-angle glaucoma (POAG) and primary angle-closure glaucoma (PACG). (B) Illustration of Mendelian randomization (MR). MR has three main assumptions: (I) the instrumental variables (IVs) are associated with the exposure of interest (i.e., each rheumatic disease in this study); (II) the IVs are independent of potential confounders; and (III) the IVs influence the outcome (i.e., the two types of glaucoma) only through the exposure. (C) Illustration of multivariable MR (MVMR). In some cases, the IVs may be related to several interactive exposures (confounders) that may influence the same outcome. MVMR can be used to adjust for these confounders (e.g., diabetes, coronary heart disease, and smoking, etc.) and then analyze whether the exposure of interest has a direct impact on the outcome.
2.1 Data sources
Data for AS, RA, and SLE were extracted from the published IEU Open GWAS database (https://gwas.mrcieu.ac.uk/). Data for SS, DM, and Gout were extracted from the FinnGen8 database (http://www.finngen.fi). The sample sizes for each rheumatic diseases were as follows: AS (9,069 cases and 1,550 controls), RA (14,361 cases and 43,923 controls), SLE (5,201 cases and 9,066 controls), SS (2,247 cases and 332,115 controls), DM (363 cases and 261,098 controls), and gout (3,768 cases and 336,797 controls). Data for glaucoma obtained from the FinnGen8 database include POAG (6,585 cases and 326,434 controls) and PACG (1,062 cases and 326,434 controls). All individuals included in the analysis were of European ancestry, and the overlap between the cohort of rheumatic diseases and the cohort of glaucoma was less than 20%.
2.2 Selection of IVs
To explore the causal association between the six rheumatic diseases and two types of glaucoma, we first screened IVs for these diseases. Only single-nucleotide polymorphisms (SNPs) according to the following criteria were selected as IVs (1): SNPs had a genome-wide p-value < 5×10−8 so that the SNPs were strongly associated with the exposures (2); SNPs should have linkage disequilibrium (LD) r2 < 0.001 and < 1,000 KB from the index variant (3); SNPs should have F-value ≥ 10, suggesting little possibility of weak IV bias. The formula is as follows: F = (βexposure/SEexposure)2, where βexposure and SEexposure were the effect value and standard error of the exposure, respectively. Moreover, outcome-related SNPs with a p-value > 1×10−5 were excluded. Then, we also checked the selected SNPs in Phenoscanner (www.phenoscanner.medschl.cam.ac.uk), a commonly used database for human genotype–phenotype associations, to test whether these SNPs were related to potential confounding factors (diabetes, hypertension, and glucocorticoid use) (20). Lastly, palindromic SNPs with intermediate allele frequencies were removed from the MR analyses.
2.3 MR analyses
First, a two-sample MR was used to detect the potential causal association between the six rheumatic diseases and the two types of glaucoma. Then, to provide a robust conclusion, we performed an MVMR to verify the results of two-sample MR by adjusting for type 2 diabetes, major coronary heart disease event, cigarette smoking, and systolic blood pressure.
We applied three different approaches to estimate the causal effects between the six rheumatic diseases and glaucoma, including MR Egger, weighted median, and the inverse-variance weighted (IVW) method. The IVW approach was used as our main analysis approach, with the other two used as auxiliary references. The three methods were based on three different assumptions. The IVW method assumes that there is no horizontal pleiotropy, either because all the SNPs used are valid IVs, or because the total pleiotropy is balanced (21). The MR-Egger method allows for horizontal pleiotropic effects under the premise that the SNPs meet the Instrument Strength Independent of Direct Effect (InSIDE) assumption (i.e., the SNP–exposure association is independent from the SNP–outcome association) (22). The slope of the MR-Egger regression provides the estimate of the causal association between the six rheumatic diseases and the two types of glaucoma. Moreover, MR-Egger also serves as a test of directional pleiotropy, namely, the MR-Egger intercept test (23). Moreover, the weighted median method can be performed to give an estimate for the causal effect when no more than half of the SNPs violate horizontal pleiotropy (24).
Several different methods for sensitivity analysis were performed to justify the MR results. The MR-Egger intercept test, as mentioned previously, was used to detect directional pleiotropy. Additionally, MR pleiotropy residual sum and outlier (MR-PRESSO) test were used to detect outlier SNPs, which would then be discarded. Furthermore, Cochran’s Q statistic was used to evaluate heterogeneity. Finally, leave-one-out analysis was performed by eliminating each SNP one by one to test if the results were driven by any single SNP.
2.4 Statistical analysis
All MR analyses were accomplished using the “TwoSampleMR” and “MRPRESSO” packages in R (version 4.2.3). The results were expressed as odds ratios (ORs) with 95% confidence intervals (95% CI) to quantify the magnitude of the causality between the six rheumatic diseases and the two types of glaucoma. The statistical significances were set as p-value < 0.05 when determining the causal effects between rheumatic diseases and glaucoma.
3 Results
3.1 IVs for rheumatic diseases and glaucoma
A total of 166 SNPs were selected to genetically predict rheumatic diseases, including 29 SNPs for AS, 82 SNPs for RA, 29 SNPs for SLE, 14 SNPs for SS, 5 SNPs for DM, and 7 SNPs for gout. Detailed information about these rheumatic disease-related SNPs are listed in Supplementary Table 1. F statistics for all SNPs were higher than 10 in this study, indicating a small chance of weak IV bias. In addition, the relationship between these SNPs and outcomes was weak, with all p-values> 1×10−5.
3.2 Two-sample MR between rheumatic diseases and glaucoma
The two-sample MR results between the six rheumatic diseases and two types of glaucoma are listed in Figure 2. The causal estimates from the IVW method showed that genetically predicted AS was positively associated with the risk of both POAG (OR: 1.28, 95% CI: 1.13 to 1.44, p = 1.1 × 10−4) and PACG (OR: 1.55, 95% CI: 1.09 to 2.09, p = 1.4 × 10−2). The other five rheumatic diseases (RA, SLE, SS, DM, and gout) showed no significant association with POAG or PACG.
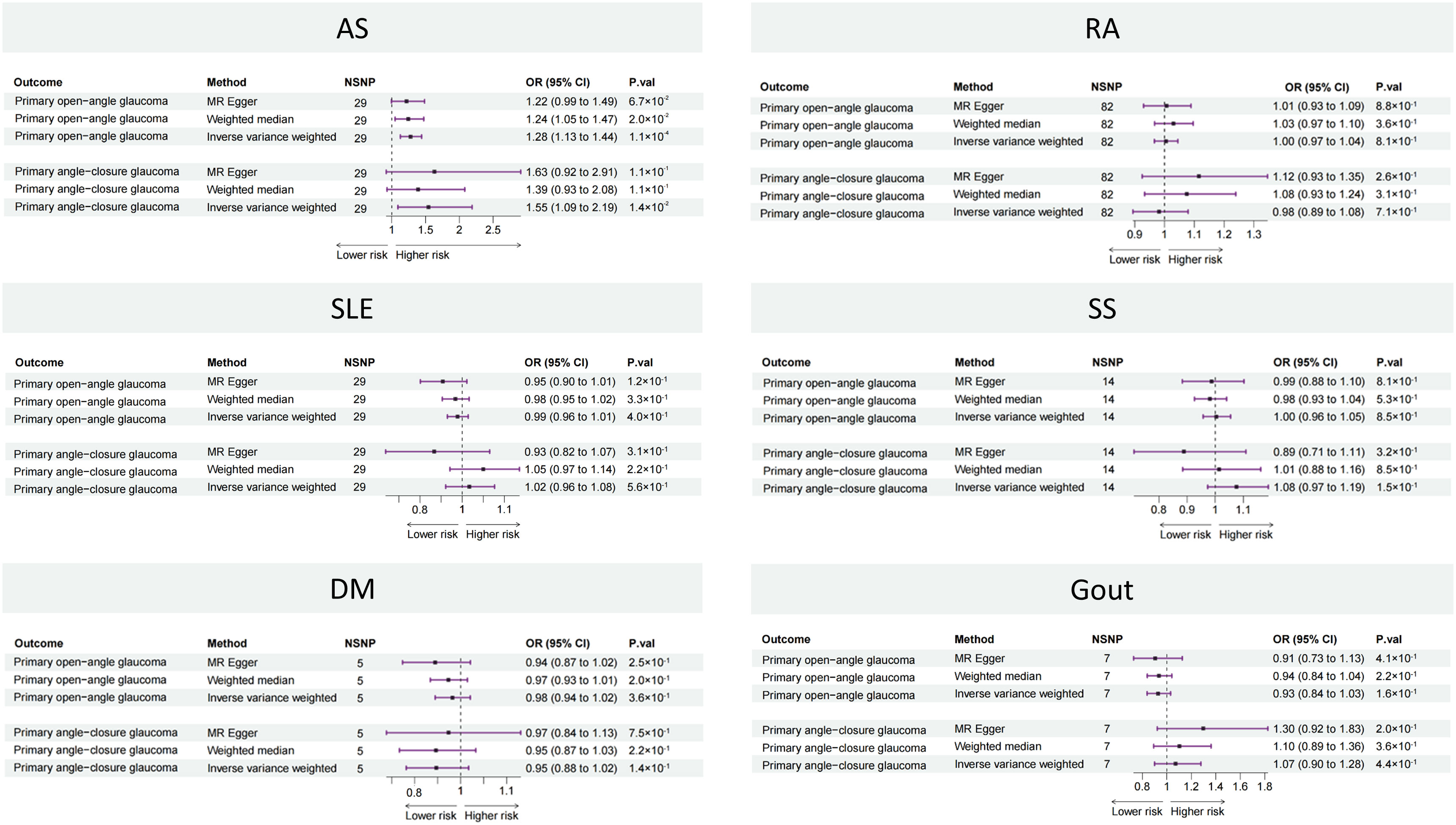
Figure 2 Forest plot showing causal estimates between rheumatic diseases and glaucoma in two-sample MR. In the forest plot, the dashed vertical line represents the ineffective line (OR = 1), the horizontal coordinate corresponding to each square represents the OR value calculated by different methods, and each horizontal solid line represents the 95% CI of the corresponding OR value. MR, Mendelian randomization; NSNP, number of single-nucleotide polymorphisms; OR, odds ratio; CI, confidence interval; AS, ankylosing spondylitis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus, SS, Sicca syndrome, DM, dermatomyositis.
The results of the Cochran’s Q statistic, MR-Egger intercept test, and MR-PRESSO test are listed in Supplementary Table 2. All Cochran’s Q-derived p-value s were > 0.05 except for estimates of SLE on POAG. All p-value s were > 0.05 in the MR-Egger intercept test, indicating that no horizontal pleiotropy existed. All p-value s were > 0.05 in the MR-PRESSO test, except for estimates of SLE on POAG. The leave-one-out analysis showed that the association between AS and glaucoma was not driven by any single SNP (Supplementary Figure 1).
The results of the sensitivity analyses indicated that the causal association between AS and glaucoma was robust.
3.3 Multivariable MR between rheumatic diseases and glaucoma
On the basis of the results of the two-sample MR, only AS was associated with the risk of POAG and PACG. Then, we further adjusted for type 2 diabetes, major coronary heart disease event, cigarette smoking, and systolic blood pressure in an MVMR to verify the reliability of the two-sample MR results (Figure 3). The MVMR results were in consistent with two-sample MR, i.e., AS was a risk factor for both POAG (OR: 1.52, 95% CI: 1.22 to 1.90, p = 1.9 × 10−4) and PACG (OR: 2.05, 95% CI: 1.06 to 3.95, p = 3.2 × 10−2) while the other five rheumatic diseases were not.
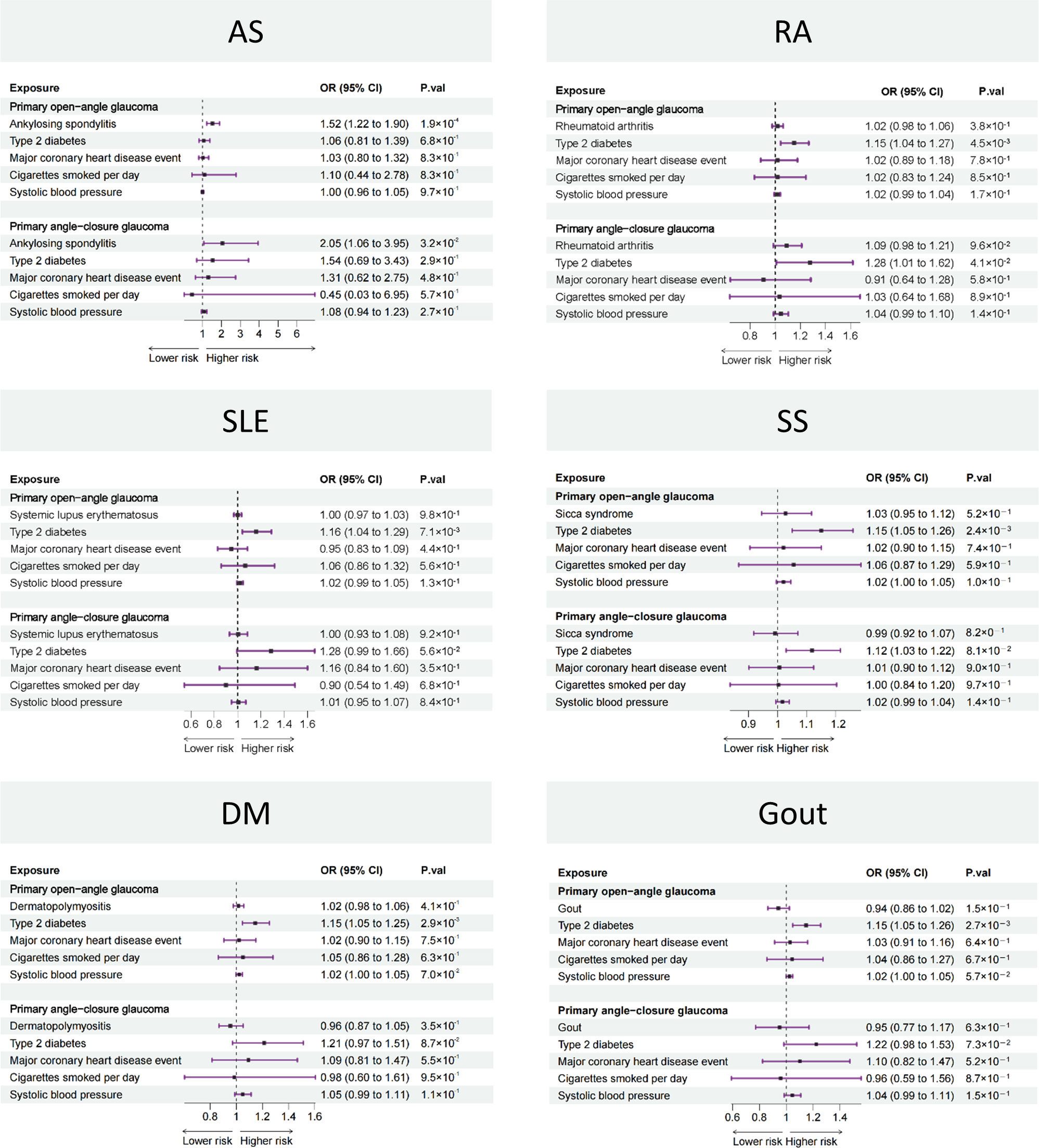
Figure 3 Forest plot showing causal estimates between rheumatic diseases and glaucoma in MVMR. In the forest plot, the dashed vertical line represents the ineffective line (OR = 1), the horizontal coordinate corresponding to each square represents the OR value calculated by different methods, and each horizontal solid line represents the 95% CI of the corresponding OR value. MVMR, multivariable Mendelian randomization; OR, odds ratio; CI, confidence interval; AS, ankylosing spondylitis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus, SS, Sicca syndrome, DM, dermatomyositis.
4 Discussion
This study, as far as we know, is the first to evaluate the causal association between the six common rheumatic diseases (AS, RA, SLE, SS, DM, and gout) and glaucoma. Using two-sample MR, we found that AS is associated with a higher risk of both POAG and PACG. Then, this finding was verified by MVMR, where several major confounders were adjusted for. In other words, AS is a risk factor for glaucoma, whereas RA, SLE, SS, DM, and gout were not.
It is well known that rheumatic diseases are a complex spectrum of diseases that affects multiple organs and systems (1). In fact, ocular involvement is a common extra-articular manifestation of several rheumatic diseases, including AS (25–28). For example, anterior uveitis is one of the most commonly seen extra-articular manifestations of AS, affecting 11.4%–15.8% of AS patients, and the prevalence of anterior uveitis can increase with a prolonged duration of AS (29–31). However, there are no published data about the prevalence of glaucoma in AS, which suggests that more attention needs to be given to this disease with irreversible vision loss in the AS group.
Owing to the limited relevant studies at present, the specific pathogenesis of glaucoma in AS is still unclear but could be contributed by three cornea-related mechanisms: central corneal thickness (CCT), corneal hysteresis (CH), and corneal stiffness. First, studies have revealed that AS patients have lower CCT when compared to healthy individuals and the CCT decreased with an increase in Bath Ankylosing Spondylitis Metrology Index score (BASMI) (32, 33). It is widely accepted that lower CCT brings a higher risk of glaucoma (34). Second, AS patients tend to have lower CH, and CH is negatively associated with the duration of disease (32, 33). Lower CH has been proven to be a risk factor for predicting both the development and progression of glaucoma (35–37). Third, it has been found that AS patients have lower corneal stiffness when compared with healthy individuals, as demonstrated by the lower stiffness parameter at first applanation (SPA1) (38). The abnormality of corneal biomechanics (including corneal stiffness) is considered to be an important risk factor for the development and progression of normal-tension glaucoma (39, 40).
AS is an inflammatory collagen connective tissue disease. The cornea, because of its high collagen content, is a target tissue of AS (41). The corneal stroma is the main part of the cornea, accounting for approximately 90% of the total corneal thickness, and is composed of collagen fibrils that are arranged in parallel into lamellae (32, 42). The lamellar organization of the collagen fibrils plays an important role in the biomechanical properties of the human cornea, such as maintaining its shape and strength (38). Thus, it is possible that collagen alterations caused by the inflammatory pathological processes in AS could affect the biomechanical parameters of the cornea, leading to a higher risk of glaucoma (38, 43).
Glaucoma is currently the leading cause of irreversible vision loss all over the world (8). Despite considerable progress in its treatment over the past few decades, the optic nerve damage and retinal ganglion cell loss caused by glaucoma are still irreversible (44). Nevertheless, diagnosis of glaucoma is often delayed since patients could remain asymptomatic until a relatively advanced stage (44). Additionally, the decrease in vision-related quality of life can be present as early as before patients are unaware of their having glaucoma, highlighting the irreplaceable role of early diagnosis and treatment (45). To sum up, recognizing the risk factors of glaucoma may aid in targeted screening among high-risk populations (e.g., patients with AS) and finally to help prevent or delay blindness in these patients. Considering the high incidence of AS in the general population (0.1%–0.5%), it is necessary to further explore the relationship between AS and glaucoma in future studies (46).
There are several strengths worth noting in this study. Above all, this is the first study to evaluate the causal association between the six common rheumatic diseases and glaucoma. Moreover, traditional observational studies are affected by reverse causality and confounding factors and, therefore, are less reliable when making causal inferences. The MR design used in this study can avoid reverse causality and most confounding factors. Moreover, an MR study is more convenient, cost-effective, and labor-saving when compared with traditional studies.
Some limitations need to be acknowledged in this study. First, although we have used the MR-PRESSO test to screen and discard outlier SNPs, the potential effect of heterogeneity on the study results cannot be completely ruled out. Second, since the enrolled participants were mainly of European ancestry, our results may not necessarily be generalizable to populations of other races. Third, although our study has revealed the causal association between AS and glaucoma, the exact pathophysiological mechanisms are still unclear, and further studies are needed. Last, it should be noticed that the causal association between AS and glaucoma might be partially mediated by some intermediate phenotypes, such as anterior uveitis. However, this does not change the finding that AS is a risk factor for glaucoma.
5 Conclusions
This is the first MR study to investigate the causal association between six common rheumatic diseases (AS, RA, SLE, SS, DM, and gout) and two types of glaucoma (POAG and PACG). We have found a causal association between AS and both types of glaucoma (i.e., AS is a risk factor for both POAG and PACG). We stress the importance of glaucoma screening for AS patients, which would help in early diagnosis and prevention of irreversible vision loss. We urge future studies to further explore the underlying mechanisms.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
YM, ZT, LL, and CC designed the study. YM, ZT, and YS analyzed and interpreted the data. YM and ZT were major contributors in writing the manuscript. LL and CC reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank the IEU Open GWAS database for providing the data. We also want to acknowledge the participants and investigators of the FinnGen study. Figure 1 was finished by Figdraw platform (www.figdraw.com).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1227138/full#supplementary-material
References
1. Rosen MJ, Dhawan A, Saeed SA. Inflammatory bowel disease in children and adolescents. JAMA Pediatr (2015) 169:1053–60. doi: 10.1001/jamapediatrics.2015.1982
3. Luo J, Xu Z, Noordam R, van Heemst D, Li-Gao R. Depression and inflammatory bowel disease: A bidirectional two-sample mendelian randomization study. J Crohns Colitis (2022) 16:633–42. doi: 10.1093/ecco-jcc/jjab191
4. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol (2015) 12:720–7. doi: 10.1038/nrgastro.2015.150
5. Gueudry J, Muraine M. Anterior uveitis. J Fr Ophtalmol (2018) 41:e11–21. doi: 10.1016/j.jfo.2017.11.003
6. Larsen S, Bendtzen K, Nielsen OH. Extraintestinal manifestations of inflammatory bowel disease: epidemiology, diagnosis, and management. Ann Med (2010) 42:97–114. doi: 10.3109/07853890903559724
7. Shah J, Shah A, Hassman L, Gutierrez A. Ocular manifestations of inflammatory bowel disease. Inflamm Bowel Dis (2021) 27:1832–8. doi: 10.1093/ibd/izaa359
8. Greuter T, Bertoldo F, Rechner R, Straumann A, Biedermann L, Zeitz J, et al. Extraintestinal manifestations of pediatric inflammatory bowel disease: prevalence, presentation, and anti-TNF treatment. J Pediatr Gastroenterol Nutr (2017) 65:200–6. doi: 10.1097/MPG.0000000000001455
9. Burisch J, Jess T, Egeberg A. Incidence of immune-mediated inflammatory diseases among patients with inflammatory bowel diseases in Denmark. Clin Gastroenterol Hepatol (2019) 17:2704–12 e3. doi: 10.1016/j.cgh.2019.03.040
10. Halling ML, Kjeldsen J, Knudsen T, Nielsen J, Hansen LK. Patients with inflammatory bowel disease have increased risk of autoimmune and inflammatory diseases. World J Gastroenterol (2017) 23:6137–46. doi: 10.3748/wjg.v23.i33.6137
11. Bowden J, Holmes MV. Meta-analysis and Mendelian randomization: A review. Res Synth Methods (2019) 10:486–96. doi: 10.1002/jrsm.1346
12. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA (2017) 318:1925–6. doi: 10.1001/jama.2017.17219
13. Sekula P, Del Greco MF, Pattaro C, Kottgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol (2016) 27:3253–65. doi: 10.1681/ASN.2016010098
14. de Lange KM, Moutsianas L, Lee JC, Lamb CA, Luo Y, Kennedy NA, et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat Genet (2017) 49:256–61. doi: 10.1038/ng.3760
15. Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner K, et al. FinnGen: Unique genetic insights from combining isolated population and national health register data. medRxiv (2022) 03:22271360. doi: 10.1101/2022.03.03.22271360
16. Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics (2019) 35:4851–3. doi: 10.1093/bioinformatics/btz469
17. Sanderson E. Multivariable mendelian randomization and mediation. Cold Spring Harb Perspect Med (2021) 11:a038984. doi: 10.1101/cshperspect.a038984
18. Rosoff DB, Davey Smith G, Mehta N, Clarke TK, Lohoff FW. Evaluating the relationship between alcohol consumption, tobacco use, and cardiovascular disease: A multivariable Mendelian randomization study. PloS Med (2020) 17:e1003410. doi: 10.1371/journal.pmed.1003410
19. Lee CH, Cook S, Lee JS, Han B. Comparison of two meta-analysis methods: inverse-variance-weighted average and weighted sum of Z-scores. Genomics Inform (2016) 14:173–80. doi: 10.5808/GI.2016.14.4.173
20. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol (2017) 32:377–89. doi: 10.1007/s10654-017-0255-x
21. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol (2016) 40:304–14. doi: 10.1002/gepi.21965
22. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
23. Jaffe GJ, Dick AD, Brezin AP, Nguyen QD, Thorne JE, Kestelyn P, et al. Adalimumab in patients with active noninfectious uveitis. N Engl J Med (2016) 375:932–43. doi: 10.1056/NEJMoa1509852
24. Nguyen QD, Merrill PT, Jaffe GJ, Dick AD, Kurup SK, Sheppard J, et al. Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): a multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet (2016) 388:1183–92. doi: 10.1016/S0140-6736(16)31339-3
25. Wright EK, Ding NS, Niewiadomski O. Management of inflammatory bowel disease. Med J Aust (2018) 209:318–23. doi: 10.5694/mja17.01001
26. Bhagat S, Das KM. A shared and unique peptide in the human colon, eye, and joint detected by a monoclonal antibody. Gastroenterology (1994) 107:103–8. doi: 10.1016/0016-5085(94)90066-3
27. Wakefield D, Clarke D, McCluskey P. Recent developments in HLA B27 anterior uveitis. Front Immunol (2020) 11:608134. doi: 10.3389/fimmu.2020.608134
28. de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol (2016) 13:13–27. doi: 10.1038/nrgastro.2015.186
29. Murdaca G, Tonacci A, Negrini S, Greco M, Borro M, Puppo F, et al. Emerging role of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun Rev (2019) 18:102350. doi: 10.1016/j.autrev.2019.102350
30. Skaaby T, Husemoen LL, Thuesen BH, Linneberg A. Prospective population-based study of the association between vitamin D status and incidence of autoimmune disease. Endocrine (2015) 50:231–8. doi: 10.1007/s12020-015-0547-4
31. Chiu ZK, Lim LL, Rogers SL, Hall AJ. Patterns of vitamin D levels and exposures in active and inactive noninfectious uveitis patients. Ophthalmology (2020) 127:230–7. doi: 10.1016/j.ophtha.2019.06.030
32. Vernia P, Burrelli Scotti G, Dei Giudici A, Chiappini A, Cannizzaro S, Afferri MT, et al. Inadequate sunlight exposure in patients with inflammatory bowel disease. J Dig Dis (2018) 19:8–14. doi: 10.1111/1751-2980.12567
33. Flynn S, Eisenstein S. Inflammatory bowel disease presentation and diagnosis. Surg Clin North Am (2019) 99:1051–62. doi: 10.1016/j.suc.2019.08.001
34. Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature (2012) 491:119–24. doi: 10.1038/nature11582
35. Wang ZK, Yang YS, Chen Y, Yuan J, Sun G, Peng LH. Intestinal microbiota pathogenesis and fecal microbiota transplantation for inflammatory bowel disease. World J Gastroenterol (2014) 20:14805–20. doi: 10.3748/wjg.v20.i40.14805
36. Sokol H, Lay C, Seksik P, Tannock GW. Analysis of bacterial bowel communities of IBD patients: what has it revealed? Inflamm Bowel Dis (2018) 14:858–67. doi: 10.1002/ibd.20392
37. Sankarasubramanian J, Ahmad R, Avuthu N, Singh AB, Guda C. Gut microbiota and metabolic specificity in ulcerative colitis and crohn's disease. Front Med (Lausanne) (2020) 7:606298. doi: 10.3389/fmed.2020.606298
38. Kemeny-Beke A, Szodoray P. Ocular manifestations of rheumatic diseases. Int Ophthalmol (2020) 40:503–10. doi: 10.1007/s10792-019-01183-9
39. Kobayashi S, Suzuki S, Ueda A, Ushiyama C, Tamura N, Inoue H, et al. Chlamydia-induced reactive arthritis–HLA-B 27 negative two patients. Ryumachi (1999) 39:11–6.
40. Key SN 3rd, Kimura SJ. Iridocyclitis associated with juvenile rheumatoid arthritis. Am J Ophthalmol (1975) 80:425–9. doi: 10.1016/0002-9394(75)90529-2
41. Patel SJ, Lundy DC. Ocular manifestations of autoimmune disease. Am Fam Physician (2002) 66:991–8. doi: 10.1016/0002-9394(75)90529-2
42. Ho LC, Sigal IA, Jan NJ, Squires A, Tse Z, Wu EX, Kim SG, Schuman JS, Chan KC. Magic angle-enhanced MRI of fibrous microstructures in sclera and cornea with and without intraocular pressure loading. Invest Ophthalmol Vis Sci (2014) 55:5662–5627. doi: 10.1167/iovs.14-14561
43. Gunes A, Erkol Inal E, Tok L, Tok O. Assessment of corneal parameters with scheimpflug imaging in patients with ankylosing spondylitis. Semin Ophthalmol (2017) 52:276–80. doi: 10.3109/08820538.2015.1068340
44. Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA (2014) 311:1901–1911. doi: 10.1001/jama.2014.3192
45. McKean-Cowdin R, Wang Y, Wu J, Azen SP, Varma R. Angeles Latino Eye Study, G. Impact of visual field loss on health-related quality of life in glaucoma: the Los Angeles Latino Eye Study. Ophthalmology (2008) 115:941–948 e941. doi: 10.1016/j.ophtha.2007.08.037
46. Ward MM, Deodhar A, Dubreuil M, Yu D, khan MA, Haroon N, Borenstein D, Wang R, Biehl A, et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Care Res (Hoboken) (2019) 71:1285–99. doi: 10.1002/acr.24025
Keywords: rheumatic diseases, Spondyloarthritis, glaucoma, genetics, single-nucleotide polymorphisms, Mendelian randomization
Citation: Meng Y, Tan Z, Su Y, Li L and Chen C (2023) Causal association between common rheumatic diseases and glaucoma: a Mendelian randomization study. Front. Immunol. 14:1227138. doi: 10.3389/fimmu.2023.1227138
Received: 22 May 2023; Accepted: 30 August 2023;
Published: 19 September 2023.
Edited by:
Alessandra Bettiol, University of Florence, ItalyReviewed by:
Elena Lastraioli, University of Florence, ItalyAugusto Vaglio, University of Parma, Italy
Michelangelo Tesi, Meyer Children's Hospital, in collaboration with reviewer AV
Copyright © 2023 Meng, Tan, Su, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu Li, bGlsdS0wMDAwMDBAMTYzLmNvbQ==; Changzheng Chen, d2h1Y2hlbmNoemhAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yang Meng1†
Yang Meng1† Zongbiao Tan
Zongbiao Tan Yu Su
Yu Su Changzheng Chen
Changzheng Chen