- 1Department of Clinical Laboratory, Hunan Provincial People’s Hospital (The First Affiliated Hospital of Hunan Normal University), Changsha, China
- 2Gastroenterology department, Hunan Provincial People’s Hospital (The First Affiliated Hospital of Hunan Normal University), Changsha, China
- 3Department of Emergency, The First Affiliated Hospital of Hunan Normal University (Hunan Provincial People’s Hospital), Changsha, China
- 4Hepatobiliary Surgery, Hunan Provincial People’s Hospital (The First Affiliated Hospital of Hunan Normal University), Changsha, China
- 5Department of Emergency Medicine, The Affiliated University of South China, Hengyang Medical School, University of South China, Changsha, China
- 6Hunan Provincial Key laboratory of Emergency and Critical Care Metabonomic, Hunan Provincial People’s Hospital (The First-Affiliated Hospital of Hunan Normal University), Changsha, China
- 7Tumor Immunity Research Center of Hunan Provincial Geriatric Institute, Hunan Provincial People’s Hospital (The First Affiliated Hospital of Hunan Normal University), Changsha, China
Objective: Effective early diagnosis and timely intervention in acute pancreatitis (AP) are essential for improving patient outcomes. This study aims to evaluate the clinical utility of the neutrophil CD64 index (nCD64) in stratifying patients with SAP and assessing mortality risk.
Methods: A total of 302 AP patients were enrolled and divided into a training cohort (n = 226) and a validation cohort (n = 76). Venous blood samples were collected within 24 hours of admission, and the nCD64 index was measured via flow cytometry. Other clinical parameters, including C-reactive protein (CRP) and procalcitonin (PCT), were also recorded. Logistic regression and receiver operating characteristic (ROC) curve analyses were performed to assess the diagnostic value of the nCD64 index and its capacity to predict mortality risk.
Results: ROC curve analysis identified a cutoff value of 1.45 for the nCD64 index. Patients with nCD64 > 1.45 had significantly higher risks of complications, including systemic inflammatory response syndrome (SIRS), acute respiratory distress syndrome (ARDS), multiple organ failure (MOF), and death. Over 65% of patients with acute pancreatitis (AP) can be effectively risk-stratified at a low cost, and it has been demonstrated that AP patients with an nCD64 value ≤ 1.45 have an extremely low mortality rate (no mortality in present training and validation cohort). Kaplan-Meier survival analysis revealed a significant survival difference between high-risk (nCD64 > 1.45) and low-risk groups (p < 0.001).
Conclusion: The nCD64 index is an effective tool for early identification of SAP patients, allowing for the classification of over 65% of cases as low-risk for mortality.
1 Introduction
Acute pancreatitis (AP) is an inflammatory disorder of the pancreas, marked by complex etiology and acute clinical progression (1). It is typically characterized by severe abdominal pain, often accompanied by nausea and vomiting (2). In severe cases, complications such as intra-abdominal infections, pancreatic hemorrhage, and necrosis may occur. While most cases are mild, self-limiting, and resolve within one week, around 20% of patients progress to moderately severe or severe acute pancreatitis (MSAP/SAP), associated with complications such as bacterial infections, multi-organ failure, and pancreatic necrosis, with a mortality rate ranging from 20% to 40% (3, 4). Given the complexity of treatment, which often necessitates multidisciplinary management, early diagnosis and timely intervention are critical for optimizing patient outcomes. Early identification of high-risk patients facilitates the implementation of targeted therapeutic strategies and critical care support, thereby improving prognosis.
Effective risk stratification is crucial in identifying patients at higher risk of mortality early in the clinical course of AP (5). Timely and accurate assessment enables clinicians to allocate critical care resources appropriately, ensuring that patients with life-threatening complications receive immediate attention (6). By distinguishing between patients likely to experience a mild disease course and those at risk of rapid deterioration or death, healthcare providers can intervene earlier with more aggressive treatments, potentially reducing the mortality rate and preventing irreversible organ damage (7). Without efficient risk stratification, delayed or insufficient interventions may lead to worse outcomes, especially in cases of severe acute pancreatitis, where time-sensitive care is essential for survival (8, 9).
Despite the availability of various biomarkers for predicting the onset of SAP, limitations in their clinical application remain. Laboratory markers such as white blood cell count, C-reactive protein (CRP), and procalcitonin (PCT) offer some diagnostic value in SAP and are relatively easy to obtain (10–12). However, these markers lack sufficient sensitivity and specificity, limiting their ability to distinguish between mild and severe cases of pancreatitis. Additionally, scoring systems like the Acute Physiology and Chronic Health Evaluation II (APACHE II), Ranson score, and the Bedside Index for Severity in Acute Pancreatitis (BISAP) aim to improve the accuracy of SAP prediction (13). However, these systems require multiple clinical parameters, making them time-consuming and complex to calculate. In emergency settings, physicians need rapid and reliable results to guide treatment decisions, but the complexity of these scoring systems can delay timely interventions. Moreover, the applicability of these scoring systems across different regions and populations remains a concern. Therefore, despite the progress made, the current biomarkers and scoring systems still fall short in clinical practice, underscoring the need for more precise and rapid diagnostic tools for SAP.
In recent years, increasing attention has been given to immune monitoring in critically ill patients, particularly the role of neutrophil-related parameters. The neutrophil CD64 index (nCD64 index), a novel blood marker, has shown superior accuracy and speed in diagnosing sepsis and other critical conditions compared to traditional blood markers (14, 15). However, the clinical utility of the nCD64 index in predicting severe acute pancreatitis remains unclear (16–18). Further research is necessary to elucidate the potential of this marker in the context of AP.
This study aims to evaluate the clinical value of nCD64 in acute pancreatitis by measuring its levels upon hospital admission and assessing its potential as a reliable biomarker for predicting the severity of AP. Furthermore, the ability of nCD64 to contribute to effective risk stratification could significantly enhance the identification of high-mortality patients, allowing for the prioritization of critical interventions and better management of resources in life-threatening cases. The findings may provide valuable insights into early diagnostic strategies for SAP and equip clinicians with an effective tool for timely intervention.
2 Materials and methods
2.1 Study design
This study was conducted from May 2021 to December 2022 at Hunan Provincial People’s Hospital and Changsha Central Hospital, enrolling patients diagnosed with acute pancreatitis. Participants were prospectively recruited and simultaneously assigned to parallel training and validation cohorts at a 3:1 ratio, a methodology designed to minimize selection bias and ensure statistical validity.
The sample size calculation was based on preliminary experiments and literature, using a one-tailed significance level (α = 0.05) and statistical power (1-β = 0.80). With an initial sensitivity of 0.857 observed in the experimental subset (n = 40) and a target sensitivity of 0.7, the required sample size for positive cases was 45. Given that positive cases represented ~20% of the population, the total estimated sample size was 226 for the training cohort. To align with the 3:1 allocation ratio and strengthen methodological rigor, an additional 76 participants were enrolled for validation, resulting in a final cohort of 302 participants: 226 in training and 76 in validation. This parallel cohort design ensured parameters derived from training were independently validated without overlap or adjustment. The detailed flow of participant screening is provided in Figure 1.
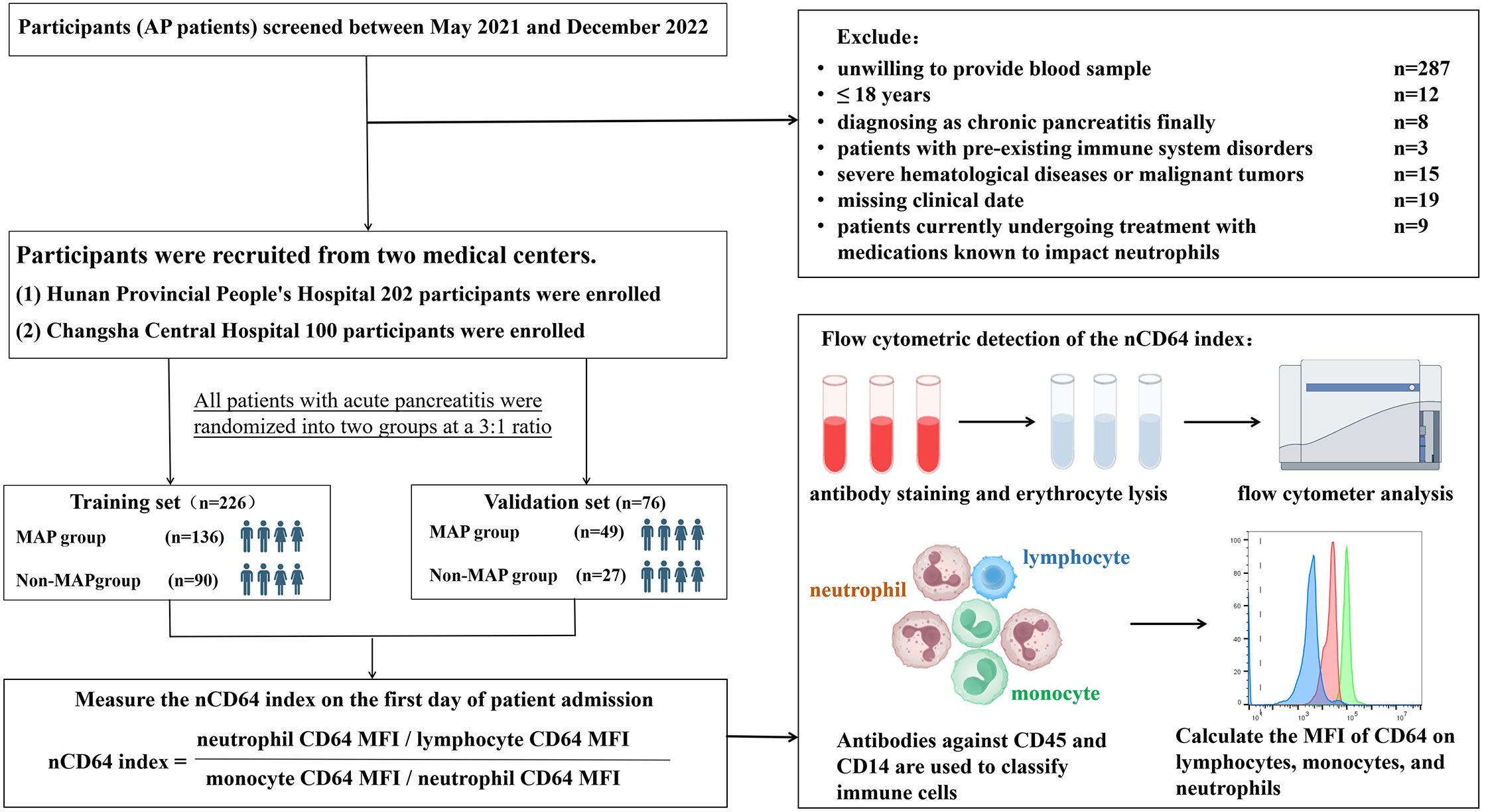
Figure 1. Study flowchart and experimental design. recruitment, exclusion, and randomization of acute pancreatitis (AP) patients into training and validation sets. The nCD64 index was measured using flow cytometry on the first day of admission, with immune cells classified by CD45 and CD14 antibodies. The nCD64 index was calculated as the ratio of neutrophil CD64 MFI to lymphocyte and monocyte CD64 MFI.
Within 24 hours of admission, venous blood samples were collected for nCD64 index quantification via flow cytometry, alongside clinical characteristics and standard laboratory biomarkers. The study adhered to the Declaration of Helsinki, with ethics approval from both institutions (Hunan Provincial People’s Hospital and Changsha Central Hospital, R201925). Written informed consent was obtained from all participants.
2.2 Study population
The diagnosis of AP was based on the diagnostic criteria, which required meeting at least two of the following three criteria: (1) persistent upper abdominal pain; (2) serum amylase and/or lipase concentration at least three times higher than the upper normal limit; (3) abdominal imaging findings consistent with acute pancreatitis (19–21).
Inclusion criteria: (1) meeting the diagnostic criteria for acute pancreatitis; (2) complete clinical data; (3) informed consent obtained; (4) age > 18 years.
Exclusion criteria: (1) patients with chronic or recurrent pancreatitis; (2) patients with a history of immune system diseases, including immunodeficiency diseases such as AIDS or systemic lupus erythematosus, rheumatoid arthritis, and systemic vasculitis; (3) patients with severe hematological disorders or malignancies; (4) patients currently undergoing treatment with drugs known to affect neutrophils, such as glucocorticoids or antithyroid drugs.
According to the revised 2012 Atlanta classification, AP patients were divided into three groups: MAP, characterized by no organ failure and no local or systemic complications; MSAP, characterized by transient (< 48 hours) organ failure and/or local complications or exacerbation of comorbidities; and SAP, characterized by persistent (> 48 hours) organ failure (4). The MSAP and SAP cases were categorized together as the MS-SAP group.
2.3 Reagents and instruments
Whole blood was collected using EDTA anticoagulant, and the nCD64 index was measured using flow cytometry (Myriad BriCyteE6, Mindray Medical, Shenzhen, China) within two hours of collection. Reagents for the nCD64 index assay included PerCP anti-human CD14, PerCP anti-human CD45, and PerCP anti-human CD64 (BioLegend, San Diego, CA, USA). The nCD64 detection procedure is summarized as follows: 50 μL of EDTA-anticoagulated blood sample was added to a test tube, along with 5 μL each of the CD14, CD45, and CD64 antibodies. The mixture was thoroughly combined and incubated in the dark for 15 min. Next, red blood cells were lysed, and the sample was processed for flow cytometry analysis. The mean fluorescence intensity (MFI) of CD64 expression on neutrophils, lymphocytes, and monocytes was determined.
nCD64 index = (neutrophil CD64 MFI/lymphocyte CD64 MFI)/(monocyte CD64 MFI/neutrophil CD64 MFI). The calculation of the nCD64 index accounted for intrinsic interindividual variability to ensure measurement stability and accuracy (since CD64 expression is highly stable on monocyte surfaces, it served as the positive control for the nCD64 index, whereas CD64 expression on lymphocytes is low, serving as the negative control). The gating strategy is illustrated in Figure 2.
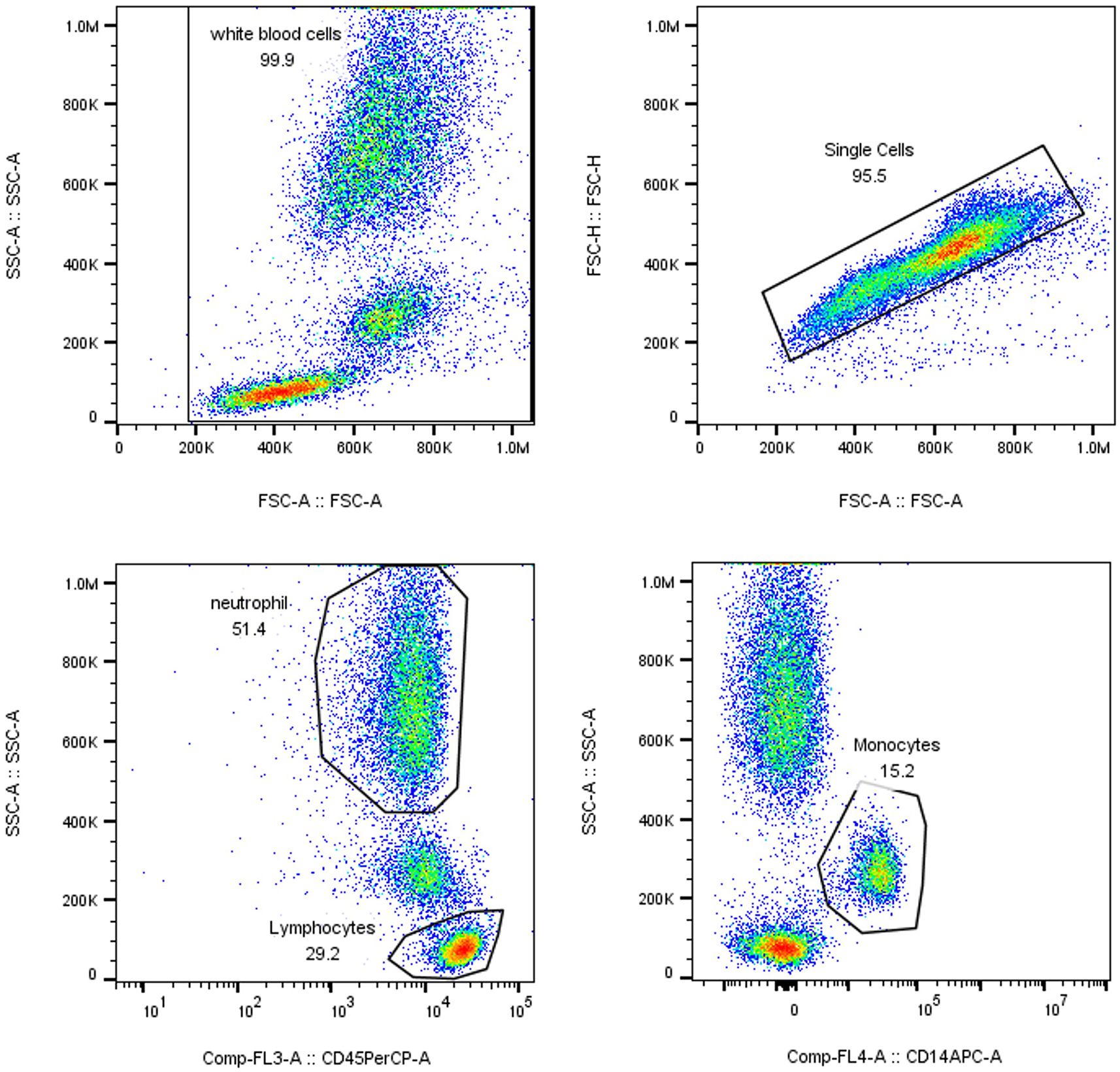
Figure 2. Gating Strategy. WBC count, immature granulocyte (IG) percentage, and neutrophil count (N) were analyzed using the XN Blood Analyzer Line and associated reagents.
2.4 Statistical analysis
Relevant clinical data, laboratory test results, and nCD64 index were analyzed using SPSS 23.0 and MedCalc software. The normality of continuous variables was assessed using SPSS. For normally distributed data, values were presented as mean ± standard deviation (x ± s). One-way analysis of variance (ANOVA) was used for comparisons among multiple groups, while the t-test was used for comparisons between two groups. For data with a skewed distribution that could be normalized, the transformed data were analyzed as normally distributed. If data could not be transformed, the median (interquartile range) (M [QL, QU]) was used, and comparisons between multiple groups and between groups were assessed using the Mann-Whitney U test. Comparisons of categorical data were conducted using the chi-square (χ²) test.
Additionally, we performed logistic regression analysis, Kaplan-Meier (K-M) curve analysis, and Spearman correlation analysis. Receiver operating characteristic (ROC) curve analysis was conducted using MedCalc software to assess the diagnostic efficacy of the nCD64 index and other indicators for severe acute pancreatitis. Subsequently, we compared the ROC curves obtained for multiple indicators in the same dataset to determine whether there were statistically significant differences in the predictive performance of each indicator. For the combined analysis of these indicators, we first conducted binary logistic regression analysis using SPSS. We then combined the two indicators, created a new composite index, and imported it into MedCalc for the aforementioned analysis.
3 Results
3.1 Baseline clinical characteristics of patients
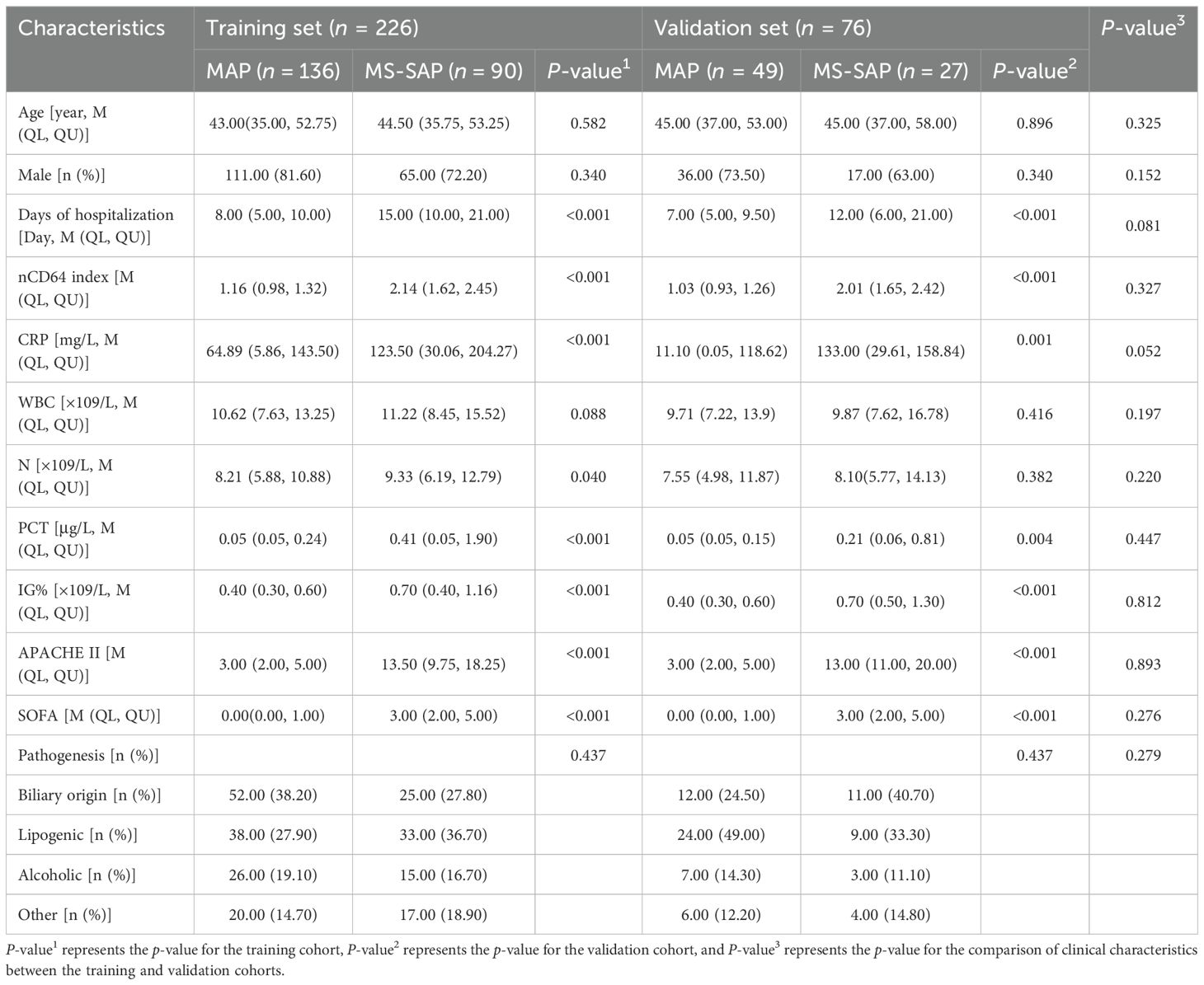
As illustrated in Table 1, the baseline clinical characteristics of patients in both the MAP and MS-SAP groups. No significant differences were observed between the two groups regarding age or gender in either the training or validation cohorts (P > 0.05). However, there were significant differences in the length of hospital stay, with MS-SAP patients requiring longer hospitalization compared to MAP patients in both the training (P < 0.001) and validation (P = 0.081) cohorts.
Inflammatory markers and severity scores also showed significant differences. The MS-SAP group had significantly higher nCD64 index, CRP, PCT, IG%, APACHE II, and SOFA scores compared to the MAP group (P < 0.001 in both cohorts). White blood cell count (WBC) and neutrophil percentage (N%) showed marginal significance in the training cohort (P = 0.088 and P = 0.040, respectively), but these differences were not statistically significant in the validation cohort (P > 0.05).
Additionally, there were no significant differences in clinical characteristics between the training and validation cohorts (P > 0.05 in both cohorts).
3.2 Logistic regression, correlation, and ROC analysis
Logistic regression analysis identified elevated nCD64 index, PCT, APACHE II, SOFA scores, and IG% as independent risk factors for the progression to severe disease (Figure 3A). Notably, a strong positive correlation was observed between nCD64 index and APACHE II scores (Figure 3B, Supplementary Table 1), reinforcing the role of nCD64 in reflecting disease severity.
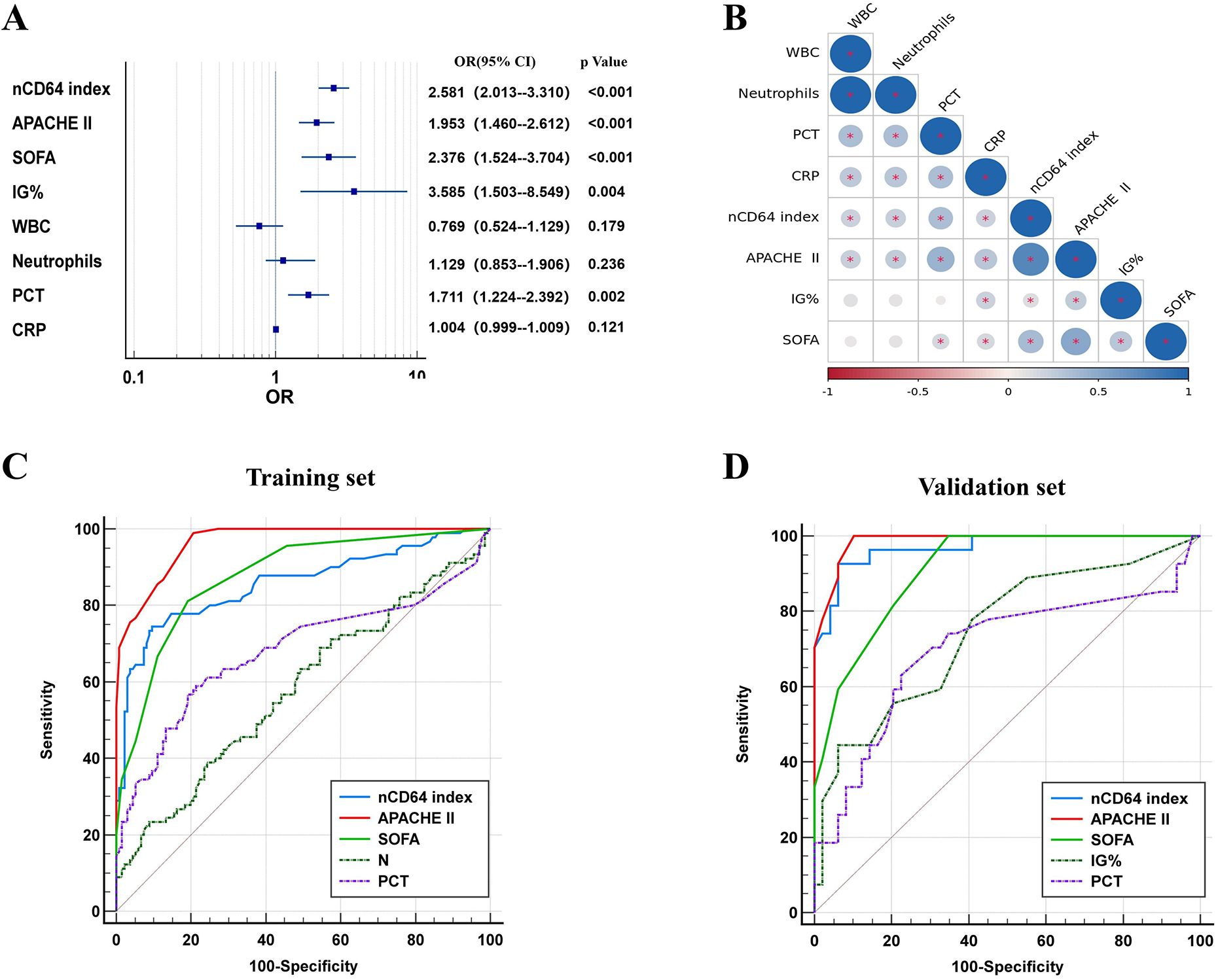
Figure 3. Logistic regression, correlation, and ROC analysis. (A) Forest map based on logstic; (B) Correlation coefficient chart; (C) ROC curve analysis of each index in the training cohort; (D) ROC curve analysis of each index in the validation cohort.
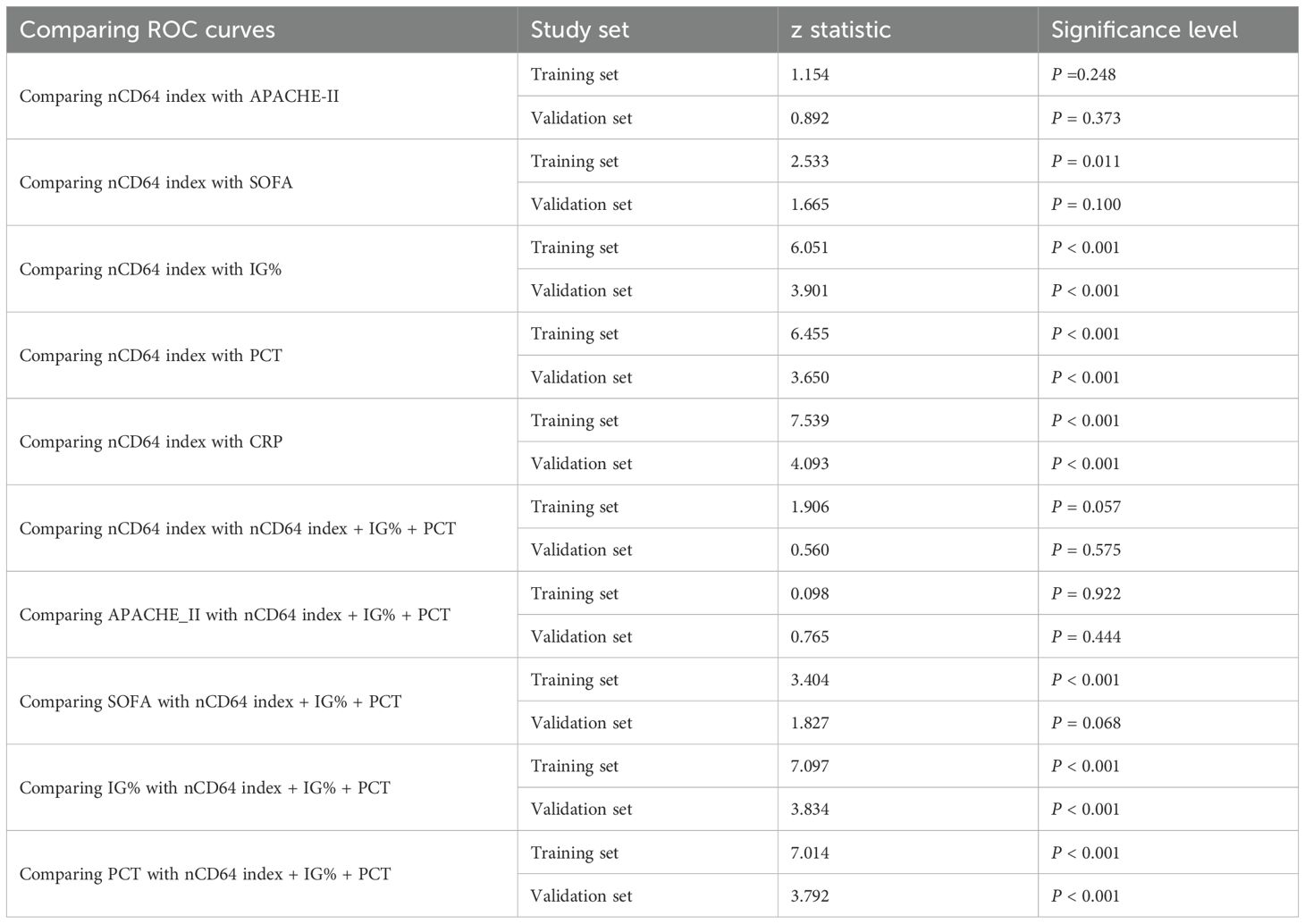
ROC curve analysis was conducted to evaluate the diagnostic performance of nCD64 index, PCT, APACHE II, SOFA scores, and IG%. The nCD64 index displayed the strongest diagnostic performance among the individual indicators. Although a combined indicator of nCD64 index, PCT, and IG% demonstrated excellent diagnostic accuracy, it did not significantly outperform the nCD64 index alone. The diagnostic efficacy of the nCD64 index was notably higher than that of IG% and PCT, and it was comparable to the APACHE II score. While the combined indicator showed superior diagnostic performance over IG% or PCT alone, it did not significantly differ from the nCD64 index when used independently (P > 0.05) (Figures 3C, D, Table 2). The cut-off value for the nCD64 index was determined to be 1.45, as presented in Supplementary Tables 2 and 3.
3.3 High-risk and low-risk group comparisons
First, we conducted relevant analyses and determined the cutoff value for the nCD64 index (1.45) in the training cohort. We further confirmed these findings in the validation cohort. Therefore, we classified patients into high-risk (nCD64 index > 1.45) and low-risk (nCD64 index ≤ 1.45) groups using a cutoff value of 1.45. In the training cohort, patients in the high-risk group exhibited significantly higher rates of complications, including acute respiratory distress syndrome (ARDS), systemic inflammatory response syndrome (SIRS), multiple organ failure (MOF), pancreatic necrosis, pancreatic infection, ICU admission, and mortality, compared to those in the low-risk group. Specifically, 9 patients (29.0%) in the high-risk group developed ARDS, while only 2 patients (4.4%) in the low-risk group experienced ARDS, demonstrating a significant difference (P = 0.004). Similarly, 19 patients (61.3%) in the high-risk group developed SIRS, compared to 10 patients (22.2%) in the low-risk group (P = 0.001). The incidence of MOF was higher in the high-risk group (5 patients, 16.1%) compared to the low-risk group (2 patients, 4.4%), although this difference did not reach statistical significance (P = 0.093) (Figure 4).
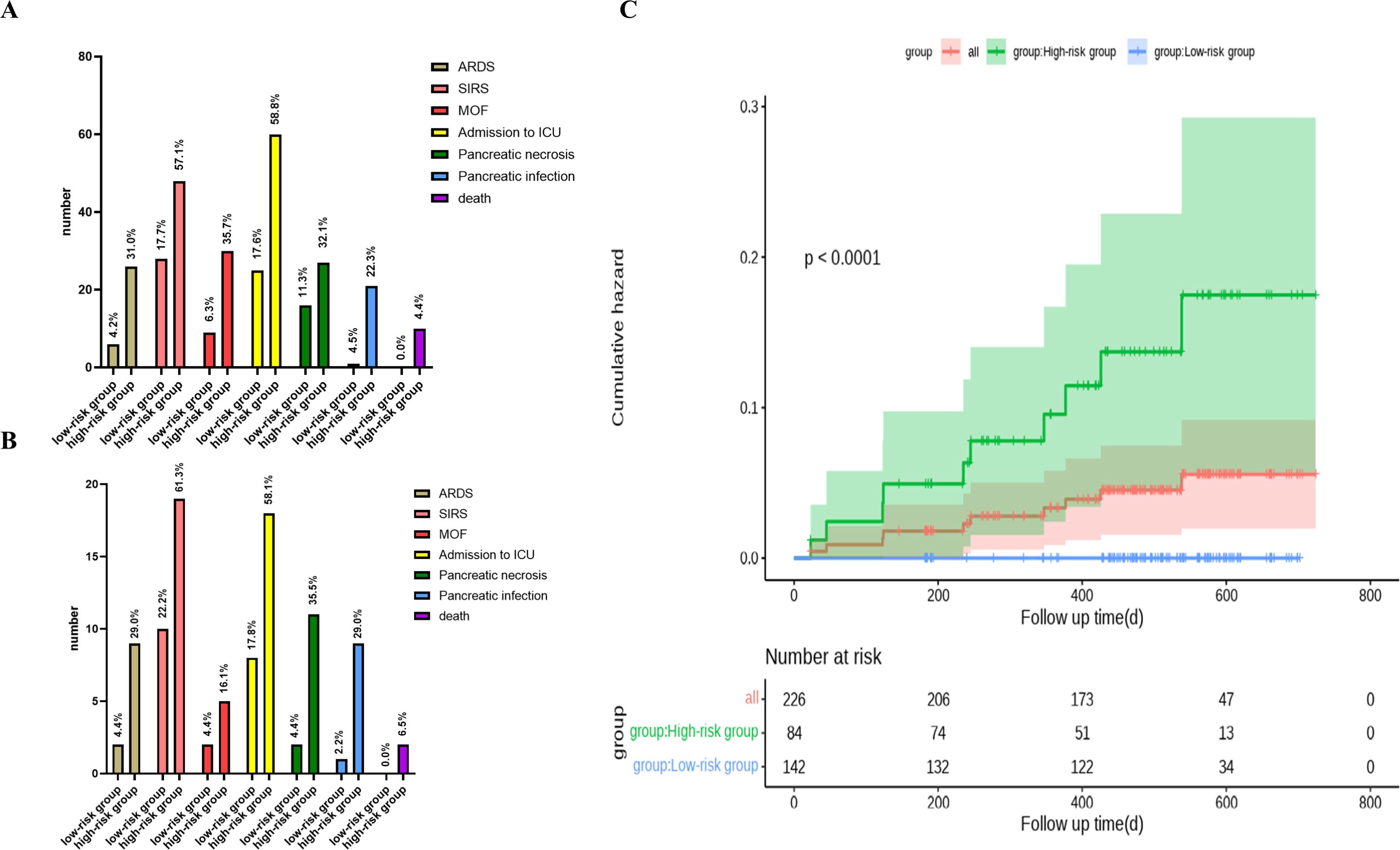
Figure 4. Clinical characteristics and cumulative hazard analysis of high-risk and low-risk patient groups. (A) Clinical features of all, high-risk, and low-risk groups. (B) Clinical features of high-risk vs. low-risk groups. (C) Kaplan-Meier plot depicting cumulative hazard for all, high-risk, and low-risk groups, with corresponding number of patients at risk over time shown below each group.
ICU admission rates were also significantly higher in the high-risk group (58.1%) compared to the low-risk group (17.8%) (P < 0.001). Similarly, the rates of pancreatic necrosis and infection were much higher in the high-risk group, with pancreatic necrosis occurring in 35.5% of high-risk patients compared to only 4.4% in the low-risk group (P = 0.001), and pancreatic infection occurring in 29.0% of high-risk patients compared to 2.2% in the low-risk group (P = 0.001) (Figure 4).
Most importantly, over 65% of patients with an nCD64 index ≤ 1.45 were correctly classified as low-risk for mortality, highlighting the effectiveness of this stratification method in identifying patients with a lower probability of death. This finding was further confirmed in the validation cohort (Figure 4).
Kaplan-Meier survival curve analysis revealed a significant difference in survival outcomes between the high-risk and low-risk groups (P < 0.001). The survival rate was significantly lower in the high-risk group, further underscoring the utility of the nCD64 index as a prognostic marker for severe outcomes in acute pancreatitis (P < 0.001). Notably, the mortality rate in the high-risk group was 6.5%, while no deaths were observed in the low-risk group (P = 0.056) (Figure 4).
These findings further emphasize the prognostic value of the nCD64 index in stratifying patients by risk and guiding clinical management strategies.
4 Discussion
In this study, we observed notable differences in clinical characteristics, inflammatory markers, and severity scores between patients with MAP and those with MS-SAP, with the neutrophil CD64 index (nCD64) standing out as a particularly rapid, convenient, and reliable marker for assessing disease severity and prognosis. Through a thorough analysis of 302 acute pancreatitis (AP) patients, the nCD64 index was demonstrated to be a simple and effective biomarker that not only accurately reflects disease severity but also enables the early identification of high-risk patients, specifically those at increased risk of mortality.
4.1 Comparison with traditional scoring systems
Our study showed no significant differences in baseline characteristics such as age, gender, or etiology between the MAP and MS-SAP groups, suggesting that demographic factors and underlying causes of pancreatitis may not significantly influence disease severity (22, 23). However, the significantly longer hospital stays in the MS-SAP group highlighted the increased burden of care required for these patients, consistent with their more severe clinical manifestations. Inflammatory markers, especially the nCD64 index, CRP, and PCT, were markedly elevated in the MS-SAP group, indicating a higher inflammatory response. Among these, the nCD64 index, which reflects neutrophil activation, aligning with previous studies that have highlighted its sensitivity in detecting systemic inflammation and infection (16).
Traditional scoring systems such as APACHE II and SOFA are time-consuming and require extensive clinical data (24–26). In contrast, the nCD64 index showed a strong positive correlation with the APACHE II score, a widely used tool for assessing disease severity, suggesting that nCD64 could offer a simpler and more accessible alternative for rapid risk stratification (27–30). Unlike these complex systems, the nCD64 index can be quickly measured through routine blood tests, delivering fast and actionable clinical insights.
4.2 Cutoff Value of nCD64 and Identification of High-Risk Patients
The study identified an optimal nCD64 cutoff value of 1.45, above which patients were significantly more likely to experience life-threatening complications, such as systemic inflammatory response syndrome (SIRS), acute respiratory distress syndrome (ARDS), and multiple organ failure (MOF). These patients also faced a notably higher risk of mortality. This finding aligns with previous studies, confirming that nCD64 is a sensitive marker for systemic inflammation and infection. By using this threshold, clinicians can effectively distinguish high-risk patients who require intensive care and timely intervention to potentially improve outcomes.
Further analysis revealed that while a composite marker (nCD64 index + PCT + IG%) improved diagnostic efficacy compared to individual markers, it did not significantly outperform the nCD64 index alone. This underscores the practicality of using the nCD64 index as a standalone marker. Moreover, we identified a cut-off value of 1.45 for the nCD64 index, which effectively differentiated between high- and low-risk groups. Kaplan-Meier survival analysis showed that patients in the high-risk group (nCD64 > 1.45) had significantly poorer survival outcomes, emphasizing the prognostic value of the nCD64 index for predicting mortality.
4.3 Application of nCD64 in Risk Stratification
Kaplan-Meier survival analysis further underscored the clinical significance of the nCD64 index. Patients in the high-risk group (nCD64 > 1.45) had significantly poorer survival outcomes compared to those in the low-risk group (p<0.001). This demonstrates that the nCD64 index can not only aid in early diagnosis but also serve as a valuable prognostic tool. Early identification of high-risk patients allows for prompt intervention, including close monitoring and early admission to the ICU, thereby improving patient survival (31, 32).
Although this study demonstrated the validity of nCD64 in a Chinese cohort, further research is needed to confirm its generalizability across different populations and healthcare systems. Future studies should also explore combining nCD64 with other biomarkers to refine diagnostic and prognostic models.
The limitations can be written in the following way in the article:This study has certain limitations in data sources and patient population. The data were derived from only two medical centers in Hunan Province (Hunan Provincial People’s Hospital and Changsha Central Hospital). Although these centers represent diversity in patient demographics and clinical expertise, the geographic and healthcare environment coverage remains limited compared to a true multicenter study. In future research, we will actively collaborate with institutions across diverse regions and healthcare settings to validate the generalizability and applicability of our findings in broader patient populations.
In conclusion, the nCD64 index is a powerful and efficient tool for identifying high-risk patients in acute pancreatitis. Its simplicity and strong correlation with established severity scores make it a valuable addition to traditional scoring systems. Incorporating the nCD64 index into standard clinical protocols could enhance early identification of high-risk patients, optimize resource allocation, and ultimately improve patient outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by Ethics Committee of Hunan Provincial People’s Hospital and the Ethics Committee of Changsha Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
MS: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Investigation, Methodology, Supervision. LW: Writing – original draft, Data curation, Methodology, Supervision. XH: Data curation, Methodology, Writing – review & editing. QO: Data curation, Writing – review & editing. YP: Formal analysis, Methodology, Writing – review & editing. SL: Methodology, Writing – review & editing. XX: Formal analysis, Methodology, Writing – review & editing. QY: Methodology, Validation, Writing – review & editing. YL: Formal analysis, Methodology, Validation, Writing – review & editing. GL: Project administration, Validation, Writing – review & editing. DN: Investigation, Methodology, Writing – review & editing. JW: Supervision, Writing – review & editing, Writing – original draft. CT: Funding acquisition, Writing – original draft, Writing – review & editing, Supervision, Methodology, Project administration, Validation. YH: Funding acquisition, Writing – review & editing, Methodology, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by Grants from China National Natural Science Fund (81600502), the Natural Science Foundation of Hunan Province (2020JJ8086, 2023JJ10024), the Project of Hunan Provincial Health Commission (202103031672), the Health Commission high-level talent project of Hunan Provincial (20240221-1010), the Project of Hunan Provincial Education Department (22A0075), the Key Cultivation Project of Renshu Fund of Hunan Provincial People’s Hospital (RS2022A15, RS2022A08), the Project of Hunan Province Science and Technology Department (2020SK3042, 2020SK50926) and the Foundation of Hunan Provincial Department of Education (Grant No. 24B0073).
Acknowledgments
This study was funded by Grants from China National Natural Science Fund (81600502), the Natural Science Foundation of Hunan Province (2020JJ8086, 2023JJ10024), the Project of Hunan Provincial Health Commission (202103031672), the Health Commission high-level talent project of Hunan Provincial (20240221-1010), the Project of Hunan Provincial Education Department (22A0075), the Key Cultivation Project of Renshu Fund of Hunan Provincial People’s Hospital (RS2022A15) and the Project of Hunan Province Science and Technology Department (2020SK3042, 2020SK50926).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1526122/full#supplementary-material
Abbreviations
Acute pancreatitis (AP), mild acute pancreatitis (MAP), moderate severe acute pancreatitis (MSAP), and severe acute pancreatitis (SAP), neutrophil CD64 index (nCD64 index), white blood cells (WBC), neutrophils (N), acute physiological and chronic health score (APACHE II), Immature granulocyte(IG), Sequential Organ Failure Assessment(SOFA), acute respiratory distress syndrome (ARDS), systemic inflammatory response syndrome (SIRS), multiple organ failure (MOF), C reactive protein (CRP), procalcitonin (PCT), area under the curve (AUC). Supplementary Notes to the Title Page.
References
1. Trikudanathan G, Yazici C, Evans Phillips A, Forsmark CE. Diagnosis and management of acute pancreatitis. Gastroenterology. (2024) 167:673–88. doi: 10.1053/j.gastro.2024.02.052
2. Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. (2019) 16:175–84. doi: 10.1038/s41575-018-0087-5
3. Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, et al. Acute pancreatitis. Lancet. (2020) 396:726–34. doi: 10.1016/S0140-6736(20)31310-6
4. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. (2013) 62:102–11.
5. Mederos MA, Reber HA, Girgis MD. Acute pancreatitis: A review. JAMA. (2021) 325:382–90. doi: 10.1001/jama.2020.20317
6. Vivian E, Cler L, Conwell D, Coté GA, Dickerman R, Freeman M, et al. Acute pancreatitis task force on quality: development of quality indicators for acute pancreatitis management. Am J Gastroenterol. (2019) 114:1322–42. doi: 10.14309/ajg.0000000000000264
7. Singhi AD, Koay EJ, Chari ST, Maitra A. Early detection of pancreatic cancer: opportunities and challenges. Gastroenterology. (2019) 156:2024–40. doi: 10.1053/j.gastro.2019.01.259
8. Żorniak M, Beyer G, Mayerle J. Risk stratification and early conservative treatment of acute pancreatitis. Visc Med. (2019) 35:82–9. doi: 10.1159/000497290
9. Hritz I, Hegyi P. Early Achievable Severity (EASY) index for simple and accurate expedite risk stratification in acute pancreatitis. J Gastrointestin Liver Dis. (2015) 24:177–82. doi: 10.15403/jgld.2014.1121.242.easy
10. Lee PJ, Papachristou GI, Speake C, Lacy-Hulbert A. Immune markers of severe acute pancreatitis. Curr Opin Gastroenterol. (2024) 40:389–95. doi: 10.1097/MOG.0000000000001053
11. Poulsen VV, Hadi A, Werge MP, Karstensen JG, Novovic S. Circulating biomarkers involved in the development of and progression to chronic pancreatitis-A literature review. Biomolecules. (2024) 14:(2). doi: 10.3390/biom14020239
12. Beyer G, Habtezion A, Werner J, Lerch MM, Mayerle J. Chronic pancreatitis. Lancet. (2020) 396:499–512. doi: 10.1016/S0140-6736(20)31318-0
13. Hu JX, Zhao CF, Wang SL, Tu XY, Huang WB, Chen JN, et al. Acute pancreatitis: A review of diagnosis, severity prediction and prognosis assessment from imaging technology, scoring system and artificial intelligence. World J Gastroenterol. (2023) 29:5268–91. doi: 10.3748/wjg.v29.i37.5268
14. Feng M, Zhang SL, Liang ZJ, Wang YL, Zhao XC, Gao C, et al. Peripheral neutrophil CD64 index combined with complement, CRP, WBC count and B cells improves the ability of diagnosing bacterial infection in SLE. Lupus. (2019) 28:304–16. doi: 10.1177/0961203319827646
15. Zhang H, Ling XL, Wu YY, Lü MH, Guo H, Zhang PB, et al. CD64 expression is increased in patients with severe acute pancreatitis: clinical significance. Gut Liver. (2014) 8:445–51. doi: 10.5009/gnl.2014.8.4.445
16. Huang X, Wu L, Ouyang Q, Huang Y, Hong L, Liu S, et al. Neutrophil CD64 index as a new early predictive biomarker for infected pancreatic necrosis in acute pancreatitis. J Transl Med. (2024) 22:218. doi: 10.1186/s12967-024-04901-9
17. Cao LL, Wang WW, Zhao L, Li JR, Kong XM, Zhu YN, et al. Neutrophil CD64 index for diagnosis of infectious disease in the pediatric ICU: a single-center prospective study. BMC Pediatr. (2022) 22:718. doi: 10.1186/s12887-022-03738-9
18. Gao Y, Lin L, Zhao J, Peng X, Li L. Neutrophil CD64 index as a superior indicator for diagnosing, monitoring bacterial infection, and evaluating antibiotic therapy: a case control study. BMC Infect Dis. (2022) 22:892. doi: 10.1186/s12879-022-07725-4
19. Gómez Pérez A, Aparicio Serrano A, Serrano Ruiz FJ. Etiological diagnosis of recurrent acute pancreatitis. Rev Esp Enferm Dig. (2024) 116:399–403. doi: 10.17235/reed.2024.10404/2024
20. Kim K, Kim HH, Joo JB, Kim OK, Park SW, Suh GH, et al. Evaluation of the clinical usefulness of pancreatic alpha amylase as a novel biomarker in dogs with acute pancreatitis: a pilot study. Vet Q. (2024) 44:1–7. doi: 10.1080/01652176.2024.2326007
21. Huang N, Chen J, Wei Y, Liu Y, Yuan K, He M, et al. Multi-marker approach using C-reactive protein, procalcitonin, neutrophil CD64 index for the prognosis of sepsis in intensive care unit: a retrospective cohort study. BMC Infect Dis. (2022) 22:662. doi: 10.1186/s12879-022-07650-6
22. Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet. (2015) 386:85–96. doi: 10.1016/S0140-6736(14)60649-8
23. Olson E, Perelman A, Birk JW. Acute management of pancreatitis: the key to best outcomes. Postgrad Med J. (2019) 95:328–33. doi: 10.1136/postgradmedj-2018-136034
24. Teng TZJ, Tan JKT, Baey S, Gunasekaran SK, Junnarkar SP, Low JK, et al. Sequential organ failure assessment score is superior to other prognostic indices in acute pancreatitis. World J Crit Care Med. (2021) 10:355–68. doi: 10.5492/wjccm.v10.i6.355
25. Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet. (1974) 139:69–81.
26. Gao W, Yang HX, Ma CE. Correction: the value of BISAP score for predicting mortality and severity in acute pancreatitis: A systematic review and meta-analysis. PloS One. (2015) 10:e0142025.
27. Silva-Vaz P, Abrantes AM, Castelo-Branco M, Gouveia A, Botelho MF, Tralhão JG. Multifactorial scores and biomarkers of prognosis of acute pancreatitis: applications to research and practice. Int J Mol Sci. (2020) 21:(1). doi: 10.3390/ijms21010338
28. Liu Q, Gao Y, Yang T, Zhou Z, Lin K, Zhang W, et al. nCD64 index as a novel inflammatory indicator for the early prediction of prognosis in infectious and non-infectious inflammatory diseases: An observational study of febrile patients. Front Immunol. (2022) 13:905060.
29. Song Y, Chen Y, Dong X, Jiang X. Diagnostic value of neutrophil CD64 combined with CRP for neonatal sepsis: A meta-analysis. Am J Emerg Med. (2019) 37:1571–6.
30. de Jong E, de Lange DW, Beishuizen A, van de Ven PM, Girbes AR, Huisman A. Neutrophil CD64 expression as a longitudinal biomarker for severe disease and acute infection in critically ill patients. Int J Lab Hematol. (2016) 38:576–84.
31. Leppäniemi A, Tolonen M, Tarasconi A, Segovia-Lohse H, Gamberini E, Kirkpatrick AW, et al. 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg. (2019) 14:27. doi: 10.1186/s13017-019-0247-0
Keywords: acute pancreatitis, severe acute pancreatitis, neutrophil CD64 index, risk stratification, death prediction
Citation: Shao M, Wu L, Huang X, Ouyang Q, Peng Y, Liu S, Xu X, Yi Q, Liu Y, Li G, Ning D, Wang J, Tan C and Huang Y (2025) Neutrophil CD64 index: a novel biomarker for risk stratification in acute pancreatitis. Front. Immunol. 16:1526122. doi: 10.3389/fimmu.2025.1526122
Received: 11 November 2024; Accepted: 31 March 2025;
Published: 16 April 2025.
Edited by:
Wandong Hong, First Affiliated Hospital of Wenzhou Medical University, ChinaReviewed by:
Vladimir Leksa, Institute of Molecular Biology (SAS), SlovakiaPeng Li, Peking University, China
Copyright © 2025 Shao, Wu, Huang, Ouyang, Peng, Liu, Xu, Yi, Liu, Li, Ning, Wang, Tan and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Wang, d2FuZ2ppYUBodW5udS5lZHUuY24=; Chaochao Tan, dGNoY2h3b2xmQDE2My5jb20=; Ying Huang, Y2FybWVuX2h1YW5nQDE2My5jb20=
†Present address: Ling Wu, Department of Clinical Laboratory, Changsha Hospital for Maternal and Child Health Care, Changsha, China
‡These authors have contributed equally to this work
 Min Shao1‡
Min Shao1‡ Ya Peng
Ya Peng Ding Ning
Ding Ning Chaochao Tan
Chaochao Tan Ying Huang
Ying Huang