- 1Department of Translational Biomedicine and Neuroscience, Institute of Respiratory Disease, University “Aldo Moro”, Bari, Italy
- 2Respiratory Medicine Unit, Policlinico “G. Rodolico-San Marco” University Hospital, Catania, Italy
- 3Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy
- 4Division of Internal Medicine and Clinical Immunology, Department of Internal Medicine and Clinical Complexity, Azienda Ospedaliera Universitaria Federico II, Napoli, Italy
- 5Department of Medicine, Surgery and Dentistry, University of Salerno, Salerno, Italy
- 6Unitá Operativa (UO) Clinica Pneumologica SUN, Dipartimento Pneumologia ed Oncologia, Azienda Ospedaliera Specialistica dei Colli, Napoli, Italy
- 7Department of Health Sciences, University “Magna Graecia” of Catanzaro, Catanzaro, Italy
- 8Department of Medical and Surgical Sciences, University of Foggia, Foggia, Italy
- 9Allergology and Pulmonology Unit, Provincial Outpatient Center of Palermo, Palermo, Italy
- 10Unitá Operativa Semplice Dipartimentale (UOSD) Malattie Respiratorie “Federico II”, Ospedale Monaldi, Azienda Ospedaliera (AO) Dei Colli, Naples, Italy
- 11Department of Medical and Surgical Sciences, School and Chair of Allergology and Clinical Immunology, University of Foggia, Foggia, Italy
- 12Division of Allergy and Clinical Immunology, University of Salerno, Salerno, Italy
- 13Division of Respiratory Diseases, Department of Health Promotion Sciences, Maternal and Infant Care, Internal Medicine and Medical Specialties (PROMISE), University of Palermo, Palermo, Italy
Introduction: Clinical remission (CliR) achievement has been recognized as a new potential outcome in severe asthma. Nevertheless, we still lack a detailed profile of what features could better identify patients undergoing clinical remission. In this study, we aim to address this issue, tracing a possible identikit of patients fulfilling remission criteria.
Methods: We enrolled 266 patients with severe eosinophilic asthma (SEA) treated with a 12-month course of anti-IL5/IL5 receptor (IL5r) monoclonal antibodies. Patients with no exacerbation, OCS withdrawal, ACT ≥ 20 and FEV1 ≥ 80% after 1 year of biologic treatment were classified as in clinical remission.
Results: 30.5% of the enrolled patients achieved remission after biologic administration. CliR group showed a lower number of baseline asthma exacerbations and better lung function parameters, with a trend for higher ACT scores and a less frequent history of a positive skin prick test. CliR achievement was unlikely in presence of a higher BMI, a positive skin prick test, an increased number of asthma exacerbations before biologic treatment, anti-muscarinic administration, and a previous diagnosis of EGPA, bronchiectasis or osteoporosis. In contrast, a better lung function, an increased blood eosinophilic count, the presence of chronic rhinosinusitis with nasal polyps and a more frequent use of reliever therapy predicts remission development. Changes in exacerbations number, OCS use, ACT scores and FEV1% between remittent and non-remittent patients arise at specific follow up timepoints and are positively associated with CliR achievement.
Discussion: anti-IL5/IL5r biologics can induce CliR in a proportion of patients with SEA. Patients achieving remission demonstrate specific clinical, functional and inflammatory features, as well as a specific moment of improvement in all the CliR items.
Introduction
In the past years, the primary focus in asthma care was centered around controlling symptoms using appropriate inhaled corticosteroid (ICS) therapy and, when needed, oral corticosteroid (OCS) (1). However, the advent of biologics has introduced a new era in asthma treatment, enabling targeted and personalized approaches. As a result of this therapeutic evolution, many patients with severe asthma experienced a significant reduction in exacerbations and OCS administration and improvement in their lung function as well as their quality of life.
These positive outcomes have given rise to a more ambitious aim: achieving potential remission of the disease. The concept of remission, similarly to other chronic conditions, is increasingly becoming widespread, and it should be regarded as a new cornerstone of asthma management. This notion is supported by the observation that spontaneous remission occurs in varying percentages (ranging from 5% to 69%) of children during their transition into adolescence and adulthood (2). Based on the recognition of this natural occurrence, some authors have extended this concept, identifying monoclonal antibodies as potentially able to induce clinical remission (CliR) in a subset of patients with type-2 severe asthma. Recently, several definitions of CliR have been proposed by different research groups, according to which subset of criteria was considered to classify patients as remittent. Menzies-Gow et al, in a three round Delphi survey involving many experts in the field of severe asthma, defined the achievement of CliR after at least 12 months of follow up according to four main criteria: 1) the absence of relevant symptoms or asthma exacerbations; 2) the complete cease of systemic corticosteroid treatments; 3) the improvement and stabilization of lung function; 4) the agreement between patients and health care practitioners on remission achievement (3). The presence of CliR along with the absence of signs related to asthma inflammation or bronchial hyperresponsiveness would then define a complete on-treatment remission. Later, Canonica and his collaborators from the Severe Asthma Network Italy (SANI) provided another definition of CliR (4), with the cessation of OCS therapies for at least 12 months as a crucial element for CliR achievement and the absence of symptoms, exacerbations, and a documented lung function stability as additional criteria to separate a partial CliR (two out of three criteria met) from a complete CliR (all three criteria met). Following this path, several studies adopted a wide range of different parameters and cut-offs for CliR definition (5–7), also revealing that patients undergoing biologic therapies and achieving remission seem to exhibit distinctive features, such as a more pronounced T-helper 2 inflammatory profile, better baseline clinical and functional performance, and a specific subset of comorbidities that could be predictive of a good treatment response (3, 4, 7–11). Despite this evidence, we need more crucial information about what clinical, functional, and biological features could predict the achievement of CliR.
Our study aimed to conduct an extensive analysis of these factors to establish a detailed patient profile that would be associated with CliR in patients treated with anti-IL5 or anti-IL5 receptor (IL-5r) monoclonal antibodies.
Materials and methods
Study design
We conducted a real-life, observational, retrospective, multicenter analysis from the “Southern Italy Network on Severe Asthma Therapy”, screening patients who underwent a one-year treatment course with Mepolizumab or Benralizumab from September 2017 to March 2022.
Study population
The “Southern Italy Network on Severe Asthma Therapy” includes 654 patients with severe asthma treated with monoclonal antibodies. For the final analysis, we included 266 patients with age > 18 years and severe eosinophilic asthma (SEA) treated with anti-IL5 or anti-IL5 receptor (IL-5r) monoclonal antibodies for at least 12 consecutive months (Figure 1). SEA diagnosis was made according to GINA recommendations and ERS guidelines (1, 12). During baseline visit (T0) we gathered anamnestic and anthropometric data, information regarding asthma exacerbation, maintenance and rescue therapies, lung function and Type-2 inflammatory biomarkers.
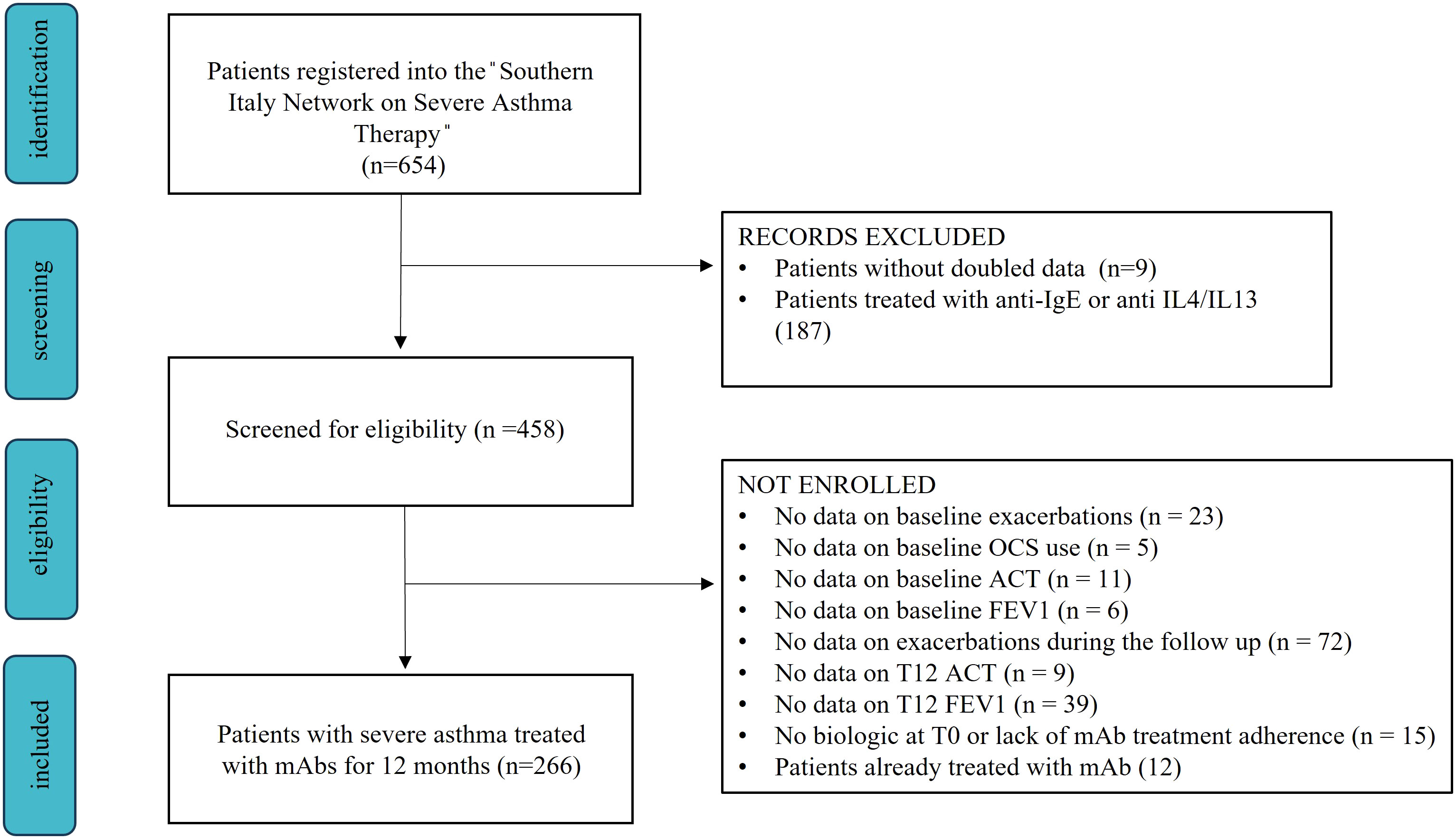
Figure 1 Flow chart showing the enrollment process from the “Southern Italy Network on Severe Asthma Therapy” database.
Asthma Control Test (ACT) and Test of Adherence to Inhalers (TAI) were used to assess symptoms control and inhaled treatment adherence, respectively. In case of poor medication adherence (TAI < 50 points), patients were re-trained on the use of their inhaler and encouraged to fulfill the prescribed therapy. Anti-IL5/IL5r biologics were prescribed according to the following criteria:
● Mepolizumab 100 mg once a month in patients with blood eosinophilic count (BEC) > 150 cells/mm3 (and a single BEC > 300 cells/mm3 in the last year) and the need of a treatment with OCS for at least 6 months or ≥ 2 exacerbations treated with OCS or hospitalization in the last year;
● Benralizumab 30 mg (once every 4 weeks for the first 3 doses, then every 8 weeks) in presence of a BEC ≥ 300 cells/mm3 and the need of a treatment with OCS or ≥ 2 exacerbations treated with OCS or hospitalization in the last year.
From a clinical standpoint, all the centers of the network agreed to evaluate a possible biologic discontinuation due to lack of efficacy only after a complete 1-year course of Mepolizumab or Benralizumab. For this reason, our final dataset could also include patients with a partial/poor response to biologics along with those achieving remission, allowing more generalizable results.
During the follow up, we performed visits after 1 (T1), 3 (T3), 6 (T6) and 12 (T12) months from the start of biologic treatment. CliR was defined after 1 year of biologic therapy when patients achieved no exacerbations, no OCS maintenance treatment, ACT ≥ 20 and FEV1 ≥ 80%.
Patients were excluded from the study in case of lack of treatment adherence, previous use of severe asthma monoclonal antibodies or missing data on items defining clinical remission (OCS administration, exacerbations, symptoms and lung function).
The study was approved by the Bari Institutional Ethics Committees (Ethical Committee number: 6313) and was conducted following the Helsinki Declaration of 1975 and the Good Clinical Practice standards. Patients signed written informed consent before enrollment.
Statistical analysis
After assessing data distribution with Kolmogorov-Smirnov test, we compared means and standard deviations (SD) of continuous variables with normal distribution using T-test, while data exhibiting non-normal distribution were analyzed with Mann-Whitney-U as medians and interquartile ranges (IQR). Discrete variables were expressed with percentages and analyzed with Chi² or Fisher exact test. In presence of missing data (see Supplementary Tables 1, 2), we performed multiple imputation analysis before processing our dataset. Then, to explore which factors could be predictive of CliR achievement, we run a multivariate logistic least absolute shrinkage and selection operator (LASSO) regression, selecting variables significantly associated with the outcome. Briefly, LASSO regression, differently from other regression analyses, allows variables selection using a penalty (λ), which reduces variance and shrinks toward zero non-relevant covariates (13). Finally, we tested the robustness of our models using receiver operating characteristics (ROC) curves with their areas under the curves (AUC). Statistical analysis was performed using SPSS Statistics 26 (IBM Corporation) and R software (version 4.0.2, R Foundation), considering a P value < 0.05 as statistically significant.
Results
Baseline features
Our study analyzed 266 patients with severe asthma (see Figure 1), 154 were treated with Mepolizumab and 112 with Benralizumab. Baseline characteristics of the enrolled patients are reported in Table 1. Our study population was predominantly female (65%), with a mean age of 58 years and a mean BMI of 26.3 kg/m2. Most patients had no smoking history (66.5%) with only 5.6% of them classified as current smokers. The most reported comorbidities were chronic rhinosinusitis with nasal polyposis (CRwNP, 50%) and gastroesophageal reflux disease (GERD, 35.3%). Nearly all patients had at least one asthma exacerbation in the year before the start of biologic treatment, 31.6% of whom required an access to emergency department (ED). Furthermore, our cohort exhibited a mean ACT score of 13.4 points and a frequent use of OCS as maintenance treatment (75.6%), with a median dose of 12.5 mg/day. Considering T-helper 2 biomarkers, fraction exhaled nitric oxide (FeNO), blood eosinophilic count (BEC) and total immunoglobulin E (IgE), median values were 36 ppb, 645 cell/mcl and 155.4 U/lt, respectively. During the follow-up, the use of biologics improved all the explored features (see Supplementary Table 3; Supplementary Figure 1). Asthma exacerbation frequency, accesses to emergency departments due to respiratory symptoms worsening and OCS administration dropped from T0 to T12 (P < 0.0001), along with FeNO (P = 0.0007) and BEC reduction (P < 0.0001). Oppositely, ACT scores and lung function rose after the start of biologic treatment (see Supplementary Figures 1, 2), confirming the improvement granted by anti-IL5/IL5r on severe asthma symptoms. None of the enrolled patients experienced any serious adverse effect due to biologic administration, defined as any reaction leading to death, hospitalization or a persistent disability during the follow up time (14).
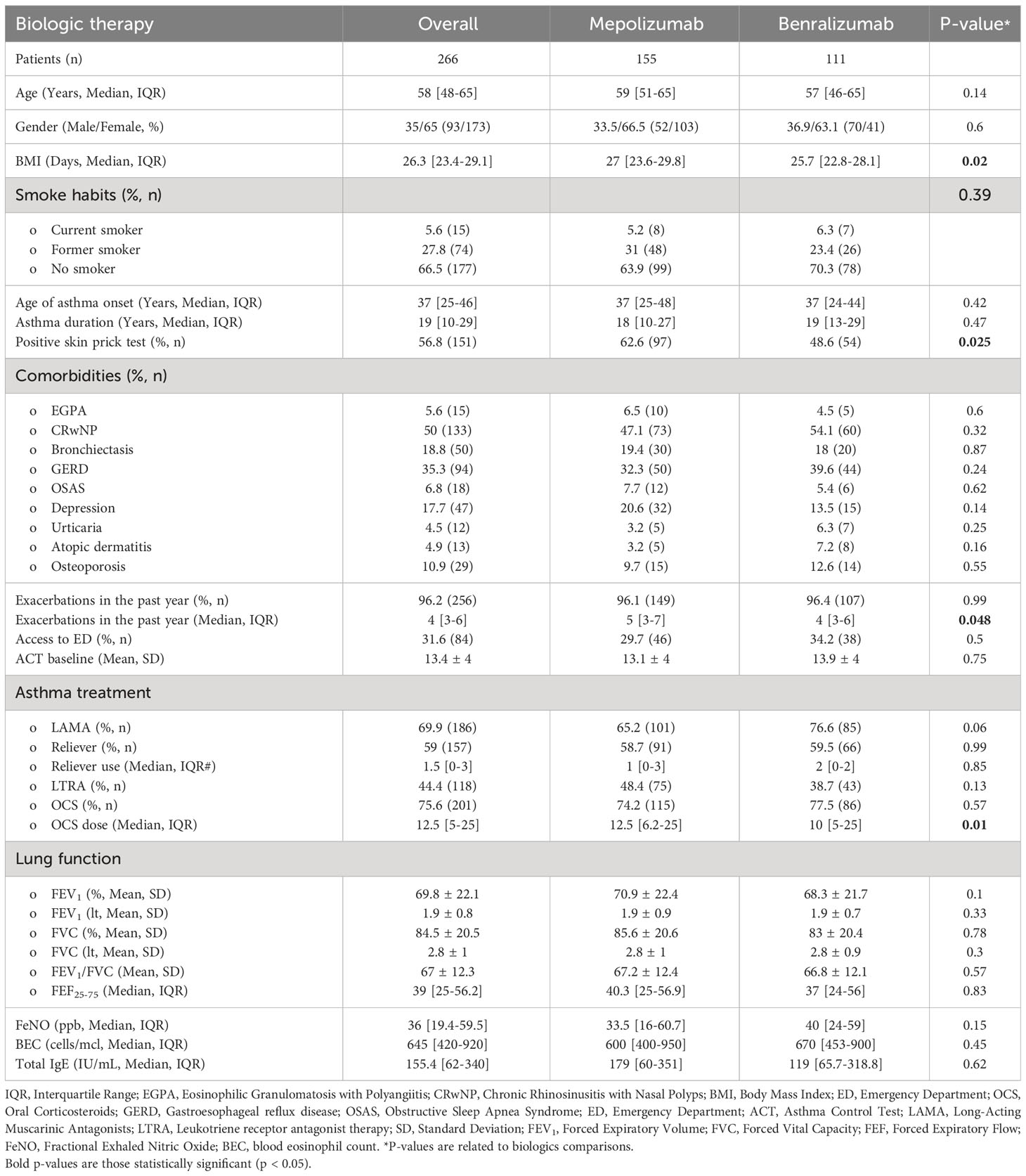
Table 1 Baseline features of the overall enrolled population according to the administered biologic treatment.
As regard main differences according to the administered biologic treatment, patients receiving Mepolizumab reported a higher BMI, were more frequently positive to the skin prick test (see Table 1, P = 0.025) and had a slightly increased number of exacerbations in the year before the enrollment (P = 0.048) requiring higher dosed of OCS (P = 0.01).
Characteristics of remittent patient
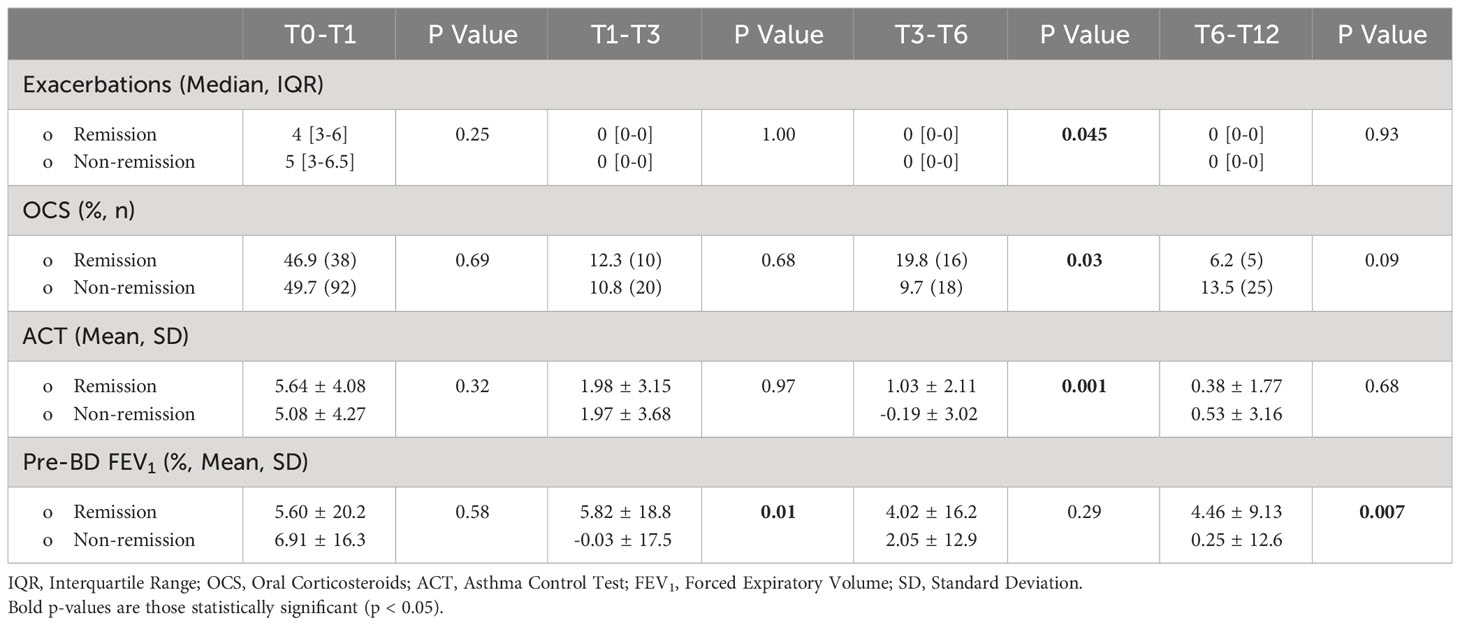
Starting from the proposed criteria for CliR definition (3, 4), 30.5% of the enrolled patients achieved this outcome, while 64.7% fulfilled ≥ 3 criteria and 84.2% at least 2 items. Notably, 77.3% of non-CliR patients still achieved at least 2 remission criteria, confirming the substantial impact of biologic treatments even in non-remittent patients (see Supplementary Table 4). Patients belonging to CliR group revealed a better baseline clinical status (see Table 2). Indeed, those achieving remission showed higher mean ACT scores (P = 0.07), reporting less exacerbations (P = 0.04), a less frequent prescription of anti-muscarinic (P = 0.014) and a better lung function at baseline, as demonstrated by higher FEV1, FVC and FEF25-75 values (P < 0.0001). Baseline clinical features of patients achieving CliR showed no substantial differences according to the choice of biologic treatment (See Supplementary Table 5), while Non-CliR patients receiving Mepolizumab had higher BMI (P = 0.03), lower levels of FeNO (P = 0.02) and a higher number of exacerbations in the year before the enrollment (P = 0.03) requiring greater OCS doses (P = 0.03). After 12 months of anti-IL5/IL5r therapy, we found a significant ACT (P < 0.0001) and FEV1 (P < 0.0001) improvement in remittent patients (see Supplementary Table 6), which could explain the simultaneous reduction in the reliever therapy use (P = 0.02) and in LAMA administration (P = 0.03). Table 3 shows main differences in CliR criteria according to remission status and follow up timepoints. Patients achieving CliR decreased the number of exacerbations and the use of OCS between T3 and T6 (P = 0.05, P = 0.03, respectively), also increasing their ACT scores in the same follow up phase (see Figure 2, P = 0.001). In contrast, FEV1 gap between patients achieving CliR and those without remission followed two phases, with a first increase between T1-T3 (P = 0.01) and a second one from T6 to T12 (P = 0.007, see Figure 3).
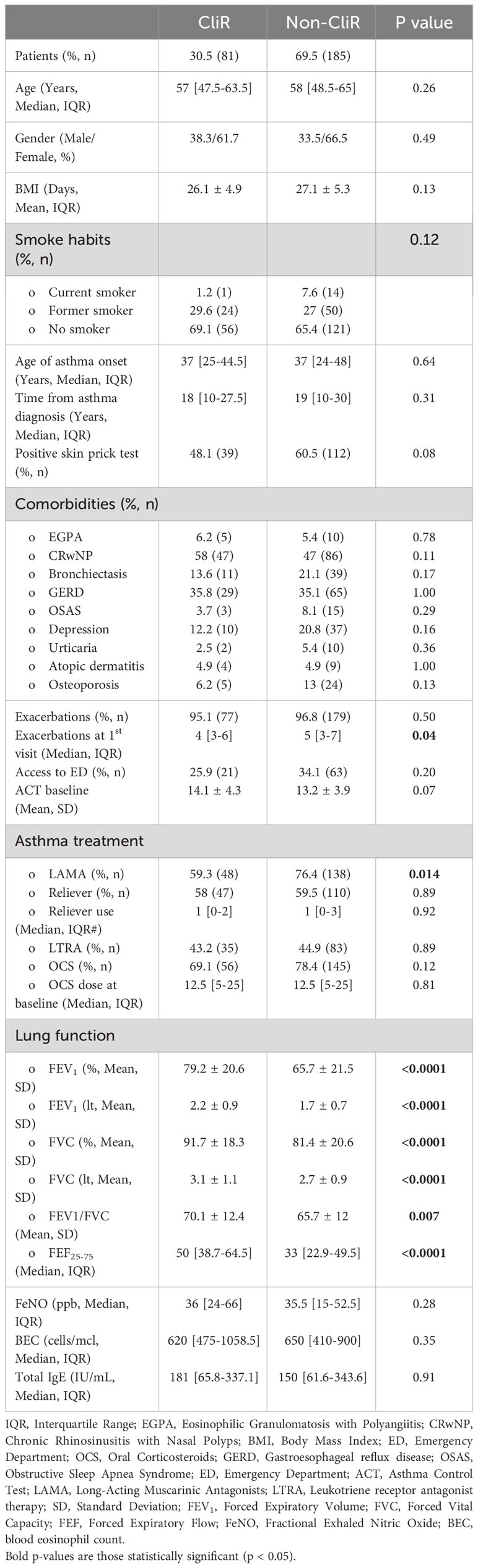
Table 2 Baseline features in patients achieving clinical remission (CliR) vs non-remittent patients (Non-CliR).
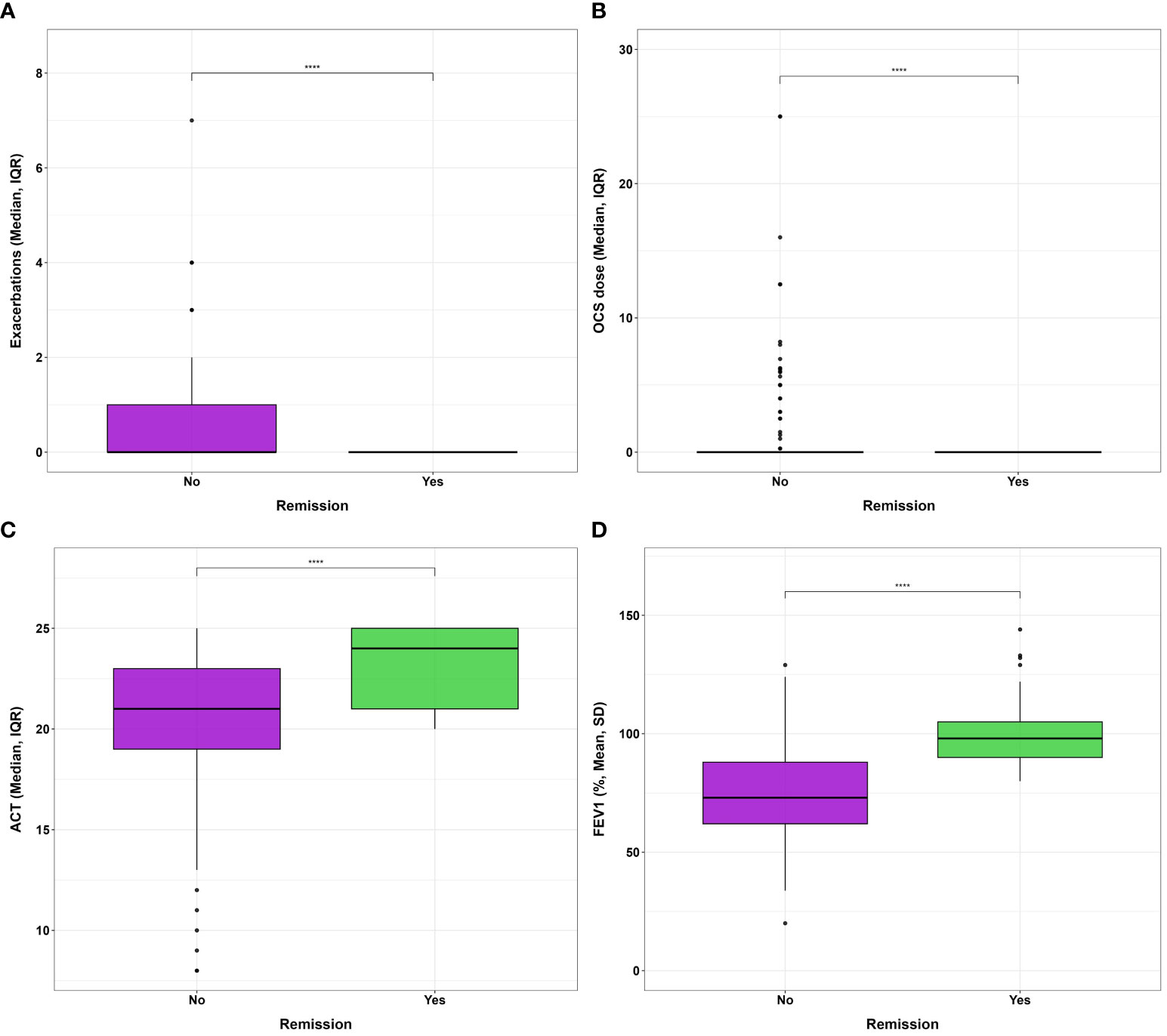
Figure 2 Differences in (A) exacerbation number, (B) oral corticosteroid administered dose, (C) ACT and (D) FEV1% after 1 year of biologic therapy according to clinical remission achievement. **** P<0.0001.
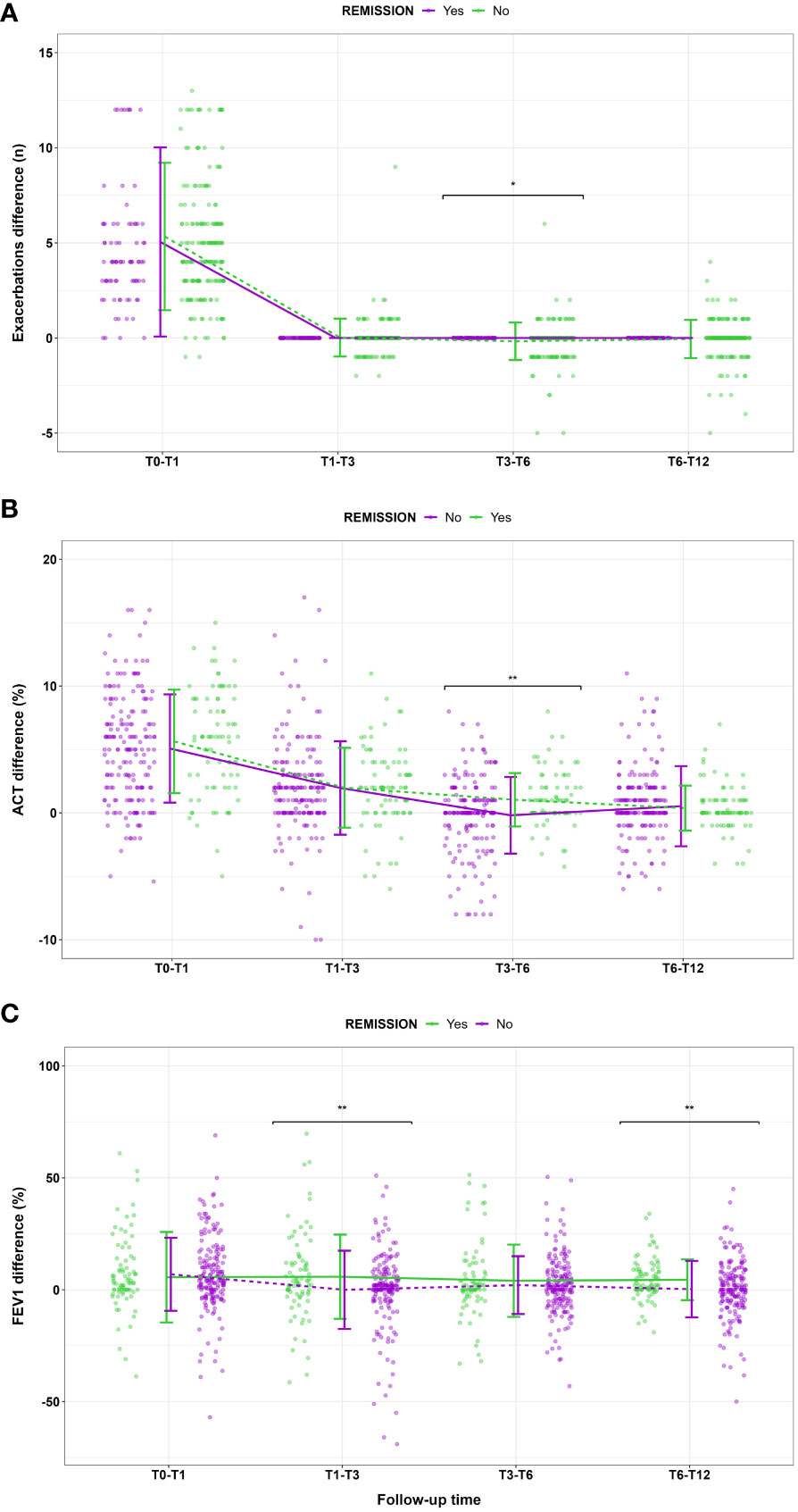
Figure 3 Main changes in (A) exacerbation number, (B) ACT score and (C) FEV1% according to clinical remission achievement at different follow up timepoints. Data are presented as means and standard deviations for graphical purposes. * P<0.05; **P<0.01.
Predictive features for CliR achievement
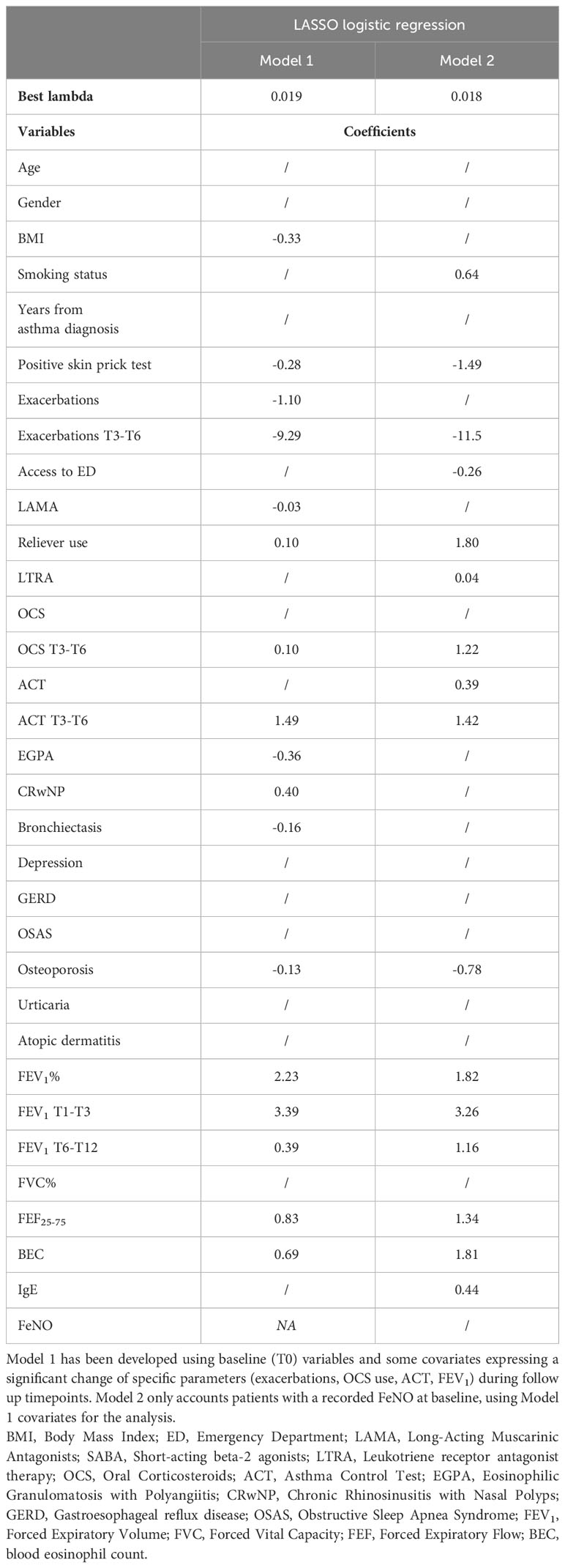
We performed a LASSO logistic regression considering several clinical, functional, and biological variables from the entire enrolled cohort (Model 1, see Table 4). Due to the amount of missing data at baseline, FeNO was excluded from the first regression analysis, being separately tested in a different LASSO regression model (Model 2). Model 1 revealed that patients with a higher baseline BEC, with better lung function, with comorbid CRwNP, with a more frequent administration of OCS or inhaled reliever therapy (as needed SABA or ICS-Formoterol) were more likely to attain this outcome. Moreover, changes in main remission items (exacerbations, OCS, ACT, FEV1) at specific follow up timepoints were all related to CliR development at T12. In contrast, the presence of a positive skin prick test, a higher Body Mass Index (BMI), a greater number of exacerbations before the start of the biologic therapy, the administration of Long-Acting Muscarinic Antagonists (LAMA), a previous diagnosis of comorbid eosinophilic granulomatosis with polyangiitis (EGPA), bronchiectasis or osteoporosis were negative predicting factors for CliR development. Model 2 did not demonstrate a significant role of FeNO on remission achievement. Finally, ROC curves analysis confirmed the robustness of our models (see Supplementary Table 7 and Figure 4, Model 1 AUC = 0.87 P < 0.0001; Model 2 AUC = 0.88, P < 0.0001).
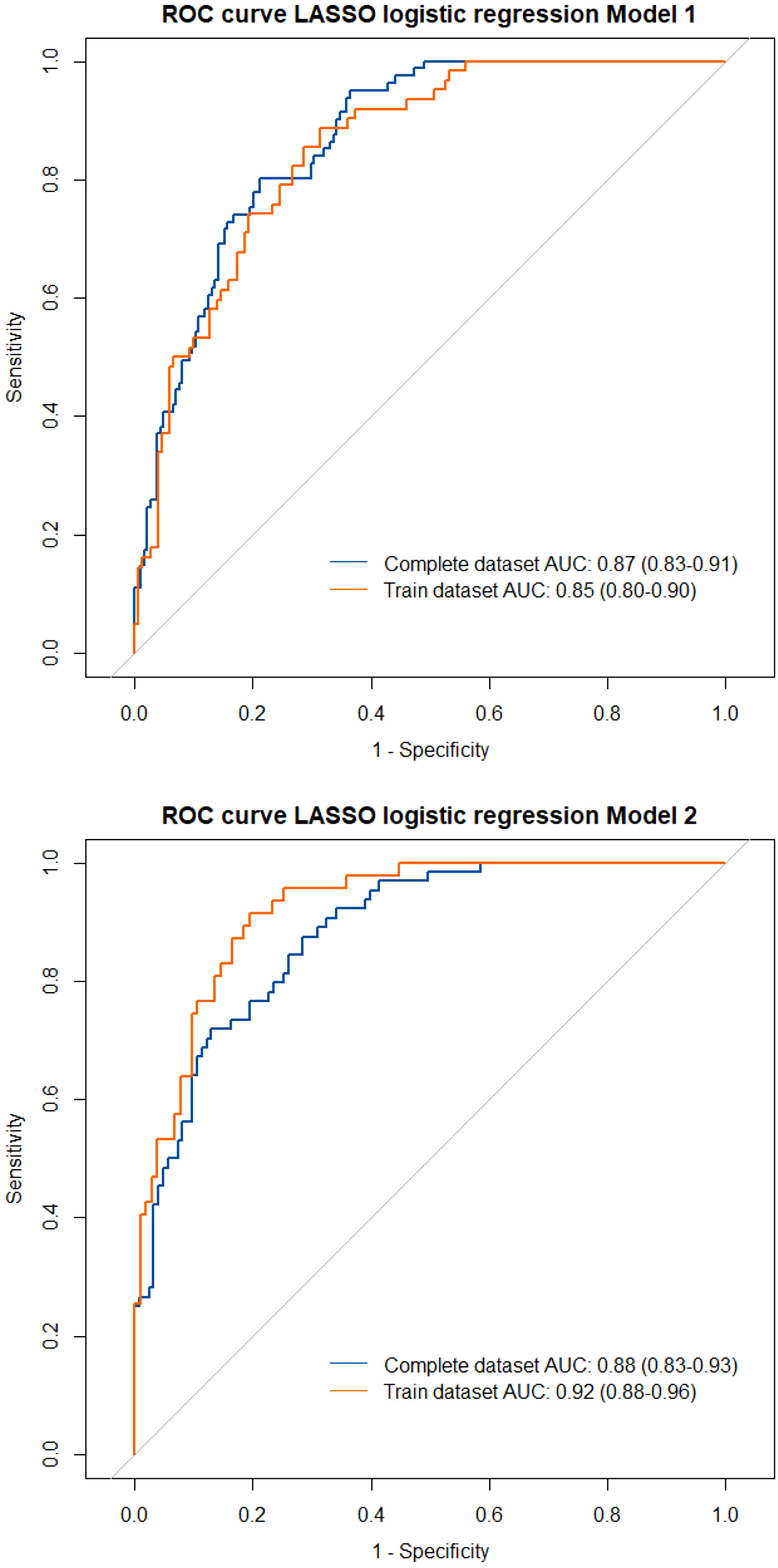
Figure 4 Receiver operating characteristics (ROC) curves with area under the curves (AUC) for the tested LASSO regression models. Model 1 included all the enrolled patients in the analysis, considering the complete dataset (blue line) and the training dataset of the LASSO regression (orange line). Model 2 only included patients with a recorded FeNO at baseline.
Discussion
Our study highlights which factors could contribute to the achievement of CliR in patients with severe asthma undergoing biologic treatment with Mepolizumab or Benralizumab. Among the enrolled population, over 30% of patients treated with Mepolizumab or Benralizumab achieved CliR at T12, a result in line with data from other real-life cohorts (7). Notably, we found no substantial differences in the remission rate (P = 0.59) and in the baseline features of patients achieving CliR according to the administered biologic (see Supplementary Table 5). These data probably reflect the similar clinical and functional effects of anti-IL5 and anti-IL5R following eosinophils depletion, which translates into comparable remission rates and CliR baseline features. Future prospective studies should assess whether biologic choice could represent a discriminating factor for CliR prediction.As reported in Table 2, remittent patients show better baseline clinical features, with a lower number of exacerbations (P = 0.04), a better ACT score (P = 0.07), less frequent use of LAMA to control severe asthma symptoms and an overall better lung function, with higher values of FEV1 (P < 0.0001), FVC (P < 0.0001) and FEF25-75 (P < 0.0001). Interestingly, the improvement of the four elements characterizing CliR is not uniform through follow up timepoints. Indeed, while the reduction in exacerbations, OCS administration and the ACT increase become evident between 3 and 6 months from the start of anti-IL5/IL-5r treatment, the gap in FEV1 response to biologic treatment has two spikes, one between 1 and 3 months from baseline and one between 6 and 12 months (Table 3). As shown in Figure 3, FEV1 improvement is sustained during the follow up only in remittent patients, dropping twice (T1-T3 and T6-T12) before the end of the study in those who did not achieve CliR. These data were also confirmed in our LASSO regression model (Table 4), where these changes in main remission items during the follow up resulted consistently associated with CliR development. Previous studies reported that several clinical, functional and biological severe asthma features could be related to biologics clinical response (5, 15, 16). Nevertheless, considering the complexity of severe asthma, we believed that tracing the trajectory of CliR only using baseline features of our cohort would have been less reliable than evaluating the changes of specific clinical and functional items of remission. For this reason, we merged baseline and follow up relevant features in our predictive model, describing how CliR features develop after the start of biologic therapies. The timely improvement in respiratory function and other observed remission features strongly indicate that these therapies have a fast-acting potential in inducing severe asthma CliR. As previously postulated, the path leading to CliR seems to follow a specific timeline since a real difference in main asthma clinical items (exacerbations, OCS, symptoms) between remittent and non-remittent patients becomes clinically visible only after at least 3 months of biologic therapy (17). Moreover, lung function improvement seems to be affected by biologic therapies earlier than other clinical features. As stated by registration trials and real-life evidence on Mepolizumab and Benralizumab (18–23), FEV1 improvement is generally reported between 4 and 12 weeks from the first monoclonal antibody administration, which is in line with our findings on the overall population and on patients with CliR. Although we are not aware of the potential mechanism explaining the double gap we found on FEV1 improvement in remittent vs non-remittent patients, it has been reported that blood and sputum eosinophilia could play a role determining lung function trajectory. Patients with a lower sputum eosinophilia, along with a more pronounced neutrophilic airway inflammation, tend to have a worse FEV1 trajectory, while those with a marked blood and sputum eosinophilia have a fast and sustained improvement in lung function (24). Moreover, we cannot also exclude that a certain degree of airway remodeling or the impact of comorbidities in non-remittent patients could have influenced the FEV1 improvement with anti IL5/IL5r therapy.
Among the other predictors included in our Model 1 LASSO regression, CliR is associated with a lower BMI, a greater BEC, a lower number of exacerbations at baseline, the absence of positive skin prick tests, a positive history of CRwNP and with the absence of a previous diagnosis of EGPA, bronchiectasis or osteoporosis. Moreover, patients who did not receive anti-muscarinic therapy but were more frequently treated with their inhaled reliever were also more frequently classified as remittent. Whereas the relationship between CliR, a lower BMI, a lower baseline exacerbation rate, an increased BEC and comorbid CRwNP have been previously identified (5, 8, 10, 15, 25), remains poorly described the impact of other comorbidities on this outcome. In patients suffering from EGPA, the add-on treatment with anti-IL5/IL-5r monoclonal antibodies provides a consistent clinical and functional improvement, allows corticosteroid tapering and favors the achievement of EGPA remission (26–28). Nevertheless, the simultaneous presence of SEA and comorbid EGPA could slow biologic treatment response (29), also influencing the achievement of CliR. Furthermore, complete OCS weaning after biologic administration in patients with EGPA could be challenging, not only due to the disease itself but also from a “cultural” standpoint, since corticosteroid cessation mainly depends on centers experience and confidence with monoclonal antibodies. Similarly to EGPA, biologics targeting IL5/IL5r have shown to improve exacerbation frequency and OCS consumption in patient with SEA and bronchiectasis (30). Nevertheless, the odds for CliR achievement in these patients seems to be lower when compared with those with SEA (31). Possible reasons for this finding could include an increased expression of non-T2 inflammation pathways, severe muco-ciliary disfunction and microbiologic colonization with multi drug resistant microorganism, which could negatively affect the clinical response to biologics (31, 32). As regards osteoporosis, there are few studies investigating its relationship with severe asthma outcomes. Considering the close causal link between osteoporosis and corticosteroid use (33), a worse clinical response to inhaled or systemic steroid-based therapies could induce patients and clinicians to increase the therapeutic corticosteroid dose, increasing the risk for osteoporosis development. This event would give birth to a distorted mechanism, where the lack of response induces the increase of inhaled or systemic steroid dosages, with negative repercussions on bone metabolism and osteopenia.
Another important aspect is the relationship between CliR and the presence of a positive skin prick test. Our results showed a negative impact of the presence of atopy on CliR, as also confirmed by the lower number of patients with an atopic trait in the remittent group (48% vs 60.5%, P = 0.08). As recently reported by Moermans and colleagues, the presence of higher sputum levels of several biomarkers related to eosinophilic inflammation (IL-5, eotaxin-1, eosinophil peroxidase) can predict CliR achievement (16). Moreover, the coexistence of the eosinophilic and the atopic traits in severe asthma patients could lead to the simultaneous activation of several cytokine pathways, whit possible drawbacks on treatment response (34).
As stated before, CliR seems also to be associated with non-biologic treatments such as LAMA and reliever therapy. To date, we are unaware of what possible reason could explain the link between the use of relievers and remission achievement. However, the negative correlation between LAMA use and CliR could be explained by the tendency to prescribe anti-muscarinics in patients with a worse FEV1, which are those who will have more difficulty in fulfilling remission criteria. Another important aspect concerns the impact of lung function over severe asthma remission. As also shown in Table 2, a better baseline lung function foreshadows CliR achievement, not only in terms of FEV1, but also considering FEF25-75. The multicentric ATLANTIS study revealed a significant association between small airway disfunction and asthma severity (35). Besides, Chan and colleagues previously highlighted the close relationship between biologic treatment response and small airways functional assessment, addressing FEF25-75 and impulse oscillometry (IOS) measures as useful tools for severe asthma management (36, 37). Our data seem to confirm these findings, not only showing a consistent improvement of FEF25-75 after 1 year of biologic therapy (see Supplementary Table 3), but also certifying its predictive role for CliR achievement (see Table 4).
Our study has several limitations, such as its retrospective design, a limited follow up time and the lack of information on other functional (i.e. IOS, bronchial challenge test) and inflammatory biomarkers (i.e. induced sputum), which would have better described what pathophysiological mechanism primary acts in the path toward CliR. Nevertheless, the multicentric nature of the study, a rigorous selection of the enrolled patients and a robust predictive model for CliR allows the generalizability of our results, strengthening the idea of a personalized clinical approach according to patient response to biologic treatments.
In conclusion, our study highlighted a possible identikit for patients achieving CliR after a 1-year course of Mepolizumab or Benralizumab. “Remission potential” seem to be characterized by a specific subset of baseline clinical, functional, and biological features, as well as a timely improvement in all main remission criteria within 24 weeks from biologic treatment start. These results could allow clinicians to better tailor their therapeutic choices in patients with severe asthma, applying the principles of precision medicine to everyday clinical practice.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Bari Institutional Ethics Committees. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
GC: Conceptualization, Project administration, Supervision, Validation, Writing – original draft. AP: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft. SN: Data curation, Methodology, Validation, Writing – original draft. AD: Data curation, Validation, Writing – review & editing. AV: Data curation, Investigation, Project administration, Writing – review & editing. CCa: Data curation, Supervision, Writing – review & editing. CP: Data curation, Supervision, Validation, Writing – review & editing. FM: Data curation, Project administration, Supervision, Visualization, Writing – original draft. GS: Data curation, Validation, Writing – review & editing. GV: Data curation, Validation, Writing – review & editing. MD: Data curation, Supervision, Validation, Writing – review & editing. MC: Data curation, Supervision, Validation, Writing – review & editing. MT: Data curation, Validation, Writing – review & editing. NS: Data curation, Supervision, Validation, Writing – review & editing. CCr: Conceptualization, Data curation, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Name of Collaborators: Nunzio Crimi: Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy; Alida Benfante: Division of Respiratory Diseases, Department of Health Promotion Sciences, Maternal and Infant Care, Internal Medicine and Medical Specialties (PROMISE), University of Palermo, Palermo, Italy; Giuseppe Spadaro: Center for Basic and Clinical Immunology Research (CISI), University of Naples Federico II, Naples, Italy; Danilo Di Bona: Department of Precision and Regenerative Medicine and Jonic Area, Unit of Allergology, University of Bari Aldo Moro, Bari, Italy; Girolamo Pelaia: Department of Health Sciences, University “Magna Graecia” of Catanzaro, Catanzaro, Italy; Maria Pia Foschino Barbaro, Department of Medical and Surgical Sciences, University of Foggia, Italy; Cristina Cardini: Department of Surgery, Medicine, Molecular Biology and Critical Care, University of Pisa, Pisa, Toscana, Italy; Angelantonio Maglio: Department of Medicine, Surgery and Dentistry, University of Salerno, 84084 Salerno, Italy. All authors would like to thank Daniela Portacci for her contribution to the graphical contents of this paper.
Conflict of interest
GC reported grants or contracts from Astrazeneca, Chiesi, GlaxoSmithKline, Sanofi, Grifols; payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events from Astrazeneca, GlaxoSmithKline, Sanofi; support for attending meetings and/or travel from Astrazeneca, Menarini, Chiesi. AP reported payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events from Astrazeneca, GlaxoSmithKline, Chiesi, Sanofi. AD reported payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events from Astrazeneca, GlaxoSmithKline, Sanofi, Novartis, Lofarma. GS reported payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events from Astrazeneca, GlaxoSmithKline, Sanofi. GV reported payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events from GlaxoSmithKline, Sanofi. MD reported consulting fees from Astrazeneca, GlaxoSmithKline, Sanofi; payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events from Astrazeneca, GlaxoSmithKline, Sanofi, Novartis; participation on Data Safety Monitoring Board or Advisory Board from Astrazeneca, GlaxoSmithKline, Sanofi. MT reported consulting fees from Astrazeneca, GlaxoSmithKline, Novartis. Nicola Scichilone reported payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events from Astrazeneca, GlaxoSmithKline, Chiesi, Sanofi. CCr reported payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events from Astrazeneca, GlaxoSmithKline, Sanofi, Menarini, ResMed, Fisher&Paykel.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1343362/full#supplementary-material
References
1. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J (2014) 43(2):343–73. doi: 10.1183/09031936.00202013
2. Cohn L. Can asthma biologics change the course of disease and induce drug-free remission? J Allergy Clin Immunol (2022) 150(1):59–61. doi: 10.1016/j.jaci.2022.04.005
3. Menzies-Gow A, Bafadhel M, Busse WW, Casale TB, Kocks JWH, Pavord ID, et al. An expert consensus framework for asthma remission as a treatment goal. J Allergy Clin Immunol (2020) 145(3):757–65. doi: 10.1016/j.jaci.2019.12.006
4. Canonica GW, Blasi F, Carpagnano GE, Guida G, Heffler E, Paggiaro P, et al. SANI definition of Clinical Remission in Severe Asthma: a Delphi consensus. J Allergy Clin Immunol Pract (2023) 11(12):3629–37. doi: 10.1016/j.jaip.2023.07.041
5. McDowell PJ, McDowell R, Busby J, Eastwood MC, Patel PH, Jackson DJ, et al. Clinical remission in severe asthma with biologic therapy: an analysis from the UK Severe Asthma Registry. Eur Respir J (2023) 62(6):2300819. doi: 10.1183/13993003.00819-2023
6. Thomas D, McDonald VM, Stevens S, Harvey ES, Baraket M, Bardin P, et al. Biologics (mepolizumab and omalizumab) induced remission in severe asthma patients. Allergy (2023). doi: 10.1111/all.15867
7. Pavord I, Gardiner F, Heaney LG, Domingo C, Price RG, Pullan A, et al. Remission outcomes in severe eosinophilic asthma with mepolizumab therapy: Analysis of the REDES study. Front Immunol (2023) 14. doi: 10.3389/fimmu.2023.1150162
8. Oishi K, Hamada K, Murata Y, Matsuda K, Ohata S, Yamaji Y, et al. A real-world study of achievement rate and predictive factors of clinical and deep remission to biologics in patients with severe asthma. J Clin Med (2023) 12(8). doi: 10.3390/jcm12082900
9. Kavanagh JE, d’Ancona G, Elstad M, Green L, Fernandes M, Thomson L, et al. Real-world effectiveness and the characteristics of a “Super-responder” to mepolizumab in severe eosinophilic asthma. Chest (2020) 158(2):491–500. doi: 10.1016/j.chest.2020.03.042
10. Kavanagh JE, Hearn AP, Dhariwal J, d’Ancona G, Douiri A, Roxas C, et al. Real-world effectiveness of benralizumab in severe eosinophilic asthma. Chest (2021) 159(2):496–506. doi: 10.1016/j.chest.2020.08.2083
11. Nannini LJ. Treat to target approach for asthma. J Asthma (2020) 57(6):687–90. doi: 10.1080/02770903.2019.1591443
13. Zou H. The adaptive lasso and its oracle properties. J Am Stat Assoc (2012) 101(476):1418–29. doi: 10.1198/016214506000000735
14. Medicines Agency E. Guideline on good pharmacovigilance practices (GVP) - Annex I - Definitions (Rev 4). (2017).
15. Harvey ES, Langton D, Katelaris C, Stevens S, Farah CS, Gillman A, et al. Mepolizumab effectiveness and identification of super-responders in severe asthma. Eur Respir J (2020) 55(5). doi: 10.1183/13993003.02420-2019
16. Moermans C, Brion C, Bock G, Graff S, Gerday S, Nekoee H, et al. Sputum type 2 markers could predict remission in severe asthma treated with anti-IL-5. Chest (2023). doi: 10.1183/13993003.congress-2023.PA1934. 163(6):1368–79. Available at: https://pubmed.ncbi.nlm.nih.gov/36740095/
17. Portacci A, Dragonieri S, Carpagnano GE. Super-responders to biologic treatment in type 2-high severe asthma: passing fad or a meaningful phenotype? J Allergy Clin Immunol Pract (2023) 11(5):1417–20. doi: 10.1016/j.jaip.2023.01.021
18. Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med (2014) 371(13):1198–207. doi: 10.1056/NEJMoa1403290
19. Chupp GL, Bradford ES, Albers FC, Bratton DJ, Wang-Jairaj J, Nelsen LM, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med (2017) 5(5):390–400. doi: 10.1016/S2213-2600(17)30125-X
20. Pelaia C, Crimi C, Pelaia G, Nolasco S, Campisi R, Heffler E, et al. Real-life evaluation of mepolizumab efficacy in patients with severe eosinophilic asthma, according to atopic trait and allergic phenotype. Clin Exp Allergy (2020) 50(7):780–8. doi: 10.1111/cea.13613
21. Harrison TW, Chanez P, Menzella F, Canonica GW, Louis R, Cosio BG, et al. Onset of effect and impact on health-related quality of life, exacerbation rate, lung function, and nasal polyposis symptoms for patients with severe eosinophilic asthma treated with benralizumab (ANDHI): a randomised, controlled, phase 3b trial. Lancet Respir Med (2021) 9(3):260–74. doi: 10.1016/S2213-2600(20)30414-8
22. Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet (2016) 388(10056):2115–27. doi: 10.1016/S0140-6736(16)31324-1
23. Varricchi G, Ferri S, Pepys J, Poto R, Spadaro G, Nappi E, et al. Biologics and airway remodeling in severe asthma. Allergy (2022) 77(12):3538–52. doi: 10.1111/all.15473
24. Kim JH, Chang HS, Shin SW, Baek DG, Son JH, Park CS, et al. Lung function trajectory types in never-smoking adults with asthma: clinical features and inflammatory patterns. Allergy Asthma Immunol Res (2018) 10(6):614–27. doi: 10.4168/aair.2018.10.6.614
25. Sposato B, Bianchi F, Ricci A, Scalese M. Clinical asthma remission obtained with biologics in real life: patients’ Prevalence and characteristics. J Pers Med (2023) 13(6). doi: 10.3390/jpm13061020
26. Wechsler ME, Akuthota P, Jayne D, Khoury P, Klion A, Langford CA, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med (2017) 376(20):1921–32. doi: 10.1056/NEJMoa1702079
27. Bettiol A, Urban ML, Dagna L, Cottin V, Franceschini F, Del Giacco S, et al. Mepolizumab for eosinophilic granulomatosis with polyangiitis: A european multicenter observational study. Arthritis Rheumatol (2022) 74(2):295–306. doi: 10.1002/art.41943
28. Nolasco S, Portacci A, Campisi R, Buonamico E, Pelaia C, Benfante A, et al. Effectiveness and safety of anti-IL-5/Rα biologics in eosinophilic granulomatosis with polyangiitis: a two-year multicenter observational study. Front Immunol (2023) 14. doi: 10.3389/fimmu.2023.1204444
29. Portacci A, Campisi R, Buonamico E, Nolasco S, Pelaia C, Crimi N, et al. Real-world characteristics of “super-responders” to Mepolizumab and Benralizumab in severe eosinophilic asthma and EGPA. ERJ Open Res (2023) 9(5). doi: 10.1183/23120541.00419-2023
30. Bendien SA, Kroes JA, van Hal LHG, Braunstahl GJ, Broeders MEAC, Oud KTM, et al. Real-world effectiveness of IL-5/5Ra targeted biologics in severe eosinophilic asthma with comorbid bronchiectasis. J Allergy Clin Immunol: In Practice (2023) 11(9):2724–2731.e2. doi: 10.1016/j.jaip.2023.05.041
31. Campisi R, Nolasco S, Pelaia C, Impellizzeri P, D’Amato M, Portacci A, et al. Benralizumab effectiveness in severe eosinophilic asthma with co-presence of bronchiectasis: A real-world multicentre observational study. J Clin Med (2023) 12(12). doi: 10.3390/jcm12123953
32. Shoemark A, Shteinberg M, De Soyza A, Haworth CS, Richardson H, Gao Y, et al. Characterization of eosinophilic bronchiectasis: A European multicohort study. Am J Respir Crit Care Med (2022) 205(8):894–902. doi: 10.1164/rccm.202108-1889OC
33. Chalitsios CV, McKeever TM, Shaw DE. Incidence of osteoporosis and fragility fractures in asthma: a UK population-based matched cohort study. Eur Respir J (2020) 57(1). doi: 10.1183/13993003.01251-2020
34. Chan R, Lipworth B. Clinical Communications The triple type 2 signature confers more frequent exacerbations and worse midexpiratory flow in moderate to severe asthma. J Allergy Clin Immunol Pract (2023) 11:2926–2928.e1. doi: 10.1016/j.jaip.2023.05.021
35. Postma DS, Brightling C, Baldi S, Van den Berge M, Fabbri LM, Gagnatelli A, et al. Exploring the relevance and extent of small airways dysfunction in asthma (ATLANTIS): baseline data from a prospective cohort study. Lancet Respir Med (2019) 7(5):402–16. doi: 10.1016/S2213-2600(19)30049-9
36. Chan R, Lipworth BJ. Impact of biologic therapy on the small airways asthma phenotype. Lung (2022) 200(6):691. doi: 10.1007/s00408-022-00579-2
Keywords: severe asthma, anti-IL5, anti-IL5 receptor, clinical remission, biologics, FEV1, exacerbations, corticosteroids
Citation: Carpagnano GE, Portacci A, Nolasco S, Detoraki A, Vatrella A, Calabrese C, Pelaia C, Montagnolo F, Scioscia G, Valenti G, D’Amato M, Caiaffa MF, Triggiani M, Scichilone N and Crimi C (2024) Features of severe asthma response to anti-IL5/IL5r therapies: identikit of clinical remission. Front. Immunol. 15:1343362. doi: 10.3389/fimmu.2024.1343362
Received: 23 November 2023; Accepted: 05 January 2024;
Published: 23 January 2024.
Edited by:
Diego Kauffmann-Guerrero, LMU Munich University Hospital, GermanyReviewed by:
Milos Jesenak, Comenius University, SlovakiaValérian Dormoy, Institut National de la Santé et de la Recherche Médicale (INSERM), France
Copyright © 2024 Carpagnano, Portacci, Nolasco, Detoraki, Vatrella, Calabrese, Pelaia, Montagnolo, Scioscia, Valenti, D’Amato, Caiaffa, Triggiani, Scichilone and Crimi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Portacci, YS5wb3J0YWNjaTAxQGdtYWlsLmNvbQ==
 Giovanna Elisiana Carpagnano
Giovanna Elisiana Carpagnano Andrea Portacci
Andrea Portacci Santi Nolasco
Santi Nolasco Aikaterini Detoraki
Aikaterini Detoraki Alessandro Vatrella
Alessandro Vatrella Cecilia Calabrese
Cecilia Calabrese Corrado Pelaia
Corrado Pelaia Francesca Montagnolo1
Francesca Montagnolo1 Giulia Scioscia
Giulia Scioscia Maria D’Amato
Maria D’Amato Massimo Triggiani
Massimo Triggiani Nicola Scichilone
Nicola Scichilone