- 1Shanghai Institute of Hematology, State Key Laboratory of Medical Genomics; National Research Center for Translational Medicine at Shanghai, Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Shanghai 411 Hospital, Shanghai, China
- 3Université Sorbonne Paris Nord, Villetaneuse, France
- 4Assistance Publique Hôpitaux de Paris, Hôpital Avicenne, Service d’Oncologie Médicale, Bobigny, France
- 5Pôle de Recherches Sino-Français en Science du Vivant et Génomique, Laboratory of Molecular Pathology, Shanghai, China
Introduction: The outcomes of refractory or relapsed diffuse large B-cell lymphoma are generally poor, especially those relapsed or progressed within 12 months from diagnosis named as early chemoimmunotherapy failure (ECF), with a 2-year OS of 24.7%. Due to the dismal outcome, early recognition of ECF and developing targeted innovative treatments to improve patient prognosis are urgent.
Methods: This study recruited 2038 newly diagnosed DLBCL patients treated with R-CHOP/RminiCHOP or R-CHOP-based immunochemotherapy in Ruijin hospital and 411 hospital from December 1997 to December 2020.
Results: Compared to the control group, ECF patients were significantly associated with elderly age, advanced Ann Arbor stage, elevated serum LDH, poor performance status, multiple extranodal involvements, double expressor lymphoma (DEL), and non-GCB subtype, as well as high frequencies of TP53, FOXO1 and FBXW7 mutations. Through multivariate analysis, elderly age, advanced stage, elevated serum LDH, DEL, and mutations of TP53 or FOXO1 were independent predictors of ECF.
Discussion: Based on these predictors, a nomogram of ECF was established, and the straining cohort of our Chinese patients as well as the external cohort from Western countries showed a good predictive power of the ECF model, indicating the efficiency of our ECF predicting model, regardless of patients' race. Our ECF model allows clinicians to early recognize ECF patients, to optimize the therapeutic strategies and to improve the outcome of those chemo-resistant patients.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma (NHL) and represents a biologically heterogeneous entity with varied clinical and molecular features (1–3). Although 60% of DLBCL patients can be cured by rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) immunochemotherapy, the other 40% either become refractory or experience disease relapse (4, 5). The outcomes of patients with refractory or relapsed (r/r) DLBCL are generally poor. According to the SCHOLAR-1, the NCIC-CTG LY.12 and the REAL-TREND studies, refractory DLBCL has a dismal outcome, with a median overall survival (OS) of 5.9- 6.1 months (5–7). Recently, patients who relapsed or progressed within 12 months from diagnosis have been identified as early chemoimmunotherapy failure (ECF) and those who relapsed after 12 months from diagnosis are classified as late chemoimmunotherapy failure (LCF) (8, 9). Patients with ECF had a worse prognosis, with a 2-year OS of 24.7%, as compared to LCF with a 2-year OS of approximately 60-70% (10). Due to the poor outcome, early recognition of ECF and developing innovative targeted treatments to improve the outcome of ECF patients are very important. However, the clinical and molecular characteristics of ECF are still unclear and a convenient method to early identify the ECF patients is an unmet need.
There are many factors indicating or affecting the prognosis of patients with DLBCL, including the international prognostic index (IPI), cell of origin (COO), gene mutations and genetic subtypes, as well as the tumor microenvironment (TME) (11–13). IPI is a powerful tool to predict the prognosis of DLBCL patients based on five clinical characteristics including age, lactate dehydrogenase (LDH), number of extranodal involvement, Ann Arbor stage, and Eastern Cooperative Oncology Group (ECOG) performance status in the rituximab era (14, 15). According to the algorithm of Hans, DLBCL is commonly classified into germinal center B-cell-like (GCB), and non-GCB (16). During the rituximab era, the GCB subgroup showed a significantly better 3-year OS than the non-GCB subgroup (17). For the genetic landscape, the mutations of genes, such as TP53, TBL1XR1, MYC, and FBXW7 have been reported to be unfavorable factors of DLBCL patients (18–20). Based on targeted sequencing and fluorescence in situ hybridization, LymphPlex classified DLBCL into seven distinct genetic subtypes with distinct prognoses, including MCD-like, BN2-like, TP53Mut, EZB-like, ST2-like, N1-like, and not otherwise specified (NOS), however, patients with MCD-like and TP53Mut had dismal outcome (21). TME plays an essential role in DLBCL progression and chemoresistance (22). Immunosuppressive TME can promote tumor growth by recruiting immunosuppressive cells such as tumor-associated macrophages (TAMs), neutrophils, and regulatory T cells (Tregs) and accumulating exhausted T-cells (23, 24). Numerous attempts have been made to incorporate clinical and genetic markers into the prognosis prediction of DLBCL, however, a useful prediction model of ECF DLBCL is still lacking (25).
The innovations of novel immunotherapies, especially the chimeric antigen receptor-T (CAR-T) cell therapies have improved the outcome of r/r DLBCL and ECF patients (26, 27). Therefore, the prognosis of DLBCL patients may be significantly improved by early identification of ECF patients and application tailored treatment strategies, based on clinical and molecular characteristics.
In this study, we conducted genomic and transcriptomic analyses to delve deeper into the molecular and microenvironmental profiles of ECF and LCF. Additionally, we constructed a nomogram model incorporating both clinical and molecular variables to predict the risk of ECF, which might help clinicians to early identify ECF and choose tailored treatment strategies to improve patients’ prognoses.
Materials and methods
Patients
The selection process for patients in this study is outlined in Figure 1. From December 1997 to December 2020, 2038 newly diagnosed DLBCL patients (1911 from Ruijin hospital, 127 from 411 hospital) with R-CHOP/R-miniCHOP or R-CHOP-based immunochemotherapy were enrolled. Among 2038 patients, 34 elderly fit patients aged ≥ 80 years received the R-miniCHOP regimen (28) and the last follow-up was September 30, 2024. Of note, all the patients in our study received six cycles of chemotherapies. In this research, patients with central nervous lymphoma, high-grade B-cell lymphoma, and primary mediastinum large B-cell lymphoma, who underwent immunochemotherapy regimens other than R-CHOP/R-miniCHOP or R-CHOP-based therapies were excluded. This study aims to evaluate the prognosis of patients who are refractory or relapsed to chemotherapy within the context of conventional chemotherapy treatment, hence the patients who received CAR-T treatment were also excluded from this research. DLBCL patients were divided into three groups according to the initial treatment response and progression time. The evaluation time of stable disease (SD) or progressive disease (PD) was after 3–4 courses of treatment (intermediate evaluation) or at the end of treatment (final evaluation). According to SCHOLAR-1, patients with SD/PD to first-line R-CHOP regimen were identified as primary refractory patients (5). However, patients achieved complete response (CR) or partial response (PR), but progressed within 1 year from diagnosis were considered as early relapse (ER). As shown in Supplemetary Figure 1, patients who obtained SD/PD after R-CHOP treatment and those who relapsed within 12 months (ER) from diagnosis had similar outcome (PFS, p=0.6476 and OS, p=0.3175, Supplementary Figure 1). Therefore, patients had SD/PD to R-CHOP or relapsed within 12 months from diagnosis were classified as ECF, and those who relapsed or progressed after 12 months from diagnosis were classified as LCF (9). Patients who achieved CR after the first-line treatment and maintained their remission status until the last follow-up were selected as control. This study was approved by the Review Boards of both Shanghai Ruijin hospital and 411 hospital, and informed consent was obtained in accordance with the Declaration of Helsinki.
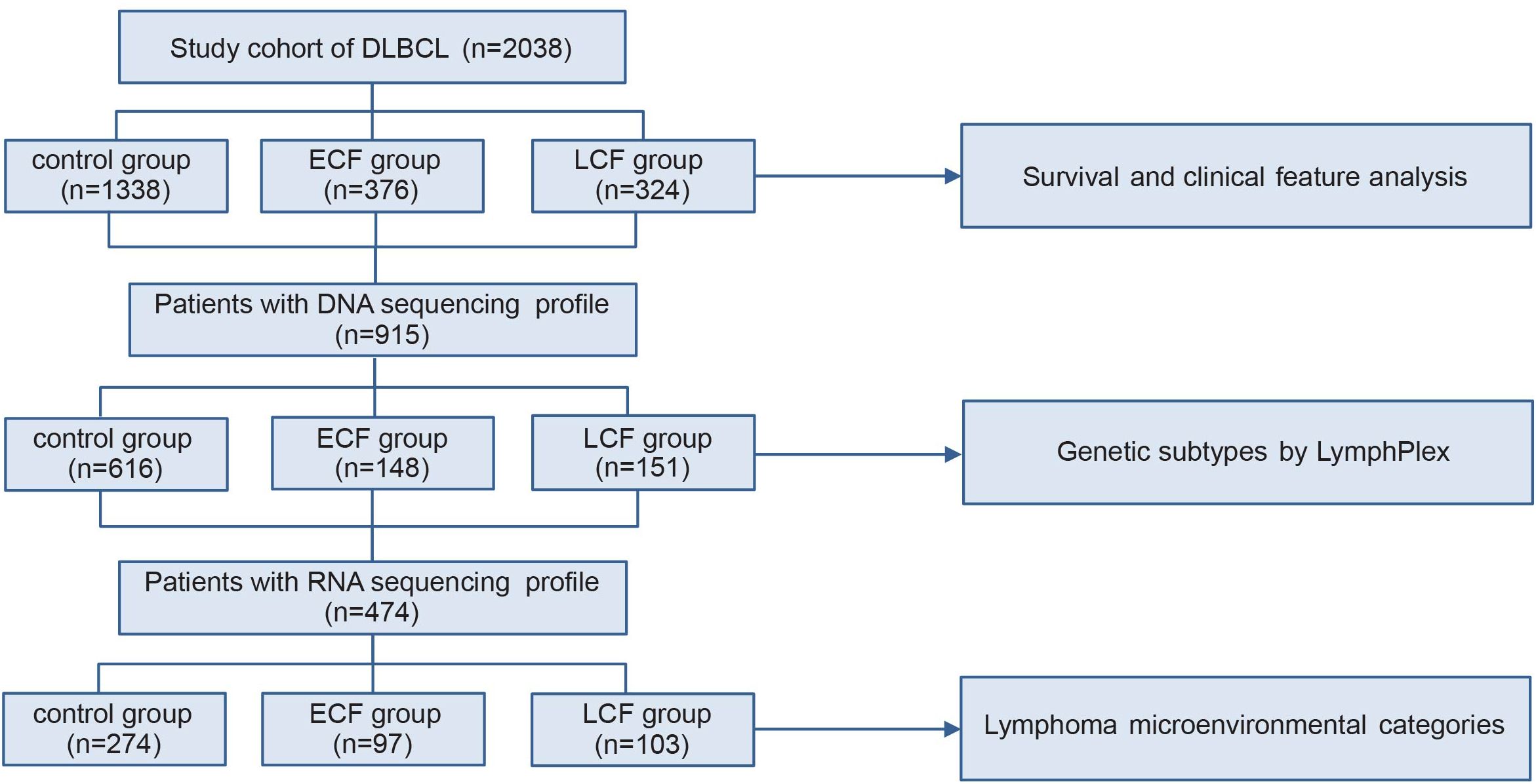
Figure 1. Patient flowchart. DLBCL, diffuse large B-cell lymphoma; ECF, early chemoimmunotherapy failure; LCF, late chemoimmunotherapy failure.
DNA sequencing
DNA sequencing was performed on 915 patients for the detection of oncogenic mutations and genetic subtypes as previously reported (29–31). Genomic DNA was extracted from frozen tumor tissue or FFPE tumor tissue by QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) and GeneRead DNA FFPE Tissue Kit (Qiagen), and whole-genome sequencing, whole-exome sequencing, and targeted sequencing covering 55 lymphoma-associated genes were performed on 67, 137, and 711 patients, respectively, as previously described (29–31). Using the GRCh37 human reference genome (version 2009-02), Samtools (version 0.1.18), Picard (version 1.93), and Genome Analysis Toolkit (version 4.1.4.0) were used for BAM file handling, local realignment, base recalibration, and calling variants, respectively. Mutations in the coding region were annotated using the Annovar software (version 2017-07-17). Variants were filtered according to the rules listed in our previous studies (32, 33).
RNA sequencing, GSEA, and tumor microenvironmental analysis
RNA-sequencing was performed on tumor samples of 474 DLBCL patients using frozen tumor tissues. RNA was extracted using Trizol (Invitrogen, California, USA) and RNeasy MinElute Cleanup Kit (Qiagen, Dusseldorf, Germany), quantified with NanoDrop. RNA purification, reverse transcription, library construction, and sequencing were performed in WuXi NextCODE according to the manufacturer’s instructions (Illumina). The details of RNA sequencing procedures and RNA sequencing data were conducted as previously reported (29–31). Bioinformatic analyses were performed by r 4.0.3 and raw reads were normalized, and differentially expressed genes were obtained with R package “limma” (v3·38·3). Gene set enrichment analysis (GSEA) was performed with the R package “clusterProfiler” (v4.0.0) based on MSigDB-curated gene sets (c5.bp.v7.0.symbols.gmt) (34). Pathways were considered statistically significant when the P value was <0.05, and the false discovery rate was <0.25. For large-scale characterization of tumor cellular heterogeneity, cell type enrichment scores were calculated by online tools ImmuCellAI (https://guolab.wchscu.cn/ImmuCellAI), which performs cell type enrichment analysis from gene expression data for immune and stroma cell types and provides a comprehensive collection of gene expression enrichment scores for cell types (35).
Construction and validation of the nomogram
The predictive model was constructed using clinical and DNA sequencing data from 2038 DLBCL patients. The candidate variables were screened by univariate and multivariate logistic regression analyses, followed by the development of a nomogram utilizing the “rms” package (https://github.com/harrelfe/rms). The external validation of the model was performed on the western DLBCL population from the BC Cancer (BCC) cohort (n=320) (36). In the external validation cohort, the progression-free survival (PFS) less than or equal to 12 months was considered as ECF.
Statistical analysis
Pearson’s χ2 test was used to analyze the clinical and molecular characteristics of patients and LymphPlex classification across different groups. PFS was calculated from the date of diagnosis to the date of disease progression or relapse, or the date of last follow-up. OS was measured from the date of diagnosis to the date of death or the last follow-up. Survival functions were analyzed using the Kaplan–Meier method and compared by the log-rank test. Differences in gene mutations, immune cell populations and normalized gene expression in two groups were assessed using the Mann–Whitney U test. The analysis of differential genes and GSEA enrichment of gene pathways were corrected by False Discovery Rate (FDR). The Tumor mutation burden of two groups of genes was analyzed by T-test. Univariate and multivariate hazards were analyzed using the logistic regression method. All statistical analyses were performed by Statistical Package for the Social Sciences (SPSS) 26.0 software or GraphPad Prism 9. Statistical significance was defined as p <0.05.
Results
Clinical characteristics of ECF
A total of 2038 patients with DLBCL were analyzed, including 1338 patients in the control group (patients without relapse or progression), 376 in the ECF group, and 324 in the LCF group (Figure 1). ECF patients were significantly associated with elderly age (p<0.001), advanced Ann Arbor stage (p<0.001), elevated serum LDH (p<0.001), poor performance status (37) (ECOG score more than 1, p<0.001), multiple extranodal involvements (p<0.001), DEL (p<0.001) and non-GCB subtype (p=0.022), as compared to the control group (Table 1). However, LCF patients were significantly associated with elderly age (p<0.001), advanced Ann Arbor stage (p<0.001), elevated serum LDH (p<0.001), multiple extranodal involvements (p<0.001), non-GCB subtype (p<0.001), poor performance status (p=0.002), and DEL (p=0.041) (Supplementary Table 1). In addition, when compared with LCF, ECF patients were also significantly associated with elevated serum LDH (p<0.001), poor performance status (p<0.001), advanced Ann Arbor stage (p=0.001), and DEL (p=0.036) (Supplementary Table 2). According to the survival analysis, the PFS of ECF patients (median PFS, 6.2 months, Figure 2A) was significantly shorter than those in the control group (median PFS, unreached, p<0.001) and LCF patients (median PFS, 24.8 months, p<0.001), while the OS of ECF patients (median OS, 13.0 months) was also significantly shorter than those in the control group (median OS, unreached, p<0.001) and LCF patients (median OS, 62.5 months, p<0.001, Figure 2B). It is noteworthy that the PFS (median PFS, 6.2 months) and OS (median OS, 13.0 months) of ECF patients were significantly shorter than those in the high-risk group according to the revised IPI score (median PFS, 24.5 months, p<0.001, median OS, 63.9 months, p<0.001). (Supplementary Figures 2A-D).
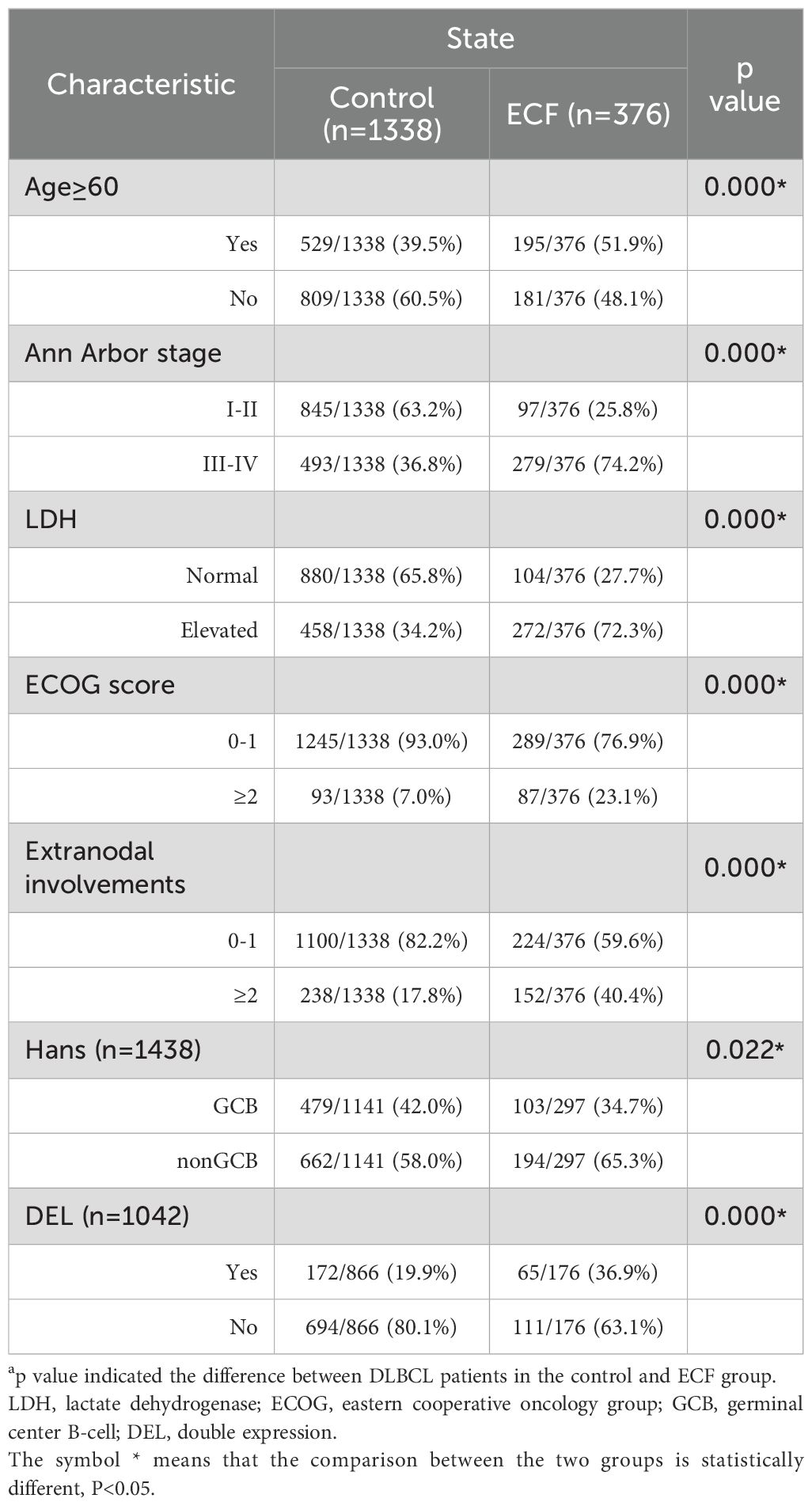
Table 1. Clinical and pathological characteristics of the patients in the control (n=1338) and ECF groups (n=376).
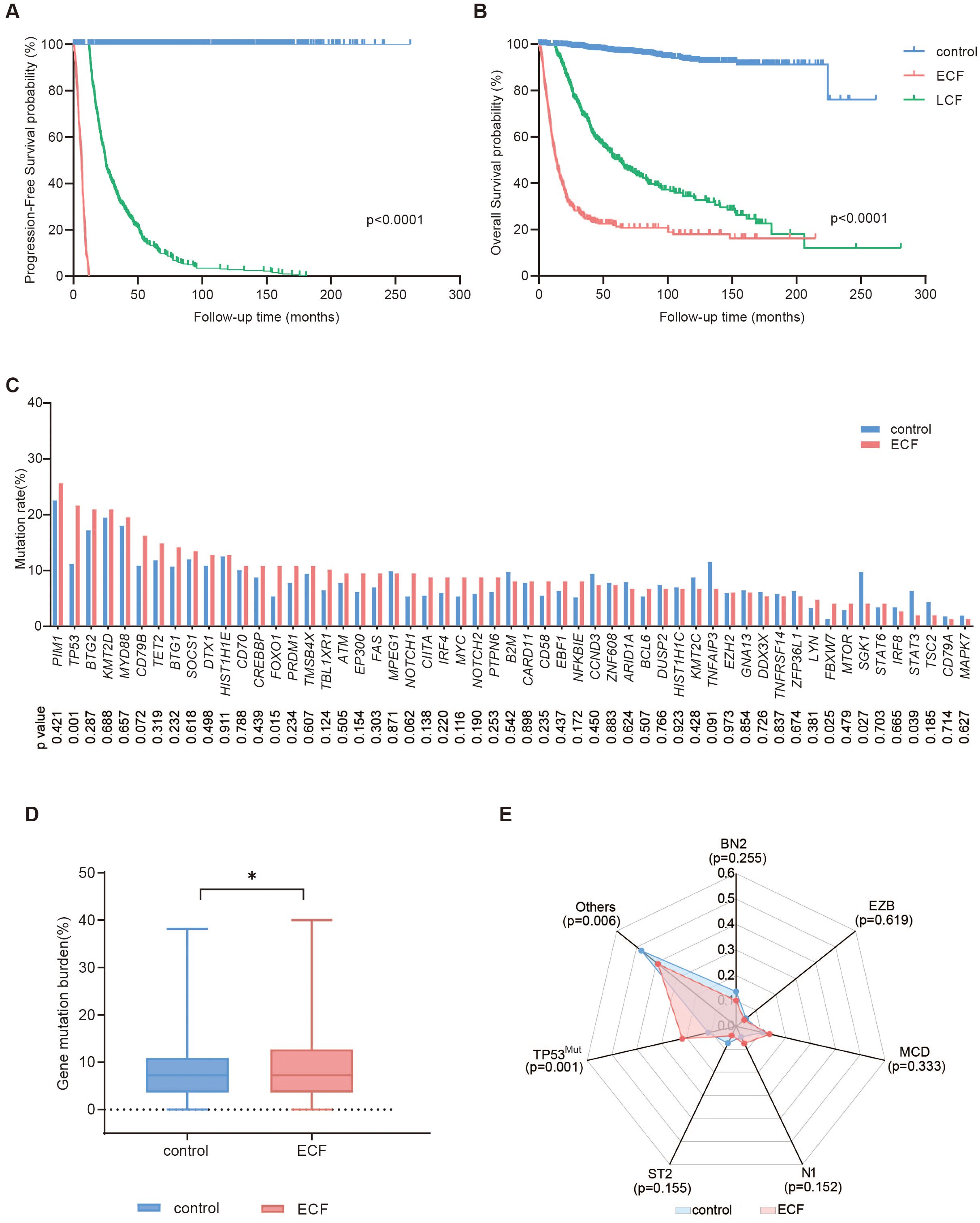
Figure 2. Survival and mutation analysis of DLBCL patients. (A, B) Progression-free survival (PFS) (A) and overall survival (OS) (B) of DLBCL patients in the control (n=1338), ECF (n=376) and LCF (n=324) groups. (C-E) Mutation profiles (C), gene mutation burden (D), and molecular subtypes (E) of patients in the control (n=616) and ECF (n=148) groups. The symbol * means that the comparison between the two groups is statistically different, P<0.05.
Mutational profile
Regarding the genetic profile, 55 genes related to the tumorigenesis of DLBCL were analyzed in 915 patients (control, n=616, ECF, n=148, and LCF, n=151, Supplementary Figure 3). The most frequently mutated genes (mutation frequency >15%) in the ECF group were PIM1 (25.7%), TP53 (21.6%), BTG2 (21.0%), KMT2D (21.0%), MYD88 (19.6%) and CD79B (16.2%). The mutation frequencies of TP53 (21.6% vs. 11.2%, p=0.001), FOXO1 (10.8% vs. 5.4%, p=0.015), FBXW7 (4.1% vs. 1.3%, p=0.025) were significantly increased in the ECF group as compared to the control group (Figure 2C). The gene mutation burden of the ECF group was higher than the control group (Figure 2D, p=0.044). As for molecular subtype, the proportion of TP53Mut subtypes in the ECF group was significantly higher than the control group (21.6% vs. 11.2%, p=0.001), and there was no statistically significant difference in the proportion of other subtypes between the two groups (Figure 2E).
As for LCF, the mutation frequencies of MYD88 (29.1% % vs. 18.0%, p=0.002), TP53 (18.5% vs. 11.2%, p=0.015), EZH2 (10.6% vs. 6.0%, p=0.046), CD79A (4.6% vs. 1.8%, p=0.038) were significantly increased in the LCF group as compared to the control group (Supplementary Figure 4A). There was no statistically significant difference in the gene mutation burden between the LCF and control groups (Supplementary Figure 4B). Regarding the LymphPlex classification, the proportion of TP53Mut subtypes (p=0.015) was significantly higher, while the proportion of BN2 subtypes (p=0.019) was markedly lower in the LCF group as compared to the control group (Supplementary Figure 4C). When compared with LCF, the mutation frequencies of HIST1H1C (6.8% % vs. 2.0%, p=0.044) and LYN (4.7% vs. 0.7%, p=0.030) were significantly increased in the ECF group. Similarly, the gene mutation burden (p=0.029) was also significantly higher in ECF Supplementary Figures 5A, B). No significant difference was observed in the molecular subtypes between LCF and ECF (Supplementary Figure 5C).
Differential gene and pathway enrichment analysis
RNA sequencing was performed on 474 lymphoma tissues, including 274 in the control group, 97 in the ECF group, and 103 in the LCF group. Compared to the control group, the ECF group differed significantly in gene expression pattern, with 530 genes differentially expressed. Of those, 183 genes were upregulated in the ECF group (Figure 3A). The GSEA analysis unveiled that the signaling pathways related to cell cycle, mismatch repair, polo-like kinase mediated events, Melanocyte Inducing Transcription Factor (MITF)-M-dependent gene expression and sumoylation were enhanced in ECF patients compared to the control group (Figure 3B). Among the genes related to the top 1 signaling pathway (cell cycle), the expression levels of GENPS-CORT, POLD1, H3C10, CDC25A and SKA1 were notably increased in ECF (Figure 3C).
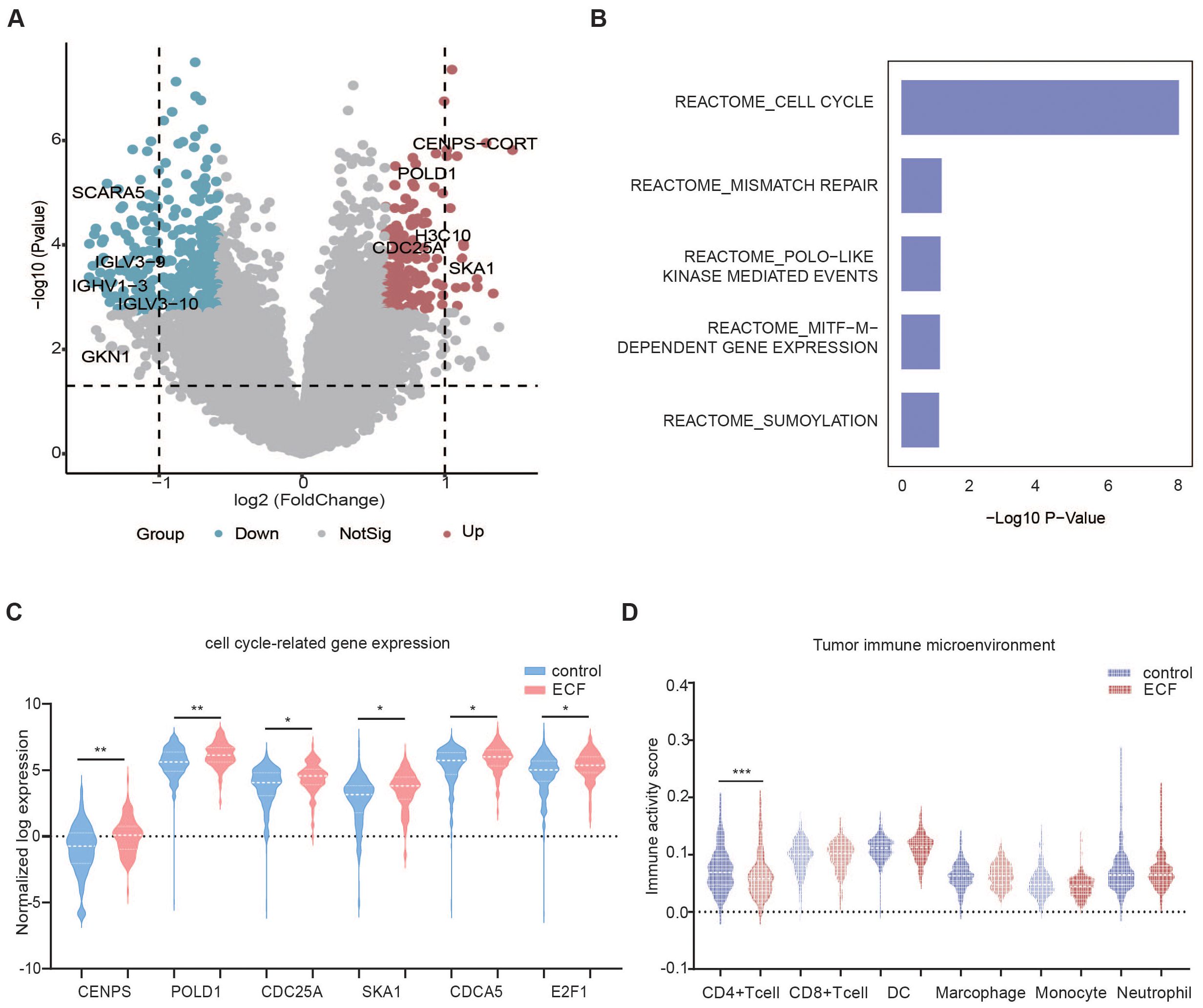
Figure 3. Differential gene expression and immune cell infiltration in the control and ECF groups. (A) The volcano plots show the differential expression of genes in the control (n=274) and ECF (n=97) groups. (B) Up-regulated pathways in ECF patients compared to the control group. (C, D) Violin plot of cell cycle-related genes and tumor microenvironment, cell cycle signaling-related genes (C) Tumor microenvironment in the control (n=274) and ECF (n=97) groups (D). The symbols *, **, and *** indicate the levels of statistical significance for each variable. Specifically, * denotes a p-value less than 0.05, suggesting the result is statistically significant at the 5% level. ** indicates a p-value less than 0.01, showing stronger significance, while *** represents a p-value less than 0.001, implying a highly significant result.
For LCF patients, a similar trend was observed in the upregulation of signaling pathways related to rRNA procession, Activated PKN1 stimulates transcription of androgen receptor (AR)-regulated genes KLK2 and KLK3, DNA replication, chromatin modifaction and rRNA expression according to the GSEA analysis (Supplementary Figures 6A, B).
Compared with the LCF group, the cell cycle checkpoints, rRNA procession, translation, DNA repair, processing of capped intron containing pre mRNA signaling pathways were upregulated in the ECF group (Supplementary Figures 6C, D).
Tumor microenvironment of ECF
TME was evaluated by a web server ImmuCellAI using RNA sequencing data (35). According to the ImmuCellAI results, patients in the control group had significantly higher CD4+ T cell infiltration in TME (p=0.001) as compared to those in the ECF group (Figure 3D). For LCF patients, cytotoxic and exhausted T cells were significantly higher in TME than in the control group (Supplementary Figure 6E). However, when compared to the LCF group, the increased recruiting activity of neutrophils (p=0.038) and the decreased recruiting activity of CD4+ T cells (p=0.001) and CD8+ T cells (p=0.012) were observed in ECF patients (Supplementary Figure 6F).
Construction and validation of the nomogram of ECF
Univariate and multivariate logistic regression analyses were performed to identify the potential risk factors for ECF patients. The univariate analysis was performed on 2038 patients, and revealed that elderly age (> 60 years), ECOG ≥2, Ann Arbor stage III/IV, elevated serum LDH level, multiple extranodal involvements, and mutations in TP53, FOXO1, and FBXW7 genes were associated with increased risk of ECF (Supplementary Table 3). Multivariate logistic regression revealed that Age >60 (OR = 1.71, 95% [CI] = 1.11 ~ 2.63, P = 0.016), Ann Arbor stage III/IV (OR = 2.08, 95% [CI] = 1.23 ~ 3.52, P =0.006), elevated serum LDH level (OR = 3.63, 95% [CI] = 2.22 ~ 5.95, P <0.001), TP53 mutation (OR = 1.91, 95% [CI] = 1.12 ~ 3.26; P =0.017) and FOXO1 mutation (OR = 2.61, 95% [CI] = 1.24 ~ 5.47; P =0.011) were independent risk factors for ECF (Supplementary Figure 7A).
Based on these independent risk factors of ECF, we developed a nomogram to predict the risk of ECF (Figure 4A). 1000 bootstrap samples were used to test the performance of the prediction model. Through bootstrap analysis, the C-index of the model was 0.768 (Figure 4B). The calibration curve (Figure 4D) also showed good agreement between the predicted and actual outcomes. To further evaluate the accuracy and reliability of our nomogram, we performed external validation using BCC cohort from Western countries (36) and the results were satisfactory. Clinical and pathological characteristics of patients in the validation cohort were listed in Supplementary Table 4. The AUC value for the external validation of the BCC cohort (n = 320) was 0.738 (Figure 4C). The nomogram was found to fit well in both training and validation cohorts according to the Hosmer-Lemeshow test (Figures 4D, E). Overall, these findings indicated that the nomogram we constructed was a feasible and effective tool for predicting the risk of ECF patients.
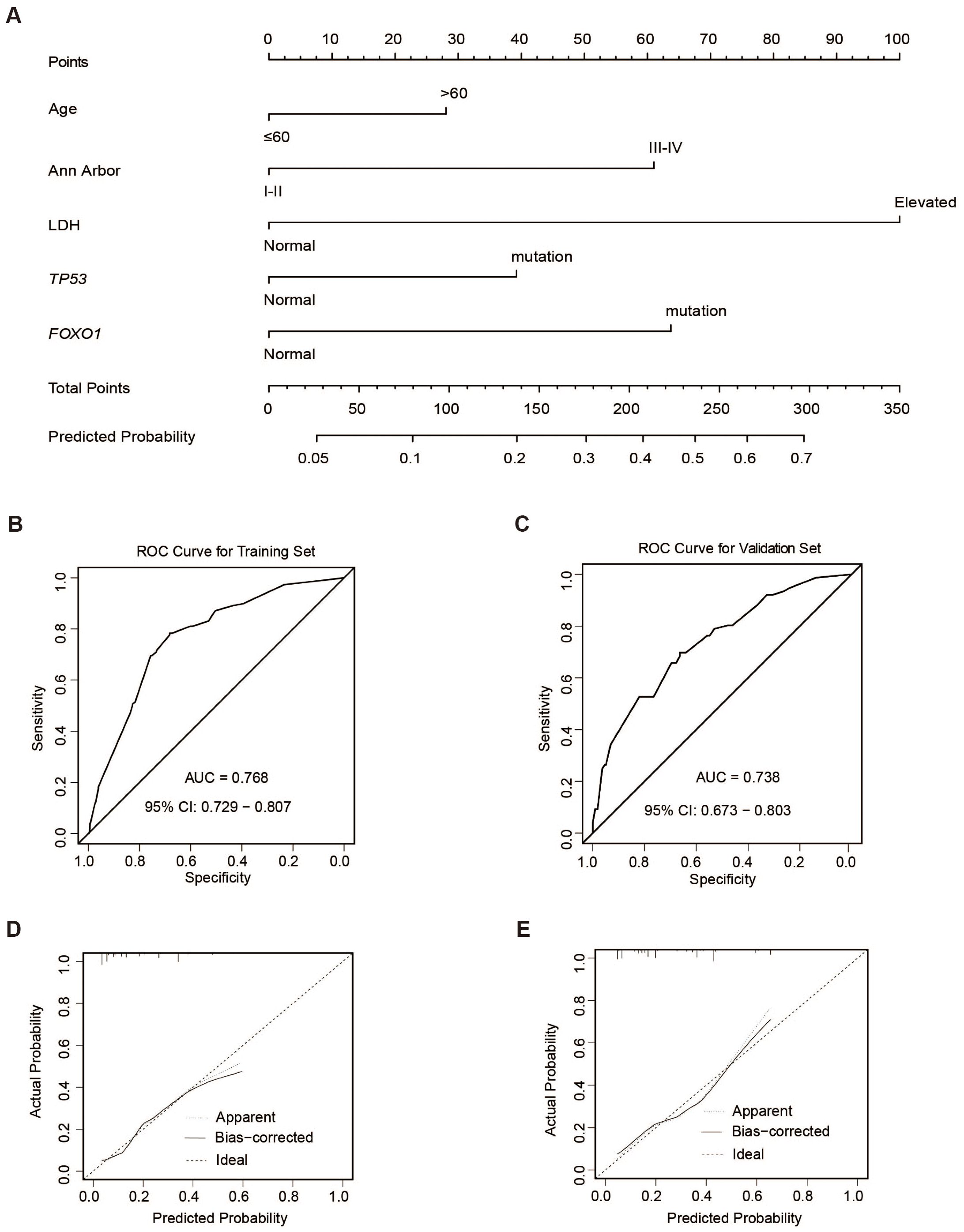
Figure 4. ECF nomogram. (A) Construction of ECF nomogram. (B, C) ROC curves of the training (n=2038) (B) and validation sets (BCC cohort n=320) (C). (D, E) Calibration curves of the training (D) and validation sets (E).
Discussion
ECF patients bear the dismal prognoses and are worthy of more attention (5, 7, 38, 39). To our knowledge, our study represents a relatively large cohort of Chinese DLBCL individuals with clinical and molecular characterizations, aiming to establish a predictive model capable of discerning ECF patients. This will provide clinicians with an efficient tool to expeditiously identify patients with the poorest survival outcomes, thus enabling the exploration of novel treatment approaches beyond R-CHOP. Multivariate analysis revealed that age >60, advanced Ann Arbor stage, elevated serum LDH, TP53, and FOXO1 mutations were independent predictors of ECF, which enabled us to construct a nomogram to predict the risk of ECF. These results are highly consistent with the worse prognosis of TP53Mut subtypes (40). Furthermore, mutated TP53 inhibited the virus response and interferon release in DLBCL, and subsequently induced the suppressive TME with the lower infiltration of T-cells, contributing to the worse outcome of TP53-mutant patients under immune-targeted therapy (41). Of note, Rushton and colleagues found that these mutations in the TP53 gene remained clonally persistent throughout treatment, explaining its role in primary treatment resistance and its resistance to subsequent high-dose chemotherapy (42) and CAR-T treatment (43). Therefore, TP53 mutations facilitate the identification of potential ECF patients and have important value in the design of future therapeutic strategies. FOXO1 is an important transcription factor, modulating the transcription of CD20. FOXO1 mutation reduces the expression level of CD20, resulting in resistance to anti-CD20 therapy, and is associated with poor prognosis of patients treated with R-CHOP regimen (44–46). Noteworthy, the training cohort of Chinese patients and the external cohort from Western countries showed a good predictive power of ECF, regardless of patients’ race. As for clinical features, ECF patients had high proportions of elderly age, advanced Ann Arbor stage, and elevated serum LDH, indicating that the high IPI score is a strong predictor of drug resistance (9, 40). For molecular characteristics, the mutation rates of TP53, FOXO1, and FBXW7 genes were high in the ECF group. In addition to TP53 and FOXO1, FBXW7, an E3 ubiquitin-protein ligase, is a notch signaling suppressor. Mutation or loss of function may lead to abnormal activation of the notch signaling pathway, which further increases the expression of CCL2 and CSF1 and promotes the transformation of tumor-associated macrophages (TAM) to M2 phenotype, thus promoting lymphoma cell proliferation (29, 47).
Regarding the TME, our results showed that the infiltration of CD4+ T cells was significantly lower in the ECF group compared to the control group, while the recruitment activity of neutrophils was higher in the ECF group as compared to the LCF group. Our results were consistent with the observations that patients with high CD4+ T cell infiltration had better survival as compared to those with low CD4+ T cell infiltration (48). Cell-mediated immunity plays a key role in controlling tumor growth and progression in DLBCL (49–52). CD4+ T cells, by activating other immune cells in the TME, are associated with a favorable prognosis in lymphoma (53). In DLBCL, neutrophils are reported to form extracellular traps, then up-regulate the Toll-like receptor 9 pathway and subsequently promote the lymphoma progression (54). Therefore, the low infiltration of CD4+ T cells and the high recruitment of neutrophils formed the immunosuppressive TME of ECF patients. Exhausted T cells were significantly increased in the LCF group, which was associated with poor prognosis of patients (55).
In conclusion, our study demonstrated the clinical and molecular profiles of ECF and LCF patients and established an ECF nomogram, which paves the way for convenient identification of patients with a high risk of ECF, thereby allowing for the development of customized therapeutic strategies beyond the conventional R-CHOP treatment in DLBCL.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.biosino.org/node, OEP001143.
Ethics statement
The studies involving humans were approved by Ruijin Hospital Ethics Committee Shanghai JiaoTong University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
Y-YD: Formal analysis, Visualization, Writing – original draft, Writing – review & editing. QS: Data curation, Writing – original draft. WW: Resources, Writing – original draft. B-BZ: Data curation, Writing – original draft. DF: Software, Writing – original draft. P-PX: Validation, Writing – original draft. SC: Resources, Writing – original draft. GB: Methodology, Writing – review & editing. W-LZ: Conceptualization, Supervision, Writing – review & editing. LW: Validation, Supervision, Writing – original draft, Writing – review & editing, Data curation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by research funding from the National Key R&D Program of China (2023YFC3605704 and 2022YFC2502600), the National Natural Science Foundation of China (82170178, 82130004, 82200201 and 82200116), the Chang Jiang Scholars Program, the Multicenter Clinical Research Project by Shanghai Jiao Tong University School of Medicine (DLY201601), the Multicenter Hematology-Oncology Program Evaluation System (M-HOPES), the Shanghai Sailing Program (22YF1425300), the Collaborative Innovation Center of Systems Biomedicine, and the Samuel Waxman Cancer Research Foundation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1553850/full#supplementary-material
Supplementary Figure 1 | Survival of DLBCL patients with SD/PD or relapsed within 1 year. (A, B) PFS (A) and OS (B) in patients with SD/PD to R-CHOP regimen (n=321) and those achieved CR/PR but relapsed within 1 year (n=81).
Supplementary Figure 2 | Survival of DLBCL patients according to different risk models. (A-B) PFS (A) and OS (B) of patients with low-risk (n=1436) or high-risk (n=602). (C-D) PFS (C) and OS (D) of patients with high-risk (n=602), ECF (n=376) and LCF (n=324).
Supplementary Figure 3 | Mutation profiles of DLBCL patients.
Supplementary Figure 4 | Gene mutations of DLBCL patients in the control and LCF groups. (A-C) Mutation rates (A), gene mutation burden (B), and molecular subtypes (C) of patients in the control (n=616) and LCF (n=151) groups.
Supplementary Figure 5 | Gene mutations of DLBCL patients in the ECF and LCF groups. (A-C) Mutation rates (A), gene mutation burden (B), and molecular subtypes (C) of patients in the ECF (n=148) and LCF (n=151) groups.
Supplementary Figure 6 | Differential gene expressions and immune cell infiltration in the control VS LCF groups and LCF VS ECF groups. (A) The volcano plots show the differential expression of genes in the control (n=274) and LCF (n=103) group. (B) Up-regulated pathways in the LCF patients compared to the control group. (C) The volcano plots show the differential expression of genes in the ECF (n=97) and LCF groups (n=103). (D) Up-regulated pathways in the ECF patients compared to the LCF patients. (E)Immune cell infiltrations in the control (n=274) and LCF (n=103) groups. (F) Immune cell infiltrations in the ECF (n=97) and LCF groups (n=103).
Supplementary Figure 7 | Multivariate analysis of ECF.
References
1. Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood J Am Soc Hematol. (2010) 116:2040–5. doi: 10.1182/blood-2010-03-276246
2. Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematol 2010 Am Soc Hematol Educ Program. (2011) 2011:498–505. doi: 10.1182/asheducation-2011.1.498
3. Li S, Young KH, and Medeiros LJ. Diffuse large B-cell lymphoma. Pathology. (2018) 50:74–87. doi: 10.1016/j.pathol.2017.09.006
4. Harrysson S, Eloranta S, Ekberg S, Enblad G, Jerkeman M, Wahlin BE, et al. Incidence of relapsed/refractory diffuse large B-cell lymphoma (DLBCL) including CNS relapse in a population-based cohort of 4243 patients in Sweden. Blood Cancer J. (2021) 11:9. doi: 10.1182/blood-2019-122014
5. Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood J Am Soc Hematol. (2017) 130:1800–8. doi: 10.1182/blood-2017-03-769620
6. Crump M, Kuruvilla J, Couban S, Macdonald DA, Kukreti V, Kouroukis CT, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY. 12. J Clin Oncol. (2014) 32:3490–6. doi: 10.1200/JCO.2013.53.9593
7. Wang S, Wang L, Hu J, Qian W, Zhang X, Hu Y, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from a multicenter real-world study in China. Cancer Commun. (2021) 41:229–39. doi: 10.1002/cac2.12126
8. Dlouhy I, Karube K, Enjuanes A, Salaverria I, Nadeu F, Ramis-Zaldivar JE, et al. Revised International Prognostic Index and genetic alterations are associated with early failure to R-CHOP in patients with diffuse large B-cell lymphoma. Br J Haematol. (2022) 196:589–98. doi: 10.1111/bjh.17858
9. Shah NN, Ahn KW, Litovich C, He Y, Sauter C, Fenske TS, et al. Is autologous transplant in relapsed DLBCL patients achieving only a PET+ PR appropriate in the CAR T-cell era? Blood J Am Soc Hematol. (2021) 137:1416–23. doi: 10.1182/blood.2020007939
10. Coiffier B, Salles G, Bosly A, Gaulard P, Haioun C, Casasnovas O, et al. Characteristics of refractory and relapsing patients with diffuse large B-cell lymphoma. Blood. (2008) 112:2589. doi: 10.1182/blood.V112.11.2589.2589
11. Gao F, Tian L, Shi H, Zheng P, Wang J, Dong F, et al. Genetic landscape of relapsed and refractory diffuse large B- cell lymphoma: a systemic review and association analysis with next-generation sequencing. Front Genet. (2021) 12:677650. doi: 10.3389/fgene.2021.677650
12. Morin RD, Assouline S, Alcaide M, Mohajeri A, Johnston RL, Chong L, et al. Genetic landscapes of relapsed and refractory diffuse large B-cell lymphomas. Clin Cancer Res. (2016) 22:2290–300. doi: 10.1158/1078-0432.CCR-15-2123
13. Wang L and Li L-R. R-CHOP resistance in diffuse large B-cell lymphoma: biological and molecular mechanisms. Chin Med J. (2021) 134:253–60. doi: 10.1097/CM9.0000000000001294
14. Project I N-H S L P F. A predictive model for aggressive non-Hodgkin’s lymphoma. New Engl J Med. (1993) 329:987–94. doi: 10.1056/NEJM199309303291402
15. Ziepert M, Hasenclever D, Kuhnt E, Glass B, Schmitz N, Pfreundschuh M, et al. Standard International prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin Oncol. (2010) 28:2373–80. doi: 10.1200/JCO.2009.26.2493
16. Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. (2000) 403:503–11. doi: 10.1038/35000501
17. Pasqualucci L, Trifonov V, Fabbri G, Ma J, Rossi D, Chiarenza A, et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet. (2011) 43:830–7. doi: 10.1038/ng.892
18. Fernández-Rodríguez C, Bellosillo B, García-García M, Sánchez-González B, Gimeno E, Vela M, et al. MYD88 (L265P) mutation is an independent prognostic factor for outcome in patients with diffuse large B-cell lymphoma. Leukemia. (2014) 28:2104–6. doi: 10.1038/leu.2014.184
19. Xu-Monette ZY, Wu L, Visco C, Tai Y C, Tzankov A, Liu W-M, et al. Mutational profile and prognostic significance of TP53 in diffuse large B-cell lymphoma patients treated with R-CHOP: report from an International DLBCL Rituximab-CHOP Consortium Program Study. Blood J Am Soc Hematol. (2012) 120:3986–96. doi: 10.1182/blood4082012-05-433334
20. Zhang X, Shi Y, Weng Y, Lai Q, Luo T, Zhao J, et al. The truncate mutation of Notch2 enhances cell proliferation through activating the NF-κB signal pathway in the diffuse large B-cell lymphomas. PloS One. (2014) 9:e108747. doi: 10.1371/journal.pone.0108747
21. Shen R, Fu D, Dong L, Zhang M-C, Shi Q, Shi Z-Y, et al. Simplified algorithm for genetic subtyping in diffuse large B- cell lymphoma. Signal Transduct Target Ther. (2023) 8:145. doi: 10.1038/s41392-023-01358-y
22. Kotlov N, Bagaev A, Revuelta MV, Phillip JM, Cacciapuoti MT, Antysheva Z, et al. Clinical and biological subtypes of B-cell lymphoma revealed by microenvironmental signatures. Cancer Discov. (2021) 11:1468–89. doi: 10.1158/2159-8290.CD-20-0839
23. Apollonio B, Ioannou N, Papazoglou D, and Ramsay AG. Understanding the immune-stroma microenvironment in B cell Malignancies for effective immunotherapy. Front Oncol. (2021) 11:626818. doi: 10.3389/fonc.2021.626818
24. Disis ML. Immune regulation of cancer. J Clin Oncol. (2010) 28:4531–8. doi: 10.1200/JCO.2009.27.2146
25. Jelicic J, Larsen TS, Frederiksen H, Andjelic B, Maksimovic M, Bukumiric Z, et al. Statistical challenges in development of prognostic models in diffuse large b-cell lymphoma: Comparison between existing models–A systematic review. Clin Epidemiol. (2020) 12:537–55. doi: 10.2147/CLEP.S244294
26. Poletto S, Novo M, Paruzzo L, Frascione PM, and Vitolo U. Treatment strategies for patients with diffuse large B-cell lymphoma. Cancer Treat Rev. (2022) 110:102443. doi: 10.1016/j.ctrv.2022.102443
27. Wang L, Li L-R, and Young KH. New agents and regimens for diffuse large B cell lymphoma. J Hematol Oncol. (2020) 13:1–23. doi: 10.1186/s13045-020-01011-z
28. Peyrade F, Jardin F, Thieblemont C, Thyss A, Emile J-F, Castaigne S, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol. (2011) 12:460–8. doi: 10.1016/S1470-2045(11)70069-9
29. Huang Y-H, Cai K, Xu P-P, Wang L, Huang C-X, Fang Y, et al. CREBBP/EP300 mutations promoted tumor progression in diffuse large B-cell lymphoma through altering tumor-associated macrophage polarization via FBXW7-NOTCH-CCL2/CSF1 axis. Signal Transduct Target Ther. (2021) 6:10. doi: 10.1038/s41392-020-00437-8
30. Qin W, Fu D, Shi Q, Dong L, Yi H, Huang H, et al. Molecular heterogeneity in localized diffuse large B-cell lymphoma. Front Oncol. (2021) 11:638757. doi: 10.3389/fonc.2021.638757
31. Shen R, Xu PP, Wang N, Yi HM, Dong L, Fu D, et al. Influence of oncogenic mutations and tumor microenvironment alterations on extranodal invasion in diffuse large B-cell lymphoma. Clin Trans Med. (2020) 10:e221. doi: 10.1002/ctm2.221
32. Huo Y-J, Xu P-P, Fu D, Yi H-M, Huang Y-H, Wang L, et al. Molecular heterogeneity of CD30+ diffuse large B-cell lymphoma with prognostic significance and therapeutic implication. Blood Cancer J. (2022) 12:48. doi: 10.1038/s41408-022-00644-2
33. Zhu Y, Fu D, Shi Q, Shi Z, Dong L, Yi H, et al. Oncogenic mutations and tumor microenvironment alterations of older patients with diffuse large B-cell lymphoma. Front Immunol. (2022) 13:842439. doi: 10.3389/fimmu.2022.842439
34. Yu G, Wang L-G, Han Y, and He Q-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics: J Integr Biol. (2012) 16:284–7. doi: 10.1089/omi.2011.0118
35. Li T, Fan J, Wang B, Traugh N, Chen Q, Liu JS, et al. TIMER: a web server for comprehensive analysis of tumor- infiltrating immune cells. Cancer Res. (2017) 77:e108–e10. doi: 10.1158/0008-5472.CAN-17-0307
36. Ennishi D, Jiang A, Boyle M, Collinge B, Grande BM, Ben-Neriah S, et al. Double-hit gene expression signature defines a distinct subgroup of germinal center B-cell-like diffuse large B-cell lymphoma. J Clin Oncol. (2019) 37:190–201. doi: 10.1200/JCO.18.01583
37. Sehn LH and Salles G. Diffuse large B-cell lymphoma. New Engl J Med. (2021) 384:842–58. doi: 10.1056/NEJMra2027612
38. Lee B, Lee H, Cho J, Yoon SE, Kim SJ, Park W-Y, et al. Mutational profile and clonal evolution of relapsed/refractory diffuse large B-cell lymphoma. Front Oncol. (2021) 11:628807. doi: 10.3389/fonc.2021.628807
39. Suzuki T, Maruyama D, Miyagi-Maeshima A, Nomoto J, Tajima K, Ito Y, et al. Clinicopathological analysis of primary refractory diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone chemoimmunotherapy. Cancer Med. (2021) 10:5101–9. doi: 10.1002/cam4.4062
40. Wang Y, Shi Q, Shi Z-Y, Tian S, Zhang M-C, Shen R, et al. Biological signatures of the International Prognostic Index in diffuse large B-cell lymphoma. Blood Adv. (2024) 8:1587–99. doi: 10.1182/bloodadvances.2023011425
41. Fang Y, Zhang M-C, He Y, Li C, Fang H, Xu P-P, et al. Human endogenous retroviruses as epigenetic therapeutic targets in TP53-mutated diffuse large B-cell lymphoma. Signal Transduct Target Ther. (2023) 8:381. doi: 10.1038/s41392-023-01626-x
42. Rushton CK, Arthur SE, Alcaide M, Cheung M, Jiang A, Coyle KM, et al. Genetic and evolutionary patterns of treatment resistance in relapsed B-cell lymphoma. Blood Adv. (2020) 4:2886–98. doi: 10.1182/bloodadvances.2020001696
43. Shi H, Zheng P, Liu R, Xu T, Yang F, Feng S, et al. Genetic landscapes and curative effect of CAR T-cell immunotherapy in patients with relapsed or refractory DLBCL. Blood Adv. (2023) 7:1070–5. doi: 10.1182/bloodadvances.2021006845
44. Kim PM, Nejati R, Lu P, Thakkar D, Mackrides N, Dupoux V, et al. Leukemic presentation and progressive genomic alterations of MCD/C5 diffuse large B-cell lymphoma (DLBCL). Mol Case Stud. (2023) 9:a006283. doi: 10.1101/mcs.a006283
45. Pyrzynska B, Dwojak M, Zerrouqi A, Morlino G, Zapala P, Miazek N, et al. FOXO1 promotes resistance of non Hodgkin lymphomas to anti-CD20-based therapy. Oncoimmunology. (2018) 7:e1423183. doi: 10.1080/2162402X.2017.1423183
46. Sablon A, Bollaert E, Pirson C, Velghe AI, and Demoulin J-B. FOXO1 forkhead domain mutants in B-cell lymphoma lack transcriptional activity. Sci Rep. (2022) 12:1309. doi: 10.1038/s41598-022-05334-4
47. Liu Q-X, Zhu Y, Yi H-M, Shen Y-G, Wang L, Cheng S, et al. KMT2D mutations promoted tumor progression in diffuse large B-cell lymphoma through altering tumor-induced regulatory T cell trafficking via FBXW7-NOTCH-MYC/TGF-β1 axis. Int J Biol Sci. (2024) 20:3972. doi: 10.7150/ijbs.93349
48. Xu-Monette Z Y, Xiao M, Au Q, Padmanabhan R, Xu B, Hoe N, et al. Immune profiling and quantitative analysis decipher the clinical role of immune-checkpoint expression in the tumor immune microenvironment of DLBCL [J. Cancer Immunol Res. (2019) 7:644–57. doi: 10.1158/2326-6066.CIR-18-0439
49. Zhang B, Liu J, Mo Y, Zhang K, Huang B, Shang D, et al. CD8+ T cell exhaustion and its regulatory mechanisms in the tumor microenvironment: key to the success of immunotherapy. Front Immunol. (2024) 15:1476904. doi: 10.3389/fimmu.2024.1476904
50. Dai E, Zhu Z, Wahed S, Qu Z, Storkus W J, Guo ZS, et al. Epigenetic modulation of antitumor immunity for improved cancer immunotherapy. Mol Cancer. (2021) 20:1–27. doi: 10.1186/s12943-021-01464-x
51. Yan Y, Huang L, Liu Y, Yi M, Chu Q, Jiao D, et al. Metabolic profiles of regulatory T cells and their adaptations to the tumor microenvironment: implications for antitumor immunity. J Hematol Oncol. (2022) 15:104. doi: 10.1186/s13045-022-01322-3
52. Zamarron BF and Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. (2011) 7:651. doi: 10.7150/ijbs.7.651
53. Ikeda D, Oura M, Uehara A, Tabata R, Narita K, Takeuchi M, et al. Prognostic relevance of tumor-infiltrating CD4 + cells and total metabolic tumor volume-based risk stratification in diffuse large B-cell lymphoma. Haematologica. (2024) 109:2822. doi: 10.3324/haematol.2024.285038
54. Nie M, Yang L, Bi X, Wang Y, Sun P, Yang H, et al. Neutrophil extracellular traps induced by IL8 promote diffuse large B-cell lymphoma progression via the TLR9 signaling. Clin Cancer Res. (2019) 25:1867–79. doi: 10.1158/1078-0432.CCR-18-1226
Keywords: DLBCL - diffuse large B cell lymphoma, early chemoimmunotherapy failure, RCHOP-like regimen, CAR- T cells, chemo-resistant, nomogram
Citation: Dong Y-Y, Shi Q, Wu W, Zhao B-B, Fu D, Xu P-P, Cheng S, Bousquet G, Zhao W-L and Wang L (2025) Characteristics and predictive model for diffuse large B-cell lymphoma with early chemoimmunotherapy failure. Front. Immunol. 16:1553850. doi: 10.3389/fimmu.2025.1553850
Received: 03 January 2025; Accepted: 28 May 2025;
Published: 16 June 2025.
Edited by:
Pier Paolo Piccaluga, University of Bologna, ItalyReviewed by:
Jing-dong Zhou, Jiangsu University Affiliated People’s Hospital, ChinaNelly Elshafie, Purdue University, United States
Copyright © 2025 Dong, Shi, Wu, Zhao, Fu, Xu, Cheng, Bousquet, Zhao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei-Li Zhao, emhhby53ZWlsaUB5YWhvby5jb20=; Li Wang, d2xfd2FuZ2RvbmdAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Ying-Yu Dong1†
Ying-Yu Dong1† Qing Shi
Qing Shi Peng-Peng Xu
Peng-Peng Xu Wei-Li Zhao
Wei-Li Zhao Li Wang
Li Wang