- 1Department of Hematology, The First Hospital of China Medical University, Shenyang, Liaoning, China
- 2Department of Pathology, The First Hospital of China Medical University, Shenyang, Liaoning, China
- 3The Second Department of Infectious Diseases, The First Hospital of China Medical University, Shenyang, Liaoning, China
Vanishing bile duct syndrome is a specific pathologic process characterized by ductopenia and intrahepatic cholestasis, which may be a unique paraneoplastic syndrome of Hodgkin’s lymphoma with an unfavorable prognosis. We report a 34-year-old woman with acute jaundice and lymphadenopathy, which was subsequently confirmed to be Hodgkin’s lymphoma with concurrent vanishing bile duct syndrome based on a liver biopsy. The patient agreed to combination chemotherapy with brentuximab vedotin and achieved a complete response. Liver function recovered within 4 months. This article reviews the literature and provides insight for addressing similar clinical challenges.
Case presentation
A 34-year-old female was admitted to the Department of Gastroenterology with rapidly progressive jaundice for 1 week and persistent lumbar pain for 1 month. She had pruritus, dizziness, fatigue, nausea, anorexia, and transient diarrhea for 2 weeks, as well as postpartum night sweats and weight loss over the preceding 6 months. She described dark urine and pale stools for 1–2 weeks. She denied fevers, dyspnea, or arthralgias. There was no personal or family history of hepatic disease and hematologic disorders. Prior to admission she was taking celecoxib (200 mg orally twice daily) and applying diclofenac diethylamine emulgel for 2 weeks to relieve the lumbar pain. Laboratory testing showed significant abnormalities in hepatic function, as follows: aspartate aminotransferase (AST), 87 U/L; alanine aminotransferase (ALT), 123 U/L; gamma-glutamyl transferase (GGT), 306 U/L; alkaline phosphatase (ALP), 997 U/L; total bilirubin, 146.2 μmol/L; direct bilirubin, 113.2 μmol/L; total cholesterol, 7.88 μmol/L; and lactate dehydrogenase (LDH), 525 U/L. During the admission, the ALT, GGT, ALP, and total bilirubin levels gradually increased to 10–20 times the upper limit of normal. A magnetic resonance cholangiopancreatography showed no evidence of biliary obstruction.
All the findings indicated intrahepatic cholestasis, but the cause was still unknown. The patient and her family denied a similar history. Laboratory examinations of rheumatism showed no apparent abnormality, except SSA IgG (95.9 U/mL). Considering the history of celecoxib use, a probable diagnosis of drug-induced liver damage was made.
Ursodeoxycholic acid (UDCA) and transmetil were used to relieve the jaundice. Furthermore, the physical examination revealed enlarged cervical lymph nodes (1–2 cm), thus neoplastic diseases were considered. Chest and abdominal computed tomography (CT) scans revealed generalized enlarged lymph nodes, splenomegaly, and bone changes in the T12 and L1 vertebrae. Positron emission tomography (PET)/CT was arranged to further assess pathologic changes in the left neck, mediastinum, retroperitoneum, liver, spleen, bone, and bone marrow. A left cervical lymph node biopsy was performed and the diagnosis of classical Hodgkin’s lymphoma (cHL) was established based on a background of diffuse inflammation and scattered large, atypical cells expressing CD15, CD30, and PAX5 (Figures 1A, B).
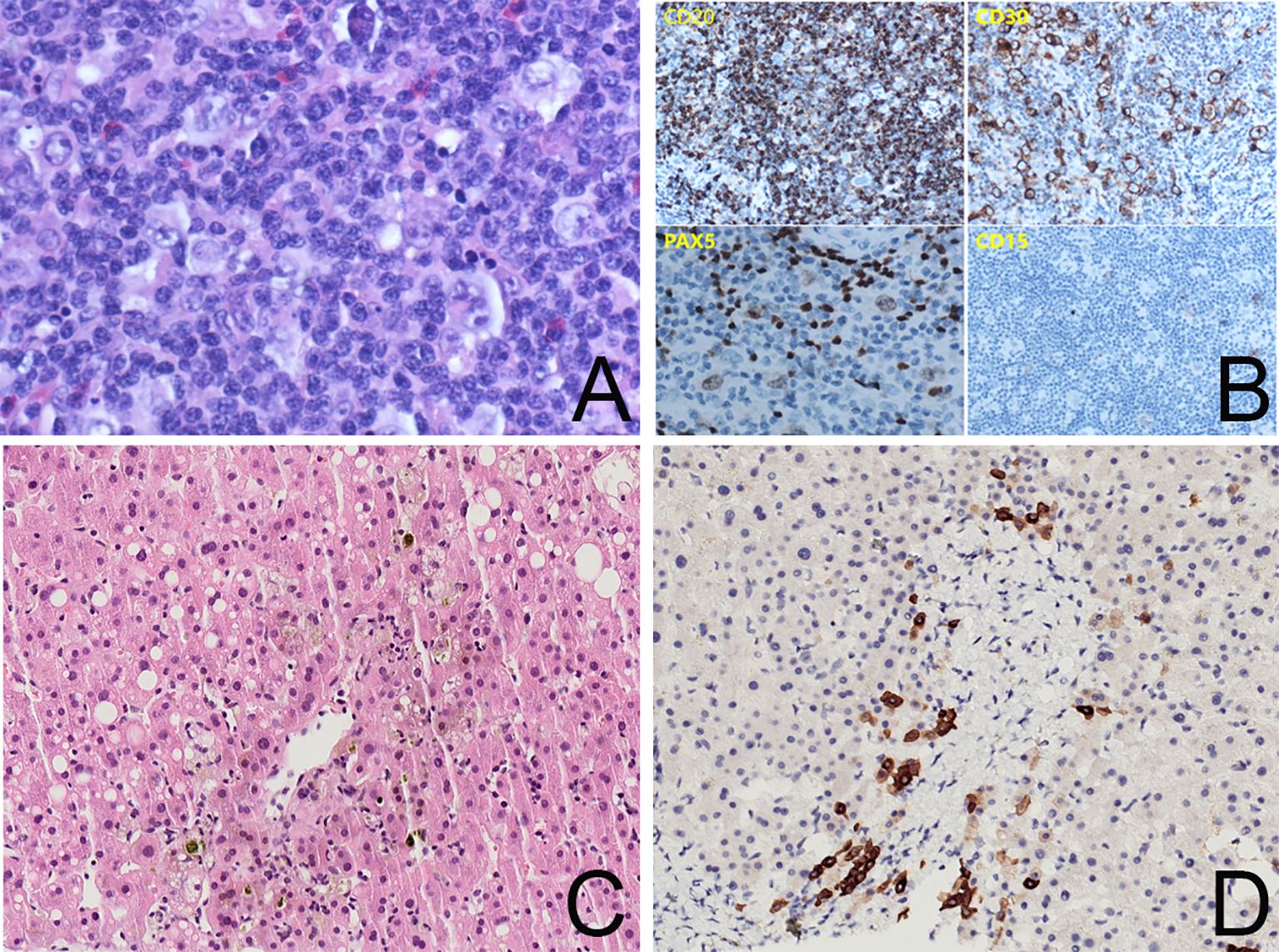
Figure 1. (A) Lymph node biopsy, ×400 magnification, showing R-S cells in a background of diffuse inflammation. (B) Immunohistochemistry staining for CD20, CD30, PAX5, and CD15 showing positive expressions in tumor cells. (C) Liver biopsy, ×200 magnification, HE immunohistochemistry stain, showing intrahepatic cholestasis around the central vein and macrovesicular steatosis in a few hepatocytes. The capillary bile ducts were blocked and dilated. There was no evidence of lymphoma. (D) Liver biopsy, ×200 magnification, CK7 immunohistochemistry stain showing hepatocytes with positive staining and loss of small bile ducts in the interstitium of the portal area. There was no sign of a bile ductular reaction.
The diagnosis was confirmed to be stage IVB cHL. The jaundice was thought to be caused by liver involvement of the lymphoma. Indeed, jaundice occurs in 3%–13% of lymphoma patients with liver involvement (1) and is usually accompanied by elevated liver enzymes and/or bilirubin. Early intervention, including combination chemotherapy, has been shown to effectively eliminate the jaundice.
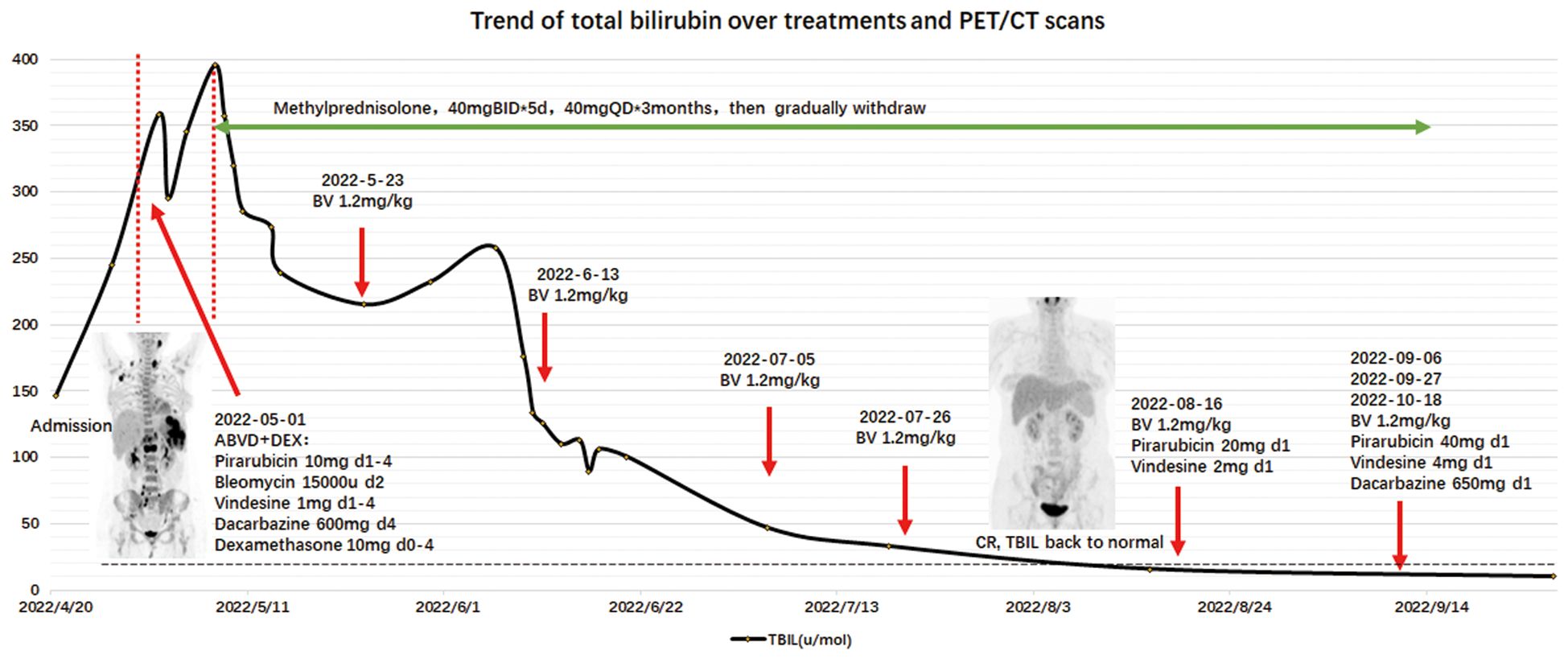
The patient received a 5-day course of dexamethasone at a dose of 10 mg followed by adriamycin, bleomycin sulfate, vinblastine sulfate, and dacarbazine (ABVD). The total bilirubin level decreased significantly the next day (358.5 to 295.1 μmol/L), then increased to 395.4 μmol/L.
The persistent, worsening jaundice led us to reconsider another possible etiology. Although the PET/CT scan suggested liver involvement, the standardized uptake value was lower than lymph nodes and other involved areas, suggesting that there would be an inadequate cause for severe intrahepatic cholestasis.
Vanishing bile duct syndrome (VBDS) was included in the differential diagnosis after a multi-disciplinary treatment discussion. The patient accepted a transjugular liver biopsy to confirm the diagnosis. The hepatic histopathologic evaluation revealed absence of bile ducts in 7 of the 11 portal areas and periportal hepatocytes with cholate stasis (Figures 1C, D). There was no evidence of lymphoma.
VBDS is a specific pathologic process that is characterized by ductopenia and intrahepatic cholestasis (2). The diagnosis is difficult to establish without performing a liver biopsy. There are many causes of VBDS, including drugs, infections, tumors, and immunologic disorders. HL has been reported to be the most common malignancy associated with VBDS. VBDS-associated HL has a poor prognosis because chemotherapy is delayed and often leads to hepatic failure or sepsis (3).
According to the recent literature, it is relatively safe for most patients to accept a modified chemotherapy regimen with low liver toxicity or molecularly targeted drugs. Brentuximab vedotin (BV) is a new and potentially effective treatment for VBDS-associated HL by avoiding hepatic damage caused by chemical drugs, as well as VBDS (4, 5).
After one-half of the ABVD regimen had been administered, the patient expressed concerns about combination chemotherapy and opted for treatment with BV with methylprednisolone (40 mg daily) and ursodeoxycholic acid (UDCA) to reduce liver damage. Given four cycles of BV (1.2 mg/kg), the bilirubin level returned to normal (18 μmol/L) and the PET/CT scan showed a complete metabolic response (CMR), suggesting that VBDS was reversed with remission of the lymphoma. Thereafter, combination chemotherapy was added (BV+AV*1, BV+AVD*3). A subsequent PET scan showed pelvic wall and mesentery lymph node progression (Deauville score = 4), so five cycles of anti-PD-1+ GVD were administered. Because the PET/CT scan showed slight uptake in the left cervical lymph nodes, the patient accepted a second biopsy to determine if regional radiation therapy should be continued. The biopsy results revealed lymphoid hyperplasia and no evidence of a lymphoma. The patient was prepared for an autologous hematopoietic stem cell transplantation with anti-PD-1 therapy as maintenance treatment. Liver function remained within normal limits until October 2023 except GGT (81 U/L) and ALP (314 U/L), as shown in Figure 2.
Discussion
VBDS associated with lymphoma was first described in 1993 (2). VBDS is mostly described as a unique paraneoplastic syndrome of Hodgkin’s lymphoma that has an unfavorable prognosis. The pathogenesis of VBDS associated with lymphoma has not been established. Some studies speculate that VBDS is relevant to the toxic cytokines released by lymphoma cells (2, 6, 7). T lymphocytes may respond to the cytokines and therefore cause adhesion and cytotoxicity to biliary epithelial cells (8, 9). This process possibly predates any other clinical signs of lymphoma, so it is difficult to identify the real illness.
We retrospectively analyzed 25 cases of lymphoma accompanied by VBDS reported in the last 10 years (4, 10–32) and came to the conclusion that due to the confidence in treating the primary disease, early recovery of liver function (represented by the decrease in bilirubin level) is the main factor that predicts the survival of patients with VBDS-associated lymphoma (Figure 3, P < 0.01). However, long-term survival rate of HL with VBDS has not been adequately addressed in the literature.
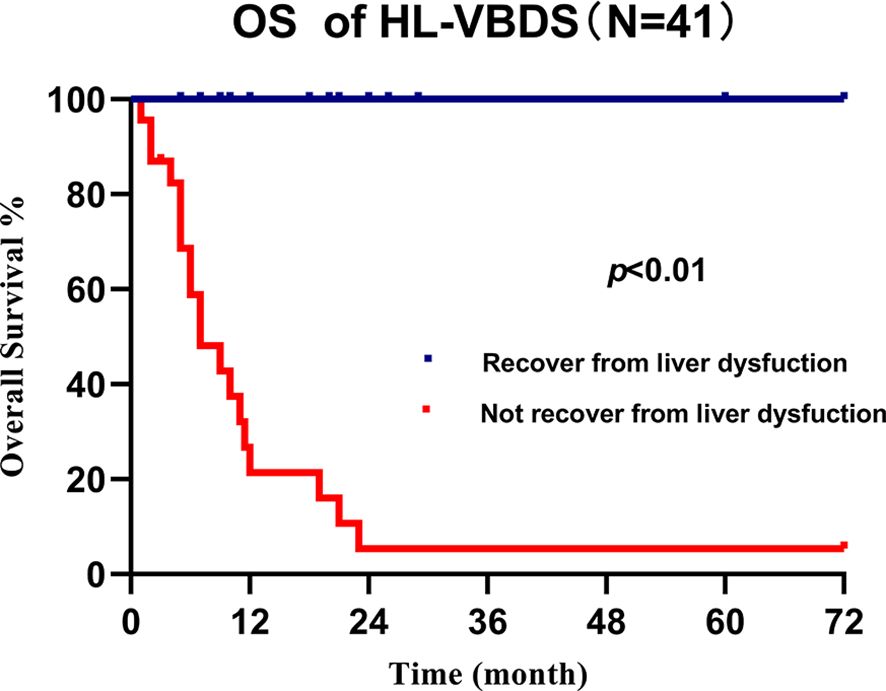
Figure 3. Overall survival of cHL-associated VBDS grouped by recovery from liver dysfunction, which showed that recovery of liver function benefits survival.
This case suggests that VBDS should be considered as a specific cause of jaundice associated with lymphoma and not only the tumor involvement. When the jaundice fails to recover quickly after systemic treatment of the primary malignancy, a liver biopsy should be performed to determine the possible cause as soon as possible.
Because the diagnosis of VBDS is confirmed by the loss of > 50% of interlobular bile ducts in an adequate biopsy specimen (containing ≥ 10 portal tracts), nearly all patients diagnosed with VBDS undergo liver biopsy. However, due to safety concerns, patients might decline a biopsy. Since some cases of intrahepatic cholestasis may only be revealed as VBDS based on liver biopsy results, the true prevalence of VBDS is very likely underestimated (2). As previously reported, ultrasound-guided percutaneous liver biopsy (PLB) is the most reliable and common method for the definitive diagnosis of VBDS in addition to liver autopsy. In our case, given the patient’s elevated bleeding risk and hyperbilirubinemia, CT-guided transjugular liver biopsy (TJLB) seemed to be a better way to reduce liver capsule damage. Although TJLB is not as accurate as PLB in patients with precisely located liver lesions, TJLB is considered more suitable for patients with diffuse liver injury.
In addition, there are many causes of VBDS, as in the current case. Drug and tumor factors are mixed, therefore it may be difficult to distinguish the chief cause of VBDS. Although coxib-induced liver injury is regarded as an uncommon event, it is undeniable that celecoxib may aggravate the side effects of chemotherapy on the liver (33). We demonstrated that the accumulation of cholate stasis in periportal hepatocytes with the absence of small bile ducts was gradually forming based on the liver pathologic findings, which was greater than what would be expected after 2 weeks of celecoxib use. Therefore, the pathologic diagnosis of this case was more appropriately referred to as VBDS caused by HL with drug-induced liver injury, rather than VBDS caused by drug-induced liver injury.
To our knowledge, VBDS may occur before, during, or after the onset of lymphoma and sometimes reappears when the lymphoma relapses (34). Assuming that the bile duct epithelial cells could recover from damage in some optimally treated patients, explains why most patients with HL-VBDS are tolerant to chemotherapy. The clinical efficacy was not significantly affected in the mid-term evaluation.
The chemotherapy interval is prolonged and the intensity is decreased due to VBDS, which may ultimately decrease patient survival. Our patient was shown to progress after the seventh cycle evaluation, which was a sign that single-agent BV was less sufficient for the treatment of stage IVB HL, even though single-agent BV had an acceptable safety profile and rapidly normalized the bilirubin level within 4 months. Combination chemotherapy might have been added as early as possible. A similar patient diagnosed with stage III HL was treated with BV, cyclophosphamide, prednisolone, and procarbazine. She also achieved a CMR in the mid-term evaluation with the total bilirubin level back to normal in approximately 5 months but relapsed 3 months after drug withdrawal. She finally achieved a PMR after 8 cycles of the ABVD regimen (17). Our treatment with single-agent BV was associated with a better safety profile, earlier recovery of liver function, and an encouraging complete remission after treatments.
Because liver failure is the leading cause of death reported previously in the literature, we should be aware of the benefits of single-agent BV in VBDS-associated lymphoma. Considering that three of four BV-involved patients achieving a CR, BV showed good survival benefits. A rapid recovery from liver lesions provides patients with an opportunity to undergo more adequate chemotherapy regimens and avoids adverse outcomes, such as severe coagulopathy or sepsis.
This case improves the clinical understanding of cHL accompanied by VBDS so that early detection with sufficient expectation of the disease episode can be made. A higher priority of BV in cHL with VBDS needs to be considered and further studies are required to determine the cause of cHL-VBDS. By summarizing the clinical features and adjusting the treatment of cHL-VBDS, we can avoid further damage of the organs and maximize the benefits.
Patient Consent Statement
Informed consent was documented by means of a written, signed, and dated informed consent form from the patient.
“We agree to publish this case report and extend heartfelt gratitude to our medical team. By prioritizing patient-centered care and scientifically optimizing the treatment plan for severe liver dysfunction, they have given the patient a new lease on life.”
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SM: Conceptualization, Data curation, Formal analysis, Writing – original draft. DC: Investigation, Project administration, Supervision, Writing – review & editing. YM: Supervision, Visualization, Writing – review & editing. BD: Supervision, Visualization, Writing – review & editing. XY: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. RG: Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the following fund projects: the National Youth Top-notch Talent of Ten Thousand Talent Program (2014-253); Translational Research Grant of HCRCH (2020ZKMB06); and Subtopic of National Basic Research Program of China (973 program [2013CB966803]).
Acknowledgments
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1561110/full#supplementary-material
References
1. Guliter S, Erdem O, Isik M, Yamac K, and Uluoglu O. Cholestatic liver disease with ductopenia (vanishing bile duct syndrome) in Hodgkin’s disease: report of a case. Tumori. (2004) 90:517–20. doi: 10.1177/030089160409000516
2. Hubscher SG, Lumley MA, and Elias E. Vanishing bile duct syndrome: a possible mechanism for intrahepatic cholestasis in Hodgkin’s lymphoma. Hepatology. (1993) 17:70–7. doi: 10.1002/hep.1840170114
3. Ballonoff A, Kavanagh B, Nash R, Drabkin H, Trotter J, Costa L, et al. Hodgkin lymphoma-related vanishing bile duct syndrome and idiopathic cholestasis: statistical analysis of all published cases and literature review. Acta Oncol. (2008) 47:962–70. doi: 10.1080/02841860701644078
4. Fong M, Boyle S, and Gutta N. Brentuximab vedotin in combination with sequential procarbazine, cyclophosphamide and prednisolone for the management of Hodgkin’s lymphoma-associated vanishing bile duct syndrome (VBDS) with severe obstructive liver failure. BMJ Case Rep. (2019) 12(2):e227676. doi: 10.1136/bcr-2018-227676
5. Papakonstantinou I, Kosmidou M, Papathanasiou K, Koumpis E, Kapsali E, Milionis H, et al. Paraneoplastic intrahepatic cholestasis in supradiaphragmatic classical Hodgkin lymphoma successfully treated with Brentuximab Vedotin: a case report and review of the literature. In Vivo. (2021) 35:1951–7. doi: 10.21873/invivo.12462
6. Gottrand F, Cullu F, Mazingue F, Nelken B, Lecomte-Houcke M, and Farriaux JP. Intrahepatic cholestasis related to vanishing bile duct syndrome in Hodgkin’s disease. J Pediatr Gastroenterol Nutr. (1997) 24:430–3. doi: 10.1097/00005176-199704000-00013
7. Yusuf MA, Elias E, and Hübscher SG. Jaundice caused by the vanishing bile duct syndrome in a child with Hodgkin lymphoma. J Pediatr Hematol Oncol. (2000) 22:154–7. doi: 10.1097/00043426-200003000-00014
8. Adams DH, Hubscher SG, Shaw J, Rothlein R, and Neuberger JM. Intercellular adhesion molecule 1 on liver allografts during rejection. Lancet. (1989) 2:1122–5. doi: 10.1016/s0140-6736(89)91489-x
9. Nakanuma Y, Tsuneyama K, and Harada K. Pathology and pathogenesis of intrahepatic bile duct loss. J Hepatobiliary Pancreat Surg. (2001) 8:303–15. doi: 10.1007/s005340170002
10. Palla Velangini S, Boddu D, Balakumar S, Premanand A, Kishore R, and Mathew LG. Vanishing bile duct syndrome secondary to Hodgkin Lymphoma in a child. J Pediatr Hematol Oncol. (2022) 44:e945–e7. doi: 10.1097/mph.0000000000002505
11. Ishitsuka K, Yokoyama Y, Baba N, Matsuoka R, Sakamoto N, Sakamoto T, et al. Administration of brentuximab vedotin to a Hodgkin lymphoma patient with liver dysfunction due to vanishing bile duct syndrome resulting in a partial response without any severe adverse events. J Clin Exp Hematop. (2022) 62:154–7. doi: 10.3960/jslrt.21035
12. Gonzalez R, Parmar P, Hardee S, Chang-Halpenny C, Titapiwatanakun R, Tcheng W, et al. Hodgkin Lymphoma-related vanishing bile duct syndrome cholestasis resolved after chemotherapy. J Pediatr Hematol Oncol. (2022) 44:e728–e32. doi: 10.1097/mph.0000000000002223
13. Gaudel P, Brown P, and Byrd K. Vanishing bile duct syndrome in the presence of Hodgkin Lymphoma. Cureus. (2022) 14:e26842. doi: 10.7759/cureus.26842
14. Boldizsár S, Rottek J, Schneider T, Varga F, and Szaleczky E. Hodgkin’s lymphoma-related vanishing bile duct syndrome. Orv Hetil. (2021) 162:884–8. doi: 10.1556/650.2021.32093
15. Greca RD, Cunha-Silva M, Costa LBE, Costa JGF, Mazo DFC, Sevá-Pereira T, et al. Vanishing bile duct syndrome related to DILI and Hodgkin lymphoma overlap: A rare and severe case. Ann Hepatol. (2020) 19:107–12. doi: 10.1016/j.aohep.2019.06.010
16. Sathyanarayanan V, Foo WC, Fanale M, and Westin J. Deeper insights into vanishing bile duct syndrome in Lymphoma: a perplexing entity. Clin Lymphoma Myeloma Leuk. (2016) 16:e65–70. doi: 10.1016/j.clml.2016.02.035
17. Bakhit M, McCarty TR, Park S, Njei B, Cho M, Karagozian R, et al. Vanishing bile duct syndrome in Hodgkin’s Lymphoma: a single center experience and clinical pearls. J Clin Gastroenterol. (2016) 50:688. doi: 10.1097/mcg.0000000000000548
18. Hallén K, Sangfelt P, Nilsson T, Nordgren H, Wanders A, and Molin D. Vanishing bile duct-like syndrome in a patient with Hodgkin lymphoma - pathological development and restitution. Acta Oncol. (2014) 53:1271–5. doi: 10.3109/0284186x.2014.897001
19. Aleem A, Al-Katari M, Alsaleh K, AlSwat K, and Al-Sheikh A. Vanishing bile duct syndrome in a Hodgkin’s lymphoma patient with fatal outcome despite lymphoma remission. Saudi J Gastroenterol. (2013) 19:286–9. doi: 10.4103/1319-3767.121037
20. Yeh P, Lokan J, Anantharajah A, and Grigg A. Vanishing bile duct syndrome and immunodeficiency preceding the diagnosis of Hodgkin lymphoma. Intern Med J. (2014) 44:1240–4. doi: 10.1111/imj.12609
21. Wong KM, Chang CS, Wu CC, and Yin HL. Hodgkin’s lymphoma-related vanishing bile duct syndrome: a case report and literature review. Kaohsiung J Med Sci. (2013) 29:636–41. doi: 10.1016/j.kjms.2013.05.002
22. Oppenheimer AP, Koh C, McLaughlin M, Williamson JC, Norton TD, Laudadio J, et al. Vanishing bile duct syndrome in human immunodeficiency virus infected adults: a report of two cases. World J Gastroenterol. (2013) 19:115–21. doi: 10.3748/wjg.v19.i1.115
23. Das A, Mitra S, Ghosh D, Modi SK, Roy P, Das J, et al. Vanishing bile duct syndrome following cytomegalovirus infection in a child with Hodgkin Lymphoma. J Pediatr Hematol Oncol. (2018) 40:83–4. doi: 10.1097/mph.0000000000001048
24. Bakhit M, McCarty TR, Park S, Njei B, Cho M, Karagozian R, et al. Vanishing bile duct syndrome in Hodgkin’s lymphoma: A case report and literature review. World J Gastroenterol. (2017) 23:366–72. doi: 10.3748/wjg.v23.i2.366
25. Nader K, Mok S, Kalra A, Harb A, Schwarting R, and Ferber A. Vanishing bile duct syndrome as a manifestation of Hodgkin’s lymphoma: a case report and review of the literature. Tumori. (2013) 99:e164–8. doi: 10.1177/030089161309900426
26. Amer S, Muqeetadnan M, Rahman A, Nusrat S, and Hassan S. Vanishing bile duct syndrome: a rare cause of jaundice in Hodgkin’s lymphoma. Turk J Gastroenterol. (2013) 24:444–6.
27. Anugwom C, Goetz G, and Hassan M. Vanishing bile duct syndrome preceding the diagnosis of Hodgkin Lymphoma. ACG Case Rep J. (2020) 7:e00336. doi: 10.14309/crj.0000000000000336
28. Rota Scalabrini D, Caravelli D, Carnevale Schianca F, D’Ambrosio L, Tolomeo F, Boccone P, et al. Complete remission of paraneoplastic vanishing bile duct syndrome after the successful treatment of Hodgkin’s lymphoma: a case report and review of the literature. BMC Res Notes. (2014) 7:529. doi: 10.1186/1756-0500-7-529
29. Zafar M, Farooq M, Butler-Manuel W, Khattak MF, Rana UI, Muhammad T, et al. Vanishing bile duct syndrome associated with non-Hodgkin’s Lymphoma and hepatitis E virus infection. Cureus. (2022) 14:e21328. doi: 10.7759/cureus.21328
30. Sreepati G, Lin J, and Liangpunsakul S. Anaplastic large cell lymphoma and vanishing bile duct syndrome. ACG Case Rep J. (2016) 3:84–5. doi: 10.14309/crj.2016.7
31. Dachy G and Connerotte T. Vanishing bile duct syndrome associated with diffuse large B-cell lymphoma. Br J Haematol. (2016) 173:505. doi: 10.1111/bjh.14004
32. Gagnon MF, Nguyen BN, Olney HJ, and Lemieux B. Vanishing bile duct syndrome arising in a patient with T-cell-rich large B-cell lymphoma. J Clin Oncol. (2013) 31:e357–9. doi: 10.1200/jco.2012.46.7787
33. Bessone F, Hernandez N, Roma MG, Ridruejo E, Mendizabal M, Medina-Cáliz I, et al. Hepatotoxicity induced by coxibs: how concerned should we be? Expert Opin Drug Saf. (2016) 15:1463–75. doi: 10.1080/14740338.2016.1225719
Keywords: jaundice, Hodgkin’s lymphoma, vanishing bile duct syndrome, liver biopsy, brentuximab vedotin (BV)
Citation: Ma S, Cai D, Miao Y, Deng B, Yan X and Gao R (2025) Case Report: Vanishing bile duct syndrome in Hodgkin’s lymphoma: a case highlighting jaundice and lymphadenopathy as early clues. Front. Immunol. 16:1561110. doi: 10.3389/fimmu.2025.1561110
Received: 15 January 2025; Accepted: 29 April 2025;
Published: 21 May 2025.
Edited by:
Narendranath Epperla, The University of Utah, United StatesReviewed by:
Cherry Bansal, Tantia University, IndiaRajesh Kumar, All India Institute of Medical Sciences Jodhpur, India
Copyright © 2025 Ma, Cai, Miao, Deng, Yan and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaojing Yan, eWFueGlhb2ppbmdfcHBAaG90bWFpbC5jb20=; Ran Gao, Y2hpbmFkcmFnb25nckAxNjMuY29t
 Shiyu Ma
Shiyu Ma Dali Cai
Dali Cai Yuan Miao2
Yuan Miao2 Baocheng Deng
Baocheng Deng Xiaojing Yan
Xiaojing Yan