- 1Medical Sciences Division, University of Oxford, Oxford, United Kingdom
- 2Experimental Tuberculosis Unit, Microbiology Department, Germans Trias i Pujol Research Institute (IGTP) and Hospital (HUGTIP), Badalona, Spain
- 3Department of Biology, University of Oxford, Oxford, United Kingdom
Introduction: BCG vaccination can have heterologous or non-specific effects (NSE) that confer resistance against pathogens other than its target Mycobacterium tuberculosis, but the underlying mechanisms are not fully understood.
Methods: We conducted a systematic review synthesising existing literature on immune mechanisms mediating the heterologous/NSE of BCG. Searches were conducted using MEDLINE and Scopus.
Results: 1032 original records were identified, of which 67 were deemed eligible. Several potentially relevant immune pathways were identified, although there may be variation by pathogen. Recent studies have focused on trained immunity whereby innate cells, or the hematopoietic stem and progenitor cells from which they are derived, undergo epigenetic and metabolic reprogramming allowing them to respond more effectively to antigen exposures unrelated to the original stimulus. However, other processes such as granulopoiesis and cross-reactive adaptive immunity may also play a role. Heterologous immunity and NSEs may be influenced by several endogenous and exogenous variables.
Discussion: We discuss the quality of available data, the importance of understanding mechanisms of heterologous protection, and its implications for vaccination strategies.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42023400375, identifier CRD42023400375.
1 Introduction
The Bacillus Calmette–Guérin (BCG) vaccine has been the only licensed vaccine for tuberculosis (TB) since 1921 (1). It is the most widely used vaccine to date, has a well-established safety profile, and confers protection against severe forms of TB and associated mortality in infants (2), although efficacy against adult pulmonary TB is highly variable. Mounting evidence suggests that BCG may also confer protection against non-mycobacterial infections (3). Calmette, co-developer of BCG, first noted a four-fold reduction in infant mortality in preliminary studies, surpassing expectations if protection were against TB alone. The British government observed a similar phenomenon following the universal introduction of BCG in 1953 (4, 5). Subsequent epidemiological and observational studies have supported heterologous benefits across different populations; a World Health Organisation (WHO) review of 17 birth cohorts found that BCG reduces infant mortality by five to ten deaths per thousand children in the first three years of life (6). This trend has been replicated across different study designs, including systematic reviews of randomised control trials (RCTs) alone (7) or cohort, case-control studies, and RCTs together (8), as well as recent gold-standard blinded RCTs (9, 10) reporting beneficial effects against heterologous infections during the neonatal period in particular.
BCG immunotherapy has been used to treat non-invasive bladder cancer for decades, though the mechanisms remain unclear (11). Additionally, a growing body of literature has suggested that BCG could confer protective benefits against a range of maladies running the gamut from infectious disease (e.g., malaria (12), influenza (13) and HIV-1 (14)); autoimmune disease (e.g., asthma (15) and type 1 diabetes (16)); as well as diseases of later life (e.g., lung cancer (17) and Alzheimer’s (18)), although much evidence remains to be validated. The recent COVID-19 pandemic sparked interest in the potential of BCG to protect against COVID-19 until specific vaccines were available. A large RCT found that BCG did not reduce risk of COVID-19, but the pandemic underscored the need to better understand heterologous immunity (19).
While the terms ‘heterologous effects’ and ‘non-specific effects’ are often used interchangeably, the latter implies the exclusion of antigen cross-reactivity and we will henceforth use ‘heterologous effects’ to encompass both antigen-independent NSE and potential cross-reactivity by cells of the adaptive arm of the immune system. The immune mechanisms mediating the heterologous effects of BCG have been under investigation for over 60 years (20). Studies on macrophage activation using BCG stimuli from the 1960s supported the idea that BCG induces non-specific antibacterial immunity (21, 22). Recently, BCG has been key to unravelling the concept of ‘trained immunity’, whereby the innate immune system retains memory via long-term reprogramming. Upregulation of innate cells and their functional mechanisms by BCG can enhance non-specific protection against unrelated secondary infections (23).
In summary, the idea that BCG vaccination confers heterologous protection against diseases other than TB – despite some uncertainties (24, 25) – is now widely accepted, with many studies exploring the mechanism(s) by which such protection occurs. A better understanding of such heterologous effects could support the development of low-cost interventions to reduce all-cause mortality in infants and could be central to rapid responses against new emerging pathogens during the lag-phase of vaccine development (26). Therefore, we conducted the first systematic review of literature describing the immune mechanism(s) mediating the heterologous effects of BCG.
2 Methods
A systematic review of the literature on immune mechanisms mediating the heterologous effects of BCG vaccination was performed in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) (Supplementary Table 1) (27). The study protocol was registered with PROSPERO prior to commencement on 13th March 2023 (ID CRD42023400375). Amendments to the initial protocol were published on 11th June 2024 and 7th April 2025 (28).
2.1 Data sources and search strategy
Searches were conducted in the MEDLINE database via PubMed and through Scopus on 6th March 2023. Relevant literature reviews were also screened for additional references. References were then compiled, de-duplicated, and screened using Rayyan, an online web tool for systematic reviews (29). The full search strategy and terms are provided in Supplementary Table 2. Eligible studies were imported into EndNote 20 for further review. To ensure the review was not outdated at time of submission, a further search was performed in PubMed using the same strategy and criteria but with a date filter of records published between 6th March 2023 and date of second search (26th September 2024). As this was not part of the core systematic review, additional papers identified are included in the discussion section.
2.2 Study selection and eligibility criteria
Studies were considered eligible if they evaluated the potential immune mechanisms of heterologous effects of BCG vaccination in protecting against infectious diseases other than TB in i) persons, cohorts or populations immunised with BCG (or biological samples thereof), ii) preclinical models whereby animals were immunised with BCG (or biological samples thereof), or iii) relevant in vitro models. Eligibility also required exposure to, or stimulation with, heterologous pathogens or their antigens in vivo or in vitro. Any experimental design, formulation, strain, dose and route of administration of the BCG vaccine was accepted.
Studies that described or observed the heterologous effects of BCG vaccination but did not evaluate the potential underlying immune mechanisms, or did so only in silico, were excluded. This review does not include studies that solely examine the role of BCG as an immunotherapy for bladder cancer or other tumours; the effect of BCG vaccination on non-infectious disease; the impact of BCG vaccination on other immunisations; or the Mantoux/Tuberculin test. Other exclusion criteria were i) studies in languages other than English, ii) pre-prints or studies that were otherwise not peer-reviewed, iii) studies for which abstracts or full papers were not available, and iv) background articles or reviews containing no original data. Records were screened by at least two authors to determine eligibility. In case of disagreement, a third author screened the record and a consensus was reached.
2.3 Data extraction
Data was collected manually from eligible papers by both NG and LT and reviewed by LT. Data was collected, where available and according to relevance, on: i) study groups analysed (species, geographical region, cohorts, age, sample size, interventions); ii) BCG vaccination strain, dose and route of administration; iii) outcomes associated with heterologous protection (or surrogates thereof); iv) immune parameters measured; and v) any associations between immune parameters measured and heterologous protection (or surrogates thereof).
2.4 Risk of bias and quality assurance
Risk of meta-biases was reduced by predefining the eligibility criteria, using broad and inclusive search terms, multiple databases, and with no restriction on date of eligibility (up to date of search). To evaluate the quality of the included studies, a quality assurance framework was applied based on previous tools developed by Tanner et al. (30), the ‘Quality Assessment of Controlled Intervention Studies’ tool developed by the National Heart, Lung and Blood Institute (NHLBI) and the ‘Quality Assessment Tool for in vitro Studies’ (QUIN) (31). Articles were assigned scores ranging from 0 to 12, classified into the following categories: 0-2 (poor), 3-5 (fair), 6-8 (good), 9-10 (very good), and 11-12 (excellent).
3 Results
3.1 Identification of studies and their characteristics
Searches identified 1032 unique records, of which 161 were sought for full-text screening. Of these, 147 studies were retrievable and were full text screened, while 14 studies could not be retrieved. 67 articles were deemed eligible and included in the review (Figure 1) (27).
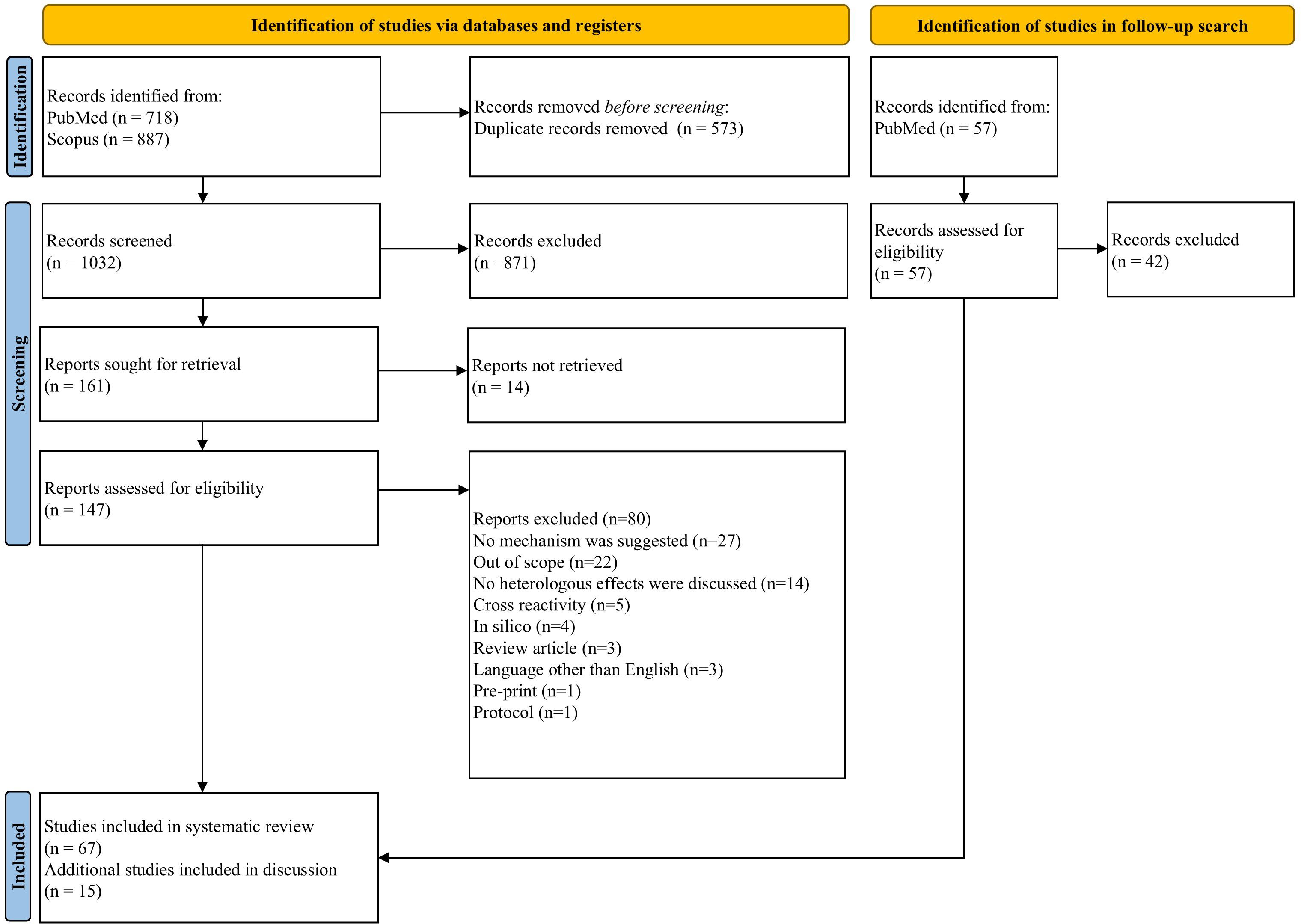
Figure 1. PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) flow diagram of search process and publication selection.
Of the 80 records excluded after full-text review, 27 were excluded because they did not consider immune mechanism(s) underlying heterologous effects. 22 articles were deemed out of the scope of this review. 14 articles did not discuss heterologous effects in detail. Five articles were related to cross-reactivity of vaccines. Four in silico papers were excluded, as were three review articles. Three non-English papers, one pre-print and an experimental protocol were also excluded.
Data extracted from eligible papers is detailed in Supplementary Table 3. 38 used human volunteers, 26 were animal studies and three used both human and animal samples. In our independent quality assessment (QA) of the included research, 11 studies (~16%) were deemed ‘excellent’, the majority of which (seven) were concerned with causes of variation in the heterologous effects of BCG vaccination. Two ‘excellent’ studies were concerned with trained innate immunity, and one each with neutrophils and T cells. 14 studies (~21%) were deemed ‘very good’, 38 (~57%) were ‘good’, and four (~6%) were of ‘fair’ quality. Each of these categories was proportionately spread across the different mechanistic categories. None of the included studies were deemed ‘poor’ (Supplementary Table 4, Supplementary Figure 1).
In the follow-up search, 57 new records were returned of which 15 were deemed eligible.
3.2 Immune mechanisms mediating the heterologous effects of BCG vaccination
Our review suggests that BCG vaccination likely confers heterologous immunity through various mechanisms that differ by context and population, and that its heterologous effects are potentially influenced by several external factors (Figure 2). This review will consider each pathway in turn.
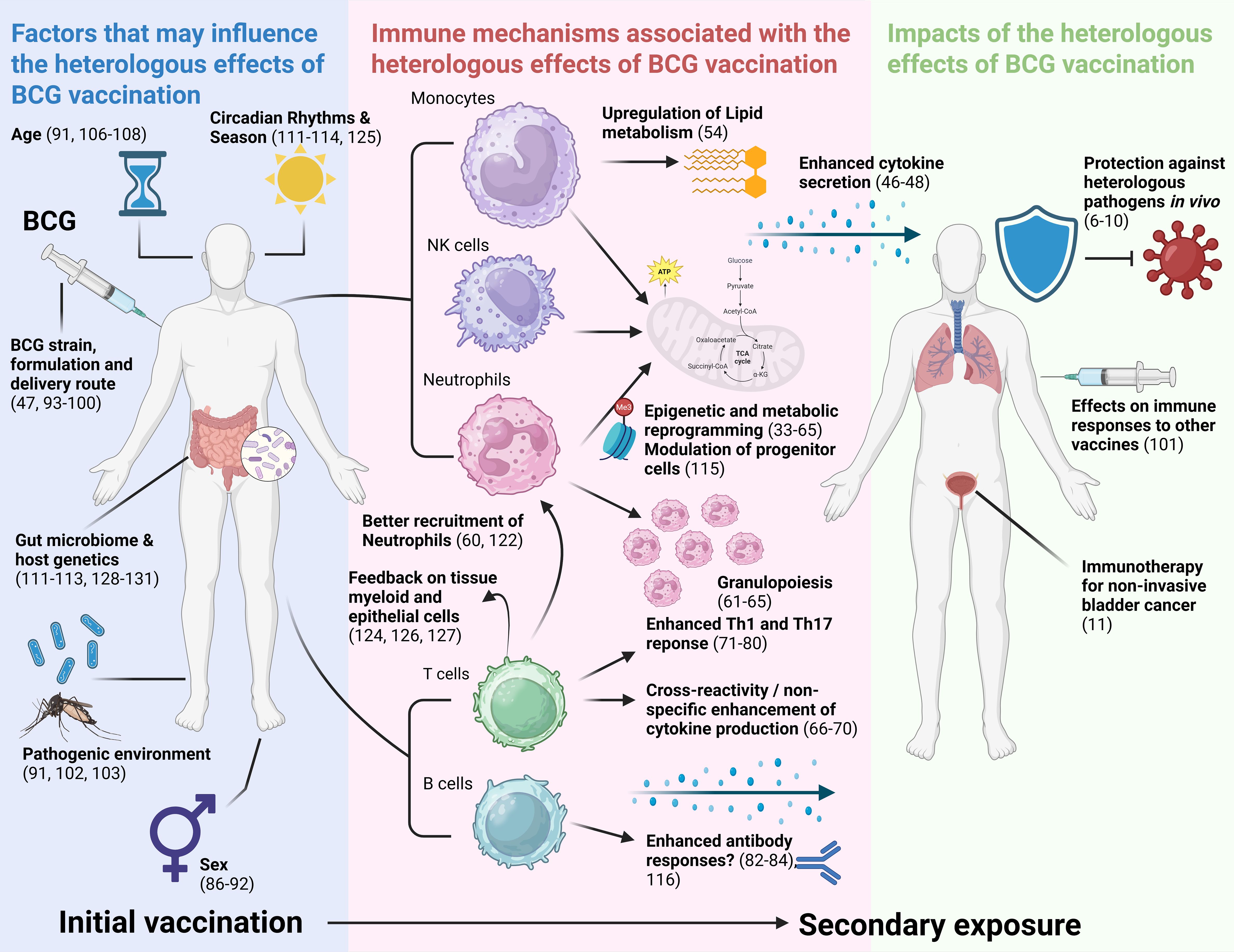
Figure 2. Visual summary of results of this review. Results are synthesised into factors that may influence the NSE of BCG vaccination, immune mechanisms associated with the NSE of BCG vaccination, and impacts of the NSE of BCG vaccination. NSE, non-specific effects; Th1, T helper 1; Th17, T helper 17. Created in BioRender.
3.2.1 Innate Immunity
3.2.1.1 Trained immunity
The concept of trained immunity – that innate cells could retain memory conferring an improved response against secondary exposures – had long been demonstrated in other species (32). This is distinct from the well-understood adaptive immune memory in which antigen-specific memory T and B cells are formed allowing for a faster and more efficient immune response upon subsequent exposure to the same pathogen. Instead, in trained immunity, innate cells undergo long-lasting changes that make them more responsive to subsequent, often unrelated, exposures.
3.2.1.1.1 Monocytes and macrophages
In 2012, Kleinnijenhuis et al. first comprehensively described this phenomenon in humans post-BCG vaccination, though post-BCG non-specific macrophage activation has been reported since 1961 (33–39). In the Kleinnijenhuis study, monocytes collected from Dutch adults vaccinated with BCG produced more cytokines (particularly IFN-γ, IL-1β, and TNF-α) in response to secondary in vitro exposure to unrelated pathogens such as Staphylococcus aureus, as well as to Mycobacterium tuberculosis (M.tb) as expected. This heterologous response was shown to be dependent on functional NOD2 proteins as well as epigenetic H3K4me3 methylation. Transcriptomic analyses of gene polymorphisms involved in autophagy also revealed that H3K4 trimethylation was significantly increased in individuals bearing the ATG2B autophagy gene (40–43). Other cytokines produced by monocytes such as IL-32 have also been implicated, particularly in the heterologous effects against parasitic infections (44). Further in vitro studies have demonstrated that similar trained immunity processes occur in human cord blood adherent monocytes, which are more representative of the monocyte processes of neonatal blood in vivo (45).
It has been shown that β-glucan stimuli drive epigenetic modifications for trained immunity through complex metabolic changes in cells (49). Studies from the 1970s noted increased metabolic rates and glycolysis in activated macrophages (50). However, the detailed metabolic effects of BCG vaccination were not reported until 2016, showing that BCG ligands detected by pattern recognition receptors (PRRs) initiate cascades in monocytes/macrophages that epigenetically upregulate glycolysis, glutamine, and glutathione metabolism, along with oxidative phosphorylation, likely via the Akt/mTOR pathway (51–53). If glycolysis or glutaminolysis is inhibited (e.g., by metformin, an mTOR prohibitor), epigenetic changes in H3K4me3 promoter sites are reversed. This is because methylation is regulated by lysine demethylases and histone methyltransferases, whose activity are influenced by metabolites functioning as co-factors. These findings point to an internal feedback loop within the epigenetic and metabolic mechanisms of trained immunity (49). A separate study of neonatal metabolomes found that BCG vaccination alters plasma lipid metabolism and these changes are correlated with blood cytokine responses to later stimulation with multiple Toll-like receptor (TLR) agonists (54). This study, the first of its kind incorporating comprehensive metabolomics in a resource-limited setting, presents a promising new lipid metabolic mechanism that may mediate heterologous effects in early life.
Finally, although hepcidin-mediated hypoferremia, effectively a limit on iron availability for pathogens, has been hypothesised as a metabolic pathway of trained immunity, studies have been unable to demonstrate this effect following BCG vaccination (9, 55). A lesser-known study of protein and zinc-deficient guinea pigs found that BCG vaccination still conferred heterologous effects (56), indicating that these metabolites may not play a mechanistic role, and lipid or glucose-based pathways seem most pertinent given their circulating levels are known predictors of trained immunity in humans (57). Other studies have investigated the role of nitric oxide production in trained immunity, but found no effect (58).
3.2.1.1.2 Natural killer cells
Experiments in natural killer (NK) cells have also demonstrated that BCG vaccination leads to trained immunity and increased secretion of the proinflammatory cytokines IL-1β, TNF-α, and IFN-γ following heterologous stimulation, indicating that this phenomenon occurs across several innate cell types (46–48).
3.2.1.1.3 Neutrophils
Early studies suggested BCG vaccination confers heterologous immunity even in granulocytopenic mouse models, suggesting a limited role for neutrophils in this phenomenon (59). However, a 1990s study found that BCG vaccination in mice enhanced the ability of macrophages and T cells to recruit neutrophils to infection sites weeks later (60), and granulopoiesis is induced through G-CSF stimulation (61). BCG vaccination is now thought to prevent neonatal sepsis mechanistically through rapid granulopoiesis (62). A study analysing hematopoietic stem cell transcriptomes of healthy human volunteers inoculated with BCG found that the genes that are most differentially expressed at three months post-vaccination are those implicated in neutrophil activation and degranulation pathways, ultimately skewing the myeloid lineage toward granulopoiesis. This may drive a phenotypic change marked by increased activation markers and reduced immunosuppression markers (63). Further studies have found BCG stimulation increases production of the antimicrobial chemokine IL-8 and the serine protease elastase in neutrophils upon ex vivo exposure to secondary pathogenic stimuli (64). In summary, BCG boosts neutrophil production, activates resting cells, and enhances their antimicrobial function.
As discussed, trained immunity in other innate cells occurs through complex metabolic and epigenetic changes (45). Similarly, neutrophil activation in this context likely involves these pathways. Neutrophils primarily use glycolysis for energy, and ex vivo-stimulated neutrophils show increased glycolytic rates following BCG vaccination, along with elevated H3K4me3 modifications at promoters of glycolysis-regulating genes such as phosphofructokinase and mTOR (64). Neutrophils only survive for a few days, yet both the epidemiological and laboratory-based epigenetic data point to months-long heterologous effects, meaning some of these modifications must be to neutrophil precursors, a process supported by current laboratory data on neutrophil activation using β-glucan (65). Further in vitro work similar to the metabolomics studies recently attempted in peripheral blood mononuclear cells (PBMCs) (43, 57) measuring epigenetic modifications could therefore be of interest in the context of neutrophils.
3.2.2 Adaptive immunity
3.2.2.1 T cells
BCG may also confer longer-term heterologous effects through adaptive immune mechanisms. Given the degenerative nature of T cell recognition, the conformational shifts a T cell receptor (TCR) undergoes to recognise a peptide/major histocompatibility complex (MHC) complex, and the conserved nature of many microbial antigens, specific memory T cells may in fact be activated by unrelated pathogens through cross-reactivity (66). However, such a phenomenon fails to explain the increased responses to pathogens such as Candida albicans or Staphylococcus aureus observed following BCG vaccination despite a lack of shared or similar epitopes. Alternatively, BCG-induced proinflammatory cytokine release by lymphocytes may act non-specifically to activate bystander macrophages resulting in a state of temporarily heightened innate immunity, although this effect wanes rapidly. Effector and memory CD8+ T cells can be non-specifically activated by IL-12 and IL-18 during early secondary infection, independent of TCR signalling, leading to IFN-γ secretion that affects innate cells. This provides a plausible explanation for longer-lived T cell-mediated heterologous effects (67).
In 1984, Orme et al. noted the emergence of a splenic T cell population that was temporally associated with the development of an acquired capacity for non-specific resistance to secondary facultative intracellular bacterial pathogens including Listeria monocytogenes following intravenous (IV) inoculation of mice with high-dose BCG (68). Further studies suggested BCG-related non-specific resistance to Listeria was lower in T cell-deficient than in intact mice (69, 70). Furthermore, Mathurin et al. found that BCG vaccination rendered mice partially resistant to infection with vaccinia virus; an effect which was lost after CD4+ T cell depletion or inhibition of TCR signalling (71). Nonetheless, the anti-malarial protection from BCG vaccination diminishes after CD8+, but not CD4+, T cell depletion (72). Although non-specifically activated, certain immune mechanisms may be more relevant for some infections than others.
Interestingly, in mice vaccinated with BCG, protection against experimental cerebral malaria after Plasmodium berghei infection has been linked to lower levels of proinflammatory mediators in the brain compared to mice infected without BCG vaccination (73). BCG vaccination was also associated with altered T cell phenotypes in the blood and spleen, and a reduced influx of T cells into the brain, which can otherwise have major pathogenic roles. The authors concluded that preventing experimental cerebral malaria results from the anti-inflammatory and T cell inhibitory functions of BCG, rather than from the hypothesised reduction in parasitaemia due to trained innate immunity (73). Such immunosuppressive functions were described over 40 years ago, including early studies describing non-specific T cell suppressor activity following IV inoculation of mice with high-dose BCG (74, 75).
Delaying BCG vaccination to 8 weeks of age does not affect overall T cell proliferation or cytokine polyfunctionality. However, infants vaccinated at birth show a significantly higher frequency of IL-2+ CD8+ T cells in response to Bordetella pertussis compared to unvaccinated infants (76). A larger multi-site study comparing BCG strains noted that infants vaccinated with BCG-Denmark mounted significantly higher magnitudes and polyfunctionality of CD4+ T cell responses to in vitro stimulation with Tetanus toxoid and B. Pertussis antigens compared to those vaccinated with BCG-Bulgaria or BCG-Russia (77). In healthy healthcare workers exposed to SARS-CoV-2, BCG vaccination was associated with enhanced central and effector memory CD4+ and CD8+ T cell subsets overall as long as 3 months later (78), although a study of Danish infants found minimal subset differences (78, 79). BCG may also enhance heterologous Th1 and Th17 immune responses for as long as 1 year after vaccination (80). Non-specific cellular cytotoxicity has been poorly studied, but an early paper measuring cytotoxicity against a non-specific target noted marked changes in NK cell, and to a lesser extent, T cell cytotoxicity following BCG vaccination (81).
3.2.2.2 B cells
Memory B cells are prone to activation by polyclonal stimulation, and it has long been suggested that mycobacterial antigens or T cell cytokines could activate pathogen-specific memory B cells in a non-specific manner, leading to expansion of antibody-secreting cells (82). Early work noted that sera from BCG vaccinated rabbits contained antibodies that bound to radiolabelled antigens from both unrelated and homologous test antigens (83). A recent study by Kimuda et al. found that active TB disease was linked to higher titres of antibodies specific to RSV and measles virus, but BCG vaccination did not affect antibodies against heterologous pathogens in the same way (84). Additional research is needed to clarify how adaptive immunity contributes to the heterologous effects of BCG and the associated mechanisms.
3.3 Causes of variation
Some of the studies in this systematic review are large RCTs characterising heterologous effects of BCG vaccination in vivo (9, 55, 85–88). The largest trial in Ugandan infants prospectively measured all-cause infectious morbidity, providing compelling evidence for heterologous effects (9). However, RCTs paint a muddled picture of the immune parameters that could mechanistically explain this phenomenon. In Uganda, BCG was associated with some minor epigenetic changes in PBMCs, but significant effects on cytokine production were not observed following stimulation with Escherichia coli and Candida albicans (9). Similarly, stimulation of cells with E. coli and C. albicans did not increase rates of cytokine secretion in Dutch infants (85). However, a retrospective study of low birth weight infants in Guinea-Bissau found that both very low and very high cytokine responses to Lipopolysaccharide (LPS) and Phytohemagglutinin (PHA) stimuli were associated with high mortality, and a balanced production was preferable (86). Two Australian studies found only decreased IFN-γ and IL-6 following stimulation with heterologous antigens (87, 88).
Given that heterologous effects in vitro are reproducible, what is causing these observed variations? What are the difficulties inherent to eliciting mechanistic trends in vivo? This review identified several factors that may explain the population-level variation observed in the heterologous effects of BCG.
3.3.1 Sex differences
There is some evidence suggesting that heterologous vaccine responses can be sex-differential (89). Two Australian infant BCG trials found a significant interaction between sex and macrophage migration inhibitory factor, as well as other cytokine secretion, following heterologous stimulation. Meanwhile, two trials in Guinea-Bissau found smaller, but still notable, variation in cytokine profiles in response to TLR agonists between sexes (86–88, 90). A separate trial measuring BCG and hepatitis B co-vaccination in neonates reported that males produced more IFN-γ and TNF-α, and less MCP-1 in response to heterologous pathogens compared with females (91). Finally, a trial of 307 healthy adults found that BCG down-regulates systemic inflammation alongside enhanced cytokine responses, but mainly in male cohorts, likely due to circulating testosterone levels (92). The mechanisms underlying the sex differential effects in heterologous immunity are largely unknown, and small demographic imbalances in study populations may drive observed variation.
3.3.2 Vaccine strain and method of delivery
A number of studies suggest that the BCG strain affects heterologous effects; specifically, slow growth and live batches elicit stronger cytokine responses in monocytes, while exact dosage seems less critical (47, 93–95). Additionally, an early 1980s study found significant variation between freeze dried and fresh liquid vaccines given either intradermally or IV (96). A recent Guinean trial found that BCG-Russia does not enhance innate immunity in the same way as BCG-Denmark, and BCG-Russia induces only short-lived effects on CD8+ T-cell reactivity to C. albicans (97). BCG-Russia is among the most widely used vaccine strains worldwide (98) but few studies used it within this review, and further research into BCG-Russia and its descendant strains is warranted. Not all included studies were methodologically clear about the BCG strain used or the preparation or immunisation methods, which should be addressed going forward, especially considering pertinent recent developments in new routes of delivery such as aerosolised BCG (99). Finally, a small trial of inactivated gamma-irradiated BCG vaccine found that it did not confer protective heterologous immunity to endotoxins, suggesting that inactivated alternatives to BCG are unlikely to deliver the same benefits as live strains (100).
3.3.3 Exogenous factors
Local environmental factors may confound the outcomes measured. Although broadly-speaking the protection BCG confers against TB increases with distance from the equator, heterologous effects appear to stratify in the opposite direction, possibly through priming from maternal vaccination in neonates or enhanced immunity resulting from continual exposure to circulating mycobacteria (25). Other research suggests that BCG might influence how the immune system responds to different neonatal vaccines (101). It is possible that the opposite effect occurs, whereby variations in individual vaccine status and the surrounding pathogenic environment result in different heterologous outcomes (91, 102, 103).
Several individual factors are posited as causes of variation, such as age at time of vaccination and sampling – the studies in this review often sampled either exclusively infants or adults. BCG vaccination RCTs in the elderly indicate that both innate immunity and adaptive mechanisms contribute to heterologous effects, especially against respiratory viruses (104, 105). However, neonatal cells respond to BCG in a fundamentally distinct way to those of adults and demonstrate shifting cytokine and epigenetic profiles throughout development (9, 91, 106, 107). Immune ontogeny is therefore likely a strong factor causing variability in heterologous immunity (108).
Research also indicates that differential levels of circulating Vitamin A metabolites down-regulate trained immunity epigenetically (109), but increased release of muramyl dipeptide, a potent adjuvant, is conversely associated with a strengthened inflammatory response (110). Finally, increasing evidence indicates that individual circadian rhythms and gut microbiota such as Roseburia may impact heterologous effects (111–113). For example, early morning vaccination produces superior cytokine production upon heterologous stimulation compared to evening vaccination (114). There is a need to better measure, control and stratify potential exogenous and endogenous confounders to fully validate heterologous mechanisms in vivo.
4 Discussion
This review is the first to systematically synthesise literature pertaining specifically to the immune mechanisms mediating the heterologous effects of the BCG vaccine and has identified several pathways that are likely non-mutually exclusive. It is now clear that BCG vaccination induces ‘training’ of innate cells including monocytes, NK cells, and neutrophils (and their precursors) via a complex interplay of metabolic and epigenetic changes conferring immunity for a year or longer. BCG may also induce heterologous reactivity of T and B cells that is not well understood. The quality of the studies included was generally ‘good’ or ‘very good’, although many were limited in their sample size. Some of the more robust preclinical studies or clinical trials of the heterologous effects of BCG vaccination were excluded as they failed to investigate the immune mechanisms involved. Those that did so almost exclusively focused on one mechanism (in the majority of cases this was trained innate immunity). A priority going forward should thus be a more comprehensive immunological analysis of samples from large in vivo studies of BCG; particularly using novel systems approaches to better understand the integration of different immune pathways.
Of the studies included herein, trained immunity in innate cells has been studied in the greatest depth and outcomes are, at least in vitro, most consistent. Recent research has also elucidated modulation of upstream pathways, focusing on hematopoietic stem and progenitor cells (115). In contrast, there is a paucity of research on the relevance of adaptive mechanisms, particularly humoral responses; much is decades old with inconsistency in results. It is important to note that different immune mechanisms (or relative contributions thereof) may underlie the heterologous effects observed against different pathogens, and in some cases these may be regulatory (73). Immune parameters mediating protection vary by pathogen life history and infection stage, and pathogens differ in their ability to evade the protective effects of heterologous immunity through strategies such as intracellular survival, antigenic variation, and immune modulation – which may also manifest differently in in vitro vs. in vivo settings. While cross-reactivity of adaptive cells is plausible for some pathogens that share molecular patterns or antigenic similarities with mycobacteria, it is a relatively rare phenomenon, and BCG epitopes associated with such protection are yet to be described (118). Trained innate immunity may be a more broadly applicable mechanism, yet also appears not to be universal.
Follow-up studies should make use of more recent technologies to gain a clearer understanding of the role of adaptive immunity (if any) in the heterologous effects of BCG vaccination, and the mechanisms by which this is mediated, including antigen identification. If, as suggested, M.tb infection can enhance heterologous antibody titres (84, 116), then it is of interest why even large doses of BCG, only attenuated by about ~9.5kb of DNA (117), appear unable to do so. Ideally such studies should be performed in vivo to avoid the pitfalls of the artificial in vitro environment. However, as discussed, a number of factors may influence the heterologous effects of BCG vaccination in vivo including the microbiome, circadian rhythms, BCG strain/formulation used, and age or sex of the individual. While difficult to implement in real-life settings, future research should aim to minimise variation and control for confounders to better delineate mediators and ensure comparability between studies. Advances in ex vivo immune organoid technology may provide an opportunity to better model the complexity of immunological outcomes following vaccination in a more controlled environment (119).
This review has several strengths and limitations. Using inclusive search terms, no limit on year of publication, and systematic methods increased the likelihood of being comprehensive and unbiased, although several older articles of interest were ultimately inaccessible for abstract or full text review. This review was also limited by excluding articles concerning the effects of BCG on cancer or other immunisations. Although of interest for understanding mechanisms of heterologous immunity, the interactions of BCG with neoplastic cells or attenuated pathogens (or their antigens) may be different to the induction of trained immunity towards secondary live infections. Future research on this topic may be relevant to understanding heterologous immunity to whole live pathogens. In silico studies (albeit small in number) were excluded to ensure the review was focussed on empirical evidence derived from experimental studies involving biological systems and to avoid the introduction of heterogeneity/bias associated with combining data across fundamentally different methodologies. However, in silico studies may become increasingly significant in future research with advances in artificial intelligence. Additionally, pre-prints and non-English articles were omitted, possibly overlooking contributions from underrepresented authors or regions.
The field is evolving rapidly, prompting us to re-execute the search for relevant records published over the 18 months since the initial search, identifying 15 new eligible papers that reinforce existing hypotheses and suggest new research directions. In vitro models of the heterologous effects of BCG are being further optimised (120), and new translational models have been proposed including pigs, although findings in this species did not corroborate the innate immunological responsiveness to BCG seen in humans and may require further optimisation (121). Notably, the role of enhanced lung immunity in mediating innate protection against heterologous respiratory bacterial infections has been highlighted, with a key role for enhanced neutrophilia that appears to be independent of centrally trained circulating monocytes (122). Additional studies have reported extensive reprogramming of lung immune cells (123), and a biphasic innate response with robust antigen-specific Th1 cell responses in the lungs following IV BCG vaccination in hamsters and mice respectively (124). Interestingly, the latter study noted a central role for CD4+ T cell feedback on tissue myeloid and epithelial cells to imprint prolonged and broad innate antiviral resistance (124). While the role of humoral immunity remains neglected, a recent NHP study found that BCG vaccination failed to enhance antibody titres against a range of heterologous pathogen antigens (116), consistent with the findings of Kimuda et al. (84).
Investigations of exogenous factors have expanded to consider how seasons influence trained immunity. Kilic et al. found that BCG vaccination during winter induced a stronger increase in pro-inflammatory cytokine production by PBMCs and NK cells following stimulation with heterologous pathogen stimuli three months later, compared to BCG vaccination in spring. Although BCG had minimal impact on the monocyte transcriptome, vaccination resulted in notable season-dependent epigenetic alterations in both monocytes and NK cells (125). The researchers suggest that BCG vaccination in winter may enhance trained immunity due to the activation and reprogramming of immune cells, especially NK cells (125). Other cell types besides monocytes, macrophages, and NK cells can contribute to trained immunity and the heterologous effects of BCG. Research shows that γδ T cells exhibit innate memory in response to BCG vaccination in healthy volunteers when stimulated by heterologous bacterial and fungal stimuli (126). Samuel et al. have also reported evidence of innate training in bovine γδ T cells following subcutaneous BCG administration and subsequent in vitro stimulation with E. coli LPS and PAM3CSK4 (127).
Specht et al. found that the genetic background of hosts affects BCG-induced antibodies that cross-react with the SARS-CoV-2 spike protein in mice (128). A more sophisticated multi-omics analysis of over 300 healthy individuals identified genetic and epigenetic predictors of baseline immunity and immune response, finding that BCG vaccination enhanced the innate immune response more-so in individuals with a dormant immune state at baseline. The authors note that epigenetic cell states function as an ‘endophenotype’ integrating signals from genotype and environment, linking them to personal immune profiles (129). A further multi-omics analysis showed that linoleic acid metabolism was correlated with the trained immunity-inducing capacity of different BCG strains, and could act as an adjuvant to enhance BCG-induced trained immunity (130). However, despite stimulation of granulopoiesis, administration of another potential adjuvant, the nitrogen-containing bisphosphonate alendronate, alongside BCG, was associated with reduced cytokine production by PBMCs against heterologous stimuli one month later in healthy individuals (131).
Recent technologies have created opportunities to enhance our understanding of monocyte functions, interactions, and gene regulation in an in vivo setting, which could be vital for comprehending clinical conditions. In a single cell transcriptional analysis of the host immune response to in vivo trained immunity by BCG, monocytes and CD8+ T cells showed heterologous transcriptional responses to LPS, with active crosstalk between the two cell types. IFN-γ was shown to play an important role in amplifying the trained immunity response, and STAT1 found to be one of the important transcription factors for trained immunity in all identified monocyte subpopulations (132). Finally, and significantly, it has been shown that BCG alters both the epigenetics and chromatic accessibility of hematopoietic stem and progenitor cells, and these effects are directly correlated with enhanced IL-1β secretion by descendant paired PBMCs following stimulation with C. albicans – strong evidence to support the concept that upstream progenitor modulation is implicated in the development of long-term trained innate immunity (115).
Kandasamy et al. (8), in a review of vaccine heterology, argue that a central challenge to understanding heterologous effects is that conventional immunological measurements produce limited mechanistic insight into the association between vaccination and immune outcomes observed epidemiologically. The mechanisms by which BCG protects against TB itself are still being uncovered (133) and future research requires improved models of vaccine-mediated immunity, such as controlled human or non-human primate infection models (2, 134). However, the innovative studies discussed also point to further discovery possibilities in designs and tools already available, such as metabolomic and epigenetic profiling techniques (43).
4.1 Conclusion
This systematic review synthesises current literature on the immune mechanisms mediating the heterologous effects of BCG vaccination. It reveals a deep and sometimes obscure history of experimental research on the impact of BCG on heterologous immunity, extending over eight decades. It finds the greatest quantity and quality of evidence for mechanisms of trained innate immunity, particularly in monocytes, NK cells and neutrophils, while the role of cross-reactive adaptive responses (in particular humoral immunity) is less clear and less well-studied.
It is striking that the BCG vaccine, painstakingly developed under the shadow of World War I (135), remains the only vaccine against the world’s leading cause of infectious disease mortality (136). Remarkably, the mechanisms of BCG-mediated protection remain unclear and defy traditional divides of innate versus adaptive immunity and the belief that immune memory is limited to the adaptive arm. Further research into this seemingly humble vaccine is therefore highly warranted.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
LT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. NG: Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. RT: Conceptualization, Funding acquisition, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. NG has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No. 847762 and Catalan Government through 2021 SGR 00920. RT is a Jenner Investigator.
Acknowledgments
We are grateful to Eli Harriss for assistance in developing the search strategies.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1567111/full#supplementary-material
References
1. Dockrell HM and McShane H. Tuberculosis vaccines in the era of Covid-19 - what is taking us so long? EBioMedicine. (2022) 79:103993. doi: 10.1016/j.ebiom.2022.103993
2. Martinez L, Cords O, Liu Q, Acuna-Villaorduna C, Bonnet M, Fox GJ, et al. Infant BCG vaccination and risk of pulmonary and extrapulmonary tuberculosis throughout the life course: a systematic review and individual participant data meta-analysis. Lancet Glob Health. (2022) 10:e1307–e16. doi: 10.1016/S2214-109X(22)00283-2
3. Freyne B, Marchant A, and Curtis N. BCG-associated heterologous immunity, a historical perspective: experimental models and immunological mechanisms. Trans R Soc Trop Med Hygiene. (2015) 109:46–51. doi: 10.1093/trstmh/tru196
4. Calmette A. Preventive vaccination against tuberculosis with BCG. Proc R Soc Med. (1931) 24:1481–90. doi: 10.1177/003591573102401109
5. D'Arcy Hart P, Charles J, Cruickshank R, Daniels M, Pointon W, Geddes DJE, et al. B.C.G. and vole bacillus vaccines in the prevention of tuberculosis in adolescents. Br Med J. (1956) 1:413–27. doi: 10.1136/bmj.1.4964.413
6. Higgins JP, Soares-Weiser K, Lopez-Lopez JA, Kakourou A, Chaplin K, Christensen H, et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ. (2016) 355:i5170. doi: 10.1136/bmj.i5170
7. Trunk G, Davidović M, and Bohlius J. Non-specific effects of bacillus calmette-guérin: A systematic review and meta-analysis of randomized controlled trials. Vaccines. (2023) 11:121–. doi: 10.3390/vaccines11010121
8. Kandasamy R, Voysey M, McQuaid F, De Nie K, Ryan R, Orr O, et al. Non-specific immunological effects of selected routine childhood immunisations: Systematic review. BMJ (Online). (2016) 355:i5225–i5225. doi: 10.1136/bmj.i5225
9. Prentice S, Nassanga B, Webb EL, Akello F, Kiwudhu F, Akurut H, et al. BCG-induced non-specific effects on heterologous infectious disease in Ugandan neonates: an investigator-blind randomised controlled trial. Lancet Infect Diseases. (2021) 21:993–1003. doi: 10.1016/S1473-3099(20)30653-8
10. Nemes E, Geldenhuys H, Rozot V, Rutkowski KT, Ratangee F, Bilek N, et al. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N Engl J Med. (2018) 379:138–49. doi: 10.1056/NEJMoa1714021
11. Pettenati C and Ingersoll MA. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat Rev Urol. (2018) 15:615–25. doi: 10.1038/s41585-018-0055-4
12. Berendsen ML, van Gijzel SW, Smits J, de Mast Q, Aaby P, Benn CS, et al. BCG vaccination is associated with reduced malaria prevalence in children under the age of 5 years in sub-Saharan Africa. BMJ Glob Health. (2019) 4:e001862. doi: 10.1136/bmjgh-2019-001862
13. Leentjens J, Kox M, Stokman R, Gerretsen J, Diavatopoulos DA, van Crevel R, et al. BCG vaccination enhances the immunogenicity of subsequent influenza vaccination in healthy volunteers: A randomized, placebo-controlled pilot study. J Infect Dis. (2015) 212:1930–8. doi: 10.1093/infdis/jiv332
14. Rieckmann A, Villumsen M, Jensen ML, Ravn H, da Silva ZJ, Sorup S, et al. The effect of smallpox and bacillus calmette-guerin vaccination on the risk of human immunodeficiency virus-1 infection in Guinea-bissau and Denmark. Open Forum Infect Dis. (2017) 4:ofx130. doi: 10.1093/ofid/ofx130
15. Abdelaziz MH, Ji X, Wan J, Abouelnazar FA, Abdelwahab SF, and Xu H. Mycobacterium-induced th1, helminths-induced th2 cells and the potential vaccine candidates for allergic asthma: imitation of natural infection. Front Immunol. (2021) 12:696734. doi: 10.3389/fimmu.2021.696734
16. Dias HF, Mochizuki Y, Kuhtreiber WM, Takahashi H, Zheng H, and Faustman DL. Bacille Calmette Guerin (BCG) and prevention of types 1 and 2 diabetes: Results of two observational studies. PloS One. (2023) 18:e0276423. doi: 10.1371/journal.pone.0276423
17. Usher NT, Chang S, Howard RS, Martinez A, Harrison LH, Santosham M, et al. Association of BCG vaccination in childhood with subsequent cancer diagnoses: A 60-year follow-up of a clinical trial. JAMA Netw Open. (2019) 2:e1912014. doi: 10.1001/jamanetworkopen.2019.12014
18. Gofrit ON, Klein BY, Cohen IR, Ben-Hur T, Greenblatt CL, and Bercovier H. Bacillus Calmette-Guerin (BCG) therapy lowers the incidence of Alzheimer's disease in bladder cancer patients. PloS One. (2019) 14:e0224433. doi: 10.1371/journal.pone.0224433
19. Pittet LF, Messina NL, Orsini F, Moore CL, Abruzzo V, Barry S, et al. Randomized trial of BCG vaccine to protect against covid-19 in health care workers. N Engl J Med. (2023) 388:1582–96. doi: 10.1056/NEJMoa2212616
20. Freyne B, Marchant A, and Curtis N. BCG-associated heterologous immunity, a historical perspective: intervention studies in animal models of infectious diseases. Trans R Soc Trop Med Hygiene. (2015) 109:52–61. doi: 10.1093/trstmh/tru197
21. Mackaness GB. The immunological basis of acquired cellular resistance. J Exp Med. (1964) 120:105–20. doi: 10.1084/jem.120.1.105
22. Mitchell MS and Murahata RI. Modulation of immunity by bacillus Calmette-Guerin (BCG). Pharmacol Ther. (1979) 4:329–53. doi: 10.1016/0163-7258(79)90141-4
23. Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunology: Nat Research;. (2020) 20:375–88. doi: 10.1038/s41577-020-0285-6
24. Pollard AJ, Finn A, and Curtis N. Non-specific effects of vaccines: plausible and potentially important, but implications uncertain. Arch Dis Child. (2017) 102:1077–81. doi: 10.1136/archdischild-2015-310282
25. Prentice S and Dockrell HM. BCG specific and nonspecific effects: different questions, similar challenges. J Infect Dis. (2021) 224:1105–8. doi: 10.1093/infdis/jiab307
26. Laxminarayan R and Bhutta ZA. Antimicrobial resistance-a threat to neonate survival. Lancet Glob Health. (2016) 4:e676–7. doi: 10.1016/S2214-109X(16)30221-2
27. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71–n71. doi: 10.1136/bmj.n71
28. Torracinta L, Gogichadze N, and Tanner R. Immune mechanisms mediating the heterologous effects of BCG vaccination: a systematic review. PROSPERO. (2023), CRD42023400375.
29. Ouzzani M, Hammady H, Fedorowicz Z, and Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. (2016) 5:210. doi: 10.1186/s13643-016-0384-4
30. Painter H, Harriss E, Fletcher HA, McShane H, and Tanner R. Development and application of the direct mycobacterial growth inhibition assay: a systematic review. Front Immunol. (2024) 15:1355983. doi: 10.3389/fimmu.2024.1355983
31. Sheth VH, Shah NP, Jain R, Bhanushali N, and Bhatnagar V. Development and validation of a risk-of-bias tool for assessing in vitro studies conducted in dentistry: The QUIN. J Prosthet Dent. (2024) 131:1038–42. doi: 10.1016/j.prosdent.2022.05.019
32. Rodrigues J, Brayner FA, Alves LC, Dixit R, and Barillas-Mury C. Hemocyte differentiation mediates innate immune memory in Anopheles Gambiae mosquitoes. Science. (2010) 329:1353–5. doi: 10.1126/science.1190689
33. Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A. (2012) 109:17537–42. doi: 10.1073/pnas.1202870109
34. Sulitzeanu D, Bekierkunst A, Groto L, and Loebel J. Studies on the mechanism of non-specific resistance to Brucella induced in mice by vaccination with BCG. Immunology. (1962) 5:116–28.
35. Blanden RV, Lefford MJ, and Mackaness GB. The host response to Calmette-Guerin bacillus infection in mice. J Exp Med. (1969) 129:1079–107. doi: 10.1084/jem.129.5.1079
36. Nacy CA and Meltzer MS. Macrophages in resistance to rickettsial infections: protection against lethal Rickettsia tsutsugamushi infections by treatment of mice with macrophage-activating agents. J Leukoc Biol. (1984) 35:385–96. doi: 10.1002/jlb.35.4.385
37. Bloksma N, de Reuver MJ, and Willers JM. Impaired macrophage functions as a possible basis of immunomodification by microbial agents, tilorone and dimethyldioctadecylammonium bromide. Antonie Van Leeuwenhoek. (1983) 49:13–22. doi: 10.1007/BF00457875
38. Monga DP. Role of macrophages in resistance of mice to experimental cryptococcosis. Infect Immun. (1981) 32:975–8. doi: 10.1128/iai.32.3.975-978.1981
39. McCuskey RS, Urbaschek R, McCuskey PA, and Urbaschek B. In vivo microscopic observations of the responses of Kupffer cells and the hepatic microcirculation to Mycobacterium bovis BCG alone and in combination with endotoxin. Infect Immun. (1983) 42:362–7. doi: 10.1128/iai.42.1.362-367.1983
40. Buffen K, Oosting M, Quintin J, Ng A, Kleinnijenhuis J, Kumar V, et al. Autophagy controls BCG-induced trained immunity and the response to intravesical BCG therapy for bladder cancer. PloS Pathog. (2014) 10:e1004485. doi: 10.1371/journal.ppat.1004485
41. Kong L, Moorlag S, Lefkovith A, Li B, Matzaraki V, van Emst L, et al. Single-cell transcriptomic profiles reveal changes associated with BCG-induced trained immunity and protective effects in circulating monocytes. Cell Rep. (2021) 37:110028. doi: 10.1016/j.celrep.2021.110028
42. Arts RJW, Moorlag S, Novakovic B, Li Y, Wang SY, Oosting M, et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe. (2018) 23:89–100 e5. doi: 10.1016/j.chom.2017.12.010
43. Bannister S, Kim B, Dominguez-Andres J, Kilic G, Ansell BRE, Neeland MR, et al. Neonatal BCG vaccination is associated with a long-term DNA methylation signature in circulating monocytes. Sci Adv. (2022) 8:eabn4002. doi: 10.1126/sciadv.abn4002
44. Silva MVT, Dos Santos JC, Figueiredo AMB, Teufel LU, Pereira JX, Matos GG, et al. The role of IL-32 in Bacillus Calmette-Guerin (BCG)-induced trained immunity in infections caused by different Leishmania spp. Microb Pathog. (2021) 158:105088. doi: 10.1016/j.micpath.2021.105088
45. Namakula R, de Bree LCJ, Netea MG, Cose S, and Hanevik K. Monocytes from neonates and adults have a similar capacity to adapt their cytokine production after previous exposure to BCG and beta-glucan. PloS One. (2020) 15:e0229287. TH AT. doi: 10.1371/journal.pone.0229287
46. Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Jacobs C, Xavier RJ, et al. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin Immunol. (2014) 155:213–9. doi: 10.1016/j.clim.2014.10.005
47. Debisarun PA, Kilic G, de Bree LCJ, Pennings LJ, van Ingen J, Benn CS, et al. The impact of BCG dose and revaccination on trained immunity. Clin Immunol. (2023) 246:109208. doi: 10.1016/j.clim.2022.109208
48. Dang Y, Souchet C, Moresi F, Jeljeli M, Raquillet B, Nicco C, et al. BCG-trained innate immunity leads to fetal growth restriction by altering immune cell profile in the mouse developing placenta. J Leukoc Biol. (2022) 111:1009–20. doi: 10.1002/JLB.4A0720-458RR
49. Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. (2014) 345:1250684. doi: 10.1126/science.1250684
50. Ratzan KR, Musher DM, Keusch GT, and Weinstein L. Correlation of increased metabolic activity, resistance to infection, enhanced phagocytosis, and inhibition of bacterial growth by macrophages from Listeria- and BCG-infected mice. Infect Immun. (1972) 5:499–504. doi: 10.1128/iai.5.4.499-504.1972
51. Ferreira AV, Koeken V, Matzaraki V, Kostidis S, Alarcon-Barrera JC, de Bree LCJ, et al. Glutathione metabolism contributes to the induction of trained immunity. Cells. (2021) 10:971-. doi: 10.3390/cells10050971
52. Zhang BZ, Shuai H, Gong HR, Hu JC, Yan B, Yuen TT, et al. Bacillus Calmette-Guerin-induced trained immunity protects against SARS-CoV-2 challenge in K18-hACE2 mice. JCI Insight. (2022) 7. doi: 10.1172/jci.insight.157393
53. Arts RJW, Carvalho A, La Rocca C, Palma C, Rodrigues F, Silvestre R, et al. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep. (2016) 17:2562–71. doi: 10.1016/j.celrep.2016.11.011
54. Diray-Arce J, Angelidou A, Jensen KJ, Conti MG, Kelly RS, Pettengill MA, et al. Bacille Calmette-Guerin vaccine reprograms human neonatal lipid metabolism in vivo and in vitro. Cell Rep. (2022) 39:110772. doi: 10.1016/j.celrep.2022.110772
55. Prentice S, Jallow MW, Prentice AM, and Group MR-IN. The effect of BCG on iron metabolism in the early neonatal period: A controlled trial in Gambian neonates. Vaccine. (2015) 33:2963–7. doi: 10.1016/j.vaccine.2015.04.087
56. McMurray DN, Carlomagno MA, and Mintzer CL. Effect of diet on non-specific antimicrobial resistance in Mycobacterium bovis BCG-vaccinated Guinea pigs. Nutr Res. (1988) 8:1171–81. doi: 10.1016/S0271-5317(88)80118-0
57. Koeken V, Qi C, Mourits VP, de Bree LCJ, Moorlag S, Sonawane V, et al. Plasma metabolome predicts trained immunity responses after antituberculosis BCG vaccination. PloS Biol. (2022) 20:e3001765. doi: 10.1371/journal.pbio.3001765
58. de Oliveira L, Borges MM, Leal RC, Assreuy J, and Kloetzel JK. Nitric oxide involvement in experimental Trypanosoma cruzi infection in Calomys callosus and Swiss mice. Parasitol Res. (1997) 83:762–70. doi: 10.1007/s004360050336
59. Buhles WC Jr. and Shifrine M. Adjuvant protection against bacterial infection in granulocytopenic mice. J Infect Dis. (1977) 136:90–5. doi: 10.1093/infdis/136.1.90
60. Appelberg R. Mycobacterial infection primes T cells and macrophages for enhanced recruitment of neutrophils. J Leukoc Biol. (1992) 51:472–7. doi: 10.1002/jlb.51.5.472
61. Urbaschek R and Urbaschek B. Ability of Post-endotoxin serum from BCG-infected mice to induce nonspecific resistance and stimulation of granulopoiesis. Infect Immun. (1983) 39:1488–90. doi: 10.1128/iai.39.3.1488-1490.1983
62. Brook B, Harbeson DJ, Shannon CP, Cai B, He D, Ben-Othman R, et al. BCG vaccination-induced emergency granulopoiesis provides rapid protection from neonatal sepsis. Sci Transl Med. (2020) 12. doi: 10.1126/scitranslmed.aax4517
63. Cirovic B, de Bree LCJ, Groh L, Blok BA, Chan J, van der Velden W, et al. BCG vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe. (2020) 28:322–34 e5. doi: 10.1016/j.chom.2020.05.014
64. Moorlag S, Rodriguez-Rosales YA, Gillard J, Fanucchi S, Theunissen K, Novakovic B, et al. BCG vaccination induces long-term functional reprogramming of human neutrophils. Cell Rep. (2020) 33:108387. doi: 10.1016/j.celrep.2020.108387
65. Mitroulis I, Ruppova K, Wang B, Chen LS, Grzybek M, Grinenko T, et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell. (2018) 172:147–61 e12. doi: 10.1016/j.cell.2017.11.034
66. Selin LK, Cornberg M, Brehm MA, Kim SK, Calcagno C, Ghersi D, et al. CD8 memory T cells: cross-reactivity and heterologous immunity. Semin Immunol. (2004) 16:335–47. doi: 10.1016/j.smim.2004.08.014
67. Berg RE, Crossley E, Murray S, and Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med. (2003) 198:1583–93. doi: 10.1084/jem.20031051
68. Orme IM, Ratcliffe MJ, and Collins FM. Acquired immunity to heavy infection with Mycobacterium bovis bacillus Calmette-Guérin and its relationship to the development of nonspecific unresponsiveness in vitro. Cell Immunol. (1984) 88:285–96. doi: 10.1016/0008-8749(84)90162-X
69. North RJ. T cell dependence of macrophage activation and mobilization during infection with Mycobacterium tuberculosis. Infect Immun. (1974) 10:66–71. doi: 10.1128/iai.10.1.66-71.1974
70. Ruitenberg EJ, Van Noorle Jansen LM, Kruizinga W, and Steerenberg PA. Effect of pretreatment with Bacillus Calmette-Guerin on the course of a Listeria monocytogenes infection in normal and congenitally athymic (nude) mice. Br J Exp Pathol. (1976) 57:310–5.
71. Mathurin KS, Martens GW, Kornfeld H, and Welsh RM. CD4 T-cell-mediated heterologous immunity between mycobacteria and poxviruses. J Virol. (2009) 83:3528–39. doi: 10.1128/JVI.02393-08
72. Parra M, Liu X, Derrick SC, Yang A, Tian J, Kolibab K, et al. Molecular analysis of non-specific protection against murine malaria induced by BCG vaccination. PloS One. (2013) 8:e66115. doi: 10.1371/journal.pone.0066115
73. Witschkowski J, Behrends J, Frank R, Eggers L, von Borstel L, Hertz D, et al. BCG provides short-term protection from experimental cerebral malaria in mice. Vaccines (Basel). (2020) 8:745. doi: 10.3390/vaccines8040745
74. Geffard M and Orbach-Arbouys S. Enhancement of T suppressor activity in mice by high doses of BCG. Cancer Immunol Immunotherapy. (1976) 1-1(1-2):1–1. doi: 10.1007/BF00205292
75. Colizzi V, Ferluga J, Garreau F, Malkovsky M, and Asherson GL. Suppressor cells induced by BCG release non-specific factors in vitro which inhibit DNA synthesis and interleukin-2 production. Immunology. (1984) 51:65–71.
76. Blakney AK, Tchakoute CT, Hesseling AC, Kidzeru EB, Jones CE, Passmore JA, et al. Delayed BCG vaccination results in minimal alterations in T cell immunogenicity of acellular pertussis and tetanus immunizations in HIV-exposed infants. Vaccine. (2015) 33:4782–9. doi: 10.1016/j.vaccine.2015.07.096
77. Kiravu A, Osawe S, Happel AU, Nundalall T, Wendoh J, Beer S, et al. Bacille calmette-guerin vaccine strain modulates the ontogeny of both mycobacterial-specific and heterologous T cell immunity to vaccination in infants. Front Immunol. (2019) 10:2307. doi: 10.3389/fimmu.2019.02307
78. Messina NL, Germano S, McElroy R, Rudraraju R, Bonnici R, Pittet LF, et al. Off-target effects of bacillus Calmette-Guerin vaccination on immune responses to SARS-CoV-2: implications for protection against severe COVID-19. Clin Transl Immunol. (2022) 11:e1387. doi: 10.1002/cti2.v11.4
79. Birk NM, Nissen TN, Kjaergaard J, Hartling HJ, Thostesen LM, Kofoed PE, et al. Effects of Bacillus Calmette-Guerin (BCG) vaccination at birth on T and B lymphocyte subsets: Results from a clinical randomized trial. Sci Rep. (2017) 7:12398. doi: 10.1038/s41598-017-11601-6
80. Kleinnijenhuis J, Quintin J, Preijers F, Benn CS, Joosten LA, Jacobs C, et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J Innate Immun. (2014) 6:152–8. doi: 10.1159/000355628
81. Thatcher N and Crowther D. Changes in nonspecific lymphoid (NK, K, T cell) cytotoxicity following BCG immunisation of healthy subjects. Cancer Immunol Immunother. (1978) 5:105–7. doi: 10.1007/BF00199983
82. Bernasconi NL, Traggiai E, and Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. (2002) 298:2199–202. doi: 10.1126/science.1076071
83. Minden P, McClatchy JK, and Farr RS. Shared antigens between heterologous bacterial species. Infect Immun. (1972) 6:574–82. doi: 10.1128/iai.6.4.574-582.1972
84. Kimuda SG, Andia-Biraro I, Sebina I, Egesa M, Nalwoga A, Smith SG, et al. Mycobacterium tuberculosis infection is associated with increased B cell responses to unrelated pathogens. Sci Reports. (2020) 10:14324–. doi: 10.1038/s41598-020-71044-4
85. Nissen TN, Birk NM, Blok BA, Arts RJW, Andersen A, Kjærgaard J, et al. Bacillus Calmette-Guérin vaccination at birth and in vitro cytokine responses to non-specific stimulation. A randomized Clin trial Eur J Clin Microbiol Infect Diseases. (2017) 37:29–41. doi: 10.1007/s10096-017-3097-2
86. Tetteh KKA, Andersen A, Jensen KJ, Erikstrup C, Ravn H, Fisker AB, et al. Both very low- and very high in vitro cytokine responses were associated with infant death in low-birth-weight children from Guinea bissau. PloS One. (2014) 9. doi: 10.1371/journal.pone.0093562
87. Curtis N, Kollmann T, Flanagan KL, Netea MG, Robins-Browne RM, Casalaz D, et al. Neonatal BCG vaccination reduces interferon-γ Responsiveness to heterologous pathogens in infants from a randomized controlled trial. J Infect Diseases. (2020) 221:1999–2009. doi: 10.1093/infdis/jiaa030
88. Freyne B, Donath S, Germano S, Gardiner K, Casalaz D, Robins-Browne RM, et al. Neonatal BCG vaccination influences cytokine responses to toll-like receptor ligands and heterologous antigens. J Infect Diseases. (2018) 217:1798–808. doi: 10.1093/infdis/jiy069
89. Flanagan KL, Klein SL, Skakkebaek NE, Marriott I, Marchant A, Selin L, et al. Sex differences in the vaccine-specific and non-targeted effects of vaccines. Vaccine. (2011) 29:2349–54. doi: 10.1016/j.vaccine.2011.01.071
90. Jensen KJ, Larsen N, Biering-Sørensen S, Andersen A, Eriksen HB, Monteiro I, et al. Heterologous immunological effects of early BCG vaccination in low-birth-weight infants in Guinea-bissau: A randomized-controlled trial. J Infect Diseases. (2015) 211:956–67. doi: 10.1093/infdis/jiu508
91. Pittet LF, Cox L, Freyne B, Germano S, Bonnici R, Gardiner K, et al. Hepatitis B vaccine co-administration influences the heterologous effects of neonatal BCG vaccination in a sex-differential manner. Vaccine. (2022) 40:1334–41. doi: 10.1016/j.vaccine.2022.01.005
92. Koeken VA, de Bree LCJ, Mourits VP, Moorlag SJ, Walk J, Cirovic B, et al. BCG vaccination in humans inhibits systemic inflammation in a sex-dependent manner. J Clin Invest. (2020) 130:5591–602. doi: 10.1172/JCI133935
93. Biering-Sørensen S, Jensen KJ, Aamand SH, Blok B, Andersen A, Monteiro I, et al. Variation of growth in the production of the BCG vaccine and the association with the immune response. An observational study within a randomised trial. Vaccine. (2015) 33:2056–65. doi: 10.1016/j.vaccine.2015.02.056
94. Yang J, Kawamura I, and Mitsuyama M. Involvement of inflammatory cytokines and nitric oxide in the expression of non-specific resistance to in mice induced by viable but not killed BCG. Microbial Pathogenesis. (1997) 22:79–88. doi: 10.1006/mpat.1996.0093
95. Angelidou A, Conti M-G, Diray-Arce J, Benn CS, Shann F, Netea MG, et al. Licensed Bacille Calmette-Guérin (BCG) formulations differ markedly in bacterial viability, RNA content and innate immune activation. Vaccine. (2020) 38:2229–40. doi: 10.1016/j.vaccine.2019.11.060
96. Brandely M, Hurtrel B, and Lagrange PH. Comparison between immunopotency tests and specific active or passive acquired resistance against Mycobacterium tuberculosis in mice induced with three different preparations of BCG pasteur vaccine. Clin Exp Immunol. (1983) 54:143–50.
97. Darboe F, Adetifa JU, Reynolds J, Hossin S, Plebanski M, Netea MG, et al. Minimal sex-differential modulation of reactivity to pathogens and toll-like receptor ligands following infant bacillus calmette–guérin Russia vaccination. Front Immunol. (2017) 8. doi: 10.3389/fimmu.2017.01092
98. Shann F. Editorial commentary: different strains of bacillus calmette–guérin vaccine have very different effects on tuberculosis and on unrelated infections. Clin Infect Diseases. (2015) 61:960–2. doi: 10.1093/cid/civ454
99. Satti I, Marshall JL, Harris SA, Wittenberg R, Tanner R, Lopez Ramon R, et al. Safety of a controlled human infection model of tuberculosis with aerosolised, live-attenuated Mycobacterium bovis BCG versus intradermal BCG in BCG-naive adults in the UK: a dose-escalation, randomised, controlled, phase 1 trial. Lancet Infect Dis. (2024) 24:909–21. doi: 10.1016/S1473-3099(24)00143-9
100. Hamers LA, Kox M, Arts RJ, Blok B, Leentjens J, Netea MG, et al. Gamma-irradiated bacille Calmette-Guerin vaccination does not modulate the innate immune response during experimental human endotoxemia in adult males. J Immunol Res. (2015) 2015:261864. doi: 10.1155/2015/261864
101. Zimmermann P and Curtis N. The influence of BCG on vaccine responses - a systematic review. Expert Rev Vaccines. (2018) 17:547–54. doi: 10.1080/14760584.2018.1483727
102. Ota MOC, Vekemans J, Schlegel-Haueter SE, Fielding K, Sanneh M, Kidd M, et al. Influence ofMycobacterium bovisBacillus calmette-gueírin on antibody and cytokine responses to human neonatal vaccination. J Immunol. (2002) 168:919–25. doi: 10.4049/jimmunol.168.2.919
103. Zimmermann P, Donath S, Perrett KP, Messina NL, Ritz N, Netea MG, et al. The influence of neonatal Bacille Calmette-Guérin (BCG) immunisation on heterologous vaccine responses in infants. Vaccine. (2019) 37:3735–44. doi: 10.1016/j.vaccine.2019.03.016
104. Giamarellos-Bourboulis EJ, Tsilika M, Moorlag S, Antonakos N, Kotsaki A, Dominguez-Andres J, et al. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell. (2020) 183:315–23 e9. doi: 10.1016/j.cell.2020.08.051
105. Moorlag S, Taks E, Ten Doesschate T, van der Vaart TW, Janssen AB, Muller L, et al. Efficacy of BCG vaccination against respiratory tract infections in older adults during the coronavirus disease 2019 pandemic. Clin Infect Dis. (2022) 75:e938–e46. doi: 10.1093/cid/ciac182
106. Sarfas C, White AD, Sibley L, Morrison AL, Gullick J, Lawrence S, et al. Characterization of the infant immune system and the influence and immunogenicity of BCG vaccination in infant and adult rhesus macaques. Front Immunol. (2021) 12. doi: 10.3389/fimmu.2021.754589
107. Smith SG, Kleinnijenhuis J, Netea MG, and Dockrell HM. Whole blood profiling of bacillus calmette–guérin-induced trained innate immunity in infants identifies epidermal growth factor, IL-6, platelet-derived growth factor-AB/BB, and natural killer cell activation. Front Immunol. (2017) 8. doi: 10.3389/fimmu.2017.00644
108. Angelidou A, Diray-Arce J, Conti M-G, Netea MG, Blok BA, Liu M, et al. Human newborn monocytes demonstrate distinct BCG-induced primary and trained innate cytokine production and metabolic activation in vitro. Front Immunol. (2021) 12. doi: 10.3389/fimmu.2021.674334
109. Arts RJW, Blok BA, van Crevel R, Joosten LAB, Aaby P, Benn CS, et al. Vitamin A induces inhibitory histone methylation modifications and down-regulates trained immunity in human monocytes. J Leukocyte Biol. (2015) 98:129–36. doi: 10.1189/jlb.6AB0914-416R
110. Mourits VP, Koeken VACM, de Bree LCJ, Moorlag SJCFM, Chu WC, Xu X, et al. BCG-induced trained immunity in healthy individuals: the effect of plasma muramyl dipeptide concentrations. J Immunol Res. (2020) 2020:1–8. doi: 10.1155/2020/5812743
111. Otasowie CO, Tanner R, Ray DW, Austyn JM, and Coventry BJ. Chronovaccination: Harnessing circadian rhythms to optimize immunisation strategies. Front Immunol. (2022) 13. doi: 10.3389/fimmu.2022.977525
112. Ciabattini A, Olivieri R, Lazzeri E, and Medaglini D. Role of the microbiota in the modulation of vaccine immune responses. Front Microbiol. (2019) 10. doi: 10.3389/fmicb.2019.01305
113. Stražar M, Mourits VP, Koeken VACM, de Bree LCJ, Moorlag SJCFM, Joosten LAB, et al. The influence of the gut microbiome on BCG-induced trained immunity. Genome Biol. (2021) 22:275. doi: 10.1186/s13059-021-02482-0
114. de Bree LCJ, Mourits VP, Koeken VACM, Moorlag SJCFM, Janssen R, Folkman L, et al. Circadian rhythm influences induction of trained immunity by BCG vaccination. J Clin Investigation. (2020) 130:5603–17. doi: 10.1172/JCI133934
115. Sun SJ, Aguirre-Gamboa R, de Bree LCJ, Sanz J, Dumaine A, van der Velden W, et al. BCG vaccination alters the epigenetic landscape of progenitor cells in human bone marrow to influence innate immune responses. Immunity. (2024) 57:2095–107 e8. doi: 10.1016/j.immuni.2024.07.021
116. Peralta Alvarez MP, Jones H, Redondo Azema H, Davis C, White AD, Sarfas C, et al. Low-dose M. tb infection but not BCG or MTBVAC vaccination enhances heterologous antibody titres in non-human primates. Front Immunol. (2024) 15:1387454. doi: 10.3389/fimmu.2024.1387454
117. Lewis KN, Liao R, Guinn KM, Hickey MJ, Smith S, Behr MA, et al. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guérin attenuation. J Infect Dis. (2003) 187:117–23. doi: 10.1086/jid.2003.187.issue-1
118. Uthayakumar D, Paris S, Chapat L, Freyburger L, Poulet H, and De Luca K. Non-specific effects of vaccines illustrated through the BCG example: from observations to demonstrations. Front Immunol. (2018) 9:2869. doi: 10.3389/fimmu.2018.02869
119. Wagar LE, Salahudeen A, Constantz CM, Wendel BS, Lyons MM, Mallajosyula V, et al. Modeling human adaptive immune responses with tonsil organoids. Nat medicine. (2021) 27:125–35. doi: 10.1038/s41591-020-01145-0
120. Lykov AP, Belogorodtsev SN, Nemkova EK, Vetlugina A, Terekhova TM, and Schwartz YS. The formation of non-specific immunological memory phenotype in human monocyte-like THP-1 and U-937 cell lines. Bull Exp Biol Med. (2023) 175:477–80. doi: 10.1007/s10517-023-05890-3
121. Jensen KJ, Hansen MS, Skovgaard K, Svensson E, Larsen LE, Heegaard PMH, et al. Immunogenicity of Bacillus Calmette-Guerin in pigs: potential as a translational model of non-specific effects of BCG. Front Immunol. (2023) 14:1219006. doi: 10.3389/fimmu.2023.1219006
122. Kang A, Ye G, Singh R, Afkhami S, Bavananthasivam J, Luo X, et al. Subcutaneous BCG vaccination protects against streptococcal pneumonia via regulating innate immune responses in the lung. EMBO Mol Med. (2023) 15:e17084. doi: 10.15252/emmm.202217084
123. Singh AK, Wang R, Lombardo KA, Praharaj M, Bullen CK, Um P, et al. Intravenous BCG vaccination reduces SARS-CoV-2 severity and promotes extensive reprogramming of lung immune cells. iScience. (2023) 26:107733. doi: 10.1016/j.isci.2023.107733
124. Lee A, Floyd K, Wu S, Fang Z, Tan TK, Froggatt HM, et al. BCG vaccination stimulates integrated organ immunity by feedback of the adaptive immune response to imprint prolonged innate antiviral resistance. Nat Immunol. (2024) 25:41–53. doi: 10.1038/s41590-023-01700-0
125. Kilic G, Debisarun PA, Alaswad A, Baltissen MP, Lamers LA, de Bree LCJ, et al. Seasonal variation in BCG-induced trained immunity. Vaccine. (2024) 42:126109. doi: 10.1016/j.vaccine.2024.07.010
126. Suen TK, Moorlag S, Li W, de Bree LCJ, Koeken V, Mourits VP, et al. BCG vaccination induces innate immune memory in gammadelta T cells in humans. J Leukoc Biol. (2024) 115:149–63. doi: 10.1093/jleuko/qiad103
127. Samuel BER, Diaz FE, Maina TW, Corbett RJ, Tuggle CK, and McGill JL. Evidence of innate training in bovine gammadelta T cells following subcutaneous BCG administration. Front Immunol. (2024) 15:1423843. doi: 10.3389/fimmu.2024.1423843
128. Specht AG, Ginese M, Kurtz SL, Elkins KL, Specht H, and Beamer G. Host genetic background influences BCG-induced antibodies cross-reactive to SARS-coV-2 spike protein. Vaccines (Basel). (2024) 12:242-. doi: 10.3390/vaccines12030242
129. Moorlag S, Folkman L, Ter Horst R, Krausgruber T, Barreca D, Schuster LC, et al. Multi-omics analysis of innate and adaptive responses to BCG vaccination reveals epigenetic cell states that predict trained immunity. Immunity. (2024) 57:171–87 e14. doi: 10.1016/j.immuni.2023.12.005
130. Xu JC, Chen ZY, Huang XJ, Wu J, Huang H, Niu LF, et al. Multi-omics analysis reveals that linoleic acid metabolism is associated with variations of trained immunity induced by distinct BCG strains. Sci Adv. (2024) 10:eadk8093. doi: 10.1126/sciadv.adk8093
131. Bulut O, Kilic G, Debisarun PA, Roring RJ, Sun S, Kolkman M, et al. Alendronate modulates cytokine responses in healthy young individuals after BCG vaccination. Immunol Lett. (2024) 267:106851. doi: 10.1016/j.imlet.2024.106851
132. Li W, Moorlag S, Koeken V, Roring RJ, de Bree LCJ, Mourits VP, et al. A single-cell view on host immune transcriptional response to in vivo BCG-induced trained immunity. Cell Rep. (2023) 42:112487. doi: 10.1016/j.celrep.2023.112487
133. Ahmed A, Rakshit S, Adiga V, Dias M, Dwarkanath P, D’Souza G, et al. A century of BCG: Impact on tuberculosis control and beyond. Immunological Rev. (2021) 301:98–121. doi: 10.1111/imr.v301.1
134. White AD, Sibley L, Sarfas C, Morrison AL, Bewley K, Churchward C, et al. Influence of aerosol delivered BCG vaccination on immunological and disease parameters following SARS-coV-2 challenge in rhesus macaques. Front Immunol. (2022) 12. doi: 10.3389/fimmu.2021.801799
135. Hawgood BJ. Albert Calmette (1863-1933) and Camille Guerin (1872-1961): the C and G of BCG vaccine. J Med Biogr. (2007) 15:139–46. doi: 10.1258/j.jmb.2007.06-15
Keywords: BCG, heterologous effects of vaccination, trained immunity, T cells, humoral immunity, tuberculosis
Citation: Torracinta L, Gogichadze N and Tanner R (2025) Immune mechanisms mediating the heterologous effects of BCG vaccination: a systematic review. Front. Immunol. 16:1567111. doi: 10.3389/fimmu.2025.1567111
Received: 26 January 2025; Accepted: 28 April 2025;
Published: 19 May 2025.
Edited by:
Andreas Kupz, James Cook University, AustraliaReviewed by:
Roopali Rajput, University of Delhi, IndiaAlissa Rothchild, University of Massachusetts Amherst, United States
Cristian Segura-Cerda, Secretaría de Ciencia Tecnología e Innovación, Mexico
Copyright © 2025 Torracinta, Gogichadze and Tanner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachel Tanner, cmFjaGVsLnRhbm5lckBiaW9sb2d5Lm94LmFjLnVr
 Louis Torracinta
Louis Torracinta Nino Gogichadze
Nino Gogichadze Rachel Tanner
Rachel Tanner