- 1Department of Orthopedics, The First People’s Hospital of Kashi Prefecture, Kashi, Xinjiang, China
- 2Department of Hand Surgery and Peripheral Neurosurgery, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, China
- 3Department of Orthopedics, Shanghai Tenth People’s Hospital, Tongji University School of Medicine, Shanghai, China
Background: Catechins are a class of natural compounds with a variety of health benefits, The relationship between catechins and the prevalence of osteoarthritis (OA) is unknown. This study investigated the associations between daily intake of catechins and the prevalence of OA among American adults and assessed the moderating effect of physical activity (PA).
Methods: This study included 10,039 participants from the National Health and Nutrition Examination Survey (2007–2010,2017-2018). The logistic regression, weighted quantile sum (WQS) regression, and restricted cubic spline (RCS) regression models were conducted to explore the associations between daily intake of catechins and the prevalence of OA. Moreover, interaction tests were performed to assess the moderating effect of PA.
Results: After multivariable adjustment, the weighted multivariable logistic regression and RCS regression analyses revealed significant J-shaped non-linear correlations between intakes of epigallocatechin and epigallocatechin 3-gallate had significant associations with the prevalence of OA among in U.S. adults. WQS regression analysis showed that excessive epigallocatechin intake was the most significant risk factor for OA among all subtypes of catechins. In the interaction assay, PA showed a significant moderating effect in the relationship between epigallocatechin intake and OA prevalence.
Conclusions: The intake of gallocatechin and gallocatechin 3-gallate had a significant negative correlation with the prevalence of OA and the dose-response relationship was J-shaped.PA below 150 MET-min/week and the threshold intakes of 32.70mg/d for epigallocatechin and 76.24mg/d for epigallocatechin 3-gallate might be the targets for interventions to reduce the risk of developing OA.
Introduction
Osteoarthritis (OA) is a degenerative and inflammatory joint disease caused by multiple factors, which is characterized by joint swelling, pain and cartilage destruction (1, 2). Although the underlying mechanism is not fully understood, its impact on the quality of life of patients is beyond doubt. According to epidemiological statistics, the age-standardized prevalence rate of OA in the United States increased by 23.2% from 1999-2017, which is one of the highest age-standardized prevalence rate increases of OA in the world (3). It is estimated that the number of OA patients in the USA will increase to 67 million by 2030 (4). The financial expenditure on OA care is estimated at US $15.5-28.6 billion per year (2). OA is becoming a disease that attracts more and more attention.
Flavonoids are a group of polyphenolic compounds derived from plant secondary metabolism. They are widely present in a variety of foods, such as vegetables, fruits, tea and wine (5, 6). According to their chemical structure, they can be divided into six subgroups, namely flavonoids, flavanones, flavonols, isoflavones, anthocyanins and flavanols (7–11). Catechins are a subgroup of flavonols. There were eight monomers of catechins, catechin (C), epicatechin (EC),gallocatechin (GC), epigallocatechin (EGC), catechin gallate (CG), epicatechin gallate (ECG), gallocatechin gallate (GCG) and epigallocatechin gallate (EGCG) (12–14). In recent years, with the deepening of research, catechins have been proven to have a kind of biological activities such as anti-inflammatory, anti-oxidation, anti-cancer and bone protection (6, 12, 15, 16). Therefore, supplemental catechins are increasingly recognized as nutritional supplements for the treatment of many diseases, including OA.
The relationship between physical activity and OA has been well-paid attention to in recent years. Homeostasis of joints and joint damage are regarded as the main causes of OA caused by PA (17–20). In addition, the loss of muscle strength caused by low-intensity PA is also the key to the elderly getting OA (21). However, for the general population, daily PA does not increase the risk of joint OA, and moderate levels of PA can also improve soft tissue ductility, blood flow, and synovial fluid mobility, maintain normal joint range of motion, and provide essential nutrients to the cartilage matrix (18, 22, 23).
Since catechins are easily accessible in daily life and have shown excellent medical effects, the present study, which used the NHANES database, attempted to investigate the relationship between daily intake of catechins and the prevalence of OA and to explore whether physical activity plays a moderating role.
Methods
Study population
The National Health and Nutrition Examination Survey (NHANES) is a periodic cross-sectional random sample survey of the non-institutional population in the United States. Survey data are compiled by professionals and released on the NHANES official website for public access (https://www.cdc.gov/nchs/nhanes/). NHANES was approved by the Division of Health and Nutrition Examination Surveys (DHANES) and the National Center for Health Statistics (NCHS), and all participants provided written informed consent.
This study included all 29,940 participants in the “2007-2008”, “2009-2010” and “2017-2018” survey cycles, which have published data on dietary flavonoid intake. After excluding individuals with missing osteoarthritis, catechins, and covariates data, a total of 10039 participants were included in this study.
OA status
Participants with OA were defined by previous physician diagnosis, and collected by trained interviewers through questionnaires. Shortly, participants aged ≥20 years were asked two questions related to arthritis: “Has a doctor or other health professional ever told you that you have arthritis?” and “Which type of arthritis was it?” Participants who responded “yes” and “osteoarthritis” were divided into the osteoarthritis group and the other participants were divided into the Non-osteoarthritis group.
Dietary catechins intake assessment
Catechins are widely found in plants and can be consumed in the daily diet through fruits, tea, coffee, and so on (24). Dietary catechins intake data were extracted from the Food and Nutrient Database for Dietary Studies (FNDDS), which used 24-hour dietary recall interview data from NHANES to calculate participants’ nutrient intakes over 24 hours. In FNDDS, flavonoids were subdivided into 29 species, of which (-)-Epicatechin, (-)-Epicatechin 3-gallate, (-)-Epigallocatechin, (-)-Epigallocatechin 3-gallate, (+)-Catechin, (+)-Gallocatechin were included in the “total catechins” category.
Physical activity assessment
Physical activity (PA) data were collected by trained interviewers through a global physical activity questionnaire survey. The metabolic equivalent (MET) of weekly PA was calculated by multiplying the weekly minutes of moderate or vigorous PA, frequency, and MET value recommended by NHANES. According to the WHO guidelines, participants who achieved moderate intensity (150 min per week) were categorized into the sufficiently PA group (>600 MET-min/wk) and the rest into the insufficiently PA group (600-150 MET-min/wk), low PA group (<150 MET-min/wk), and inactive PA group (PA=0) (25).
Covariates
Demographic information, including age, gender, ethnicity, education level, marital status, and poverty income ratio (PIR), was collected by trained interviewers using a questionnaire standardized. History of hypertension and diabetes were determined by the use of prescribed medications and previous physician diagnosis. Body mass index (BMI) was measured by professionals in the Mobile Examination Center (MEC). Defined as a drinker based on ≥ 12 drinks per year and < 12 drinks per year as a non-drinker. Smokers were defined as having smoked more than 100 cigarettes in the past.
Statistical analyses
Considering the complex sampling design of NHANES, the dietary day one sample weights were included in all analyses of this study. In the characterization of osteoarthritis and non-osteoarthritis participants, normally distributed continuous variables were presented as mean (standard deviation), catechins intake was presented as median (25th, 75th) due to non-normal distribution, and categorical variables were expressed as absolute values (weighted percentages). Statistical differences between the two groups were tested by t-tests, Wilcoxon rank sum tests, and Rao-Scott Chi-square tests., respectively. Univariate and multivariate logistic regression analyses adjusting for the confounding factors and trend tests for the Q group of catechins were used to investigate the 6 catechins and total catechins intake in relation to the prevalence of OA. Model 1 was a univariate logistic regression model without adjustment. Model 2 was the multivariate logistic regression model adjusted for age (20–59 years, ≥60 years), gender (male, female), and ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American, Other race). Model 3 was additionally adjusted for education level (less than high school, high school or equivalent, and college or above), marital status (married/cohabiting, widowed/divorced/separated, never married), PIR, BMI, smoke, alcohol drinking, and history of diabetes or hypertension. The restricted cubic spline (RCS) regression model was performed to investigate the non-linear relationship between the intake of Epigallocatechin and Epigallocatechin 3 gallate with the risk of OA. The number of knots for the RCS regression was three, with the smallest Akaike information criterion to ensure the best fit. Weighted quantile sum (WQS) regression models were performed to evaluate the relationship between indexes representing six catechins or Epigallocatechin and Epigallocatechin 3 gallate co-exposure and the risk of OA. The likelihood ratio test was used to test the significance of the effect on the risk of OA caused by the multiplicative interaction term of PA with Epigallocatechin or Epigallocatechin 3 gallate. Multiple sensitivity analyses are conducted to check the robustness of our results, including logistic regression models adjusting for different covariates, weighted regression models, stratified analyses, and interaction tests. All analyses were conducted using R software (version 4.2.1), and a bilateral P value less than 0.05 was considered statistically significant.
Result
Basic characteristics and catechins intake of study participants
As shown in Table 1, a total of 10,039 volunteers from the NHANES were enrolled in our study, including 8839 non-osteoarthritis and 1,200 osteoarthritis patients. Interestingly, in addition to catechin and gallocatechin, we found that intakes of epigallocatechin, epigallocatechin 3-gallate, epicatechin, epicatechin 3-gallate, and total Catechins were statistically different between the OA and non-OA populations (P < 0.05). In addition, there were significant differences in age, gender, ethnicity, marital status, BMI, poverty income ratio, smoke, diabetes, hypertension, and physical activity between the OA and non-OA populations (P < 0.05). Among them, those aged ≥60 years old, female, divorced, widowed, obesity, smoke, diabetes, and hypertension were more likely to have osteoarthritis.
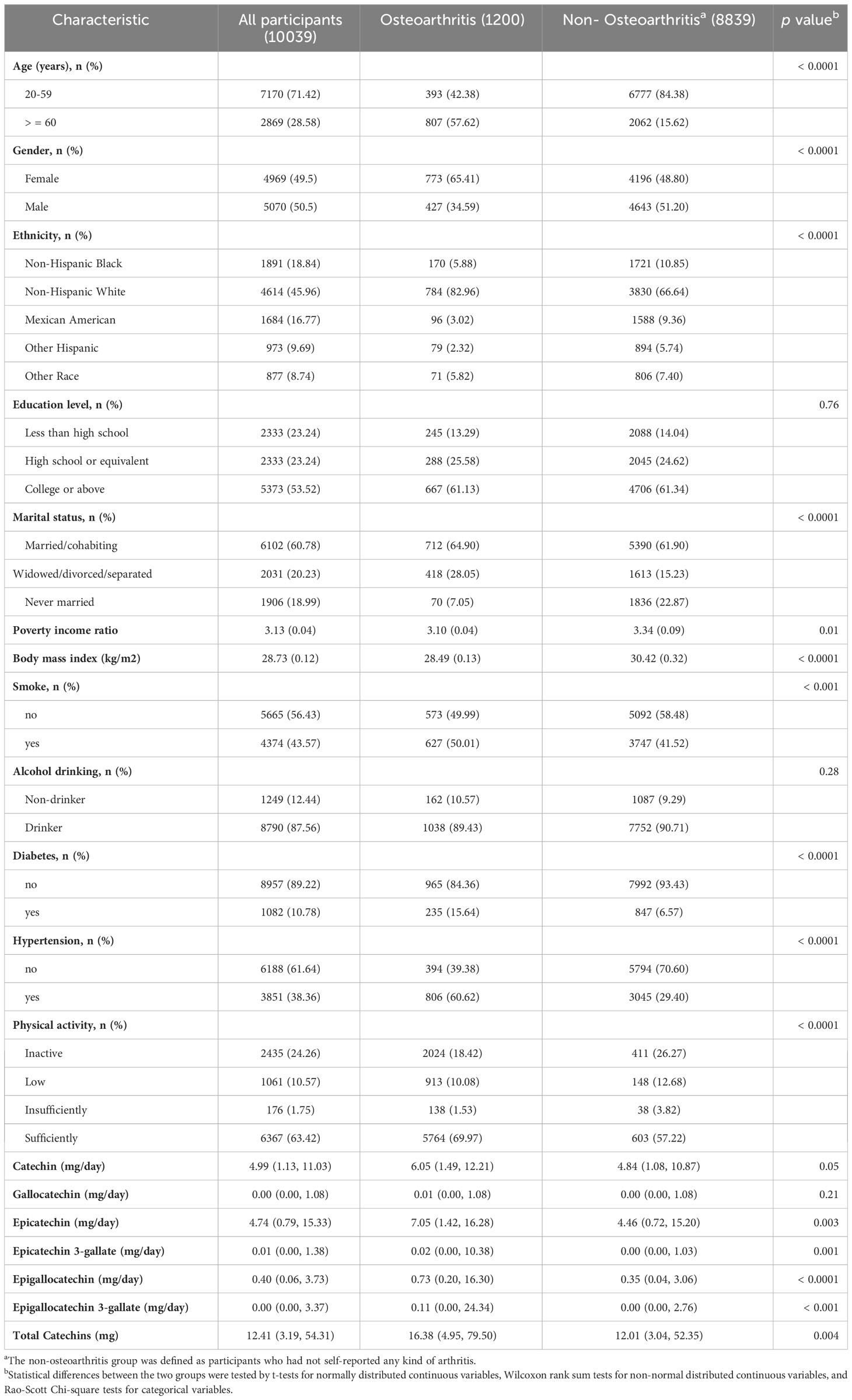
Table 1 Characteristics and catechins intake in participants stratified by OA in the US (NHANES 2007-2010 and 2017-2018).
The associations between six catechins subclass intakes and the prevalence of OA
Firstly, we used logistic regression models to analyze the single effect of each catechin subclass on the prevalence of OA (Table 2). Interestingly, we found, in model 3 adjusted for all covariates, intakes of epigallocatechin (P for trend = 0.02) and epigallocatechin 3-gallate (P for trend = 0.01) showed significant associations with OA in the third Qs. Compared to the Q 1 group, epigallocatechin (OR: 1.76, 95% CI: 1.28, 2.42) and epigallocatechin 3-gallate (OR: 1.338, 95% CI: 1.08, 1.77) in the Q 3 group presented a higher risk of OA. In addition, we further used the WQS regression model to analyze the mixed effects of total catechins subclass intakes on the risk of OA (Table 3), and the results showed that the WQS index had no statistical significance with OA risk reduction. Subsequently, we selected epigallocatechin and epigallocatechin 3-gallate for re-analysis, and the results showed that the WQS index was significantly correlated with increased OA risk (OR: 1.04, 95% CI: 1.00,1.08). The estimated weights of epigallocatechin and epigallocatechin 3-gallate in the WQS regression model are shown in Figure 1B.
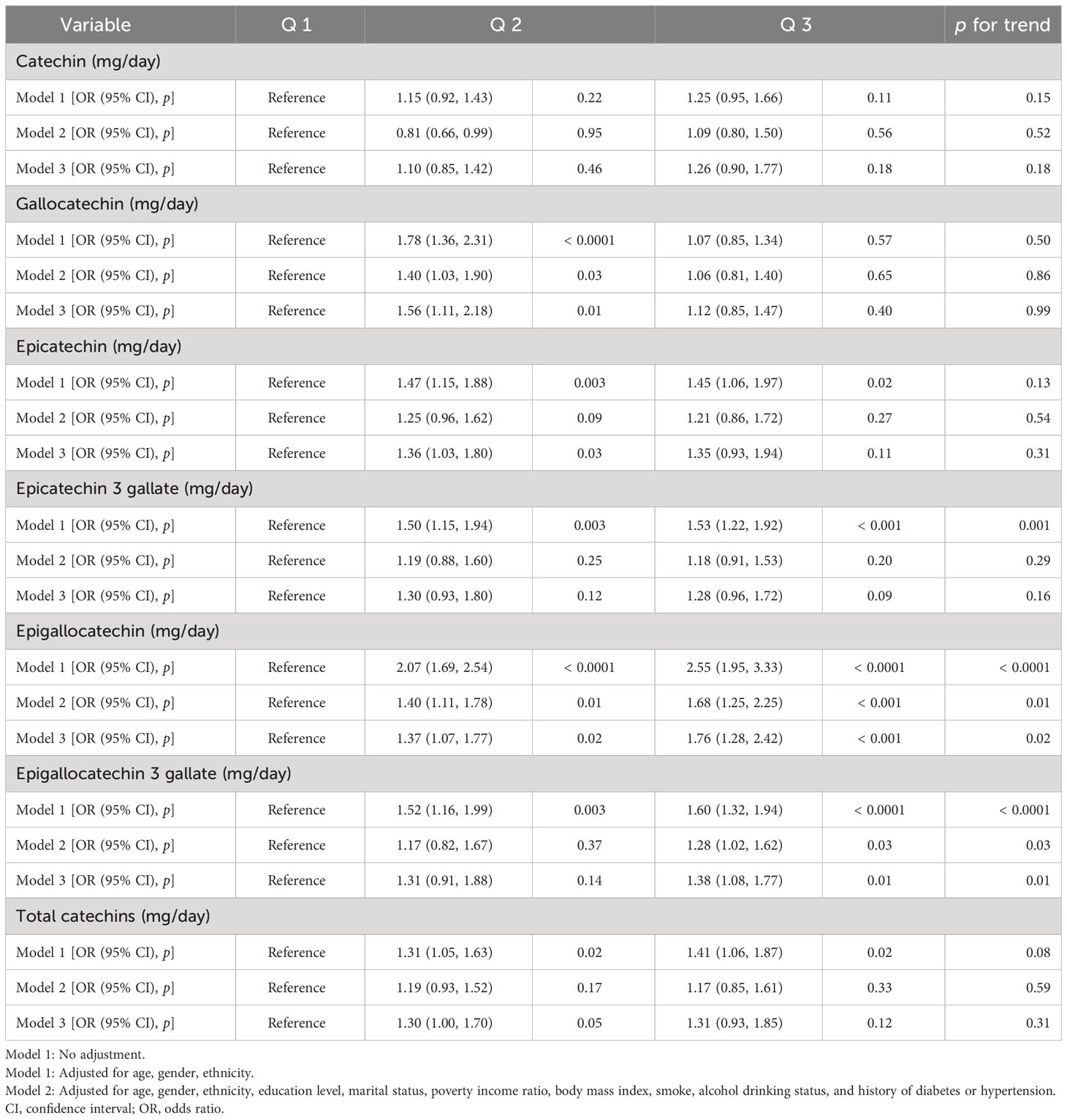
Table 2 The association between the catechins intake and osteoarthritis in the US (NHANES 2007-2010 and 2017-2018).
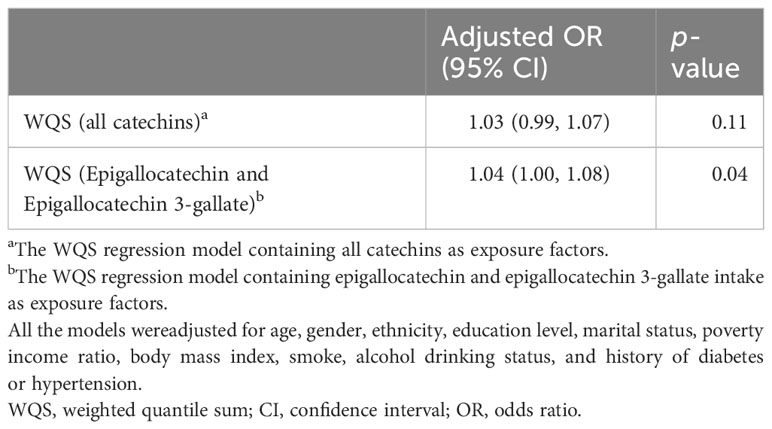
Table 3 The association between the catechins intake and osteoarthritis in the US by WQS analysis in the US (NHANES 2007-2010 and 2017-2018).
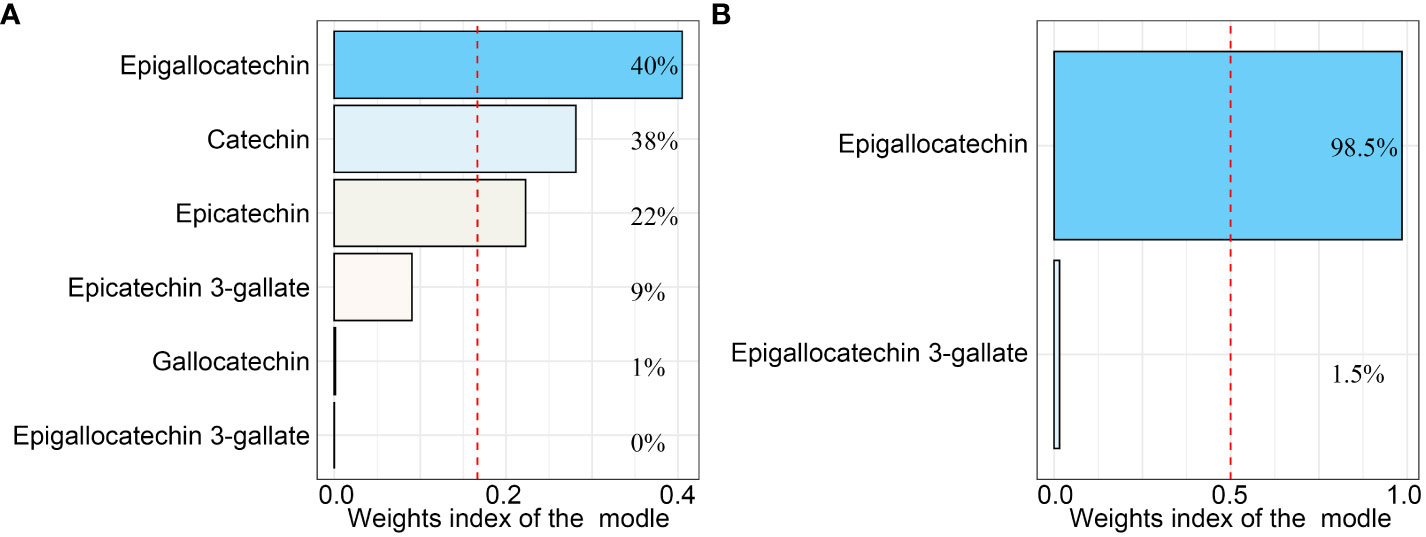
Figure 1 The weighted quantile sum (WQS) regression model index weights for the WQS regression model that included (A) all catechins and (B) only Epigallocatechin and Epigallocatechin 3-gallate. The red dashed line indicates the inverse of the number of exposed variables in the model. All the models were adjusted for age, gender, ethnicity, education level, marital status, poverty income ratio, body mass index, serum cotinine, alcohol drinking status, and history of diabetes or hypertension.
As shown in Figure 1, in the WQS regression model containing all catechins, the highest contribution to the WQS index was made by epigallocatechin (40%), and in the WQS regression model consisting of intakes of epigallocatechin and epigallocatechin 3-gallate, the highest contribution to the WQS index was still made by epigallocatechin (98.5%). Moreover, we further explored the associations with the regression coefficients assumed to be negative in the two WQS models. Unsurprisingly, the weight of Epigallocatechin in the models was zero and neither model is statistically significant (Figure S1 and Table S3). Furthermore, the restricted cubic spline (RCS) modes adjusted for all confounders demonstrated a nonlinear and J-shaped association between the intakes of epigallocatechin (P for non-linearity = 0.0016) and Epigallocatechin 3-gallate (P for non-linearity = 0.0004) and risk of OA (Figure 2). The risk of OA reached a nadir when epigallocatechin at approximately 32.70 mg/day and epigallocatechin 3-gallate at approximately 76.24 mg/day, followed by a gradual increase in OR with increasing daily intake.
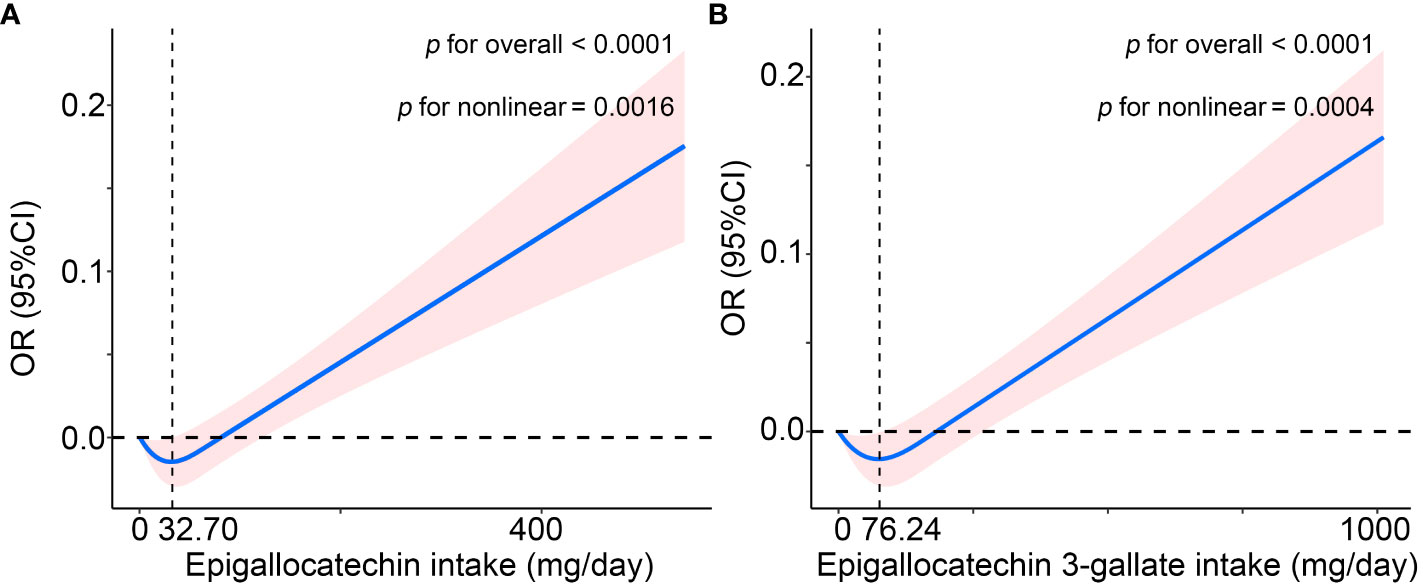
Figure 2 The non-linear association of osteoarthritis with (A) Epigallocatechin intake and (B) Epigallocatechin 3-gallate intake in the US (NHANES 2007-2010 and 2017-2018), using the restricted cubic spline (RCS) regression analysis. The solid red line is the OR value and the shadowed area is the corresponding 95% CI. The model was adjusted for age, gender, ethnicity, education level, marital status, poverty income ratio, body mass index, serum cotinine, alcohol drinking status, and history of diabetes or hypertension. CI, confidence interval; OR, odds ratio.
Subgroup analysis
In the subgroup analyses, we stratified all covariates and used multifactorial logistic regression models adjusted for all confounders except the variables themselves to analyze the association between daily dietary epigallocatechin and epigallocatechin 3-gallate intake and the prevalence of OA. In addition, a multiplicative interaction term was added to each model for testing potential interactions, and the results indicated no significant interactions between daily dietary intake of epigallocatechin and epigallocatechin 3-gallate and the stratification variables (Tables S1, 2).
Interaction effect between physical activity and epigallocatechin intake on the prevalence of OA
In the interaction assay, as shown in Table 4, we found a significant moderating effect of PA in the relationship between epigallocatechin intake and the prevalence of OA (P for interaction = 0.03), whereas this interaction was not observed in the relationship between epigallocatechin 3-gallate and OA prevalence (P for interaction = 0.58). Specifically, in the Low PA group, the results of the multivariate-adjusted logistic regression model showed ORs (95%CI) were 0.02 (0.00, 0.21) and 0.05 (0.01, 0.27) for Q 2 and 3, respectively, compared to the Q 1 group (Figure 3). While in the sufficiently PA group, with Q 1 group being the reference, the ORs (95%CI) were Q 2 1.55 (1.09, 2.22) and Q 3 2.03 (1.25, 3.30). In addition, the results of the trend test were statistically significant in the Low PA group (P for trend = 0.004) and sufficiently PA group (P for trend = 0.03).
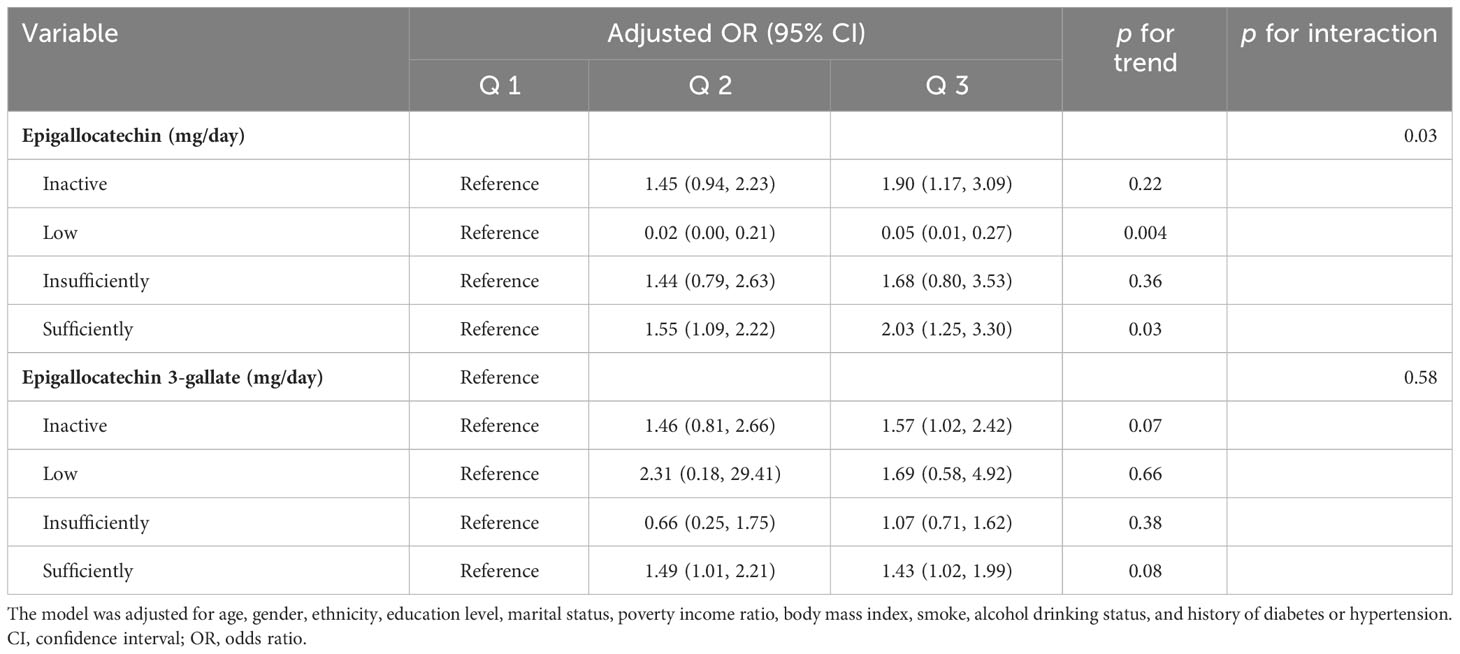
Table 4 Interaction effect between physical activity and Epigallocatechin or Epigallocatechin 3-gallate intake on the risk of osteoarthritis in the US (NHANES 2007-2010 and 2017-2018).
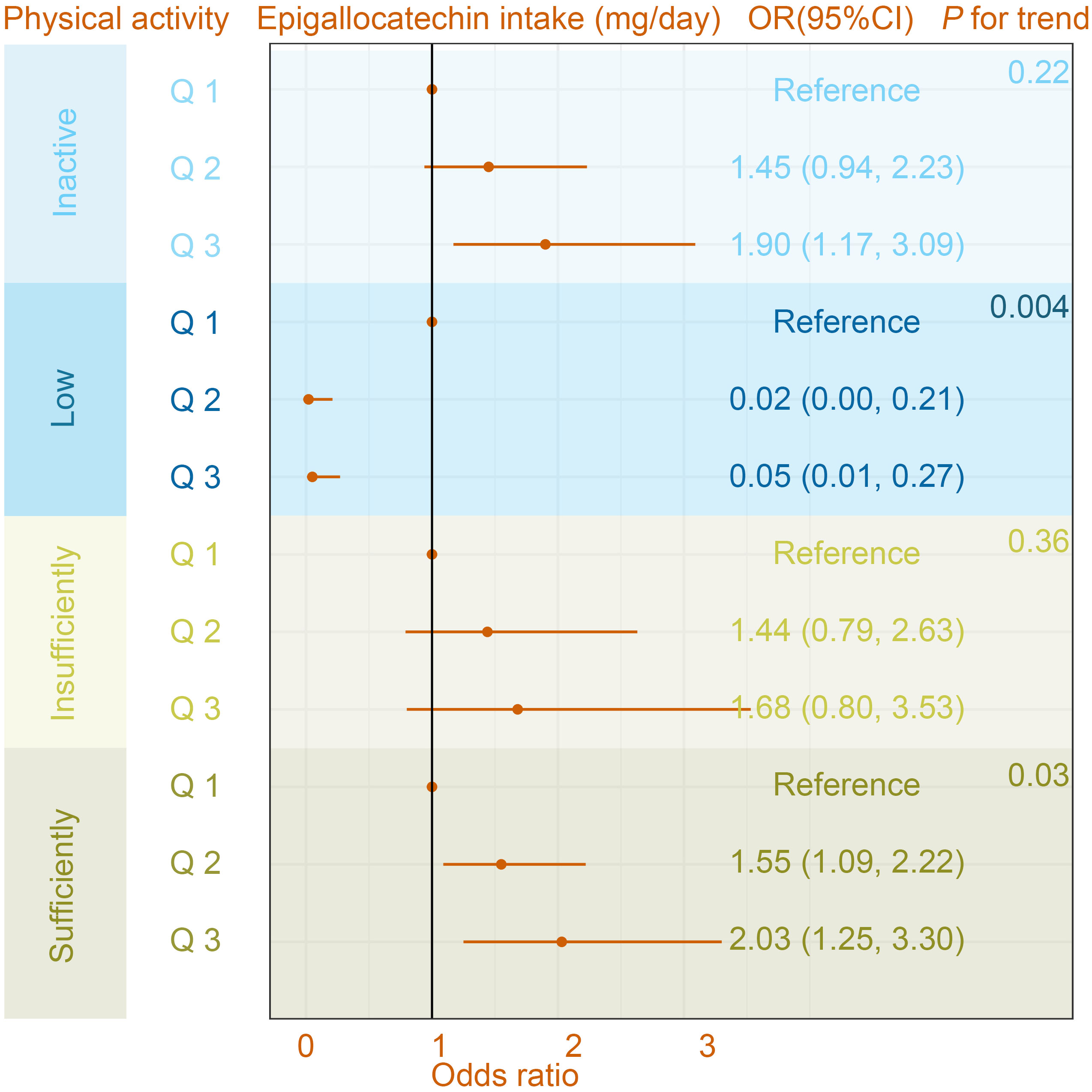
Figure 3 The association between osteoarthritis and Epigallocatechin intake in different physical activity subgroups. The model was adjusted for age, gender, ethnicity, education level, marital status, poverty income ratio, body mass index, serum cotinine, alcohol drinking status, and history of diabetes or hypertension. CI, confidence interval; OR, odds ratio.
Discussion
In this study, the relationship between dietary catechins intake and the prevalence of OA was investigated for the first time, using the study cohort of 10039 participants from the NHANES database, including both OA and non-OA individuals. Our results suggest that a J-shaped nonlinearly correlation between the intakes of epigallocatechin and epigallocatechin 3-gallate with the risk of OA, in which PA played a significant moderating effect.
Most studies have found that catechins have a positive effect on OA treatment. The mechanisms are not fully understood, but several possible mechanisms have been suggested. Catechins could up-regulate the expression of nuclear factor erythrocyte 2-related factor 2 (Nrf2), oxygenase 1 (HO-1), NADPH quinone oxidoreductase 1 (NQO1), and other antioxidant enzymes, and improve the oxidative stress-induced chondrocyte dysfunction (26). Moreover, catechins can effectively clear excessive ROS in cells, significantly reduce the expression of pro-inflammatory cytokines, reduce the expression of M1-type macrophages, and show an excellent promotion effect on the transformation of macrophages to M2 phenotype (27, 28). However, the relationship between catechins intake and the risk of OA has not been evaluated in any study. In this research, we explored the associations between six catechins subclasses and the prevalence of OA, with multifactorial logistic regression and WQS regression model, and found that excessive intake of epigallocatechin and epigallocatechin 3-gallate increases the risk of OA in the general US population.
Several experimental animal studies and epidemiological studies have shown that tea polyphenols have dose-dependent toxicology and low and medium doses (0.01-0.25%) of tea polyphenols show beneficial effects in the large intestine, liver, and kidney (29). Conversely, a high dietary dose (0.5-1%) of GTP reduced the expression of antioxidant enzymes and heat shock protein (HSP), leading to the worsening of colitis and colorectal cancer in mice, and also causing liver and kidney dysfunction (30). In addition, there have been case reports that excessive consumption of tea extract can cause liver damage (31, 32). It has been suggested that this may be related to the properties of tea polyphenols. Mechanistically, studies have found that tea polyphenols can produce reactive oxygen species (ROS) through auto-oxidation. Low and medium doses of tea polyphenols produce low levels of ROS, which can activate Nrf2 to reduce oxidative stress, while high doses of tea polyphenols produce high levels of ROS, leading to apoptosis and tissue damage (33–35). All the above research evidence suggests that the daily intake of dietary catechins should take into account the complementary and toxicological effects of dose relationships. Noteworthy, our findings suggest that although catechins have some adjunctive therapeutic effects in the OA population, gallocatechin intake greater than 32.70 mg/d or gallocatechin 3-gallate intake greater than 76.24 mg/d significantly increased the risk of OA in the general population.
Interestingly, in the present study, using the WQS regression model, we first explored the mixed effect of intake of all catechins on the prevalence of OA and the results showed that this model was not associated with the prevalence of OA. Subsequently, we focused on the mixed effect of intake of epigallocatechin and epigallocatechin 3-gallate, and unsurprisingly, there was significance between the model and the prevalence of OA. Therefore, the results of the WQS regression model showed that the overall effect of all six catechins subclasses or epigallocatechin and epigallocatechin 3-gallate mainly resulted from Epigallocatechin suggesting that Epigallocatechin intakes are more important for studying the prevalence of OA in the general U.S. population. Not alone, we found that there was no significant interaction effect in the intake of epigallocatechin and OA prevalence in age, gender, and ethnicity subgroups, while a significant interaction effect was found in the PA subgroup. According to the 2018 Physical Activity (PA) Guidelines from the U.S. Department of Health and Human Services (DHHS), maintaining a moderate intensity of physical activity each week can reduce OA risk and also have a positive effect on OA recovery (18, 36). Many reports have shown that high-intensity exercise itself is easy to cause joint strain, which has shown that high-intensity exercise is an increased risk of OA (19, 37, 38). Similarly, our study found that intake of epigallocatechin hardly affected the protective effect of low PA on the risk of OA in the Low PA group. However, in the Sufficiently PA group, the prevalence of OA was significantly higher in the epigallocatechin Q 3 group compared to the Q 1 group, and OA prevalence increased with the higher daily intake of epigallocatechin.
This study is a relatively large population study using three complementary methods to reveal the relationship between dietary catechin intake and the prevalence of OA in American adults. Under the premise that dietary catechins are now recommended as natural health products in daily life, we first found that excessive daily catechins intake will lead to an increase in the risk of OA. However, further large-scale prospective studies and clinical trials are needed to confirm our findings and their underlying mechanisms. In addition, there are some limitations to our study. First, we used data from a cross-sectional survey. The assessment of dietary catechin intake in this study can only reflect current intake status, but OA is a long-term developing disease, which may have biased our results. Second, dietary catechins intake data were collected through a 24-hour dietary recall survey, which could lead to recall bias. Third, our analysis was unable to conclude a causal relationship between dietary catechin intake and OA. Fourth, our population inclusion is limited by the NHANES database. It is unclear whether the relationship between dietary catechin intake and OA applies to other populations.
Conclusion
In summary, the results of this study suggested that epigallocatechin intake greater than 32.70 mg/d or epigallocatechin 3-gallate intake greater than 76.24 mg/d significantly increases the risk of OA in the general US population. In addition, PA showed a significant moderating effect on the relationship between epigallocatechin intake and the prevalence of OA.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
YF: Conceptualization. LL: Conceptualization. JG: Writing – original draft. FW: Writing – review & editing. ZZ: Writing – review & editing. YZ: Project administration.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Project supported by: Science&Technology Department of Xinjiang Uygur Autonomous Region (Grant No. 22022D01F100).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1287856/full#supplementary-material
References
1. Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol (2016) 12(10):580–92. doi: 10.1038/nrrheum.2016.136
2. Krasnokutsky S, Attur M, Palmer G, Samuels J, Abramson SB. Current concepts in the pathogenesis of osteoarthritis. Osteoarthritis Cartilage (2008) 16 Suppl 3:S1–3. doi: 10.1016/j.joca.2008.06.025
3. Safiri S, Kolahi AA, Smith E, Hill C, Bettampadi D, Mansournia MA, et al. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis (2020) 79(6):819–28. doi: 10.1136/annrheumdis-2019-216515
4. Cisternas MG, Murphy L, Sacks JJ, Solomon DH, Pasta DJ, Helmick CG, et al. Alternative methods for defining osteoarthritis and the impact on estimating prevalence in a US population-based survey. Arthritis Care Res (Hoboken) (2016) 68(5):574–80. doi: 10.1002/acr.22721
5. Middleton E Jr. Effect of plant flavonoids on immune and inflammatory cell function. Adv Exp Med Biol (1998) 439:175–82. doi: 10.1007/978-1-4615-5335-9_13
6. Patane GT, Putaggio S, Tellone E, Barreca D, Ficarra S, Maffei C, et al. Catechins and proanthocyanidins involvement in metabolic syndrome. Int J Mol Sci (2023) 24(11). doi: 10.3390/ijms24119228
7. Russo C, Maugeri A, Lombardo GE, Musumeci L, Barreca D, Rapisarda A, et al. The second life of citrus fruit waste: A valuable source of bioactive compounds. Molecules (2021) 26(19). doi: 10.3390/molecules26195991
8. Abbate F, Maugeri A, Laurà R, Levanti M, Navarra M, Cirmi S, et al. Zebrafish as a useful model to study oxidative stress-linked disorders: focus on flavonoids. Antioxid (Basel) (2021) 10(5). doi: 10.3390/antiox10050668
9. Barreca D, et al. Flavanones: Citrus phytochemical with health-promoting properties. Biofactors (2017) 43(4):495–506. doi: 10.1002/biof.1363
10. Barreca D, Mandalari G, Calderaro A, Smeriglio A, Trombetta D, Felice MR, et al. Citrus flavones: an update on sources, biological functions, and health promoting properties. Plants (Basel) (2020) 9(3). doi: 10.3390/plants9030288
11. Durazzo A, Lucarini M, Souto EB, Cicala C, Caiazzo E, Izzo AA, et al. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother Res (2019) 33(9):2221–43. doi: 10.1002/ptr.6419
12. Farhan M. Green tea catechins: nature’s way of preventing and treating cancer. Int J Mol Sci (2022) 23(18). doi: 10.3390/ijms231810713
13. Ahmad N, Mukhtar H. Green tea polyphenols and cancer: biologic mechanisms and practical implications. Nutr Rev (1999) 57(3):78–83. doi: 10.1111/j.1753-4887.1999.tb06927.x
14. Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med (1992) 21(3):334–50. doi: 10.1016/0091-7435(92)90041-F
15. Ye Y, Zhou J. The protective activity of natural flavonoids against osteoarthritis by targeting NF-kappaB signaling pathway. Front Endocrinol (Lausanne) (2023) 14:1117489. doi: 10.3389/fendo.2023.1117489
16. Monika P, Chandraprabha MN, Murthy KNC. Catechin, epicatechin, curcumin, garlic, pomegranate peel and neem extracts of Indian origin showed enhanced anti-inflammatory potential in human primary acute and chronic wound derived fibroblasts by decreasing TGF-beta and TNF-alpha expression. BMC Complement Med Ther (2023) 23(1):181. doi: 10.1186/s12906-023-03993-y
17. Arokoski JP, Jurvelin JS, Väätäinen U, Helminen HJ. Normal and pathological adaptations of articular cartilage to joint loading. Scand J Med Sci Sports (2000) 10(4):186–98. doi: 10.1034/j.1600-0838.2000.010004186.x
18. Buckwalter JA, Martin JA. Sports and osteoarthritis. Curr Opin Rheumatol (2004) 16(5):634–9. doi: 10.1097/01.bor.0000132647.55056.a9
19. Lefèvre-Colau M-M, Nguyen C, Haddad R, Delamarche P, Paris G, Palazzo C, et al. Is physical activity, practiced as recommended for health benefit, a risk factor for osteoarthritis? Ann Phys Rehabil Med (2016) 59(3):196–206. doi: 10.1016/j.rehab.2016.02.007
20. Papavasiliou KA, Kenanidis EI, Potoupnis ME, Kapetanou A, Sayegh FE. Participation in athletic activities may be associated with later development of hip and knee osteoarthritis. Phys Sportsmed (2015) 39(4):51–9. doi: 10.3810/psm.2011.11.1939
21. Øiestad BE, Holm I, Gunderson R, Myklebust G, Risberg MA. Quadriceps muscle weakness after anterior cruciate ligament reconstruction: a risk factor for knee osteoarthritis? Arthritis Care Res (Hoboken) (2010) 62(12):1706–14. doi: 10.1002/acr.20299
22. Hall AC, Urban JP, Gehl KA. The effects of hydrostatic pressure on matrix synthesis in articular cartilage. J Orthop Res (1991) 9(1):1–10. doi: 10.1002/jor.1100090102
23. James MJ, Cleland LG, Gaffney RD, Proudman SM, Chatterton BE. Effect of exercise on 99mTc-DTPA clearance from knees with effusions. J Rheumatol (1994) 21(3):501–4.
24. Gouveia HJCB, Urquiza-Martínez MV, Manhaes-de-Castro R, Costa-de-Santana BJR, Villarreal JP, Mercado-Camargo R, et al. Effects of the treatment with flavonoids on metabolic syndrome components in humans: A systematic review focusing on mechanisms of action. Int J Mol Sci (2022) 23(15). doi: 10.3390/ijms23158344
25. Chen L, Cai M, Li HT, Wang XJ, Tian F, Wu YL, et al. Risk/benefit tradeoff of habitual physical activity and air pollution on chronic pulmonary obstructive disease: findings from a large prospective cohort study. BMC Med (2022) 20(1):70. doi: 10.1186/s12916-022-02274-8
26. Zhu WR, Tang H, Cao L, Zhang J, Li JC, Ma D, et al. Epigallocatechin-3-O-gallate ameliorates oxidative stress-induced chondrocyte dysfunction and exerts chondroprotective effects via the Keap1/Nrf2/ARE signaling pathway. Chem Biol Drug Des (2022) 100(1):108–20. doi: 10.1111/cbdd.14056
27. Li H, Xiang D, Gong C, Wang X, Liu L. Naturally derived injectable hydrogels with ROS-scavenging property to protect transplanted stem cell bioactivity for osteoarthritic cartilage repair. Front Bioeng Biotechnol (2022) 10:1109074. doi: 10.3389/fbioe.2022.1109074
28. Wei H, Qin J, Huang QX, Jin ZQ, Zheng L, Zhao JM, et al. Epigallocatechin-3-gallate (EGCG) based metal-polyphenol nanoformulations alleviates chondrocytes inflammation by modulating synovial macrophages polarization. BioMed Pharmacother (2023) 161:114366. doi: 10.1016/j.biopha.2023.114366
29. Bonkovsky HL. Hepatotoxicity associated with supplements containing Chinese green tea. Ann Intern Med (2006) 144:380–380. doi: 10.7326/0003-4819-144-1-200601030-00020
30. Murakami A. Dose-dependent functionality and toxicity of green tea polyphenols in experimental rodents. Arch Biochem Biophys (2014) 557:3–10. doi: 10.1016/j.abb.2014.04.018
31. Mazzanti G, Menniti-Ippolito F, Moro PA, Cassetti F, Raschetti R, Santuccio C, et al. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur J Clin Pharmacol (2009) 65(4):331–41. doi: 10.1007/s00228-008-0610-7
32. Salminen WF, Yang X, Shi Q, Greenhaw J, Davis K, Ali AA. Green tea extract can potentiate acetaminophen-induced hepatotoxicity in mice. Food Chem Toxicol (2012) 50(5):1439–46. doi: 10.1016/j.fct.2012.01.027
33. Song S, Huang Y-W, Tian Y, Wang X-J, Sheng J. Mechanism of action of (-)-epigallocatechin-3-gallate: auto-oxidation-dependent activation of extracellular signal-regulated kinase 1/2 in Jurkat cells. Chin J Nat Med (2014) 12(9):654–62. doi: 10.1016/S1875-5364(14)60100-X
34. Wei YQ, Chen PP, Ling TJ, Wang YJ, Dong RX, Zhang C, et al. Certain (-)-epigallocatechin-3-gallate (EGCG) auto-oxidation products (EAOPs) retain the cytotoxic activities of EGCG. Food Chem (2016) 204:218–26. doi: 10.1016/j.foodchem.2016.02.134
35. Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer (2009) 9(6):429–39. doi: 10.1038/nrc2641
36. Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) (2020) 72(2):149–62. doi: 10.1002/acr.24131
37. U.S. Department of Health and Human Services. Physical Activity Guidelines Advisory Committee report, 2008. To the Secretary of Health and Human Services. Part A: executive summary. Nutr Rev (2009) 67(2):114–20. doi: 10.1111/j.1753-4887.2008.00136.x
Keywords: osteoarthritis, catechins, physical activity, epigallocatechin, epigallocatechin 3-gallate
Citation: Fu Y, Li L, Gao J, Wang F, Zhou Z and Zhang Y (2024) J-shaped association of dietary catechins intake with the prevalence of osteoarthritis and moderating effect of physical activity: an American population-based cohort study. Front. Immunol. 14:1287856. doi: 10.3389/fimmu.2023.1287856
Received: 02 September 2023; Accepted: 15 December 2023;
Published: 08 January 2024.
Edited by:
Lei Zhang, University of Waterloo, CanadaReviewed by:
Owen Kelly, Sam Houston State University, United StatesJingmou Yu, Huzhou University, China
Copyright © 2024 Fu, Li, Gao, Wang, Zhou and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fazheng Wang, MTM4OTkxNTM3NjZAMTYzLmNvbQ==; Zihan Zhou, enpoMjExMDAxMTI1QHdtdS5lZHUuY24=; Yiwei Zhang, emhhbmd5aXdlaTIyNkAxNjMuY29t
†These authors have contributed equally to this work
 Yuesong Fu1†
Yuesong Fu1† Yiwei Zhang
Yiwei Zhang