- 1Department of Oncology, The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), Hangzhou, China
- 2Cancer Institute of Integrated Traditional Chinese & Western Medicine, Zhejiang Chinese Medical University, Hangzhou, China
Background: The pre-metastatic niche (PMN) represents the microenvironment established in target organs before primary tumor metastasis, playing a crucial role in organ-specific metastasis. Understanding and preventing PMN formation holds promise for enhancing immunotherapy efficacy and reducing cancer-related mortality. Despite the significance of this field, a comprehensive bibliometric analysis is lacking. This study aims to identify global research trends and hotspots in PMN through a systematic bibliometric evaluation, providing a foundation for future advancements in this field.
Methods: Publications related to PMN research from 2005 to 2024 were retrieved from the Web of Science Core Collection database. Bibliometric analyses and visualizations were conducted using VOSviewer, CiteSpace, Microsoft Excel, ArcGIS, Scimago Graphica, and Microsoft Charticulator.
Results: The study included 1,303 publications authored by 7,955 researchers from 1,627 institutions across 62 countries, with articles published in 400 journals. China and the United States emerged as central contributors to global PMN research. China has led in publication volume and institutional representation, while the United States has produced the most high-quality papers and impactful authors. Cancers published the most PMN-related papers, while Cancer Research had the most citations and co-citations. Professor David Lyden of Cornell University, USA, was identified as the most influential scholar in the field. Analysis of references and keywords suggests future research will focus on metastatic organotropism, extracellular vesicles, innate immunocytes (e.g., macrophages and neutrophils), and immunotherapy.
Conclusion: This bibliometric study represents the first comprehensive analysis of global scientific output in PMN research over the past two decades. By summarizing the current status and identifying trends in the field, this study provides valuable insights and a reference point for researchers aiming to prevent and treat tumor metastasis effectively.
1 Introduction
Tumor metastasis poses a significant challenge in oncology, serving as a primary cause of treatment failure and mortality among cancer patients (1). Biologically, metastasis involves tumor cells invading distant organs from their primary site via direct invasion, bloodstream dissemination, lymphatic spread, or adjacent structures (2). This process is driven by both the genetic characteristics of tumor cells, which enhance their metastatic potential, and specific alterations in the microenvironment of distant organs (3). Before circulating tumor cells (CTCs) reach target organs, factors produced by the primary tumor induce the formation of a “pre-metastatic niche” (PMN), a concept first proposed by David Lyden’s team in 2005 (4).
The PMN concept stems from Paget’s 1889 “seed-and-soil” theory, which suggests that tumor cells (seeds) metastasize in environments (soil) that promote colonization (5). Unlike the metastatic niche, which forms upon the arrival of CTCs, PMN represents a pro-tumorigenic microenvironment devoid of cancer cells (6). Emerging evidence highlights PMN’s pivotal role in organ-specific metastasis (7). For instance, primary pancreatic tumors are associated with heightened liver PMN inflammation and metabolic alterations that may indicate future liver metastasis (8). The formation of PMN requires tumor-derived secreted factors (TDSFs) and extracellular vesicles (EVs) to mediate changes in resident cells (e.g., macrophages, fibroblasts, endothelial cells). It also affects infiltrating immune cells (e.g., innate and adaptive immunocytes) within distant organs (9).
Together, these components create an inflammatory and immunosuppressive microenvironment. This microenvironment promotes the formation of new blood and lymphatic vessels (angiogenesis and lymphangiogenesis) and facilitates extracellular matrix (ECM) remodeling, ultimately enabling PMN formation (10).
As research advances, specific biomarkers of PMN and its organ-specific tendencies are becoming increasingly understood. Recent research increasingly focuses on clinical strategies to prevent and treat tumor metastasis, including inhibiting PMN-promoting signals, disrupting tumor-derived EV signaling, and modulating immune cell infiltration (11). Although previous reviews have addressed PMN research from various perspectives—such as formation, characteristics, and diagnostic or therapeutic potential (6, 9–14)—no comprehensive analysis highlights the evolution of PMN research or its future priorities.
Bibliometric analysis is a method that applies mathematical and statistical techniques to review systematically, and qualitatively and quantitatively analyze research within a specific field over a defined period (15). This study provides an objective overview of global PMN research through bibliometric analysis, integrating statistical data and visualization to highlight key contributors, institutions, and countries. We also explore research trends and future directions in the PMN field. Our findings aim to offer valuable insights for preventing PMN formation and metastasis while guiding researchers toward new research topics and strategic planning.
2 Materials and methods
2.1 Data retrieval and literature screening
All publication data for this study were obtained from the Web of Science Core Collection (WOSCC) database. In the WOSCC database, TS represents a topic search. The following search strategy was applied in this study: TS = (pre-metasta* OR premetasta*) AND TS = (niche* OR microenvironment*). The search period spanned January 1, 2005, to September 18, 2024. Original research articles and reviews published in English were included, while early-access papers and other document types were excluded (Figure 1). All data were downloaded as “plain text” files.
2.2 Data analysis and visualization
Two authors independently extracted bibliometric parameters, including titles, keywords, countries or regions, institutions, authors, journals, year of publication, citations, and cited references. The Impact Factor (IF) and H-index (16) were used to assess the academic influence of publications and researchers. Journal Citation Reports (JCR) 2023 was used to calculate the IF. A researcher’s H-index indicates that at least h papers have been cited at least h times. Additionally, the H-index was also used to evaluate the productivity and influence of countries, institutions, and journals.
VOSviewer 1.6.20 was employed to analyze and visualize the collected data, focusing on country cooperation, institutional and author cooperation, journals publishing and being cited, and keyword co-occurrence. Journal overlay dual maps, keyword time zone maps, and reference and keyword burst analyses were generated using CiteSpace 6.3.R3. In these visualizations, each node represents a distinct parameter, such as a country, institution, author, or keyword. The size of each node reflects its weight, which is determined by parameters like the number of publications (NP), number of citations (NC), or frequency of occurrence. A higher weight corresponds to a larger node. Nodes and lines were colored according to their cluster, while lines between nodes indicated links. The total strength of co-authorship and co-citation links between countries, institutions and authors was represented by the Total Link Strength (TLS).
Several additional tools were used to present and map the data. Microsoft Excel 2019 was used to depict publication and citation trends in the PMN field over the past 20 years. ArcGIS 10.8 facilitated the mapping of the geographic distribution of global publication output. Scimago Graphica 1.0.43 displayed the annual citation frequency of the top 10 articles based on total citations. Finally, Microsoft Charticulator (https://donghaoren.org/charticulator/index.html) was employed to create a chord diagram illustrating country cooperation.
2.3 Research ethics
All raw data used in this study were obtained from publicly accessible databases and did not involve animal or human experimentation. As such, no ethical approval was required.
3 Results
3.1 Publication output and citation trends
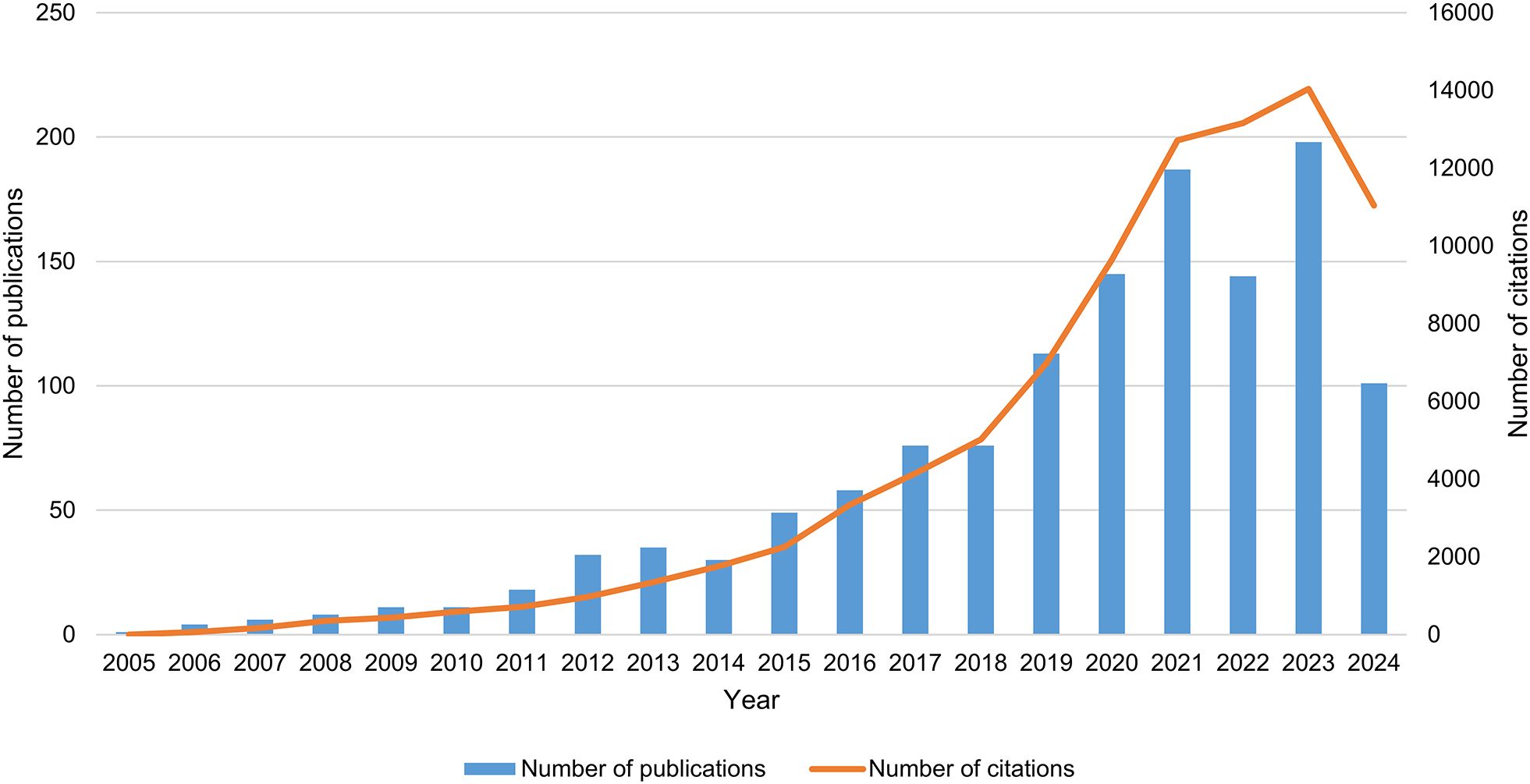
As of September 18, 2024, a total of 1,303 articles on PMN were identified in the WOSCC database, comprising 704 original research articles and 599 reviews. Between 2005 and 2021, the NP in this field increased significantly (Figure 2). From 2022 to 2023, publication numbers fluctuated but trended upward, peaking in 2023 with 198 papers published (2024 data includes publications only up to September 18). Additionally, the annual NC has risen markedly over the past decade, reaching approximately 8,237.7 citations compared to an average of 641.1 citations during the first ten years of the study period. These trends indicate a growing interest in PMN research, highlighting the increasing academic value and potential impact of this field.
3.2 Distribution and cooperation of countries
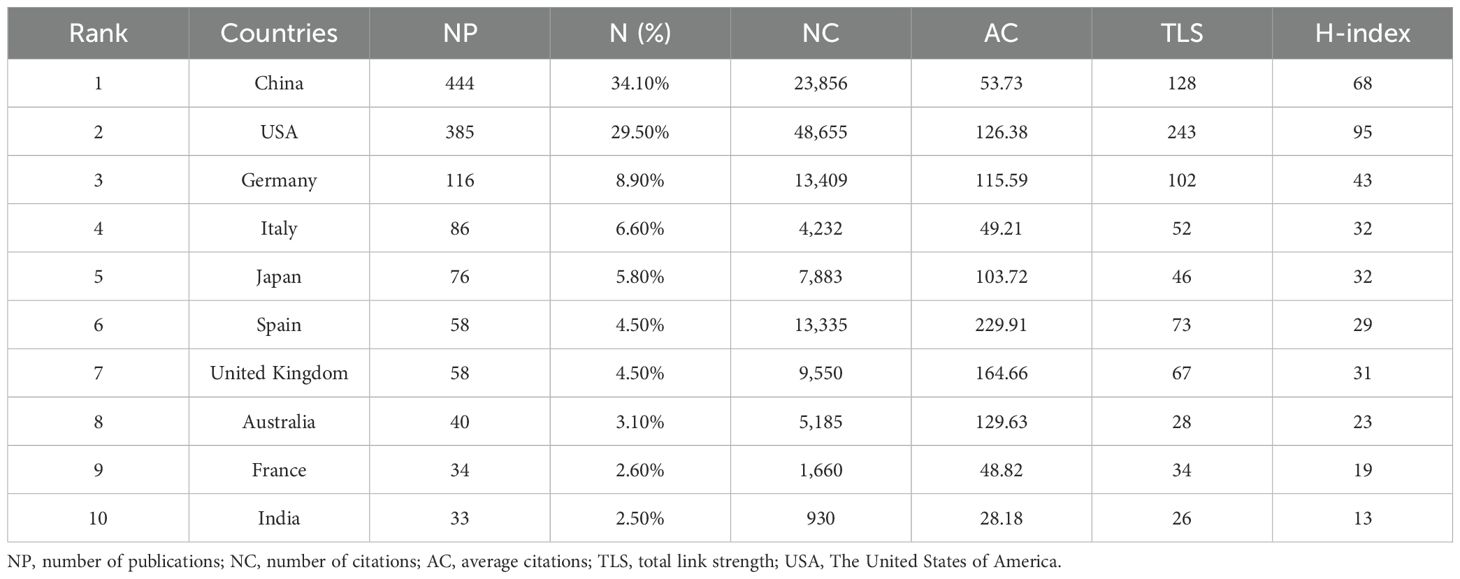
A total of 62 countries contributed to research in the PMN field. The geographic distribution of publications is presented in Figure 3A, where darker colors on the world map indicate higher numbers of publications. Table 1 and Figure 3B rank the top 10 countries by the number of articles published on PMN. China accounted for the largest number of publications, contributing 34.1% (444/1,303) of the total, followed by the United States (29.5%, 385/1,303) and Germany (8.9%, 116/1,303). The United States had the highest total citations (48,655) and H-index (95), while articles from Spain achieved the highest average citations per article (229.91).
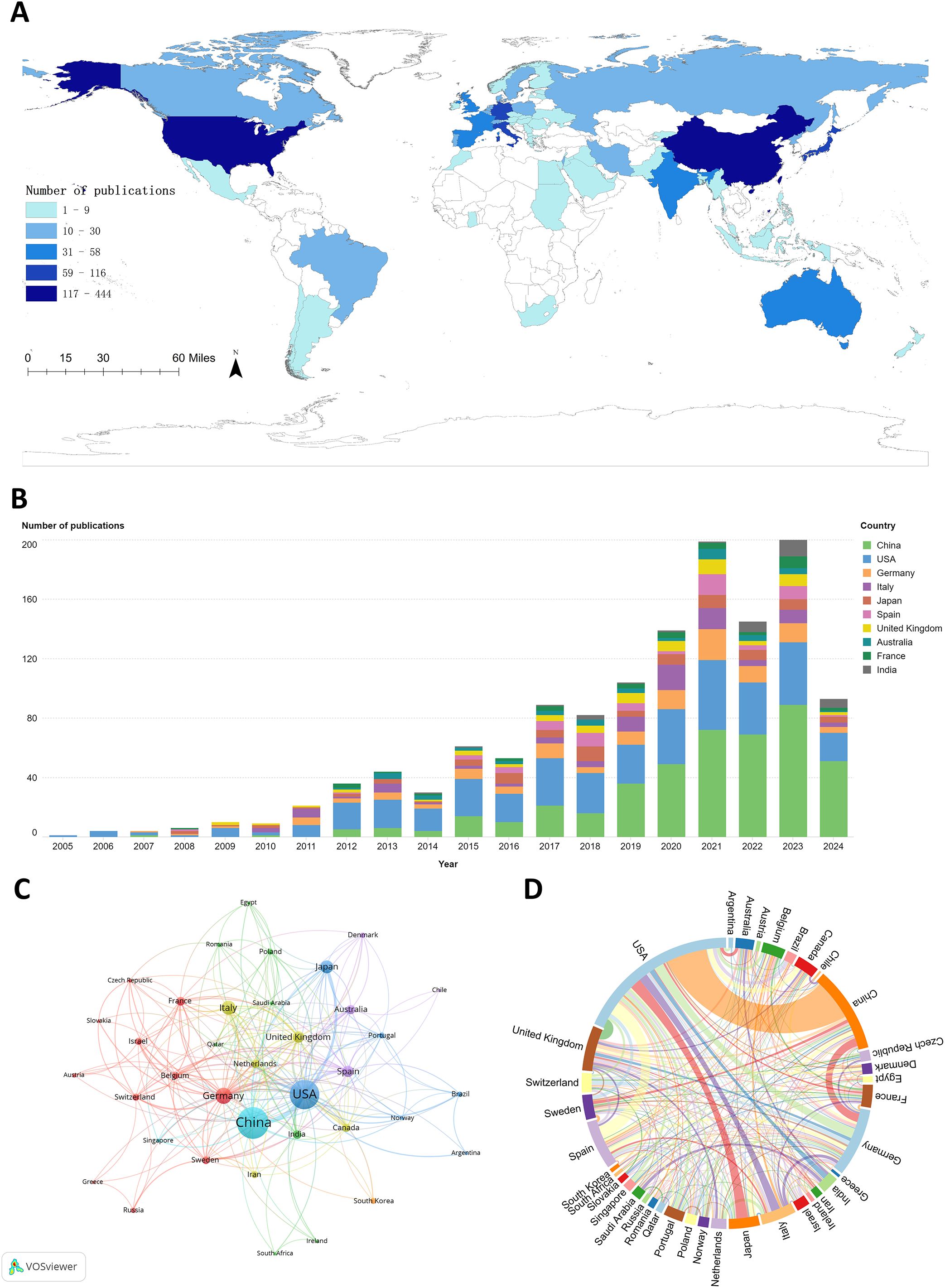
Figure 3. (A) The geographic distribution of global publications on pre-metastatic niches is shown in, highlighting publication density by country. (B) illustrates annual publication trends for the top 10 contributing countries. (C) presents the country cooperation network visualization map, generated using VOSviewer (version 1.6.20), depicting collaborations among countries through node-link relationships. (D) features the country cooperation chord diagram created with Microsoft Charticulator, where node size indicates the total number of collaborations for each country, and line thickness represents the frequency of collaboration between countries.
Figure 3C illustrates international collaboration among the top 37 countries, each with at least five publications. The collaboration network shows that the United States (TLS = 243), China (TLS = 128), and Germany (TLS = 102) collaborated most frequently with other countries, with the highest number of collaborations occurring between China and the United States (Figure 3D).
3.3 Distribution and cooperation of institutions
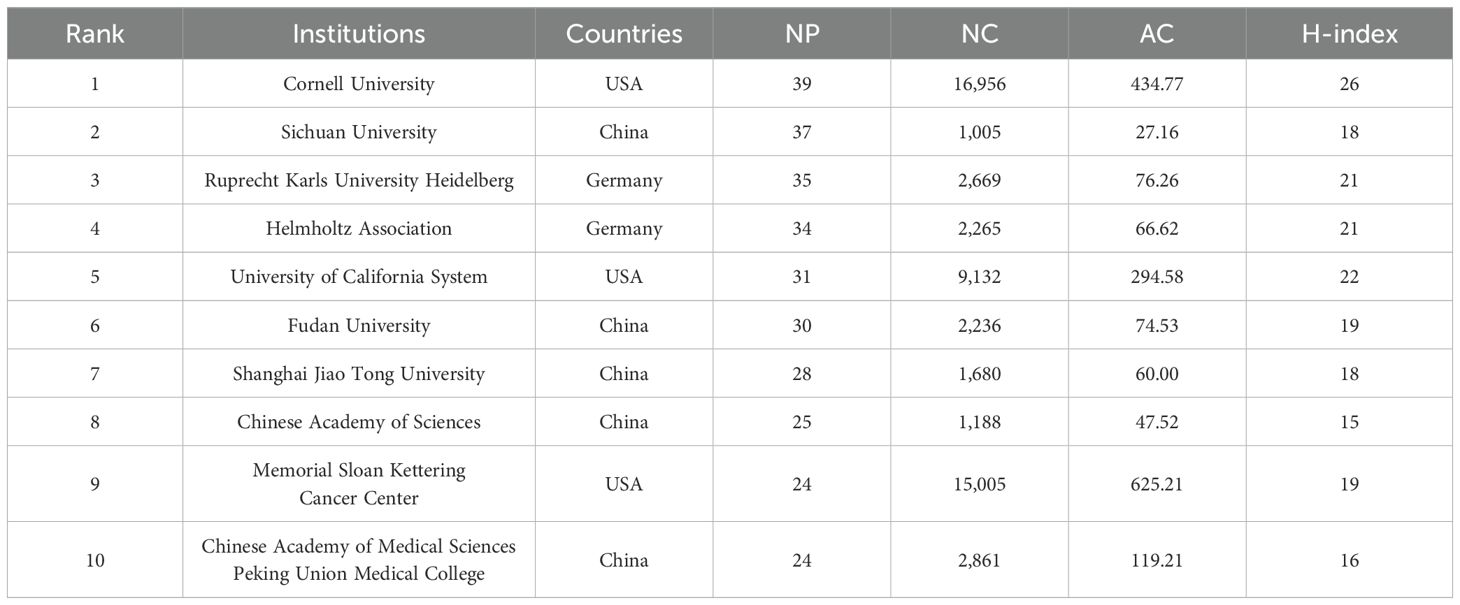
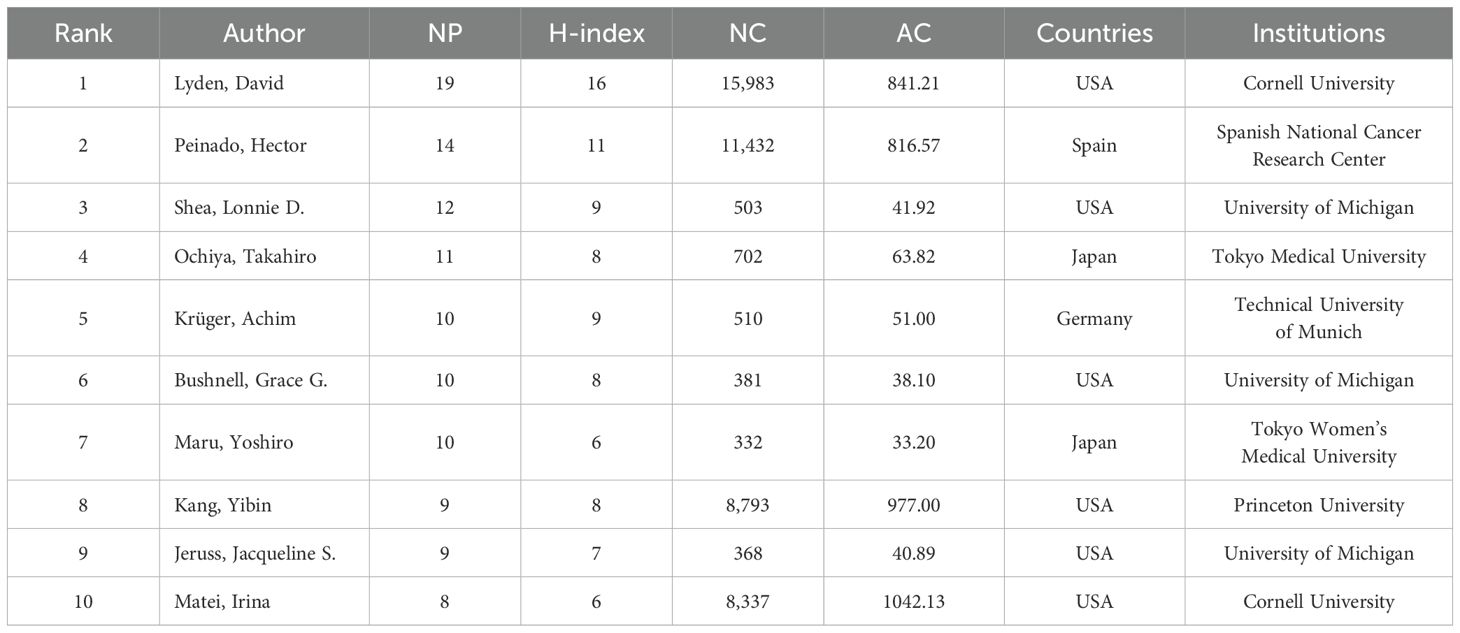
A total of 1,627 academic institutions contributed to PMN research. Table 2 lists the top 10 institutions ranked by the number of publications, with institutions from the United States, China, and Germany dominating the list. Cornell University had the highest number of publications, closely followed by Sichuan University and Ruprecht Karls University Heidelberg. Cornell University also achieved the highest H-index, indicating its significant impact on the field, while Memorial Sloan Kettering Cancer Center reported the highest average citations per paper.
Figure 4A illustrates the collaboration network among the top 78 institutions, each with at least seven publications. These institutions were divided into 12 clusters based on their collaboration patterns, with closely linked institutions indicating active partnerships that drive the PMN field forward. Figure 4B presents a citation network map showing 44 items and 837 links. Cornell University had the highest TLS (1,942), followed by Memorial Sloan Kettering Cancer Center (TLS = 1,310) and the Spanish National Cancer Research Center (TLS = 954).
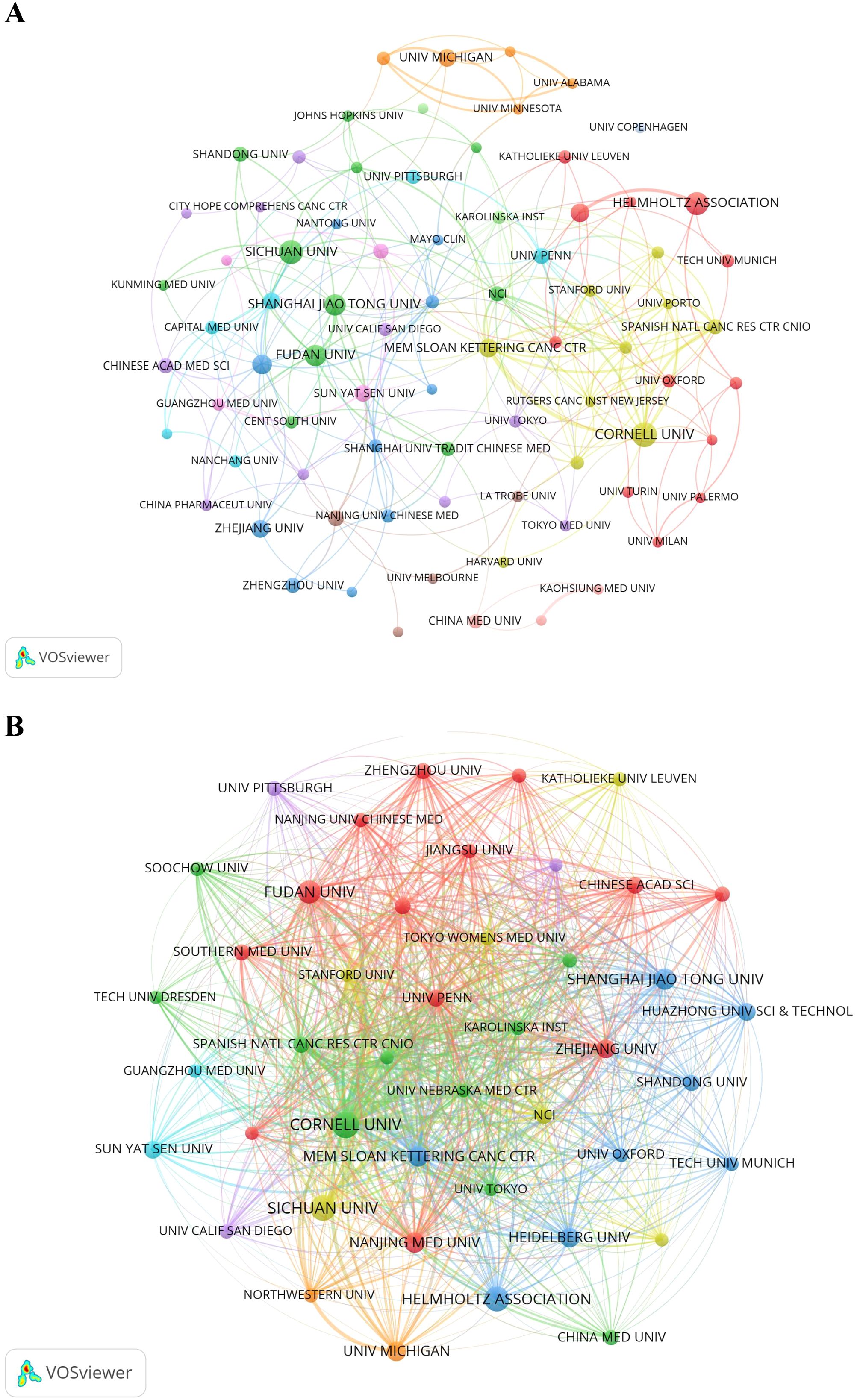
Figure 4. (A) The institution cooperation network visualization map generated by VOSviewer (version 1.6.20). (B) The institution citation network visualization map was generated by VOSviewer (version 1.6.20).
3.4 Authors and co-cited authors
A total of 7,955 authors contributed to the 1,303 publications in the PMN field. Table 3 lists the top 10 most productive authors ranked by the number of publications. Six were from the United States, two from Japan, while Spain and Germany contributed one researcher each. David Lyden (Cornell University, USA) was the most prolific author, with 19 publications and the highest H-index (16), followed by Hector Peinado (Spanish National Cancer Research Center, Spain) and Lonnie D Shea (University of Michigan, USA). Irina Matei (USA) achieved the highest average citations per article, with an impressive 1,042.13.
Figure 5A illustrates the collaboration network of 57 authors who each published at least five articles. Prolific authors like David Lyden, Hector Peinado, Yibin Kang, Achim Krüger, and Grace G Bushnell had the active network of collaborators. The network also includes independent authors, such as Ya-Ling Hsu, Dmitry I Gabrilovich and Jochen Utikal. Figure 5B presents the co-citation network, which contains 184 items, 3 clusters, and 15,908 links. Hector Peinado (TLS = 14,853), Rosandra N Kaplan (TLS = 10,363), and Sachie Hiratsuka (TLS = 9,841) had the highest TLS, indicating their significant influence in the PMN field.
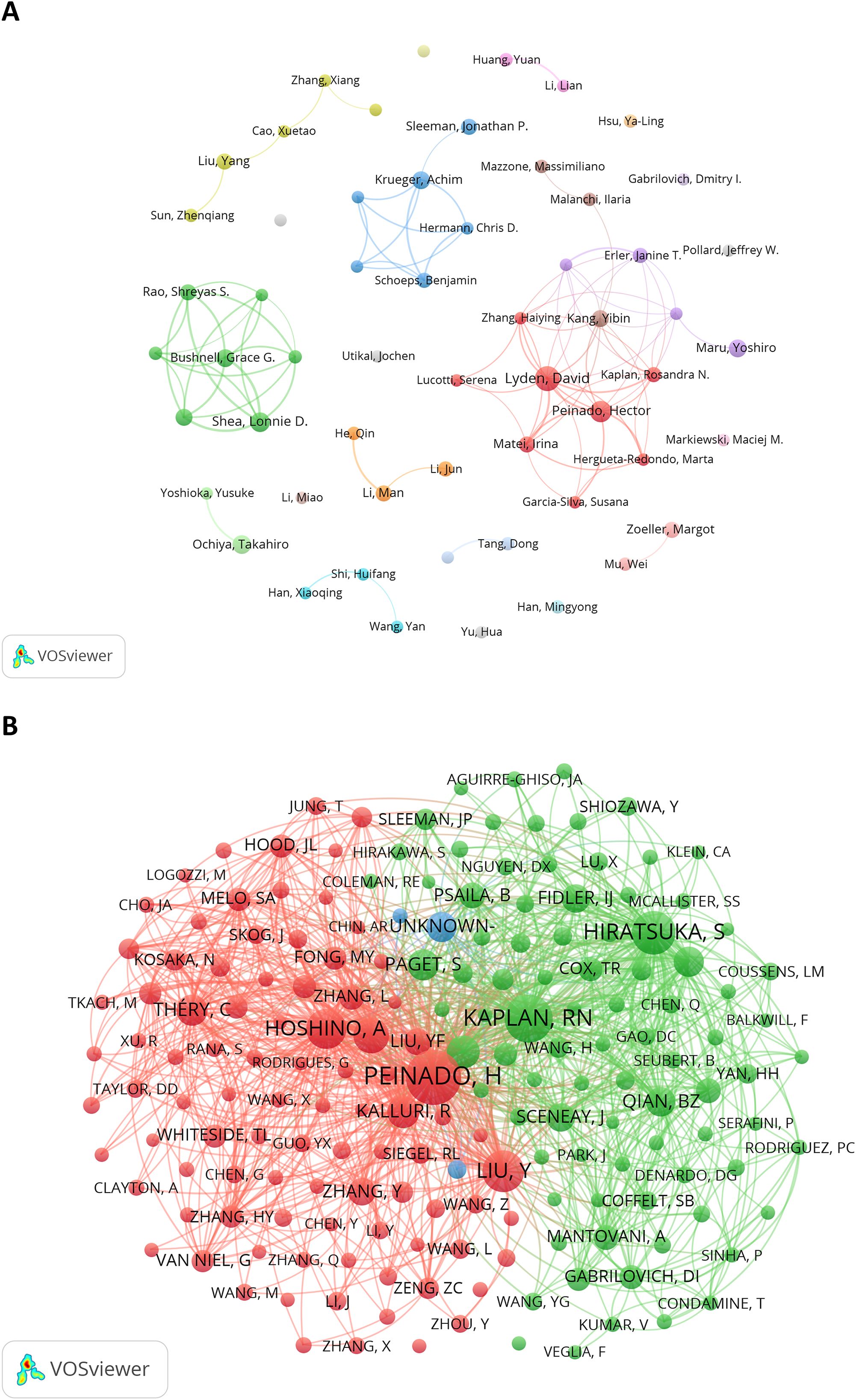
Figure 5. (A) The author cooperation network visualization map generated by VOSviewer (version 1.6.20). (B) The author co-citation network visualization map generated by VOS viewer (version 1.6.20).
3.5 Journals and co-cited journals
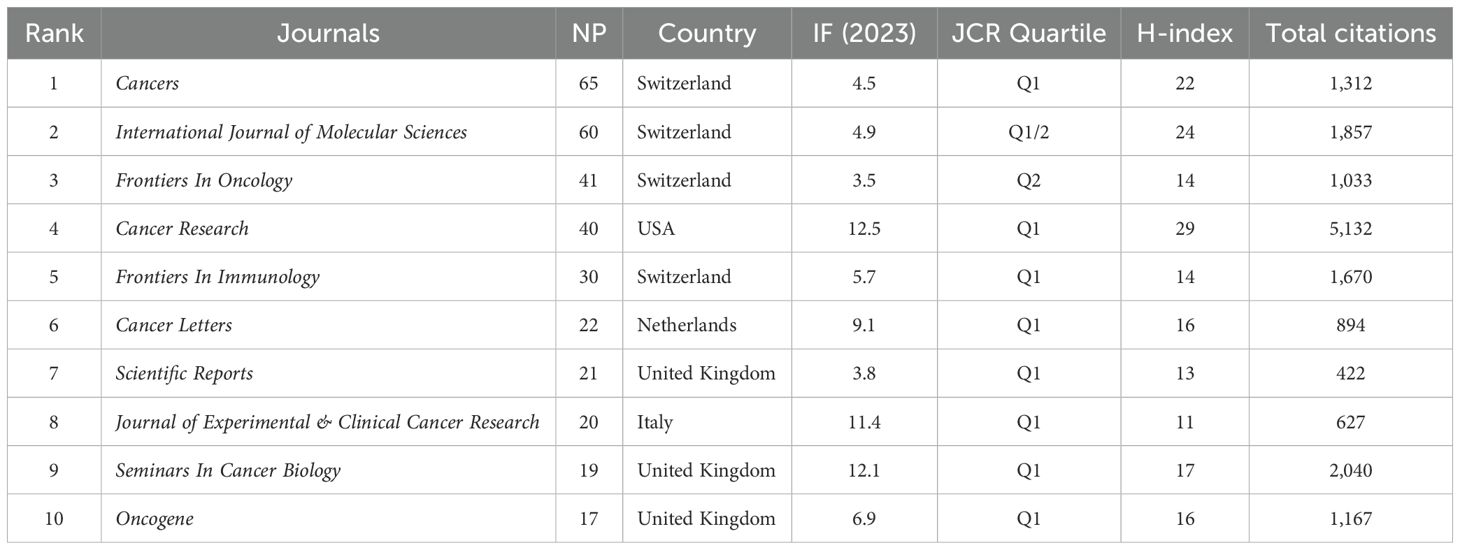
Altogether, 400 journals published PMN-related articles, with journal IF and Journal Citation Report (JCR) quartile serving as key indicators of their influence. Table 4 lists the top 10 journals by the number of publications, accounting for 25.7% (n=335) of all articles in the PMN field. Among these, three Swiss journals occupied the top three spots: Cancers (70 articles, IF 2023 = 4.5, Q1), International Journal of Molecular Sciences (60 articles, IF 2023 = 4.9, Q1/2), and Frontiers in Oncology (41 articles, IF 2023 = 3.5, Q2).
Supplementary Table 1 provides additional insights into the top 10 co-cited journals, ranked by co-citation frequency, IF (2023), and JCR quartile. The most frequently co-cited journals included Cancer Research (IF 2023 = 12.5, Q1, 5,889 co-citations) and Nature (IF 2023 = 50.5, Q1, 4,142 co-citations). Of the top 10 co-cited journals, nine were ranked in Q1 of the JCR, and seven had an IF exceeding 10. Notably, Cancer Research achieved the highest H-index (29) among all journals, along with the most citations (5,132) and co-citations (5,889), underscoring its significant impact in the field.
As shown in Figure 6, citing journals were predominantly spread across subjects #2 (Medicine, Medical, Clinical) and #4 (Molecular, Biology, Immunology), while cited journals were mainly centered in subject #8 (Molecular, Biology, Genetics). Subsets of cited journals also extended into subjects #2 (Environmental, Toxicology, Nutrition), #4 (Chemistry, Materials, Physics), and #5 (Health, Nursing, Medicine). The strongest connection, represented by the thickest orange line, highlights the significant influence of Molecular Biology and Genetics on Molecular Biology and Immunology (z = 6.22, f = 16119).
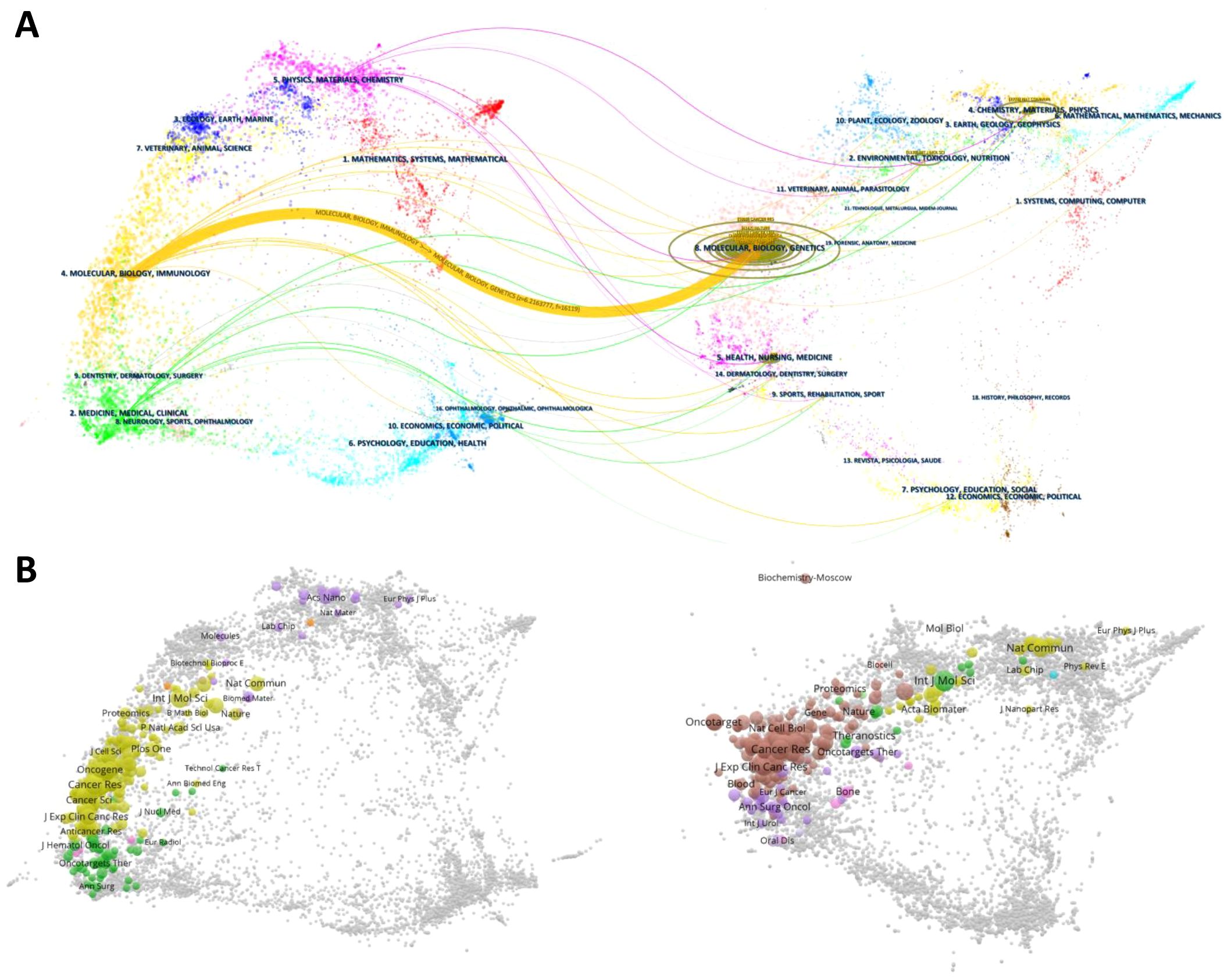
Figure 6. (A) presents a double-map overlay of citing and cited journals for the 1,303 PMN-related articles generated by CiteSpace (version 6.3.R3). This visualization maps citation relationships across related fields. The left side represents citing journals, which reflect applied research in PMN, while the right side represents cited journals, serving as the research foundation. Lines connecting the maps indicate citation paths across disciplines, with different colors representing subject areas and thicker lines denoting stronger links. (B) displays the overlay of citing and cited journals, where the dual maps can explore specific journal relationships and connections.
3.6 Cited and co-cited references
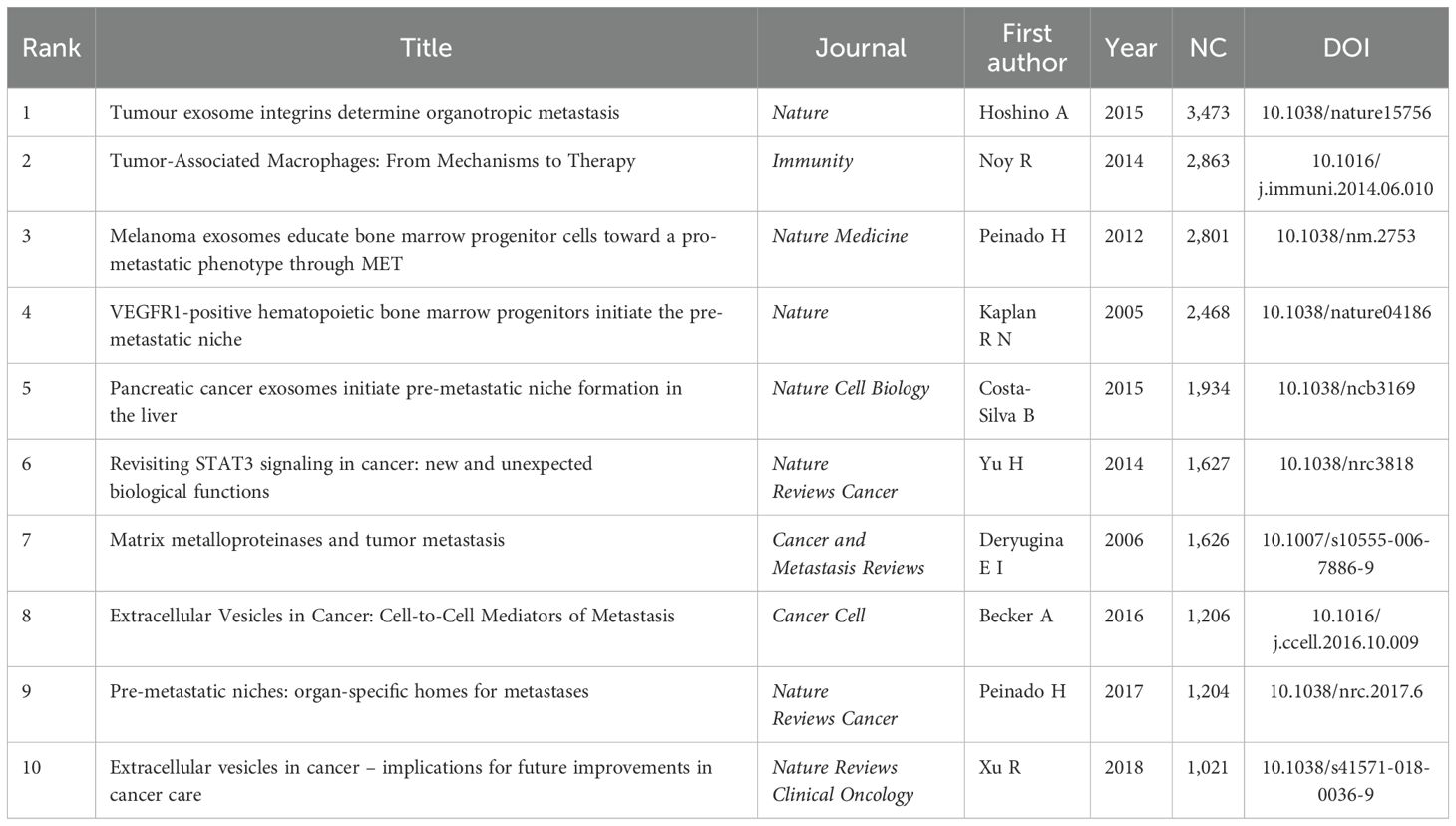
The impact of an article in the PMN field is often reflected by its citation count. Table 5 and Figure 7A highlight the top 10 most cited articles, with two published in Nature and 5 in Nature-affiliated journals. The most frequently cited article was “Tumor exosome integrins determine organotropic metastasis” (Ayuko Hoshino et al., 2015), with 3,473 citations. This groundbreaking study demonstrated how tumor exosome integrins establish PMN by interacting with organ-specific cells, paving the way for diagnostic and therapeutic innovations in organotropic metastasis (17).
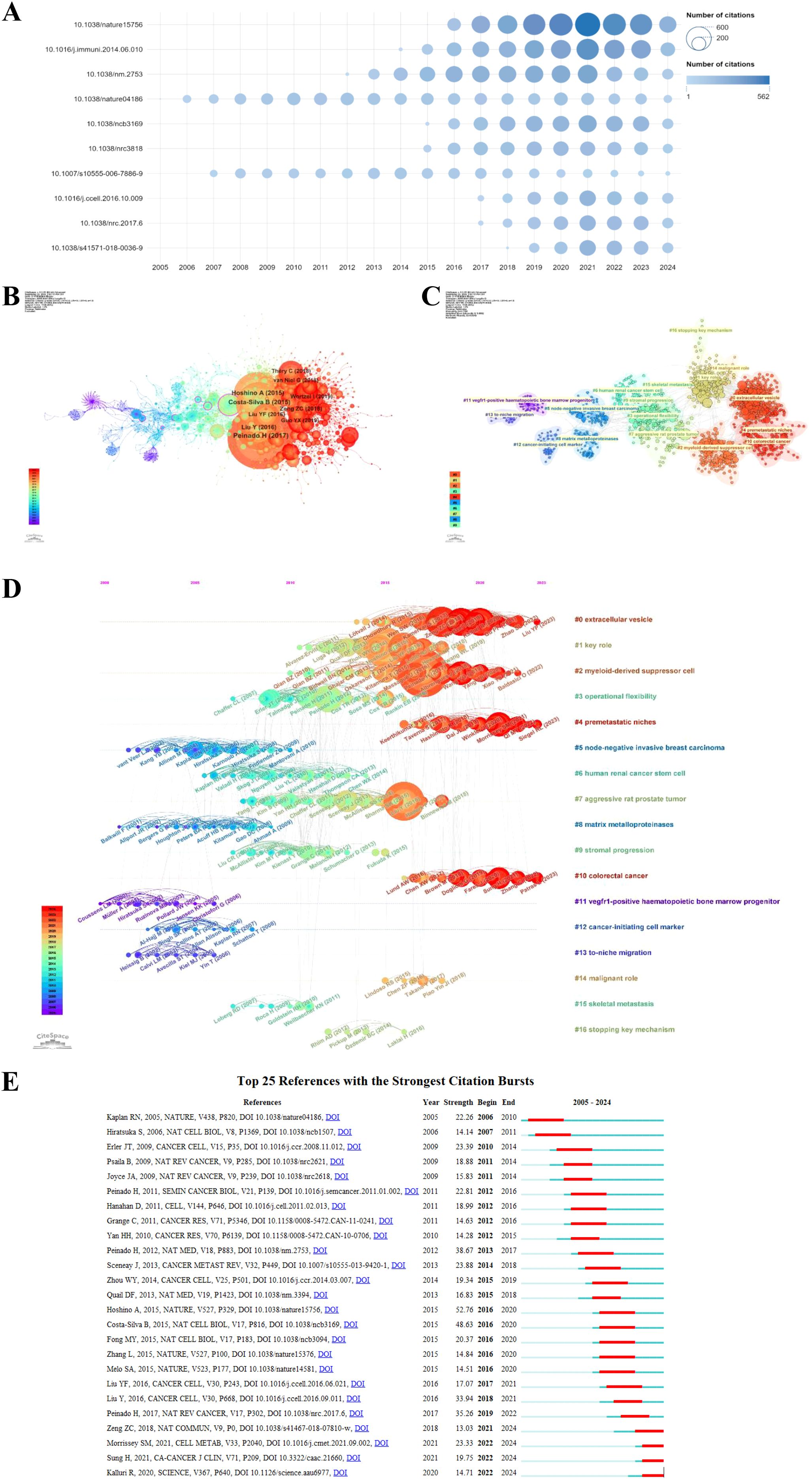
Figure 7. The top 10 most cited articles in the PMN field are shown in (A), with bubble size representing citation frequency and colors ranging from light blue to dark blue to indicate increasing citation counts. DOI numbers replace article titles for clarity. (B) illustrates the co-citation network map of the top 10 references, generated using CiteSpace (version 6.3.R3), where node size corresponds to the number of co-citations, and labels include the first author and publication year. (C) presents the clustering network map of references, with clusters labeled using reference keywords and numbered ascendingly, such that lower numbers indicate clusters with more studies. (D) displays the timeline view of co-cited references, with nodes arranged by publication year; older references are on the left, and newer ones on the right. Nodes aligned horizontally represent clusters, with the duration of each cluster indicated by the length of its horizontal line. (E) highlights the top 25 references with the strongest citation bursts, where blue segments represent time intervals, and red segments indicate active periods of citation bursts, showcasing rapidly growing research areas in the PMN field.
Using a scaling factor of k = 25, we retrieved 1,303 articles with the g-index and identified 1,180 cited references. Co-citation and cluster analysis revealed core knowledge and evolutionary hotspots in the PMN field. Supplementary Table 2 lists the top 10 co-cited references, with the co-citation network visualized in Figure 7B. The most co-cited reference, “Pre-metastatic niches: organ-specific homes for metastases” (Hector Peinado et al., 2017), summarized key mechanisms of PMN formation and its novel processes.
The co-citation analysis identified 17 clusters, with modularity Q and mean profile S above 0.75, indicating strong clustering effects (Figure 7C). The timeline view (Figure 7D) illustrates the evolution of research hotspots over time. Early clusters, such as #5 (node-negative invasive breast carcinoma), #8 (matrix metalloproteinases), #11 (vegfr1-positive hematopoietic bone marrow progenitor), #12 (cancer-initiating cell marker), and #13 (to-niche migration), represent initial research topics in the PMN field. However, these hotspots have since shifted. Current clusters, such as #0 (extracellular vesicle), #2 (myeloid-derived suppressor cell), #4 (PMN), and #10 (colorectal cancer), reflect emerging areas of interest. Additionally, the research now encompasses key topics linked to other cancers, including breast cancer, pancreatic cancer, and hepatocellular carcinoma, highlighting the growing scope of PMN-related studies.
Citation burst analysis provides insight into rapidly growing research areas. The top 25 references with the highest citation bursts are presented in Figure 7E. The earliest burst, attributed to Rosandra N Kaplan et al.’s 2005 study, proposed the concept of PMN, demonstrating that bone marrow-derived hematopoietic progenitor cells create favorable microenvironments for tumor cells. This seminal work sparked interest in PMN interventions to prevent metastasis. The strongest citation burst (strength = 52.76) was observed in Ayuko Hoshino et al.’s 2015 study in Nature, which lasted until 2020. Notably, 2016 had the highest number of new citation bursts (5), followed by 2012 (4), indicating that high-burst papers drove significant research activity during these years. The most recent citation burst, observed in 2022 and still ongoing, is driven primarily by three influential articles: Samantha M Morrissey et al. (2021) in Cell Metabolism (18), Hyuna Sung et al. (2021) in CA: a Cancer Journal for Clinicians (19), and Raghu Kalluri et al. (2020) in Science (20). These results underscore the dynamic and evolving nature of research in the PMN field.
3.7 Keywords analysis of research hotspots
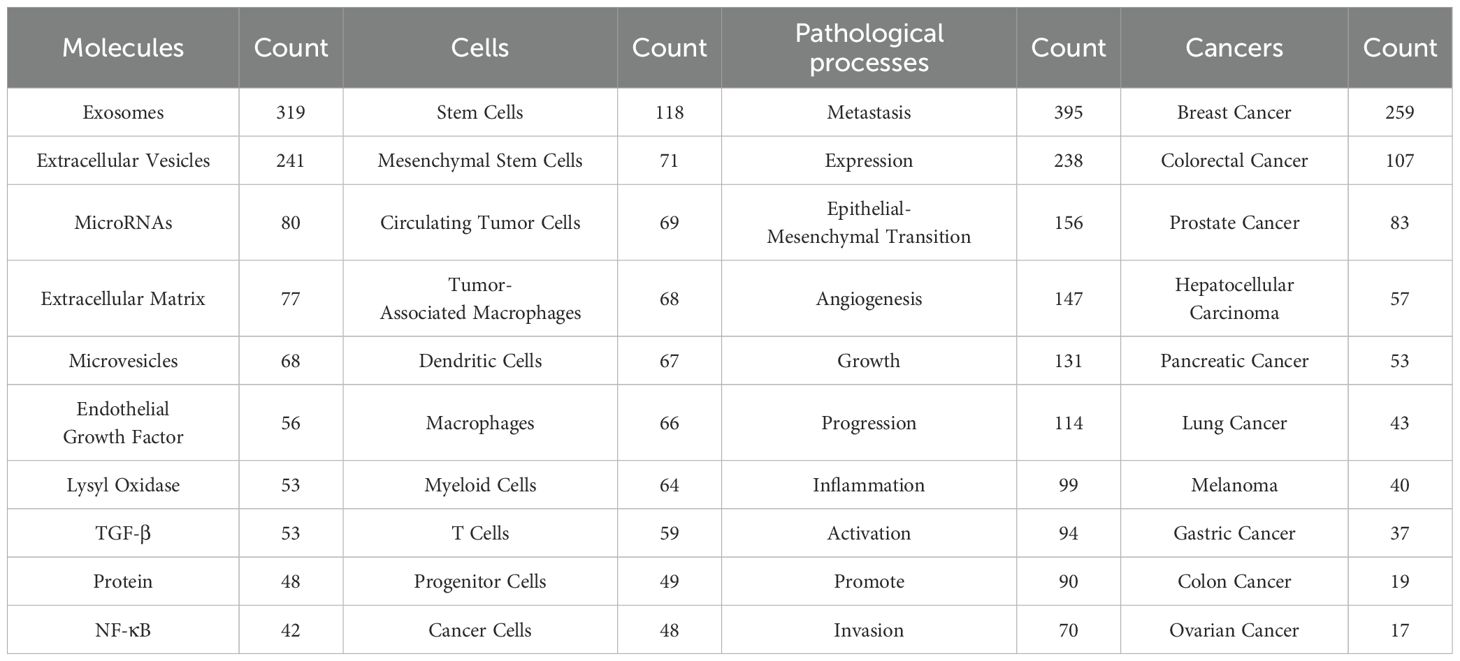
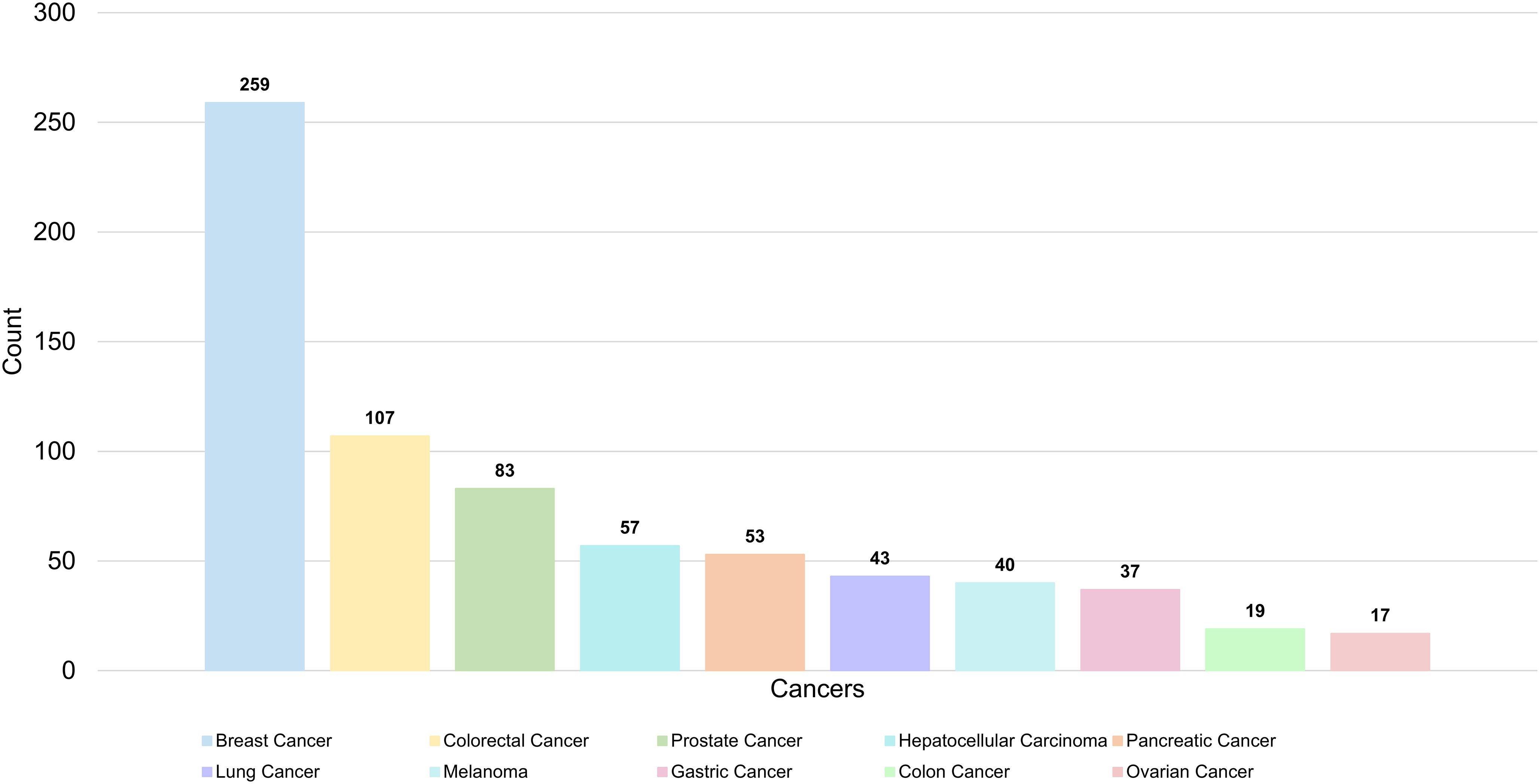
A total of 336 keywords were identified from 1,303 articles in the PMN field. The most commonly used molecular keywords included exosomes (N = 319), EVs (N = 241), and microRNAs (N = 80), while stem cells (N = 118), mesenchymal stem cells (N = 71), and CTCs (N = 71) were the most frequently used cellular keywords. Pathological processes frequently referenced included metastasis (N = 395), expression (N = 238), and epithelial-mesenchymal transition (N = 156). Among cancers, the most studied were breast cancer (N = 259), colorectal cancer (N = 107), and prostate cancer (N = 83) (Table 6, Figure 8).
We performed a cluster analysis of keywords to explore the distribution of topics within the PMN research field. Figure 9A shows a keyword network map revealing three distinct clusters. The central red cluster focuses on the study of PMN and organotropic metastases, their relationship with pathological processes in the microenvironment, and the role of associated cells, including bone metastasis, lung metastasis, brain metastasis, growth, progression, inflammation, immunosuppression, circulating tumor cells, and immune cells (e.g., macrophages, neutrophils, T cells, dendritic cells). The green cluster highlights PMN associations with specific diseases, pathological processes of tumor metastasis, and related molecular proteins, including breast cancer, colorectal cancer, pancreatic cancer, metastasis, tumor microenvironment, epithelial-mesenchymal transition, transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), and nuclear factor kappa-B (NF-κB). The blue cluster centers on EVs and their connection to PMN, along with their diagnostic, therapeutic, and prognostic roles in tumor metastasis, encompassing terms like exosomes, microvesicles, intercellular transfer, communication, microRNAs, biomarkers, liquid biopsy, proteomic analysis, and delivery.
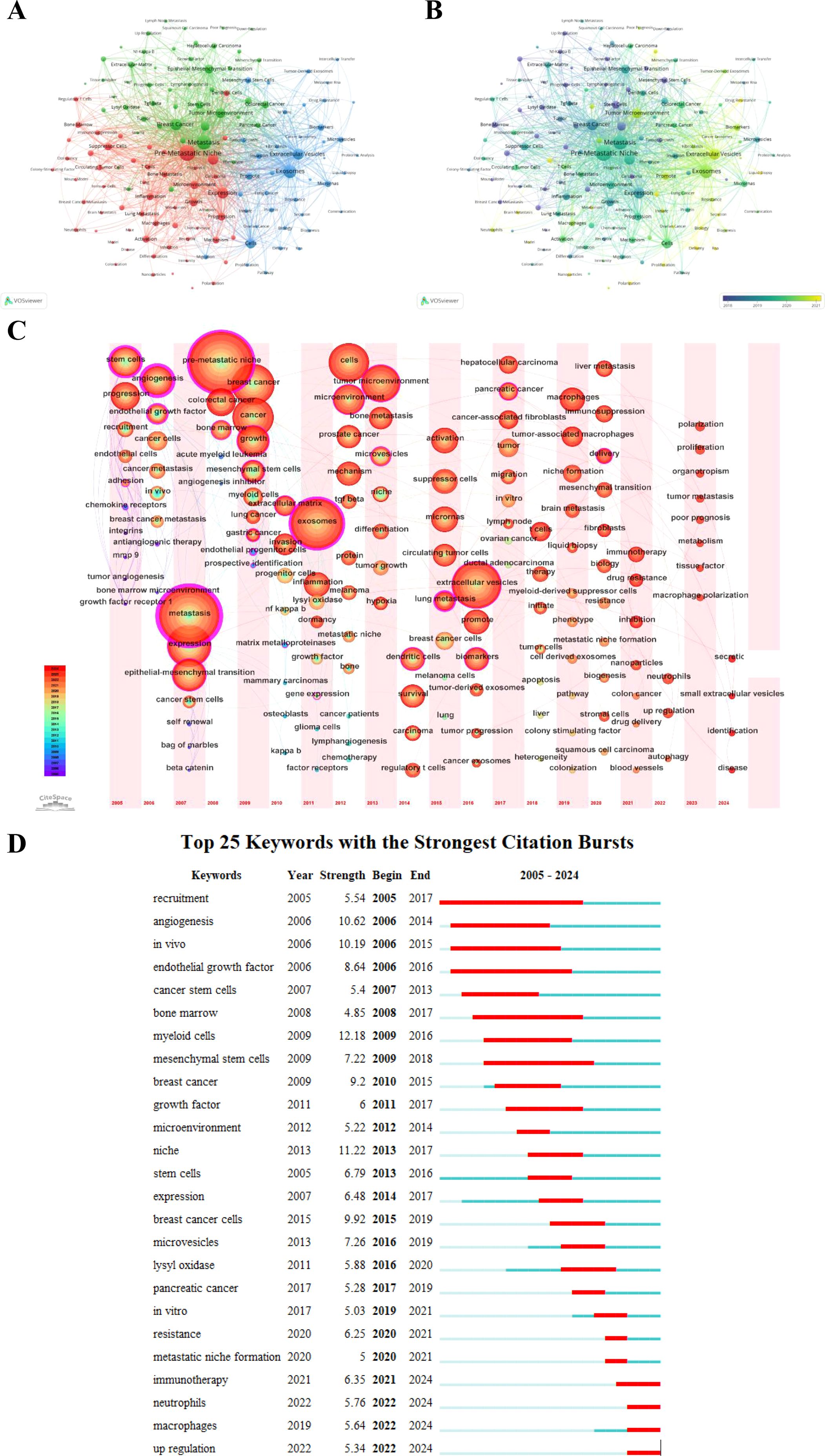
Figure 9. (A) shows the network visualization map of keywords generated by VOSviewer (version 1.6.20), highlighting clusters of interconnected terms that represent distinct research areas. (B) illustrates the evolution of keywords over time, where nodes are colored based on their average appearance year, with earlier terms in purple and more recent ones in yellow. (C) provides a time zone view of keywords generated by CiteSpace (version 6.3.R3), displaying the chronological emergence of key terms and their contribution to research evolution. (D) highlights the top 25 keywords with the strongest citation bursts, where blue line segments represent time intervals, and red segments indicate periods of heightened activity. These bursts reflect rapidly growing interest in specific topics and emerging hotspots in PMN research.
We mapped keywords to a timeline using VOSviewer (version 1.6.20) and CiteSpace (version 6.3.R3), resulting in two time-varying keyword visualization maps. Figure 9B illustrates the evolution of PMN research clusters over time. Purple nodes represent keywords that first emerged, on average, before 2018, while yellow nodes indicate keywords that have gained prominence in recent years, particularly after 2021. Figure 9C, a keyword timezone map, shows the chronological progression of research hotspots. Between 2005 and 2024, research trends have shifted from stem cell and bone marrow progenitor cell studies and TDSFs to EVs and immunotherapy, reflecting the dynamic evolution of the field.
Citation bursts provide another perspective on academic hotspots and emerging research trends. Figure 9D displays the top 25 keywords with the highest citation burst intensities. Notable examples include “myeloid cells” (12.18), “niche” (11.22), “angiogenesis” (10.62), “in vivo” (10.19), and “breast cancer cells” (9.92). Recent bursts observed in 2024 for keywords such as immunotherapy, neutrophils, macrophages, and upregulation highlight growing areas of interest that may define future PMN research directions.
4 Discussion
Bibliometric maps and visualizations of 1,303 PMN-related publications extracted from the WOSCC database (2005–2024) were analyzed using more than five advanced scientific tools. This study systematically evaluated the current state of PMN research and identified promising future directions in the field.
4.1 General distribution
This study analyzed 1,303 PMN-related articles published between January 1, 2005, and September 18, 2024, sourced from 1,627 institutions and authored by 7,955 researchers. The data reveal the increasing global attention to PMN research. Since Rosandra N Kaplan et al. introduced the concept of the “pre-metastatic niche” in Nature in 2005, the field has experienced significant growth. By 2023, the NP was approximately five times that of 2013, and the total NC had increased tenfold over the same period, underscoring rapid advancements in this area (Figure 2).
China and the United States were the leading contributors to PMN research. China ranked first in publication output, accounting for 34.1% of the total (444 articles), while the United States ranked second with 29.5% (385 articles). However, the United States led in the NC (48,655) and H-index (95), highlighting the superior quality and impact of its research. Spain’s articles had the highest average citations per article (229.91). The USA-China collaboration was the most prominent, with other countries, including Germany, the United Kingdom, Spain, Italy, and Japan, also playing significant roles in advancing the PMN field and fostering international partnerships.
Institutional contributions reflected these national trends. Among the top ten institutions, five were based in China, three in the United States, and two in Germany. United States institutions, including Cornell University, the University of California System, and Memorial Sloan Kettering Cancer Center, demonstrated the highest citation counts and H-index values. These results underscore the pivotal role of first-class colleges and research institutions in elevating a country’s academic standing and global influence.
Six of the ten most productive scientists were from the United States, two were from Japan, while Spain and Germany contributed one researcher each. David Lyden of Cornell University pioneered the PMN field, introducing the concept in 2005 and consistently producing influential research. His foundational study, “VEGFR1-positive hematopoietic bone marrow progenitors initiate the pre-metastatic niche,” remains a cornerstone of PMN research. For nearly 20 years, his research has driven key advancements and breakthroughs in the PMN field, serving as a foundational reference for other scientists. Other prominent contributors include Hector Peinado from the Spanish National Cancer Research Center, Lonnie D Shea from the University of Michigan, and Rosandra N Kaplan from the National Institutes of Health. These researchers, along with their extensive network of collaborators, have significantly shaped the PMN research landscape (Figure 5).
These individuals and their groups were more likely to publish impactful research on PMN. While China ranked first in the NP and demonstrated extensive international collaboration, the data suggest a relative lack of high-level scholars and research teams in comparison to other leading countries.
Key journals such as Cancers, International Journal of Molecular Sciences, Frontiers in Oncology, Cancer Research, and Frontiers in Immunology have been instrumental in disseminating PMN-related findings. Cancers published the highest number of PMN-related articles, while Cancer Research had the highest total citations and co-citations, indicating that these journals are well-positioned to continue publishing significant discoveries in PMN research. High-impact journals such as Nature, Nature Reviews Cancer, Cancer Cell, and Cell (all JCR Q1) further underscore the PMN field’s significance in tumor metastasis research (Supplementary Table 1).
The dual-map overlay analysis (Figure 6) highlights that PMN field studies are primarily published in molecular biology, immunology, and clinical medicine journals. Advances in foundational sciences (such as molecular biology, genetics, chemistry, materials, physics, etc.) continue to provide critical support for understanding PMN mechanisms and translating findings into clinical applications. The multidisciplinary collaboration between these fields is poised to accelerate research progress and deliver impactful patient outcomes.
4.2 Hotspots and frontiers
Co-citation analysis of references and keyword co-occurrence networks provide a comprehensive overview of the structure of PMN research. Tools like timeline views of cited references and timezone maps of publication keywords illustrate the dynamic evolution of research hotspots in the PMN field. This analysis reveals key focus areas, including metastatic organotropism, extracellular vesicles, innate immunocytes (e.g., macrophages and neutrophils), and immunotherapy. These insights highlight current research priorities and emerging academic frontiers. The following subsections delve deeper into these areas, exploring their significance for the field and their potential to shape future research.
4.2.1 Metastatic organotropism
The ability of certain tumors to spread and invade specific distant organs, including the liver, lung, bone, and brain, is known as metastatic organotropism (21). Metastatic organotropism is among PMN’s defining characteristics (10). Studies have shown that PMN composition in particular organs significantly influences organ-specific metastatic spread and determines cancer cell organotropism (6, 22). Bone, lung, liver, brain, and lymph node metastases are the most common organotropic metastases in PMN research, as shown in Table 6 and Figure 9. PMN characteristics are shared across different organs; however, unique cell types and microenvironments result in some organ-specific PMN traits. For example, bone metastasis is common in patients with lung, breast, and prostate cancer; the formation of bone PMNs is driven by osteoclast and osteoblast activation, along with angiogenesis, to provide a growth-permissive environment for CTCs (23). Lung metastasis frequently occurs in patients with breast cancer, upper gastrointestinal cancer, and renal cell carcinoma; the formation of lung PMNs recruits bone marrow-derived cells to support inflammation and immunosuppression, and this state is exacerbated by reprogrammed lung fibroblasts, alveolar epithelial cells, and macrophages (24). Liver metastasis is commonly seen in patients with pancreatic, colorectal, and gastric cancers, among others. Liver PMNs show the influx of bone marrow-derived cells, fibrosis, and immunosuppression in which hepatic stellate and Kupffer cells play an essential role (25). Brain metastasis is common in patients with lung cancer, breast cancer, and melanoma. Brain PMNs have higher blood-brain barrier permeability and local immunosuppression, and are rich in neurons and glial cells, providing a suitable environment for the growth of CTCs (26). Lymph node PMNs exhibit lymphangiogenesis and remodeling of high endothelial venules, facilitating tumor dissemination through the lymphatic system (27).
The connection between PMN and metastatic organotropism is attracting increasing attention. Early studies mainly focused on how TDSFs influence adhesion molecules and ECM in distal organs to promote organotropic metastasis (28, 29). More recent researches, however, have identified tumor-derived EVs as pivotal factors in determining metastatic organotropism (30–33).
Exosomes, a subset of EVs approximately 30–150 nm in diameter (34), play a critical role in this process. Ayuko Hoshino et al. were the first to demonstrate the decisive role of tumor exosome integrins (ITGs) in organotropic metastasis (17). Their findings showed that the specific fusion of organ-resident cells with tumor exosome ITGs activates Src phosphorylation and upregulates pro-inflammatory S100 expression, promoting PMN formation and organ-specific metastasis. Notably, specific associations have been identified: exosomal ITGs α6β4 and α6β1 predominantly target lung tissues, facilitating lung metastases, while ITGs αvβ5 demonstrate a similar role in liver metastases. The relationship between exosomal ITGs and metastases in lymph nodes (35), bone (36), and brain (37) has also been reported. Another critical facet of metastatic organotropism is that tumor-derived EVs are taken up by specific cell types in individual organs, creating a microenvironment conducive to metastasis (38). For example, fibroblasts (39) and epithelial cells (40) in the lung internalize exosomes from lung-tropic tumor cells, Kupffer cells (32) in the liver internalize exosomes from liver-tropic tumors, and microglia and brain endothelial cells take up exosomes from brain-tropic tumors (41).
The VEGF, TGF-β, and C-X-C chemokine ligand 12 (CXCL12)/C-X-C chemokine receptor type 4 (CXCR4) signaling pathways are pivotal in PMN formation and organotropic metastasis. The VEGF signaling primarily regulates vascular endothelial cell proliferation and migration, promotes angiogenesis in PMNs, increases vascular permeability, and facilitates CTC invasion and metastasis. Wei Mu et al. demonstrated that benzo[a]pyrene exposure induces hepatocellular carcinoma exosome-derived circular RNA, which activates VEGF expression in lung fibroblasts, promoting lung metastasis (42). The TGF-β pathway controls critical cellular processes such as migration, invasion, ECM remodeling, and immunosuppression. Studies by Anyi Liu et al. (43), Fangting Liu et al. (44), and Xing-Ning Lai et al. (45) indicate that aberrant TGF-β activation enhances tissue-specific metastasis by upregulating organ-specific metastasis-related genes. The CXCL12-CXCR4 axis is a critical mediator of metastasis, recruiting CTCs and immune cells within the PMN (46). Zhen Wang et al. reported that CD62Ldim neutrophils aggregate in the pre-metastatic lung microenvironment via the CXCL12-CXCR4 pathway, promoting lung metastasis in breast cancer (47).
Targeting these PMN-promoting pathways can inhibit PMN formation and reduce organotropic metastasis risk. The TGF-β inhibitors, such as monoclonal antibodies or soluble TGF-β receptors, block TGF-β signaling (48), and have shown promising results in preclinical and early clinical trials (49). The VEGF inhibitors, including anti-VEGF drugs such as bevacizumab and sunitinib, and VEGF receptor (VEGFR) inhibitors such as sorafenib, suppress tumor angiogenesis, growth, and PMN formation by blocking VEGF-A binding to VEGFR and subsequent VEGF signal transduction (50). NOX-A12, a polyethylene glycolated mirror oligonucleotide that binds CXCL12 to inhibit its interaction with CXCR4. Meggy Suarez-Carmona et al. reported its high safety and tolerability in patients with advanced metastatic colorectal and pancreatic cancer (51). The CXCR4-targeting therapies include non-peptide antagonists (such as AMD3100) and peptide-based antagonists (such as PL-Peptide R) (52). AMD3100 (Plerixafor) is the only FDA-approved CXCR4 antagonist. Jian Jiang et al. demonstrated that combining AMD3100 with combretastatin A4 nanodrug, a neovascularization disruptor, significantly inhibited tumor growth and lung metastasis in mice with breast cancer (53). Moreover, Caterina Ieranò et al. developed PL-Peptide R, a peptide R-modified stealth liposome, which inhibited CXCR4-dependent migration in vitro, reduced lung metastasis, and improved survival in melanoma-affected mice (54).
Despite these advancements, several key questions remain, including the impact of tumor heterogeneity on organ-specific PMN formation, the regulatory mechanisms of ECM remodeling in PMN, and the influence of PMN evolution over time on organotropic metastasis. Further exploration of these areas is essential. Emerging advances in science and technology have greatly facilitated the systematic study of PMN’s key biological characteristics and organotropism. First, developments in materials technology have enabled researchers to biologically engineer 3D models mimicking the complexity of tissues (55–57). For instance, Ryan A Carpenter et al. seeded human bone marrow stromal cells onto a hydrogel scaffold, which was then implanted subcutaneously in mice to induce vascularization and a pro-inflammatory microenvironment, forming a tissue-engineered PMN that subsequently recruited CTCs from orthotopic prostate tumor xenografts (58). These bioengineered PMNs have significantly advanced our understanding of PMN development and metastatic organotropism, while also facilitating the testing of anti-tumor therapies in high-throughput settings (59). Second, the advent of spatial transcriptomics, single-cell sequencing, advanced imaging techniques, and artificial intelligence has enabled researchers to analyze PMN components comprehensively and predict metastatic organotropism (60). For example, Joseph J Zhao et al. revealed spatially resolved ecological niches and altered tumor microenvironments associated with peritoneal metastasis in gastric cancer (61). Linda Bojmar et al. developed a pre-metastatic liver multiparameter profile capable of predicting early metastasis outcomes after pancreatic cancer surgery with an accuracy of 78% (8). Third, it is important to highlight that microphysiological systems (MPS) have emerged as powerful tools in organotropic metastasis research. These systems encompass 3D cell co-culture platforms and organ-on-a-chip models that integrate microfluidics, biology, and engineering to replicate complex organ-specific microenvironments in vitro (62). The MPS enables real-time monitoring, morphological analysis, and protein quantification, enhancing our understanding of tumor migration, angiogenesis, and PMN formation (63).
4.2.2 Extracellular vesicles
EVs are heterogeneous lipid bilayer membrane structures secreted by cells (64) and are commonly found in human body fluids such as blood, urine, and saliva. Based on their size and biogenic mechanism, EVs can be classified into exosomes, microvesicles, apoptotic bodies, and oncosomes (64, 65). Over the past decade, evidence has increasingly pointed to EVs as essential mediators of cancer progression and metastasis through cell-cell communication (66–68). Tumor-derived EVs (TDEs) are the primary focus in PMN research, as their cargo—comprising proteins, mRNAs, miRNAs, small RNAs, DNA fragments, lipids, and metabolites—has been shown to alter the host microenvironment and promote PMN formation, driving organ-specific metastasis (13, 25, 69, 70).
TDEs mediate PMN formation through various mechanisms, including promoting inflammation (71, 72), increasing angiogenesis and vascular permeability (73, 74), stimulating lymphangiogenesis (75), reprogramming stromal cells (76, 77), remodeling the ECM (78, 79), inducing immunosuppression (9, 80), and altering the metabolic milieu (81, 82). Recognizing their pivotal role, researchers have developed targeted strategies to block TDE-mediated metastasis, including inhibiting TDEs secretion (83), removing or destroying circulating TDEs (84), blocking TDEs interactions with recipient cells (85), and using TDEs as biomolecule or drug delivery tools to inhibit metastasis (86). For example, Niamh McNamee et al. demonstrated that calpeptin, Y27632, manumycin A, GW4869, and their combinations inhibited EV release from triple-negative breast cancer cells in in vitro studies (87). Eun-Ju Im et al. identified the oral antibiotic sulfisoxazole as an EV secretion inhibitor in breast cancer cells through screening and validation in animal models (88). Qunfei Tai et al. developed an antibody-functionalized capillary device for in situ exosome capture and quantification, successfully trapping Michigan Cancer Foundation-7 (MCF-7)-derived exosomes and inhibiting epithelial-mesenchymal transition in MCF-10A epithelial cells in vitro (89). Emma Rigg et al. reported that miR-146a-5p was highly expressed in brain tissue of patients with melanoma brain metastases and in melanoma cell-derived EVs, where it stimulated pro-tumorigenic cytokine secretion by cerebral astrocytes, promoting tumor progression. Deserpidine suppressed miR-146a-5p expression in EVs in both in vivo and in vitro experiments, reducing the incidence of melanoma brain metastasis (90). Moreover, as natural carriers, exosomes can be engineered as drug delivery vehicles, transporting exogenous RNAs, such as small interfering RNAs (siRNAs) and microRNAs, to target tissues or cells in vivo, modulating gene expression and inhibiting tumor metastasis (91). Liuwan Zhao et al. demonstrated in animal models that exosome membrane-encapsulated biomimetic nanoparticles (CBSA/siS100A4@Exosome) effectively delivered therapeutic siS100A4 to the lungs of mice, inhibiting lung metastasis post-breast cancer surgery (92). Sushrut Kamerkar et al. at The University of Texas MD Anderson Cancer Center engineered exosomes carrying siRNA targeting the KRASG12D oncogene, significantly suppressing pancreatic cancer progression and metastasis, thereby improving survival in mice (93). Based on these findings, MD Anderson Cancer Center is conducting a Phase I clinical trial (NCT03608631) to evaluate the safety and efficacy of EVs loaded with KRASG12D-targeted siRNA in patients with metastatic pancreatic cancer.
However, clinical applications of TDE-targeted therapies still face challenges such as potential interference with normal cell functions, technical difficulties in isolating circulating TDEs, the possibility of immune responses triggered by TDEs destruction, and the need for large-scale production of stable, bioavailable TDEs carriers (94). Despite these obstacles, researchers agree that targeting TDEs is a promising avenue for inhibiting PMN formation and tumor metastasis (95).
In parallel, advances in liquid biopsy technology have highlighted TDEs as biomarkers for early detection, metastasis prediction, and tumor prognosis (96). Liquid biopsy leverages humoral fluids to detect tumor-related information, predict treatment responses, and guide the selection of personalized treatment options (97). Current biomarker research focuses on miRNAs and proteins within TDEs (70). For example, studies have shown that upregulated miR-223-3p, miR-92a-3p, miR-21-5p, and miR-342-3p in exosomes from gastric cancer ascites are associated with peritoneal metastasis (98). Similarly, high expression of cadherin 11 and integrin α5 in blood exosomes from breast cancer models correlated with bone metastasis (99). Lymph node metastasis is a strong prognostic indicator of poor outcomes in intrahepatic cholangiocarcinoma. A Phase I clinical trial (NCT06381648), initiated by Kyushu University and other universities in Japan, is actively recruiting patients to evaluate the use of exosomal biomarkers in liquid biopsy for preoperative detection of lymph node metastasis, aiding in treatment decision-making.
However, the lack of robust methods for isolating and analyzing TDE components limits further clinical application of liquid biopsy (100). As detection techniques improve and our understanding of TDE biology deepens, liquid biopsy is expected to play an increasingly significant role in PMN and metastasis research.
4.2.3 Innate immunocytes
The coexistence of inflammation and immunosuppression is a hallmark of PMN and a major driver of cancer progression and metastasis (10). Innate immunocytes play a crucial role in mediating PMN-related immunosuppression and inflammation (9). Among them, macrophages and neutrophils have garnered significant research attention in recent years due to their pivotal functions in tumor metastasis.
4.2.3.1 Macrophages
Macrophages are mononuclear phagocytic cells derived from monocytes (101). In PMN, the macrophage population includes bone marrow-derived macrophages (BMDMs) and tissue-resident macrophages, such as liver Kupffer cells, lung interstitial and alveolar macrophages, and brain microglia (102). TDSFs and TDEs secreted by primary tumors recruit BMDMs to future metastatic organs. These factors foster an inflammatory microenvironment, inducing the accumulation and proliferation of additional BMDMs (103, 104).
Furthermore, the macrophage phenotype is regulated by TDSFs and TDEs. Macrophages exhibit different functional subsets, including anti-tumor (M1 phenotype) and pro-tumor (M2 phenotype) subsets, which exert opposing effects (105). Current research focuses on the regulation of tissue-resident macrophages phenotypes and their roles in PMN formation and metastasis. For instance, Samantha M Morrissey et al. demonstrated that exosomes shed by lung cancer cells polarize alveolar interstitial macrophages into an immunosuppressive phenotype via NF-κB-dependent glycolysis-dominant metabolic reprogramming pathways. This process upregulates programmed cell death ligand 1 (PD-L1) expression and excretes large amounts of lactic acid, thereby creating an immunosuppressive niche that promotes tumor metastasis (18). Similarly, Hao Sun et al. observed that exosomes with high expression of miR-135a-5p are phagocytized by liver Kupffer cells. These exosomes promote the production of matrix metalloproteinase 7 and related signaling, decrease CD4+ T cells in the liver, and facilitate hepatic PMN formation (106). In summary, macrophages are influenced by their local microenvironment and exhibit remarkable functional plasticity. By fostering tumor-associated inflammation, remodeling the extracellular matrix, and inducing immunosuppression, they play pivotal roles in PMN formation and ultimately contribute to metastasis.
4.2.3.2 Neutrophils
Neutrophils are the predominant component of peripheral white blood cells and are closely associated with inflammation and immune function (107). Advances in high-throughput sequencing have revealed the significant accumulation and critical functions of neutrophils in PMN. Neutrophil heterogeneity and neutrophil extracellular traps (NETs) are emerging as key research areas in the PMN field.
Neutrophils in PMN exhibit two phenotypic states, N1 (anti-tumor) and N2 (pro-tumor), influenced by the complex surrounding microenvironment (108, 109). Studies by Yanfang Liu et al. and Ching-Fang Wu et al. revealed that the lack of type I interferon in PMN, combined with abundant granulocyte colony-stimulating factor (G-CSF), IL-6, and IL-10 secretion by tumors, promotes N2 polarization (40, 110). These N2 neutrophils contribute to oxidative stress at the site of inflammation through the secretion of cytokines, such as tumor necrosis factor α, shaping the PMN’s inflammatory response, activating endothelial and stromal cells, and increasing circulating tumor cell adhesion (111, 112). Furthermore, N2 neutrophils suppress immune responses by mechanisms such as secreting type 1 arginase (ARG1) to deplete arginine, impairing T cell function, as demonstrated by Huajia Zhang et al. (113), and increasing PD-L1 expression under GM-CSF stimulation to inhibit T cell activation (114).
NETs, reticular structures composed of DNA, histones, and granular proteins, are released by neutrophils in response to stimuli (115). Evidence strongly links NETs to metastasis (116). Inflammatory factors like IL-8 and high mobility group box protein 1 in PMN, along with tumor-derived G-CSF, promote NETs formation (117). Mechanistic studies show that NETs contribute to metastasis in two main ways. First, WonJae Lee et al., Luyu Yang et al., and Álvaro Teijeira et al. demonstrated that NETs attract and capture CTCs through β-integrin-mediated adhesion and shield them from immune attack by cytolytic T lymphocytes and natural killer cells, facilitating colonization in PMN (118–120). Second, NET components indirectly influence tumor cell metastasis. Linbin Yang et al. found that NET DNA binds to transmembrane protein CCDC25 on breast cancer cells, enhancing motility (121). Additionally, Hamza O Yazdani et al. showed that neutrophil elastase activates the toll-like receptor-4 signaling pathway in colon cancer cells, promoting mitochondrial biogenesis and tumor growth (122). PD-L1 on NETs also contributes to immune evasion by depleting and impairing CD4+ and CD8+ T cells, as observed by Christof Kaltenmeier et al. (123).
4.2.4 Immunotherapy
Immune checkpoint inhibitors (ICIs) are among the most successful types of immunotherapies and represent a prominent treatment for metastatic cancer. They target T cell checkpoints such as PD-L1, programmed cell death protein 1, and cytotoxic T lymphocyte antigen 4. However, the therapeutic success of ICIs has been limited to cancers with high mutation rates and is further hampered by the emergence of treatment resistance (124). Currently, no tailored therapies exist for preventing PMN formation, but innovative immune-based approaches are undergoing investigation and validation (9, 125).
The rapid development of advanced technologies, including single-cell sequencing, liquid biopsy, spatiotemporal omics, nanotechnology, and artificial intelligence, provides scientists with deeper insights into the immune landscape of PMN. These technologies facilitate the identification of immune determinants that drive the temporal and spatial evolution of PMN (9, 126–128). In the near future, these advancements are anticipated to inspire the development of next-generation immunotherapies that target critical immune cells involved in PMN formation. Such therapies aim to disrupt immunosuppressive environments within PMN and activate early anti-tumor immune responses, offering more effective strategies to combat metastasis (125).
4.2.4.1 Targeting innate immunocytes
Blocking the recruitment of innate immunocytes like macrophages, neutrophils, and myeloid-derived suppressor cells (MDSCs) to PMN, inducing their depletion, or altering their immunosuppressive activity by reprogramming are currently common targeting strategies (129). Research to block macrophage recruitment is focused on inhibiting the cyclooxygenase-1/thromboxane A2 (130) and colony-stimulating factor-1/colony-stimulating factor-1 receptor (131) pathways and targeting the CXCL12/CXCR4 (132) and angiopoietin-2/TIE2 (133) axes. Methods to induce macrophage depletion include the use of liposomal clodronate (134) and trabectedin (135). CD40 agonists in combination with CSF-1R-targeting drugs (136), selective class IIa histone deacetylase inhibitors (137), and toll-like receptor agonists (138) can reprogram macrophages to enhance anti-tumor immunity.
Regarding neutrophil-targeted therapies, Zhiyuan Zheng et al. demonstrated that complement component C3 promotes neutrophil recruitment and NET formation, and that targeting the Th2 cell cytokine-STAT6-C3-NET axis can block neutrophil recruitment and NET formation in PMN (139). Other strategies to inhibit NET formation include blocking IL-17 (140), targeting tissue C5a receptor-1 and eliminating C5a (141), and using PAD4 drug inhibitors (118). Furthermore, Abhishek Tyagi et al. revealed that low concentrations of salidroside can inhibit N2 phenotype polarization of neutrophils in PMN and promote N1 phenotype polarization (142).
MDSCs, immature myeloid cells derived from the bone marrow, have immunosuppressive functions and are involved in multiple mechanisms of PMN generation. They represent a recent hotspot in immunotherapy research (143). Studies have shown that CXCR2 antagonists (144) and C‐C motif chemokine 2 or C‐C motif chemokine 2 receptor inhibitors (145, 146) can block MDSCs recruitment to PMN, and their combination with ICIs can improve the efficacy of immunotherapy. Research by Zhihao Lu et al. demonstrated that low-dose adjuvant epigenetic therapy can block MDSCs recruitment and promote their differentiation into a mesenchymal macrophage phenotype, disrupting the generation of postoperative lung PMN (147). Additionally, Traditional Chinese Medicine has shown beneficial effects in inhibiting MDSCs recruitment and tumor metastasis (148, 149).
Weakening the immunosuppressive function of MDSCs is another important approach in immunotherapy. Phosphodiesterase 5 inhibitors, including tadalafil and sildenafil, can downregulate ARG1 and inducible nitric oxide synthase expression, reverse MDSCs-induced immunosuppression, enhance anti-tumor immunity, and reduce the incidence of postoperative metastasis (150, 151).
4.2.4.2 Targeting adaptive immunocytes
The immune surveillance roles of T-cells and B-cells, categorized as adaptive immunocytes, are frequently suppressed by innate immunocytes within PMN. This suppression is driven by TDSFs and EVs, leading to a reduction in cytotoxic and effector T cells and an expansion of regulatory T cells and regulatory B cells, thereby exacerbating PMN immunosuppression and promoting metastasis (152, 153).
Sabina Kaczanowska et al. utilized genetically engineered myeloid cells to deliver IL-12, inducing adaptive immune responses in tumor-bearing mice. Their findings demonstrated antigen presentation and T-cell activation, which reversed pulmonary PMN immunosuppression and significantly reduced lung metastasis incidence (154).
Additionally, the metastatic S100A4 protein facilitates T cell recruitment to the lung PMN, inducing a Th1/Th2 imbalance that shifts toward a pro-tumor Th2 phenotype. In a study involving mice with spontaneous breast cancer, Birgitte Grum-Schwensen et al. reported that the 6B12 antibody effectively blocked S100A4 activity, restored T cell polarization balance, and inhibited lung metastasis (155).
Regarding B cell research, Yan Gu et al. observed that tumor-domesticated B cells aggregate within lymph nodes and secrete pathological antibodies targeting the glycosylated membrane protein HSPA4. This interaction facilitates lymph node PMN formation and promotes breast cancer metastasis by activating the CXCR4/SDF1α axis (156). However, immunotherapy strategies targeting HSPA4 remain in the early stages and require further investigation.
4.2.4.3 Nanotechnology-enhanced immunotherapies
To date, nanotechnology has demonstrated significant potential in enhancing immunotherapy for metastatic cancer, offering promising avenues for disrupting the biological processes underlying tumor metastasis (157). Strategically designed nanomaterials can inhibit metastasis-related immunosuppressive cells, reshape the immune microenvironment of the PMN, and reduce the likelihood of tumor metastasis (158). Several successful attempts have already been reported. For instance, Weiya Zeng et al. developed activated neutrophil membrane-coated nanoparticles, which disrupt PMN recruitment of neutrophils and simultaneously block neutrophil adhesion to CTCs as nano-baits, effectively reducing organ metastases in mice with breast cancer tumors (159). Micellar nanoparticles designed by Yang Long et al. (160) and Zhengze Lu et al. (161, 162), as well as the sponge-like nano-system studied by Chunyu Xia et al. (163), have shown efficacy in inhibiting pulmonary PMN formation by interfering with MDSC recruitment and mitigating MDSC-mediated immunosuppression. These innovations exemplify the potential for nanotechnology to reshape PMN-related immunotherapies. The eventual translation of these technologies into clinical applications is highly anticipated.
Additionally, emerging approaches such as leveraging gut microbiota to enhance immunotherapies (164), vaccine-based anti-tumor strategies (165), and TDEs-based immunotherapies (166) are under investigation, further broadening the horizon of PMN-targeted treatments.
4.3 Limitations
To the best of our knowledge, this study represents the first bibliometric assessment of current research on PMN. However, several limitations should be acknowledged. First, the literature data were exclusively sourced from the WOSCC database, potentially omitting relevant publications that were not indexed within this platform (such as in PubMed and Scopus database). Second, the analysis was limited to English-language literature, which may exclude significant studies published in other languages. Third, both original research articles and reviews were included in our analysis, which may have inflated the publication count for institutes that primarily produce reviews, thereby diluting the impact of original research. Lastly, publications from 2024 were not fully included, as the database was incomplete at the time of the data retrieval (September 18, 2024).
5 Conclusion
Over the past two decades, research in the field of PMN has witnessed a remarkable surge in activity. Significant contributions have been made by scholars from China and the United States, with Professor David Lyden of Cornell University recognized as the founder and pioneer of this field. Cornell University, Sichuan University, and Ruprecht Karls University Heidelberg stand out as the most prolific institutions driving advancements in PMN research. Key journals such as Cancers and Cancer Research have served as pivotal platforms for disseminating findings in this domain.
Furthermore, the prevailing research trends and advancements in the field were critically examined. Several key challenges persist in this discipline. First, the potential for PMNs to continue forming in distant organs after surgical resection of the primary tumor remains unclear. Accurate methods for detecting PMNs and determining which PMNs eventually lead to metastasis are still being explored. Moreover, the role of primary tumor heterogeneity in organ-specific PMN formation requires further investigation, as not all primary tumors may generate PMNs. The heterogeneity of EV cargo presents another challenge, necessitating strategies to precisely target tumor-associated EVs to inhibit PMN formation. Current PMN-related microbiome research primarily focuses on intestinal microorganisms, whereas the role of the tumor microbiome in PMN formation and immunotherapy remains underexplored. Although metabolic reprogramming is increasingly recognized as a key factor in cancer progression and metastasis, research on PMN immunometabolic reprogramming is still limited. A deeper understanding of its role in PMN formation is needed. Despite significant preclinical efforts to target PMNs, the effective translation of these findings into clinical applications remains uncertain. Continued research is necessary to bridge this gap. These challenges are still being addressed in basic and preclinical research, and have yet to be widely implemented in clinical practice. Emerging technologies are essential for advancing PMN research. Innovations in single-cell sequencing, spatial transcriptomics, liquid biopsies, advanced imaging techniques, nanotechnology, and artificial intelligence have significantly enhanced our understanding of the temporal evolution of organotropic metastasis. Integrating MPS-based in vitro models with large-scale omics analyses and in vivo genetic knockout models will be crucial for studying organotropic metastasis. Future research should focus on predicting metastatic risk and site-specific metastasis formation, preventing PMN-driven metastasis, and developing targeted therapeutic strategies.
Moving forward, topics like metastatic organotropism, extracellular vesicles, innate immunocytes (including macrophages and neutrophils), and immunotherapy are anticipated to remain at the forefront of PMN research, guiding the exploration of innovative solutions to combat cancer metastasis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
MC: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. QS: Data curation, Investigation, Methodology, Visualization, Writing – original draft. HD: Data curation, Methodology, Visualization, Writing – original draft. CC: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Young Scientists Fund of National Natural Science Foundation of China (No.81904101), the Young Eagle Program of China Association of Chinese Medicine and Training Project of Clinical Young Talents of Traditional Chinese Medicine (Phase I), training personnel of medical rookies in Zhejiang Province (Office of health commission of Zhejiang province [2022]32), school-level scientific research project of Zhejiang Chinese Medical University (No.2023JKJNTZ06 and 2023JKZKTS35).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1552053/full#supplementary-material
Abbreviations
AC, average citations; ARG1, type 1 arginase; BMDMs, bone marrow-derived macrophages; CTCs, circulating tumor cells; CXCL12, C-X-C chemokine ligand 12; CXCR4, C-X-C chemokine receptor type 4; ECM, extracellular matrix; EVs, extracellular vesicles; G-CSF, granulocyte colony-stimulating factor; ICIs, immune checkpoint inhibitors; IF, impact factor; JCR, journal citation report; MDSCs, myeloid-derived suppressor cells; MCF, Michigan cancer foundation; NC, number of citations; NETs, neutrophil extracellular traps; NF-κB, nuclear factor kappa-B; NP, number of publications; PD-L1, programmed cell death ligand 1; PMN, pre-metastatic niche; siRNAs, small interfering RNAs; TDEs, tumor-derived extracellular vesicles; TDSFs, tumor-derived secreted factors; TGF-β, transforming growth factor-β; TLS, total link strength; TS, topic search; VEGF, vascular endothelial growth factor; WOSCC, Web of Science Core Collection.
References
1. Wan L, Pantel K, and Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nat Med. (2013) 19:1450–64. doi: 10.1038/nm.3391
2. Gerstberger S, Jiang Q, and Ganesh K. Metastasis. Cell. (2023) 186:1564–79. doi: 10.1016/j.cell.2023.03.003
3. Massagué J and Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. (2016) 529:298–306. doi: 10.1038/nature17038
4. Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. (2005) 438:820–7. doi: 10.1038/nature04186
5. Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. (1989) 8:98–101.
6. Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. (2017) 17:302–17. doi: 10.1038/nrc.2017.6
7. Dunbar KJ, Efe G, Cunningham K, Esquea E, Navaridas R, and Rustgi AK. Regulation of metastatic organotropism. Trends Cancer. (2025) 11:216–31. doi: 10.1016/j.trecan.2024.11.012
8. Bojmar L, Zambirinis CP, Hernandez JM, Chakraborty J, Shaashua L, Kim J, et al. Multi-parametric atlas of the pre-metastatic liver for prediction of metastatic outcome in early-stage pancreatic cancer. Nat Med. (2024) 30:2170–80. doi: 10.1038/s41591-024-03075-7
9. Patras L, Shaashua L, Matei I, and Lyden D. Immune determinants of the pre-metastatic niche. Cancer Cell. (2023) 41:546–72. doi: 10.1016/j.ccell.2023.02.018
10. Liu Y and Cao X. Characteristics and significance of the pre-metastatic niche. Cancer Cell. (2016) 30:668–81. doi: 10.1016/j.ccell.2016.09.011
11. Liu Z, Chen J, Ren Y, Liu S, Ba Y, Zuo A, et al. Multi-stage mechanisms of tumor metastasis and therapeutic strategies. Signal Transduct Target Ther. (2024) 9:270. doi: 10.1038/s41392-024-01955-5
12. Wang Y, Jia J, Wang F, Fang Y, Yang Y, Zhou Q, et al. Pre-metastatic niche: formation, characteristics and therapeutic implication. Signal Transduct Target Ther. (2024) 9:236. doi: 10.1038/s41392-024-01937-7
13. Li Y, Zheng Y, Tan X, Du Y, Wei Y, and Liu S. Extracellular vesicle-mediated pre-metastatic niche formation via altering host microenvironments. Front Immunol. (2024) 15:1367373. doi: 10.3389/fimmu.2024.1367373
14. Zhou Y, Han M, and Gao J. Prognosis and targeting of pre-metastatic niche. J Control Release. (2020) 325:223–34. doi: 10.1016/j.jconrel.2020.06.037
15. Hicks D, Wouters P, Waltman L, de Rijcke S, and Rafols I. Bibliometrics: The Leiden Manifesto for research metrics. Nature. (2015) 520:429–31. doi: 10.1038/520429a
16. Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. (2005) 102:16569–72. doi: 10.1073/pnas.0507655102
17. Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. (2015) 527:329–35. doi: 10.1038/nature15756
18. Morrissey SM, Zhang F, Ding C, Montoya-Durango DE, Hu X, Yang C, et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. (2021) 33:2040–58 e10. doi: 10.1016/j.cmet.2021.09.002
19. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
20. Kalluri R and LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. (2020) 367:eaau6977. doi: 10.1126/science.aau6977
21. Gao Y, Bado I, Wang H, Zhang W, Rosen JM, and Zhang XHF. Metastasis organotropism: redefining the congenial soil. Dev Cell. (2019) 49:375–91. doi: 10.1016/j.devcel.2019.04.012
22. Carrolo M, Miranda JAI, Vilhais G, Quintela A, Sousa MFE, Costa DA, et al. Metastatic organotropism: a brief overview. Front Oncol. (2024) 14:1358786. doi: 10.3389/fonc.2024.1358786
23. Yue Z, Niu X, Yuan Z, Qin Q, Jiang W, He L, et al. RSPO2 and RANKL signal through LGR4 to regulate osteoclastic premetastatic niche formation and bone metastasis. J Clin Invest. (2022) 132:e144579. doi: 10.1172/JCI144579
24. Gong Z, Li Q, Shi J, Wei J, Li P, Chang C-H, et al. Lung fibroblasts facilitate pre-metastatic niche formation by remodeling the local immune microenvironment. Immunity. (2022) 55:1483–1500.e9. doi: 10.1016/j.immuni.2022.07.001
25. Dong Q, Liu X, Cheng K, Sheng J, Kong J, and Liu T. Pre-metastatic niche formation in different organs induced by tumor extracellular vesicles. Front Cell Dev Biol. (2021) 9:733627. doi: 10.3389/fcell.2021.733627
26. Geissler M, Jia W, Kiraz EN, Kulacz I, Liu X, Rombach A, et al. The brain pre-metastatic niche: biological and technical advancements. Int J Mol Sci. (2023) 24:236. doi: 10.3390/ijms241210055
27. Gillot L, Baudin L, Rouaud L, Kridelka F, and Noël A. The pre-metastatic niche in lymph nodes: formation and characteristics. Cell Mol Life Sci. (2021) 78:5987–6002. doi: 10.1007/s00018-021-03873-z
28. Liu S, Jiang M, Zhao Q, Li S, Peng Y, Zhang P, et al. Vascular endothelial growth factor plays a critical role in the formation of the pre-metastatic niche via prostaglandin E2. Oncol Rep. (2014) 32:2477–84. doi: 10.3892/or.2014.3516
29. Peinado H, Lavotshkin S, and Lyden D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol. (2011) 21:139–46. doi: 10.1016/j.semcancer.2011.01.002
30. Hu M, Kenific CM, Boudreau N, and Lyden D. Tumor-derived nanoseeds condition the soil for metastatic organotropism. Semin Cancer Biol. (2023) 93:70–82. doi: 10.1016/j.semcancer.2023.05.003
31. Qi M, Xia Y, Wu Y, Zhang Z, Wang X, Lu L, et al. Lin28B-high breast cancer cells promote immune suppression in the lung pre-metastatic niche via exosomes and support cancer progression. Nat Commun. (2022) 13:897. doi: 10.1038/s41467-022-28438-x
32. Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. (2015) 17:816–26. doi: 10.1038/ncb3169
33. Yang X, Zhang Y, Zhang Y, Li H, Li L, Wu Y, et al. Colorectal cancer-derived extracellular vesicles induce liver premetastatic immunosuppressive niche formation to promote tumor early liver metastasis. Signal Transduct Target Ther. (2023) 8:102. doi: 10.1038/s41392-023-01384-w
34. ELA S, Mäger I, Breakefield XO, and Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. (2013) 12:347–57. doi: 10.1038/nrd3978
35. Lin Y, Zheng H, Jia L, Luo Y, Zhang D, An M, et al. Integrin α6-containing extracellular vesicles promote lymphatic remodelling for pre-metastatic niche formation in lymph nodes via interplay with CD151. J Extracell Vesicles. (2024) 13:e12518. doi: 10.1002/jev2.12518
36. Qiu R, Deng Y, Lu Y, Liu X, Huang Q, and Du Y. ITGB3-enriched extracellular vesicles mediate the formation of osteoclastic pre-metastatic niche to promote lung adenocarcinoma bone metastasis. Mol Carcinog. (2024) 63:2190–204. doi: 10.1002/mc.23803
37. Chen GY, Cheng JC, Chen YF, Yang JC, and Hsu FM. Circulating exosomal integrin beta3 is associated with intracranial failure and survival in lung cancer patients receiving cranial irradiation for brain metastases: A prospective observational study. Cancers. (2021) 13:380. doi: 10.3390/cancers13030380
38. Urabe F, Patil K, Ramm GA, Ochiya T, and Soekmadji C. Extracellular vesicles in the development of organ-specific metastasis. J Extracell Vesicles. (2021) 10:e12125. doi: 10.1002/jev2.12125
39. Fang T, Lv H, Lv G, Li T, Wang C, Han Q, et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun. (2018) 9:191. doi: 10.1038/s41467-017-02583-0
40. Liu Y, Gu Y, Han Y, Zhang Q, Jiang Z, Zhang X, et al. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell. (2016) 30:243–56. doi: 10.1016/j.ccell.2016.06.021
41. Rodrigues G, Hoshino A, Kenific CM, Matei IR, Steiner L, Freitas D, et al. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nat Cell Biol. (2019) 21:1403–12. doi: 10.1038/s41556-019-0404-4
42. Mu W, Gu P, Li H, Zhou J, Jian Y, Jia W, et al. Exposure of benzo[a]pyrene induces HCC exosome-circular RNA to activate lung fibroblasts and trigger organotropic metastasis. Cancer Commun (Lond). (2024) 44:718–38. doi: 10.1002/cac2.12574
43. Liu A, Yu C, Qiu C, Wu Q, Huang C, Li X, et al. PRMT5 methylating SMAD4 activates TGF-β signaling and promotes colorectal cancer metastasis. Oncogene. (2023) 42:1572–84. doi: 10.1038/s41388-023-02674-x
44. Liu F, Shi Z, Bao W, Zheng J, Chen K, Lin Z, et al. ZIC2 promotes colorectal cancer growth and metastasis through the TGF-β signaling pathway. Exp Cell Res. (2022) 415:113118. doi: 10.1016/j.yexcr.2022.113118
45. Lai X-N, Li J, Tang L-B, Chen W-T, Zhang L, and Xiong L-X. MiRNAs and lncRNAs: dual roles in TGF-β Signaling-regulated metastasis in lung cancer. Int J Mol Sci. (2020) 21:1193. doi: 10.3390/ijms21041193
46. Mortezaee K. CXCL12/CXCR4 axis in the microenvironment of solid tumors: A critical mediator of metastasis. Life Sci. (2020) 249:117534. doi: 10.1016/j.lfs.2020.117534
47. Wang Z, Yang C, Li L, Zhang Z, Pan J, Su K, et al. CD62Ldim neutrophils specifically migrate to the lung and participate in the formation of the pre-metastatic niche of breast cancer. Front Oncol. (2020) 10:540484. doi: 10.3389/fonc.2020.540484
48. Jaschinski F, Rothhammer T, Jachimczak P, Seitz C, Schneider A, and Schlingensiepen KH. The antisense oligonucleotide trabedersen (AP 12009) for the targeted inhibition of TGF-beta2. Curr Pharm Biotechnol. (2011) 12:2203–13. doi: 10.2174/138920111798808266
49. Akhurst RJ. Targeting TGF-β Signaling for therapeutic gain. Cold Spring Harb Perspect Biol. (2017) 9:a022301. doi: 10.1101/cshperspect.a022301
50. Waldner MJ and Neurath MF. Targeting the VEGF signaling pathway in cancer therapy. Expert Opin Ther Targets. (2012) 16:5–13. doi: 10.1517/14728222.2011.641951
51. Suarez-Carmona M, Williams A, Schreiber J, Hohmann N, Pruefer U, Krauss J, et al. Combined inhibition of CXCL12 and PD-1 in MSS colorectal and pancreatic cancer: modulation of the microenvironment and clinical effects. J Immunother Cancer. (2021) 9:e002505. doi: 10.1136/jitc-2021-002505
52. Yang Y, Li J, Lei W, Wang H, Ni Y, Liu Y, et al. CXCL12-CXCR4/CXCR7 axis in cancer: from mechanisms to clinical applications. Int J Biol Sci. (2023) 19:3341–59. doi: 10.7150/ijbs.82317
53. Jiang J, Shen N, Song W, Yu H, Sakurai K, Tang Z, et al. Combretastatin A4 nanodrug combined plerixafor for inhibiting tumor growth and metastasis simultaneously. Biomater Sci. (2019) 7:5283–91. doi: 10.1039/c9bm01418g
54. Ieranò C, Portella L, Lusa S, Salzano G, D’Alterio C, Napolitano M, et al. CXCR4-antagonist Peptide R-liposomes for combined therapy against lung metastasis. Nanoscale. (2016) 8:7562–71. doi: 10.1039/c5nr06335c
55. Colombo MV, Bersini S, Arrigoni C, Gilardi M, Sansoni V, Ragni E, et al. Engineering the early bone metastatic niche through human vascularized immuno bone minitissues. Biofabrication. (2021) 13:035036. doi: 10.1088/1758-5090/abefea
56. Kwak TJ and Lee E. Rapid multilayer microfabrication for modeling organotropic metastasis in breast cancer. Biofabrication. (2020) 13:015002. doi: 10.1088/1758-5090/abbd28
57. Ji X, Bei H-P, Zhong G, Shao H, He X, Qian X, et al. Premetastatic niche mimicking bone-on-A-chip: A microfluidic platform to study bone metastasis in cancer patients. Small. (2023) 19:e2207606. doi: 10.1002/smll.202207606
58. Carpenter RA, Kwak J-G, Peyton SR, and Lee J. Implantable pre-metastatic niches for the study of the microenvironmental regulation of disseminated human tumour cells. Nat BioMed Eng. (2018) 2:915–29. doi: 10.1038/s41551-018-0307-x
59. Morris AH, Orbach SM, Bushnell GG, Oakes RS, Jeruss JS, and Shea LD. Engineered niches to analyze mechanisms of metastasis and guide precision medicine. Cancer Res. (2020) 80:3786–94. doi: 10.1158/0008-5472.CAN-20-0079
60. Zhang X, Xiao K, Wen Y, Wu F, Gao G, Chen L, et al. Multi-omics with dynamic network biomarker algorithm prefigures organ-specific metastasis of lung adenocarcinoma. Nat Commun. (2024) 15:9855. doi: 10.1038/s41467-024-53849-3
61. Zhao JJ, Ong CJ, Srivastava S, Chia DKA, Ma H, Huang K, et al. Spatially resolved niche and tumor microenvironmental alterations in gastric cancer peritoneal metastases. Gastroenterology. (2024) 167:1384–98.e4. doi: 10.1053/j.gastro.2024.08.007
62. Jackson CE, Green NH, English WR, and Claeyssens F. The use of microphysiological systems to model metastatic cancer. Biofabrication. (2024) 16:89654. doi: 10.1088/1758-5090/ad3b70
63. Ko J, Song J, Lee Y, Choi N, and Kim HN. Understanding organotropism in cancer metastasis using microphysiological systems. Lab Chip. (2024) 24:1542–56. doi: 10.1039/d3lc00889d
64. Welsh JA, Goberdhan DCI, O’Driscoll L, Buzas EI, Blenkiron C, Bussolati B, et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J Extracell Vesicles. (2024) 13:e12404. doi: 10.1002/jev2.12404
65. O’Brien K, Breyne K, Ughetto S, Laurent LC, and Breakefield XO. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol. (2020) 21:585–606. doi: 10.1038/s41580-020-0251-y
66. Kalluri R and McAndrews KM. The role of extracellular vesicles in cancer. Cell. (2023) 186:1610–26. doi: 10.1016/j.cell.2023.03.010
67. Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, and Simpson RJ. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat Rev Clin Oncol. (2018) 15:617–38. doi: 10.1038/s41571-018-0036-9
68. Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, and Lyden D. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. (2016) 30:836–48. doi: 10.1016/j.ccell.2016.10.009
69. Wortzel I, Dror S, Kenific CM, and Lyden D. Exosome-mediated metastasis: communication from a distance. Dev Cell. (2019) 49:347–60. doi: 10.1016/j.devcel.2019.04.011
70. Bayat M and Sadri Nahand J. Exosomal miRNAs: the tumor’s trojan horse in selective metastasis. Mol Cancer. (2024) 23:167. doi: 10.1186/s12943-024-02081-0
71. Gener Lahav T, Adler O, Zait Y, Shani O, Amer M, Doron H, et al. Melanoma-derived extracellular vesicles instigate proinflammatory signaling in the metastatic microenvironment. Int J Cancer. (2019) 145:2521–34. doi: 10.1002/ijc.32521
72. Blavier L, Nakata R, Neviani P, Sharma K, Shimada H, Benedicto A, et al. The capture of extracellular vesicles endogenously released by xenotransplanted tumours induces an inflammatory reaction in the premetastatic niche. J Extracell Vesicles. (2023) 12:e12326. doi: 10.1002/jev2.12326
73. Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. (2018) 9:5395. doi: 10.1038/s41467-018-07810-w
74. Ma Z, Wei K, Yang F, Guo Z, Pan C, He Y, et al. Tumor-derived exosomal miR-3157-3p promotes angiogenesis, vascular permeability and metastasis by targeting TIMP/KLF2 in non-small cell lung cancer. Cell Death Dis. (2021) 12:840. doi: 10.1038/s41419-021-04037-4
75. García-Silva S, Benito-Martín A, Nogués L, Hernández-Barranco A, Mazariegos MS, Santos V, et al. Melanoma-derived small extracellular vesicles induce lymphangiogenesis and metastasis through an NGFR-dependent mechanism. Nat Cancer. (2021) 2:1387–405. doi: 10.1038/s43018-021-00272-y
76. Ji Q, Zhou L, Sui H, Yang L, Wu X, Song Q, et al. Primary tumors release ITGBL1-rich extracellular vesicles to promote distal metastatic tumor growth through fibroblast-niche formation. Nat Commun. (2020) 11:1211. doi: 10.1038/s41467-020-14869-x
77. Mo Y, Leung LL, Mak CSL, Wang X, Chan WS, Hui LMN, et al. Tumor-secreted exosomal miR-141 activates tumor-stroma interactions and controls premetastatic niche formation in ovarian cancer metastasis. Mol Cancer. (2023) 22:4. doi: 10.1186/s12943-022-01703-9
78. Patras L, Paul D, and Matei IR. Weaving the nest: extracellular matrix roles in pre-metastatic niche formation. Front Oncol. (2023) 13:1163786. doi: 10.3389/fonc.2023.1163786
79. Deep G, Jain A, Kumar A, Agarwal C, Kim S, Leevy WM, et al. Exosomes secreted by prostate cancer cells under hypoxia promote matrix metalloproteinases activity at pre-metastatic niches. Mol Carcinog. (2020) 59:323–32. doi: 10.1002/mc.23157
80. Sharma P, Diergaarde B, Ferrone S, Kirkwood JM, and Whiteside TL. Melanoma cell-derived exosomes in plasma of melanoma patients suppress functions of immune effector cells. Sci Rep. (2020) 10:92. doi: 10.1038/s41598-019-56542-4
81. Yan W, Wu X, Zhou W, Fong MY, Cao M, Liu J, et al. Cancer-cell-secreted exosomal miR-105 promotes tumour growth through the MYC-dependent metabolic reprogramming of stromal cells. Nat Cell Biol. (2018) 20:597–609. doi: 10.1038/s41556-018-0083-6
82. Bergers G and Fendt S-M. The metabolism of cancer cells during metastasis. Nat Rev Cancer. (2021) 21:162–80. doi: 10.1038/s41568-020-00320-2
83. Catalano M and O’Driscoll L. Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. J Extracell Vesicles. (2020) 9:1703244. doi: 10.1080/20013078.2019.1703244
84. Nishida-Aoki N, Tominaga N, Takeshita F, Sonoda H, Yoshioka Y, and Ochiya T. Disruption of circulating extracellular vesicles as a novel therapeutic strategy against cancer metastasis. Mol Ther. (2017) 25:181–91. doi: 10.1016/j.ymthe.2016.10.009
85. Gobbo J, Marcion G, Cordonnier M, Dias AMM, Pernet N, Hammann A, et al. Restoring anticancer immune response by targeting tumor-derived exosomes with a HSP70 peptide aptamer. J Natl Cancer Inst. (2016) 108:djv330. doi: 10.1093/jnci/djv330
86. Kar R, Dhar R, Mukherjee S, Nag S, Gorai S, Mukerjee N, et al. Exosome-based smart drug delivery tool for cancer theranostics. ACS Biomater Sci Eng. (2023) 9:577–94. doi: 10.1021/acsbiomaterials.2c01329
87. McNamee N, Catalano M, Mukhopadhya A, and O’Driscoll L. An extensive study of potential inhibitors of extracellular vesicles release in triple-negative breast cancer. BMC Cancer. (2023) 23:654. doi: 10.1186/s12885-023-11160-2
88. Im E-J, Lee C-H, Moon P-G, Rangaswamy GG, Lee B, Lee JM, et al. Sulfisoxazole inhibits the secretion of small extracellular vesicles by targeting the endothelin receptor A. Nat Commun. (2019) 10:1387. doi: 10.1038/s41467-019-09387-4
89. Tai Q, Yu H, Gao M, and Zhang X. In situ capturing and counting device for the specific depletion and purification of cancer-derived exosomes. Anal Chem. (2023) 95:13113–22. doi: 10.1021/acs.analchem.3c01670
90. Rigg E, Wang J, Xue Z, Lunavat TR, Liu G, Hoang T, et al. Inhibition of extracellular vesicle-derived miR-146a-5p decreases progression of melanoma brain metastasis via Notch pathway dysregulation in astrocytes. J Extracell Vesicles. (2023) 12:e12363. doi: 10.1002/jev2.12363
91. Deng M, Wu S, Huang P, Liu Y, Li C, and Zheng J. Engineered exosomes-based theranostic strategy for tumor metastasis and recurrence. Asian J Pharm Sci. (2023) 18:100870. doi: 10.1016/j.ajps.2023.100870
92. Zhao L, Gu C, Gan Y, Shao L, Chen H, and Zhu H. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J Control Release. (2020) 318:1–15. doi: 10.1016/j.jconrel.2019.12.005
93. Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. (2017) 546:498–503. doi: 10.1038/nature22341
94. Zhang C, Qin C, Dewanjee S, Bhattacharya H, Chakraborty P, Jha NK, et al. Tumor-derived small extracellular vesicles in cancer invasion and metastasis: molecular mechanisms, and clinical significance. Mol Cancer. (2024) 23:18. doi: 10.1186/s12943-024-01932-0
95. Chen H, Chengalvala V, Hu H, and Sun D. Tumor-derived exosomes: Nanovesicles made by cancer cells to promote cancer metastasis. Acta Pharm Sin B. (2021) 11:2136–49. doi: 10.1016/j.apsb.2021.04.012
96. Hoshino A, Kim HS, Bojmar L, Gyan KE, Cioffi M, Hernandez J, et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell. (2020) 182:1044-1061.e18. doi: 10.1016/j.cell.2020.07.009
97. Lone SN, Nisar S, Masoodi T, Singh M, Rizwan A, Hashem S, et al. Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments. Mol Cancer. (2022) 21:79. doi: 10.1186/s12943-022-01543-7
98. Ohzawa H, Kumagai Y, Yamaguchi H, Miyato H, Sakuma Y, Horie H, et al. Exosomal microRNA in peritoneal fluid as a biomarker of peritoneal metastases from gastric cancer. Ann Gastroenterol Surg. (2020) 4:84–93. doi: 10.1002/ags3.12296
99. Li XQ, Zhang R, Lu H, Yue XM, and Huang YF. Extracellular vesicle-packaged CDH11 and ITGA5 induce the premetastatic niche for bone colonization of breast cancer cells. Cancer Res. (2022) 82:1560–74. doi: 10.1158/0008-5472.Can-21-1331
100. Yu D, Li Y, Wang M, Gu J, Xu W, Cai H, et al. Exosomes as a new frontier of cancer liquid biopsy. Mol Cancer. (2022) 21:56. doi: 10.1186/s12943-022-01509-9
101. Varol C, Mildner A, and Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. (2015) 33:643–75. doi: 10.1146/annurev-immunol-032414-112220
102. Bied M, Ho WW, Ginhoux F, and Blériot C. Roles of macrophages in tumor development: a spatiotemporal perspective. Cell Mol Immunol. (2023) 20:983–92. doi: 10.1038/s41423-023-01061-6
103. Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. (2011) 475:222–5. doi: 10.1038/nature10138
104. Bretz NP, Ridinger J, Rupp AK, Rimbach K, Keller S, Rupp C, et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J Biol Chem. (2013) 288:36691–702. doi: 10.1074/jbc.M113.512806
105. Zhang J, Zhou X, and Hao H. Macrophage phenotype-switching in cancer. Eur J Pharmacol. (2022) 931:175229. doi: 10.1016/j.ejphar.2022.175229
106. Sun H, Meng Q, Shi C, Yang H, Li X, Wu S, et al. Hypoxia-inducible exosomes facilitate liver-tropic premetastatic niche in colorectal cancer. Hepatology. (2021) 74:2633–51. doi: 10.1002/hep.32009
107. Jia J, Wang Y, Li M, Wang F, Peng Y, Hu J, et al. Neutrophils in the premetastatic niche: key functions and therapeutic directions. Mol Cancer. (2024) 23:200. doi: 10.1186/s12943-024-02107-7
108. Giese MA, Hind LE, and Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood. (2019) 133:2159–67. doi: 10.1182/blood-2018-11-844548
109. Hedrick CC and Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol. (2022) 22:173–87. doi: 10.1038/s41577-021-00571-6
110. Wu CF, Andzinski L, Kasnitz N, Kröger A, Klawonn F, Lienenklaus S, et al. The lack of type I interferon induces neutrophil-mediated pre-metastatic niche formation in the mouse lung. Int J Cancer. (2015) 137:837–47. doi: 10.1002/ijc.29444
111. Zelová H and Hošek J. TNF-α signalling and inflammation: interactions between old acquaintances. Inflammation Res. (2013) 62:641–51. doi: 10.1007/s00011-013-0633-0
112. Coussens LM and Werb Z. Inflammation and cancer. Nature. (2002) 420:860–7. doi: 10.1038/nature01322
113. Zhang H, Zhu X, Friesen TJ, Kwak JW, Pisarenko T, Mekvanich S, et al. Annexin A2/TLR2/MYD88 pathway induces arginase 1 expression in tumor-associated neutrophils. J Clin Invest. (2022) 132:e153643. doi: 10.1172/jci153643
114. Wang TT, Zhao YL, Peng LS, Chen N, Chen W, Lv YP, et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut. (2017) 66:1900–11. doi: 10.1136/gutjnl-2016-313075
115. Ravindran M, Khan MA, and Palaniyar N. Neutrophil extracellular trap formation: physiology, pathology, and pharmacology. Biomolecules. (2019) 9:365. doi: 10.3390/biom9080365
116. Yang D and Liu J. Neutrophil extracellular traps: A new player in cancer metastasis and therapeutic target. J Exp Clin Cancer Res. (2021) 40:233. doi: 10.1186/s13046-021-02013-6
117. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. (2018) 18:134–47. doi: 10.1038/nri.2017.105
118. Lee W, Ko SY, Mohamed MS, Kenny HA, Lengyel E, and Naora H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J Exp Med. (2019) 216:176–94. doi: 10.1084/jem.20181170
119. Yang L, Liu L, Zhang R, Hong J, Wang Y, Wang J, et al. IL-8 mediates a positive loop connecting increased neutrophil extracellular traps (NETs) and colorectal cancer liver metastasis. J Cancer. (2020) 11:4384–96. doi: 10.7150/jca.44215
120. Teijeira Á, Garasa S, Gato M, Alfaro C, Migueliz I, Cirella A, et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity. (2020) 52:856–71.e8. doi: 10.1016/j.immuni.2020.03.001
121. Yang L, Liu Q, Zhang X, Liu X, Zhou B, Chen J, et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. (2020) 583:133–8. doi: 10.1038/s41586-020-2394-6
122. Yazdani HO, Roy E, Comerci AJ, van der Windt DJ, Zhang H, Huang H, et al. Neutrophil extracellular traps drive mitochondrial homeostasis in tumors to augment growth. Cancer Res. (2019) 79:5626–39. doi: 10.1158/0008-5472.Can-19-0800
123. Kaltenmeier C, Yazdani HO, Morder K, Geller DA, Simmons RL, and Tohme S. Neutrophil extracellular traps promote T cell exhaustion in the tumor microenvironment. Front Immunol. (2021) 12:785222. doi: 10.3389/fimmu.2021.785222
124. Wang DR, Wu XL, and Sun YL. Therapeutic targets and biomarkers of tumor immunotherapy: response versus non-response. Signal Transduct Target Ther. (2022) 7:331. doi: 10.1038/s41392-022-01136-2
125. Edwards SC, Hoevenaar WHM, and Coffelt SB. Emerging immunotherapies for metastasis. Br J Cancer. (2021) 124:37–48. doi: 10.1038/s41416-020-01160-5
126. Zhu C, Liao JY, Liu YY, Chen ZY, Chang RZ, Chen XP, et al. Immune dynamics shaping pre-metastatic and metastatic niches in liver metastases: from molecular mechanisms to therapeutic strategies. Mol Cancer. (2024) 23:254. doi: 10.1186/s12943-024-02171-z
127. Bao R, Hutson A, Madabhushi A, Jonsson VD, Rosario SR, Barnholtz-Sloan JS, et al. Ten challenges and opportunities in computational immuno-oncology. J Immunother Cancer. (2024) 12:e009721. doi: 10.1136/jitc-2024-009721
128. Liu L, Chen A, Li Y, Mulder J, Heyn H, and Xu X. Spatiotemporal omics for biology and medicine. Cell. (2024) 187:4488–519. doi: 10.1016/j.cell.2024.07.040
129. DeNardo DG and Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. (2019) 19:369–82. doi: 10.1038/s41577-019-0127-6
130. Lucotti S, Cerutti C, Soyer M, Gil-Bernabé AM, Gomes AL, Allen PD, et al. Aspirin blocks formation of metastatic intravascular niches by inhibiting platelet-derived COX-1/thromboxane A2. J Clin Invest. (2019) 129:1845–62. doi: 10.1172/jci121985
131. Swierczak A, Cook AD, Lenzo JC, Restall CM, Doherty JP, Anderson RL, et al. The promotion of breast cancer metastasis caused by inhibition of CSF-1R/CSF-1 signaling is blocked by targeting the G-CSF receptor. Cancer Immunol Res. (2014) 2:765–76. doi: 10.1158/2326-6066.Cir-13-0190
132. Sánchez-Martín L, Estecha A, Samaniego R, Sánchez-Ramón S, Vega M, and Sánchez-Mateos P. The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood. (2011) 117:88–97. doi: 10.1182/blood-2009-12-258186
133. Schmittnaegel M, Rigamonti N, Kadioglu E, Cassará A, Wyser Rmili C, Kiialainen A, et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci Transl Med. (2017) 9:eaak9670. doi: 10.1126/scitranslmed.aak9670
134. Piaggio F, Kondylis V, Pastorino F, Di Paolo D, Perri P, Cossu I, et al. A novel liposomal Clodronate depletes tumor-associated macrophages in primary and metastatic melanoma: Anti-angiogenic and anti-tumor effects. J Control Release. (2016) 223:165–77. doi: 10.1016/j.jconrel.2015.12.037
135. Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. (2013) 23:249–62. doi: 10.1016/j.ccr.2013.01.008
136. Wiehagen KR, Girgis NM, Yamada DH, Smith AA, Chan SR, Grewal IS, et al. Combination of CD40 agonism and CSF-1R blockade reconditions tumor-associated macrophages and drives potent antitumor immunity. Cancer Immunol Res. (2017) 5:1109–21. doi: 10.1158/2326-6066.Cir-17-0258
137. Guerriero JL, Sotayo A, Ponichtera HE, Castrillon JA, Pourzia AL, SChad S, et al. Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature. (2017) 543:428–32. doi: 10.1038/nature21409
138. Vidyarthi A, Khan N, Agnihotri T, Negi S, Das DK, Aqdas M, et al. TLR-3 stimulation skews M2 macrophages to M1 through IFN-αβ Signaling and restricts tumor progression. Front Immunol. (2018) 9:1650. doi: 10.3389/fimmu.2018.01650
139. Zheng Z, Li YN, Jia S, Zhu M, Cao L, Tao M, et al. Lung mesenchymal stromal cells influenced by Th2 cytokines mobilize neutrophils and facilitate metastasis by producing complement C3. Nat Commun. (2021) 12:6202. doi: 10.1038/s41467-021-26460-z
140. Zhang Y, Chandra V, Riquelme Sanchez E, Dutta P, Quesada PR, Rakoski A, et al. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J Exp Med. (2020) 217:e20190354. doi: 10.1084/jem.20190354
141. Ortiz-Espinosa S, Morales X, Senent Y, Alignani D, Tavira B, Macaya I, et al. Complement C5a induces the formation of neutrophil extracellular traps by myeloid-derived suppressor cells to promote metastasis. Cancer Lett. (2022) 529:70–84. doi: 10.1016/j.canlet.2021.12.027
142. Tyagi A, Sharma S, Wu K, Wu SY, Xing F, Liu Y, et al. Nicotine promotes breast cancer metastasis by stimulating N2 neutrophils and generating pre-metastatic niche in lung. Nat Commun. (2021) 12:474. doi: 10.1038/s41467-020-20733-9
143. Law AMK, Valdes-Mora F, and Gallego-Ortega D. Myeloid-derived suppressor cells as a therapeutic target for cancer. Cells. (2020) 9:561. doi: 10.3390/cells9030561
144. Sun L, Clavijo PE, Robbins Y, Patel P, Friedman J, Greene S, et al. Inhibiting myeloid-derived suppressor cell trafficking enhances T cell immunotherapy. JCI Insight. (2019) 4:e126853. doi: 10.1172/jci.insight.126853
145. Masuda T, Noda M, Kogawa T, Kitagawa D, Hayashi N, Jomori T, et al. Phase I dose-escalation trial to repurpose propagermanium, an oral CCL2 inhibitor, in patients with breast cancer. Cancer Sci. (2020) 111:924–31. doi: 10.1111/cas.14306
146. Mu XY, Wang RJ, Yao ZX, Zheng Z, Jiang JT, Tan MY, et al. RS 504393 inhibits M-MDSCs recruiting in immune microenvironment of bladder cancer after gemcitabine treatment. Mol Immunol. (2019) 109:140–8. doi: 10.1016/j.molimm.2019.02.014
147. Lu Z, Zou J, Li S, Topper MJ, Tao Y, Zhang H, et al. Epigenetic therapy inhibits metastases by disrupting premetastatic niches. Nature. (2020) 579:284–90. doi: 10.1038/s41586-020-2054-x
148. Li H, Yang F, Zhang L, Zhao R, Li X, and Li H. Xiaoliu pingyi pecipe inhibits lung pre-metastatic niche formation and prevents myeloid-derived suppressor cells recruitment. Integr Cancer Ther. (2023) 22:15347354231187000. doi: 10.1177/15347354231187000
149. Tian S, Song X, Wang Y, Wang X, Mou Y, Chen Q, et al. Chinese herbal medicine Baoyuan Jiedu decoction inhibits the accumulation of myeloid derived suppressor cells in pre-metastatic niche of lung via TGF-β/CCL9 pathway. BioMed Pharmacother. (2020) 129:110380. doi: 10.1016/j.biopha.2020.110380
150. Hassel JC, Jiang H, Bender C, Winkler J, Sevko A, Shevchenko I, et al. Tadalafil has biologic activity in human melanoma. Results of a pilot trial with Tadalafil in patients with metastatic Melanoma (TaMe). Oncoimmunology. (2017) 6:e1326440. doi: 10.1080/2162402x.2017.1326440
151. Tai LH, Alkayyal AA, Leslie AL, Sahi S, Bennett S, Tanese de Souza C, et al. Phosphodiesterase-5 inhibition reduces postoperative metastatic disease by targeting surgery-induced myeloid derived suppressor cell-dependent inhibition of Natural Killer cell cytotoxicity. Oncoimmunology. (2018) 7:e1431082. doi: 10.1080/2162402x.2018.1431082
152. Kitamura T, Qian BZ, and Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol. (2015) 15:73–86. doi: 10.1038/nri3789
153. Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells. Cancer Res. (2011) 71:3505–15. doi: 10.1158/0008-5472.Can-10-4316
154. Kaczanowska S, Beury DW, Gopalan V, Tycko AK, Qin H, Clements ME, et al. Genetically engineered myeloid cells rebalance the core immune suppression program in metastasis. Cell. (2021) 184:2033–52.e21. doi: 10.1016/j.cell.2021.02.048
155. Grum-Schwensen B, Klingelhöfer J, Beck M, Bonefeld CM, Hamerlik P, Guldberg P, et al. S100A4-neutralizing antibody suppresses spontaneous tumor progression, pre-metastatic niche formation and alters T-cell polarization balance. BMC Cancer. (2015) 15:44. doi: 10.1186/s12885-015-1034-2
156. Gu Y, Liu Y, Fu L, Zhai L, Zhu J, Han Y, et al. Tumor-educated B cells selectively promote breast cancer lymph node metastasis by HSPA4-targeting IgG. Nat Med. (2019) 25:312–22. doi: 10.1038/s41591-018-0309-y
157. Zhang P, Meng J, Li Y, Yang C, Hou Y, Tang W, et al. Nanotechnology-enhanced immunotherapy for metastatic cancer. Innovation (Camb). (2021) 2:100174. doi: 10.1016/j.xinn.2021.100174
158. Cai Q, He Y, Zhou Y, Zheng J, and Deng J. Nanomaterial-based strategies for preventing tumor metastasis by interrupting the metastatic biological processes. Adv Healthc Mater. (2024) 13:e2303543. doi: 10.1002/adhm.202303543
159. Zeng W, Wang Y, Zhang Q, Hu C, Li J, Feng J, et al. Neutrophil nanodecoys inhibit tumor metastasis by blocking the interaction between tumor cells and neutrophils. ACS Nano. (2024) 18:7363–78. doi: 10.1021/acsnano.3c08946
160. Long Y, Lu Z, Xu S, Li M, Wang X, Zhang Z, et al. Self-delivery micellar nanoparticles prevent premetastatic niche formation by interfering with the early recruitment and vascular destruction of granulocytic myeloid-derived suppressor cells. Nano Lett. (2020) 20:2219–29. doi: 10.1021/acs.nanolett.9b03883
161. Lu Z, Ma L, Mei L, Ren K, Li M, Zhang L, et al. Micellar nanoparticles inhibit the postoperative inflammation, recurrence and pulmonary metastasis of 4T1 breast cancer by blocking NF-κB pathway and promoting MDSCs depletion. Int J Pharm. (2022) 628:122303. doi: 10.1016/j.ijpharm.2022.122303
162. Lu Z, Liu H, Ma L, Ren K, He Z, Li M, et al. Micellar nanoparticles inhibit breast cancer and pulmonary metastasis by modulating the recruitment and depletion of myeloid-derived suppressor cells. Nanoscale. (2022) 14:17315–30. doi: 10.1039/d2nr03880c
163. Xia C, Bai W, Deng T, Li T, Zhang L, Lu Z, et al. Sponge-like nano-system suppresses tumor recurrence and metastasis by restraining myeloid-derived suppressor cells-mediated immunosuppression and formation of pre-metastatic niche. Acta Biomater. (2023) 158:708–24. doi: 10.1016/j.actbio.2023.01.009
164. Qiu M, Huang K, Liu Y, Yang Y, Tang H, Liu X, et al. Modulation of intestinal microbiota by glycyrrhizic acid prevents high-fat diet-enhanced pre-metastatic niche formation and metastasis. Mucosal Immunol. (2019) 12:945–57. doi: 10.1038/s41385-019-0144-6
165. Sahin U, Oehm P, Derhovanessian E, Jabulowsky RA, Vormehr M, Gold M, et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. (2020) 585:107–12. doi: 10.1038/s41586-020-2537-9
Keywords: pre-metastatic niche, PMN, metastasis, bibliometric analysis, research hotspots, immunotherapy, metastatic organotropism, extracellular vesicles
Citation: Cheng M, Sun Q, Duan H and Chen C (2025) Global hotspots and trends in pre-metastatic niche research: a bibliometric analysis(2005-2024). Front. Immunol. 16:1552053. doi: 10.3389/fimmu.2025.1552053
Received: 27 December 2024; Accepted: 12 May 2025;
Published: 29 May 2025.
Edited by:
Irina R. Matei, Cornell University, United StatesReviewed by:
Abhishek Tyagi, Wake Forest University, United StatesDalia Ramírez-Ramírez, Biomedical Research Center of East (CIBIOR), Mexico
Linda Bojmar, Linköping University, Sweden
Copyright © 2025 Cheng, Sun, Duan and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cihui Chen, Y2lodWljaGVuQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Mengqi Cheng
Mengqi Cheng Qianhui Sun
Qianhui Sun Hua Duan
Hua Duan Cihui Chen
Cihui Chen