- Department of Pathobiology, College of Veterinary Medicine, University of Illinois Urbana-Champaign, Urbana, IL, United States
Pulmonary diseases, such as cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), and ventilator-associated pneumonia (VAP), are attributed to the prolonged infection of the airway and hypersecretion of mucus. Pseudomonas aeruginosa (PA) is one of the most common nosocomial pathogens in these diseased airways, secreting a wide spectrum of metabolites and volatile organic compounds (VOCs) that significantly impact the respiratory epithelium, including disruption of mucus homeostasis and inflammatory responses of the diseased lungs. In this review, we highlighted the major metabolites and VOCs produced by PA and the mechanisms by which they modulate inflammation, cellular injury, and mucus hypersecretion in respiratory epithelium.
1 Introduction
Pseudomonas aeruginosa (PA) is a common Gram-negative opportunistic bacterial pathogen that colonizes the respiratory tracts of individuals suffering from chronic lung diseases such as cystic fibrosis (CF), advanced stages of chronic obstructive pulmonary disease (COPD), bronchiectasis, and chronic bronchitis (CB); as well as ventilator-associated pneumonia (VAP) (1). Infections are most commonly associated with increased morbidity, pulmonary function deterioration, and prolonged hospitalization (2, 3). PA is extremely versatile metabolically, and is capable of producing a plethora of virulence factors, volatile organic compounds (VOCs), and secondary metabolites, which contribute to its pathogenicity in mammalian hosts, environmental adaptability, and interactions with other microorganisms. The persistent presence of PA is often linked to poor clinical outcomes. In this review, we describe the effects of various PA secondary metabolites and VOCs on respiratory epithelial cells and local lung inflammation. In particular, it examines how these metabolites participate in mucus imbalance, epithelial injury, and pneumonic inflammation, providing clues about the disease pathogenesis during PA infections.
2 Pseudomonas aeruginosa-derived volatile organic compounds
As is the case with all living creatures, bacteria emit a wide variety of VOCs. Some of these VOCs are unique to specific bacterial species and are useful biomarkers for pathogen identification. These VOCs encompass a diverse range of metabolites generated through microbial growth, serving as indicators of cellular signaling and metabolic activities (4). PA emits a list of unique VOCs during lung infections that are identifiable through recently improved detection methodologies, and have sparked growing interest in associating the presence of specific VOC profiles for clinical applications (5–7), aiming to improve diagnostic accuracy for disease detection and monitoring. The swift and precise identification of the causative pathogen is essential for the effective administration of targeted, narrow-spectrum antimicrobial treatment. Additionally, early diagnosis of PA infection, combined with appropriate antibiotic treatment, may facilitate the eradication of the pathogen before the infection progresses to a chronic state. However, current diagnostic methods are primarily based on the microbiological culture of respiratory specimens (8). This approach is often protracted, typically requiring three days, invasive, and not routinely performed following initial clinical suspicion of PA infection (9, 10). In fact, both the detection and monitoring of PA lung infections traditionally rely on sputum cultures. However, with advancements in highly effective modulator therapy in CF, sputum production has decreased, even though the risk of lung infections remains. A promising alternative to address these limitations is to monitor shifts in the molecular phenotype of either the host or the bacterial metabolism by analyzing distinct VOC profiles (11). Consequently, many laboratories have attempted to identify such biomarkers by analyzing VOCs released from in vitro PA cultures and in patients, as detailed in Tables 1, 2. These investigations are largely based on gas chromatography-mass spectrometry (GC-MS), frequently coupled with solid-phase microextraction (SPME), and on selected ion flow tube mass spectrometry (SIFT-MS) and proton transfer reaction mass spectrometry (PTR-MS) (6, 7). Notably, the assessment of VOCs from respiratory samples in human subjects, such as bronchoalveolar lavage fluid (BALF), sputum, sinus mucus, and exhaled breath, has been suggested as a minimally invasive method for diagnosis and monitoring of PA lung and sinus infections, particularly in conditions such as CF (12–22).

Table 1. Summary of volatile organic compounds (VOCs) detected in in vitro studies involving P. aeruginosa.
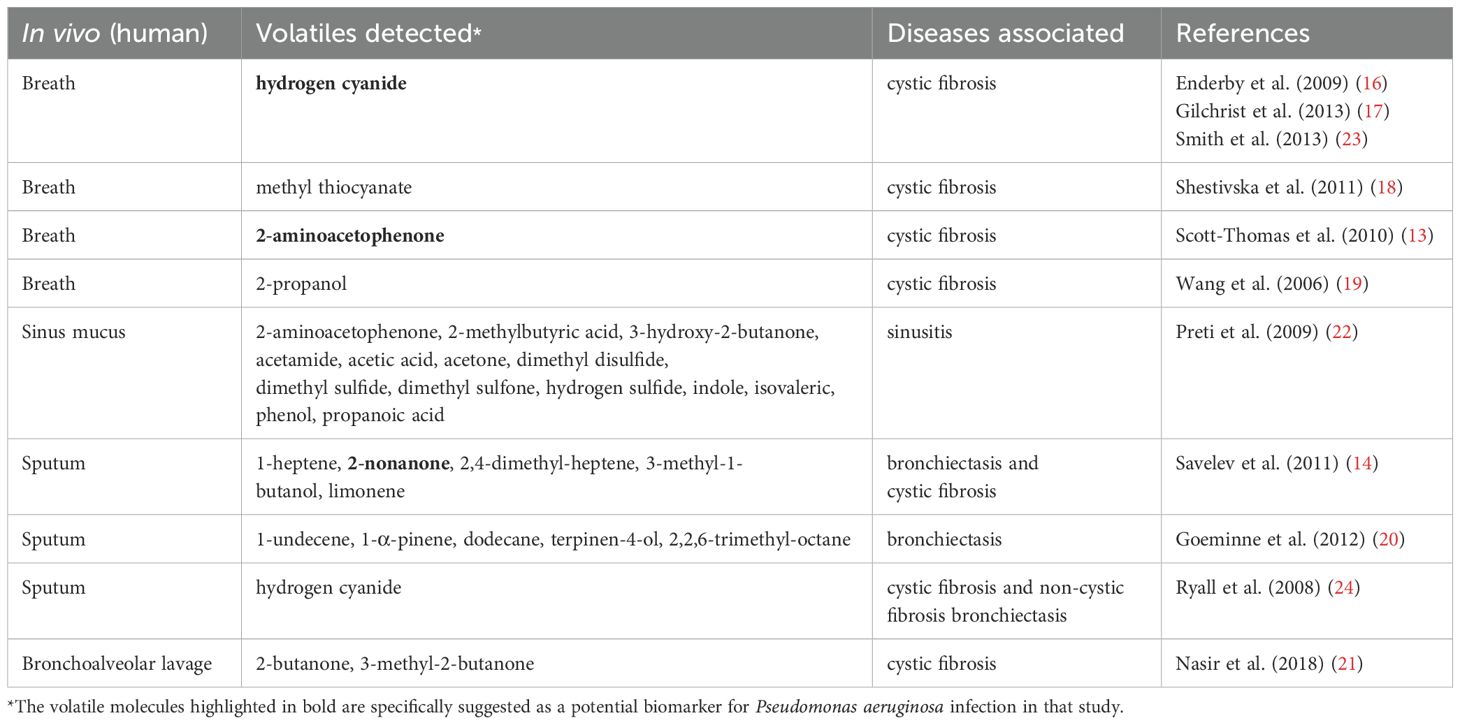
Table 2. Literature overview of volatile organic compounds (VOCs) detected in in vivo studies involving P. aeruginosa.
2.1 PA-derived VOCs detected in vitro and in vivo
Key VOCs identified for PA include hydrogen cyanide (HCN), a well-known compound that has been consistently detected in the breath and sputum volatilome of individuals infected with PA (16, 17, 23, 24), as well as under specific bacterial culture conditions (25, 26). Consequently, it has been suggested as a potential non-invasive diagnostic biomarker for PA colonization. Methyl thiocyanate has emerged as an additional biomarker, exhibiting concentrations ranging from 2 to 21 ppbv in the exhaled breath of CF patients infected with PA, as well as in the bacterial culture headspace (18). Notably, the observed parallel correlation between HCN levels and methyl thiocyanate suggests that the synthesis of methyl thiocyanate by PA strains is contingent upon the production of HCN (18). Another VOC found in the breath of CF patients (13) and in the headspace of bacterial cultures (22, 27–32), is 2-aminoacetophenone (2-AA). This molecule, which imparts a distinctive ‘grape-like’ fruity odor on PA cultures, is known to modulate the virulence of PA by promoting a shift toward a chronic infection phenotype in lungs (33). Methyl ketones, such as 2-nonanone and 2-undecanone (30, 34) are likewise released by PA cultures in vitro. 2-nonanone, in particular, can be detected in vitro in bacterial cultures (30, 34–37) and in vivo as a marker for the detection of PA in the breath of bronchiectasis and CF septum samples (14).This detection sensitivity can be further enhanced by 19% when 2-nonanone is combined with 17 other detected VOCs in a sputum library (14). Other VOCs associated with PA under in vitro and in vivo conditions include hydrocarbons (e.g., 1-undecene (20, 22, 30, 32, 34, 35), 1-dodecene (35)), ketones (e.g., acetone (22, 27, 31, 37–39)), aldehydes (e.g., 3-methyl-1-butanol (14, 30, 34–36, 38)), acids (e.g., acetic acid (22, 27, 39)), alcohols (e.g., ethanol (27, 28, 31, 35, 38, 39), 1-butanol (34)), sulfur compounds (e.g., dimethyl sulfide (22, 32, 35, 37, 39), dimethyl disulfide (22, 30, 35, 37, 39), dimethyl trisulfide (30, 35, 37)), terpenes (e.g., 1-α-pinene (20), terpinen-4-ol (20)), and other compounds (e.g., 2,2,6-trimethyl-octane (20), indole (22, 27)). The identification of overlapping biomarkers among corroborating reports provides considerable encouragement that these VOCs are potentially PA-specific. A comprehensive list of core VOCs derived from PA has been compiled in Tables 1, 2, incorporating both in vitro and in vivo published literatures, with associated diseases listed alongside.
2.2 Discrepancies and confounding factors between in vitro and in vivo findings on PA volatilome profiles
Collectively, the above studies suggest that PA-related VOC profiles may serve as sensitive and specific biomarkers for its identification and detection in human specimens (in/ex vivo), as well as in pure and mixed bacterial cultures. Despite these advances, integrating these biomarkers into the clinical diagnosis of PA lung infections remains challenging due to multiple confounding factors including differences in culture conditions, bacterial strains and phenotypes, host factors, the non-specific origins of many VOCs, and discrepancies between in vitro and in vivo research findings. Thus far, comprehensive translational research bridging in vitro and in vivo studies in human patients—an essential step for biomarker validation—remains limited. In 2013, Zhu et al. made the first attempt at comparing the in vivo and in vitro volatile profiles from the same PA and Staphylococcus aureus strains using a murine infection model (40). They showed a low similarity (25-34%) between VOC profiles of PA and S. aureus cultures in vitro to in vivo (40). Nevertheless, the VOC profiles were able to differentiate between mice with and without infection, between mice infected by PA versus S. aureus, and infection by different PA strains. In addition, the host immune response has a significant impact on the VOC profile. Bean et al., who reported the presence of unique breath prints including host-derived volatiles of inflammation that allow discrimination between healthy, active PA infection, and convalescent state (41). Furthermore, Fenn et al found that PA emitted fewer pathogen-specific VOCs when co-cultured with alveolar A549 human epithelial cells as compared to when PA was grown alone (42). All together, these findings suggest that VOC biomarkers are modulated by the availability of host environment, an essential consideration for understanding their biochemical origins.
Previous studies (29, 43) have also demonstrated how the bacterial culture environment (e.g., pH, CO2/O2 ratio, nutrient availability, and medium composition) influences the observed VOC profiles, highlighting PA’s ability to produce diverse VOCs while also posing a challenge in establishing a consensus panel of biomarkers for reliable in vivo detection. Moreover, it’s important to note that the VOC profile of PA can shift longitudinally, correlating with the adaptation of infection phenotypes (early vs. chronic), thus indicating the diagnostic potential for monitoring chronic CF lung infections through breath analysis (36). Overall, various confounding factors, including PA strains (31), bacterial culture media (29, 43), growth stage (biofilm vs. planktonic) (44), bacterial phenotypes (mucoid vs. non-mucoid) (45), and individual patient’s factors such as the stage of infection (36), diet (13, 46), and smoking (35), have all been shown to influence the composition of volatilome of PA.
2.3 Recent advances and concepts in PA volatilome profiling
As discussed above, many in vitro studies aimed at identifying distinct PA VOC biomarkers have not successfully translated into in vivo contexts for the identification of analogous volatilomes in clinical patients. The variability in VOC species observed in different studies, as outlined in Tables 1, 2, raises the question of whether a single VOC is indicative of PA presence or if a distinct “pattern” of collective VOCs is, in fact, more reflective of this pathogen. Due to the limited success in developing clinical diagnostics based on selected in vitro volatile biomarkers, several techniques are now being explored to capture more comprehensive bacterial volatilomes for diagnostic purposes. Volatile profiling, also known as fingerprinting, is being explored through the application of chemical sensors along with gas chromatography (GC) and mass spectrometry (MS) techniques (12, 47–50). Since then, there has been notable success in utilizing the entire volatilome fingerprint for PA detection in both human (51) and murine models (40). The literature on this topic converges on the fact that volatile metabolites are related to infection pathogenesis as a whole, which may include both physiological and host response factors. Hence, it is generally a “pattern” of VOCs that signifies the presence of PA in clinical specimens, rather than the detection of an individual compound. The combination of multiple GC or GC-MS breath biomarkers, along with the use of the entire volatilome fingerprint, has proven to be a reliable strategy for diagnosing PA lung infections (12, 14, 52–54). Advances in analysis-methods and particularly in small and VOC-specific sensor-arrays resulted in cost-effective, miniaturized ‘eNose’ sensors. These devices, among other possible applications, have been used in pilot clinical studies to detect bacterial colonization in CF patients with bronchiectasis (55, 56), representing non-invasive diagnostic and monitoring tools for PA lung infections.
3 PA-derived secondary metabolites
In addition to the aforementioned VOCs, PA produces numerous important exoproducts and secondary metabolites that play a role in its pathogenicity and in the persistence of PA in the lung. These comprise the redox-active tricyclic phenazines, the quorum sensing (QS) molecules, siderophores, and exopolysaccharides that all have essential functions in the modulation of host cell behaviors. Some of the essential metabolites are listed below:
3.1 Phenazines
Phenazines represent a substantial category of nitrogen-containing heterocyclic compounds, which include the redox-active pyocyanin (PYO), phenazine-1-carboxylic acid (PCA), phenazine-1-carboxamide (PCN), 1-hydroxyphenazine, and 5-methylphenazine-1-carboxylic acid betaine (57, 58). These compounds are recognized as critical virulence factors of PA, playing significant roles in quorum sensing, biofilm formation, virulence expression, iron acquisition, oxidative stress, competition against other microbes within the same niche, and modulation of host responses (58–60). Through these multifaceted activities, phenazines greatly enhance the pathogenic potential and ecological adaptability of PA. Their detection in clinical specimens correlates with heightened virulence and adverse patient outcomes, particularly in cases of CF (61, 62).
3.2 Quorum sensing molecules
The QS systems in PA are a hierarchical network that orchestrates virulence factor expression and biofilm formation. This regulation is mediated by a variety of signaling molecules, including N-3-oxo-dodecanoyl homoserine lactone (3-oxo-C12-HSL), N-butanoyl-L-homoserine lactone (C4-HSL), Pseudomonas quinolone signal (PQS), 2-heptyl-4-hydroxyquinoline (HHQ), 2-(2-hydroxyphenyl)-thiazole-4-carbaldehyde (IQS), and 2-heptyl-4-hydroxyquinoline N-oxide (HQNO). Two acyl-homoserine lactone (AHL) QS systems, the Las and Rhl, are closely connected, and are involved in the synthesis of a variety of virulence factors such elastases, alkaline protease, rhamnolipids, phenazines, lectins, superoxide dismutase, and biofilm formation (63). The more recently identified PQS and IQS systems contribute additional layers of complexity to PA’s QS network (64). Notably, PQS, along with its precursor HHQ and the derivative HQNO, is frequently found in the sputum, bronchoalveolar fluid, and mucopurulent secretions of people with CF (65). In brief, QS systems allow PA to modulate gene expression in response to cell density, thus controlling important functions such as virulence, antibiotic resistance, and biofilm formation (64, 66). This intricate communication network significantly enhances the adaptability and pathogenic potential of PA in diverse environments.
3.3 Siderophores
The siderophores pyoverdine and pyochelin chelate iron from the host microenvironments and lysed RBCs. This system is not only essential for bacteria survival but also enhances pathogenicity during lung infection processes (67, 68).
3.4 Exopolysaccharides
Extracellular polysaccharides provide a barrier protecting bacteria against environmental factors, such as dehydration, bacteriophages and the host immune factors. PA synthesizes three main polysaccharides, including alginate, PSL, and PEL, all of which are important components of in vitro biofilms (69). The production of alginate is particularly noteworthy, as it imparts the mucoid phenotype of clinical PA isolates from CF lungs (70). These polysaccharides are important for the establishment of PA biofilms, providing a shield against host defenses such as reactive oxygen species (ROS) and phagocytosis (71–73), as well as enhancing resistance to antibiotics (74–76).
4 Effects of PA-derived metabolites on respiratory epithelial cells
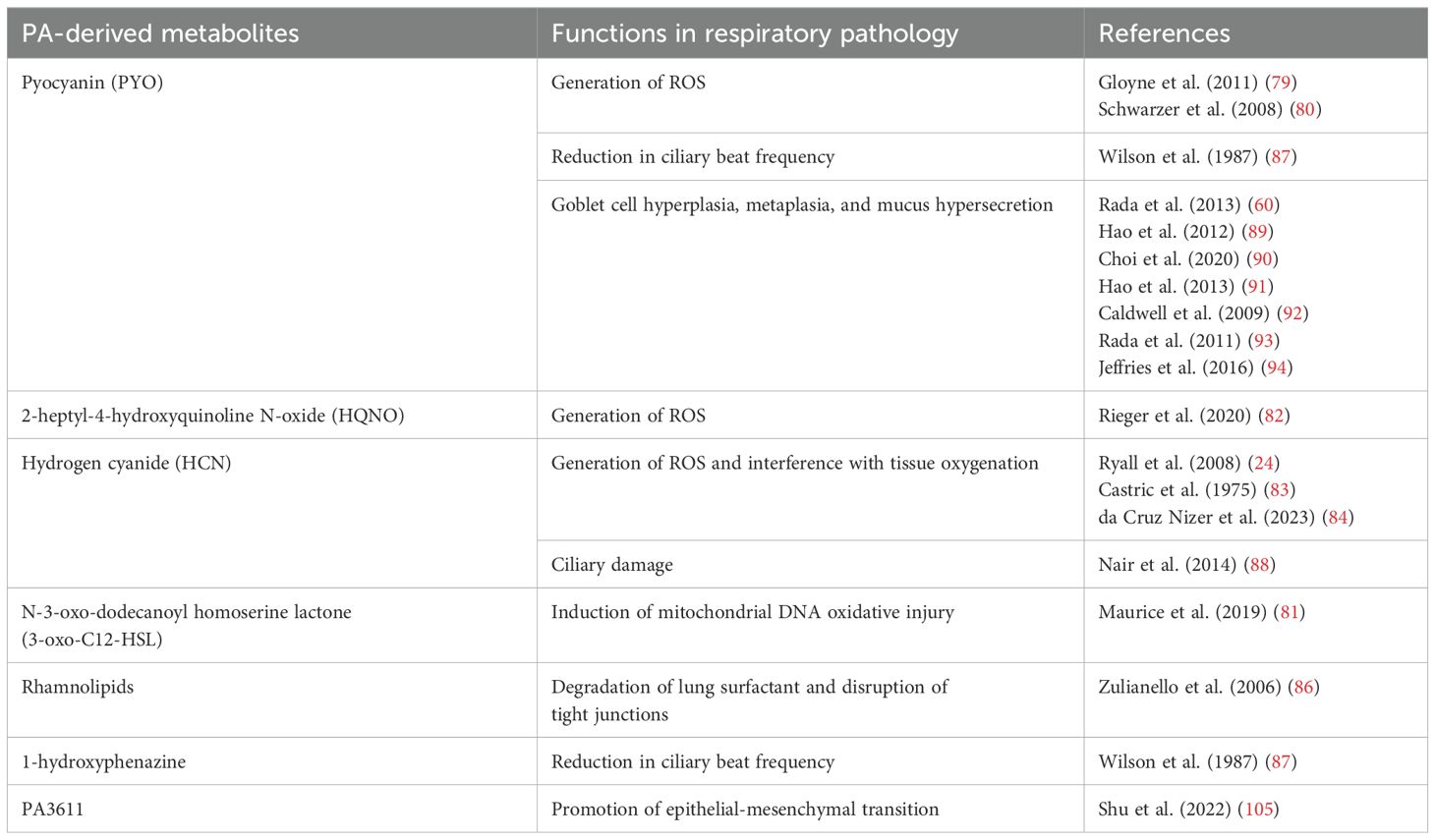
The respiratory epithelium of human lung is the body’s first line of defense against inhaled germs, allergens, and pollutants, and plays a crucial role in the initiation of immune responses. Its primary innate immune functions encompass: (i) the production of mucus to ensnare pathogens; (ii) the expulsion of inhaled bacteria via ATP-dependent, coordinated mucociliary escalator; (iii) the release of antibacterial peptides and ROS; (iv) the initiation of wound healing processes after epithelial damage; and (v) the secretion of cytokines and chemokines to signal the immune system (77). The structural integrity of the epithelium, coupled with mucociliary clearance, pollutant metabolism, and production of antimicrobial and immune mediators, is essential for protecting the gas exchange units (alveoli) and submucosal layers from environmental inhalants (78). The integrity and function of respiratory epithelial cells are hence crucial for maintaining airway homeostasis. PA-derived metabolites and VOCs can disrupt airway epithelial functions in several ways summarized below (Figure 1).
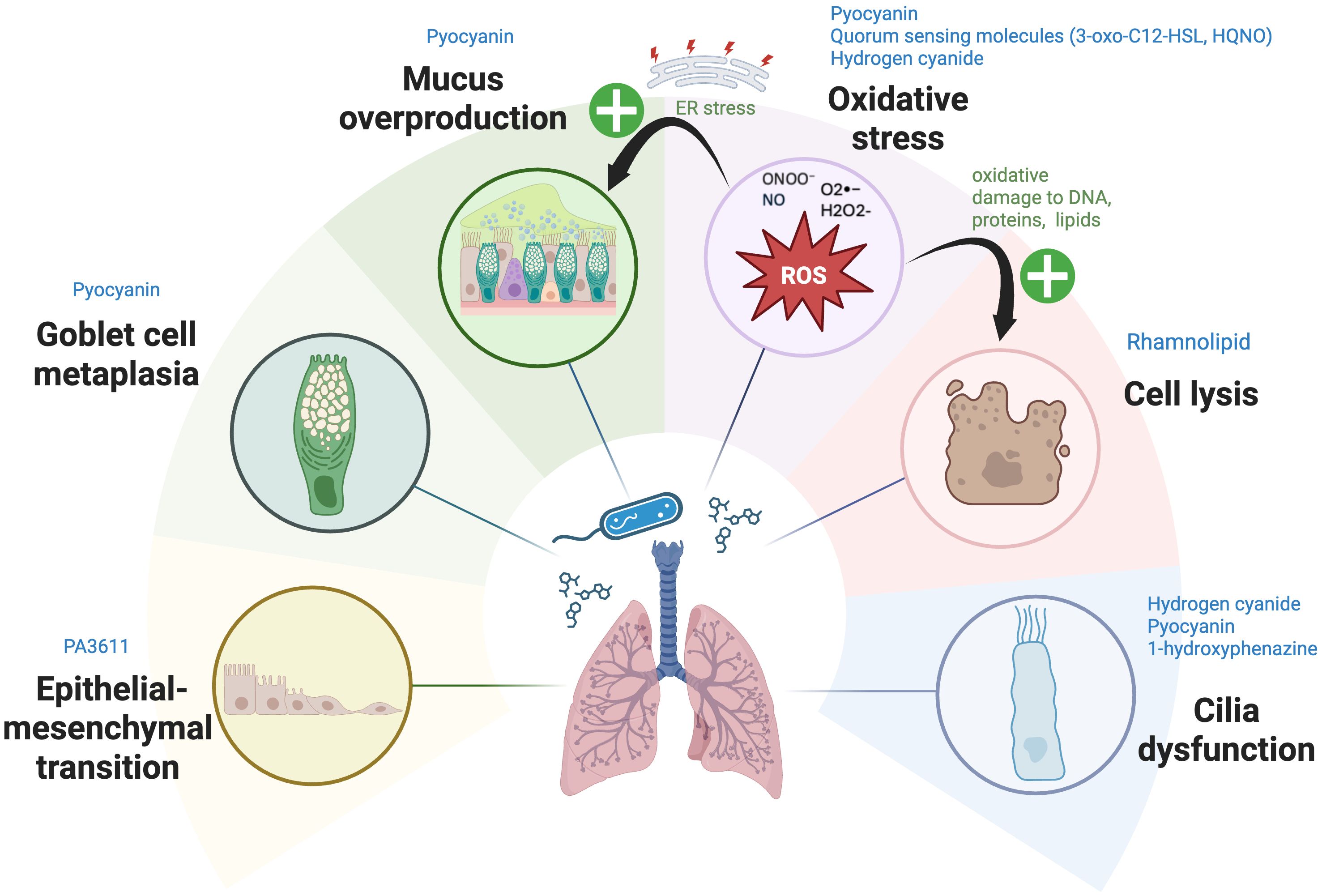
Figure 1. The mechanisms by which Pseudomonas aeruginosa-derived secondary metabolites affect respiratory epithelial cells. Pseudomonas aeruginosa (PA) employs multiple mechanisms to disrupt respiratory epithelial cells. Metabolites such as pyocyanin (PYO), hydrogen cyanide (HCN), and quorum-sensing (QS) molecules (3-oxo-C12-HSL, HQNO) generate reactive oxygen and nitrogen species (ROS, RNS), including superoxide (O2•−), hydrogen peroxide (H2O2), peroxynitrite (ONOO−), and nitric oxide (NO). These ROS and RNS disrupt mitochondrial electron transport, causing oxidative stress and damage to DNA, proteins, and lipids. Furthermore, excessive ROS and RNS generated by PA metabolites—such as PYO, 3-oxo-C12-HSL, HQNO, and HCN—perturb the respiratory epithelial barrier via activation of apoptosis pathways in epithelial cells and induce excessive mucus production by inducing endoplasmic reticulum (ER) stress. This stress further exacerbates mucus production and contributes to chronic inflammatory conditions. Rhamnolipid induce direct cell lysis, while HCN, PYO, and 1-hydroxyphenazine impair ciliary function, with PYO also driving mucus overproduction and promoting goblet cell metaplasia. Furthermore, PA3611, a quorum-sensing-regulated protein expressed by PA during infection, promotes epithelial-mesenchymal transition (EMT) in bronchial epithelial cells—a tissue remodeling process wherein epithelial cells lose their characteristics and differentiate into myofibroblasts. Image created with BioRender.com. Kuo, S. (2025) https://BioRender.com/f5fss63
4.1 Cytotoxicity via oxidative stress and direct cell lysis
PA metabolites can damage cellular and mitochondrial components, leading to cell death or dysfunction by generating excessive ROS and causing oxidative stress. These ROS interfere with multiple cellular functions in host cells, including electron transport, cellular respiration, and energy metabolism (60). PYO, a redox-active pigment and major virulence factor produced by PA, plays a significant role in oxidative stress generation by elevating intracellular levels of ROS, particularly superoxide (O2•−) and hydrogen peroxide (H2O2) via consumption of catalase-associated NADPH (60). These ROS cause oxidative damage to DNA, proteins, and lipids, thereby inhibiting key cellular enzymes and disrupting normal cellular functions (79, 80). Similarly, QS molecules such as 3-oxo-C12-HSL (81) and HQNO (82), along with the VOC HCN (24, 83, 84), disrupt electron transport in mitochondria, attenuating cellular respiration and inducing the generation of ROS. This, in turn, triggers apoptotic pathways in epithelial cells and compromises the integrity of the epithelial barrier (81, 85). Furthermore, rhamnolipids degrade lung surfactant and disrupt tight junctions, causing direct injury to tracheal and lung epithelial barrier (86).
4.2 Ciliary dysfunction
Phenazines and HCN, have detrimental effects on mucociliary clearance by directly impairing ciliary function. PYO and 1-hydroxyphenazine reduce ciliary beat frequency in the lungs, weakening the cilia’s ability to clear mucus and trapped particles from the airways (87). Likewise, HCN produced by PA, which is also a principal ‘ciliatoxic’ component found in cigarette smoke, significantly damages the cilia, disrupting their synchronized beating and hindering the efficient escalator movement of mucus that clears entrapped particles out of the respiratory system (88).
4.3 Goblet cell hyperplasia and mucus hypersecretion
PYO plays a significant and multifaceted role in enhancing mucus hypersecretion and goblet cell metaplasia and hyperplasia during infections. PYO inactivates FOXA2, a transcriptional regulator of airway mucus homeostasis which ordinarily inhibits excessive goblet cell hyperplasia and metaplasia and mucus production (89, 90). Additionally, the ROS and reactive nitrogen species (RNS) generated by PYO also cause post-translational modifications of FOXA2, including nitrosylation, acetylation, and ubiquitination, which impair its capacity to bind to the promoter of the MUC5B gene (91). Subsequent investigations utilizing normal and CF and COPD-diseased primary and immortalized human airway cells, along with studies in mice, reveal that PYO inhibits FOXA2 expression via the activation of antagonistic signaling cascades, among others, EGFR-PI3K-AKT, EGFR-MEK-ERK, and IL-13R-STAT6-SPDEF, leading to goblet cell hyperplasia and metaplasia and overexpression and hypersecretion of mucus (89, 90, 92). Moreover, the ROS associated with PYO stimulate the release of inflammatory cytokines and growth factors that promote EGFR-dependent mucin secretion in airway epithelial cells (60, 93). Long-term chronic exposure (12 weeks) to PYO in murine airways results in goblet cell hyperplasia, airway fibrosis, destruction of alveolar spaces, and a shift towards a Th2 immune response marked by increased levels of Th2 cytokines IL-4 and IL-13. These cytokines further activate the STAT6 signaling pathway, exacerbating goblet cell hyperplasia and promoting excessive mucus production (92). Besides, PYO has been found to upregulate expression of sialyl-Lewis(x), a sugar modification of airway mucins to which PA preferentially adheres, utilizing this as part of its strategy to condition the airway for chronic infection (94). Consequently, a sophisticated interplay of autocrine and paracrine signaling pathways facilitates the mucin secretion induced by PYO in respiratory epithelial cells (Figure 2). Additionally, prolonged oxidative stress leads to an accumulation of improperly folded proteins within the endoplasmic reticulum (ER), resulting in ‘ER stress’ and the subsequent activation of the ‘unfolded protein response’ (UPR). This mechanism can further exacerbate mucus production and contribute to chronic inflammatory conditions (95–98) characterized by the secretion of proinflammatory cytokines. This release further escalates ER stress, creating a feedback loop that amplifies the inflammatory response (95, 99). Also, ER stress has been implicated in the initiation and progression of pulmonary fibrosis, with growing evidence suggesting that it also plays a role in obstructive lung diseases, pulmonary infections associated with CF, and lung cancer (100).
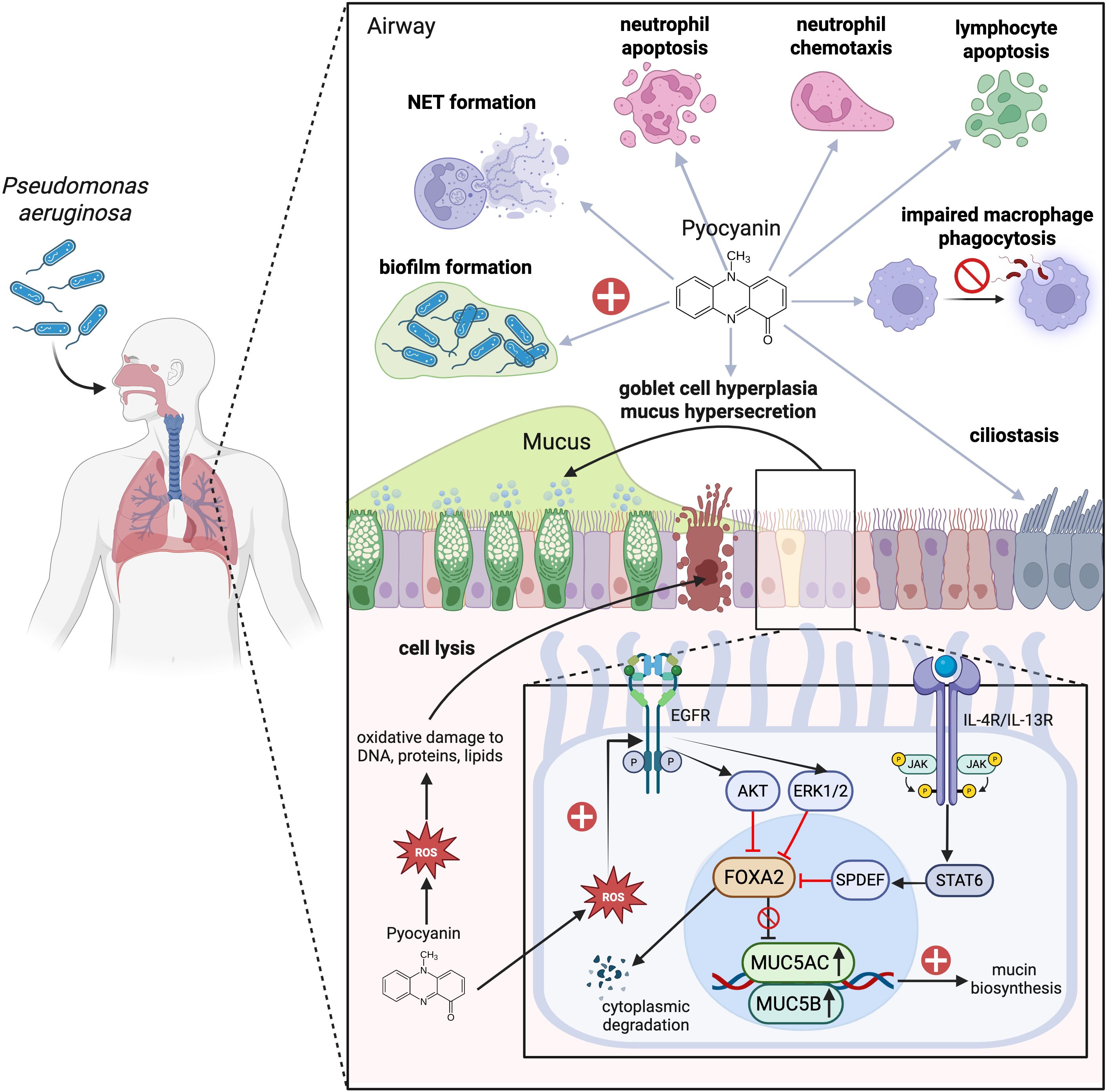
Figure 2. Mechanisms of respiratory impact and immune modulation by pyocyanin during Pseudomonas aeruginosa (PA) infection. Pyocyanin (PYO), a chemical redox pigment and the major virulence factor in PA, generates an oxidative burst through the increased production of intracellular reactive oxygen species (ROS) that damage DNA, protein, and phospholipids. These damages initiate apoptotic cascades and disruption of the respiratory barrier. Also, PYO reduces ciliary beats frequency, which has a detrimental effect on mucociliary clearance. Moreover, PYO causes goblet cell hyperplasia and mucus hypersecretion by suppressing FOXA2, a master regulator of mucus homeostasis, through activation of the EGFR-PI3K-AKT, EGFR-MEK-ERK, and IL-4/IL-13R-JAK-STAT6-SPDEF pathways. The ROS generated by PYO is additionally responsible for the promotion of chemokine and growth factor release which augment EGFR-induced mucin hyperproduction. In addition to the above effects, PYO also modulates both pro-inflammatory and anti-inflammatory immune responses. On the one hand, it increases neutrophil chemotaxis, and, on the other hand, it inhibits macrophage phagocytosis and activates the apoptosis of neutrophils, T lymphocytes, and B lymphocytes. Neutrophils drawn to tissue following chemotaxis exacerbate tissue damage via the release of ROS, proteases, and pro-inflammatory cytokines. PYO also induces the release of extracellular DNA with neutrophil extracellular traps (NETs) formation, which contribute to biofilm formation and persistent infection of PA in tissue. Image created with BioRender.com. Kuo, S. (2025) https://BioRender.com/5y1ycg1.
4.4 Epithelial-mesenchymal transition
As aforementioned, PA infects chronically diseased lungs (1). Epithelial injury triggers a sustained immune response, leading to emphysema and airway remodeling, which involves peribronchial fibrosis and possibly increased airway smooth muscle mass (101, 102). Pulmonary fibrosis develops as a complication of repeated PA infections, epithelial damage, and tissue repair. The EMT in bronchial epithelial cells—a tissue remodeling process where epithelial cells lose their characteristics and differentiate into myofibroblasts—plays a pivotal role in the progression of bronchial and pulmonary fibrosis and obliterative bronchiolitis (OB). These changes in cell proportions can result in goblet cell metaplasia/hyperplasia and increase mucus production, a hallmark of chronic bronchitis in COPD (103). Prolonged exposure to PYO has been shown to induce peribronchial fibrosis (92). PA3611, a putative QS-regulated protein produced by PA during infection (104), has been shown to promote EMT by integrin αvβ6-mediated activation of the TGF-β1-induced p38/NF-κB pathway, which causes mesenchymal markers to be upregulated and epithelial markers to be downregulated (105). In line with this, Borthwick et al. demonstrated that PA-activated monocytic cells can enhance TGF-β1-driven EMT in primary bronchial epithelial cells (106). These observations shed light on the association between PA infection and the increased likelihood of developing obliterative bronchiolitis following lung transplantation.
Overall, PA exerts its pathogenic effects through a multifaceted approach, including the production of ROS, inhibition of mucociliary blanket, and induction of goblet cell hyperplasia and metaplasia, mucus hypersecretion, and the promotion of EMT. A brief overview of documented PA-derived metabolites and VOCs, along with their implicated roles in respiratory epithelial pathology, is summarized in Table 3.
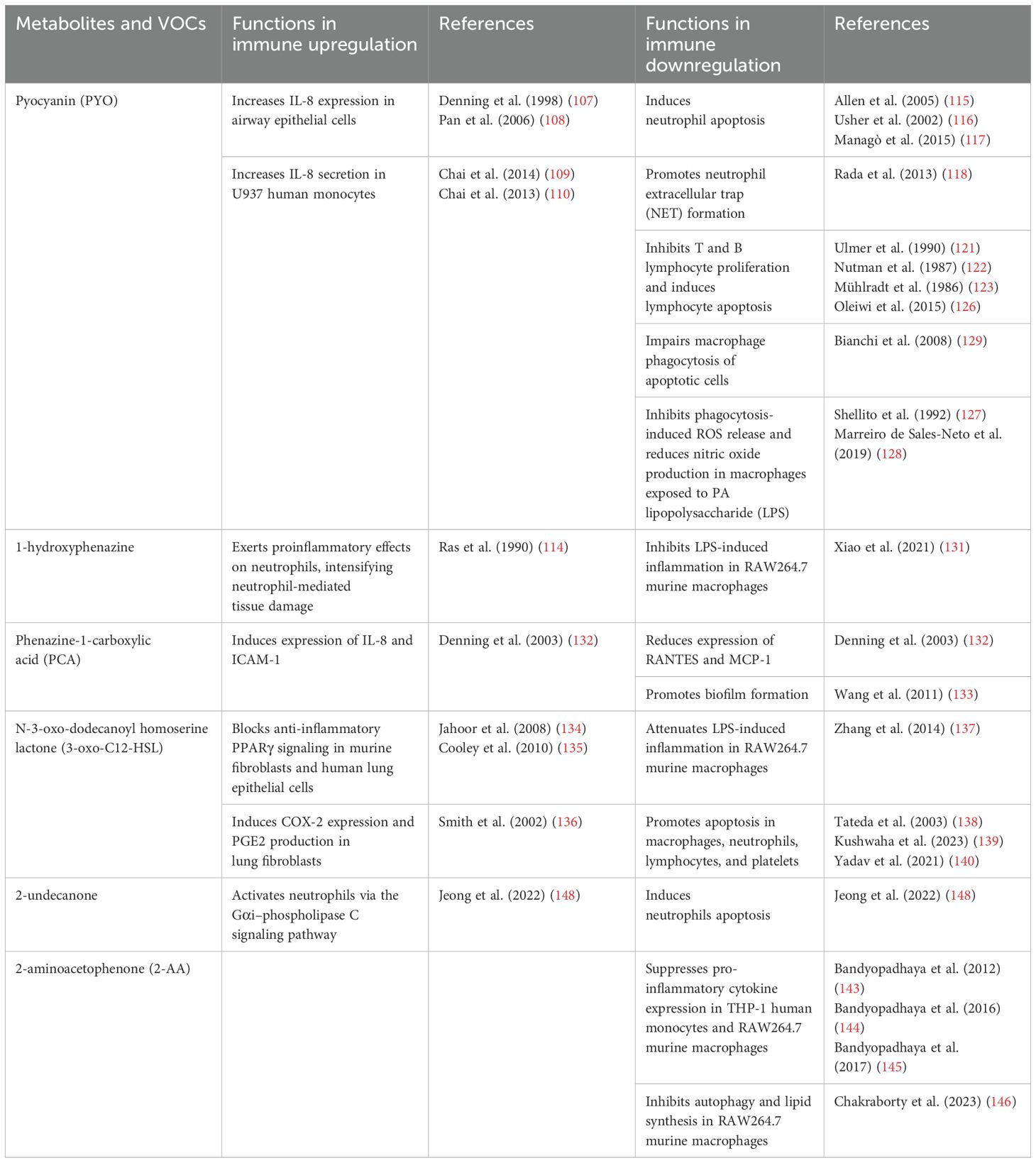
5 Influence of PA-derived metabolites on lung inflammation
Chronic inflammation is interconnected with mucus dysregulation and has a bidirectional relationship, that is, each of the two events serves as a cause for the other. Thus, PA-derived metabolites exacerbate pulmonary inflammation and disease courses through multiple mechanisms. It was noted that from the point of their role in lung inflammation, several metabolites have a significant influence on respiratory health and disease, as will be further discussed in the following section. Interestingly, these metabolites often exert a dual role in modulating inflammation during PA infection, promoting neutrophil chemotaxis while concurrently impairing host defense mechanisms. A comparison of PA-derived metabolites and VOCs involved in immune modulation during infection is presented in Table 4.
5.1 Pyocyanin
PYO, in particular, plays a complex role in modulating inflammation during PA infection. First, PYO has several mechanisms that promote inflammation, and is known to increase the expression of interleukin-8 (IL-8) in airway epithelial cells that involve oxidative stress and kinase signaling pathways (107, 108). Additionally, it acts in synergy with pro-inflammatory cytokines such as TNF-α and IL-1α resulting in an amplified production of IL-8 (107). Chai et al. conducted further studies that indicated PYO significantly increases IL-8 secretion in U937 cells, a human monocyte cell line, in a time- and concentration-dependent fashion. Their research suggests that this effect is mediated through the activation of specific signaling pathways, including protein kinase C (PKC), p38, and ERK mitogen-activated protein kinases (MAPKs), in addition to nuclear factor-kappa B (NF-κB) (109, 110). The antioxidant N-acetyl cysteine was found to effectively inhibit the expression of IL-8, suggesting a ROS-dependent mechanism (109). As a potent neutrophil chemoattractant, elevated IL-8 levels play a crucial role in driving the pronounced neutrophil infiltration frequently observed in PA infections. Neutrophils are central to the pathogenesis of CF and other respiratory disorders, where their elevated presence in lung tissue often intensifies the inflammatory response (111). Their accumulation, while aimed at clearing bacterial infections, inadvertently contributes to lung damage through the release of proteases, ROS, and pro-inflammatory cytokines, which can harm the surrounding tissues and exacerbate disease progression (112). In particular, neutrophils release neutrophil elastase, myeloperoxidase and H2O2, which are key components of the peroxidase system and potent contributors to oxidative stress (113). This oxidative stress, in turn, amplifies cellular damage and further escalates inflammatory responses in the lungs (114).
While PYO possesses pro-inflammatory properties, it is also able to inhibit various arms of the immune responses in neutrophils, lymphocytes, and macrophages. Even as it is extremely neutrophilic, PYO can induce neutrophil apoptosis, thereby hampering their host defense mechanisms and allowing PA to evade immune clearance (115, 116) through stimulation of mitochondrial ROS release and activation of mitochondrial acid sphingomyelinase (117). Moreover, PYO induces extracellular DNA (eDNA) and neutrophil extracellular traps (NETs) release in a dose-dependent manner, a process that requires NADPH oxidase and involves c-Jun N-terminal kinase (JNK) and phosphatidylinositol 3-kinase (PI3K) pathways (118). NETs in their turn escalate biofilm formation (119) the latter is a well-established driver of persistent infections that are difficult to eradicate (120). Besides, PYO exhibits dose-dependent effects on B and T lymphocyte function (121). PYO inhibits T lymphocyte proliferation by blocking the release of IL-2 and reducing IL-2 receptor expression on T cells (121–123). This inhibition reduces immunoglobulin secretion by B lymphocytes and decreases lymphocyte proliferation, ultimately leading to a diminished immune response against PA (123–125). Of note, it was shown that toxic effects on T and B lymphocyte proliferation could be induced by PYO concentrations as low as 0.5 µg/mL (121). This is further supported by subsequent study showing that PYO induces DNA fragmentation in human peripheral blood lymphocytes, leading to their apoptosis (126). PYO suppresses phagocytosis-induced ROS generation and subsequently decreases the production of nitric oxide in macrophages upon the treatment of PA’s lipopolysaccharides (LPS) (127). These results were further supported by an independent study showing that PYO exerts anti-inflammatory effects by downregulating the production of nitric oxide, TNF-α, and IL-1β in LPS-activated murine macrophages (128). Additionally, macrophage phagocytosis of apoptotic cells was also impaired by the presence of PYO, which was related to the generation of intracellular ROS and alterations in small GTPase signaling (129). These multiple effects of PYO on immune cells contribute to PA’s ability to evade host defenses and establish chronic infections, particularly in immunocompromised individuals (130). The important roles of PYO in infection and pulmonary inflammation are also summarized in Figure 2.
5.2 1-hydroxyphenazine and phenazine-1-carboxylic acid
Other phenazine compounds, such as 1-hydroxyphenazine and PCA, have also been reported to interfere with the host mucosal immune responses. Similar to PYO, 1-hydroxyphenazine has proinflammatory effects on neutrophils that may intensify neutrophil-mediated tissue damage during infection (114). Intriguingly, 1-hydroxyphenazine was later characterized as having anti-inflammatory activity toward murine macrophages, inhibiting LPS-induced inflammation in RAW264.7 cells in vitro (131). PCA has been found to induce expression of both IL-8 and ICAM-1, but simultaneously reduces the expression of RANTES and monocyte chemoattractant protein-1 (MCP-1) (132). In human airway epithelial cells, PCA is also linked to heightened intracellular oxidant generation (132). These activities are further inhibited by antioxidants, suggesting that oxidative stress is integral to these mechanisms (132). Furthermore, PCA is implicated in promoting bacterial biofilm formation through the acquisition of ferrous iron in the later stages of infection (133).
5.3 N-3-oxo-dodecanoyl homoserine lactone
The PA QS signaling molecule 3-oxo-C12-HSL can also modulate the function of a variety of mammalian cell types, including lymphocytes, macrophages, neutrophils, platelets, fibroblasts, and respiratory epithelial cells. By acting as an agonist of PPARβ/δ and antagonist of PPARγ, 3-oxo-C12-HSL induces proinflammatory responses in host cells by blocking anti-inflammatory PPARγ signaling in murine fibroblasts and human lung epithelial cells (134, 135). It also stimulates the formation of cyclooxygenase-2 and prostaglandin E2 production in lung fibroblasts, hence contributing to inflammation and lung pathology (136). Conversely, 3-oxo-C12-HSL attenuates LPS-induced inflammation in RAW264.7 mouse macrophage cell by activating the unfolded protein response, which suppresses NF-κB activation (137). 3-oxo-C12-HSL particularly facilitates the induction of apoptosis in diverse immune cells, including macrophages (138), neutrophils (138), lymphocytes (139), and platelets (140). These studies further reinforce the concept that QS AHLs not only regulate bacterial virulence but also modulates various cellular functions that are essential for host inflammation and immune defenses.
5.4 2-aminoacetophenone
The PA VOC, 2-AA, plays a complex role in inflammation and infection. 2-AA silences acute virulence functions while promoting chronic infection phenotypes in PA by modulating the virulence regulator MvfR and inducing biostability (33). Although not demonstrated in lungs, 2-AA has been shown to trigger mitochondrial dysfunction in skeletal muscle, reducing the rate of ATP synthesis and compromises muscle function (141, 142). A decline in energy production, coupled with mitochondrial dysfunction, may create conditions that favor infections and contribute to host tolerance of pathogens, promoting their persistence—an important step in establishing chronic infections (33, 141). Additionally, 2-AA has been found to regulate HDAC1 activity and NF−KB interactions, suppressing pro-inflammatory cytokine expression in human monocytes THP-1 cells and mouse macrophage RAW264.7 cell (143–145). Recently, Chakraborty et al. found that 2−AA inhibits murine macrophage processes such as autophagy and lipid synthesis (146) and re-wiring cellular bioenergetics through the PGC−1/ERR axis, reducing bacterial clearance (146, 147). Moreover, in mouse models of PA infection, pretreatment with 2-AA yields a higher survival rate compared to control mice, even with increased bacterial burden (143). Collectively, these observations suggest that 2-AA has a multifunctional role in PA infection, regulating immunological and metabolic processes to promote host tolerance and bacterial persistence, promoting chronic infection.
5.5 2-undecanone
Another VOC 2-undecanone, which is produced by PA during infection, has recently been identified as a potent activator of neutrophils through the Gαi-phospholipase C pathway. However, this activation subsequently leads to a reduction in the bactericidal capabilities and promotes apoptosis of neutrophils, potentially aiding PA in escaping immune detection (148).
6 Conclusion
There is a burgeoning interest in microbial VOCs, with a growing number of research efforts focused on understanding their production and functional roles. In this review, we summarize the major species of PA-derived VOCs and discuss the potential and limitations of VOCs in the non-invasive diagnosis of chronic lung infections, calling for more intensified translational research to bridge in vitro and in vivo findings. Advances in analytical techniques are enabling increasingly broader VOC profiling, steering away from individual biomarkers and towards more comprehensive metabolic profiles that better represent PA infections in the clinical niche. PA-derived secondary metabolites, including VOCs, initiate a multifaceted array of signaling pathways and molecular events in airway epithelial cells, leading to epithelial and ciliary injury, mucostasis, EMT, and disturbed local immune responses. These mechanisms include the activation of oxidative stress pathways, ER stress, inflammatory signaling, mucin gene regulation, and more. In addition, the influence of PA metabolites on lung inflammation presents multifaceted interactions between pathogenicity and the host immune response. The phenazines PYO, 1-hydroxyphenazine, and PCA represent how PA-metabolites can worsen and moderate inflammatory processes in the various subpopulations of immune cells in lungs. PYO, through its twin role in triggering neutrophil infiltration and simultaneously inactivating their host defense functions, highlights the complexities of the inflammatory response to PA infection. Also, 3-oxo-C12-HSL as well as 2-AA are other metabolites that showcase the delicate connection between the host immune system and the QS communication of bacteria. This review also highlights PA-derived metabolites’ participation in chronic lung inflammation and development of the disease course. Deeper insights into these complex interactions and disease mechanisms opens avenues for targeting PA metabolites and virulence factors in therapeutic and diagnostic strategies, improving outcomes in PA infections.
Author contributions
SK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. GL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the United States National Institute of Health grants 1 R01HL142626 and 1 R21AI171524A1, and the United States Department of Defense US Army Medical Research Acquisition Peer Reviewed Medical Research Program grant HT9425-23-1-0372 to GL.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Garcia-Clemente M, de la Rosa D, Máiz L, Girón R, Blanco M, Olveira C, et al. Impact of pseudomonas aeruginosa infection on patients with chronic inflammatory airway diseases. J Clin Med. (2020) 9:3800. doi: 10.3390/jcm9123800
2. Mayer-Hamblett N, Kronmal RA, Gibson RL, Rosenfeld M, Retsch-Bogart G, Treggiari MM, et al. Initial Pseudomonas aeruginosa treatment failure is associated with exacerbations in cystic fibrosis. Pediatr Pulmonol. (2012) 47:125–34. doi: 10.1002/ppul.21525
3. Nixon GM, Armstrong DS, Carzino R, Carlin JB, Olinsky A, Robertson CF, et al. Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J Pediatr. (2001) 138:699–704. doi: 10.1067/mpd.2001.112897
4. Schmidt R, Cordovez V, De Boer W, Raaijmakers J, and Garbeva P. Volatile affairs in microbial interactions. ISME J. (2015) 9:2329–35. doi: 10.1038/ismej.2015.42
5. Sethi S, Nanda R, and Chakraborty T. Clinical application of volatile organic compound analysis for detecting infectious diseases. Clin Microbiol Rev. (2013) 26:462–75. doi: 10.1128/CMR.00020-13
6. Ahmed WM, Lawal O, Nijsen TM, Goodacre R, and Fowler SJ. Exhaled volatile organic compounds of infection: a systematic review. ACS Infect Dis. (2017) 3:695–710. doi: 10.1021/acsinfecdis.7b00088
7. Ratiu IA, Bocos-Bintintan V, Monedeiro F, Milanowski M, Ligor T, and Buszewski B. An optimistic vision of future: diagnosis of bacterial infections by sensing their associated volatile organic compounds. Crit Rev Anal Chem. (2020) 50:501–12. doi: 10.1080/10408347.2019.1663147
8. Jünger M, Vautz W, Kuhns M, Hofmann L, Ulbricht S, Baumbach JI, et al. Ion mobility spectrometry for microbial volatile organic compounds: a new identification tool for human pathogenic bacteria. Appl Microbiol Biotechnol. (2012) 93:2603–14. doi: 10.1007/s00253-012-3924-4
9. Huang S, Wang X, Chen X, Liu X, Xu Q, Zhang L, et al. Rapid and sensitive detection of Pseudomonas aeruginosa by isothermal amplification combined with Cas12a-mediated detection. Sci Rep. (2023) 13:19199. doi: 10.1038/s41598-023-45766-0
10. Smith WD, Bardin E, Cameron L, Edmondson CL, Farrant KV, Martin I, et al. Current and future therapies for Pseudomonas aeruginosa infection in patients with cystic fibrosis. FEMS Microbiol Lett. (2017) 364:fnx121. doi: 10.1093/femsle/fnx121
11. Bos LDJ, Sterk PJ, and Schultz MJ. Volatile metabolites of pathogens: a systematic review. PloS Pathog. (2013) 9:e1003311. doi: 10.1371/journal.ppat.1003311
12. Robroeks CMHHT, Van Berkel JJBN, Dallinga JW, Jöbsis Q, Zimmermann LJI, Hendriks HJE, et al. Metabolomics of volatile organic compounds in cystic fibrosis patients and controls. Pediatr Res. (2010) 68:75–80. doi: 10.1203/PDR.0b013e3181df4ea0
13. Scott-Thomas AJ, Syhre M, Pattemore PK, Epton M, Laing R, Pearson J, et al. 2-Aminoacetophenone as a potential breath biomarker for Pseudomonas aeruginosa in the cystic fibrosis lung. BMC Pulm Med. (2010) 10:56. doi: 10.1186/1471-2466-10-56
14. Savelev SU, Perry JD, Bourke SJ, Jary H, Taylor R, Fisher AJ, et al. Volatile biomarkers of Pseudomonas aeruginosa in cystic fibrosis and noncystic fibrosis bronchiectasis. Lett Appl Microbiol. (2011) 52:610–3. doi: 10.1111/j.1472-765X.2011.03049.x
15. Gilchrist FJ, Belcher J, Jones AM, Smith D, Smyth AR, Southern KW, et al. Exhaled breath hydrogen cyanide as a marker of early pseudomonas aeruginosa infection in children with cystic fibrosis. ERJ Open Res. (2015) 1:00044–2015. doi: 10.1183/23120541.00044-2015
16. Enderby B, Smith D, Carroll W, and Lenney W. Hydrogen cyanide as a biomarker for Pseudomonas aeruginosa in the breath of children with cystic fibrosis. Pediatr Pulmonol. (2009) 44:142–7. doi: 10.1002/ppul.20963
17. Gilchrist FJ, Bright-Thomas RJ, Jones AM, Smith D, Španěl P, Webb AK, et al. Hydrogen cyanide concentrations in the breath of adult cystic fibrosis patients with and without Pseudomonas aeruginosa infection. J Breath Res. (2013) 7:26010. doi: 10.1088/1752-7155/7/2/026010
18. Shestivska V, Nemec A, Dřevínek P, Sovová K, Dryahina K, and Spaněl P. Quantification of methyl thiocyanate in the headspace of Pseudomonas aeruginosa cultures and in the breath of cystic fibrosis patients by selected ion flow tube mass spectrometry. Rapid Commun Mass Spectrom. (2011) 25:2459–67. doi: 10.1002/rcm.5146
19. Wang T, Carroll W, Lenny W, Boit P, and Smith D. The analysis of 1-propanol and 2-propanol in humid air samples using selected ion flow tube mass spectrometry. Rapid Commun Mass Spectrom. (2006) 20:125–30. doi: 10.1002/rcm.2285
20. Goeminne PC, Vandendriessche T, Van Eldere J, Nicolai BM, Hertog MLATM, and Dupont LJ. Detection of Pseudomonas aeruginosa in sputum headspace through volatile organic compound analysis. Respir Res. (2012) 13:87. doi: 10.1186/1465-9921-13-87
21. Nasir M, Bean HD, Smolinska A, Rees CA, Zemanick ET, and Hill JE. Volatile molecules from bronchoalveolar lavage fluid can “rule-in” Pseudomonas aeruginosa and “rule-out” Staphylococcus aureus infections in cystic fibrosis patients. Sci Rep. (2018) 8:826. doi: 10.1038/s41598-017-18491-8
22. Preti G, Thaler E, Hanson CW, Troy M, Eades J, and Gelperin A. Volatile compounds characteristic of sinus-related bacteria and infected sinus mucus: analysis by solid-phase microextraction and gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol BioMed Life Sci. (2009) 877:2011–8. doi: 10.1016/j.jchromb.2009.05.028
23. Smith D, Španěl P, Gilchrist FJ, and Lenney W. Hydrogen cyanide, a volatile biomarker of Pseudomonas aeruginosa infection. J Breath Res. (2013) 7:44001. doi: 10.1088/1752-7155/7/4/044001
24. Ryall B, Davies JC, Wilson R, Shoemark A, and Williams HD. Pseudomonas aeruginosa, cyanide accumulation and lung function in CF and non-CF bronchiectasis patients. Eur Respir J. (2008) 32:740–7. doi: 10.1183/09031936.00159607
25. Carrol W, Lenney W, Wang T, Španěl P, Alcock A, and Smith D. Detection of volatile compounds emitted by Pseudomonas aeruginosa using selected ion flow tube mass spectrometry. Pediatr Pulmonol. (2005) 39:452–6. doi: 10.1002/ppul.20170
26. Gilchrist FJ, Alcock A, Belcher J, Brady M, Jones A, Smith D, et al. Variation in hydrogen cyanide production between different strains of Pseudomonas aeruginosa. Eur Respir J. (2011) 38:409–14. doi: 10.1183/09031936.00166510
27. Zhu J, Bean HD, Kuo YM, and Hill JE. Fast detection of volatile organic compounds from bacterial cultures by secondary electrospray ionization-mass spectrometry. J Clin Microbiol. (2010) 48:4426–31. doi: 10.1128/JCM.00392-10
28. Thorn RMS, Reynolds DM, and Greenman J. Multivariate analysis of bacterial volatile compound profiles for discrimination between selected species and strains in vitro. J Microbiol Methods. (2011) 84:258–64. doi: 10.1016/j.mimet.2010.12.001
29. Cox CD and Parker J. Use of 2-aminoacetophenone production in identification of Pseudomonas aeruginosa. J Clin Microbiol. (1979) 9:479–84. doi: 10.1128/jcm.9.4.479-484.1979
30. Labows JN, McGinley KJ, Webster GF, and Leyden JJ. Headspace analysis of volatile metabolites of Pseudomonas aeruginosa and related species by gas chromatography-mass spectrometry. J Clin Microbiol. (1980) 12:521–6. doi: 10.1128/jcm.12.4.521-526.1980
31. Shestivska V, Španěl P, Dryahina K, Sovová K, Smith D, Musílek M, et al. Variability in the concentrations of volatile metabolites emitted by genotypically different strains of Pseudomonas aeruginosa. J Appl Microbiol. (2012) 113:701–13. doi: 10.1111/j.1365-2672.2012.05370.x
32. Ahmed WM, Fenn D, White IR, Dixon B, Nijsen TME, Knobel HH, et al. Microbial volatiles as diagnostic biomarkers of bacterial lung infection in mechanically ventilated patients. Clin Infect Dis. (2023) 76:1059–66. doi: 10.1093/cid/ciac859
33. Kesarwani M, Hazan R, He J, Que Y, Apidianakis Y, Lesic B, et al. A quorum sensing regulated small volatile molecule reduces acute virulence and promotes chronic infection phenotypes. PloS Pathog. (2011) 7:e1002192. doi: 10.1371/journal.ppat.1002192
34. Zechman JM and Labows JN. Volatiles of Pseudomonas aeruginosa and related species by automated headspace concentration - gas chromatography. Can J Microbiol. (1985) 31:232–7. doi: 10.1139/m85-045
35. Filipiak W, Sponring A, Baur MM, Filipiak A, Ager C, Wiesenhofer H, et al. Molecular analysis of volatile metabolites released specifically by Staphylococcus aureus and Pseudomonas aeruginosa. BMC Microbiol. (2012) 12:113. doi: 10.1186/1471-2180-12-113
36. Bean HD, Rees CA, and Hill JE. Comparative analysis of the volatile metabolomes of Pseudomonas aeruginosa clinical isolates. J Breath Res. (2016) 10:47102. doi: 10.1088/1752-7155/10/4/047102
37. Davis TJ, Karanjia AV, Bhebhe CN, West SB, Richardson M, and Bean HD. Pseudomonas aeruginosa volatilome characteristics and adaptations in chronic cystic fibrosis lung infections. mSphere. (2020) 5:e00843–20. doi: 10.1128/msphere.00843-20
38. Lawal O, Knobel H, Weda H, Bos LD, Nijsen TME, Goodacre R, et al. Volatile organic compound signature from co-culture of lung epithelial cell line with: Pseudomonas aeruginosa. Analyst. (2018) 143:3148–55. doi: 10.1039/c8an00759d
39. Allardyce RA, Langford VS, Hill AL, and Murdoch DR. Detection of volatile metabolites produced by bacterial growth in blood culture media by selected ion flow tube mass spectrometry (SIFT-MS). J Microbiol Methods. (2006) 65:361–5. doi: 10.1016/j.mimet.2005.09.003
40. Zhu J, Bean HD, Wargo MJ, Leclair LW, and Hill JE. Detecting bacterial lung infections: In vivo evaluation of in vitro volatile fingerprints. J Breath Res. (2013) 7:16003. doi: 10.1088/1752-7155/7/1/016003
41. Bean HD, Jiménez-Díaz J, Zhu J, and Hill JE. Breathprints of model murine bacterial lung infections are linked with immune response. Eur Respir J. (2015) 45:181–90. doi: 10.1183/09031936.00015814
42. Fenn D, Ahmed WM, Lilien TA, Kos R, Tuip de Boer AM, Fowler SJ, et al. Influence of bacterial and alveolar cell co-culture on microbial VOC production using HS-GC/MS. Front Mol Biosci. (2023) 10:1160106. doi: 10.3389/fmolb.2023.1160106
43. Lawal O, Muhamadali H, Ahmed WM, White IR, Nijsen TME, Goodacre R, et al. Headspace volatile organic compounds from bacteria implicated in ventilator-associated pneumonia analysed by TD-GC/MS. J Breath Res. (2018) 12:026002. doi: 10.1088/1752-7163/aa8efc
44. Gilchrist FJ, Sims H, Alcock A, Belcher J, Jones AM, Smith D, et al. Quantification of hydrogen cyanide and 2-aminoacetophenone in the headspace of Pseudomonas aeruginosa cultured under biofilm and planktonic conditions. Anal Methods. (2012) 4:3661–5. doi: 10.1039/c2ay25652e
45. Koehler T, Wingender J, Lueling M, Meckelmann SW, Telgheder U, and Schmitz OJ. Characterization of the extracellular volatile metabolome of Pseudomonas aeruginosa applying an in vitro biofilm model under cystic fibrosis-like conditions. Front Biosci (Landmark Ed). (2022) 27:156. doi: 10.31083/j.fbl2705156
46. Scott-Thomas A, Pearson J, and Chambers S. Potential sources of 2-aminoacetophenone to confound the Pseudomonas aeruginosa breath test, including analysis of a food challenge study. J Breath Res. (2011) 5:46002. doi: 10.1088/1752-7155/5/4/046002
47. Wilson AD and Baietto M. Advances in electronic-nose technologies developed for biomedical applications. Sensors. (2011) 11:1105–76. doi: 10.3390/s110101105
48. Ding J, Yang S, Liang D, Chen H, Wu Z, Zhang L, et al. Development of extractive electrospray ionization ion trap mass spectrometry for in vivo breath analysis. Analyst. (2009) 134:2040–50. doi: 10.1039/b821497b
49. Smith D and Španěl P. Direct, rapid quantitative analyses of BVOCs using SIFT-MS and PTR-MS obviating sample collection. Trends Anal Chem. (2011) 30:945–59. doi: 10.1016/j.trac.2011.05.001
50. Thorn RMS and Greenman J. Microbial volatile compounds in health and disease conditions. J Breath Res. (2012) 6:24001. doi: 10.1088/1752-7155/6/2/024001
51. Kramer R, Sauer-Heilborn A, Welte T, Guzman CA, Höfle MG, and Abraham WR. A rapid method for breath analysis in cystic fibrosis patients. Eur J Clin Microbiol Infect Dis. (2015) 34:745–51. doi: 10.1007/s10096-014-2286-5
52. Fowler SJ, Basanta-Sanchez M, Xu Y, Goodacre R, and Dark PM. Surveillance for lower airway pathogens in mechanically ventilated patients by metabolomic analysis of exhaled breath: A case-control study. Thorax. (2015) 70:320–5. doi: 10.1136/thoraxjnl-2014-206273
53. Schnabel R, Fijten R, Smolinska A, Dallinga J, Boumans ML, Stobberingh E, et al. Analysis of volatile organic compounds in exhaled breath to diagnose ventilator-associated pneumonia. Sci Rep. (2015) 5:17179. doi: 10.1038/srep17179
54. Van Oort PMP, Nijsen TM, White IR, Knobel HH, Felton T, Rattray N, et al. Untargeted molecular analysis of exhaled breath as a diagnostic test for ventilator-associated lower respiratory tract infections (BreathDx). Thorax. (2022) 77:79–81. doi: 10.1136/thoraxjnl-2021-217362
55. Suarez-Cuartin G, Giner J, Merino JL, Rodrigo-Troyano A, Feliu A, Perea L, et al. Identification of Pseudomonas aeruginosa and airway bacterial colonization by an electronic nose in bronchiectasis. Respir Med. (2018) 136:111–7. doi: 10.1016/j.rmed.2018.02.008
56. Joensen O, Paff T, Haarman EG, Skovgaard IM, Jensen P, Bjarnsholt T, et al. Exhaled breath analysis using electronic nose in cystic fibrosis and primary ciliary dyskinesia patients with chronic pulmonary infections. PloS One. (2014) 9:e115584. doi: 10.1371/journal.pone.0115584
57. Gonçalves T and Vasconcelos U. Colour me blue: The history and the biotechnological potential of pyocyanin. Molecules. (2021) 26:927. doi: 10.3390/molecules26040927
58. Schneider S, Ettenauer J, Pap IJ, Aspöck C, Walochnik J, and Brandl M. Main metabolites of pseudomonas aeruginosa: A study of electrochemical properties. Sensors. (2022) 22:4694. doi: 10.3390/s22134694
59. Lau GW, Hassett DJ, Ran H, and Kong F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol Med. (2004) 10:599–606. doi: 10.1016/j.molmed.2004.10.002
60. Rada B and Leto TL. Pyocyanin effects on respiratory epithelium: Relevance in Pseudomonas aeruginosa airway infections. Trends Microbiol. (2013) 21:73–81. doi: 10.1016/j.tim.2012.10.004
61. Murray TS, Egan M, and Kazmierczak BI. Pseudomonas aeruginosa chronic colonization in cystic fibrosis patients. Curr Opin Pediatr. (2007) 19:83–8. doi: 10.1097/MOP.0b013e3280123a5d
62. Courtney JM, Bradley J, Mccaughan J, O’connor TM, Shortt C, Bredin CP, et al. Predictors of mortality in adults with cystic fibrosis. Pediatr Pulmonol. (2007) 42:525–32. doi: 10.1002/ppul.20619
63. Smith RS and Iglewski BHP. aeruginosa quorum-sensing systems and virulence. Curr Opin Microbiol. (2003) 6:56–60. doi: 10.1016/S1369-5274(03)00008-0
64. Lee J and Zhang L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell. (2015) 6:26–41. doi: 10.1007/s13238-014-0100-x
65. Collier DN, Anderson L, McKnight SL, Noah TL, Knowles M, Boucher R, et al. A bacterial cell to cell signal in the lungs of cystic fibrosis patients. FEMS Microbiol Lett. (2002) 215:41–6. doi: 10.1016/S0378-1097(02)00937-0
66. Venturi V. Regulation of quorum sensing in Pseudomonas. FEMS Microbiol Rev. (2006) 30:274–91. doi: 10.1111/j.1574-6976.2005.00012.x
67. Qin S, Xiao W, Zhou C, Pu Q, Deng X, Lan L, et al. Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct Target Ther. (2022) 7:199. doi: 10.1038/s41392-022-01056-1
68. Cox CD and Adams P. Siderophore activity of pyoverdin for Pseudomonas aeruginosa. Infect Immun. (1985) 48:130–8. doi: 10.1128/iai.48.1.130-138.1985
69. Diggle SP and Whiteley M. Microbe profile: Pseudomonas aeruginosa: Opportunistic pathogen and lab rat. Microbiol (United Kingdom). (2020) 166:30–3. doi: 10.1099/mic.0.000860
70. Hentzer M, Teitzel GM, Balzer GJ, Heydorn A, Molin S, Givskov M, et al. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J Bacteriol. (2001) 183:5395–401. doi: 10.1128/JB.183.18.5395-5401.2001
71. Cabral DA, Loh BA, and Speert DP. Mucoid Pseudomonas aeruginosa resists nonopsonic phagocytosis by human neutrophils and macrophages. Pediatr Res. (1987) 22:429–31. doi: 10.1203/00006450-198710000-00013
72. Pier GB, Coleman F, Grout M, Franklin M, and Ohman DE. Role of alginate O acetylation in resistance of mucoid Pseudomonas aeruginosa to opsonic phagocytosis. Infect Immun. (2001) 69:1895–901. doi: 10.1128/IAI.69.3.1895-1901.2001
73. Grant Stiver H, Zachidniak K, and Speert DP. Inhibition of polymorphonuclear leukocyte chemotaxis by the mucoid exopolysaccharide of Pseudomonas aeruginosa. Clin Invest Med. (1988) 11:247–52.
74. Anwar H, Dasgupta M, Lam K, and Costerton JW. Tobramycin resistance of mucoid Pseudomonas aeruginosa biofilm grown under iron limitation. J Antimicrob Chemother. (1989) 24:647–55. doi: 10.1093/jac/24.5.647
75. Hodges NA and Gordon CA. Protection of Pseudomonas aeruginosa against ciprofloxacin and β-lactams by homologous alginate. Antimicrob Agents Chemother. (1991) 35:2450–2. doi: 10.1128/AAC.35.11.2450
76. Nichols WW, Dorrington SM, Slack MPE, and Walmsley HL. Inhibition of tobramycin diffusion by binding to alginate. Antimicrob Agents Chemother. (1988) 32:518–23. doi: 10.1128/AAC.32.4.518
77. Parker D and Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol. (2011) 45:189–201. doi: 10.1165/rcmb.2011-0011RT
78. Aghapour M, Raee P, Moghaddam SJ, Hiemstra PS, and Heijink IH. Airway epithelial barrier dysfunction in chronic obstructive pulmonary disease: Role of cigarette smoke exposure. Am J Respir Cell Mol Biol. (2018) 58:157–69. doi: 10.1165/rcmb.2017-0200TR
79. Gloyne LS, Grant GD, Perkins AV, Powell KL, McDermott CM, Johnson PV, et al. Pyocyanin-induced toxicity in A549 respiratory cells is causally linked to oxidative stress. Toxicol In Vitro. (2011) 25:1353–8. doi: 10.1016/j.tiv.2011.05.004
80. Schwarzer C, Fischer H, Kim EJ, Barber KJ, Mills AD, Kurth MJ, et al. Oxidative stress caused by pyocyanin impairs CFTR Cl- transport in human bronchial epithelial cells. Free Radic Biol Med. (2008) 45:1653–62. doi: 10.1016/j.freeradbiomed.2008.09.011
81. Maurice NM, Bedi B, Yuan Z, Goldberg JB, Koval M, Hart CM, et al. Pseudomonas aeruginosa induced host epithelial cell mitochondrial dysfunction. Sci Rep. (2019) 9:11929. doi: 10.1038/s41598-019-47457-1
82. Rieger B, Thierbach S, Ommer M, Dienhart FSV, Fetzner S, and Busch KB. Pseudomonas quinolone signal molecule PQS behaves like a B class inhibitor at the IQ site of mitochondrial complex I. FASEB Bioadv. (2020) 2:188–202. doi: 10.1096/fba.2019-00084
83. Castric PA. Hydrogen cyanide, a secondary metabolite of Pseudomonas aeruginosa. Can J Microbiol. (1975) 21:613–18. doi: 10.1139/m75-088
84. da Cruz Nizer WS, Adams ME, Inkovskiy V, Beaulieu C, and Overhage J. The secondary metabolite hydrogen cyanide protects Pseudomonas aeruginosa against sodium hypochlorite-induced oxidative stress. Front Microbiol. (2023) 14:1294518. doi: 10.3389/fmicb.2023.1294518
85. Chadha J, Harjai K, and Chhibber S. Revisiting the virulence hallmarks of Pseudomonas aeruginosa: a chronicle through the perspective of quorum sensing. Environ Microbiol. (2022) 24:2630–56. doi: 10.1111/1462-2920.15784
86. Zulianello L, Canard C, Köhler T, Caille D, Lacroix JS, and Meda P. Rhamnolipids are virulence factors that promote early infiltration of primary human airway epithelia by Pseudomonas aeruginosa. Infect Immun. (2006) 74:3134–47. doi: 10.1128/IAI.01772-05
87. Wilson R, Pitt T, Taylor G, Watson D, MacDermot J, Sykes D, et al. Pyocyanin and 1-hydroxyphenazine produced by Pseudomonas aeruginosa inhibit the beating of human respiratory cilia. vitro. J Clin Invest. (1987) 79:221–9. doi: 10.1172/JCI112787
88. Nair C, Shoemark A, Chan M, Ollosson S, Dixon M, Hogg C, et al. Cyanide levels found in infected cystic fibrosis sputum inhibit airway ciliary function. Eur Respir J. (2014) 44:1253–61. doi: 10.1183/09031936.00097014
89. Hao Y, Kuang Z, Walling BE, Bhatia S, Sivaguru M, Chen Y, et al. Pseudomonas aeruginosa pyocyanin causes airway goblet cell hyperplasia and metaplasia and mucus hypersecretion by inactivating the transcriptional factor FoxA2. Cell Microbiol. (2012) 14:401–15. doi: 10.1111/j.1462-5822.2011.01727.x
90. Choi W, Choe S, Lin J, Borchers MT, Kosmider B, Vassallo R, et al. Exendin-4 restores airway mucus homeostasis through the GLP1R-PKA-PPARγ-FOXA2-phosphatase signaling. Mucosal Immunol. (2020) 13:637–51. doi: 10.1038/s41385-020-0262-1
91. Hao Y, Kuang Z, Xu Y, Walling BE, and Lau GW. Pyocyanin-induced mucin production is associated with redox modification of FOXA2. Respir Res. (2013) 14:82. doi: 10.1186/1465-9921-14-82
92. Caldwell CC, Chen Y, Goetzmann HS, Hao Y, Borchers MT, Hassett DJ, et al. Pseudomonas aeruginosa exotoxin pyocyanin causes cystic fibrosis airway pathogenesis. Am J Pathol. (2009) 175:2473–88. doi: 10.2353/ajpath.2009.090166
93. Rada B, Gardina P, Myers TG, and Leto TL. Reactive oxygen species mediate inflammatory cytokine release and EGFR-dependent mucin secretion in airway epithelial cells exposed to Pseudomonas pyocyanin. Mucosal Immunol. (2011) 4:158–71. doi: 10.1038/mi.2010.62
94. Jeffries JL, Jia J, Choi W, Choe S, Miao J, Xu Y, et al. Pseudomonas aeruginosa pyocyanin modulates mucin glycosylation with sialyl-Lewis x to increase binding to airway epithelial cells. Mucosal Immunol. (2016) 9:1039–50. doi: 10.1038/mi.2015.119
95. Grootjans J, Kaser A, Kaufman RJ, and Blumberg RS. The unfolded protein response in immunity and inflammation. Nat Rev Immunol. (2016) 16:469–84. doi: 10.1038/nri.2016.62
96. Wang X, Yang X, Li Y, Wang X, Zhang Y, Dai X, et al. Lyn kinase represses mucus hypersecretion by regulating IL-13-induced endoplasmic reticulum stress in asthma. EBioMedicine. (2017) 15:137–49. doi: 10.1016/j.ebiom.2016.12.010
97. van ‘t Wout EFA, van SChadewijk A, van Boxtel R, Dalton LE, Clarke HJ, Tommassen J, et al. Virulence factors of Pseudomonas aeruginosa induce both the unfolded protein and integrated stress responses in airway epithelial cells. PloS Pathog. (2015) 11:e1004946. doi: 10.1371/journal.ppat.1004946
98. Kim MH, Bae CH, Choi YS, Na HG, Song SY, and Kim YD. Endoplasmic reticulum stress induces MUC5AC and MUC5B expression in human nasal airway epithelial cells. Clin Exp Otorhinolaryngol. (2019) 12:181–9. doi: 10.21053/ceo.2018.00493
99. Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. (2006) 124:587–99. doi: 10.1016/j.cell.2005.11.040
100. Bradley KL, Stokes CA, Marciniak SJ, Parker LC, and Condliffe AM. Role of unfolded proteins in lung disease. Thorax. (2021) 76:92–9. doi: 10.1136/thoraxjnl-2019-213738
101. Carlier FM, de Fays C, and Pilette C. Epithelial barrier dysfunction in chronic respiratory diseases. Front Physiol. (2021) 12:691227. doi: 10.3389/fphys.2021.691227
102. Rout-Pitt N, Farrow N, Parsons D, and Donnelley M. Epithelial mesenchymal transition (EMT): A universal process in lung diseases with implications for cystic fibrosis pathophysiology. Respir Res. (2018) 19:136. doi: 10.1186/s12931-018-0834-8
103. Raby KL, Michaeloudes C, Tonkin J, Chung KF, and Bhavsar PK. Mechanisms of airway epithelial injury and abnormal repair in asthma and COPD. Front Immunol. (2023) 14:1201658. doi: 10.3389/fimmu.2023.1201658
104. Das D, Chiu HJ, Farr CL, Grant JC, Jaroszewski L, Knuth MW, et al. Crystal structure of a putative quorum sensing-regulated protein (PA3611) from the Pseudomonas-specific DUF4146 family. Proteins. (2014) 82:1086–92. doi: 10.1002/prot.24455
105. Shu L, Chen S, Lin S, Lin H, Shao Y, Yao J, et al. The Pseudomonas aeruginosa secreted protein PA3611 promotes bronchial epithelial cell epithelial-mesenchymal transition via integrin αvβ6-mediated TGF-β1-induced p38/NF-κB pathway activation. Front Microbiol. (2022) 12:763749. doi: 10.3389/fmicb.2021.763749
106. Borthwick LA, Sunny SS, Oliphant V, Perry J, Brodlie M, Johnson GE, et al. Pseudomonas aeruginosa accentuates epithelial-to-mesenchymal transition in the airway. Eur Respir J. (2011) 37:1237–47. doi: 10.1183/09031936.00088410
107. Denning GM, Wollenwebber LA, Railsback MA, Cox CD, Stoll LL, and Britigan BE. Pseudomonas pyocyanin increases interleukin-8 expression by human airway epithelial cells. Infect Immun. (1998) 66:5777–84. doi: 10.1128/iai.66.12.5777-5784.1998
108. Pan NY, Hui WS, Tipoe GL, Taylor GW, Leung RYH, Lam WK, et al. Inhibition of pyocyanin-potentiated IL-8 release by steroids in bronchial epithelial cells. Respir Med. (2006) 100:1614–22. doi: 10.1016/j.rmed.2005.12.003
109. Chai W, Zhang J, Duan Y, Pan D, Liu W, Li Y, et al. Pseudomonas pyocyanin stimulates IL-8 expression through MAPK and NF-κB pathways in differentiated U937 cells. BMC Microbiol. (2014) 14:26. doi: 10.1186/1471-2180-14-26
110. Chai W, Zhang J, Zhu Z, Liu W, Pan D, Li Y, et al. Pyocyanin from Pseudomonas induces IL-8 production through the PKC and NF-κB pathways in U937 cells. Mol Med Rep. (2013) 8:1404–10. doi: 10.3892/mmr.2013.1662
111. Cabrini G, Rimessi A, Borgatti M, Lampronti I, Finotti A, Pinton P, et al. Role of cystic fibrosis bronchial epithelium in neutrophil chemotaxis. Front Immunol. (2020) 11:1438. doi: 10.3389/fimmu.2020.01438
112. Lauredo IT, Sabater JR, Ahmed A, Botvinnikova Y, and Abraham WM. Mechanism of pyocyanin- and 1-hydroxyphenazine-induced lung neutrophilia in sheep airways. J Appl Physiol. (1998) 85:2298–304. doi: 10.1152/jappl.1998.85.6.2298
113. Reszka KJ, O’Malley Y, McCormick ML, Denning GM, and Britigan BE. Oxidation of pyocyanin, a cytotoxic product from Pseudomonas aeruginosa, by microperoxidase 11 and hydrogen peroxide. Free Radic Biol Med. (2004) 36:1448–59. doi: 10.1016/j.freeradbiomed.2004.03.011
114. Ras GJ, Anderson R, Taylor GW, Savage JE, Van Niekerk E, Wilson R, et al. Proinflammatory interactions of pyocyanin and 1-hydroxyphenazine with human neutrophils in vitro. J Infect Dis. (1990) 162:178–85. doi: 10.1093/infdis/162.1.178
115. Allen L, Dockrell DH, Pattery T, Lee DG, Cornelis P, Hellewell PG, et al. Pyocyanin production by Pseudomonas aeruginosa induces neutrophil apoptosis and impairs neutrophil-mediated host defenses. vivo. J Immunol. (2005) 174:3643–9. doi: 10.4049/jimmunol.174.6.3643
116. Usher LR, Lawson RA, Geary I, Taylor CJ, Bingle CD, Taylor GW, et al. Induction of neutrophil apoptosis by the Pseudomonas aeruginosa exotoxin pyocyanin: a potential mechanism of persistent infection. J Immunol. (2002) 168:1861–8. doi: 10.4049/jimmunol.168.4.1861
117. Managò A, Becker KA, Carpinteiro A, Wilker B, Soddemann M, Seitz AP, et al. Pseudomonas aeruginosa pyocyanin induces neutrophil death via mitochondrial reactive oxygen species and mitochondrial acid sphingomyelinase. Antioxid Redox Signal. (2015) 22:1097–110. doi: 10.1089/ars.2014.5979
118. Rada B, Jendrysik MA, Pang L, Hayes CP, Yoo DG, Park JJ, et al. Pyocyanin-enhanced neutrophil extracellular trap formation requires the NADPH oxidase. PloS One. (2013) 8:e54205. doi: 10.1371/journal.pone.0054205
119. Papayannopoulos V. Neutrophils facing biofilms: the battle of the barriers. Cell Host Microbe. (2019) 25:477–9. doi: 10.1016/j.chom.2019.03.014
120. Abdelaziz AA, Kamer AMA, Al-Monofy KB, and Al-Madboly LA. Pseudomonas aeruginosa’s greenish-blue pigment pyocyanin: its production and biological activities. Microb Cell Fact. (2023) 22:110. doi: 10.1186/s12934-023-02122-1
121. Ulmer AJ, Pryjma J, Tarnok Z, Ernst M, and Flad HD. Inhibitory and stimulatory effects of Pseudomonas aeruginosa pyocyanine on human T and B lymphocytes and human monocytes. Infect Immun. (1990) 58:808–15. doi: 10.1128/iai.58.3.808-815.1990
122. Nutman J, Berger M, Chase PA, Dearborn DG, Miller KM, Waller RL, et al. Studies on the mechanism of T cell inhibition by the Pseudomonas aeruginosa phenazine pigment pyocyanine. J Immunol. (1987) 138:3481–7. doi: 10.4049/jimmunol.138.10.3481
123. Mühlradt PF, Tsai H, and Conradt P. Effects of pyocyanine, a blue pigment from Pseudomonas aeruginosa, on separate steps of T cell activation: Interleukin 2 (IL 2) production, IL 2 receptor formation, proliferation and induction of cytolytic activity. Eur J Immunol. (1986) 16:434–40. doi: 10.1002/eji.1830160421
124. Ran H, Hassett DJ, and Lau GW. Human targets of Pseudomonas aeruginosa pyocyanin. Proc Natl Acad Sci. (2003) 100:14315–20. doi: 10.1073/pnas.2332354100
125. Hall S, McDermott C, Anoopkumar-Dukie S, McFarland AJ, Forbes A, Perkins AV, et al. Cellular effects of pyocyanin, a secreted virulence factor of Pseudomonas aeruginosa. Toxins. (2016) 8:236. doi: 10.3390/toxins8080236
126. Oleiwi SR. Study the Effect of pyocyanin extracted from Pseudomonas aeruginosa on DNA fragmentation of human lymphocytes cells. Iraqi J Sci. (2015) 56:1366–71.
127. Shellito J, Nelson S, and Sorensen RU. Effect of pyocyanine, a pigment of pseudomonas aeruginosa, on production of reactive nitrogen intermediates by murine alveolar macrophages. Infect Immun. (1992) 60:3913–5. doi: 10.1128/iai.60.9.3913-3915.1992
128. Marreiro de Sales-Neto J, Lima ÉA, Cavalcante-Silva LHA, Vasconcelos U, and Rodrigues-Mascarenhas S. Anti-inflammatory potential of pyocyanin in LPS-stimulated murine macrophages. Immunopharmacol Immunotoxicol. (2019) 41:102–8. doi: 10.1080/08923973.2018.1555845
129. Bianchi SM, Prince LR, McPhillips K, Allen L, Marriott HM, Taylor GW, et al. Impairment of apoptotic cell engulfment by pyocyanin, a toxic metabolite of Pseudomonas aeruginosa. Am J Respir Crit Care Med. (2008) 177:35–43. doi: 10.1164/rccm.200612-1804OC
130. Mudaliar SB and Bharath Prasad AS. A biomedical perspective of pyocyanin from Pseudomonas aeruginosa: its applications and challenges. World J Microbiol Biotechnol. (2024) 40:90. doi: 10.1007/s11274-024-03889-0
131. Xiao J, Thwe AA, Liu T, Gong D, Lin W, Shang C, et al. Anti-inflammatory effects of an extract from Pseudomonas aeruginosa and its purified product 1-hydroxyphenazine on RAW264.7 Cells. Curr Microbiol. (2021) 78:2762–73. doi: 10.1007/s00284-021-02544-3
132. Denning GM, Iyer SS, Reszka KJ, O’Malley Y, Rasmussen GT, and Britigan BE. Phenazine-1-carboxylic acid, a secondary metabolite of Pseudomonas aeruginosa, alters expression of immunomodulatory proteins by human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. (2003) 285:L584–92. doi: 10.1152/ajplung.00086.2003
133. Wang Y, Wilks JC, Danhorn T, Ramos I, Croal L, and Newman DK. Phenazine-1-carboxylic acid promotes bacterial biofilm development via ferrous iron acquisition. J Bacteriol. (2011) 193:3606–17. doi: 10.1128/JB.00396-11
134. Jahoor A, Patel R, Bryan A, Do C, Krier J, Watters C, et al. Peroxisome proliferator-activated receptors mediate host cell proinflammatory responses to Pseudomonas aeruginosa autoinducer. J Bacteriol. (2008) 190:4408–15. doi: 10.1128/JB.01444-07
135. Cooley MA, Whittall C, and Rolph MS. Pseudomonas signal molecule 3-oxo-C12-homoserine lactone interferes with binding of rosiglitazone to human PPARγ. Microbes Infect. (2010) 12:231–7. doi: 10.1016/j.micinf.2009.12.009
136. Smith RS, Kelly R, Iglewski BH, and Phipps RP. The pseudomonas autoinducer N -(3-oxododecanoyl) homoserine lactone induces cyclooxygenase-2 and prostaglandin E2 production in human lung fibroblasts: implications for inflammation. J Immunol. (2002) 169:2636–42. doi: 10.4049/jimmunol.169.5.2636
137. Zhang J, Gong F, Li L, Zhao M, and Song J. Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl) homoserine lactone attenuates lipopolysaccharide-induced inflammation by activating the unfolded protein response. BioMed Rep. (2014) 2:233–8. doi: 10.3892/br.2014.225
138. Tateda K, Ishii Y, Horikawa M, Matsumoto T, Miyairi S, Pechere JC, et al. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect Immun. (2003) 71:5785–93. doi: 10.1128/IAI.71.10.5785-5793.2003
139. Kushwaha A and Agarwal V. Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)-L-homoserine lactone mediates Ca+2 dysregulation, mitochondrial dysfunction, and apoptosis in human peripheral blood lymphocytes. Heliyon. (2023) 9:e21462. doi: 10.1016/j.heliyon.2023.e21462
140. Yadav VK, Singh PK, Sharma D, Pandey H, Singh SK, and Agarwal V. Autoinducer N-(3-oxododecanoyl)-L-homoserine lactone induces calcium and reactive oxygen species-mediated mitochondrial damage and apoptosis in blood platelets. Microb Pathog. (2021) 154:104792. doi: 10.1016/j.micpath.2021.104792
141. Bandyopadhaya A, Tzika AA, and Rahmea LG. Pseudomonas aeruginosa quorum sensing molecule alters skeletal muscle protein homeostasis by perturbing the antioxidant defense system. mBio. (2019) 10:e02211–19. doi: 10.1128/mBio.02211-19
142. Tzika AA, Constantinou C, Bandyopadhaya A, Psychogios N, Lee S, Mindrinos M, et al. A small volatile bacterial molecule triggers mitochondrial dysfunction in murine skeletal muscle. PloS One. (2013) 8:e74528. doi: 10.1371/journal.pone.0074528
143. Bandyopadhaya A, Kesarwani M, Que Y-A, He J, Padfield K, Tompkins R, et al. The quorum sensing volatile molecule 2-amino acetophenon modulates host immune responses in a manner that promotes life with unwanted guests. PloS Pathog. (2012) 8:e1003024. doi: 10.1371/journal.ppat.1003024
144. Bandyopadhaya A, Tsurumi A, Maura D, Jeffrey KL, and Rahme LG. A quorum-sensing signal promotes host tolerance training through HDAC1-mediated epigenetic reprogramming. Nat Microbiol. (2016) 1:16174. doi: 10.1038/nmicrobiol.2016.174
145. Bandyopadhaya A, Tsurumi A, and Rahme LG. NF-κBp50 and HDAC1 interaction is implicated in the host tolerance to infection mediated by the bacterial quorum sensing signal 2-aminoacetophenone. Front Microbiol. (2017) 8:1211. doi: 10.3389/fmicb.2017.01211
146. Chakraborty A, Kabashi A, Wilk S, and Rahme LG. Quorum-sensing signaling molecule 2-aminoacetophenone mediates the persistence of Pseudomonas aeruginosa in macrophages by interference with autophagy through epigenetic regulation of lipid biosynthesis. mBio. (2023) 14:e0015923. doi: 10.1128/mbio.00159-23
147. Chakraborty A, Bandyopadhaya A, Singh VK, Kovacic F, Cha S, Oldham WM, et al. The bacterial quorum sensing signal 2’-aminoacetophenone rewires immune cell bioenergetics through the Ppargc1a/Esrra axis to mediate tolerance to infection. Elife. (2024) 13:RP97568. doi: 10.7554/eLife.97568
Keywords: Pseudomonas aeruginosa, volatile organic compounds, bacterial metabolites, airway mucus dysregulation, pulmonary immunity
Citation: Kuo SH and Lau GW (2025) Pseudomonas aeruginosa-derived metabolites and volatile organic compounds: impact on lung epithelial homeostasis and mucosal immune response. Front. Immunol. 16:1553013. doi: 10.3389/fimmu.2025.1553013
Received: 29 December 2024; Accepted: 22 May 2025;
Published: 09 June 2025.
Edited by:
Fang He, Southwest University, ChinaReviewed by:
Ana-Maria Dragoi, Ochsner LSU Health, United StatesJean-Michel Sallenave, INSERM U1152 Physiopathologie et Epidémiologie des Maladies Respiratoires, France
Copyright © 2025 Kuo and Lau. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gee W. Lau, Z2VlbGF1QGlsbGlub2lzLmVkdQ==
†Present address: Shanny Hsuan Kuo, Department of Comparative Medicine, School of Medicine, University of Washington, Seattle, WA, United States
 Shanny Hsuan Kuo
Shanny Hsuan Kuo Gee W. Lau
Gee W. Lau