- 1Department of Hematology, Affiliated Hospital of Nantong University, Nantong, China
- 2TriArm Therapeutics (Shanghai), Shanghai, China
- 3Shanghai First Song Therapeutics, Shanghai, China
- 4TriArm Inc., San Mateo, CA, United States
- 5Department of General Surgery, Affiliated Hospital of Nantong University, Nantong, China
CD19-directed CAR T-cell therapy is a breakthrough immunotherapy for B-cell malignancies. However, CD19 loss-mediated relapsed/refractory disease continues to pose a significant challenge, highlighting the urgent need for CAR T cells targeting alternative antigens. To address this issue, we developed a CD20-directed CAR T incorporated with an additional CD30-directed binder to enhance cytotoxicity toward cancer cells. Here, we report that a patient with bulky transformed follicular lymphoma was successfully treated with CD20/CD30-directed CAR-T cells. The patient received two doses of anti-CD20/CD30-CAR-T therapy administered one month apart. Complete metabolic remission was achieved 1 month after the first infusion without evidence of cytokine release syndrome (CRS) or immune effector cell-associated neurotoxicity syndrome (ICANS). The second dose was given as a consolidation therapy with sustained disease-free survival exceeding 12 months to date. The report underscores the promising therapeutic potential and safety profile of CD20/CD30-directed CAR T-cell therapy.
Clinical Trial Registration: https://www.clinicaltrials.gov, identifier NCT06756321.
Introduction
Chimeric antigen receptor (CAR) T-cell therapy has transformed the clinical landscape of hematologic malignancies. Currently, FDA has approved 5 CAR T-cell products for the management of B-cell malignancies, demonstrating remarkable efficacy with an overall remission of 71-81% in B-cell acute lymphoblastic leukemia (1–3), and 52-97% in B-cell non-Hodgkin lymphoma (B-NHL) (4–10). Despite the high initial response rate, relapsed or refractory disease following CAR T still represents a challenge, with subsequent salvage treatments providing less than 3 months of progression-free survival benefit (11). The loss of CD19 antigen, which is the target of all 5 commercial products, was reported as one of the major contributors to disease relapse (12–16), emphasizing the need for alternative targets to be explored.
CD20 is a B cell marker universally expressed on the surface of normal B cells and most mature B-cell malignancies. For several years, CD20-targeted therapy involving monoclonal antibodies and T cell engagers have served as the cornerstone in the management of B-NHL. In clinical trials, CD20-targeted CAR T-cell therapies have exhibited potent anti-lymphoid effect in various B-NHL cancers (17–22), particularly in cases of relapsed or refractory disease following CD19-directed CAR T treatment (17, 22). In one study, anti-CD20 CAR T-cell therapy generated a complete remission rate of 57.1% even after failure of anti-CD19 CAR-T treatment (17), which is comparable to the efficacy in CAR T-naïve NHLs (4, 7, 9). The above studies suggested that CD20 could be a promising target for CAR T development.
CD30 is mostly found to express in a subset of activated lymphocytes and lymphomas, including classical Hodgkin lymphoma (cHL), anaplastic large-cell lymphoma (ALCL) (23, 24). Brentuximab Vedotin (BV), a CD30-targeted antibody drug conjugate, has been approved for the treatment and maintenance of cHL, and for combo therapy with lenalidomide and rituximab against large B-cell lymphoma. Given its success in various lymphoid malignancies, CD30 has emerged as a promising target for CAR T-cell therapy. Most anti-CD30-CAR T-cell studies have demonstrated remarkable efficacy against CD30+ lymphomas, with objective response rates ranging from 37.5% to 91.7% (25–29). Notably, Kochenderfer et al. reported a 43% transient anti-lymphoid response in CD30+ lymphomas, with extensive rash and prolonged hematologic toxicities (30). Despite these challenges, anti-CD30-CAR-T have shown clinical responses in some CD30+ lymphomas, solidifying CD30 as a compelling target for further CAR T-cell development.
Multi-specific CAR T is a potential strategy to enhance the potency of CAR T-cell therapies. By targeting 2 or more antigens on tumor cells, tumor microenvironment (TME), or immune cells, multi-specific CAR design can potentially prevent antigen escape-mediated relapse (12, 31–36), remodel the tumor microenvironment (37–39), boost CAR T-cell expansion (40), and enhance CAR T-cell function (41). In 2019, Abken et al. reported that co-targeting of CD30 in CEA- and TAG72-targeted CAR T cells enhanced T cell activity against CD30-negative tumor cells via elimination of CD30+ T cells, which suppress the cytotoxic T cell response. Moreover, CD30 is expressed in 10-30% of NHL cancers (42–44). Beyond the direct activity against CD30+ NHL, BV combined therapy with rituximab, a CD20-targeted agents, produce robust clinical response in large B-cell lymphoma regardless of CD30 expression (45, 46), suggesting a potential synergistic antitumor effects with CD20/CD30 dual targeting therapies. The dual-targeting strategy may improve therapeutic outcomes and reduce the risk of relapse due to antigen escape.
Therefore, we developed a CAR T-cell product targeting both CD20 and CD30, for patients with relapsed/refractory B-cell non-Hodgkin lymphoma, including patients who progressed following conventional CD19-targeted CAR T cells. Here we report a case using the CD20/CD30 bispecific CAR T to treat a bulky transformed follicular lymphoma.
Case description
A 60-year-old female patient with extensive lymphadenopathy (cervical, supraclavicular and abdominal nodes) was diagnosed with grade 1 follicular lymphoma (stage III) in January 2019 (Figure 1A). She was first treated with 6 cycles rituximab combined with cyclophosphamide, pirarubicin, vincristine, and prednisone (R-CHOP) and then obtained a partial response by CT-scan. As the patient had no symptoms at the time, she refused further treatment.
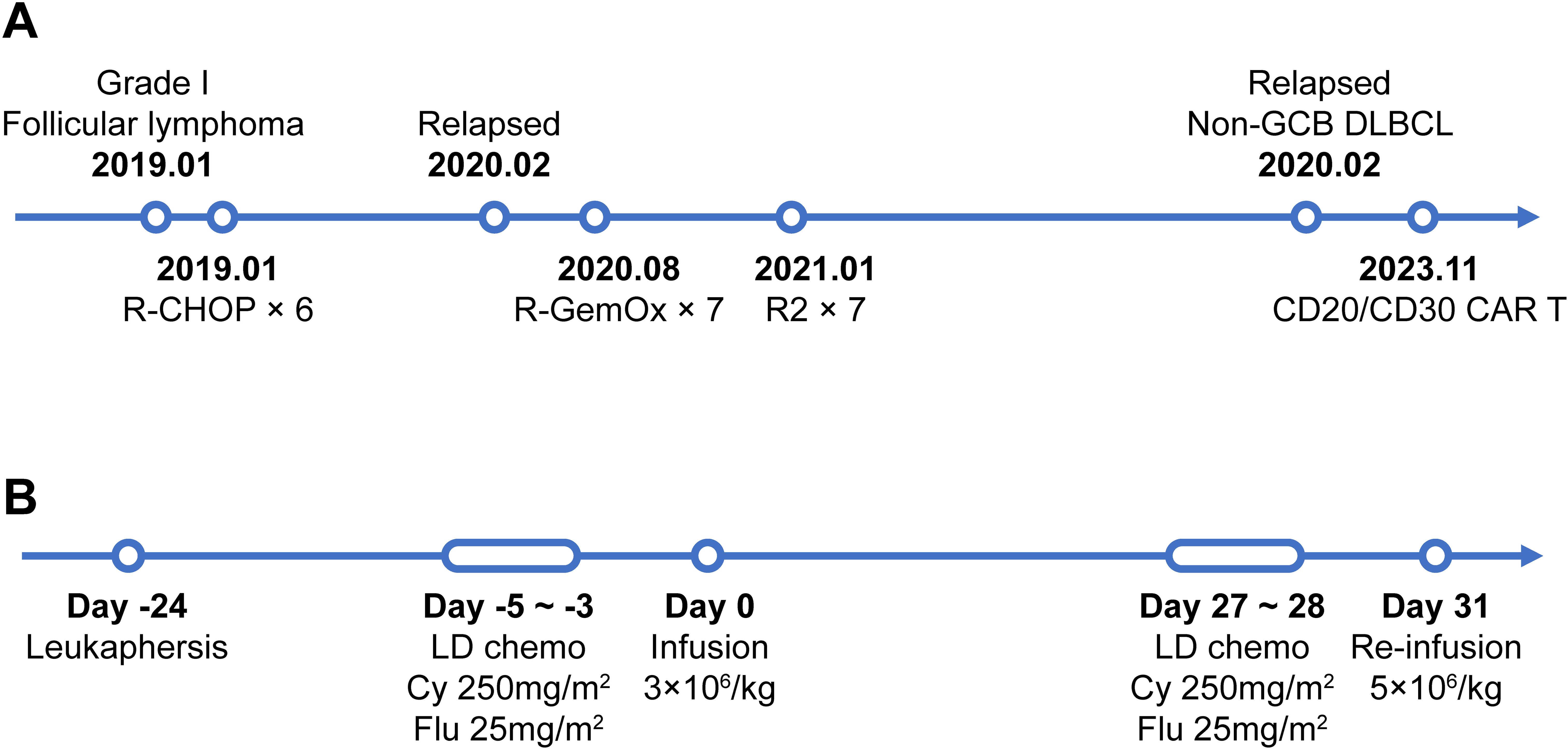
Figure 1. The timeline of patients’ treatment and trial intervention. (A) Flow chart of the disease process and therapeutic modalities before CAR T treatment. (B) The timeline of CAR T preparation and administration from leukapheresis to the 2nd dose of CAR T infusion. Abbreviations: Non-GCB DLBCL, Non-germinal center diffuse large B cell lymphoma; LD chemo, lymphodepleting chemotherapy; Cy, cyclophasphomide; Flu, fludarabine.
The first relapse occurred approximately 3 months after the last chemotherapy. She developed right inguinal lymphadenopathy, and further core needle aspiration revealed a B cell lymphoma with the following immunohistochemistry (IHC) results: CD20+ (3+), CD30-, Bcl-2+ (3+), Bcl-6+ (3+), Ki-67+ (40%+), and MUM1-. She was subsequently treated with R-GemOx (consisting of rituximab, gemcitabine and oxaliplatin). Two months into her treatment, her abdominal lymph nodes have significantly reduced in size, but there has been little change in the lesions in other areas. Unfortunately, PET-CT scans after 7 cycles R-GemOx demonstrated progressive disease with enlarged lymph nodes and increased FDG uptake (Deauville 5) in left cervical, left supraclavicular, left axilla, para-aortic, presacral, left obturator, right inguinal, and left gluteal intermuscular area. The treatment was then switched to R-DHAP (Rituximab, Dexamethasone, High-dose Cytarabine, Cisplatin) but was discontinued due to intolerance. Rituximab combined with lenalidomide (R2 regimen) was then administered for 5 cycles. No further treatment or evaluation was performed.
Approximately 1.5 years later, the patient was admitted with left gluteal pain and limited physical activity for 7 months. Her MRI scan showed an occupying lesion (73×66 mm) in the left gluteus maximus and partial mid-arm muscle area. The third pathological investigation revealed non-germinal center B cell like (GCB) diffuse large B cell lymphoma (DLBCL) with invasion of striated muscle tissue, positive for Bcl-2 (90%+), Bcl-6 (60%+), CD20 (3+), Ki67 (80%+), MUM1 (80%+) and c-myc (50%+). PET-CT showed a bulky mass (68×64×101mm) in the left buttocks with increased SUV uptake (SUVmax=30) and an enlarged left inguinal lymph node with slightly increased SUV uptake (SUVmax=5.6). Similarly, the extremely high LDH concentration (>3 × upper limit of normal) also reflected an extensive tumor burden. In light of the patient’s history of FL, this DLBCL was diagnosed as a transformed lymphoma.
Due to ineffective treatment approaches, the patient visited the affiliated hospital of Nantong University in search of a CAR T-cell therapy. Immunohistochemistry (IHC) analysis revealed diffuse positivity for CD20, partial positivity (80%) for CD19, and scattered/weak positivity for CD30 in the tumor cells (Supplementary Figure S1). Following a comprehensive discussion of available therapies, the patient opted to participate in an investigator-initiated clinical trial of tandem anti-CD20/CD30 CAR T-cell therapy (NCT No.: NCT06756321) instead of pursuing CD19-directed CAR T-cell therapy. Leukapheresis was successfully performed and peripheral blood mononuclear cells (PBMCs) were collected for manufacturing the 3rd generation tandem anti-CD20/CD30 CAR T-cells (The CAR construct and characterization is given in Supplementary Figure S2). After a mild lymphodepleting chemotherapy (cyclophosphamide 250mg/m2 d1-d3, fludarabine 25mg/m2 d1-d3), a total dose of 1.7×108 CAR T-cells (3×106/kg) were then infused (Figure 1B).
Following the infusion, her left gluteal pain was soon relieved and no longer affected her daily life. In parallel, the serum LDH remarkably decreased and returned to normal range within 2 weeks (Figure 2A). At month 1 after infusion, PET-CT showed a clear regression of FDG uptake in the left buttocks and inguinal lymph node (Figure 3). Notably, the patient did not experience cytokine release syndrome (CRS) or neurotoxicity or notable changes in inflammatory markers or cytokines (Figures 2B–D) despite a rapid therapeutic response. Except hematologic toxicity (Grade 4 lymphocytopenia and grade 3 neutropenia), no other adverse events were observed.
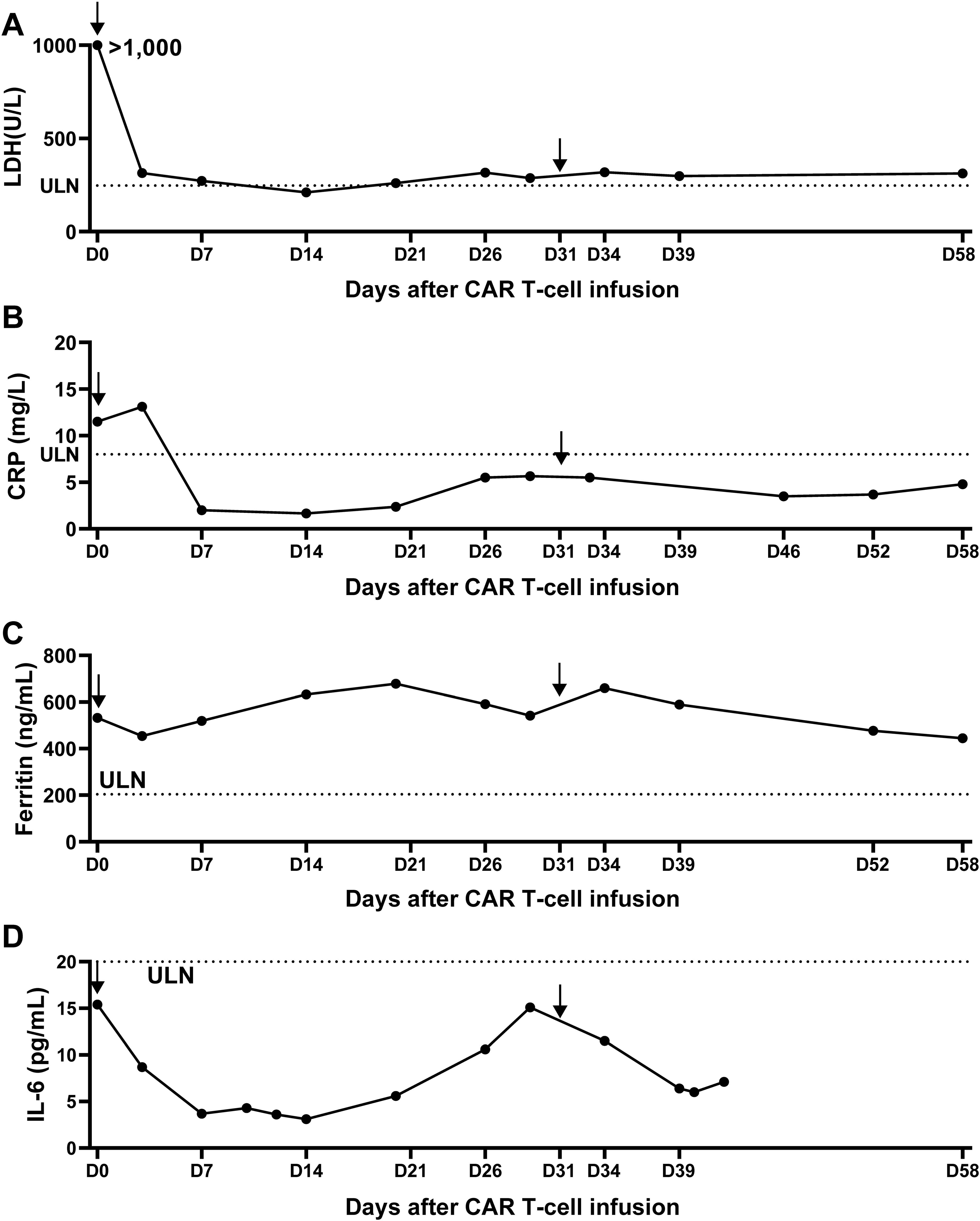
Figure 2. Measurement of lactate dehydrogenase and CRS-related indicators over 2 months after anti-CD20/CD30-CAR-T infusion. (A) The serum lactate dehydrogenase (LDH) was remarkably decreased after CAR T infusion. (B-D) Serum C-reactive protein (B), serum ferritin (C) and IL-6 (D) showed no significant changes over the first 2 months after CAR T infusion, consistent with the absence of CRS. Arrow indicates the timepoint of CAR T infusion and the doted lines indicate the lower limits of normal ranges.
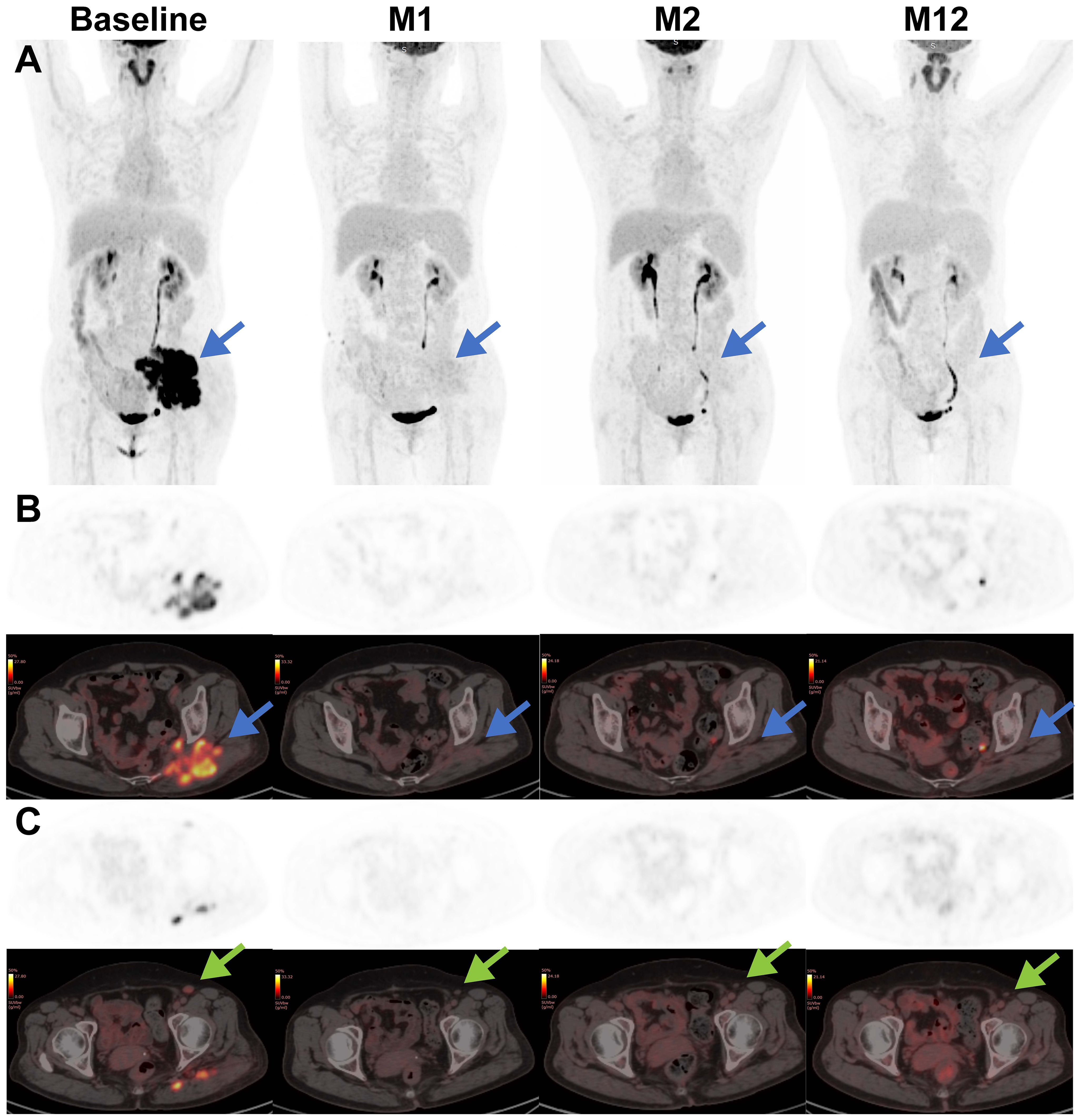
Figure 3. Representative coronal-view and axial-view images of serial 18F-FDG PET/CT scans before and after CAR T treatment. The baseline PET-CT shows a bulky mass (blue arrow) in the left buttocks with SUVmax of 30 and an enlarged left inguinal lymph node (green arrow) with SUVmax of 5.6. After CAR T infusion, the lymphoma lesions showed a rapid decrease in metabolic activity and a slow reduction in size, supporting the conclusion of complete remission. (A) Maximum intensity projection PET images; (B) axial images of lymphoma lesion in the left buttocks; (C) axial images of enlarged left inguinal lymph node.
After T cell infusion, CAR T expansion was analyzed at the indicated time points via quantitative polymerase chain reaction (qPCR). As demonstrated in Figure 4A, CAR T-cells began to expand in vivo after 3 days, peaked on day 7 and then gradually decreased, with a Cmax of 11,228.9 CAR copies/μg DNA. Meanwhile, circulating B cells remained at extremely low levels after infusion.
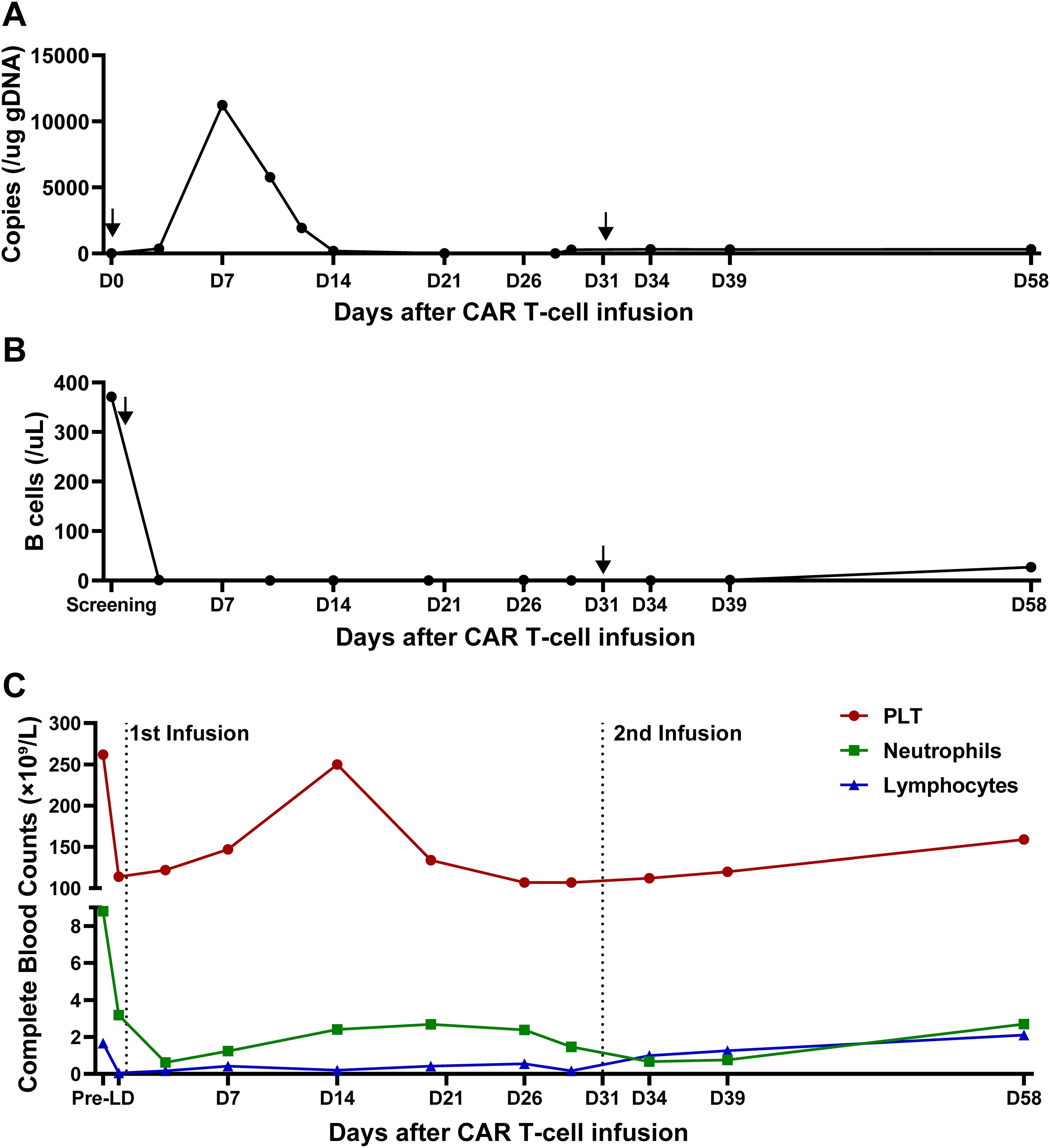
Figure 4. CAR T cells and other blood cells dynamics after infusion. (A) Quantification of anti-CD20/CD30 CAR copies detected by qPCR after infusion. Arrows indicate the timepoint of CAR T infusion. (B) B cell reconstitution after CAR T infusion. Data is shown as B cell counts detected by flow cytometry in peripheral blood. Arrows indicate the timepoint of CAR T infusion. (C) The recovery of platelet counts, absolute neutrophil counts and absolute lymphocyte counts during CAR T treatment. Dot lines indicate the timepoint of CAR T infusion.
At month 1, the patient experienced complete metabolic response and had hypometabolic lesions (Deaville score 3). Considering that circulating CAR T cells were no longer detectable at month 1 and the patient still experienced mild edema in the left gluteus maximus (with the possibility of residual tumor), an additional 2.8×108 CAR T-cells (5×106/kg) were given as a consolidative treatment following a reduced-dose LD regimen (cyclophosphamide 250mg/m2 d1-d2, fludarabine 25mg/m2 d1-d2) (Figure 1B). Although the patient developed mild fever and fatigue after reinfusion, the 2nd CAR T failed to engraft with minimal levels of CAR copies detected in the peripheral blood (Figure 4A). B cells, neutrophils, and lymphocytes began to rebound 1 month after the 2nd CAR T re-infusion (Figures 4B, C). At month 2, the patient continued to have complete metabolic response which was maintained for approximately 12 months following initial CAR T infusion (Figure 3).
Discussion
Here we report the treatment of a relapsed/refractory transformed follicular lymphoma patient with anti-CD20/CD30 bispecific CAR T-cells. The treatment was well tolerated with no CRS or ICANS and expected hematologic toxicities. Peak levels of CAR-T cells occurred on day 7 following CAR T-cell infusion and the patient reached CMR at 1 month, which was maintained for over 12 months following infusion.
In this case, while the 1st CAR T infusion expanded significantly in vivo, the 2nd consolidative CAR T cells appeared to fail to engraft. While re-infusion often exhibiting poor expansion and suboptimal efficacy (47, 48), CAR T-cell expansion is in general related with various factors, including target antigen burden and CAR design. Following the 1st CAR T dosed, the patient experienced rapid resolution of symptoms, accompanied by normalization of LDH levels within 2 weeks. The PET-CT scan confirmed a complete metabolic response at Month 1. The absence of target antigen may explain both the failed expansion of the 2nd CAR T dose and the rapid disappearance of the 1st CAR T cells from Day 14 to Month 1. Furthermore, CD30 has been reported to be expressed on activated T cells (24). The introduction of an additional anti-CD30 CAR might have induced fratricide during late-phase of CAR T expansion, contributing to their poor persistence. Notably, the suboptimal CAR T persistence did not compromise tumor control in this case - a finding seemingly contradictory to the conventional paradigm that durable tumor control requires long-term CAR T persistence. Further studies may provide more insights on expansion and persistence of anti-CD20-CD30 CAR T cells vs clinical responses.
It has been well-established that CD30 is a biomarker of classic Hodgkin lymphoma (cHL) (49). In contrast, CD30 expression can be detected at various frequencies in NHL, including primary mediastinal B-cell lymphoma (PMBCL) (50) and mediastinal gray-zone lymphoma (MGZL) (51). BV monotherapy in NHL has been disappointing. However, the combination of BV and rituximab plus lenalidomide had a significant improvement in overall survival compared with rituximab and lenalidomide plus placebo in patients with relapsed and refractory DLBCL (45, 46). The overall survival benefit suggests potential synergy of CD20- and CD30-targeted treatment in NHL. The quick metabolic response in our case after bispecific anti-CD20-CD30 CAR T cell infusion seems consistent with this hypothesis.
Bispecific CAR T has several advantages over monospecific CAR-T. First, bispecific anti-CD20 and -CD30 CAR-T can be administered to patients with NHL and HL because expression of CD20 and CD30 can be detected in both diseases. Second, multi-specific CAR-T may potentially mitigate antigen escape mediated tumor relapse (52, 53), which is frequently observed after monospecific CAR T treatment in clinical trials. However, multi-targeting CAR-T may also have its drawbacks. The clinical efficacy of dual-targeting CAR T-cell therapy has been inconsistent. Several studies have reported that CD19/CD22 dual-targeting CAR T cells exhibit non-superior objective response rates and shorter response durations in ALL or LBCL, potentially due to inadequate CAR T-cell persistence or pharmacokinetics (12, 54, 55). Furthermore, multi-targeting CAR T-cell may potentially result in the loss of multiple targetable antigens (12, 56, 57), thereby restricting further therapeutic options. Another concern is that conventional dual-targeting CAR T strategies often rely on co-transduction with separate viral vectors, which may lead to large burden of vector integration and increase genotoxicity concerns. To mitigate these risks, our study utilizes a tandem CAR design delivered via a single vector, substantially minimizing potential safety issues.
A caveat of the study is that the patient did not receive a CD19-targeted therapy before CAR T-cell infusion. Although CD19 CAR-T has been approved for the treatment of NHL, up to 30% patients relapse due to antigen loss, mutation, or other reasons. We aim to investigate the efficacy of anti-CD20/CD30 bispecific CAR T-cells in patients who relapse after CD19 CAR T therapy. We also plan to evaluate escalating doses of bispecific CD20/CD30 CAR T-cells in future.
Conclusion remarks
This case report demonstrates the safety and efficacy of CD20/CD30 CAR-T cell therapy in the treatment of transformed follicular lymphoma. Our results provide evidence that bispecific CAR T-cell therapy may be a promising strategy for relapsed and refractory B-cell malignancy. Further research efforts will focus on potential efficacy in patients with heterogenous expression of CD20 and CD30 and with prior CD19-directed CAR T-cell treatment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Institutional Ethics Committee of the Affiliated Hospital of Nantong University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YH: Writing – review & editing. YG: Writing – review & editing. XL: Writing – review & editing. HR: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft. JL: Writing – review & editing. HK: Writing – review & editing. HL: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. HW: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Shanghai First Song Therapeutics and grants from the Nantong Health Commission Project (QN2023007).
Acknowledgments
We thank the patient and her family along with the staff at Affiliated Hospital of Nantong University for the participation in this study.
Conflict of interest
The authors disclose the following: HR: Employee of Shanghai First Song Therapeutics; HK-M: Employee of TriArm Inc.; YG, XL, and JL: Employee of TriArm Therapeutics Shanghai.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this work received funding from Shanghai First Song Therapeutics. The funder was involved in the preparation of the manuscript.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1567149/full#supplementary-material
References
1. Shah BD, Ghobadi A, Oluwole OO, Logan AC, Boissel N, Cassaday RD, et al. Kte-X19 for relapsed or refractory adult B-cell acute lymphoblastic le ukaemia: phase 2 results of the single-arm, open-label, multicentre zu. Lancet (London England). (2021) 398(10299):491–502. doi: 10.1016/S0140-6736(21)01222-8
2. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. (2018) 378:439–48. doi: 10.1056/NEJMoa1709866
3. Roddie C, Sandhu KS, Tholouli E, Logan AC, Shaughnessy P, Barba P, et al. Obecabtagene autoleucel in adults with B-cell acute lymphoblastic leukemia. N Engl J Med. (2024) 391:2219–30. doi: 10.1056/NEJMoa2406526
4. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel car T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. (2017) 377:2531–44. doi: 10.1056/NEJMoa1707447
5. Jacobson CA, Chavez JC, Sehgal AR, William BM, Munoz J, Salles G, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-hodgkin lymphoma (Zuma-5): A single-arm, multicentre, phase 2 trial. Lancet Oncol. (2022) 23:91–103. doi: 10.1016/S1470-2045(21)00591-X
6. Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. Kte-X19 car T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. (2020) 382:1331–42. doi: 10.1056/NEJMoa1914347
7. Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (Transcend nhl 001): A multicentre seamless design study. Lancet. (2020) 396:839–52. doi: 10.1016/s0140-6736(20)31366-0
8. Morschhauser F, Dahiya S, Palomba ML, Martin Garcia-Sancho A, Reguera Ortega JL, Kuruvilla J, et al. Lisocabtagene maraleucel in follicular lymphoma: the phase 2 transcend fl study. Nat Med. (2024) 30:2199–207. doi: 10.1038/s41591-024-02986-9
9. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. (2019) 380:45–56. doi: 10.1056/NEJMoa1804980
10. Fowler NH, Dickinson M, Dreyling M, Martinez-Lopez J, Kolstad A, Butler J, et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 elara trial. Nat Med. (2022) 28:325–32. doi: 10.1038/s41591-021-01622-0
11. Di Blasi R, Le Gouill S, Bachy E, Cartron G, Beauvais D, Le Bras F, et al. Outcomes of patients with aggressive B-cell lymphoma after failure of anti-cd19 car T-cell therapy: A descar-T analysis. Blood. (2022) 140:2584–93. doi: 10.1182/blood.2022016945
12. Spiegel JY, Patel S, Muffly L, Hossain NM, Oak J, Baird JH, et al. Car T cells with dual targeting of cd19 and cd22 in adult patients with recurrent or refractory B cell Malignancies: A phase 1 trial. Nat Med. (2021) 27:1419–31. doi: 10.1038/s41591-021-01436-0
13. Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, et al. Convergence of acquired mutations and alternative splicing of cd19 enables resistance to cart-19 immunotherapy. Cancer Discov. (2015) 5:1282–95. doi: 10.1158/2159-8290.Cd-15-1020
14. Pan J, Tan Y, Deng B, Tong C, Hua L, Ling Z, et al. Frequent occurrence of cd19-negative relapse after cd19 car T and consolidation therapy in 14 tp53-mutated R/R B-all children. Leukemia. (2020) 34:3382–7. doi: 10.1038/s41375-020-0831-z
15. Schneider D, Xiong Y, Wu D, Nölle V, Schmitz S, Haso W, et al. A tandem cd19/cd20 car lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines. J Immunother Cancer. (2017) 5:42. doi: 10.1186/s40425-017-0246-1
16. Plaks V, Rossi JM, Chou J, Wang L, Poddar S, Han G, et al. Cd19 target evasion as a mechanism of relapse in large B-cell lymphoma treated with axicabtagene ciloleucel. Blood. (2021) 138:1081–5. doi: 10.1182/blood.2021010930
17. Li P, Liu W, Zhou L, Ye S, Zhu D, Huang J, et al. C-car066, a novel fully human anti-cd20 car-T therapy for relapsed or refractory large B-cell lymphoma after failure of anti-cd19 car-T therapy: A phase I clinical study. Am J Hematol. (2024) 99:2306–12. doi: 10.1002/ajh.27488
18. Cheng Q, Tan J, Liu R, Kang L, Zhang Y, Wang E, et al. Cd20-specific chimeric antigen receptor-expressing T cells as salvage therapy in rituximab-refractory/relapsed B-cell non-hodgkin lymphoma. Cytotherapy. (2022) 24:1026–34. doi: 10.1016/j.jcyt.2022.05.001
19. Shadman M, Caimi PF, O’Brien SM, Reagan PM, Dezube B, Navaratnarajah P, et al. Efficacy and safety of a third generation cd20 car-T (Mb-106) for treatment of relapsed/refractory indolent B-cell non-hodgkin lymphoma: phase-1 results from a multicenter trial. Blood. (2023) 142:2102. doi: 10.1182/blood-2023-175007
20. Shadman M, Yeung C, Redman MW, Yun Lee S, Hoon Lee D, Ra S, et al. P1097: cd20 car-T therapy with mb-106 for btk inhibitor-refractory waldenstrÖm macroglobulinemia (Wm)/lymphoplasmacytic lymphoma (Lpl) – single institution study. HemaSphere. (2023) 7:e68877ca. doi: 10.1097/01.HS9.0000971284.68877.ca
21. Shadman M, Yeung C, Redman M, Lee SY, Lee DH, Ra S, et al. S207: efficacy and safety of a third generation cd20 cart (Mb-106) for treatment of relapsed/refractory follicular lymphoma (Fl). HemaSphere. (2022) 6:108–9. doi: 10.1097/01.HS9.0000843720.23634.a9
22. Xue F, Zheng P, Yang F, Liu R, Feng S, Guo Y, et al. Salvage cd20-sd-cart therapy in aggressive B-cell lymphoma after cd19 cart treatment failure. Front Oncol. (2024) 14:1376490. doi: 10.3389/fonc.2024.1376490
23. Schwab U, Stein H, Gerdes J, Lemke H, Kirchner H, Schaadt M, et al. Production of a monoclonal antibody specific for hodgkin and sternberg-reed cells of hodgkin’s disease and a subset of normal lymphoid cells. Nature. (1982) 299:65–7. doi: 10.1038/299065a0
24. Horie R, Watanabe T. Cd30: expression and function in health and disease. Semin Immunol. (1998) 10:457–70. doi: 10.1006/smim.1998.0156
25. Ramos CA, Ballard B, Zhang H, Dakhova O, Gee AP, Mei Z, et al. Clinical and immunological responses after cd30-specific chimeric antigen receptor-redirected lymphocytes. J Clin Invest. (2017) 127:3462–71. doi: 10.1172/jci94306
26. Ramos CA, Grover NS, Beaven AW, Lulla PD, Wu MF, Ivanova A, et al. Anti-cd30 car-T cell therapy in relapsed and refractory hodgkin lymphoma. J Clin Oncol. (2020) 38:3794–804. doi: 10.1200/jco.20.01342
27. Sang W, Wang X, Geng H, Li T, Li D, Zhang B, et al. Anti-pd-1 therapy enhances the efficacy of cd30-directed chimeric antigen receptor T cell therapy in patients with relapsed/refractory cd30+ Lymphoma. Front Immunol. (2022) 13:858021. doi: 10.3389/fimmu.2022.858021
28. Wang CM, Wu ZQ, Wang Y, Guo YL, Dai HR, Wang XH, et al. Autologous T cells expressing cd30 chimeric antigen receptors for relapsed or refractory hodgkin lymphoma: an open-label phase I trial. Clin Cancer Res. (2017) 23:1156–66. doi: 10.1158/1078-0432.Ccr-16-1365
29. Tschernia NP, Heiling H, Deal AM, Cheng C, Babinec C, Gonzalez M, et al. Patient-reported outcomes in cd30-directed car-T cells against relapsed/refractory cd30+ Lymphomas. J Immunother Cancer. (2023) 11:e006959. doi: 10.1136/jitc-2023-006959
30. Brudno JN, Natrakul DA, Karrs J, Patel N, Maass-Moreno R, Ahlman MA, et al. Transient responses and significant toxicities of anti-cd30 car T cells for cd30+ Lymphomas: results of a phase 1 trial. Blood Adv. (2024) 8:802–14. doi: 10.1182/bloodadvances.2023011470
31. Shah NN, Johnson BD, Schneider D, Zhu F, Szabo A, Keever-Taylor CA, et al. Bispecific anti-cd20, anti-cd19 car T cells for relapsed B cell Malignancies: A phase 1 dose escalation and expansion trial. Nat Med. (2020) 26:1569–75. doi: 10.1038/s41591-020-1081-3
32. Tong C, Zhang Y, Liu Y, Ji X, Zhang W, Guo Y, et al. Optimized tandem cd19/cd20 car-engineered T cells in refractory/relapsed B-cell lymphoma. Blood. (2020) 136:1632–44. doi: 10.1182/blood.2020005278
33. Schneider D, Xiong Y, Wu D, Hu P, Alabanza L, Steimle B, et al. Trispecific cd19-cd20-cd22-targeting duocar-T cells eliminate antigen-heterogeneous B cell tumors in preclinical models. Sci Transl Med. (2021) 13:eabc6401. doi: 10.1126/scitranslmed.abc6401
34. Zhang Y, Wang Y, Liu Y, Tong C, Wang C, Guo Y, et al. Long-term activity of tandem cd19/cd20 car therapy in refractory/relapsed B-cell lymphoma: A single-arm, phase 1–2 trial. Leukemia. (2022) 36:189–96. doi: 10.1038/s41375-021-01345-8
35. Zurko JC, Fenske TS, Johnson BD, Bucklan D, Szabo A, Xu H, et al. Long-term outcomes and predictors of early response, late relapse, and survival for patients treated with bispecific lv20.19 car T-cells. Am J Hematol. (2022) 97:1580–8. doi: 10.1002/ajh.26718
36. Ghorashian S, Lucchini G, Richardson R, Nguyen K, Terris C, Guvenel A, et al. Cd19/cd22 targeting with cotransduced car T cells to prevent antigen-negative relapse after car T-cell therapy for B-cell all. Blood. (2024) 143:118–23. doi: 10.1182/blood.2023020621
37. Li D, Qin J, Zhou T, Li Y, Cheng X, Chen Z, et al. Bispecific gpc3/pd−1 car−T cells for the treatment of hcc. Int J Oncol. (2023) 62:53. doi: 10.3892/ijo.2023.5501
38. Zannikou M, Duffy JT, Levine RN, Seblani M, Liu Q, Presser A, et al. Il15 modification enables car T cells to act as a dual targeting agent against tumor cells and myeloid-derived suppressor cells in gbm. J Immunother Cancer. (2023) 11:e006239. doi: 10.1136/jitc-2022-006239
39. Hou AJ, Shih RM, Uy BR, Shafer A, Chang ZL, Comin-Anduix B, et al. Il-13rα2/tgf-β Bispecific car-T cells counter tgf-β-mediated immune suppression and potentiate anti-tumor responses in glioblastoma. Neuro Oncol. (2024) 26:1850–66. doi: 10.1093/neuonc/noae126
40. Chen N, Pu C, Zhao L, Li W, Wang C, Zhu R, et al. Chimeric antigen receptor T cells targeting cd19 and gcc in metastatic colorectal cancer: A nonrandomized clinical trial. JAMA Oncol. (2024) 10:1532–6. doi: 10.1001/jamaoncol.2024.3891
41. Hombach AA, Rappl G, Abken H. Blocking cd30 on T cells by a dual specific car for cd30 and colon cancer antigens improves the car T cell response against cd30(-) tumors. Mol Ther. (2019) 27:1825–35. doi: 10.1016/j.ymthe.2019.06.007
42. Gardner LJ, Polski JM, Evans HL, Perkins SL, Dunphy CH. Cd30 expression in follicular lymphoma. Arch Pathol Lab Med. (2001) 125:1036–41. doi: 10.5858/2001-125-1036-ceifl
43. Campuzano-Zuluaga G, Cioffi-Lavina M, Lossos IS, Chapman-Fredricks JR. Frequency and extent of cd30 expression in diffuse large B-cell lymphoma and its relation to clinical and biologic factors: A retrospective study of 167 cases. Leuk Lymphoma. (2013) 54:2405–11. doi: 10.3109/10428194.2013.778407
44. Hu S, Xu-Monette ZY, Balasubramanyam A, Manyam GC, Visco C, Tzankov A, et al. Cd30 expression defines a novel subgroup of diffuse large B-cell lymphoma with favorable prognosis and distinct gene expression signature: A report from the international dlbcl rituximab-chop consortium program study. Blood. (2013) 121:2715–24. doi: 10.1182/blood-2012-10-461848
45. Bartlett NL, Hahn U, Kim WS, Fleury I, Laribi K, Bergua JM, et al. Brentuximab vedotin combination for relapsed diffuse large B-cell lymphoma. J Clin Oncol. (2025) 43(9):1061–72. doi: 10.1200/jco-24-02242
46. Jacobsen ED, Sharman JP, Oki Y, Advani RH, Winter JN, Bello CM, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory dlbcl with variable cd30 expression. Blood. (2015) 125:1394–402. doi: 10.1182/blood-2014-09-598763
47. Gauthier J, Bezerra ED, Hirayama AV, Fiorenza S, Sheih A, Chou CK, et al. Factors associated with outcomes after a second cd19-targeted car T-cell infusion for refractory B-cell Malignancies. Blood. (2021) 137:323–35. doi: 10.1182/blood.2020006770
48. Holland EM, Molina JC, Dede K, Moyer D, Zhou T, Yuan CM, et al. Efficacy of second car-T (Cart2) infusion limited by poor cart expansion and antigen modulation. J Immunother Cancer. (2022) 10:e004483. doi: 10.1136/jitc-2021-004483
49. Dürkop H, Latza U, Hummel M, Eitelbach F, Seed B, Stein H. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for hodgkin’s disease. Cell. (1992) 68:421–7. doi: 10.1016/0092-8674(92)90180-k
50. Higgins JP, Warnke RA. Cd30 expression is common in mediastinal large B-cell lymphoma. Am J Clin Pathol. (1999) 112:241–7. doi: 10.1093/ajcp/112.2.241
51. Hutchinson CB, Wang E. Primary mediastinal (Thymic) large B-cell lymphoma: A short review with brief discussion of mediastinal gray zone lymphoma. Arch Pathol Lab Med. (2011) 135:394–8. doi: 10.5858/2009-0463-rsr.1
52. Hamieh M, Dobrin A, Cabriolu A, van der Stegen SJC, Giavridis T, Mansilla-Soto J, et al. Car T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature. (2019) 568:112–6. doi: 10.1038/s41586-019-1054-1
53. Hegde M, Corder A, Chow KK, Mukherjee M, Ashoori A, Kew Y, et al. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol Ther. (2013) 21:2087–101. doi: 10.1038/mt.2013.185
54. Larson SM, Walthers CM, Ji B, Ghafouri SN, Naparstek J, Trent J, et al. Cd19/cd20 bispecific chimeric antigen receptor (Car) in naive/memory T cells for the treatment of relapsed or refractory non-hodgkin lymphoma. Cancer Discov. (2023) 13:580–97. doi: 10.1158/2159-8290.Cd-22-0964
55. Shalabi H, Qin H, Su A, Yates B, Wolters PL, Steinberg SM, et al. Cd19/22 car T cells in children and young adults with B-all: phase 1 results and development of a novel bicistronic car. Blood. (2022) 140:451–63. doi: 10.1182/blood.2022015795
56. Dai H, Wu Z, Jia H, Tong C, Guo Y, Ti D, et al. Bispecific car-T cells targeting both cd19 and cd22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J Hematol Oncol. (2020) 13:30. doi: 10.1186/s13045-020-00856-8
Keywords: CAR T-cell therapy, transformed follicular lymphoma, CD20, CD30, bispecific CAR T
Citation: Huang Y, Gong Y, Liu X, Ruan H, Lu J, Kouros-Mehr H, Liu H and Wang H (2025) Case Report: Bispecific CD20/CD30-targeted chimeric antigen receptor T-cell therapy for non-Hodgkin’s lymphoma. Front. Immunol. 16:1567149. doi: 10.3389/fimmu.2025.1567149
Received: 26 January 2025; Accepted: 14 April 2025;
Published: 08 May 2025.
Edited by:
Hamid Reza Mirzaei, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Reshmi Parameswaran, Case Western Reserve University, United StatesRoman H. Khadka, University of Pennsylvania, United States
Copyright © 2025 Huang, Gong, Liu, Ruan, Lu, Kouros-Mehr, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Liu, aG9uZ2xpdTYzQDEyNi5jb20=; Han Wang, d3doaDE5OTFAYWxpeXVuLmNvbQ==
 Yuejiao Huang
Yuejiao Huang Yiming Gong2
Yiming Gong2 Xiang Liu
Xiang Liu Hosein Kouros-Mehr
Hosein Kouros-Mehr Han Wang
Han Wang