- 1NHC Key Laboratory of Prevention and Treatment of Central Asia High Incidence Diseases, The First Affiliated Hospital/Shihezi University School of Medicine, Shihezi, China
- 2Department of Pathology, First Affiliated Hospital of Xinjiang Medical University, Urumqi, China
- 3Department of Cardiothoracic Surgery, Zhuhai People's Hospital, Zhuhai Hospital Affiliated with Jinan University, Zhuhai, China
- 4Department of Medical Record Statistics, Northeast Yunnan Regional Central Hospital, Zhaotong, China
- 5Department of Neurology, People’s Hospital of Xinjiang Uygur Autonomous Region, Urumqi, China
Introduction: In 2021, the World Health Organization published a new classification system for central nervous system tumors. This study reclassified the adult diffuse glioma (ADG) into astrocytoma, oligodendroglioma, and glioblastoma (GBM) according to the new tumor classification.
Methods: The association of TERT promoter (pTERT) mutation, MGMT methylation, and CD47/TIGIT expression with patient prognosis was investigated.
Results: Immunohistochemical analysis showed that the expression levels of CD47 and TIGIT in tumor tissues were significantly higher than those in normal brain tissues. CD47 levels were higher in GBM and grade 4 astrocytoma tissues. TIGIT expression was also higher in patients with GBM. The high expressions of CD47, TIGIT, and CD47/TIGIT were positively correlated with MGMT unmethylation but not pTERT mutation. Moreover, MGMT unmethylation was associated with poor overall survival in astrocytoma. High CD47, TIGIT, and CD47/TIGIT levels were associated with significantly reduced survival in ADG and GBM. GBM, MGMT unmethylation, and high CD47 expression were independent prognostic factors for overall survival in ADG.
Discussion: Collectively, these results showed that the MGMT unmethylation and high levels of CD47 and TIGIT are associated with a poor prognosis in ADG. Patients with high CD47 and TIGIT expression may benefit from anti-CD47 and TIGIT immunotherapy.
1 Introduction
According to the Global Cancer Statistics, brain and nervous system tumors accounted for 310,000 new cases worldwide and 250,000 deaths (1). The survey report of the National Cancer Center of China showed that nervous system tumors caused 70,000 deaths in 2020, of which glioma accounted for about 60% (2). In recent years, considerable progress has been made in treating gliomas by surgical resection, radiotherapy, and chemotherapy. However, the clinical efficacy of these conventional treatments is still far from satisfactory. Studies have revealed various driver genes in glioma that may be closely associated with prognosis and have demonstrated that prognosis cannot be predicted exclusively based on morphological characteristics. In 2021, the World Health Organization (WHO) published the Classification of Tumors of the Central Nervous System (CNS), fifth edition, which introduced a new tumor classification system (3). The system involves an integrated diagnostic model based on morphological characteristics and gene expression levels in tumors, serving as a guide for clinical diagnosis, treatment, and prognosis.
Epigenetic silencing of the DNA repair enzyme O6-methylguanine-DNA methyltransferase (MGMT) has emerged as a prognostic and predictive marker in patients with glioblastomas (GBM) (4). However, the diagnostic criteria for GBM have shifted from purely histomorphological to molecular characteristics. In the 2021 WHO classification of tumors of the central nervous system, GBM in adult-type diffuse gliomas (ADG) is defined as the absence of mutations in the isocitrate dehydrogenase gene (i.e., IDH wild-type) (3). Meanwhile, patients with IDH wild-type and TERT promoter (pTERT) mutant gliomas in the new classification are also diagnosed with GBM. Studies on the status of pTERT and MGMT in GBM have also been reported (5), but there are few studies on pTERT mutations and MGMT methylation status in other types of ADG in the new classification.
Immune checkpoint inhibitors (ICIs) are a promising treatment for glioma (6, 7). Programmed death-1 (PD-1)/programmed death ligand-1 (PD-L1) inhibitors are the most widely used inhibitors in immunotherapy. Although ICIs offer hope to many patients with advanced malignancies, their efficacies range between 15% and 60%, with many patients showing no benefit (8, 9). Gliomas, especially GBMs, are immunosuppressive tumors. Therefore, the identification of relevant biomarkers, as well as effective immunosuppressants for treating gliomas, are important areas of research. Differentiation cluster 47 (CD47), a glycoprotein widely expressed on the cell surface, regulates tumor invasion and metastasis (10). CD47 inhibitors have been tested in clinical trials for the treatment of ovarian cancer, colorectal cancer, and leukemia (11, 12). However, studies related to their effect on glioma remain limited. T cell immune receptor with Ig and ITIM domains (TIGIT) is a novel checkpoint inhibitor molecule expressed on a variety of immune cells, including T cells, regulatory T cells (Tregs), and natural killer cells (NK) (13). They are highly expressed in colon cancer, multiple myeloma, breast cancer, and prostate cancer and are closely related to prognosis (14). Targeting TIGIT may restore T-cell function, leading to antitumor effects (15). Therefore, in ADG, exploring the expression levels of CD47 and TIGIT and their prognostic relationships, as well as the relationship between pTERT mutations and MGMT methylation status and prognosis, may provide some valuable clues for the therapy of ADG.
2 Materials and methods
2.1 Human tumor samples
The study was approved by the Ethics Committee of the First Affiliated Hospital of Shihezi University, and signed informed consent forms were obtained from patients. Glioma and normal brain tissues were acquired from participants who had received excision surgery from May 2012 to October 2021. Patients who had undergone previous adjuvant radiotherapy or chemotherapy were excluded. A total of 125 patients diagnosed with astrocytic and oligodendroglial tumors according to the Classification of Tumors (fourth edition and fourth edition revision) were included in the study. These 125 cases included 35 cases of diffuse astrocytoma, 35 cases of anaplastic astrocytoma, 42 cases of GBM, 8 cases of oligodendroglioma, and 5 cases of anaplastic oligodendroglioma. Reclassification of ADG was performed by two neuropathologists who independently reviewed histological sections and various test findings of this group of patients according to the diagnostic criteria of the WHO Classification of Tumors (fifth edition). Patients with gliomas were followed up starting from October 1, 2016. Patient survival data were obtained via outpatient examinations and telephone follow-ups. Overall survival (OS) was defined as the duration from surgery to death or the last follow-up. The review deadline was January 15, 2023.
2.2 Immunohistochemical analysis
Tissue samples were fixed in 10% neutral formalin and embedded in paraffin. All tissue samples were stained with antibodies against IDH1 R132H (H9), CD47 (mouse anti-human, ready-to-use type, OT13B10; Beijing Zhongshan Co.), and TIGIT (rabbit anti-human, 1:100, BLR047F; Abcam). A Leica Bond HRP Poly Kit (Shanghai Gene Company) was used following standard procedures. All immunohistochemical (IHC) sections were scanned using a digital pathology slide scanner (KF-PRO-005; Ningbo Jiangfeng Biological Information Technology Co., Ltd.). The scanned images were scored by two neuropathologists. Ten different tumor areas were selected from each specimen, and the percentage and intensity of positive tumor cells were observed and recorded (16). Positive percentage scores were 0 (0-5%), 1 (6-25%), 2 (26-50%), and 3 (more than 51%); positive intensity according to 0 (no staining), 1 (light brown), 2 (brown), 3 (dark brown); the two scores were multiplied and considered the final score. The scoring results of two pathologists were summarized, and the samples with inconsistent scoring results were discussed and scored again. All cases had a final score greater than the median and were considered high expression; ≤ the median is low expression. Among the 115 ADG cases, the median CD47 and TIGIT score was 2, and a score greater than 2 was a high expression. A score of less than or equal to 2 is a low expression.
2.3 Detection of IDH1/2 mutation
A DNA Extraction Kit (QIAamp Paraffin Tissue) was used to extract genomic DNA from all glioma tissues. IDH1 R132H mutations were detected by qPCR (7500fast, ABI) using the detection reagents from Beijing Fanshengzi Gene Technology Co, Ltd. The experiment was performed according to the manufacturer’s instructions, and ΔCT ≤ 8 was interpreted as positive. First-generation sequencing was performed in cases showing inconsistent results between IHC and qPCR, in cases showing negative results by both methods and in patients younger than 55 (17). For gene sequencing, primers for IDH1 (R132H, R132C, and R132S) and IDH2R172K were designed by Shanghai Bioengineering Co., Ltd (18). Primer sequences are shown in the Supplementary Material. Amplification and sequencing were completed by the Sequencing Department of Shanghai Bioengineering Co., Ltd.
2.4 Detection of pTERT mutant and MGMT methylation
The qPCR reagents for detecting the pTERT mutation (C228T and C250T) were provided by Beijing Fanshengzi Gene Technology Co., Ltd. and were used according to the instructions provided. Values of ΔCT ≤ 9 were interpreted as positive; otherwise, the experimental results were invalid. MGMT methylation reagents were obtained from Shanghai Gene Corporation. First, bisulfite conversion was performed, and then qPCR amplification to detect fluorescence signals. When ΔCT ≤ 7, the methylation level of the MGMT gene in the sample was ≥1%, and MGMT methylation was positive.
2.5 Fluorescence in situ hybridization
To evaluate the gene copy number status, fluorescence in situ hybridization (FISH) was used to analyze chromosome arms 1p and 19q, CDKN2A (Vysis), EGFR, chromosome 7, and chromosome 10 (Anbiping). The pretreatment kit was obtained from Beijing Yakangbo Biotechnology Co., Ltd. The experiment was performed according to the instructions accompanying each reagent. In brief, green and orange fluorescence signals were observed under a fluorescence microscope (ZEISS Image A2) with appropriate filters. The detection thresholds for 1p/19q co-deletion (19), CDKN2A deletion (20), EGFR amplification, chromosome 7 polysomy, and chromosome 10 monosomy (21) are detailed in Supplementary Material.
2.6 Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics, version 26.0. Normally distributed data are expressed as the mean ± standard deviation, while count data are expressed as a frequency or percentage. Group comparisons were performed using the chi-square test or Fisher’s test. Survival rates were evaluated using the Kaplan-Meier method, and differences between the survival curves for each group were assessed using the log-rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using Cox proportional hazards regression models. Statistical significance was set at P < 0.05.
3 Results
3.1 IHC combined with qPCR and sequencing could accurately detect IDH1/2 mutation
IHC results indicated that the positive rate for IDH1 R123H in 125 glioma cases was 49.6% (62/125), while qPCR and first-generation sequencing showed that the mutation rate of the IDH1/2 gene was 46.4% (58/125). Among 42 cases successfully sequenced for IDH1, seven were positive for R132H, while R132C and R132S were not detected. In contrast, IDH2 R172K mutation was not seen among the 39 successfully sequenced cases. The consistency rate of IDH1/2 detection by IHC, qPCR, and sequencing techniques was 85.6% (107/125), and discordance between IHC and qPCR results was evident in 18 cases. Eleven cases were IHC-positive but IDH1 wildtype by qPCR and sequencing. Seven samples were negative for IDH1 R132H via IHC, while IDH1 mutations were detected via the other two methods (Figures 1A–C). The concordance rate between qPCR and sequencing techniques was 100%.

Figure 1 Three representative cases of ADG were evaluated by HE, IHC, and molecular tests. Case 1 was a 49-year-old male; HE (A); IDH1 R132H negative (200×magnification) (B); sequencing results indicated IDH1 R132H, 395 G>A (C). Case 2 was a 36-year-old male; HE morphology was characterized as a low-grade pattern (D); IDH1 R132H positive (200×magnification) (E); FISH showed CDKN2A homozygous deletion (loss of red CDKN2A signals, white arrow) (1000×magnification) (F). Case 3 was a 46-year-old female; HE showed obvious cellular atypia and proliferation of blood vessels (200×magnification) (G); FISH test showed EGFR amplification (red EGFR signals are seen, white arrow) (H), chromosome 7 gain (green chromosome 7 signals are seen, white arrow; 1000×magnification) (I).
3.2 Reclassification of ADG
In 125 gliomas, we detected the IDH1/2 mutation in 58 cases, co-deletion of chromosome arms 1p and 19q (1p/19q co-deletion) in 22 cases (22/125, 17.6%), IDH1/2 mutation and 1p/19q codeletion in 16 cases, wildtype IDH1/2 with 1p/19q- co-deletion in six patients, and the pTERT mutation in 64 cases (64/125; 51.2%), with C228T and C250T mutation rates of 76.56 (49/64) and 23.44% (15/64), respectively. Histomorphology and molecular detection results revealed eight cases each of grades 2 and 3 oligodendroglioma carrying mutant IDH with the 1p/19q-codeletion; 18, 11, and 13 cases of grades 2, 3, and 4 astrocytoma carrying mutant IDH. One case of grade 2 astrocytoma with homozygous deletion of CDKN2A, according to the new tumor classification, this patient should be IDH-mutant, grade 4 (Figures 1D–F). In 45 of 67 wild-type IDH samples, high cell density and frequent mitotic signs with angiogenesis or necrosis were observed, with one case of EGFR amplification and chromosome 7 polysomy (Figures 1G–I). Among 22 cases of low-grade glioma (LGG) with wild-type IDH, 12 patients had a median age of 53 (37–73) and were diagnosed with molecularly characterized GBM (10 cases had a pTERT mutation; two carried the EGFR amplification, one of which showed a chromosome 7 gain) (22). The other 10 LGGs with wild-type IDH and pTERT had no EGFR amplification or chromosome 7 or 10 changes. After combining the molecular markers with clinical characteristics and follow-up information of the patients, 7 cases were low-grade pediatric glioma, and the other three cases were diffuse midline glioma with the H3K27 variant. Therefore, they were excluded from this study.
3.3 Clinical and molecular information of ADG
The results of HE, IHC, and molecular tests for 115 cases with ADG were reviewed based on the new tumor classification by neuropathologists. The cases were reclassified as follows: 42 (23 males and 19 females; average age 45.26 ± 12.01) cases of astrocytoma carrying mutant IDH where 17, 11, and 14 cases were grades 2, 3, and 4, respectively; 16 (12 males and four females; average age 46.19 ± 12.12) cases of oligodendroglioma carrying mutant IDH with 1p/19q-codeleted, where 8 cases each were grades 2 and 3; and 57 (31 males and 26 females; average age 54.75 ± 13.08) cases of GBM, IDH-wildtype. Clinical and molecular information of the 115 ADG are shown in Supplementary Table 1. pTERT mutations and MGMT methylation accounted for 55.7% (64/115) and 61.7% (71/115) of ADG tissues, respectively. IHC results for CD47 and TIGIT revealed high and low expression (Figure 2) for CD47 in ADG in 47.0% (54/115) and 53.0% (61/115) of cases, respectively; high and low TIGIT expression in ADG was observed in 42.6% (49/115) and 57.4% (66/115) of cases, respectively.
3.4 pTERT mutation and MGMT methylation status in ADG
The frequency of mutant pTERT in GBM was significantly higher than that in astrocytoma (P < 0.0001) (Supplementary Table 2). pTERT mutations and MGMT methylation were detected in grade 2 and 3 oligodendroglioma. The rate of MGMT methylation in astrocytoma was significantly higher than that in GBM (P < 0.0001). In addition, levels of MGMT methylation in grade 2 astrocytoma tissue were higher than in grades 3 and 4 (P = 0.014).
3.5 Overexpression of CD47 and TIGIT in ADG tissues
IHC was used to detect CD47 and TIGIT expression in ADG tissues. The expression levels of CD47 and TIGIT in tumor tissues (n = 115) were significantly higher than those in normal brain tissues (P = 0.0073, P = 0.0064) (Figure 3). Twenty ADG tumors with matched normal brain tissues were further analyzed; the expression levels of CD47 and TIGIT in tumor tissues were higher than those in matched adjacent normal brain tissues (P < 0.0001). The expression of CD47 in GBM was higher than that in astrocytoma and oligodendroglioma (P = 0.004) (Supplementary Table 3) and was higher in astrocytoma grade 4 than that in grade 2 or 3 (P = 0.006). There were no significant differences in the expression levels of TIGIT among the three subtypes or between astrocytoma and oligodendroglioma grades 2, 3, and 4 and grades 2 and 3.
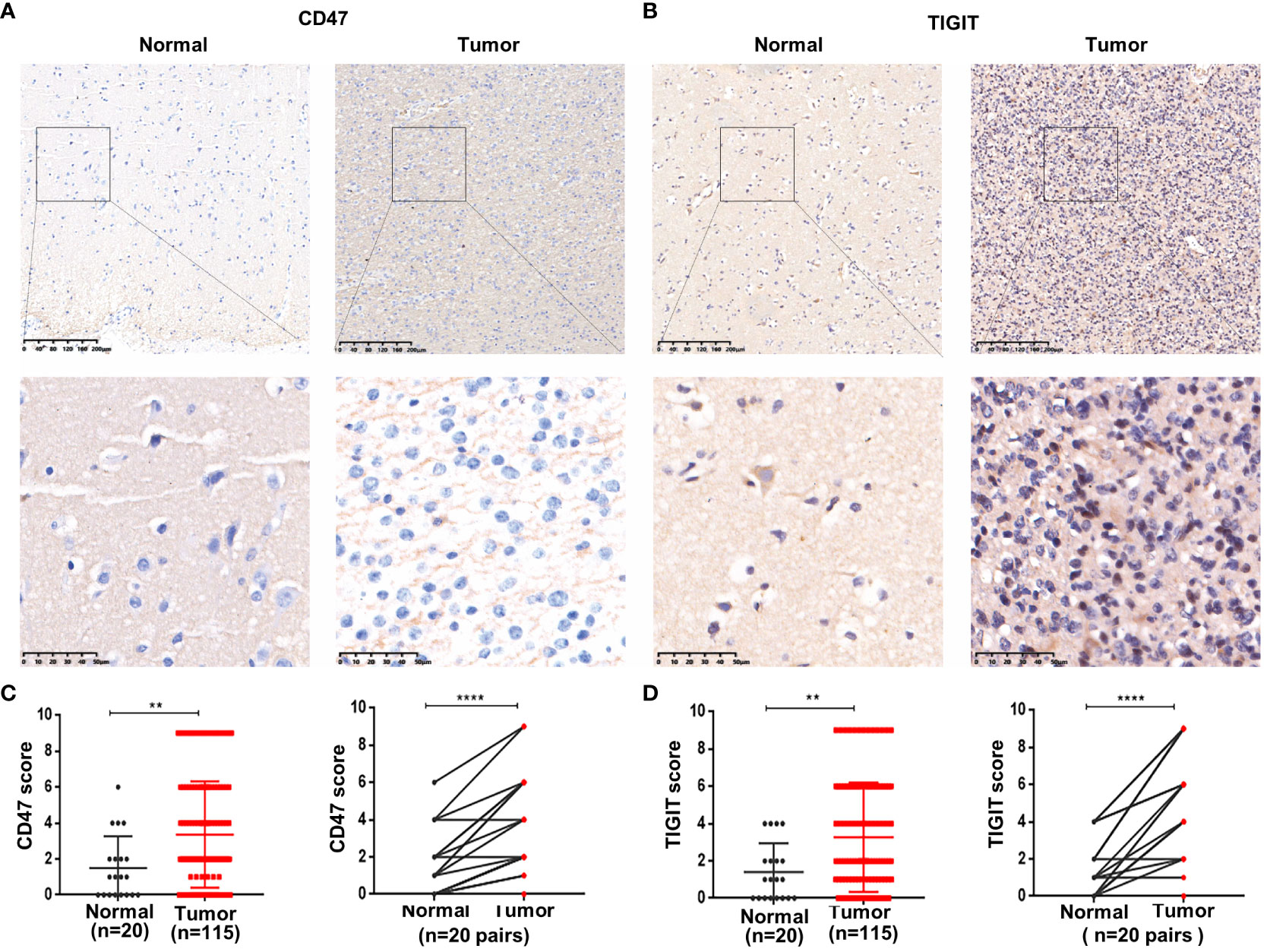
Figure 3 Overexpression of CD47 and TIGIT in ADG tissues. Representative images of CD47 and TIGIT in ADG and matched adjacent tissues (100× and 400×magnification) (A, B). Statistical of CD47 (C) and TIGIT (D) expression in ADG and normal brain tissues. **P<0.01, ****P<0.0001.
3.6 Relationships between CD47 and TIGIT expression and the clinicopathological characteristics of patients with ADG
CD47 expression was not correlated with sex or pTERT mutations (Table 1); however, CD47 expression was higher in patients older than 50 years of age (P < 0.05) and in patients classified as GBM (P < 0.05) and was significantly associated with grade 4 in astrocytoma, whereas high expression of CD47 and TIGIT were related to the MGMT unmethylation status, respectively (P < 0.05). High CD47 expression was positively correlated with high TIGIT expression (P < 0.05). High TIGIT expression was not associated with age, sex, astrocytoma grade, or the pTERT mutation status. However, TIGIT expression was higher in patients with GBM (P < 0.05). Dual CD47/TIGIT high expression was not correlated with age, sex, astrocytoma of all grades, or pTERT mutations. However, dual CD47/TIGIT high expression was higher in patients with GBM and associated with MGMT unmethylation (P < 0.05).
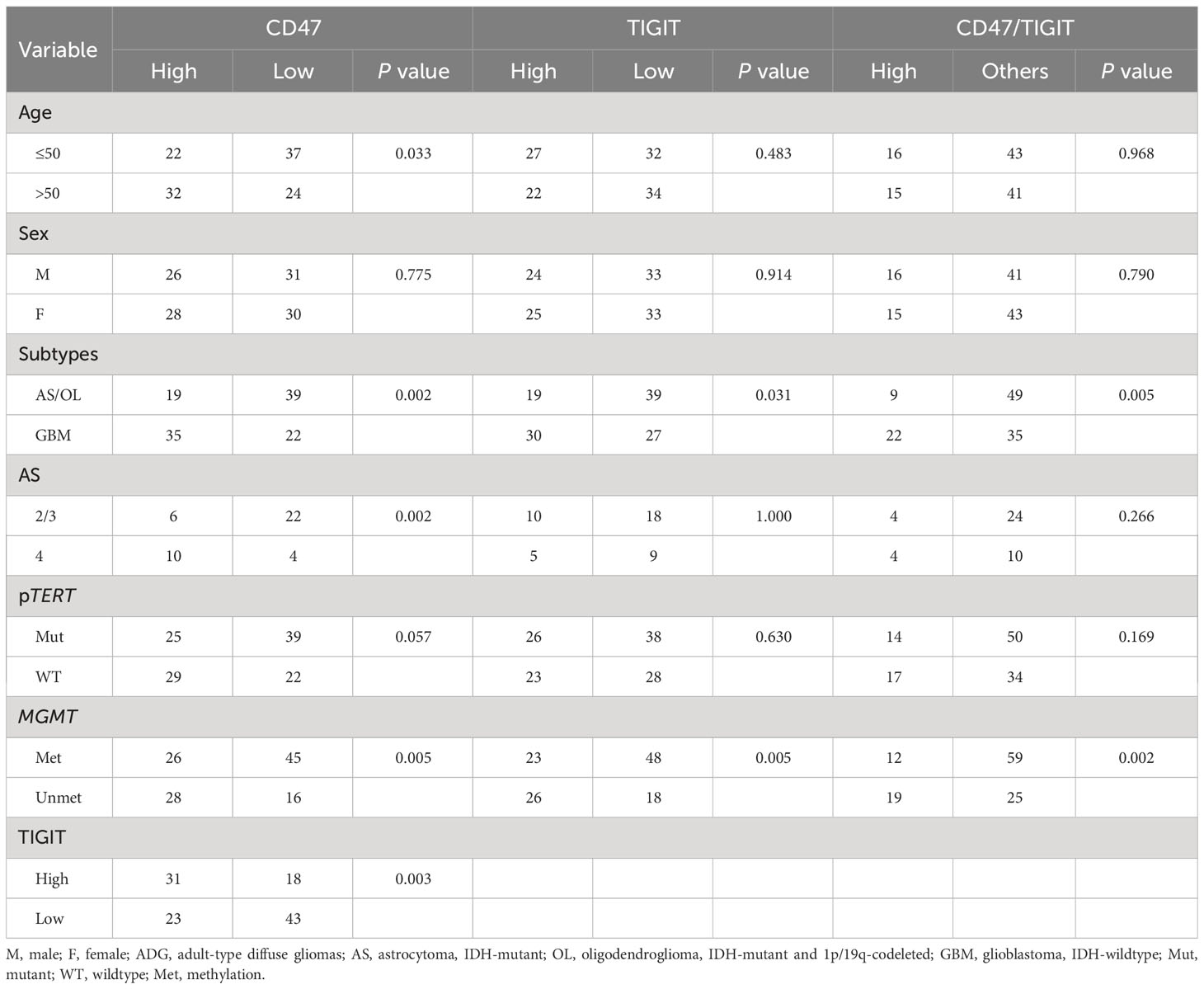
Table 1 Correlation between clinicopathological characteristics and TIGIT/CD47 expression in ADG cases (n=115).
3.7 MGMT unmethylation and high levels of CD47 and TIGIT are associated with poor prognosis in ADG and subtypes
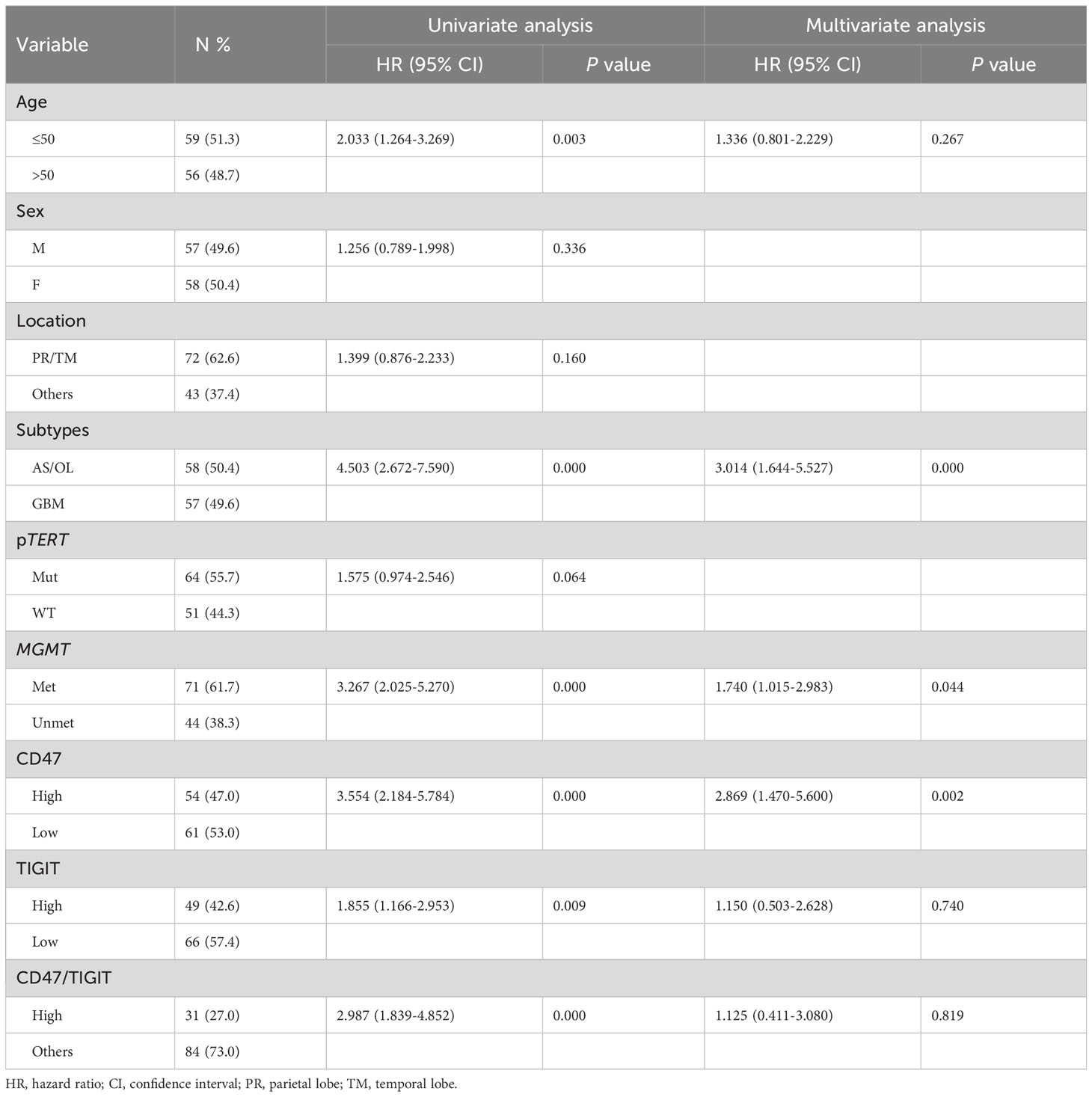
Next, we used univariate and multivariate Cox regression analyses to identify prognostic factors in patients with ADG in Table 2. Univariate analyses showed that age, tumor type, MGMT unmethylation, CD47, and high TIGIT expression were all associated with OS, whereas gender, tumor location, and pTERT mutations were unrelated to OS. Multivariate analysis indicated that GBM, MGMT unmethylation, and high CD47 expression were independent prognostic factors for OS. Furthermore, we found that groups with GBM and astrocytoma grade 4 had a lower OS than the other groups (P < 0.0001, Figures 4A, B). For pTERT mutation and MGMT methylation in astrocytoma and GBM, only the MGMT unmethylated group showed a shorter OS in astrocytomas (P < 0.0001, Figures 4C–F).
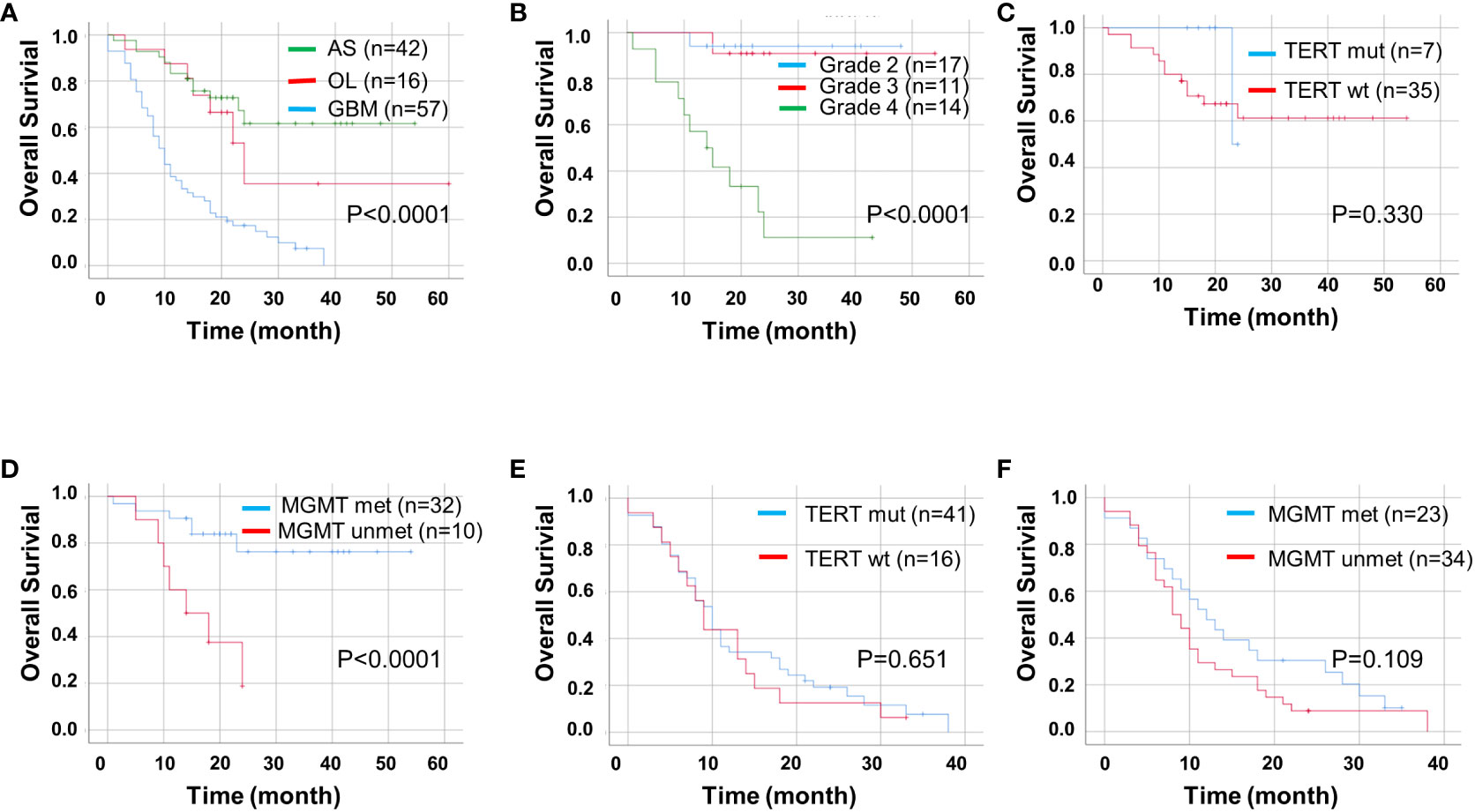
Figure 4 Kaplan-Meier curves of OS for ADG (n = 115). OS for ADG (A) and astrocytoma, IDH⁃ mutant (B), and for pTERT mutant and MGMT methylation status in astrocytoma (C, D) and GBM (E, F). AS, astrocytoma; OL, oligodendroglioma; GBM, glioblastoma; pTERT, TERT promoter; mut, mutant; wt, wildtype.
We found that high expression levels of CD47 and TIGIT and double high expression of CD47/TIGIT were related to a significantly reduced OS in patients with ADG (P < 0.05, Figures 5A–C). High CD47 expression, but not TIGIT and CD47/TIGIT, significantly reduced the OS of patients with astrocytoma (P < 0.05, Figures 5D–F). In patients with GBM, high expression of CD47, TIGIT, and both CD47/TIGIT significantly decreased OS (P < 0.005, Figures 5G–I), indicating high expression levels of CD47 and TIGIT may be valuable indicators of a poor prognosis in patients with ADG.
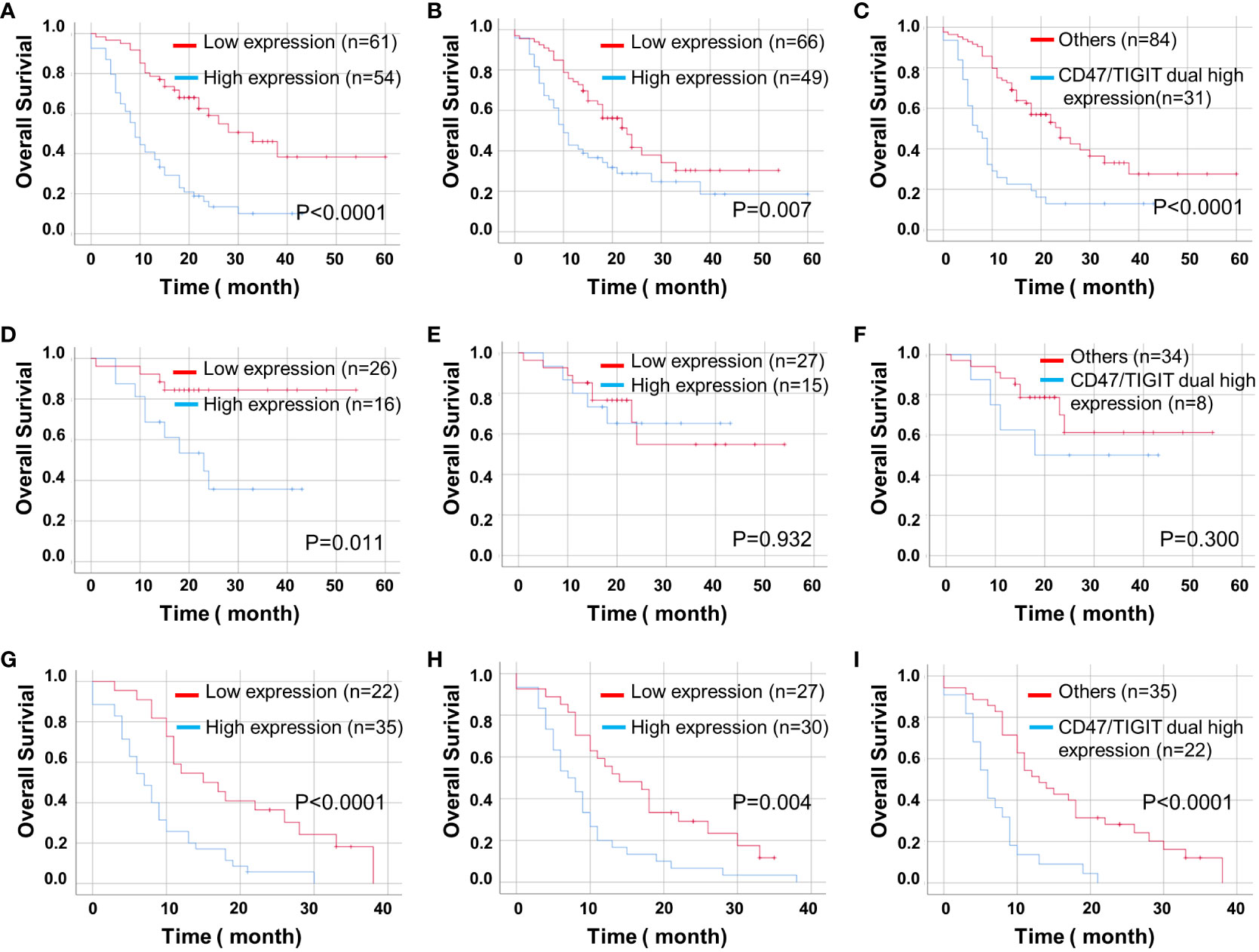
Figure 5 Kaplan-Meier curves of OS for CD47 and TIGIT expression and their combination in ADG (n = 115). OS for CD47 (A) and TIGIT (B) expression and their combination (C) in ADG. OS for CD47 (D) and TIGIT (E) expression and their combination (F) in astrocytoma, IDH⁃mutant (n = 42). OS for the expression of CD47 (G) and TIGIT (H) and their combination (I) in GBM, IDH⁃wildtype (n = 57).
4 Discussion
The new cancer classification system integrates molecular and clinical pathological diagnoses. The treatment of diffuse gliomas also requires further stratification according to the new classification. In this study, 115 cases of ADG were reclassified according to the new tumor classification, and a survival analysis of patients with ADG enrolled in the study further demonstrated that patients with GBM may experience a shorter survival than patients with astrocytoma. Patients with grade 4 astrocytoma also showed a poor prognosis. The frequencies of pTERT mutations were 55.7% (64/115) in ADG, 16.7% (7/42) in astroglioma, and 71.9% (41/57) in GBM. In ADG, there was no significant difference in OS between pTERT mutation and wild-type cases; similar results were obtained for astrocytoma and GBM. A study of the pTERT mutation status in GBM has indicated that while pTERT mutations account for about 70–80% of GBM, they are generally regarded as a late or terminal event in GBM (23), and thus their prognostic significance remains uncertain. Another study of GBM (72 patients) compared survival based on The Cancer Genome Atlas (TCGA) adult GBM cohort (24); in GBM, the pTERT mutant and wild-type status were not associated with OS, consistent with our results. In our study, the methylation level of MGMT in astrocytoma was significantly higher than that in GBM, and the MGMT methylation level in grade 2 was significantly higher than those of grades 3 and 4 in astrocytoma. Univariate and multivariate analyses showed that MGMT unmethylation may act as an independent prognostic factor for OS in ADG. A subsequent analysis of ADG subtypes revealed that patients with astrocytoma in the MGMT unmethylated group had a shorter OS than those in the MGMT methylated group. However, the difference in OS in patients with GBM between the MGMT-methylated and unmethylated groups was insignificant. In this study, the number of patients with oligodendroglioma was small, and no patients had the MGMT unmethylated status; therefore, survival analyses could not be performed. This result was further confirmed by another study showing that MGMT methylation was significantly associated with a longer PFS and OS in prospectively collected grade II gliomas treated with radiotherapy combined with temozolomide (25), suggesting that MGMT methylation may be a better prognostic biomarker in IDH-mutant LGGs.
There is an urgent need to explore new therapeutic approaches to improve the survival of patients with glioma. Although anti-PD-1/PD-L1 and Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) monoclonal antibodies are studied extensively in glioma, most are in phase I or phase II clinical research stages (26, 27). In some clinical trials, anti-PD-L1 therapy showed slight improvements and was more effective in patients with high PD-L1 expression. However, more targets are needed due to the rapid development of drug tolerance and adverse reactions. Our study showed that CD47 and TIGIT expression levels in GBM were significantly higher than in astrocytomas and oligodendrogliomas. High expression of CD47, TIGIT, and CD47/TIGIT was all associated with poor survival in ADG and GBM, whereas high expression of CD47 was associated with shorter OS in astrocytoma.
CD47 is expressed in many tumors, such as breast cancer and anaplastic thyroid cancer, among others (28, 29). Recent studies have indicated that CD47 expression in malignant meningiomas was increased while the number of T cells was decreased, and the number of macrophages expressing CD68 was increased (30). Blocking CD47 with an anti-CD47 antibody inhibited the growth and movement of malignant meningioma cells and promoted phagocytosis mediated by macrophages (30). Liu et al. (31) showed that compared with normal controls, CD47 was more highly expressed in GBM tissues and various GBM cell lines. CD47 downregulation via siRNA suppressed invasion in vitro, whereas CD47 overexpression exerted the opposite effect. These results suggested CD47 may be a valuable predictor of a poor prognosis.
TIGIT is a co-inhibitory receptor that is expressed on a variety of immune cells. TIGIT interacts with different ligands, including CD155 expressed on dendritic cells, thereby inhibiting tumor killing by NK cells and antigen presentation by dendritic cells, thus weakening the anti-tumor effect of T cells (14). In recent years, TIGIT has been investigated as an important checkpoint in cancer research focused on esophageal small cell carcinoma (32) and lung adenocarcinoma (33), where TIGIT-positive patients showed a shorter OS and a lower PFS than those of TIGIT-negative patients. In the present study, TIGIT expression was high in patients with poor prognoses. High CD47/TIGIT co-expression was also associated with a poor prognosis, supporting the feasibility of using CD47 and TIGIT ICIs in treating ADG.
Our study showed an association of MGMT unmethylation with CD47 and TIGIT high expression in ADG. The addition of Temozolomide (TMZ) chemotherapy improves survival in patients with GBM containing a methylated MGMT promoter (34). A randomized phase 3 study showed that MGMT promoter methylation was also associated with better outcomes among patients with recurrent glioblastoma treated with nivolumab targeting PD-1 (35). However, for GBM patients with an unmethylated MGMT promoter, the benefit of TMZ is marginal and increasingly questioned. Therefore, radiotherapy is preferred when tolerable for patients with MGMT unmethylation (36). In contrast, for elderly, frail patients who cannot tolerate radiotherapy, the choice of anti-CD47 and TIGIT immunotherapy might be a better approach.
However, this study was affected by several limitations. Samples of the three subtypes of ADG included in the study were not balanced because the number of patients with oligodendroglioma was small, preventing evaluations of the relationship between relevant indicators and prognosis. Although we predicted the efficacy of immunotherapy using new targets for ADG, we did not include treated patients. Therefore, we were unable to draw firm conclusions regarding efficacy prediction. In addition, although we described the association of MGMT unmethylation, the expression levels of CD47 and TIGIT with patient prognosis, their therapeutic role and potential interconnections in ADG warrant to be further demonstrated by in vivo and in vitro experiments.
5 Conclusions
Unmethylated MGMT in astrocytomas and the overexpression of CD47 and TIGIT in ADG tissues are associated with a poor prognosis. Thus, patients with ADG showing high CD47 and TIGIT expression levels may benefit from anti-CD47 and TIGIT immunotherapy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of Shihezi University. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
LM: Writing – original draft, Data curation. YS: Formal analysis, Writing – review & editing. CL: Formal analysis, Writing – review & editing. BD: Writing – review & editing, Data curation. JJ: Methodology, Writing – review & editing. YC: Supervision, Writing – review & editing. HL: Resources, Supervision, Writing – original draft. LW: Investigation, Project administration, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2020-PT330-003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1323307/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (2022) 135(5):584–90. doi: 10.1097/CM9.0000000000002108
3. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro-oncology (2021) 23(8):1231–51. doi: 10.1093/neuonc/noab106
4. Binabaj MM, Bahrami A, ShahidSales S, Joodi M, Joudi Mashhad M, Hassanian SM, et al. The prognostic value of MGMT promoter methylation in glioblastoma: A meta-analysis of clinical trials. J Cell Physiol (2018) 233(1):378–86. doi: 10.1002/jcp.25896
5. Tunthanathip T, Sangkhathat S, Tanvejsilp P, Kanjanapradit K. Prognostic impact of the combination of MGMT methylation and TERT promoter mutation in glioblastoma. J Neurosci Rural Practice (2021) 12(4):694–703. doi: 10.1055/s-0041-1735821
6. Kelly PN. The cancer immunotherapy revolution. Sci (New York NY) (2018) 359(6382):1344–5. doi: 10.1126/science.359.6382.1344
7. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Sci (New York NY) (2018) 359(6382):1350–5. doi: 10.1126/science.aar4060
8. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet (London England) (2017) 389(10088):2492–502. doi: 10.1016/S0140-6736(17)31046-2
9. Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, MaChado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol (2018) 4(5):e180013. doi: 10.1001/jamaoncol.2018.0013
10. Tan W, Tang H, Jiang X, Ye F, Huang L, Shi D, et al. Metformin mediates induction of miR-708 to inhibit self-renewal and chemoresistance of breast cancer stem cells through targeting CD47. J Cell Mol Med (2019) 23(9):5994–6004. doi: 10.1111/jcmm.14462
11. Shimizu A, Sawada K, Kobayashi M, Yamamoto M, Yagi T, Kinose Y, et al. Exosomal CD47 plays an essential role in immune evasion in ovarian cancer. Mol Cancer research: MCR (2021) 19(9):1583–95. doi: 10.1158/1541-7786.MCR-20-0956
12. Jiang Z, Sun H, Yu J, Tian W, Song Y. Targeting CD47 for cancer immunotherapy. J Hematol Oncol (2021) 14(1):180. doi: 10.1186/s13045-021-01197-w
13. Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol (2009) 10(1):48–57. doi: 10.1038/ni.1674
14. Harjunpää H, Guillerey C. TIGIT as an emerging immune checkpoint. Clin Exp Immunol (2020) 200(2):108–19. doi: 10.1111/cei.13407
15. Thibaudin M, Limagne E, Hampe L, Ballot E, Truntzer C, Ghiringhelli F. Targeting PD-L1 and TIGIT could restore intratumoral CD8 T cell function in human colorectal cancer. Cancer Immunol Immunother: CII (2022) 71(10):2549–63. doi: 10.1007/s00262-022-03182-9
16. Wang Y, Deng J, Wang L, Zhou T, Yang J, Tian Z, et al. Expression and clinical significance of PD-L1, B7-H3, B7-H4 and VISTA in craniopharyngioma. J Immunother Cancer (2020) 8(2):e000406. doi: 10.1136/jitc-2019-000406
17. Sporikova Z, Slavkovsky R, Tuckova L, Kalita O, Megova Houdova M, Ehrmann J, et al. IDH1/2 mutations in patients with diffuse gliomas: A single centre retrospective massively parallel sequencing analysis. Appl immunohistochemistry Mol Morphology: AIMM (2022) 30(3):178–83. doi: 10.1097/PAI.0000000000000997
18. Waitkus MS, Diplas BH, Yan H. Isocitrate dehydrogenase mutations in gliomas. Neuro-oncology (2016) 18(1):16–26. doi: 10.1093/neuonc/nov136
19. Pinto LW, Araújo MB, Vettore AL, Wernersbach L, Leite AC, Chimelli LM, et al. Glioblastomas: correlation between oligodendroglial components, genetic abnormalities, and prognosis. Virchows Archiv: an Int J Pathol (2008) 452(5):481–90. doi: 10.1007/s00428-007-0562-9
20. Reis GF, Pekmezci M, Hansen HM, Rice T, Marshall RE, Molinaro AM, et al. CDKN2A loss is associated with shortened overall survival in lower-grade (World Health Organization Grades II-III) astrocytomas. J Neuropathol Exp Neurol (2015) 74(5):442–52. doi: 10.1097/NEN.0000000000000188
21. Koshiyama DB, Trevisan P, Graziadio C, Rosa RFM, Cunegatto B, Scholl J, et al. Frequency and clinical significance of chromosome 7 and 10 aneuploidies, amplification of the EGFR gene, deletion of PTEN and TP53 genes, and 1p/19q deficiency in a sample of adult patients diagnosed with glioblastoma from Southern Brazil. J Neuro-oncol (2017) 135(3):465–72. doi: 10.1007/s11060-017-2606-6
22. Brat DJ, Aldape K, Colman H, Figrarella-Branger D, Fuller GN, Giannini C, et al. cIMPACT-NOW update 5: recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathologica (2020) 139(3):603–8. doi: 10.1007/s00401-020-02127-9
23. Stead LF, Verhaak RGW. Doomed from the TERT? A two-stage model of tumorigenesis in IDH-wild-type glioblastoma. Cancer Cell (2019) 35(4):542–4. doi: 10.1016/j.ccell.2019.03.009
24. Liu EM, Shi ZF, Li KK, Malta TM, Chung NY, Chen H, et al. Molecular landscape of IDH-wild type, pTERT-wild type adult glioblastomas. Brain Pathol (Zurich Switzerland) (2022) 32(6):e13107. doi: 10.1111/bpa.13107
25. Bell EH, Zhang P, Fisher BJ, Macdonald DR, McElroy JP, Lesser GJ, et al. Association of MGMT promoter methylation status with survival outcomes in patients with high-risk glioma treated with radiotherapy and temozolomide: an analysis from the NRG oncology/RTOG 0424 trial. JAMA Oncol (2018) 4(10):1405–9. doi: 10.1001/jamaoncol.2018.1977
26. Lukas RV, Rodon J, Becker K, Wong ET, Shih K, Touat M, et al. Clinical activity and safety of atezolizumab in patients with recurrent glioblastoma. J Neuro-oncol (2018) 140(2):317–28. doi: 10.1007/s11060-018-2955-9
27. Gorsi HS, Malicki DM, Barsan V, Tumblin M, Yeh-Nayre L, Milburn M, et al. Nivolumab in the treatment of recurrent or refractory pediatric brain tumors: A single institutional experience. J Pediatr Hematology/oncology (2019) 41(4):e235–e41. doi: 10.1097/MPH.0000000000001339
28. Betancur PA, Abraham BJ, Yiu YY, Willingham SB, Khameneh F, Zarnegar M, et al. A CD47-associated super-enhancer links pro-inflammatory signalling to CD47 upregulation in breast cancer. Nat Commun (2017) 8:14802. doi: 10.1038/ncomms14802
29. Schürch CM, Roelli MA, Forster S, Wasmer MH, Brühl F, Maire RS, et al. Targeting CD47 in anaplastic thyroid carcinoma enhances tumor phagocytosis by macrophages and is a promising therapeutic strategy. Thyroid: Off J Am Thyroid Assoc (2019) 29(7):979–92. doi: 10.1089/thy.2018.0555
30. Liu X, Zhang H, Wang C, Li Z, Zhu Q, Feng Y, et al. Tumor-selective blockade of CD47 signaling with CD47 antibody for enhanced antitumor activity in Malignant meningioma. Curr Neuropharmacol (2023) 21(10):2159–73. doi: 10.21203/rs.3.rs-2002161/v1
31. Liu X, Wu X, Wang Y, Li Y, Chen X, Yang W, et al. CD47 promotes human glioblastoma invasion through activation of the PI3K/Akt pathway. Oncol Res (2019) 27(4):415–22. doi: 10.3727/096504018X15155538502359
32. Zhao K, Ma L, Feng L, Huang Z, Meng X, Yu J. CD155 overexpression correlates with poor prognosis in primary small cell carcinoma of the esophagus. Front Mol Biosciences (2020) 7:608404. doi: 10.3389/fmolb.2020.608404
33. Sun Y, Luo J, Chen Y, Cui J, Lei Y, Cui Y, et al. Combined evaluation of the expression status of CD155 and TIGIT plays an important role in the prognosis of LUAD (lung adenocarcinoma). Int Immunopharmacol (2020) 80:106198. doi: 10.1016/j.intimp.2020.106198
34. Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. New Engl J Med (2005) 352(10):997–1003. doi: 10.1056/NEJMoa043331
35. Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the checkMate 143 phase 3 randomized clinical trial. JAMA Oncol (2020) 6(7):1003–10. doi: 10.1001/jamaoncol.2020.1024
Keywords: adult-type diffuse glioma, IDH mutant, pTERT mutation, MGMT methylation, CD47, TIGIT
Citation: Ma L, Shi Y, Li C, Deng B, Jiang J, Cao Y, Wang L and Li H (2024) MGMT unmethylation and high levels of CD47 and TIGIT indicate a poor prognosis in adult diffuse gliomas. Front. Immunol. 15:1323307. doi: 10.3389/fimmu.2024.1323307
Received: 17 October 2023; Accepted: 23 January 2024;
Published: 09 February 2024.
Edited by:
Guoyi Yan, Xinxiang University, ChinaCopyright © 2024 Ma, Shi, Li, Deng, Jiang, Cao, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongyan Li, bGh5eHh5QHZpcC4xNjMuY29t; Lianghai Wang, bGhfd2FuZ0BzaHp1LmVkdS5jbg==
†These authors have contributed equally to this work
 Lingbo Ma1†
Lingbo Ma1† Lianghai Wang
Lianghai Wang Hongyan Li
Hongyan Li