- 1Department of Pharmacy, Fuzhou University Affiliated Provincial Hospital, Fuzhou, Fujian, China
- 2Department of Clinical Nutrition, Zhangzhou Affiliated Hospital of Fujian Medical University, Zhangzhou, Fujian, China
- 3Department of Pharmacy, Mindong Hospital Affiliated to Fujian Medical University, Ningde, Fujian, China
Background: The COMPASSION-15 trial demonstrated that cadonilimab plus chemotherapy (CAD-CHM) confers clinical benefits over placebo plus chemotherapy (PLA-CHM) as a first-line treatment for human epidermal growth factor receptor 2 (HER2)-negative advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma. However, the introduction of cadonilimab substantially elevates treatment costs, and its cost-effectiveness relative to PLA-CHM remains undetermined. This study evaluates the cost-effectiveness of CAD-CHM compared with PLA-CHM from the perspective of the Chinese healthcare system.
Methods: A Markov model with three health states was developed to assess the cost-effectiveness of CAD-CHM in HER2-negative advanced G/GEJ adenocarcinoma. Clinical efficacy data were sourced from the COMPASSION-15 trial, while drug costs were calculated based on national tender prices, and additional costs and utility values were extracted from published literature. The analysis encompassed the overall population, as well as subgroups stratified by programmed death ligand 1 (PD-L1) combined positive score (CPS) ≥ 5 and CPS < 5. Outcomes included total costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs). Sensitivity analyses were conducted to evaluate model robustness.
Results: The ICER of CAD-CHM was $67,378.09 per QALY in the overall population, $48,433.34 per QALY in the PD-L1 CPS ≥ 5 subgroup, and $78,463.86 per QALY in the PD-L1 CPS < 5 subgroup. Key determinants influencing model outcomes included patient weight, cadonilimab cost, and the utility value of progression-free survival. Across all groups, CAD-CHM resulted in an ICER exceeding the willingness-to-pay threshold of $41,511 per QALY, with a 0% probability of cost-effectiveness compared with PLA-CHM.
Conclusion: From the perspective of the Chinese healthcare system, CAD-CHM is not cost-effective as a first-line treatment for HER2-negative advanced G/GEJ adenocarcinoma, either in the overall population or in subgroups stratified by PD-L1 CPS status, compared with chemotherapy alone.
1 Introduction
Gastric cancer, including gastroesophageal junction (GEJ) cancer, remains a major global health challenge, ranking fifth in both incidence and mortality among malignant tumors. In 2022, over 968,000 new cases and nearly 660,000 deaths were reported worldwide (1, 2). China bears a disproportionately high gastric or GEJ (G/GEJ) burden, accounting for 42% of global new cases and 45% of gastric cancer-related deaths (3). Due to the lack of specific clinical symptoms, nearly 90% of patients with G/GEJ cancer are diagnosed at an advanced stage (4), resulting in a dismal prognosis, with a five-year survival rate below 5% (5). Adenocarcinoma is the predominant histological subtype, representing over 90% of G/GEJ cancers, and the majority of these tumors are human epidermal growth factor receptor 2 (HER2)-negative (6, 7). For decades, platinum- and fluorouracil-based combination chemotherapy has remained the standard first-line treatment for HER2-negative advanced G/GEJ adenocarcinoma (8). However, therapeutic outcomes remain suboptimal, with a median survival of less than one year (9).
Recent clinical trials have demonstrated that combining programmed cell death protein 1 (PD-1) inhibitors with chemotherapy improves survival in patients with HER2-negative G/GEJ adenocarcinoma (10–14). Moreover, evidence suggests that dual blockade of PD-1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) enhances antitumor responses across multiple solid tumors (15–17). Cadonilimab, a tetravalent bispecific human antibody targeting PD-1 and CTLA-4, exhibits enhanced binding activity in tumor tissues (18–20). The COMPASSION-15 phase III trial recently evaluated the efficacy and safety of cadonilimab plus chemotherapy (CAD-CHM) as a first-line treatment for HER2-negative advanced G/GEJ adenocarcinoma (21). The results demonstrated a significant improvement in median overall survival (OS) (14.1 vs. 11.1 months) and median progression-free survival (PFS) (7.0 vs. 5.3 months) compared with placebo plus chemotherapy (PLA-CHM), with manageable safety. These findings suggest CAD-CHM as a potential first-line treatment option for HER2-negative advanced G/GEJ adenocarcinoma.
Despite its promising clinical efficacy, the incorporation of cadonilimab into combination therapy substantially increases treatment costs, particularly drug-related expenses, imposing a significant financial burden. In resource-limited settings such as China, the cost-effectiveness of CAD-CHM remains a critical consideration for clinicians and policymakers. To date, no comprehensive economic evaluation has assessed CAD-CHM as a first-line treatment for HER2-negative advanced G/GEJ adenocarcinoma. The absence of such analyses may hinder its adoption in healthcare systems with constrained resources. Therefore, this study aims to evaluate the cost-effectiveness of CAD-CHM compared with PLA-CHM as a first-line treatment for HER2-negative advanced G/GEJ adenocarcinoma from the perspective of the Chinese healthcare system.
2 Methodology
This study was conducted following the Consolidated Health Economic Evaluation Reporting Standards 2022 (Supplementary Table 1) (22).
2.1 Model development
A Markov model was developed using TreeAge 2022 software to assess the cost-effectiveness of CAD-CHM versus PLA-CHM as first-line treatments for HER2-negative advanced G/GEJ adenocarcinoma (Figure 1). The model comprised three health states: PFS, progressive disease (PD), and death. All patients entered the model in the PFS state, with death as the absorbing state (23). During each cycle, patients could either remain in their current state or transition to the next state, with no possibility of reversal. The cycle length was set at 21 days to align with the treatment cycle, and the model was run for 160 cycles (approximately 9.2 years), by which point 99% of patients were expected to have died. The primary model outcomes included total costs, quality-adjusted life-years (QALYs), and the incremental cost-effectiveness ratios (ICERs). In accordance with the Chinese Guidelines for Pharmacoeconomic Evaluation, the willingness-to-pay (WTP) threshold was set at three times the per capita GDP of China in 2024 ($41,511 per QALY) (24), with an ICER below this threshold considered cost-effective.
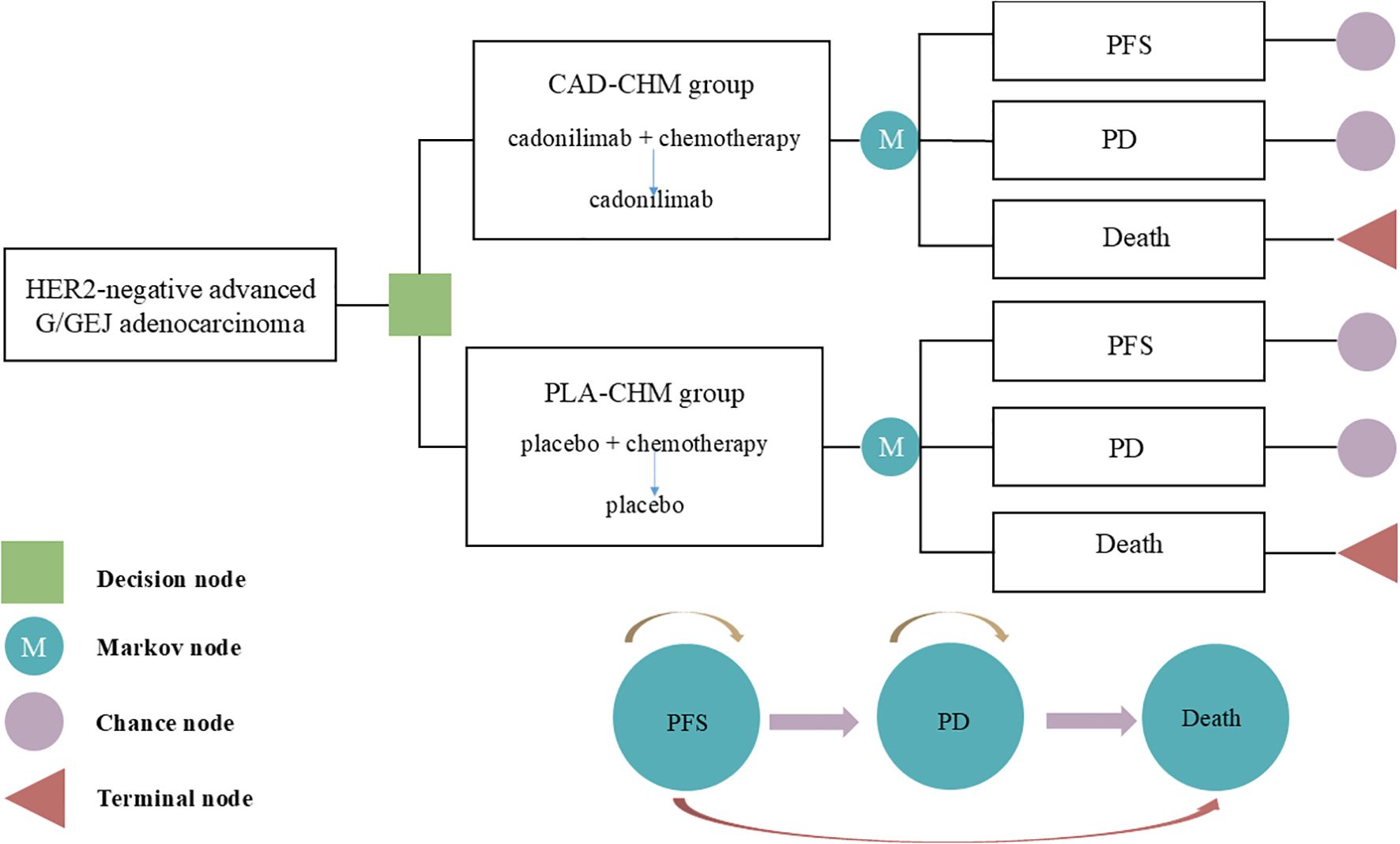
Figure 1. The Markov model simulating outcomes for the COMPASSION-15 trial. All patients started with PFS state and received treatment with CAD-CHM or PLA-CHM. CAD-CHM, cadonilimab plus chemotherapy; G/GEJ, gastric or gastroesophageal junction; HER2, human epidermal growth factor receptor 2; PD, progressive disease; PFS, progression-free survival; PLA-CHM, placebo plus chemotherapy.
2.2 Patient clinical treatment data
Clinical treatment data were derived from the COMPASSION-15 trial (21), a multicenter, randomized, phase III trial conducted across 75 hospitals in China. Eligible patients were aged 18–75 years, had histologically confirmed unresectable, locally advanced, or metastatic HER2-negative G/GEJ adenocarcinoma, and had not received prior systemic anticancer therapy. A total of 610 patients were enrolled in the COMPASSION-15 trial, including 256 patients with a PD-L1 Combined Positive Score (CPS) ≥ 5 and 304 patients with a PD-L1 CPS < 5. Patients were randomized to receive either cadonilimab or placebo in combination with chemotherapy (oxaliplatin and capecitabine) (CAD-CHM or PLA-CHM). Specifically, cadonilimab (10 mg/kg) or placebo was administered via intravenous infusion on Day 1 of each cycle, oxaliplatin (130 mg/m2) via intravenous injection on Day 1 of each cycle, and capecitabine (1,000 mg/m2) orally twice daily on Days 1–14 of each cycle, with each cycle lasting 3 weeks for up to 6 cycles. Thereafter, patients continued maintenance therapy with cadonilimab or placebo until disease progression or intolerable toxicity. According to the COMPASSION-15 trial (21), the median treatment duration for cadonilimab in the CAD-CHM group was 5.62 months, while oxaliplatin and capecitabine were administered for a median of 4.14 months and 4.17 months, respectively. In the PLA-CHM group, oxaliplatin and capecitabine were administered for a median of 4.14 months. As post-progression treatment details were not provided in the COMPASSION-15 trial, it was assumed that all patients received the best supportive care following disease progression. The study population included the overall population as well as subgroups stratified by PD-L1 CPS (≥ 5 and < 5).
2.3 Survival transition probabilities
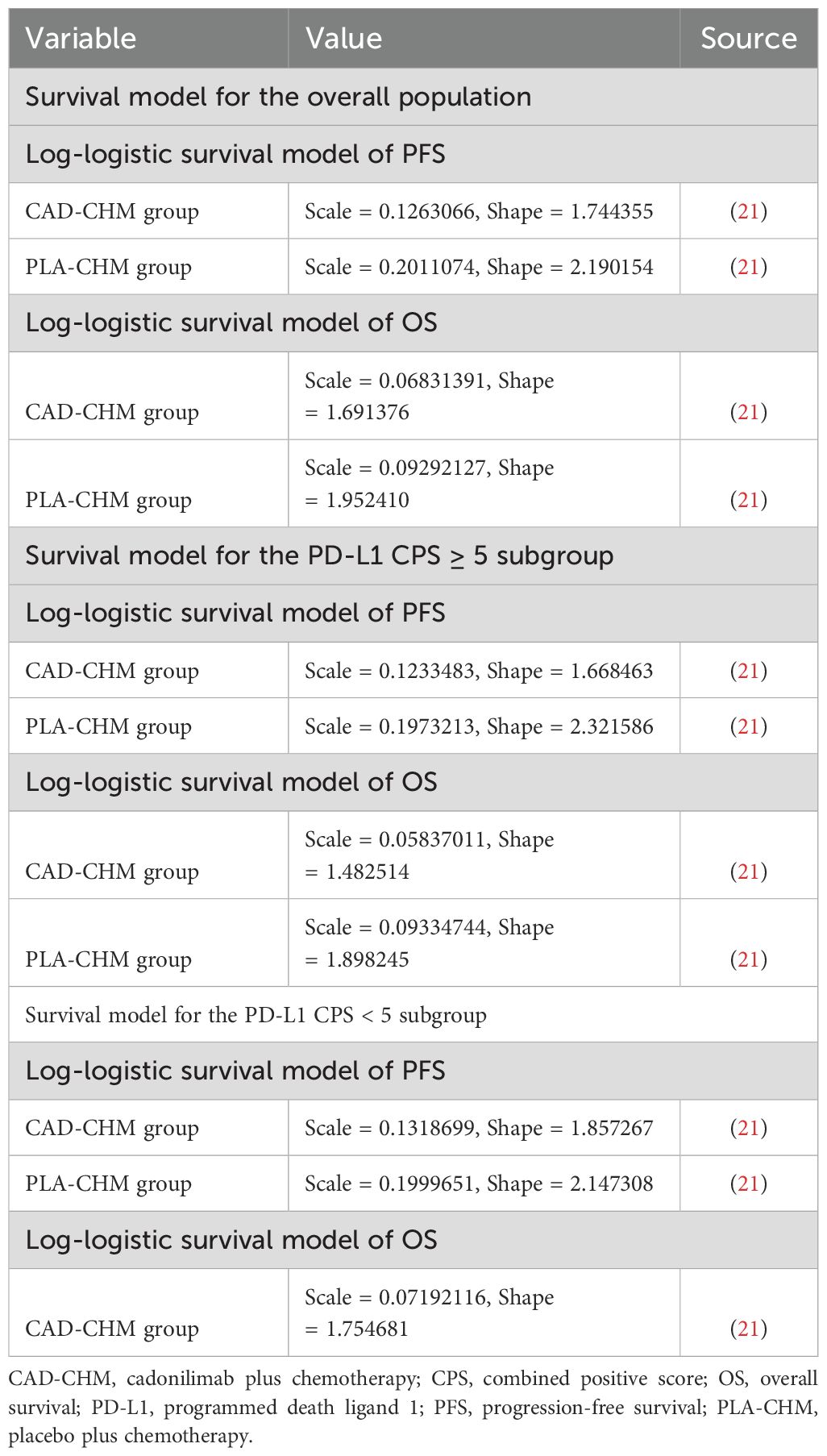
GetData Graph Digitizer (version 2.26) was used to digitize the PFS and OS curves from the COMPASSION-15 trial. Patient survival data were reconstructed in R software following the method outlined by Guyot et al. (25), and various survival distributions were fitted to extrapolate survival curves beyond the clinical trial follow-up period. The evaluated distributions included exponential, gamma, generalized F, generalized gamma, Gompertz, Weibull, log-logistic, and log-normal models (26, 27). The optimal survival distribution was selected based on Akaike and Bayesian information criteria (28, 29) and subsequently used to estimate transition probabilities between health states (Supplementary Table 2). The best-fitting survival distributions and their parameters are detailed in Table 1.
2.4 Costs and utilities
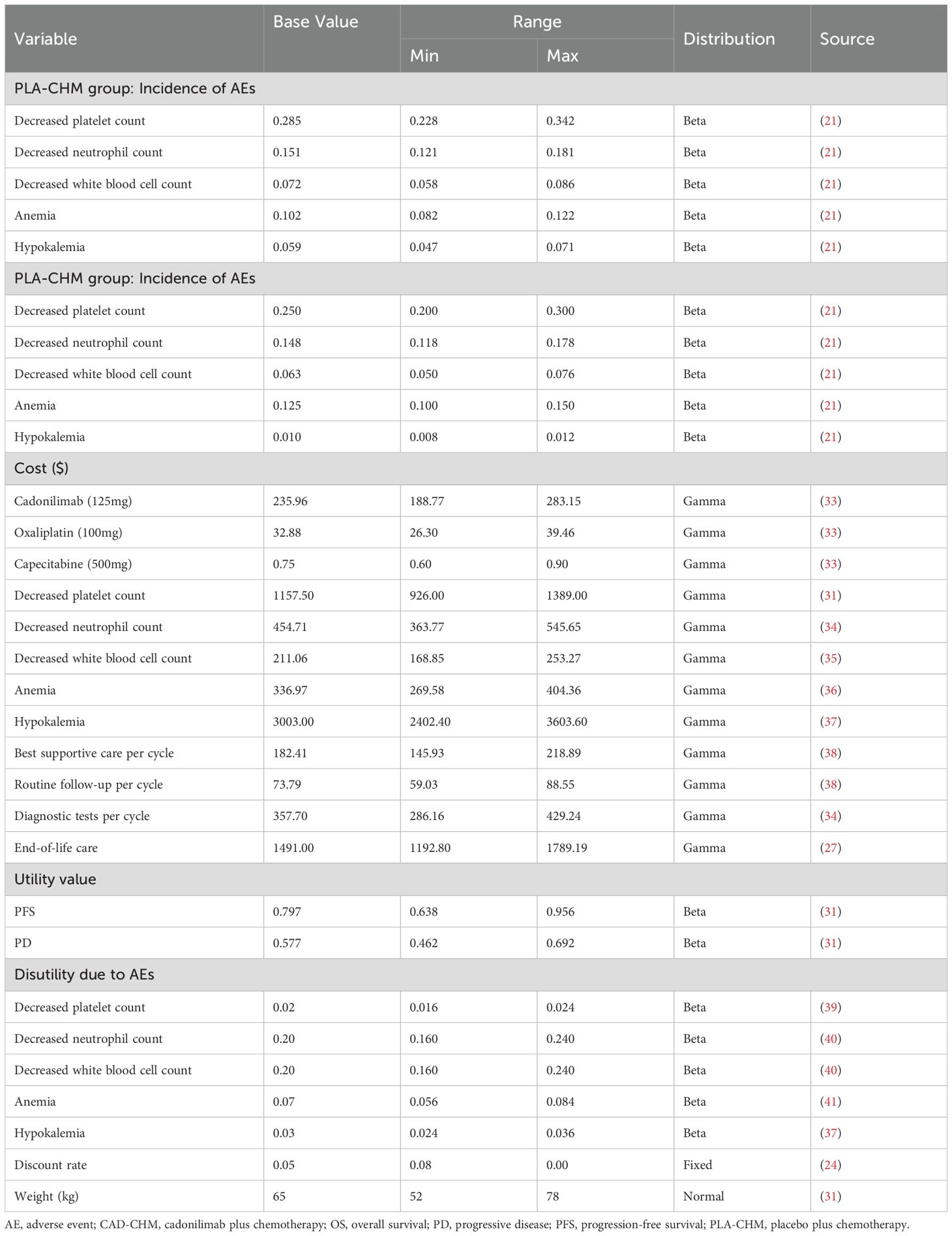
This study exclusively considered direct medical costs, including drug expenses, diagnostic tests, routine follow-up, best supportive care, management of grade 3 or higher adverse events with an incidence exceeding 5%, and end-of-life care (Table 2). Drug costs were sourced from national tender prices, while other expenditures were obtained from published literature and adjusted to 2024 values using the medical price index from the National Bureau of Statistics of China (30). All costs were reported in US dollars and converted at the 2024 average exchange rate (1 USD = 7.12 CNY). As the COMPASSION-15 trial did not provide quality-of-life data, utility values for PFS and PD were derived from published studies in China (31). To address the potential bias arising from the use of identical utility values for the CAD-CHM and PLA-CHM groups, we considered the disutility of grade 3 or higher adverse events with an incidence exceeding 5% in each treatment group, to improve the accuracy of the health utility values for each treatment group. All costs and utility values were discounted at an annual rate of 5%, in line with pharmacoeconomic guidelines (24). Drug dosages were calculated based on an assumed patient weight of 65 kg and a body surface area of 1.72 m2 (32).
2.5 Sensitivity analysis
A one-way sensitivity analysis was performed to evaluate the impact of parameter variations on model outcomes. Each parameter was adjusted within its reported 95% confidence interval, and where unavailable, a ±20% range around the baseline value was applied. The discount rate was varied from 0% to 8% (Table 2). The results were visualized using a tornado diagram. To assess the combined effect of parameter uncertainty, a probabilistic sensitivity analysis was conducted using 1,000 Monte Carlo simulations, with each parameter assigned a specific probability distribution (Table 2). The results were illustrated in a scatter plot. Additionally, the ICER of CAD-CHM compared with PLA-CHM was iteratively recalculated while progressively reducing the price of cadonilimab to determine the threshold at which CAD-CHM becomes cost-effective.
2.6 Scenario analysis
Two scenario analyses were performed for the overall population. In Scenario 1, the model duration was adjusted to 2, 4, and 6 years to assess its influence on model outcomes. In Scenario 2, it was assumed that only 30% or 50% of patients received the best supportive care after disease progression, simulating real-world scenarios where some patients discontinue treatment for various reasons.
3 Results
3.1 Results of the base case analysis
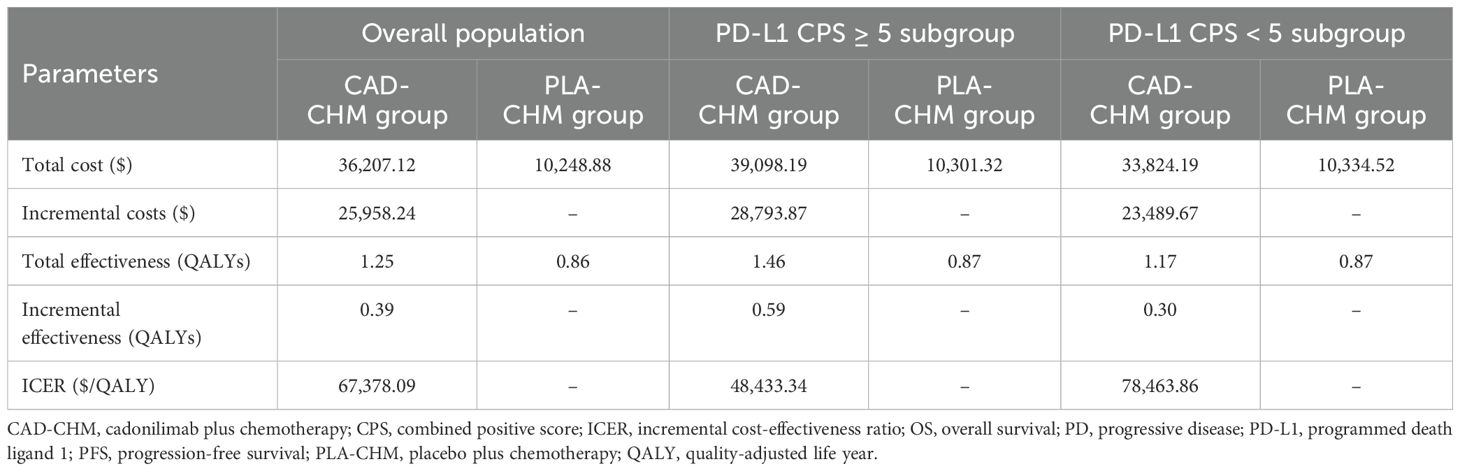
In the overall population, CAD-CHM incurred a total cost of $36,207.12 and yielded 1.25 QALYs, while PLA-CHM had a total cost of $10,248.88 and provided 0.86 QALYs, resulting in an ICER of $67,378.09 per QALY. In the PD-L1 CPS ≥ 5 subgroup, CAD-CHM cost $39,098.19 and achieved 1.46 QALYs, whereas PLA-CHM cost $10,301.32 and yielded 0.87 QALYs, with an ICER of $48,433.34 per QALY. In the PD-L1 CPS < 5 subgroup, CAD-CHM incurred a total cost of $33,824.19 and provided 1.17 QALYs, while PLA-CHM cost $10,334.52 and generated 0.87 QALYs, resulting in an ICER of $78,463.86 per QALY (Table 3). All ICERs exceeded the WTP threshold of $41,511 per QALY, indicating that CAD-CHM is not cost-effective compared with chemotherapy alone for HER2-negative advanced G/GEJ adenocarcinoma from the perspective of the Chinese healthcare system.
3.2 Sensitivity analysis
One-way sensitivity analysis results, visualized in a tornado diagram (Figures 2–4), indicated that patient weight, cadonilimab cost, and PFS utility value were the most influential parameters in the overall population and the PD-L1 CPS < 5 subgroup. However, even under parameter variations, the ICER remained above the WTP threshold, suggesting minimal impact on model conclusions. In contrast, in the PD-L1 CPS ≥ 5 subgroup, CAD-CHM became cost-effective when patient weight and cadonilimab cost approached their lower limits. Probabilistic sensitivity analysis results, illustrated in a scatter plot (Figures 5–7), showed that the probability of CAD-CHM being cost-effective at a WTP threshold of $41,511 per QALY was 6.4% in the overall population, 31.0% in the PD-L1 CPS ≥ 5 subgroup, and 2.4% in the PD-L1 CPS < 5 subgroup. Additionally, cost-reduction analysis revealed that CAD-CHM would only become a cost-effective first-line option for HER2-negative advanced G/GEJ adenocarcinoma in the overall population if the cost of cadonilimab (150 mg) dropped below $129.5.
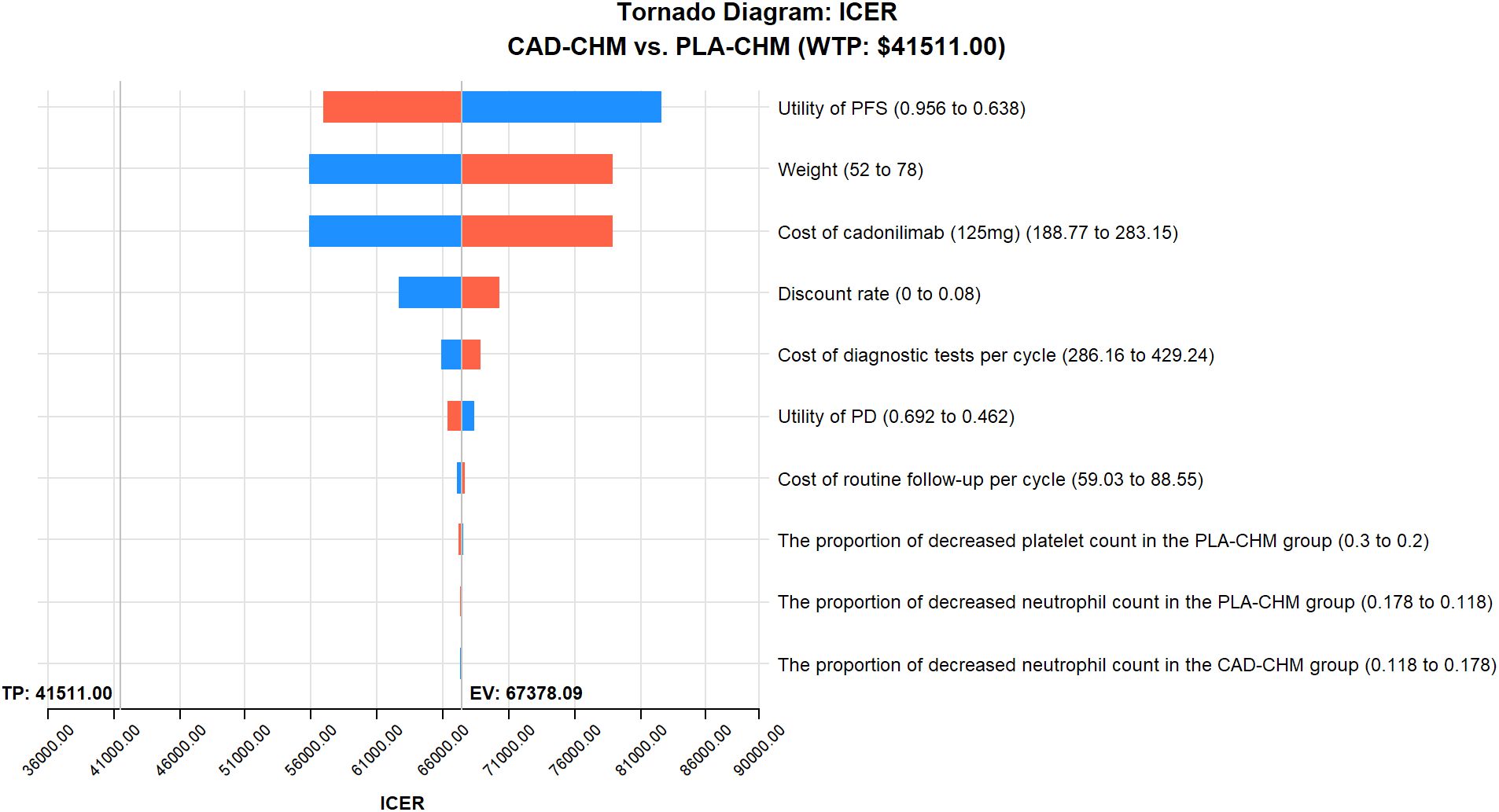
Figure 2. One-way sensitivity analyses in the overall population. CAD-CHM, cadonilimab plus chemotherapy; ICER, incremental cost-effectiveness ratio; PD, progressive disease; PFS, progression-free survival; PLA-CHM, placebo plus chemotherapy.
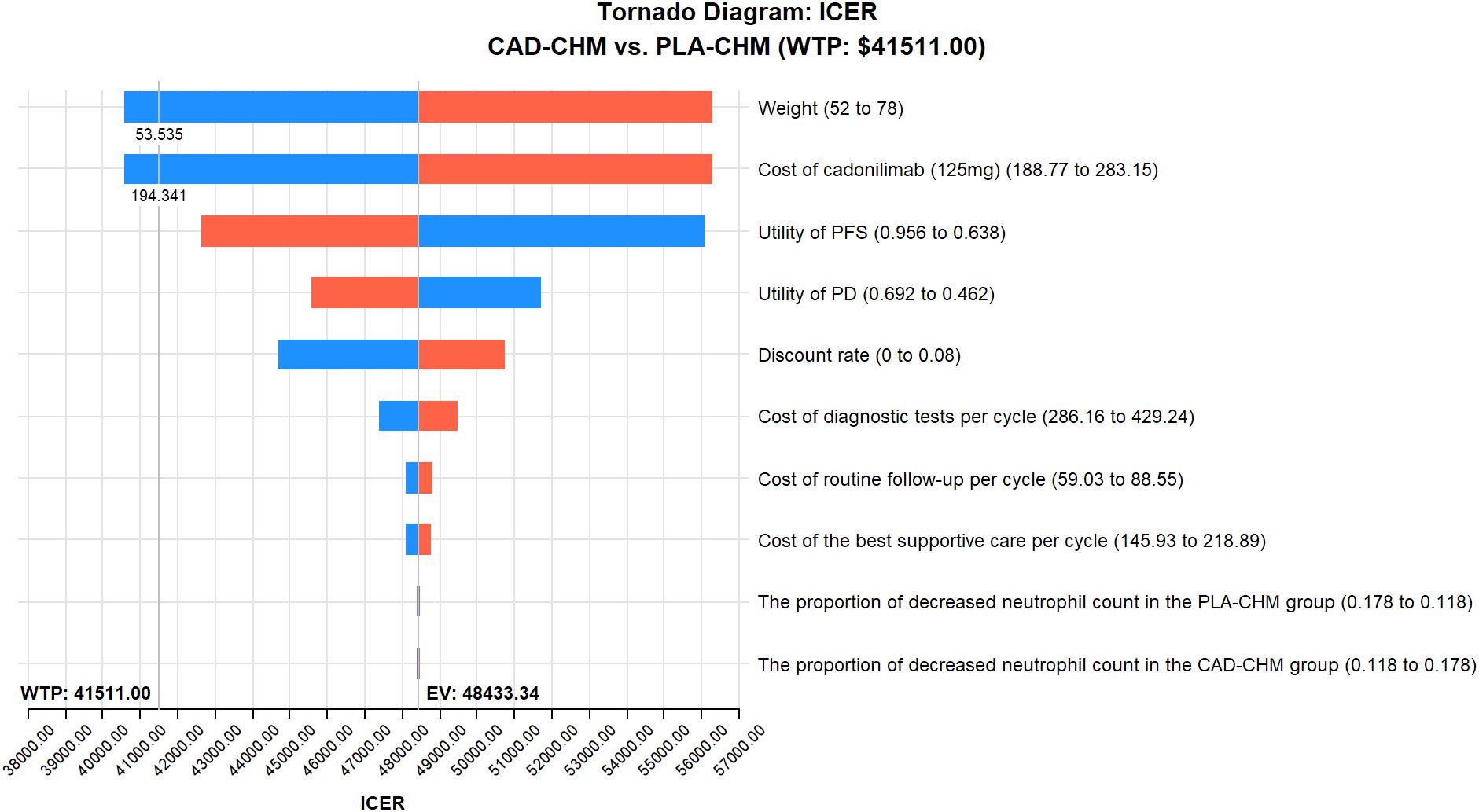
Figure 3. One-way sensitivity analyses in PD-L1 CPS ≥ 5 subgroup. CAD-CHM, cadonilimab plus chemotherapy; ICER, incremental cost-effectiveness ratio; PD, progressive disease; PFS, progression-free survival; PLA-CHM, placebo plus chemotherapy.
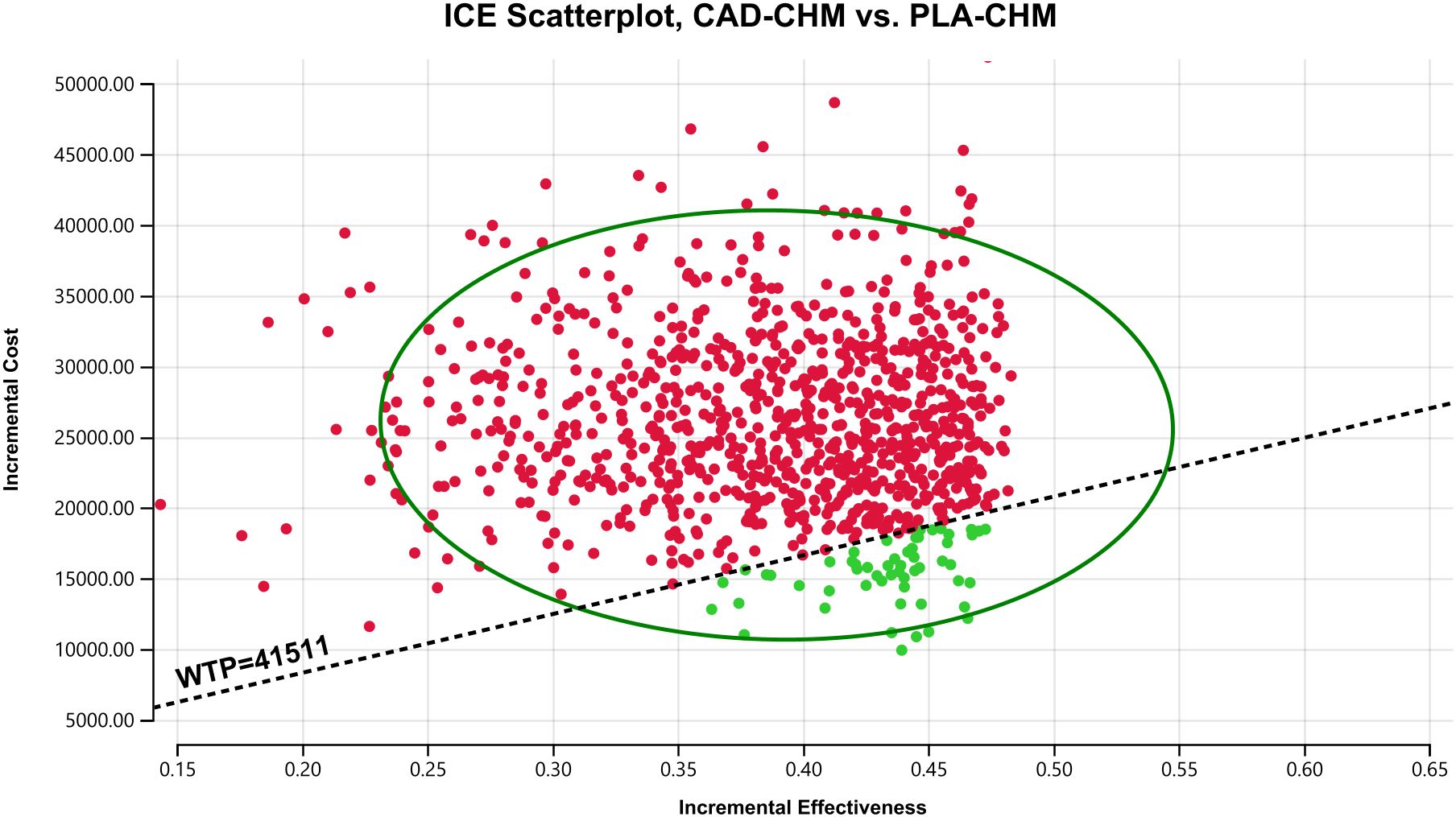
Figure 4. One-way sensitivity analyses in the PD-L1 CPS < 5 subgroup. CAD-CHM, cadonilimab plus chemotherapy; ICER, incremental cost-effectiveness ratio; PD, progressive disease; PFS, progression-free survival; PLA-CHM, placebo plus chemotherapy.
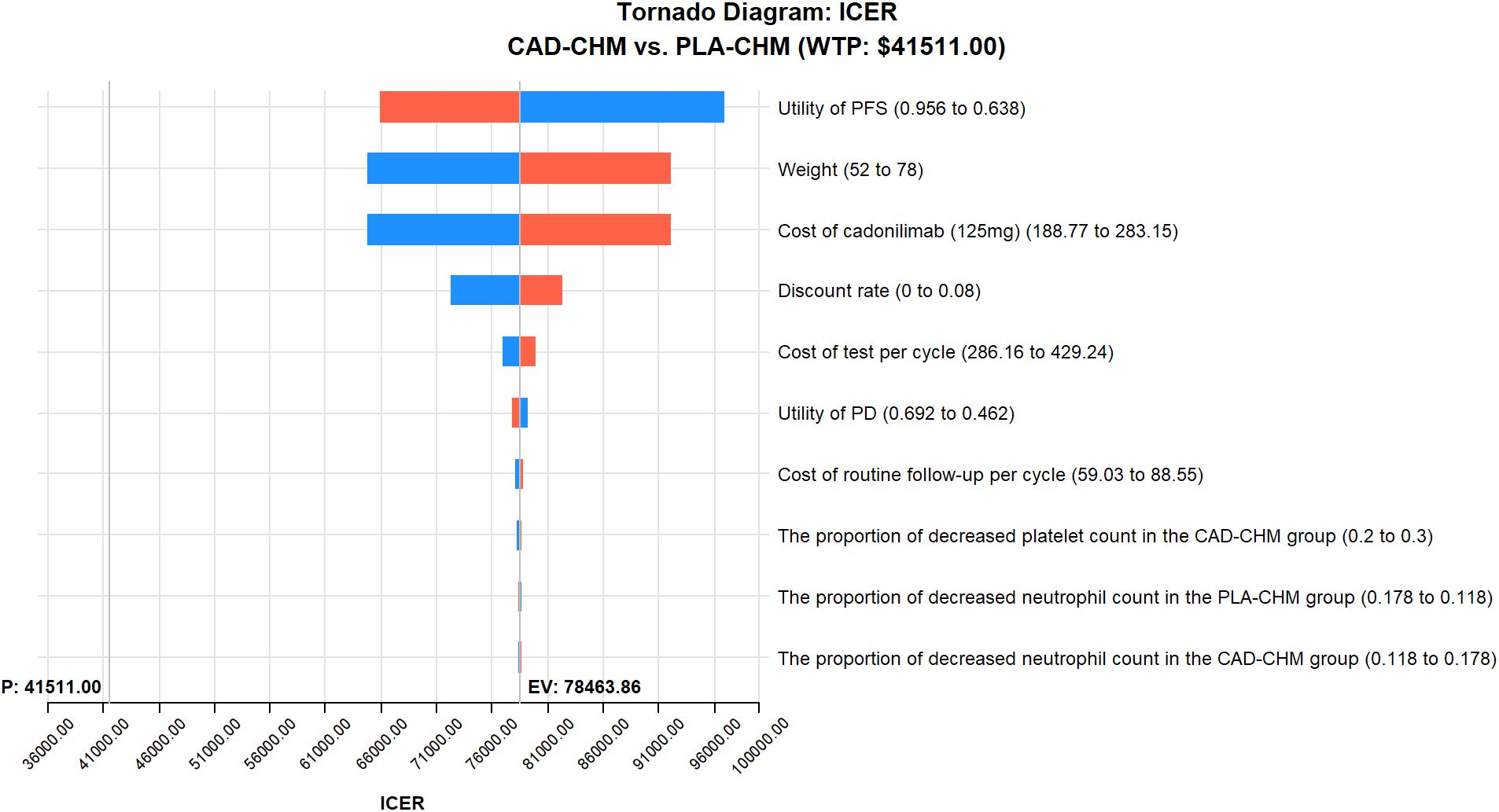
Figure 5. Probabilistic sensitivity analysis in the overall population. CAD-CHM, cadonilimab plus chemotherapy; ICE, incremental cost-effectiveness; PLA-CHM, placebo plus chemotherapy; WTP, willingness-to-pay.
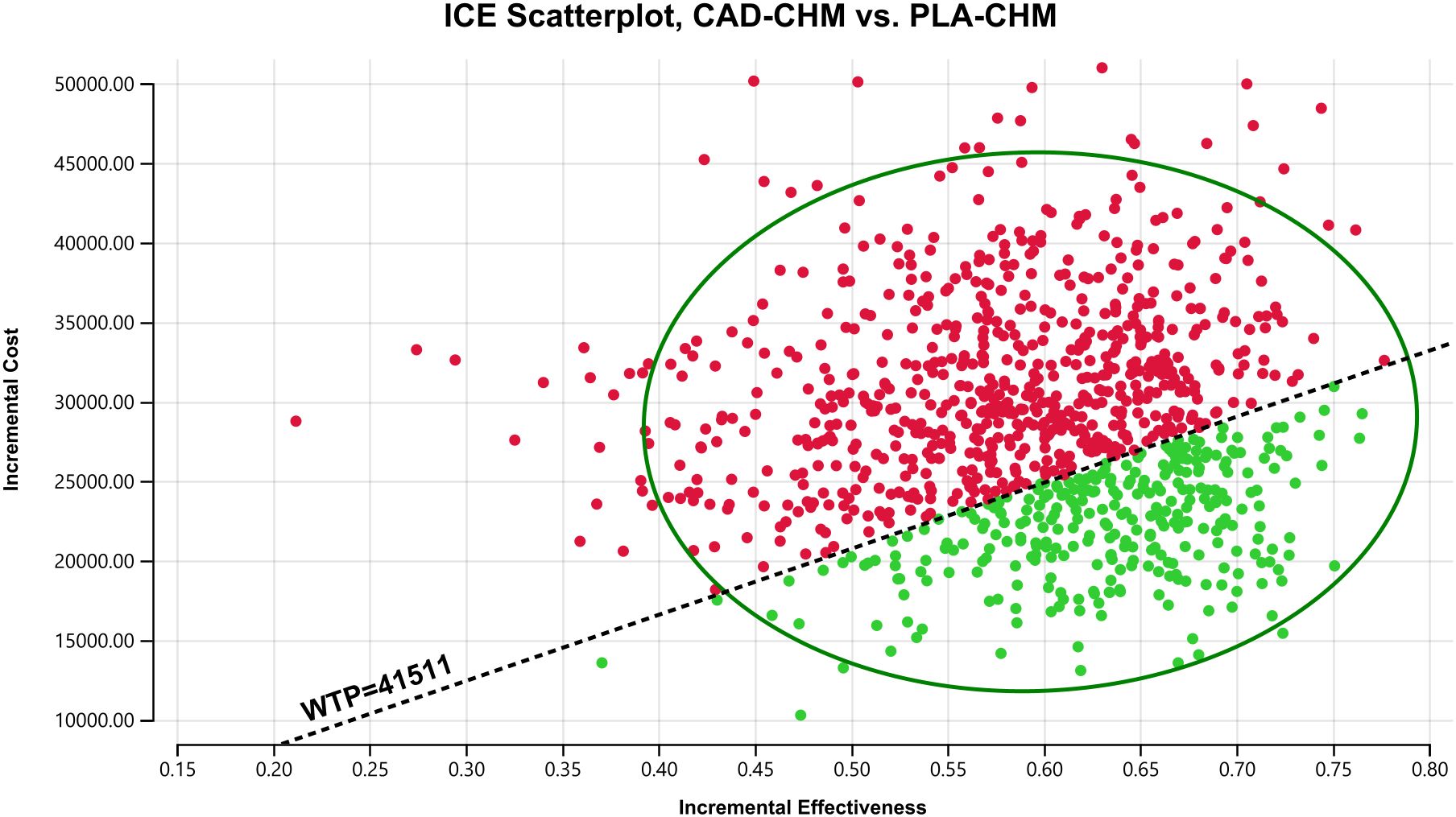
Figure 6. Probabilistic sensitivity analysis in PD-L1 CPS ≥ 5 subgroup. CAD-CHM, cadonilimab plus chemotherapy; ICE, incremental cost-effectiveness; PLA-CHM, placebo plus chemotherapy; WTP, willingness-to-pay.
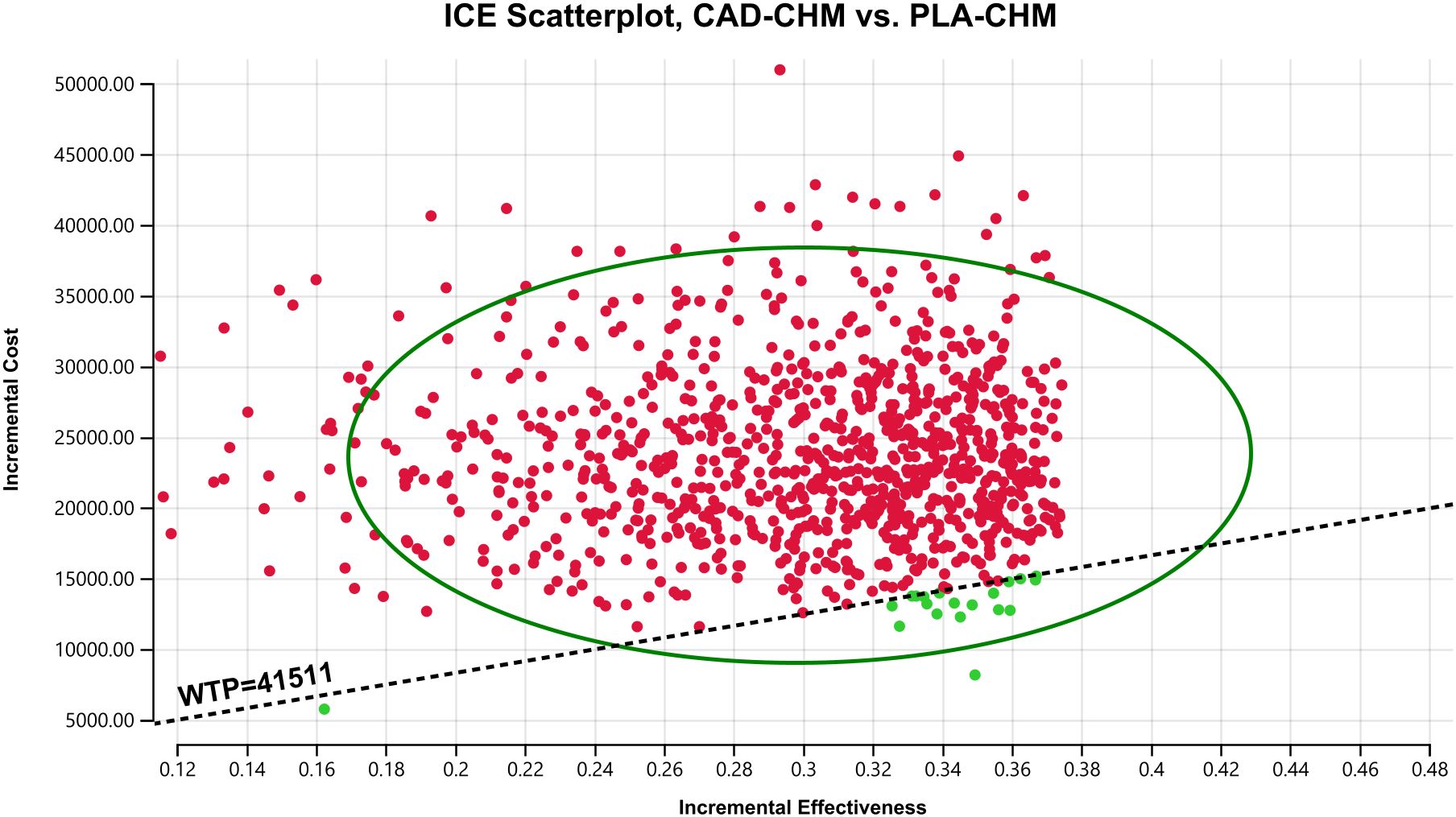
Figure 7. Probabilistic sensitivity analysis in PD-L1 CPS < 5 subgroup. CAD-CHM, cadonilimab plus chemotherapy; ICE, incremental cost-effectiveness; PLA-CHM, placebo plus chemotherapy; WTP, willingness-to-pay.
3.3 Scenario analysis
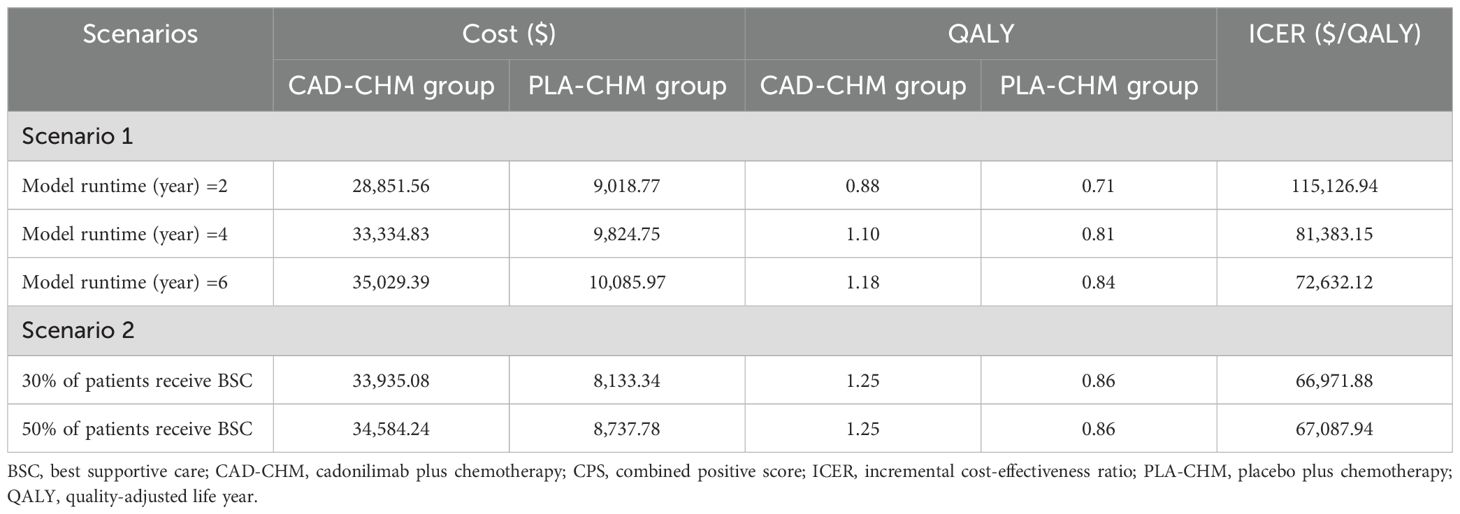
Scenario analysis results are presented in Table 4. In Scenario 1, when the model duration was adjusted to 2, 4, and 6 years, the ICERs of CAD-CHM compared with PLA-CHM were $115,126.94/QALY, $81,383.15/QALY, and $72,632.12/QALY, respectively. This indicates a progressive decline in ICER with increasing model duration. In Scenario 2, when the proportion of patients receiving the best supportive care was set at 30% and 50%, the ICERs of CAD-CHM compared with PLA-CHM were $66,971/QALY and $67,087.94/QALY, respectively. This indicates that the model results are relatively insensitive to assumptions regarding changes in the proportion of patients receiving the best supportive care.
4 Discussion
Co-inhibition of PD-1 and CTLA-4 induces a synergistic anti-tumor response by reshaping the tumor immune microenvironment into a more immunogenic phenotype (42). The complementary effects of CTLA-4 and PD-1 inhibitors have been well established (43). Cadonilimab, the first bispecific immune checkpoint inhibitor approved globally, simultaneously targets PD-1 and CTLA-4, enhancing anti-tumor efficacy through dual-pathway blockade (18). The COMPASSION-15 trial (21) demonstrated that CAD-CHM significantly improves survival in patients with HER2-negative advanced G/GEJ adenocarcinoma, underscoring its clinical potential. However, the escalating costs of novel cancer therapies pose a substantial challenge to healthcare system sustainability. As previously reported (23, 44), comprehensive cost-effectiveness evaluations are crucial for guiding policy decisions and optimizing healthcare resource allocation. Given the high cost of CAD-CHM, a thorough assessment of its cost-effectiveness as a first-line treatment for HER2-negative advanced G/GEJ adenocarcinoma is imperative to ensure both economic feasibility and equitable access within resource-constrained healthcare systems.
This study represents the first cost-effectiveness analysis of CAD-CHM as a first-line treatment for HER2-negative advanced G/GEJ adenocarcinoma within the Chinese healthcare system. Beyond its domestic significance, the findings offer valuable insights for the global medical community, marking a core innovation of this research. Compared with PLA-CHM, the incremental cost per additional QALY gained with CAD-CHM amounts to $67,378.09 in the overall population, $48,433.34 in the PD-L1 CPS ≥ 5 subgroup, and $78,463.86 in the PD-L1 CPS < 5 subgroup—substantially exceeding the predefined WTP threshold of $41,511 per QALY. Consequently, CAD-CHM does not demonstrate cost-effectiveness as a first-line therapy for HER2-negative advanced G/GEJ adenocarcinoma, irrespective of PD-L1 CPS status, from the perspective of the Chinese healthcare system. This outcome likely stems from the prolonged maintenance therapy required for cadonilimab and its substantially higher cost relative to oxaliplatin and capecitabine, leading to significantly elevated drug expenditures without providing sufficient incremental survival benefits. One-way sensitivity analysis identified patient weight (which determines cadonilimab dosing) and drug cost as the most influential factors in the cost-effectiveness of CAD-CHM, further supporting this conclusion. These findings highlight an urgent need to reduce the cost of cadonilimab to improve the affordability of the CAD-CHM regimen. Policy interventions should be implemented to enhance the cost-effectiveness of these promising treatments, ensuring broader patient access. Pharmaceutical companies can mitigate costs by optimizing manufacturing processes, improving supply chain efficiency, and refining pricing strategies, thereby enhancing the economic viability of this regimen and facilitating the widespread adoption of innovative therapies. Additionally, the analysis suggests that the cost of cadonilimab (125 mg) must be reduced to below 54.89% of its current price—specifically, under $129.50—for CAD-CHM to become a cost-effective first-line option for HER2-negative advanced G/GEJ adenocarcinoma in the overall population. This threshold provides a critical pricing benchmark for both healthcare policymakers and pharmaceutical manufacturers.
Subgroup analysis revealed that the ICER in the PD-L1 CPS ≥ 5 subgroup was substantially lower than that in the overall population, whereas the ICER in the PD-L1 CPS < 5 subgroup exceeded that of the overall population. Although neither subgroup achieved cost-effectiveness, the CAD-CHM regimen demonstrated greater economic viability in the PD-L1 CPS ≥ 5 subgroup. This underscores the critical role of PD-L1 expression level detection, which may serve as a strategy to enhance the cost-effectiveness of CAD-CHM in treating HER2-negative advanced G/GEJ adenocarcinoma. These findings provide valuable guidance for Chinese medical insurance policymakers in defining appropriate reimbursement criteria for cadonilimab.
Scenario analysis has proven instrumental in evaluating drug cost-effectiveness by accounting for varying assumptions and uncertainties, thereby better approximating real-world complexities. Accordingly, two scenario analyses were conducted. Scenario 1 demonstrated that prolonged treatment duration improves the cost-effectiveness of CAD-CHM. Scenario 2 indicated that following disease progression, an increased proportion of patients receiving the best supportive care does not substantially impact the ICER of CAD-CHM, suggesting that continued supportive care does not significantly diminish the cost-effectiveness of CAD-CHM. These analyses suggest that extended treatment adherence may optimize therapeutic value, aligning with the interests of clinicians, patients, and their families, as well as broader ethical and societal considerations.
To date, only two cost-effectiveness studies have assessed the use of immune checkpoint inhibitors as first-line treatments for HER2-negative advanced G/GEJ adenocarcinoma within the Chinese healthcare system. Lang et al. (45) and Zhang et al. (46) concluded that pembrolizumab plus chemotherapy is not cost-effective as a first-line option for treating HER2-negative advanced G/GEJ adenocarcinoma. These findings are consistent with the present study, which similarly identified no economic advantage of CAD-CHM over chemotherapy alone.
This study possesses several notable strengths. First, it leverages the most recent data from the COMPASSION-15 trial, incorporating nearly two years of survival analysis to compare the efficacy of cadonilimab plus chemotherapy with chemotherapy alone, thereby providing the latest clinical evidence. Second, as all participants in the COMPASSION-15 trial were Chinese patients, the findings exhibit strong population-specific applicability, offering a more accurate reflection of treatment outcomes and economic benefits within the Chinese healthcare system. Lastly, through comprehensive subgroup and scenario analyses, the study evaluates economic impacts across diverse patient cohorts and treatment conditions, furnishing critical insights for clinicians, patients, and policymakers in making personalized treatment decisions.
However, certain limitations should be acknowledged. First, given that the COMPASSION-15 trial remains ongoing, long-term survival data are currently unavailable. This study extrapolated survival beyond the follow-up period using survival models, which may introduce some deviation from actual outcomes. For instance, the tail of the survival curve for patients receiving immunotherapy may exhibit a plateau (47). Our model does not account for the possibility of long-term survival and may therefore underestimate the efficacy of immunotherapy. Future studies should validate these findings using real-world data for cost-effectiveness analysis. Second, post-progression treatment details were not reported in the COMPASSION-15 trial, necessitating the assumption that all patients received the best supportive care after first-line treatment failure, which may not fully align with real-world clinical practice. In reality, the selection of subsequent treatment regimens is individualized based on each patient’s specific circumstances. Fortunately, the results of the one-way sensitivity analysis provided reassurance, as they consistently indicated that altering the estimated range of subsequent treatments would not change the model’s outcomes. Third, due to the absence of quality-of-life data in the trial, health utility values were sourced from Chinese literature, potentially introducing bias into the model; however, sensitivity analysis confirmed that this does not alter the study’s overall conclusions. However, it must be acknowledged that obtaining more accurate health utility values is crucial for enhancing the accuracy of our model outcomes. Should future clinical studies report health-related quality-of-life outcomes specific to the Chinese population, incorporating these reliable data would optimize our model results. Lastly, this analysis focused solely on the cost-effectiveness of CAD-CHM relative to chemotherapy alone, without considering alternative treatment regimens such as pembrolizumab plus chemotherapy, owing to the lack of direct comparative data. However, the studies by Lang et al. (45) and Zhang et al. (46) suggest that pembrolizumab combined with chemotherapy is not cost-effective compared to chemotherapy alone. Therefore, we believe that selecting chemotherapy as the comparator in the cost-effectiveness analysis of CAD-CHM is reasonable. Despite these limitations, the findings remain highly informative for healthcare policymakers, clinicians, and patients.
5 Conclusion
The study results indicate that, from the perspective of the Chinese healthcare system, CAD-CHM as a first-line treatment for HER2-negative advanced G/GEJ adenocarcinoma lacks cost-effectiveness compared with chemotherapy alone, irrespective of PD-L1 CPS subgroup stratification.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
ZZ: Writing – original draft, Writing – review & editing. YY: Writing – original draft, Writing – review & editing. SC: Writing – original draft, Writing – review & editing. MY: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported in part by grants from the Natural Science Foundation of Fujian Province (Grant number: 2023J011188); the Natural Science Foundation of Ningde (Grant number: 2022J29). This study was not supported by any pharmaceutical company.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1575627/full#supplementary-material
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. (2020) 396:635–48. doi: 10.1016/S0140-6736(20)31288-5
3. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
4. Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. (2018) 6:e555–555e567. doi: 10.1016/S2214-109X(18)30127-X
5. Ajani JA, Lee J, Sano T, Janjigian YY, Fan D, Song S. Gastric adenocarcinoma. Nat Rev Dis Primers. (2017) 3:17036. doi: 10.1038/nrdp.2017.36
6. Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. (2015) 18:476–84. doi: 10.1007/s10120-014-0402-y
7. Abrahao-MaChado LF, Scapulatempo-Neto C. HER2 testing in gastric cancer: An update. World J Gastroenterol. (2016) 22:4619–25. doi: 10.3748/wjg.v22.i19.4619
8. Wang FH, Zhang XT, Tang L, Wu Q, Cai MY, Li YF, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2023. Cancer Commun (Lond). (2024) 44:127–72. doi: 10.1002/cac2.12516
9. Shitara K, Lordick F, Bang YJ, Enzinger P, Ilson D, Shah MA, et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet. (2023) 401:1655–68. doi: 10.1016/S0140-6736(23)00620-7
10. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. (2021) 398:27–40. doi: 10.1016/S0140-6736(21)00797-2
11. Xu J, Jiang H, Pan Y, Gu K, Cang S, Han L, et al. Sintilimab plus chemotherapy for unresectable gastric or gastroesophageal junction cancer: the ORIENT-16 randomized clinical trial. JAMA. (2023) 330:2064–74. doi: 10.1001/jama.2023.19918
12. Qiu MZ, Oh DY, Kato K, Arkenau T, Tabernero J, Correa MC, et al. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first line treatment for advanced gastric or gastro-oesophageal junction adenocarcinoma: RATIONALE-305 randomised, double blind, phase 3 trial. BMJ. (2024) 385:e078876. doi: 10.1136/bmj-2023-078876
13. Shitara K, Ajani JA, Moehler M, Garrido M, Gallardo C, Shen L, et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature. (2022) 603:942–8. doi: 10.1038/s41586-022-04508-4
14. Rha SY, Oh DY, Yañez P, Bai Y, Ryu MH, Lee J, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. (2023) 24:1181–95. doi: 10.1016/S1470-2045(23)00515-6
15. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. (2015) 373:23–34. doi: 10.1056/NEJMoa1504030
16. Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. (2016) 17:883–95. doi: 10.1016/S1470-2045(16)30098-5
17. Overman MJ, Lonardi S, Wong K, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. (2018) 36:773–9. doi: 10.1200/JCO.2017.76.9901
18. Pang X, Huang Z, Zhong T, Zhang P, Wang ZM, Xia M, et al. Cadonilimab, a tetravalent PD-1/CTLA-4 bispecific antibody with trans-binding and enhanced target binding avidity. MAbs. (2023) 15:2180794. doi: 10.1080/19420862.2023.2180794
19. Hou X, Liu S, Zeng Z, Wang Z, Ding J, Chen Y, et al. Preclinical imaging evaluation of a bispecific antibody targeting hPD1/CTLA4 using humanized mice. BioMed Pharmacother. (2024) 175:116669. doi: 10.1016/j.biopha.2024.116669
20. Caushi JX, Zhang J, Ji Z, Vaghasia A, Zhang B, Hsiue EH, et al. Transcriptional programs of neoantigen-specific TIL in anti-PD-1-treated lung cancers. Nature. (2021) 596:126–32. doi: 10.1038/s41586-021-03752-4
21. Shen L, Zhang Y, Li Z, Zhang X, Gao X, Liu B, et al. First-line cadonilimab plus chemotherapy in HER2-negative advanced gastric or gastroesophageal junction adenocarcinoma: a randomized, double-blind, phase 3 trial. Nat Med. (2025) 31(4):1163–70. doi: 10.1038/s41591-024-03450-4
22. Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health. (2022) 25:3–9. doi: 10.1016/j.jval.2021.11.1351
23. Zhang J, Lei J, You C, Fu W, Zheng B, Cai H, et al. Cost-effectiveness analysis of durvalumab with chemotherapy and maintenance durvalumab with or without olaparib for advanced endometrial cancer. Sci Rep. (2025) 15:2497. doi: 10.1038/s41598-025-86021-y
24. Yue X, Li Y, Wu J, Guo JJ. Current development and practice of pharmacoeconomic evaluation guidelines for universal health coverage in China. Value Health Reg Issues. (2021) 24:1–5. doi: 10.1016/j.vhri.2020.07.580
25. Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. (2012) 12:9. doi: 10.1186/1471-2288-12-9
26. Su D, Wu B, Shi L. Cost-effectiveness of atezolizumab plus bevacizumab vs sorafenib as first-line treatment of unresectable hepatocellular carcinoma. JAMA Netw Open. (2021) 4:e210037. doi: 10.1001/jamanetworkopen.2021.0037
27. Liu L, Wang L, Chen L, Ding Y, Zhang Q, Shu Y. Cost-effectiveness of sintilimab plus chemotherapy versus chemotherapy alone as first-line treatment of locally advanced or metastatic oesophageal squamous cell carcinoma. Front Immunol. (2023) 14:1092385. doi: 10.3389/fimmu.2023.1092385
28. Ishak KJ, Kreif N, Benedict A, Muszbek N. Overview of parametric survival analysis for health-economic applications. Pharmacoeconomics. (2013) 31:663–75. doi: 10.1007/s40273-013-0064-3
29. Williams C, Lewsey JD, Mackay DF, Briggs AH. Estimation of survival probabilities for use in cost-effectiveness analyses: A comparison of a multi-state modeling survival analysis approach with partitioned survival and Markov decision-analytic modeling. Med Decis Making. (2017) 37:427–39. doi: 10.1177/0272989X16670617
30. Compiled by national bureau of statistics of China. Available online at: https://www.stats.gov.cn/sj/ndsj/2024/indexch.htm (Accessed February 1, 2024).
31. Xu L, Long Y, Yao L, Wang H, Ge W. Updated cost-effectiveness analysis of tislelizumab in combination with chemotherapy for the first-line treatment of advanced gastric cancer or gastroesophageal junction adenocarcinoma. Front Oncol. (2024) 14:1477722. doi: 10.3389/fonc.2024.1477722
32. Zhang PF, Xie D, Li Q. Cost-effectiveness analysis of nivolumab in the second-line treatment for advanced esophageal squamous cell carcinoma. Future Oncol. (2020) 16:1189–98. doi: 10.2217/fon-2019-0821
33. Yao ZH. The big data service platform for China’s health industry: Information Query of Drug Bid Winning . Available online at: https://data.yaozh.com/ (Accessed February 1, 2024).
34. Liu S, Dou L, Wang K, Shi Z, Wang R, Zhu X, et al. Cost-effectiveness analysis of nivolumab combination therapy in the first-line treatment for advanced esophageal squamous-cell carcinoma. Front Oncol. (2022) 12:899966. doi: 10.3389/fonc.2022.899966
35. Shen J, Du Y, Shao R, Jiang R. First-line sintilimab plus chemotherapy in locally advanced or metastatic esophageal squamous cell carcinoma: A cost-effectiveness analysis from China. Front Pharmacol. (2022) 13:967182. doi: 10.3389/fphar.2022.967182
36. Zhan M, Xu T, Zheng H, He Z. Cost-effectiveness analysis of pembrolizumab in patients with advanced esophageal cancer based on the KEYNOTE-181 study. Front Public Health. (2022) 10:790225. doi: 10.3389/fpubh.2022.790225
37. Liu S, Dou L, Li S. Cost-effectiveness analysis of PD-1 inhibitors combined with chemotherapy as first-line therapy for advanced esophageal squamous-cell carcinoma in China. Front Pharmacol. (2023) 14:1055727. doi: 10.3389/fphar.2023.1055727
38. Zhang Q, Wu P, He X, Ding Y, Shu Y. Cost-effectiveness analysis of camrelizumab vs. Placebo added to chemotherapy as first-line therapy for advanced or metastatic esophageal squamous cell carcinoma in China. Front Oncol. (2021) 11:790373. doi: 10.3389/fonc.2021.790373
39. Ionova Y, Vuong W, Sandoval O, Fong J, Vu V, Zhong L, et al. Cost-effectiveness analysis of atezolizumab versus durvalumab as first-line treatment of extensive-stage small-cell lung cancer in the USA. Clin Drug Investig. (2022) 42:491–500. doi: 10.1007/s40261-022-01157-3
40. Nafees B, Lloyd AJ, Dewilde S, Rajan N, Lorenzo M. Health state utilities in non-small cell lung cancer: An international study. Asia Pac J Clin Oncol. (2017) 13:e195–195e203. doi: 10.1111/ajco.12477
41. Cai H, Xu B, Li N, Zheng B, Zheng Z, Liu M. Cost-effectiveness analysis of camrelizumab versus chemotherapy as second-line treatment of advanced or metastatic esophageal squamous cell carcinoma. Front Pharmacol. (2021) 12:732912. doi: 10.3389/fphar.2021.732912
42. Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res. (2019) 38:255. doi: 10.1186/s13046-019-1259-z
43. Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. (2018) 378:1277–90. doi: 10.1056/NEJMoa1712126
44. Lang W, He Y, Hou C, Li H, Jiang Q, Mei L. Cost-effectiveness analysis of pembrolizumab plus chemotherapy versus chemotherapy in untreated advanced pleural mesothelioma in the Chinese healthcare system. Front Pharmacol. (2024) 15:1402423. doi: 10.3389/fphar.2024.1402423
45. Lang W, Deng L, Lu M, Ouyang M. Cost-effectiveness analysis of pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric/gastroesophageal junction cancer in the Chinese healthcare system. Expert Rev Pharmacoecon Outcomes Res. (2024) 24:1027–42. doi: 10.1080/14737167.2024.2378983
46. Zheng Z, Song X, Cai H, Zhu H. Pembrolizumab combined with chemotherapy versus placebo combined with chemotherapy for HER2-negative advanced gastric cancer in China: a cost-effectiveness analysis. Expert Rev Pharmacoecon Outcomes Res. (2024) 24:1017–25. doi: 10.1080/14737167.2024.2378986
47. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. (2018) 391:1023–75. doi: 10.1016/S0140-6736(17)33326-3
Keywords: cadonilimab, chemotherapy, cost-effectiveness, first-line treatment, HER2-negative, gastric or gastroesophageal junction adenocarcinoma
Citation: Zhou Z, Yang Y, Chen S and You M (2025) Cost-effectiveness analysis of first-line cadonilimab plus chemotherapy in HER2-negative advanced gastric or gastroesophageal junction adenocarcinoma. Front. Immunol. 16:1575627. doi: 10.3389/fimmu.2025.1575627
Received: 12 February 2025; Accepted: 24 April 2025;
Published: 13 May 2025.
Edited by:
Stavros P. Papadakos, Laiko General Hospital of Athens, GreeceReviewed by:
Xuwei Shao, Pace Analytical labs, United StatesIoannis Katsaros, National and Kapodistrian University of Athens, Greece
Copyright © 2025 Zhou, Yang, Chen and You. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maojin You, eW91bWFvamluQDE2My5jb20=; Yanqing Yang, eWFuZ3lhbnFpbmcyMDI1QDE2My5jb20=
†These authors have contributed equally to this work
 Zhifeng Zhou1
Zhifeng Zhou1 Maojin You
Maojin You