- 1Oncology, Graduate School of Bengbu Medical University, Bengbu, Anhui, China
- 2Department of Radiation Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, China
Purpose: This study evaluates the impact of thoracic radiotherapy (TRT) combined with immune checkpoint inhibitors (ICIs) treatment-related pneumonitis on tumor progression and prognosis in patients with non-small cell lung cancer (NSCLC).
Methods: Data were collected retrospectively from NSCLC patients treated with TRT and ICIs between January 2019 and August 2023. Treatment-related pneumonitis (TRP) was assessed and graded using the Common Terminology Criteria for Adverse Events (CTCAE) and the Chinese Society of Clinical Oncology Guidelines for Managing Immunotherapy-Related Toxicities. Kaplan-Meier curves and log-rank tests examined associations between pneumonitis with local recurrence-free survival (LRFS), distant metastasis-free survival (DMFS), progression-free survival (PFS), and overall survival (OS). COX regression identified prognostic factors in the pneumonitis group.
Results: Among 86 patients, 58 (67.4%) developed TRP, including 37.2% with grade 2 pneumonitis, and no grade ≥3 cases. 12 patients (14.0%) developed mixed radiation and ICIs pneumonitis. The pneumonitis group had significantly shorter DMFS (12.07 vs not reached, p = 0.028) and PFS (9.53 vs 14.27 months, p = 0.040), shorter LRFS compared to the non-pneumonitis group, but similar OS. High-grade pneumonitis correlated with worse outcomes, especially DMFS (p = 0.031), basically no differences among pneumonitis types. Multivariate COX analysis identified solitary pulmonary nodules or masses as independent negative prognostic factors for PFS, while higher MLD (mean lung dose) independently predicted reduced OS.
Conclusion: Pneumonitis resulting from TRT combined with ICIs was associated with shorter PFS but did not affect OS in NSCLC patients. Mixed pneumonitis did not worsen outcomes. Larger prospective studies are needed to validate these findings.
1 Introduction
According to the latest GLOBOCAN2022 data, lung cancer remains the leading malignant tumor globally in both morbidity and mortality (1). Non-small cell lung cancer (NSCLC) is the predominant histological type, accounting for 85% of all lung cancer cases. In recent years, immune checkpoint inhibitors (ICIs) have revolutionized NSCLC treatment, establishing it as the first-line standard for advanced driver-negative cases. Moreover, following the landmark PACIFIC trial, durvalumab as a consolidation treatment following concurrent chemoradiotherapy (CCRT) has become the new standard for unresectable locally advanced NSCLC (2).
The combination of radiotherapy (RT) and ICIs therapy has shown a synergistic effect. However, whether used as consolidation in locally advanced settings, or palliative RT for oligo-metastatic cases, this combination increased the risk of toxicity, particularly pneumonitis, which has emerged as a key challenge in clinical practice (3). In the PACIFIC study, combining RT with durvalumab led to a higher incidence of pneumonitis compared to the placebo group (any grade: 33.9% vs. 24.8%; grade 3-4: 3.4% vs. 2.6%) (2). Similarly, in a large cohort of 1994 patients, the 2-year cumulative incidence of grades ≥2 pneumonitis was significantly higher with CRT plus durvalumab (22.1%) than with CRT alone (13.9%; P ≤ 0.001) (4). Grade 2 or higher pneumonitis during durvalumab consolidation therapy after chemoradiotherapy in stage III NSCLC was revealed to be associated with poorer progression-free survival (PFS) and overall survival (OS) (5).
However, reports on the impact of pneumonitis following radiotherapy or checkpoint inhibitors are inconsistent. Severe acute radiation pneumonitis (SARP), defined as grade ≥3, is a potentially life-threatening side effect of thoracic radiotherapy (TRT), diminishing survival benefits and impacting survivors’ quality of life (6–8). The prognostic significance of CIP is controversial. Suresh et al. (9) reported that CIP correlated with worse survival outcomes in advanced NSCLC, while Haratani et al. (10) indicated that irAEs, including CIP, were associated with better OS and PFS.
There is a distinction in the type of pneumonitis that develops in patients receiving a combination of RT and ICIs therapy in a clinical setting. So in this study, we conducted a retrospective analysis to explore the impact of the occurrence, type, and classification of pneumonitis on tumor progression and survival. Additionally, we assessed clinical and imaging predictors of poor prognosis in this patient population.
2 Materials and methods
2.1 Patients and treatment
This retrospective study was approved by the Ethics Committee of Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences. It included NSCLC patients who underwent TRT in combination with ICIs from January 2019 to August 2023. The inclusion criteria were as follows: (1) a confirmed initial NSCLC pathologically diagnosed; (2) administration of TRT and ICIs before the onset of pneumonitis; (3) availability of clinical, pathological, and dosimetric data. The exclusion criteria included: (1) absence of laboratory and imaging evaluations at the time of diagnosing treatment-related pneumonitis (TRP); (2) loss to follow-up; (3) prior TRT for other conditions; (4) asymptomatic pulmonary fibrosis six months after RT without a prior history of acute pneumonitis. Given the favorable prognosis associated with oligometastatic disease, in addition to locally advanced NSCLC, we included stage IV patients with 1–2 oligometastatic lesions.
2.2 Data collection and follow-up
Demographic data, clinical characteristics, details on pneumonitis and tumor treatments, radiotherapy dosimetry parameters, inflammation-related testing and imaging within a week of pneumonitis onset and throughout the treatment process were gathered from the patients’ electronic medical records. All patients underwent chest computed tomography scans before and after treatment every 1–3 months, continuing for up to 6 months prior to the final follow-up. Follow-up visits on disease progression and survival, either in-person or via telephone, were conducted every 3 months starting from the initiation of RT. Survival tracking began on the date RT was initiated, with endpoints defined as the time to tumor progression, last follow-up, or death. The primary endpoints were progression-free survival (PFS) and overall survival (OS), while secondary endpoints included local recurrence-free survival (LRFS) and distant metastasis-free survival (DMFS). The final follow-up was scheduled for May 2024.
Additionally, complete blood count data obtained within a week of pneumonitis onset were used to calculate the systemic immune-inflammation index (SII), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) using the following formulas: SII = platelet count × neutrophil count/lymphocyte count, NLR = neutrophil count/lymphocyte count, and PLR = platelet count/lymphocyte count.
2.3 Diagnosis and assessment of treatment-related pneumonitis
A multidisciplinary oncology team, comprising at least one radiation oncologist and one imaging expert, established the diagnosis, types (radiation pneumonitis, checkpoint inhibitors pneumonitis, and mixed pneumonitis), grading, and imaging characteristics of pneumonitis. The staging followed our previously defined criteria (11). Radiation pneumonitis (RP) typically occurs within six months after RT and is generally aligned with the dose distribution curve, while checkpoint inhibitors pneumonitis (CIP) that arises following ICIs appears more diffuse on CT scans and exhibits various imaging patterns, although the pneumonitis area rarely crosses lung fissures. Mixed pneumonitis is classified when both RP and CIP features are present, cannot be distinctly differentiated, and other causes such as heart failure, infection, and tumor progression have been excluded (12). The Common Terminology Criteria for Adverse Events (CTCAE, v5.0) was utilized to grade pneumonitis, categorizing symptomatic TRP as grade ≥2. All suspected pneumonitis diagnoses were evaluated by multiple physicians, ensuring a consensus conclusion. Imaging characteristics of TRP were categorized based on the International Multidisciplinary Classification Criteria established by the American Thoracic Society and the European Respiratory Society (ATS/ERS). Following prior research, pneumonitis-related imaging patterns were classified into organizing pneumonitis (OP), ground glass opacities (GGO), acute interstitial pneumonitis (AIP), hypersensitivity pneumonitis (HP), non-specific interstitial pneumonitis (NSIP) (13).
2.4 Statistical analysis
The data from this study were analyzed using SPSS 27.0 software. Descriptive analysis was conducted for all variables. Categorical variables were summarized as frequencies and percentages, while continuous variables were presented as medians. Receiver Operating Characteristic (ROC) curve analysis was utilized to determine the optimal critical values for each immunoinflammatory index and target area plan parameter. The χ2 test was used for comparing categorical variables, while continuous variables were analyzed using either the independent samples t-test or the nonparametric Mann-Whitney U test. Survival analysis of pneumonitis-related factors was evaluated using the Log-rank test, along with the Kaplan-Meier method for survival curves plotting survival curves. Cox proportional hazards models were applied for multivariate regression analyses to identify potential prognostic factors in the pneumonitis group. Potential interactions between variables were evaluated using the interactionR package in R (version 4.0.0). A p-value of less than 0.05 was deemed statistically significant.
3 Results
3.1 Patient characteristics
This study included 86 patients with NSCLC who underwent TRT combined with ICIs. The median age of the cohort was 65 years (range: 40–82). Among them, 52 patients (60.5%) had a smoking history, 16 patients (18.6%) had previously undergone lobectomy, and the predominant histological type was squamous cell carcinoma (48,55.8%). Additionally, 54 patients (62.8%) were diagnosed with stage III disease, and 30.2% were in stage IV. In terms of treatment modalities, 25 (29.1%) received CCRT, and 32 (37.2%) underwent RT concurrent ICIs. The clinical characteristics of all patients are presented in Table 1.
3.2 Features of treatment-related pneumonitis
58 patients (67.4%) experienced TRP, with a median onset time of 3.58 months (ranging from 0.47 to 11.77 months) from the initiation of the first RT. Supplementary Table 1 outlines the characteristics of TRP: 40 cases (46.5%) were classified as RP, 6 cases (6.98%) as CIP, and 12 cases (14.0%) as mixed pneumonitis. Of these, 32 cases (37.2%) had grade 2 symptomatic pneumonitis, and the remaining 30.2% had grade 1 pneumonitis. No pneumonitis-related deaths were observed. We subsequently compared the clinical features of patients with pneumonitis to those without, as detailed in Supplementary Table 2. The median RT dose for the pneumonitis group was 54 Gy (ranging from 30 to 66 Gy), which was comparable to that of patients without pneumonitis. However, both the MLD (P = 0.026) and V20 (P = 0.037) were associated with the development of TRP, showing significantly higher values in the pneumonitis group compared to the non-pneumonitis group. Concurrent chemotherapy or ICIs were also risk factors for pneumonitis.
Patients with TRP exhibited a variety of CT imaging findings (as illustrated in Supplementary Table 3). The five most prevalent CT signs were patch (100%), lung consolidation (87.9%), strip shape (79.3%), ground-glass opacity (62.1%), and honeycomb (42.1%). In cases of TRP, synchronous tumor progression was observed in 12.1% of patients, pleural effusion in 31.0%, and enlarged non-neoplastic lymph nodes in hilar and mediastinal regions of the lungs in 84.5%. The predominant imaging pattern was OP (100%), followed by GGO (63.8%), NSIP (13.8%), lung nodules or mass-like (5.2%), HP (3.4%), and only one case (1.7%) of bronchitis. No cases of pneumonitis were presented as AIP or DAD.
3.3 Features of treatment-related pneumonitis
The median follow-up period after the initial RT was 24.1 months (ranging from 9.93 to 66.47 months), with a median OS of 20.47 months (95% CI: 20.85-27.03 months) and PFS of 9.70 months (95% CI: 10.13-14.67 months) for all patients.
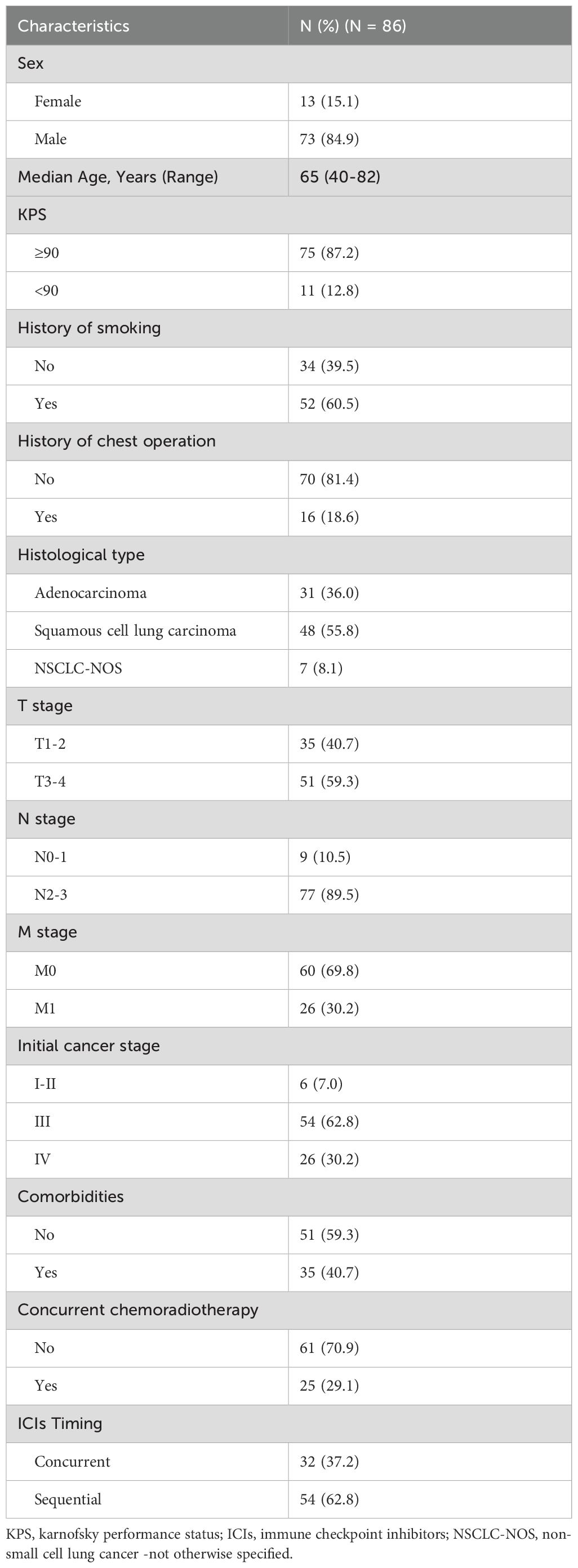
Patients in the pneumonitis group had a median PFS of 9.53 months (95% CI: 6.90-12.16 months), compared to 14.27 months (95% CI: 0.00-33.96 months) in the non-pneumonitis group (P=0.040; Figure 1A). There was no statistically significant difference in PFS among the three types of pneumonitis (Figure 1B). Both grade 1 and grade 2 pneumonitis significantly reduced PFS, with no difference between the grades. (Figure 1C).
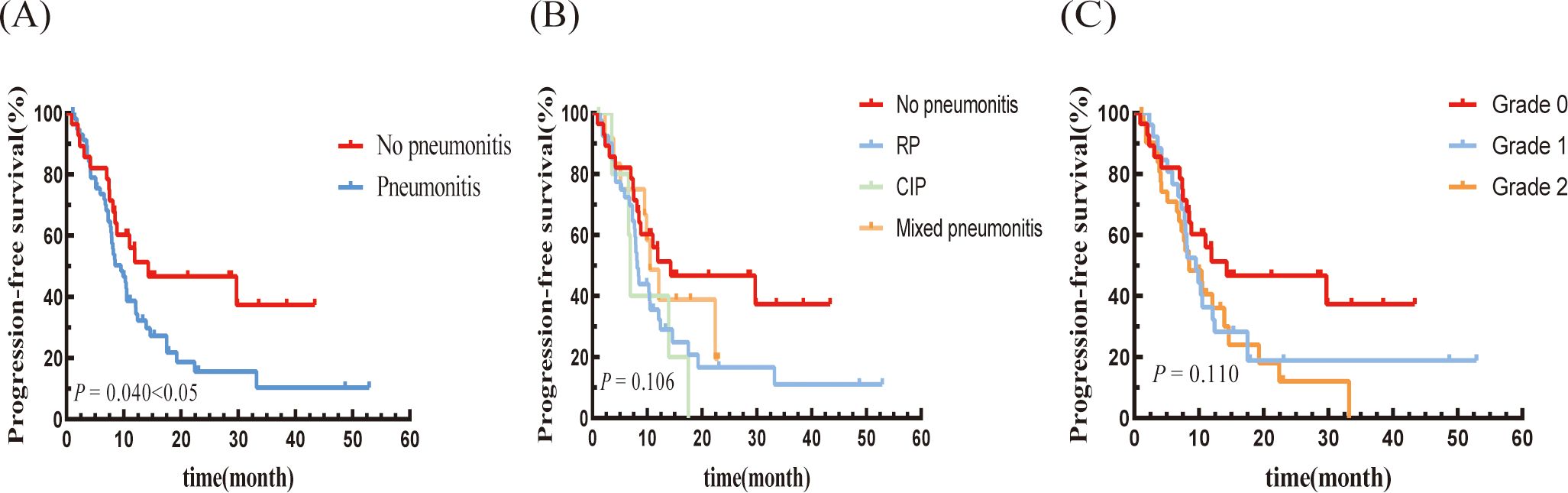
Figure 1. Kaplan–Meier graph of PFS for patients according to (A) Pneumonitis or not, (B) Type of pneumonitis, and (C) Pneumonitis classification.
The median LRFS for the pneumonitis group was 13.9 months (95% CI: 6.139-21.661 months), which was significantly shorter than that of the non-pneumonitis group (not reached). Although the difference was not statistically significant, it showed a notable downward trend (P=0.084; Figure 2A). In terms of pneumonitis type, the CIP group exhibited a lower trend compared to the other two groups. the LRFS trends were consistent with the PFS trends across pneumonitis grades (Figures 2B, C).
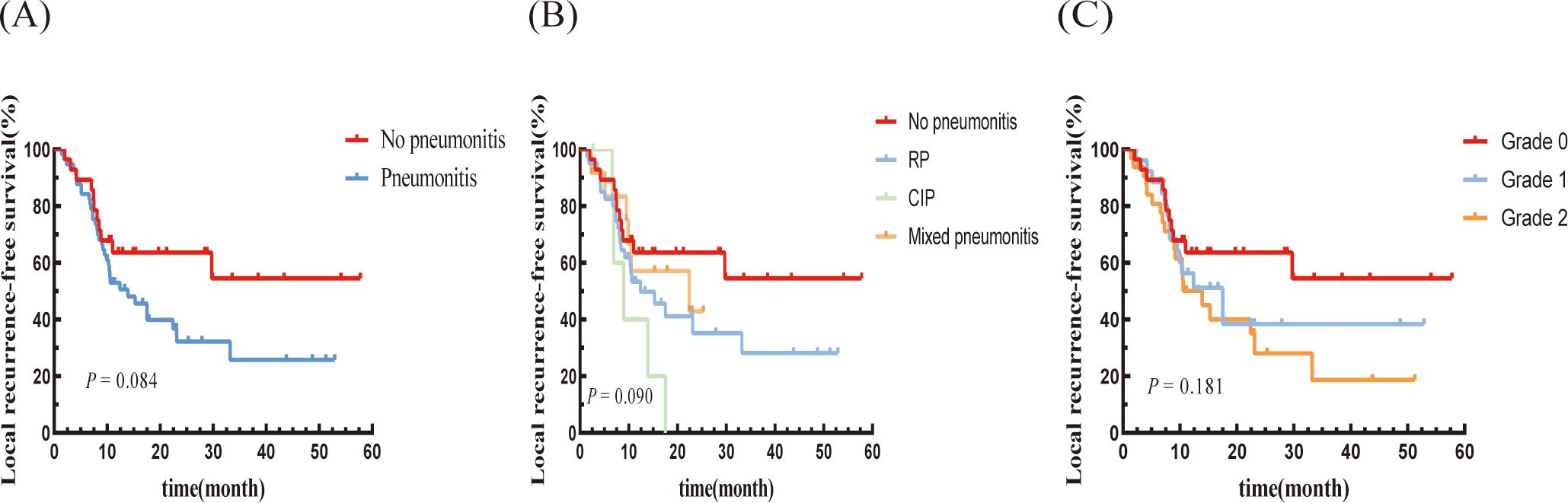
Figure 2. Kaplan–Meier graph of LRFS for patients according to (A) Pneumonitis or not, (B) Type of pneumonitis, and (C) Pneumonitis classification.
The median DMFS was 12.07 months (95% CI: 3.67-20.47 months) in the pneumonitis group, which was shorter than in the non-pneumonitis group (not reached), with a statistically significant difference (P=0.028; Figure 3A). The mixed pneumonitis group showed a higher trend in DMFS compared to the other two groups. However, patients with grade 2 pneumonitis had a lower DMFS compared to those with grade 1 pneumonitis and those without pneumonitis, with all differences reaching statistical significance (Figures 3B, C).
Figure 3. Kaplan–Meier graph of DMFS for patients according to (A) Pneumonitis or not, (B) Type of pneumonitis, and (C) Pneumonitis classification.
There was no significant difference in OS between the pneumonitis group and the non-pneumonitis group (P = 0.971; Figure 4A). Moreover, there were no significant differences in OS based on the type or grade of pneumonitis (Figures 4B, C).
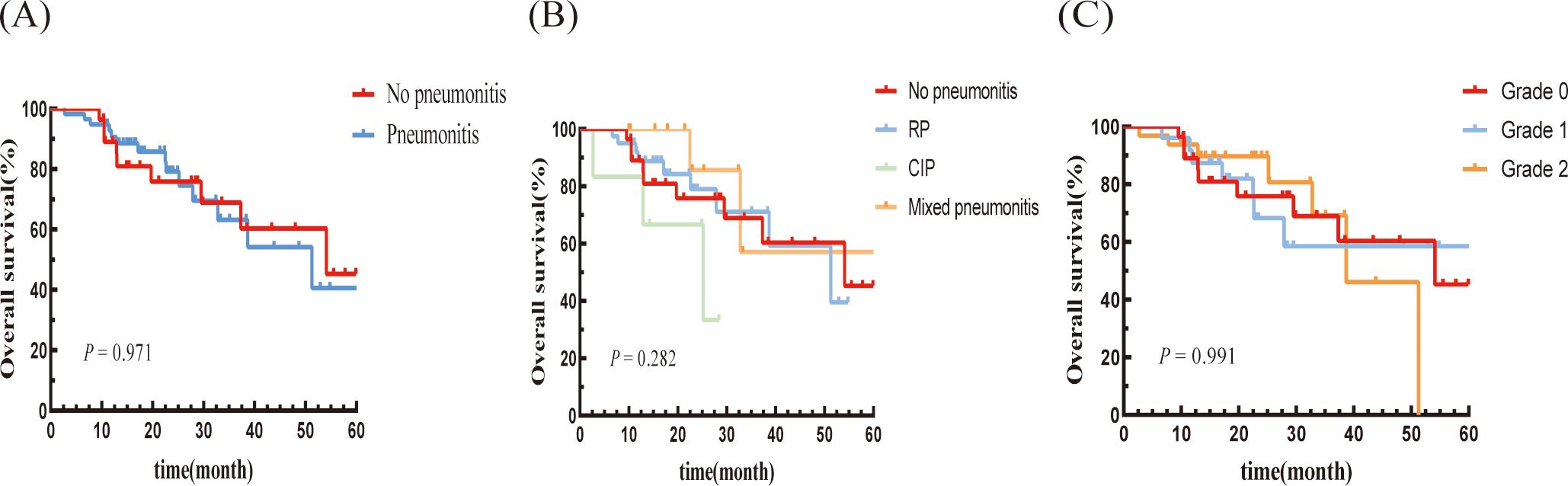
Figure 4. Kaplan–Meier graph of OS for patients according to (A) Pneumonitis or not, (B) Type of pneumonitis, and (C) Pneumonitis classification.
3.4 Prognostic factors for patients in the pneumonitis group
In 58 patients with pneumonitis, the progression group had a significantly higher NLR within one week (4.41 vs. 2.78, P = 0.02) and a larger PTV for RT (208.6 cm³ vs. 143.1 cm³, P = 0.044) compared to the non-progression group (Table 2). No significant associations were observed between tumor progression and clinical features, tumor status, treatment, dosimetric parameters and imaging characteristics (Supplementary Table 4).
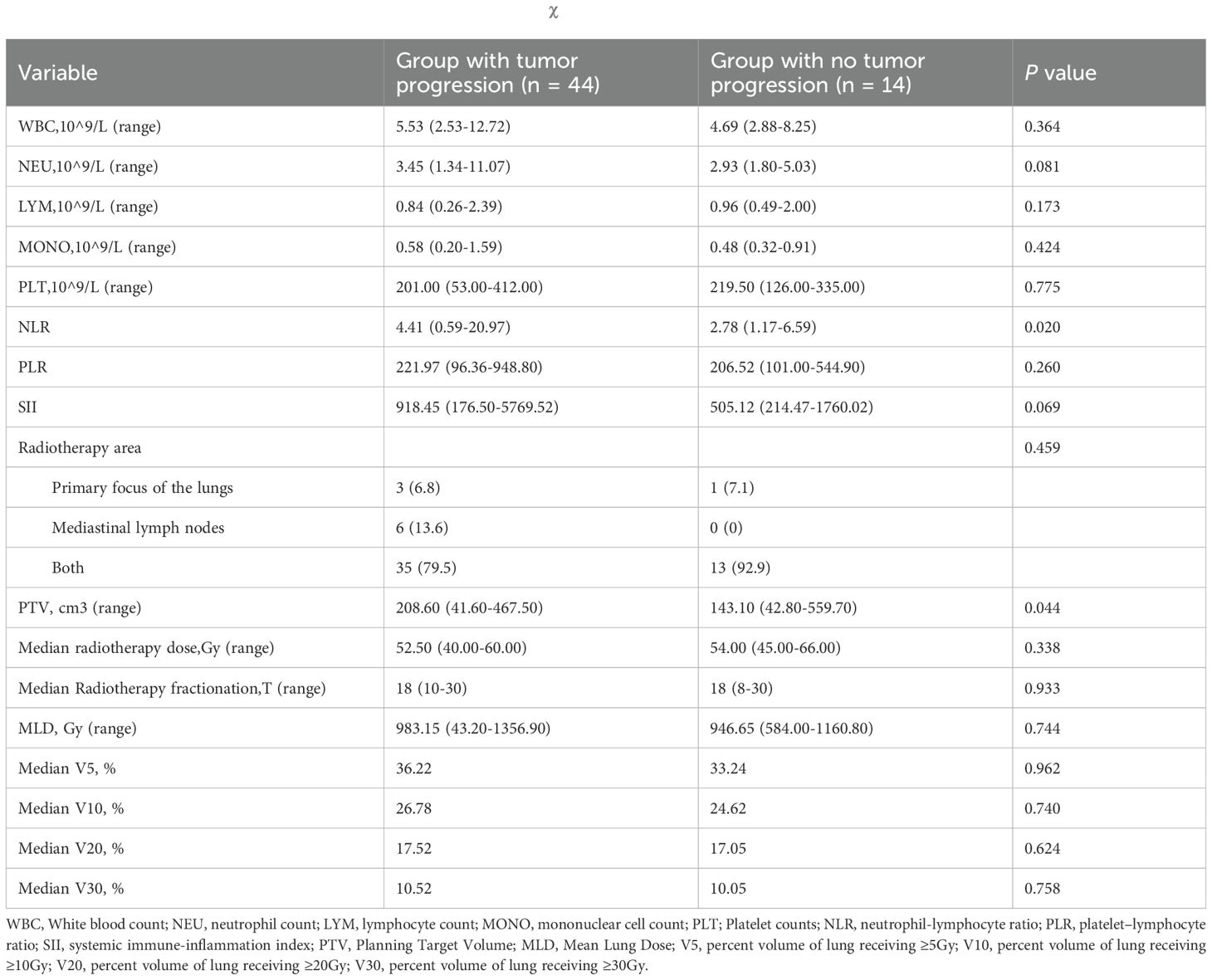
Table 2. Characteristics of patients in the two groups and with the χ2 test for categorical variables.
Univariate analysis revealed that NLR ≥ 3.605 (HR: 2.520; 95% CI 1.288-4.931; p = 0.007), SII ≥ 712.46 (HR: 2.058; 95% CI 1.034-4.096; p = 0.04), and the presence of a solitary pulmonary nodule or mass at pneumonitis onset (HR: 2.552, 95% CI: 1.054–6.176, P = 0.038) were factors associated with poor PFS. However, given the existence of interactions, only NLR were included in the multivariate Cox proportional hazards regression based on the interaction results. In the multivariate Cox proportional hazards regression model, a high NLR (HR = 2.841; 95% CI 1.428-5.649; p= 0.003) and the solitary pulmonary nodule or mass (HR: 3.360, 95.0% CI: 1.354-8.340; p=0.009) were identified as independent prognostic factors for PFS (Table 3).
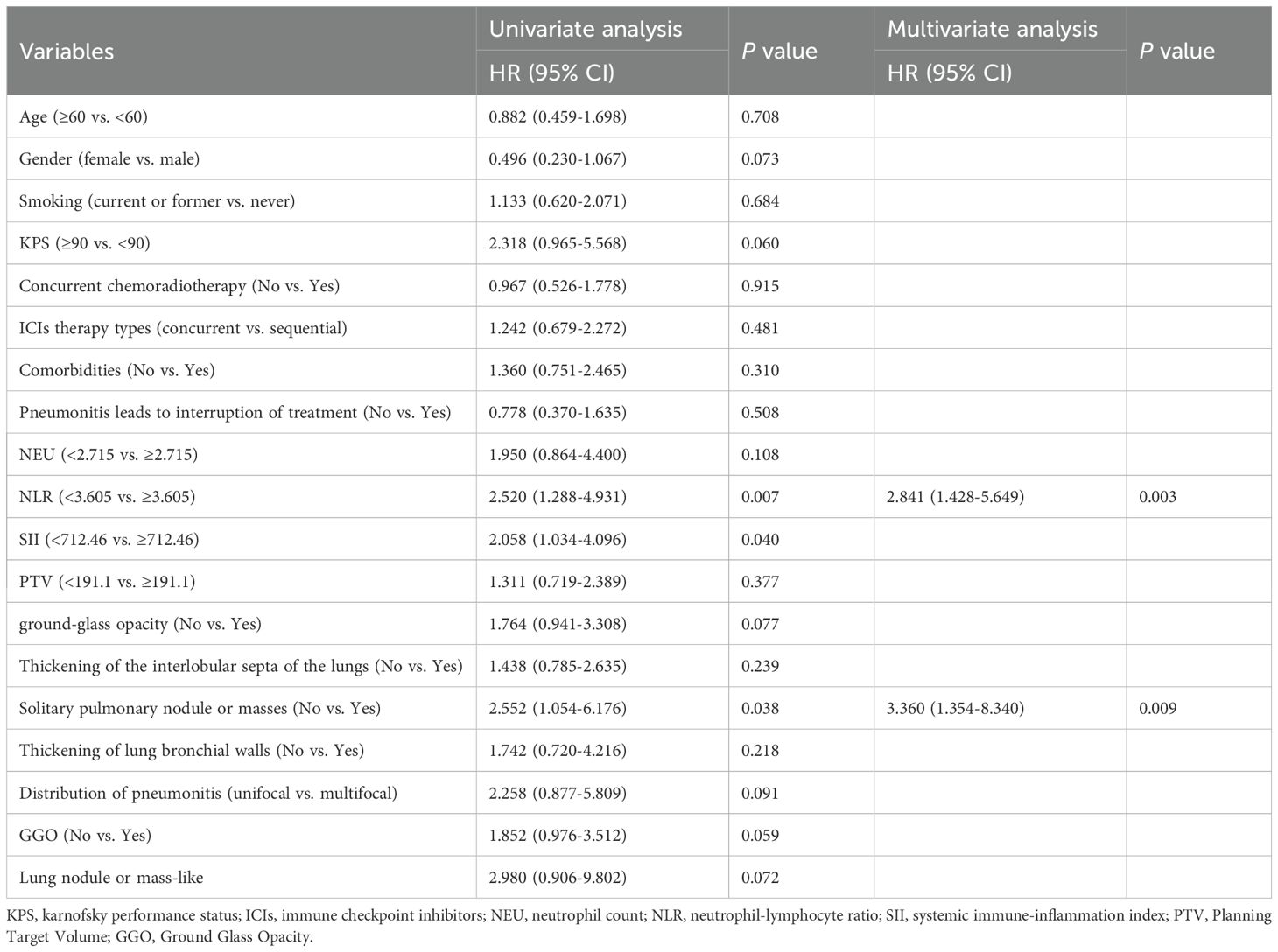
Table 3. Univariate and multivariate Cox proportional hazards regression for progression free survival in the group with pneumonitis.
Univariate analysis showed that a MLD ≥ 1091.25 (HR = 4.590; 95% CI 1.427-14.768; p= 0.011), a KPS score < 90 (HR = 3.577; 95% CI 1.092-11.583; p= 0.035), and multifocal pneumonitis distribution (No vs Yes; HR: 6.815, 95.0% CI: 1.679-27.661; p=0.007) were associated with poor OS. In the multivariate analysis, only a high MLD (HR = 4.076; 95% CI 1.186-14.005; p= 0.026) remained an independent prognostic factor for OS in patients with pneumonitis (Table 4).
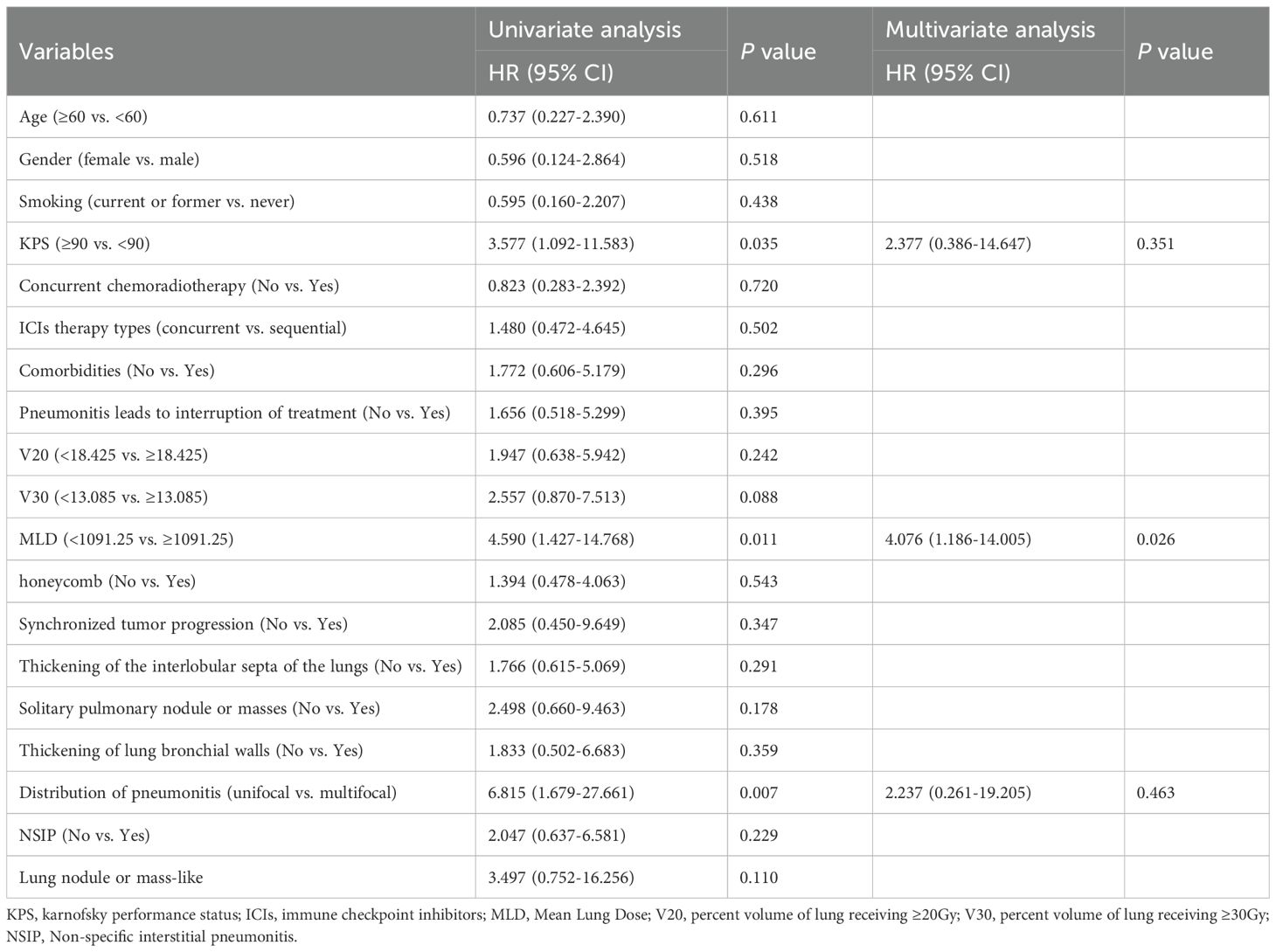
Table 4. Univariate and multivariate Cox proportional hazards regression for Overall survival in the group with pneumonitis.
4 Discussion
Our findings, together with that of Kinehara Y et al. (5) both highlighted the adverse impact of RT combined with ICIs therapy-related pneumonitis on PFS. A plausible immunological mechanism underlying this phenomenon involves radiotherapy-induced inflammation triggering systemic immune hyperactivation, leading to excessive cytokine release. This pathological process can severely impair the function of tumor-infiltrating lymphocytes (TILs), result in elevated immunosuppressive factors, and promote a more immunosuppressive microenvironment (14–16). Further mechanistic exploration is needed to validate this hypothesis, particularly regarding immune microenvironment dynamics and cytokine profiling. However, accumulating evidence has consistently demonstrated the detrimental effects of inflammation on tumor progression, underscoring the clinical importance of pneumonitis prevention and active management.
Regarding the impact of pneumonitis on OS, we do not fully align with the conclusions from Kinehara Y et al. (5) While our findings indicate that pneumonitis correlates with poorer progression in NSCLC, its association with OS appears less pronounced. In our study, the absence of grade 3 or higher pneumonitis may account for the absence of survival detriment effects. This observation aligns with previous studies about CIP, where grade 1–2 CIP was reported to be associated with favorable OS, whereas grade 3–4 CIP is not (17), indirectly supporting the above rationale.
Our study indicated a trend towards a relatively poorer prognosis in cases of pure CIP, while mixed pneumonitis did not significantly worsen tumor prognosis. In our research, among the six CIP patients, most (83.3%) had grade 2 symptomatic pneumonitis, whereas RP and mixed pneumonitis were predominantly low-grade and asymptomatic. So this discrepancy may be partly attributed to the frequent treatment interruption or discontinuation in CIP patients, as well as the use of glucocorticoid which has been reported to compromise survival benefits (18).
Follow-up analyses of the PACIFIC study showed that Grade 2+ pneumonitis/radiation pneumonitis (RP) does not compromise the clinical benefits of durvalumab in terms of PFS and OS in patients with unresectable stage III NSCLC (19). This aligns with our findings, indicating that while TRP may increase treatment-related risk and complicated management, it does not significantly affect the long-term OS for patients with NSCLC undergoing RT combined with ICIs. These findings suggested that while proactive management of TRP is essential, the therapeutic potential of this combination strategy should not be disregarded in eligible patient populations.
Our findings revealed that elevated NLR and SII at the onset of pneumonitis were associated with poorer PFS. Both NLR and SII are well-established markers of inflammatory response and immune status. Numerous studies have demonstrated that elevated pretreatment NLR and PLR correlate with shorter PFS and OS in lung cancer patients receiving ICIs (20, 21). Similarly, the predictive value of SII in the PFS of lung cancer patients treated with ICIs was also reported (22). Our previous work using animal models found that the combination of radiotherapy and ICIs exaggerated pulmonary inflammation, with significantly increased levels of neutrophilic infiltration (23).
Furthermore, the study identified that pneumonitis presenting as solitary pulmonary nodule or masses was a predictor of shorter PFS. Solitary pulmonary nodules or masses may be associated with the persistent stimulation of immune responses, but the mechanistic characteristics of TRP with different imaging features remain unclear. Additionally, solitary pulmonary nodules or masses appear to be more commonly observed in CIP compared to RP or mixed-pattern pneumonitis. In this study, the relatively higher severity and poorer prognosis of CIP may explain the correlation between this imaging feature and prognosis. However, due to the small sample size, we may not have identified the true radiological features associated with poor prognosis. Larger sample studies are needed to provide more definitive insights for future clinical practice.
Multifocal imaging patterns have been identified as a prognostic factor for poorer OS in patients with pneumonitis. The multifocal distribution of pneumonitis leads to more extensive and widespread inflammation compared to unifocal patterns, which not only exert multifaceted effects on the tumor immune microenvironment and systemic immunity but also complicates treatment approaches. Furthermore, it increases the risk of complications and secondary health issues, collectively contributing to the observed decline in survival rates.
Consistent with previous reports, CCRT and RT concurrent ICIs therapy in the treatment of NSCLC is more likely to result in TRP (24, 25). However, when dosimetric high-risk factors such as V20 and MLD are controlled, the overall severity of pneumonitis remains mild. According to our findings and previous studies, the impact of mild pneumonitis on tumor progression and survival is acceptable (4, 18). Research indicates that compared to the pre-immunotherapy era, where V20 limits were strictly controlled during CCRT, a V20 exceeding 20% in RT combined with ICIs significantly increases the risk of pneumonitis (26, 27). Therefore, while there is no need for undue hesitation, careful and cautious advancement is still necessary.
However, this study has several limitations that should be acknowledged: the limited number of patients in this study, with even six patients in CIP subgroups, affected the ability to confirm statistical significance, which will result in regarding potential type II errors and the reduced generalizability of subgroup analyses. The small number of cases with different types of pneumonitis and varying pneumonitis characteristics precluded drawing definitive clinical implications. Additionally, as a retrospective analysis, this study has inherent limitations. For example, to mitigate the toxicity associated with the combination of RT and ICIs therapy, clinical practice naturally involved cautious strategies to minimize normal lung dose exposure. As a result, the overall severity of pneumonitis observed in this study was relatively mild, with few cases of grade 2 pneumonitis. The true impact of pneumonitis on tumor progression and prognosis, therefore, requires further validation. Furthermore, to increase the sample size, the study included both locally advanced patients and stage IV oligometastatic patients, whose differing disease stages could potentially influence the prognostic data.
5 Conclusion
Pneumonitis resulting from the combination of RT and ICIs therapy did not impact OS in NSCLC patients, although it was associated with shorter PFS. Mixed pneumonitis did not further deteriorate the patient’s prognosis. Nevertheless, due to the limited number of cases analyzed thus far, further large-scale prospective studies are necessary to validate these findings and to comprehensively explore risk factors predicting poor prognosis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZW: Conceptualization, Methodology, Data curation, Investigation, Writing – original draft, Writing – review & editing. KX: Conceptualization, Methodology, Data curation, Investigation, Writing – review & editing. HS: Methodology, Investigation, Writing – review & editing. JL: Investigation, Resources, Writing – review & editing. WJ: Supervision, Resources, Writing – review & editing. LW: Supervision, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the National Natural Science Foundation of China (82202938); National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen (SZ2020ZD002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1578057/full#supplementary-material
Abbreviations
TRT, thoracic radiotherapy; ICIs, immune checkpoint inhibitors; NSCLC, non-small cell lung cancer; TRP, treatment-related pneumonitis; CTCAE, common terminology criteria for adverse events; LRFS, local recurrence-free survival; DMFS, distant metastasis-free survival; PFS, progression-free survival; OS, overall survival; CCRT, concurrent chemoradiotherapy; RT, radiotherapy; SARP, severe acute radiation pneumonitis; CIP, checkpoint inhibitor pneumonitis; irAEs, immune-related adverse events; SII, systemic immune-inflammation index; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; RP, radiation pneumonitis; OP, organizing pneumonitis; GGO, ground glass opacities; AIP, acute interstitial pneumonitis; HP, hypersensitivity pneumonitis; NSIP, non-specific interstitial pneumonitis; MLD, mean lung dose.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clinicians. (2024) 74(3):229–63. doi: 10.3322/caac.21834
2. Shintani T, Kishi N, Matsuo Y, Ogura M, Mitsuyoshi T, Araki N, et al. Incidence and risk factors of symptomatic radiation pneumonitis in non–small-cell lung cancer patients treated with concurrent chemoradiotherapy and consolidation durvalumab. Clin Lung Cancer. (2021) 22(5):401–10. doi: 10.1016/j.cllc.2021.01.017
3. Zhuang L, Bai X, Chen Y, Zhang D, Sheng L, Du X. Analysis of the risk factors of radiation pneumonitis and the predictive ability of dosiomics in non-small-cell lung cancer. Future Oncol. (2023) 19(32):2157–69. doi: 10.2217/fon-2023-0316
4. Edwards DM, Sankar K, Alseri A, Jiang R, Schipper M, Miller S, et al. Pneumonitis after chemoradiotherapy and adjuvant durvalumab in stage III non-small cell lung cancer. Int J Radiat OncologyBiologyPhysics. (2024) 118(4):963–70. doi: 10.1016/j.ijrobp.2023.09.050
5. Kinehara Y, Shiroyama T, Tamiya A, Tamiya M, Minami S, Kanazu M, et al. Pneumonitis during durvalumab consolidation therapy affects survival in stage III NSCLC. JTO Clin Res Rep. (2023) 4(11):100586. doi: 10.1016/j.jtocrr.2023.100586
6. Niu L, Chu X, Yang X, Zhao H, Chen L, Deng F, et al. A multiomics approach-based prediction of radiation pneumonia in lung cancer patients: impact on survival outcome. J Cancer Res Clin Oncol. (2023) 149(11):8923–34. doi: 10.1007/s00432-023-04827-7
7. Zha Y, Zhang J, Yan X, Yang C, Wen L, Li M. A dynamic nomogram predicting symptomatic pneumonia in patients with lung cancer receiving thoracic radiation. BMC Pulm Med. (2024) 24(1):99. doi: 10.1186/s12890-024-02899-w
8. Zhou Y, Yan T, Zhou X, Cao P, Luo C, Zhou L, et al. Acute severe radiation pneumonitis among non-small cell lung cancer (NSCLC) patients with moderate pulmonary dysfunction receiving definitive concurrent chemoradiotherapy: Impact of pre-treatment pulmonary function parameters. Strahlenther Onkol. (2020) 196(6):505–14. doi: 10.1007/s00066-019-01552-4
9. Suresh K, Psoter KJ, Voong KR, Shankar B, Forde PM, Ettinger DS, et al. Impact of checkpoint inhibitor pneumonitis on survival in NSCLC patients receiving immune checkpoint immunotherapy. J Thoracic Oncol. (2019) 14(3):494–502. doi: 10.1016/j.jtho.2018.11.016
10. Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of immune-related adverse events with nivolumab efficacy in non–small-cell lung cancer. JAMA Oncol. (2018) 4(3):374–8. doi: 10.1001/jamaoncol.2017.2925
11. Lu X, Wang J, Zhang T, Zhou Z, Deng L, Wang X, et al. Comprehensive pneumonitis profile of thoracic radiotherapy followed by immune checkpoint inhibitor and risk factors for radiation recall pneumonitis in lung cancer. Front Immunol. (2022) 13:918787. doi: 10.3389/fimmu.2022.918787
12. Naidoo J, Nishino M, Patel SP, Shankar B, Rekhtman N, Illei P, et al. Immune-related pneumonitis after chemoradiotherapy and subsequent immune checkpoint blockade in unresectabl e stage III non-small-cell lung cancer. Clin Lung Cancer. (2020) 21(5):e435–44. doi: 10.1016/j.cllc.2020.02.025
13. on behalf of the Society for Immunotherapy of Cancer Toxicity Management Working Group, Puzanov I, Diab A, Abdallah K, CO B, Brogdon C, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J immunotherapy Cancer. (2017) 5(1):95. doi: 10.1186/s40425-017-0300-z
14. Zhang A, Yang F, Gao L, Shi X, Yang J. Research progress on radiotherapy combined with immunotherapy for associated pneumonitis during treatment of non-small cell lung cancer. CMAR. (2022) 14:2469–83. doi: 10.2147/CMAR.S374648
15. Yin Z, Zhang H, Zhang K, Yue J, Tang R, Wang Y, et al. Impacts of Combining PD-L1 inhibitor and Radiotherapy on the Tumour immune microenvironment in a Mouse Model of Esophageal Squamous Cell Carcinoma. (2024) 25(1):474. doi: 10.21203/rs.3.rs-4338719/v1.
16. Kong P, Wang J, Song Z, Liu S, He W, Jiang C, et al. Circulating lymphocytes, PD-L1 expression on tumor-infiltrating lymphocytes, and survival of colorectal cancer patients with different mismatch repair gene status. J Cancer. (2019) 10(7):1745–54. doi: 10.7150/jca.25187
17. Tone M, Izumo T, Awano N, Kuse N, Inomata M, Jo T, et al. High mortality and poor treatment efficacy of immune checkpoint inhibitors in patients with severe grade checkpoint inhibitor pneumonitis in non-small cell lung cancer. Thoracic Cancer. (2019) 10(10):2006–12. doi: 10.1111/1759-7714.13187
18. Liu X, Hao N, Yang S, Li J, Wang L. Predictive factors and prognosis of immune checkpoint inhibitor-related pneumonitis in non-small cell lung cancer patients. Front Oncol. (2023) 13:1145143. doi: 10.3389/fonc.2023.1145143
19. Vansteenkiste JF, Naidoo J, Faivre-Finn C, Özgüroğlu M, Villegas A, Daniel D, et al. Symptomatic pneumonitis with durvalumab after concurrent chemoradiotherapy in unresectable stage III NSCLC. JTO Clin Res Rep. (2024) 5(3):100638. doi: 10.1016/j.jtocrr.2024.100638
20. Diem S. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. (2017) 111:176–81. doi: 10.1016/j.lungcan.2017.07.024
21. Mandaliya H, Jones M, Oldmeadow C, Nordman II. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Trans Lung Cancer Res. (2019) 8(6):886–94. doi: 10.21037/tlcr.2019.11.16
22. Yang Y, Li J, Wang Y, Luo L, Yao Y, Xie X. Prognostic value of the systemic immune-inflammation index in lung cancer patients receiving immune checkpoint inhibitors: A meta-analysis. PloS One. (2024) 19(11):e0312605. doi: 10.1371/journal.pone.0312605
23. Sheng Y, Chen K, Jiang W, Wu Z, Zhang W, Jing H, et al. PD-1 restrains IL-17A production from γδ T cells to modulate acute radiation-induced lung injury. Transl Lung Cancer Res. (2021) 10(2):685–98. doi: 10.21037/tlcr-20-838
24. Deutsch E, Chargari C, Galluzzi L, Kroemer G. Optimising efficacy and reducing toxicity of anticancer radioimmunotherapy. Lancet Oncol. (2019) 20(8):e452–63. doi: 10.1016/S1470-2045(19)30171-8
25. Balasubramanian A, Onggo J, Gunjur A, John T, Parakh S. Immune checkpoint inhibition with chemoradiotherapy in stage III non–small-cell lung cancer: A systematic review and meta-analysis of safety results. Clin Lung Cancer. (2021) 22(2):74–82. doi: 10.1016/j.cllc.2020.10.023
26. Yan Y, Zhu Y, Yang S, Qian C, Zhang Y, Yuan X, et al. Clinical predictors of severe radiation pneumonitis in patients undergoing thoracic radiotherapy for lung cancer. Transl Lung Cancer Res. (2024) 13(5):1069–83. doi: 10.21037/tlcr-24-328
Keywords: treatment-related pneumonitis, NSCLC, thoracic radiotherapy, immune checkpoint inhibitors therapy, prognosis
Citation: Wang Z, Xu K, Sun H, Liang J, Jiang W and Wang L (2025) Impact of pneumonitis from radiotherapy combined with immune checkpoint inhibitors therapy on tumor progression and survival in patients with non-small cell lung cancer. Front. Immunol. 16:1578057. doi: 10.3389/fimmu.2025.1578057
Received: 17 February 2025; Accepted: 18 April 2025;
Published: 09 May 2025.
Edited by:
Vinay Kumar, The Pennsylvania State University, United StatesReviewed by:
Lukasz Chlewicki, Eli Lilly, United StatesJianzhong Cao, Shanxi Provincial Cancer Hospital, China
Copyright © 2025 Wang, Xu, Sun, Liang, Jiang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Jiang, amlhbmd3ZWlAY3Njby5vcmcuY24=; Luhua Wang, d2FuZ2x1aHVhQGNzY28ub3JnLmNu
 Ziwei Wang1,2
Ziwei Wang1,2 Jun Liang
Jun Liang Luhua Wang
Luhua Wang