- 1National Clinical Research Center for Kidney Diseases, Jinling Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, China
- 2The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi People’s Hospital, Department of Nephrology, Wuxi Medical Center, Nanjing Medical University, Wuxi, China
- 3National Clinical Research Center for Kidney Diseases, Jinling Hospital, Nanjing Medical University, Nanjing, China
Aims: To explore the clinicopathological features and renal outcome in patients with cryoglobulinemic glomerulonephritis (Cryo-GN) without confirmed systemic autoimmune diseases.
Methods: Sixty-nine patients with Cryo-GN from a single center were recruited in this retrospective study. Their clinical, pathologic, and follow-up data were collected and analyzed. According to whether the serum monoclonal immunoglobulin (MIg) and HBV-DNA/HBV markers or HCV-RNA/anti-HCV antibodies were positive or not, they were classified into four groups: positive serum MIg only (MIg group), positive HBV-DNA/HBV markers or HCV-RNA/anti-HCV antibodies (HBV/HCV) only (HBV/HCV group), positive serum MIg and HBV/HCV (MIg+HBV/HCV group), and all MIg/HBV/HCV negative group.
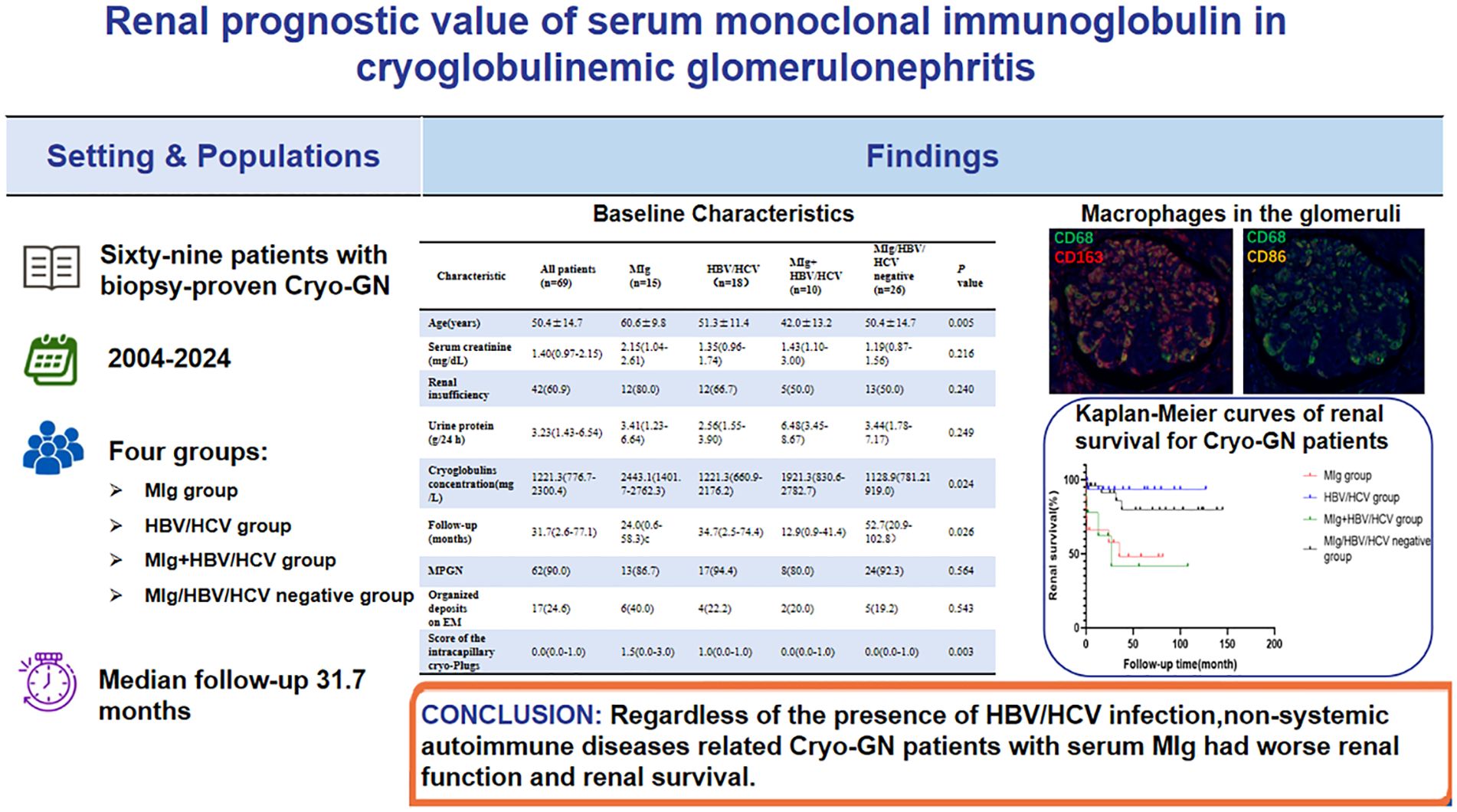
Results: The male-to-female ratio was 1.38:1 with a mean age of 50.4 ± 14.7 years in the patient cohort. Hypertension was presented in 59.4% of cases, anemia in 73.9%, renal insufficiency in 60.9%, nephrotic proteinuria in 44.9% and microscopic hematuria in 94.2%. The MIg group had significantly lower eGFR levels, higher cryoglobulin levels, and higher rates of abnormal serum-free light chain ratios than the MIg/HBV/HCV negative group. The most common histological pattern of Cryo-GN was membranoproliferative glomerulonephritis (MPGN), and the MIg group had significantly higher scores of the severity of intracapillary cryo-Plugs than the MIg+HBV/HCV group and the MIg/HBV/HCV negative group. Immunohistochemical staining of 29 patients revealed a significant infiltration of CD68+ cells within the glomeruli. Further multiplex immunohistochemical staining of 4 of these patients showed that the infiltrating cells within the glomeruli in Cryo-GN were predominantly CD68+CD163+ cells. Sixty-seven patients had a median follow-up of 31.7 months, and 23.9% of them progressed to end-stage renal disease (ESRD). The renal survival was inferior for MIg group than HBV/HCV group. Multivariate analysis showed that serum MIg and eGFR were independent prognostic factors.
Conclusion: Regardless of the presence of HBV/HCV infection, non-systemic autoimmune diseases related Cryo-GN patients with serum MIg had worse renal function and renal survival. Patients with a large number of pseudothrombi in the glomerular capillary lumens tend to have worse renal outcomes. Serum MIg and eGFR were independent risk factors for renal survival in Cryo-GN patients without autoimmune diseases.
Introduction
Cryoglobulins are serum immunoglobulins that precipitate at temperatures below 37°C and redissolve on warming. Cryoglobulinemia is divided into three types based on immunoglobulin composition: type I (composed of a single monoclonal immunoglobulin, typically associated with lymphoproliferative disorders such as multiple myeloma or Waldenström macroglobulinemia), type II (mixed cryoglobulins consisting of monoclonal IgM with rheumatoid factor activity and polyclonal IgG, most commonly linked to hepatitis C virus infection), and type III (polyclonal IgM and IgG, often related to chronic infections or autoimmune diseases) (1, 2). Precipitating in the microcirculation and immune-complex-mediated inflammation of blood vessels are the two major pathogenic mechanisms of cryoglobulins. The skin, kidneys, nervous system, and joints can be involved. Cryoglobulinaemia is associated with many diseases, which can be generally grouped into infections, autoimmune disorders, and malignancies (2, 3).
Kidney is one of the most involved organs in cryoglobulinemia, named as cryoglobulinemic glomerulonephritis (Cryo-GN) (2.3). Cryo-GN is a recognized independent risk factor of the poor prognosis of cryoglobulinemia (4–6). Several studies have indicated that 9-14% of patients with Cryo-GN may progress to end-stage renal disease (ESRD) (7–10). Previous studies had shown that hepatitis B virus (HBV) infection is more common than hepatitis C virus (HCV) infection among Chinese patients with cryoglobulinemia, and there is relatively less research on HBV-related cryoglobulinemic glomerulonephritis (Cryo-GN) (11, 12). According to the new consensus on monoclonal gammopathy of renal significance, some type I and type II cryoglobulinemia-related glomerulonephritis are classified as monoclonal gammopathy of renal significance (MGRS)-related diseases (13). However, the differences in clinical and renal histopathological features of cryoglobulinemia caused by different etiologies have not been fully elucidated. This study retrospectively analyzed 69 Chinese patients who had biopsy-proven Cryo-GN and explored the clinical and pathological differences of Cryo-GN caused by different etiologies, to deepen the understanding of Cryo-GN, improve the clinical diagnosis, treatment level, and the prognosis of patients.
Materials and methods
Patient selection
Sixty-nine patients with Cryo-GN were identified by retrospective review of all native renal biopsies received at the National Clinical Research Center for Kidney Diseases, Jinling Hospital, from January 2004 to December 2024 (Figure 1). Inclusion criteria were (1): Serum cryoglobulins test ≥192mg/dL (2); Accompanied by clinical manifestations of renal damage such as proteinuria, hematuria, and renal dysfunction (3); Kidney biopsy findings showing MPGN or endocapillary proliferative glomerulonephritis patterns excluding other defined causes such as acute poststreptococcal glomerulonephritis, lupus nephritis, IgA nephropathy, C3 glomerulopathy, monoclonal immunoglobulin deposition, and genetic glomerulonephritis. We excluded patients diagnosed with autoimmune conditions, including systemic lupus erythematosus, rheumatoid arthritis, or Sjögren’s syndrome, from subsequent analyses owing to their distinctive disease mechanisms, therapeutic regimens, and clinical outcomes.
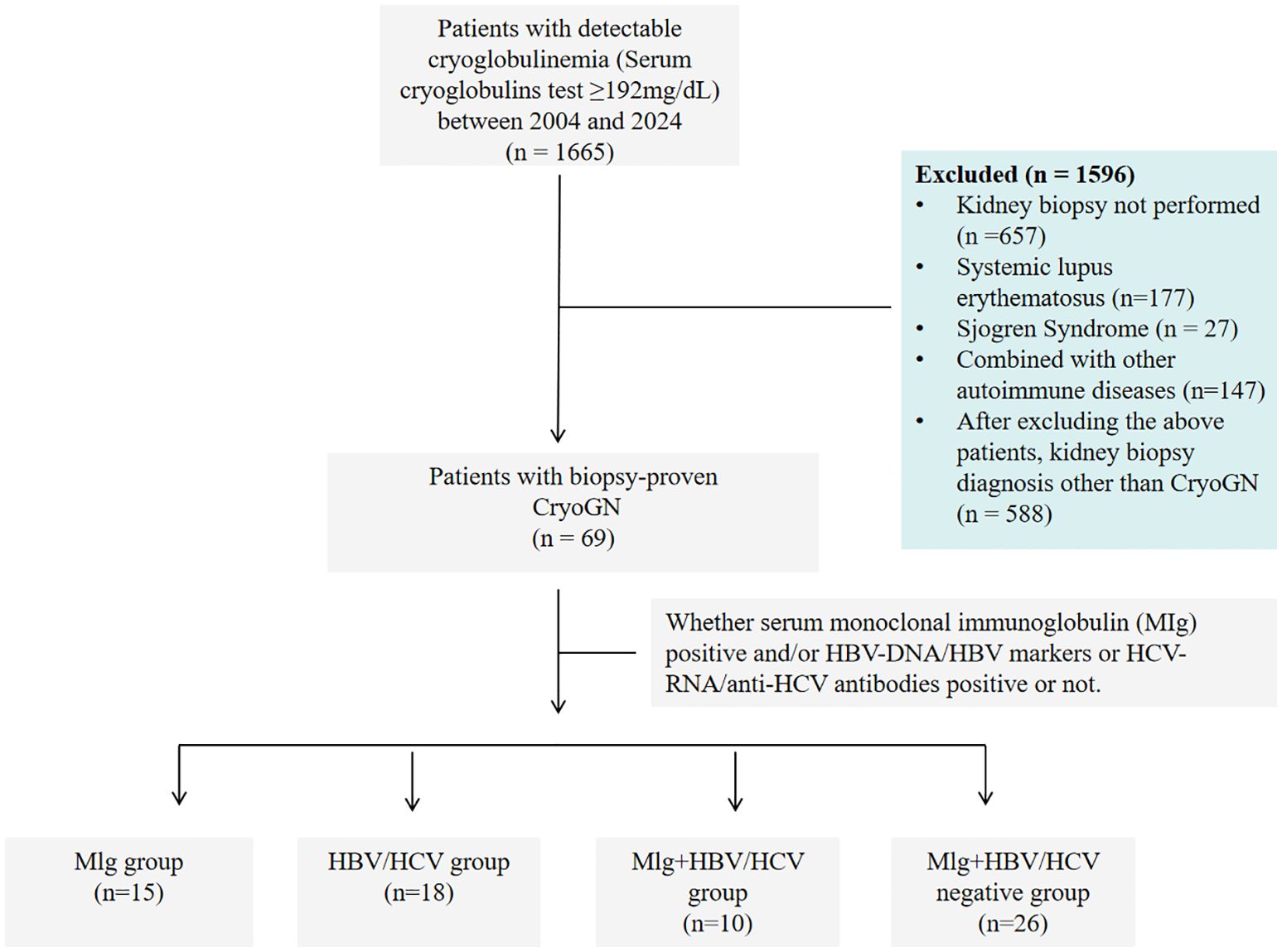
Figure 1. Study flow chart of patients with cryoglobulinemia who underwent a kidney biopsy from 2004 to 2024.
According to whether serum monoclonal immunoglobulin (MIg) was positive and/or HBV-DNA/HBV markers or HCV-RNA/anti-HCV antibodies positive or not, all participants were classified into four groups: Only positive serum MIg (MIg group), only positive for HBV-DNA/HBV markers or HCV-RNA/anti-HCV antibodies (HBV/HCV group), both positive for MIg and HBV/HCV (MIg+HBV/HCV positive group), both negative for MIg and HBV/HCV (MIg/HBV/HCV negative group).
Patient’s medical records were reviewed for demographic information, clinical and laboratory data at the time of biopsy, treatment and follow-up. The cause of cryoglobulinemia was established according to the research of Brouet et al (1).
The clinical manifestations were defined as follows: hypertension: systolic blood pressure >140 mmHg or diastolic blood pressure>90mmHg or ongoing use of antihypertensive medications; anemia: hemoglobin (Hb) <120 g/L in males, Hb <110 g/L in females (14); nephrotic-range proteinuria (NRP): proteinuria≥3.5g/24h; microscopic hematuria: urinary sediment erythrocyte count > 12/μL(SysmexUF-1000 urine analyzer); renal insufficiency: estimated glomerular filtration rate (eGFR) (EPI)<60 mL/min/1.73 m² for ≥3 months; abnormal serum free light chain (FLC) ratio: κ/λ<0.26 or>1.65(Freelite, BindingSite, UK); RF positive (RF > 20 (IU/ml). ESRD: eGFR (EPI)<15ml/(min.1.73m2) or being dependent on renal replacement therapy (15). The serum anti-HCV antibody, HBV markers, HCV-RNA, and HBV-DNA were also obtained. Definition of renal response to treatment (10): Complete remission (CR) (1): Urinary protein <0.4 g/24 h (2), Disappearance of hematuria (3). When the baseline eGFR is lower than 70 ml/(min.1.73m2), the eGFR after treatment increases by 20% compared with the baseline level. Partial remission (PR): At least one of the criteria for complete remission is met. No response (NR): None of the criteria for complete remission is met. Follow-up endpoints: progress to ESRD (estimated glomerular filtration rate(eGFR)(EPI)<15ml/(min.1.73m2) or being dependent on renal replacement therapy).
Detection of cryoglobulins
(1) Syringes and test tubes were prewarmed to 37°C. 15ml of venous blood were collected into warmed tubes, 37°C water bath until clotted (2). After the separation of serum, serum was centrifuged at 37°C, 2000rpm (3). 5ml of supernatant was accurately collected and stored at 4°C for precipitation for 5–7 days (4). After precipitation and centrifugation, cryoprecipitate was isolated and washed with cold PBS (< 4°C) for 3 times (5). After dissolution in 3 mL sodium acetate buffer, the absorbance was measured at OD280 and OD260 with a microplate reader. Cryoglobulin concentration =1550×OD280- 770×OD260. The cryoglobulin detection method in this study has a sensitivity of 0.1–0.5 mg/mL and a positive threshold set at 0.192 mg/mL (192 mg/L), it can effectively identify clinically significant cryoglobulinemia.
Pathology studies
All renal biopsies were processed using the standard techniques of light microscopy (LM), immunofluorescence (IF), and electron microscope (EM). Acute tubular injury was defined by the presence of tubular simplification, loss or attenuation of the brush border. Interstitial fibrosis/tubular atrophy (IF/TA) and acute tubular injury (ATI) were scored semiquantitatively based on the percentage of the tubulointerstitial compartment affected and recorded as 0% (0), 1%-25% (1), 26%-50% (2), or >50% (3). The severity of intracapillary thrombi in glomeruli was scored according to the proportion of glomeruli affected: 0 (0), <25% (1); 25-75% (2); >75% (3). IF was performed on cryosections (4μm) using polyclonal FITC-conjugated antibodies against IgG, IgM, IgA, C3, C1q, κ, and λ light chains (Dako Corp., Glostrup, Denmark). Cases with IgG deposits underwent IgG subtype staining. Ultrastructural evaluation was performed using a FEI Tecnai G2 Spirit transmission electron microscope.
Immunohistochemical staining
Formalin-fixed, paraffin-embedded kidney tissue sections were processed according to routine clinical practice. Kidney tissue sections were stained for CD3 (Clone 565, Newcastle, 1:100) as a T-cell marker, CD4 (Clone L26, Dako) as a CD4+ T-cell marker, and CD8 (Clone L26, Dako) as a CD8+ T-cell marker. The staining was performed on consecutive sections of patient renal tissue using a standard automated immunostaining device (Leica BOND, United States). Positive cell counts were determined for tangential and non-sclerotic glomeruli.
Multiplex immunohistochemical staining
Cryopreserved 3.5-μm paraffin tissue sections from renal biopsy specimens were sequentially placed into xylene I for 10 min, xylene II for 10 min, xylene III for 10 min, anhydrous ethanol I for 5 min, anhydrous ethanol II for 5 min, 90% ethanol for 5 min, 70% ethanol for 5 min, and rinsed in distilled water for 3 min. Antigen retrieval was then performed using citrate buffer (pH 6.0) (Akoya Biosciences, AR600250ML). Endogenous peroxidase activity was blocked with a peroxidase-blocking solution (Beyotime Biotechnology, P0100B), followed by serum blocking with goat serum. The sections were incubated with a primary antibody or an isotype-matched control antibody at room temperature for 1 hour. After incubation with a universal secondary antibody for mouse and rabbit (Akoya Biosciences, ARH1001EA) at room temperature for 30 minutes, corresponding dyes were applied according to the primary antibody staining sequence (CD68 (Abcam, ab955, pan-macrophage marker), CD163 (Abcam, ab182422, M2 macrophage marker), CD86 (CST, 91882S, M1 macrophage/antigen-presenting cell marker), CD206 (CST, 24595S, M2 macrophage mannose receptor), CD56 (CST, 99746S), CD3 (Abcam, ab11089), CD8 (Abcam, ab199016)), and incubated for 10 min. Slides were then washed three times in TBST for 5 min each. The above steps were repeated according to the primary antibody staining sequence until all markers were completed. Subsequently, the slides were counterstained with DAPI, mounted, and imaged using a slide scanner. Using Qupath-0.5.0 software for image analysis, select a kidney tissue and count all types of marker cells within the glomeruli, taking the average and maximum values.
Statistical analyses
Continuous variables are presented as the means ± SD and non-normally distributed data are expressed as the median(interquartile rang(IQR). Survival analysis was performed by univariate survival analysis, multivariable Cox regression models, and Kaplan–Meier curves. Most clinical variables are assessed in continuous ways while pathological parameters in categorical ways. Kruskal-Wallis test and nonparametric Mann–Whitney test were used for the comparison of different groups, followed by Dunn-Bonferroni post hoc test for pairwise comparisons. Statistical significance was assumed at P<0.05. All statistical analysis was a two-sided test. Statistical analysis was performed with SPSS (version 27.0, SPSS, Chicago, IL, USA).
Results
General demographics and clinical characteristics
The clinical characteristics of the patients at kidney biopsy are described in Table 1. There were 40 males and 29 females, mean age 50.4 ± 14.7 years. Twenty-one patients (30.4%) were positive for serum HBV markers or HBV-DNA and 7 patients (10.1%) were positive for anti-HCV antibodies or HCV-RNA. The median 24-h urine protein was 3.23g/d (1.43-6.54g/d) and 44.9% of patients had nephrotic proteinuria. Renal dysfunction was observed in 60.9% of patients, with a median serum creatine of 1.40mg/dL (0.97-2.15mg/dL). The median concentration of cryoglobulin in the 69 patients was 1221.3mg/L (776.7-2300.4mg/L). Sixty-four patients underwent routine RF testing, with a median RF level of 128.5 IU/ml (19.9-555.0 IU/ml).
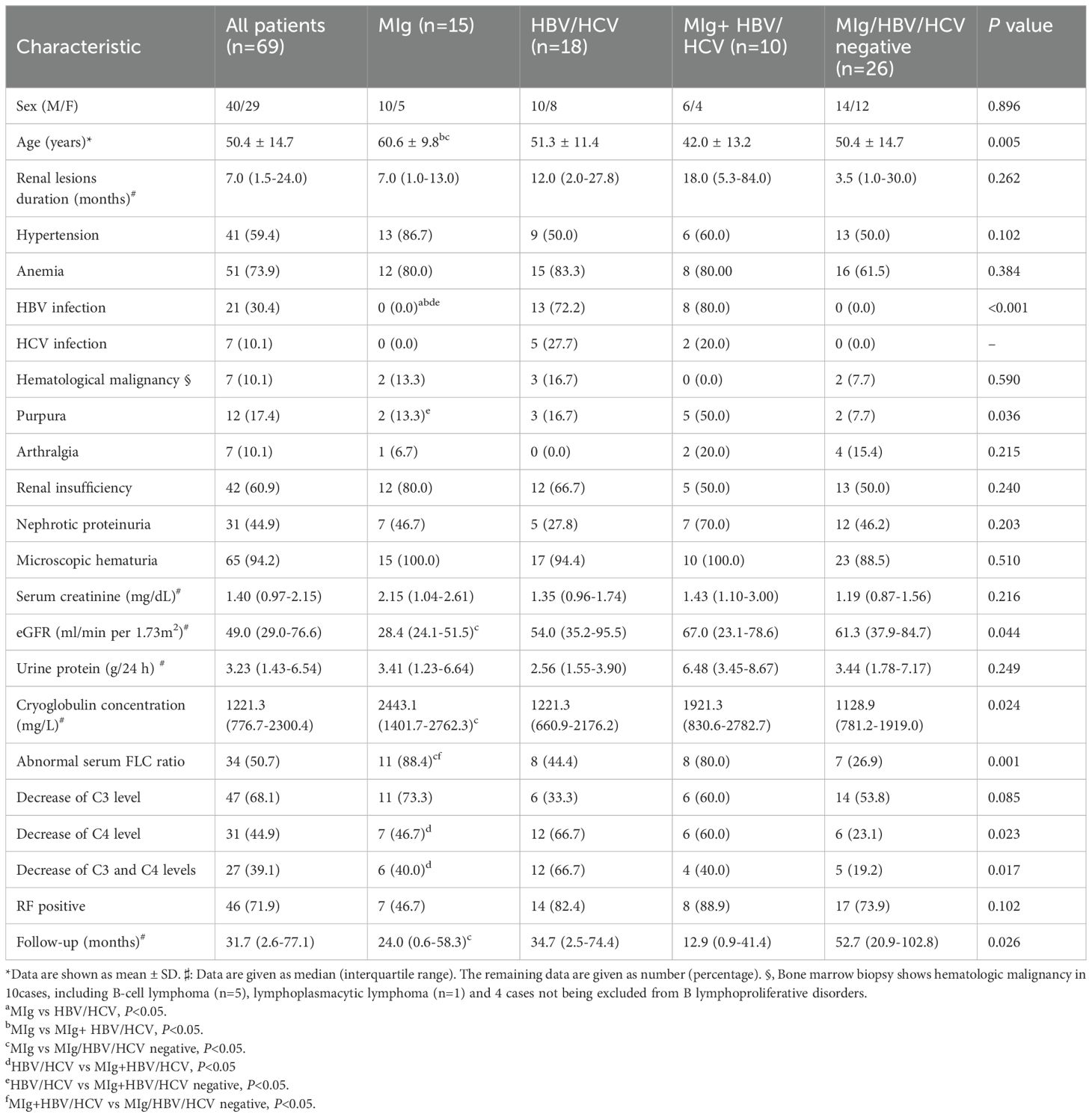
Table 1. Demographic and clinical data in Cryo-GN patients without autoimmune diseases at renal biopsy.
Serum immunofixation electrophoresis (SIFE) detection was performed in all patients, and 25 cases (35.0%) were positive, of which 15 were IgM κ (60.0%), 4 were IgG κ (16.0%), 3 were IgG λ (12.0), 2 were κ alone and 1 were λ alone. A total of 66 patients underwent the detection of serum free light chain κ and λ, of which 34 (50.7%) had an abnormal ratio. Forty-three cases underwent bone marrow biopsy after admission, and 5 cases showed hematologic malignancy including 2 diffuse large B-cell lymphoma, 2 small B-cell lymphoma, 1 lymphoplasmacytic lymphoma. Besides, 4 patients showed B-lymphoproliferative disorder and 1 Plasmacytoplastic lesions by bone marrow biopsy. Two cases were diagnosed with B cell lymphoma prior to admission (MIg/HBV/HCV negative group).
Clinical data in different groups
In this study, all patients were classified into four groups, 15 in MIg group, 18 in HBV/HCV group, 10 in MIg+HBV/HCV positive group, and 26 in MIg/HBV/HCV negative group. The clinical data of different groups are summarized in Table 1. The level of cryoglobulin and the rate of abnormal serum FLC ratio in MIg group were significantly higher than those in MIg/HBV/HCV negative group (P<0.05). Besides, the eGFR level and the renal survival time in MIg group were lower than those in MIg/HBV/HCV negative group (P<0.05).
Pathologic characteristics
Light microscopy
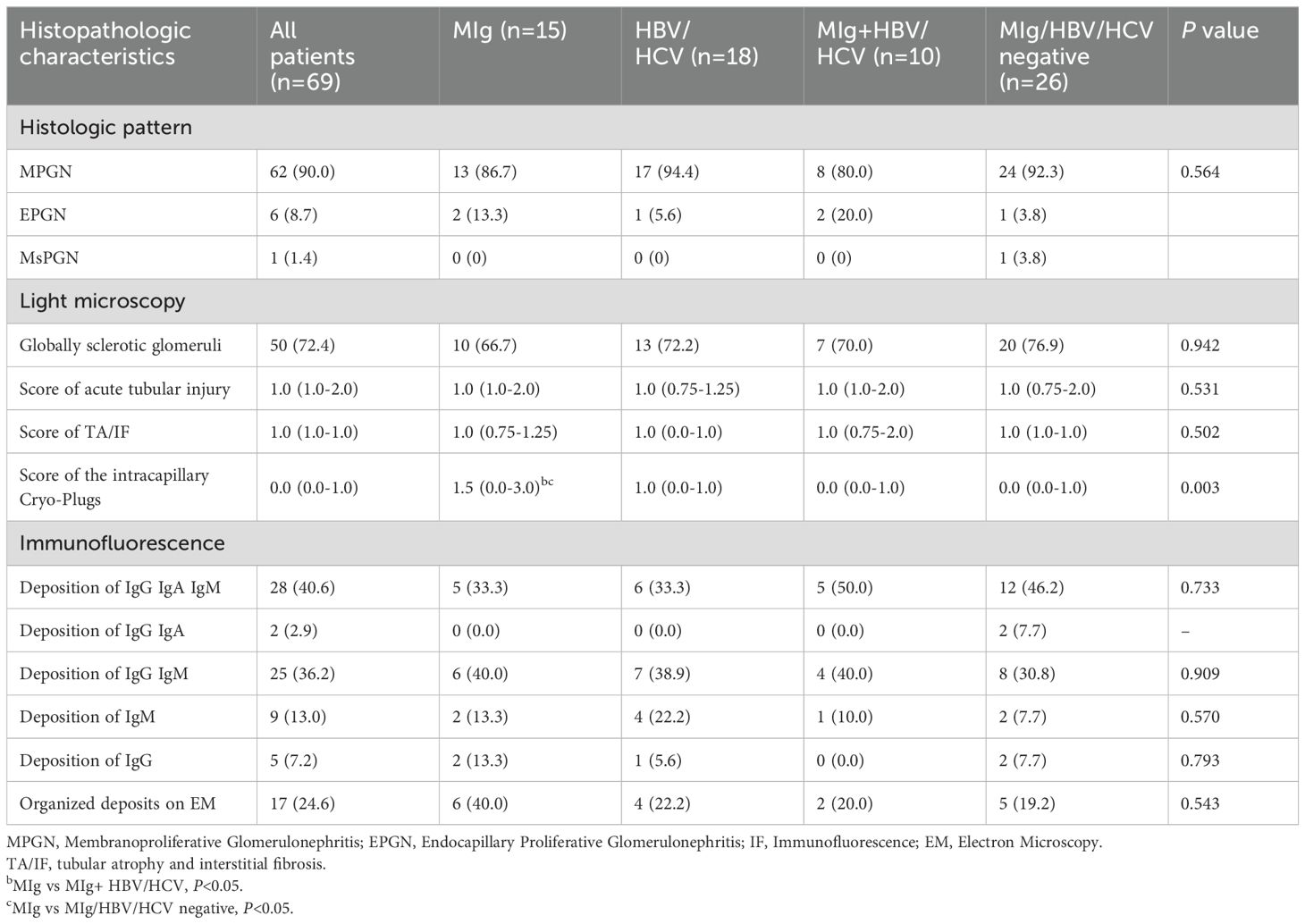
All kidney biopsy samples were available for review (Table 2). The median number of glomeruli obtained by light microscopy was 27 (20–36). Twenty-eight patients (40.6%) had glomerular crescent formation. Membranoproliferative GN (MPGN) was the predominant histologic pattern, seen in 62 (90.0%) of cases (shown in Figure 2). Strongly PAS positive and fuchsinophilic/eosinophilic intracapillary cryo-plugs were observed in 42% of patients. The serum MIg-positive group had a significantly higher score of the intracapillary cryo-Plugs than the MIg+ HBV/HCV group and the MIg/HBV/HCV negative group.
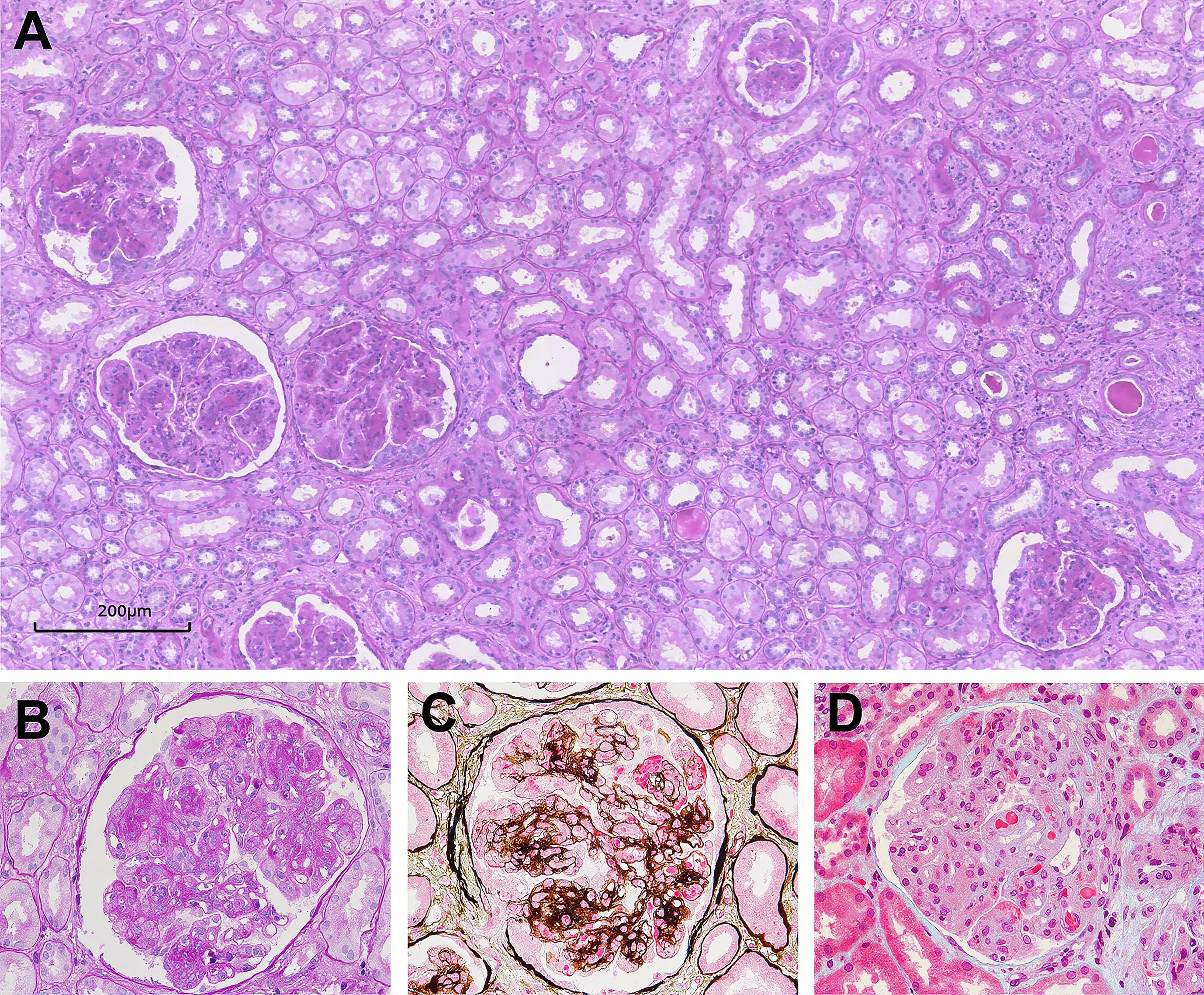
Figure 2. Glomerular lesions in Cryo-GN on light microscopy. (A–D) Light microscopy examination showing typical membranoproliferative glomerulonephritis (PAS, 100×, PAS, 400×, PASM-Masson, 400×, and Masson, 400× respectively).
Immunofluorescence
Fifty-five out of 69 cases had multiple Igs deposition in kidney biopsy samples, 5 cases had IgG deposition only, and 9 cases had IgM deposition only (shown in Supplementary Figure 1, Table 2). There is positive staining for IgG in 60 patients. Positive staining for C3 was observed in 90.0% of the cases, whereas positive staining for C1q was reported in 53.6% of the cases. In 25 patients with serum MIg (the Mlg group and Mlg+HBV/HCV group), only 4 patients exhibited monoclonal immunoglobulin deposition in renal tissue. In 44 patients with negative serum MIg (the HBV/HCV and Mlg+HBV/HCV negative groups), 9 patients showed positive staining for immunoglobulin of single heavy chain class in renal tissue, including 2 patients with monoclonal immunoglobulin deposition. Seven patients showed single IgG subtype in glomeruli, 4 had IgG3 subtype and 3 had IgG1 subtype. Two patients with IgG3 subclass had single κ light chain positivity, while the remaining patients exhibited polyclonal immunoglobulin deposition. Supplementary Table 1 shows the results of immunofluorescence in patients with serum MIg and/or single Ig or IgG subtype deposits in renal tissue.
Electron microscopy
Sixty-three patients underwent electron microscopy examination, but glomeruli were not obtained in the electron microscopy kidney tissue sections of 6 patients. Electron dense deposits were found in subendothelial, subepithelial, mesangial areas and intracapillary lumens. Organized structures were observed in 17 cases, among which 10 had curved microtubular structures with a diameter of about 10–49 nm (shown in Figure 3A), 4 had fibrils about 6–30 nm in diameter (shown in Figures 3B, C), 2 had fingerprint structures (shown in Figure 3D), and 1 had hollow lattice substructure curved at both ends with some concentric circle-like cross sections.
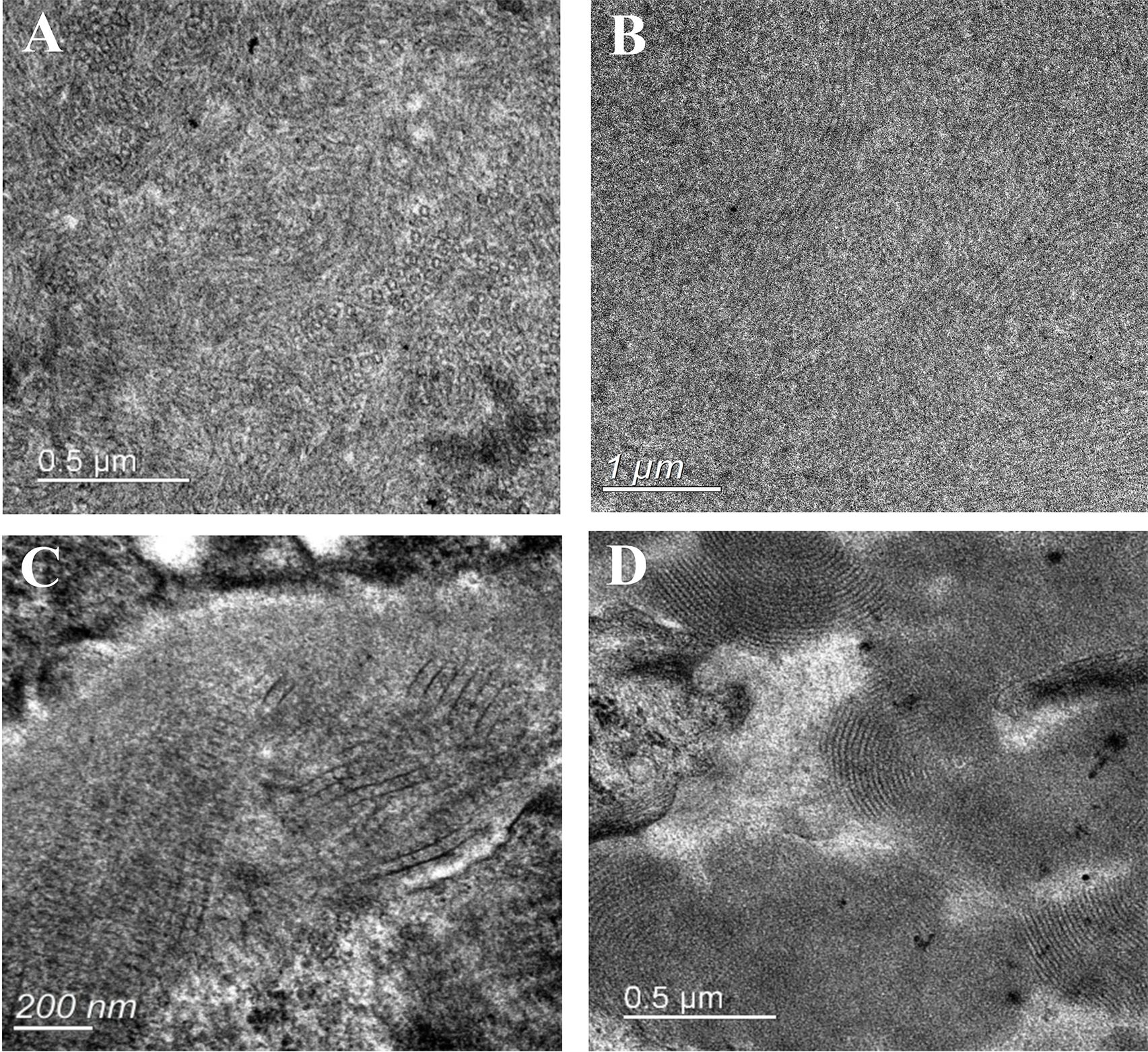
Figure 3. Electron microscopy analysis showing deposits with organized substructure. (A) Substructure of curved microtubules in 30-40nm diameter; (B) Substructure of fibers with a diameter of 14-30nm; (C) Fibers are arranged in a herringbone or grid pattern, with fiber diameters of 6-12nm; (D) Substructure of fingerprint.
Results of immunohistochemical and multiplex immunofluorescence staining
We performed immunohistochemical staining for CD68, CD3, CD4, and CD8 on 29 cases. The majority of Cryo-GN patients exhibited a significant infiltration of CD68+ cells within the glomeruli, with a median count of 38 (15–59)/glomeruli (Supplementary Figures 2A, B). There were no significant differences in the number of infiltrating macrophages within the glomeruli among the four groups (p=0.107, Highest; p=0.281, Average). Besides, only seven patients demonstrated a sparse infiltration of CD3+ cells within the glomeruli. We selected one case from each of the four groups for multiple immunofluorescence staining, and the results showed that the infiltrating cells within the glomeruli in Cryo-GN were predominantly CD68+CD163+ cells, with a small number of CD68+CD206+ cells and CD3+ cells infiltrating, and CD68+CD86+ cells were rarely observed (Figure 4).
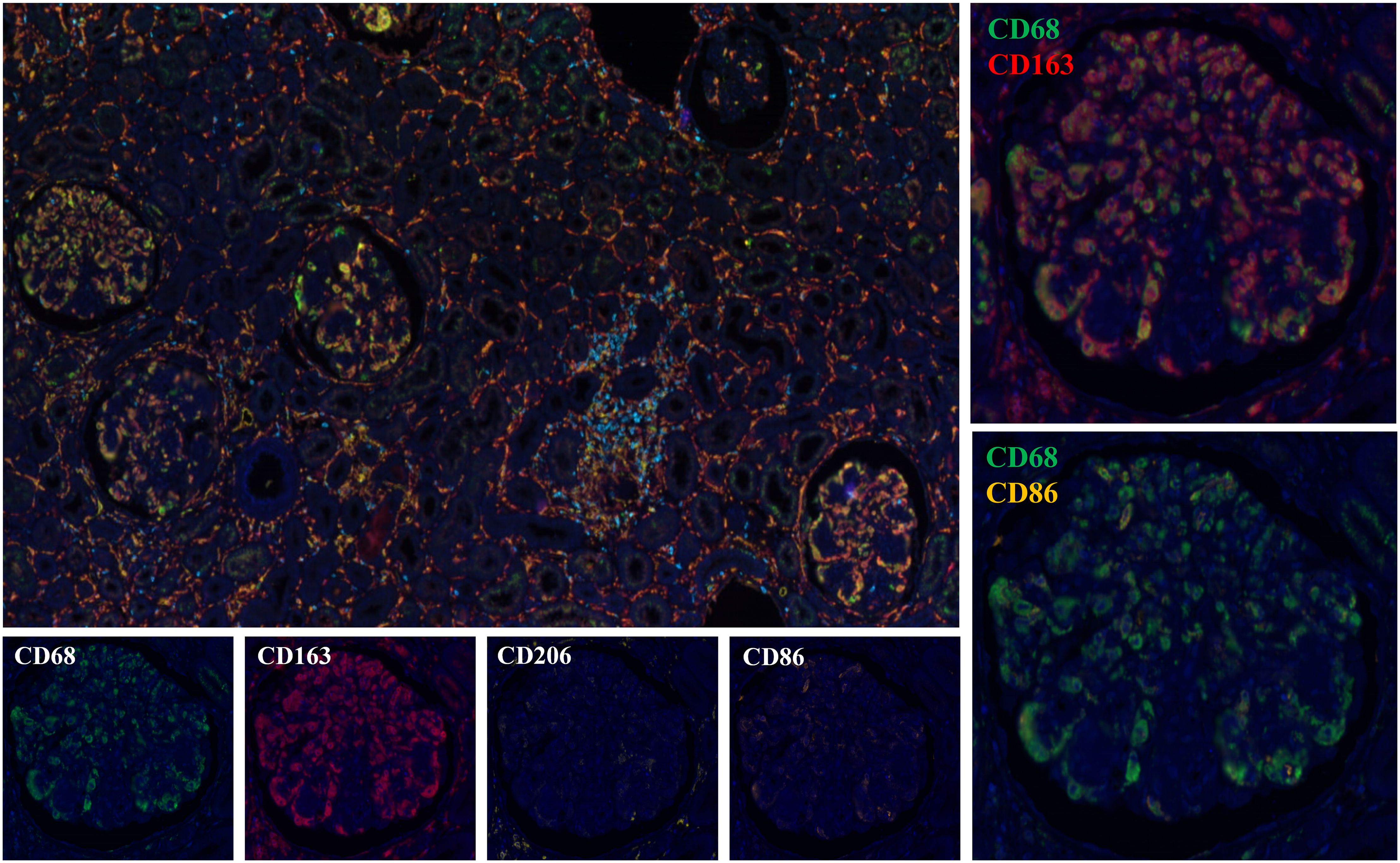
Figure 4. Multiplex immunohistochemical staining analysis results (Figures 2, 4 are from the same patient).
Treatment and renal outcome
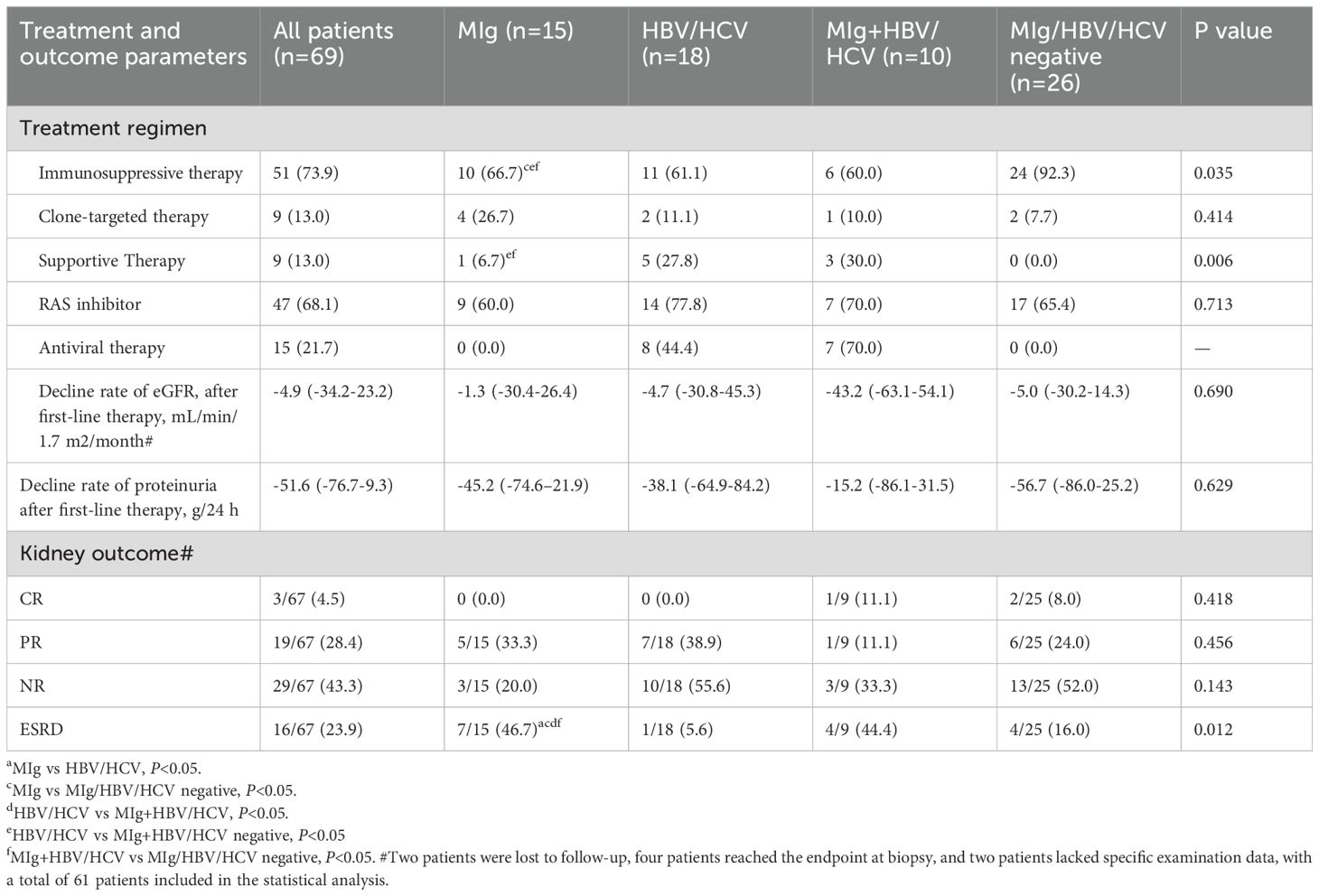
The treatment and renal outcome are shown in Table 3. Fifty-one patients (73.9%) received immunosuppressive therapy (steroids with or without cytotoxic drugs), 9 patients (13.0%) received clonal-targeted therapy (bortezomib or CD20 monoclonal antibody therapy), and 9 patients (21.7%) received only supportive therapy (including blood pressure control, sodium restriction, renin-angiotensin system (RAS) inhibitors, antiviral or antibacterial therapy when necessary). Forty-seven patients (68.1%) received RAS inhibitors, while 10 patients (14.5%) with HBV and 5 patients (7.2%) with HCV received antiviral therapy.
Two patients were lost to follow-up after renal biopsy. The remaining 67 patients had a median follow-up of 31.7 months (2.6-77.1 months). After the first-line treatment, three out of 67 patients (4.5%) achieved complete remission and 19 patients (28.4%) achieved partial remission. At the end of follow-up, 16 patients (23.9%) reached end-stage renal disease (ESRD), of whom 7 patients (46.7%) were in the MIg group. The serum MIg-positive group and the MIg+HBV/HCV negative group had a significantly worse renal prognosis than the HBV/HCV group (p=0.007)(p=0.010) and the MIg+HBV/HCV positive group (p=0.022)(p=0.023) (Figure 5A).
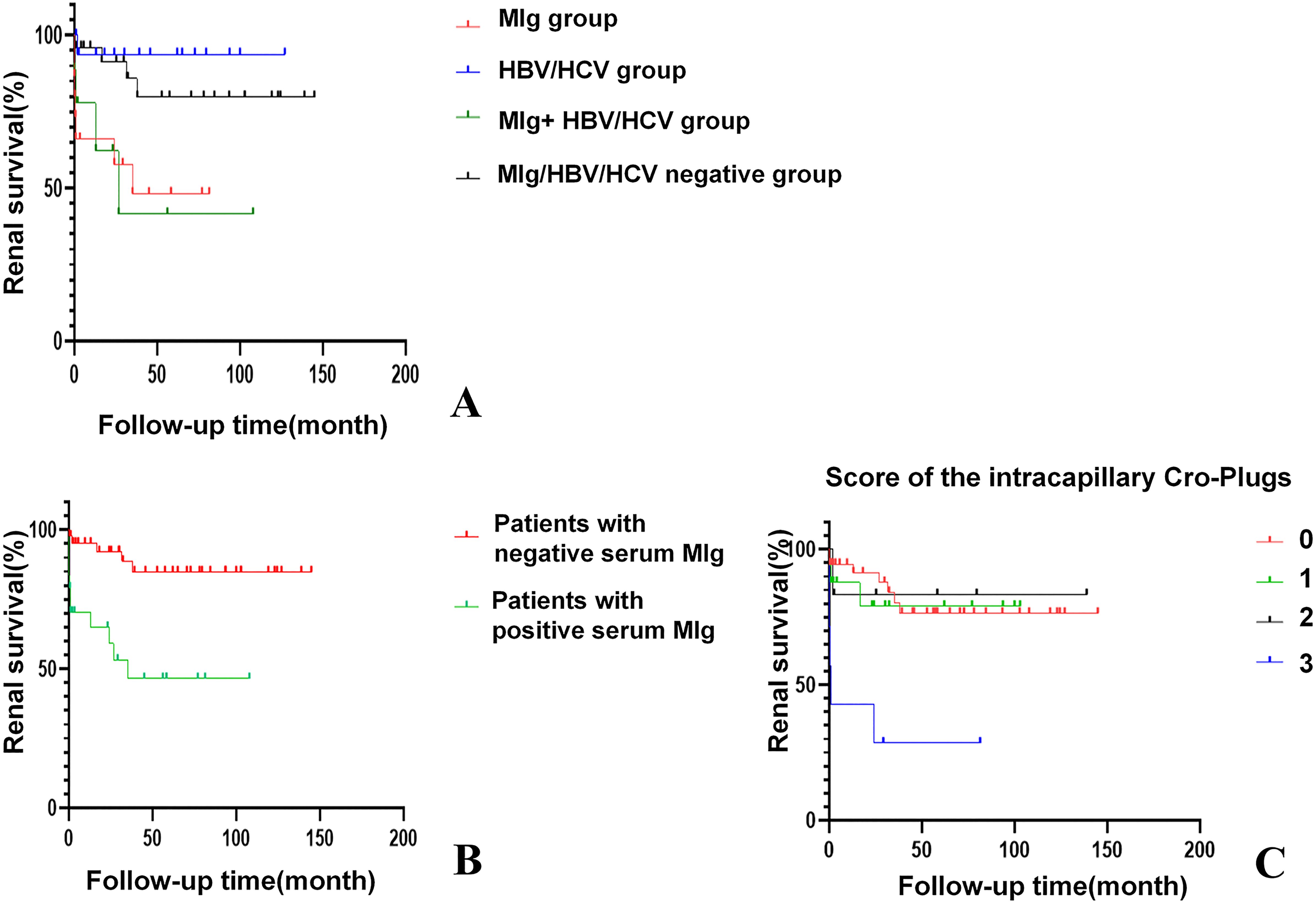
Figure 5. Kaplan-Meier curves of renal survival for Cryo-GN patients. (A) Renal survival in patients of different groups. (p=0.007); (B) Patients with positive serum MIg had worse renal outcomes. (p<0.001). (C) Patients with an intracapillary cryo-Plugs score of 3 demonstrated poorer renal outcomes. (p<0.001) [The severity of intracapillary thrombi in glomeruli was scored according to the proportion of glomeruli affected: 0 (0), <25% (1); 25-75% (2); >75% (3)].
Univariate analysis identified six factors associated with ESRD: positive serum MIg, age, eGFR, intracapillary cryo-plugs score, renal insufficiency, and acute tubular injury score. Variable selection in this study was based on univariate Cox regression, clinical significance prior assumptions, and LASSO regression. By multivariate Cox analysis, positive serum MIg and eGFR were independent factors associated with ESRD at last follow-up (Supplementary Table 2). The serum MIg-positive patients had worse renal prognosis than the MIg-negative patients (P<0.001) (Figure 5B). Patients with the severity of intracapillary cryo-Plugs score of 3 had worse renal outcomes than those with a score of 0, 1, and 2. (P<0.001) (P=0.002) (P=0.020) (Figure 5C).
Discussion
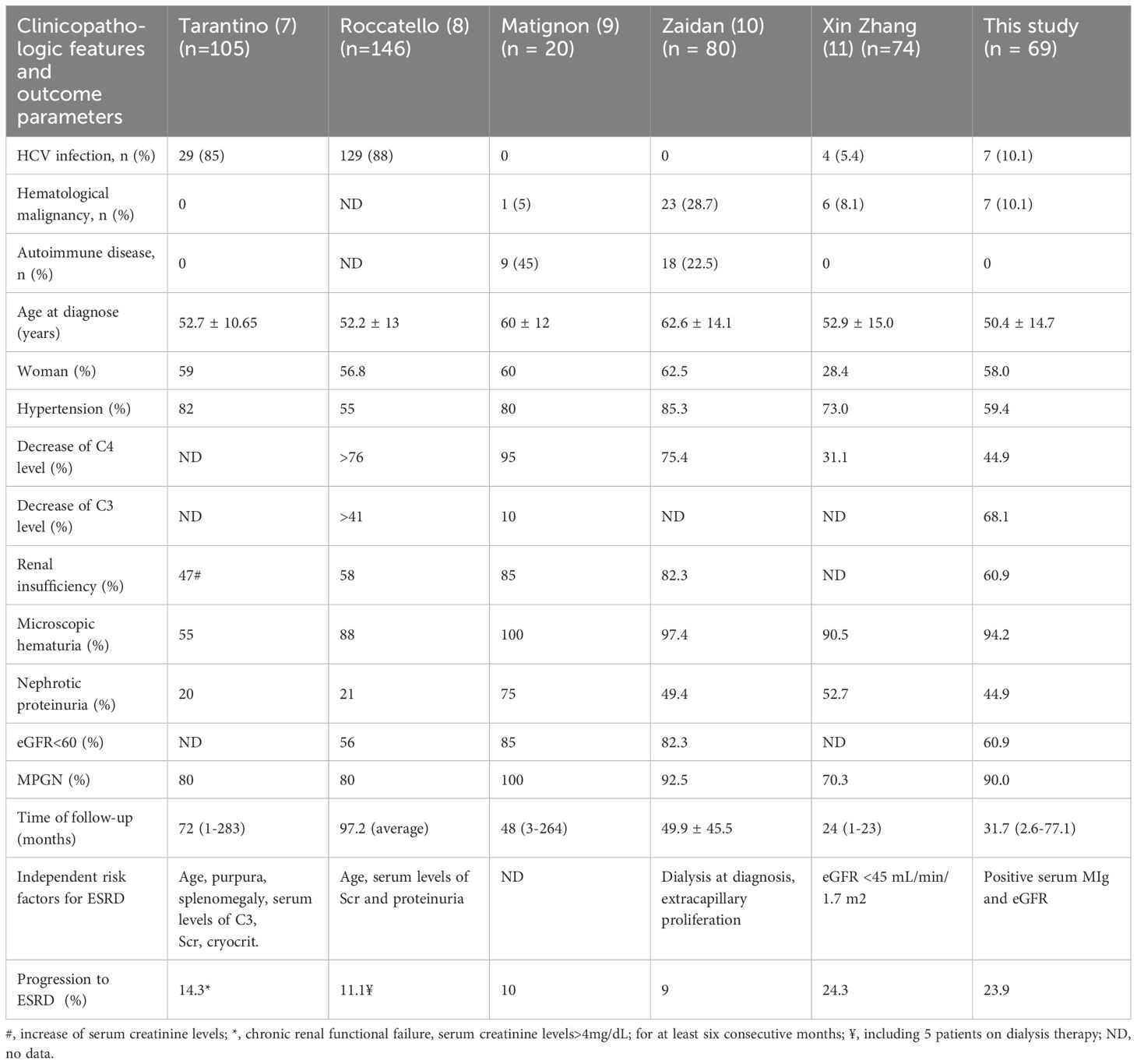
The clinical characteristics of Cryo-GN are various, mainly characterized by purpura, arthralgia and peripheral neuropathy, which are more common in mixed cryoglobulinemia (2, 4, 16, 17). Renal involvement (renal dysfunction or abnormal urinalysis) can be the only clinical manifestation of cryoglobulinemia (9, 10). Based on kidney biopsy data from our hospital, non-autoimmune diseases related Cryo-GN accounts for a low rate of native kidney biopsy (0.08%). The exceptionally low biopsy prevalence of 0.08% in our cohort starkly contrasts with the 1.2-6% reported in HCV-endemic regions like Italy and France (2, 7, 16). This discrepancy may reflect the potential underdiagnosis of atypical presentations, as most of our cases lacked classical cryoglobulinemia symptoms. These findings advocate for earlier nephrological evaluation in patients with unexplained proteinuria or hematuria.
The causes of Cryo-GN are varied, including infection, autoimmune disease and lymphoproliferative disorders. According to the previous studies, HCV infection is the main cause of mixed cryoglobulinemic vasculitis (MCV) and Cryo-GN (18–20). HBV-related MCV in Western countries is rare, and HBV-Cryo-GN has predominantly been reported in small-sample retrospective studies or case series (21–24). In China, 7.18% of the population under 60 years old carry HBsAg (25), while the incidence of HCV infection in mainland China was only 0.43% (26). Some small-sample cohort studies on HBV-Cryo-GN have been reported in China previously (11, 21, 27). In our study, HBV infection was more common than HCV infection in Cryo-GN patients, which was considered to be related to the high prevalence of HBV infection in China.
In the present study, serum MIg was found in 36.2% of Cryo-GN patients without autoimmune diseases. It is worth noting that 40% of the patients with serum MIg had HBV/HCV infection simultaneously. Moreover, the RF positivity rate was significantly higher in the HBV/HCV infection-related groups (HBV/HCV group and MIg+HBV/HCV group). This observation may reflect specific immunopathological mechanisms involving cryoglobulins of different types. Type I cryoglobulins consist of single monoclonal immunoglobulins (typically IgM or IgG) produced by B-cell proliferative disorders including MGUS, multiple myeloma, lymphoma, and Waldenström’s macroglobulinemia. Type II cryoglobulins contain monoclonal IgM with RF activity bound to polyclonal IgG, while type III comprise entirely polyclonal immunoglobulins. In HBV/HCV-associated cases, viral infection can drive monoclonal expansion of innate B cells that produce IgM with RF activity (23, 28), while RF, as a core component of cryoglobulin complexes, may further exacerbate cryoglobulin deposition (28). In addition, Ishitoku et al. found that MGUS-induced cryoglobulinemic vasculitis may occur even after HCV elimination (29). Although HBV/HCV infection may contribute to monoclonal B-cell proliferation, it’s important to recognize that serum MIg in our cohort could also derive from other lymphoproliferative disorders (30, 31). This distinction is crucial as it impacts therapeutic strategy. Therefore, comprehensive cryoglobulin typing and examination for HBsAg/HCsAg in cryoglobulins remains critical for treatment planning.
The severity of intracapillary cryo-plugs was a critical prognostic determinant in our cohort. In glomeruli, elevated protein concentration and fluctuations in anion concentration can promote cryoglobulin aggregation, leading to their deposition on glomerular membranes and subsequent formation of thrombi within glomerular capillaries (32). Besides, the capillary luminal deposits may be related to the molecular characteristics of IgM, which has a larger pentameric structure and a higher intrinsic viscosity, making it more prone to precipitation than other immunoglobulin subtypes (33). Due to the increased protein concentration caused by ultrafiltration, IgM is more likely to deposit in the glomerular capillary lumens (34, 35). By Kaplan-Meier curve, we found that patients with a large number of pseudothrombi in the glomerular capillary lumens tend to have a shorter renal survival time, indicating that the deposition of cryo-Plugs in the lumen may have a special pathogenic mechanism that further aggravates the renal burden. In clinical practice, we believe that the pathology of massive cryo-plugs in the glomerular capillary lumens should be actively paid attention to.
Interestingly, most patients with positive serum MIg showed polyclonal immunoglobulin deposition in renal tissue, while some patients with negative serum MIg had monoclonal immunoglobulin deposition detected in their renal tissue. Moreover, we validated this result by performing monoclonal antibody-specific staining on paraffin sections from the same kidney tissue. This inconsistency essentially reflects the complex immunopathology of Cryo-GN. Several mechanisms may explain this phenomenon: Firstly, serum MIg could act as type II cryoglobulin components inducing deposition of polyclonal Ig in renal tissue, masking the originally monoclonal components (2, 3, 30). Secondly, HBV/HCV infection can trigger B cell oligoclonal expansion, generating antibodies against similar variable regions in the kidneys, appearing monoclonal-like without detectable serum MIg (27, 28). Finally, the methods for detecting monoclonal immunoglobulins also have limitations. In clinical practice, a comprehensive assessment integrating serological tests (cryoglobulin typing, HCV infection), renal pathological presentations, and clinical manifestations is required, rather than relying on a single indicator.
In this study, the etiology of Cryo-GN patients negative for MIg/HBV/HCV remains incompletely defined, possibly associated with occult lymphoproliferative disorders or clonal plasma cell diseases, and these patients may develop lymphoproliferative diseases during follow-up with detectable monoclonal immunoglobulins. However, other infections (such as EBV, cytomegalovirus, or bacterial infections) or unrecognized inflammatory states may also contribute to the development of cryoglobulinemia in these patients (2, 3). While serum MIg detection typically coincides with the diagnosis of underlying hematological malignancies (e.g., lymphoma, myeloma), certain hematologic tumors may not produce monoclonal immunoglobulins, leading to false-negative serological results (36). Moreover, some studies have shown that after the diagnosis of cryoglobulinemia, there was an increased risk of developing hematologic malignancy, especially non-Hodgkin lymphoma (NHL) (37, 38). Although patients with Sjögren’s syndrome-associated cryoglobulinemic glomerulonephritis were not included in this study, clinicians should remain vigilant about the potential risk of lymphoma in patients with Sjögren’s syndrome-associated cryoglobulinemia (39). The identification of monoclonal cryoglobulins (type I or type II with monoclonal IgM-RF) mandates a thorough evaluation for underlying hematological malignancies, including serum/urine immunofixation electrophoresis, serum free light chain assay, and bone marrow biopsy. This is critical because type I cryoglobulinemia is invariably associated with clonal lymphoproliferation (e.g., WM, lymphoma), and even type II cryoglobulinemia may reflect occult B-cell dysregulation in virus-negative cases.
Under electron microscopy, ultrastructure of curved microtubules and fibrils was observed in some Cryo-GN patients, and it is significant for the differential diagnosis of Cryo-GN with fibrillary glomerulonephritis and immunotactoid glomerulopathy (40, 41). It is found that electron-dense deposits with ultrastructure were more common in patients with serum MIg or monotypic IgG deposition in kidney, which indicated that monoclonal Ig-related cryoglobulin may be more likely to form ultrastructure. Previous studies also showed monoclonal cryoglobulins were easy to form ultrastructure, often presenting with microtubule substructures, crystal/crystalloid substructures with parallel arrangement of the filaments, etc. (41, 42).
Macrophage infiltration in the glomeruli is a distinctive feature of cryoglobulinemic MPGN, which was also confirmed in this study. Guo et al. have confirmed that macrophages are essential contributors to kidney injury in murine cryoglobulinemic MPGN (43), where they observed high expression of M1 macrophage-related markers. Meanwhile, M2 macrophages with marker of CD206 were also found in the majority of glomeruli. Differently, we found less CD68+CD86+ cells in the glomeruli, a large number of CD68+CD163+ cells in the glomeruli, and a large number of CD68+CD206+ cells in the interstitium of Cryo-GN patients through multiple immunohistochemical staining. Previous studies have found that CD163+ and CD206+ cells can promote matrix proliferation and interstitial fibrosis in chronic kidney disease, and M2 macrophages are associated with the formation of crescents in IgA nephropathy and the chronicity of the disease (44, 45). We speculate that the disease has entered a chronic phase at the time of renal biopsy in our patient corhort.
Due to the complexity of Cryo-GN, the treatment of Cryo-GN is various according to associated disease. For the treatment of Cryo-GN associated with lymphoproliferative disorders, chemotherapy was adopted for hematopoietic malignancies that producing cryoglobulin or non-malignant proliferative MGRS: the plasma cell derived (monoclonal IgG) were treated with anti-plasma cell drugs such as bortezomib, and/or thalidomide, lenalidomide; lymphoplasmacytoid cell lymphoma can produce monoclonal IgM and often take the rituximab(RTX)-containing treatments (46). Antiviral therapy should be employed in patients with HBV or HCV infection-related Cryo-GN. In addition, immunosuppressants (corticosteroids, CTX, etc.) are important for the treatment of cryoglobulinaemic vasculitis. Studies have shown that RTX combined with antiviral drugs or prednisone is more effective than antiviral or corticosteroids alone, and patients can achieve higher and faster renal complete remission (47–49).
It’s reported that around 10% of the Cryo-GN patients would progress to ESRD (7–11). Previous studies on Cryo-GN showed a large variation in the rate of progression to ESRD due to different follow-up time and etiologies, and identified different independent risk factors for ESRD (Table 4). The differences in renal injury among various types of cryoglobulinemia are determined by the composition of immune complexes and the degree of complement activation: Type I shows direct monoclonal immunoglobulin deposition with minimal inflammation; Type II involves monoclonal IgM-polyclonal IgG complexes causing strong complement activation and membranoproliferative glomerulonephritis; Type III features polyclonal immune complexes producing milder mesangioproliferative lesions (2, 50, 51). By grouping the patients according to the presence or absence of serum MIg and HBV/HCV infection, we can better reveal the impact of HBV/HCV infection and serum MIg on the development and prognosis of Cryo-GN. Multivariate Cox analysis revealed that serum MIg and eGFR are independent risk factors for the renal prognosis of Cryo-GN. Coliche et al. also found that elevated cryglobulin concentration and IgG κ monoclonal components were independent predictors of renal involvement (6). This suggests that we should pay more attention to the relationship between serum MIg and Cryo-GN in clinical practice. However, we think further studies are also needed to reveal the role of serum MIg in the occurrence and development of Cryo-GN.
The study has limitations. Firstly, it is a retrospective study, so the analysis is hampered by incomplete data, short follow-up time, and unstandardized therapy. Secondly, some patients lacked IgG subtype staining due to the unavailability of kidney biopsy samples for immunofluorescence (IF) examination. Finally, the number of cases for multiplex immunohistochemical staining is relatively limited.
Conclusion
In conclusion, whether or not there is a HBV/HCV infection, patients with serum MIg in Cryo-GN have worse renal function and outcomes, and patients with a large number of pseudothrombi in the glomerular capillary lumens tend to have a shorter renal survival time; serum immunofixation electrophoresis and immunoglobulin typing in kidney samples are important for finding the cause and guiding the treatment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by The Ethics Committee of Jinling Hospital (2019NZKYKS-009-01). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
LM: Data curation, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. YX: Data curation, Resources, Writing – original draft, Writing – review & editing. YF: Data curation, Methodology, Software, Writing – review & editing. DZ: Data curation, Investigation, Methodology, Writing – original draft. XY: Data curation, Software, Validation, Writing – review & editing. YZ: Data curation, Methodology, Writing – review & editing. FY: Resources, Validation, Writing – original draft. FX: Data curation, Writing – original draft. SL: Data curation, Writing – review & editing. YW: Data curation, Writing – original draft. XZ: Data curation, Writing – review & editing. DC: Data curation, Writing – original draft. RT: Data curation, Writing – original draft. ZZ: Data curation, Writing – review & editing. DL: Supervision, Writing – review & editing. CZ: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82070793, 81800629) and the Medical Scientific Research Project of Jiangsu Provincial Health Commission (No. ZD2021018).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1578295/full#supplementary-material
References
1. Brouet JC, Clauvel JP, Danon F, Klein M, and Seligmann M. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am J Med. (1974) 57:775–88. doi: 10.1016/0002-9343(74)90852-3
2. Ramos-Casals M, Stone JH, Cid MC, and Bosch X. The cryoglobulinaemias. Lancet. (2012) 379(9813):348–60. doi: 10.1016/S01406736(11)60242-0
3. Cacoub P, Vieira M, and Saadoun D. Cryoglobulinemia - one name for two diseases. N Engl J Med. (2024) 391:15. doi: 10.1056/NEJMra2400092
4. Cacoub P, Comarmond C, Domont F, Savey L, and Saadoun D. Cryoglobulinemia vasculitis. Am J Med. (2015) 128:950–5. doi: 10.1016/j.amjmed.2015.02.017
5. Terrier B, Krastinova E, Marie I, Launay D, Lacraz A, Belenotti P, et al. Management of noninfectious mixed cryoglobulinemia vasculitis: data from 242 cases included in the CryoVas survey. Blood. (2012) 119:5996–6004. doi: 10.1182/blood-2011-12-396028
6. Coliche V, Sarda M-N, Laville M, Chapurlat R, Rheims S, Seve P, et al. Predictive factors of renal involvement in cryoglobulinaemia: a retrospective study of 153 patients. Clin Kidney J. (2018) 12:365–72. doi: 10.1093/ckj/sfy096
7. Tarantino AC, Banfi G, Confalonieri R, Confalonieri R, Bucci A, Montoli A, et al. Long-term predictors of survival in essential mixed cryoglobulinemic glomerunephritis. Kidney Int. (1995) 47:618–23. doi: 10.1038/ki.1995.78
8. Roccatello D, Fornasieri A, Giachino O, Rossi D, Beltrame A, Banf G, et al. Multicenter study on hepatitis C virus-related cryoglobulinemic glomerulonephritis. Am J Kidney Dis. (2007) 49:69–82. doi: 10.1053/j.ajkd.2006.09.015
9. Matignon M, Cacoub P, Colombat M, Saadoun D, Brocheriou I, Mougenot B, et al. Clinical and morphologic spectrum of renal involvement in patients with mixed cryoglobulinemia without evidence of hepatitis C virus infection. Med (Baltimore). (2009) 88:341–8. doi: 10.1097/MD.0b013e3181c1750f
10. Zaidan M, Terrier B, Pozdzik A, Frouget T, Rioux-Leclercq N, Combe C, et al. Spectrum and prognosis of noninfectious renal mixed cryoglobulinemic GN. J Am Soc Nephrol. (2016) 27:1213–24. doi: 10.1681/ASN.2015020114
11. Zhang X, Yu XJ, An CW, Yong Zh, Wang SX, Zhou Fd, et al. Clinicopathological spectrum of cryoglobulinemic glomerulonephritis without evidence of autoimmunity disorders: A retrospective study from a single institute of China. Kidney Dis (Basel). (2022) 8:253–63. doi: 10.1159/000522537
12. Li C, Li H, Su W, Wen Yb, Ye W, Ye Wl, et al. Clinicopathological study of mixed cryoglobulinemic glomerulonephritis secondary to hepatitis B virus infection. BMC Nephrol. (2020) 21:395. doi: 10.1186/s12882-020-02057-4
13. Leung N, Bridoux F, Batuman V, Chaidos A, Cockwell P, DAgati V, et al. The evaluation of monoclonal gammopathy of renal significance: a consensus report of the International Kidney and Monoclonal Gammopathy Research Group. Nat Rev Nephrol. (2019) 15:45–59. doi: 10.1038/s41581-018-0077-4
14. Wang Y, Chen D, Hu R, Zhang Y, Liang Dd, Xu F, et al. Clinicopathological characteristics of light and heavy chain deposition disease: A case series. Am J Kidney Dis. (2024) 84:447–56. doi: 10.1053/j.ajkd.2024.03.021
15. Rovin BH, Adler SG, Barratt J, Bridoux F, Burdge KA, Chan TM, et al. Executive summary of the KDIGO 2021 Guideline for the Management of Glomerular Diseases. Kidney Int. (2021) 100:753–9. doi: 10.1016/j.kint.2021.05.015
17. Tedeschi AB, Minola E, and Morra E. Cryoglobulinemia. Blood Rev. (2007) 21:183–200. doi: 10.1016/j.blre.2006.12.002
18. NéelA PF, Perrin F, Decaux O, Dejoie T, Tessoulin B, Halliez M, et al. Long-term outcome of monoclonal (type 1) cryoglobulinemia. Am J Hematol. (2014) 89:156–61. doi: 10.1002/ajh.23608
19. Beddhu S BS and Johnson JP. The clinical and morphologic spectrum of renal cryoglobulinemia. Med (Baltimore). (2002) 81:398–409. doi: 10.1097/00005792-200209000-00005
20. Trejo O, RAMOS-CASALS M, García-Carrasco M, Yagüe J, JIMÉNEZ S, Red GDL, et al. Cryoglobulinemia: study of etiologic factors and clinical and immunologic features in 443 patients from a single center. Med (Baltimore). (2001) 2001:80: 252–262. doi: 10.1097/00005792-200107000-00004
21. Wang C, Ye ZY, Zeng DH, Xie FL, Qu LJ, Zheng ZY, et al. Clinicopathological features of cryoglobulinemic glomerulonephritis associated with HBV infection: a retrospective analysis of 8 cases in China. Int J Clin Exp Pathol. (2015) 8:10475–81. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC4637572/.
22. Han HX, Su W, Zhou DB, Li J, and Cao XX. Hepatttts B virus-related cryoglobulinemia: Clinical characteristtcs, virological features, and treatment. Virus Res. (2023) 336:199212. doi: 10.1016/j.virusres.2023.199212
23. Terrier B, Marie I, Lacraz A, Belenotti P, Bonnet F, Chiche L, et al. Non HCV-related infectious cryoglobulinemia vasculitis: Results from the French nationwide CryoVas survey and systematic review of the literature. J Autoimmun. (2015) 65:74–81. doi: 10.1016/j.jaut.2015.08.008
24. Spatola L, Generali E, Angelini C, Badalamenti S, and Selmi C. HCV-negative mixed cryoglobulinemia and kidney involvement: in-depth review on physiopathological and histological bases. Clin Exp Med. (2018) 18:465–71. doi: 10.1007/s10238-018-0514-5
25. Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, et al. Epidemiological serosurvey of hepatitis B in China–declining HBV prevalence due to hepatitis B vaccination. Vaccine. (2009) 27:6550–7. doi: 10.1016/j.vaccine.2009.08.048
26. Cui Y and Jia J. Update on epidemiology of hepatitis B and C in China. J Gastroenterol Hepatol. (2013) 28 Suppl 1:7–10. doi: 10.1111/jgh.12220
27. Li SJ, Xu ST, Chen HP, Zhang MC, Xu F, Cheng SQ, et al. Clinical and morphologic spectrum of renal involvement in patients with HBV-associated cryoglobulinaemia. Nephrol (Carlton). (2017) 22:449–55. doi: 10.1111/nep.12795
28. Visentini M, Pascolini S, Mitrevski M, Marrapodi R, Del Padre M, Todi L, et al. Hepatitis B virus causes mixed cryoglobulinaemia by driving clonal expansion of innate B-cells producing a VH1-69-encoded antibody. Clin Exp Rheumatol. (2015) 33:000–0. Available online at: https://www.clinexprheumatol.org/abstract.asp?a=9455.
29. Ishitoku M, Yoshida Y, Matsubara T, Fujii K, Yorishima A, Oka N, et al. Cryoglobulinemic vasculitis associated with monoclonal gammopathy of undetermined significance developed after sustained virologic response of hepatitis C. Intern med. (2023) 62:1999–2004. doi: 10.2169/internalmedicine.9768-22
30. Fabrizi F, Plaisier E, Saadoun D, Martin P, Messa P, and Cacoub P. Hepatitis C virus infection, mixed cryoglobulinemia, and kidney disease. Am J Kidney Dis. (2013) 61:623–37. doi: 10.1053/j.ajkd.2012.08.040
31. Roccatello D, Saadoun D, Ramos-Casals M, Tzioufas AG, Fervenza FC, Cacoub P, et al. Cryoglobulinaemia. Nat Rev Dis Primers. (2018) 4:11. doi: 10.1038/s41572-018-0009-4
32. Kolopp Sarda MN and Miossec P. Cryoglobulinemic vasculitis: pathophysiological mechanisms and diagnosis. Curr Opin Rheumatol. (2021) 33:1–7. doi: 10.1097/BOR.0000000000000757
33. Girard LP, Soekojo CY, Ooi M, Chng WJ, and Mel Sd. Immunoglobulin M. Immunoglobulin M monoclonal gammopathies of clinical significance. Front Oncol. (2022) 12:905484. doi: 10.3389/fonc.2022.905484
34. Morel-Maroger L BA, Danon F, Verroust P, and Richet G. Pathology of the kidney in Waldenström’s macroglobu-linemia. N Engl J Med. (1970) 283:123–9. doi: 10.1056/NEJM197007162830304
35. Ma L, Liang D, Yao X, Yang X, Li S, Adelibiekeet Y, et al. Intracapillary monoclonal IgM deposits disease with massive pseudothrombi: A clinicopathologic study of 4 cases and literature review. Am J Clin Pathol. (1970) 283:123–9. doi: 10.1093/ajcp/aqae109
36. Campo E, Swerdlow S, Harris N, Pileri S, Stein H, and Jaffe E. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. (2011) 117:5019–32. doi: 10.1182/blood-2011-01-293050
37. Monti G, Pioltelli P, Saccardo F, Campanini M, Candela M, Cavallero G, et al. Incidence and characteristics of non-Hodgkin lymphomas in a multicenter case file of patients with hepatitis C virus-related symptomatic mixed cryoglobulinemias. Arch Intern Med. (2005) 165:01–5. doi: 10.1001/archinte.165.1.101
38. Saadoun D, Sellam J, Ghillani-Dalbin P, Crecel R, Piette JC, Cacoub P, et al. Increased risks of lymphoma and death among patients with non-hepatitis C virus-related mixed cryoglobulinemia. Arch Intern Med. (2006) 166:2101–8.
39. Longhino S, Chatzis LG, Dal Pozzolo R, Peretti S, Fulvio G, La Rocca G, et al. Sjögren’s syndrome: one year in review 2023. Clin Exp Rheumatol. (2023) 41:2343–56. doi: 10.55563/clinexprheumatol/255qsx
40. Motwani SS, Herlitz L, Monga D, Jhaveri KD, and Lam AQ. American society of nephrology onco-nephrology F. Paraprotein-related kidney disease: glomerular diseases associated with paraproteinemias. Clin J Am Soc Nephrol. (2016) 11:2260–72. doi: 10.2215/CJN.02980316
41. Javaugue V, Valeri AM, Sathick IJ, Said SM, and Damgard SE. The characteristics of seronegative and seropositive non-hepatitis-associated cryoglobulinemic glomerulonephritis. Kidney Int. (2022) 102:382–94. doi: 10.1016/j.kint.2022.03.030
42. Ogihara T, Saruta T, Saito I, Abe S, Ozawa Y, Kato E, et al. Finger print deposits of the kidney in pure monoclonal IgG kappa cryoglobulinemia. Clin Nephrol. (1979) 12:186–90.
43. Guo S, Wietecha TA, Hudkins KL, Kida Y, Spencer MW, Pichaiwong W, et al. Macrophages are essential contributors to kidney injury in murine cryoglobulinemic membranoproliferative glomerulonephritis. Kidney Int. (2011) 80:946–58. doi: 10.1038/ki.2011.249
44. Moll S, Angeletti A, Scapozza L, Cavalli A, Ghiggeri GM, Prunotto M, et al. Glomerular macrophages in human auto- and allo-immune nephritis. Cells. (2021) 10(3):603. doi: 10.3390/cells10030603
45. Luo L, Wang S, Hu Y, Wang L, Jiang X, Zhang J, et al. Precisely regulating M2 subtype macrophages for renal fibrosis resolution. ACS Nano. (2023) 17:22508–26. doi: 10.1021/acsnano.3c05998
46. Fermand JP, Bridoux F, Kyle RA, Kastritis E, Weiss BM, Cook MA, et al. How I treat monoclonal gammopathy of renal significance (MGRS). Blood. (2013) 122:3583–90. doi: 10.1182/blood-2013-05-495929
47. Terrier B, Saadoun D, Sene D, Sellam J, Perard L, Coppere B, et al. Efficacy and tolerability of rituximab with or without PEGylated interferon alfa-2b plus ribavirin in severe hepatitis C virus-related vasculitis: a long-term followup study of thirty-two patients. Arthritis Rheumatol. (2009) 60:2531–40. doi: 10.1002/art.24703
48. Dammacco F, Tucci FA, Lauletta G, Gatti P, Re VD, Conteduca V, et al. Pegylated interferon-alpha, ribavirin, and rituximab combined therapy of hepatitis C virus-related mixed cryoglobulinemia: a long-term study. Blood. (2010) 116:343–53. doi: 10.1182/blood-2009-10-245878
49. Saadoun D, Resche Rigon M, Sene D, Terrier B, Karras A, Perard L, et al. Rituximab plus Peg-interferon-alpha/ribavirin compared with Peg-interferon-alpha/ribavirin in hepatitis C-related mixed cryoglobulinemia. Blood. (2010) 116:326–34. doi: 10.1182/blood-2009-10-248518
50. Gorevic PD and Ghebrehiwet B. Complement activation in mixed cryoglobulinemia. Front Immunol. (2021) 12:724322. doi: 10.1016/j.dld.2021.04.021
Keywords: clinicopathological features, monoclonal immunoglobulin, cryoglobulinemic glomerulonephritis, prognostic factors, macrophage - cell
Citation: Ma L, Xia Y, Fan Y, Zhou D, Yao X, Zhong Y, Yang F, Xu F, Liang S, Wang Y, Zhu X, Chen D, Tan R, Zhu Z, Liang D and Zeng C (2025) Renal prognostic value of serum monoclonal immunoglobulin in cryoglobulinemic glomerulonephritis. Front. Immunol. 16:1578295. doi: 10.3389/fimmu.2025.1578295
Received: 17 February 2025; Accepted: 30 June 2025;
Published: 29 July 2025.
Edited by:
Titto Augustine, Indiana University, Purdue University Indianapolis, United StatesReviewed by:
Nicolas Kozakowski, Medical University of Vienna, AustriaElena Zakharova, S.P. Botkin Clinical Hospital, Russia
Hoda Mahmoud, Mansoura University, Egypt
Rajib Gupta, University of California, Davis, United States
Copyright © 2025 Ma, Xia, Fan, Zhou, Yao, Zhong, Yang, Xu, Liang, Wang, Zhu, Chen, Tan, Zhu, Liang and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dandan Liang, bGRkMTExMUBob3RtYWlsLmNvbQ==; Caihong Zeng, emVuZ2NhaWhvbmdAbmp1LmVkdS5jbg==
†These authors have contributed equally to this work
‡ORCID: Dandan Liang, orcid.org/0009-0007-3066-7112
Caihong Zeng, orcid.org/0000-0002-3816-5164
 Lei Ma
Lei Ma Yuanyuan Xia1†
Yuanyuan Xia1† Feng Xu
Feng Xu Shaoshan Liang
Shaoshan Liang Xiaodong Zhu
Xiaodong Zhu Caihong Zeng
Caihong Zeng