- 1Department of Emergency, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, China
- 3Department of Pediatrics, West China Second University Hospital, Sichuan University, Chengdu, China
Objective: Cow’s milk allergy (CMA) is one of the most common causes of food allergies (FA) in children. There have been studies on the use of immunotherapy in cow’s milk protein allergy, with oral immunotherapy (OIT) being the most extensively researched. We conducted a comprehensive analysis of randomized controlled trials (RCTs) to explore the efficacy and safety of OIT to manage cow’s milk allergy in children.
Methods: PubMed, EMBASE, Cochrane Library, and Scopus databases were searched from their inception until August 2024. Randomized controlled trials that reported on the efficacy or safety of IT for CMA were included. Two investigators independently extracted data on regimen of intervention, outcomes, number of cases and gender ratio. Pooled estimates of relative risks or standardized mean differences with 95% confidence intervals were calculated from the included studies for dichotomous and continuous outcomes.
Results: Nineteen RCT articles (815 participants) were included. The meta-analysis indicated that oral immunotherapy significantly facilitated desensitization in patients with cow’s milk allergy in children (relative risk [RR] 2.51, 95% CI: 1.54–4.09, I²=84.4%). Tolerance threshold at oral food challenges (OFC) increased following oral immunotherapy compared with a standard mean difference (SMD) of 3.58 (2.82–4.33). After oral immunotherapy, the antibody titers of cow milk protein sIgE (SMD -0.42, 95% CI: -0.72 to -0.11, I²=28.8%) and casein sIgE (SMD -0.54, 95% CI: -0.97 to -0.11, I²=0%) decreased. The risk of adverse reactions with immunotherapy was not higher than that in the control group, with an RR of 2.05 (95% CI 0.96–4.37, I²=81.5%).
Conclusions: Oral immunotherapy, is associated with desensitization to CMA in children, without increased risk of short-term adverse events, but late complications such as eosinophilic esophagitis require caution. More high-quality studies are needed to explore the long-term efficacy of OIT for CMA.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/recorddashboard, identifier CRD42024541769.
1 Introduction
Food allergy (FA) as a common disease in children with increasing prevalence rates over the past two to three decades. In some countries, FA prevalence has reached as high as 10% (1). A systematic review estimated that direct medical costs for patients with FA in the United States alone amount to $4.3 billion annually (2). Cow’s milk allergy is one of the most common causes of FAs in children. The reported prevalence of CMA varies due to differences in diagnostic methods and epidemiological survey designs. CMA prevalence range of 1.8%–7.5% in the first year of life was reported (3). Some adult patients with CMA had allergies since childhood, but some studies have also reported adult-onset CMA (4).
Management of cow milk protein allergy involves strict avoidance of food allergens and the use of pharmacological treatments to address reactions from accidental exposure. In infancy, CMA is often managed by substituting cow milk with extensively hydrolyzed protein or amino acid-based formulas. However, these alternatives have poor taste and are costly, imposing a significant financial burden on families (4). Alongside the persistent risk of systemic allergic reactions, avoiding milk consumption may also have nutritional implications. Sinai et al. compared the adult height of CMA patients with a control group without dietary restrictions and found that CMA patients were, on average, 3.8 cm shorter and had a higher rate of vitamin D deficiency (5). Recently, there has been an increasing research focus on immunotherapy for CMA, aiming to achieve desensitization as early as possible (6). Various immunotherapy approaches for milk protein desensitization, including oral immunotherapy, sublingual immunotherapy, and subcutaneous immunotherapies, and OIT is the most common approach. OIT is a treatment method that induces immune tolerance by gradually increasing the intake of allergens. OIT likely induces immune tolerance via repeated allergen exposure by rebalancing the Th2-driven allergic response toward a Th1/Treg-dominated profile (7). However, findings regarding their effectiveness are inconsistent. Some studies have found that immunotherapy can facilitate desensitization to cow milk protein (8), while others have reported no such effects (9). Therefore, we aimed to conduct a systematic review and meta-analysis of oral immunotherapy for CMA in children to provide clinical recommendations for managing CMA.
2 Materials and methods
This review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (10). The protocol and search strategies were registered with PROSPERO (York. ac. uk) (CRD42024541769).
2.1 Retrieval of studies
A systematic search of PubMed, EMBASE, the Cochrane Library, and Scopus was conducted to identify relevant studies from inception to August 2024. The search strategy is detailed in Supplementary Material. Additional studies were identified by manually searching the references of included research articles, meta-analyses, and reviews.
2.2 Eligibility criteria
The eligibility criteria were as follows: (1) participants were under 18 years of age with a confirmed IgE-mediated milk protein allergy; (2) the intervention involved IT; (3) studies reported on at least one of the following outcomes: efficacy (desensitization or sustained unresponsiveness [SU]), changes in reaction thresholds during oral food challenges (OFC), laboratory parameters, changes in quality of life, or adverse reactions (ARs); and (4) studies had a RCT design.
Exclusion criteria were: (1) non-IgE-mediated milk protein allergy; (2) absence of a control group; (3) case-control studies, case reports, conferences, reviews, and animal studies; (4) overlapping data or lack of raw data; and (5) participants over 18 years of age.
2.3 Data extraction
Two independent reviewers extracted data on the first author, publication year, country, study design, intervention, comparator, sex ratio, number of cases and total sample population, OIT regimen, and outcomes.
2.4 Quality assessment
The Cochrane risk-of-bias tool was used to assess the quality of randomized trials. RoB grades bias of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases of low, unclear, or high risk.
2.5 Statistical analysis
The meta-analysis was conducted using Stata version 18.0 (Stata Corporation, College Station, TX, USA). For trials with multiple reports, we included all relevant data. A random-effects model was employed as anticipated. Pooled estimates of relative risks (RRs) with 95% confidence intervals (CIs) were calculated for dichotomous outcomes. We combined continuous outcomes across studies using standardized mean differences. The heterogeneity between studies was assessed using the I2 statistic (significance level >50%). We also performed sensitivity analysis by sequentially excluding each study. Publication bias was visually assessed using Egger’s and Begg’s tests (significance level: P<0.05). Review manager 5.4.1 was used to evaluate the certainty of the evidence.
3 Results
3.1 Search results and study characteristics
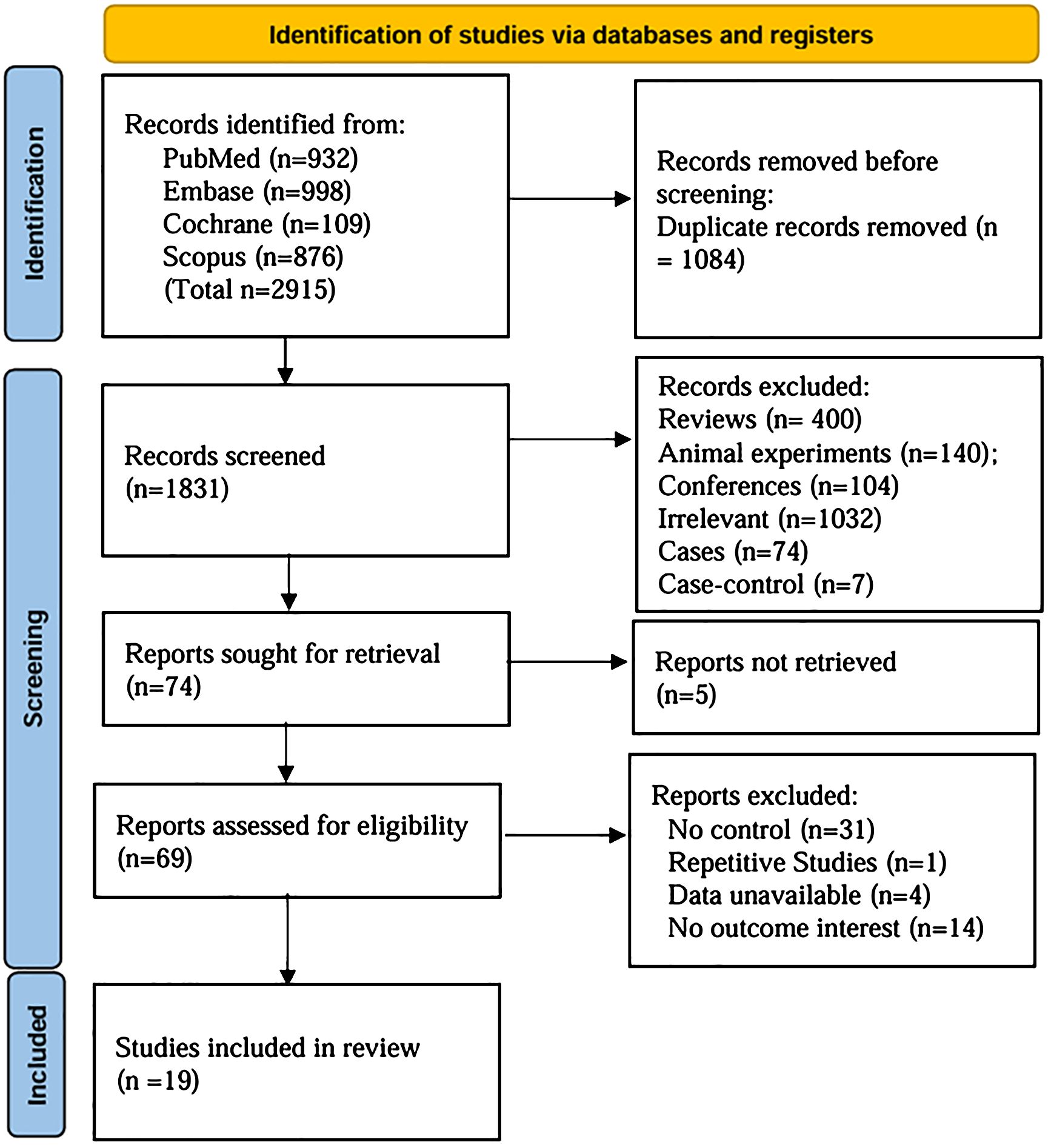
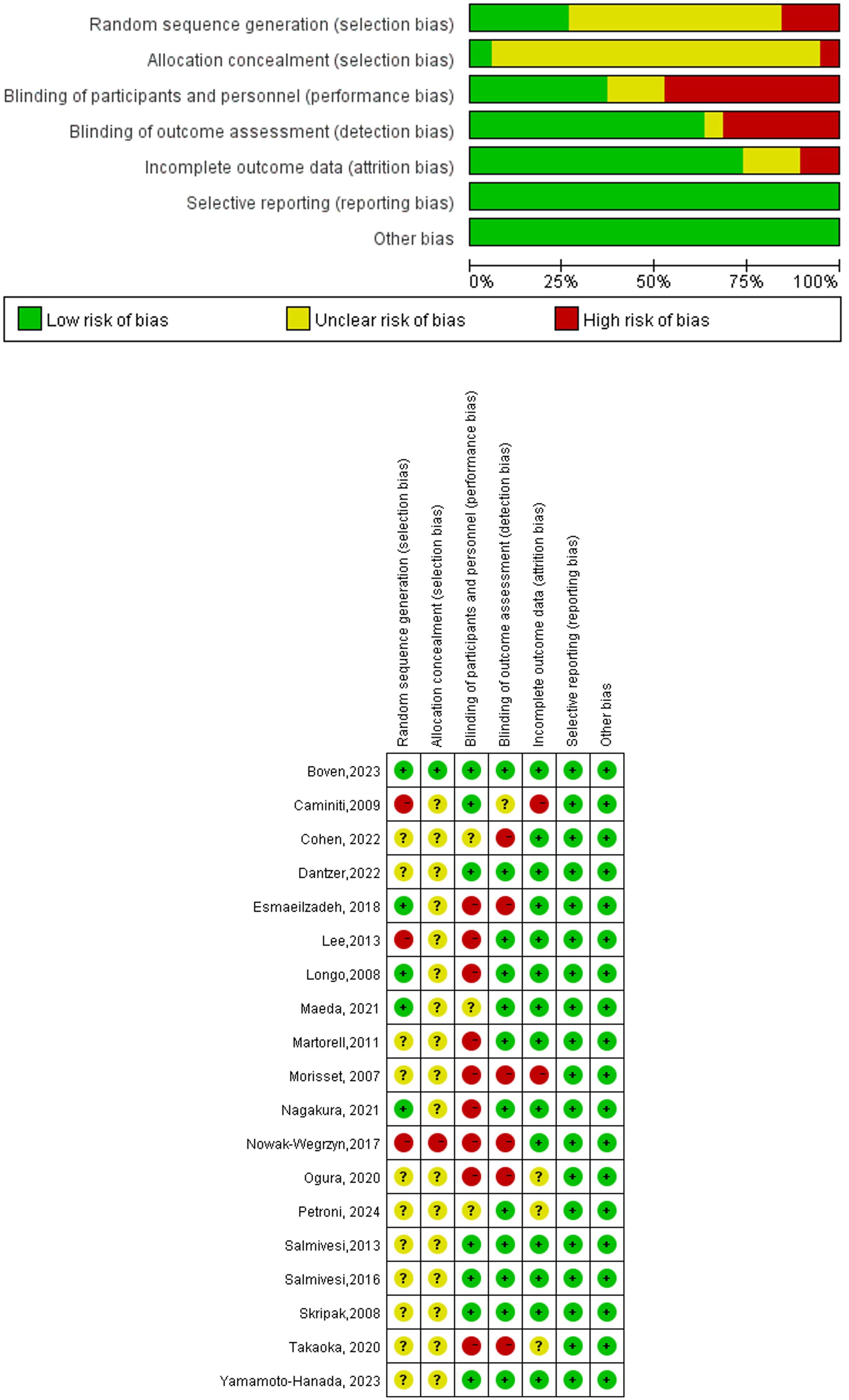
A comprehensive search identified 2915 articles (PubMed: 932; EMBASE: 998; Cochrane Library: 109; Scopus: 876). After careful screening, 19 studies (8, 9, 11–27) were selected for inclusion (Figure 1). The characteristics of the 19 selected studies are presented in Supplementary Table S1. A total of 815 participants were enrolled, with 367 assigned to the control group and 448 to the OIT group. The ages of the study population ranged from 3 month to 18 years. Geographically, the studies were conducted in North America (5) (9, 13, 19, 23, 24), Europe (7) (8, 11, 20, 22, 25–27), and Asia (7) (12, 14–18, 21). Overall, the quality of the RCTs was low, with many studies showing unclear or high risk of bias in various methodological domains. The quality of evidence in the study was primarily influenced by allocation concealment and blinding procedures (Figure 2).
3.2 Primary outcomes
3.2.1 Desensitization
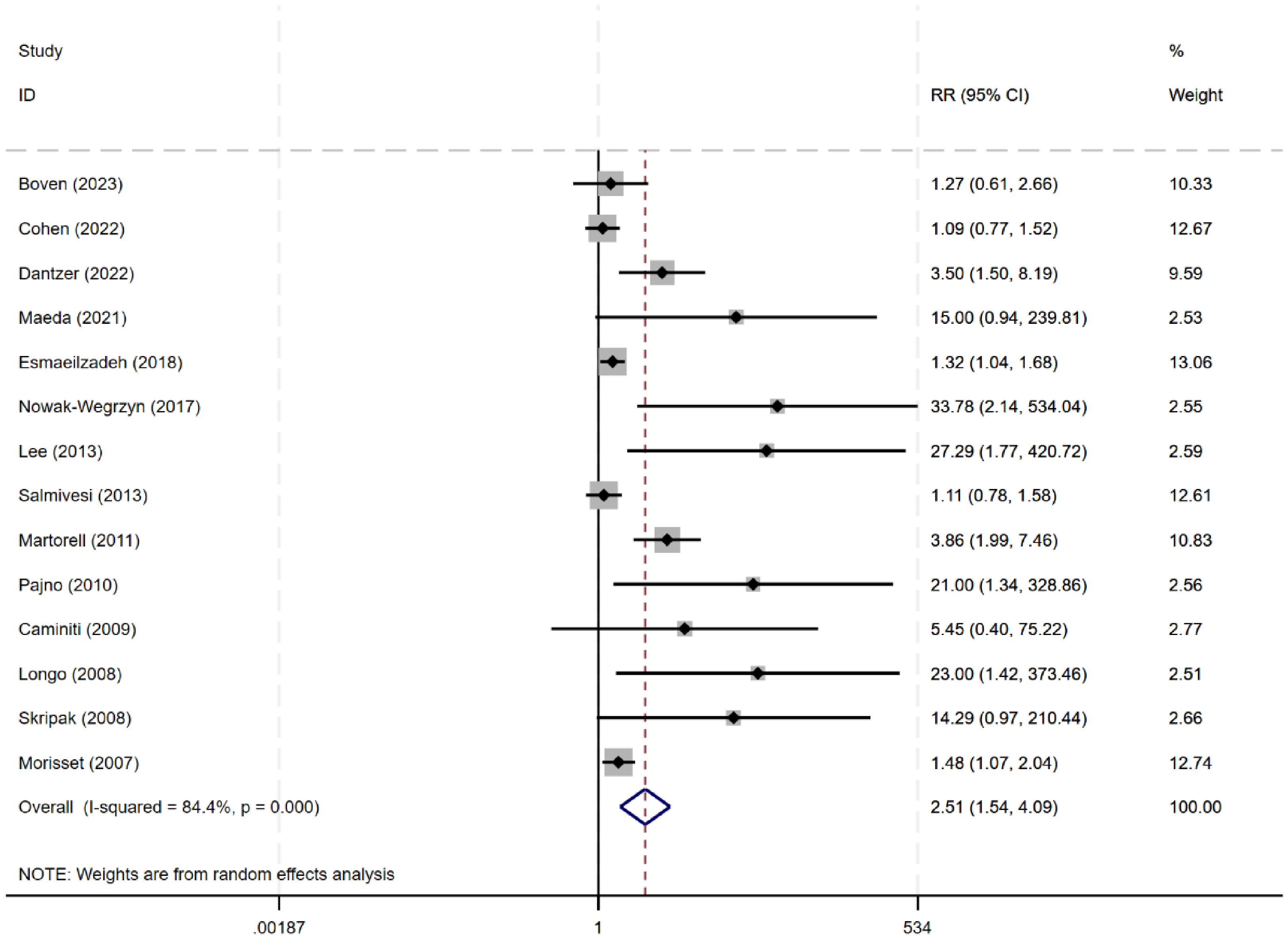
Fourteen studies (n=663) (8, 9, 11, 13, 14, 18–26) evaluated the efficacy of desensitization across a wide range of thresholds (264 mg to 7250 mg, or 3–250 ml). The meta-analysis showed a significant increase in desensitization among patients allergic to milk (RR 2.51, 95% CI: 1.54–4.09), with an I² of 84.4%, indicating high heterogeneity (Figure 3). Further subgroup analyses were performed based age group, control group type, type of milk intervention, and treatment duration.
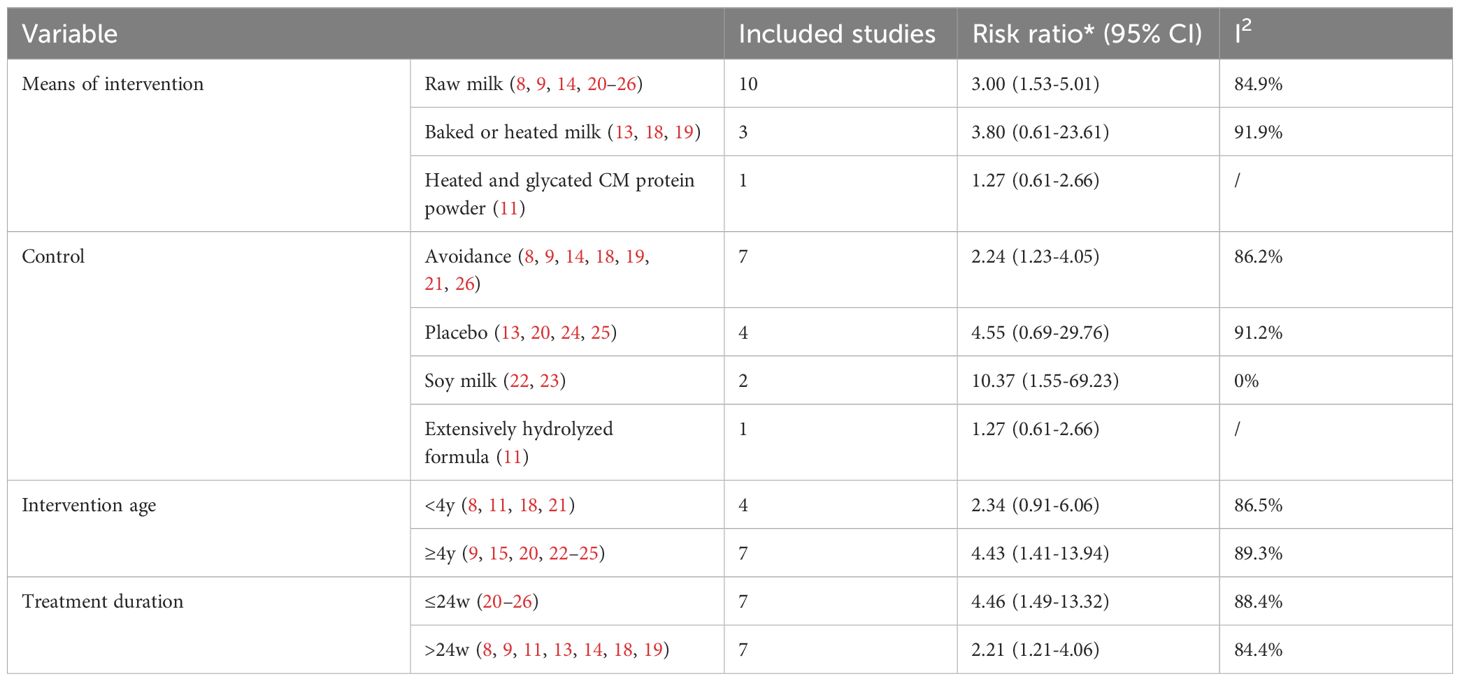
For children under 4 years of age, oral immunotherapy did not demonstrate effectiveness in the desensitization to cow’s milk protein (RR: 2.34, 95% CI: 0.91–6.06). However, for children over 4 years old, OIT was effective in inducing desensitization to cow’s milk protein (RR: 4.43, 95% CI: 1.41–13.94). This may be related to the fact that cow’s milk protein allergy in younger children has a certain degree of self-limiting nature, whereas the likelihood of spontaneous resolution of cow’s milk protein allergy in older children is lower. Compared to avoidance of cow’s milk protein intake (RR: 2.23, 95% CI: 1.23-4.05) or using soy milk as a control (RR: 10.37, 95% CI: 1.55-69.23), OIT achieved desensitization effects; but compared to a placebo control, OIT did not show effectiveness (RR: 4.55, 95% CI: 0.69-29.76). The combined data showed a statistically significant tolerance to cow’s milk in the OIT group using raw milk compared to the control group (RR: 3.00, 95% CI: 1.53-5.91, I²=84.9%), but using baked milk (RR: 3.80, 95% CI: 0.61-23.61) or heated milk (RR: 1.27, 95% CI: 0.61-2.66) as intervention methods did not show an advantage in desensitization (Table 1).
3.2.2 Change in reaction thresholds at OFC
A meta-analysis (n=3) (13, 14, 24) indicated that the tolerance threshold at OFC increased following immunotherapy compared to before treatment, with a SMD of 3.58 (2.82–4.33) and an I² of 0% (Figure 4).
3.2.3 Adverse reactions
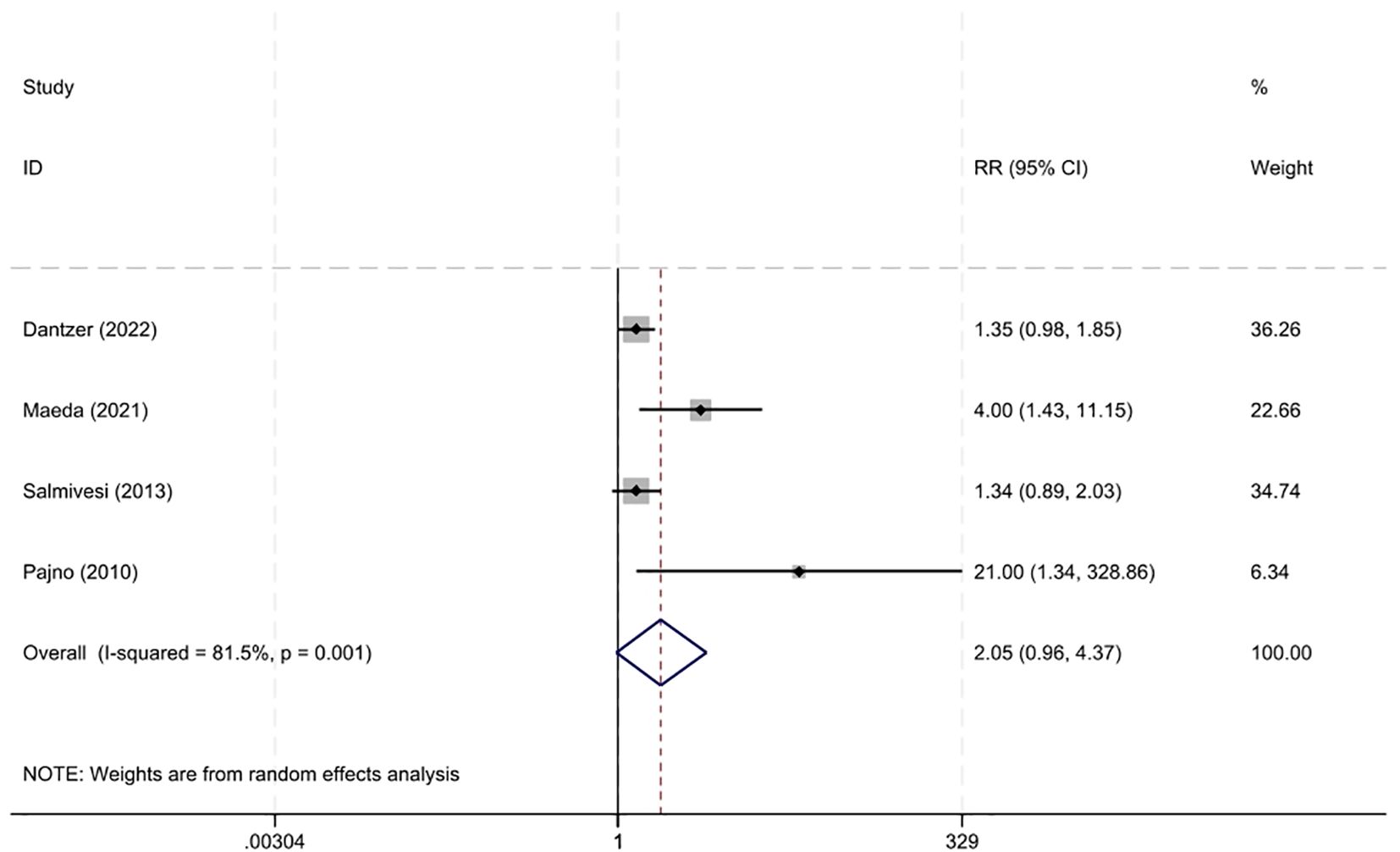
Four studies (13, 14, 20, 22) documented the occurrence of adverse events. The risk of adverse reactions with immunotherapy was not higher than that in the control group, with an RR of 2.05 (95% CI 0.96–4.37, I²=81.5%) (Figure 5). Most adverse reactions were mild, and there was no significant difference in the risk of severe adverse reactions between the two groups, with an RR of 2.65 (95% CI 0.79–16.90, I²=0%) (13, 14, 22).
Eosinophilic esophagitis (EoE) is an allergen-mediated inflammatory disorder of the esophagus characterized by progressive esophageal dysfunction. EoE has been observed in patients receiving OIT for IgE-mediated food allergies and may represent a late complication of OIT treatment. Among the studies included in this meta-analysis, only one (15) reported a single case of EoE. After expanding our database search, we included 11 cohort studies (15, 28–37) and found that 4.0% (95% CI:2%-7%) of children receiving OIT had biopsy-confirmed EoE (Supplementary Figure S1).
3.3 Secondary outcomes
3.3.1 Immunological changes
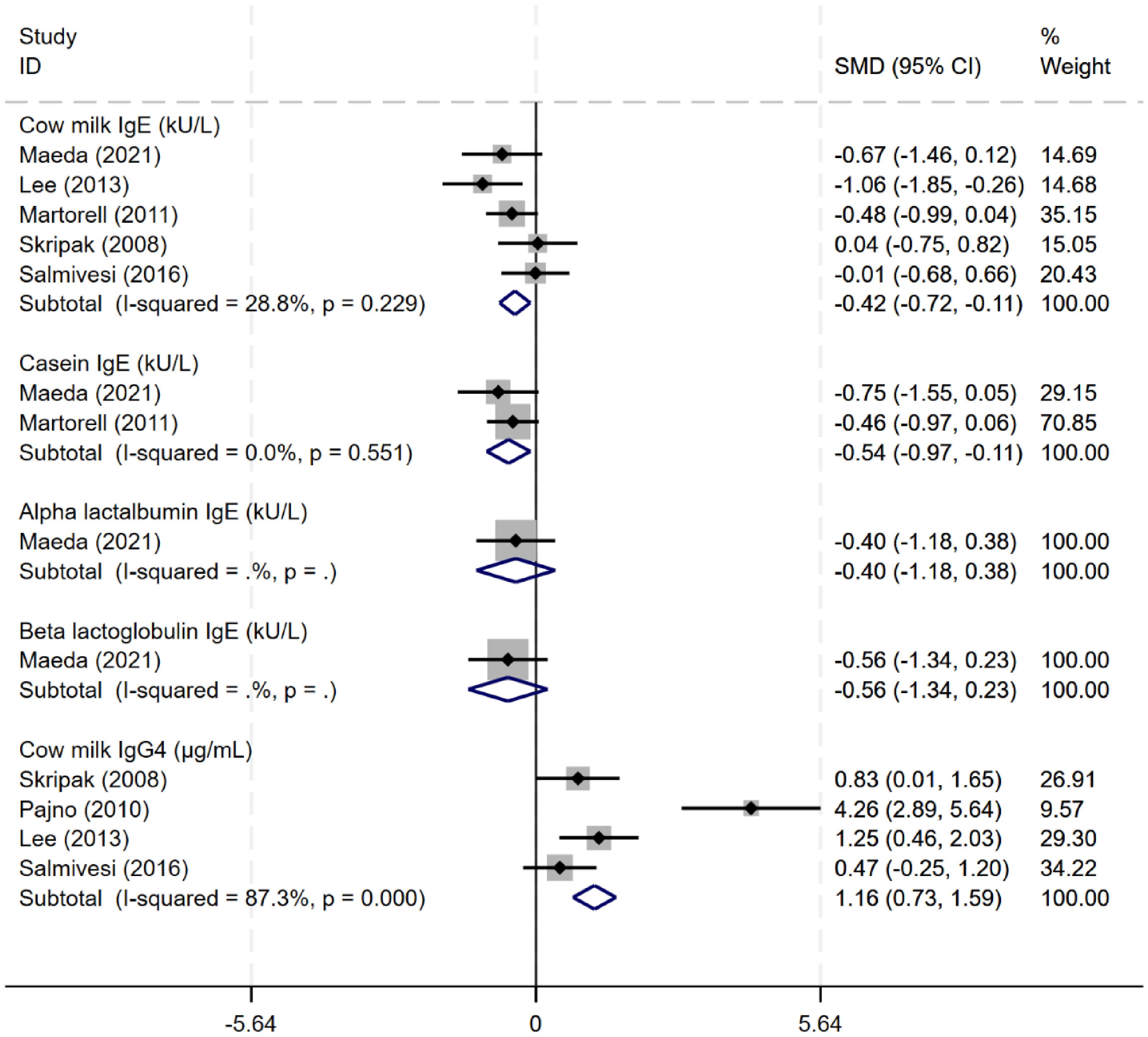
Five studies (8, 14, 21, 22, 24, 27) evaluated the effects of immunotherapy on immunological changes. Oral immunotherapy reduced the levels of serum cow milk protein-related (CM-sIgE) and casein-specific IgE (CN-sIgE) antibodies (CM-sIgE: SMD -0.42, 95% CI: -0.72 to -0.11, I²=28.8%; CN-sIgE: SMD -0.54, 95% CI: -0.97 to -0.11, I²=0%). After immunotherapy, cow milk protein IgG4 levels were higher than before treatment, with an SMD of 2.01 (95% CI 0.34 to 3.69, I²=89.1%). Only one study evaluated the change of α-lactalbumin and β-lactoglobulin IgE levels (Figure 6).
Only one study (27) in the literature reported the effects of OIT on cytokines. The analysis demonstrated that OIT did not significantly alter the levels of cytokines IL-4, IL-5, IL-6, or IL-10 (Supplementary Figure S2).
3.3.2 Quality of life assessment
Only one study used a standardized scale to evaluate the impact of immunotherapy on the quality of life of patients (13), using improved Food Allergy Quality of Life-Parent Form (FAQOL-PF) or Food Allergy Quality of Life-Child Form (FAQOL-CF) scores (≥0.5-minimal clinically important difference) from baseline to month 12 as indicators. The RR was 0.58 (95% CI 0.18–1.85) for FAQOL-PF and 1.40 (95% CI 0.23–8.44) for FAQOL-CF.
3.3.3 Other studies
Two studies (16, 17) compared the impact of different target doses on the efficacy of desensitization. No significant differences were observed between the low-dose (20–25%) and high-dose (100%) groups in the treatment of cow’s milk protein desensitization (RR 1.10, 95% CI 0.56–2.16). One study investigated the effect of adding probiotics to oral immunotherapy. The study found that the effect of OIT combined with the probiotic LP013216 on cow’s milk protein desensitization was not significantly different from that of OIT alone (RR 1.17, 95% CI 0.83–1.65). Only one study (15) compared the effects of heated milk versus raw milk on desensitization, showing no significant difference (RR 1.26, 95% CI 0.92-1.71).
3.4 Sensitivity analysis and publication bias
Sensitivity analysis was performed for the main outcomes with high heterogeneity. The results of sensitivity analysis confirmed stable results in the efficacy of OIT for CMA and adverse events of OIT (Supplementary Figures S3, S4). A funnel plot suggested a potential publication bias in relation to desensitization of OIT for CMA (Egger, P<0.001). We further undertook a sensitivity analysis by using the trim-and-fill method to estimate the number of missing studies that may have caused funnel plot asymmetry, and we imputed the hypothetical studies to produce a symmetrical funnel plot. After adjusting for potential publication bias, the impact of the intervention remained statistically significant (RR1.59, 95% CI 1.08-2.36) (Supplementary Figure S5).
4 Discussion
Our meta-analysis included 19 studies to comprehensively evaluate the overall efficacy and safety of immunotherapy in patients with IgE-mediated cow milk protein allergy. Despite the high heterogeneity and generally low evidence levels among these studies, immunotherapy was more effective for achieving desensitization in patients with CMA compared to avoidance therapy, and it significantly altered the cumulative tolerance dose. The immunotherapy group did not show a higher risk of adverse effects than the control group, and severe adverse events were rare.
Like the Riggioni (38) study, oral immunotherapy was effective in the treatment of cow’s milk allergy compared to avoidance of milk protein, with the results exhibiting considerable heterogeneity. Unlike the Riggioni study, which included eight RCT studies and two case-control studies and did not perform subgroup analysis, our study exclusively included RCTs, yet it still exhibited a high degree of heterogeneity, suggesting that the experimental design is not the source of heterogeneity. The differences may be related to the high heterogeneity in study populations, intervention protocols, outcome measures, follow-up periods, and other methodological variations. Despite the heterogeneity, sensitivity analysis suggested the reliability of the results. Standardization of trial design, outcome measurement, and reporting practices is crucial to address these issues and enhance the reliability and applicability of research findings.
CMA pathogenesis can be mediated by IgE, non-IgE, or both. Most cow milk protein allergies are mediated by IgE. Cow milk contains caseins and whey proteins, which account for approximately 80% and 20% of the total milk protein, respectively. Most patients with cow milk protein allergy are allergic to multiple proteins in cow milk, with casein, β-lactoglobulin, and α-lactalbumin being the major allergens (39). Immunological evaluation in this meta-analysis showed that immunotherapy reduced cow milk protein-specific IgE and casein-specific IgE levels. Levels of cow milk-related IgG4 increased, likely due to enhanced IgG4 binding to milk epitopes, which in turn reduces IgE binding to these epitopes and induces tolerance (40).
This meta-analysis also examined how intervention type and dosage affect desensitization efficacy. Among the included studies, four types of milk interventions were used: baked milk, heated milk, raw cow milk, and glycated milk, with most studies using raw cow milk. Heating or baking milk at high temperatures can degrade certain structural epitopes, rendering them unrecognizable by allergen-specific IgE. Although one study (18) suggested that frequent consumption of baked products accelerates tolerance to cow milk protein, our study did not find this relationship, which is consistent with the meta-analysis results of Anagnostou et al. (41). Tolerance to heated or baked milk may be temporary and diminish within a few years. Dantzer et al. found that a protocol involving gradually increasing doses of baked milk was effective in promoting desensitization. This suggests that the initial dose may influence the efficacy of desensitization. Two studies evaluated the effects of low-dose and high-dose target protocols, and a pooled results indicated that the maximum dose did not appear to be associated with the efficacy of tolerance. Furthermore, baked or heated milk products are not standardized, and the extent and consistency of the allergen quantity or epitope structure in each preparation method are unknown, necessitating further research.
Although children with cow’s milk allergy have a higher prevalence of EoE than the general population, Ferreira Martins et al. (33) reported cases of children with CMA who had no pre-existing EoE developing EoE following oral immunotherapy. This suggests that EoE may represent a potential complication of OIT treatment. EoE typically emerges approximately 2.8 years after initiating the maintenance phase of OIT in CM-allergic patients (28). Therefore, studies with short follow-up periods may underestimate the true incidence of EoE. In our meta-analysis, the observation periods in the included RCTs ranged from 23 to 96 weeks, with only one study following patients for 96 weeks and the remainder not exceeding 52 weeks. This relatively short follow-up duration may have led to underestimation. After expanding our search to include non-RCT studies, we found that 4% of CM-allergic patients developed biopsy-confirmed EoE following OIT - a rate consistent with previous reports (42).
This study has several strengths: 1) inclusion of the latest research on immunotherapy and biological therapy for cow milk protein allergy, encompassing many participants, and 2) a comprehensive analysis with stratified evaluations of immunotherapy for CMA by region, age of inclusion, intervention methods, and control groups. However, the high heterogeneity across studies, including differences in study population, intervention protocols, outcome measures, and other methodological variations, presents a limitation. Nevertheless, the large sample size and the stability of sensitivity analysis results add robustness to our findings for clinical decision-making.
Limitations of this study included: 1) the included studies had a small sample size; 2) the exclusion of studies without a control group, potentially introducing bias in the evaluation of indicators before and after immunotherapy or biological therapy; 3) high heterogeneity among included studies and the failure to identify the sources of heterogeneity through subgroup analyses and other methods. The heterogeneity may be attributed to variations in target doses and maintenance duration across studies. The included studies in this analysis employed varying protocols, with target doses ranging from 3 mL to 250 mL. The high-dose group demonstrated a higher incidence of severe adverse reactions during the maintenance phase compared to the low-dose group. Longer durations of OIT were associated with higher rates of desensitization or non-responsiveness (43). Consequently, the treatment duration significantly impacts efficacy evaluation. The double-blind, placebo-controlled food challenge (DBPCFC) is the gold standard for diagnosing food allergies and is an objective tool for assessing desensitization therapy, allowing for the evaluation of changes from before to after immunotherapy and the assessment of thresholds. Notably, many articles did not use DBPCFC as an outcome measure, limiting the interpretation of the results. Inconsistent follow-up periods and potential publication bias further complicate the interpretation and generalization of the results. Optimizing the included population, standardizing trial design, and outcome measurements may help improve heterogeneity; 4) Sustained unresponsiveness is defined as the absence of allergic reactions upon food reintroduction after a prescribed period of allergen avoidance following OIT discontinuation, serving as a key metric for assessing long-term therapeutic success. However, none of the included RCTs reported sustained unresponsiveness rates, underscoring the need for future trials to incorporate this clinically meaningful endpoint;5) the lack of a systematic evaluation of long-term effects or side effects. The outcome measures included in this study had a relatively short duration, mostly within one year, and lacked long-term outcome evaluations, and 6) Among the included studies, only one assessed cytokine changes during OIT without in-depth analysis of the underlying immunomodulatory mechanisms. There are reports (44) that OIT may have long-term side effects, including eosinophilic esophagitis; however, most are case reports, and further attention is needed to monitor these side effects.
Future studies should establish standardized definitions for initial dosing (differentiating low versus standard starting doses), maintenance dosing (distinguishing low versus high maintenance doses), and efficacy evaluation criteria to reduce inter-study heterogeneity. A severity-stratified design should be implemented, with conservative low starting doses adopted for children with severe allergies. Furthermore, extended follow-up periods are recommended to properly evaluate long-term therapeutic efficacy and safety profiles.
5 Conclusion
This meta-analysis suggests that oral immunotherapy can effectively desensitize milk proteins in children with cow protein milk allergy, though data on long-term follow-up remains incomplete. Currently, there are limited data on the long-term use of immunotherapy agents as well as the impact of treatment interruption on milk allergy status, immunological changes, and improvements in quality of life. Although the reported adverse events are mainly mild to moderate, comprehensive studies on long-term safety are still lacking.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
YW: Conceptualization, Formal analysis, Writing – original draft, Methodology, Data curation, Investigation. SL: Methodology, Writing – original draft, Software, Supervision, Validation. ML: Methodology, Supervision, Data curation, Writing – review & editing. JG: Methodology, Data curation, Resources, Software, Writing – original draft. CL: Methodology, Software, Investigation, Project administration, Writing – review & editing. LH: Methodology, Project administration, Writing – review & editing, Conceptualization, Formal analysis, Validation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to thank Zhao Ningyuan for figure editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1570050/full#supplementary-material
References
1. Loh W and Tang MLK. The epidemiology of food allergy in the global context. Int J Environ Res Public Health. (2018) 15:2043. doi: 10.3390/ijerph15092043
2. Bilaver LA, Chadha AS, Doshi P, O’Dwyer L, and Gupta RS. Economic burden of food allergy: A systematic review. Ann Allergy Asthma Immunol. (2019) 122:373–80.e1. doi: 10.1016/j.anai.2019.01.014
3. Luyt D, Ball H, Makwana N, Green MR, Bravin K, Nasser SM, et al. BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin Exp Allergy. (2014) 44:642–72. doi: 10.1111/cea.2014.44.issue-5
4. Stöger P and Wüthrich B. Type I allergy to cow milk proteins in adults. A retrospective study of 34 adult milk- and cheese-allergic patients. Int Arch Allergy Immunol. (1993) 102:399–407. doi: 10.1159/000236589
5. Sinai T, Goldberg MR, Nachshon L, Amitzur-Levy R, Yichie T, Katz Y, et al. Reduced final height and inadequate nutritional intake in cow’s milk-allergic young adults. J Allergy Clin Immunol Pract. (2019) 7:509–15. doi: 10.1016/j.jaip.2018.11.038
6. Riggioni C, Oton T, Carmona L, Du Toit G, Skypala I, and Santos AF. Immunotherapy and biologics in the management of IgE-mediated food allergy: Systematic review and meta-analyses of efficacy and safety. Allergy. (2024) 79:2097–127. doi: 10.1111/all.16129
7. Gernez Y and Nowak-Węgrzyn A. Immunotherapy for food allergy: are we there yet? J Allergy Clin Immunol Pract. (2017) 5:250–72. doi: 10.1016/j.jaip.2016.12.004
8. Martorell A, de la Hoz B, Ibáñez MD, Bone J, Terrados MS, Michavila A, et al. Oral desensitization as a useful treatment in 2-year-old children with cow’s milk allergy. Clin Exp Allergy. (2011) 41:1297–304. doi: 10.1111/j.1365-2222.2011.03749.x
9. Cohen CG, Zhao WW, Ke D, Beaudette L, Lejtenyi D, McCusker C, et al. Elevated cow’s milk-specific igE levels prior to oral immunotherapy decrease the likelihood of reaching the maintenance dose. J Allergy Clin Immunol Pract. (2022) 10:215–21.e2. doi: 10.1016/j.jaip.2021.11.005
10. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160. doi: 10.1136/bmj.n160
11. van Boven FE, Arends NJT, Sprikkelman AB, Emons JAM, Hendriks AI, van Splunter M, et al. Tolerance induction in cow’s milk allergic children by heated cow’s milk protein: the iAGE follow-up study. Nutrients. (2023) 15:1181. doi: 10.3390/nu15051181
12. Yamamoto-Hanada K, Sato M, Toyokuni K, Irahara M, Hiraide-Kotaki E, Harima-Mizusawa N, et al. Combination of heat-killed Lactiplantibacillus plantarum YIT 0132 (LP0132) and oral immunotherapy in cow’s milk allergy: a randomised controlled trial. Beneficial Microbes. (2023) 14:17–29. doi: 10.3920/BM2022.0064
13. Dantzer J, Dunlop J, Psoter KJ, Keet C, and Wood R. Efficacy and safety of baked milk oral immunotherapy in children with severe milk allergy: a randomized, double-blind, placebo-controlled phase 2 trial. J Allergy Clin Immunol. (2022) 149:1383–91.e17. doi: 10.1016/j.jaci.2021.10.023
14. Maeda M, Imai T, Ishikawa R, Nakamura T, Kamiya T, Kimura A, et al. Effect of oral immunotherapy in children with milk allergy: the ORIMA study. Allergol Int. (2021) 70:223–8. doi: 10.1016/j.alit.2020.09.011
15. Nagakura KI, Sato S, Miura Y, Nishino M, Takahashi K, Asaumi T, et al. A randomized trial of oral immunotherapy for pediatric cow’s milk-induced anaphylaxis: heated vs unheated milk. Pediatr Allergy Immunol. (2021) 32:161–9. doi: 10.1111/pai.13352
16. Takaoka Y, Yajima Y, Ito YM, Kumon J, Muroya T, Tsurinaga Y, et al. Single-center noninferiority randomized trial on the efficacy and safety of low-and high-dose rush oral milk immunotherapy for severe milk allergy. Int Arch Allergy Immunol. (2020) 181:699–705. doi: 10.1159/000508627
17. Ogura K, Yanagida N, Sato S, Imai T, Ito K, Kando N, et al. Evaluation of oral immunotherapy efficacy and safety by maintenance dose dependency: A multicenter randomized study. World Allergy Organ J. (2020) 13. doi: 10.1016/j.waojou.2020.100463
18. Esmaeilzadeh H, Alyasin S, Haghighat M, Nabavizadeh H, Esmaeilzadeh E, and Mosavat F. The effect of baked milk on accelerating unheated cow’s milk tolerance: a control randomized clinical trial. Pediatr Allergy Immunol. (2018) 29:747–53. doi: 10.1111/pai.2018.29.issue-7
19. Nowak-Wegrzyn A, Lawson K, Masilamani M, Kattan J, Bahnson HT, and Sampson HA. Increased tolerance to less extensively heat-denatured (Baked) milk products in milk-allergic children. J Allergy Clin Immunol Practice. (2018) 6:486–95.e5. doi: 10.1016/j.jaip.2017.10.021
20. Salmivesi S, Korppi M, Mäkelä MJ, and Paassilta M. Milk oral immunotherapy is effective in school-aged children. Acta Paediatr. (2013) 102:172–6. doi: 10.1111/j.1651-2227.2012.02815.x
21. Lee JH, Kim WS, Kim H, and Hahn YS. Increased cow’s milk protein-specific IgG4 levels after oral desensitization in 7- to 12-month-old infants. Ann Allergy Asthma Immunol. (2013) 111:523–8. doi: 10.1016/j.anai.2013.09.001
22. Pajno G, Caminiti L, Ruggeri P, De Luca R, Vita D, La Rosa M, et al. Oral immunotherapy for cow’s milk allergy with a weekly updosing regimen: A randomised double blind controlled study. Allergy: Eur J Allergy Clin Immunol. (2010) 65:370. doi: 10.1016/j.anai.2010.03.015
23. Caminiti L, Passalacqua G, Barberi S, Vita D, Barberio G, De Luca R, et al. A new protocol for specific oral tolerance induction in children with IgE-mediated cow’s milk allergy. Allergy Asthma Proc. (2009) 30:443–8. doi: 10.2500/aap.2009.30.3221
24. Skripak JM, Nash SD, Rowley H, Brereton NH, Oh S, Hamilton RG, et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol. (2008) 122:1154–60. doi: 10.1016/j.jaci.2008.09.030
25. Longo G, Barbi E, Berti I, Meneghetti R, Pittalis A, Ronfani L, et al. Specific oral tolerance induction in children with very severe cow’s milk-induced reactions. J Allergy Clin Immunol. (2008) 121:343–7. doi: 10.1016/j.jaci.2007.10.029
26. Morisset M, Moneret-Vautrin DA, Guenard L, Cuny JM, Frentz P, Hatahet R, et al. Oral desensitization in children with milk and egg allergies obtains recovery in a significant proportion of cases. A randomized study in 60 children with cow’s milk allergy and 90 children with egg allergy. Eur Ann Allergy Clin Immunol. (2007) 39:12–9.
27. Salmivesi S, Paassilta M, Huhtala H, Nieminen R, Moilanen E, and Korppi M. Changes in biomarkers during a six-month oral immunotherapy intervention for cow’s milk allergy. Acta Paediatr. (2016) 105:1349–54. doi: 10.1111/apa.2016.105.issue-11
28. Morales-Cabeza C, Infante S, Cabrera-Freitag P, Fuentes-Aparicio V, Zubeldia JM, and Álvarez-Perea A. Oral immunotherapy and risk of eosinophilic esophagitis in children: 15 years’ Experience. J Pediatr Gastroenterol Nutr. (2023) 76:53–8. doi: 10.1097/MPG.0000000000003631
29. Arasi S, Caminiti L, Crisafulli G, Panasiti I, Barbalace A, Passalacqua G, et al. The safety of oral immunotherapy for food allergy during maintenance phase: Effect of counselling on adverse reactions. World Allergy Organ J. (2019) 12:100010. doi: 10.1016/j.waojou.2018.11.008
30. Yonekura Anagusko CL, Mendonca J, Lopes MM, Torres P, Ferreira Martins CS, Castro FM, et al. Specific IgG4 to milk proteins during oral immunotherapy for milk allergy: relationship to eosinophilic esophagitis. J Allergy Clin Immunol. (2019) 143:AB138. doi: 10.1016/j.jaci.2018.12.419
31. De Schryver S, Mazer B, Clarke AE, St Pierre Y, Lejtenyi D, Langlois A, et al. Adverse events in oral immunotherapy for the desensitization of cow’s milk allergy in children: A randomized controlled trial. J Allergy Clin Immunol Pract. (2019) 7:1912–9. doi: 10.1016/j.jaip.2019.02.007
32. Aquilante BP, Faion MCN, Marcelino F, Veiga S, Soldateli G, Yonamine GH, et al. Eosinophilic esophagitis during milk oral immunotherapy: A growing concern. J Allergy Clin Immunol. (2018) 141:AB135. doi: 10.1016/j.jaci.2017.12.431
33. Ferreira Martins CS, Torres P, Zanandrea A, Penido A, Mouco CC, Pereira da Silva L, et al. EoE, OIT and Anaphylaxis: an unsolved puzzle. J Allergy Clin Immunol. (2019) 143:AB136.
34. Gómez Torrijos E, Mendez Díaz Y, Moreno Lozano L, Extremera Ortega AM, Borja Segade J, Feo Brito JF, et al. Frequency and course of eosinophilic esophagitis during oral immunotherapy for cow’s milk allergy in a series of 57 children. J Investig Allergol Clin Immunol. (2017) 27:132–3. doi: 10.18176/jiaci.0130
35. Babaie D, Nabavi M, Arshi S, Mesdaghi M, Chavoshzadeh Z, Bemanian MH, et al. Cow’s milk desensitization in anaphylactic patients: A new personalized-dose method. Iran J Allergy Asthma Immunol. (2017) 16:45–52.
36. Echeverría-Zudaire L, Fernández-Fernández S, Rayo-Fernández A, Muñóz-Archidona C, and Checa-Rodriguez R. Primary eosinophilic gastrointestinal disorders in children who have received food oral immunotherapy. Allergol Immunopathol (Madr). (2016) 44:531–6. doi: 10.1016/j.aller.2016.05.002
37. Sanchez-Garcia S, Rodriguez Del Rio P, Escudero C, Costa M, Jurado F, and Ibañez M. Efficacy and safety of milk oral immunotherapy: Five years of experience in treatment and follow-up. Allergy: Eur J Allergy Clin Immunol. (2011) 66:225.
38. Riggioni C, Ricci C, Moya B, Wong D, van Goor E, Bartha I, et al. Systematic review and meta-analyses on the accuracy of diagnostic tests for IgE-mediated food allergy. Allergy: Eur J Allergy Clin Immunol. (2024) 79:324–52. doi: 10.1111/all.15939
39. Tsabouri S, Douros K, and Priftis KN. Cow’s milk allergenicity. Endocr Metab Immune Disord Drug Targets. (2014) 14:16–26. doi: 10.2174/1871530314666140121144224
40. Savilahti EM, Rantanen V, Lin JS, Karinen S, Saarinen KM, Goldis M, et al. Early recovery from cow’s milk allergy is associated with decreasing IgE and increasing IgG4 binding to cow’s milk epitopes. J Allergy Clin Immunol. (2010) 125:1315–21.e9. doi: 10.1016/j.jaci.2010.03.025
41. Anagnostou A, Mack DP, Johannes S, Shaker M, Abrams EM, DeSanto K, et al. The safety and efficacy of baked egg and milk dietary advancement therapy: A systematic review and meta-analysis. J Allergy Clin Immunol Pract. (2024) 12:2468–80. doi: 10.1016/j.jaip.2024.06.016
42. Bognanni A, Chu DK, Firmino RT, Arasi S, Waffenschmidt S, Agarwal A, et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk&xa0;Allergy (DRACMA) guideline update &x2013; XIII &x2013; Oral immunotherapy for CMA &x2013; Systematic review. World Allergy Organ J. (2022) 15. doi: 10.1016/j.waojou.2022.100682
43. Takahashi M, Taniuchi S, Soejima K, Hatano Y, Yamanouchi S, and Kaneko K. Two-weeks-sustained unresponsiveness by oral immunotherapy using microwave heated cow’s milk for children with cow’s milk allergy. Allergy Asthma Clin Immunol. (2016) 12:44. doi: 10.1186/s13223-016-0150-0
Keywords: oral immunotherapy, cow’s milk allergy, children, desensitization, adverse reaction
Citation: Wang Y, Liu S, Lu M, Guo J, Lv C and Huang L (2025) Oral immunotherapy for cow’s milk allergy in children: a systematic review and meta-analysis. Front. Immunol. 16:1570050. doi: 10.3389/fimmu.2025.1570050
Received: 02 February 2025; Accepted: 20 May 2025;
Published: 04 June 2025.
Edited by:
Annette Kuehn, Luxembourg Institute of Health, LuxembourgReviewed by:
Marit Zuurveld, Utrecht University, NetherlandsSait Karaman, Manisa City Hospital, Türkiye
Copyright © 2025 Wang, Liu, Lu, Guo, Lv and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lan Huang, aHVhbmdsYW4zMDFAMTI2LmNvbQ==
 Yan Wang1,2
Yan Wang1,2 Shunli Liu
Shunli Liu Meizhu Lu
Meizhu Lu Lan Huang
Lan Huang