- Department of Orthopedics, Second Affiliated Hospital, Chongqing Medical University, Chongqing, China
Background: Intervertebral disc degeneration (IVDD) is a prominent contributor to chronic low back pain, impacting millions of individuals annually. Current research on disc degeneration is placing a growing emphasis on the role of the immune system in this process. Nevertheless, the precise relationship between immunity and disc degeneration remains to be fully elucidated.
Method: We obtained GWAS data for immune cells from the latest summary-level GWAS, including 6,620 individuals from Sardinian and 746,667 individuals from five global populations. Summary results for IVDD were sourced from the FinnGen consortium, comprising 20,001 cases and 164,682 controls. We conducted a comprehensive univariable Mendelian randomization (MR) analysis to explore the potential causal relationship between immune cells and IVDD. Primary estimation was carried out using Inverse-Variance Weighting (IVW). To ensure robustness, we employed additional MR methods such as MR-Egger, Weighted Median, Weighted Mode, and Simple Mode. Various tests were employed to assess pleiotropy and heterogeneity, including the Cochran Q test, leave-one-out test, MR-Egger intercept analysis and MR-PRESSO test. To account for potential confounding factors among the immune cells, we conducted a multivariable MR analysis. Finally, we investigated the possibility of a reverse association between immune cells and IVDD through bidirectional MR.
Result: In total, our study identified 15 immune cells significantly associated with IVDD through univariable MR. Among these, 9 immune cell types were indicated as potential contributors to IVDD, while 6 were found to have protective effects. Importantly, we observed no evidence of heterogeneity or pleiotropy, signifying the robustness of our results. To mitigate confounding among immune cells, we utilized multivariable MR, leading to the discovery that only 9 immune cell types exerted independent effects on IVDD. These encompassed 7 as risk factors and 2 as protective factors. Additionally, our analysis revealed a bidirectional causal relationship between CD39+ CD4+ T cell %CD4+ T cell and IVDD.
Conclusion: Our findings suggest a connection between immune cells and the risk of IVDD, shedding light on potential therapeutic avenues for modulating immune cell function in individuals with IVDD. However, the specific underlying mechanisms warrant further investigation in future experiments.
1 Introduction
Intervertebral disc degeneration (IVDD) is a prevalent degenerative disease, with an incidence of 40-50 cases per 10,000 people (1). IVDD is a pathological condition that can lead to persistent back pain or neurological deficits. The incidence of IVDD is gradually increasing, posing an escalating healthcare burden and economic pressure (2, 3). Unfortunately the etiology of IVDD still remains unclear (4–6). It is reported that intervertebral disc belongs to the immune-privileged tissue (7–9). Extrinsic factors, such as external forces and mechanical loads, play a role in annulus fibrosus rupture, with the subsequent immune response triggered by nucleus pulposus antigen exposure serving as the initiator of disc degeneration (10–13). Nevertheless, the association between immune cells and IVDD remains unconfirmed due to the absence of randomized controlled trials (RCTs).
Mendelian Randomization (MR), a method examining potential causal relationships between exposure factors and disease outcomes, has gained popularity in recent years (14). Consequently, we chose to employ univariable, multivariable, and bidirectional MR approaches to identify potential protective and risk factors for IVDD. This study focused on exploring the possible causal links between immune cells and IVDD, with the aim of uncovering novel targets for IVDD prevention and treatment.
2 Materials and methods
2.1 Study design
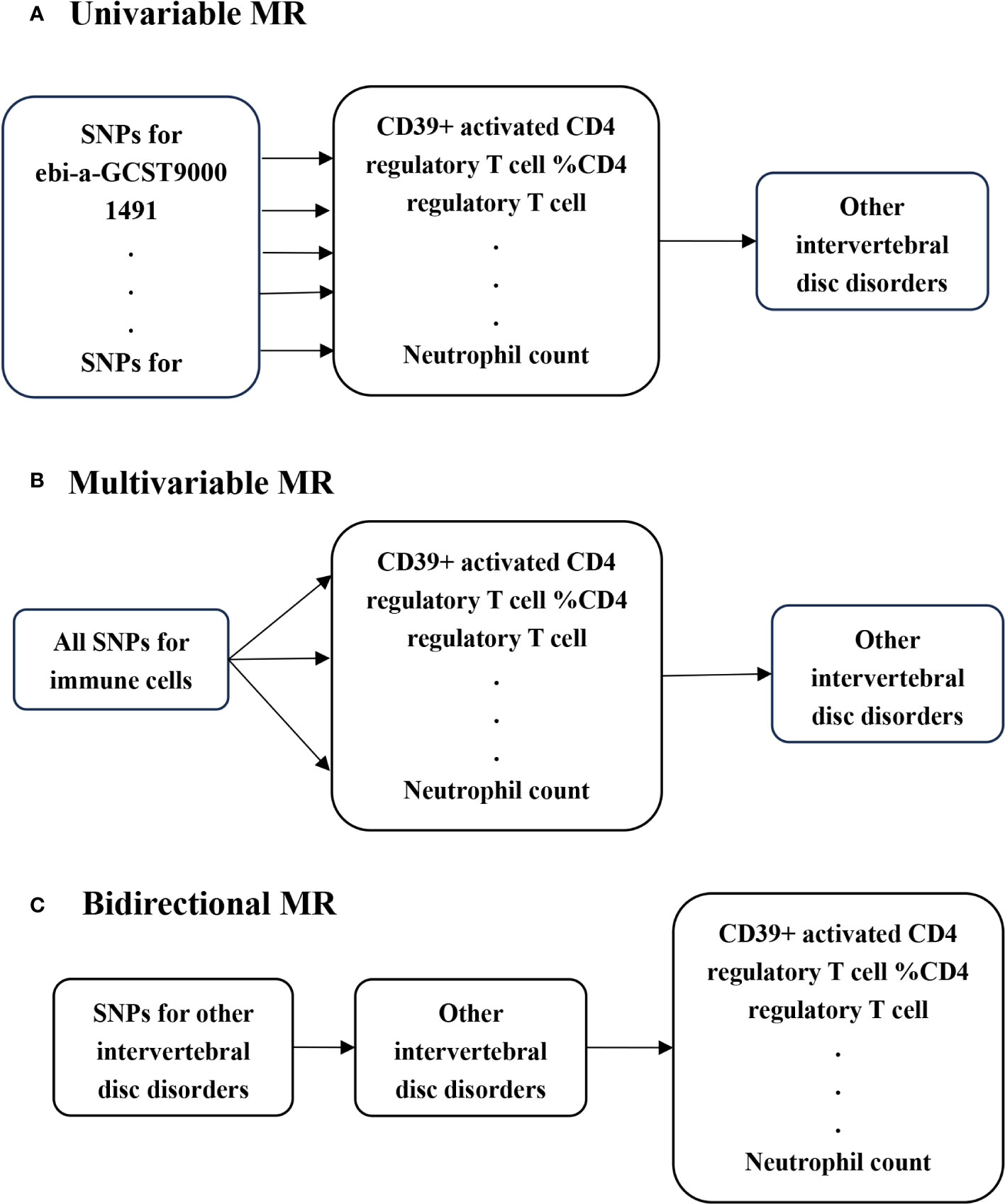
In this study, we conducted univariable MR to investigate potential causal associations between various immune cells and IVDD, utilizing genome-wide association studies (GWAS) summary statistics (Figure 1A). To ensure unbiased causal effects, MR analysis must adhere to three core assumptions: (I) the relevance assumption, where genetic variants are strongly associated with the exposure of interest; (II) the independence assumption, ensuring genetic variants are not associated with potential confounders; and (III) the exclusion restriction, stipulating that genetic variants affect outcomes solely through the exposure of interest (15). To further explore the link between immune cells and IVDD, we employed multivariable MR, assessing the independent beta of different immune cells, as these cells can influence each other’s effector values (Figure 1B). Additionally, we conducted bidirectional MR to investigate the possibility of reverse causality between the exposure and the outcome (14) (Figure 1C).
2.2 GWAS data source
The GWAS data on immune cells were obtained from the latest summary-level GWAS, which involved 6,620 individuals from Sardinian descent within the European population and 746,667 individuals from five diverse global populations (16, 17). The former study comprised 3,757 cases and 3,027 controls, with a gender distribution of 43% males and 57% females, and age ranges spanning from 18 to 102 years (16). The latter dataset included 408,961 individuals of European ancestry from the UK Biobank (UKBB), 143,988 individuals of Japanese ancestry from the Biobank of Japan (BBJ), and 5,275 African Americans from the Vanderbilt University Biobank (BioVU) (17).
The summary results for Intervertebral Disc Degeneration (IVDD) were acquired from the FinnGen consortium, specifically from the R9 release (https://www.finngen.fi/fi). This dataset encompasses 20,001 cases and 164,682 controls. IVDD diagnoses were based on the International Classification of Diseases, specifically ICD-10 (M51), ICD-9 722, and ICD-8 725 coding standards.
2.3 Instrumental variable selection
Genetic instrumental variables for immune cell exposures were carefully chosen based on a genome-wide significance threshold (p<5×10–8) (14). We employed the PLINK clumping method to assess the linkage disequilibrium (LD) among the selected SNPs, with LD defined as SNPs having an R2>0.001 and being within a 10,000 kb physical distance (14). SNPs identified to be in LD were subsequently excluded from further analysis. Additionally, to eliminate weak instrumental variables, we calculated F-statistics for the SNPs using a previously described approximation method (18, 19). Importantly, all included exposures exhibited F-statistics exceeding 10 [Supplementary Table 1].
2.4 Statistical analysis
We primarily employed the inverse-variance weighted (IVW) method as our main approach in Mendelian randomization (MR). In addition, we conducted MR-Egger, weighted median, weighted mode, and simple mode analyses to ensure the robustness of our results. To assess heterogeneity among summary estimates, Cochran’s Q test was utilized. Moreover, we utilized the MR-Egger intercept to address and account for pleiotropy (14). To further assess the robustness of our findings, we implemented a leave-one-out analysis. The MR-PRESSO test was employed to identify and address pleiotropy, and to remove outliers (20).
We conducted all statistical analyses using the “TwoSampleMR” (version 0.5.7) packages and MR-PRESSO package within the R statistical software (version 4.3.1). A significance level of p < 0.05 was chosen to denote statistical significance.
3 Result
3.1 Causal effect from blood immune cells on intervertebral disc degeneration
We undertook a comprehensive Mendelian randomization study aimed at uncovering the causal relationship between 744 immune cell types and IVDD [Supplementary Table 2]. In our analysis, we identified 24 specific blood types of immune cells that demonstrated a causal association with disc degeneration through univariable MR [Supplementary Table 3], including Memory B cell %B cell, Naive-mature B cell %B cell, IgD-CD38dim B cell %lymphocyte, CD39+activated CD4 regulatory T cell %CD4 regulatory T cell, CD39+ secreting CD4 regulatory T cell %CD4 regulatory T cell, CD45RA+ CD8+ T cell %CD8+ T cell, CD8+ T cell %leukocyte, CD39+CD4+ T cell %T cell, CD39+ CD4+ T cell %CD4+ T cell,CD39+ CD8+ T cell %T cell, CD39+ CD8+ T cell Absolute Count, CD127- CD8+ T cell Absolute Count, CD28- CD8+ T cell Absolute Count, CD19 on IgD+ CD38+ B cell, CD19 on IgD+ CD38dim B cell, CD19 on IgD- CD38- B cell, CD19 on unswitched memory B cell, CD19 on transitional B cell, CD24 on IgD+ CD38+ B cell,CD28 on CD39+ secreting CD4 regulatory T cell, CD25 on CD39+ CD4+ T cell, CD4 on CD39+ CD4+ T cell, CD4 on CD39+ resting CD4 regulatory T cell and Neutrophil count. We excluded certain exposures due to a limited number of available SNPs to prevent potential bias, including Memory B cell %B cell, Naive-mature B cell %B cell, IgD-CD38dim B cell %lymphocyte, CD8+T cell %leukocyte, CD127-CD8+ T cell Absolute Count, CD19 on IgD-CD38-B cell, CD24 on IgD+ CD38+ B cell, CD28 on CD39+ secreting CD4 regulatory T cell and CD4 on CD39+ resting CD4 regulatory T cell.
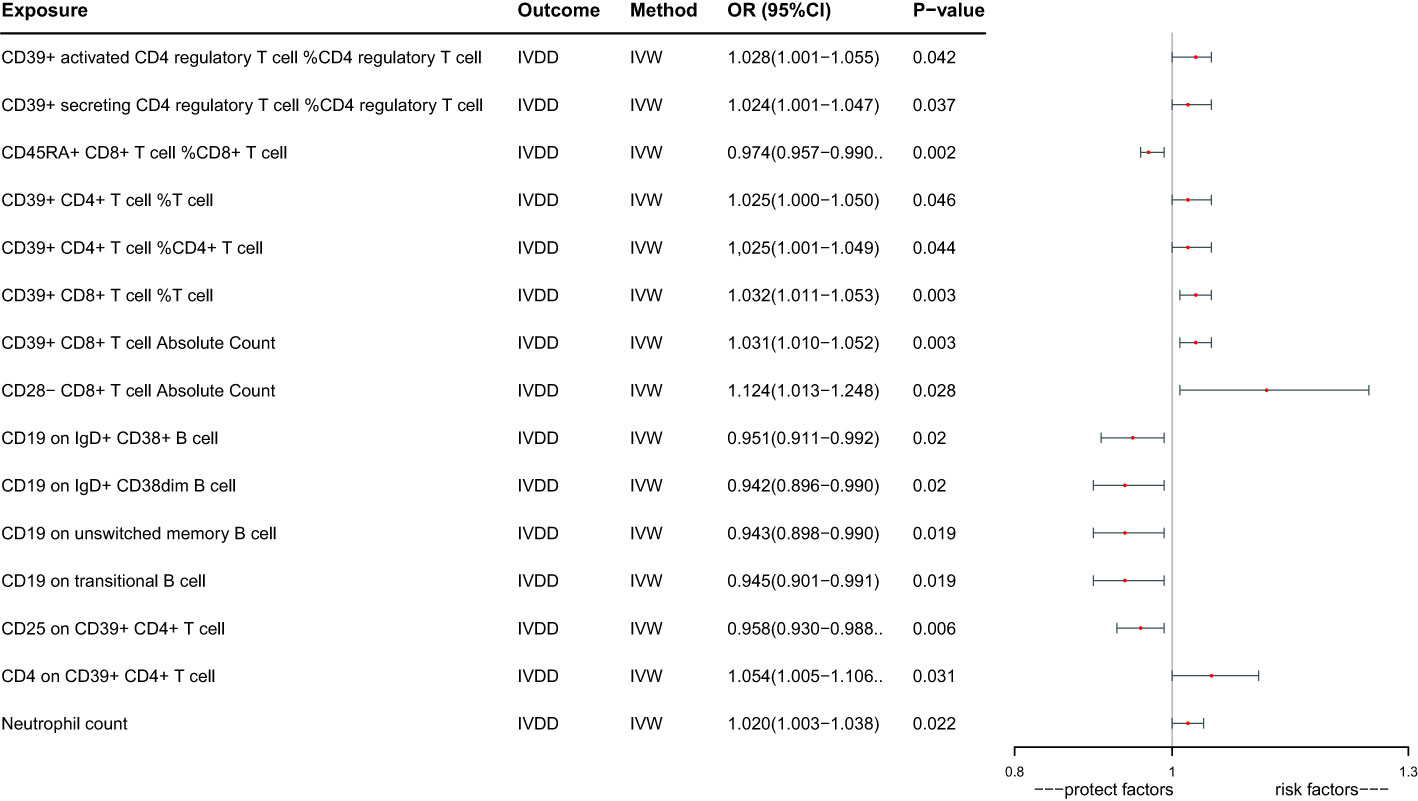
Ultimately, we identified 15 immune cells that have a potential causal relationship with IVDD (Figure 2). CD39+ activated CD4 regulatory T cell %CD4 regulatory T cell increase the risk of IVDD significantly (OR: 1.028, 95%CI:1.001-1.055, P=0.042), CD39+ secreting CD4 regulatory T cell %CD4 regulatory T cell increase the risk of IVDD significantly (OR: 1.024, 95%CI:1.001-1.047, P=0.037), CD45RA+ CD8+ T cell %CD8+ T cell decrease the risk of IVDD significantly(OR:0.974,95%CI: 0.957 -0.990, P=0.002),CD39+ CD4+ T cell %T cell increase the risk of IVDD significantly (OR: 1.025, 95%CI:1.000-1.050, P=0.046), CD39+ CD4+ T cell %CD4+ T cell increase the risk of IVDD significantly (OR:1.025, 95%CI:1.001-1.049, P=0.044), CD39+ CD8+ T cell %T cell increase the risk of IVDD significantly (OR:1.032, 95%CI:1.011-1.053,P=0.003), CD39+ CD8+ T cell Absolute Count increase the risk of IVDD significantly (OR:1.031, 95%CI:1.010-1.052,P=0.003), CD28- CD8+ T cell Absolute Count increase the risk of IVDD significantly (OR:1.124, 95%CI:1.013-1.248, P=0.028), CD19 on IgD+ CD38+ B cell decrease the risk of IVDD significantly (OR:0.951,95%CI:0.911-0.992,P=0.020), CD19 on IgD+ CD38dim B cell decrease the risk of IVDD significantly (OR:0.942, 95%CI:0.896- 0.990,P=0.020), CD19 on unswitched memory B cell decrease the risk of IVDD significantly(OR:0.943,95%CI:0.898 -0.990, P=0.019), CD19 on transitional B cell decrease the risk of IVDD significant (OR:0.945, 95%CI:0.901-0.991,P=0.019), CD25 on CD39+ CD4+ T decrease the risk of IVDD significantly (OR:0.958,95%CI:0.930 -0.988,P=0.006), CD4 on CD39+ CD4+ T cell increase the risk of IVDD significantly (OR:1.054, 95%CI:1.005-1.106,P=0.031), Neutrophil count increase the risk of IVDD significantly (OR:1.020, 95%CI:1.003-1.038, P=0.022).
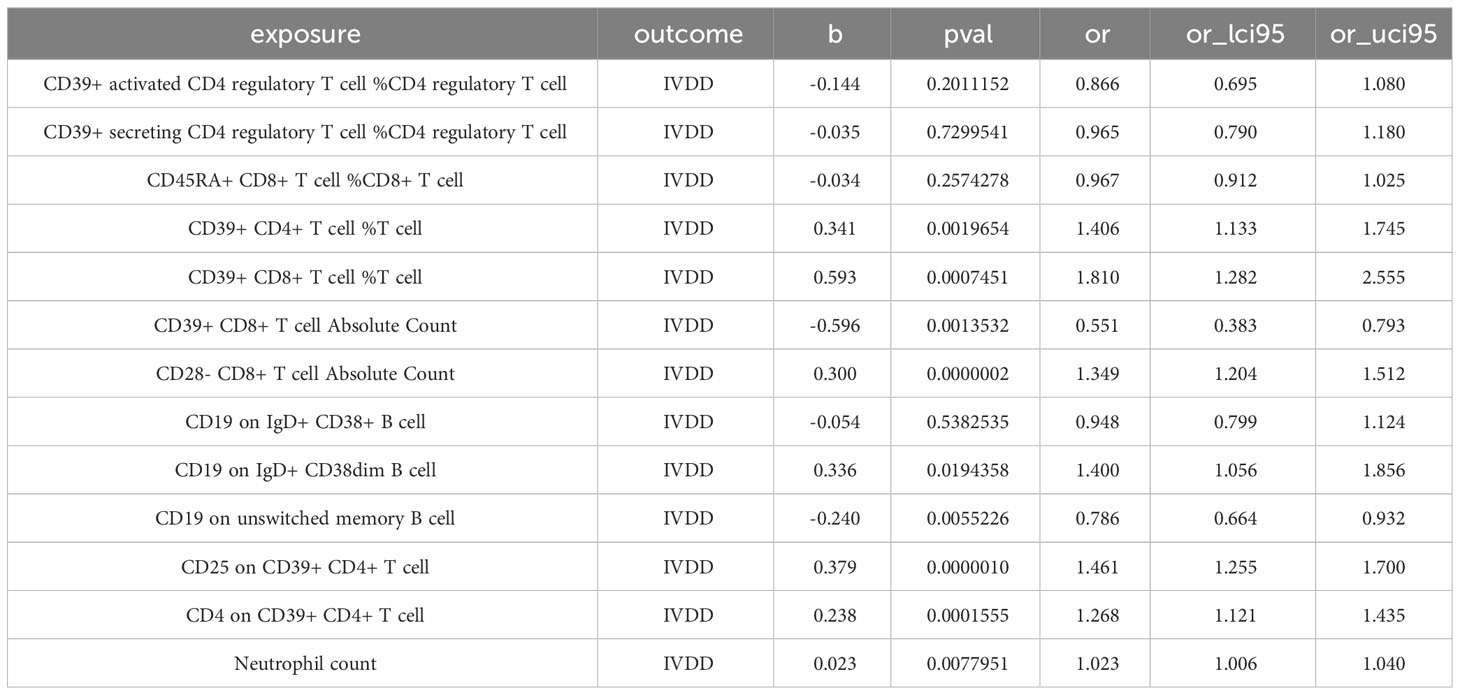
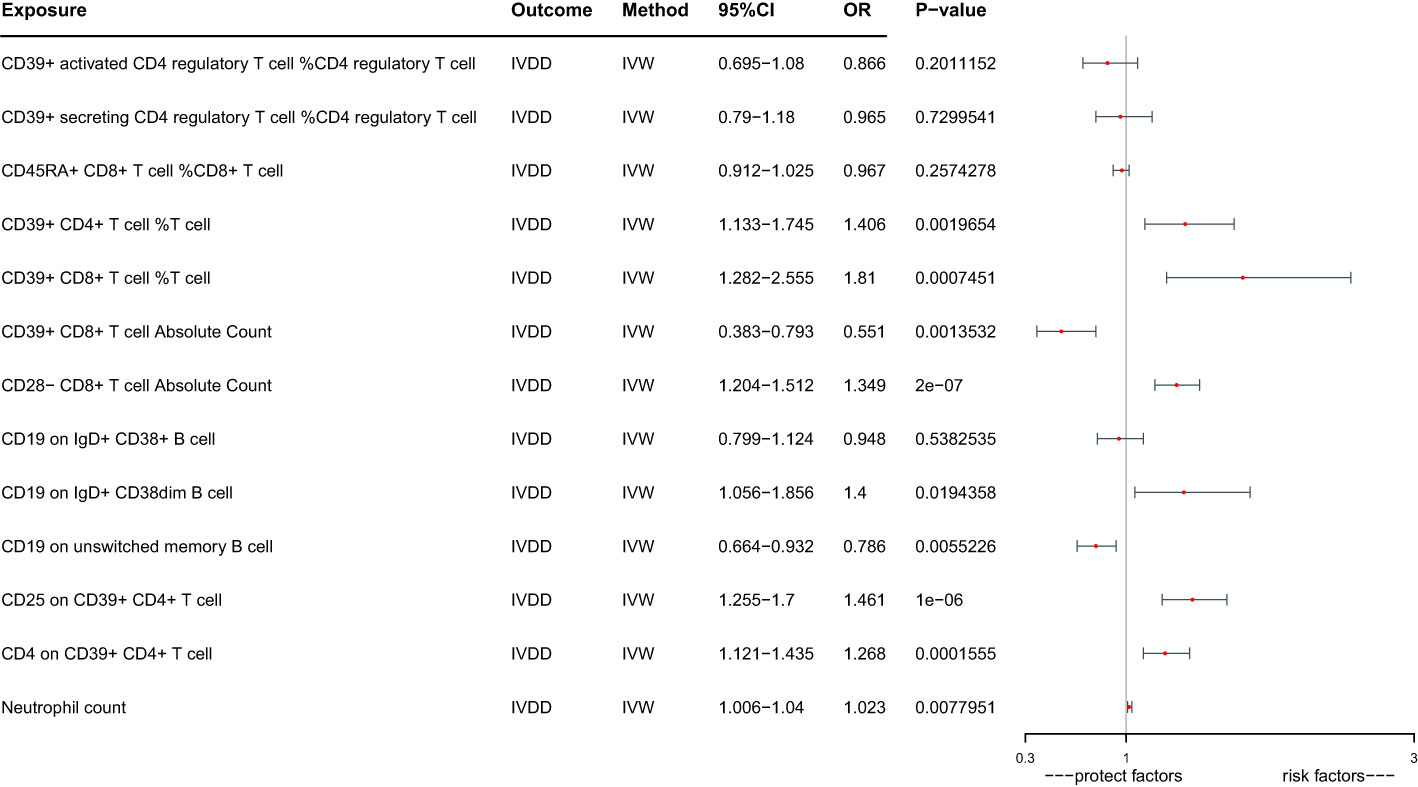
Given the interrelated nature of various immune cells, they have the potential to act as confounding factors for each other. Hence, we employed multivariable MR to estimate the independent direct effect of each exposure on the outcome. Our analysis revealed that only 9 immune cell types maintained an independent role when accounting solely for the effect of each exposure on the outcome (Table 1; Figure 3). CD39+ CD4+ T cell %T cell increase the risk of IVDD significantly (OR:1.406, 95%CI: 1.133-1.745). CD39+ CD8+ T cell %T cell increase the risk of IVDD significantly (OR: 1.810, 95%CI: 1.282-2.555). CD39+ CD8+ T cell Absolute Count decrease the risk of IVDD significantly (OR: 0.551, 95%CI: 0.383-0.793). CD28- CD8+ T cell Absolute Count increase the risk of IVDD significantly (OR: 1.394, 95%CI: 1.204-1.512). CD19 on IgD+ CD38dim B cell increase the risk of IVDD significantly (OR: 1.400, 95%CI: 1.056-1.856). CD19 on unswitched memory B cell decrease the risk of IVDD significantly (OR: 0.786, 95%CI: 0.664-0.932). CD25 on CD39+ CD4+ T cell increase the risk of IVDD significantly (OR: 1.461, 95%CI: 1.255-1.700).CD4 on CD39+ CD4+ T cell increase the risk of IVDD significantly (OR:1.268, 95%CI: 1.121-1.435). Neutrophil count increases the risk of IVDD significantly (OR: 1.023, 95%CI: 1.006-1.040). After controlling for confounding factors, the remaining six immune cell types no longer exhibit independent effects. Interestingly, in the aftermath of multivariate Mendelian Randomization, CD39+ CD8+ T cell Absolute Count, CD19 on IgD+ CD38dim B cell, and CD25 on CD39+ CD4+ T cell exhibited opposing effects on IVDD (Figures 2, 3).
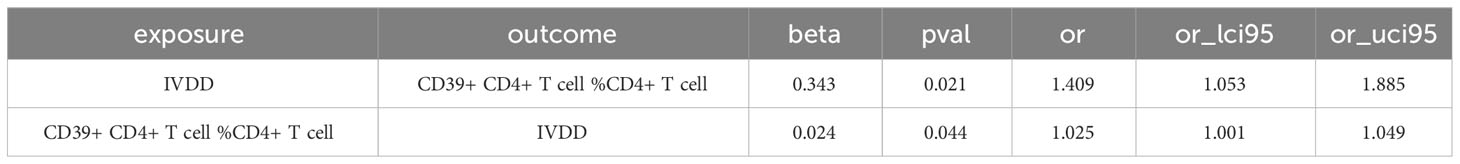
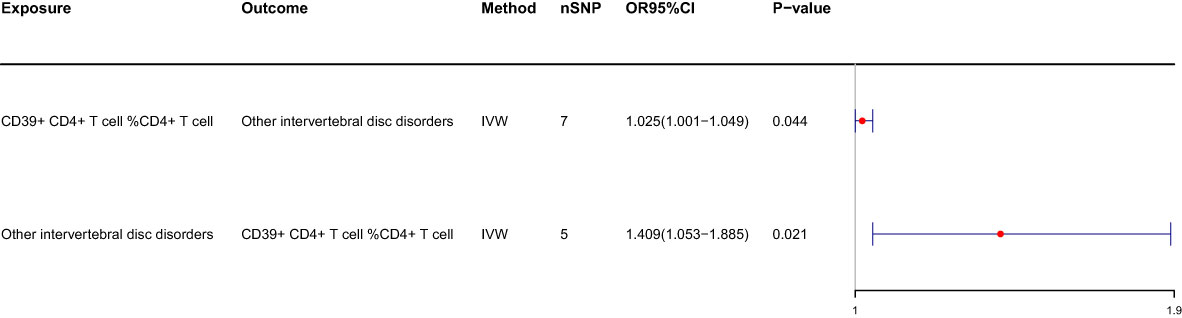
We conducted a reverse MR analysis and identified reverse causality specifically between CD39+ CD4+ T cells %CD4+ T cells and IVDD. Additionally, we observed that other intervertebral disc disorders significantly increase the risk of CD39+ CD4+ T cell %CD4+ T cell (OR: 1.409, 95%CI: 1.053-1.885, p=0.021) (Table 2; Figure 4). The causal relationship between these factors appears to be bidirectional.
3.2 Sensitivity analyses
To ensure the robustness of our MR estimates, we conducted several sensitivity analyses. We employed Cochran’s Q test, MR-Egger intercept test, and a leave-one-out test. Notably, all MR-Egger intercept tests yielded P-values greater than 0.05, indicating the absence of pleiotropy. Additionally, the Cochran’s Q test revealed no significant heterogeneity between immune cells and IVDD (Table 3). Furthermore, the results remained stable in the leave-one-out test (Figure 5). The MR-PRESSO analysis revealed the absence of outliers and pleiotropy, as illustrated in Table 3. However, the MR-PRESSO test could not be applied to the CD28-CD8+ T cell Absolute Count due to the limited number of SNPs. In summary, the results of the univariable MR analysis are deemed acceptable.
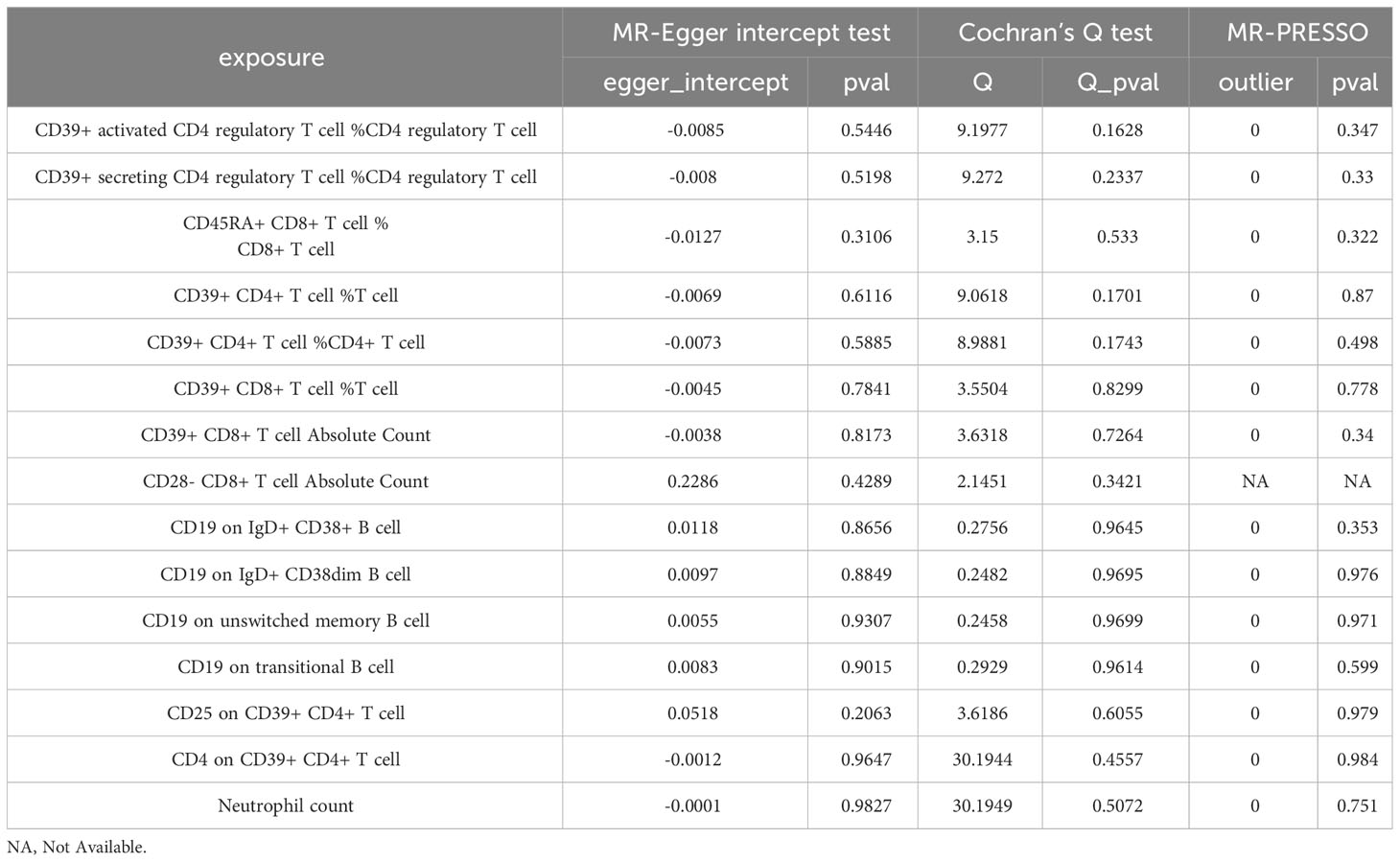
Table 3 Results of sensitivity analysis of blood immune cells on the risk of intervertebral disc disorder.
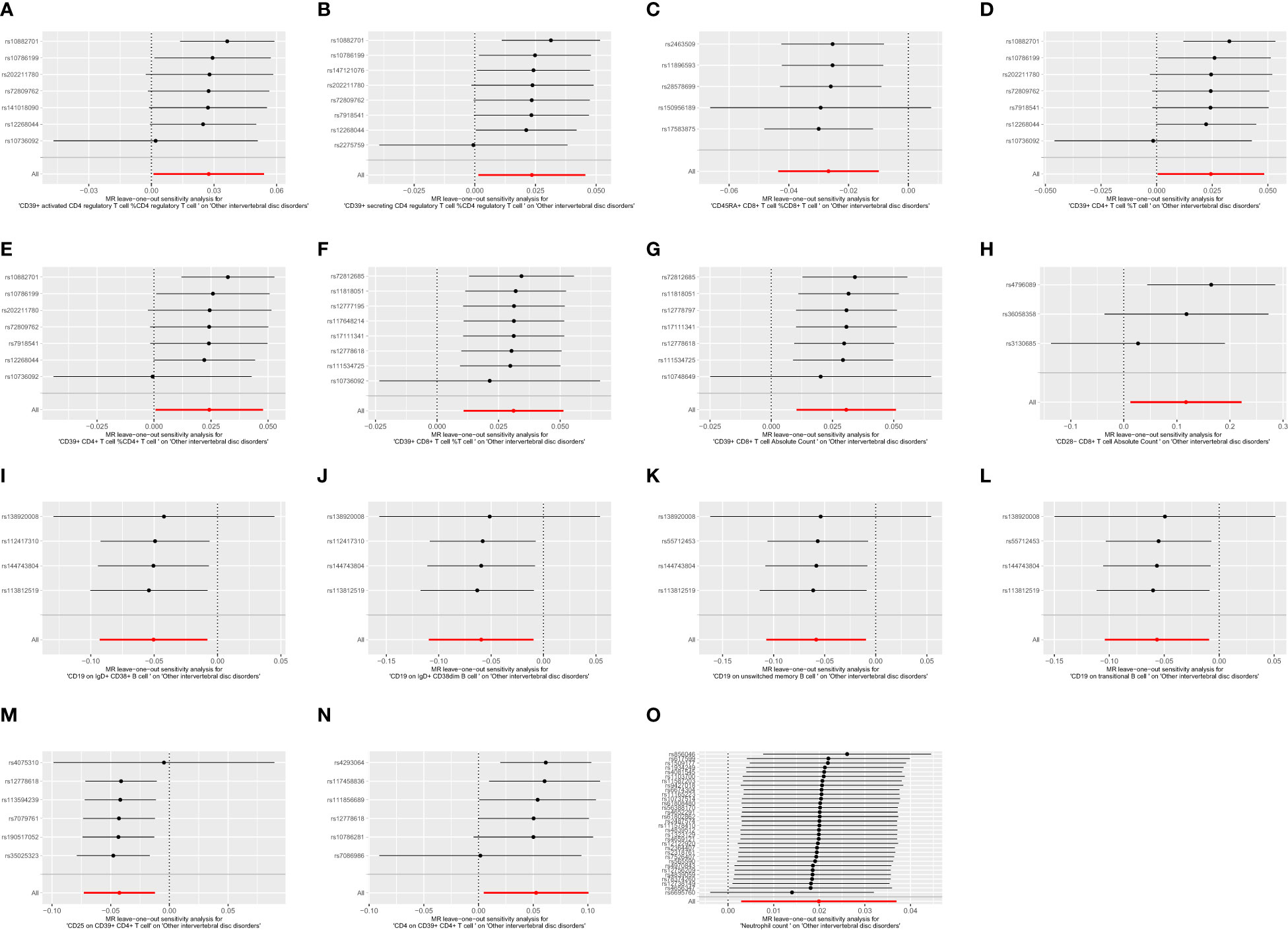
Figure 5 The leave one out plot of Mendelian randomization analyses. (A):CD39+ activated CD4 regulatory T cell %CD4 regulatory T cell; (B):CD39+ secreting CD4 regulatory T cell %CD4 regulatory T cell; (C):CD45RA+ CD8+ T cell %CD8+ T cell; (D):CD39+ CD4+ T cell %T cell; (E):CD39+ CD4+ T cell %CD4+ T cell; (F):CD39+ CD8+ T cell %T cell; (G):CD39+ CD8+ T cell Absolute Count; (H):CD28- CD8+ T cell Absolute Count; (I):CD19 on IgD+ CD38+ B cell; (J):CD19 on IgD+ CD38dim B cell; (K):CD19 on unswitched memory B cell; (L):CD19 on transitional B cell; (M):CD25 on CD39+ CD4+ T cell; (N):CD4 on CD39+ CD4+ T cell; (O):Neutrophil count.
For the bidirectional MR analysis between CD39+ CD4+ T cell %CD4+ T cell and other intervertebral disc disorders, both the p-values of Cochran’s Q and MR-Egger intercept were greater than 0.05 (Table 4), suggesting the absence of heterogeneity and pleiotropy. The leave-one-out test reaffirmed the stability of these results (Figure 6). Pleiotropy and outliers were not observed in our results (Table 4). In summary, the bidirectional results remained stable.
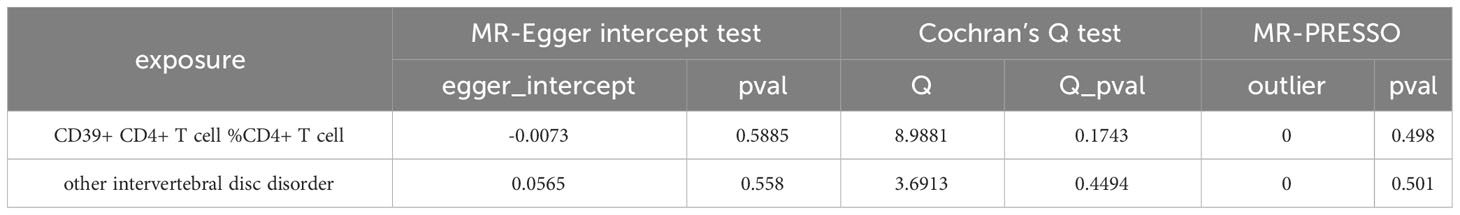
Table 4 Results of sensitivity analysis between other intervertebral disc disorder and the risk of CD39+ CD4+ T cell %CD4+ T cell.
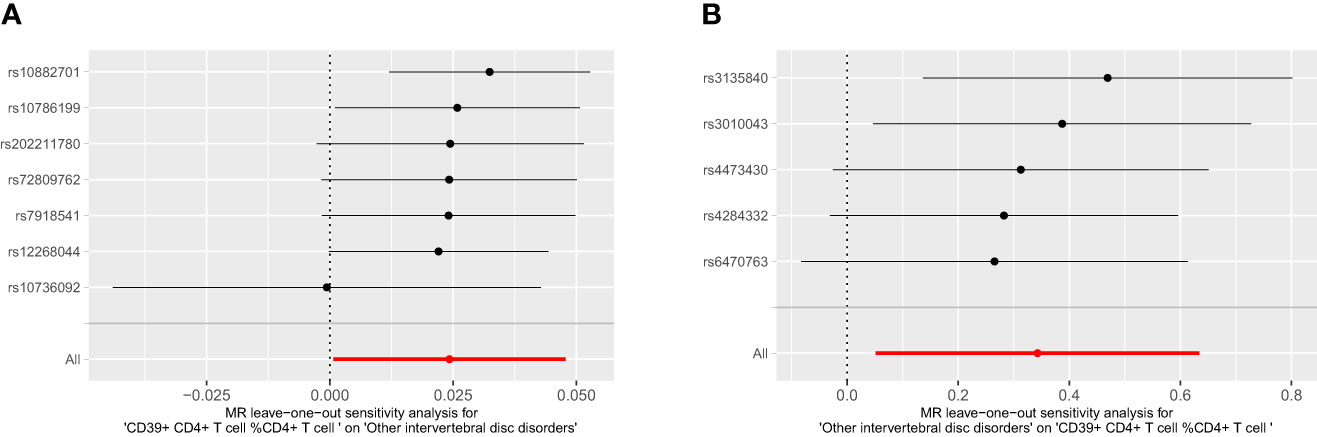
Figure 6 The leave one out plot of bidirectional MR between CD39+ CD4+ T cell % CD4+ T cell and IVDD. (A): CD39+ CD4+ T cell %CD4+ T cell, (B): other intervertebral disc disorder.
4 Discussion
In our comprehensive study, we conducted MR to investigate the causal relationship between 744 immune cells and IVDD. Our analysis identified 15 immune cells significantly associated with IVDD, encompassing 6 protective factors and 9 risk factors. We utilized multivariable MR to eliminate the potential confounding factors, revealing that only 9 immune cells, including 7 risk factors and 2 protective factors. Furthermore, a bidirectional causal relationship between IVDD and CD39+ CD4+ T cell %CD4+ T cell was established through bidirectional MR analysis.Our results indicated that immune cells also played protective factors for IVDD. These findings provided a brand new understanding of the relationship between immunity and IVDD.
First, we observed a significant relationship between CD39+ CD4+ T cell %T cell, CD39+ CD8+ T cell %T cell, CD4 on CD39+ CD4+ T cell, CD25 on CD39+ CD4+ T cell, and CD39+ CD8+ T cell Absolute Count with IVDD. CD39 plays a crucial role in the immune system. CD39+ CD4+ T cells are linked to the effector functions of T helper (Th)17, known for their role in fostering chronic inflammation and contributing to matrix degradation (21–23). The collaboration of CD39 and CD73 leads to the conversion of ATP to ADP and cAMP, ultimately generating adenosine (24). Adenosine can interact with multiple receptors, such as A1, A2A, A2B, and A3, inducing various immune responses (25). Adenosine stimulates immune responses through A1 and A3 receptors, while it has immunosuppressive effects when interacting with A2A and A2B receptors (25–27). These findings might be the reasons that CD39+ CD4+ T cell %T cell, CD39+ CD8+ T cell %T cell, CD4 on CD39+ CD4+ T cell and CD25 on CD39+ CD4+ T cell can accelerate the degeneration and CD39+ CD8+ T cell Absolute Count plays a protective role in IVDD. However, further exploration of underlying mechanisms is needed.
Furthermore, our findings indicated associations between CD19 on unswitched memory B cells and CD19 on IgD+ CD38dim B cells with IVDD. CD19 is essential for B-cell maturation and function (28, 29). Reduced CD19 expression can result in decreased immune function (30). CD19 is increasing in the patient of IVDD (31). However, unswitched memory B cells lack the ability to produce antibodies, making them incapable of initiating an immune attack on the disc. CD38 facilitates inflammation, cell migration, phagocytosis, and antigen presentation (32). CD19 on IgD+ CD38dim B cells appears to activate the immune response to the disc, thereby contributing to IVDD.
In our study, we found that a type of immune cell count called CD28- CD8+ T cells could increase the risk of IVDD.CD28 usually helps activate CD8 cells to boost the immune response (33). Surprisingly, even without CD28, CD8+ still contributes to IVDD (34). So we suppose that CD8 plays a more crucial role and CD28 might not be the main factor in IVDD.
Adjusted by multivariate MR, CD39+ CD8+ T cell Absolute Count and CD25 on CD39+ CD4+ T cells changed from protective to facilitating factors. CD19 on IgD+ CD38dim B cells changed from facilitating to protective factors. This may be due to the fact that the interference between immune cells leads to the masking of their true role for IVDDD. Consequently, it was only through multivariate MR that we unveiled their true independent effects.
We also discovered a bidirectional potential causal link between CD39+ CD4+ T cell %CD4+ T cell and IVDD. It is suggested that there is a vicious cycle between immune cells and IVDD. This is a consequence of the immune system being initiated by the deteriorated disc and the activated immune system have irreversible damage to the disc (10, 12, 35).
Our MR analysis offers several advantages. Firstly, we employed univariable, multivariable, and bidirectional MR, mitigating confounding factors and reverse causality. Secondly, we conducted numerous sensitivity analyses to validate our hypotheses and minimize bias. Thirdly, we addressed population stratification issues by restricting the test population.
However, our study has limitations. It may not be directly applicable to other populations. The sample size was limited, potentially introducing bias, necessitating larger samples for robust results. Through we have utilized the univariable MR, bidirectional MR and multivariable MR, there is also unmeasured pleiotropy. Lastly, we refrained from using multiple analysis corrections as our primary goal was to identify potential biomarkers or therapeutic targets for IVDD. We opted not to apply the Bonferroni correction in our analysis since our primary focus was to identify potential therapeutic and prophylactic targets associated with IVDD. Bonferroni’s criteria are quite stringent and could have resulted in the exclusion of meaningful indicators.
5 Conclusion
Our comprehensive MR analysis revealed 15 immune cells associated with IVDD risk through univariable MR. After accounting for confounding effects between immune cells, our multivariable MR identified only 9 immune cells as independent factors for IVDD, comprising 7 risk factors and 2 protective factors. These 9 immune cells can serve as potential biomarkers for IVDD, offering new perspectives on its treatment and prevention. Nonetheless, further experiments are needed to elucidate the underlying mechanisms.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
CQ: Formal analysis, Software, Data curation, Investigation, Resources, Validation, Visualization, Writing – original draft. MC: Writing – review & editing, Investigation, Software, Validation. QY: Methodology, Project administration, Writing – review & editing. XW: Software, Writing – review & editing, Supervision. TH: Writing – review & editing, Investigation, Software. BL: Investigation, Writing – review & editing, Resources. ZY: Writing – review & editing, Conceptualization, Methodology, Project administration. SC: Writing – review & editing, Conceptualization, Formal analysis, Methodology, Software.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1321295/full#supplementary-material
References
1. Court C, Mansour E, Bouthors C. Thoracic disc herniation: Surgical treatment. Orthop Traumatol Surg Res (2018) 104(1):S31–40. doi: 10.1016/j.otsr.2017.04.022
2. Wu A, March L, Xuanqi Z, Jinfeng H, Xiangyang W, Zhao J, et al. Global low back pain prevalence and years lived with disability from 1990 to 2017: estimates from the Global Burden of Disease Study 2017 - PMC. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7186678/.
3. Deyo RA, Cherkin D, Conrad D, Volinn E. Cost, controversy, crisis: low back pain and the health of the public. Annu Rev Public Health (1991) 12(1):141–56. doi: 10.1146/annurev.pu.12.050191.001041
4. Wang Y, Videman T, Battié MC. ISSLS prize winner: lumbar vertebral endplate lesions. Spine (2012) 37(17):1490–6. doi: 10.1097/BRS.0b013e3182608ac4
5. Shirazi-Adl A, Taheri M, Urban JPG. Analysis of cell viability in intervertebral disc: Effect of endplate permeability on cell population. J Biomech (2010) 43(7):1330–6. doi: 10.1016/j.jbiomech.2010.01.023
6. Adams MA, Freeman BJC, Morrison HP, Nelson IW, Dolan P. Mechanical initiation of intervertebral disc degeneration. Spine (2000) 25(13):1625–36. doi: 10.1097/00007632-200007010-00005
7. Di Martino A, Merlini L, Faldini C. Autoimmunity in intervertebral disc herniation: from bench to bedside. Expert Opin Ther Targets (2013) 17(12):1461–70. doi: 10.1517/14728222.2013.834330
8. Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol (2014) 10(1):44–56. doi: 10.1038/nrrheum.2013.160
9. Tian P, Ma Xl, Wang T, Ma Jx, Yang X. Correlation between radiculalgia and counts of T lymphocyte subsets in the peripheral blood of patients with lumbar disc herniation. Orthop Surg (2009) 1(4):317–21. doi: 10.1111/j.1757-7861.2009.00052.x
10. Park MS, Lee HM, Hahn SB, Moon SH, Kim YT, Lee CS, et al. The association of the activation-inducible tumor necrosis factor receptor and ligand with lumbar disc herniation. Yonsei Med J (2007) 48(5):839. doi: 10.3349/ymj.2007.48.5.839
11. Hirsch C, Schajowicz F. Studies on structural changes in the lumbar annulus fibrosus. Acta Orthop Scand (1952) 22(1–4):184–231. doi: 10.3109/17453675208989006
12. Geha RS, Leung DYM. Cellular abnormalities in patients with elevated serum IgE levels. J Allergy Clin Immunol (1986) 78(5):995–9. doi: 10.1016/0091-6749(86)90291-5
13. Wu J, Chen Y, Liao Z, Liu H, Zhang S, Zhong D, et al. Self-amplifying loop of NF-κB and periostin initiated by PIEZO1 accelerates mechano-induced senescence of nucleus pulposus cells and intervertebral disc degeneration. Mol Ther J Am Soc Gene Ther (2022) 30(10):3241–56. doi: 10.1016/j.ymthe.2022.05.021
14. Guidelines for performing Mendelian randomization investigations: update for summer 2023 - PMC (2023). Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7384151/.
15. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians - PMC (2023). Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6041728/.
16. Orrù V, Steri M, Sidore C, Marongiu M, Serra V, Olla S, et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat Genet (2020) 52(10):1036–45. doi: 10.1038/s41588-020-0684-4
17. Chen MH, Raffield LM, Mousas A, Sakaue S, Huffman JE, Moscati A, et al. Trans-ethnic and ancestry-specific blood-cell genetics in 746,667 individuals from 5 global populations. Cell (2020) 182(5):1198–213. doi: 10.1016/j.cell.2020.06.045
18. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the [ … formula … ] statistic - PMC (2023). Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5446088/.
19. Burgess S, Thompson SG. CRP CHD Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol (2011) 40(3):755–64. doi: 10.1093/ije/dyr036
20. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet (2018) 50(5):693–8. doi: 10.1038/s41588-018-0099-7
21. Song C, Zhou Y, Cheng K, Liu F, Cai W, Zhou D, et al. Cellular senescence – Molecular mechanisms of intervertebral disc degeneration from an immune perspective. BioMed Pharmacother (2023) 162:114711. doi: 10.1016/j.biopha.2023.114711
22. Schenk U, Frascoli M, Proietti M, Geffers R, Traggiai E, Buer J, et al. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci Signal (2011) 4(162):ra12. doi: 10.1126/scisignal.2001270
23. Suyama K, Sakai D, Watanabe M. The role of IL-17-mediated inflammatory processes in the pathogenesis of intervertebral disc degeneration and herniation: A comprehensive review. Front Cell Dev Biol (2022) 10:857164. doi: 10.3389/fcell.2022.857164
24. Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature (2001) 414(6866):916–20. doi: 10.1038/414916a
25. Moesta AK, Li XY, Smyth MJ. Targeting CD39 in cancer. Nat Rev Immunol (2020) 20(12):739–55. doi: 10.1038/s41577-020-0376-4
26. Mittal D, Sinha D, Barkauskas D, Young A, Kalimutho M, Stannard K, et al. Adenosine 2B receptor expression on cancer cells promotes metastasis. Cancer Res (2016) 76(15):4372–82. doi: 10.1158/0008-5472.CAN-16-0544
27. Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood (1997) 90(4):1600–10. doi: 10.1182/blood.V90.4.1600
28. Engel P, Zhou LJ, Ord DC, Sato S, Koller B, Tedder TF. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity (1995) 3(1):39–50. doi: 10.1016/1074-7613(95)90157-4
29. van Zelm MC, Reisli I, van der Burg M, Castaño D, van Noesel CJM, van Tol MJD, et al. An antibody-deficiency syndrome due to mutations in the CD19 gene. N Engl J Med (2006) 354(18):1901–12. doi: 10.1056/NEJMoa051568
30. Li X, Ding Y, Zi M, Sun L, Zhang W, Chen S, et al. CD19, from bench to bedside. Immunol Lett (2017) 183:86–95. doi: 10.1016/j.imlet.2017.01.010
31. Akyol S, Eraslan BS, Etyemez H, Tanriverdi T, Hanci M. Catabolic cytokine expressions in patients with degenerative disc disease. Turk Neurosurg (2010) 20(4):492–9. doi: 10.5137/1019-5149.JTN.3394-10.1
32. Piedra-Quintero ZL, Wilson Z, Nava P, Guerau-de-Arellano M. CD38: an immunomodulatory molecule in inflammation and autoimmunity. Front Immunol (2020) 11:597959. doi: 10.3389/fimmu.2020.597959
33. Huff WX, Kwon JH, Henriquez M, Fetcko K, Dey M. The evolving role of CD8+CD28- immunosenescent T cells in cancer immunology. Int J Mol Sci (2019) 20(11):2810. doi: 10.3390/ijms20112810
34. Geiss A, Larsson K, Rydevik B, Takahashi I, Olmarker K. Autoimmune properties of nucleus pulposus: an experimental study in pigs. Spine (2007) 32(2):168–73. doi: 10.1097/01.brs.0000251651.61844.2d
Keywords: immune cells, intervertebral disc degeneration, multivariable mendelian randomization, univariable mendelian randomization, bidirectional mendelian randomization
Citation: Qin C, Chen M, Yu Q, Wang X, Hu T, Lei B, Yan Z and Cheng S (2024) Causal relationship between the blood immune cells and intervertebral disc degeneration: univariable, bidirectional and multivariable Mendelian randomization. Front. Immunol. 14:1321295. doi: 10.3389/fimmu.2023.1321295
Received: 13 October 2023; Accepted: 19 December 2023;
Published: 10 January 2024.
Edited by:
Sadiq Umar, University of Illinois Chicago, United StatesReviewed by:
Imran Jamal, University of Pittsburgh Medical Center, United StatesPeihao Jin, Beijing Jishuitan Hospital, China
Copyright © 2024 Qin, Chen, Yu, Wang, Hu, Lei, Yan and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Si Cheng, MzA0MjM4QGNxbXUuZWR1LmNu; Zhengjian Yan, eWFuempAaG9zcGl0YWwuY3FtdS5lZHUuY24=
†These authors share first authorship
 Chaofan Qin†
Chaofan Qin† Qingshuai Yu
Qingshuai Yu Si Cheng
Si Cheng