- Department of Rheumatology and Immunology, Laboratory of Rheumatology and Immunology, Institutes for Systems Genetics, West China Hospital, Sichuan University, Chengdu, China
Introduction: Anti-synthetase syndrome (ASS) is a subtype of idiopathic inflammatory myopathy (IIM) characterized by characteristic rash, myositis, and interstitial lung disease (ILD). The etiology of ASS is unknown, and patients have a poor quality of life and are prone to pulmonary infection. Recent studies have elucidated the potential role of abnormal glycosylation of immunoglobulin G (IgG) in the pathogenesis of autoimmune diseases. However, the pattern of patient-specific IgG N-glycosylation in ASS has not been fully elucidated.
Methods: the GlycoQuant method was used to quantify the intact N-glycopeptides of IgG from 30 ASS patients and 30 healthy controls (HCs).
Results and Discussion: Thirteen differentially expressed intact N-glycopeptides were identified (p<0.05). Notably, we observed increased fucosylation (p<0.0001) and decreased N-acetylneuraminic acid (p<0.05) in ASS patients. In addition, specific glycosylation patterns correlated with lung function parameters. Our study revealed the IgG glycosylation profile in ASS patients and provided a valuable reference for further investigation of its potential diagnostic and prognostic applications.
Introduction
Anti-synthetase syndrome (ASS) represents a distinct clinical type of idiopathic inflammatory myopathy (IIM), characterized by myopathic manifestations (muscle weakness and myalgia) and systemic involvement, including characteristic rash, arthritis, interstitial lung disease (ILD), and cardiac complications. A defining feature of ASS is the presence of anti-transfer RNA synthetases (ARSs) (1). The enzyme family includes anti-Jo-1, anti-PL-7, anti-PL-12, anti-EJ, anti-OJ, anti-KS, anti-Zo, anti-Ha, anti-JS, anti-SC, and anti-YRS, but data on the screening of anti-ARS in non- systemic autoimmune rheumatic disease (SARD) patients are often limited (2). The detection of certain anti-ARS agents, such as antibodies against PL-12 and KS in patients with idiopathic ILD has been reported (3, 4). One study reported that 10% of patients with idiopathic ILD had anti-ARS (5). At present, the pathogenesis of ASS is not clear, the quality of life of ASS patients has decreased, and the prognosis of patients with ILD has worsened. Therefore, in addition to the anti-ARS family, other in-depth studies are urgently needed to enhance the understanding of ASS and find new therapeutic targets (6).
Immunoglobulins (Igs) are a class of glycoproteins that play critical roles in immune function (7, 8). Their Fc region, which is responsible for their effector functions, is significantly influenced by the attached N-glycans. Among the five isotypes (IgM, IgD, IgG, IgA, and IgE) of Igs, IgG is the most prevalent serum autoantibody found in autoimmune disorders (9). Research has shown that variations in the glycosylation patterns of IgG are linked to various diseases (10, 11). A decrease in galactosylation is correlated with heightened inflammatory responses, and this connection is evident in the progression or flare-ups of autoimmune diseases (12, 13), such as systemic lupus erythematosus (SLE) (14), rheumatoid arthritis (RA) (15), inflammatory bowel disease (IBD) (16, 17), Sjögren’s syndrome (SS) (18), anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) (19), myasthenia gravis and Guillain–Barré syndrome (20). Similar patterns have been observed in chronic infectious diseases such as tuberculosis (12), HIV infection (21), and hepatitis B and C virus infection (22). Therefore, analyzing IgG glycosylation offers a promising avenue for predicting the severity of these diseases (23, 24). Earlier investigations revealed the distinct FC-glycan profiles of IgGs in ASS patients with anti-Jo1 antibodies compared with those in healthy individuals (25). However, a detailed quantitative exploration of the site-specific N-glycosylation of IgGs in the peripheral blood of ASS patients remains unexplored. This study aimed to explore a novel aspect of ASS by examining global IgG glycosylation patterns and provide a new perspective on immune dysregulation in ASS, which current antibody-based studies may not fully capture.
The specificity and complexity of glycosylation patterns make them promising biomarkers, but accurate measurement of intact N-glycopeptides in many clinical samples is highly challenging. A quantitative pipeline, GlycoQuant, has been confirmed to effectively identify intact N-glycopeptides, which combines mass spectrometry fragmentation technology (EThcD-sceHCD) with intact N-glycopeptide batch quantification software (PANDA v.1.2.5). In this study, we attempted to identify the N-glycosylation pattern of plasma IgG in ASS patients via this method and lay a framework for future mechanistic investigations.
Materials and methods
Biospecimen collection
Our study identified and recruited thirty patients diagnosed with ASS within the years 2021 and 2022 from the Department of Rheumatology, West China Hospital, Sichuan University. Patients with malignancies, serious infections, or any other autoimmune disease were excluded from this study. The diagnosis of ASS for each participant was rigorously determined following the diagnostic guidelines established by Solomon et al. in 2011 (26), and a diagnosis of ASS was confirmed if a patient tested positive for anti-ARS antibodies and met either two major criteria or one major criterion alongside two minor criteria. The major criteria include ILD (with exclusions for other causes) and polymyositis/dermatomyositis. The minor criteria include arthritis, Raynaud’s phenomenon, and mechanic’s hands. The diagnosis of myositis must adhere to the criteria recommended by Bohan and Peter in 1975, which are: symmetrical proximal muscle weakness, elevated serum muscle enzymes, electromyography indicating muscle origin damage, and a muscle biopsy supporting the diagnosis of inflammatory myopathy. Confirmation of the diagnosis is achievable by meeting any three of these four criteria. This research was granted approval by the Ethics Committee of West China Hospital, Sichuan University, and all participants provided informed consent prior to specimen collection. We selected 30 healthy controls (HCs) from the family members of the hospitalized patients whose age and sex did not differ from those of the ASS patients. A 5-milliliter sample of peripheral blood was obtained and stored in tubes containing ethylenediaminetetraacetic acid disodium salt (EDTA). Following collection, these samples were centrifuged at 1,200 g for 10 minutes at 4 °C. This process was aimed at isolating the plasma, which was then preserved at -80 °C, until further analysis.
Clinical and laboratory data collection
The necessary data were retrospectively obtained from patient medical records. These data included demographic characteristics, medical history, complications, comorbidities, medication utilization, blood routine parameters, liver and kidney function assessments, autoantibody profiles, lung function measurements, and inflammatory markers.
Isolation, purification and digestion
The process of isolating, purifying, and digesting IgGs is a detailed procedure that involves several precise steps (27). Briefly, 40 μL of immobilized protein A/G agarose and 200 μL of binding buffer were incubated with 20 μL of plasma on a rotator for 2 h at 4°C. Next, any proteins that were not bound were washed away with 500 μL of binding buffer. Subsequently, 50 μL of elution buffer containing 0.1 M formic acid was added to release the IgGs. To neutralize the mixture, 50 μL of neutralization buffer was added until the mixture reached a physiological pH. The protein concentration was subsequently quantified via a bicinchoninic acid (BCA) protein assay (Thermo Fisher Scientific, USA). Purified IgGs (20 μg) were denatured by heating at 95°C for 10 min, followed by reduction with 20 mM dithiothreitol (DTT) at 56°C for 30 min and alkylation with 50 mM iodoacetamide (IAA) at 25°C in the dark for 30 min. The mixture was transferred to a 30 kDa ultrafiltration tube (Millipore, Bedford, MA, USA) and replaced with 50 mM ammonium bicarbonate (NH4HCO3) buffer (pH 8.5). Finally, 0.5 μg of sequence-grade trypsin (Promega, Madison, WI, USA) was added, and the mixture was incubated for 2 h at 37°C. The obtained IgG peptides were quantified via peptide colorimetry (Thermo Fisher Scientific, USA).
LC-MS/MS analysis
As previously described (27), we utilized an advanced EASY-nLC 1200 HPLC system connected to an Orbitrap Fusion Lumos mass spectrometer (Thermo Fisher Scientific, USA) to conduct peptide analysis. Specifically, the peptides were dissolved in buffer A (0.1% FA in water) at a flow rate of 350 nL/min and separated on a 20-centimeter column (ReproSil-Pur C18-AQ, 1.9 μm, 75 μm inner diameter; Dr Maisch) with a 30-min gradient. The separated samples were analyzed via EThcD-sceHCD-MS/MS. The parameter settings were outlined following previously established methods (28).
Data analysis and bioinformatics
Intact N-glycopeptides were identified via Byonic software (version 3.10, Protein Metrics, Inc.) targeting the human IgG database with a mass tolerance of ±6 parts per million (ppm) for precursor ions and ±20 ppm for fragment ions. Up to two cleavage sites were allowed for trypsin digestion. Fixed modifications included amino methylation (C), whereas variable modifications consisted of oxidation (M) and acetylation (N-term). Additionally, 182 human N-glycans were designated N-glycan modifications. The proteome was filtered to a false discovery rate of 1%, and confident intact N-glycopeptides required a score of at least 200 and at least six amino acids. All glycopeptide profile matches (GPSMs) were performed manually. The quantification of intact N-glycopeptides was conducted via PANDA software (v 1.2.5) in label-free quantification mode with default values used for all other parameters. The selection of highly reliable and reproducible N-glycans is based on three main criteria: 1) analytical reproducibility across multiple sample preparations and instrument runs, where glycans with coefficient of variation (CV) values less than 15% in repeated measurements are prioritized; 2) statistical significance in their abundance differences between ASS patients and controls, with a p-value threshold of less than 0.05 after adjusting for multiple comparisons via the false discovery rate (FDR) method; and 3) biological relevance, where we refer to literature on the role of specific N-glycans in immune-related processes and autoimmune diseases.
R programming software was utilized for data analysis. The differentially expressed intact N-glycopeptides were identified via a moderated t test in the limma package following median normalization of the data. Additionally, R programming software and GraphPad Prism 9.5 software were used for data visualization. The supplementary data were analyzed via SigmaPlot 12.5, GraphPad Prism 9.5, and SPSS Statistics 29.0.1.09(171). The results are presented as the mean ± standard deviation (M ± SD) or median (Q25, Q75), with count data expressed as n (%). Intergroup comparisons were conducted via Student’s t test. Correlation analyses employ Pearson or Spearman coefficients with the choice of test determined by data distribution, followed by multivariate analysis via the appropriate regression model (linear or binary logistic). A p-value of less than 0.05 was considered statistically significant. Statistical differences between the ASS and HC groups are indicated as follows: *p<0.05, **p<0.005, ***p<0.001, **** p<0.0001.
Results and discussion
Experimental design
This study profiles the IgG glycosylation patterns in patients with anti-synthetase syndrome (ASS) and explores their clinical and laboratory correlations through a systematic workflow (Figure 1). To do this, plasma IgG molecules were isolated, purified, and digested from 30 ASS patients and 30 healthy controls (HCs). All the peptides were subsequently analyzed via the mass spectrometry technique EThcD-sceHCD-MS/MS. Finally, the intact N-glycopeptides were identified with Byonic software and quantified via PANDA software. This study shed light on the patterns of IgG glycosylation in ASS and its potential implications for understanding this disease.
Figure 1. Schematic illustration of the workflow for the analysis of IgG intact N-glycopeptides via the GlycoQuant method.
Baseline demographics and clinical characteristics
The demographic and clinical characteristics of the study participants are presented in detail in Supplementary Table S1. The ASS group consisted of 30 patients, and the HC group also included 30 participants. The average age of the HC group was 50 ± 4 years, while that of the ASS group was 50 ± 8 years. The median disease duration among ASS patients was 12 months, and their average body mass index (BMI) was 23.238 ± 3.657 kg/m (2). Among the participants, 4 had hypertension, 3 had diabetes, and 7 had hyperlipidemia. The mean myositis disease activity assessment visual analogue scale (MYOACT) score was 0.197 ± 0.094, and the mean myositis intention to treat activity index (MITAX) score was 0.181 ± 0.149. The clinical manifestations included rash in 26.7% of the cases, Mechanic’s hand in 40%, muscle weakness in 23.3%, myalgia in 40%, dyspnea in 73.3%, cough in 86.7%, expectoration in 73.3%, arthralgia in 43.3%, the Velco sign in 30%, and Raynaud’s phenomenon in 10%. In terms of the antibody test results, 16 patients were positive for anti-jo-1 antibody; 7 patients were positive for anti-PL7 antibody; 5 patients were positive for anti-PL12 antibody; 3 patients were positive for anti-EJ antibody; 1 patient was positive for anti-OJ antibody; and 1 patient was positive for anti-HA antibody. All participants were administered glucocorticoids, with a mean dose of 43.5 mg, and 22 of them also received cyclophosphamide.
N-glycosylation patterns of plasma IgG in patients with ASS
We conducted qualitative and quantitative analyses of the IgG intact N-glycopeptides from the ASS and HC groups. A total of 11 highly reliable and reproducible N-glycans were quantified in both groups (Supplementary Table S2). The quantitative results of the IgG N-glycans in these two groups were subsequently compared. As shown in Figure 2, in comparison with those in HCs, 2 N-glycans were increased (Figures 2A, B) and 7 were decreased (Figures 2C-I) among patients with ASS. In addition, the levels of the 2 N-glycans did not differ significantly between the two groups (Supplementary Figure S1). In addition, we selected N-glycans containing F (fucose) and A (N-acetylneuraminic acid) from the 11 highly reliable and reproducible N-glycans, added them and found that fucose was significantly increased, whereas N-acetylneuraminic acid was significantly decreased in ASS patients (Figure 3). The binding of IgG to FcγRIIIA on NK cells activates antibody-dependent cell cytotoxicity (ADCC) (23). In in-vitro models using monoclonal antibodies and affinity studies employing surface plasmon resonance, afucosylated antibodies have been demonstrated to possess an affinity for FcγRIIIA that is up to 100-fold greater than that of fucosylated antibodies, regardless of the subclass (29, 30). In the field of therapeutics, afucosylation serves as an essential means of enhancing the capacity of monoclonal antibodies to induce ADCC and thereby improving treatment efficacy (31). In contrast to previous findings that afucosylation promotes inflammation in in-vitro models, some studies have reported an increase in fucosylation among JO-1+ myositis patients (25), SLE patients (32) and RA patients (33), which is consistent with our study. Notably, the level of fucosylation in SLE patients was positively correlated with the SLEDAI score, suggesting that the influence of disease status and/or disease type on the effect of N-glycosylation requires further investigation. Our study also revealed a reduction in sialylation among ASS patients, which is in line with findings from other studies on autoimmune diseases (14, 15). Sialylation is widely acknowledged for its anti-inflammatory properties, and its impact on the effector function of IgG has been extensively investigated. Sialylated IgG interacts with CD23, a C-type lectin receptor, leading to the upregulation of inhibitory FcγRIIB on B cells and triggering a negative signaling cascade that counteracts the activating signal following BCR ligation. Moreover, sialylated IgG also engages with CD22, inducing a tolerogenic state in dendritic cells and resulting in the generation of regulatory T (Treg) cells expressing forkhead box P3+ that suppress immune responses (23).
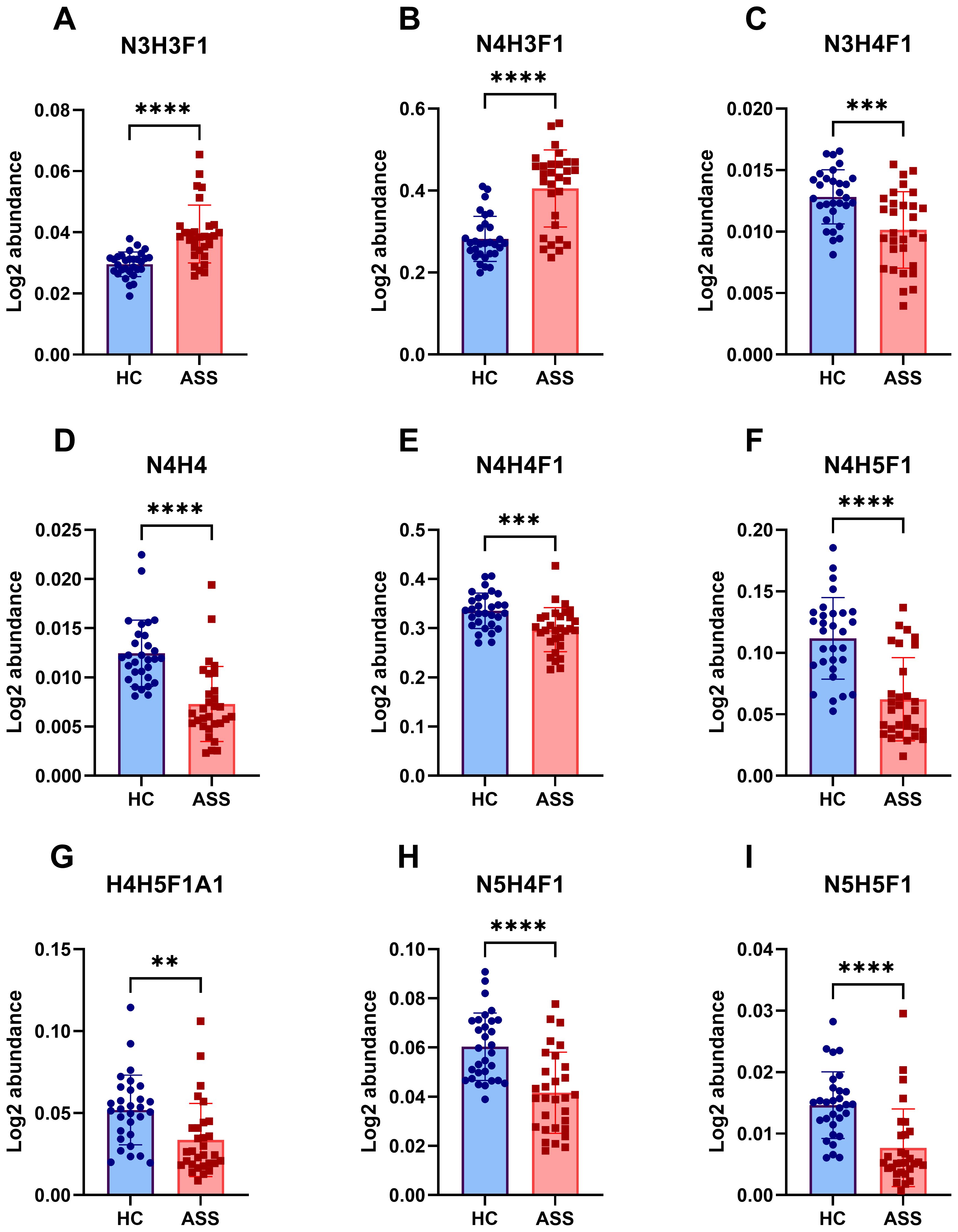
Figure 2. Differences in IgG N-glycans between the ASS and HC groups. This figure highlights the significant variations in N-glycosylation patterns observed between the ASS and HC groups. (A) N3H3F1, (B)N4H3F1, (C)N3H4F1, (D) N4H4, (E) N4H4F1, (F) N4H5F1, (G) N4H5F1A1, (H) N5H4F1, (I) N5H5F1. H, hexose; N, N-acetylhexosamine; F, fucose; A, N-acetylneuraminic acid. **p<0.005, ***p<0.001, ****p<0.0001.
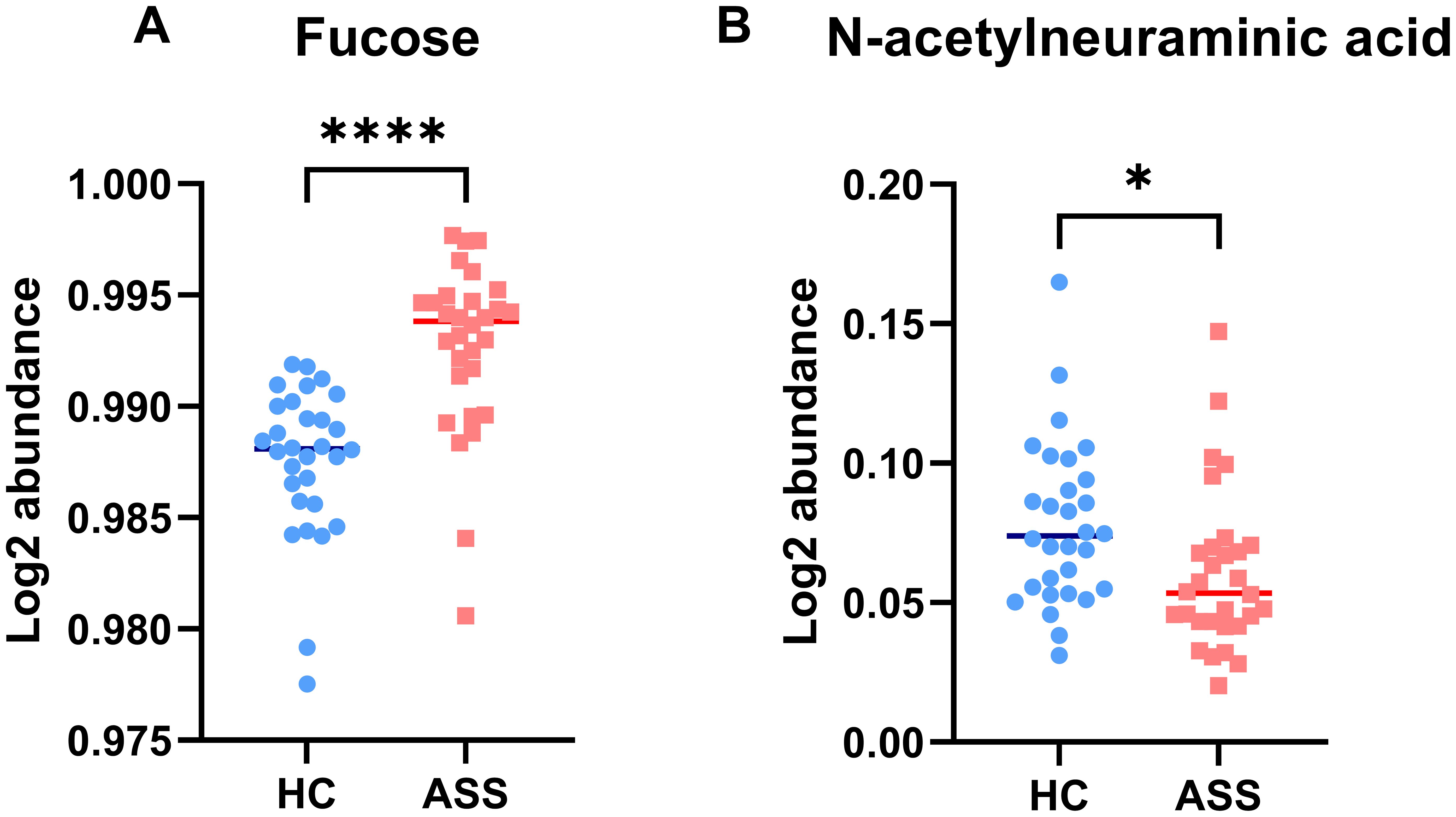
Figure 3. Characteristics of IgG N-glycans containing fucose (A) and N-acetylneuraminic acid (B) in the ASS and HC groups. *p <0.05, **** p<0.0001.
Furthermore, a total of 15 IgG intact N-glycopeptides were quantified from the two groups (Supplementary Table S3). The principal component analysis (PCA) plot demonstrated that these IgG intact N-glycopeptides could effectively distinguish between the ASS and HC groups (Supplementary Figure S2). Heatmap analysis revealed differences in intact N-glycopeptides between the ASS and HC groups (Supplementary Figure S3). Upon further analysis of the intact N-glycopeptides of the IgG subclasses, 13 differentially expressed intact N-glycopeptides were detected (Figure 4). The results indicated that six intact N-glycopeptides were significantly increased (Figures 4A-F). Another set of 7 intact N-glycopeptides was significantly decreased (Figures 4G-M). Additionally, the levels of IgG2-N4H4F1A1 and IgG2-N5H3F1 did not significantly differ between the two groups (Supplementary Figure S4). The diverse IgG subclasses, namely, IgG1, IgG2, IgG3, and IgG4, display significant variations in their biological activities. This includes the capacity for complement fixation (IgG3 >IgG1 >IgG2 >IgG4) as well as the ability to bind to Fc receptors. Among these subclasses (34), IgG2 is distinguished by its somewhat restricted capacity in terms of complement and FC receptor binding. Our study revealed that seven intact N-glycopeptides were derived from the IgG2 subclass. Nevertheless, individuals who are deficient in certain immunoglobulin subclasses appear to be capable of making up for it with the remaining set of antibodies used to maintain overall health (35). Therefore, we hypothesize that the reduction in glycosylation of IgG2 in ASS patients might be associated with its transformation into other subclasses. However, further research is needed to confirm this hypothesis.
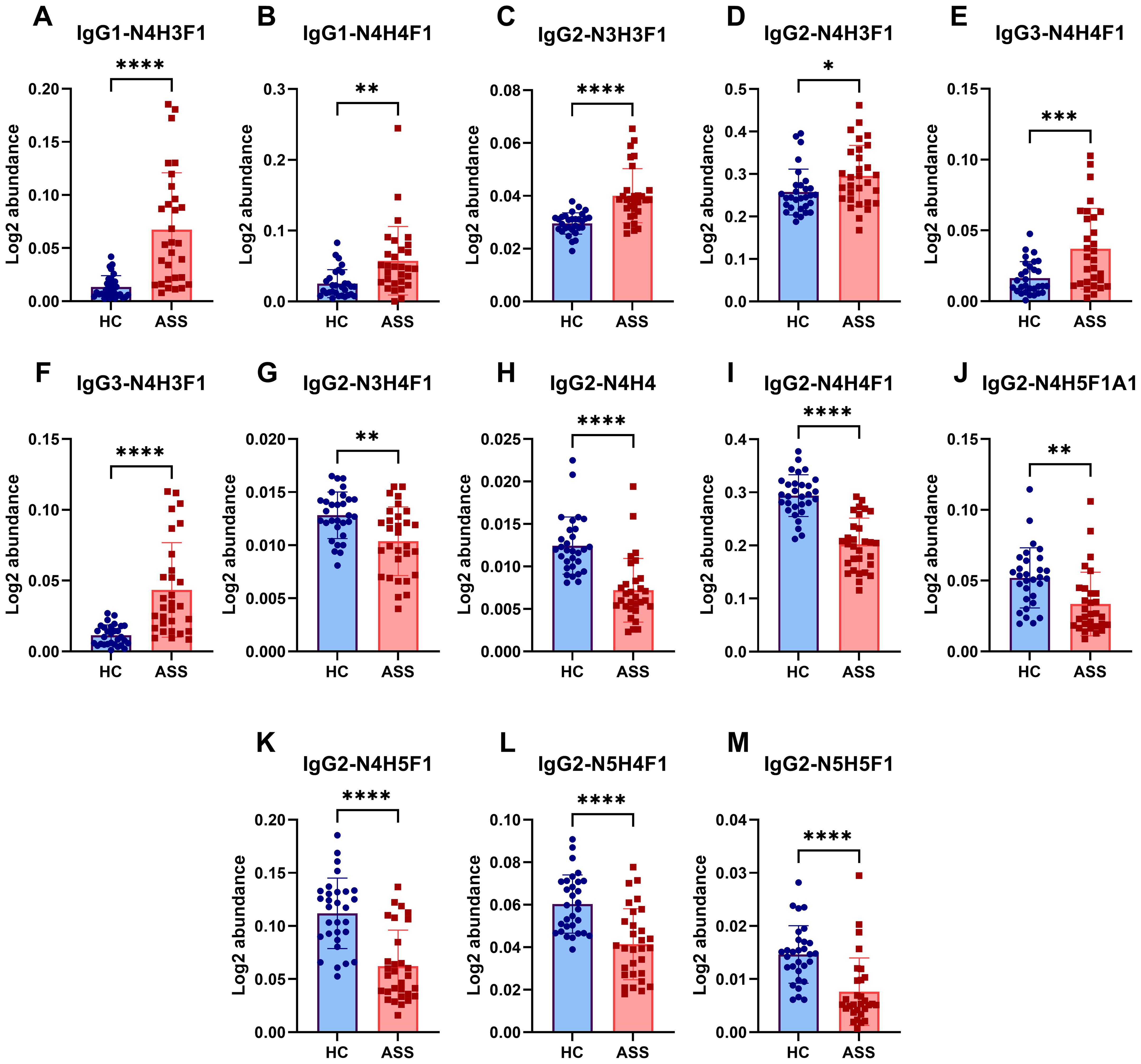
Figure 4. Differences in intact N-glycopeptides of IgG between the ASS and HC groups. (A) IgG1-N4H3F1, (B) IgG1-N4H4F1, (C) IgG2-N3H3F1, (D) IgG2-N4H3F1, (E) IgG3-N4H4F1, (F) IgG3-N4H3F1, (G) IgG2-N3H4F1, (H) IgG2-N4H4, (I) IgG2-N4H4F1, (J) IgG2-N4H5F1A1, (K) IgG2-N4H5F1, (L) IgG2-N5H4F1, (M) IgG2-N5H5F1. *p <0.05, **p<0.005, ***p<0.001, **** p<0.0001.
IgG glycosylation patterns associated with the clinical manifestations of ASS
To explore the potential clinical applications of various glycosylation patterns, we conducted a correlation analysis between the clinical manifestations and IgG intact N-glycopeptides in ASS patients. As shown in Figure 5, Supplementary Tables S4, and S5, our finding that N-glycosylation is significantly associated with specific clinical manifestations (such as rash, muscle weakness, Raynaud’s phenomenon, and arthralgia), disease activity, and even the development of interstitial lung disease in ASS patients implies a potential role for glycosylation in the pathogenesis and progression of this disease. The multivariate adjustment of clinical parameters that showed strong associations with two or more glycosylation features revealed persistent correlations. Nineteen clinical outcomes maintained their significance (Supplementary Table S6), although the effect sizes were reduced compared with the univariate findings, and no binary outcome associations were found to be significant. Previous research has revealed a correlation between the JO-1 titer and the severity and prognosis of ASS disease (36, 37). Moreover, studies have reported elevated fucosylation levels in JO-1 positive patients compared with both healthy controls and JO-1 negative patients (25), a finding that aligns with our own study. These findings suggest that the influence of the JO-1 titer on ASS disease activity might be related to glycosylation. Currently, the relationship between clinical manifestations and glycosylation in autoimmune diseases has been most extensively studied in rheumatoid arthritis. Studies have demonstrated that glycosylation plays a role in synovitis in rheumatoid arthritis and is correlated with disease activity (38). Consequently, exploring glycosylation is crucial for the monitoring of disease activity and potentially for the treatment of ASS.
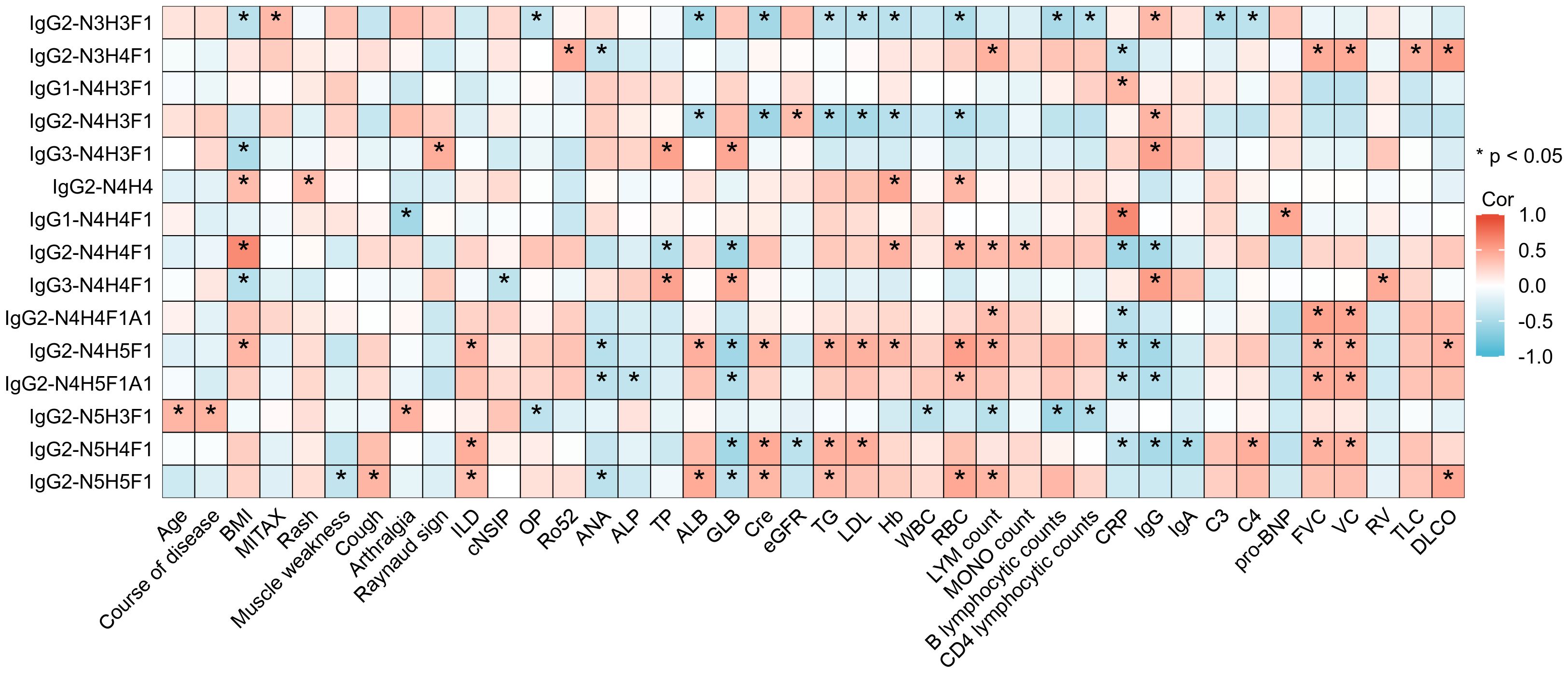
Figure 5. Heatmap depicting the correlation between IgG intact N-glycopeptides and the clinical manifestations of ASS. *Indicates a statistically significant correlation between the N-glycopeptide and the clinical indicators in ASS patients, with *p <0.05. BMI, bode-mass index; MITAX, myositis intention to treat activity index; ILD, interstitial lung disease; cNSIP, cell nonspecific interstitial pneumonia; OP, organizing pneumonia; Ro52, anti-cytoplasmic ribonucleoprotein of 52 kDa; ANA, antinuclear antibody; ALP, alkaline phosphatase; TP, total protein; ALB, albumin; GLB, globulin; Cre, creatinine; eGFR, estimated glomerular filtration rate; TG, triglyceride; LDL, low-density lipoprotein; Hb, hemoglobin; WBC, white blood cell count; RBC, red blood cell count; LYM, lymphocyte; MONO, monocyte; CRP, C-reactive protein; IgG, immunoglobulin G; IgA, immunoglobulin A; C3, complement C3; C4, complement C4; pro-BNP, pro-brain natriuretic peptide; FVC, forced vital capacity; VC, vital capacity; RV, residual volume; TLC, total lung capacity; DLCO, diffusing capacity of the lung for carbon monoxide.
Our study has certain limitations. First, the relatively small sample size makes it impossible to conduct in-depth group discussions among ASS patients with different ARSs. Second, since the theoretical sequence and structure of N-glycosylation are based on currently known biosynthetic rules and reverse synthesis strategies, it is possible that the glycopeptide structures we identified are not comprehensive enough (39). Third, our research lacks validation, even though numerous studies have been carried out, the functions of these glycopeptides are still somewhat of a mystery, and their potential clinical application value demands further validation through additional fundamental research. In the future, relevant ASS animal models or in vitro models, such as cell lines expressing ASS-associated autoantigens, can be used to further explore the functional significance of these glycosylation changes. Additionally, the glycosylation map of anti-ARS is a highly meaningful research direction, and research on this topic might explain the relationship of anti-ARS with the pathogenesis of ASS.
Conclusion
In this study, we revealed distinct IgG N-glyco-signatures that differentiate ASS patients from HCs. Notable disease-specific changes included elevated fucosylation (p<0.0001) and reduced N-acetylneuraminic acid (p<0.05). Furthermore, distinct intact N-glycopeptides of IgGs were found to be correlated with pulmonary function metrics and inflammatory markers. These preliminary findings highlight specific IgG glycosylation alterations in ASS, suggesting potential links between certain glycosylation characteristics and clinical parameters associated with the disease.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: http://www.proteomexchange.org/, PXD058077.
Ethics statement
The studies involving humans were approved by Ethics Committee of West China Hospital Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JZ: Investigation, Visualization, Writing – original draft, Writing – review & editing, Software, Validation. YHL: Methodology, Resources, Writing – review & editing. YYL: Data curation, Formal Analysis, Software, Writing – review & editing. TW: Validation, Visualization, Writing – review & editing. YW: Supervision, Visualization, Writing – review & editing. CT: Data curation, Writing – review & editing, Formal Analysis, Methodology. LC: Visualization, Writing – review & editing, Formal Analysis, Methodology, Supervision, Validation. DH: Investigation, Writing – review & editing, Validation. YL: Funding acquisition, Resources, Supervision, Writing – review & editing, Conceptualization, Methodology, Visualization. YZ: Data curation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. We are grateful for the financial support from the National Natural Science Foundation of China (92478101, 82172731) and the National Key R&D Program of China (2022YFF0608401).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1538219/full#supplementary-material
References
1. Lundberg IE, Fujimoto M, Vencovsky J, Aggarwal R, Holmqvist M, Christopher-Stine L, et al. Idiopathic inflammatory myopathies. Nat Rev Dis Primers. (2021) 7,. doi: 10.1038/s41572-021-00321-x
2. Witt LJ, Curran JJ, and Strek ME. The diagnosis and treatment of antisynthetase syndrome. Clin Pulm Med. (2016) 23,:218–26. doi: 10.1097/CPM.0000000000000171
3. Targoff IN and Arnett FC. Clinical manifestations in patients with antibody to PL-12 antigen (alanyl-tRNA synthetase). Am J Med. (1990) 88,:241–51. doi: 10.1016/0002-9343(90)90149-8
4. Hirakata M, Suwa A, Nagai S, Kron MA, Trieu EP, Mimori T, et al. Anti-KS: identification of autoantibodies to asparaginyl-transfer RNA synthetase associated with interstitial lung disease. J Immunol. (1999) 162,:2315–20. doi: 10.4049/jimmunol.162.4.2315
5. Nakashima R, Imura Y, Hosono Y, Seto M, Murakami A, Watanabe K, et al. The multicenter study of a new assay for simultaneous detection of multiple anti-aminoacyl-tRNA synthetases in myositis and interstitial pneumonia. PLoS One. (2014) 9,:e85062. doi: 10.1371/journal.pone.0085062
6. Zhang Y, Zeng W, Zhao Y, and Yang H. Editorial: New methods, techniques and applications in clinical glycoproteomics. Front Mol Biosci. (2023) 10:1170818. doi: 10.3389/fmolb.2023.1170818
7. Wang Y, Lei K, Zhao L, and Zhang Y. Clinical glycoproteomics: methods and diseases. MedComm (2020). (2024) 5,:e760. doi: 10.1002/mco2.v5.10
8. Plomp R, Bondt A, de Haan N, Rombouts Y, and Wuhrer M. Recent advances in clinical glycoproteomics of immunoglobulins (Igs). Mol Cell Proteomics. (2016) 15,:2217–28. doi: 10.1074/mcp.O116.058503
9. Schroeder HW Jr. and Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. (2010) 125,:S41–52. doi: 10.1016/j.jaci.2009.09.046
10. Luo M, Mao Y, Zeng W, Zheng S, Li H, Hu J, et al. Site-specific N-glycosylation characterization of micro monoclonal immunoglobulins based on EThcD-sceHCD-MS/MS. Front Immunol. (2022) 13:1013990. doi: 10.3389/fimmu.2022.1013990
11. Jin Y, Hu R, Gu Y, Wei A, Li A, and Zhang Y. Quantitative site-specific N-glycosylation analysis reveals IgG glyco-signatures for pancreatic cancer diagnosis. Clin Proteomics. (2024) 21,:68. doi: 10.1186/s12014-024-09522-4
12. Lu LL, Chung AW, Rosebrock TR, Ghebremichael M, Yu WH, Grace PS, et al. A functional role for antibodies in tuberculosis. Cell. (2016) 167,:433–443.e14. doi: 10.1016/j.cell.2016.08.072
13. Pilkington C, Basaran M, Barlan I, Costello AM, and Rook GA. Raised levels of agalactosyl IgG in childhood tuberculosis. Trans R Soc Trop Med Hyg. (1996) 90,:167–8. doi: 10.1016/S0035-9203(96)90124-8
14. Sjöwall C, Zapf J, von Löhneysen S, Magorivska I, Biermann M, Janko C, et al. Altered glycosylation of complexed native IgG molecules is associated with disease activity of systemic lupus erythematosus. Lupus. (2015) 24,:569–81. doi: 10.1177/0961203314558861
15. Gudelj I, Salo PP, Trbojević-Akmačić I, Albers M, Primorac D, Perola M, et al. Low galactosylation of IgG associates with higher risk for future diagnosis of rheumatoid arthritis during 10 years of follow-up. Biochim Biophys Acta Mol Basis Dis. (2018) 1864,:2034–9. doi: 10.1016/j.bbadis.2018.03.018
16. Šimurina M, de Haan N, Vučković F, Kennedy NA, Štambuk J, Falck D, et al. Glycosylation of immunoglobulin G associates with clinical features of inflammatory bowel diseases. Gastroenterology. (2018) 154,:1320–1333.e10. doi: 10.1053/j.gastro.2018.01.002
17. Trbojević Akmačić I, Ventham NT, Theodoratou E, Vučković F, Kennedy NA, Krištić J, et al. Inflammatory bowel disease associates with proinflammatory potential of the immunoglobulin G glycome. Inflammation Bowel Dis. (2015) 21,:1237–47. doi: 10.1097/MIB.0000000000000372
18. Bond A, Alavi A, Axford JS, Bourke BE, Bruckner FE, Kerr MA, et al. A detailed lectin analysis of IgG glycosylation, demonstrating disease specific changes in terminal galactose and N-acetylglucosamine. J Autoimmun. (1997) 10,:77–85. doi: 10.1006/jaut.1996.0104
19. Kemna MJ, Plomp R, van Paassen P, Koeleman CAM, Jansen BC, Damoiseaux J, et al. Galactosylation and sialylation levels of igG predict relapse in patients with PR3-ANCA associated vasculitis. EBioMedicine. (2017) 17:108–18. doi: 10.1016/j.ebiom.2017.01.033
20. Fokkink WJ, Selman MH, Dortland JR, Durmuş B, Kuitwaard K, Huizinga R, et al. IgG Fc N-glycosylation in Guillain-Barré syndrome treated with immunoglobulins. J Proteome Res. (2014) 13,:1722–30. doi: 10.1021/pr401213z
21. Vaccari M, Gordon SN, Fourati S, Schifanella L, Liyanage NP, Cameron M, et al. Adjuvant-dependent innate and adaptive immune signatures of risk of SIVmac251 acquisition. Nat Med. (2016) 22,:762–70. doi: 10.1038/nm.4105
22. Ho CH, Chien RN, Cheng PN, Liu JH, Liu CK, Su CS, et al. Aberrant serum immunoglobulin G glycosylation in chronic hepatitis B is associated with histological liver damage and reversible by antiviral therapy. J Infect Dis. (2015) 211,:115–24. doi: 10.1093/infdis/jiu388
23. Beyze A, Larroque C, and Le Quintrec M. The role of antibody glycosylation in autoimmune and alloimmune kidney diseases. Nat Rev Nephrol. (2024) 20,:672–89. doi: 10.1038/s41581-024-00850-0
24. Li H, Lu W, Jin Q, Sun J, Gao L, Hu J, et al. Deciphering N-glycosylation dynamics of serum monoclonal immunoglobulins in multiple myeloma via EThcD-sceHCD-MS/MS. J Proteome Res. (2025) 24,:2553–63. doi: 10.1021/acs.jproteome.5c00253
25. Fernandes-Cerqueira C, Renard N, Notarnicola A, Wigren E, Gräslund S, Zubarev RA, et al. Patients with anti-Jo1 antibodies display a characteristic IgG Fc-glycan profile which is further enhanced in anti-Jo1 autoantibodies. Sci Rep. (2018) 8,:17958. doi: 10.1038/s41598-018-36395-z
26. Solomon J, Swigris JJ, and Brown KK. Myositis-related interstitial lung disease and antisynthetase syndrome. J Bras Pneumol. (2011) 37,:100–9. doi: 10.1590/S1806-37132011000100015
27. Yu G, Zhang Y, Meng B, Xie X, Wang Z, Ying W, et al. O-glycoforms of polymeric immunoglobulin A1 in the plasma of patients with IgA nephropathy are associated with pathological phenotypes. Nephrol Dial Transplant. (2021) 37,:33–41. doi: 10.1093/ndt/gfab204
28. Zhao Y, Zhang Y, Meng B, Luo M, Li G, Liu F, et al. A novel integrated pipeline for site-specific quantification of N-glycosylation. Phenomics. (2024) 4,:213–26. doi: 10.1007/s43657-023-00150-w
29. Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. (2002) 277,:26733–40. doi: 10.1074/jbc.M202069200
30. Dekkers G, Treffers L, Plomp R, Bentlage AEH, de Boer M, Koeleman CAM, et al. Decoding the human immunoglobulin G-glycan repertoire reveals a spectrum of fc-receptor- and complement-mediated-effector activities. Front Immunol. (2017) 8:877. doi: 10.3389/fimmu.2017.00877
31. Shrivastava A, Joshi S, Guttman A, and Rathore AS. N-Glycosylation of monoclonal antibody therapeutics: A comprehensive review on significance and characterization. Anal Chim Acta. (2022) 1209:339828. doi: 10.1016/j.aca.2022.339828
32. Han J, Zhou Z, Zhang R, You Y, Guo Z, Huang J, et al. Fucosylation of anti-dsDNA IgG1 correlates with disease activity of treatment-naïve systemic lupus erythematosus patients. EBioMedicine. (2022) 77:103883. doi: 10.1016/j.ebiom.2022.103883
33. Roy S, Biswas S, Saroha A, Sahu D, and Das HR. Enhanced expression and fucosylation of ficolin3 in plasma of RA patients. Clin Biochem. (2013) 46,:160–3. doi: 10.1016/j.clinbiochem.2012.10.028
34. Liu H and May K. Disulfide bond structures of IgG molecules: structural variations, chemical modifications and possible impacts to stability and biological function. MAbs. (2012) 4,:17–23. doi: 10.4161/mabs.4.1.18347
35. Lefranc G, Chaabani H, Van Loghem E, Lefranc MP, De Lange G, and Helal AN. Simultaneous absence of the human IgG1, IgG2, IgG4 and IgA1 subclasses: immunological and immunogenetical considerations. Eur J Immunol. (1983) 13,:240–4. doi: 10.1002/eji.1830130312
36. Yang H, Chen Q, Sun C, Jin Q, Zhang L, Liu Q, et al. Clinical and prognostic associations of anti-Jo-1 antibody levels in patients with antisynthetase syndrome. Respir Res. (2024) 25,:222. doi: 10.1186/s12931-024-02851-w
37. Stone KB, Oddis CV, Fertig N, Katsumata Y, Lucas M, Vogt M, et al. Anti-Jo-1 antibody levels correlate with disease activity in idiopathic inflammatory myopathy. Arthritis Rheum. (2007) 56,:3125–31. doi: 10.1002/art.22865
38. Kissel T, Toes REM, Huizinga TWJ, and Wuhrer M. Glycobiology of rheumatic diseases. Nat Rev Rheumatol. (2023) 19,:28–43. doi: 10.1038/s41584-022-00867-4
Keywords: autoimmune disease, anti-synthetase syndrome (ASS), N-glycosylation, intact N-glycopeptide, immunoglobulin g (IgG)
Citation: Zhao J, Li Y, Ling Y, Wu T, Wu Y, Tan C, Cheng L, Huang D, Liu Y and Zhang Y (2025) N-glycosylation patterns of plasma immunoglobulin G in anti-synthetase syndrome disease. Front. Immunol. 16:1538219. doi: 10.3389/fimmu.2025.1538219
Received: 02 December 2024; Accepted: 03 June 2025;
Published: 18 June 2025.
Edited by:
Alfred Hyoungju Kim, Washington University in St. Louis, United StatesReviewed by:
Sheng-Yan Lin, Huazhong University of Science and Technology, ChinaGeorgios E Manousakis, University of Minnesota, United States
Weihua Tian, Technical University of Denmark, Denmark
Copyright © 2025 Zhao, Li, Ling, Wu, Wu, Tan, Cheng, Huang, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Zhang, bmFua2FpMTk4OUBmb3htYWlsLmNvbQ==; emhhbmd5b25nMDgwOUB3Y2hzY3UuY24=; Yi Liu, eWlsaXU4OTk5QHdjaHNjdS5jbg==
†These authors have contributed equally to this work
 Jing Zhao
Jing Zhao Yanhong Li
Yanhong Li Yingying Ling†
Yingying Ling† Tong Wu
Tong Wu Yinlan Wu
Yinlan Wu Chunyu Tan
Chunyu Tan Lu Cheng
Lu Cheng Deying Huang
Deying Huang Yi Liu
Yi Liu Yong Zhang
Yong Zhang