- 1The First Clinical Medical School, Hubei University of Chinese Medicine, Wuhan, Hubei, China
- 2Department of Urology, Hubei Provincial Hospital of Traditional Chinese Medicine, Affiliated Hospital of Hubei University of Chinese Medicine, Wuhan Hubei, China
- 3Hubei Key Laboratory of Theory and Application Research of Liver and Kidney in Traditional Chinese Medicine, Affiliated Hospital of Hubei University of Chinese Medicine, Wuhan, Hubei, China
- 4Hubei Provincial Institute of Traditional Chinese Medicine, Wuhan, Hubei, China
- 5Department of Orthopedics II, Xingguo County Hospital of Traditional Chinese Medicine, Ganzhou, Jiangxi, China
Introduction: The prognostic significance of platelet-to-lymphocyte ratio (PLR) in non-muscle invasive bladder cancer (NMIBC) remains controversial despite numerous investigations. This study aimed to systematically evaluate the prognostic value of PLR in NMIBC.
Materials and methods: An extensive systematic search was executed utilizing four major electronic databases: Embase, PubMed, Web of Science, and Cochrane Library. The prognostic significance of PLR was assessed using pooled hazard ratios (HRs) with 95% CIs. Forest plots were used to present data visualization and statistical summaries, illustrating the effects of individual studies and the reliability of the pooled results. Funnel plot analysis and Egger’s test were employed to evaluate the potential presence of publication bias. Sensitivity analysis was performed to assess the robustness of the pooled findings. Subgroup analysis and meta-regression were used to identify sources of heterogeneity.
Results: Eleven retrospective studies encomprising 3,566 patients met the inclusion criteria. Elevated PLR notably correlated with inferior progression-free survival (PFS) (HR=2.132, 95% CI: 1.146-3.967, p=0.017) and relapse-free survival (RFS) (HR=1.732, 95% CI: 1.174-2.554, p=0.006). No statistically meaningful correlation emerged in cancer-specific survival (CSS) (HR=1.218, 95% CI: 0.800-1.854, p=0.358) or overall survival (OS) (HR=1.350, 95% CI: 0.611-2.983, p=0.459). Publication bias was detected in RFS analyses (Egger’s test, P=0.010). Sensitivity analysis demonstrated that the pooled results were robust. Subgroup analysis and meta-regression identified geographic differences and patient characteristics as key sources of heterogeneity in RFS outcomes. Subgroup analysis indicated that geographic differences and sample size were potential sources of heterogeneity in PFS results.
Discussion: This study comprehensively analyzed 11 studies and 3,566 NMIBC cases and found that elevated PLR was significantly associated with poorer RFS and PFS, suggesting that PLR can be used as a prognostic biomarker for the management of NMIBC. The prognostic value of PLR may be related to immune regulation and inflammatory response in the tumor microenvironment; nevertheless, further studies are needed to elucidate its mechanism and establish its clinical application.
Conclusions: This study demonstrates that elevated PLR serves as an independent predictor of poor PFS and RFS in NMIBC patients. As a cost-effective biomarker, PLR shows promise in risk stratification and treatment planning. However, large-scale prospective studies are warranted to validate these findings and establish standardized cut-off values.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024621307, identifier CRD42024621307.
1 Introduction
In the global cancer landscape of 2023, urinary bladder malignancy emerged as the fourth most common cancer affecting males, representing 6% of new cancer cases and contributing to 4% of cancer-associated mortality worldwide (1). This disease not only elevates the risk of mortality, but also incurs significant healthcare costs and substantially affects patient outcomes and quality of life (2). The clinical classification of bladder cancer follows an invasion-based staging system, distinguishing between muscle-invasive bladder cancer (MIBC) and non-muscle-invasive bladder cancer (NMIBC) (3). NMIBC constitutes 70-75% of all bladder cancer cases (4), characterized by neoplastic involvement limited to either the mucosa layer (Ta stage or carcinoma in situ) or submucosa tissue (T1 stage) (3–5). Despite its initial containment, NMIBC demonstrates concerning clinical behavior, with approximately one-fifth of cases advancing to muscle-invasive disease (6), which carries a markedly poorer prognosis (7). Clinical management strategies employ a three-tiered risk classification system - low, intermediate, and high-risk - according to specific clinicopathological criteria (8). For patients classified as intermediate or high-risk, the established treatment protocol combines surgical intervention through transurethral tumor resection with subsequent intravesical immunotherapy using Bacillus Calmette-Guerin (9, 10). Although early detection facilitates surgical management in most cases (70-80%), the disease exhibits concerning patterns of recurrence and progression. Studies indicate that 50-80% of NMIBC cases recur within a five-year window, with 15-30% progressing to advanced stages with poor survival outcomes (11). These statistics highlight the urgent need for reliable, accessible biomarkers to predict disease behavior and improve patient outcomes.
The tumor microenvironment (TME) has received significant attention in cancer research, with a particular focus on its role in tumor development and progression (12). The immune environment is an important component of the TME. The location, density, and phenotype of various immune cells, as well as secreted chemokines and cytokines, comprise the “immune contexture”, which has prognostic significance (13). In addition, inflammatory cell infiltration occurs within the TME, and there is interaction between the immune environment and inflammatory cell infiltration. Across various solid tumors, both inflammatory cell infiltration and the immune environment within the TME have demonstrated substantial prognostic value (14). Among systemic inflammatory markers, the platelet-to-lymphocyte ratio (PLR) has gained substantial recognition in oncological investigations (15). This parameter, derived from the ratio between platelet and lymphocyte counts, serves as a valuable prognostic indicator in multiple cancer types, including non-small cell lung cancer (NSCLC), breast cancer, hepatocellular carcinoma, colorectal cancer, and prostate cancer (16–23).
Nevertheless, the prognostic utility of PLR in NMIBC remains a subject of debate. For example, Caglayan et al. (24) claimed that PLR has limited value in predicting postoperative recurrence in NMIBC patients. Conversely, L. Ding et al. (25) reported that PLR holds prognostic significance in this patient population. In view of these conflicting results, our meta-analysis systematically reviewed 11 cohort studies to quantify the prognostic value of PLR at different levels in NMIBC, using outcome measures such as RFS and PFS. This analysis aims to establish robust evidence for optimizing clinical management strategies and healthcare decision-marking.
2 Methods
2.1 Study registration
This study was conducted in alignment with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (26). The protocol was pre-registered with the International Prospective Register of Systematic Reviews (PROSPERO) under the identifier CRD42024621307.
2.2 Literature search strategy
We performed a systematic search spanning from database inception to November 27, 2024, utilizing Embase, PubMed, Web of Science, and Cochrane Library. Search terms incorporated variations of “non-muscle invasive bladder cancer,” “NMIBC,” “platelet-to-lymphocyte ratio,” and “PLR”. Supplementary searches examined reference lists and grey literature sources. The detailed search strategies is presented in Supplementary Table S1.
2.3 Inclusion and exclusion criteria
Inclusion criteria: (1) Pathologically confirmed diagnosis of NMIBC; (2) Investigation of PLR as an exposure variable; (3) Provision of sufficient data for hazard ratio (HR) calculation with 95% confidence interval (CI); (4) Publications in English; (5) Observational study design; (6) Assessment of progression-free survival (PFS), recurrence-free survival (RFS), cancer-specific survival (CSS), or overall survival (OS).
Exclusion criteria: (1) Cell experiments, animal studies, case reports, protocols, conference abstracts, editorials, letters, or reviews; (2) Incomplete or erroneous data presentation; (3) Duplicate publications; (4) Inaccessible full-text articles; (5) Overlapping study populations.
2.4 Data extraction
Literature management was facilitated through EndNote 21 software. Two independent researchers (Hu and Liang) carried out the initial browse of abstracts and titles according to the predetermined eligibility criteria, followed by full-text reviews of pertinent literature. In cases of selection disagreement, a third researcher (Zhou) provided additional evaluation to reach a final decision. The reviewers independently documented study information using a predefined extraction template, capturing key parameters including publication year, author information, research design, study location, sample characteristics (size, sex ratio, age), PLR cut-off values, patient classification, therapeutic interventions, follow-up duration, survival analysis, and outcome measures.
2.5 Quality assessment
Study quality underwent independent evaluation by two investigators using the Newcastle-Ottawa Scale (NOS) (27), examining three domains: selection, comparability, and exposure. Studies scoring ≥6 points (maximum 9) were designated as high-quality investigations.
2.6 Statistical analysis
STATA 15.1 software was used for statistical analysis. The correlation of PLR to RFS, PFS, OS, and CSS was assessed by calculating HR and 95% CI. Heterogeneity assessment combined I2 statistics and Cochrane’s Q test (significant: I2>50% or P<0.10). The choice between random-effects and fixed-effects models was contingent upon heterogeneity assessment, with the former employed in cases of significant heterogeneity. Robustness of findings was evaluated through sensitivity analyses. Heterogeneity sources were examined via predefined subgroup analyses (stratified by region, sample size, patient characteristics, immunotherapy status, NOS score, PLR cut-off value, and cut-off determination) and meta-regression. Publication bias evaluation combined visual funnel plot assessment with Egger’s test quantification (significance: P<0.05).
3 Results
3.1 Literature search outcomes
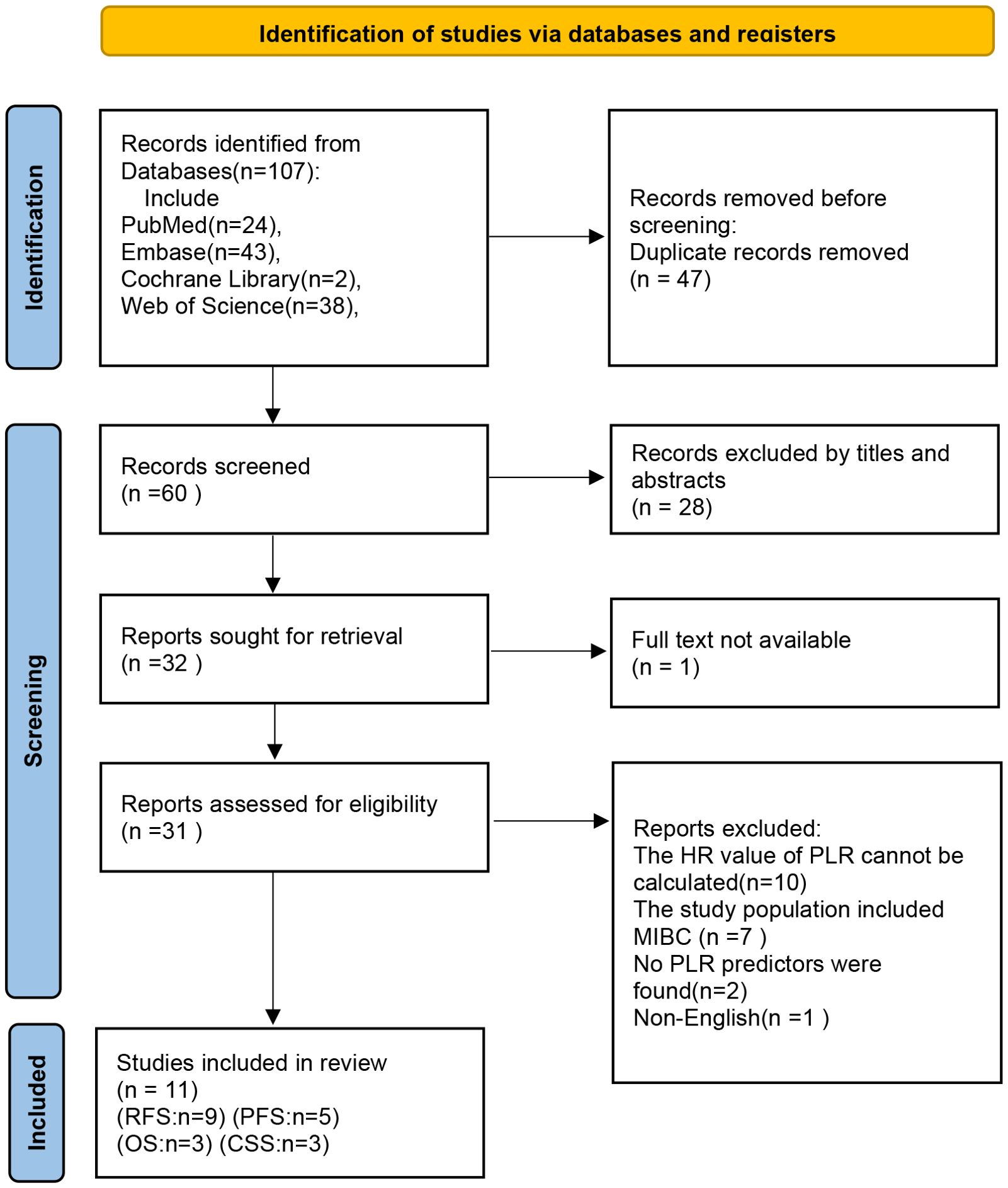
Initial database searches identified 107 publications. Upon removing 47 duplicates and screening titles and abstracts, 32 articles qualified for full-text evaluation. Based on eligibility criteria, 32 articles were further excluded (Supplementary Table S2). Finally, 11 studies were included in the meta-analysis. The complete selection workflow is documented in Figure 1.
3.2 Studies characteristics and quality assessment
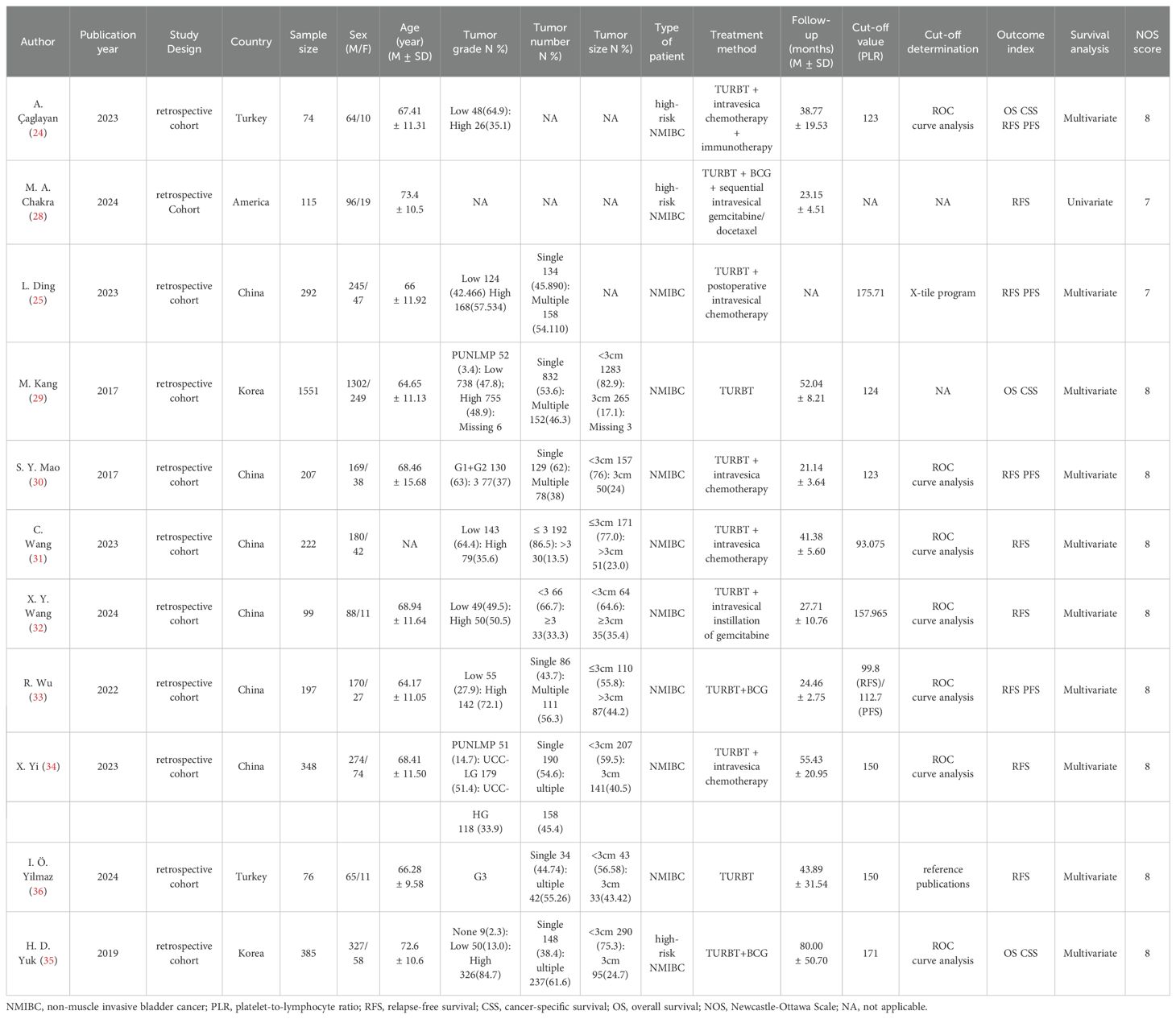
The analysis incorporated 11 retrospective cohort studies (24, 25, 28–36) conducted across four countries (China, America, Turkey, and Korea), encompassing 3,566 patients (2,980 males, 586 females). The studies, published between 2017 and 2024, employed PLR cut-off values ranging from 93.075 to 175.71. The quality assessment revealed that NOS scores for all included studies exceeded 6 (Supplementary Table S3), confirming their high methodological quality. Detailed study characteristics are summarized in Table 1.
3.3 Prognostic significance of PLR for RFS
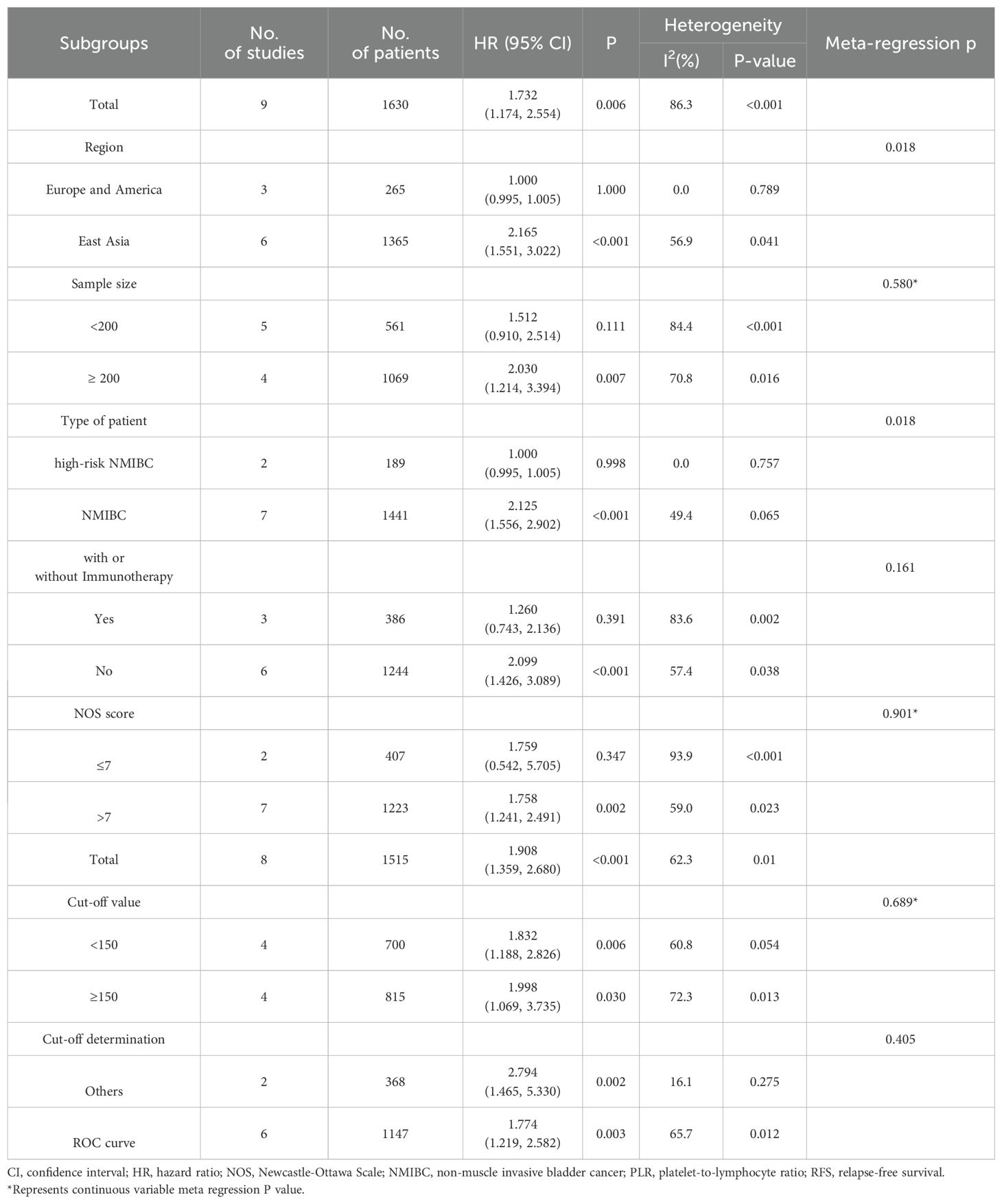
Nine studies (24, 25, 28, 30–34, 36) involving 1,630 patients investigated the relationship between RFS and PLR in NMIBC patients. Meta-analysis using a random-effects model (necessitated by substantial heterogeneity: I2 = 86.3%, P <0.001) demonstrated significantly inferior RFS in patients with elevated PLR (HR=1.732, 95% CI: 1.174-2.554, p=0.006). Subsequent subgroup analyses and meta-regression identified geographical variation and patient characteristics as potential sources of heterogeneity. These findings are visualized in Figure 2A and detailed in Table 2.
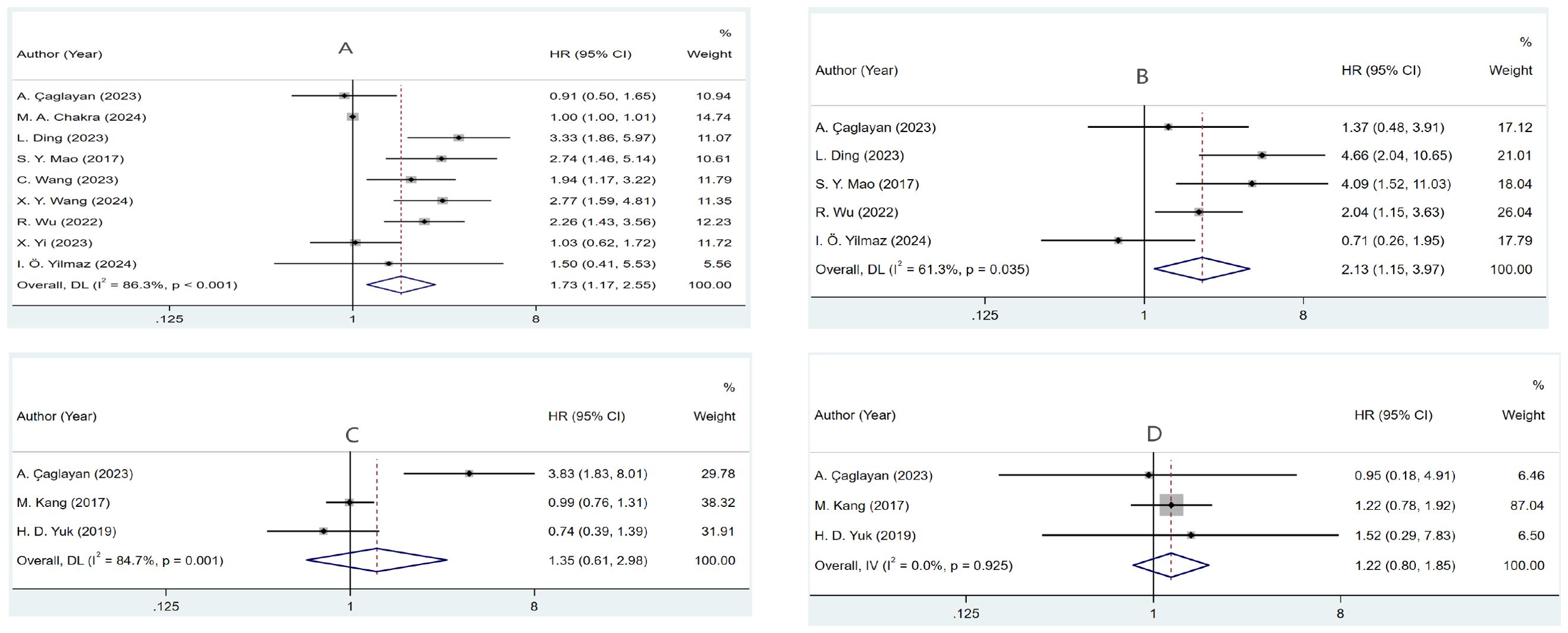
Figure 2. Forest plots of the prognostic role of PLR for (A) RFS, (B) PFS, (C) OS, and (D) CSS in NMIBC patients.
3.4 Prognostic significance of PLR for PFS
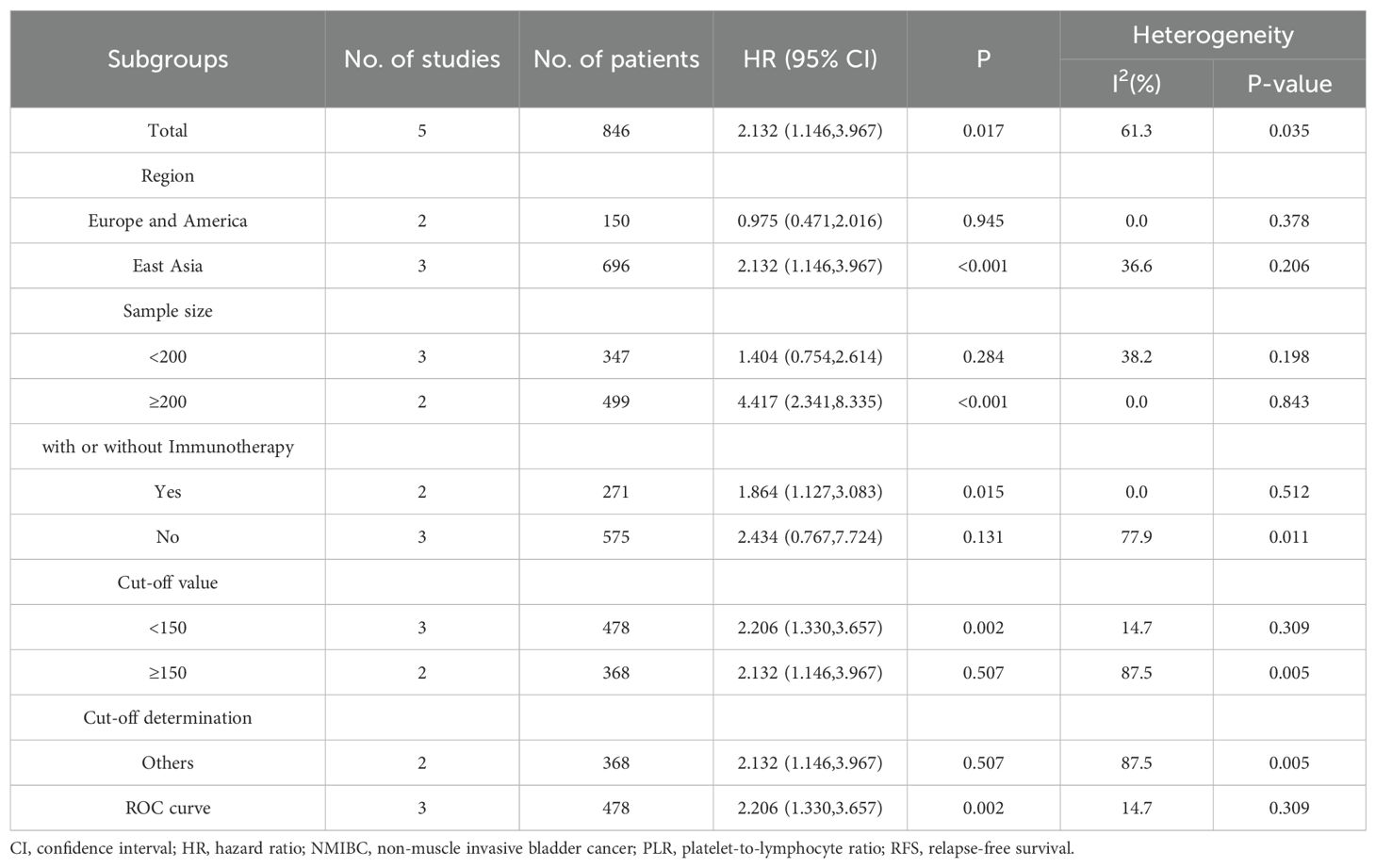
Analysis of five studies (24, 25, 30, 33, 36) revealed that increased PLR values were associated with reduced PFS outcomes (HR=2.132, 95% CI: 1.146-3.967, p=0.017). Notable between-study variance (I2 = 61.3%, p=0.035) warranted random-effects modeling. Further investigation through subgroup analyses identified geographical variation and sample size as key sources of heterogeneity (Figure 2B, Table 3).
3.5 Prognostic significance of PLR for OS
Three studies (24, 29, 35) examined PLR’s relationship with OS. Statistical analysis utilizing random-effects modeling, prompted by marked heterogeneity (I²=84.7%, P=0.001), found no definitive link between PLR and OS (HR=1.350, 95% CI: 0.611–2.983, p=0.459) (Figure 2C).
3.6 Prognostic significance of PLR for CSS
Examination of CSS across three studies (24, 29, 35) showed minimal statistical heterogeneity (I2 = 0.0%, P=0.925), supporting fixed-effects analysis. The data indicated no substantial relationship between PLR and CSS (HR=1.218, 95% CI: 0.800–1.854, p=0.358) (Figure 2D).
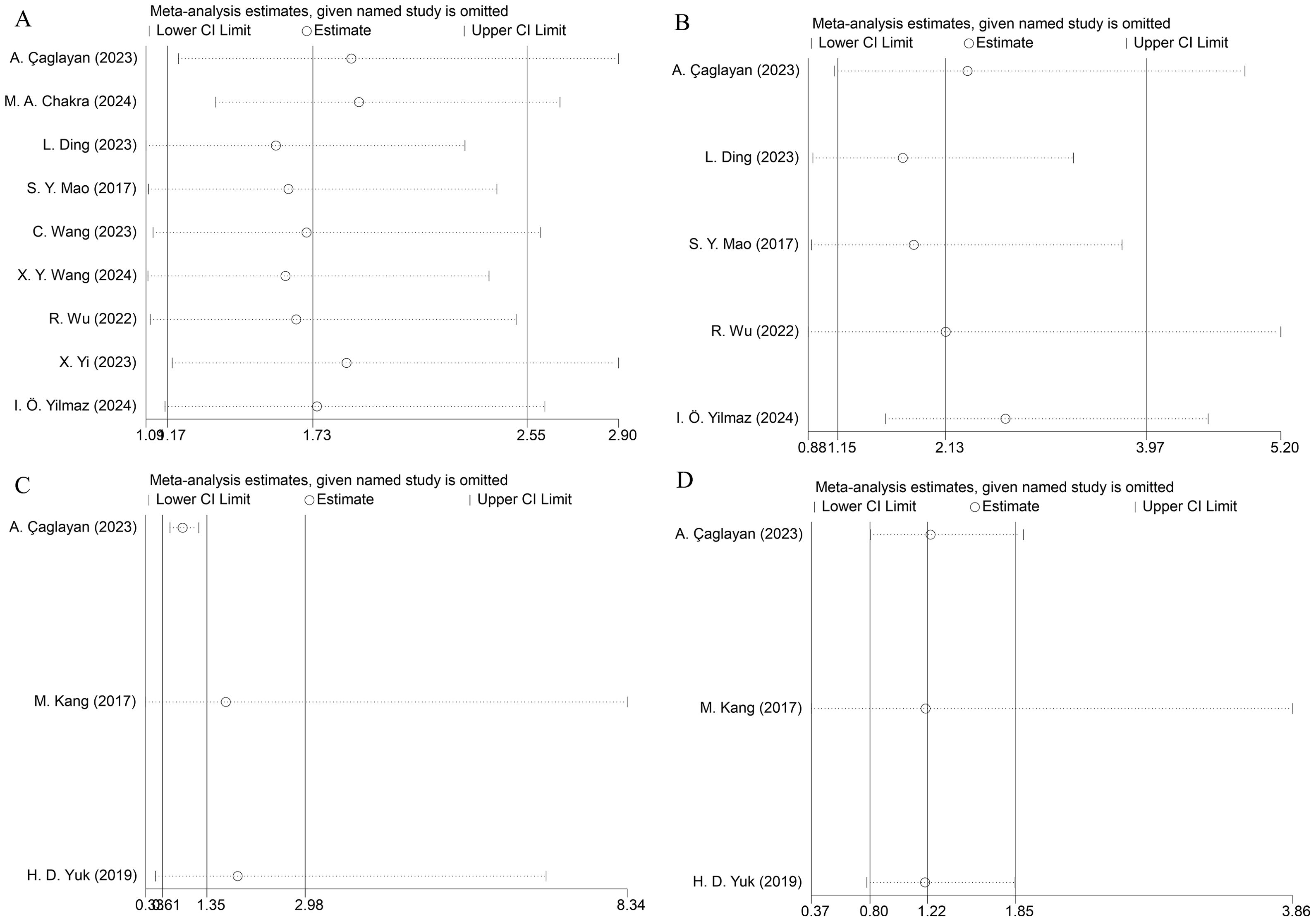
3.7 Sensitivity analysis
To verify result stability, we executed leave-one-out sensitivity analyses. Sequential omission of individual studies demonstrated no substantial alterations in the pooled effect estimates, confirming the robustness of our findings (Figure 3).
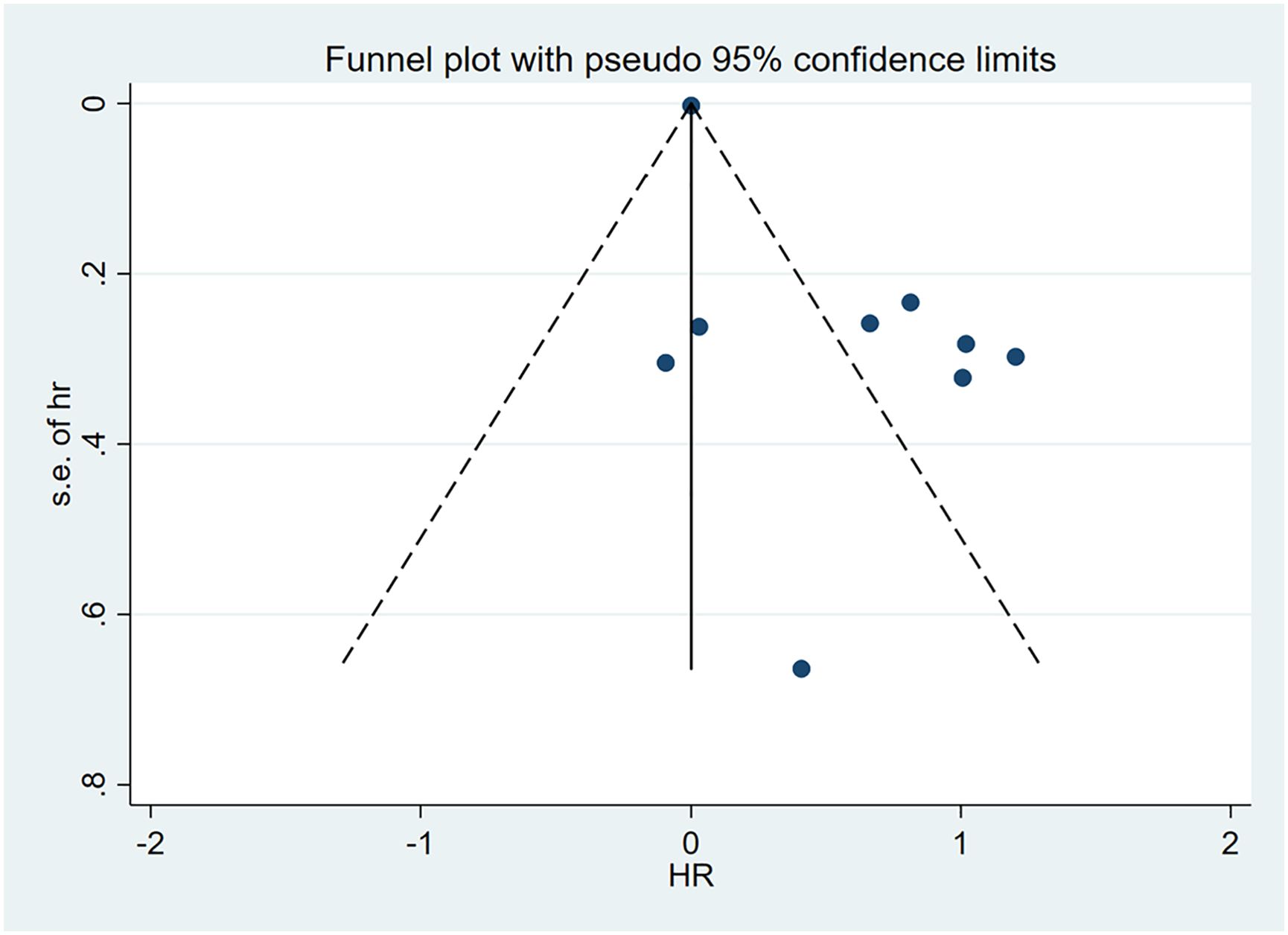
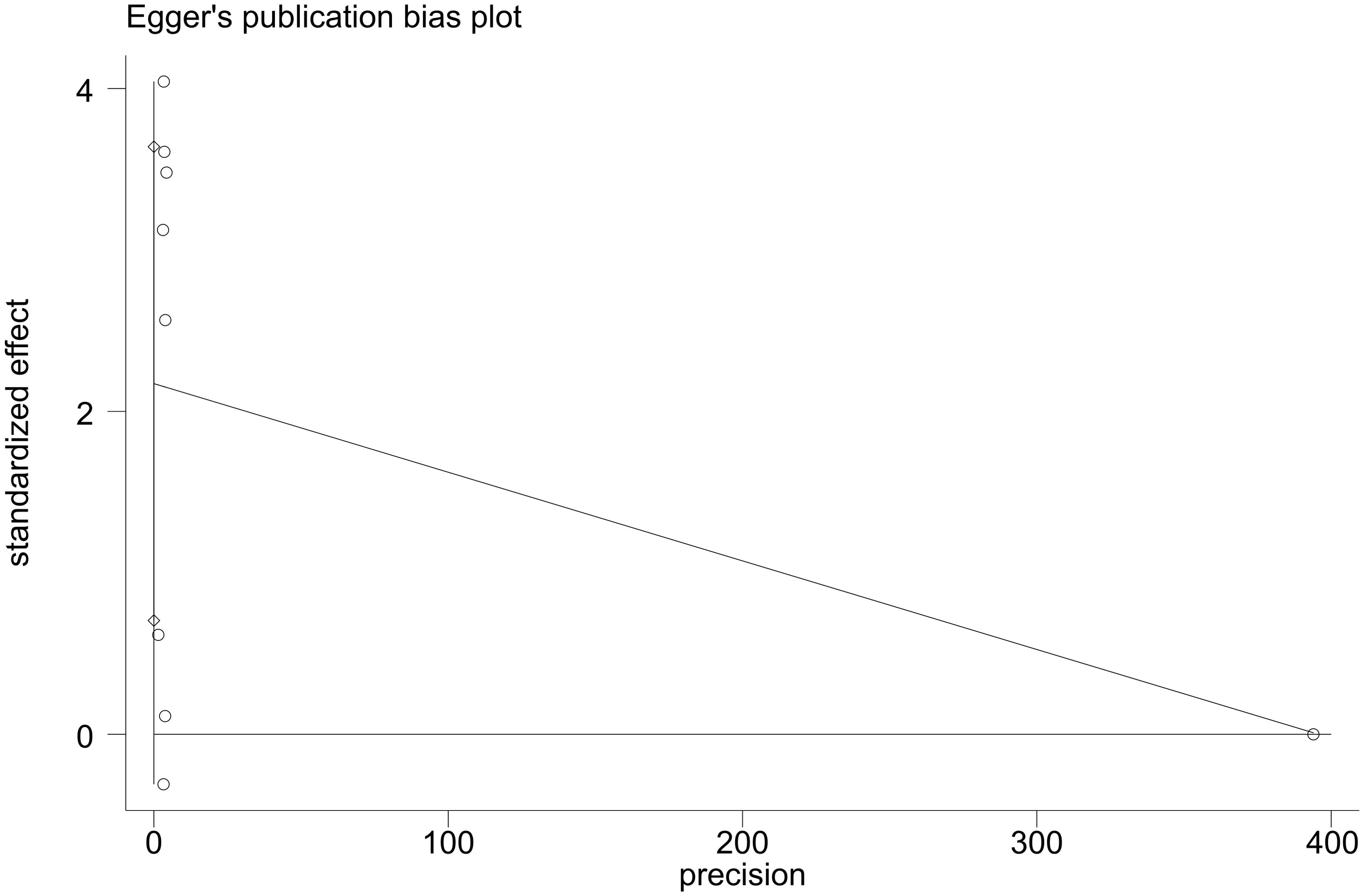
3.8 Publication bias
Visual examination of funnel plot demonstrated asymmetrical distribution, and Egger’s test (p=0.010) suggested possible reporting bias within the analyzed literature (Figures 4, 5).
4 Discussion
This systematic analysis consolidated evidence from 11 studies, comprising 3,566 NMIBC cases, to establish PLR’s prognostic implications in NMIBC. Sensitivity analysis demonstrated that the results of pooling these 11 independent studies were robust, thereby enhancing the reliability of our conclusions. Our findings revealed that elevated PLR correlates significantly with inferior RFS and PFS outcomes, suggesting its potential utility as a readily accessible and reliable prognostic biomarker in NMIBC management.
The prognostic value of PLR has been well-documented across various cancers (37, 38). Recent investigations have demonstrated significant associations between elevated PLR and adverse outcomes in multiple cancer types. For instance, elevated PLR levels correlate with decreased RFS in ovarian cancer (P=0.018) (39) and biliary tract cancer (HR=1.53, 95% CI: 1.16-2.00, p=0.002) (40). Similarly, meta-analyses have shown that higher PLR is associated with shorter PFS in both immunotherapy-treated gastric cancer patients (HR=1.52, 95% CI: 1.20-1.94, P<0.01) (41) and NSCLC patients undergoing immune checkpoint inhibitor therapy (HR=1.57, 95% CI: 1.30-1.90, P < 0.001) (42). The results of our study regarding the prognostic relevance of PLR in NMIBC are consistent with these earlier findings.
The biological basis for PLR’s prognostic value likely stems from the inflammatory nature and immune regulation of the TME, which critically influences tumor initiation, progression, and metastasis (43, 44). The intricate relationship between inflammatory responses and tumor progression has been well-established (45), though the precise mechanisms underlying PLR’s impact on NMIBC survival remain incompletely understood. Platelets contribute to tumor progression through multiple pathways, including the secretion of pro-angiogenic factors (vascular endothelial growth factor, platelet-derived growth factor) (46–48) and the release of microvesicles that activate mitogen-activated protein kinases signaling (49). Research has shown that nucleotides released by platelets promote cancer cell migration across endothelial barriers and enhance metastatic spread through P2Y2 receptor-mediated mechanisms (50). Furthermore, inflammation initiates the recruitment and activation of numerous immune cells, which produce and release a variety of inflammatory mediators, generating an autonomous loop of chronic inflammatory responses. As chronic inflammation progresses, an immunosuppressive microenvironment is induced, characterized by the accumulation of immune suppressor cells, such as MDSCs, pro-inflammatory cytokines, and growth and angiogenic factors that suppress T-cell and natural killer (NK) cells activity (51). Platelet-derived transforming growth factor-beta down-regulates NKG2D, thereby inhibiting NK cell antitumor reactivity (52). Platelets and fibrin (ogen) further increase metastatic potential by impeding NK cell-mediated elimination of tumor cells (53). Conversely, lymphocytes execute crucial anti-tumor functions through suppression of tumor cell division and direct cytotoxic effects (54, 55). NK cells and cytotoxic T lymphocytes (CD8+ T cells) contribute to the antitumor response (13). NK cells recognize tumor cell surface stress molecules (such as MICA/B) via NKG2D receptors, releasing perforin and granzyme to mediate tumor inhibition. CD8+ T cells specifically recognize the antigenic peptide-MHC-I complex through the T cell receptor (TCR), triggering TCR signaling and inhibiting tumor cells; CD8+ T cells can also directly induce tumor cells apoptosis through two main pathways: perforin-granase pathway and death receptor pathway. In addition, they secrete IFN-γ and TNF-α, which indirectly enhance antitumor immunity. Clinical evidence demonstrates that higher levels of lymphocyte infiltration within tumor sites correlate with better treatment response, while reduced lymphocyte presence diminishes anti-tumor immunity, potentially leading to immune escape (56). Tumor infiltrating lymphocytes (TIL) particularly demonstrate significant anti-tumor activity, with higher TIL densities associated with improved patient survival (57, 58). These complementary roles of platelet and lymphocytes underscore PLR’s potential as an integrated prognostic indicator, suggesting that patients with elevated PLR may benefit from more intensive therapy and surveillance protocols.
Certain limitations must be acknowledged when interpreting our results. First, the inclusion of only retrospective studies may introduce inherent biases in our analysis, and the relatively modest sample size precluded comprehensive subgroup analyses. Further prospective investigations are essential for validation. Second, studies currently employ diverse approaches to establish threshold values, introducing potential methodological inconsistencies influenced by demographic and clinical variables. Future research would benefit from developing either universal standards or specialized cutoff criteria that account for specific patient populations and clinical contexts. Third, the observed publication bias in RFS analyses, referring to the omission of studies with negative or inconclusive results due to their lower likelihood of publication, may have impact the reliability of our findings. This bias may be attributable to factors such as small sample sizes. Finally, the limited number of studies reporting certain survival outcomes (PFS, OS, and CSS) necessitates additional research to strengthen these findings.
5 Conclusion
This meta-analysis provides evidence that elevated PLR notably correlates with RFS and PFS in NMIBC patients. Given its economic efficiency and routine availability in clinical settings, PLR shows promise as a valuable marker for NMIBC patient care decisions. Nevertheless, robust prospective studies involving larger populations are essential to validate these initial observations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
WH: Writing – original draft, Writing – review & editing. JL: Data curation, Writing – review & editing. HH: Data curation, Writing – review & editing. JF: Formal Analysis, Investigation, Writing – review & editing. JL: Formal Analysis, Investigation, Writing – review & editing. XW: Formal Analysis, Investigation, Writing – review & editing. PZ: Software, Writing – review & editing. XZ: Software, Writing – review & editing. JZ: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Natural Science Joint Foundation Project of Hubei Province (Grant No.2024AFD262).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1578069/full#supplementary-material
References
1. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
2. Lopez-Beltran A, Cookson MS, Guercio BJ, Cheng L. Advances in diagnosis and treatment of bladder cancer. BMJ. (2024) 384:e076743. doi: 10.1136/bmj-2023-076743
3. Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, et al. European association of urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ). Eur Urol. (2022) 81:75–94. doi: 10.1016/j.eururo.2021.08.010
4. Bourlotos G, Baigent W, Hong M, Plagakis S, Grundy L. BCG induced lower urinary tract symptoms during treatment for NMIBC-Mechanisms and management strategies. Front Neurosci. (2023) 17:1327053. doi: 10.3389/fnins.2023.1327053
5. Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder cancer: A review. Jama. (2020) 324:1980–91. doi: 10.1001/jama.2020.17598
6. van den Bosch S, Alfred Witjes J. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: a systematic review. Eur Urol. (2011) 60:493–500. doi: 10.1016/j.eururo.2011.05.045
7. Patel VG, Oh WK, Galsky MD. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J Clin. (2020) 70:404–23. doi: 10.3322/caac.21631
8. Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol. (2016) 196:1021–9. doi: 10.1016/j.juro.2016.06.049
9. Lobo N, Brooks NA, Zlotta AR, Cirillo JD, Boorjian S, Black PC, et al. 100 years of Bacillus Calmette-Guérin immunotherapy: from cattle to COVID-19. Nat Rev Urol. (2021) 18:611–22. doi: 10.1038/s41585-021-00481-1
10. Thyavihally YB, Dev P, Waigankar S, Pednekar A, Athikari N, Raut A, et al. Intravesical bacillus Calmette-Guerin (BCG) in treating non-muscle invasive bladder cancer-analysis of adverse effects and effectiveness of two strains of BCG (Danish 1331 and Moscow-I). Asian J Urol. (2022) 9:157–64. doi: 10.1016/j.ajur.2021.05.002
11. Yeary KHK, Yu H, Kuliszewski MG, Li Q, McCann SE, Pratt R, et al. Outcomes of a dietary intervention to reduce bladder cancer recurrence and progression in survivors of non-muscle-invasive bladder cancer. J Natl Compr Canc Netw. (2024) 22. doi: 10.6004/jnccn.2023.7086
13. Pottier C, Wheatherspoon A, Roncarati P, Longuespée R, Herfs M, Duray A, et al. The importance of the tumor microenvironment in the therapeutic management of cancer. Expert Rev Anticancer Ther. (2015) 15:943–54. doi: 10.1586/14737140.2015.1059279
14. Richards CH, Flegg KM, Roxburgh CS, Going JJ, Mohammed Z, Horgan PG, et al. The relationships between cellular components of the peritumoural inflammatory response, clinicopathological characteristics and survival in patients with primary operable colorectal cancer. Br J Cancer. (2012) 106:2010–5. doi: 10.1038/bjc.2012.211
15. Li B, Zhou P, Liu Y, Wei H, Yang X, Chen T, et al. Platelet-to-lymphocyte ratio in advanced Cancer: Review and meta-analysis. Clin Chim Acta. (2018) 483:48–56. doi: 10.1016/j.cca.2018.04.023
16. Hu G, Liu Q, Ma JY, Liu CY. Prognostic significance of platelet-to-lymphocyte ratio in cholangiocarcinoma: A meta-analysis. BioMed Res Int. (2018) 2018:7375169. doi: 10.1155/2018/7375169
17. Hamid HKS, Emile SH, Davis GN. Prognostic significance of lymphocyte-to-monocyte and platelet-to-lymphocyte ratio in rectal cancer: A systematic review, meta-analysis, and meta-regression. Dis Colon Rectum. (2022) 65:178–87. doi: 10.1097/DCR.0000000000002291
18. Wang X, Ni X, Tang G. Prognostic role of platelet-to-lymphocyte ratio in patients with bladder cancer: A meta-analysis. Front Oncol. (2019) 9:757. doi: 10.3389/fonc.2019.00757
19. Zhao Y, Si G, Zhu F, Hui J, Cai S, Huang C, et al. Prognostic role of platelet to lymphocyte ratio in hepatocellular carcinoma: a systematic review and meta-analysis. Oncotarget. (2017) 8:22854–62. doi: 10.18632/oncotarget.15281
20. Zhang M, Huang XZ, Song YX, Gao P, Sun JX, Wang ZN. High platelet-to-lymphocyte ratio predicts poor prognosis and clinicopathological characteristics in patients with breast cancer: A meta-analysis. BioMed Res Int. (2017) 2017:9503025. doi: 10.1155/2017/9503025
21. Min GT, Wang YH, Yao N, Zhao JM, Wang J, Wang HP, et al. The prognostic role of pretreatment platelet-to-lymphocyte ratio as predictors in patients with colorectal cancer: a meta-analysis. biomark Med. (2017) 11:87–97. doi: 10.2217/bmm-2016-0181
22. Wang J, Zhou X, He Y, Chen X, Liu N, Ding Z, et al. Prognostic role of platelet to lymphocyte ratio in prostate cancer: A meta-analysis. Med (Baltimore). (2018) 97:e12504. doi: 10.1097/MD.0000000000012504
23. Zhao QT, Yuan Z, Zhang H, Zhang XP, Wang HE, Wang ZK, et al. Prognostic role of platelet to lymphocyte ratio in non-small cell lung cancers: A meta-analysis including 3,720 patients. Int J Cancer. (2016) 139:164–70. doi: 10.1002/ijc.v139.1
24. Caglayan A, Horsanali MO. Can peripheral blood systemic immune response parameters predict oncological outcomes in patients with non-muscle-invasive bladder cancer? Niger J Clin Pract. (2023) 26:591–8. doi: 10.4103/njcp.njcp_399_22
25. Ding L, Deng X, Wang K, Xia W, Zhang Y, Zhang Y, et al. Preoperative systemic inflammatory markers as a significant prognostic factor after TURBT in patients with non-muscle-invasive bladder cancer. J Inflammation Res. (2023) 16:283–96. doi: 10.2147/JIR.S393511
26. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
27. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
28. Chakra MA, Lassila R, El Beayni N, Mott SL, O’Donnell MA. Prognostic role of the neutrophil/lymphocyte ratio in high-risk BCG-naïve non-muscle-invasive bladder cancer treated with intravesical gemcitabine/docetaxel. BJU Int. (2025) 135:125–32. doi: 10.1111/bju.16486
29. Kang M, Jeong CW, Kwak C, Kim HH, Ku JH. Preoperative neutrophil-lymphocyte ratio can significantly predict mortality outcomes in patients with non-muscle invasive bladder cancer undergoing transurethral resection of bladder tumor. Oncotarget. (2017) 8:12891–901. doi: 10.18632/oncotarget.14179
30. Shi-Yu Mao T-BH, Xiong D-D, Liu M-N, Cai K-K, Yao X-D. Prognostic value of preoperative systemic inflammatory responses in patients with non-muscle invasive bladder cancer undergoing transurethral resection of bladder tumor. Int J Clin Exp Pathol. (2017) 5:5799–810.
31. Wang C, Jin W, Ma X, Dong Z. The different predictive value of mean platelet volume-to-lymphocyte ratio for postoperative recurrence between non-muscular invasive bladder cancer patients treated with intravesical chemotherapy and intravesical chemohyperthermia. Front Oncol. (2022) 12:1101830. doi: 10.3389/fonc.2022.1101830
32. Wang X, Zhang S, Sun Y, Cai L, Zhang J. The prognostic significance of preoperative platelet-to-lymphocyte ratio and interleukin-6 level in non-muscle invasive bladder cancer. Int J Biol Markers. (2024) 39:255–64. doi: 10.1177/03936155241261719
33. Wu R, Li D, Zhang F, Bai Y, Wang X, Han P. Prognostic value of platelet-to-lymphocyte ratio in non-muscle invasive bladder cancer patients: intravesical bacillus calmette-guerin treatment after transurethral resection of bladder tumor. Front Surg. (2022) 9:907485. doi: 10.3389/fsurg.2022.907485
34. Yi X, Pi J, Liu C, Xiong Y, Liu J, Fu W, et al. The relationship between inflammatory response markers and the prognosis of non-muscle invasive bladder cancer and the development of a nomogram model. Front Oncol. (2023) 13:1189086. doi: 10.3389/fonc.2023.1189086
35. Yuk HD, Jeong CW, Kwak C, Kim HH, Ku JH. Elevated neutrophil to lymphocyte ratio predicts poor prognosis in non-muscle invasive bladder cancer patients: initial intravesical bacillus calmette-guerin treatment after transurethral resection of bladder tumor setting. Front Oncol. (2018) 8:642. doi: 10.3389/fonc.2018.00642
36. Yilmaz İÖ, Değer M, Akdoğan N, Aridogan IA, Bayazıt Y, Izol V. Prognostic values of inflammatory markers in patients with high-grade lamina propria-invasive bladder cancer. Bull Urooncol. (2024) 23:16–21. doi: 10.4274/uob.galenos.2023.2023.2.1
37. Zhou X, Du Y, Huang Z, Xu J, Qiu T, Wang J, et al. Prognostic value of PLR in various cancers: a meta-analysis. PloS One. (2014) 9:e101119. doi: 10.1371/journal.pone.0101119
38. Templeton AJ, Ace O, McNamara MG, Al-Mubarak M, Vera-Badillo FE, Hermanns T, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. (2014) 23:1204–12. doi: 10.1158/1055-9965.EPI-14-0146
39. Song Q, Xu SX, Wu JZ, Ling L, Wang S, Shu XH, et al. The preoperative platelet to neutrophil ratio and lymphocyte to monocyte ratio are superior prognostic indicators compared with other inflammatory biomarkers in ovarian cancer. Front Immunol. (2023) 14:1177403. doi: 10.3389/fimmu.2023.1177403
40. Zhou LH, Luo XF. Platelet to lymphocyte ratio in biliary tract cancer: Review and meta-analysis. Clin Chim Acta. (2017) 474:102–7. doi: 10.1016/j.cca.2017.09.006
41. Tan S, Zheng Q, Zhang W, Zhou M, Xia C, Feng W. Prognostic value of inflammatory markers NLR, PLR, and LMR in gastric cancer patients treated with immune checkpoint inhibitors: a meta-analysis and systematic review. Front Immunol. (2024) 15:1408700. doi: 10.3389/fimmu.2024.1408700
42. Zhang N, Jiang J, Tang S, Sun G. Predictive value of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in non-small cell lung cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Int Immunopharmacol. (2020) 85:106677. doi: 10.1016/j.intimp.2020.106677
43. Coussens LM, Werb Z. Inflammation and cancer. Nature. (2002) 420:860–7. doi: 10.1038/nature01322
44. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. (2008) 454:436–44. doi: 10.1038/nature07205
45. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
46. Goubran HA, Burnouf T, Radosevic M, El-Ekiaby M. The platelet-cancer loop. Eur J Intern Med. (2013) 24:393–400. doi: 10.1016/j.ejim.2013.01.017
47. Goubran HA, Stakiw J, Radosevic M, Burnouf T. Platelets effects on tumor growth. Semin Oncol. (2014) 41:359–69. doi: 10.1053/j.seminoncol.2014.04.006
48. Goubran HA, Stakiw J, Radosevic M, Burnouf T. Platelet-cancer interactions. Semin Thromb Hemost. (2014) 40:296–305. doi: 10.1055/s-0034-1370767
49. Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. (2005) 113:752–60. doi: 10.1002/ijc.v113:5
50. Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell. (2013) 24:130–7. doi: 10.1016/j.ccr.2013.05.008
51. Meyer C, Sevko A, Ramacher M, Bazhin AV, Falk CS, Osen W, et al. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc Natl Acad Sci U S A. (2011) 108:17111–6. doi: 10.1073/pnas.1108121108
52. Kopp HG, Placke T, Salih HR. Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res. (2009) 69:7775–83. doi: 10.1158/0008-5472.CAN-09-2123
53. Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. (2005) 105:178–85. doi: 10.1182/blood-2004-06-2272
54. Paijens ST, Vledder A, de Bruyn M, Nijman HW. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol Immunol. (2021) 18:842–59. doi: 10.1038/s41423-020-00565-9
55. Chen KJ, Zhou L, Xie HY, Ahmed TE, Feng XW, Zheng SS. Intratumoral regulatory T cells alone or in combination with cytotoxic T cells predict prognosis of hepatocellular carcinoma after resection. Med Oncol. (2012) 29:1817–26. doi: 10.1007/s12032-011-0006-x
56. Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. (2011) 105:93–103. doi: 10.1038/bjc.2011.189
57. Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. (2013) 13:759–71. doi: 10.1038/nrc3611
Keywords: platelet-to-lymphocyte ratio, prognosis, biomarker, meta-analysis, non-muscle invasive bladder cancer
Citation: Hu W, Liang J, Luo J, Fan J, Hu H, Wang X, Zhou P, Zhang X and Zhou J (2025) Elevated platelet-to-lymphocyte ratio predicts poor clinical outcomes in non-muscle invasive bladder cancer: a systematic review and meta-analysis. Front. Immunol. 16:1578069. doi: 10.3389/fimmu.2025.1578069
Received: 17 February 2025; Accepted: 21 April 2025;
Published: 13 May 2025.
Edited by:
Vinay Kumar, The Pennsylvania State University, United StatesReviewed by:
Suman Asalla, The Ohio State University, United StatesManav Jain, The University of Utah, United States
Copyright © 2025 Hu, Liang, Luo, Fan, Hu, Wang, Zhou, Zhang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Zhou, emhvdWppZXVzZXJAMTYzLmNvbQ==
†These authors contributed equally to this work and share first authorship
 Wenfeng Hu
Wenfeng Hu Jinze Liang1†
Jinze Liang1†