- 1Department of Integrative Oncology, Fudan University Shanghai Cancer Center, Shanghai, China
- 2Department of Minimally Invasive Therapy Center, Fudan University Shanghai Cancer Center, Shanghai, China
- 3Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
- 4School of Traditional Chinese Medicine, Naval Medical University, Shanghai, China
Background: Transarterial chemoembolization (TACE) is recommended for intermediate-stage hepatocellular carcinoma (HCC). However, several therapies have shown better efficacy than TACE, meaning that the optimal therapy is unclear. We addressed this uncertainty using network meta-analysis (NMA).
Methods: A literature review was performed up to March 15, 2024. Efficacy was evaluated using overall survival (OS) and progression-free survival (PFS). The hazard ratios (HRs) and 95% confidence intervals (CIs) were extracted from the Kaplan–Meier curves. A random-effects NMA was conducted, and subgroup analysis was performed according to the tumor number, tumor size, viral etiology, and alpha fetoprotein (AFP) level. The efficacy of the different therapies was ranked based on the P-score.
Results: A total of 38 studies, 10,972 patients, and 13 therapeutic regimens were eligible. Seven therapies showed OS benefit over TACE, including TACE plus microwave ablation (MWA) (HR = 0.24, 95%CI = 0.06–0.91), TACE plus liver resection (HR = 0.35, 95%CI = 0.22–0.57), liver resection plus RFA (HR =0.49,95%CI=0.35-0.70), TACE plus immune checkpoint inhibitors (ICIs) plus tyrosine kinase inhibitors (TKIs) (HR = 0.51, 95%CI = 0.27–0.95), liver resection (HR = 0.54, 95%CI = 0.45–0.65), and TACE plus radiofrequency ablation (RFA) (HR = 0.57, 95%CI = 0.36–0.93). However, no therapies improved the PFS better than TACE alone. Subgroup analysis indicated that liver resection plus TACE showed the best OS for patients with hepatitis B virus (HBV) infection.
Conclusions: Seven therapies showed better efficacy than TACE alone for particular patients with intermediate-stage HCC.
Systematic review registration: https://www.crd.york.ac.uk/, PROSPERO CRD42023459740.
Introduction
Globally, primary liver cancer (PLC) is the sixth most frequent malignancy and the third leading cause of cancer-related death. In particular, there were approximately 860,000 new cases of PLC in 2023 (1). Hepatocellular carcinoma (HCC) accounts for nearly 80% of PLC (2). Hepatitis virus infection, aflatoxin, and metabolic dysfunction are the main risk factors of HCC. Notably, due to the lack of obvious symptoms in the early stage, nearly 80% of patients with HCC are diagnosed in the middle–advanced stage (3). Exacerbating this problem is the high resistance of some patients in the intermediate-advanced stage to current therapies and the limited survival benefits.
Presently, the Barcelona Clinic Liver Cancer (BCLC) classification, which is both a treatment strategy and a staging system, has been externally confirmed and is widely endorsed by a series of liver disease associations, including the European Association for the Study of Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) (3–5). Patients in the BCLC-B stage (intermediate stage) are defined as those with multifocal tumors with preserved liver function, good performance status, and without macrovascular invasion and extrahepatic spread (6). Therefore, intermediate-stage HCC is characterized by a high heterogeneity with extensive tumor number, tumor size, and different liver function.
Transarterial chemoembolization (TACE) is recommended as the standard therapy for patients with BCLC-B (7). The anticancer mechanism of TACE involves not only blocking the blood supply of the embolization area but also directly killing tumor cells through chemotherapy drugs. However, the median overall survival (OS) of patients treated with TACE ranges from 14 to 45 months due to the high heterogeneity of both the patient population and the TACE technique (8, 9). Partial patients are unsuitable for or are refractory to TACE (10). In addition, a considerable number of patients suffer from serious post-embolization syndromes (PES) after TACE, leading to a reduction in treatment compliance (11). Furthermore, only half of patients with HCC could benefit from a single TACE, in particular patients with large and/or multifocal tumors, which might be caused by the incomplete necrosis of the target lesions after a single TACE (12). Therefore, this procedure is repeatedly performed several times in the clinic. However, repeated TACE is usually accompanied with hepatic injury and induces the formation of an ischemic/hypoxia microenvironment, subsequently activating the hypoxia-induced factor 1 alpha (HIF-1α) pathway and modulating angiogenesis, tumor invasion, and metastasis (13, 14). Therefore, the efficacy of a single TACE in the treatment of patients with BCLC-B stage HCC is unsatisfactory. Accordingly, several randomized controlled trials (RCTs) have been conducted to compare the efficacy of TACE plus molecular targeted therapy (TACE+MTT) with that of TACE alone (15–17). Regrettably, these trials did not achieve positive results.
Presently, the efficacy and the safety of immune checkpoint inhibitors (ICIs) in the treatment of HCC have been confirmed. The IMbrave150 trial confirmed that atezolizumab (ICI) plus bevacizumab significantly prolonged the OS and PFS compared with sorafenib in patients with unresected HCC (18). Subgroup analysis also showed a positive trend in patients with BCLC-B stage HCC (19). Interestingly, Pinato et al. found that TACE could induce immunogenic cell death and release tumor antigens, which may enhance the efficacy of ICIs (20). The CHANCE001 trial, a multicenter retrospective cohort study in China, demonstrated that TACE with ICIs plus MTT showed better OS and PFS than TACE alone in patients with advanced HCC; however, the trial did not improve the OS and PFS in patients with intermediate-stage HCC (21). In addition, the EMERALD-1 trial, a global three-phase study on patients with unresected HCC, showed a significant benefit in PFS using TACE combined with durvalumab (ICI) plus bevacizumab compared with TACE alone. Notably, this trial showed positive results in patients with BCLC-B stage HCC (22). Recently, the LEAP-012 trial evaluated the efficacy of TACE plus lenvatinib plus pembrolizumab vs. TACE alone in patients with intermediate-stage HCC. The PFS was significantly prolonged in the triple therapy group compared with the monotherapy group. At the same time, the OS did not show positive results (23). In addition, an RCT found that partial resection showed better OS than TACE in patients with resectable multiple HCC out of the Milan criteria (24). Furthermore, several therapies, including TACE plus thermal ablation, TACE plus liver resection, liver resection plus TACE, and liver resection plus ablation, showed therapeutic benefits in patients with BCLC-B stage HCC (25–27). However, most of these clinical trials have a small sample size and are retrospective in nature.
As described above, there exists a series of potential therapies for patients with intermediate-stage HCC. However, due to the lack of direct comparisons, the optimal therapy is controversial. Therefore, we conducted a network meta-analysis (NMA) to indirectly compare the efficacy of these therapies.
Materials and methods
We searched and extracted relative data according to the latest Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) and Assessing the Methodological Quality of Systematic Reviews (AMSTAR) guidelines (28). The results are shown in Supplementary Tables S1, S2. This study is registered in the PROSPERO database (CRD42023459740).
Search strategy and selection criteria
We searched relevant databases, including PubMed, Embase, Web of Science, and the Cochrane Library. In addition, the references of the retrieved articles and meta-analyses were manually searched. The last search date was up to March 15, 2024. The relative search items for each database are shown in Supplementary Table S3.
Participants
BCLC-B stage HCC was defined according to the following criteria: 1) two or three nodules and with at least one tumor larger than 3 cm; 2) four or more tumor nodules, any size; 3) without the presentation of extrahepatic metastases; 4) without tumor thrombus in the portal vein or other major vascular structures; 6) Child–Pugh (Child–Turcotte–Pugh, CTP) A–B class; and additionlly, samle size ≥25.
Interventions
Interventions included the therapies for intermediate-stage HCC.
Comparator
TACE was used as the reference.
Outcomes
The primary endpoint was OS. The secondary outcome was PFS.
Study selection
Firstly, two authors independently read and reviewed the titles and abstracts according to the search strategy. On this basis, the selected studies were further confirmed by downloading and reviewing the full texts. Subsequently, relative data were extracted into a pre-designed Excel sheet, including the PMID number; author; publication year; sample size; follow-up duration; study design; efficacy outcomes; and clinical parameters such as age, sex, tumor size, tumor number, hepatitis B virus (HBV)/hepatitis C virus (HCV) infection status, Child–Pugh score, and alpha fetoprotein (AFP) level ≥400 ng/ml.
Data extraction
The hazard ratios (HRs) and relative 95% confidence intervals (CIs) of the individual study were pooled for the survival data. If the HR and 95% CIs were reported, these data were directly extracted for further analysis; otherwise, the HR and 95%CIs were extracted from the survival curves using the method reported by Parmar et al. (29).
Risk of bias assessment
Two authors independently evaluated the risk of bias (RoB) of the included studies. The assessment tools used were based on the study design. For RCTs, the Risk of Bias 2 (RoB2) tool was used (30). For observational studies, the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) was used. Discrepancies were resolved through discussion with another author.
Design-adjusted analysis
Considering the potential confounding of non-randomized studies (NRS), the inclusion of these studies in the NMA may influence the transitivity and consistency assumed by the method. To minimize bias, a design-adjusted analysis was performed to combine both randomized and non-randomized evidence in the NMA (31). As described above, ROBINS-I was used to evaluate the RoB of the NRS. Studies with a higher RoB were assigned a lower weight, while studies with a lower RoB were assigned a higher weight. In detail, for observational studies with low, moderate, and high RoB, weights of 25%, 50%, and 75%, respectively, were assigned. For RCTs, the assigned weight was 1.
Standard meta-analysis
STATA (version 12.0; Stata Corp, College Station, TX, USA) was used for pairwise meta-analysis. Firstly, statistical heterogeneity was evaluated using the I2 value. An I2 ≥ 50% meant that there exists significant heterogeneity; therefore, the random-effects model was used. Otherwise, the fixed-effects model was utilized for pooling the HR, relative risk (RR), and relative 95%CIs. Egger’s regression test was conducted to evaluate publication bias, and a funnel plot was used for visual assessment.
Network meta-analysis
A frequentist model NMA was performed using the “netmeta” package in R software (version 4.3.1; R Foundation for Statistical Computing, Vienna, Austria). A random-effects NMA model was used, and the network plots were drawn accordingly. For the evaluation and ranking of the efficacy of each treatment, the P-scores of each therapy were calculated and accordingly ranked. In terms of efficacy, a P-score of “0” denotes worst relative therapy, while “1” indicates that the treatment therapy is the best, which is contrary to the terms of adverse events.
Inconsistency assessment
The consistency of the NMA is crucial for the evaluation of the stability of the transitivity assumption. Firstly, the back-calculation method was used to assess the existence of local inconsistency. In addition, a design-by-treatment interaction was determined to evaluate the global inconsistency of the model (32). The “netmeta” package in R was used to accomplish the above analysis.
Subgroup analysis
For the primary outcomes, subgroup analysis was conducted to explore the source of heterogeneity according to the following parameters: AFP level, Child–Pugh class, tumor size, tumor number, HCV and HBV infection status, and sample size. The median value of the continuous variable was calculated for further subgroup analysis.
Credibility of evidence
The evidence credibility of the NMA results was evaluated using CINeMA (http://cinema.ispm.ch/) (33), which is composed of six domains: within-study bias, across-study bias, indirectness, heterogeneity, imprecision, and incoherence. The degree of evidence of the NMA results was summarized based on these six domains.
Results
Study selection and characteristics
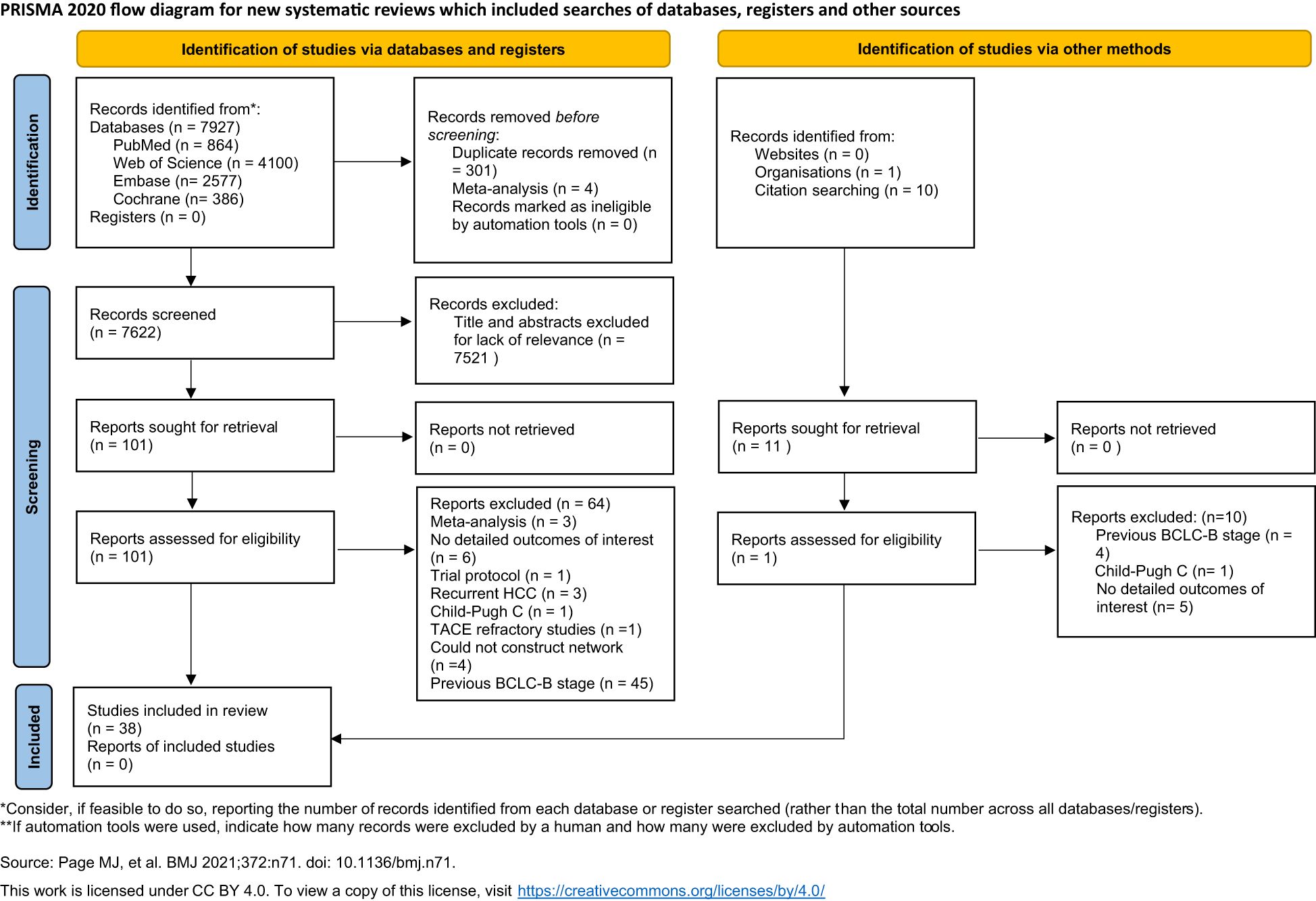
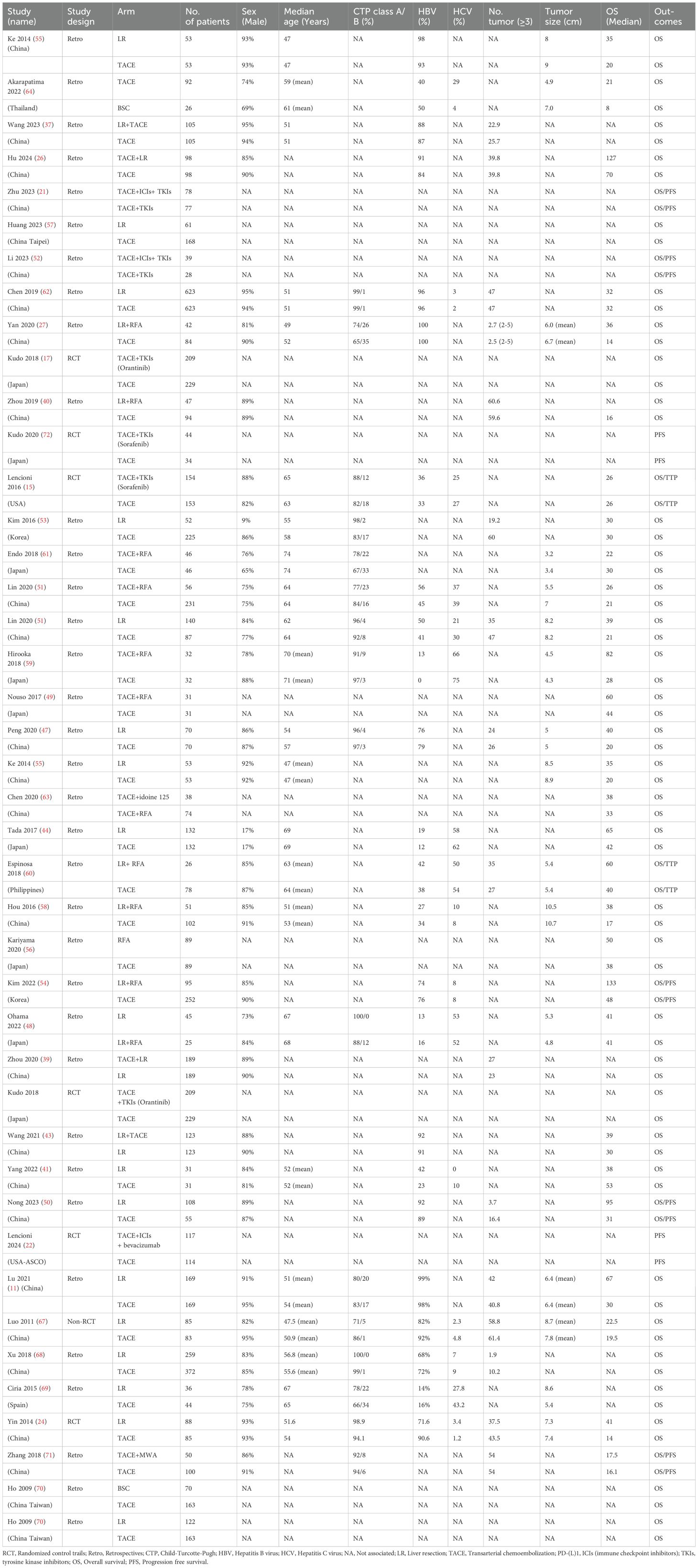
Based on the search strategy, a total of 7,927 potential citations were identified. Accordingly, 301 studies with duplicate records and four meta-analyses were excluded. A total of 7,521 studies were excluded after reading the titles and abstracts. One article was excluded due to the inclusion of Child–Pugh class C patients (34), while three studies were excluded due to the enrolled participants not fulfilling the criteria: three had recurrent HCC (35–37) and one was refractory to TACE (38). There were 40 studies that reported data on OS (15, 17, 18, 21, 24, 26, 27, 39–71) and 12 studies that reported data on PFS (15, 18, 21, 22, 45, 46, 50, 52, 54, 60, 71, 72). Four studies were excluded due to the therapies not being able to form a network (18, 45, 46, 65). Finally, a total of 38 studies were found eligible for inclusion in this systematic review. The flowchart of the included studies is presented in Figure 1. Detailed characteristics of the included studies are shown in Table 1.
Risk of bias assessment
ROBINS-I is composed of confounding, selection of participants, classification of interventions, deviation from intended interventions, missing data, measurement of the outcome, and selection of the reported results. Five studies were considered to be at high risk, 12 studies were identified as moderate risk, and 21 studies were considered to be at low risk. Details of the assessments are shown in Supplementary Tables S4, S5.
Primary outcome: overall survival
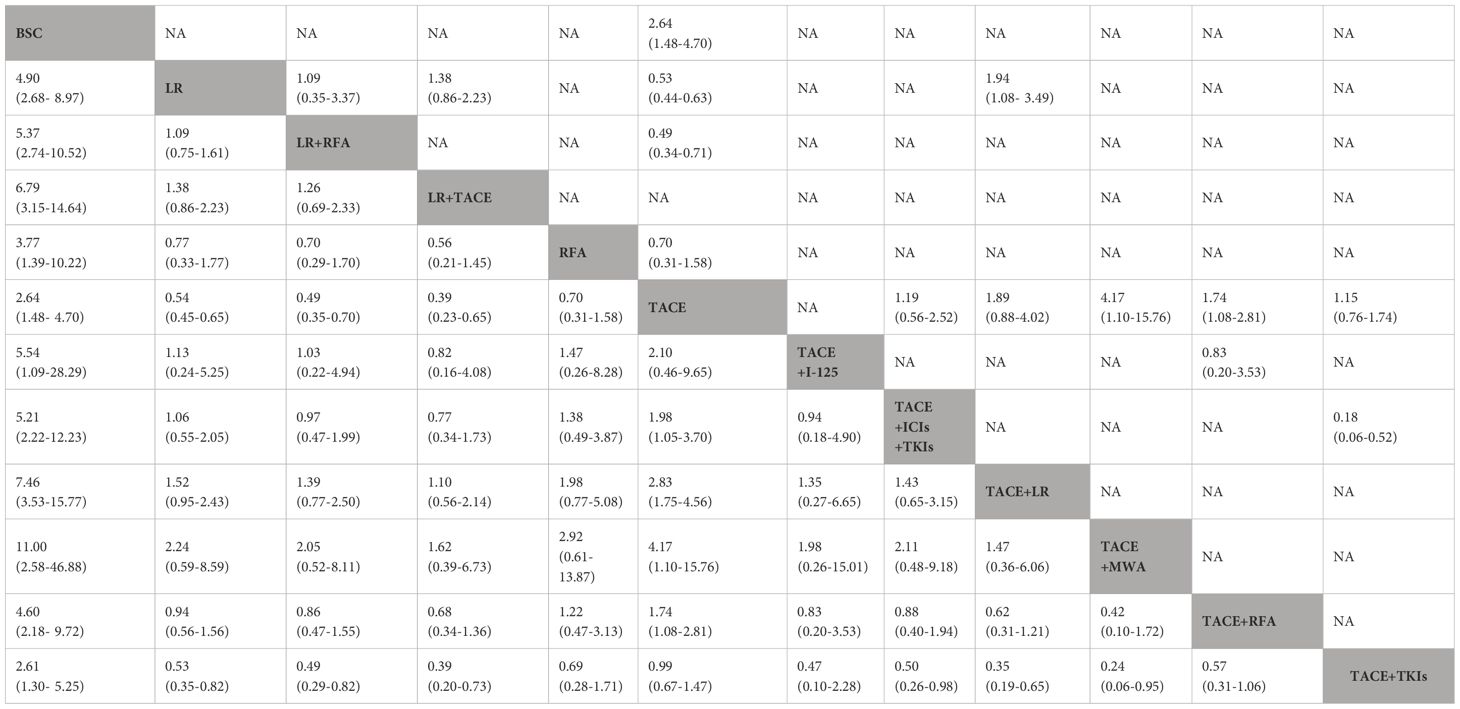
There were a total of 36 studies (one prospective non-randomized analysis, three RCTs, and 32 retrospective studies) and 12 treatment therapies. The pairwise meta-analysis showed that several regimens had better OS benefit than TACE alone, including TACE plus radiofrequency ablation (RFA) [adjusted HR (aHR) = 0.57, 95%CI = 0.42–0.78], liver resection (aHR = 0.53, 95%CI = 0.44–0.62), liver resection plus RFA (aHR = 0.52, 95%CI = 0.41–0.66), and TACE plus tyrosine kinase inhibitors (TKIs) (aHR = 0.86, 95%CI = 0.68–1.09). The results are presented in Table 2.
The results of the NMA are shown in Figure 2, Table 2. Several therapeutic regimens had significantly better OS than TACE alone, including TACE plus microwave ablation (MWA) (aHR = 0.24, 95%CI = 0.06–0.91, P-score = 0.87; low confidence), TACE plus liver resection (aHR = 0.35, 95%CI = 0.22–0.57, P-score = 0.82; high confidence), liver resection plus TACE (aHR = 0.39, 95%CI = 0.23–0.65, P-score = 0.76; low confidence), liver resection plus RFA (aHR = 0.49, 95%CI = 0.35–0.70, P-score = 0.61; high confidence), TACE combined with ICIs with TKIs (aHR = 0.51, 95%CI = 0.27–0.95, P-score = 0.59; very low confidence), liver resection (aHR = 0.54, 95%CI = 0.45–0.65, P-score = 0.52; low confidence), and TACE plus RFA (aHR = 0.57, 95%CI = 0.36–0.93, P-score = 0.49; very low confidence). There were no significant differences in OS improvement between TACE and the following therapies: TACE plus I-125, TACE plus TKIs, and best supportive care (BSC). Notably, TACE plus MWA ranked the highest out of all 12 treatment therapies for prolonging OS. TACE plus liver resection and liver resection plus TACE were the second and third best therapies, respectively. The evidence credibility of the NMA results is shown in Supplementary Table S7, Supplementary Figure S1.
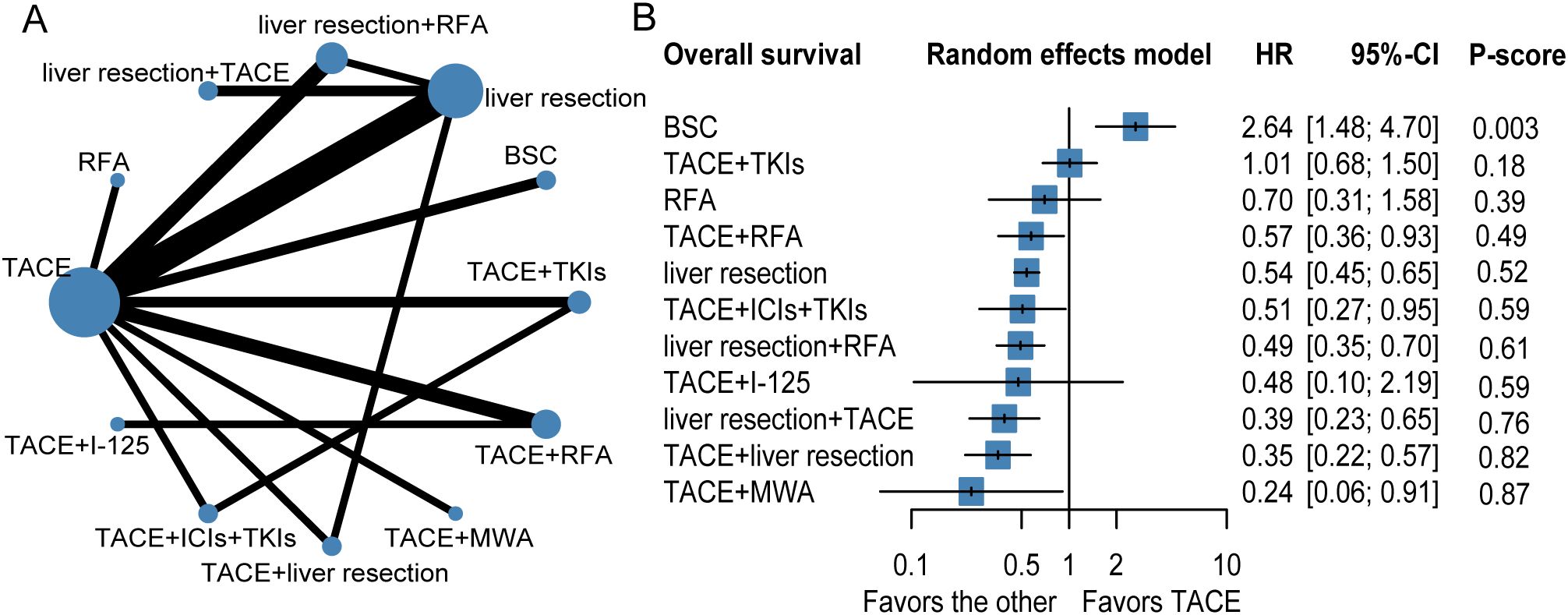
Figure 2. Network plot (A), forest plot (B) for overall survival. The therapeutic regimens with direct comparisons are linked by lines, the width of lines is proportional to the number of trials comparing each pair of interventions. The size of each node is proportional to the number of sample size. BSC, best supportive care; TKIs, tyrosine kinase inhibitors; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; ICIs, immune checkpoint inhibitors; I-125, iodine 125 seeds.
Secondary outcome: progression-free survival
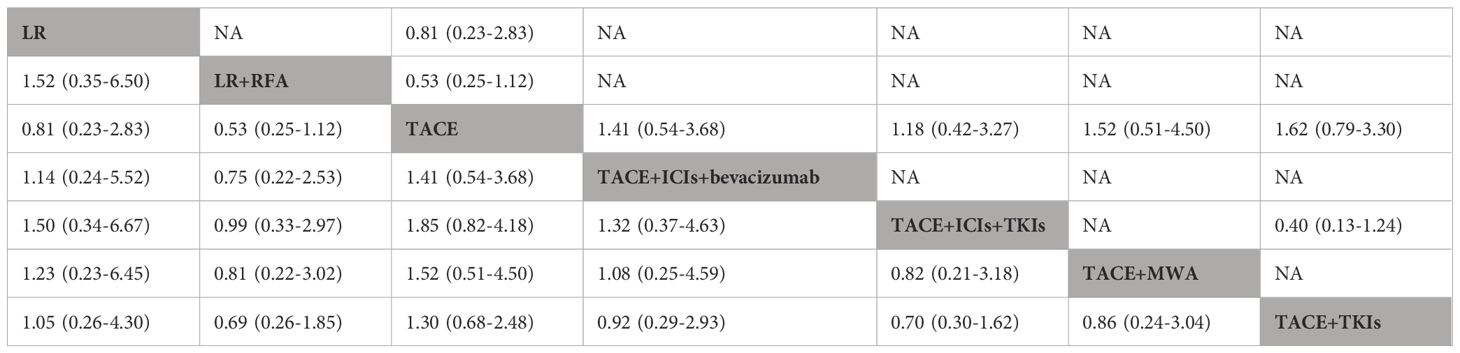
To evaluate progression-free survival (PFS), nine studies and seven therapeutic regimens in 1,776 patients with HCC were selected for further analysis (Figure 3, Table 3). No therapies showed better PFS benefit than TACE alone. Liver resection plus TACE (P-score = 0.71) and TACE combined with ICIs and TKIs (P-score = 0.70) were ranked first and second, respectively. In addition, pairwise treatment comparisons also indicated that no therapies improved PFS compared with TACE alone (Supplementary Table S8). The evidence credibility of the NMA results is shown in Supplementary Table S9, Supplementary Figure S2.
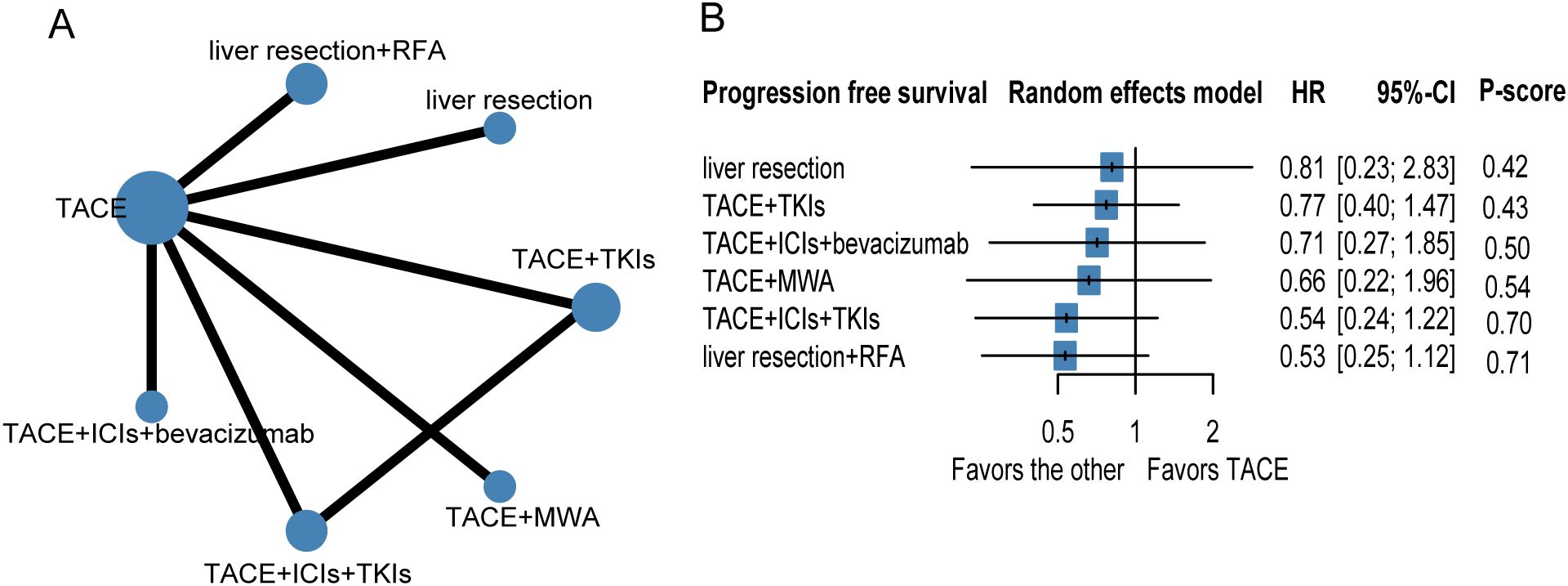
Figure 3. Network plot (A) and forest plot (B) for progression free survival. Circle sizes reflect numbers of participants, while line widths reflect numbers of direct comparisons. The absence of a connecting line between two treatments indicates that there was no direct comparison. TKIs, tyrosine kinase inhibitors; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; ICIs, immune checkpoint inhibitors.
Subgroup analysis
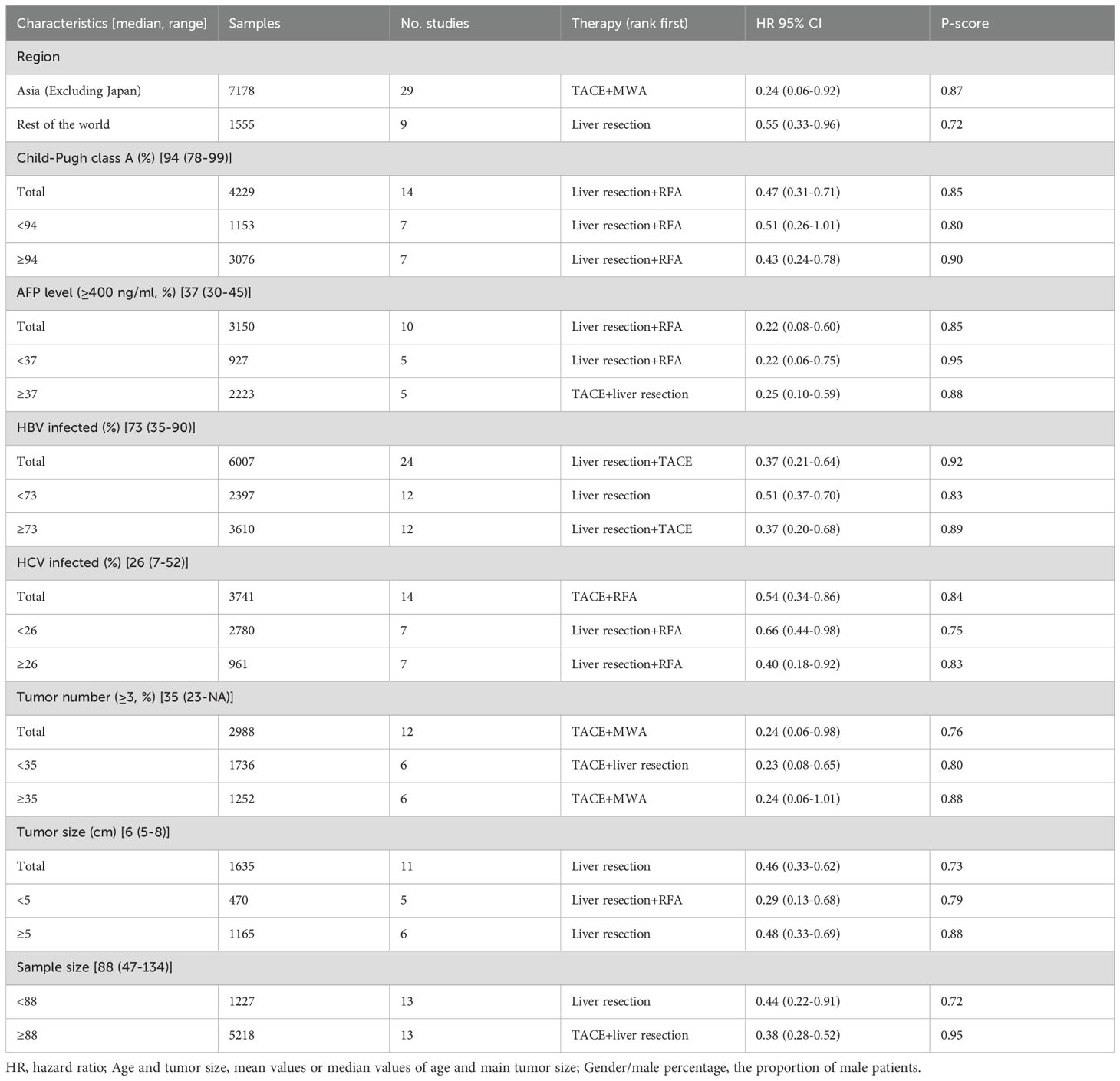
Intermediate-stage (BCLC-B) HCC is characterized by substantial heterogeneity. Therefore, subgroup analysis was performed to identify the optimal patient group for these effective regimens. In terms of the primary endpoint (i.e., OS), subgroup analysis was performed according to a series of clinical parameters, including region, Child–Pugh class, AFP level, etiology (HBV or HCV infection), tumor number, and tumor size. As shown in Table 1, there was significant heterogeneity in terms of etiology among the included studies. Subgroup analysis was performed according to the median value of these clinical parameters (Table 4).
Subgroup analysis was also performed according to the region of origin. TACE plus MWA (aHR = 0.24, 95%CI = 0.06–0.92) was associated with the highest OS benefit in Asia (excluding Japan) (Supplementary Table S10, Supplementary Figure S3), while liver resection ranked first in the rest of the region (aHR = 0.55, 95%CI = 0.33–0.96). The results are shown in Supplementary Table S11, Supplementary Figure S4. In terms of AFP level (Supplementary Figure S8), eight studies with 2,836 patients were included. Liver resection plus RFA ranked highest in patients with lower AFP levels (aHR = 0.22, 95%CI = 0.06–0.75) (Supplementary Figure S9, Supplementary Table S14). For the subgroup with higher AFP levels (Supplementary Figure S10, Supplementary Table S15), the combination of TACE and liver resection was associated with the greatest reduction in risk of death (aHR = 0.25, 95%CI = 0.10–0.59).
For the subgroup analysis of HBV infection (Supplementary Figure S11), 24 studies with 6,007 patients were included. Liver resection ranked highest in patients with lower HBV infection (aHR = 0.51, 95%CI = 0.37–0.70) (Supplementary Figure S12, Supplementary Table S16), while liver resection plus TACE ranked first in terms of improving OS in patients with higher HBV infection (aHR = 0.37, 95%CI = 0.20–0.68) (Supplementary Figure S13, Supplementary Table S17). In addition, a total of 14 studies with 3,741 patients showed the HCV infection status (Supplementary Figure S14). Subgroup analysis showed that liver resection plus RFA was reported as the highest therapy with the highest improvement in OS in patients with HCV infection (Supplementary Table S18, Supplementary Figure S16). In addition, 11 studies with 1,635 patients reported data on tumor size (Supplementary Figure S19), with the median value of tumor size being 6 cm. Liver resection plus RFA ranked highest in patients with smaller tumor size (aHR = 0.29, 95%CI = 0.13–0.68) (Supplementary Figure S20, Supplementary Table S21). For the subgroup with bigger tumor size, liver resection was significantly associated with the greatest reduction in risk of death (aHR = 0.48, 95%CI = 0.33–0.69) (Supplementary Figure S21, Supplementary Table S22). Notably, in the subgroup of bigger sample size, a total of 13 studies with 5,218 patients were included. TACE plus liver resection had the highest OS benefit in this subgroup (HR = 0.38, 95%CI = 0.28–0.52). Details of the subgroup analysis are shown in Supplementary Table S24, Supplementary Figure S24.
Assessment of inconsistency
Overall, the results of the design-by-treatment interaction test indicated a slight inconsistency in terms of OS (p = 0.039). There was no significant difference for PFS in terms of inconsistency between direct and indirect estimates in the back-calculation and design-by-treatment interaction test (p = 0.099).
Discussion
To date, BCLC classification is the most widely used treatment algorithm for HCC around the world. TACE is still the recommended standard therapy for BCLC-B stage HCC. However, this stage is characterized by substantial heterogeneity. In addition, patients treated with several therapy combinations have shown longer survival times compared with those with TACE alone. To compare the efficacy of these therapies, NMA was performed, which included 10,972 patients who received 13 different therapies across 38 clinical studies.
The results of the NMA revealed that TACE plus thermal ablation, TACE plus resection, TACE plus ICIs plus TKIs, liver resection, liver resection plus TACE, and liver resection plus RFA significantly improved OS compared with TACE alone. On the other hand, no monotherapies or combination treatments significantly prolonged the PFS compared with TACE alone. Notably, TACE plus MWA showed the best efficacy in improving OS. The superiority of TACE plus MWA can be explained as follows: 1) chemoembolization could significantly reduce the tumor size, then increase the complete ablation rate; 2) the undetected micronodules and the margin of active tumors can be identified by hepatic artery angiography, which then guides further ablation; and 3) the blood supply artery of the tumor can be embolized using TACE, which then reduces the cooling effect of blood flow and enhances the efficacy of ablation. In addition, the efficacy of TACE plus MWA is superior to TACE plus RFA in terms of OS, which can be attributed to MWA able to result in higher intratumor temperature, larger necrosis area, and deeper tissue penetration than RFA. Consistently, TACE plus RFA is suitable for patients in the BCLC-B stage up to seven (51, 73). Moreover, patients with two or three tumors, and intermediate tumor size (3–5 cm), could benefit from TACE plus MWA (74–76). Subgroup analysis showed that TACE plus RFA significantly prolonged the OS in patients with poor liver function, no history of HBV infection, HCV infection, and in old patients (Table 5).
Our study showed that liver resection provides significant OS improvement compared with TACE alone, which is consistent with a previous meta-analysis (77). Notably, we excluded patients with solitary large HCC (>5 cm), classified as BCLC-A stage in the updated BCLC classification. Huang et al. confirmed that liver resection showed better OS than TACE in patients with middle-high tumor burden (up to 7), but with tumor numbers ≤3 and preserved liver function (57). Consistently, subgroup analysis also found that liver resection ranked first among patients with intermediate tumor size (6 cm, range = 5–8 cm), a lower proportion of HBV-infected patients, and non-Asian areas (excluding Japan). Most of the patients with HCC had a history of HBV infection in Asia, but non-viral or HCV infection in Japan and in western countries (78). Moreover, the HBV-associated HCC patients with positive HBcAb showed a higher risk of recurrence after liver resection (79); hence, postoperative therapies are needed for these patients. Notably, subgroup analysis found that adjuvant TACE showed the best efficacy in prolonging OS in patients with positive HBcAb. Therefore, liver resection is suitable for intermediate-stage patients with two or three nodules, median tumor size (5–8 cm), preserved liver function, and negative HBcAb (Table 5).
Preoperative TACE ranked second in terms of prolonging OS in the NMA. TACE was performed as a neoadjuvant and transformative therapy in the clinic, the aim of which is to reduce the tumor size, downstage the cancer, and eventually enhance the efficacy of surgery, as well as even creating opportunities for resection. However, the clinical value of preoperative TACE in large (≥5 cm) resectable HCC is still uncertain. A prospective study confirmed that preoperative TACE did not improve OS in patients with resectable large HCC (≥5 cm, BCLC-A/B stage). This study was not included in our NMA due to the lack of subgroup analysis. Notably, the included studies also found that intemediate-stage patients with up to 7 (two tumors, tumor size ≤5 cm) could benefit from preoperative TACE (26, 39). Consistently, subgroup analysis found that preoperative TACE ranked first in terms of prolonging OS in patients in the BCLC-B stage with two nodules (Table 5).
Notably, our NMA found that adjuvant TACE significantly prolonged OS compared with TACE alone. Its efficacy ranked third among all therapies. The 5-year postoperative recurrence rate of HCC is up to 75% (80). Theoretically, TACE is performed to identify and eliminate latent micro-metastasis after resection, then to prevent HCC recurrence. However, the efficacy of adjuvant TACE for HCC is controversial. Some clinical studies have suggested that patients with intermediate (solitary tumor ≥5 cm without microvascular invasion) or high risk (a single tumor with microvascular invasion, two or three tumor nodules) of recurrence could benefit from adjuvant TACE (43, 81), particularly for HBV-related HCC (82). Consistently, subgroup analysis also showed that patients with two or three tumors, elevated AFP (≥400 ng/ml), and HBV infection and those aged <58 years could benefit from adjuvant TACE (Table 5).
As described above, curative resection provided better efficacy than TACE for a particular subgroup of BCLC-B stage patients. However, considerable intermediate-stage HCC patients showed no chance of resection due to the unfavorable tumor location, insufficient future liver remnant (FLR), and excessive tumors (four or more). To strive for curative chances for patients who can benefit from hepatectomy, partial hepatectomy plus RFA was introduced (58). This combination therapy is performed simultaneously to eradicate all tumor nodules through hepatectomy of the main tumor and RFA of the residual lesions. The included studies found that this combination therapy may be suitable for a particular subgroup of BCLC-B stage HCC patients, including those with preserved liver function, resectable dominant lesions restricted to one lobe, ≤4 or ≤5 tumors (with the main tumor size being <6 cm), and limited ablated tumor size (≤2 cm) (27, 58). Consistently, subgroup analysis also confirmed that patients with preserved liver function (Child–Pugh class A), dominant tumor size <6 cm, low AFP (<400 ng/ml), no history of HBV and HCV infection, are men, and aged >58 years could benefit from this combination therapy (Table 5).
With the advent of the immune targeting era, several RCTs and real-world studies have been conducted to evaluate the efficacy of TACE combined with ICIs and MTTs for the treatment of HCC. Our NMA found that this triple therapy could significantly prolong OS. However, the included participants of an RCT (EMERALD-1) and a real-world study (CHANCE001) were unresectable or were BCLC-B/C stage HCC patients. Consistently, the latest phase 3 LEAP-012 study (83) confirmed that TACE plus lenvatinib plus pembrolizumab significantly prolonged the PFS in unresected, non-metastatic HCC compared with TACE. Subgroup analysis also obtained positive results in unresectable BCLC-B stage HCC patients (HR = 0.57, 95%CI = 0.41–0.77), although not significant in terms of OS. Longer follow-up is needed. Thus, participants with unresectable BCLC-B stage HCC comprised one part of the included patients. TACE plus TKIs plus ICIs may be suitable for partial unresectable BCLC-B stage HCC. We could not further perform detailed subgroup analysis based on tumor size, tumor number, etc. The optimal BCLC-B stage patients who could benefit from this triple therapy need further validation (Table 5).
Intermediate-stage (BCLC-B) HCC is characterized by substantial heterogeneity. TACE is the standard therapeutic approach for patients in this stage. However, TACE is possibly overused and may not be appropriate for all patients. Our results revealed that seven therapies showed OS benefit over TACE alone: liver resection, liver resection plus TACE, liver resection plus RFA, TACE plus ICIs plus TKIs, TACE plus RFA, TACE plus MWA, and TACE plus liver resection showed better OS benefit than TACE alone. Subgroup analysis indicated that these therapies are suitable for particular intermediate-stage HCC patients. These results could be used to guide further clinical studies and facilitate clinical decision-making. However, only a portion of the included studies reported detailed clinical parameters. Due to the limited number of studies and the small sample size of the subgroups, the efficacy of TACE plus another therapy among all subtypes could not be accurately evaluated. Further high-quality clinical studies are needed to further verify these findings.
This NMA has several limitations. Firstly, all NMA studies are limited by assumptions of transitivity and similarity. The methodologies in the included studies were comparable, and the characteristics of the patients were similar. Several retrospective studies were included in this NMA. To reduce the RoB of retrospective studies, only the results of the propensity score matching (PSM) analysis were included. In addition, design-adjusted analysis was performed according to the RoB (studies with a higher bias risk were assigned a lower weight). Secondly, the HR and 95%CI were not reported in some studies. We extracted these data from the Kaplan–Meier curves. Thirdly, there was a lack of detailed clinical characteristics in some studies; therefore, the subgroup analysis could not be performed accordingly. Finally, there exists heterogeneity among the included studies. The median follow-up time of the included trials differed. Moreover, the procedures for TACE and thermal ablation varied across the included studies.
Conclusion
In summary, it was found that a series of therapeutic regimens, including TACE plus MWA, preoperative TACE, TACE plus TKIs plus ICIs, TACE plus RFA, liver resection, and adjuvant therapies (TACE and RFA) showed better OS benefit than TACE alone. This might have implications in informed decision-making when considering the optimal treatment regimen for patients with intermediate-stage HCC. Despite confidence in the findings being high for preoperative TACE and liver resection plus RFA, low for TACE plus MWA and adjuvant RFA and very low for all others. Therefore, more therapeutic regimens and high-quality studies are needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
SZ: Conceptualization, Data curation, Funding acquisition, Validation, Writing – original draft. YY: Formal Analysis, Investigation, Methodology, Software, Writing – original draft. ZY: Formal Analysis, Funding acquisition, Investigation, Visualization, Writing – review & editing. LF: Methodology, Project administration, Software, Writing – review & editing. KW: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. ZC: Formal Analysis, Project administration, Supervision, Validation, Writing – review & editing. HC: Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. ZM: Conceptualization, Supervision, Validation, Writing – review & editing. YH: Conceptualization, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Financial support was provided by the Natural Science Foundation of Shanghai (No. 21ZR1479500), Natural Science Foundation of China (No. 82405144).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1577614/full#supplementary-material
Abbreviations
HCC, hepatocellular carcinoma; BCLC, Barcelona Clinic Liver Cancer; TACE, transarterial chemoembolization; OS, overall survival; PFS, progression-free survival; CI, confidence interval; MWA, microwave ablation; PLC, primary liver cancer; CNLC, China Liver Cancer; PES, post-embolization syndromes; RFA, radiofrequency ablation; TARE-Y90, radioembolization with yttrium 90; ICIs, immune checkpoint inhibitors; RCT, randomized controlled trial.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Asafo-Agyei KO and Samant H. Hepatocellular Carcinoma. In: StatPearls. StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC, Treasure Island (FL (2024).
3. Vogel A, Meyer T, Sapisochin G, Salem R, and Saborowski A. Hepatocellular carcinoma. Lancet. (2022) 400:1345–62. doi: 10.1016/s0140-6736(22)01200-4
4. Bruix J and Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. (2011) 53:1020–2. doi: 10.1002/hep.24199
5. European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer(EASL-EORTC). EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. (2012) 56:908–43. doi: 10.1016/j.jhep.2011.12.001
6. Forner A, Reig M, and Bruix J. Hepatocellular carcinoma. Lancet. (2018) 391:1301–14. doi: 10.1016/s0140-6736(18)30010-2
7. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. (2018) 68:723–50. doi: 10.1002/hep.29913
8. Sieghart W, Hucke F, and Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol. (2015) 62:1187–95. doi: 10.1016/j.jhep.2015.02.010
9. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. (2022) 76:681–93. doi: 10.1016/j.jhep.2021.11.018
10. Mason MC, Massarweh NN, Salami A, Sultenfuss MA, and Anaya DA. Post-embolization syndrome as an early predictor of overall survival after transarterial chemoembolization for hepatocellular carcinoma. HPB (Oxford). (2015) 17:1137–44. doi: 10.1111/hpb.12487
11. Lu H, Zheng C, Liang B, and Xiong B. Efficacy and safety analysis of dexamethasone-lipiodol emulsion in prevention of post-embolization syndrome after TACE: a retrospective analysis. BMC Gastroenterol. (2021) 21:256. doi: 10.1186/s12876-021-01839-w
12. Lencioni R, de Baere T, Soulen MC, Rilling WS, and Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology. (2016) 64:106–16. doi: 10.1002/hep.28453
13. Zhong C, Niu Y, Liu W, Yuan Y, Li K, Shi Y, et al. S100A9 derived from chemoembolization-induced hypoxia governs mitochondrial function in hepatocellular carcinoma progression. Adv Sci (Weinh). (2022) 9:e2202206. doi: 10.1002/advs.202202206
14. Zong S, Huang G, Pan B, Zhao S, Ling C, and Cheng B. A hypoxia-related miRNA-mRNA signature for predicting the response and prognosis of transcatheter arterial chemoembolization in hepatocellular carcinoma. J Hepatocell Carcinoma. (2024) 11:525–42. doi: 10.2147/jhc.S454698
15. Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. (2016) 64:1090–8. doi: 10.1016/j.jhep.2016.01.012
16. Meyer T, Fox R, Ma YT, Ross PJ, James MW, Sturgess R, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. (2017) 2:565–75. doi: 10.1016/s2468-1253(17)30156-5
17. Kudo M, Cheng AL, Park JW, Park JH, Liang PC, Hidaka H, et al. Orantinib versus placebo combined with transcatheter arterial chemoembolisation in patients with unresectable hepatocellular carcinoma (ORIENTAL): a randomised, double-blind, placebo-controlled, multicentre, phase 3 study. Lancet Gastroenterol Hepatol. (2018) 3:37–46. doi: 10.1016/s2468-1253(17)30290-x
18. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
19. Kudo M, Finn RS, Galle PR, Zhu AX, Ducreux M, Cheng AL, et al. IMbrave150: Efficacy and Safety of Atezolizumab plus Bevacizumab versus Sorafenib in Patients with Barcelona Clinic Liver Cancer Stage B Unresectable Hepatocellular Carcinoma: An Exploratory Analysis of the Phase III Study. Liver Cancer. (2023) 12:238–50. doi: 10.1159/000528272
20. Pinato DJ, Murray SM, Forner A, Kaneko T, Fessas P, Toniutto P, et al. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J Immunother Cancer. (2021) 9. doi: 10.1136/jitc-2021-003311
21. Zhu HD, Li HL, Huang MS, Yang WZ, Yin GW, Zhong BY, et al. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Signal Transduct Target Ther. (2023) 8:58. doi: 10.1038/s41392-022-01235-0
22. Lencioni R, Kudo M, Erinjeri J, Qin S, Ren Z, Chan SL, et al. EMERALD-1: A phase 3, randomized, placebo-controlled study of transarterial chemoembolization combined with durvalumab with or without bevacizumab in participants with unresectable hepatocellular carcinoma eligible for embolization. (2024).
23. Llovet JM, Finn RS, Ren Z, Guo Y, Han G, Lin H, et al. LEAP-012: A phase 3, study of lenvatinib plus pembrolizumab plus transarterial chemoembolization for intermediate-stage hepatocellular carcinoma. (2024).
24. Yin L, Li H, Li AJ, Lau WY, Pan ZY, Lai EC, et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol. (2014) 61:82–8. doi: 10.1016/j.jhep.2014.03.012
25. Yang Y, Yu H, Qi L, Liu C, Feng Y, Qi J, et al. Combined radiofrequency ablation or microwave ablation with transarterial chemoembolization can increase efficiency in intermediate-stage hepatocellular carcinoma without more complication: a systematic review and meta-analysis. Int J Hyperthermia. (2022) 39:455–65. doi: 10.1080/02656736.2022.2048095
26. Hu Z, Wang X, Fu Y, Yang D, Zhou Z, Chen M, et al. Survival benefit of liver resection following complete response to transarterial chemoembolization for intermediate-stage hepatocellular carcinoma: a retrospective, multicenter, cohort study. Int J Surg. (2024) 110:1019–27. doi: 10.1097/js9.0000000000000942
27. Yan J, Man Z, Lu Q, and Ma K. Long-term survival in patients receiving combination therapy with resection and radiofrequency ablation for multi-focal hepatocellular carcinoma classified as Barcelona clinic liver cancer stage B: A retrospective controlled study. Cancer Manag Res. (2020) 12:2613–21. doi: 10.2147/cmar.S237635
28. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. doi: 10.1136/bmj.n71
29. Parmar MK, Torri V, and Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. (1998) 17:2815–34.
30. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. (2019) 366:l4898. doi: 10.1136/bmj.l4898
31. Efthimiou O, Mavridis D, Debray TP, Samara M, Belger M, Siontis GC, et al. Combining randomized and non-randomized evidence in network meta-analysis. Stat Med. (2017) 36:1210–26. doi: 10.1002/sim.7223
32. White IR, Barrett JK, Jackson D, and Higgins JP. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. (2012) 3:111–25. doi: 10.1002/jrsm.1045
33. Salanti G, Del Giovane C, Chaimani A, Caldwell DM, and Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PloS One. (2014) 9:e99682. doi: 10.1371/journal.pone.0099682
34. Dai WC, Cheung TT, Chok KS, Chan AC, Sharr WW, Tsang SH, et al. Radiofrequency ablation versus transarterial chemoembolization for unresectable solitary hepatocellular carcinomas sized 5-8 cm. HPB (Oxford). (2015) 17:226–31. doi: 10.1111/hpb.12324
35. Kaibori M, Matsushima H, Ishizaki M, Kosaka H, Matsui K, Kariya S, et al. The impact of sorafenib in combination with transarterial chemoembolization on the outcomes of intermediate-stage hepatocellular carcinoma. Asian Pac J Cancer Prev. (2021) 22:1217–24. doi: 10.31557/apjcp.2021.22.4.1217
36. Wang C, Liao Y, Qiu J, Yuan Y, Zhang Y, Li K, et al. Transcatheter arterial chemoembolization alone or combined with ablation for recurrent intermediate-stage hepatocellular carcinoma: a propensity score matching study. J Cancer Res Clin Oncol. (2020) 146:2669–80. doi: 10.1007/s00432-020-03254-2
37. Wang XH, Duan WB, Liang W, Li H, Xie XY, Li SQ, et al. Efficacy of radiofrequency ablation following transarterial chemoembolisation combined with sorafenib for intermediate stage recurrent hepatocellular carcinoma: a retrospective, multicentre, cohort study. EClinicalMedicine. (2023) 56:101816. doi: 10.1016/j.eclinm.2022.101816
38. Huang WK, Yang SF, You LN, Liu M, Liu DY, Gu P, et al. Transcatheter arterial chemoembolisation (TACE) plus S-1 for the treatment of BCLC stage B hepatocellular carcinoma refractory to TACE. Contemp Oncol (Pozn). (2016) 20:468–74. doi: 10.5114/wo.2016.65607
39. Zhou Q, Tuo F, Li R, Wang X, Wang J, Huang Z, et al. Transarterial chemoembolization combined with hepatectomy for the treatment of intermediate-stage hepatocellular carcinoma. Front Oncol. (2020) 10:578763. doi: 10.3389/fonc.2020.578763
40. Zhou C, Peng Y, Zhou K, Zhang L, Zhang X, Yu L, et al. Surgical resection plus radiofrequency ablation for the treatment of multifocal hepatocellular carcinoma. Hepatobiliary Surg Nutr. (2019) 8:19–28. doi: 10.21037/hbsn.2018.11.19
41. Yang B, Yuan M, Yang T, Liao Z, and Wu H. Transarterial chemoembolization vs. liver resection as initial treatment for hepatocellular carcinoma occurring exclusively in caudate lobe: A retrospective propensity matching analysis. Oncol Res. (2022) 30:23–33. doi: 10.32604/or.2022.026044
42. Wang XH, Zhou QF, Wang CM, Xiang CL, Song YH, Li SQ, et al. Adjuvant transarterial chemoembolization for intermediate-stage hepatocellular carcinoma with microvascular invasion. Br J Surg. (2023) 110:913–6. doi: 10.1093/bjs/znac376
43. Wang L, Lin N, Lin K, Xiao C, Wang R, Chen J, et al. The clinical value of postoperative transarterial chemoembolization for resectable patients with intermediate hepatocellular carcinoma after radical hepatectomy: a propensity score-matching study. J Gastrointest Surg. (2021) 25:1172–83. doi: 10.1007/s11605-020-04588-5
44. Tada T, Kumada T, Toyoda H, Tsuji K, Hiraoka A, Itobayashi E, et al. Role of hepatic resection in patients with intermediate-stage hepatocellular carcinoma: A multicenter study from Japan. Cancer Sci. (2017) 108:1414–20. doi: 10.1111/cas.13257
45. Tada T, Kumada T, Hiraoka A, Hirooka M, Kariyama K, Tani J, et al. Comparison of prognostic impact of atezolizumab plus bevacizumab versus lenvatinib in patients with intermediate-stage hepatocellular carcinoma. Liver Int. (2024) 44:113–24. doi: 10.1111/liv.15753
46. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2–3 study. Lancet Oncol. (2021) 22:977–90. doi: 10.1016/s1470-2045(21)00252-7
47. Peng Y, Liu F, Xu H, Wei Y, and Li B. Is laparoscopic liver resection suitable for selected patients with BCLC stage B HCC? A propensity score-matched analysis. HPB (Oxford). (2020) 22:595–602. doi: 10.1016/j.hpb.2019.08.016
48. Ohama H, Hiraoka A, Tada F, Kato K, Fukunishi Y, Yanagihara E, et al. Clinical usefulness of surgical resection including the complementary use of radiofrequency ablation for intermediate-stage hepatocellular carcinoma. Cancers (Basel). (2022) 15. doi: 10.3390/cancers15010236
49. Nouso K, Kariyama K, Nakamura S, Oonishi A, Wakuta A, Oyama A, et al. Application of radiofrequency ablation for the treatment of intermediate-stage hepatocellular carcinoma. J Gastroenterol Hepatol. (2017) 32:695–700. doi: 10.1111/jgh.13586
50. Nong X, Zhang Y, Xie J, Liang J, Xie A, and Zhang Z. Evaluation of the up-to-7 criterion for determining the treatment of hepatocellular carcinoma in Barcelona Clinic Liver Cancer stage B: a single-center retrospective cohort study. J Gastrointest Oncol. (2023) 14:768–79. doi: 10.21037/jgo-23-69
51. Lin CW, Chen YS, Lo GH, Hsu YC, Hsu CC, Wu TC, et al. Comparison of overall survival on surgical resection versus transarterial chemoembolization with or without radiofrequency ablation in intermediate stage hepatocellular carcinoma: a propensity score matching analysis. BMC Gastroenterol. (2020) 20:99. doi: 10.1186/s12876-020-01235-w
52. Li H, Wang J, Zhang G, Kuang D, Li Y, He X, et al. Transarterial chemoembolization combined donafenib with/without PD-1 for unresectable HCC in a multicenter retrospective study. Front Immunol. (2023) 14:1277329. doi: 10.3389/fimmu.2023.1277329
53. Kim JY, Sinn DH, Gwak GY, Choi GS, Saleh AM, Joh JW, et al. Transarterial chemoembolization versus resection for intermediate-stage (BCLC B) hepatocellular carcinoma. Clin Mol Hepatol. (2016) 22:250–8. doi: 10.3350/cmh.2016.0015
54. Kim GH, Kim JH, Ko HK, Chu HH, Kim SH, Shin JH, et al. Surgical Resection plus Intraoperative Radiofrequency Ablation versus Chemoembolization for the Treatment of Intermediate-Stage (BCLC B) Hepatocellular Carcinoma with Preserved Liver Function: A Propensity Score-Matched Analysis. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14102440
55. Ke Y, Zhong J, Guo Z, Liang Y, Li L, and Xiang B. Comparison liver resection with transarterial chemoembolization for Barcelona Clinic Liver Cancer stage B hepatocellular carcinoma patients on long-term survival after SPSS propensity score matching. Zhonghua Yi Xue Za Zhi. (2014) 94:747–50.
56. Kariyama K, Nouso K, Wakuta A, Oonishi A, Toyoda H, Tada T, et al. Treatment of intermediate-stage hepatocellular carcinoma in Japan: position of curative therapies. Liver Cancer. (2020) 9:41–9. doi: 10.1159/000502479
57. Huang CT, Chu YL, Su TH, Huang SC, Tseng TC, Hsu SJ, et al. Optimizing survival benefit by surgical resection by the seven-eleven criteria in Barcelona clinic liver cancer stage A/B hepatocellular carcinoma beyond the Milan criteria. Liver Cancer. (2023) 12:539–49. doi: 10.1159/000529143
58. Hou YF, Wei YG, Yang JY, Wen TF, Xu MQ, Yan LN, et al. Combined hepatectomy and radiofrequency ablation versus TACE in improving survival of patients with unresectable BCLC stage B HCC. Hepatobiliary Pancreat Dis Int. (2016) 15:378–85. doi: 10.1016/s1499-3872(16)60089-9
59. Hirooka M, Hiraoka A, Ochi H, Kisaka Y, Joko K, Michitaka K, et al. Transcatheter arterial chemoembolization with or without radiofrequency ablation: outcomes in patients with barcelona clinic liver cancer stage B hepatocellular carcinoma. AJR Am J Roentgenol. (2018) 210:891–8. doi: 10.2214/ajr.17.18177
60. Espinosa W, Liu YW, Wang CC, Lin CC, Wang JH, Lu SN, et al. Combined resection and radiofrequency ablation versus transarterial embolization for intermediate-stage hepatocellular carcinoma: A propensity score matching study. J Formos Med Assoc. (2018) 117:197–203. doi: 10.1016/j.jfma.2017.03.014
61. Endo K, Kuroda H, Oikawa T, Okada Y, Fujiwara Y, Abe T, et al. Efficacy of combination therapy with transcatheter arterial chemoembolization and radiofrequency ablation for intermediate-stage hepatocellular carcinoma. Scand J Gastroenterol. (2018) 53:1575–83. doi: 10.1080/00365521.2018.1548645
62. Chen S, Jin H, Dai Z, Wei M, Xiao H, Su T, et al. Liver resection versus transarterial chemoembolization for the treatment of intermediate-stage hepatocellular carcinoma. Cancer Med. (2019) 8:1530–9. doi: 10.1002/cam4.2038
63. Chen L, Kan X, Sun T, Ren Y, Cao Y, Yan L, et al. Transarterial chemoembolization combined with iodine 125 seeds versus transarterial chemoembolization combined with radiofrequency ablation in the treatment of early- and intermediate-stage hepatocellular carcinoma. BMC Gastroenterol. (2020) 20:205. doi: 10.1186/s12876-020-01355-3
64. Akarapatima K, Chang A, Prateepchaiboon T, Pungpipattrakul N, Songjamrat A, Pakdeejit S, et al. Comparison of overall survival between transarterial chemoembolization and best supportive care in intermediate- stage hepatocellular carcinoma. Asian Pac J Cancer Prev. (2022) 23:3173–8. doi: 10.31557/apjcp.2022.23.9.3173
65. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. (2022) 1:EVIDoa2100070. doi: 10.1056/EVIDoa2100070
66. Lu L, Zheng P, Wu Z, and Chen X. Hepatic resection versus transarterial chemoembolization for intermediate-stage hepatocellular carcinoma: A cohort study. Front Oncol. (2021) 11:618937. doi: 10.3389/fonc.2021.618937
67. Luo J, Peng ZW, Guo RP, Zhang YQ, Li JQ, Chen MS, et al. Hepatic resection versus transarterial lipiodol chemoembolization as the initial treatment for large, multiple, and resectable hepatocellular carcinomas: a prospective nonrandomized analysis. Radiology. (2011) 259:286–95. doi: 10.1148/radiol.10101072
68. Xu W, Rao Q, An Y, Li M, Xu G, Sang X, et al. Proposal for subclassification to select patients for hepatectomy with intermediate hepatocellular carcinoma and Child-Pugh A liver function: A double-center study from China. Med (Baltimore). (2018) 97:e11800. doi: 10.1097/md.0000000000011800
69. Ciria R, López-Cillero P, Gallardo AB, Cabrera J, Pleguezuelo M, Ayllón MD, et al. Optimizing the management of patients with BCLC stage-B hepatocellular carcinoma: Modern surgical resection as a feasible alternative to transarterial chemoemolization. Eur J Surg Oncol. (2015) 41:1153–61. doi: 10.1016/j.ejso.2015.05.023
70. Ho MC, Huang GT, Tsang YM, Lee PH, Chen DS, Sheu JC, et al. Liver resection improves the survival of patients with multiple hepatocellular carcinomas. Ann Surg Oncol. (2009) 16:848–55. doi: 10.1245/s10434-008-0282-7
71. Zhang R, Shen L, Zhao L, Guan Z, Chen Q, and Li W. Combined transarterial chemoembolization and microwave ablation versus transarterial chemoembolization in BCLC stage B hepatocellular carcinoma. Diagn Interv Radiol. (2018) 24:219–24. doi: 10.5152/dir.2018.17528
72. Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. (2020) 69:1492–501. doi: 10.1136/gutjnl-2019-318934
73. Li L, Xin Y, Zhang X, Chen Y, Yang Y, Zhou X, et al. The effectiveness of radiofrequency ablation for patients with BCLC B1 stage hepatocellular carcinoma downgraded by transarterial chemoembolization. Clin Res Hepatol Gastroenterol. (2022) 46:101878. doi: 10.1016/j.clinre.2022.101878
74. Ni JY, Fang ZT, Sun HL, An C, Huang ZM, Zhang TQ, et al. A nomogram to predict survival of patients with intermediate-stage hepatocellular carcinoma after transarterial chemoembolization combined with microwave ablation. Eur Radiol. (2020) 30:2377–90. doi: 10.1007/s00330-019-06438-8
75. Smolock AR, Cristescu MM, Hinshaw A, Woo KM, Wells SA, Ziemlewicz TJ, et al. Combination transarterial chemoembolization and microwave ablation improves local tumor control for 3- to 5-cm hepatocellular carcinoma when compared with transarterial chemoembolization alone. Abdom Radiol (NY). (2018) 43:2497–504. doi: 10.1007/s00261-018-1464-9
76. Zaitoun MMA, Elsayed SB, Zaitoun NA, Soliman RK, Elmokadem AH, Farag AA, et al. Combined therapy with conventional trans-arterial chemoembolization (cTACE) and microwave ablation (MWA) for hepatocellular carcinoma >3-<5 cm. Int J Hyperthermia. (2021) 38:248–56. doi: 10.1080/02656736.2021.1887941
77. Labgaa I, Taffé P, Martin D, Clerc D, Schwartz M, Kokudo N, et al. Comparison of partial hepatectomy and transarterial chemoembolization in intermediate-stage hepatocellular carcinoma: A systematic review and meta-analysis. Liver Cancer. (2020) 9:138–47. doi: 10.1159/000505093
78. Lau G, Abou-Alfa GK, Cheng AL, Sukeepaisarnjaroen W, Van Dao T, Kang YK, et al. Outcomes in the Asian subgroup of the phase III randomised HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. J Hepatol. (2024) 82(2):258–267. doi: 10.1016/j.jhep.2024.07.017
79. Li T, Wang SK, Zhou J, Sun HC, Qiu SJ, Ye QH, et al. Positive HBcAb is associated with higher risk of early recurrence and poorer survival after curative resection of HBV-related HCC. Liver Int. (2016) 36:284–92. doi: 10.1111/liv.12898
80. de Lope CR, Tremosini S, Forner A, Reig M, and Bruix J. Management of HCC. J Hepatol. (2012) 56 Suppl 1:S75–87. doi: 10.1016/s0168-8278(12)60009-9
81. Ren ZG, Lin ZY, Xia JL, Ye SL, Ma ZC, Ye QH, et al. Postoperative adjuvant arterial chemoembolization improves survival of hepatocellular carcinoma patients with risk factors for residual tumor: a retrospective control study. World J Gastroenterol. (2004) 10:2791–4. doi: 10.3748/wjg.v10.i19.2791
82. Wang Z, Ren Z, Chen Y, Hu J, Yang G, Yu L, et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: A randomized controlled study. Clin Cancer Res. (2018) 24:2074–81. doi: 10.1158/1078-0432.Ccr-17-2899
83. Kudo M, Ren Z, Guo Y, Han G, Lin H, Zheng J, et al. Transarterial chemoembolisation combined with lenvatinib plus pembrolizumab versus dual placebo for unresectable, non-metastatic hepatocellular carcinoma (LEAP-012): a multicentre, randomised, double-blind, phase 3 study. Lancet. (2025) 405:203–15. doi: 10.1016/s0140-6736(24)02575-3
Keywords: intermediate stage, hepatocellular carcinoma, TACE, efficacy, network meta-analysis
Citation: Zong S, Yang Y, Yin Z, Feng L, Wang K, Chen H, Chen Z, Meng Z and Hua Y (2025) Efficacy of therapies for intermediate-stage hepatocellular carcinoma: systematic review and network meta-analysis. Front. Immunol. 16:1577614. doi: 10.3389/fimmu.2025.1577614
Received: 16 February 2025; Accepted: 16 June 2025;
Published: 09 July 2025.
Edited by:
Maria Lina Tornesello, G. Pascale National Cancer Institute Foundation (IRCCS), ItalyReviewed by:
Marco Fiore, Università degli Studi della Campania ‘Luigi Vanvitelli’, ItalyDino Bekric, Paracelsus Medical University, Austria
Copyright © 2025 Zong, Yang, Yin, Feng, Wang, Chen, Chen, Meng and Hua. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongqiang Hua, a2VxaWFuZzEyMTVAMTI2LmNvbQ==; Zhiqiang Meng, bWVuZ3NoY2FAZnVkYW4uZWR1LmNu; Zhen Chen, emNoZW56bEBmdWRhbi5lZHUuY24=; Hao Chen, Y2hlbmdrbGxAc2luYS5jb20=
†These authors have contributed equally to this work
 Shaoqi Zong
Shaoqi Zong Yifan Yang1,2,3†
Yifan Yang1,2,3† Hao Chen
Hao Chen Zhen Chen
Zhen Chen Zhiqiang Meng
Zhiqiang Meng