- 1Interventional Therapy Center for Oncology, Beijing You’an Hospital, Capital Medical University, Beijing, China
- 2Research Center for Biomedical Resources, Beijing You’an Hospital Capital Medical University, Beijing, China
- 3National Center for Infectious Diseases, Beijing Ditan Hospital, Capital Medical University, Beijing, China
- 4Interventional Radiology Department, Beijing Friendship Hospital, Capital Medical University, Beijing, China
- 5Research Center for Biomedical Resources, Beijing You’an Hospital, Capital Medical University, Beijing, China
Background: Hepatitis B surface antigen (HBsAg) clearance is associated with improved long-term outcomes and reduced risk of complications. The aim of our study was to identify the effects of levels of HBsAg in HCC patients undergoing TACE and sequential ablation. In addition, we created a nomogram to predict the prognosis of HCC patients with high levels of HBsAg (≥1000U/L) after local treatment.
Method: This study retrospectively evaluated 1008 HBV-HCC patients who underwent TACE combined with ablation at Beijing Youan Hospital and Beijing Ditan Hospital from January 2014 to December 2021, including 334 patients with low HBsAg levels and 674 patients with high HBsAg levels. The high HBsAg group was divided into the training cohort (N=385), internal validation cohort (N=168), and external validation cohort (N=121). The clinical and pathological features of patients were collected, and independent risk factors were identified using Lasso-Cox regression analysis for developing a nomogram. The performance of the nomogram was evaluated by C-index, receiver operating characteristic (ROC) curves, calibration curves, and decision curve analysis (DCA) curves in the training and validation cohorts. Patients were classified into high-risk and low-risk groups based on the risk scores of the nomogram.
Result: After PSM, mRFS was 28.4 months (22.1-34.7 months) and 21.9 months (18.5-25.4 months) in the low HBsAg level and high HBsAg level groups (P<0.001). The content of the nomogram includes age, BCLC stage, tumor size, globulin, GGT, and bile acids. The C-index (0.682, 0.666, and 0.740) and 1-, 3-, and 5-year AUCs of the training, internal validation, and external validation cohorts proved good discrimination of the nomogram. Calibration curves and DCA curves suggested accuracy and net clinical benefit rates. The nomogram enabled to classification of patients with high HBsAg levels into low-risk and high-risk groups according to the risk of recurrence. There was a statistically significant difference in RFS between the two groups in the training, internal validation, and external validation cohorts (P<0.001).
Conclusion: High levels of HBsAg were associated with tumor progression. The nomogram developed and validated in the study had good predictive ability for patients with high HBsAg levels.
1 Introduction
Primary liver cancer is the sixth most common cancer and the second leading cause of cancer death worldwide, which poses a huge economic and disease burden worldwide due to its high morbidity and mortality rates (1, 2). China is the country with the highest hepatocellular carcinoma (HCC) occurrence and the overall incidence of HCC is expected to continue to climb (3). HCC occurs most often in the setting of chronic liver inflammation and is mainly induced by hepatitis B virus (HBV) infection (4), which is a key risk factor for liver cirrhosis and HCC, capable of increasing the risk of HCC approximately 20-fold (5–7). For early HCC, surgical resection, liver transplantation, and ablation are recommended treatments. Studies have shown that ablation has similar five-survival rates compared to surgical treatment, and fewer complications than surgery (8, 9). However, the recurrence rate after ablation remains high, with a five-year recurrence rate of 50-70% (10). Transcatheter arterial chemoembolization (TACE) is the only guideline-recommended global standard of care for intermediate-stage HCC, and the median progression-free survival time (mPFS) is only 5 months (11). Therefore, diagnosis and treatment of HCC is an increasingly important public health problem.
The first serologic marker of HBV infection is Hepatitis B surface antigen (HBsAg), which can be detected from 2 to 12 weeks after infection with HBV (12). HBsAg clearance, which is currently regarded as the functional cure of chronic hepatitis (CHB), is associated with improved long-term outcomes and reduced risk of complications (13, 14). The decline in HBsAg during antiviral therapy is relatively slow, and the seroclearance rate is faster at low serum HBsAg expression (<1000U/L) (15, 16). Previous studies revealed that high serum levels of HBsAg increase the risk of developing HCC and have a worse prognosis for patients who have already developed HCC (17). Nevertheless, the prognostic impact of serum HBsAg levels in patients after TACE sequential ablation therapy needs to be further confirmed.
HBV-HCC prognosis is linked to several factors, including tumor burden, AFP, disease stage, ALBI, and NLR (18, 19), and there are also nomograms about HBV-HCC (20–22). However, no nomogram for HCC patients with high HBsAg expression after local treatment has been available to our knowledge. We compared the effects of high levels of HBsAg (≥1000U/L) and low levels of HBsAg (<1000U/L) in HCC patients undergoing TACE and sequential ablation and utilized propensity score matching to minimize selection bias. In addition, we created a nomogram to predict the prognosis of HCC patients with high levels of HBsAg after local treatment to more accurately guide the clinical decision.
2 Materials and methods
2.1 Patient selection
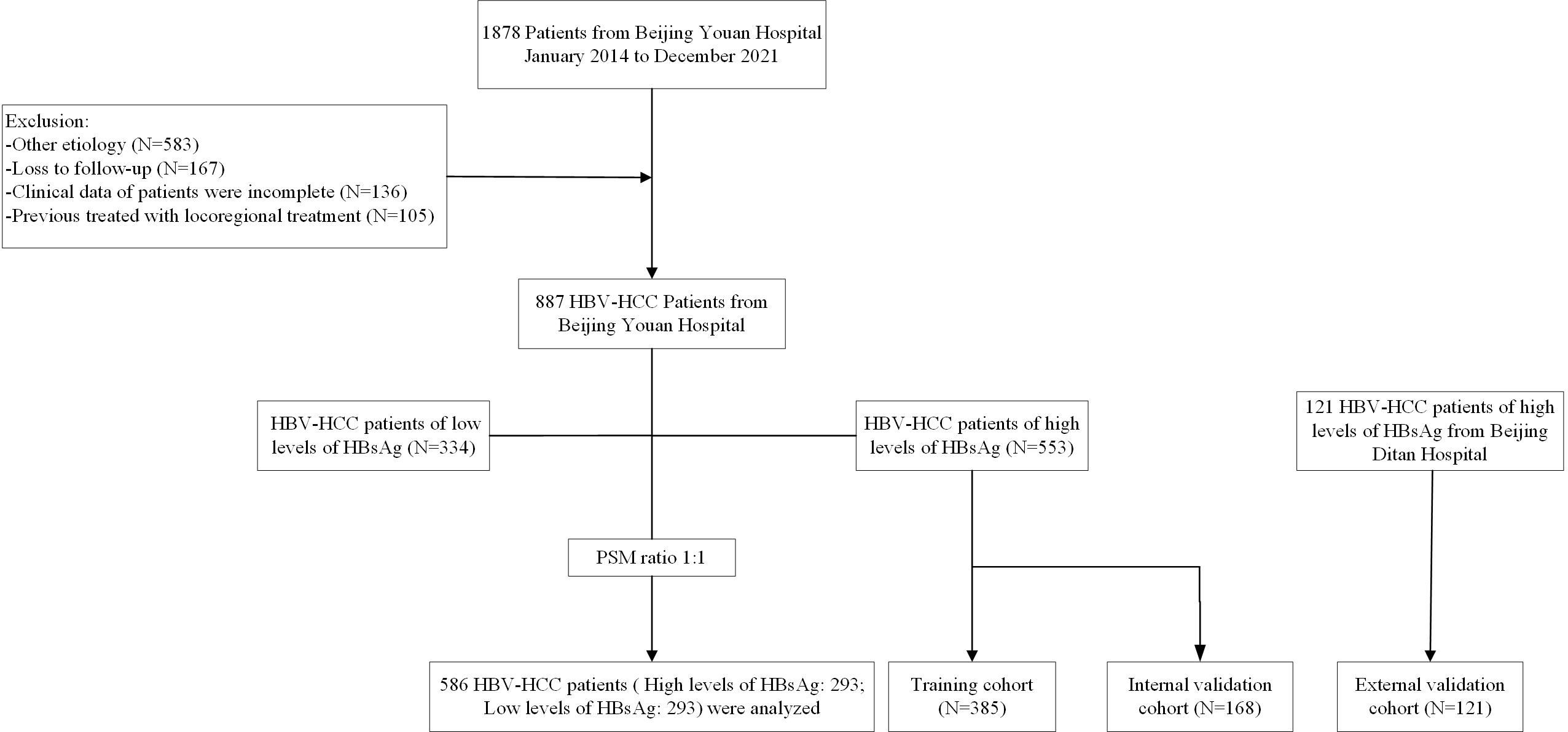
This study retrospectively evaluated 1008 HBV-HCC patients who underwent TACE combined with ablation at Beijing Youan Hospital and Beijing Ditan Hospital from January 2014 to December 2021. The diagnosis of HCC was based on the guideline of the America Association for the Study of Liver Diseases (ASSLD) (1, 23). The patients at Youan Hospital consisted of 553 patients with a high level of HBsAg and 334 patients with a low level of HBsAg. In order to build a reliable model, the patients from Youan Hospital were divided into the training cohort (N=385) and the validation cohort (N=168). Furthermore, 121 patients from Ditan Hospital were used as an independent external verification cohort to verify the external applicability of the nomogram. The inclusion criteria of patients were as follows (1): Aged 18-80 years (2). received TACE combined ablation (3). Child-Pugh classification was class A or B (4). all patients had not received any other therapeutics before ablation. Exclusion criteria were listed as follows (1): with second primary malignant tumors (2). clinical follow-up data incomplete (3). advanced HCC. (Figure 1).
The study was approved by the Medical Ethics Committee of Youan Hospital and Ditan Hospital and was performed in compliance with the standards of the Helsinki Declaration. The requirement for informed consent was waived because the study was deemed to pose no additional risk to patients and the data were deidentified.
2.2 Clinicopathologic characteristics
The demographic, clinical, and histopathologic data of patients were collected. Demographics included age, sex, drinking history, smoking history, hypertension and diabetes. Clinical and pathological data was composed of tumor size, tumor number, alpha-fetoprotein (AFP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyl transferase (GGT), albumin (ALB), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and gamma glutamyl transferase to lymphocyte ratio (GLR).
2.3 Treatment received
2.3.1 TACE procedure
TACE was conducted by experienced interventional radiologists. Under local anesthesia, percutaneous right femoral artery puncture with a modified Seldinger technique was performed. Angiography was conducted by the 5-F (Terumo, Tokyo, Japan) catheter to identify arterial supply to tumors and to assess the patency of the portal vein. When applicable, a microcatheter was inserted into the blood-supply artery of the carcinoma to inject a mixture of doxorubicin (Pfizer Inc., New York, NY, USA) and lipiodol (Guerbet, Villepinte, France), followed by embolization using embolic materials, such as gelfoam or polyvinyl alcohol particles. The blood flow was monitored until complete vessel occlusion was observed. TACE was repeated thereafter if the lesion is not completely necrotic and the active portion exceeds 50% of the baseline value.
2.3.2 Ablation procedure
Performed under the guidance of computed tomography (CT) and magnetic resonance imaging (MRI) by a qualified interventionalist. The size of the tumor decided the number of electrodes. Routine disinfection and intravenous anesthesia were applied around the puncture points. During RFA, after measuring the baseline impedance, the power was gradually increased from 80w to 200w to reach the maximum impedance. The electrode tip temperature was kept below 20°C by the pump injected cold brine into the electrode chamber. Moreover, to achieve complete ablation, the safe margin for complete ablation of the tumor was 0.5cm. After ablation, the needle track was ablated to prevent postoperative bleeding and tumor implantation along the needle track. Arteriography-enhanced CT was performed immediately after treatment to evaluate the success of the procedure and its complications.
2.3 Follow-up
All patients underwent regular follow-ups at the outpatient clinics. Tumor responses were evaluated at approximately 4-6 weeks after ablation by using CT or MRI. For the follow-up protocol, patients were examined every 3 months during the first year and every 6 months thereafter. The contents of the follow-up included blood tests, liver function, and imaging examination to detect tumor recurrence. The study endpoint was recurrence-free survival (RFS), defined as the time from ablation to the first recurrence.
2.4 Statistical analysis
Differences between the groups were compared through the t-test, chi-square test, Mann-Whitney U test, and Kruskal-Wallis test, with the purpose of providing median or counts and percentages to summarize baseline variables. Survival and recurrence were calculated using the Kaplan-Meier method, and the log-rank test was used for comparison. Lasso regression was performed for risk factor selection and identified independent risk factors for tumor recurrence were used in Multivariate Cox regression analysis. A nomogram based on independent risk factors to predict recurrence. Subsequently, the performance of the nomogram was validated in the internal validation and the external validation cohort. According to the nomogram scores, the patients were classified as low-risk and high-risk groups, and their recurrence rates were predicted. The receiver operating characteristic (ROC) curves were plotted and the area under the curves (AUCs) was calculated to evaluate prognostic value. Calibration curves and the Hosmer-Lemeshow test were conducted to assess the predictive ability of the nomogram. To estimate the clinical utility of the nomogram, decision curve analysis (DCA) was conducted by calculating the net benefits for a range of threshold probabilities.
To reduce the potential selection bias, 1:1 propensity score matching (PSM) was conducted, with a matching tolerance was 0.1. Matches were made in baseline variables that were previously considered clinically relevant in the literature, comprising age, sex, Child-pugh classification, BCLC stage, tumor size, tumor number, ALT, AST, and AFP.
All data were analyzed with SPSS (version 26.0, IBM, Armonk, NY, USA) and R software (version 4.1.3) in this study, and a P-value less than 0.05 was considered statistically significant (two-tailed tests).
3 Result
A total of 1008 HBV-HCC patients from Beijing Youan Hospital and Beijing Ditan Hospital were screened between January 1, 2014, to December 31, 2021, including 334 patients with low HBsAg levels and 674 patients with high HBsAg levels. After PSM, 293 patients were included in each group (Figure 1). The high levels of HBsAg groups were divided into the training cohort (N=385), internal validation cohort (N=168), and external validation cohort (N=121). The last follow-up until July 1, 2023, and the median follow-up time was 4.05 years (25~75th percentiles, 2.68~7.05 years).
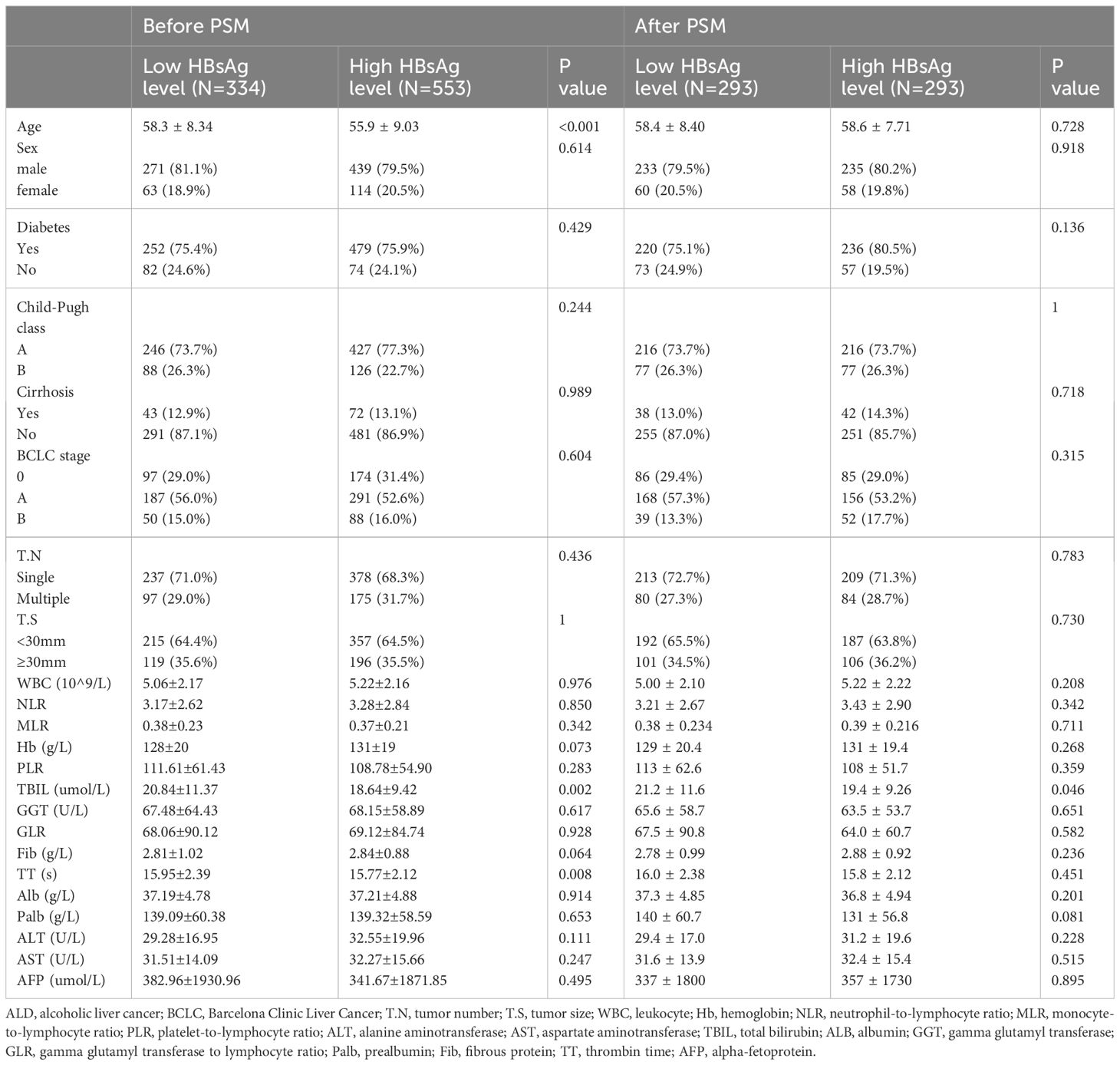
Before PSM, baseline data showed that compared to the low HBsAg level group, the high HBsAg level group had a younger age (55.9 ± 9.03 VS. 58.3 ± 8.34, P<0.001), lower levels of TBIL (18.64 ± 9.42 VS. 20.84 ± 11.37, P=0.002), and shorter TT (15.77 ± 2.12VS. 15.95 ± 2.39, P=0.008). After PSM, all demographic and clinicopathologic data were well balanced between the two groups (Table 1).
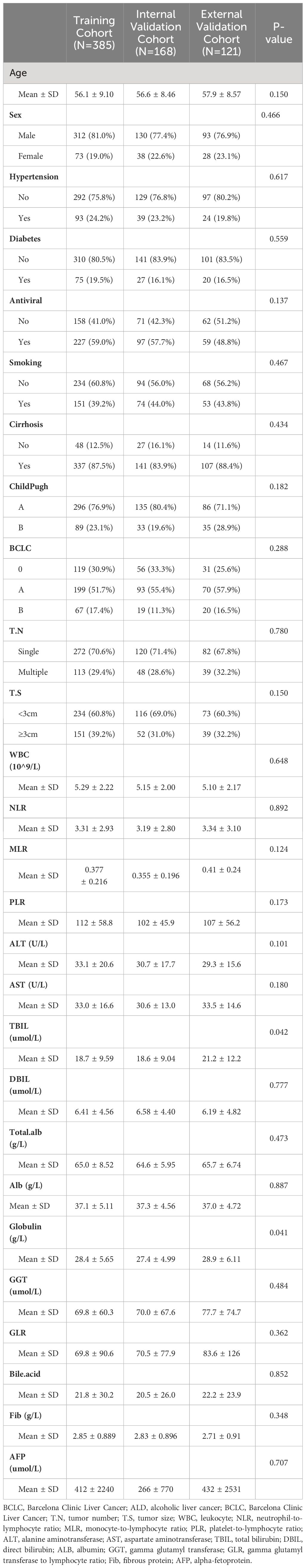
The internal validation cohort and the external validation cohort had similar baseline characteristics to the training cohort. In the three cohorts, the majority of the patients were male (81.0%VS. 77.4%VS. 76.9%, p=0.466), and the average age was over 50 years(56.1 ± 9.10 VS. 56.6 ± 8.46 VS. 57.9 ± 8.57, P=0.466). Most patients were Child-Pugh A (76.9%VS. 80.4% VS., P=0.427), suggesting that the patients had good liver function. BCLC A had the highest percentage of patients (51.7% VS. 55.4% VS. 71.7%, P=0.182). Regarding tumor characteristics, most tumors were solitary (70.6% vs.71.4% VS. 67.8%, P=0.780) and tumor size was less than 3cm (70.6% vs. 69.0% VS. 67.8%, P=0.150) (Table 2).
3.1 Efficacy
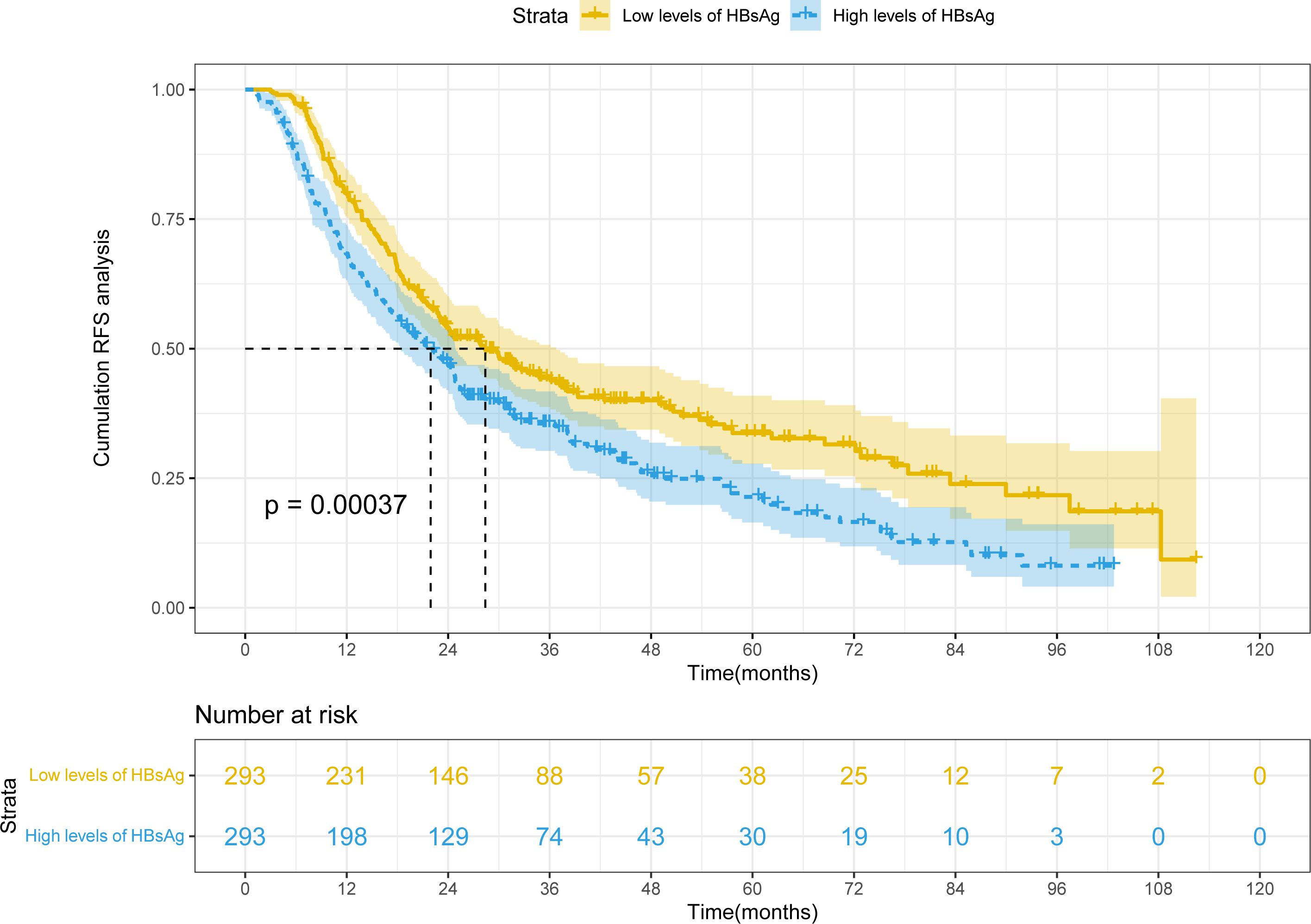
After PSM, mRFS was 28.4 months (22.1-34.7 months) and 21.9 months (18.5-25.4 months) in the high HBsAg level and low HBsAg level groups, respectively (Figure 2). Because mRFS were significantly shorter in the high HBsAg level (P<0.001), a nomogram for predicting recurrence needs to be developed for the high HBsAg group in order to prompt clinical interventions.
3.2 The prediction model was built based on the Lasso-Cox regression
3.2.1 Independent prognostic factors of RFS
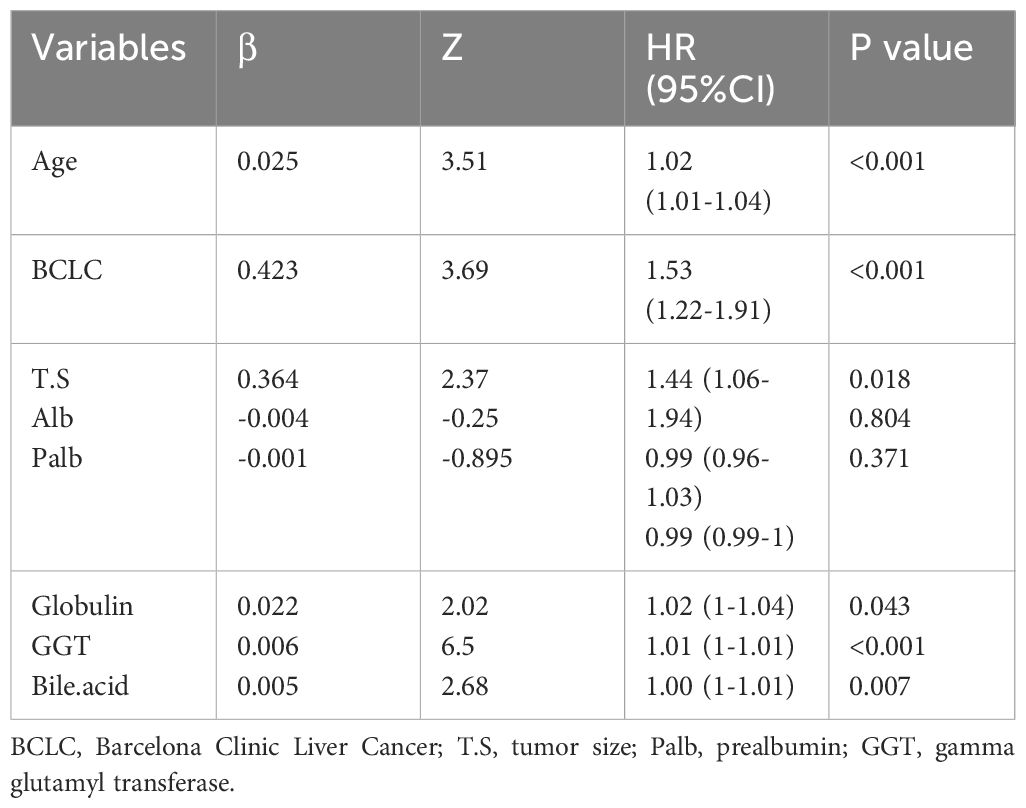
The cohort in Beijing Youan Hospital was randomly split in a 7:3 ratio into the training (N=385) and internal validation (N=168) sets. The external validation cohort consisted of patients from Beijing Ditan Hospital. There were no statistical differences between the three groups (P<0.05), which showed that the data grouping was random and reasonable. Lasso regression was used to screen parameters, and the variation characteristics of the coefficient of these variables were shown in Figure 3A. The model exhibited outstanding performance and the least number of independent variables (Figure 3B). The screened variables included age, BCLC stage, tumor size, ALB, Palb, GLB, GGT, and bile acids. Variables screened based on Lasso regression were further subjected to multifactorial COX regression analysis to screen independent risk factors associated with recurrence (Table 3). The final results obtained were age (HR: 1.02, 95% CI: 1.01-1.04), BCLC stage (HR: 1.53, 95% CI: 1.22-1.91), tumor size (HR: 1.44, 95% CI: 1.06-1.94), globulin (HR: 1.02, 95% CI: 1-1.04), GGT (HR: 1.01, 95% CI: 1-1.01), and bile acids (HR: 1, 95% CI: 1-1.01).
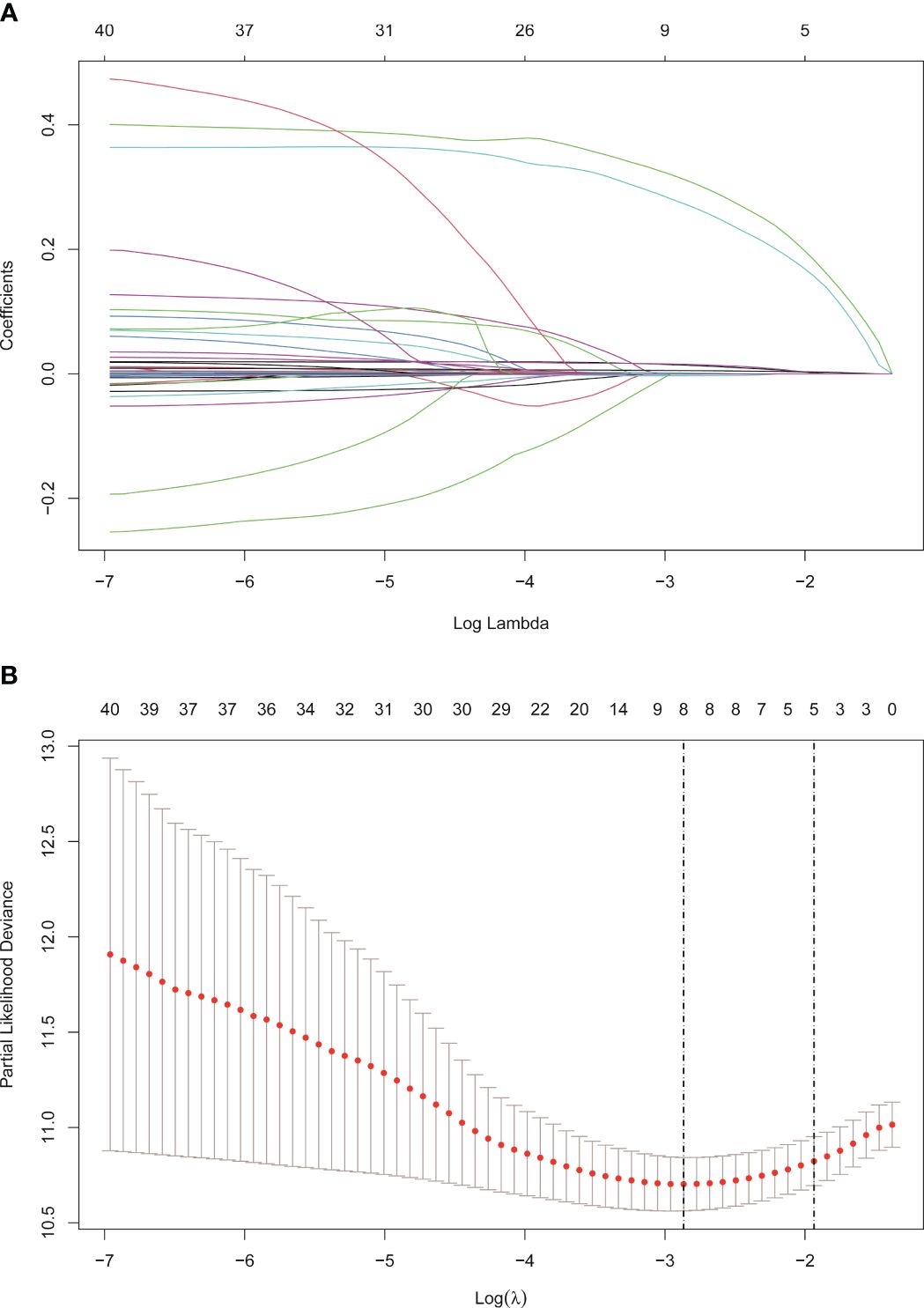
Figure 3 Screening of variables based on Lasso regression. (A) The variation characteristics of the coefficient of variables. (B) the selection process of the optimum value of the parameter λ in the Lasso regression model by cross-validation method.
3.2.2 Develop the nomogram
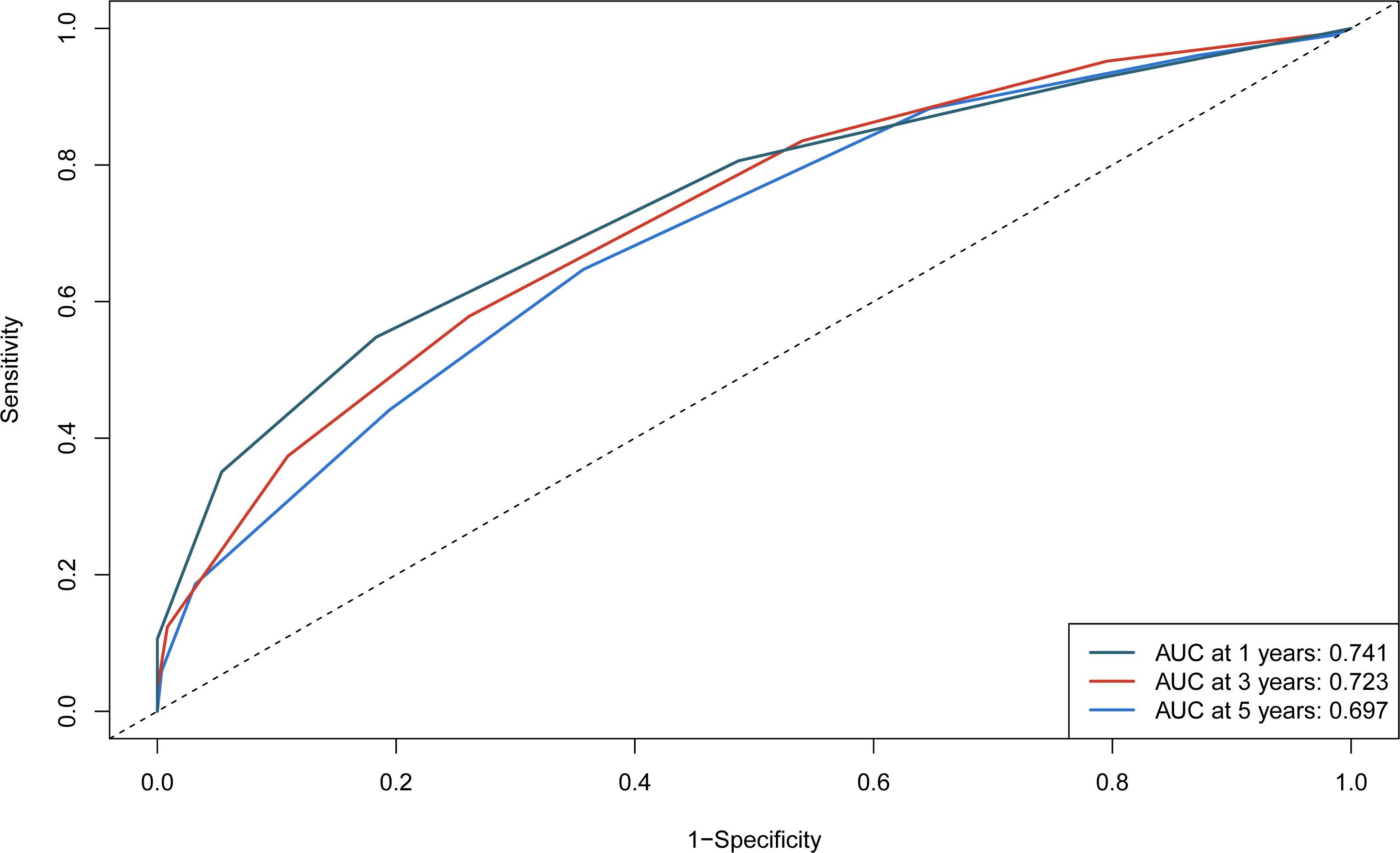
The independent predictors found by the Lasso-Cox regression analysis were used to construct a nomogram (Figure 4). In the training cohort, the C-index was 0.682(95%CI: 0.639-0.725), and the time-dependent ROC curve demonstrated that AUCs of 1-, 3-, and 5-year were 0.741, 0.723, and 0.687 (Figure 5). It indicated the good predicting ability of our nomogram. The calibration curves of 1-, 3-, and 5-year demonstrated satisfactory accordance between the nomogram prediction and actual observation. In addition, the clinical value of the nomogram was evaluated using DCA, which provided the net benefits in reasonable threshold probability (Figure 6).
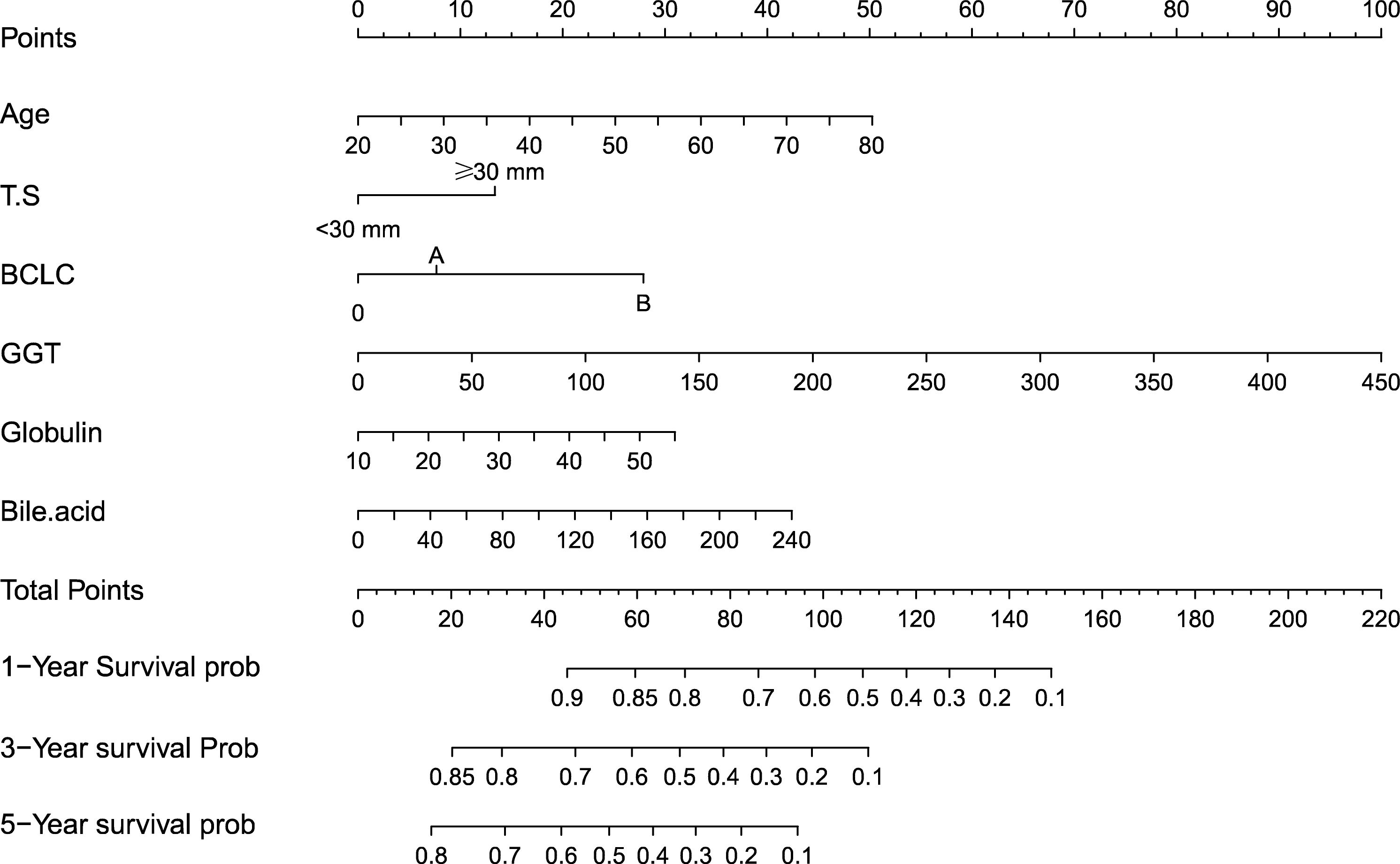
Figure 4 Nomogram, including Age, tumor number, BCLC stage, Globulin, GGT, and Bile acid for 1-, 3-, and 5- years recurrence free survival (RFS) in HCC patients with high HBsAg levels in AFP. The nomogram is valued to obtain the probability of 1-, 3-, and 5- years recurrence by adding up the points identified on the points scale for each variable. T.S, tumor size; BCLC, Barcelona Clinic Liver Cancer; GGT, gamma glutamyl transferase.
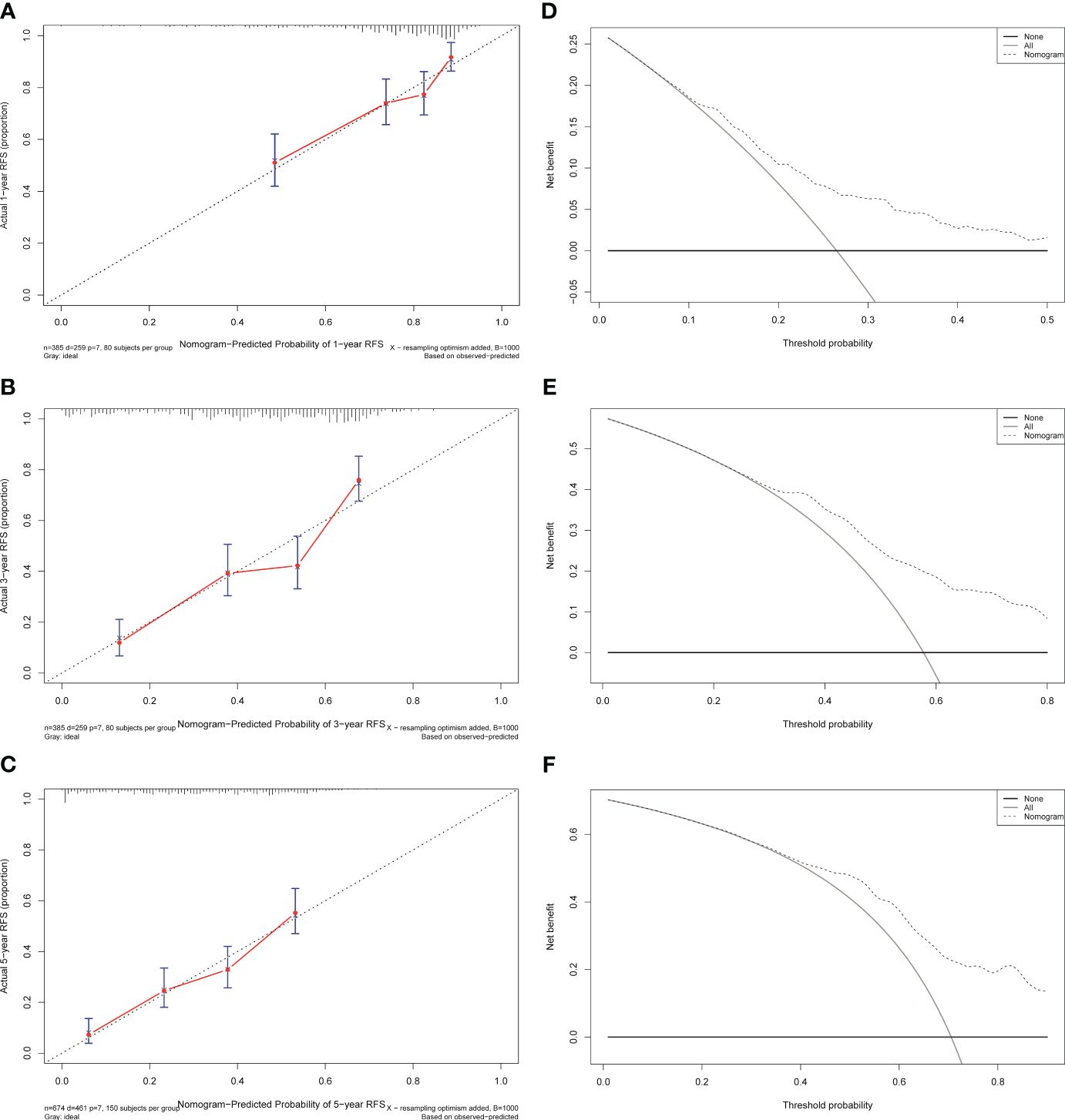
Figure 6 Calibration curves and decision curves analysis for recurrence of the nomogram in the training cohort. (A) One-year calibration curve in the training cohort. (B) Three-year calibration curve in the training cohort. (C) Five-year calibration curve in the training cohort. (D) One-year decision curve analysis in the training cohort. (E) Three-year decision curve analysis in the training cohort. (F) Five-year decision curve analysis in the training cohort.
Patients were classified into two groups according to the score of the nomogram: low-risk group and high-risk group. In the training cohort, there were apparent variances in RFS (Figure 7) between the low-risk group (N=193) and high-risk group (N=192) (P<0.001).
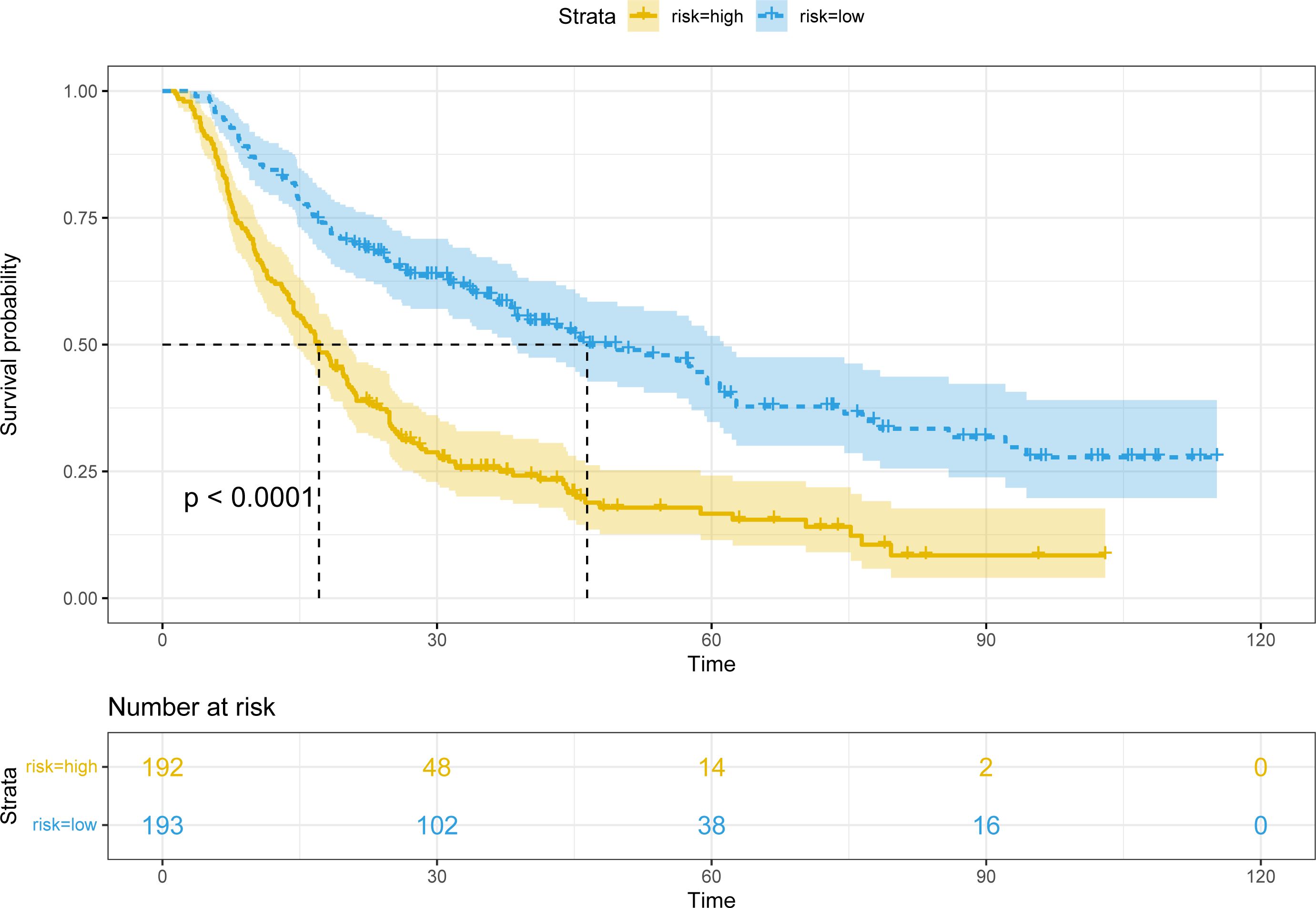
Figure 7 Kaplan-Meier plots of RFS for the low-risk group and high-risk group in the training cohort.
3.2.3 Validate the nomogram
To further test the efficacy of the reliability and robustness of our prognostic nomogram, internal and external validations were conducted on the nomogram. In the internal and external validation cohorts, the C-indexes of the nomogram for predicting the RFS were 0.666 (95%CI: 0.613-0.719) and 0.74 (95%CI: 0.696-0.783). The time-dependent ROC revealed that the AUCs of 1-, 3-, and 5-year were 0.702, 0.704, 0.684, 0.792, 0.734, and 0.770 in the internal and external validation cohorts (Supplementary Figure S1). The calibration curves also matched well (Supplementary Figure S2), and the DCA curves of 1-, 3-, and 5-year had good clinical practicability (Supplementary Figure S3).
The patients in two validation cohorts were also divided into high-risk and low-risk groups. The recurrence rates in the high-risk groups were significantly higher in the low-risk groups (P<0.001) (Supplementary Figure S4).
4 Discussion
HCC is one of the most common malignant tumors in the world. In China, the major etiology of the HCC is the HBV infection, which can promote the development and metastasis of the HCC (10, 24, 25). With the use of 1:1 PSM, our study found that the high level of HBsAg had a higher risk of recurrence than the low level of HBsAg. Consequently, our study is the first to focus on the high level of HBsAg patients who underwent TACE combined ablation to develop and validate a nomogram, which will hopefully predict the recurrence in H-HBsAg patients (High level of HBsAg). At present, there is a lack of a recurrence prediction model for H-HBsAg. We simultaneously created a nomogram by Lasso-Cox regression to accurately predict the prognosis of H-HBsAg patients.
The nomogram contains seven factors to produce the probability of an individual-specific clinical event, including age, tumor size, BCLC stage, globulin, GGT, and bile acid. The scores of the nomogram were obtained by drawing a vertical line at the location of the corresponding total score so that it intersected the three lines predicting the risk of recurrence, and the values shown at the intersection were predicted RFS at 1, 3, and 5 years. The C-index and AUCs of the training cohort and validation cohorts were similar, demonstrating adequate discrimination ability. The calibration curves presented the good prediction performance of the nomogram. Moreover, the nomogram indicated reliable clinical applicability by DCA curves. Patients were divided into two different risk groups according to the nomogram, and RFS was clearly different(P<0.001), which illustrated that our nomogram had a better ability to distinguish H-HBsAg patients to determine the risk of relapse after ablation therapy.
The number and size of tumors suggested strong tumor aggressiveness and poor prognosis of HCC, which was currently uncontroversial and needed not to be described here. Liver weight and portal blood flow velocity are reduced in the elderly, resulting in less reparability of the young body. Elderly people have lower immunity and faster tumor progression after treatment, leading to higher recurrence rates and worse prognosis (26, 27). At present, the BCLC system is regarded as an optimal staging system for tumor stage, treatment regimens, and expected survival. The expected survival rate is 50-70% for patients who are BCLC A at 5 years (28, 29). When we combined BCLC with other independent prognostic factors, the predictive value for prognosis could improve. GGT may be involved in the balance of oxidant and anti-oxidation, leading to sustained oxidative stress in tumor cells, which can contribute to the process of cancer (30, 31). Various proinflammatory proteins, including immunoglobulins, C-reactive protein, α2 macroglobulin, and fibrinogen are globulins (32, 33). Since human immunoglobulins are mainly metabolized by the liver, patients with severe hepatic dysfunction have a reduced ability to clear immunoglobulins, causing hyperglobulinemia (34, 35). Bile acid synthesis occurs in liver cells and is the end product of cholesterol metabolism (36). The Systemic homeostasis of bile acid mainly depends on its enterohepatic circulation process, which is of great significance for nutrient absorption and distribution, metabolic regulation, and homeostasis (37). Bile acid metabolism is implicated in tumor progression and hydrophobic bile acids are promoters of HCC (38, 39). Besides, reduced Farnesoid X (FXR) receptor signaling during hepatic inflammation induces to decrease in bile acid transporter proteins, resulting in elevated bile acids and persistent hepatic inflammation, which promote the development of HCC (40, 41).
The presence of HBsAg is a serologic marker of HBV infection and is used in clinical diagnosis (42, 43). HBsAg appears 1-2 weeks after exposure to HBV and precedes the onset of clinical symptoms and other serologic biochemical indicators of infection. There are still 257 million carriers of HBsAg despite the availability of antiviral therapeutics (44, 45). Many studies showed that the spontaneous HBsAg seroconversion rate was 1% and the presence of persistent HBsAg was associated with a high risk of HCC and a worse prognosis (46, 47). Previous studies by our team have also reported that the prognosis of HCC patients with negative HBsAg expression was better than that with positive HBsAg expression (48). In our study, we investigated the role of HBsAg levels in the recurrence of HCC after local treatment and used PSM to reduce bias. The results revealed that HBV-HCC patients with high HBsAg levels have worse prognosis than those with low HBsAg levels.
In the BCLC Guideline, TACE is recommended for BCLC intermediate stage B HCC. For early-stage HCC, TACE can mark the tumor and achieve tumor downstaging, thereby declining the time and increasing the success rate of ablation (49). Foreign and domestic studies have suggested that combination therapy by TACE and ablation improved overall and progression-free survival compared with TACE alone (50, 51). Unlike the conventional univariate analysis, the LASSO regression that we used aimed to select variables for Cox regression to avoid overfitting. Also, the nomogram can be validated by both internal and external validation because our study was a multicenter retrospective study. Simultaneous examination of comprehensive patient features covering demographics, liver function, tumor load, tumor markers, and inflammatory markers was a major strength of our study. The consists of our nomogram are simple and easy to obtain so that the clinicians are able to evaluate the patient’s condition in a timely and effective manner.
Several limitations of our study should be addressed. The first one of them is the retrospective nature and it is necessary to strengthen the conclusions by further validations in large prospective studies. Because as a retrospective study, there is inevitable selection bias. Although internal and external validations were conducted by a larger multicenter sample, external validations from other centers are still required in the future. Besides, the patients included in our study all received TACE combined with ablation. Whether the nomogram would be suitable for other treatments such as surgery and liver transplantation requires further investigation. Lastly, the study was conducted only in China, where hepatitis B virus is the principal cause of HCC. Thus, generalizing to other populations in which HBV is not a major causative factor for HCC must be carried out with caution. Nevertheless, we used up to eight years of follow-up to create an accurate and reliable nomogram to better guide clinical practice for this group of HCC patients with high levels of HBsAg. In general, high-risk patients needed more frequent clinical surveillance and appropriate interventions to prevent recurrence and progression.
5 Conclusion
In summary, high levels of HBsAg were associated with tumor progression and poor prognosis. For high levels of HBsAg patients, we created an accurate and reliable nomogram to predict recurrence based on the Lasso-Cox regression analysis. The nomogram, including age, BCLC stage, tumor size, globulin, GGT, and bile acids, demonstrated adequate discrimination ability, which could better guide the clinical decisions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
YX: Data curation, Writing – original draft, Writing – review & editing. WQ: Validation, Visualization, Writing – review & editing. QW: Data curation, Writing – review & editing. KL: Supervision, Writing – review & editing. RJ: Resources, Supervision, Writing – review & editing. YZ: Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by a grant Beijing Municipal Natural Science Foundation (7191004), Capital health development project (2020-1-2182 and 2020-2-1153), Beijing Key Laboratory (BZ0373).
Acknowledgments
The authors are highly grateful to all the patients who participated in this study and to our team from Beijing Youan Hospital and Beijing Ditan Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1357496/full#supplementary-material
Supplementary Figure 1 | A. 1-, 3-, and 5-year ROC curves of the nomogram in the internal validation cohort. B. 1-, 3-, and 5-year ROC curves of the nomogram in the external validation cohort.
Supplementary Figure 2 | Calibration curves for recurrence of the nomogram in the validation cohorts. (A) One-year calibration curve in the internal validation cohort. (B) Three-year calibration curve in the internal validation cohort. (C) Five-year calibration curve in the internal validation cohort. (D) One-year calibration curve in the external validation cohort. (E) Three-year calibration curve in the external validation cohort. (F) Five-year calibration curve in the external validation cohort.
Supplementary Figure 3 | Decision curves analysis for recurrence in the internal validation cohort. (A) Decision curve analysis for one-year RFS in the internal validation cohort. (B) Decision curve analysis for three-year RFS in the internal validation cohort. (C) Decision curve analysis for five- year RFS in the internal validation cohort. (D) Decision curve analysis for one-year RFS in the external validation cohort. (E) Decision curve analysis for three-year RFS in the external validation cohort. (F) Decision curve analysis for five- year RFS in the external validation cohort.
Supplementary Figure 4 | (A) Kaplan-Meier plots of RFS for the low-risk group and high-risk group in the internal validation cohort. (B) Kaplan-Meier plots of RFS for the low-risk group and high-risk group in the external validation cohort.
References
1. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. (2022) 400:1345–62. doi: 10.1016/s0140-6736(22)01200-4
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, et al. Changing cancer survival in China during 2003-15: A pooled analysis of 17 population-based cancer registries. Lancet Glob Health. (2018) 6:e555–e67. doi: 10.1016/s2214-109x(18)30127-x
4. Asafo-Agyei KO, Samant H. Hepatocellular carcinoma. In: Statpearls. StatPearls Publishing LLC, Treasure Island (FL (2023). StatPearls Publishing Copyright © 2023.
5. Iannacone M, Guidotti LG. Immunobiology and pathogenesis of hepatitis B virus infection. Nat Rev Immunol. (2022) 22:19–32. doi: 10.1038/s41577-021-00549-4
6. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. (2007) 132:2557–76. doi: 10.1053/j.gastro.2007.04.061
7. Xue M, Lin X, Lin QX, Pu X, Liu J, Li XF, et al. Association between hepatitis B and E virus infection and hepatocellular carcinoma risk. Int J Cancer. (2021) 148:2974–81. doi: 10.1002/ijc.33505
8. Bai XM, Cui M, Yang W, Wang H, Wang S, Zhang ZY, et al. The 10-year survival analysis of radiofrequency ablation for solitary hepatocellular carcinoma 5 cm or smaller: primary versus recurrent hcc. Radiology. (2021) 300:458–69. doi: 10.1148/radiol.2021200153
9. Kang TW, Kim JM, Rhim H, Lee MW, Kim YS, Lim HK, et al. Small hepatocellular carcinoma: radiofrequency ablation versus nonanatomic resection–propensity score analyses of long-term outcomes. Radiology. (2015) 275:908–19. doi: 10.1148/radiol.15141483
10. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. (2021) 7:6. doi: 10.1038/s41572-020-00240-3
11. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. Bclc strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. (2022) 76:681–93. doi: 10.1016/j.jhep.2021.11.018
12. Broquetas T, Carrión JA. Past, present, and future of long-term treatment for hepatitis B virus. World J Gastroenterol. (2023) 29:3964–83. doi: 10.3748/wjg.v29.i25.3964
13. Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. Aasld guidelines for treatment of chronic hepatitis B. Hepatology. (2016) 63:261–83. doi: 10.1002/hep.28156
14. Easl 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. (2017) 67:370–98. doi: 10.1016/j.jhep.2017.03.021
15. García-López M, Lens S, Pallett LJ, Testoni B, Rodríguez-Tajes S, Mariño Z, et al. Viral and immune factors associated with successful treatment withdrawal in hbeag-negative chronic hepatitis B patients. J Hepatol. (2021) 74:1064–74. doi: 10.1016/j.jhep.2020.11.043
16. Sonneveld MJ, Chiu SM, Park JY, Brakenhoff SM, Kaewdech A, Seto WK, et al. Probability of hbsag loss after nucleo(S)Tide analogue withdrawal depends on hbv genotype and viral antigen levels. J Hepatol. (2022) 76:1042–50. doi: 10.1016/j.jhep.2022.01.007
17. Anderson RT, Choi HSJ, Lenz O, Peters MG, Janssen HLA, Mishra P, et al. Association between seroclearance of hepatitis B surface antigen and long-term clinical outcomes of patients with chronic hepatitis B virus infection: systematic review and meta-analysis. Clin Gastroenterol Hepatol. (2021) 19:463–72. doi: 10.1016/j.cgh.2020.05.041
18. Liang X, Liangliang X, Peng W, Tao Y, Jinfu Z, Ming Z, et al. Combined prognostic nutritional index and albumin-bilirubin grade to predict the postoperative prognosis of hbv-associated hepatocellular carcinoma patients. Sci Rep. (2021) 11:14624. doi: 10.1038/s41598-021-94035-5
19. Zheng X, Ye B, Gou Y, Li Z, Chen C, Liao F, et al. Neutrophil to lymphocyte and platelet to lymphocyte ratios as biomarkers to predict relapse and survival in posthepatectomy hbv-related hepatocellular carcinoma: A meta-analysis and preliminary immune perspective. Transl Cancer Res. (2021) 10:1261–72. doi: 10.21037/tcr-20-3125
20. Su K, Shen Q, Tong J, Gu T, Xu K, Li H, et al. Construction and validation of a nomogram for hbv-related hepatocellular carcinoma: A large, multicenter study. Ann Hepatol. (2023) 28:101109. doi: 10.1016/j.aohep.2023.101109
21. Wang Q, Qiao W, Zhang H, Liu B, Li J, Zang C, et al. Nomogram established on account of lasso-cox regression for predicting recurrence in patients with early-stage hepatocellular carcinoma. Front Immunol. (2022) 13:1019638. doi: 10.3389/fimmu.2022.1019638
22. Wang Q, Guo D, Gao W, Yuan C, Li J, Zhang Y, et al. Individual surveillance by competing risk model for patients with hepatocellular carcinoma occurrence in all-cause cirrhosis. J Cancer Res Clin Oncol. (2023) 149:13403–16. doi: 10.1007/s00432-023-04911-y
23. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. Aasld guidelines for the treatment of hepatocellular carcinoma. Hepatology. (2018) 67:358–80. doi: 10.1002/hep.29086
24. Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: A time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond). (2021) 41:1037–48. doi: 10.1002/cac2.12197
25. Lu L, Mullins CS, Schafmayer C, Zeißig S, Linnebacher M. A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun (Lond). (2021) 41:1137–51. doi: 10.1002/cac2.12220
26. Schmucker DL. Aging and the liver: an update. J Gerontol A Biol Sci Med Sci. (1998) 53:B315–20. doi: 10.1093/gerona/53a.5.b315
27. Zoli M, Iervese T, Abbati S, Bianchi GP, Marchesini G, Pisi E. Portal blood velocity and flow in aging man. Gerontology. (1989) 35:61–5. doi: 10.1159/000213000
28. Tsilimigras DI, Bagante F, Sahara K, Moris D, Hyer JM, Wu L, et al. Prognosis after Resection of Barcelona Clinic Liver Cancer (Bclc) Stage 0, a, and B Hepatocellular Carcinoma: A Comprehensive Assessment of the Current Bclc Classification. Ann Surg Oncol. (2019) 26:3693–700. doi: 10.1245/s10434-019-07580-9
29. Wang J, Wang K, Chen C, Xiong Y, Guo C, Wang C, et al. Survival analysis and development of a prognostic nomogram for patients with hepatitis B virus-associated hepatocellular carcinoma. Heliyon. (2023) 9:e20850. doi: 10.1016/j.heliyon.2023.e20850
30. Yang JG, He XF, Huang B, Zhang HA, He YK. Rule of changes in serum ggt levels and ggt/alt and ast/alt ratios in primary hepatic carcinoma patients with different afp levels. Cancer biomark. (2018) 21:743–6. doi: 10.3233/cbm-170088
31. Zhang LX, Lv Y, Xu AM, Wang HZ. The prognostic significance of serum gamma-glutamyltransferase levels and ast/alt in primary hepatic carcinoma. BMC Cancer. (2019) 19:841. doi: 10.1186/s12885-019-6011-8
32. Deng Y, Pang Q, Miao RC, Chen W, Zhou YY, Bi JB, et al. Prognostic significance of pretreatment albumin/globulin ratio in patients with hepatocellular carcinoma. Onco Targets Ther. (2016) 9:5317–28. doi: 10.2147/ott.S109736
33. Xing Y, Guo ZN, Yan S, Jin H, Wang S, Yang Y. Increased globulin and its association with hemorrhagic transformation in patients receiving intra-arterial thrombolysis therapy. Neurosci Bull. (2014) 30:469–76. doi: 10.1007/s12264-013-1440-x
34. Doi H, Hayashi E, Arai J, Tojo M, Morikawa K, Eguchi J, et al. Enhanced B-cell differentiation driven by advanced cirrhosis resulting in hyperglobulinemia. J Gastroenterol Hepatol. (2018) 33(9):1667–76. doi: 10.1111/jgh.14123
35. Li J, Li Z, Hao S, Wang J, Chen W, Dai S, et al. Inversed albumin-to-globulin ratio and underlying liver disease severity as a prognostic factor for survival in hepatocellular carcinoma patients undergoing transarterial chemoembolization. Diagn Interv Radiol. (2023) 29:520–8. doi: 10.5152/dir.2022.211166
36. Boyer JL. Bile formation and secretion. Compr Physiol. (2013) 3:1035–78. doi: 10.1002/cphy.c120027
37. Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. (2016) 24:41–50. doi: 10.1016/j.cmet.2016.05.005
38. Režen T, Rozman D, Kovács T, Kovács P, Sipos A, Bai P, et al. The role of bile acids in carcinogenesis. Cell Mol Life Sci. (2022) 79:243. doi: 10.1007/s00018-022-04278-2
39. Nguyen PT, Kanno K, Pham QT, Kikuchi Y, Kakimoto M, Kobayashi T, et al. Senescent hepatic stellate cells caused by deoxycholic acid modulates Malignant behavior of hepatocellular carcinoma. J Cancer Res Clin Oncol. (2020) 146:3255–68. doi: 10.1007/s00432-020-03374-9
40. Fuchs CD, Trauner M. Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat Rev Gastroenterol Hepatol. (2022) 19:432–50. doi: 10.1038/s41575-021-00566-7
41. Shi Q, Yuan X, Xue C, Gu X, Li L. Establishment and validation of a novel risk score for hepatocellular carcinoma based on bile acid and bile salt metabolism-related genes. Int J Mol Sci. (2023) 24(10):8579. doi: 10.3390/ijms24108597
42. Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: aasld 2018 hepatitis B guidance. Hepatology. (2018) 67:1560–99. doi: 10.1002/hep.29800
43. Liaw YF. Clinical utility of hepatitis B surface antigen quantitation in patients with chronic hepatitis B: A review. Hepatology. (2011) 53:2121–9. doi: 10.1002/hep.24364
44. Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013. Lancet. (2015) 386:1546–55. doi: 10.1016/s0140-6736(15)61412-x
45. Yuen MF, Chen DS, Dusheiko GM, Janssen HLA, Lau DTY, Locarnini SA, et al. Hepatitis B virus infection. Nat Rev Dis Primers. (2018) 4:18035. doi: 10.1038/nrdp.2018.35
46. Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. (2014) 384:2053–63. doi: 10.1016/s0140-6736(14)60220-8
47. Moini M, Fung S. Hbsag loss as a treatment endpoint for chronic hbv infection: hbv cure. Viruses. (2022) 14(4):657. doi: 10.3390/v14040657
48. Liu B, Wang Q, Mei T, Zheng J, Gao W, Yuan C, et al. Effect of hbsag expression in liver tissue on prognosis of hepatocellular carcinoma after minimally invasive interventional therapy. Front Oncol. (2023) 13:1106333. doi: 10.3389/fonc.2023.1106333
49. Wang Q, Qiao W, Liu B, Li J, Yuan C, Long J, et al. The Monocyte to Lymphocyte Ratio Not Only at Baseline but Also at Relapse Predicts Poor Outcomes in Patients with Hepatocellular Carcinoma Receiving Locoregional Therapy. BMC Gastroenterol. (2022) 22:98. doi: 10.1186/s12876-022-02180-6
50. Chen S, Zeng X, Su T, Xiao H, Lin M, Peng Z, et al. Combinatory local ablation and immunotherapies for hepatocellular carcinoma: rationale, efficacy, and perspective. Front Immunol. (2022) 13:1033000. doi: 10.3389/fimmu.2022.1033000
Keywords: hepatocellular carcinoma, hepatitis B surface antigen (HBsAg), TACE, ablation, nomogram, recurrence
Citation: Xiong Y, Qiao W, Wang Q, Li K, Jin R and Zhang Y (2024) Construction and validation of a machine learning-based nomogram to predict the prognosis of HBV associated hepatocellular carcinoma patients with high levels of hepatitis B surface antigen in primary local treatment: a multicenter study. Front. Immunol. 15:1357496. doi: 10.3389/fimmu.2024.1357496
Received: 18 December 2023; Accepted: 19 March 2024;
Published: 27 March 2024.
Edited by:
Tinka Vidovic, University of Zagreb, CroatiaReviewed by:
Cemil Çolak, İnönü University, TürkiyeDillip Kumar Bishi, Rama Devi Women’s University, India
Copyright © 2024 Xiong, Qiao, Wang, Li, Jin and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonghong Zhang, emhhbmd5aEBjY211LmVkdS5jbg==; Ronghua Jin, cm9uZ2h1YWppbkBjY211LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Yiqi Xiong1†
Yiqi Xiong1† Wenying Qiao
Wenying Qiao Qi Wang
Qi Wang Kang Li
Kang Li Ronghua Jin
Ronghua Jin Yonghong Zhang
Yonghong Zhang