Abstract
Background:
Locoregional treatment combined with systemic therapy is expected to play a synergistic anticancer role. We conducted this systemic meta-analysis to examine the efficacy and safety of transarterial chemoembolization (TACE) plus lenvatinib with or without programmed cell death protein-1 (PD-1) inhibitors (TLP group) compared with TACE + lenvatinib (TL group) for unresectable hepatocellular carcinoma (uHCC).
Methods:
From the inception date to April 2024, the data from PubMed, EMBASE, the Cochrane Library, Ovid, Web of Science, and Clinical Trials. gov were used for meta-analysis. All clinical outcomes of interest included overall survival (OS), progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), and adverse events (AEs). The hazard ratio (HR) and risk ratio (RR) with 95% confidence intervals (CI) were used to measure the pooled effect.
Results:
This study included 10 retrospective cohort studies, including 1128 patients. The OS (HR=0.51; 95% CI: 0.43–0.60, P < 0.05), PFS (HR=0.52; 95% CI: 0.45–0.61, P < 0.05), ORR (RR = 1.58; 95% CI: 1.37–1.83; P < 0.05) and DCR (RR = 1.31; 95% CI: 1.20–1.43; P < 0.05) were significantly higher in TLP group than in the TL group. The incidence of AEs was acceptable. Prognostic factor analysis identified that ECOG PS (1/0), Child-Pugh class (B/A), BCLC stage (C/B) and main portal vein invasion (yes/no) were independent prognostic factors for OS. BCLC stage (C/B) and main portal vein invasion (yes/no) were independent prognostic factors for PFS.
Conclusion:
The TLP group had better efficacy for uHCC than that of the TL group, with acceptable safety.
Systematic review registration:
PROSPERO, identifier (CRD42023420093).
Introduction
The morbidity and mortality of hepatocellular carcinoma (HCC) remain high worldwide, among which primary liver cancer is the most common. It ranks fifth in the incidence rate and is the third leading cause of cancer death in the world (1). Because of the strong compensatory ability of liver, most patients are diagnosed with HCC when the disease progresses to the middle and late stage. Patients eventually lost the opportunity of surgery, ablation and liver transplantation, resulting in poor prognosis (2). Therefore, the combined treatment of unresectable HCC (uHCC) has attracted much attention.
In SHARP and REFLECT trials, tyrosine kinase inhibitors (TKIs) sorafenib and lenvatinib were recommended as the first-line treatment drugs for advanced HCC respectively (3, 4). Among them, subsequent trials proved that the effect of lenvatinib was not inferior to sorafenib for HCC (5). However, it is found that systemic therapy alone cannot achieve satisfactory survival time for advanced HCC. According to the treatment strategy of Barcelona Clinic Liver Cancer (BCLC), transitional chemoembolization (TACE) is recognized as the first preferred treatment for uHCC (6). But TACE alone has certain limitations. Subsequent clinical trials have proved that the combination of TACE and lenvatinib is more effective than single therapy, with potential effectiveness and safety (7).
With the emergence of refractory and drug-resistant HCC, blocking immune checkpoint pathway by immune checkpoint inhibitors (ICIs) has been found to be a new cancer treatment strategy (8). At present, the most widely studied ICIs is anti-programmed cell death 1 (PD-1)/programmed cell death protein ligand 1 (PD-L1), which can enhance the function of T cells and exert an anti-tumor activity (9). A recent clinical trial (IMbrave150) showed that compared with sorafenib or lenvatinib, the combination of atilizumab (PD-L1) and bevacizumab (anti-vascular endothelial growth factor, VEGF) was a better first-line choice for advanced HCC, and the overall survival(OS) of HCC was significantly prolonged (10). Since then, the beginning of targeted immunotherapy for HCC has begun, and PD-1 has become the second-line therapy for advanced HCC. Therefore, adding PD-1 on the basis of TACE + lenvatinib may optimize the efficacy of the triple therapy and produce more desirable synergistic effect (11, 12).
In the past two years, the efficacy of TACE + lenvatinib + PD-1 triple therapy has achieved encouraging results in many trials. Therefore, we conducted this systemic meta-analysis to compare the efficacy and safety of TACE + lenvatinib + PD-1 triple therapy and TACE + lenvatinib dual therapy in multiple trials, so as to determine a better treatment plan for uHCC patients.
Materials and methods
Ethical approval was not required for this study, and the article has been reported in line with the PRISMA (Preferred Reporting Items for systemic Reviews and Meta Analyses) checklist (13). This meta-analysis was registered at PROSPERO (CRD42023420093).
Literature search strategy
The publication time was limited to when the databases were established until April, 2024. We conducted a systemic search of PubMed, EMBASE, the Cochrane Library, Ovid, Web Of Science, and Clinical Trials.gov databases to identify useful literatures related to this meta-analysis. The MESH terms used in these databases included (“carcinoma, hepatocellular” or “liver cancer” or “HCC” or “liver neoplasm”), (“transarterial chemoembolization” or “TACE”), (“PD-1” or “immunotherapy therapies”) (“Lenvatinib” or “Lenvima”). There are no restrictions on the language of selected literatures. After that, two authors (XX and XX) independently extracted and confirmed relevant data. The flowchart of the article screening and selection process is presented in the Figure 1.
Figure 1
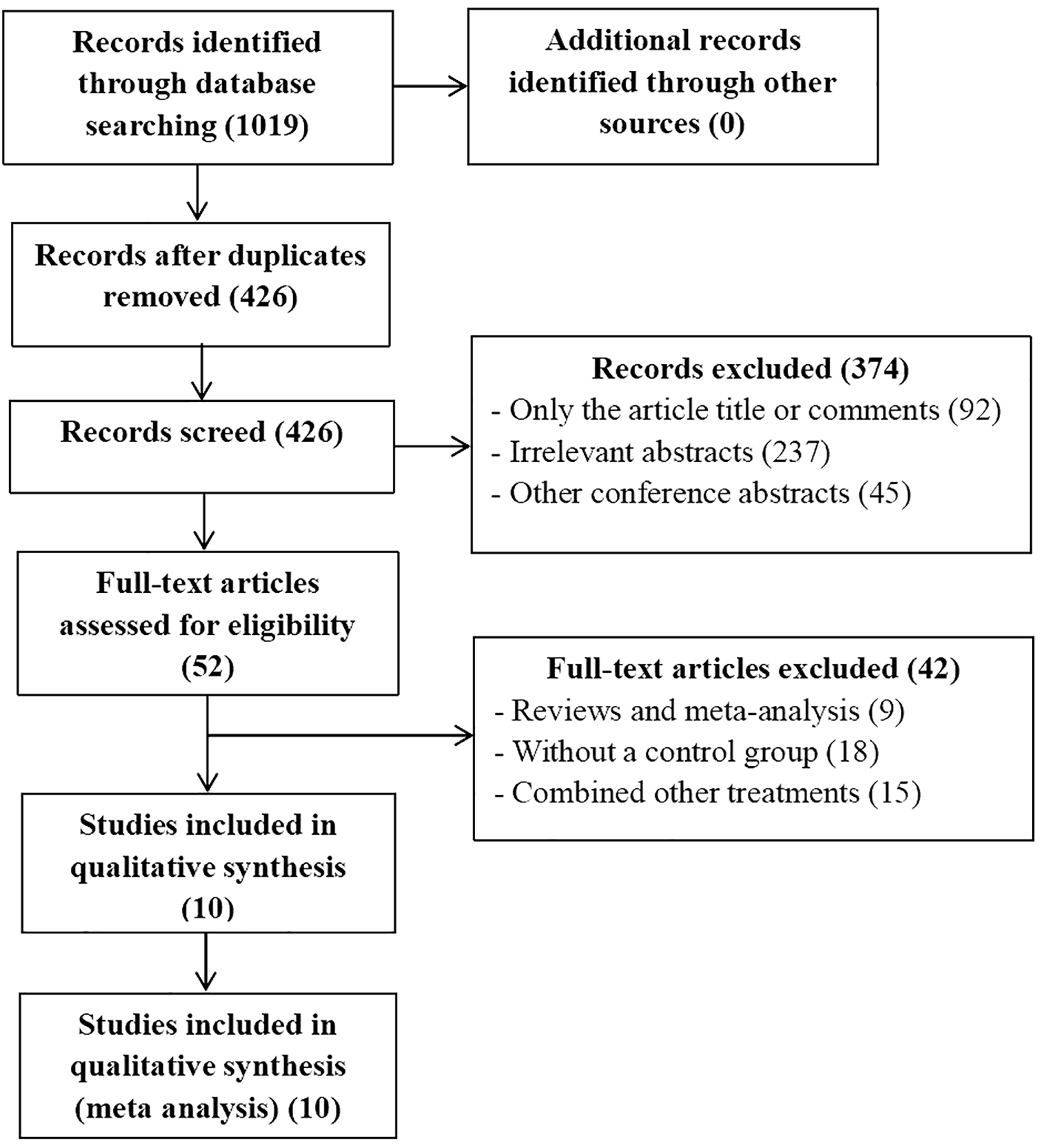
Flowchart of article screening and selection process.
Study selection
Inclusion criteria: 1) the patients diagnosed with uHCC by imaging and biopsy evidence; 2) the uHCC patients received TACE + lenvatinib + PD-1(TLP) group compared with TACE + lenvatinib (TL) group; 3) the types of study include randomized controlled trials (RCT) and retrospective cohort studies (RCS); 4) the clinical outcomes evaluated were OS, progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), and adverse events (AEs), which include at least one valuable survival outcome.
Exclusion criteria: 1) the study types included a review, a meta-analysis, a conference abstract, a letter, and a case report; 2) the study lacked effective outcomes data or reported irrelevant outcomes; 3) There is no control group.
Data extraction and quality assessment
Two reviewers (XX and XX) independently screened the studies and evaluated the quality of the included studies in a standardized way. Any discrepancy was resolved through a discussion. A third reviewer (XX) would decide if necessary. The data extracted from each study include the name of the first author, the year of publication, the design of the study population, the nationality and the clinical characteristics of patients (including sex, age, Child-Pugh class, ECOG PS, BCLC stage). The Newcastle-Ottawa Scale (NOS) was applied for RCS (14). Additionally, tumor responses were determined by the modified response evaluation criteria in solid tumors (mRECIST) or RECIST. The quality assessment of each literature is presented in Table 1.
Table 1
| Study | Country | Design | Patients (M/F) |
Age (years) |
Child-Pugh class (A/B) |
ECOG PS (0/1/2) |
BLCL (B/C) |
PD-1 | NOS SCORE |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TACE-L-P | L-P | TACE-L-P | L-P | TACE-L-P | L-P | TACE-L-P | L-P | TACE-L-P | L-P | |||||
| Cai 2022 (15) |
China | RCS | 41 (37/4) |
40 (33/7) |
51.9 ± 10.3 | 54.6 ± 11.0 | 37/4 | 33/7 | 33/8/0 | 28/12/0 | / | / | Sintilimab, Tislelizumab, Camrelizumab | 8 |
| Chen 2021 |
China | RCS | 70 (37/33) |
72 (38/34) |
54.2 | 50 | / | / | 47/23/0 | 30/42 | 47/23 | 45/27 | Pembrolizumab | 8 |
| Guo 2022 (16) |
China | RCS | 75 (65/10) |
91 (88/3) |
≤60: 66 >60: 25 |
≤60: 60 >60: 15 |
13/78 | 2/73 | 58/33/0 | 69/6/0 | 20/71 | 20/55 | Camrelizumab, Sintilimab | 8 |
| Qu 2022 (17) |
China | RCS | 30 (26/4) |
21 (20/1) |
55.5 (47.8, 64.3) |
50.0 (45.0, 61.0) |
28/2 | 21/0 | / | / | 1/29 | 3/18 | / | 7 |
| Sheng 2024 (18) |
China | RCS | 113 (95/18) |
128 (108/20) |
64.48 ± 10.83 | 62.59 ± 10.58 | 88/25 | 99/29 | 62/36/15 | 66/42/20 | 54/59 | 63/65 | Sintilimab, Camrelizumab, Tislelizumab | 8 |
| Wang yy 2023 (19) |
China | RCS | 45 (42/3) |
20 (15/5) |
54 (18 - 79) | 62 (26 - 75) | 30/15 | 18/2 | 26/19/0 | 7/13/0 | 11/34 | 5/15 | Camrelizumab, Sintilimab, Pembrolizumab, Tislelizumab, Nivolumab | 7 |
| Wang wj 2023 (20) |
China | RCS | 54 (49/5) |
45 (43/2) |
57.0 ± 9.9 | 60.8 ± 9.4 | 49/5 | 42/3 | 46/8/0 | 41/4/0 | / | / | Sintilimab, Camrelizumab, Toripalimab | 8 |
| Wu 2024 (21) |
China | RCS | 18 (15/3) |
23 (18/5) |
56.9 ± 8.1 | 58.1 ± 9.4 | 18/0 | 21/2 | 7/11/0 | 7/16/0 | / | / | Sintilimab, Camrelizumab, Nivolumab, Tislelizumab | 8 |
| Xiang 2023 (22) |
China | RCS | 33 (28/5) |
49 (45/4) |
51.0 ± 12.2 | 51.7 ± 11.2 | 25/8 | 41/8 | 22/11 | 38/11 | 10/23 | 22/27 | camrelizumab | 8 |
| Zou 2023 (23) |
China | RCS | 70 (59/11) |
90 (77/13) |
53.6 ± 15.1 | 52.3 ± 14.8 | 46/24 | 61/29 | 17/53/0 | 28/62/0 | 0/70 | 0/90 | Pembrolizumab, Sintilimab | 8 |
Baseline characteristics of the studies.
M, male; F, female; TACE; transarterial chemoembolization; L, lenvatinib; P, programmed cell death protein-1; ECOG, Eastern Cooperative Oncology Group; BCLC, Barcelona Clinic Liver Cancer; NOS, the Newcastle–Ottawa Scale; RCS, retrospective cohort study; /, not reported.
Statistical analysis
Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated to analyze OS and PFS. The risk ratios (RR) with 95% CIs were calculated to analyze ORR, DCR and AEs. A fixed effect model was used for data pooling if no significant heterogeneity among included trials was observed. Otherwise, a random effect model was used. The I2 statistic (I2 > 50% was deemed to have significant heterogeneity) and χ2 test (P < 0.10 was deemed to suggest significant heterogeneity) were used to assess the heterogeneity among the trials. The funnel plots were performed to detect the existence of publication bias (P < 0.10 was deemed to represent significant publication bias). All analyses were performed using the Revman5.4 software. Statistical test was a two-tailed test, and p < 0.05 was statistically significant.
Results
Search results
A total of 10 articles met the inclusion and exclusion criteria, of which 426 were selected after removing duplicates. We excluded 374 articles after reviewing the titles and abstracts of 426 articles. The full text of the remaining 52 articles were evaluated. According to the inclusion and exclusion criteria, 42 studies were excluded. Ultimately, 10 articles including 1128 patients were included in the current meta-analysis, which all studies included were RCS (15–24).
Study characteristics
The included study characteristics are summarized in Table 1. All included articles were published in China from 2022 to 2024. Of the 1128 patients included in our meta-analysis, 848 were male and 280 were female. Furthermore, 549 patients with uHCC received TLP triple therapy, while 579 patients received TL dual therapy. The PD-1 included in all selected articles were mainly Sintilimab, Tislelizumab, Camrelizumab, Pembrolizumab and Nivolumab, which was also depicted in Table 1. There were some significant variables which were analyzed in subgroups in the two groups.
Risk of bias
NOS was used to assess RCS. It contains the selection of subjects, comparability of the groups, and assessment of outcomes, with a maximum of 9 points. Studies with a score of more than 6 were determined to be high quality.
Meta-analysis outcomes
Overall survival and progression-free survival
Only nine articles provided the outcomes of OS and 10 articles provided the outcomes of PFS for the two groups, including the point estimate (HR) and its 95% CI. The meta-analysis indicated that patients with uHCC in the TLP group had significantly longer OS (HR=0.51; 95% CI: 0.43–0.60, P < 0.05) and PFS (HR=0.52; 95% CI: 0.45–0.61, P < 0.05) than those in the TL group. The findings indicated that the TLP triple therapy could significantly prolonged survival time (Figure 2).
Figure 2
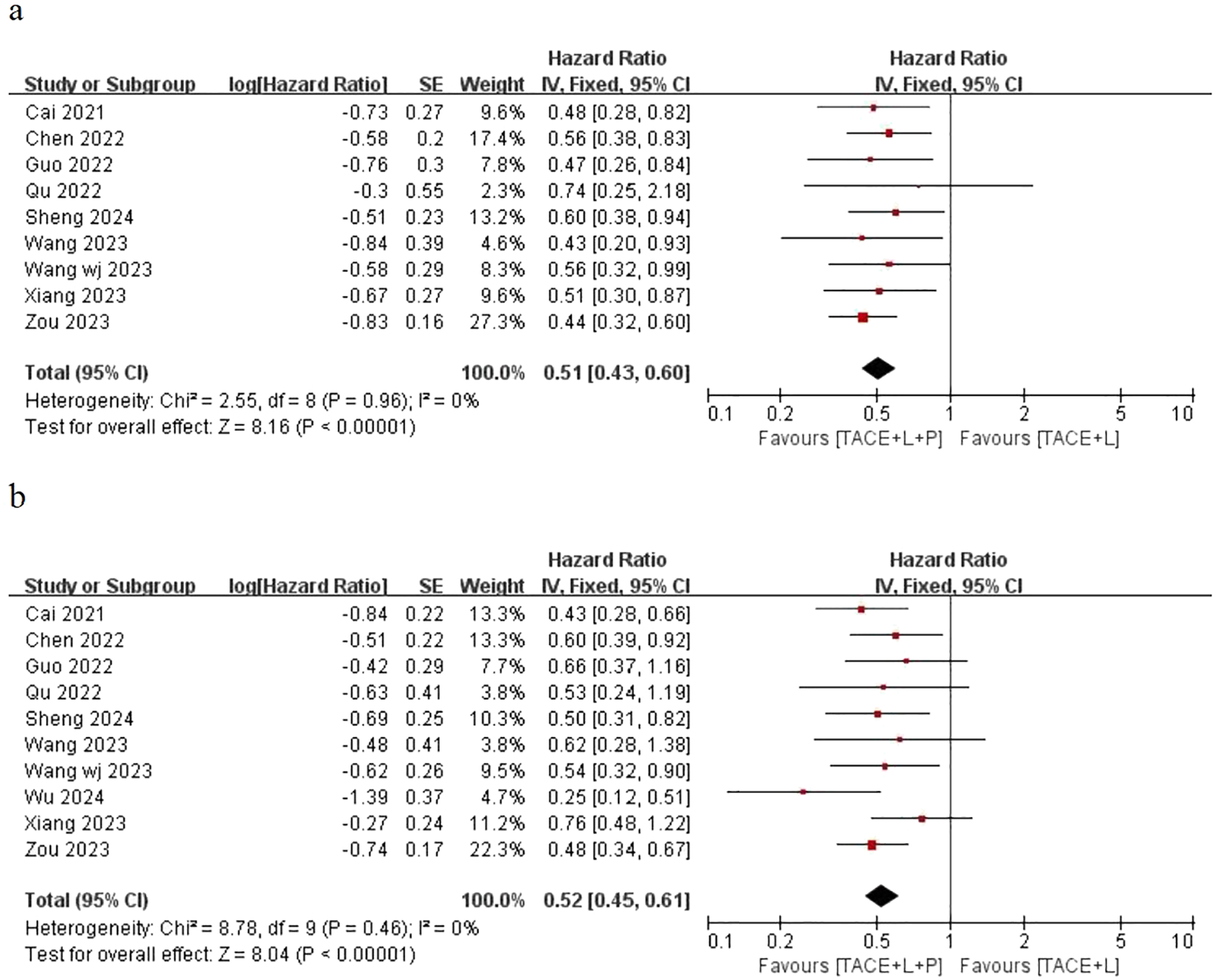
Fixed effect model of OS (A) and PFS (B) for uHCC with TLP vs TL. OS: overall survival, PFS: progression-free survival, uHCC: unresectable hepatocellular carcinoma, T: transarterial chemoembolization, L: lenvatinib, P: programmed cell death protein-1, CI: confidence intervals.
Disease control rate and objective response rate
Ten articles have provided the outcomes of ORR and only nine articles have provided the outcomes of DCR for the two groups, including the point estimate (RR) and its 95% CI. The meta-analysis indicated that patients with uHCC in the TLP group had significantly better ORR (RR = 1.58; 95% CI: 1.37–1.83; P < 0.05) and DCR (RR = 1.31; 95% CI: 1.20–1.43; P < 0.05) than those in the TL group (Figure 3). Similarly, the results also showed that the TLP triple therapy was more effective than the TL dual therapy.
Figure 3
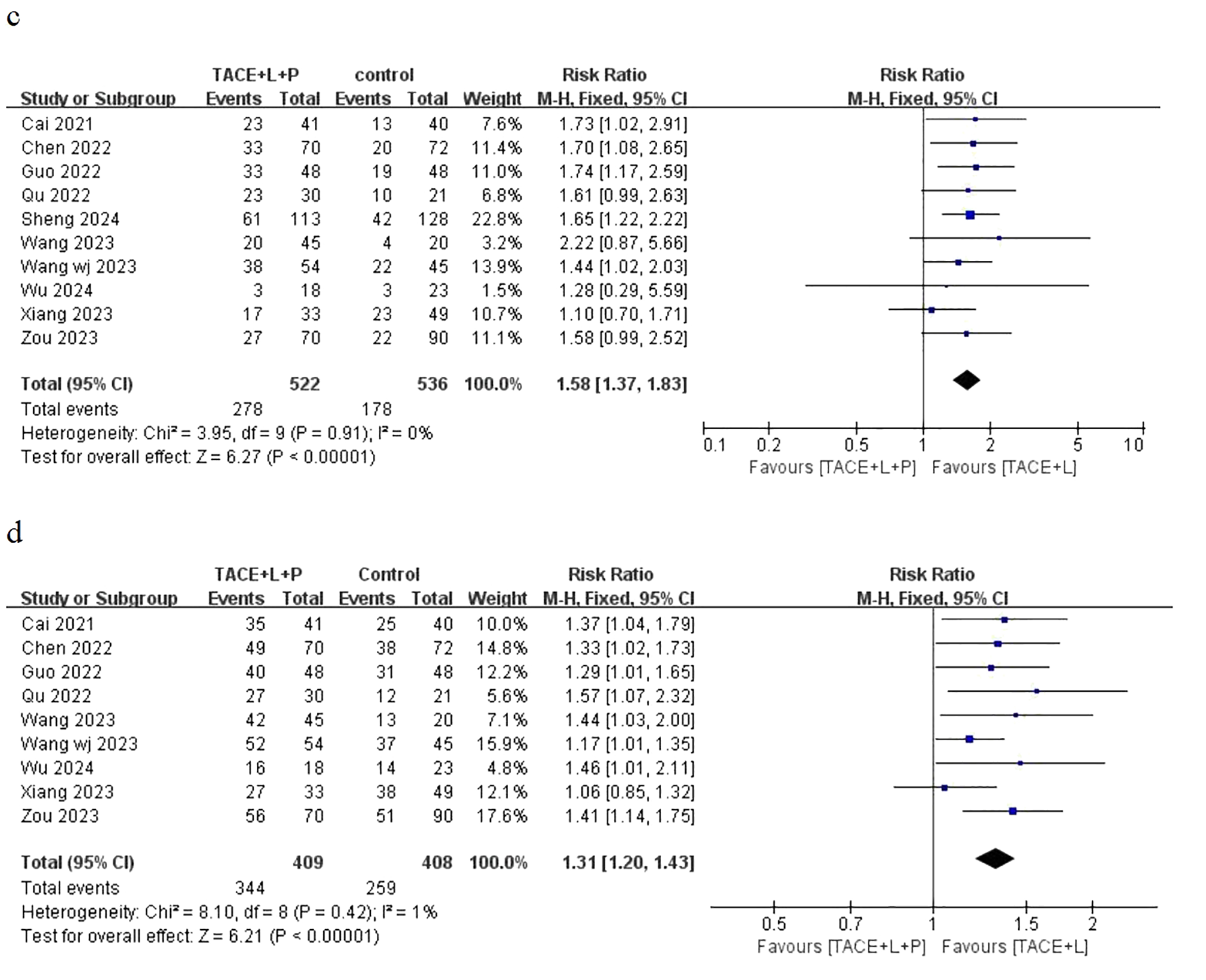
Fixed effect model of ORR (C) and DCR (D) for uHCC with TLP vs TL. ORR: objective response rate, DCR: disease control rate, uHCC: unresectable hepatocellular carcinoma, T: transarterial chemoembolization, L: lenvatinib, P: programmed cell death protein-1, CI: confidence intervals.
Prognostic factor analysis for overall survival and progression-free survival
The results based on univariate and multivariate analysis data from included trials in both groups showed that ECOG PS (1/0): (HR=1.18; 95% CI: 1.02-1.36, P < 0.05), Child-Pugh class (B/A): (HR=1.83; 95% CI: 1.54-2.17, P < 0.05), BCLC stage (C/B): (HR=1.85; 95% CI: 1.29-2.66, P < 0.05) and main portal vein invasion (yes/no): (HR=1.25; 95% CI: 1.05-1.50, P < 0.05) were independent prognostic factors for OS. Similarly, the results showed that BCLC stage (C/B): (HR=1.60; 95% CI: 1.04-2.46, P < 0.05) and main portal vein invasion (yes/no): (HR=1.27; 95% CI: 1.02-1.59, P < 0.05) were independent prognostic factors for PFS (Table 2).
Table 2
| Factor | Analysis of OS | Analysis of PFS | ||||
|---|---|---|---|---|---|---|
| No. | HR [95% CI] | P | No. | HR [95% CI] | P | |
| AFP((μg/L) ≥400/<400 |
4 | 1.23 [0.96, 1.56] | 0.1 | 5 | 1.05 [0.86, 1.29] | 0.62 |
| ECOG PS 1/0 |
4 | 1.18 [1.02, 1.36] | 0.03 | 5 | 1.45 [0.96, 2.19] | 0.07 |
| Child-Pugh class B/A |
5 | 1.83 [1.54, 2.17] | <0.001 | 4 | 1.32 [0.81, 2.14] | 0.27 |
| BCLC stage C/B |
3 | 1.85 [1.29, 2.66] | 0.001 | 2 | 1.60 [1.04, 2.46] | 0.03 |
| main portal vein invasion yes/no |
4 | 1.25 [1.05, 1.50] | 0.01 | 5 | 1.27 [1.02, 1.59] | 0.03 |
| Extrahepatic metastasis yes/no |
4 | 1.42 [0.89, 2.26] | 0.15 | 3 | 1.44 [0.88, 2.33] | 0.14 |
Analyses of prognostic factors for OS and PFS of TLP group vs TL group.
OS, overall survival; PFS, progression-free survival; T; transarterial chemoembolization; L, lenvatinib; P, programmed cell death protein-1; AFP, alpha fetoprotein; ECOG, Eastern Cooperative Oncology Group; BCLC, Barcelona Clinic Liver Cancer; No., number; HR, hazard ratio; CI, confidence intervals.
Bold values indicate significant risk factors affecting OS and PFS for uHCC patients in the TLP group vs. TLP group.
Adverse events
Ten included studies reported the incidence of grade 3/4 AEs and only 9 studies reported all grades AEs. The common incidence of grade 3-4 AEs and all grade AEs included abdominal pain, decreased appetite, hypertension, nausea, diarrhea, rash, hand-foot syndrome, elevated aspartate aminotransferase (AST), elevated alanine aminotransferase (ALT), thrombocytopenia, thyroid dysfunction (Table 3). The frequency of all grades AEs was similar in both groups. The incidence of 3/4 grade nausea (RR = 4.40; 95% CI: 1.42-13.61; P < 0.05), rash (RR = 2.75; 95% CI: 1.13-6.70; P < 0.05), hand-foot syndrome (RR = 2.51; 95% CI: 1.29-4.89; P < 0.05) and thyroid dysfunction (RR = 6.12; 95% CI: 1.49, 25.19; P < 0.05) were more frequent in the TLP group than in the TL group.
Table 3
| Adverse Events | All grades | Grade 3/4 | ||||
|---|---|---|---|---|---|---|
| No. | RR [95% CI] | P | No. | RR [95% CI] | P | |
| Abdominal pain | 7 | 0.92 [0.73, 1.17] | 0.52 | 7 | 1.00 [0.47, 2.11] | 0.99 |
| Decreased appetite | 6 | 1.22 [0.55, 2.72] | 0.63 | 7 | 1.33 [0.65, 2.70] | 0.44 |
| Hypertension | 8 | 1.23 [0.96, 1.59] | 0.1 | 8 | 1.74 [1.10, 2.74] | 0.51 |
| Nausea | 5 | 0.84 [0.50, 1.40] | 0.5 | 6 | 4.40 [1.42, 13.61] | 0.01 |
| Diarrhea | 9 | 1.09 [0.80, 1.48] | 0.58 | 10 | 1.45 [0.77, 2.71] | 0.25 |
| Rash | 6 | 1.39 [0.90, 2.15] | 0.14 | 7 | 2.75 [1.13, 6.70] | 0.03 |
| Hand-foot syndrome | 5 | 1.00 [0.70, 1.42] | 1 | 6 | 2.51 [1.29, 4.89] | 0.007 |
| Elevated AST | 5 | 1.18 [0.90, 1.56] | 0.23 | 6 | 1.32 [0.76, 2.31] | 0.32 |
| Elevated ALT | 5 | 1.05 [0.83, 1.32] | 0.71 | 6 | 0.95 [0.47, 1.93] | 0.89 |
| Thrombocytopenia | 7 | 1.19 [0.84, 1.70] | 0.32 | 8 | 1.31 [0.70, 2.46] | 0.40 |
| Thyroid dysfunction | 7 | 1.48 [0.89, 2.47] | 0.13 | 7 | 6.12 [1.49, 25.19] | 0.01 |
Adverse events of TLP group vs TL group.
T, transarterial chemoembolization; L, lenvatinib; P, programmed cell death protein-1; AST, aspartate aminotransferase; ALT, alanine aminotransferase; No., number; RR, relative risk; CI, confidence intervals.
The bold values indicate that compared to the TLP group, the TL group of uHCC patients experienced more frequent and severe grade 3-4 adverse events, which should be taken seriously.
Publication bias
The funnel plots were applied to show the publication bias of this meta-analysis. The funnel plots of outcomes for OS, PFS, ORR, and DCR are shown in the Supplementary Materials. In general, the probability of publication bias is low as the scatter points were distributed symmetrically in the inverted funnel.
Discussion
With the widespread concern of locoregional treatment combined with systemic targeted immunotherapy in the treatment of uHCC, more experiments have been conducted to study the efficacy of TACE plus lenvatinib combined with PD-1 for advanced HCC. The results of our meta-analysis showed that compared with the TL group, the TLP group achieved longer OS and PFS, better ORR and DCR, with low heterogeneity. Treatment-related AEs were acceptable. Prognostic factor analysis identified ECOG PS (1/0), Child Pugh class (B/A), BCLC stage (C/B), and main portal vein invasion (yes/no) as independent prognostic factors for OS. BCLC stage (C/B) and main portal vein invasion (yes/no) were identified as independent prognostic factors for PFS. As far as we know, our meta-analysis is a relatively comprehensive study at present, providing more reliable evidence for uHCC patients.
Lenvatinib is a multi-targeted receptor TKI that targets VEGFR1-3, as well as fibroblast growth factor receptors (FGFR)1-4 (25). The LAUNCH trial showed TACE + lenvatinib had longer OS and PFS than lenvatinib alone for uHCC (7). Experts agree that hypoxia caused by TACE therapy can up-regulate VEGF. TACE combined with lenvatinib can reverse the high secretion of antigen factors and inhibit the recurrence and metastasis of residual tumors (26, 27).
Currently, our meta-analysis compared TLP triple therapy with TL dual therapy, and the latter had better results in TLP group. The results of pooled analyses showed a low degree of heterogeneity for the outcomes, using the fixed effect models. The results are consistent with previous studies comparing the combination of TLP versus TL. In a real-world study, TACE combined with PD-1 and TKI significantly improved OS, PFS and ORR for Chinese patients with advanced HCC, with acceptable safety (28). Recently, a prospective study has shown that the triple therapy of TACE + lenvatinib + camrelizumab had satisfactory clinical effect and controllable safety, which further confirmed the clinical efficacy of locoregional treatment combined with targeted immunotherapy for uHCC (29). Better outcomes of TACE + lenvatinib + PD-1 are considered to be attributed to the synergistic antitumor activity of the three combination therapies. PD-1 was added to the TACE + lenvatinib combination therapy to enhance the anti-tumor immune effect. On the one hand, the possible mechanism is that lenvatinib can inhibit IFN-γ signal transduction in tumor cells by targeting FGFR, and the combination of PD-1 and lenvatinib can reverse the immunosuppressive state of the tumor microenvironment, thus improving the immune response rate of PD-1 inhibitors (12, 30). On the other hand, TACE can cause ischemic necrosis of tumor tissue and release a large number of tumor-specific antigens, thus enhancing the anti-tumor immune effect of PD-1 inhibitors (31, 32). In addition, TACE has relatively reduced the adverse reactions of HCC patients to systemic targeted immunotherapy and avoided the frequent drug resistance (33).
In a pooled analysis of all the studies we included, multiple factors were found to be risk factors for OS and PFS for uHCC, which was consistent with previous studies (34). For patients with uHCC, BCLC stage C and major portal vein invasion are both risk factors for OS and PFS of TLP group vs TP group. Both ECOG PS 0 and Child Pugh class A are applied to uHCC patients of TLP group vs TP group. Of note, liver function was evaluated with Child Pugh scores after the treatment procedure, which is significant for prognosis (35). Only patients with better liver function have the ability to tolerate subsequent target immunotherapy and fully utilize the advantages of combination therapy (36).
In addition, the treatment-related AEs were controllable and acceptable in both groups. It is worth noting that the results of our meta-analysis showed that the occurrence frequency of grade 3-4 AEs was higher in the TLP group than that in the TL group, which was safe with appropriate symptomatic treatment (37). The reason why the degree of nausea is more severe in the TLP group is that the triple combination therapy has greater toxic effects. Rash, hand-foot syndrome, and thyroid dysfunction are the most common immune-related adverse events (irAEs), mainly related to the therapeutic mechanism of PD-1 blockade of immune checkpoints. Importantly, the combination therapy for uHCC can lead to inevitable adverse reactions, and its long-term safety requires ongoing attention.
Although the results of this meta-analysis are satisfactory, there are still several limitations. Firstly, due to all the included literatures are retrospective studies, it is difficult to avoid recall bias. Secondly, only 10 studies were included, and there were not enough cases to analyze. Thirdly, considering that all published studies are from China, the conclusion is not applicable to western populations. Last but not least, most of the etiology of HCC are mainly related to hepatitis B virus infection in China. The benefits of combined PD-1 treatment may be related to the etiologies of disease in our study. Therefore, independent prospective clinical trials are needed to further verify the evaluation results.
Conclusion
This meta-analysis demonstrated that the triple therapy of TACE + lenvatinib +PD-1 was superior to the dual therapy of TACE + lenvatinib with respect to OS, PFS, ORR and DCR, with less occurrence of AEs.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
YC: Writing – original draft, Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software. LJ: Data curation, Writing – review & editing. YL: Data curation, Writing – review & editing. WC: Data curation, Writing – review & editing. CZ: Data curation, Writing – review & editing. JW: Data curation, Writing – review & editing. CB: Supervision, Writing – review & editing. TL: Validation, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank all teams and individuals which involved in this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1466113/full#supplementary-material
References
1
Sung H Ferlay J Siegel RL Laversanne M Soerjomataram I Jemal A et al . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 counties. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2
Zhou J Sun H Wang Z Cong W Wang J Zeng M et al . Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. (2020) 9:682–720. doi: 10.1159/000509424
3
Llovet JM Ricci S Mazzaferro V Hilgard P Gane E Blanc JF et al . Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. (2008) 359:378–90. doi: 10.1056/NEJMoa0708857
4
Llovet JM Montal R Sia D Finn RS . Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. (2018) 15:599–616. doi: 10.1038/s41571-018-0073-4
5
Kudo M Finn RS Qin S Han KH Ikeda K Piscaglia F et al . Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. (2018) 391:1163–73. doi: 10.1016/S0140-6736(18)30207-1
6
Reig M Forner A Rimola J Ferrer-F à brega J Ferrer-Fàbrega J Burrel M Garcia-Criado Á et al . BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. (2022) 76:681–93. doi: 10.1016/j.jhep.2021.11.018
7
Peng Z Fan W Zhu B Wang G Sun J Xiao C et al . Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: A phase III, randomized clinical trial (LAUNCH). J Clin Oncol. (2023) 41:117–27. doi: 10.1200/JCO.22.00392
8
Cheng AL Hsu C Chan SL Choo SP Kudo M . Challenges of combination therapy with immune checkpoint inspectors for hepatocellular carcinoma. J Hepatol. (2020) 72:307–19. doi: 10.1016/j.jhep.2019.09.025
9
Blank C Gajewski TF Mackensen A . Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. (2005) 54:307–14. doi: 10.1007/s00262-004-0593-x
10
Finn RS Qin S Ikeda M Galle PR Ducreux M Kim TY et al . Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
11
Rimassa L Finn RS Sangro B . Combination immunotherapy for hepatocellular carcinoma. J Hepatol. (2023) 79:506–15. doi: 10.1016/j.jhep.2023.03.003
12
Yi C Chen L Lin Z Liu L Shao W Zhang R et al . Lenvatinib targets FGF receptor 4 to enhance antitumor immune response of anti-programmed cell death-1 in HCC. Hepatology. (2021) 74:2544–60. doi: 10.1002/hep.31921
13
Page MJ Moher D Bossuyt PM Liu L Shao W Zhang R et al . PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160. doi: 10.1136/bmj.n160
14
Lo CK Mertz D Loeb M . Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. (2014) 14:45. doi: 10.1186/1471-2288-14-45
15
Cai M Huang W Huang J Shi W Guo Y Liang L et al . Transarterial chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: A retrospective cohort study. Front Immunol. (2022) 13:848387. doi: 10.3389/fimmu.2022.848387
16
Guo P Pi X Gao F Li Q Li D Feng W et al . Transarterial chemoembolization plus lenvatinib with or without programmed death-1 inhibitors for patients with unresectable hepatocellular carcinoma: A propensity score matching study. Front Oncol. (2022) 12:945915. doi: 10.3389/fonc.2022.945915
17
Qu WF Ding ZB Qu XD Tang Z Zhu GQ et al . Conversion therapy for initially unresectable hepatocellular carcinoma using a combination of toripalimab, lenvatinib plus TACE: real-world study. BJS Open. (2022) 6:zrac114. doi: 10.1093/bjsopen/zrac114
18
Sheng Y Wang Q Liu H Wang Q Chen W Xing W . Prognostic nomogram model for selecting between transarterial chemoembolization plus lenvatinib, with and without PD-1 inhibitor in unresectable hepatocellular carcinoma. Br J Radiol. (2024) 97:668–79. doi: 10.1093/bjr/tqae018
19
Wang YY Yang X Wang YC Long JY Sun HS Li YR et al . Clinical outcomes of lenvatinib plus transarterial chemoembolization with or without programmed death receptor-1 inhibitors in unresectable hepatocellular carcinoma. World J Gastroenterol. (2023) 29:1614–26. doi: 10.3748/wjg.v29.i10.1614
20
Wang WJ Liu ZH Wang K Yu HM Cheng YQ Xiang YJ et al . Efficacy and safety of TACE combined with lenvatinib and PD-1 inhibitors for unresectable recurrent HCC: A multicenter, retrospective study. Cancer Med. (2023) 12:11513–24. doi: 10.1002/cam4.5880
21
Wu HX Ding XY Xu YW Yu MH Li XM Deng N et al . Transcatheter arterial chemoembolization combined with PD-1 inhibitors and Lenvatinib for hepatocellular carcinoma with portal vein tumor thrombus. World J Gastroenterol. (2024) 30:843–54. doi: 10.3748/wjg.v30.i8.843
22
Xiang Z Li G Mu L Wang H Zhou C Yan H et al . TACE combined with lenvatinib and camrelizumab for unresectable multiple nodular and large hepatocellular carcinoma (>5 cm). Technol Cancer Res Treat. (2023) 22:15330338231200320. doi: 10.1177/15330338231200320
23
Zou X Xu Q You R Yin G . Correlation and efficacy of TACE combined with lenvatinib plus PD-1 inhibitor in the treatment of hepatocellular carcinoma with portal vein tumor thrombus based on immunological features. Cancer Med. (2023) 12:11315–33. doi: 10.1002/cam4.5841
24
Chen S Wu Z Shi F Mai Q Wang L Wang F et al . Lenvatinib plus TACE with or without pembrolizumab for the treatment of initially unresectable hepatocellular carcinoma harbouring PD-L1 expression: a retrospective study. J Cancer Res Clin Oncol. (2022) 148:2115–25. doi: 10.1007/s00432-021-03767-4
25
Yamamoto Y Matsui J Matsushima T Obaishi H Miyazaki K Nakamura K et al . Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. (2014) 6:18. doi: 10.1186/2045-824X-6-18
26
Nahm JH Rhee H Kim H Yoo JE San Lee J Jeon Y et al . Increased expression of stemness markers and altered tumor stroma in hepatocellular carcinoma under TACE-induced hypoxia: A biopsy and resection matched study. Oncotarget. (2017) 8:99359–71. doi: 10.18632/oncotarget.22078
27
Shim JH Park JW Kim JH An M Kong SY Nam BH et al . Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci. (2008) 99:2037–44. doi: 10.1111/j.1349-7006.2008.00909.x
28
Zhu HD Li HL Huang MS Yang WZ Yin GW Zhong BY et al . Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Signal Transduct Target Ther. (2023) 8:58. doi: 10.1038/s41392-022-01235-0
29
Wu XK Yang LF Chen YF Chen ZW Lu H Shen XY et al . Transcatheter arterial chemoembolisation combined with lenvatinib plus camrelizumab as conversion therapy for unresectable hepatocellular carcinoma: a single-arm, multicentre, prospective study. EClinicalMedicine. (2023) 67:102367. doi: 10.1016/j.eclinm.2023.102367
30
Huinen ZR Huijbers EJM van Beijnum JR Nowak-Sliwinska P Griffioen AW . Anti-angiogenic agents - overcoming tumour endothelial cell anergy and improving immunotherapy outcomes. Nat Rev Clin Oncol. (2021) 18:527–40. doi: 10.1038/s41571-021-00496-y
31
Cheu JW Wong CC . Mechanistic rationales guiding combination hepatocellular carcinoma therapies involving immune checkpoint inhibitors. Hepatology. (2021) 74:2264–76. doi: 10.1002/hep.31840
32
Fukumura D Kloepper J Amoozgar Z Duda DG Jain RK . Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. (2018) 15:325–40. doi: 10.1038/nrclinonc.2018.29
33
Sangro B Chan SL Meyer T Reig M El-Khoueiry A Galle PR . Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J Hepatol. (2020) 72:320–41. doi: 10.1016/j.jhep.2019.10.021
34
Duan X Li H Kuang D Chen P Zhang K et al . Transcatheter arterial chemoembolization plus apatinib with or without camrelizumab for unresectable hepatocellular carcinoma: a multicenter retrospective cohort study. Hepatol Int. (2023) 17:915–26. doi: 10.1007/s12072-023-10519-8
35
Katzke V Johnson T Sookthai D Hüsing A Kühn T Kaaks R . Circulating liver enzymes and risks of chronic diseases and mortality in the prospective EPIC-Heidelberg case-cohort study. BMJ Open. (2020) 10:e033532. doi: 10.1136/bmjopen-2019-033532
36
D'Avola D Granito A Torre-Aláez M Piscaglia F . The importance of liver functional reserve in the non-surgical treatment of hepatocellular carcinoma. J Hepatol. (2022) 76:1185–98. doi: 10.1016/j.jhep.2021.11.013
37
Khoja L Day D Wei-Wu Chen T Siu LL Hansen AR . Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. (2017) 28:2377–85. doi: 10.1093/annonc/mdx286
Summary
Keywords
transarterial chemoembolization, lenvatinib, programmed cell death protein-1 inhibitors, unresectable, hepatocellular carcinoma
Citation
Chen Y, Jia L, Li Y, Cui W, Wang J, Zhang C, Bian C and Luo T (2024) Efficacy and safety of transarterial chemoembolization plus lenvatinib combined with PD-1 inhibitors versus transarterial chemoembolization plus lenvatinib for unresectable hepatocellular carcinoma: a meta-analysis. Front. Immunol. 15:1466113. doi: 10.3389/fimmu.2024.1466113
Received
17 July 2024
Accepted
15 August 2024
Published
30 August 2024
Volume
15 - 2024
Edited by
Yan-Ru Lou, University of Helsinki, Finland
Reviewed by
Elena Niculet, Dunarea de Jos University, Romania
Kazuto Tajiri, University of Toyama University Hospital, Japan
Updates
Copyright
© 2024 Chen, Jia, Li, Cui, Wang, Zhang, Bian and Luo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Luo, Taoluo35@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.