- Department of Neurology, Houston Methodist Neurological Institute, Houston Methodist Research Institute, Houston Methodist Hospital, Houston, TX, United States
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by dopaminergic neuron loss in the substantia nigra, which is accompanied by immune dysfunction and chronic inflammation. Peripheral monocytes, key players in systemic inflammation, cross the blood-brain barrier and alter PD etiology and progression. To define the role of peripheral monocytes, cross-sectional studies of RNA transcripts isolated from PD monocytes were compared with age- and sex-matched control monocytes. After stratification by Hoehn & Yahr (H&Y) stage, inflammatory transcripts IL-6, IL-1β, ARG1, CD163, and CCR2 were upregulated in PD monocytes and increased with disease burden. Furthermore, PPARGC1A (PGC-1α), GPX4, NFE2L2 (NRF2), and SIRT3 decreased with increasing disease burden, while only SIRT1 expression increased, reflecting oxidative stress and mitochondrial dysregulation. Overall, the PD monocyte transcripts correlated with PD disease burden as monitored by H&Y, UPDRS total, UPDRS Part 3, ADL, and disease duration. This study demonstrated that dysregulation of inflammation and oxidative stress pathways contributed to disease progression in PD. Monocytes may serve as biomarkers for tracking clinical symptoms and could be leveraged as targets for therapeutic intervention.
Introduction
Parkinson’s disease (PD) is one of the most prevalent neurodegenerative disorders, marked by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc). This degeneration leads to motor symptoms such as tremors, rigidity, and bradykinesia, as well as non-motor symptoms, including cognitive impairment, autonomic dysfunction, and psychiatric disturbances (1–3). Clinical and preclinical studies of PD indicate that immune system dysfunction actively contributes to disease progression (4–10). The full impact of these dysfunctional immune constituents on the central nervous system (CNS) in PD remains unclear.
The interplay between these immune pathways culminates in neuroinflammation that can drive neurodegeneration. Imaging studies have documented early microglial activation in the SNpc, which is hypothesized to precede significant dopaminergic neuronal loss (11). The early neuroinflammatory response of microglia, marked by the release of cytokines such as interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor (TNF), along with the subsequent generation of reactive oxygen species (ROS), creates a toxic microenvironment (6, 12–18). Additionally, reactive astrocyte activation in response to inflammatory cytokines shifts astrocytes into a neurotoxic A1 phenotype (18, 19).
Peripheral immune dysfunction, characterized by altered cytokine levels and dysregulated immune cell function, plays a significant role in PD pathogenesis. This dysfunction is evident in monocytes, T cells, and neutrophils, which exhibit abnormal activity and contribute to elevated serum pro-inflammatory cytokine levels (6, 20, 21). CD4+ T cell subsets are dysregulated in PD, with decreases in Th2, Th17, and Treg cells, and an increase in the pro-inflammatory Th1 subset (6, 18, 20, 22–28). More importantly, Treg populations are reduced in PD patients, showing decreased expression of IL2RA/CD25 and FOXP3, along with impaired suppressive function compared to controls. The extent of this Treg dysfunction correlates with the Hoehn & Yahr (H&Y) disease stage (25). Furthermore, major histocompatibility complex class II (MHCII) expression, particularly HLA-DR, is elevated on microglia in neurodegenerative brain regions and increased on peripheral monocytes. This upregulation, along with the presentation of α-synuclein-specific peptides via MHCII, contributes to T cell activation and neuroinflammation (8, 25, 29, 30).
Monocytes play a significant role in peripheral and central inflammation and have been implicated in the neurodegeneration process in PD. Circulating monocyte populations exhibit heightened pro-inflammatory responses to inflammatory stimuli in vitro, producing higher levels of IL-1β, IL-6, and TNF (25, 31–34). Genetic studies have linked multiple PD-associated risk loci to monocyte activation, including variants in the human leukocyte antigen (HLA) system, LRRK2, and GBA1 (35, 36). Additionally, transcriptomic analyses of peripheral monocytes from PD patients have revealed dysregulated gene expression patterns (31, 37). In preclinical PD models, modulating monocyte activation or blocking their recruitment into the CNS reduces neuroinflammation and confers neuroprotection (38, 39).
As peripheral monocytes serve as a critical link between systemic inflammation and neurodegeneration, investigating their phenotypic and transcriptional changes in PD patients across disease progression is warranted. Understanding the role of monocytes in PD pathogenesis may facilitate the identification of biomarkers and the development of therapeutic strategies aimed at modulating neuroinflammation.
Methods
Recruitment of patients with PD and controls
Patients with PD and age-matched healthy controls were recruited to the study by Dr. Eugene C. Lai at the Houston Methodist Neurological Institute Neurodegenerative Disease Clinic. Written informed consent was obtained from all participants in accordance with a protocol approved by the Houston Methodist Institutional Review Board (IRB ID: PRO00026718), with initial approval granted on July 29, 2020. This protocol has been reviewed annually to ensure continued compliance with institutional and federal research regulations. All PD patients were evaluated using the Movement Disorder Society clinical diagnostic criteria for PD. Disease burden was assessed using validated clinical scales, including the Hoehn and Yahr (H&Y) staging scale, the Unified Parkinson’s Disease Rating Scale (UPDRS), and the Activities of Daily Living (ADL) scale. At the time of blood collection, all PD patients were undergoing standard-of-care dopaminergic therapy. No patients were drug-naïve or required to withhold treatment for the purposes of this study. This approach reflects the real-world clinical heterogeneity of a chronically treated PD population. A total of 62 PD patients (average age 73.3 ± 7.76 years) and 16 age-matched controls (71.9 ± 7.49 years) were included in the study. Participant demographics and clinical characteristics are further detailed in Supplementary Table 1.
Pan monocyte cell isolations
Following peripheral blood collection from PD patients and healthy controls, peripheral blood mononuclear cells (PBMCs) were isolated using a Lymphoprep (Stemcell) density gradient, followed by indirect magnetic isolation of pan-monocytes using the Pan Monocyte Isolation Kit (Miltenyi Biotec), which enriches for classical (CD14++CD16−), non-classical (CD14+CD16++), and intermediate (CD14++CD16+) monocytes.
RNA purification and RT-PCR analysis
Following pan-monocyte isolation, RNA was extracted using TRIzol reagent, followed by purification with the Direct-zol RNA MiniPrep Plus Kit (Zymo Research). RNA concentration and purity were assessed using a NanoDrop spectrophotometer. Quantitative RT-PCR (qRT-PCR) was performed using a one-step SYBR Green-based RT-PCR kit (Bio-Rad) on a Bio-Rad iQ5 Multicolor Real-Time PCR Detection System. All primers used in this study were commercially validated PrimePCR SYBR Green Assays obtained from Bio-Rad. Primer sequences, assay IDs, and links to validation data, including melt curve profiles and amplification efficiency, are provided in Supplementary Table 4 to facilitate transparency and replication of results. Relative gene expression was calculated using the 2^ΔΔCt method, with normalization to beta-actin (ACTB) as the reference gene. Fold change values were calculated relative to healthy controls and transformed into log2 fold change (log2FC). Log2FC values and standard errors of the mean for all analyzed transcripts are reported in Supplementary Table 2.
Statistics and analysis
Statistical analyses were performed using GraphPad Prism software. Transcript log2FC data were visualized using box plots and analyzed using Welch’s t-test with Holm-Sidak p-value correction for comparisons between two groups. Analysis of multiple groups was conducted using one-way ANOVA with Sidak’s multiple comparisons test. *Data are presented as mean ± SEM, and p-values were reported following New England Journal of Medicine conventions (p < 0.05, p < 0.01, p < 0.001). Correlations were analyzed using simple linear regression modeling, and Spearman’s correlation coefficient was calculated with corresponding p-values for each correlation measure.
Results
Differential expression of inflammatory and immunoregulatory transcripts in peripheral blood monocytes isolated from patients with PD
RNA transcripts involved in inflammation and immunoregulatory pathways were analyzed in peripheral blood monocytes from patients with PD and age-matched controls. These transcripts were then analyzed relative to H&Y stages of disease. Upregulation of the pro-inflammatory cytokine IL-6 transcript was observed in PD monocytes compared to control monocytes (Figure 1A), and its expression increased with advancing H&Y stages of PD. Early-stage disease (H&Y 1–1.5) exhibited a modest upregulation of IL-6 compared to controls, while IL-6 RNA levels progressively increased in H&Y 2–2.5, H&Y 3, H&Y 4, and H&Y 5. The pro-inflammatory cytokine IL-1β transcript was also elevated in monocytes from patients with PD and increased with advancing disease (Figure 1B), while TNF transcripts displayed a moderate increase but did not change significantly with disease progression (Figure 1C). The chemokine receptor C-C chemokine receptor type 2 (CCR2), which facilitates monocyte migration to sites of inflammation, was upregulated in PD monocytes compared to controls (Figure 1D). Additionally, CCR2 expression was increased in early PD and continued to rise with advancing disease stages.
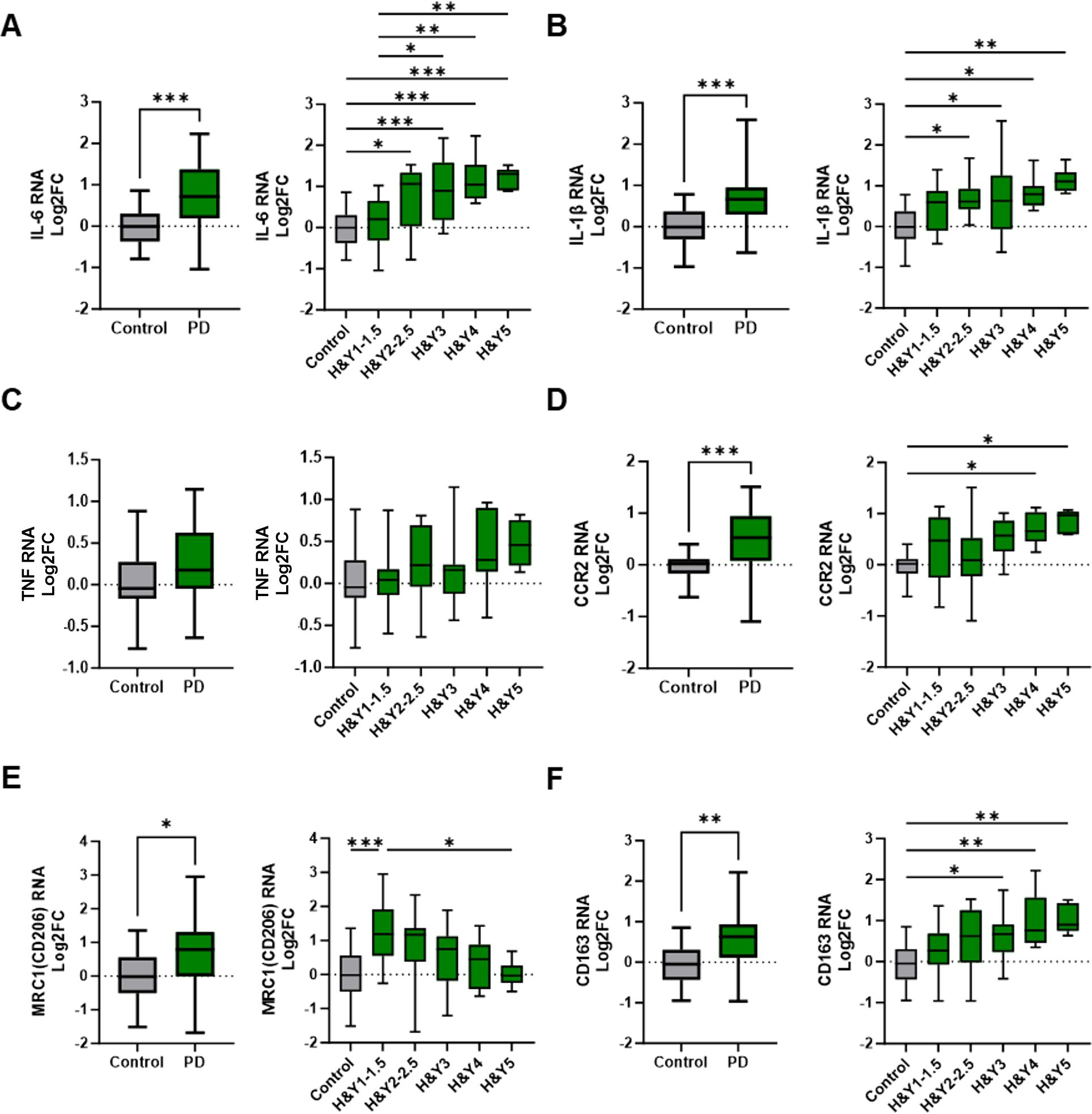
Figure 1. Differential expression of inflammatory and immunoregulatory transcripts in peripheral monocytes from PD patients and through disease progression. Peripheral pan monocytes were isolated from blood of PD patients and controls. (A–C) Increased IL-6 and IL-1β pro-inflammatory transcripts in PD monocytes and with increased H&Y stage scoring. (D) Cell migration and chemokine receptor CCR2 transcripts increased in PD monocytes. (E, F) Increased levels of immunoregulatory markers in PD monocytes compared to controls. Decreasing MRC1/CD206 transcripts with increased H&Y scoring while CD163 transcripts increase with advancing H&Y PD staging. Data shown in Figure 1 represent log2FC transcript values depicted via box plot summary statistics and analyzed using Welch’s t test with Holm-Sidak p-value correction (PD n=60-62; C n= 16). H&Y data analyzed using ordinary one-way ANOVA with Sidak’s multiple comparisons testing (C n=16; H&Y1-1.5 n=14-17; H&Y2-2.5 n=12-13; H&Y3 n=12-15; H&Y4 n=6-9; H&Y5 n=5-6). Statistical significance read as *p<0.05, **p<0.01, and ***p<0.001).
Transcripts associated with anti-inflammatory and immunoregulatory functions were also examined in monocytes isolated from patients with PD and healthy controls. Transcripts of the mannose receptor (MRC1/CD206), a marker of alternatively activated (M2) myeloid cells, were upregulated in early-stage PD monocytes but declined with disease progression, resulting in decreased expression in late-stage disease (H&Y 5) (Figure 1E). CD163, a scavenger receptor associated with immunoregulation and protection from oxidative stress, was increased in monocytes from PD patients. CD163 transcripts were low in early-stage PD but increased with disease progression (Figure 1F). Arginase 1 transcripts (ARG1) were elevated in PD monocytes relative to controls, but their expression varied across disease stages (data not shown).
Alterations in metabolic, oxidative stress, and mitochondrial regulatory transcripts in peripheral monocytes of PD patients
PD is directly associated with aberrant oxidative stress mechanisms, mitochondrial dysfunction, and metabolic dysregulation. Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PPARGC1A or PGC-1α), an important transcriptional activator, exhibited a slight but non-significant increase in early-stage PD monocytes. However, its transcript levels progressively declined as the disease advanced, with the most pronounced reductions occurring in H&Y 4 and H&Y 5 stages (Figure 2A). Glutathione peroxidase 4 (GPX4), an antioxidant enzyme implicated in PD, was elevated in PD monocytes compared to controls (Figure 2B). When stratified by H&Y stage, GPX4 expression increased early but declined in later stages of disease.
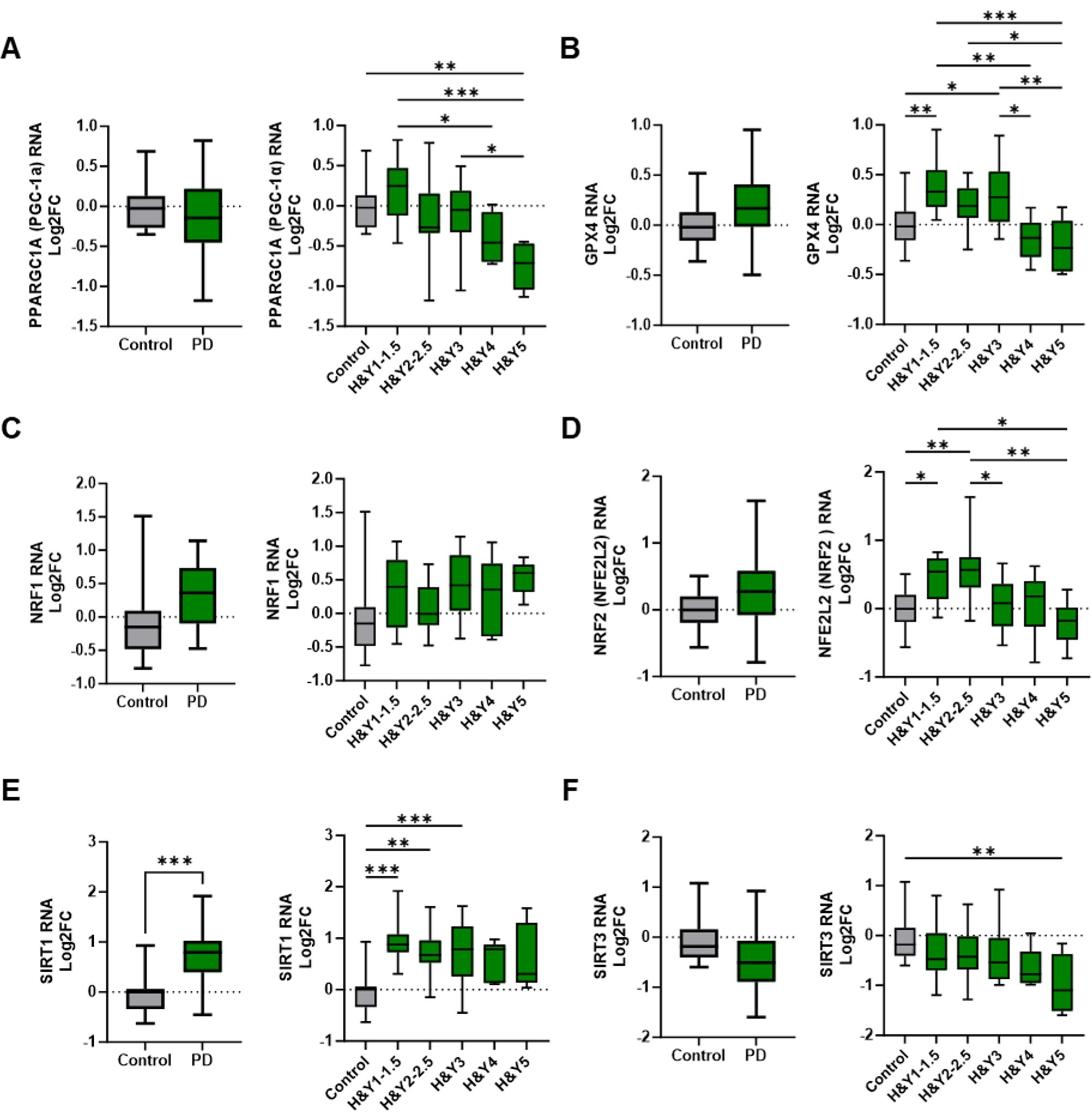
Figure 2. Alterations in metabolic, oxidative stress, and mitochondrial regulatory gene expression in peripheral monocytes of Parkinson’s disease patients. (A) Changes in PD monocyte PPARGC1A/PGC-1α gene transcripts compared with controls and through H&Y disease progression. (B) GPX4 transcripts in PD vs control monocytes that increased early in PD progression staging. (C, D) NRF1 and NRF2 transcripts in PD monocytes and through disease progression staging. Antioxidant NRF2 increased early in PD staging but significantly decreased in later stages. (E) Increased SIRT1 expression in PD monocytes with elevations early in PD progression and remaining elevated through intermediate disease. (F) SIRT3 RNA is slightly decreased early in PD with a significant drop in late stage of disease. Data shown in Figure 2 represent log2FC transcript values depicted via box plot summary statistics and analyzed using Welch’s t test with Holm-Sidak p-value correction for PD vs Control analysis (PD n=60-62; C n= 16). Ordinary one-way ANOVA with Sidak’s multiple comparisons testing utilized for H&Y staging of disease analysis (C n=16; H&Y1-1.5 n=14-17; H&Y2-2.5 n=12-13; H&Y3 n=12-15; H&Y4 n=6-9; H&Y5 n=5-6). Statistical significance read as *p<0.05, **p<0.01, and ***p<0.001).
Nuclear respiratory factor 1 (NRF1) and nuclear factor erythroid 2–related factor 2 (NFE2L2/NRF2) regulate oxidative stress and mitochondrial function, with impairments linked to mitochondrial dysfunction, oxidative damage, and inflammation. While NRF1 transcripts were increased in early-stage PD, overall NRF1 expression did not significantly differ in PD monocytes compared to controls (Figure 2C). NRF2 transcripts initially increased at H&Y 1 and H&Y 2–2.5 but declined in stages H&Y 3 through 5 (Figure 2D).
Sirtuin 1 (SIRT1) and Sirtuin 3 (SIRT3) are NAD+-dependent deacetylases with critical roles in oxidative stress responses, mitochondrial regulation, and inflammation. SIRT1 transcripts were upregulated in PD monocytes relative to controls; expression increased during early and intermediate disease stages but declined with disease progression (Figure 2E). Conversely, SIRT3 transcripts were reduced in PD monocytes, with early stage decreases that became more pronounced as disease advanced (Figure 2F).
Correlations between monocyte gene expression and clinical parameters in PD patients
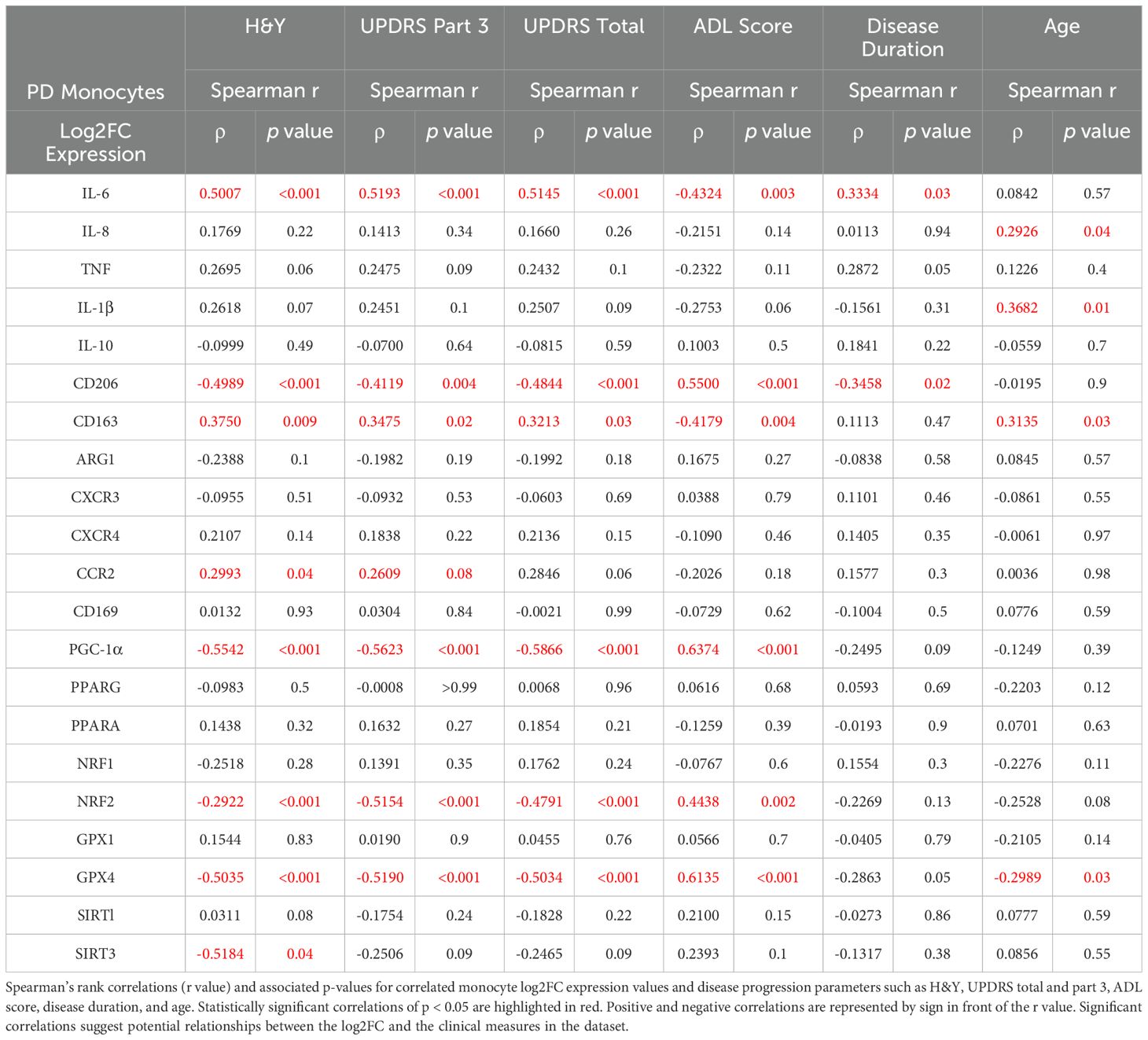
Spearman correlations of monocyte transcripts from patients with PD were assessed using multiple clinical measures of disease burden, including H&Y scoring, the Unified Parkinson’s Disease Rating Scale (UPDRS), the Parkinson’s Disease Activities of Daily Living Scale (ADL), as well as measures of disease duration and age (Figure 3, Table 1). IL-6 transcripts correlated with H&Y progression (ρ= 0.5007, p<0.001), UPDRS Part 3 scoring (ρ= 0.5193, p<0.001), UPDRS total scoring (ρ= 0.5145, p<0.001), and the ADL scoring (ρ= -0.4324, p=0.003) (Table 1, Supplementary Figure 1A). With respect to anti-inflammatory and phagocytic transcripts, there were negative correlations for MRC1/CD206 transcripts including H&Y scale (ρ= -0.4989, p<0.001), UPDRS part 3 (ρ= -0.4119, p=0.004), UPDRS total scoring (ρ = -0.4844, p<0.001), and disease duration (ρ= -0.3458, p=0.02) (Table 1, Figure 1B). Conversely, CD163 transcripts had positive correlations with H&Y (ρ= 0.3750, p=0.009), UPDRS part 3 (ρ= 0.3475, p=0.02), and UPDRS total scoring (ρ =0.3213, p=0.03) (Table 1, Supplementary Figure 1C).
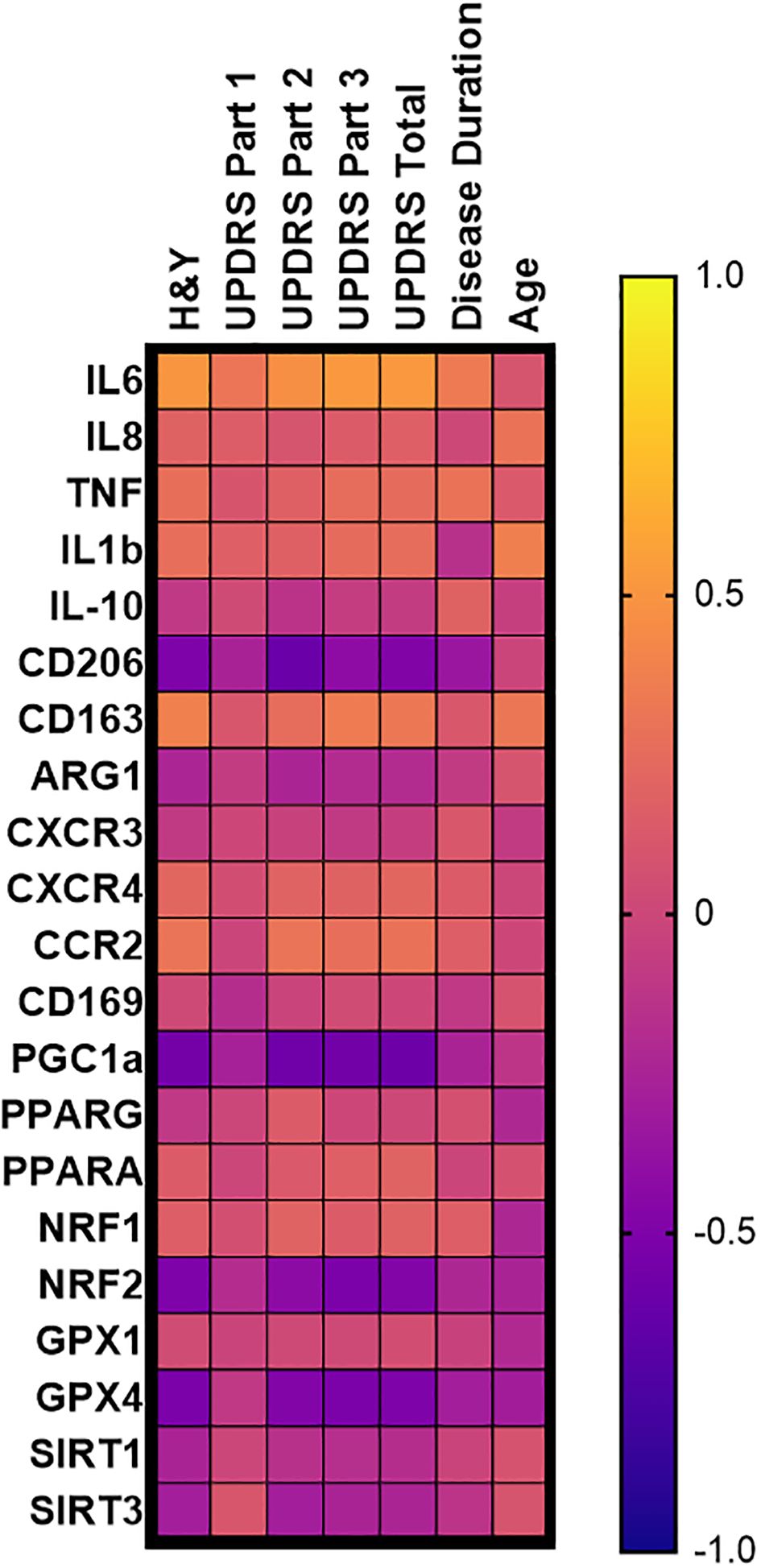
Figure 3. PD monocyte transcript correlation matrix. Correlation matrix of PD monocyte inflammatory and oxidative stress transcripts as a function of common disease burden assessments done in PD patients. Spearman r correlation values calculated using PD monocyte log2FC expression against patient testing parameters of H&Y, UPDRS, disease duration, and age of the patient. Exact correlation values and significance shown in Table 1.
The oxidative stress PPARGC1A/PGC-1α transcripts were negatively correlated for H&Y (ρ= -0.5542, p<0.001), UPDRS part 3 and total (ρ = -0.5623, p<0.001; ρ= -0.5866, p<0.001), and ADL scoring (ρ= 0.6374, p<0.001) (Table 1, Supplementary Figure 2A). There was no correlation for disease duration (ρ= -0.2495; p=0.09). As for NFE2L2/NRF2 antioxidant transcripts, similar negative correlations were found with H&Y (ρ= -0.2922, p<0.001), UPDRS part 3 (ρ= -0.5154, p<0.001), UPDRS total score (ρ= -0.4791, p<0.001), and ADL score (ρ= 0.4438, p=0.002) (Table 1, Supplementary Figure 2B). A similar observation was found for GPX4 transcripts with H&Y (ρ = -0.5035, p<0.001), UPDRS part 3 (ρ = -0.5190, p<0.001), UPDRS total score (ρ= -0.5034, p<0.001), and ADL score (ρ = 0.6135, p<0.001) (Table 1, Supplementary Figure 2C). PPARGC1A/PGC-1α, NFE2L2/NRF2, and GPX4 did not correlate with disease duration (Table 1).
Most transcripts did not correlate with patient age. Specifically, IL-6 and MRC1/CD206 had correlations with disease duration, but they did not correlate with patients’ age. PPARGC1A/PGC-1α and NFE2L2/NRF2 had correlated with disease progression, but there was no correlation with age. The monocyte transcripts that did significantly correlate with age were CD163 (ρ = 0.3135, p=0.03) and GPX4 (ρ= -0.2989, p=0.03) while the cytokine transcript IL-1β (ρ= 0.3682, p=0.01) did correlate with patient age; however, it did not correlate with disease progression (Table 1). Stratifying the Log2FC and disease progression correlations into male and female outputs, it was revealed that females retained a stronger correlative inflammatory signature while both males and females retained strong oxidative stress and mitochondrial dysfunction monocyte correlations (Supplementary Table 3).
Discussion
Immune dysfunction and chronic inflammation contribute to PD pathogenesis and progression, yet identifying peripheral, non-invasive indicators of disease onset and progression remains a challenge. In this cross-sectional study, differential expression of inflammatory, immunoregulatory, and chemotactic receptor transcripts was observed in peripheral blood monocytes isolated from patients with PD, and age- and sex-matched controls. Specifically, there was an upregulation of IL-1β, IL-6, IL-10, MRC1/CD206, CD163, and CCR2 transcripts in PD monocytes. IL-1β, IL-6, CD163, and CCR2 transcripts increased with advancing disease while MRC1/CD206 transcripts were elevated early in disease but declined as disease advanced. Metabolic, oxidative stress, and mitochondrial transcript analysis showed an upregulation of SIRT1 in PD monocytes. Dysregulation of PPARGC1A/PGC-1α, GPX4, and NFE2L2/NRF2 transcripts was evident in disease burden analyses and was increased in early disease PD monocytes but declined in later stages; SIRT1 transcripts were elevated early and throughout disease staging, while SIRT3 declined later in disease. Finally, correlation analyses of monocyte transcripts across multiple PD progression parameters revealed significant correlations with respect to H&Y, UPDRS Part 3, UPDRS Total, and ADL staging and disease duration, but no correlation with the patients’ age.
There is now increased interest in peripheral blood monocytes as both a potential biomarker and a target for therapeutic intervention in PD. Gene expression studies in PD patients have demonstrated that PD-associated risk genes are highly expressed in monocytes, leading to downstream immune-altering effects. Previous studies have reported altered numbers and shifts in peripheral blood monocyte populations, along with upregulated surface expression of HLA-DR. These findings are consistent with a shift toward more pro-inflammatory monocyte subsets (25, 34). These alterations were also shown to be increased with respect to increasing disease burden. When isolated and stimulated ex vivo, PD monocytes exhibit a hyperactive, pro-inflammatory response, producing higher levels of pro-inflammatory cytokines than monocytes from healthy controls (33). In preclinical PD models, peripheral monocyte infiltration into the CNS is a critical step in α-synuclein-mediated neuroinflammation and subsequent neurodegeneration (38, 39). Their entry is thought to exacerbate inflammation, which is toxic to neurons, and blocking their migration into the CNS has been shown to be neuroprotective in these models.
The observed upregulation of pro-inflammatory cytokine transcripts IL-1β, IL-6, and TNF aligns with previous reports documenting the upregulation of these cytokines in the peripheral blood of patients with PD (20, 21). The progressive rise in IL-1β and IL-6 transcripts with increasing disease burden supports the notion that chronic and exacerbating pro-inflammatory signaling occurs in PD. This may serve as a proxy indicator of the toxic microenvironment in the CNS of these patients.
The upregulation of anti-inflammatory transcripts such as ARG1, MRC1/CD206, and CD163 in isolated PD monocytes suggests a potential compensatory response to disease, particularly in early stages. MRC1/CD206 is predominantly expressed on the surface of activated myeloid cells and plays a critical role in the immune response by both enhancing phagocytosis and modulating immune responses to an alternative anti-inflammatory/resolution state (40). CD163 is increased on myeloid cells near neurodegenerative pathology, including PD, while soluble CD163 correlates with exacerbation of inflammation and worsening disease outcomes in neurodegenerative, inflammatory, and autoimmune diseases. Interestingly, CD163 expression on isolated PD monocytes remains steady in early stages of disease and increases with advanced disease burden. This paradoxical effect of CD206 and CD163 may signal dynamic shifts in monocyte activation states during the course of disease. CD206 is associated with an anti-inflammatory phenotype, and the increasing levels of this transcript may reflect an initial anti-inflammatory response aimed at counteracting early neuroinflammation. CD163, also linked to anti-inflammatory phenotypes, does not increase until late stage of disease, and this late response may indicate compensatory mechanisms mitigating oxidative stress and pro-inflammatory responses (40–42). Our finding of increased CD163 transcripts in PD monocytes with advancing disease may reflect a compensatory upregulation in response to enhanced proteolytic shedding of membrane-bound CD163 into the circulation. This mechanism may serve to replenish surface expression amid ongoing monocyte activation. Prior studies have demonstrated that soluble CD163 (sCD163) levels rise progressively with PD severity and correlate with markers of neuroinflammation and neurodegeneration, further supporting the role of CD163 as a dynamic indicator of monocyte activation and disease progression (43).
Additionally, CCR2 expression on isolated peripheral blood PD monocytes was most pronounced in advanced stages of disease. Increased CCR2 expression is associated with enhanced migratory capacity of monocytes to the CNS. CCR2 ligands are elevated in patients with PD and lead to enhanced migration and infiltration of pro-inflammatory monocytes to the CNS which may contribute to toxic neuroinflammation with subsequent neurodegeneration (44–48). Blocking CCR2 recruitment pathways to the CNS reduces monocyte infiltration to the CNS, abrogates neuroinflammation, and prevents neurodegeneration (39).
PGC-1α is a master regulator of mitochondrial biogenesis and antioxidant defenses, protecting myeloid cells from pro-inflammatory activation and promoting an anti-inflammatory, M2 phenotype (49–51). The current data demonstrated that PGC-1α transcripts were elevated early but declined with increased disease burden. PGC-1α is known to be decreased in CNS tissues from patients with PD and in preclinical models of disease which may drive mitochondrial dysfunction, increasing oxidative stress and pro-inflammation responses. Targeting PGC-1α in preclinical models of PD induces neuroprotective benefits, suggestive of a promising therapeutic intervention for PD (49, 50, 52).
Oxidative stress, mitochondrial dysfunction, and lipid peroxidation are documented driving forces in PD pathogenesis and progression (53–56). Examination of these pathways in myeloid cells is limited, but monocytes have been shown to enter and directly contribute to CNS pathology (46–48). Myeloid cells are early and preferentially vulnerable to oxidative and ferroptotic stress, and may serve as a proxy for the CNS microenvironment while sampled from the blood (57). Of significance is the current demonstration that GPX4 and NRF2 transcripts were increased in early disease but then declined at later stages. GPX4 is an essential enzyme that protects cells from lipid peroxidation, ferroptosis, and subsequent cell death. A functional loss of GPX4 in late-stage PD may exacerbate lipid peroxidation and ferroptotic stress (58, 59). Early increases in NRF2 transcripts may be indicative of an adaptive response to early oxidative stress, but its decline may signal a compromised antioxidant defense system (60).
Sirtuin family transcripts are also altered in isolated PD monocytes. SIRT1 is increased early in PD, but its expression is lost by mid to late-stage disease resulting in loss of its anti-inflammatory effects on NF-kB signaling and autophagy. SIRT3, another sirtuin deacetylase known to mitigate mitochondrial dysfunction and promote antioxidant activity, is modestly decreased early, and is further decreased in the late-stage disease. Transcriptional changes in sirtuins have been reported to contribute to PD pathogenesis through their roles in inflammation regulation, oxidative stress mitigation, and mitochondrial enhancement/maintenance (61–63). SIRT1 and SIRT3 dysregulation have been primarily studied in dopaminergic neurons, but their altered expression in peripheral monocytes suggests their potential as biomarkers and therapeutic targets.
Patient sex differences are a biological variable that affects the immune system, whether that be its function or potential dysfunction in disease-associated patient monocytes (64–66). The current study had a relatively equal male-to-female ratio. When stratifying the correlation parameters based on sex, male monocytes lost some of the strength in the inflammatory correlations (Supplementary Table 2). Conversely, females retained and strengthened their correlative Spearman r value with respect to the inflammatory monocyte transcripts. In a different study examining peripheral immune cells in PD, female monocytes demonstrated greater inflammatory activation, while the male monocyte activation patterns were more heterogeneous (67). The predominance of males over females in a PD diagnosis, along with the heterogeneity of myeloid cell activation states in the males, may complicate the tracking of peripheral myeloid inflammatory changes in patients with PD. The observed sex-specific transcriptional correlations likely reflect fundamental immunological dimorphisms, including the influence of sex hormones, differential regulation of immune signaling pathways, and X-linked gene expression. These dimorphisms have been reported in both health and disease, with female immune cells often exhibiting heightened inflammatory responsiveness and more robust transcriptional activity (68, 69). Clinically, such differences may contribute to sex-based variability in PD disease presentation or trajectory, as males are more commonly diagnosed, yet some studies suggest females may experience more rapid cognitive and functional decline (70). These findings underscore the importance of considering sex as a biological variable in PD immune biomarker research. Interestingly, the oxidative stress and mitochondrial transcript signatures remained significant across both sexes, supporting the hypothesis that mitochondrial dysfunction in PD monocytes represents a sex-independent and potentially CNS-relevant marker of disease. Future studies are warranted to further define these sex-specific immune alterations through monocyte subset stratification, transcriptomic and epigenetic profiling, and integration with clinical phenotyping to clarify their mechanistic and therapeutic relevance in PD.
These data generate a hypothesis that pro-inflammatory mechanisms and oxidative stress generate a toxic microenvironment that can inhibit anti-inflammatory mechanisms, cause cellular dysfunction of tolerogenic immune cells such as Tregs, and even drive polarization of anti-inflammatory cells to pro-inflammatory responses. With myeloid cells being the main driver of pro-inflammatory mechanisms in the brain and periphery, targeting these cells and enhancing Treg function would be a promising strategy to overcome immune dysfunction.
The current study has several limitations. First, the number of healthy controls was smaller than the PD cohort, which may reduce statistical power for some comparisons; however, the groups were age- and sex-matched, and statistical methods were used to mitigate the effects of unequal sample sizes and variances. Future longitudinal studies and expanded recruitment through Parkinson’s disease consortia will improve group matching and enable more robust analysis of clinical trajectory and immune changes. Second, the use of a pan-monocyte isolation approach effectively captures total monocyte transcriptional responses but precludes analysis of subset-specific changes in classical, intermediate, and/or non-classical monocytes. Future studies incorporating subset-specific sorting and/or single-cell RNA profiling will be important for refining these findings and correlations. Third, all PD patients were on dopaminergic therapy at the time of sampling. While these therapies are not thought to substantially alter adaptive T cell polarization, their effects on peripheral monocytes are less well understood. Although we did not assess dopamine receptor expression on monocytes, prior studies have shown dopaminergic modulation of T cell receptor profiles during PD progression, and in vitro data suggest dopamine can alter monocyte chemotaxis and activation (71, 72). The transcriptomic changes reported here occurred despite ongoing therapy, suggesting persistent monocyte dysregulation. Preclinical models support a role for dopaminergic signaling in promoting myeloid cell trafficking into the CNS and inflammation, and blocking monocyte recruitment in PD models, especially CCR2+ subsets, has shown neuroprotective effects (39, 73–75). These findings support a role for CCR2+ monocytes in PD-related neuroinflammation and highlight the need for future longitudinal studies to track dopaminergic therapy initiation, dosage, and regimen changes to clarify its influence on monocyte phenotypes and their association with disease progression. Lastly, although this cross-sectional study identified correlations between monocyte transcripts and clinical parameters, causality cannot be inferred, and further mechanistic studies are required to determine how these immune changes contribute to PD pathophysiology.
Overall, these data highlight a dysregulation in peripheral monocytes in PD patients, evidenced by both pro-inflammatory transcript activation and dysregulation of oxidative stress and mitochondrial dysfunction transcripts. This dysregulation is particularly striking when looking at the changes across different stages of disease burden; there is an early anti-inflammatory and antioxidant response in the monocytes followed by a more pro-inflammatory phenotype in late-stage disease. These data suggest the importance of myeloid cells in the pathogenesis of PD and the value of circulating cells to monitor inflammation and oxidative stress pathways. Peripheral monocytes may serve as viable biomarkers of both disease and progression, supporting the development of therapeutic agents that target and inhibit their pro-inflammatory activity.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Houston Methodist Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AT: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. JW: Data curation, Investigation, Methodology, Validation, Writing – review & editing. FA: Methodology, Project administration, Writing – review & editing. JT: Formal Analysis, Investigation, Writing – review & editing. AF: Data curation, Investigation, Writing – review & editing. WZ: Formal Analysis, Investigation, Writing – review & editing. DB: Formal Analysis, Investigation, Writing – review & editing. EL: Formal Analysis, Investigation, Project administration, Supervision, Writing – review & editing. SA: Conceptualization, Funding acquisition, Investigation, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Research supported by a sponsored research agreement in place with Houston Methodist Hospital Research Institute and Coya Therapeutics, Inc. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Acknowledgments
We sincerely thank our Parkinson’s patients, their families, and caregivers for their time and dedication to advancing research and therapy development. We also appreciate the contributions of the Stanley H. Appel Department of Neurology, Dr. Eugene Lai and his team, and Houston Methodist Hospital to this study.
Conflict of interest
AT is a consultant for Coya Therapeutics, Inc. JT transitioned employment from Houston Methodist to Director of Clinical Development at Coya Therapeutics, Inc. during preparation of the manuscript. AF is a consultant for Coya Therapeutics, Inc. SHA is Chair of the Coya Scientific Advisory Committee. Coya Therapeutics, Inc. has licensed research from Houston Methodist Research Institute.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1571074/full#supplementary-material
Supplementary Figure 1 | PD monocyte inflammatory transcripts correlate with different PD progression parameters. To evaluate correlations with different PD progression parameters, we plotted data and applied simple linear regression modeling to visual trend. Spearman’s correlation coefficient (r) with corresponding p value depicted in each graph with respect to H&Y progression staging, UPDRS scoring, and disease duration via years. Examination of previously significant inflammatory or immunoregulatory transcripts of (A) IL-6, (B) MRC1/CD206, and (C) CD163.
Supplementary Figure 2 | PD monocyte oxidative stress transcripts correlate with PD progression parameters. Evaluation of correlations with different PD progression parameters were plotted with applied simple linear regression modeling done to visual trend data. Spearman’s correlation coefficient (r) with corresponding p value depicted in each graph with respect to H&Y progression staging, UPDRS scoring, and disease duration via years. Examination of previously significant inflammatory or immunoregulatory transcripts of (A) PGC-1a, (B) NRF2, and (C) GPX4.
Supplementary Table 1 | Demographics and Clinical Characteristics of PD Patients Demographic summary table of age, sex, and disease progression of PD patients recruited to the study. Demographics detailed as PD cohort as a whole and separated into male vs female. Disease progression stratified by Hoehn and Yahr (H&Y) scale. Age represented as mean ± standard deviation (SD) and disease duration reported as average years.
Supplementary Table 2 | Log2FC values for PD monocytes and H&Y Log2FC values. Summary table of Log2FC averages and standard error of the mean (SEM) of monocyte gene expression levels across different stages of PD via H&Y scale.
Supplementary Table 3 | Patient sex stratified correlation table. Sex stratification of the correlation data. Spearman’s rank correlations (r value) and associated p-values for correlated monocyte log2FC expression values and disease progression parameters such as H&Y, UPDRS total and part 3, ADL score, disease duration, and age. Statistically significant correlations of p < 0.05 are highlighted in red. Positive and negative correlations are represented by sign in front of the r value. Significant correlations suggest potential relationships between the log2FC and the clinical measures in the dataset.
Supplementary Table 4 | Primer information for transcript analysis. Table describes commercially validated PrimePCR SYBR Green Assays (Bio-Rad) used for quantitative RT-PCR analysis of monocyte RNA transcripts. For each target gene, the table provides the gene symbol, full gene name, Bio-Rad unique assay ID, and link to online validation (sequences, melt curves, performance metrics, etc.). These primers were used to quantify gene expression changes across patient and control samples in this study.
References
1. Armstrong MJ and Okun MS. Diagnosis and treatment of parkinson disease: A review. JAMA. (2020) 323:548–60. doi: 10.1001/jama.2019.22360
2. Dehay B, Bourdenx M, Gorry P, Przedborski S, Vila M, Hunot S, et al. Targeting alpha-synuclein for treatment of Parkinson's disease: mechanistic and therapeutic considerations. Lancet Neurol. (2015) 14:855–66. doi: 10.1016/S1474-4422(15)00006-X
3. Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. (2001) 2:492–501. doi: 10.1038/35081564
4. Appel SH. Inflammation in Parkinson's disease: cause or consequence? Mov Disord. (2012) 27:1075–7. doi: 10.1002/mds.25111
5. Fathi M, Vakili K, Yaghoobpoor S, Qadirifard MS, Kosari M, Naghsh N, et al. Pre-clinical studies identifying molecular pathways of neuroinflammation in parkinson's disease: A systematic review. Front Aging Neurosci. (2022) 14:855776. doi: 10.3389/fnagi.2022.855776
6. Harms AS, Yang YT, and Tansey MG. Central and peripheral innate and adaptive immunity in Parkinson's disease. Sci Transl Med. (2023) 15:eadk3225. doi: 10.1126/scitranslmed.adk3225
7. Hirsch EC and Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol. (2009) 8:382–97. doi: 10.1016/S1474-4422(09)70062-6
8. McGeer PL, Itagaki S, Boyes BE, and McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. (1988) 38:1285–91. doi: 10.1212/WNL.38.8.1285
9. Samant RR, Standaert DG, and Harms AS. The emerging role of disease-associated microglia in Parkinson's disease. Front Cell Neurosci. (2024) 18:1476461. doi: 10.3389/fncel.2024.1476461
10. Schonhoff AM, Williams GP, Wallen ZD, Standaert DG, and Harms AS. Innate and adaptive immune responses in Parkinson's disease. Prog Brain Res. (2020) 252:169–216. doi: 10.1016/bs.pbr.2019.10.006
11. Yacoubian TA, Fang YD, Gerstenecker A, Amara A, Stover N, Ruffrage L, et al. Brain and systemic inflammation in de novo parkinson's disease. Mov Disord. (2023) 38:743–54. doi: 10.1002/mds.29363
12. Ho MS. Microglia in parkinson's disease. Adv Exp Med Biol. (2019) 1175:335–53. doi: 10.1007/978-981-13-9913-8_13
13. Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, et al. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett. (1994) 180:147–50. doi: 10.1016/0304-3940(94)90508-8
14. Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, and Nagatsu T. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. (1994) 165:208–10. doi: 10.1016/0304-3940(94)90746-3
15. Blesa J, Trigo-Damas I, Quiroga-Varela A, and Jackson-Lewis VR. Oxidative stress and Parkinson's disease. Front Neuroanat. (2015) 9:91. doi: 10.3389/fnana.2015.00091
16. Dias V, Junn E, and Mouradian MM. The role of oxidative stress in Parkinson's disease. J Parkinsons Dis. (2013) 3:461–91. doi: 10.3233/JPD-130230
17. Puspita L, Chung SY, and Shim JW. Oxidative stress and cellular pathologies in Parkinson's disease. Mol Brain. (2017) 10:53. doi: 10.1186/s13041-017-0340-9
18. Roodveldt C, Bernardino L, Oztop-Cakmak O, Dragic M, Fladmark KE, Ertan S, et al. The immune system in Parkinson's disease: what we know so far. Brain. (2024) 147:3306–24. doi: 10.1093/brain/awae177
19. Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. (2017) 541:481–7. doi: 10.1038/nature21029
20. Qin XY, Zhang SP, Cao C, Loh YP, and Cheng Y. Aberrations in peripheral inflammatory cytokine levels in parkinson disease: A systematic review and meta-analysis. JAMA Neurol. (2016) 73:1316–24. doi: 10.1001/jamaneurol.2016.2742
21. Reale M, Iarlori C, Thomas A, Gambi D, Perfetti B, Di Nicola M, et al. Peripheral cytokines profile in Parkinson's disease. Brain Behav Immun. (2009) 23:55–63. doi: 10.1016/j.bbi.2008.07.003
22. Garretti F, Agalliu D, Lindestam Arlehamn CS, Sette A, and Sulzer D. Autoimmunity in parkinson's disease: the role of alpha-synuclein-specific T cells. Front Immunol. (2019) 10:303. doi: 10.3389/fimmu.2019.00303
23. Bhatia D, Grozdanov V, Ruf WP, Kassubek J, Ludolph AC, Weishaupt JH, et al. T-cell dysregulation is associated with disease severity in Parkinson's Disease. J Neuroinflammation. (2021) 18:250. doi: 10.1186/s12974-021-02296-8
24. Sun C, Zhao Z, Yu W, Mo M, Song C, Si Y, et al. Abnormal subpopulations of peripheral blood lymphocytes are involved in Parkinson's disease. Ann Transl Med. (2019) 7:637. doi: 10.21037/atm.2019.10.105
25. Thome AD, Atassi F, Wang J, Faridar A, Zhao W, Thonhoff JR, et al. Ex vivo expansion of dysfunctional regulatory T lymphocytes restores suppressive function in Parkinson's disease. NPJ Parkinsons Dis. (2021) 7:41. doi: 10.1038/s41531-021-00188-5
26. He Y, Peng K, Li R, Zhang Z, Pan L, Zhang T, et al. Changes of T lymphocyte subpopulations and their roles in predicting the risk of Parkinson's disease. J Neurol. (2022) 269:5368–81. doi: 10.1007/s00415-022-11190-z
27. Stevens CH, Rowe D, Morel-Kopp MC, Orr C, Russell T, Ranola M, et al. Reduced T helper and B lymphocytes in Parkinson's disease. J Neuroimmunol. (2012) 252:95–9. doi: 10.1016/j.jneuroim.2012.07.015
28. Yan Z, Yang W, Wei H, Dean MN, Standaert DG, Cutter GR, et al. Dysregulation of the adaptive immune system in patients with early-stage parkinson disease. Neurol Neuroimmunol Neuroinflamm. (2021) 8. doi: 10.1212/NXI.0000000000001036
29. Fiszer U, Mix E, Fredrikson S, Kostulas V, and Link H. Parkinson's disease and immunological abnormalities: increase of HLA-DR expression on monocytes in cerebrospinal fluid and of CD45RO+ T cells in peripheral blood. Acta Neurol Scand. (1994) 90:160–6. doi: 10.1111/j.1600-0404.1994.tb02699.x
30. Tansey MG and Romero-Ramos M. Immune system responses in Parkinson's disease: Early and dynamic. Eur J Neurosci. (2019) 49:364–83. doi: 10.1111/ejn.2019.49.issue-3
31. Schlachetzki JCM, Prots I, Tao J, Chun HB, Saijo K, Gosselin D, et al. A monocyte gene expression signature in the early clinical course of Parkinson's disease. Sci Rep. (2018) 8:10757. doi: 10.1038/s41598-018-28986-7
32. Nissen SK, Shrivastava K, Schulte C, Otzen DE, Goldeck D, Berg D, et al. Alterations in blood monocyte functions in parkinson's disease. Mov Disord. (2019) 34:1711–21. doi: 10.1002/mds.v34.11
33. Grozdanov V, Bliederhaeuser C, Ruf WP, Roth V, Fundel-Clemens K, Zondler L, et al. Inflammatory dysregulation of blood monocytes in Parkinson's disease patients. Acta Neuropathol. (2014) 128:651–63. doi: 10.1007/s00401-014-1345-4
34. Su Y, Shi C, Wang T, Liu C, Yang J, Zhang S, et al. Dysregulation of peripheral monocytes and pro-inflammation of alpha-synuclein in Parkinson's disease. J Neurol. (2022) 269:6386–94. doi: 10.1007/s00415-022-11258-w
35. Strader S and West AB. The interplay between monocytes, alpha-synuclein and LRRK2 in Parkinson's disease. Biochem Soc Trans. (2023) 51:747–58. doi: 10.1042/BST20201091
36. Raj T, Rothamel K, Mostafavi S, Ye C, Lee MN, Replogle JM, et al. Polarization of the effects of autoimmune and neurodegenerative risk alleles in leukocytes. Science. (2014) 344:519–23. doi: 10.1126/science.1249547
37. Navarro E, Udine E, Lopes KP, Parks M, Riboldi G, Schilder BM, et al. Dysregulation of mitochondrial and proteolysosomal genes in Parkinson's disease myeloid cells. Nat Aging. (2021) 1:850–63. doi: 10.1038/s43587-021-00110-x
38. Harms AS, Cao S, Rowse AL, Thome AD, Li X, Mangieri LR, et al. MHCII is required for alpha-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J Neurosci. (2013) 33:9592–600. doi: 10.1523/JNEUROSCI.5610-12.2013
39. Harms AS, Thome AD, Yan Z, Schonhoff AM, Williams GP, Li X, et al. Peripheral monocyte entry is required for alpha-Synuclein induced inflammation and Neurodegeneration in a model of Parkinson disease. Exp Neurol. (2018) 300:179–87. doi: 10.1016/j.expneurol.2017.11.010
40. Roszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. (2015) 2015:816460. doi: 10.1155/2015/816460
41. Tan HY, Wang N, Li S, Hong M, Wang X, and Feng Y. The reactive oxygen species in macrophage polarization: reflecting its dual role in progression and treatment of human diseases. Oxid Med Cell Longev. (2016) 2016:2795090. doi: 10.1155/2016/2795090
42. Zhang Y, Choksi S, Chen K, Pobezinskaya Y, Linnoila I, and Liu ZG. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. (2013) 23:898–914. doi: 10.1038/cr.2013.75
43. Nissen SK, Ferreira SA, Nielsen MC, Schulte C, Shrivastava K, Hennig D, et al. Soluble CD163 changes indicate monocyte association with cognitive deficits in parkinson's disease. Mov Disord. (2021) 36:963–76. doi: 10.1002/mds.28424
44. Huerta C, Alvarez V, Mata IF, Coto E, Ribacoba R, Martinez C, et al. Chemokines (RANTES and MCP-1) and chemokine-receptors (CCR2 and CCR5) gene polymorphisms in Alzheimer's and Parkinson's disease. Neurosci Lett. (2004) 370:151–4. doi: 10.1016/j.neulet.2004.08.016
45. Jiao Y, Zhu X, Zhou X, Li Y, Zhou L, Zhao A, et al. Collaborative plasma biomarkers for Parkinson disease development and progression: A cross-sectional and longitudinal study. Eur J Neurol. (2023) 30:3090–7. doi: 10.1111/ene.v30.10
46. Mahad D, Callahan MK, Williams KA, Ubogu EE, Kivisakk P, Tucky B, et al. Modulating CCR2 and CCL2 at the blood-brain barrier: relevance for multiple sclerosis pathogenesis. Brain. (2006) 129:212–23. doi: 10.1093/brain/awh655
47. Ransohoff RM. Microglia and monocytes: 'tis plain the twain meet in the brain. Nat Neurosci. (2011) 14:1098–100. doi: 10.1038/nn.2917
48. Ransohoff RM and Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. (2010) 468:253–62. doi: 10.1038/nature09615
49. Han B, Jiang W, Cui P, Zheng K, Dang C, Wang J, et al. Microglial PGC-1alpha protects against ischemic brain injury by suppressing neuroinflammation. Genome Med. (2021) 13:47. doi: 10.1186/s13073-021-00863-5
50. Lv J, Jiang S, Yang Z, Hu W, Wang Z, Li T, et al. PGC-1alpha sparks the fire of neuroprotection against neurodegenerative disorders. Ageing Res Rev. (2018) 44:8–21. doi: 10.1016/j.arr.2018.03.004
51. Rius-Perez S, Torres-Cuevas I, Millan I, Ortega AL, and Perez S. PGC-1alpha, inflammation, and oxidative stress: an integrative view in metabolism. Oxid Med Cell Longev. (2020) 2020:1452696. doi: 10.1155/2020/1452696
52. Panes JD, Wendt A, Ramirez-Molina O, Castro PA, and Fuentealba J. Deciphering the role of PGC-1alpha in neurological disorders: from mitochondrial dysfunction to synaptic failure. Neural Regener Res. (2022) 17:237–45. doi: 10.4103/1673-5374.317957
53. Bhat AH, Dar KB, Anees S, Zargar MA, Masood A, Sofi MA, et al. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. BioMed Pharmacother. (2015) 74:101–10. doi: 10.1016/j.biopha.2015.07.025
54. Chang KH and Chen CM. The role of oxidative stress in parkinson's disease. Antioxidants (Basel). (2020) 9. doi: 10.3390/antiox9070597
55. Jenner P and Olanow CW. Oxidative stress and the pathogenesis of Parkinson's disease. Neurology. (1996) 47:S161–70. doi: 10.1212/WNL.47.6_Suppl_3.161S
56. Subramaniam SR and Chesselet MF. Mitochondrial dysfunction and oxidative stress in Parkinson's disease. Prog Neurobiol. (2013) 106-107:17–32. doi: 10.1016/j.pneurobio.2013.04.004
57. Liddell JR, Hilton JBW, Kysenius K, Billings JL, Nikseresht S, McInnes LE, et al. Microglial ferroptotic stress causes non-cell autonomous neuronal death. Mol Neurodegener. (2024) 19:14. doi: 10.1186/s13024-023-00691-8
58. Chu J, Li J, Sun L, and Wei J. The role of cellular defense systems of ferroptosis in parkinson's disease and alzheimer's disease. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms241814108
59. Dar NJ, John U, Bano N, Khan S, and Bhat SA. Oxytosis/ferroptosis in neurodegeneration: the underlying role of master regulator glutathione peroxidase 4 (GPX4). Mol Neurobiol. (2024) 61:1507–26. doi: 10.1007/s12035-023-03646-8
60. Parga JA, Rodriguez-Perez AI, Garcia-Garrote M, Rodriguez-Pallares J, and Labandeira-Garcia JL. NRF2 activation and downstream effects: focus on parkinson's disease and brain angiotensin. Antioxidants (Basel). (2021) 10. doi: 10.3390/antiox10111649
61. Li X, Feng Y, Wang XX, Truong D, and Wu YC. The critical role of SIRT1 in parkinson's disease: mechanism and therapeutic considerations. Aging Dis. (2020) 11:1608–22. doi: 10.14336/AD.2020.0216
62. Kandy AT, Chand J, Baba MZ, and Subramanian G. Is SIRT3 and mitochondria a reliable target for parkinson's disease and aging? A narrative review. Mol Neurobiol. (2024) 62(6):6898–912. doi: 10.1007/s12035-024-04486-w
63. Shen Y, Wu Q, Shi J, and Zhou S. Regulation of SIRT3 on mitochondrial functions and oxidative stress in Parkinson's disease. BioMed Pharmacother. (2020) 132:110928. doi: 10.1016/j.biopha.2020.110928
64. Calabro A, Accardi G, Aiello A, Caruso C, and Candore G. Sex and gender affect immune aging. Front Aging. (2023) 4:1272118. doi: 10.3389/fragi.2023.1272118
65. Marquez EJ, Chung CH, Marches R, Rossi RJ, Nehar-Belaid D, Eroglu A, et al. Sexual-dimorphism in human immune system aging. Nat Commun. (2020) 11:751. doi: 10.1038/s41467-020-14396-9
66. Sciarra F, Campolo F, Franceschini E, Carlomagno F, and Venneri MA. Gender-specific impact of sex hormones on the immune system. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24076302
67. Carlisle SM, Qin H, Hendrickson RC, Muwanguzi JE, Lefkowitz EJ, Kennedy RE, et al. Sex-based differences in the activation of peripheral blood monocytes in early Parkinson disease. NPJ Parkinsons Dis. (2021) 7:36. doi: 10.1038/s41531-021-00180-z
68. Gal-Oz ST, Maier B, Yoshida H, Seddu K, Elbaz N, Czysz C, et al. ImmGen report: sexual dimorphism in the immune system transcriptome. Nat Commun. (2019) 10:4295. doi: 10.1038/s41467-019-12348-6
69. Klein SL and Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. (2016) 16:626–38. doi: 10.1038/nri.2016.90
70. Georgiev D, Hamberg K, Hariz M, Forsgren L, and Hariz GM. Gender differences in Parkinson's disease: A clinical perspective. Acta Neurol Scand. (2017) 136:570–84. doi: 10.1111/ane.12796
71. Kustrimovic N, Comi C, Magistrelli L, Rasini E, Legnaro M, Bombelli R, et al. Parkinson's disease patients have a complex phenotypic and functional Th1 bias: cross-sectional studies of CD4+ Th1/Th2/T17 and Treg in drug-naive and drug-treated patients. J Neuroinflammation. (2018) 15:205. doi: 10.1186/s12974-018-1248-8
72. Kustrimovic N, Rasini E, Legnaro M, Bombelli R, Aleksic I, Blandini F, et al. Dopaminergic receptors on CD4+ T naive and memory lymphocytes correlate with motor impairment in patients with parkinson's disease. Sci Rep. (2016) 6:33738. doi: 10.1038/srep33738
73. Calderon TM, Williams DW, Lopez L, Eugenin EA, Cheney L, Gaskill PJ, et al. Dopamine increases CD14(+)CD16(+) monocyte transmigration across the blood brain barrier: implications for substance abuse and HIV neuropathogenesis. J Neuroimmune Pharmacol. (2017) 12:353–70. doi: 10.1007/s11481-017-9726-9
74. Coley JS, Calderon TM, Gaskill PJ, Eugenin EA, and Berman JW. Dopamine increases CD14+CD16+ monocyte migration and adhesion in the context of substance abuse and HIV neuropathogenesis. PloS One. (2015) 10:e0117450. doi: 10.1371/journal.pone.0117450
Keywords: Parkinson’s disease, monocytes, inflammation, neuroinflammation, oxidative stress, peripheral biomarkers, neurodegeneration, neuroimmunology
Citation: Thome AD, Wang J, Atassi F, Thonhoff JR, Faridar A, Zhao W, Beers DR, Lai EC and Appel SH (2025) Peripheral monocyte transcriptional signatures of inflammation and oxidative stress in Parkinson’s disease. Front. Immunol. 16:1571074. doi: 10.3389/fimmu.2025.1571074
Received: 04 February 2025; Accepted: 02 July 2025;
Published: 23 July 2025.
Edited by:
Wendy Watford, University of Georgia, United StatesReviewed by:
Stanislava Stanojevic, University of Belgrade, SerbiaCong Jin, Chinese Center For Disease Control and Prevention, China
Copyright © 2025 Thome, Wang, Atassi, Thonhoff, Faridar, Zhao, Beers, Lai and Appel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stanley H. Appel, c2FwcGVsQGhvdXN0b25tZXRob2Rpc3Qub3Jn
 Aaron D. Thome
Aaron D. Thome Jinghong Wang
Jinghong Wang Alireza Faridar
Alireza Faridar Weihua Zhao
Weihua Zhao David R. Beers
David R. Beers Eugene C. Lai
Eugene C. Lai Stanley H. Appel
Stanley H. Appel