- 1Department of Pulmonary Medicine, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan
- 2Department of Thoracic Oncology, Osaka International Cancer Institute, Osaka, Japan
- 3Department of Respiratory Medicine and Hematology, School of Medicine, Hyogo Medical University, Nishinomiya, Japan
- 4Department of Respiratory Medicine, Fujita Health University School of Medicine, Toyoake, Japan
- 5Department of Respiratory Medicine, Fukuoka University Hospital, Nanakuma, Japan
- 6Department of Respiratory Medicine, Japanese Red Cross Kyoto Daiichi Hospital, Kyoto, Japan
- 7Department of Respiratory Medicine, Japanese Red Cross Kyoto Daini Hospital, Kyoto, Japan
- 8Department of Respiratory Medicine, Saiseikai Suita Hospital, Suita, Japan
- 9Department of Medical Oncology, Fukuchiyama City Hospital, Fukuchiyama, Japan
- 10Department of Pulmonary Medicine, Kyoto Chubu Medical Center, Nantan, Japan
- 11Department of Respiratory Medicine, Uji-Tokushukai Medical Center, Uji, Japan
- 12Department of Respiratory Medicine, Saiseikai Shigaken Hospital, Rittou, Japan
- 13Department of Respiratory Medicine, Rakuwakai Otowa Hospital, Kyoto, Japan
Introduction: The proportion of older patients diagnosed with advanced-stage non-small cell lung cancer (NSCLC) has been increasing. Immune checkpoint inhibitor (ICI) monotherapy (MONO) and combination therapy of ICI and chemotherapy (COMBO) are standard treatments for patients with NSCLC and programmed cell death ligand-1 (PD-L1) tumor proportion scores (TPS) ≥ 50%. However, evidence from the clinical trials specifically for older patients is limited. Thus, it is unclear which older patients benefit more from COMBO than MONO.
Methods: We retrospectively analyzed 199 older NSCLC patients of Eastern Cooperative Oncology Group performance status (ECOG PS) 0-1 and PD-L1 TPS ≥ 50% who were treated with MONO or COMBO. We analyzed the association between treatment outcomes and baseline patient characteristics in each group, using propensity score matching.
Results: Of the 199 patients, 131 received MONO, and 68 received COMBO. The median overall survival (OS; MONO: 25.2 vs. COMBO: 42.2 months, P = 0.116) and median progression-free survival (PFS; 10.9 vs. 11.8 months, P = 0.231) did not significantly differ between MONO and COMBO group. In the MONO group, OS was significantly shorter in patients without smoking history compared to those with smoking history [HR for smoking history against non-smoking history: 0.36 (95% CI: 0.16-0.78), P = 0.010]. In the COMBO group, OS was significantly shorter in patients with PS 1 than those with PS 0 [HR for PS 0 against PS 1: 3.84 (95% CI: 1.44-10.20), P = 0.007] and for patients with squamous cell carcinoma (SQ) compared to non-squamous cell carcinoma (non-SQ) [HR for SQ against non-SQ: 0.17 (95% CI: 0.06-0.44), P < 0.001]. For patients with ECOG PS 0 (OS: 26.1 months vs. not reached, P = 0.0031, PFS: 6.5 vs. 21.7 months, P = 0.0436) or non-SQ (OS: 23.8 months vs. not reached, P = 0.0038, PFS: 10.9 vs. 17.3 months, P = 0.0383), PFS and OS were significantly longer in the COMBO group.
Conclusions: ECOG PS and histological type should be considered when choosing MONO or COMBO treatment in older patients with NSCLC and PD-L1 TPS ≥ 50%.
1 Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide (1). Non-small-cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancer cases, with the majority of NSCLC cases being diagnosed at an advanced, unresectable, and metastatic disease stage (2). Immune checkpoint inhibitors (ICIs), such as programmed death 1 (PD-1) or programmed death ligand 1 (PD-L1) antibodies, have improved the prognosis of advanced NSCLC. The PD-L1 tumor proportion score (TPS) is used as a predictor associated with treatment response (3, 4). In several phase 3 clinical trials, ICI monotherapy (MONO) has shown outstanding efficacy compared to chemotherapy in patients with NSCLC and PD-L1 TPS ≥ 50% and has been established as a standard first-line treatment option for such patients (5, 6). Concurrently, numerous phase 3 clinical trials for advanced NSCLC, regardless of PD-L1 TPS, have reported significantly improved clinical outcomes in patients treated with combination therapy of ICI and chemotherapy (COMBO) compared to those treated with chemotherapy alone. COMBO is thus considered as a standard first-line treatment for NSCLC (7–10). Similar to MONO, an association between PD-L1 TPS and therapeutic response has been reported in patients who received COMBO. Long-term follow-up analyses have shown favorable treatment responses particularly in the PD-L1 TPS ≥50% population (11, 12). Therefore, both MONO and COMBO are effective first-line treatment options for NSCLC patients with PD-L1 TPS ≥ 50%.
The proportion of older patients diagnosed with advanced-stage lung cancer has been increasing owing to the aging of the population (13), with almost half of all lung cancer cases occurring in patients aged 70 years or more (14). Consequently, formulating a treatment strategy for older patients with NSCLC is essential. However, evidence based on the clinical trials specifically for older patients is limited.
Pooled analyses of clinical trials involving pembrolizumab monotherapy in patients with PD-L1-positive advanced NSCLC reported a longer overall survival (OS) and superior safety profiles compared to standard chemotherapy in older patients with PD-L1 TPS ≥ 50%. However, approximately 40% of the older patients with NSCLC still experience mortality within 1 year, thus limiting the benefits of pembrolizumab monotherapy in this subgroup (15). Nonetheless, data on the efficacy and safety of COMBO for older patients are lacking, as COMBO may lead to more severe adverse events in older patients and is currently recommended only for a select group of patients (16). As such, the question of whether MONO or COMBO should be the preferred treatment choice for older patients with NSCLC and PD-L1 TPS ≥ 50% remains unanswered.
To this end, in this retrospective study, we conducted a real-world assessment to compare the efficacy and safety of MONO and COMBO in older patients with NSCLC and PD-L1 TPS ≥ 50%. In this study, we aimed to provide clarity on which clinical populations benefit from the addition of chemotherapy to ICI treatment.
2 Materials and methods
2.1 Patient population
This retrospective multicenter cohort study was conducted across 13 institutions in Japan. We collected the information on consecutive cases of advanced NSCLC (stage IV, including postoperative recurrence, according to the American Joint Committee on Cancer Staging Manual, version 8) in patients with PD-L1 TPS ≥ 50% who received first-line MONO or COMBO treatment between March 2017 and June 2021. We analyzed the treatment outcomes in older patients with PD-L1 TPS ≥ 50% and an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–1, since patients with NSCLC with poor ECOG are generally ineligible for several phase 3 clinical trials involving COMBO (7–10). In this study, ‘older patients’ were defined as those aged 70 years or older, following criteria established in previous clinical trials (17–20). Clinical data relevant to first-line treatment were obtained from electronic medical records. PD-L1 TPS in tumor cells was assessed using PD-L1 immunohistochemistry with the 22C3 pharmDx antibody (clone 22C3; Dako North America, Inc, Carpinteria, CA, USA), in accordance with the regulations of each facility.
This study was approved by the Ethics Review Board of the Kyoto Prefectural University of Medicine and was conducted with consent from the Ethics Review Board of each hospital (approval no. ERB-C-2113). Informed patient consent was not required owing to the retrospective nature of the study.
2.2 Assessments of efficacy and safety
Initially, we investigated the association between patient characteristics, including OS, in patients receiving either MONO or COMBO. Furthermore, we identified factors significantly associated with MONO or COMBO outcomes and compared the outcomes between patients administered with MONO and those administered with COMBO, with or without the identified factors. Treatment response was evaluated using the Response Evaluation Criteria in Solid Tumors version 1.1. Progression-free survival (PFS) was defined as the time from the start of first-line treatment until the occurrence of progressive disease or death from any cause. OS was defined as the time from the start of first-line treatment until death from any cause. The incidence of adverse events was assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Data were collected until February 28, 2023.
For comparing treatment outcomes between the MONO and COMBO groups, we adjusted for significant differences in the baseline characteristics of patients using propensity score matching (PSM) for the following variables: age, sex, smoking status, ECOG PS, histologic profile, PD-L1 TPS, and cancer stage. Nearest neighbor matching was performed at a ratio of 1:1 without replacement and a caliper of 0.2.
2.3 Statistical analysis
The relationship between age and other patient characteristics was examined using the Fisher’s exact test or the chi-square test. PFS and OS were calculated using the Kaplan–Meier method and were compared using the log-rank test. The hazard ratios (HRs) and their corresponding 95% confidence intervals (Cis) were determined using a Cox proportional hazard model in both univariate and multivariate analyses. All statistical analyses were performed using EZR version 1.61 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (version 4.2.2; The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R designed to add statistical functions frequently used in biostatistics. Statistical significance was set at P < 0.05.
3 Results
3.1 Patient characteristics
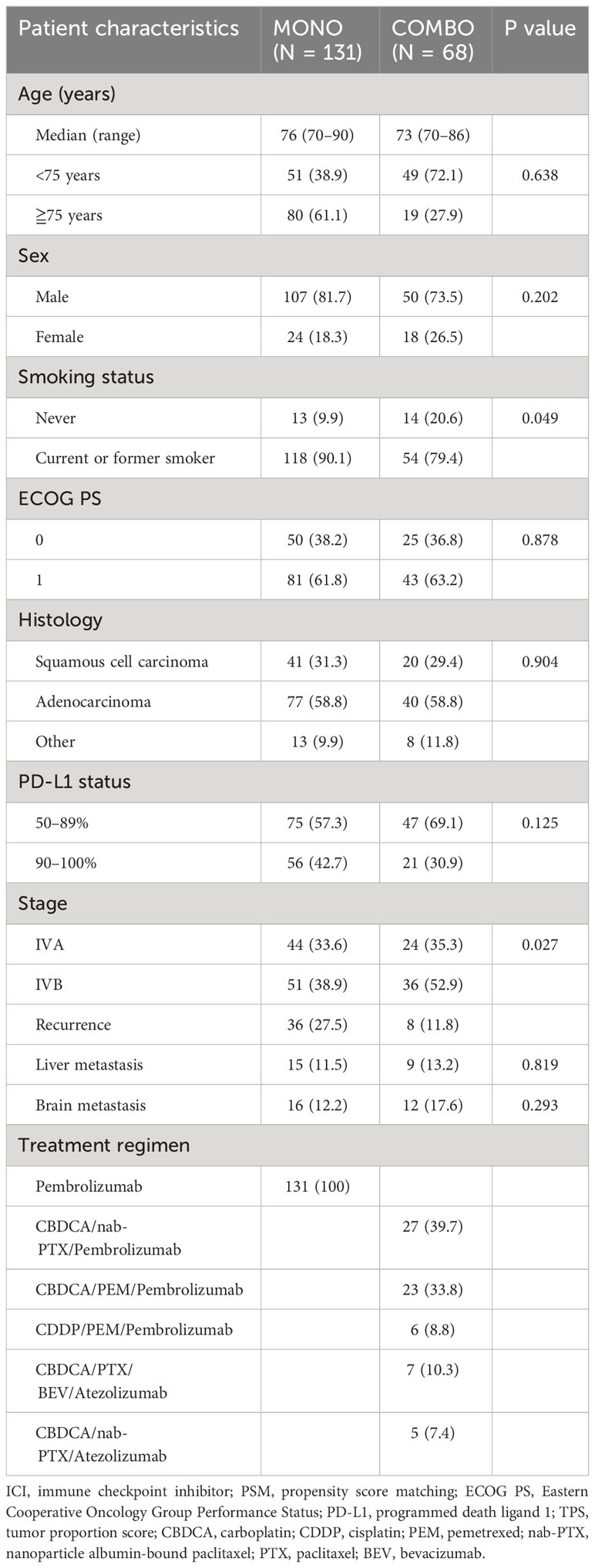
A total of 446 patients with PD-L1 TPS ≥ 50% who received MONO or COMBO were screened for enrollment. Among them, 204 patients younger than 70 years were excluded. In addition, 43 patients with poor PS (PS 2–4) were excluded, as only patients with PS 0–1 were examined in clinical trials for treatment with COMBO. Finally, a total of 199 patients aged 70 years or more with PD-L1 TPS ≥ 50% were enrolled in this study, of which 131 received MONO while 68 received COMBO as the first-line treatment (Supplementary Figure 1). All patients in the MONO group were treated with pembrolizumab monotherapy. In the COMBO group, 56 patients were treated with a regimen that included pembrolizumab and 12 patients were treated with a regimen that included atezolizumab. 29 patients were treated with a regimen that included pemetrexed. Five patients with epidermal growth factor receptor mutations and two with anaplastic lymphoma kinase fusion were included. The median follow-up time was 21.9 months for both treatment groups.
The baseline characteristics of the patients are summarized in Table 1. Compared with the COMBO group, the MONO group had a significantly higher number of patients with a history of smoking (54/68 patients or 79.4% vs. 118/131 patients or 90.1%, P = 0.049), and there were significant differences in cancer stage between the two groups. After PSM weighting, each group contained 52 matched patients, with no significant differences in baseline characteristics observed between the two matched groups (Supplementary Table 1). The median follow-up time was 22.4 months for both groups.
3.2 Treatment outcomes in all patients
Overall, the median OS in the COMBO group appeared to be longer than that in the MONO group, though the difference was not statistically significant (42.2 vs. 25.0 months, P = 0.0502. Similarly, the median PFS (11.2 vs. 11.5 months, P = 0.683) did not significantly differ between the MONO and COMBO groups (Supplementary Figure 2). Adverse effects of CTCAE grade ≥ 3 were more frequently observed in the COMBO group (31/68, 45.6%) compared to the MONO group (39/131, 29.8%, P = 0.030). Analysis of the treatment outcomes after PSM showed that the median OS was similar between the MONO and COMBO groups (25.2 vs. 42.2 months, P = 0.116; Figure 1A). The median PFS was also similar between the two groups (10.9 vs. 11.8 months, P = 0.231; Figure 1B).
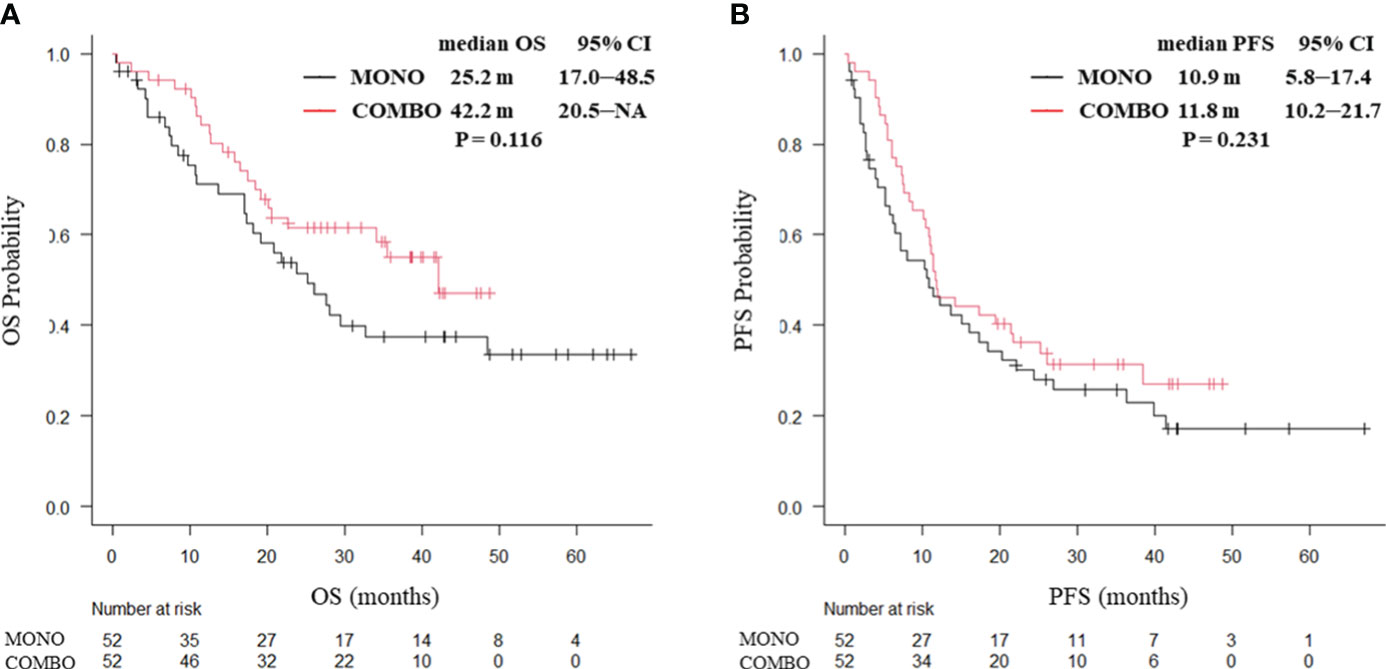
Figure 1 Kaplan–Meier curves for (A) OS and (B) PFS of patients aged ≥70 years with PD-L1 TPS ≥ 50% who received ICI monotherapy or combination therapy of ICI and chemotherapy after PSM (N = 104). OS, overall survival; PFS, progression-free survival; CI, confidence interval; PD-L1 TPS, programmed cell death ligand-1 tumor proportion score; ICI, immune checkpoint inhibitor; PSM, propensity score matching; MONO, ICI monotherapy; COMBO, ICI and chemotherapy combination therapy.
3.3 Treatment outcomes according to patient characteristics in each treatment group
We further analyzed the association of OS with patient characteristics within each treatment group. The results for the MONO group are presented in Table 2. Univariate analysis using Cox proportional hazards models indicated that none of the factors were significantly associated with OS. However, the multivariate Cox proportional hazards regression model revealed that smoking status (HR 0.36 [95% CI, 0.16–0.78], P = 0.010) was an independent predictor of OS.
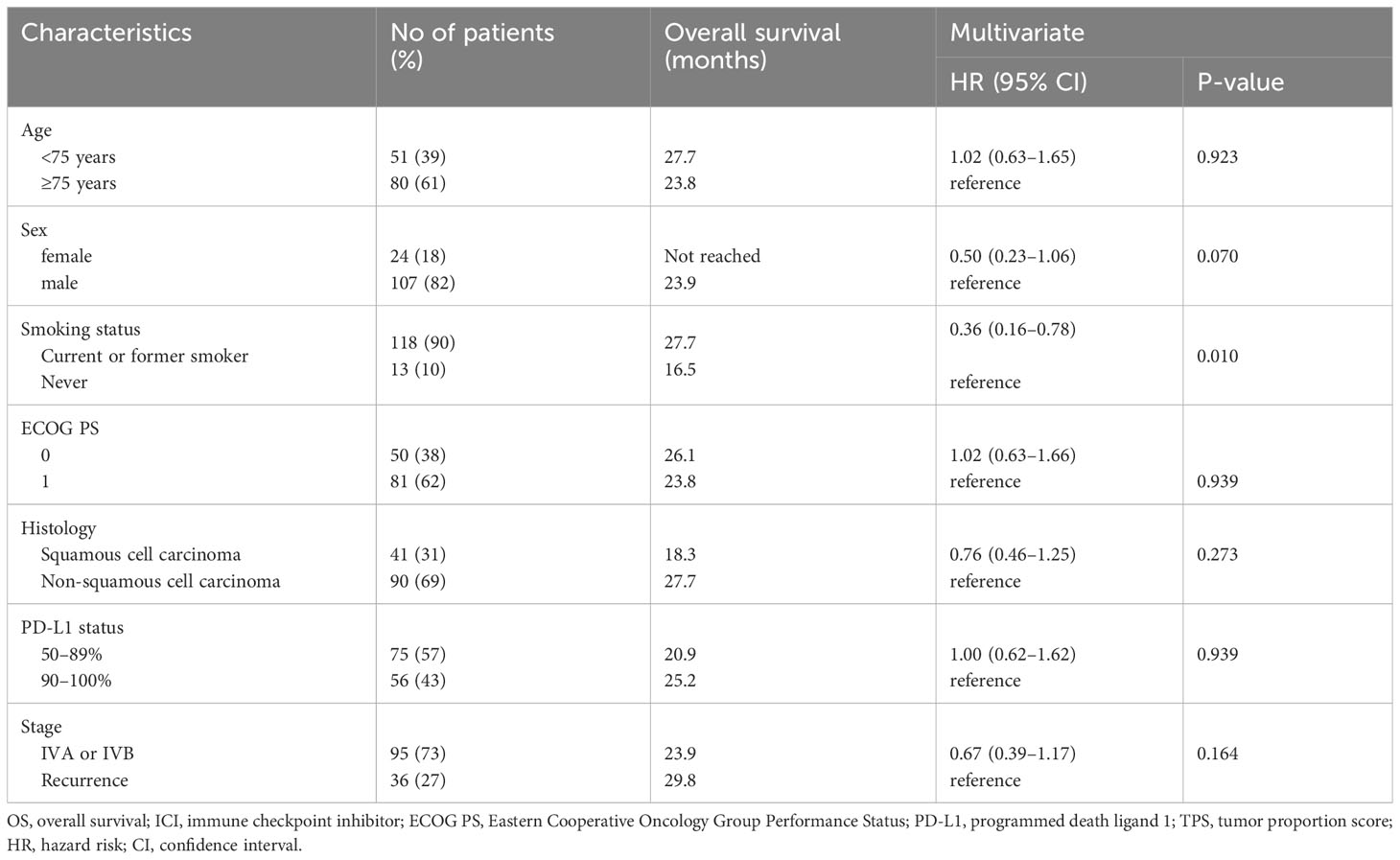
Table 2 Cox proportional hazard models (multivariate analyses) for overall survival of patients aged ≥70 years with PD-L1 TPS ≥ 50% treated with ICI monotherapy (N = 131).
OS according to patient characteristics in the COMBO group is presented in Table 3. Univariate analysis using Cox proportional hazards models indicated that ECOG PS (HR 2.84 [95% CI, 1.16–6.98], P = 0.018) and histology (HR 0.32 [95% CI, 0.15–0.66], P = 0.0013) were significantly associated with OS. Similarly, the multivariate Cox proportional hazard regression model showed that ECOG PS (HR 3.84 [95% CI, 1.44¬10.20], P = 0.007) and histology (HR 0.17 [95% CI, 0.06–0.44, P < 0.001) were independent predictors of OS.
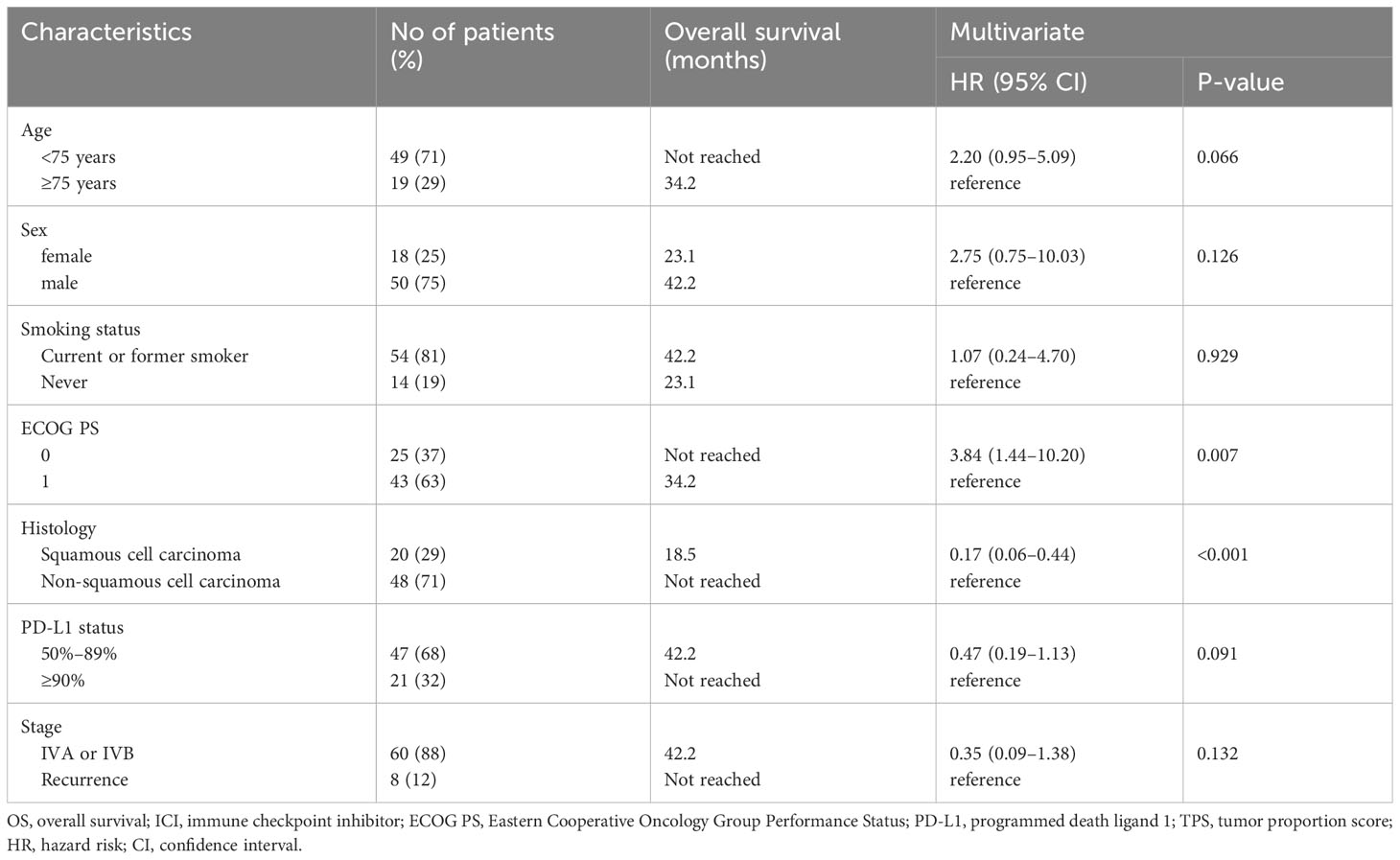
Table 3 Cox proportional hazard models (multivariate analyses) for overall survival in patients ≥70 years old with PD-L1 TPS ≥ 50% treated with combination therapy of ICI and chemotherapy (N = 68).
3.4 Treatment outcomes in patients with or without each independent predictor
We then compared the treatment outcomes of MONO and COMBO in patients with or without each identified independent clinical factor (smoking status, ECOG PS, and histology).
3.4.1 Treatment outcomes in patients with or without smoking history
After PSM weighting, 92 patients with smoking history and 12 without smoking history were included. No significant differences in baseline characteristics of the patients were found between the two groups. In patients with a smoking history, no significant differences in the median OS (27.7 months vs. not reached, P = 0.084; Figure 2A) and median PFS (10.6 vs. 11.8 months, P = 0.208; Figure 2B) were observed between the MONO and COMBO groups. Similarly, in patients without a smoking history, no significant differences in the median OS (15.6 vs. 20.1 months, P = 0.252) and the median PFS (10.9 vs. 7.4 months, P = 0.43; Supplementary Figure 3) were observed.
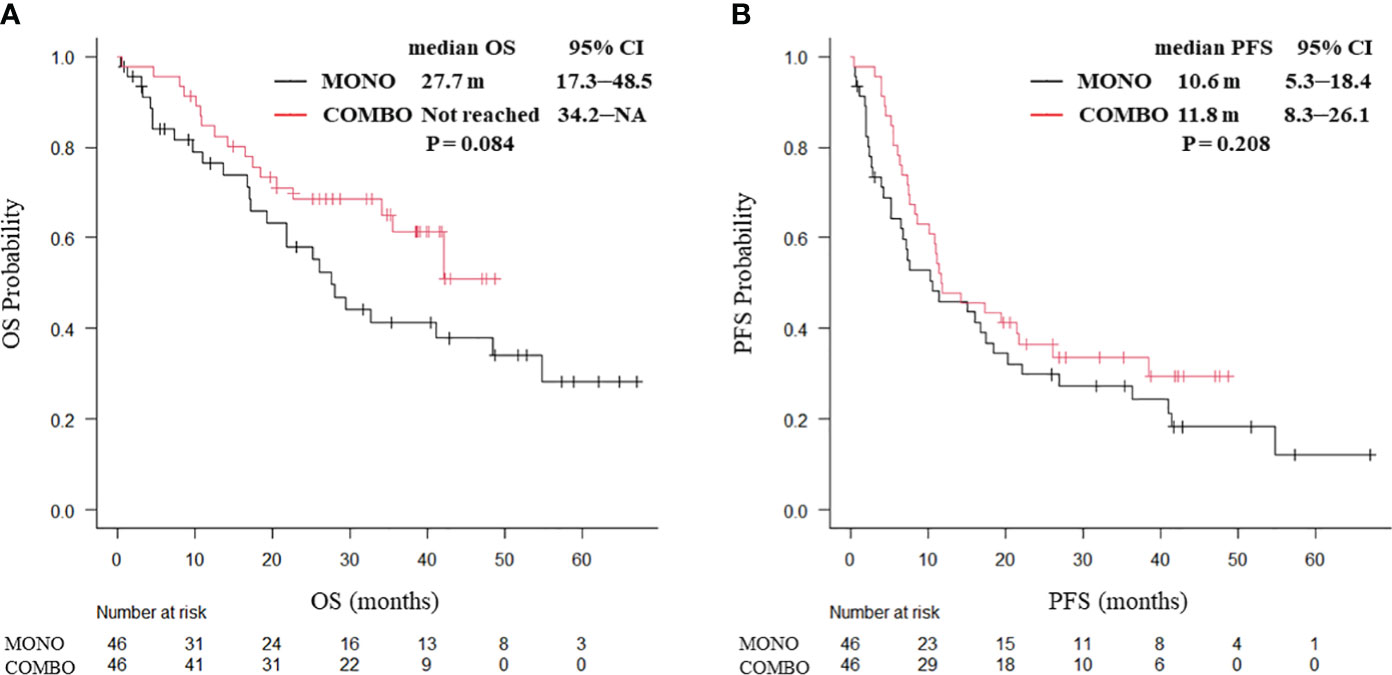
Figure 2 Kaplan–Meier curves for (A) OS and (B) PFS of patients aged ≥70 years with PD-L1 TPS ≥ 50% and with a smoking history, who received ICI monotherapy or combination therapy of ICI and chemotherapy after PSM (N = 92). OS, overall survival; PFS, progression-free survival; CI, confidence interval; PD-L1 TPS, programmed cell death ligand-1 tumor proportion score; ICI, immune checkpoint inhibitor; PSM, propensity score matching; MONO, ICI monotherapy; COMBO, ICI and chemotherapy combination therapy.
3.4.2 Treatment outcomes in patients with ECOG PS 0 or 1
PSM weighting, 38 patients with ECOG PS 0 and 62 patients with ECOG PS 1 were included. In ECOG PS 0 patients, both the median OS (not reached vs. 26.1 months, P = 0.0031; Figure 3A) and median PFS (21.7 vs. 6.5 months, P = 0.0436; Figure 3B) were significantly longer in the COMBO group than in the MONO group. By contrast, in ECOG PS1 patients, the median OS (25.5 vs. 35.5 months, P = 0.544) and the median PFS (11.5 vs. 12.0 months, P = 0.406; Supplementary Figure 4) were similar between the two groups.
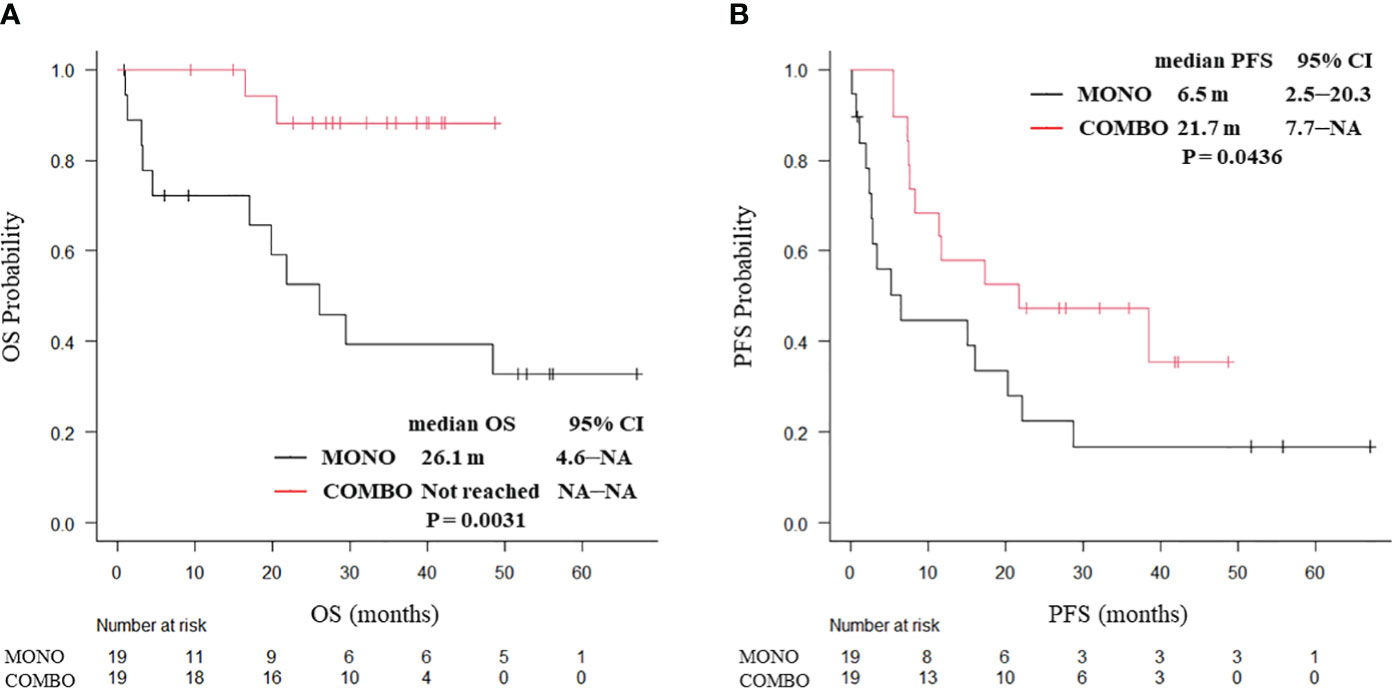
Figure 3 Kaplan–Meier curves for (A) OS and (B) PFS of ECOG PS 0 patients aged ≥70 years with PD-L1 TPS ≥ 50% who received ICI monotherapy or combination therapy of ICI and chemotherapy after PSM (N = 38). OS, overall survival; PFS, progression-free survival; CI, confidence interval; PD-L1 TPS, programmed cell death ligand-1 tumor proportion score; ICI, immune checkpoint inhibitor; PSM, propensity score matching; MONO, ICI monotherapy; COMBO, ICI and chemotherapy combination therapy.
3.4.3 Treatment outcomes in patients with squamous cell carcinoma or non-squamous cell carcinoma
After PSM weighting, 20 patients with squamous cell carcinoma and 78 with non-squamous cell carcinoma were included. In patients with squamous cell carcinoma, no significant differences were observed in the median OS (25.2 vs. 16.5 months, P = 0.566) and median PFS (9.0 vs. 10.2 months, P = 0.993; Supplementary Figure 5) between the MONO and COMBO groups. By contrast, both the median OS (not reached vs. 23.8 months, P = 0.0038; Figure 4A) and median PFS (17.3 vs. 10.9 months, P = 0.0383; Figure 4B) were significantly longer in the COMBO group than in the MONO group in patients with non-squamous cell carcinoma.
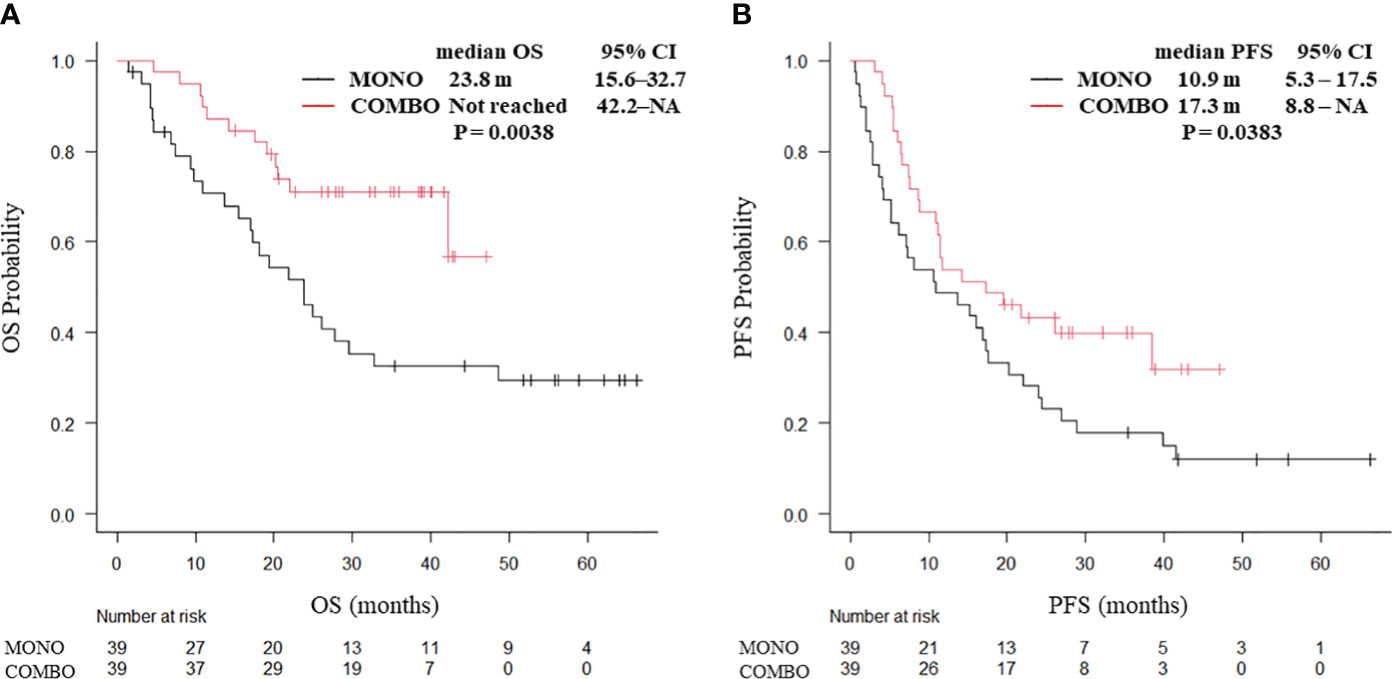
Figure 4 Kaplan–Meier curves for (A) OS and (B) PFS of patients with non-squamous cell carcinoma aged ≥70 years with PD-L1 TPS ≥ 50% who received ICI monotherapy or combination therapy of ICI and chemotherapy after PSM (N = 78). OS, overall survival; PFS, progression-free survival; CI, confidence interval; PD-L1 TPS, programmed cell death ligand-1 tumor proportion score; ICI, immune checkpoint inhibitor; PSM, propensity score matching; MONO, ICI monotherapy; COMBO, ICI and chemotherapy combination therapy.
4 Discussion
Aging is a complex process characterized by the accrual of genetic and environmental factors, leading to a diverse array of alterations, such as diminished telomeres, genomic instability, impaired mitochondrial function, disruption of protein homeostasis, epigenetic modifications, declining immune system functionality, and the onset of cellular senescence. Furthermore, increasing age heightens the susceptibility to developing lung cancer, a phenomenon that is exacerbated by the growing aging population worldwide (21). Aging has also been reported to reduce the generation of new T cells from the thymus and impair T cell diversity, indicating that the adverse consequences of aging on T cells could potentially influence therapeutic efficacy (22).
In this study, we retrospectively analyzed the treatment outcomes in older patients with NSCLC and with PD-L1 TPS ≥ 50% who received MONO or COMBO as first-line treatment, both of which are recognized as standard first-line treatment options for patients with NSCLC and high PD-L1 TPS. Our findings indicated that there were no significant differences in PFS and OS between MONO and COMBO in patients with NSCLC aged ≥ 70 years with PD-L1 TPS ≥ 50%. However, notably, in patients with an ECOG PS 0 or those with non-squamous cell carcinoma, both PFS and OS were significantly longer in the COMBO group compared to the MONO group. To the best of our knowledge, this study is the first to identify clinical factors in an older NSCLC population that may benefit from COMBO rather than from MONO, which may be applied as predictors for clinical decision-making in this population.
Despite the valuable insights provided by our study, several limitations should be acknowledged. First, this study was a retrospective, nonrandomized study. Thus, the possibility of selection bias cannot be ruled out. However, we conducted PSM to reduce selection bias. Second, adverse events and complications are always a concern in chemotherapy for older patients, but this study did not mention either in detail. Third, in the MONO group, subsequent therapy after progression of first line pembrolizumab monotherapy may have affected the prognosis. However, our study had a lack of data about the detail of second line treatment, and we could not investigate these association. Finally, the study included only Japanese patients, and patient sample size was limited, essentializing the need for a global larger cohort to validate our novel findings.
In this study, the treatment efficacy did not differ between MONO and COMBO in the older population. A pooled analysis of phase 3 clinical trials previously reported that most patient subgroups with NSCLC and PD-L1 TPS ≥ 50% may achieve comparable or superior OS and PFS outcomes compared to MONO, whereas the outcomes of the subgroup analysis based on age in this study indicate that elderly patients receiving COMBO may not have improved outcomes over MONO (23). Additionally, recent analysis and real-world data reported MONO is generally preferred in older patients with NSCLC and PD-L1 TPS ≥ 50% (24, 25). In our subgroup analysis of patients ≥70 years, COMBO also did not show an improvement over MONO in OS or PFS outcomes, suggesting that the benefits of adding chemotherapy to MONO might be more limited in older patients with NSCLC compared to that in their younger counterparts. Based on these results, when considering the addition of chemotherapy to MONO for older NSCLC patients with high PD-L1 TPS, it becomes critical to appropriately select patients with predictive factors for better efficacy, particularly in this older demographic. Further studies are needed to identify clinical biomarkers that correlate with the treatment outcome for the efficacy and tolerability of COMBO in older patients.
Our study showed that COMBO yielded better clinical outcomes than MONO in patients with ECOG PS 0, while no significant difference was observed in patients with ECOG PS 1, suggesting that a more detailed assessment of general conditions at pretreatment may be useful for treatment selection in older patients. Similarly, the JCOG1210/WJOG7813L trial, in which carboplatin/pemetrexed treatment was compared with docetaxel treatment in older patients with NSCLC, indicated that the OS benefit for carboplatin/pemetrexed was notable in patients with ECOG PS 0 at baseline but not in those with ECOG PS 1 (26).
Given the results of this study, it may be necessary to subdivide PS and patient background when considering treatment strategies for older patients. ECOG PS is a simple and useful scoring system in a clinical setting. However, it is subject to high interobserver variability, and clinicians are more likely to evaluate better ECOG PS than patients themselves. ECOG PS can only assess patients from a functional perspective and may miss important impairments identified by the Geriatric Assessment (GA) (27). In recent years, several guidelines have recommended GA over ECOG PS for older patients, as it provides a comprehensive evaluation including physical, social, and spiritual aspects (28–30). Screening tools such as the G8, the Flemish version of the Triage Risk Screening Tool, and the Clinical Frailty Scale allow for a more detailed assessment of daily living and frailties of older patients (31, 32). In the field of geriatric oncology, several studies have reported the usefulness of these screening tools in prognosis prediction (33–35). Therefore, utilizing these tools may strongly assist in selecting better treatment choices for older patients with cancer.
The present study indicates that COMBO may be more effective than MONO in patients with non-squamous cell carcinoma. The tumor immune microenvironment, including the composition of CD8+ lymphocytes infiltrating the tumor, differs between squamous cell lung cancer and non-squamous cell carcinoma (36). In addition, the poor treatment efficacy of concomitant chemotherapy to MONO in older patients with squamous cell carcinoma is presumably based on a heavier smoking history and higher prevalence of comorbidities in these patients (37). Squamous cell carcinoma is strongly associated with aging and smoking; thus, patients with this carcinoma type are often prone to comorbidities, the most frequent of which are cardiovascular diseases and chronic obstructive pulmonary diseases (38). These comorbidities not only affect treatment choice and adherence to treatment but are also associated with poor survival outcome as comorbidity-related symptoms affect OS (39). Patients with severe comorbidities are more likely to experience hematologic toxicity after chemotherapy (40). For these reasons, the potential prognostic impact of comorbidities in squamous cell carcinoma of the lung is multifactorial, limiting the benefit of concomitant chemotherapy.
5 Conclusions
In conclusion, our study revealed that both PFS and OS were similar between MONO and COMBO treatments for patients with NSCLC aged ≥ 70 years with high PD-L1 TPS ≥ 50% but were significantly longer for COMBO than MONO in patients with ECOG PS 0 or non-squamous cell carcinoma. Therefore, ECOG PS and histological type may be important factors to consider when choosing between MONO or COMBO treatment in this population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Review Board of the Kyoto Prefectural University of Medicine and the Ethics Review Board of each hospital (approval no. ERB-C-2113). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because owing to the retrospective nature of the study.
Author contributions
ST: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. HK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. TY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. Writing – review & editing. MT: Data curation, Writing – review & editing. YN: Data curation, Writing – review & editing. YG: Data curation, Writing – review & editing. AN: Data curation, Writing – review & editing. SS: Data curation, Writing – review & editing. KT: Data curation, Writing – review & editing. TT: Data curation, Writing – review & editing. AO: Data curation, Writing – review & editing. TH: Data curation, Writing – review & editing. KD: Data curation, Writing – review & editing. YC: Data curation, Writing – review & editing. IH: Data curation, Writing – review & editing. NT: Data curation, Writing – review & editing. YK: Writing – review & editing. NN: Writing – review & editing. KM: Writing – review & editing. MI: Writing – review & editing. ST: Writing – review & editing. TK: Writing – review & editing. KT: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank the patients, their families, and all the investigators involved in this study. Additionally, we thank Editage (www.editage.jp) for their help with English language editing.
Conflict of interest
HK received personal fees from Ono Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., AstraZeneca KK, Taiho Pharmaceutical Co. Ltd., Eli Lilly Japan KK, and MSD KK outside the purview of the submitted work. TY received research grants from Ono Pharmaceutical, Janssen, AstraZeneca, and Takeda Pharmaceutical, and has received speaking honoraria from Eli Lilly outside the purview of the submitted work. MT received research grants from Boehringer Ingelheim, Ono Pharmaceutical, Bristol-Myers Squibb, MSD, Daiichi-Sankyo, Eisai, Chugai Pharmaceutical Co. Ltd., and Janssen and personal fees from Chugai Pharmaceutical Co. Ltd., Boehringer Ingelheim, AstraZeneca, Taiho Pharmaceutical, Eli Lilly, Novartis, Pfizer, Asahi Kasei Pharmaceutical, Ono Pharmaceutical, Bristol-Myers Squibb, MSD, Bayer, Amgen, Kyowa-Kirin, and Nippon Kayaku outside the purview of the submitted work. AO received personal fees from Chugai-Roshe, AstraZeneca, Boehringer Ingelheim, Eli Lilly Japan, Nippon Kayaku, and Bristol-Myers Squibb outside the purview of the submitted work. TK received personal fees from Chugai Pharmaceutical Co. Ltd. and MSD KK outside the purview of the submitted work. KT received research grants from Chugai Pharmaceutical Co. Ltd. and Ono Pharmaceutical and personal fees from AstraZeneca, Chugai Pharmaceutical Co. Ltd., MSD-Merck, Eli Lilly, Boehringer Ingelheim, and Daiichi-Sankyo outside the purview of the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1348034/full#supplementary-material
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
2. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. (2008) 83:584–94. doi: 10.4065/83.5.584
3. Abdel-Rahman O. Correlation between PD-L1 expression and outcome of NSCLC patients treated with anti-PD-1/PD-L1 agents: A meta-analysis. Crit Rev Oncol Hematol. (2016) 101:75–85. doi: 10.1016/j.critrevonc.2016.03.007
4. Carbognin L, Pilotto S, Milella M, Vaccaro V, Brunelli M, Caliò A, et al. Differential activity of nivolumab, pembrolizumab and MPDL3280A according to the tumor expression of programmed death-Ligand-1 (PD-L1): Sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PloS One. (2015) 10:e0130142. doi: 10.1371/journal.pone.0130142
5. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
6. Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. (2020) 383:1328–39. doi: 10.1056/NEJMoa1917346
7. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. (2018) 378:2078–92. doi: 10.1056/NEJMoa1801005
8. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. (2018) 379:2040–51. doi: 10.1056/NEJMoa1810865
9. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. (2018) 378:2288–301. doi: 10.1056/NEJMoa1716948
10. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2019) 20:924–37. doi: 10.1016/S1470-2045(19)30167-6
11. Garassino MC, Gadgeel S, Speranza G, Felip E, Esteban E, Dómine M, et al. Pembrolizumab plus pemetrexed and platinum in nonsquamous non-small-cell lung cancer: 5-year outcomes from the phase 3 KEYNOTE-189 study. J Clin Oncol. (2023) 41:1992–8. doi: 10.1200/JCO.22.01989
12. Novello S, Kowalski DM, Luft A, Gümüş M, Vicente D, Mazières J, et al. Pembrolizumab plus chemotherapy in squamous non-small-cell lung cancer: 5-year update of the phase III KEYNOTE-407 study. J Clin Oncol. (2023) 41:1999–2006. doi: 10.1200/JCO.22.01990
13. Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. (2022) 72:409–36. doi: 10.3322/caac.21731
14. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
15. Nosaki K, Saka H, Hosomi Y, Baas P, de Castro G, Reck M, et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: Pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer. (2019) 135:188–95. doi: 10.1016/j.lungcan.2019.07.004
16. Tagliamento M, Frelaut M, Baldini C, Naigeon M, Nencioni A, Chaput N, et al. The use of immunotherapy in older patients with advanced non-small cell lung cancer. Cancer Treat Rev. (2022) 106:102394. doi: 10.1016/j.ctrv.2022.102394
17. Soubeyran P, Bellera C, Goyard J, Heitz D, Cure H, Rousselot H, et al. Validation of the G8 screening tool in geriatric oncology: The ONCODAGE project. J Clin Oncol. (2011) 29:9001. doi: 10.1200/jco.2011.29.15_suppl.9001
18. Soubeyran P, Bellera C, Goyard J, Heitz D, Curé H, Rousselot H, et al. Screening for vulnerability in older cancer patients: The ONCODAGE prospective multicenter cohort study. PloS One. (2014) 9:e115060. doi: 10.1371/journal.pone.0115060
19. Quoix E, Zalcman G, Oster JP, Westeel V, Pichon E, Lavolé A, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet. (2011) 378:1079–88. doi: 10.1016/S0140-6736(11)60780-0
20. Abe T, Takeda K, Ohe Y, Kudoh S, Ichinose Y, Okamoto H, et al. Randomized phase III trial comparing weekly docetaxel plus cisplatin versus docetaxel monotherapy every 3 weeks in elderly patients with advanced non-small-cell lung cancer: The intergroup trial JCOG0803/WJOG4307L. J Clin Oncol. (2015) 33:575–81. doi: 10.1200/JCO.2014.55.8627
21. Song S, Lam EW, Tchkonia T, Kirkland JL, Sun Y. Senescent cells: Emerging targets for human aging and age-related diseases. Trends Biochem Sci. (2020) 45:578–92. doi: 10.1016/j.tibs.2020.03.008
22. Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, et al. The influence of age on T cell generation and TCR diversity. J Immunol. (2005) 174:7446–52. doi: 10.4049/jimmunol.174.11.7446
23. Akinboro O, Vallejo JJ, Nakajima EC, Ren Y, Mishra-Kalyani PS, Larkins EA, et al. Outcomes of anti–PD-(L)1 therapy with or without chemotherapy (chemo) for first-line (1L) treatment of advanced non–small cell lung cancer (NSCLC) with PD-L1 score ≥ 50%: FDA pooled analysis. J Clin Oncol. (2022) 40:9000. doi: 10.1200/JCO.2022.40.16_suppl.9000
24. Gridelli C, Peters S, Velcheti V, Attili I, de Marinis F. Immunotherapy in the first-line treatment of elderly patients with advanced non-small-cell lung cancer: results of an International Experts Panel Meeting by the Italian Association of Thoracic Oncology (AIOT). ESMO Open. (2023) 8:101192. doi: 10.1016/j.esmoop.2023.101192
25. Uematsu M, Tsukita Y, Tozuka T, Kushiro K, Hosokawa S, Sumi T. First-line immune checkpoint inhibitors alone or in combination with chemotherapy in real-life elderly patients with advanced non-small cell lung cancer (NEJ057). J Clin Oncol. (2023) 41, no. 16_suppl. doi: 10.1200/JCO.2023.41.16_suppl.9012. ASCO 2023 annual meeting.
26. Okamoto I, Nokihara H, Nomura S, Niho S, Sugawara S, Horinouchi H, et al. Comparison of carboplatin plus pemetrexed followed by maintenance pemetrexed with docetaxel monotherapy in elderly patients with advanced nonsquamous non-small cell lung cancer: A phase 3 randomized clinical trial. JAMA Oncol. (2020) 6:e196828. doi: 10.1001/jamaoncol.2019.6828
27. Galvin A, Soubeyran P, Brain E, Cheung KL, Hamaker ME, Kanesvaran R, et al. Assessing patient-reported outcomes (PROs) and patient-related outcomes in randomized cancer clinical trials for older adults: Results of DATECAN-ELDERLY initiative. J Geriatr Oncol. (2023) 15(1):101611. doi: 10.1016/j.jgo.2023.101611
28. Mohile SG, Dale W, Somerfield MR, Schonberg MA, Boyd CM, Burhenn PS, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. (2018) 36:2326–47. doi: 10.1200/JCO.2018.78.8687
29. Puts MT, Hardt J, Monette J, Girre V, Springall E, Alibhai SM. Use of geriatric assessment for older adults in the oncology setting: A systematic review. J Natl Cancer Inst. (2012) 104:1133–63. doi: 10.1093/jnci/djs285
30. Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen ML, Extermann M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. (2014) 32:2595–603. doi: 10.1200/JCO.2013.54.8347
31. Rockwood K, Theou O. Using the Clinical Frailty Scale in allocating scarce health care resources. Can Geriatr J. (2020) 23:210–5. doi: 10.5770/cgj.23.463
32. Bellera CA, Rainfray M, Mathoulin-Pélissier S, Mertens C, Delva F, Fonck M, et al. Screening older cancer patients: First evaluation of the G-8 geriatric screening tool. Ann Oncol. (2012) 23:2166–72. doi: 10.1093/annonc/mdr587
33. Wu X, Kumar R, Milner-Watts C, Walder D, Battisti NML, Minchom A, et al. The predictive value of the G8 questionnaire in older patients with lung cancer or mesothelioma before systemic treatment. Clin Oncol (R Coll Radiol). (2023) 35:e163–72. doi: 10.1016/j.clon.2022.10.020
34. Kenis C, Decoster L, Van Puyvelde K, De Grève J, Conings G, Milisen K, et al. Performance of two geriatric screening tools in older patients with cancer. J Clin Oncol. (2014) 32:19–26. doi: 10.1200/JCO.2013.51.1345
35. Morimoto K, Yamada T, Takeda T, Shiotsu S, Date K, Harada T, et al. Prospective observational study evaluating the prognostic value of the G8 screening tool for extensive-stage small cell lung cancer patients who received programmed death-ligand 1 inhibitor plus platinum-etoposide chemotherapy. Drugs Aging. (2023) 40:563–71. doi: 10.1007/s40266-023-01034-4
36. Meng X, Gao Y, Yang L, Jing H, Teng F, Huang Z, et al. Immune microenvironment differences between squamous and non-squamous non-small-cell lung cancer and their influence on the prognosis. Clin Lung Cancer. (2019) 20:48–58. doi: 10.1016/j.cllc.2018.09.012
37. Socinski MA, Obasaju C, Gandara D, Hirsch FR, Bonomi P, Bunn P, et al. Clinicopathologic features of advanced squamous NSCLC. J Thorac Oncol. (2016) 11:1411–22. doi: 10.1016/j.jtho.2016.05.024
38. Janssen-Heijnen ML, Schipper RM, Razenberg PP, Crommelin MA, Coebergh JW. Prevalence of co-morbidity in lung cancer patients and its relationship with treatment: A population-based study. Lung Cancer. (1998) 21:105–13. doi: 10.1016/s0169-5002(98)00039-7
39. Asmis TR, Ding K, Seymour L, Shepherd FA, Leighl NB, Winton TL, et al. Age and comorbidity as independent prognostic factors in the treatment of non-small-cell lung cancer: A review of National Cancer Institute of Canada Clinical Trials Group trials. J Clin Oncol. (2008) 26:54–9. doi: 10.1200/JCO.2007.12.8322
40. Grønberg BH, Sundstrøm S, Kaasa S, Bremnes RM, Fløtten O, Amundsen T, et al. Influence of comorbidity on survival, toxicity and health-related quality of life in patients with advanced non-small-cell lung cancer receiving platinum-doublet chemotherapy. Eur J Cancer. (2010) 46:2225–34. doi: 10.1016/j.ejca.2010.04.009
Keywords: chemoimmunotherapy, immunochemotherapy, immune checkpoint inhibitor, non-small-cell lung cancer, PD-L1
Citation: Takei S, Kawachi H, Yamada T, Tamiya M, Negi Y, Goto Y, Nakao A, Shiotsu S, Tanimura K, Takeda T, Okada A, Harada T, Date K, Chihara Y, Hasegawa I, Tamiya N, Katayama Y, Nishioka N, Morimoto K, Iwasaku M, Tokuda S, Kijima T and Takayama K (2024) Prognostic impact of clinical factors for immune checkpoint inhibitor with or without chemotherapy in older patients with non-small cell lung cancer and PD-L1 TPS ≥ 50%. Front. Immunol. 15:1348034. doi: 10.3389/fimmu.2024.1348034
Received: 01 December 2023; Accepted: 12 February 2024;
Published: 23 February 2024.
Edited by:
Sung Yong Lee, Korea University Guro Hospital, Republic of KoreaReviewed by:
Chi Young Jung, Catholic University of Daegu, Republic of KoreaSeong Hoon Yoon, Pusan National University Yangsan Hospital, Republic of Korea
Copyright © 2024 Takei, Kawachi, Yamada, Tamiya, Negi, Goto, Nakao, Shiotsu, Tanimura, Takeda, Okada, Harada, Date, Chihara, Hasegawa, Tamiya, Katayama, Nishioka, Morimoto, Iwasaku, Tokuda, Kijima and Takayama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tadaaki Yamada, dGF5YW1hZGFAa290by5rcHUtbS5hYy5qcA==
 Shota Takei
Shota Takei Hayato Kawachi
Hayato Kawachi Tadaaki Yamada
Tadaaki Yamada Motohiro Tamiya2
Motohiro Tamiya2 Shinsuke Shiotsu
Shinsuke Shiotsu Keiko Tanimura
Keiko Tanimura Takayuki Takeda
Takayuki Takeda Kenji Morimoto
Kenji Morimoto Takashi Kijima
Takashi Kijima Koichi Takayama
Koichi Takayama