- 1Department of Immunology and Rheumatology, Division of Advanced Preventive Medical Sciences, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan
- 2Department of Pathology, Sasebo City General Hospital, Sasebo, Japan
- 3Division of Functional Genomics, Nagasaki University Graduate School of Medical Sciences, Nagasaki, Japan
Background: Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by loss of immune tolerance, leading to systemic inflammation and organ damage. The Mediterranean fever (MEFV) gene, primarily linked to familial Mediterranean fever (FMF), has been suggested to have a protective role against SLE. However, comprehensive whole-exon analyses of MEFV and research on MEFV or FMF in non-Mediterranean populations, where MEFV exon 10 mutations are relatively rare, are limited.
Methods: We conducted a whole-exon analysis of the MEFV gene in 55 Japanese patients with SLE. Patients were classified based on the presence or absence of MEFV variants, and their clinical characteristics were compared. In addition, we generated MRL/lpr mice with the human MEFV E148Q variant using CRISPR technology to examine its impact on disease phenotypes. Disease activity and kidney pathology were assessed using the established clinical and histological scoring systems.
Results: Among the 55 patients, those carrying MEFV variants exhibited a significantly lower prevalence of lupus nephritis than non-carriers (P = 0.007). The number of MEFV variants was inversely associated with the risk of lupus nephritis (P = 0.03). In MRL/lpr mice, the E148Q variant was associated with reduced anti-dsDNA antibody production, reduced formation of memory B cells, and milder kidney pathology, indicating a shift from adaptive immunity to innate immune responses.
Conclusions: Our findings suggest that MEFV variants, particularly the E148Q variant, may play a protective role against lupus nephritis in Japanese patients with SLE by modulating immune responses. These results provide valuable insights into the genetic factors influencing SLE severity.
1 Introduction
Systemic lupus erythematosus (SLE) is a heterogeneous disease characterized by loss of immune tolerance and autoantibody production against elements of the cell nucleus followed by immune-mediated tissue damage and systemic inflammation (1, 2). More than 100 susceptibility loci have been identified through genome-wide association studies as potential genetic factors contributing to the development of SLE (3–9).
The prevalence of Mediterranean fever (MEFV) gene variants is lower in adult patients with SLE than in healthy individuals (10). The prevalence of SLE is also lower in several large cohorts of patients with familial Mediterranean fever (FMF) compared with healthy individuals (11–14). These results suggest a potential protective effect of MEFV variants and/or FMF against SLE. MEFV variants may modify clinical SLE phenotypes (10, 15, 16). In particular, the E148Q variant in the MEFV exon 2 may prevent the development of lupus nephritis in adult patients with SLE (15). However, reports showing a relationship between SLE and MEFV gene variants/mutations are limited to patients in the Mediterranean area, where MEFV exon 10 mutations are relatively common. In addition, whole-exon analyses to determine the relationship between MEFV gene variants and SLE have not been reported.
A relatively high proportion of healthy individuals in East Asian population including Japan have MEFV exon 2 and exon 3 variants (especially E148Q variants in MEFV exon 2) as follows: minor allele frequency (MAF) for E148Q, 1443 out of 5160 alleles (28.0%) in East Asian population and 3555 out of 147018 (2.4%) in non-East Asian population based on a genome database from gnomAD v3.1.2 (https://gnomad.broadinstitute.org). The genetic characteristics of the MEFV gene in Japan and the Mediterranean region are different. We performed a whole-exon analysis of the MEFV gene in 55 Japanese patients with SLE and compared the clinical characteristics of SLE in patients with and without MEFV variants. MRL/lpr mice with the human MEFV E148Q variant were generated to confirm the contribution of MEFV variants with the E148Q to SLE clinical phenotypes.
2 Methods
2.1 Study design
This study included 55 Japanese patients with SLE treated between January 2008 and April 2020 at Nagasaki University Hospital. All patients met the American College of Rheumatology 1997 revised criteria (17) and/or the Systemic Lupus International Collaborating Clinics 2012 criteria (18) for SLE classification and were diagnosed with SLE based on their clinical courses. Patients were clinically observed for ≥12 months. This study was approved by the Institutional Review Board of Nagasaki University (approval no. 18070919), and all patients provided signed informed consent to participate in the study.
2.2 Genetic analysis
Genomic DNA was extracted from whole blood using the Promega Wizard® Genomic DNA Purification Kit (Promega, Madison, WI, USA). Whole-exon analyses was performed to detect single nucleotide variants and short insertions and deletions in MEFV gene via targeted next-generation sequencing. Next-generation sequencing (NGS) was performed on an Ion Personal Genome Machine (Thermo Fisher Scientific, Waltham, MA, USA) using an Ion AmpliSeq Library Kit Plus (Thermo Fisher Scientific). Each depth of coverage of the variants detected by NGS was analyzed to show the quality. The variant allele frequencies in the patients were almost 50% or almost 100%. No chimeras or mosaics were identified. The number of MEFV variants for each patient was counted; heterozygous variants were counted as one and homozygous variants were counted as two.
2.3 Clinical and laboratory assessments
Demographic data, laboratory results, and disease activity at the time of SLE diagnosis were obtained from patient records. Laboratory assays were performed in a routine clinical setting. Disease activity was objectively measured at the time of SLE diagnosis using the Safety of Estrogens in Lupus Erythematosus National Assessment–Systemic Lupus Erythematosus Disease Activity Index (SELENA-SLEDAI) score. The score range for the SELENA-SLEDAI is 0–105, and higher scores indicate increased disease activity (19).
Clinical characteristics and comorbidities were assessed during the clinical course of the disease. Lupus nephritis was diagnosed based on pathological findings from renal biopsies and/or the clinical course. Renal pathologists classified renal biopsies according to the International Society of Nephrology/Renal Pathology Society guidelines (20).
2.4 Generation of human MEFV exon 2 encoding wild-type and E148Q knock-in MRL/lpr mice
Wild-type MRL.lpr mice were obtained from Jackson Laboratory and maintained under specific pathogen-free conditions at the animal facility of Nagasaki University. The animal study protocol was approved by the Institutional Animal Care and Use Committee of Nagasaki University.
The following guide RNA targeting exon 2 of mouse MEFV was designed using CRISPR Design Tools (https://horizondiscovery.com/en/ordering-and-calculation-tools/crispr-design-tool): 5′-CCATCCAGCCAGGCTGAAGTGTTTTAGAGCTATGCT-3′. The following single-strand DNA oligonucleotides (ssODN) were used to introduce the human MEFV exon 2 sequence into the mouse counterpart: Wild-type human MEFV exon 2: 5′-CCTGAAGGTGATGGGACACAGCAAAACAATGATGAATCAGACACCCTTCCATCCAGCCAGCCCGAGGCCGGGAGGGGGCTGTCGAGGAAGTCACTGACCAAAAGGAAGGATCAGAGGGGCCCCGAGAGCCTGGACTCACAGACCAAG-3′, human MEFV E148Q variant: 5′-CCTGAAGGTGATGGGACACAGCAAAACAATGATGAATCAGACACCCTTCCATCCAGCCAGCCCCAGGCCGGGAGGGGGCTGTCGAGGAAGTCACTGACCAAAAGGAAGGATCAGAGGGGCCCCGAGAGCCTGGACTCACAGACCAAG-3′. A mixture of CAS9 nuclease mRNA (10 ng/μL), crRNA (100 ng/μL), tracrRNA (200 ng/μL), and ssODN (10 μM) was injected into the cytoplasm of MRL/lpr zygotes using an Olympus IX70 Fluorescence Microscope (Olympus, Tokyo, Japan), TransferMan NK2 (Eppendorf, Hamburg, Germany), FemtoJet 4i (Eppendorf), and CellTram 4r Oil (Eppendorf). CAS9 nuclease mRNA was purchased from GE Healthcare Dharmacon (Lafayette, CO, USA), and other reagents were purchased from Integrated DNA Technologies (Coralville, IA, USA). Zygotes were cultured until the two-cell stage in vitro before transferring to recipient pseudopregnant female oviducts. MRL/lpr exhibiting the homozygous wild-type human MEFV exon 2 (MRL/lprhMEFV WT) and the homozygous human MEFV E148Q variant (MRL/lprhMEFV E148Q) were used for comparisons.
2.5 Proteinuria in MRL/lpr mice
Urine was collected at 8, 10, and 16 weeks from each female MRL/lprhMEFV WT and MRL/lprhMEFV E148Q mice (n = 3 per group). Urinary creatinine (urinary) was measured with a colorimetric assay kit (Cayman Chemical) and mouse albumin was measured with an ELISA kit (Bethyl Laboratories). Proteinuria was calculated as the albumin-to-creatinine ratio.
2.6 Serum autoantibody in MRL/lpr mice
Serum samples were collected at 8 and 16 weeks from female mice in each group (n = 3 per group). Serum anti-dsDNA IgG levels were measured using a mouse anti-dsDNA IgG ELISA kit (Alpha Diagnostic Intl. Inc.) according to the manufacturer’s instructions.
2.7 In vitro bone marrow-derived macrophage differentiation
Bone marrow-derived macrophages (BMDMs) were obtained by differentiating bone marrow progenitors from the tibia and femur of 8-week-old female mice for 7 days in Iscove’s Modified Dulbecco’s Medium containing 20 ng/ml M-CSF (Peprotech), 10% heat-inactivated fetal bovine serum (Invitrogen), 1 mM sodium pyruvate, 100 U/ml penicillin, and 100 Mg/ml streptomycin (Invitrogen). BMDMs were plated in 12-well plates 24 h before experiments and stimulated with LPS (1,000 ng/ml) for 5 h. The supernatants of culture media were stored at −80°C until the following assessment. Cytokines were measured in the supernatants from each group (n = 3 per group) using a cytokine bead assay and magnetic beads (Millipore, Billerica, MA, USA), according to the manufacturer’s instructions.
2.9 Flow cytometry
Spleens were excised from 16-week-old female mice (n = 5 per group), and cell suspensions were obtained by teasing the organs through a nylon mesh. Isolated cells were stained for 30 min at 4°C with the following antibodies: anti-mouse CD3 (clone 17A2, BioLegend), anti-mouse CD19 (clone 6D5, BioLegend), and anti-mouse IgD (clone 11-26c.2a, BioLegend). Data were acquired using an LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo v. 7.6.1.
2.10 Assessment of skin and kidney pathology
The skin and kidneys were collected from 16-week-old female mice in each group (n = 5–6 per group). Tissues were stored in 4% paraformaldehyde, sectioned, stained with hematoxylin and eosin (H&E), and scored by a certified pathologist. The skin severality score was calculated as the sum of cellular infiltration (score 0–3), epidermal hyperplasia (score 0–2), and epidermal ulcerations (score 0–2) based on a previously described method (21). The kidney severality scores were determined using a previously described method (https://www.inotiv.com/solutions/mouse-lupus-methods). Subsequently, glomerular scores, focusing on the glomerular area including the crescent, were also assessed according to previously described criteria (22). Briefly, twenty glomeruli were randomly observed per mouse using H&E-stained slides. Each glomerulus was scored using a semi-quantitative scale, as follows: 0 normal (35–40 cells/gcs), 1 mild (glomeruli with slight proliferative changes and mild hypercellularity, 41–50 cells/gcs, and/or minor exudation), 2 moderate (glomeruli with moderate hypercellularity, 50–60 cells/gcs, including segmental and/or diffuse proliferative changes, hyalinosis, and/or moderate exudates), and 3 severe (glomeruli with segmental or global sclerosis and/or exhibiting severe hypercellularity, 60 cells/gcs, crescent formation, and/or heavy exudation).
2.11 Statistical analysis
Categorical variables are presented as frequencies and quantitative variables are presented as medians and interquartile ranges. Categorical variables were compared using Fisher’s exact tests, and quantitative variables were compared using Wilcoxon rank sum tests. Multiple logistic regression analysis was performed to identify independent factors associated with lupus nephritis as the primary endpoint. Clinically important variables were included in the model. Mouse groups were compared using unpaired Student’s t-tests after the assumption of normality was tested for the datasets using the Shapiro-Wilk test. All statistical analyses were performed using JMP Pro 18.1.1 software (SAS Institute, Cary, NC, USA). P < 0.05 (two-tailed) was considered statistically significant. GraphPad Prism version 8.0 was used to create figures. As this was an exploratory study aimed at identifying potential associations and generating hypotheses, no corrections for multiple comparisons were applied. Results should therefore be interpreted with caution, and future confirmatory studies are warranted to validate the findings.
3 Results
3.1 Characteristics and MEFV variants in patients with SLE
The demographic and clinical characteristics and mutational pattern of the MEFV gene in patients with SLE are summarized in Supplementary Table 1. All patients with SLE and MEFV variants had one or more of the following variants in exons 1–5 of MEFV: E84K, L110P, E148Q, R202Q, G304R, P369S, R408Q, and S503C. None of the patients had exon 10 mutations. The depth of coverage of all MEFV variants detected by NGS was 736 ± 74.45 (Average ± SEM).
3.2 Characteristics of SLE patients with MEFV variants
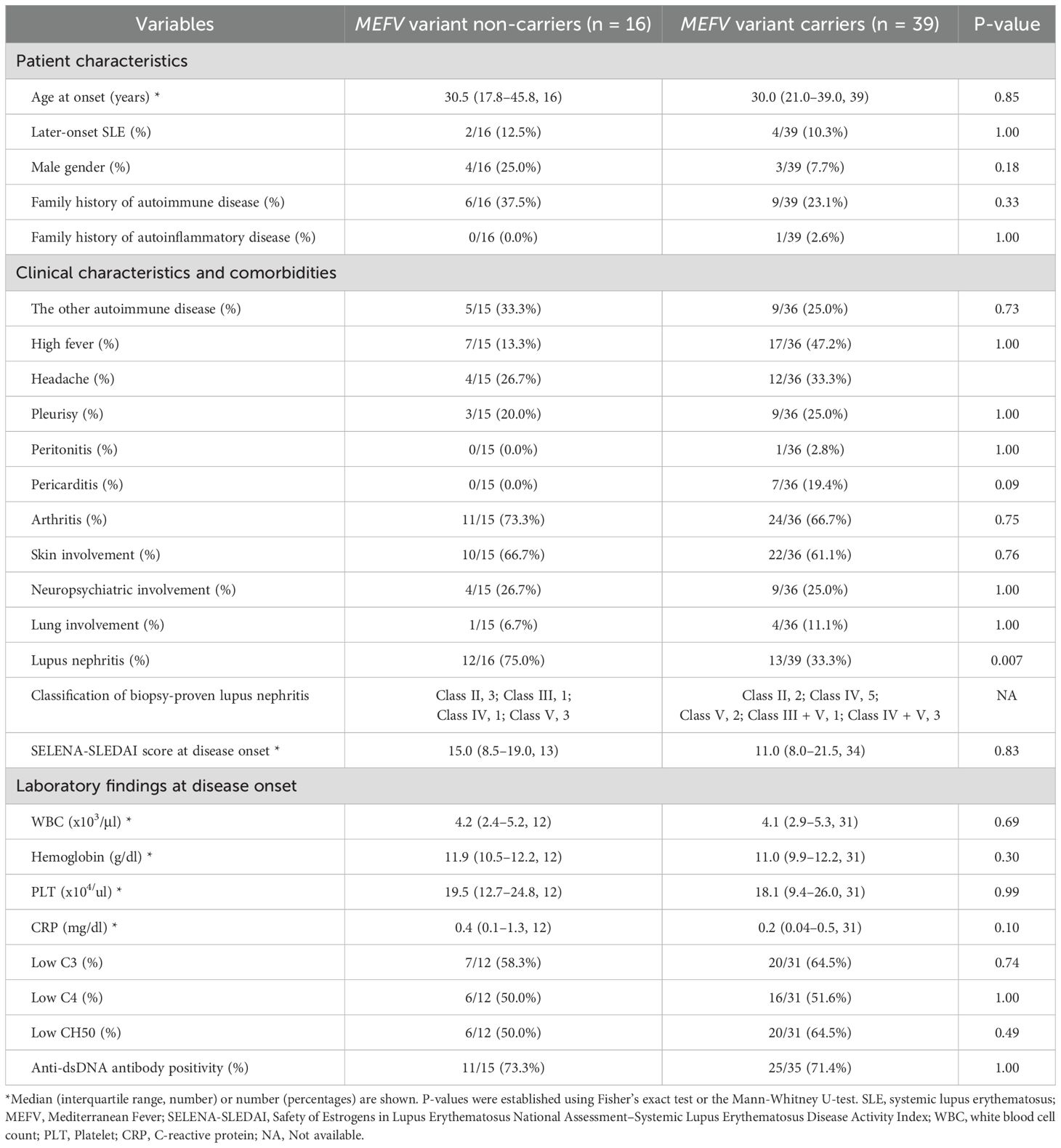
The demographic and clinical characteristics of patients with SLE without MEFV variants (MEFV variant non-carriers, n = 16) and patients with SLE and MEFV variants (MEFV variant carriers, n = 39) were compared (Table 1). MEFV variant carriers exhibited a significantly lower prevalence of lupus nephritis compared with non-carriers (P = 0.007). No significant differences in other parameters were detected between carriers and non-carriers. The demographic and clinical characteristics of SLE in patients with (n = 22) or without (n = 33) the E148Q variant in exon 2 of MEFV were also compared. No significant difference in the prevalence of lupus nephritis was detected between MEFV E148Q carriers and non-carriers (39.4% vs. 54.6%, P = 0.29, not shown).
3.3 Number of MEFV variants according to the presence of lupus nephritis
The number of MEFV variants in patients with lupus nephritis was significantly lower than the number of MEFV variants in patients without lupus nephritis (Mean ± SD; 0.80 ± 0.87 vs. 1.53 ± 0.94, = 0.006, not shown). The number of MEFV variants, but not low CH50 or anti-dsDNA antibody positivity, was significantly associated with lupus nephritis in patients with SLE (odds ratio 0.351, P = 0.03; Table 2).
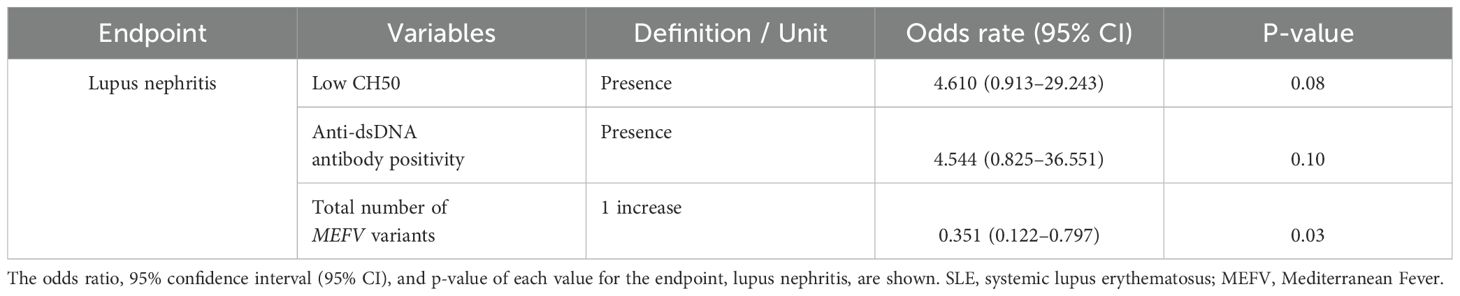
Table 2. Multiple regression analysis of factors associated with the development of lupus nephritis in patients with systemic lupus erythematosus.
3.4 MRL/lpr mice with human exon 2 of the MEFV gene
Successful insertion of the human MEFV exon 2 sequence into the mouse counterpart was confirmed via Sanger sequencing (Figure 1A). IL-1α levels were significantly higher in the media of BMDMs from MRL/lprhMEFVE148Q mice compared with the levels in the supernatants of BMDMs from MRL/lprhMEFV WT mice (Figure 1B). In contrast, anti-dsDNA antibody production (Figure 1C) and memory B cell formation in spleens were reduced in MRL/lprhMEFVE148Q mice compared with MRL/lprhMEFV WT mice (Figure 1D). No significant differences in the skin severity scores and proteinuria levels were detected between the groups (Supplementary Figures S1A, B). However, kidney severity scores in MRL/lprhMEFV E148Q mice were lower than kidney scores from MRL/lprhMEFV WT mice, particularly in the glomerular region, including reduced crescent formation (Supplementary Figure S1C). Glomerular severity scores were also significantly lower in the MRL/lprhMEFV E148Q mice (Figure 1E).
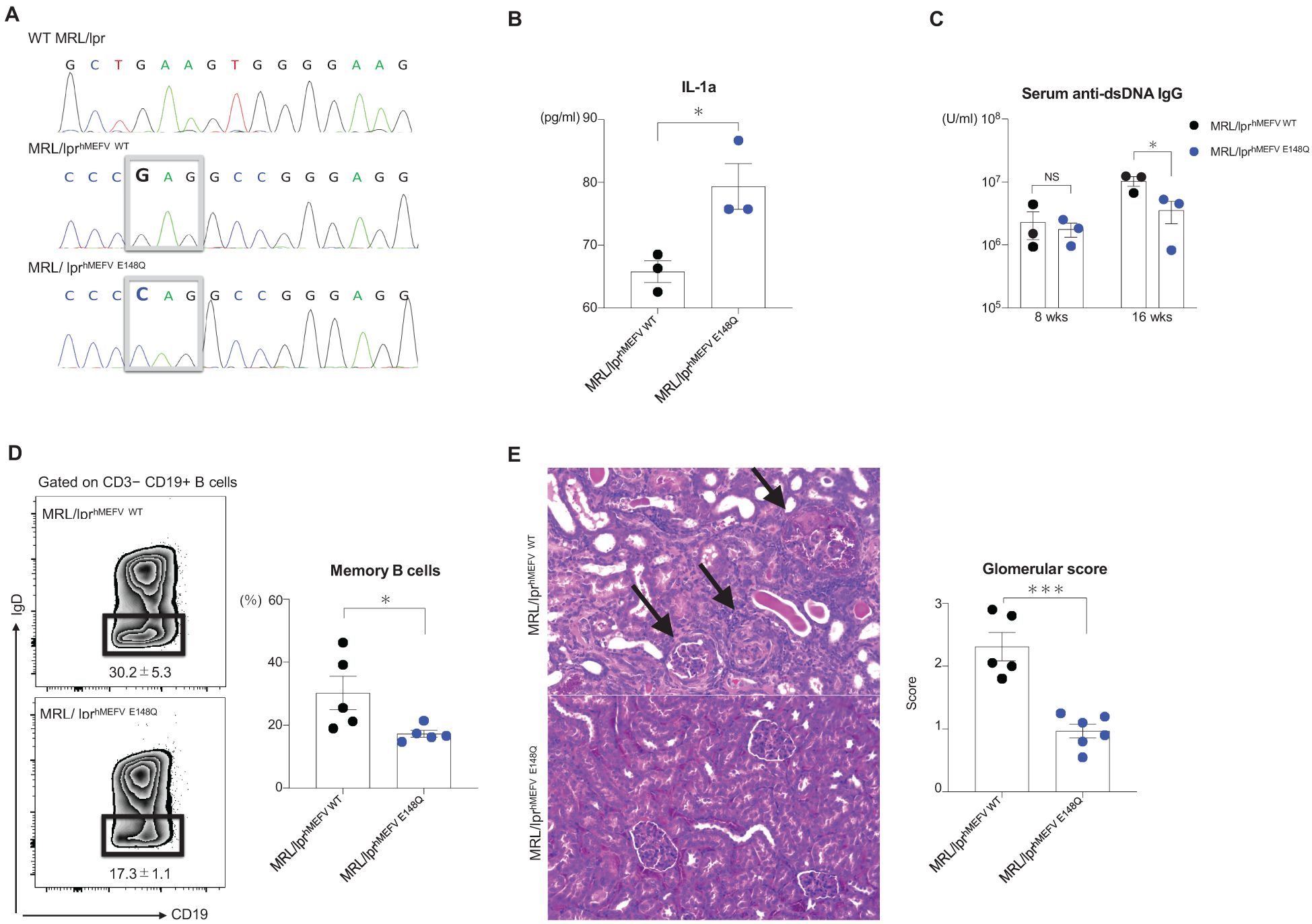
Figure 1. MRL/lpr mice with human exon 2 of the MEFV gene. MRL/lprhMEFV WT and MRL/lprhMEFV E148Q mice were compared. (A) Sanger sequencing results and parts of MEFV exon 2 in wild-type MRL/lpr, MRL/lprhMEFV WT, and MRL/lprhMEFV E148Q. The boxed sequences represent glutamic acid in MRL/lprhMEFV WT and glutamine in MRL/lprhMEFV E148Q. (B) Supernatant IL-1α levels from cultured BMDMs isolated from MRL/lprhMEFV WT and MRL/lprhMEFV E148Q mice. (C) Anti-dsDNA IgG levels at 8 and 16 weeks. (D) Representative flow cytometric plot of splenic class-switched memory B cells (CD3−CD19+IgD+) and the percentage of splenic memory B cells in each group. (E) Representative H&E-stained kidney sections and glomerular severity scores in each group. The black arrows show crescent formations in the glomerulus. (B-E) Unpaired two-tailed Student’s t-test. Data represent means ± SEMs (NS; not significant, *P < 0.05, ***P < 0.005.
4 Discussion
Our results provide compelling evidence for the protective role of MEFV variants, including the E148Q variant, in shaping the clinical trajectory of SLE; MEFV variants may mitigate the severity of lupus nephritis. Clinical observations in Japanese patients with SLE combined with experimental results from a murine model highlight the contribution of the E148Q variant in redirecting immune responses from adaptive immunity toward innate immune mechanisms.
The role of MEFV variants (excluding homozygous exon10 mutations but including the E148Q variant) in the development of autoinflammation is controversial. However, previous reports suggest that MEFV variants contribute to the pathogenesis of other autoinflammatory diseases (23–25). Our clinical findings demonstrate a significantly lower prevalence of lupus nephritis in SLE patients carrying MEFV variants compared with non-carriers. Of note, the number of MEFV variants inversely associated with the risk of lupus nephritis in patients with SLE, underscoring the protective effects of MEFV variants. These results align with a previous study suggesting that the E148Q variant prevents lupus nephritis (15). These results suggest a shift in immune responses from autoimmunity in SLE to autoinflammation. Notably, the protective effects of MEFV variants in this cohort were not solely due to the E148Q variant, emphasizing the need for larger-scale investigations using comprehensive whole-exon analyses of the MEFV gene in patients with SLE, similar to our approach.
Based on the kidney pathology in lupus-prone MRL/lpr mice, the severity of lupus nephritis was reduced in mice carrying the human MEFV E148Q variant. Notably, anti-dsDNA antibody production and memory B cell formation in the spleen were reduced in MRL/lprhMEFV E148Q mice, indicating suppression of adaptive immune responses. Conversely, IL-1α production by bone marrow-derived macrophages (BMDMs) from these mice increased compared with MRL/lprhMEFV WT mice, indicating that the variant enhanced the innate immune response. These results support the hypothesis that the E148Q variant promotes a shift from pathogenic adaptive immunity in SLE to innate immune activation, which may mitigate autoimmune-driven kidney damage. This shift from adaptive to innate immunity, mediated by MEFV variants such as E148Q, may reduce the severity of lupus nephritis by reducing autoantibody production.
This study provides valuable insights, but several limitations should be acknowledged. First, the size of the clinical cohort was small, limiting the generalizability of our results. No MEFV exon 10 mutations were detected among the patients enrolled in this study. While this finding may raise the hypothesis that MEFV exon 10 mutations could be protective against SLE, such an association is difficult to assess in the Japanese population due to the extremely low prevalence of these mutations. For example, the M694I mutation—the most frequently reported MEFV exon 10 variant in Japan—has a minor allele frequency of approximately 0.15% according to the Tohoku Medical Megabank Organization database (https://jmorp.megabank.tohoku.ac.jp). Given this rarity, it is not feasible to perform robust statistical analyses, such as Hardy–Weinberg equilibrium testing, in our cohort. Therefore, we believe that it is difficult to draw reliable conclusions regarding the association between exon 10 mutations and SLE susceptibility in this population. Larger, multicenter studies are necessary to confirm the protective role of MEFV variants and mutations in SLE among diverse populations.
Second, the experimental mouse model was informative but may not fully replicate the complexity and heterogeneity of patients with SLE. Finally, the precise molecular pathways mediating the protective effects of the E148Q variant against nephritis are unclear. The future investigations may provide insights into novel therapeutic strategies for SLE.
Finally, this study was exploratory in nature and intended to generate hypotheses regarding the potential involvement of MEFV variants in SLE pathogenesis. Given this objective, we did not apply corrections for multiple testing, as such adjustments may increase the risk of type II errors and obscure meaningful associations, especially when analyzing rare variants. Nonetheless, we acknowledge that the lack of multiple testing correction may increase the risk of false-positive findings. This limitation has been clearly stated, and our findings should be interpreted with appropriate caution. Future studies with larger cohorts and confirmatory designs are needed to validate these results.
In summary, the E148Q variant of the MEFV gene may protect patients with lupus nephritis by modulating immune responses and shifting the balance from adaptive to innate immunity. These findings highlight the protective role of MEFV variants and provide the foundation for further research on the innate immune mechanisms underlying SLE.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by Yushiro Endo and Institutional Review Board of Nagasaki University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The animal studies were approved by Yushiro Endo and Institutional Animal Care and Use Committee of Nagasaki University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
YE: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. TKo: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LM: Writing – review & editing. YT: Writing – review & editing. MU: Writing – review & editing. HH: Writing – review & editing. TKi: Writing – review & editing. AK: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by JSPS KAKENHI [Grant Numbers 19K17888 to YE].
Acknowledgments
We thank Kaori Furukawa (Nagasaki University, Nagasaki, Japan) and Yuko Mizunoo (Nagasaki University) for their technical assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1571208/full#supplementary-material
Supplementary Figure 1 | Comparison of MRL/lprhMEFV WT and MRL/lprhMEFV E148Q mice. (A) Skin severity scores based on hematoxylin and eosin (H&E)-stained skin sections. (B) Proteinuria levels are indicated by albumin-to-creatinine ratios at 8, 10, and 16 weeks. (C) Kidney severity scores based on H&E-stained kidney sections. Unpaired two-tailed Student’s t-test. Data and bars represent means ± SEM (NS; Not significant).
Abbreviations
BMDM, Bone marrow-derived macrophages; FMF, Familial Mediterranean fever; H&E, Hematoxylin and eosin; MEFV, MEFV variants; SLE, Systemic lupus erythematosus.
References
1. Benseler SM and Silverman ED. Systemic lupus erythematosus. Rheum Dis Clin North Am. (2007) 33:471–98, vi. doi: 10.1016/j.rdc.2007.07.008
2. Tsokos GC. Systemic lupus erythematosus. N Engl J Med. (2011) 365:2110–21. doi: 10.1056/NEJMra1100359
3. Teruel M and Alarcon-Riquelme ME. The genetic basis of systemic lupus erythematosus: What are the risk factors and what have we learned. J Autoimmun. (2016) 74:161–75. doi: 10.1016/j.jaut.2016.08.001
4. Yin X, Kim K, Suetsugu H, Bang SY, Wen L, Koido M, et al. Meta-analysis of 208370 East Asians identifies 113 susceptibility loci for systemic lupus erythematosus. Ann Rheum Dis. (2021) 80:632–40. doi: 10.1136/annrheumdis-2020-219209
5. Gateva V, Sandling JK, Hom G, Taylor KE, Chung SA, Sun X, et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat Genet. (2009) 41:1228–33. doi: 10.1038/ng.468
6. Han JW, Zheng HF, Cui Y, Sun LD, Ye DQ, Hu Z, et al. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat Genet. (2009) 41:1234–7. doi: 10.1038/ng.472
7. Cunninghame Graham DS, Morris DL, Bhangale TR, Criswell LA, Syvanen AC, Ronnblom L, et al. Association of NCF2, IKZF1, IRF8, IFIH1, and TYK2 with systemic lupus erythematosus. PloS Genet. (2011) 7:e1002341. doi: 10.1371/journal.pgen.1002341
8. Okada Y, Shimane K, Kochi Y, Tahira T, Suzuki A, Higasa K, et al. A genome-wide association study identified AFF1 as a susceptibility locus for systemic lupus eyrthematosus in Japanese. PloS Genet. (2012) 8:e1002455. doi: 10.1371/journal.pgen.1002455
9. Akizuki S, Ishigaki K, Kochi Y, Law SM, Matsuo K, Ohmura K, et al. PLD4 is a genetic determinant to systemic lupus erythematosus and involved in murine autoimmune phenotypes. Ann Rheum Dis. (2019) 78:509–18. doi: 10.1136/annrheumdis-2018-214116
10. Shinar Y, Kosach E, Langevitz P, Zandman-Goddard G, Pauzner R, Rabinovich E, et al. Familial Mediterranean FeVer gene (MEFV) mutations as a modifier of systemic lupus erythematosus. Lupus. (2012) 21:993–8. doi: 10.1177/0961203312441048
11. Ozen S and Bakkaloglu A. C reactive protein: protecting from lupus in familial Mediterranean fever. Ann Rheum Dis. (2005) 64:786–7. doi: 10.1136/ard.2004.027037
12. Lidar M, Zandman-Goddard G, Shinar Y, Zaks N, Livneh A, and Langevitz P. Systemic lupus erythematosus and familial Mediterranean fever: a possible negative association between the two disease entities–report of four cases and review of the literature. Lupus. (2008) 17:663–9. doi: 10.1177/0961203308089403
13. Tunca M, Akar S, Onen F, Ozdogan H, Kasapcopur O, Yalcinkaya F, et al. Familial Mediterranean fever (FMF) in Turkey: results of a nationwide multicenter study. Med (Baltimore). (2005) 84:1–11. doi: 10.1097/01.md.0000152370.84628.0c
14. Atas N, Armagan B, Bodakci E, Satis H, Sari A, Bilge NSY, et al. Familial Mediterranean fever is associated with a wide spectrum of inflammatory disorders: results from a large cohort study. Rheumatol Int. (2020) 40:41–8. doi: 10.1007/s00296-019-04412-7
15. Deniz R, Ozen G, Yilmaz-Oner S, Alibaz-Oner F, Erzik C, Aydin SZ, et al. Familial Mediterranean fever gene (MEFV) mutations and disease severity in systemic lupus erythematosus (SLE): implications for the role of the E148Q MEFV allele in inflammation. Lupus. (2015) 24:705–11. doi: 10.1177/0961203314560203
16. Erer B, Cosan F, Oku B, Ustek D, Inanc M, Aral O, et al. MEFV gene variations in patients with systemic lupus erythematosus. Mod Rheumatol. (2014) 24:93–6. doi: 10.3109/14397595.2013.854049
17. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. (1997) 40:1725. doi: 10.1002/art.1780400928
18. Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. (2012) 64:2677–86. doi: 10.1002/art.34473
19. Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. (2005) 353:2550–8. doi: 10.1056/NEJMoa051135
20. Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. (2004) 15:241–50. doi: 10.1097/01.ASN.0000108969.21691.5D
21. Yang JQ, Chun T, Liu H, Hong S, Bui H, Van Kaer L, et al. CD1d deficiency exacerbates inflammatory dermatitis in MRL-lpr/lpr mice. Eur J Immunol. (2004) 34:1723–32. doi: 10.1002/eji.200324099
22. Kikawada E, Lenda DM, and Kelley VR. IL-12 deficiency in MRL-Fas(lpr) mice delays nephritis and intrarenal IFN-gamma expression, and diminishes systemic pathology. J Immunol. (2003) 170:3915–25. doi: 10.4049/jimmunol.170.7.3915
23. Migita K, Uehara R, Nakamura Y, Yasunami M, Tsuchiya-Suzuki A, Yazaki M, et al. Familial mediterranean fever in Japan. Med (Baltimore). (2012) 91:337–43. doi: 10.1097/MD.0b013e318277cf75
24. Kucukali B, Bayraktar EO, Yildiz C, Gonen S, Kutlar M, Karacayir N, et al. Clinical associations of E148Q heterozygosity: what to expect from E148Q? J Clin Rheumatol. (2024) 30:229–34. doi: 10.1097/RHU.0000000000002119
Keywords: systemic lupus erythematosus, Mediterranean fever variant, lupus nephritis, adaptive immunity, innate immunity
Citation: Endo Y, Koga T, Mohamed L, Tsuji Y, Umeda M, Hayashi H, Kishino T and Kawakami A (2025) Mediterranean fever gene variants may prevent the development of lupus nephritis in Japanese patients with systemic lupus erythematosus. Front. Immunol. 16:1571208. doi: 10.3389/fimmu.2025.1571208
Received: 05 February 2025; Accepted: 05 June 2025;
Published: 07 July 2025.
Edited by:
Jonathan A. Harton, Albany Medical College, United StatesReviewed by:
Davide Martorana, University Hospital of Parma, ItalyPanagiotis Skendros, Democritus University of Thrace, Greece
Copyright © 2025 Endo, Koga, Mohamed, Tsuji, Umeda, Hayashi, Kishino and Kawakami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tomohiro Koga, dGtvZ2FAbmFnYXNha2ktdS5hYy5qcA==
 Yushiro Endo
Yushiro Endo Tomohiro Koga
Tomohiro Koga Lamiaa Mohamed1
Lamiaa Mohamed1 Yoshika Tsuji
Yoshika Tsuji Masataka Umeda
Masataka Umeda Tatsuya Kishino
Tatsuya Kishino Atsushi Kawakami
Atsushi Kawakami