- 1Department of Hepatobiliary and Pancreatic Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, Guangdong, China
- 2Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, Guangdong, China
Background: Combination of multiple therapies is a common approach to treating patients with unresectable hepatocellular carcinoma (uHCC). The impact of immune checkpoint inhibitors (ICIs) on prognosis in uHCC patients treated with transarterial chemoembolization (TACE) and lenvatinib remains unclear.
Aim: The purpose of this study was to compare the efficacy and safety of TACE plus lenvatinib plus ICIs (TACE+L+I) with TACE plus lenvatinib (TACE+L) in the treatment of patients with uHCC.
Methods: Publicly available studies comparing the efficacy and safety of TACE+L+I and TACE+L in the treatment of uHCC were collected from the databases PubMed, Embase and Cochrane Library, with a cut-off date of November 1, 2024. Stata SE 15 software was used for analysis.
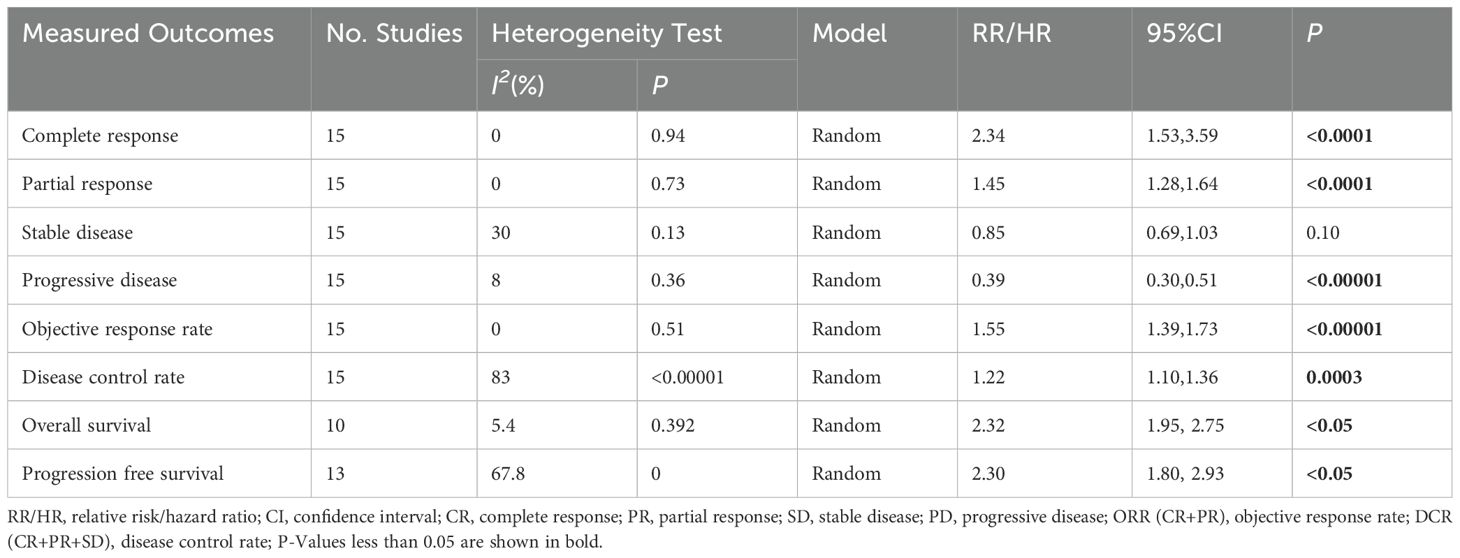
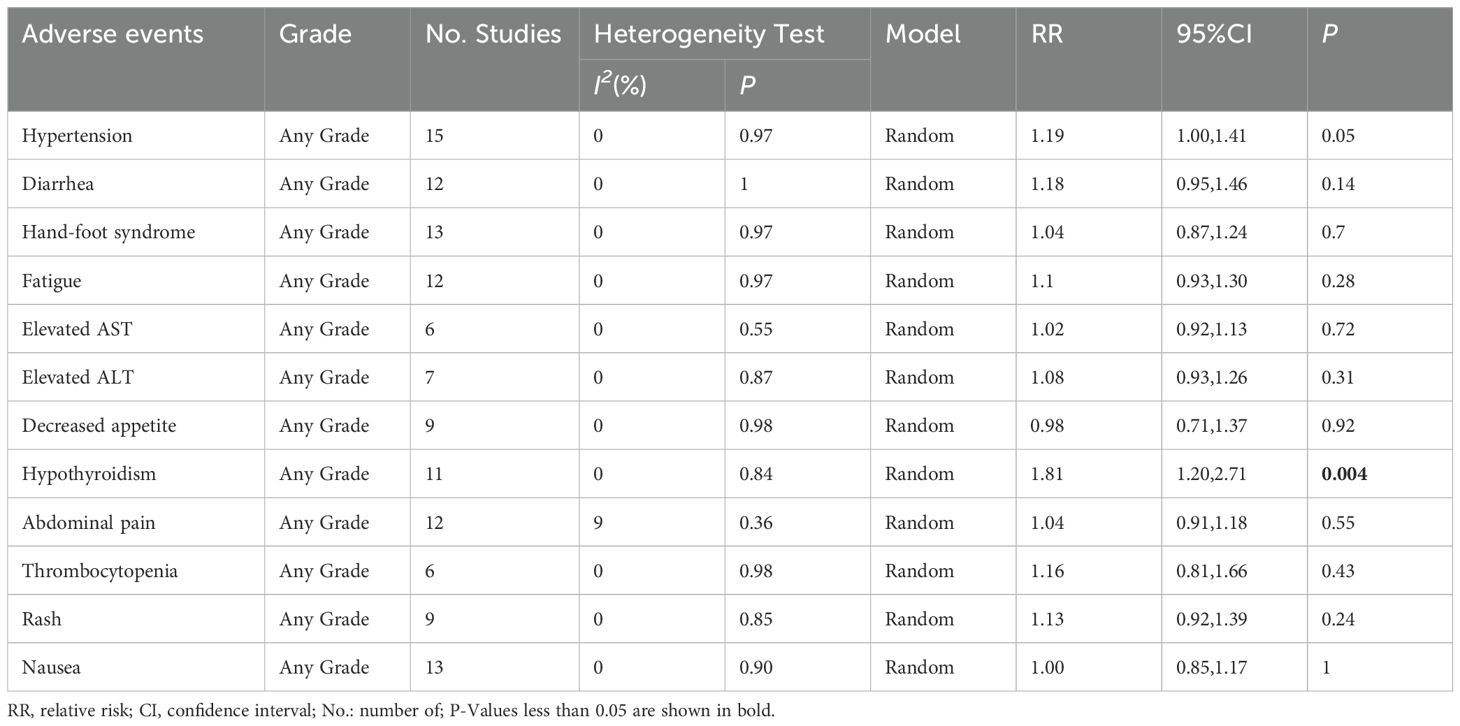
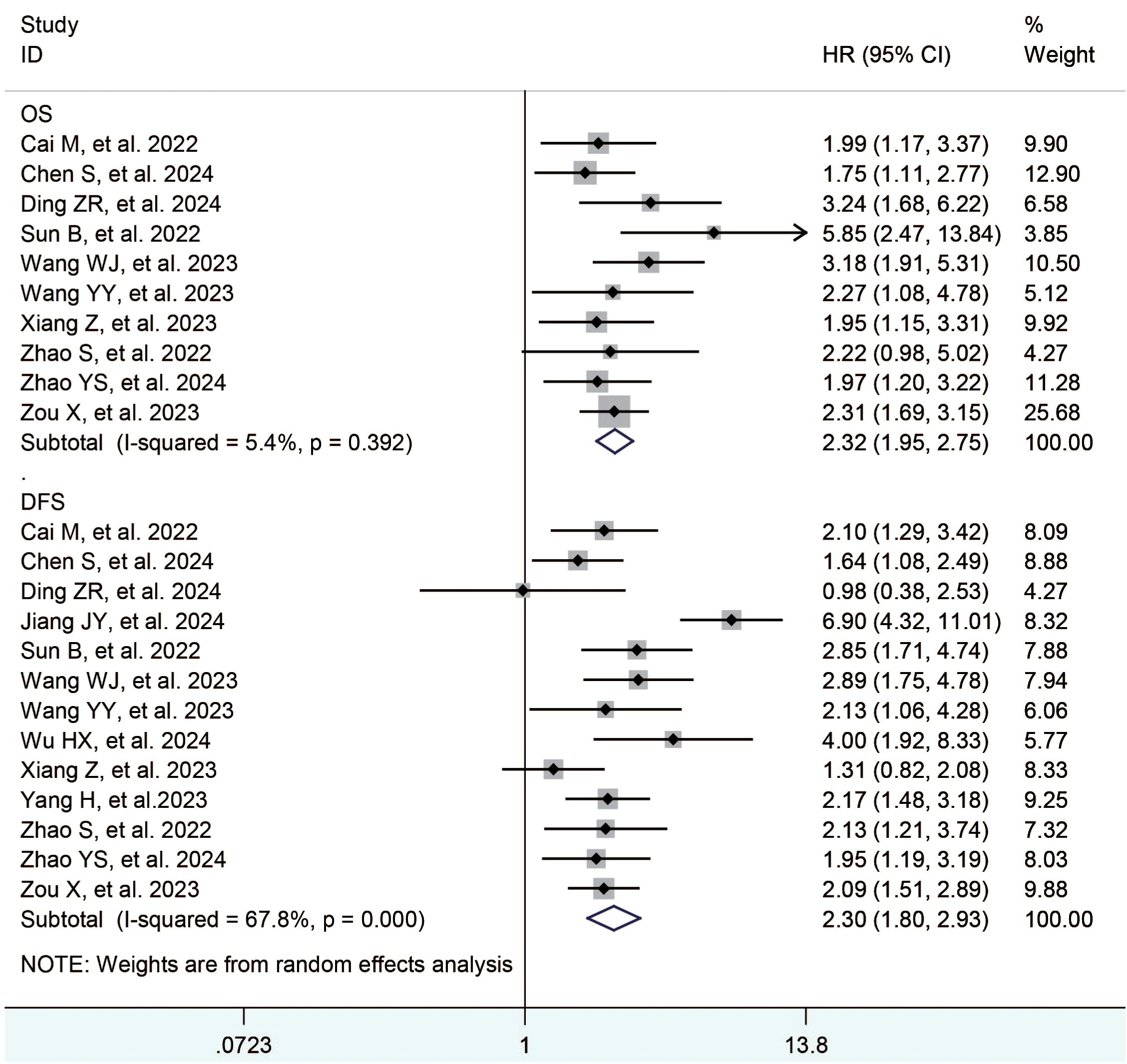
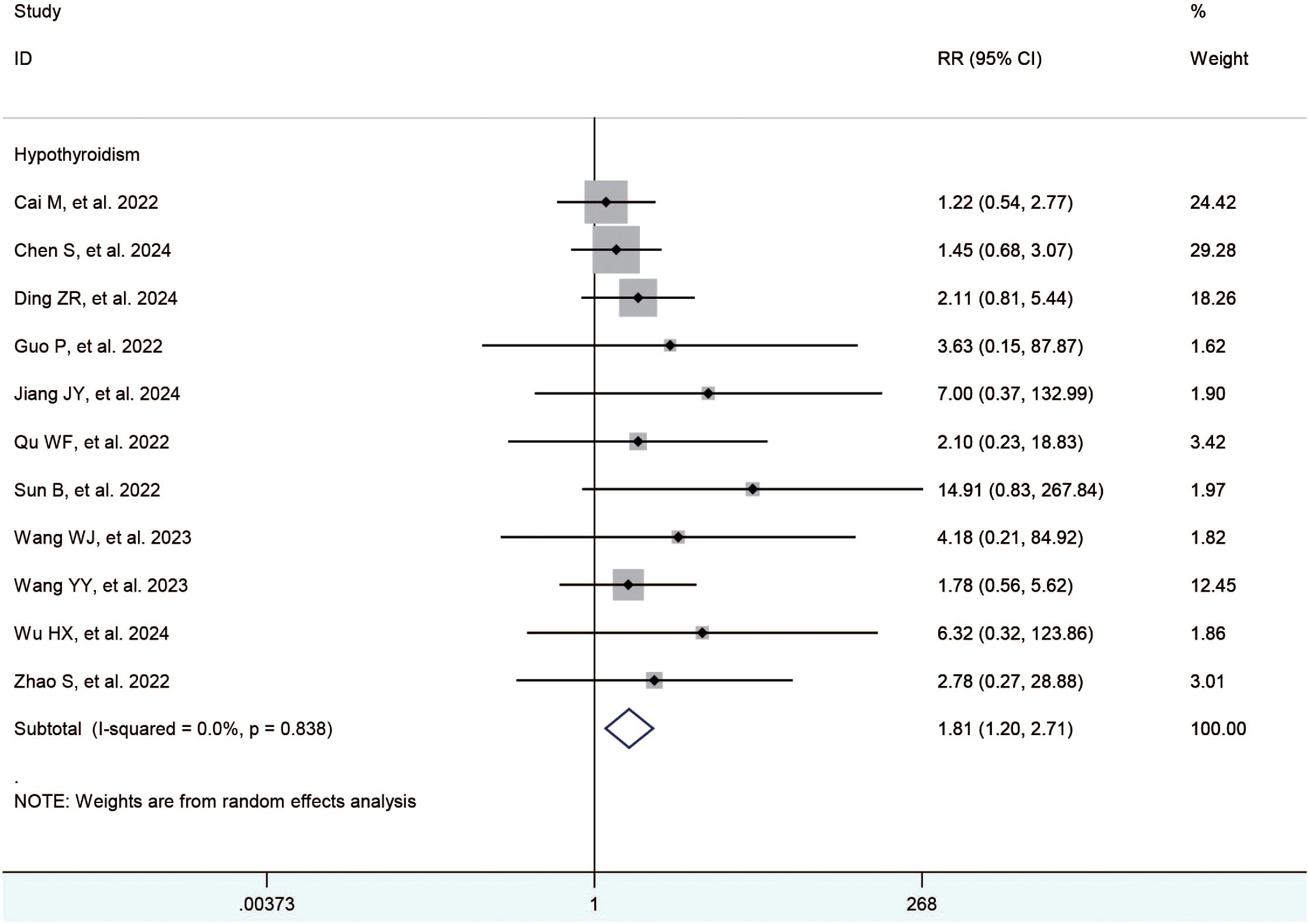
Results: Fifteen studies with a total of 1365 patients were included, 688 in the TACE+L+I group and 677 in the TACE+L group. Meta-analysis showed that the TACE+L+I group was significantly higher than the TACE+L group in complete response (RR = 2.34, 95%CI:1.53, 3.59, p < 0.0001), partial response (RR = 1.45, 95%CI:1.28, 1.64, p < 0.0001), objective response rate (RR = 1.55, 95%CI:1.39, 1.73, p < 0.00001), and disease control rate (RR = 1.22, 95%CI:1.10, 1.36, p = 0.0003). The TACE+L+I group was significantly lower than the TACE+L group in progression of disease (RR = 0.39, 95%CI:0.30, 0.51, p < 0.00001). Moreover, TACE+L+I group was not significantly different from TACE+L group in stable disease (RR = 0.85, 95%CI:0.69, 1.03, p = 0.10). The TACE+L+I group was significantly higher than the TACE+L group in overall survival (HR = 2.32, 95%CI:1.95, 3.15, p<0.05) and progression-free survival (HR = 2.30, 95%CI:1.80, 2.93, p<0.05). The TACE+L+I group had a significantly higher incidence of hypothyroidism compared to the TACE+L group (RR = 1.81, 95%CI:1.20, 2.71, p<0.05), but there was no significant difference in other adverse events, such as hypertension, diarrhea, hand-foot syndrome, fatigue, elevated AST, elevated ALT, decreased appetite, hypothyroidism, abdominal pain, thrombocytopenia, rash, and nausea.
Conclusion: ICIs significantly improved the survival outcome of uHCC treated with TACE+L, and increased the incidence of hypothyroidism. However, this conclusion still needs further validation in the future with more high-quality randomized controlled trials and longer follow-up.
1 Introduction
Hepatocellular carcinoma (HCC), one of the most common fatal malignancies, accounts for 75-85% of primary liver malignant tumors (1). Although surgical resection, ablation, and liver transplantation are effective on early-stage HCC, most patients with HCC are diagnosed with advanced disease and have a poor prognosis, with an expected median survival of 6–8 months (2–4). Transarterial chemoembolization (TACE) is recommended by various guidelines for the treatment of unresectable HCC (uHCC) (2–4). The use of TACE can effectively slow down the local progression of intrahepatic tumors, but may not be as effective in treating extrahepatic metastases, making it a less satisfactory treatment option (5). Hypoxia occurs in liver after TACE, which induce tumor angiogenesis and potentially lead to tumor recurrence and progression. The combination of anti-angiogenic drugs with TACE can effectively counteract the angiogenesis caused by hypoxia after TACE, resulting in better inhibition of HCC (6, 7). Lenvatinib is a new tyrosine kinase inhibitor approved in 2018 as a first-line treatment for uHCC (8). Some studies have shown that the combination of TACE and lenvatinib can induce a satisfactory effect in the treatment of uHCC (9–13), and the combination of TACE and antiangiogenic drugs has become a promising choice for the treatment of advanced-stage HCC.
Immune checkpoint inhibitors (ICIs), including programmed death 1 (PD-1) and programmed death ligand 1 (PD-L1) inhibitors, have recently shown clinical benefit in patients with a variety of solid tumors (14). Some encouraging results suggest that the combination of TACE, lenvatinib, and ICIs has promising therapeutic potential for patients with HCC (15, 16). In theory, hypoxia following TACE promotes angiogenesis and disrupts antitumor immunity. However, lenvatinib not only inhibits angiogenesis but also normalizes vasculature and reduces the immunosuppressive environment of tumors, creating a favorable setting for T cell trafficking into tumors, thereby enabling the efficacy of ICIs (17–19); thus the combination of TACE, lenvatinib, and ICIs may induce a synergistic antitumor effect on HCC, improving clinical outcomes and inducing manageable side effects. Some studies comparing the efficacy and safety of TACE+L+I versus TACE combined with lenvatinib (TACE+L) in the treatment of patients with uHCC are available, but with inconsistent conclusions. Therefore, the purpose of this meta-analysis was to evaluate the efficacy and safety of ICIs in patients with uHCC treated with TACE+L, to be used as a clinical reference.
2 Materials and methods
A systematic evaluation and meta-analysis of preferred reporting items was performed according to the PRISMA guidelines (20). This study did not require formal institutional review board approval or patient informed consent because it was a secondary study using publicly available data.
2.1 Search strategy
A literature search on the EMBASE, PubMed, and Cochrane Library databases was performed to identify relevant available articles up to November 1, 2024. The search strategy for each database is shown in the Supplementary File S1. The authors were contacted to obtain extra information if necessary. If multiple studies were performed by the same authors or medical centers with duplicates in patients, the highest quality study was selected.
2.2 Inclusion criteria
(1) Study population: confirmed diagnosis of uHCC; (2) Publicly available literature reporting comparative efficacy of TACE+L+I and TACE+L; (3) No restriction on the study sample size; (4) No restriction on the duration of the follow-up; (5) No restriction on the type of language used to write the articles; (6) Human studies only; and (7) Study results were evaluated by the Response to Criteria for Evaluation of the Efficacy of Solid Tumors (RECIST), Common Terminology Criteria for Adverse Events v5.0.
2.3 Extraction criteria
(1) Studies with incomplete information, no access to valid data, no response from the authors after contacting them, duplicates and unpublished studies; (2) single-arm studies of TACE+L+I or TACE+L; (3) other treatments such as radiofrequency ablation; and (4) reviews, case reports, and animal experiments.
2.4 Quality assessment
In all the included studies, RCTs conducted a risk assessment of the risk according to the “risk assessment tool” recommended by the Cochrane Collaboration Network. The Cohort Studies are based on the Newcastle-Ottawa Scale. The results are shown in the Supplementary File S2.
2.5 Statistical analysis
Statistical analysis in this meta-analysis was performed using Stata SE 15 software. Relative risk (RR) was calculated in comparison of dichotomous variables by Mantel-Haenszel method, and hazard ratio (HR) was calculated in the comparison of survival variables by Inverse Variance method. The level of heterogeneity among studies was evaluated using I2 statistics. A randomized model was used in this study. Sensitivity analysis was performed by removing 1 study at a time to assess whether the results were markedly affected by a single study. Funnel plots were used to qualitatively assess publication bias, and the results are shown in the Supplementary File S3. Begg’s test and Egger’s test were quantitatively used to assess publication bias in the included studies, and their significance level was limited to 0.05, as shown in the Supplementary File S4.
3 Results
3.1 Search results and study selection
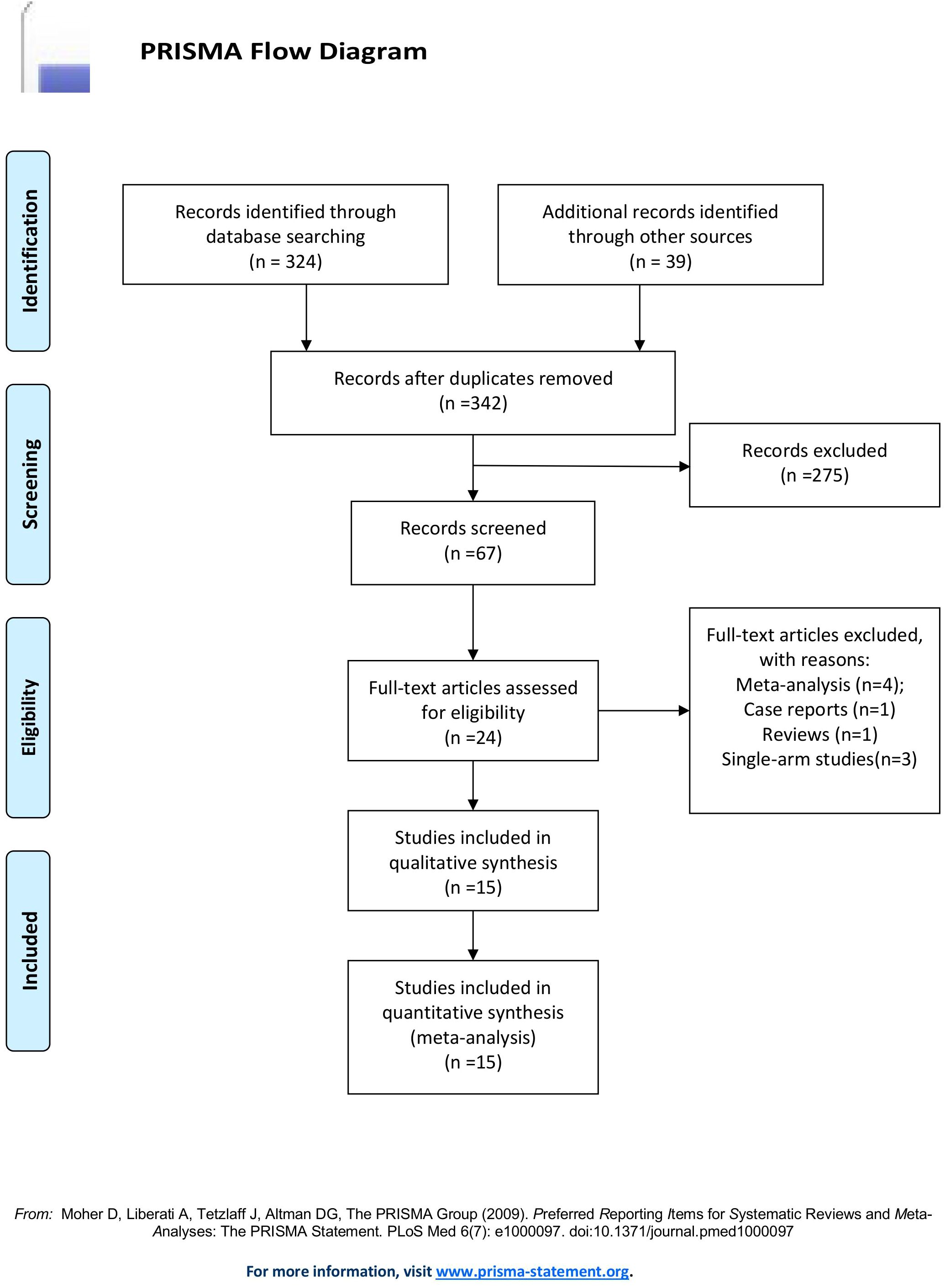
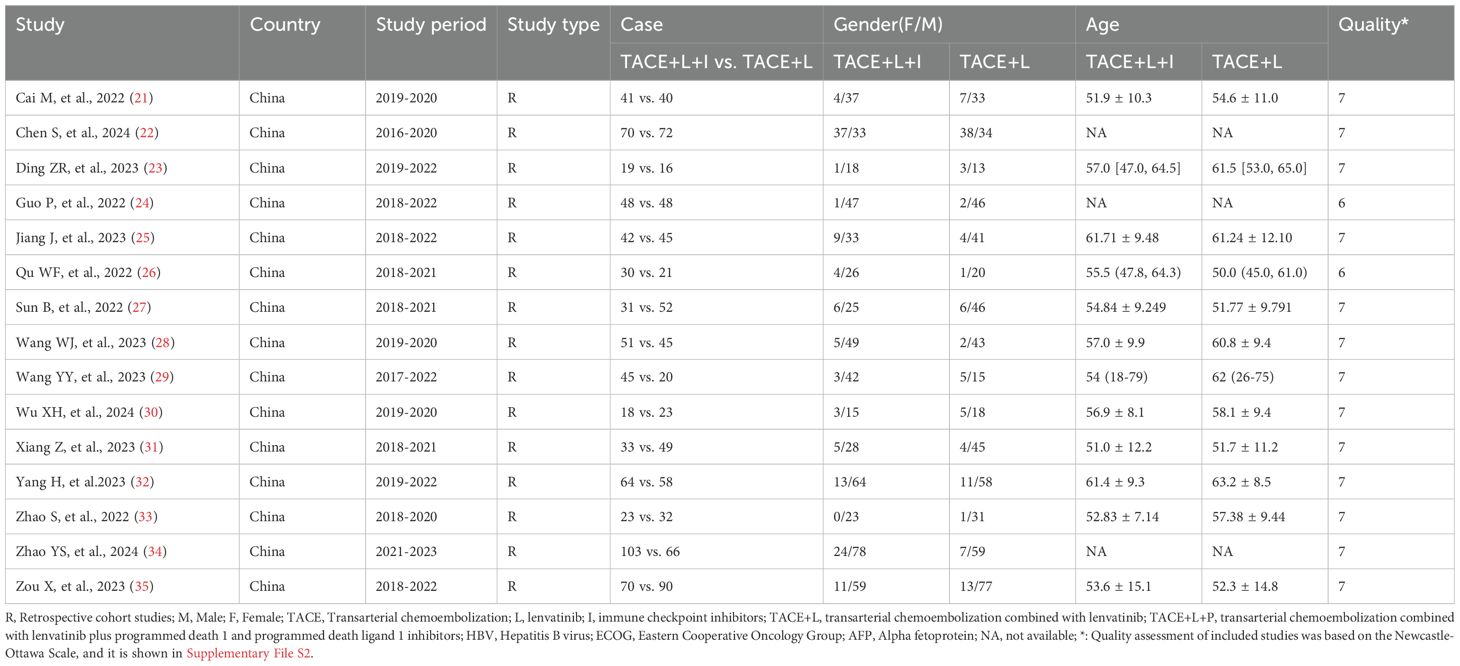
A total of 363 articles were collected. The duplicates were excluded; then 342 articles remained. Then, reviews, case reports, and other types of articles were excluded. Finally, 15 articles remained (21–35). The detailed steps of our literature search are shown in Figure 1. Fifteen studies with a total of 1365 patients were included in the final analysis. A total of 688 patients (50.40%) received TACE+L+I, and 677 (49.60%) patients received TACE+L. The characteristics of these studies are listed in Table 1.
3.2 Meta-analysis results
The treatment effects of TACE+L+I and TACE+L were compared by assessing tumor response, long-term survival outcome, and adverse events. The results are listed in Tables 2, 3.
3.2.1 Tumor response
Fifteen studies (21–35) reported complete response, partial response, stable disease, progression of disease, objective response rate, and disease control rate. The meta-analysis showed that the TACE+L+I group was significantly better than the TACE+L group in complete response (RR = 2.34, 95%CI:1.53, 3.59, p < 0.0001), partial response (RR = 1.45, 95%CI:1.28, 1.64, p < 0.0001), progression of disease (RR = 0.39, 95%CI:0.30, 0.51, p < 0.00001), objective response rate (RR = 1.55, 95%CI:1.39, 1.73, p < 0.00001), disease control rate (RR = 1.22, 95%CI:1.10, 1.36, p = 0.0003), whereas the TACE+L+I group was not significantly different from the TACE+L group in stable disease (RR = 0.85, 95%CI:0.69, 1.03, p = 0.10). As shown in Table 2.
3.2.2 Long-term survival outcome
Thirteen studies (23, 25, 27–35) reported the overall survival and progression free survival. The meta-analysis showed that the TACE+L+I group was significantly better than the TACE+L group in terms of overall survival (HR = 2.32, 95%CI:1.95, 3.15, p < 0.05) and progression free survival (HR = 2.30, 95%CI:1.80, 2.93, p < 0.05), as shown in Table 2 and Figure 2.
3.2.2.1 Adverse events
The included studies (21–35) reported the hypertension, diarrhea, hand-foot syndrome, fatigue, elevated ast, elevated alt, decreased appetite, hypothyroidism, abdominal pain, thrombocytopenia, rash, and nausea. The meta-analysis showed no significant difference between the TACE+L+I and TACE+L group regarding hypertension (RR = 1.19, 95%CI:1.00, 1.41, P = 0.05), diarrhea (RR = 1.18, 95%CI:0.95,1.46, P = 0.14), hand-foot syndrome (RR = 1.04, 95%CI:0.87,1.24, P = 0.70), fatigue (RR = 1.10, 95%CI:0.93, 1.30, P = 0.28), elevated AST (RR = 1.02, 95%CI:0.92,1.13, P = 0.72), elevated ALT (RR = 1.08, 95%CI:0.93,1.26, P = 0.31), decreased appetite (RR = 0.98, 95%CI:0.71,1.37, P = 0.92), abdominal pain (RR = 1.04, 95%CI:0.91, 1.18, P = 0.55), thrombocytopenia (RR = 1.16, 95% CI:0.81,1.66, P = 0.43), rash (RR = 1.13, 95%CI. 0.92, 1.39, P = 0.24), and nausea (RR = 1.00, 95%CI:0.85,1.17, P = 1.00). However, hypothyroidism was significantly higher in the TACE+L+I group (RR = 1.81, 95%CI: 1.20, 2.71, P = 0.004) than in the TACE+L group, as shown in Table 3 and Figure 3.
3.3 Sensitivity analysis and publication bias
The results of each meta-analysis were stable when the sensitivity analysis was performed. Begg’s funnel plot with pseudo 95% confidence limits is shown in Supplementary File S3. Publication bias was not found using Begg’s test and Egger’s test, as shown in Supplementary File S4.
4 Discussion
HCC is the fifth most common cancer worldwide and the second most common cause of cancer-related deaths (1). First-line treatment options for HCC include surgical resection, ablation, liver transplantation, TACE, and drugs, but despite that, the prognosis of uHCC remains poor (36). The TACE is the standard treatment recommended by the European Association for the Study of the Liver guidelines and the Barcelona Clinic Liver Cancer (BCLC) treatment strategy for intermediate and advanced HCC (37, 38), and it may result in tumor response in up to 50% of HCC, resulting in a survival benefit. However, TACE alone has limited therapeutic efficacy as a local-regional therapy, which may promote anti-tumor immunity by releasing tumor antigens and inducing damage-associated molecules that induce “immunogenic cell death” (39, 40). Furthermore, the hypoxic microenvironment after TACE may result in the expression of VEGF and PD-L1 (39–42). Antiangiogenic drugs combined with ICIs represent a promising addition to TACE (43–48). So far, the conclusions of various studies regarding the survival benefit from TACE+L+I of patients with uHCC are inconsistent.
The results of our meta-analysis found that complete response, partial response, objective response rate, and disease control rate were significantly higher in the TACE+L+I group, while progression of disease was significantly lower in the TACE+L+I group than in the TACE+L group, suggesting that the addition of ICIs has a significantly enhanced the tumor response rate. Different therapeutic approaches at different clinical stages are needed to combat HCC, and combination therapy may be used (49, 50). Although tumor response rates suggest that the TACE+L+I is superior to the TACE+L, the effectiveness of antitumor treatments should be based on more direct evidence of clinical benefit, such as prolonged survival, improved quality of life, or reduction of associated symptoms. These clinical benefits are sometimes not predicted by tumor response rates. Therefore, the survival data were analyzed, revealing that the overall survival and progression-free survival were longer in the TACE+L+I group than in the TACE+L group.
Currently, the combination of TACE and lenvatinib is considered clinically safe. Our meta-analysis found that the TACE+L+I group had a significantly higher incidence of hypothyroidism compared to the TACE+L group, while the TACE+L+I group was not significantly different from the TACE+L group in other adverse events, such as hypertension, diarrhea, hand-foot syndrome, fatigue, elevated ast, elevated alt, decreased appetite, hypothyroidism, abdominal pain, thrombocytopenia, and rash, nausea. Common adverse events with lenvatinib included hand and foot skin reactions, diarrhea, and hypertension, while common adverse events with TACE included pain and transient transaminase elevations. Fortunately, treatment-related adverse events were predominantly grade 1 or 2 and were resolved or eliminated after appropriate and prompt management. Therefore, the adverse events associated with TACE+L+I were acceptable.
Several meta-analyses are available on similar topics, but all of them have limitations. Gao Y, et al. (51) performed a meta-analysis and concluded that TACE or hepatic arterial infusion chemotherapy (HAIC) combined with lenvatinib plus PD-1 inhibitor could effectively delays the progression of HCC, prolong the survival, and improve the quality of life of HCC patients with portal vein thrombosis. The intervention in that study included HAIC in addition to TACE. In another meta-analysis by Liu J, et al. (52), the authors compared the efficacy of TACE plus tyrosine kinase inhibitors and ICIs (T+T+I) with that of TACE plus tyrosine kinase inhibitors (T+T) for the treatment of uHCC, concluding that T+T+I for advanced HCC had better objective response rate, as well as longer progression-free survival and overall survival than TACE+T, with no significant increase in adverse events. They did not distinguish between lenvatinib and other tyrosine kinase inhibitors in the study, making the conclusions too broadly applicable. The first systematic review on T+L+I for uHCC was conducted by Sun L, et al. (53), but no further meta-analysis was performed. The first meta-analysis on the same topic was conducted by Liu J, et al. (54) in 2023. Eight cohort studies on TACE plus lenvatinib with or without ICIs for uHCC were included. The T+L+I group had significantly longer overall and progression-free survival in that meta-analysis, as well as higher objective response and disease control rates, which was consistent with our findings; however, there was a higher incidence of hypertension, vomiting or nausea, and hypothyroidism in the T+L+I group, which was inconsistent with our study. Our meta-analysis only found that the T+L+I group was significantly higher than the T+L group in hypothyroidism. The conclusions of our meta-analysis may be more in line with the first-line clinic, as the latest studies we included allowed for a larger number of patients to reduce the potential bias in previous meta-analyses.
This is the most comprehensive meta-analysis available assessing TACE+L+I for uHCC, and although most of the included studies were retrospective, which would be inherently subjected to selection and publication bias, retrospective studies can be reflective of the real world. Our findings assessed the real-world clinical efficacy of TACE+L+I for the treatment of uHCC, and provided a guidance for subsequent clinical studies, although this combination therapy needs further exploration in future randomized controlled trials.
5 Conclusions
TACE+L+I for advanced HCC resulted in significantly better tumor response rates, overall survival, and disease-free survival than TACE+L, while the incidence of hypothyroidism was higher in the TACE+L+I group than in the TACE+L group. The adverse events of ICIs were acceptable compared to the survival benefit of ICIs. These conclusions still need to be further confirmed in the future with high-quality randomized controlled trials.
6 Limitations
Our study has several limitations. First, this was a retrospective study, which may lead to selection bias. Second, there was significant heterogeneity across the included studies in terms of disease control rate. By exploring the source of the heterogeneity, we found that it was not originated from a particular study or studies, which may be related to the data distribution characteristics, because disease control rate corresponds to progressive disease, and inter-study heterogeneity is low in progressive disease. Therefore, subsequent scholars should be cautious in applying the outcome. Finally, most of the included studies were from a single institution with a limited number of cases and most came from China, leading to conclusions with little explanatory power.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
WZ: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Writing – original draft. ZL: Resources, Validation, Visualization, Writing – review & editing. HL: Conceptualization, Methodology, Writing – original draft. ZH: Investigation, Project administration, Resources, Writing – review & editing. XH: Project administration, Resources, Writing – review & editing. LX: Conceptualization, Funding acquisition, Project administration, Writing – review & editing. XC: Conceptualization, Data curation, Funding acquisition, Supervision, Writing – review & editing. ZZ: Conceptualization, Data curation, Formal analysis, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Shenzhen High-Level Hospital Construction Fund, the Sanming Project of Medicine in Shenzhen (SZSM202011010), National Natural Science Foundation of China (82072728), Medical Science and Technology Research Fund of Guangdong Province (No. A2023292), and National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen (E010122001 and SZ2020MS011).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1573505/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. (2020) 9:682–720. doi: 10.1159/000509424
3. Benson AB, D’Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:541–65. doi: 10.6004/jnccn.2021.0022
4. European Association For The Study Of The Liver. Corrigendum to ‘EASL recommendations on treatment of hepatitis C: Final update of the series’ [J Hepatol 73 (2020) 1170-1218. J Hepatol. (2023) 78:452. doi: 10.1016/j.jhep.2022.10.006
5. Fu Z, Li X, Zhong J, Chen X, Cao K, Ding N, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int. (2021) 15:663–75. doi: 10.1007/s12072-021-10184-9
6. Chang Y, Jeong SW, Young Jang J, and Jae Kim Y. Recent updates of transarterial chemoembolilzation in hepatocellular carcinoma. Int J Mol Sci. (2020) 21:8165. doi: 10.3390/ijms21218165
7. Kishore SA, Bajwa R, and Madoff DC. Embolotherapeutic strategies for hepatocellular carcinoma: 2020 update. Cancers (Basel). (2020) 12(4):791. doi: 10.3390/cancers12040791
8. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. (2018) 391:1163–73. doi: 10.1016/S0140-6736(18)30207-1
9. Chen S, Wu Z, Shi F, Mai Q, Wang L, Wang F, et al. Lenvatinib plus TACE with or without pembrolizumab for the treatment of initially unresectable hepatocellular carcinoma harbouring PD-L1 expression: a retrospective study. J Cancer Res Clin Oncol. (2022) 148:2115–25. doi: 10.1007/s00432-021-03767-4
10. Kudo M. A new treatment option for intermediate-stage hepatocellular carcinoma with high tumor burden: initial lenvatinib therapy with subsequent selective TACE. Liver Cancer. (2019) 8:299–311. doi: 10.1159/000502905
11. Yuan P, Song J, Wang F, Zhu G, and Chen B. Combination of TACE and Lenvatinib as a promising option for downstaging to surgery of initially unresectable intrahepatic cholangiocarcinoma. Invest New Drugs. (2022) 40:1125–32. doi: 10.1007/s10637-022-01257-z
12. Cao F, Yang Y, Si T, Luo J, Zeng H, Zhang Z, et al. The efficacy of TACE combined with lenvatinib plus sintilimab in unresectable hepatocellular carcinoma: A multicenter retrospective study. Front Oncol. (2021) 11:783480. doi: 10.3389/fonc.2021.783480
13. Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao C, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: A phase III, randomized clinical trial (LAUNCH). J Clin Oncol. (2023) 41:117–27. doi: 10.1200/JCO.22.00392
14. Cheng AL, Hsu C, Chan SL, Choo SP, and Kudo M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol. (2020) 72:307–19. doi: 10.1016/j.jhep.2019.09.025
15. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. (2020) 38:2960–70. doi: 10.1200/JCO.20.00808
16. Qu S, Zhang X, Wu Y, Meng Y, Pan H, Fang Q, et al. Efficacy and safety of TACE combined with lenvatinib plus PD-1 inhibitors compared with TACE alone for unresectable hepatocellular carcinoma patients: A prospective cohort study. Front Oncol. (2022) 12:874473. doi: 10.3389/fonc.2022.874473
17. Guo X, Nie H, Zhang W, Li J, Ge J, Xie B, et al. Contrasting cytotoxic and regulatory T cell responses underlying distinct clinical outcomes to anti-PD-1 plus lenvatinib therapy in cancer. Cancer Cell. (2025) 43:248–68. doi: 10.1016/j.ccell.2025.01.001
18. Kimura T, Kato Y, Ozawa Y, Kodama K, Ito J, Ichikawa K, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1–6 hepatocellular carcinoma model. Cancer Sci. (2018) 109:3993–4002. doi: 10.1111/cas.2018.109.issue-12
19. Yi C, Chen L, Lin Z, Liu L, Shao W, Zhang R, et al. Lenvatinib targets FGF receptor 4 to enhance antitumor immune response of anti-programmed cell death-1 in HCC. Hepatology. (2021) 74:2544–60. doi: 10.1002/hep.31921
20. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006
21. Cai M, Huang W, Huang J, Shi W, Guo Y, Liang L, et al. Transarterial chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: A retrospective cohort study. Front Immunol. (2022) 13:848387. doi: 10.3389/fimmu.2022.848387
22. Chen S, Shuangyan T, Shi F, Cai H, Wu Z, Wang L, et al. TACE plus lenvatinib and tislelizumab for intermediate-stage hepatocellular carcinoma beyond up-to-11 criteria: a multicenter cohort study. Front Immunol. (2024) 15:1430571. doi: 10.3389/fimmu.2024.1430571
23. Ding Z, Fang G, Tang Y, and Zeng Y. The impact of PD-1 inhibitors on prognosis in unresectable hepatocellular carcinoma treated with TACE and lenvatinib: a retrospective study. Sci Rep. (2024) 14:14334. doi: 10.1038/s41598-024-63571-1
24. Guo P, Pi X, Gao F, Li Q, Li D, Feng W, et al. Transarterial chemoembolization plus lenvatinib with or without programmed death-1 inhibitors for patients with unresectable hepatocellular carcinoma: A propensity score matching study. Front Oncol. (2022) 12:945915. doi: 10.3389/fonc.2022.945915
25. Jiang J, Zhang H, Lai J, Zhang S, Ou Y, Fu YN, et alEfficacy and safety of transarterial chemoembolization plus lenvatinib with or without tislelizumab as the first-Line treatment for unresectable hepatocellular carcinoma: a propensity score matching analysis. J Hepatocell Carcinoma. (2024) 1:1607–22. doi: 10.2147/JHC.S472286
26. Qu WF, Ding ZB, Qu XD, Tang Z, Zhu GQ, Fu XT, et al. Conversion therapy for initially unresectable hepatocellular carcinoma using a combination of toripalimab, lenvatinib plus TACE: real-world study. BJS Open. (2022) 6:zrac114. doi: 10.1093/bjsopen/zrac114
27. Sun B, Zhang L, Sun T, Ren Y, Cao Y, Zhang W, et al. Safety and efficacy of lenvatinib combined with camrelizumab plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: A two-center retrospective study. Front Oncol. (2022) 12:982948. doi: 10.3389/fonc.2022.982948
28. Wang WJ, Liu ZH, Wang K, Yu HM, Cheng YQ, Xiang YJ, et al. Efficacy and safety of TACE combined with lenvatinib and PD-1 inhibitors for unresectable recurrent HCC: A multicenter, retrospective study. Cancer Med. (2023) 12:11513–24. doi: 10.1002/cam4.v12.10
29. Wang YY, Yang X, Wang YC, Long JY, Sun HS, Li YR, et al. Clinical outcomes of lenvatinib plus transarterial chemoembolization with or without programmed death receptor-1 inhibitors in unresectable hepatocellular carcinoma. World J Gastroenterol. (2023) 29:1614–26. doi: 10.3748/wjg.v29.i10.1614
30. Wu HX, Ding XY, Xu YW, Yu MH, Li XM, Deng N, et al. Transcatheter arterial chemoembolization combined with PD-1 inhibitors and Lenvatinib for hepatocellular carcinoma with portal vein tumor thrombus. World J Gastroenterol. (2024) 30:843–54. doi: 10.3748/wjg.v30.i8.843
31. Xiang Z, Li G, Mu L, Wang H, Zhou C, Yan H, et al. and camrelizumab for unresectable multiple nodular and large hepatocellular carcinoma (>5 cm). Technol Cancer Res Treat. (2023) 22:15330338231200320. doi: 10.1177/15330338231200320
32. Yang H, Yang T, Qiu G, and Liu J. Efficacy and safety of TACE combined with lenvatinib and PD-(L)1 inhibitor in the treatment of unresectable hepatocellular carcinoma: A retrospective study. J Hepatocell Carcinoma. (2023) :10:1435–1443. doi: 10.2147/JHC.S423684
33. Zhao S, Zhou M, Wang P, Yang J, Zhang D, Yin F, et al. Sorafenib, lenvatinib, or lenvatinib combining PD-1 inhibitors plus TACE in unresectable hepatocellular carcinoma: A retrospective analysis. Technol Cancer Res Treat. (2022) 21:15330338221133640. doi: 10.1177/15330338221133640
34. Zhao Y, Wen S, Xue Y, Dang Z, Nan Z, Wang D, et al. Transarterial chemoembolization combined with lenvatinib plus tislelizumab for unresectable hepatocellular carcinoma: a multicenter cohort study. Front Immunol. (2024) 15:1449663. doi: 10.3389/fimmu.2024.1449663
35. Zou X, Xu Q, You R, and Yin G. Correlation and efficacy of TACE combined with lenvatinib plus PD-1 inhibitor in the treatment of hepatocellular carcinoma with portal vein tumor thrombus based on immunological features. Cancer Med. (2023) 12:11315–33. doi: 10.1002/cam4.v12.10
36. Lau WY, Leung TW, Lai BS, Liew CT, Ho SK, Yu SC, et al. Preoperative systemic chemoimmunotherapy and sequential resection for unresectable hepatocellular carcinoma. Ann Surg. (2001) 233:236–41. doi: 10.1097/00000658-200102000-00013
37. Bruix J, Sherman M, and American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. (2011) 53:1020–2. doi: 10.1002/hep.24199
38. Forner A, Reig M, and Bruix J. Hepatocellular carcinoma. Lancet. (2018) 391:1301–14. doi: 10.1016/S0140-6736(18)30010-2
39. Galluzzi L, Buqué A, Kepp O, Zitvogel L, and Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. (2017) 17:97–111. doi: 10.1038/nri.2016.107
40. Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 edition). Liver Cancer. (2018) 7:235–60. doi: 10.1159/000488035
41. Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. (2008) 103:914–21. doi: 10.1111/j.1572-0241.2007.01712.x
42. Pinato DJ, Murray SM, Forner A, Kaneko T, Fessas P, Toniutto P, et al. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J Immunother Cancer. (2021) 9:e003311. doi: 10.1136/jitc-2021-003311
43. Pinato DJ, Howell J, Ramaswami R, and Sharma R. Review article: delivering precision oncology in intermediate-stage liver cancer. Aliment Pharmacol Ther. (2017) 45:1514–23. doi: 10.1111/apt.2017.45.issue-12
44. Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. (2011) 47:2117–27. doi: 10.1016/j.ejca.2011.05.007
45. Kudo M, Han G, Finn RS, Poon RT, Blanc JF, Yan L, et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: A randomized phase III trial. Hepatology. (2014) 60:1697–707. doi: 10.1002/hep.27290
46. Meyer T, Fox R, Ma YT, Ross PJ, James MW, Sturgess R, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. (2017) 2:565–75. doi: 10.1016/S2468-1253(17)30156-5
47. Kudo M, Cheng AL, Park JW, Park JH, Liang PC, Hidaka H, et al. Orantinib versus placebo combined with transcatheter arterial chemoembolisation in patients with unresectable hepatocellular carcinoma (ORIENTAL): a randomised, double-blind, placebo-controlled, multicentre, phase 3 study. Lancet Gastroenterol Hepatol. (2018) 3:37–46. doi: 10.1016/S2468-1253(17)30290-X
48. Park JW, Kim YJ, Kim DY, Bae SH, Paik SW, Lee YJ, et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: The phase III STAH trial. J Hepatol. (2019) 70:684–91. doi: 10.1016/j.jhep.2018.11.029
49. Xiang YJ, Wang K, Yu HM, Li XW, Cheng YQ, Wang WJ, et al. Transarterial chemoembolization plus a PD-1 inhibitor with or without lenvatinib for intermediate-stage hepatocellular carcinoma. Hepatol Res. (2022) 52:721–9. doi: 10.1111/hepr.13773
50. Bruix J, Gores GJ, and Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. (2014) 63:844–55. doi: 10.1136/gutjnl-2013-306627
51. Yuanren G, Yin L, Liu R, and Cui Y. The treatment of transarterial chemoembolization/hepatic arterial infusion chemotherapy combined with lenvatinib and PD-1 inhibitor is effective against hepatocellular carcinoma with portal vein tumor thrombus: A systematic review. Front Oncol. (2023) 13:1054072. doi: 10.3389/fonc.2023.1054072
52. Liu J, Wang P, Shang L, Zhang Z, Tian Y, Chen X, et al. TACE plus tyrosine kinase inhibitors and immune checkpoint inhibitors versus TACE plus tyrosine kinase inhibitors for the treatment of patients with hepatocellular carcinoma: a meta-analysis and trial sequential analysis. Hepatol Int. (2024) 18:595–609. doi: 10.1007/s12072-023-10591-0
53. Sun L, Xu X, Meng F, Liu Q, Wang H, Li X, et al. Lenvatinib plus transarterial chemoembolization with or without immune checkpoint inhibitors for unresectable hepatocellular carcinoma: A review. Front Oncol. (2022) 12:980214. doi: 10.3389/fonc.2022.980214
54. Liu J, Wei S, Yang L, Yu J, Yan D, and Yi P. Efficacy and safety of transarterial chemoembolization plus lenvatinib with or without programmed death-1 inhibitors in the treatment of unresectable hepatocellular carcinoma: a systematic review and meta-analysis. J Cancer Res Clin Oncol. (2023) 149:14451–61. doi: 10.1007/s00432-023-05231-x
Keywords: hepatocellular carcinoma, transarterial chemoembolization, lenvatinib, immune checkpoint inhibitor, PD-1 inhibitor
Citation: Zhang W, Liu Z, Liu H, Huang Z, Huang X, Xu L, Che X and Zhan Z (2025) The impact of immune checkpoint inhibitors on prognosis in unresectable hepatocellular carcinoma treated with TACE and lenvatinib: a meta-analysis. Front. Immunol. 16:1573505. doi: 10.3389/fimmu.2025.1573505
Received: 09 February 2025; Accepted: 25 April 2025;
Published: 21 May 2025.
Edited by:
Jun Li, Shanghai Jiao Tong University, ChinaReviewed by:
Kratika Singh, Centre of Bio-Medical Research (CBMR), IndiaMaria A. Clavijo-Salomon, National Institutes of Health (NIH), United States
Copyright © 2025 Zhang, Liu, Liu, Huang, Huang, Xu, Che and Zhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengyin Zhan, enp5MDEyMEAxMjYuY29t; Xu Che, eWl4dWV0Z0Bmb3htYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Wei Zhang1†
Wei Zhang1† Xu Che
Xu Che