- Department of Pharmacy, Shanghai Public Health Clinical Center, Fudan University, Shanghai, China
Objective: The application of Traditional Chinese Medicine (TCM) in treating cancer by regulating the immune system has garnered significant attention in the academic community. However, comprehensive quantitative analyses in this field remain limited. This study aims to assess the research progress and key trends over the past decade, providing a framework for future studies.
Methods: A comprehensive literature search was conducted on the application of TCM in treating cancer by regulating the immune system from 2015 to 2025 using the Web of Science database. The search terms mainly included cancer, Traditional Chinese Medicine, immunity and so on. Data were analyzed and visualized using Origin, R software, VOSviewer, and CiteSpace.
Results: A total of 2,459 articles were included in the analysis. The number of related publications has steadily increased since 2015. China leads in publication volume and plays a crucial role in international collaboration. The Journal of Ethnopharmacology is the leading journal in this field, publishing a substantial number of highly cited studies. Key research areas include keywords such as “apoptosis,” “expression,” “inflammation,” “extract,” “in vitro,” “activation,” “antioxidant,” and “NF-kappa B,” focusing on exploring the role, mechanisms, and efficacy of TCM in modulating immune responses.
Conclusion: Research interest in TCM’s role in treating cancer through immune system regulation continues to grow, underscoring its potential in cancer therapy. Current research primarily focuses on the mechanisms by which TCM treats cancer through the modulation of immune cell functions, inhibition of tumor immune evasion, and regulation of immune-related signaling pathways. It also explores its clinical applications and the potential for enhancing the efficacy of immunotherapy.
1 Introduction
Cancer is one of the leading causes of death worldwide and has long been a focal point of medical research (1). In recent years, immunotherapy has emerged as a promising treatment modality, achieving remarkable clinical outcomes by activating the human immune system to fight tumors (2). Technologies such as immune checkpoint inhibitors (e.g., PD-1/PD-L1 inhibitors) (3)and cancer vaccines (4) have been widely adopted in the treatment of various cancers and have demonstrated great clinical potential. However, despite the success of immunotherapy in some patients, many still face challenges, including limited efficacy, drug resistance, and immune-related side effects (5). These issues have prompted the medical community to continue exploring new treatments and adjuvant therapies to optimize the effects of immunotherapy and minimize side effects.
TCM, a medical system with thousands of years of history and extensive clinical experience, has garnered increasing global attention in recent years (6). TCM has shown significant potential in enhancing immune function and reducing immunotherapy-related side effects through its unique approach of holistic regulation and syndrome differentiation. Numerous studies have indicated that TCM can support cancer immunotherapy by regulating the immune system, enhancing immune cell activity, inhibiting tumor immune escape mechanisms, and promoting tumor cell apoptosis (7). Additionally, certain components of Chinese herbal medicines have been found to enhance the efficacy of immune checkpoint inhibitors and alleviate immunotherapy-related adverse reactions (8). For example, herbal medicines such as Ganoderma lucidum, Astragalus membranaceus, and Atractylodes macrocephala have been shown to regulate immune responses, improve immune cell function, and alleviate side effects, such as immune rash and liver damage, during immunotherapy (9, 10).
Moreover, Chinese medicine plays a critical role in regulating the tumor microenvironment. Immune escape in the tumor microenvironment is one of the main challenges facing cancer immunotherapy (11). Chinese medicine can enhance the effects of immunotherapy by improving the tumor microenvironment, inhibiting angiogenesis, and regulating immune cell infiltration (12). Compounds such as tanshinone in Danshen and astragalus polysaccharides in Astragalus membranaceus not only directly target tumor cells but also promote immunotherapy efficacy by modulating immune responses and enhancing immune system function (9, 13).
Despite increasing interest in this topic, current research is primarily centered on individual herbal components or limited experimental studies, with a lack of comprehensive, systematic evaluations of the field’s development. This has created a gap in understanding the overall research landscape, key contributors, collaboration networks, and emerging research hotspots in the field of TCM-based immunotherapy for cancer. Bibliometric analysis provides a powerful and quantitative approach to address this gap (14). By statistically analyzing a large volume of scientific literature, bibliometrics can uncover knowledge structures and research dynamics in a given field. It enables researchers to identify influential authors, institutions, countries, high-frequency keywords, citation networks, and trending topics, thus helping to map the evolution of research and guide future investigations (15).
Therefore, this study aims to conduct a bibliometric analysis of research on TCM in cancer immunotherapy from 2015 to 2025. Specifically, we will (1) identify publication trends and core contributors in this field, (2) explore major research themes, mechanisms of action, and representative herbal components, and (3) analyze emerging trends and potential research directions. By addressing the existing research gap, this study seeks to provide a systematic overview of the current landscape, support the scientific integration of TCM with modern immunotherapy, and offer theoretical foundations for innovative cancer treatment strategies.
2 Materials and methods
2.1 Data collection
The data for this study were obtained from the Web of Science Core Collection (WOSCC). The search was conducted on January 15, 2025. A comprehensive search was conducted in the WOSCC database using keywords related to “Traditional Chinese Medicine,” “Immunotherapy,” and “Cancer.” A total of 12558 articles were retrieved. The search strategy was as follows:
((((TS=(“immune” OR “immunotherapy” OR “immunosuppression” OR “autoimmunity” OR “PD-1” OR “PD-L1” OR “CTLA-4” OR “CAR-T”)) AND TS=(cancer* OR tumor* OR neoplasm OR neoplasia* OR “malignant neoplasm*” OR malignanc*)) AND TS=(“Chinese medicine” OR “traditional Chinese medicine” OR TCM OR “herbal medicine” OR “botanical medicine” OR “material medicine” OR decoction OR tang OR powder OR san OR pill OR extract OR “Chinese proprietary medicine” OR “Chinese patent medicine” OR granules OR plaster OR plasters OR “oral liquid” OR “oral liquids” OR gels OR capsule OR syrup))).
Initially, 12558 articles were retrieved from the WOSCC database using keywords related to “traditional Chinese medicine,” “immunotherapy,” and “cancer,” with a time range from January 15, 2015, to January 15, 2025, and restricted to English-language literature. During the screening process, 3,483 articles were excluded for reasons such as non-compliance with literature type (e.g., conference abstracts, case reports), non-English language, and publication dates outside the specified range. A further 6616 articles were removed due to weak relevance to cancer treatment, Chinese medicine, or immunotherapy. Following these exclusions, 2459 valid articles remained for analysis. Data visualization and analysis were conducted using Origin 2018, CiteSpace(version 6.1.4), VOSviewer(version 1.6.17), and R software(version 3.6.3), focusing on institutions, countries, authors, journals, and keywords. A literature screening flowchart is provided in Annex 1.
2.2 Data analysis
To analyze the number of publications per year, Origin 2018 software was used in this study. Data visualization and the creation of scientific knowledge maps were performed using R software (version 3.6.3) with the bibliometrix package (version 4.0), VOSviewer (version 1.6.17), and CiteSpace (version 6.1.4). To ensure the accuracy and reliability of the data, data extraction and analysis were conducted independently by two authors.
VOSviewer was employed to visualize country/institution collaboration networks, source co-citation analysis, and keyword co-occurrence analysis. For the collaboration network analysis, the following parameters were set: a minimum of five publications for countries and a minimum of five publications for institutions. In the source co-citation analysis, the minimum number of citations for a source was set to 150. For the keyword co-occurrence analysis, the parameters included a minimum of 20 keyword occurrences. The following terms were excluded from the analysis: “cancer,” “cells,” “traditional Chinese medicine,” “immunomodulatory,” “mechanism,” “responses,” “immune response,” “immune,” “medicine,” “cell,” and “immune function.” These terms were already included in the search query, and their exclusion helps to better understand current research trends by avoiding redundancy and repetitive analysis.
The Impact Factor (IF) data for journals was obtained from the 2023 Journal Citation Reports (JCR) and used to assess the academic influence of the journals.
3 Results
3.1 Literature overview of TCM treats cancer by regulating the immune system
A total of 12558 articles were initially collected from the WoSCC database. After removing duplicates and retracted articles, 2459 valid articles remained. As shown in Figure 1A, the number of publications related to TCM for cancer treatment through immune system modulation has steadily increased from 2015 to 2024, indicating a growing interest in this field over the years. A notable observation is the sharp increase in the number of publications after 2020, which may be associated with the surge in healthcare demand driven by the COVID-19 pandemic. Overall, this upward trend can be attributed to the growing global interest in tumor immunotherapy, advances in immunology and omics technologies, and increasing evidence supporting the immunomodulatory effects of TCM. In addition, national policy support, clinical demand for low-toxicity adjuvant therapies, and the internationalization of TCM research have further contributed to the academic output in this field.
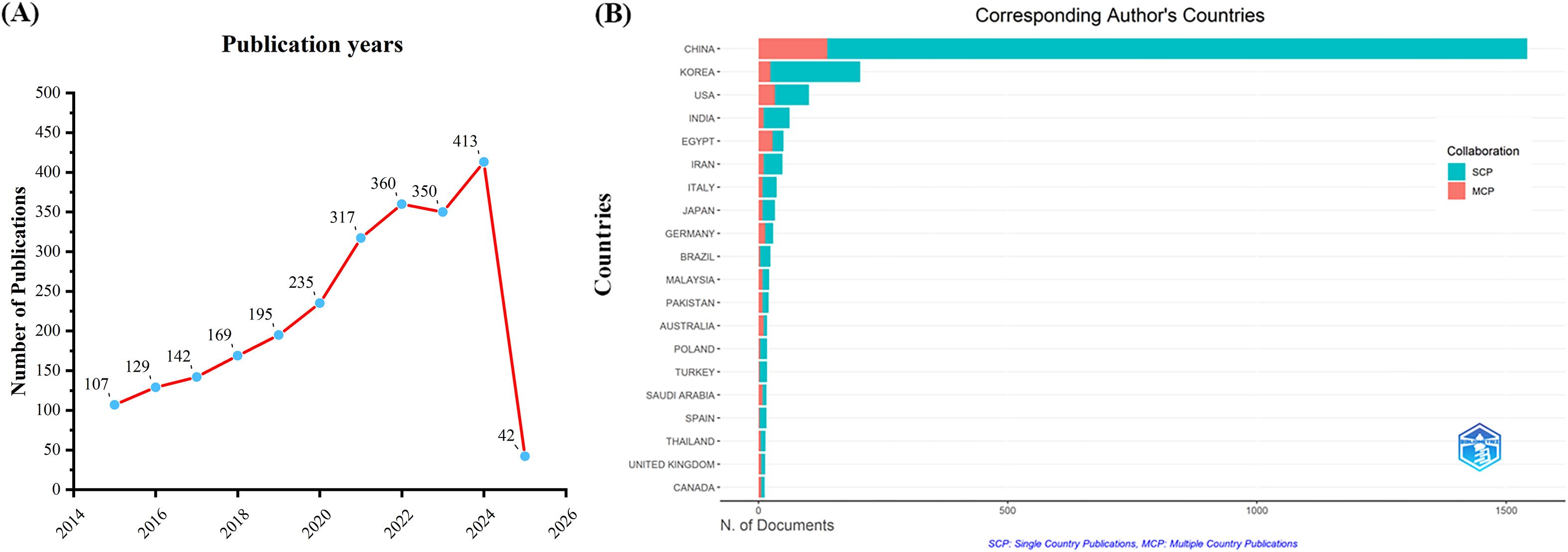
Figure 1. Trends in annual publication outputs TCM in treating cancer by regulating the immune system. (A) Trends of annual publication outputs. (B) Distribution of corresponding authors’ countries and cooperation.
Analysis of publication volume by the corresponding author’s country of origin reveals that China (n = 1,542) leads in publication output, followed by South Korea (n = 204), the United States (n = 101), India (n = 62), and Egypt (n = 50). Among the top five countries in terms of publication volume, China and South Korea exhibit relatively low cross-border publication proportions (MCPs), at 8.9% and 11.8%, respectively. These MCPs are significantly lower than those of the United States and Egypt, where the MCP ratios are 32.7% and 56.0%, respectively (Figure 1B, Table 1). This indicates that research in China and South Korea is predominantly focused on domestic publications, while the United States and Egypt demonstrate a stronger inclination toward international collaboration and transnational academic exchange.
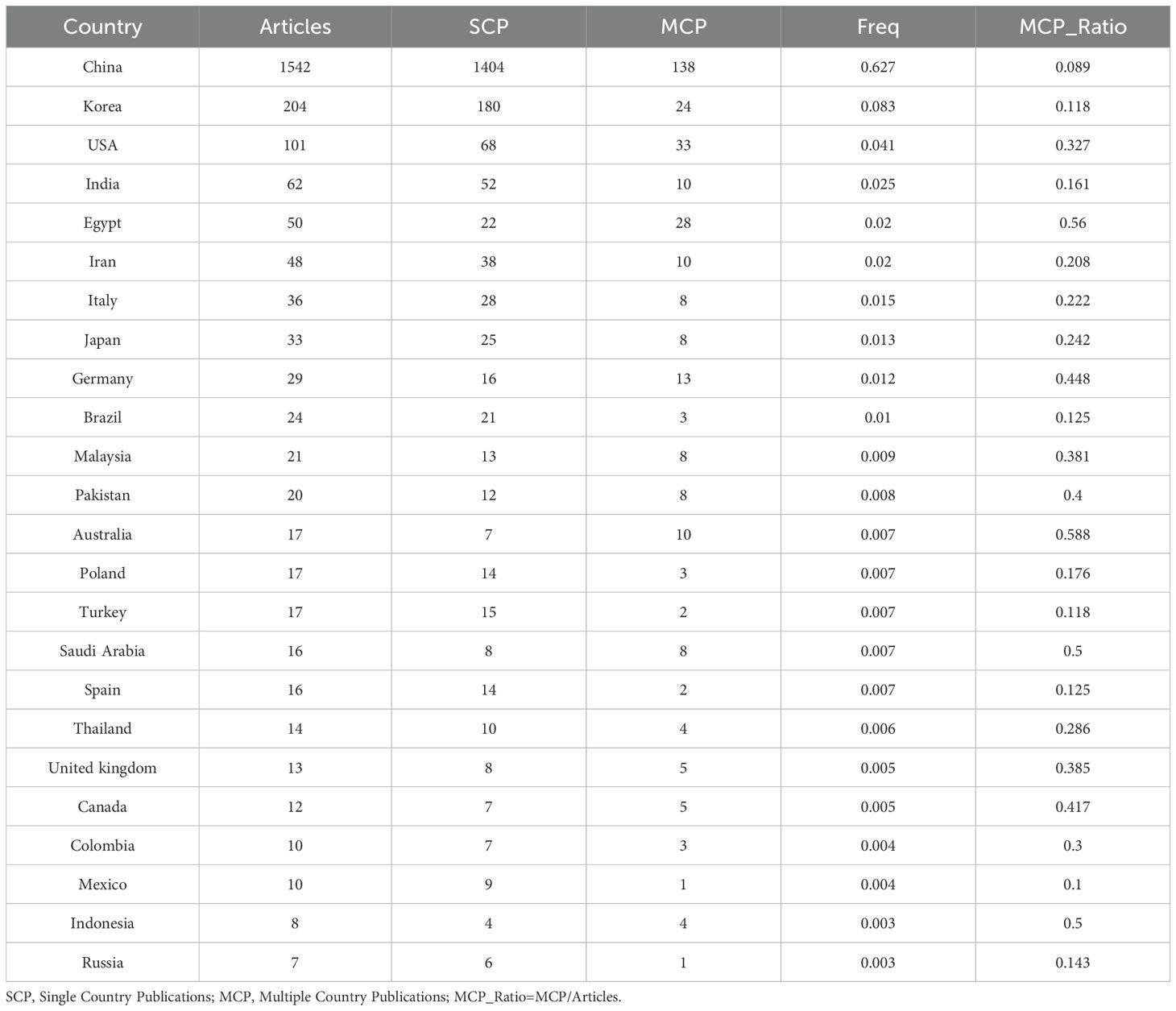
Table 1. Most relevant countries by corresponding authors of Application of TCM in treating cancer by regulating the immune system.
Further analysis of the collaboration network, depicted in Figure 2A, reveals that China (links:34) has extensive research collaborations with other countries, particularly in Asia, Europe, and North America. The visualized collaboration network highlights the Shanghai University of Traditional Chinese Medicine (n = 86) and Nanjing University of Chinese Medicin (n = 72) as key players in global research collaboration, establishing them as important centers of research collaboration (Figure 2B, Table 2). This underscores the critical role of TCM treats cancer by regulating the immune system, particularly in fostering international collaboration.
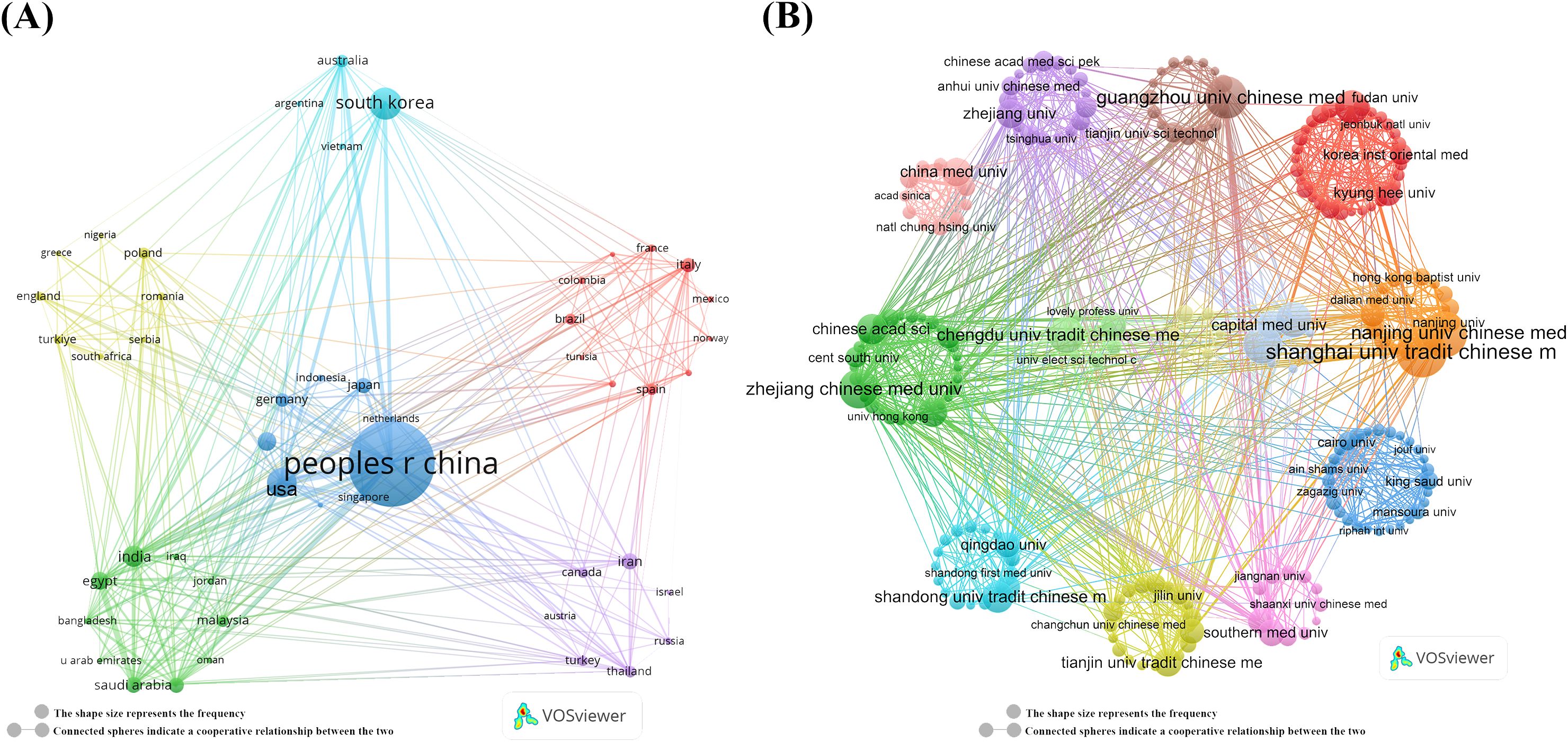
Figure 2. Map of countries/regions and institutions TCM in treating cancer by regulating the immune system. (A) Map of cooperation between different countries. (B) Map of cooperation between different institutions.
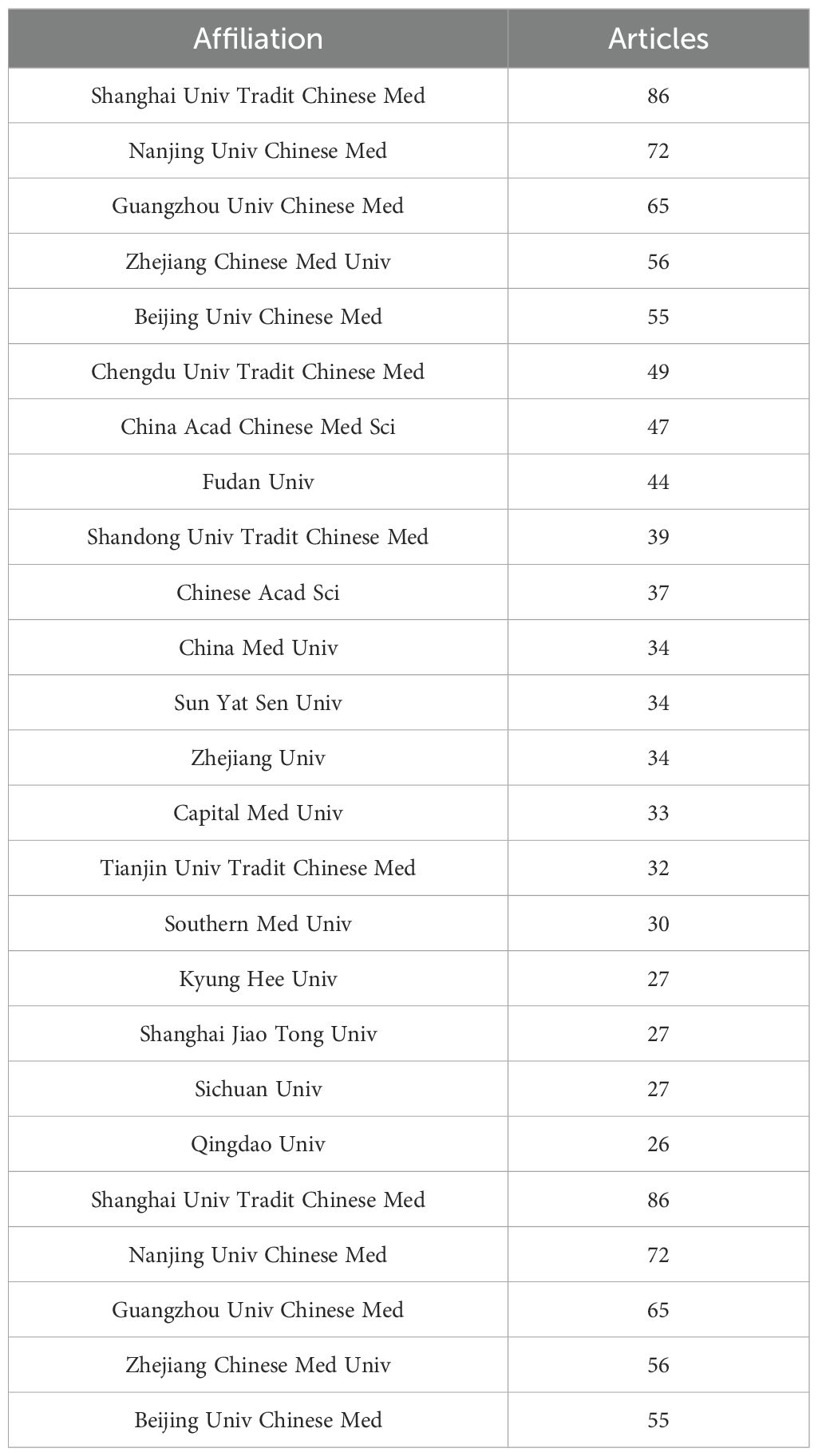
Table 2. Most relevant affiliations of Application of TCM in treating cancer by regulating the immune system.
3.2 Analysis of journals and co-citation journals
This study employed R software (version 3.6.3) with the Bibliometrix and ggplot2 packages to analyze journals in the field of TCM treats cancer by regulating the immune system. The analysis focused on identifying journals with the highest number of published articles and the most citations. Additionally, VOSviewer (version 1.6.17) was used for co-citation analysis. The results showed that 2,459 documents were distributed across 606 academic journals.
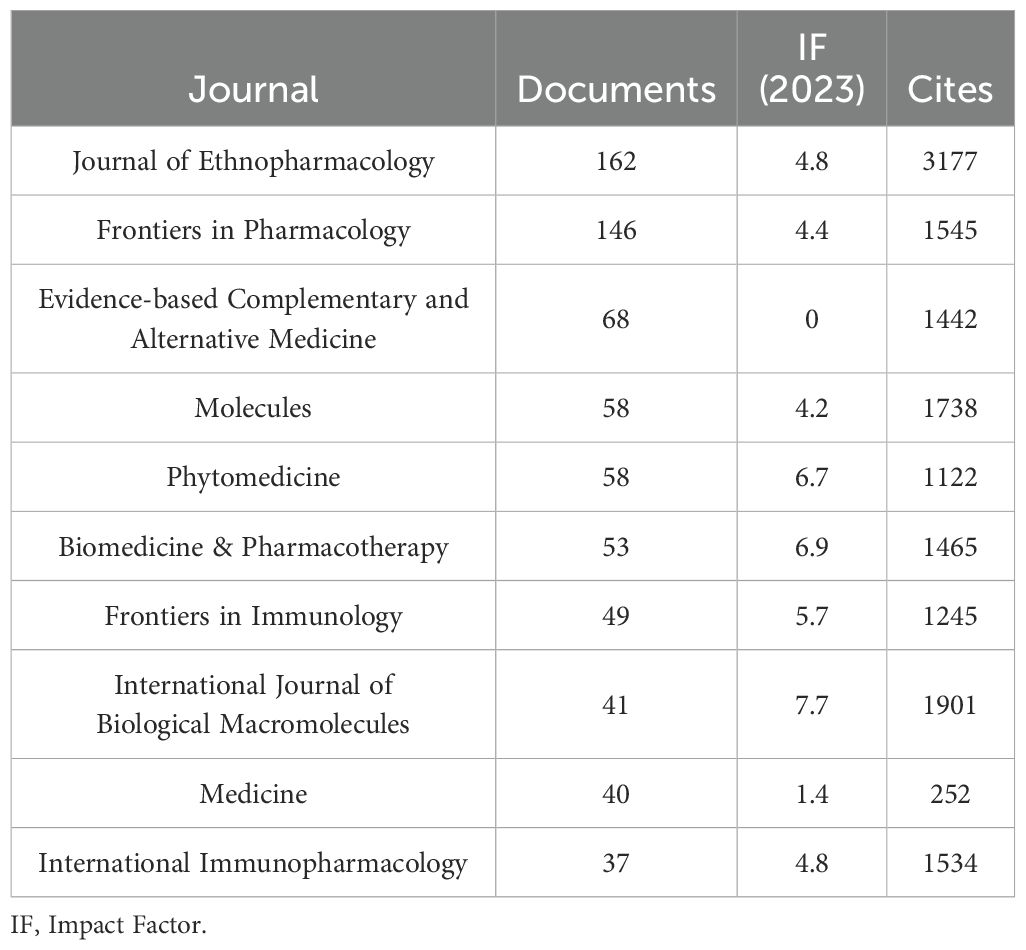
As shown in Table 3 and Figure 3A, the journal with the largest number of published articles is Journal of Ethnopharmacology (n = 162, IF = 4.8), followed by Frontiers in Pharmacology (n = 146, IF = 4.4), Evidence-Based Complementary and Alternative Medicine (n = 68, IF = 0), Molecules (n = 58, IF = 4.2), and Phytomedicine (n = 58, IF = 6.7). These journals have significant impact in the fields of TCM treats cancer by regulating the immune system, reflecting key research trends in the field.
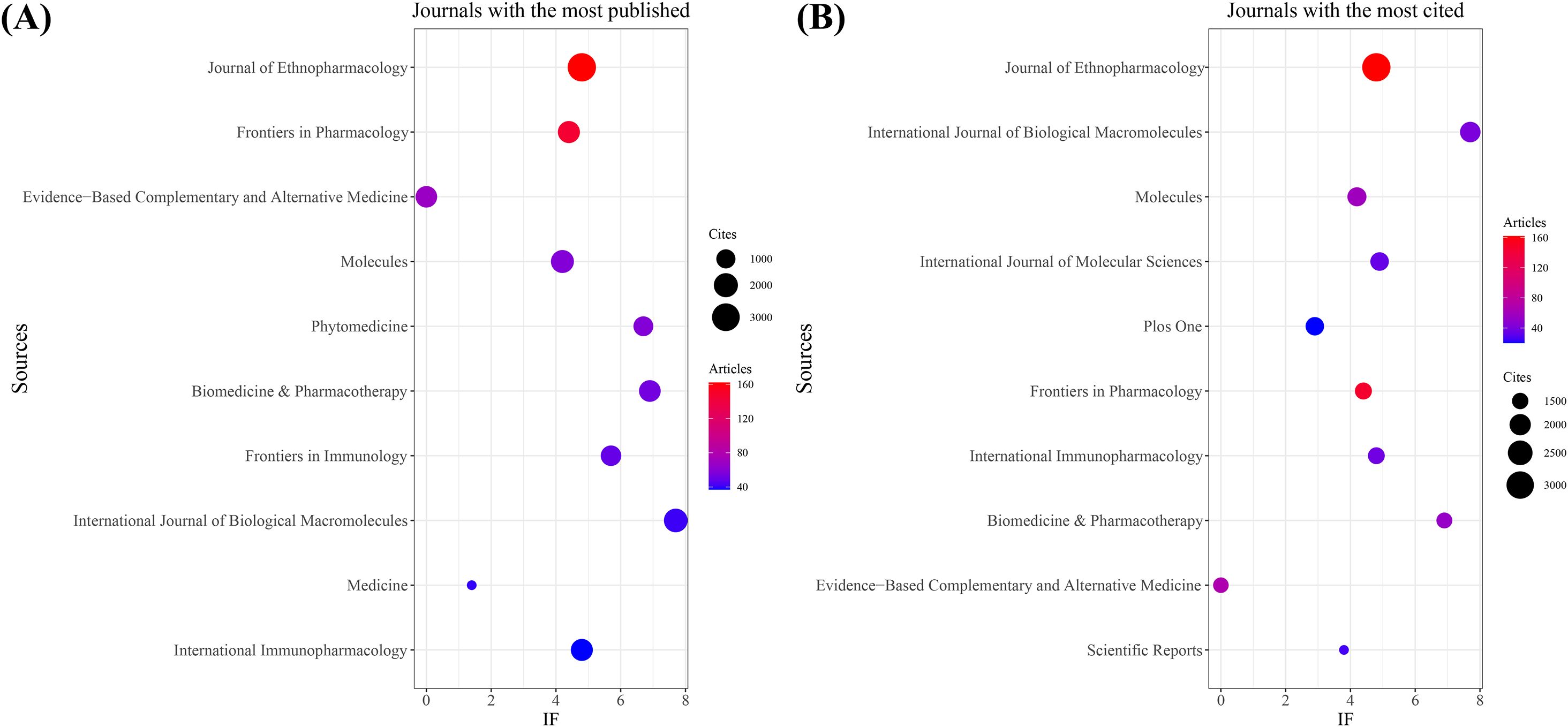
Figure 3. Journal with the largest number of articles published and the journal with the largest number of citations. (A) Journal with the largest number of articles published. (B) Journals with the largest number of citations.
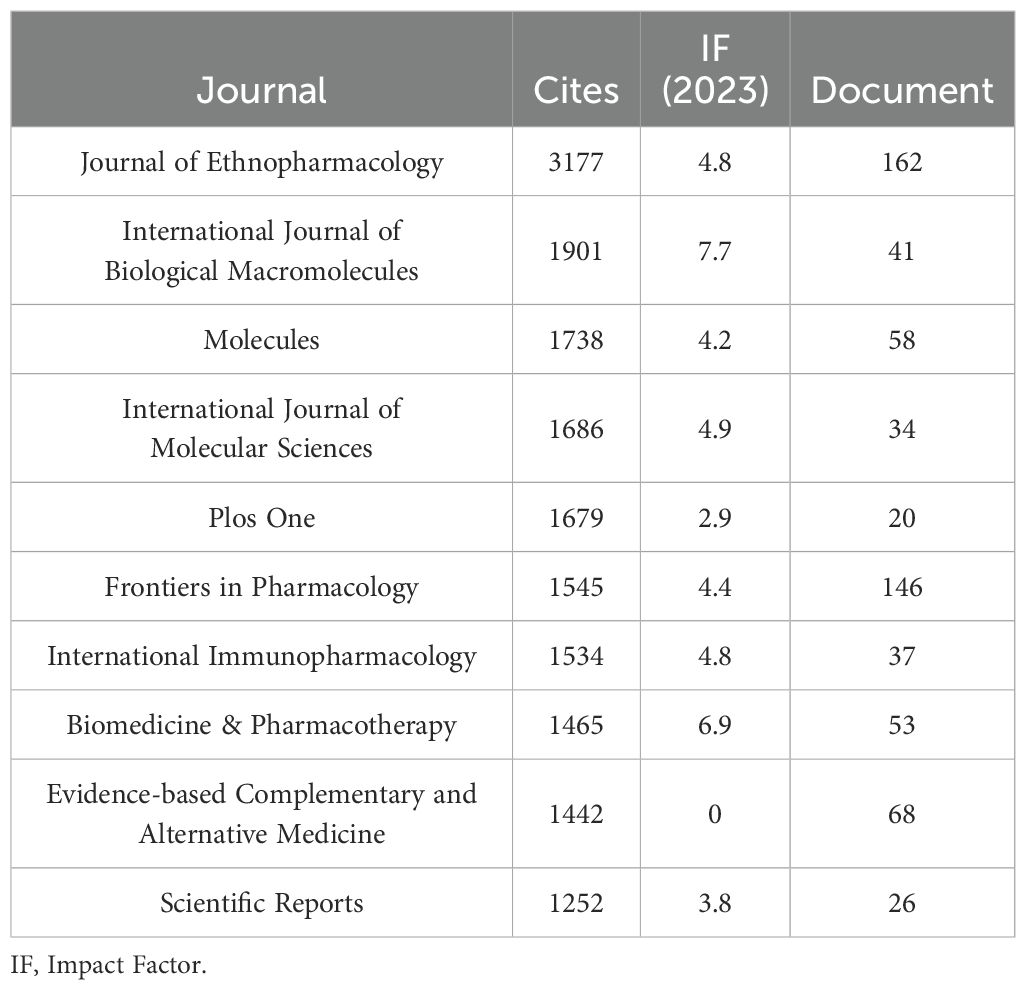
In terms of citation count, Table 4 and Figure 3B show that Journal of Ethnopharmacology ranks first (n = 3,177, IF = 4.8), followed by International Journal of Biological Macromolecules (n = 1,901, IF = 7.7), Molecules (n = 1,738, IF = 4.2), International Journal of Molecular Sciences (n = 1,686, IF = 4.9), and PLOS ONE (n = 1,679, IF = 2.9). The citation frequency of these journals highlights their academic influence and the broad dissemination of their research.
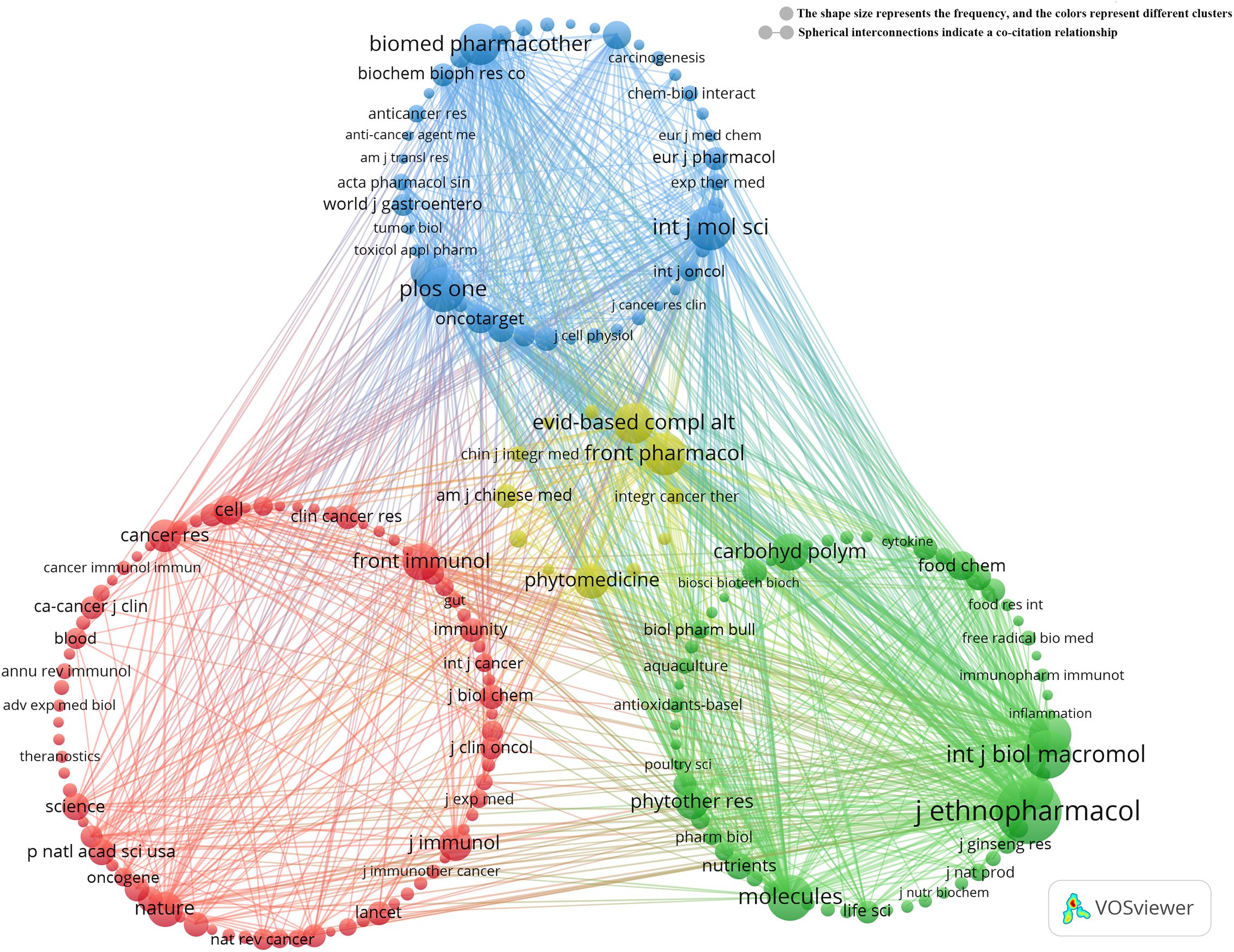
Co-citation analysis further reveals that Journal of Ethnopharmacology and Frontiers in Pharmacology serve as central hubs for research on TCM treats cancer by regulating the immune system (Figure 4). These journals, along with Molecules, lead in both publication volume and citation count. These findings suggest that Journal of Ethnopharmacology, Frontiers in Pharmacology, and Molecules are among the most representative and influential journals in this field.
However, despite the strong performance of these journals in terms of publications and citations, it is noteworthy that the concentration of research related to TCM treats cancer by regulating the immune system remains relatively low in these leading journals. This phenomenon highlights a gap in the research depth and quality in the field, indicating the need for a stronger focus on fostering deeper research, interdisciplinary collaboration, and innovative studies, particularly in the area of TCM for cancer treatment.
3.3 Citation burst
To identify citation bursts, we used CiteSpace to analyze the top 25 papers with citation bursts lasting at least two years. A total of 36 papers with significant citation bursts were identified, with the top 25 shown in Figure 5. The titles of these citations and their respective DOIs are listed in Annexes 2. The three papers with the most active citation bursts are:(1)”Cancer Statistics in China, 2015” (intensity: 10.1)(2)”Cancer Statistics, 2020” (intensity: 6.68)(3)” Astragalus membranaceus: A Review of its Protection Against Inflammation and Gastrointestinal Cancers” (intensity: 5.69).

Figure 5. Top 25 references with the strongest citation bursts on TCM in treating cancer by regulating the immune system.
Further analysis reveals that the following three articles have experienced a notable surge in citations in recent years:(1)”Ginseng Polysaccharides Alter the Gut Microbiota and Kynurenine/Tryptophan Ratio, Potentiating the Antitumor Effect of Anti-PD-1/PD-L1 Immunotherapy” (intensity: 3.83)(2)”Research Status and Molecular Mechanism of the TCM and Antitumor Therapy Combined Strategy Based on Tumor Microenvironment” (intensity: 4.08)(3)”Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries” (intensity: 5.4).
The surge in citations of these articles underscores their importance in tumor immunotherapy, the combination of TCM with antitumor therapy, and the tumor microenvironment.
Through citation burst analysis, several key research frontiers and hotspots in the field of TCM treats cancer by regulating the immune system were identified, particularly regarding its role in cancer treatment. These research directions include: (1) Clinical Research and Application of TCM in Cancer Treatment, which focuses on the use of TCM in clinical practice, especially during the perioperative period; (2) Immune and Inflammatory Response Mechanisms of TCM in Cancer Treatment, which explores how TCM can enhance cancer immunotherapy by modulating immune responses and inflammatory pathways; and (3) The Influence of Traditional Chinese Medicine on Immune Function, examining how TCM affects overall immune function in cancer therapy.
3.4 Keyword clustering and topic evolution
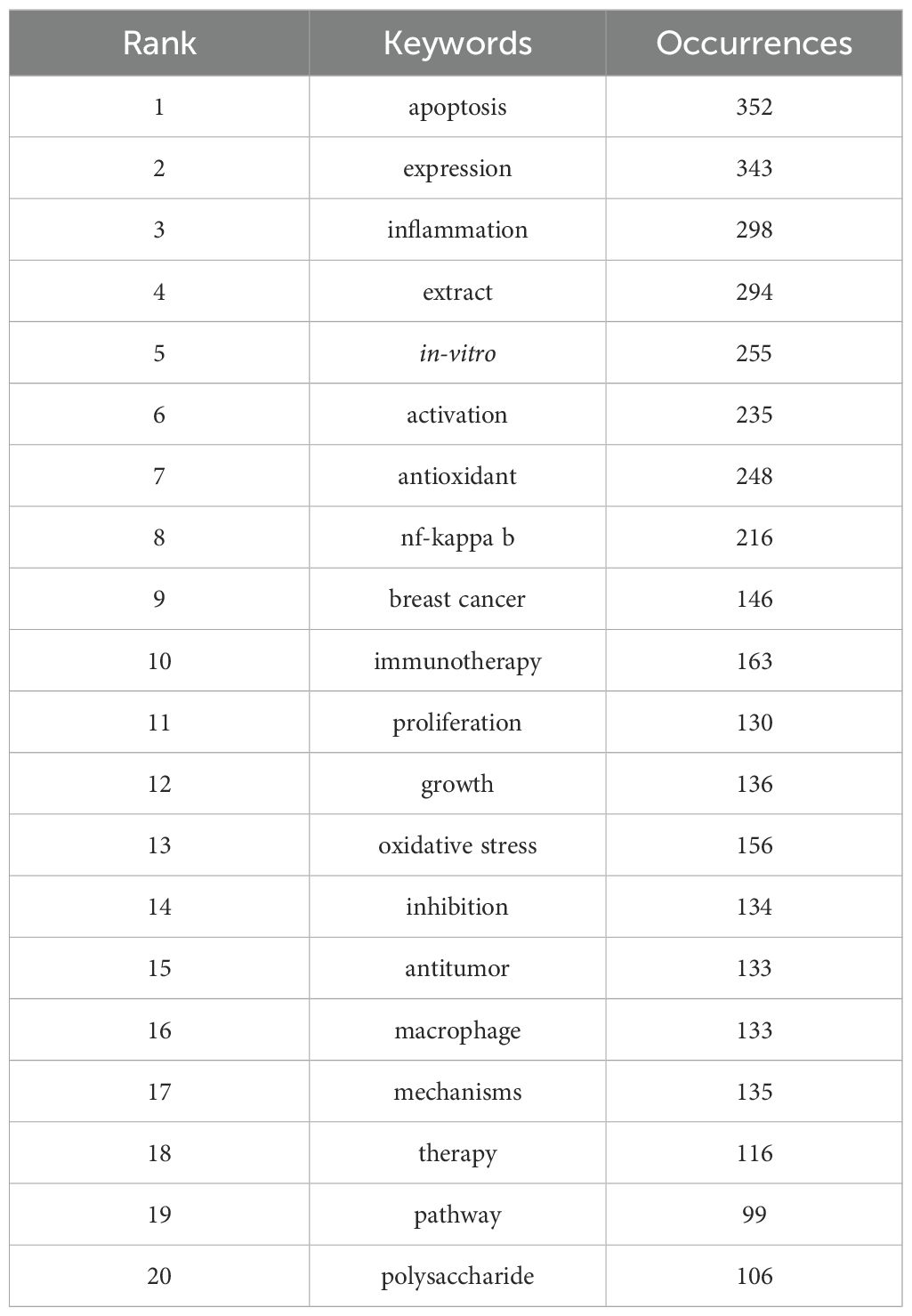
Keyword clustering analysis is crucial for identifying core topics and emerging trends in a research field. In this study, we used the VOSviewer tool to analyze 10,299 keywords and selected the top 20 keywords that appeared more than 20 times (Table 5). These keywords represent the primary research directions in the field of TCM treats cancer by regulating the immune system The most frequent keyword was “apoptosis” (n = 352), followed by “expression” (n = 343), “inflammation” (n = 298), “extract” (n = 294), “in-vitro” (n = 255), “activation” (n = 235), “antioxidant” (n = 248), and “NF-kappa B” (n = 216).Through cluster analysis, we identified five major research clusters, shown in Figure 6:
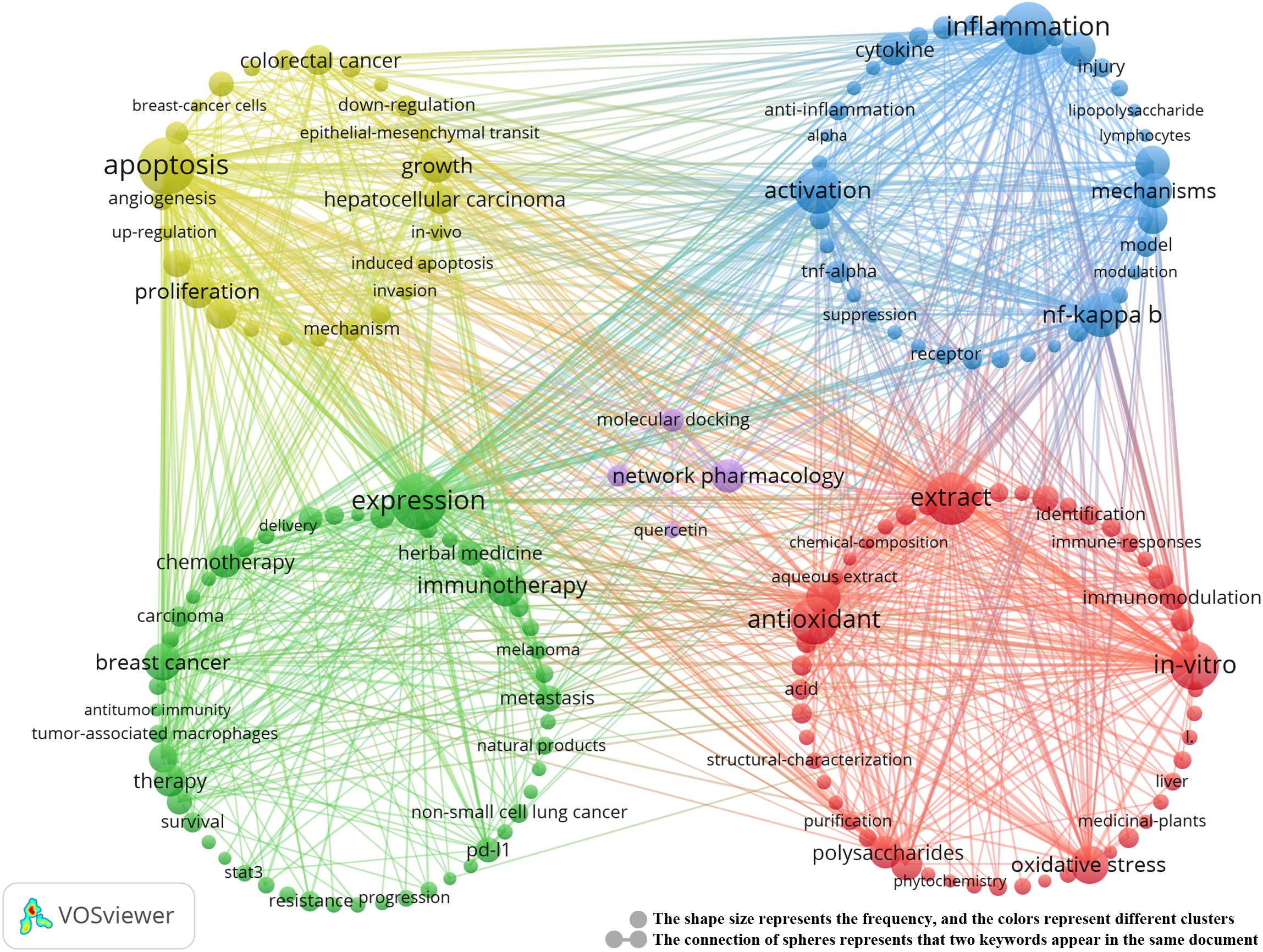
Figure 6. Keyword co-occurrence map of publications on TCM in treating cancer by regulating the immune system.
Biological Activity and Immunomodulation of TCM (red dots): (1) This cluster includes 52 keywords, such as anti-inflammatory, antioxidant, anti-tumor, flavonoids, polysaccharides, and saponins, highlighting the potential of TCM in immunomodulation and anti-tumor effects. (2) Cancer Immunotherapy and Clinical Application (yellow dots): Comprising 51 keywords, this cluster focuses on cancer immunotherapy, including specific cancers like breast and lung cancer, chemotherapy, and immune checkpoint inhibitors, reflecting the latest advances in cancer immunotherapy. (3) TCM Regulates Immune Inflammation and Immune Diseases (green dots): Containing 35 keywords, this cluster explores pathways such as cytokines, immune cells, inflammation, NF-kappa B, and TNF-alpha, emphasizing TCM’s unique role in treating immune diseases. (4) Mechanisms of Action of TCM in Cancer Cells (blue dots): With 26 keywords, this cluster includes terms like angiogenesis, apoptosis, tumor cells, cancer cells, natural products, and cancer treatment mechanisms, illustrating the complexity of TCM’s mechanisms in cancer treatment. (5) Molecular Docking and Network Pharmacology (purple dots): This cluster includes 4 keywords focusing on molecular docking, network pharmacology, and compounds such as quercetin, reflecting the growing interest in applying these techniques in TCM cancer immunotherapy. For detailed information on the keywords within each cluster, refer to Annexes 3.
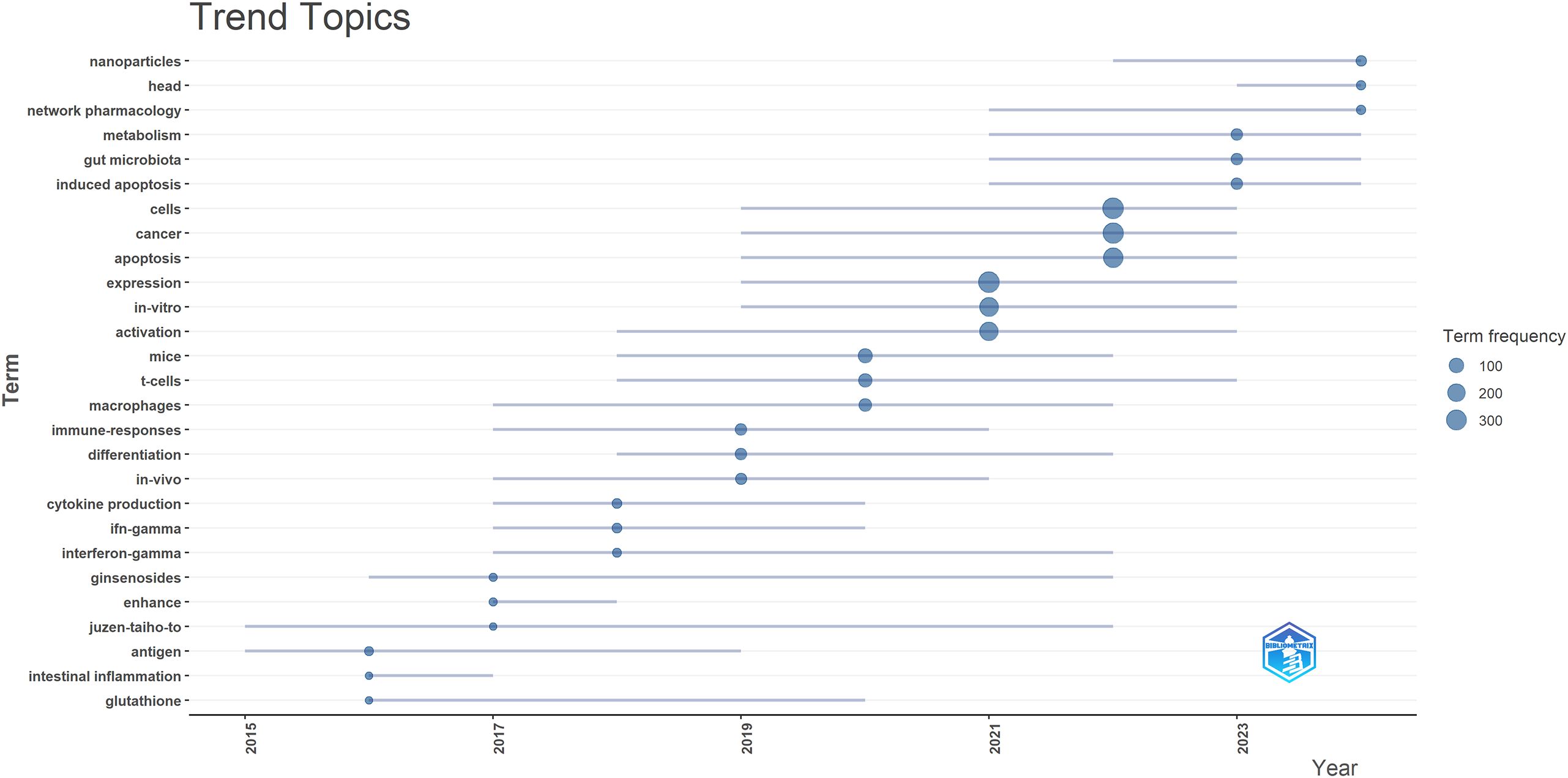
To predict future trends in this field, the Bibliometrix package in R software was used to create a topic evolution diagram (Figure 7), enabling a chronological examination of specific research topics and revealing the evolutionary trajectory of TCM in cancer treatment by regulating the immune system. The trend topic map in Figure 7 highlights key research areas, such as nanoparticles, head, and network pharmacology. The analysis identifies three main research directions in TCM treats cancer by regulating the immune system: (1) Mechanism of Action of TCM treats cancer by regulating the immune system, with ongoing research focusing on understanding how TCM influences cancer immunotherapy at a fundamental level; (2) Combination of Cancer Immunotherapy with TCM, as there is growing interest in combining TCM with conventional cancer immunotherapy in clinical practice; and (3) Application of Molecular Docking and Network Pharmacology in TCM Cancer Immunotherapy, where the use of molecular docking and network pharmacology has become a key frontier in studying TCM’s effects on cancer treatment.
4 Discussion
4.1 Overview
This study analyzed a total of 2,459 articles published between 2015 and 2025. The data revealed an overall upward trend in publications related to how TMC treats cancer by regulating the immune system.
China remains the largest contributor to research on TCM treats cancer by regulating the immune system, reflecting the country’s deep-rooted history in traditional medicine. The prominence of Chinese researchers underscores the ongoing significance of TCM in China. China and its institutions have played a leading role in this field primarily due to the historical, cultural, and policy-driven foundations of traditional Chinese medicine (TCM). TCM has been an integral part of China’s healthcare system for thousands of years, with well-established theories and practices that emphasize holistic and immune-based approaches to disease management. In recent years, the Chinese government has significantly increased investment in TCM modernization and integration with Western medicine, particularly through national strategies such as the “Healthy China 2030” initiative and inclusion of TCM in national cancer treatment guidelines. Substantial research funding, dedicated TCM research institutes, and supportive regulatory frameworks have empowered Chinese institutions to lead large-scale, interdisciplinary studies exploring the immunomodulatory role of TCM in cancer therapy. Consequently, China has emerged as a global hub for research in this domain.
Of the 2459 articles published across 606 journals, high-impact journals such as Journal of Ethnopharmacology, Frontiers in Pharmacology, and Molecules have played a pivotal role in advancing the field. The Journal of Ethnopharmacology, in particular, stood out due to its high volume of published articles and citations, reinforcing its central role in the dissemination of TCM-related research. This journal’s significant influence highlights its importance in academic discussions, particularly regarding TCM’s role in cancer immunotherapy and treatment.
4.2 Hotspots and development trends
Through a comprehensive analysis of literature clustering, keyword frequency, keyword clustering, and theme evolution, the main research hotspots and development trends of TCM treats cancer by regulating the immune system have been identified. The key research areas include the following three points:
4.2.1 TCM treats cancer by regulating the immune system in regulating immunotherapy
The mechanisms through which TCM regulates cancer immunotherapy are multifaceted, involving multiple targets and components to enhance anti-tumor immune responses. Below are the detailed mechanisms, along with specific examples:
(1) Regulation of Immune Cell Function
TCM enhances anti-tumor immunity by regulating the function of various immune cells, including T-cells, NK cells, and macrophages. For instance, Astragalus polysaccharides stimulate CD4+ and CD8+ T-cells proliferation and cytokine secretion via the JAK/STAT pathway, thereby enhancing T-cells-mediated anti-tumor responses (16, 17). Similarly, ginsenosides in Ginseng promote T-cells proliferation and improve tumor-specific activity, thereby enhancing the effectiveness of cancer immunotherapy (18). Additionally, TCM strengthens the activity of NK cells, which are crucial for directly killing tumor cells. Ganoderma lucidum polysaccharides activate NK cells through Toll-like receptor (TLR) signaling, stimulating the release of cytokines such as IFN-γ and TNF-α, which are especially beneficial in liver and lung cancer treatments (19, 20). Danshen (Salvia miltiorrhiza) also enhances NK cell cytotoxicity, particularly in breast cancer (21). Macrophages, key players in immune defense, can be activated by TCM to further enhance their anti-tumor activity (22). Lycium barbarum (Goji berries) boosts macrophage phagocytosis and cytokine secretion, while Astragalus stimulates macrophage activity by promoting pro-inflammatory cytokines like TNF-α and IL-6, improving immune responses within the tumor microenvironment, particularly in liver and lung cancer (23, 24)
(2) Inhibition of Tumor Immune Escape Mechanisms
TCM plays a crucial role in inhibiting tumor immune escape mechanisms, which often reduce the effectiveness of immunotherapy (25). Tumor cells evade immune surveillance through various immune escape strategies, and TCM helps regulate these mechanisms through multiple pathways. One key approach is the regulation of regulatory T-cells (Tregs), which suppress anti-tumor immune responses by secreting immunosuppressive cytokines such as TGF-β and IL-10 (26). TCM ingredients such as curcumin and resveratrol can alleviate the immunosuppressive effects of Tregs, thereby enhancing the immune system’s ability to fight tumors (27). For instance, curcumin, when combined with chemotherapy, has been shown to reduce Tregs-mediated immunosuppression, improving the overall efficacy of immunotherapy (28). Additionally, TCM can inhibit the production of immunosuppressive factors like TGF-β and IL-10, which are prominent in the tumor microenvironment. Herbs such as curcumin and resveratrol have been found to reduce TGF-β levels, enhancing T-cells anti-tumor activity, reducing immune escape, and further improving the efficacy of immunotherapy (29, 30). Furthermore, TCM can modulate tumor-associated macrophages (TAMs), which are key regulators of tumor immune escape. TAMs predominantly exhibit an M2 phenotype within the tumor microenvironment, characterized by the secretion of anti-inflammatory cytokines (e.g., IL-10, TGF-β), expression of immune checkpoint molecules, and promotion of regulatory T cell (Treg) recruitment. These features collectively contribute to an immunosuppressive milieu that facilitates tumor immune evasion, growth, and metastasis (31). TCM has been shown to reprogram TAMs toward the M1 phenotype, which is associated with pro-inflammatory cytokine production (e.g., IL-12, TNF-α), enhanced antigen presentation, and activation of cytotoxic T lymphocytes. This phenotypic shift disrupts the immunosuppressive microenvironment, restores anti-tumor immunity, and impairs the mechanisms by which tumor cells escape immune surveillance. For example, herbs such as Lycium barbarum and Astragalus membranaceus have been reported to promote M1 polarization of TAMs, thereby inhibiting tumor progression and enhancing immune-mediated tumor clearance (32, 33).
(3) Regulation of Immune-Related Signaling Pathways
TCM enhances immune responses and inhibits tumor growth by modulating immune-related signaling pathways, which are pivotal in tumor immune escape and immune tolerance (34, 35). TCM holds significant potential in regulating these pathways, thereby improving the efficacy of immunotherapy. For example, TCM can modulate the NF-κB pathway, which plays a critical role in both immune escape and tumor cell growth. Herbs such as Astragalus and Salvia miltiorrhiza have been shown to inhibit NF-κB activation, reduce tumor cell tolerance to immune responses, and enhance anti-tumor immunity (36, 37). Astragalus polysaccharide, an active ingredient of Astragalus, has been demonstrated to significantly inhibit NF-κB activation, enhance immune cell anti-tumor functions, reduce immune escape, and promote tumor control (38). Furthermore, TCM can regulate the JAK/STAT signaling pathway, which plays a central role in immune cell function and tumor immune escape (39). Studies have shown that compounds like curcumin and resveratrol can inhibit JAK/STAT pathway activation, reduce tumor cell evasion of immune surveillance, and enhance immune cell-mediated anti-tumor responses (40, 41). Curcumin, in particular, regulates the JAK/STAT3 pathway, inhibits tumor growth, and improves immunotherapy efficacy (42). Additionally, TCM can influence the MAPK signaling pathway, which is linked to tumor cell proliferation, survival, and immune response (43). Herbs like Lycium barbarum (wolfberry) and Ganoderma lucidum have been shown to regulate the MAPK pathway, enhancing the anti-tumor function of immune cells and inhibiting tumor cell proliferation (44, 45). For instance, Lycium barbarum significantly inhibits tumor growth and enhances immune cell activity by modulating the activity of ERK1/2 proteins in the MAPK pathway, thereby improving the overall effectiveness of immunotherapy (46).
In summary, TCM offers a multifaceted approach to cancer immunotherapy, enhancing immune cell activity, inhibiting immune escape mechanisms, and modulating key signaling pathways, thereby improving the overall effectiveness of immunotherapy.
4.2.2 Clinical application of cancer immunotherapy combined with traditional Chinese medicine
Cancer immunotherapy, particularly the use of immune checkpoint inhibitors (e.g., PD-1/PD-L1 antibodies), has significantly advanced cancer treatment by activating immune cells and enhancing their ability to target and eliminate tumor cells (47). Despite its success, challenges such as treatment resistance, immune-related side effects, and variations in treatment responses persist (48). Thus, improving the efficacy of immunotherapy, reducing adverse effects, and prolonging the duration of therapeutic responses have become critical objectives in clinical cancer treatment.
TCM, with its multi-component and multi-target approach, can complement cancer immunotherapy to address these challenges. TCM can enhance the effectiveness of immunotherapy, mitigate its side effects, and improve patients’ quality of life by regulating various immune system components (49). The synergistic effects of combining TCM with immunotherapy can lead to improved therapeutic outcomes. The following section delves into the specific ways in which TCM can enhance the efficacy of cancer immunotherapy.
(1) Improving the efficacy of immunotherapy
The success of immunotherapy relies on activating the immune system to accurately identify and eliminate tumor cells, and TCM plays a crucial role in supporting this process through multiple mechanisms. Numerous studies have shown that TCM significantly enhances the effectiveness of immunotherapy by improving T-cells function. Herbs such as Astragalus, Ganoderma lucidum, and Wolfberry promote immune responses by regulating T-cells activity and cytokine production (23, 50, 51). For instance, Astragalus polysaccharide, an active ingredient in Astragalus, activates T-cells and enhances their anti-tumor function by stimulating the secretion of immune factors such as IL-2 and IFN-γ (50). Clinical studies have demonstrated that combining Astragalus with anti-PD-1 antibodies enhances immune cell activity and strengthens anti-tumor immune responses (52). This combination therapy has shown positive results in treating liver cancer and non-small cell lung cancer (53). Natural Killer (NK) cells are essential for immune defense, as they directly recognize and kill tumor cells. TCMs, including Ganoderma lucidum and Salvia miltiorrhiza, enhance NK cell activity and, consequently, their anti-tumor effects (54, 55). The polysaccharide components in Ganoderma lucidum significantly boost NK cell activity and tumor-killing ability. When used in conjunction with immune checkpoint inhibitors, Ganoderma lucidum extracts can significantly increase NK cell activity and improve immune responses. For example, combining Ganoderma lucidum with anti-PD-1 antibodies significantly improved the efficacy of immunotherapy in lung cancer patients (56). Moreover, TCM supports the persistence and enhancement of immune responses by modulating the secretion of key immune factors. Herbs such as Wolfberry and Astragalus further stimulate immune cell activation by promoting the production of cytokines such as IL-6 and IL-2 (57, 58). These immunomodulatory properties enhance the overall effectiveness of immunotherapy by stimulating various components of the immune system. In conclusion, TCM improves immunotherapy outcomes by enhancing immune cell function, bolstering immune responses, and prolonging therapeutic effects, making it a valuable complementary approach in cancer treatment.
(2) Alleviating the Side Effects of Immunotherapy
Although cancer immunotherapy has made significant advances in therapeutic efficacy, its side effects remain a major challenge in clinical practice (5). Immune-related adverse reactions, such as immune pneumonia, hepatitis, colitis, and rashes, can occur during treatment, and some patients may even experience persistent inflammatory responses (59–62). TCM offers valuable support in alleviating these side effects through multiple mechanisms:
Immunotherapy can sometimes lead to the overactivation of immune cells, which in turn triggers immune-related adverse reactions such as immune pneumonia and hepatitis. Chinese herbs like resveratrol and astragalus possess potent anti-inflammatory and immunomodulatory properties that help mitigate these reactions. By regulating immune responses, these herbs prevent the immune system from attacking healthy tissues, thus alleviating side effects like rashes and liver toxicity. Resveratrol, a natural antioxidant, plays a critical role in inhibiting immune cell overactivation. It helps reduce immune-related liver damage and prevent immune hepatitis (63). Similarly, Astragalus supports immune regulation, reducing overactivation during immunotherapy. It protects normal tissues, enhances immune responses, and effectively controls inflammation and immune-related damage (64).
TCM also enhances the body’s tolerance to the side effects of immunotherapy by regulating the function of internal organs such as the spleen, stomach, and liver. By improving the functions of these organs, TCM optimizes the immune system and strengthens the body’s resilience during treatment. Herbs such as Astragalus and Codonopsis help improve the qi and blood functions of the spleen and stomach, boost overall immunity, and increase the body’s tolerance to the stress of immunotherapy (65). These herbs align with the TCM principle of strengthening the body’s “positive energy” to better combat external pathogens. Clinical studies have shown that Astragalus, when combined with chemotherapy or immunotherapy, can improve immune tolerance, reduce adverse reactions, and prolong the efficacy of immunotherapy (66).
Furthermore, TCM works by regulating internal organ function, balancing qi and blood, and enhancing immune function to restore overall health. This holistic approach not only boosts the effects of immunotherapy but also helps minimize its side effects. By methods such as replenishing qi and blood, regulating liver qi, and other therapeutic techniques, TCM helps patients regain strength and enhance immune function. It is effective in alleviating common immunotherapy side effects, such as fatigue, loss of appetite, and indigestion. These benefits are achieved through herbal treatments, acupuncture, and other holistic therapies, all of which improve patients’ quality of life and help them better tolerate treatment (67).
In summary, TCM plays a supportive role in cancer immunotherapy by managing and relieving side effects, improving overall health, and promoting better treatment outcomes. By combining TCM with modern immunotherapy, patients can better cope with treatment, improve their quality of life, and prolong their response time to treatment.
4.2.3 Application of traditional Chinese medicine in treating various cancers through the immune system
In recent years, TCM has garnered increasing attention for its potential role in cancer immunotherapy. TCM has shown promising results in enhancing the body’s anti-tumor immune response and improving the tumor microenvironment, thus offering new avenues for cancer treatment. For instance, in colorectal cancer, herbs like Astragalus, Codonopsis, and Ganoderma lucidum have been found to bolster immune responses by modulating T-cells activity and boosting NK cell function (54, 68, 69). Clinical studies indicate that combining these herbs with chemotherapy can enhance immune cell activity and promote tumor cell apoptosis (70). Moreover, combining TCM with immune checkpoint inhibitors such as PD-1 antibodies has proven effective in amplifying immune responses and inhibiting tumor growth (71).
In addition, TCM is increasingly being incorporated into treatment strategies for cholangiocarcinoma, a challenging bile duct cancer (72). Herbs such as Atractylodes macrocephala and Bupleurum chinense enhance immune responses by stimulating T-cells activity and inhibiting immunosuppressive cells. These herbs improve the immune microenvironment, enhance NK cell activity, and reduce tumor growth (73, 74). In breast cancer, TCM helps regulate immune responses by promoting T-cells activation, NK cell proliferation, and macrophage function, and shows promise when combined with modern immunotherapies like PD-1 inhibitors. Clinical studies have demonstrated that TCM combined with immunotherapy can significantly enhance the immune system’s ability to fight breast cancer (75, 76).
For lung cancer, one of the most lethal cancers worldwide (77), herbs like Ganoderma lucidum, bitter melon, and Codonopsis pilosula improve NK and T-cells function, modulate the immune microenvironment, and enhance immune responses (78–80). Gastric cancer, another prevalent malignancy, benefits from TCM-enhanced immunotherapy. Herbs such as Astragalus and Hedyotis diffusa have shown positive effects by boosting T-cells and NK cell activity, inhibiting tumor metastasis, and modulating the immune environment (66, 81). Lastly, ovarian cancer treatment benefits from TCM’s immunomodulatory effects, with herbs like Astragalus, Codonopsis pilosula, and Ganoderma lucidum improving immune cell function and reducing tumor recurrence during chemotherapy (82, 83).
In conclusion, TCM’s ability to boost immune function, regulate the tumor microenvironment, and address challenges like immune escape and drug resistance positions it as a valuable complementary approach to modern cancer therapies, improving outcomes for patients across a range of cancers.
4.3 Limitations and future directions
4.3.1 Limitations
(1) Data Source Limitations
This study primarily relies on the Web of Science (WoS) database, which may have overlooked relevant literature from other important databases such as PubMed, Scopus, and CNKI. While WoS plays a key role in bibliometric analysis, restricting the data source to only one database could limit the comprehensiveness of the research findings. However, the WoSCC provides comprehensive bibliometric indicators (e.g., h-index, citation counts) and structured metadata, which are essential for conducting rigorous bibliometric analyses. Its standardized format also facilitates cross-study comparisons. Limiting the data source to WoS ensures that our study can be replicated using a widely recognized and accessible database. Expanding to multiple databases may introduce variability due to indexing overlaps and inconsistencies. Including multiple databases such as Scopus and PubMed would significantly increase the complexity of data extraction and deduplication processes, potentially leading to errors. Several databases, such as PubMed, do not support the export of full records and cited references. Given the trade-off between data breadth and depth, we prioritize data depth. Moreover, in the context of medical research, there is a high degree of overlap between records indexed in WoS and those in databases like PubMed. Accordingly, we are confident that the results of our study are both methodologically sound and reliable.
(2) Language Bias
This study focused on analyzing English-language literature, which may have missed significant studies published in other languages (such as Chinese). This language bias could impact the completeness and accuracy of the research conclusions.
4.3.2 Future research directions
Future research on TCM in cancer immunotherapy should focus on the following key areas to enhance its potential application:
(1) Mechanisms of Action of Active Ingredients
Further research should delve into the specific mechanisms by which active ingredients in TCM affect immune responses, inflammation, and the intestinal microbiota. Understanding these biological effects will help improve their clinical therapeutic applications and enhance their potential for cancer treatment.
(2) Efficacy and Safety in Diverse Populations
Future studies should assess the efficacy and safety of TCM across different patient populations with varying pathological characteristics and genetic backgrounds. This will ensure that TCM treatments are not only universally applicable but also personalized to better meet individual patient needs.
(3) Long-Term Effects of TCM
It is important to evaluate the long-term effects of TCM, including disease relief, improvements in patients’ quality of life, and any potential side effects. This will help determine the effectiveness of TCM in practical, long-term cancer care.
(4) Precision Cancer Treatment
Research should move toward precision cancer treatment, aiming to address the complex biological characteristics of cancer and improve treatment efficacy. The development of more targeted therapies using TCM could significantly enhance treatment outcomes.
(5) Combination Therapies with Modern Medicine
Exploring combinations of TCM with modern medicine, particularly Western medicine, could improve treatment efficacy. This approach offers a more comprehensive strategy for cancer treatment and may unlock new synergies in therapeutic regimens.
By addressing these key areas, future research will strengthen the scientific foundation of TCM, promote its integration into modern medicine, and advance its development as a viable treatment option in cancer therapy.
5 Conclusion
This study highlights the key research hotspots and emerging trends in TCM treats cancer by regulating the immune system. The main findings include:
(1) Global Attention to TCM Immunotherapy
There is growing global interest in the application of TCM in immunotherapy, with China, the United States, South Korea, Japan, and Brazil leading research efforts. International cooperation in this field is vibrant and driving significant progress.
(2) Important Journals
The Journal of Ethnopharmacology and Frontiers in Pharmacology are leading journals in TCM-based cancer immunotherapy research. The Journal of Ethnopharmacology is particularly influential, with the highest number of citations in this area.
(3) Mechanistic Insights
Regulation of immune cell function, inhibition of tumor immune escape mechanisms, and regulation of immune - related signaling pathways.
(4) Translational Focus
Recent research increasingly integrates molecular mechanism studies with clinical investigations, reflecting a growing emphasis on evidence-based application and translational relevance.
(5) Treatment Strategies
The combination of TCM with anti-inflammatory and antioxidant properties in cancer treatment has emerged as a key strategy to enhance the efficacy of immunotherapy. At the same time, the potential of TCM in alleviating the side effects of immunotherapy has garnered widespread attention.
This study broadens the research perspective in cancer immunotherapy, offering valuable insights into future research directions and clinical applications. Based on these findings, we recommend that future research focus on integrating TCM with advanced immunological and multi-omics technologies to clarify underlying mechanisms and enhance clinical translation. High-quality clinical trials and international, interdisciplinary collaboration should be prioritized to maximize scientific and therapeutic value. For policymakers, sustained investment, supportive regulatory frameworks, and initiatives that promote global cooperation are essential to facilitate the development and integration of TCM-based immunotherapies into standardized cancer treatment protocols.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: Web of Science.
Author contributions
YL: Data curation, Software, Visualization, Writing – original draft. CC: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1581885/full#supplementary-material
References
1. Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. (2021) 127:3029–30. doi: 10.1002/cncr.v127.16
2. Liu C, Yang M, Zhang D, Chen M, Zhu D. Clinical cancer immunotherapy: Current progress and prospects. Front Immunol. (2022) 13:961805. doi: 10.3389/fimmu.2022.961805
3. Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. (2017) 8:561. doi: 10.3389/fphar.2017.00561
4. Saxena M, van der Burg SH, Melief CJ, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. (2021) 21:360–78. doi: 10.1038/s41568-021-00346-0
5. Kroschinsky F, Stölzel F, von Bonin S, Beutel G, Kochanek M, Kiehl M, et al. New drugs, new toxicities: severe side effects of modern targeted and immunotherapy of cancer and their management. Crit Care. (2017) 21:1–11. doi: 10.1186/s13054-017-1678-1
6. Tang J-L, Liu B-Y, Ma K-W. Traditional chinese medicine. Lancet. (2008) 372:1938–40. doi: 10.1016/S0140-6736(08)61354-9
7. Xiang Y, Guo Z, Zhu P, Chen J, Huang Y. Traditional Chinese medicine as a cancer treatment: Modern perspectives of ancient but advanced science. Cancer medicine. (2019) 8:1958–75. doi: 10.1002/cam4.2019.8.issue-5
8. Yu Y-X, Wang S, Liu Z-N, Zhang X, Hu Z-X, Dong H-J, et al. Traditional Chinese medicine in the era of immune checkpoint inhibitor: theory, development, and future directions. Chin medicine. (2023) 18:59. doi: 10.1186/s13020-023-00751-7
9. Li C-X, Liu Y, Zhang Y-Z, Li J-C, Lai J. Astragalus polysaccharide: a review of its immunomodulatory effect. Arch Pharmacal Res. (2022) 45:367–89. doi: 10.1007/s12272-022-01393-3
10. Li X, Rao Z, Xie Z, Qi H, Zeng N. Isolation, structure and bioactivity of polysaccharides from Atractylodes macrocephala: A review. J Ethnopharmacol. (2022) 296:115506. doi: 10.1016/j.jep.2022.115506
11. Kerkar SP, Restifo NP. Cellular constituents of immune escape within the tumor microenvironment. Cancer Res. (2012) 72:3125–30. doi: 10.1158/0008-5472.CAN-11-4094
12. Zhang Y, Lou Y, Wang J, Yu C, Shen W. Research status and molecular mechanism of the traditional Chinese medicine and antitumor therapy combined strategy based on tumor microenvironment. Front Immunol. (2021) 11:609705. doi: 10.3389/fimmu.2020.609705
13. Sun K, Wu L, Wang S, Deng W. Antitumor effects of Chinese herbal medicine compounds and their nano-formulations on regulating the immune system microenvironment. Front oncology. (2022) 12:949332. doi: 10.3389/fonc.2022.949332
14. Moral-Muñoz JA, Herrera-Viedma E, Santisteban-Espejo A, Cobo MJ. Software tools for conducting bibliometric analysis in science: An up-to-date review. Profesional la Información. (2020) 29:e290103. doi: 10.3145/epi.2020.ene.03
15. Derviş H. Bibliometric analysis using bibliometrix an R package. J scientometric Res. (2019) 8:156–60. doi: 10.5530/jscires.8.3.32
16. Han X-Y, Xu N, Yuan J-F, Wu H, Shi H-L, Yang L, et al. Total flavonoids of Astragalus inhibit activated CD4+ T cells and regulate differentiation of Th17/Th1/Treg cells in experimental autoimmune encephalomyelitis mice by JAK/STAT and NF κ B signaling pathways. Am J Chin Medicine. (2023) 51:1233–48. doi: 10.1142/S0192415X23500568
17. Yang Y, Xiao G, Cheng P, Zeng J, Liu Y. Protective application of Chinese herbal compounds and formulae in intestinal inflammation in humans and animals. Molecules. (2023) 28:6811. doi: 10.3390/molecules28196811
18. Vinh LB, Park JU, Duy LX, Nguyet NTM, Yang SY, Kim YR, et al. Ginsenosides from Korean red ginseng modulate T cell function via the regulation of NF-AT-mediated IL-2 production. Food Sci Biotechnol. (2019) 28:237–42. doi: 10.1007/s10068-018-0428-8
19. Marcus A, Gowen BG, Thompson TW, Iannello A, Ardolino M, Deng W, et al. Recognition of tumors by the innate immune system and natural killer cells. Adv Immunol. (2014) 122:91–128. doi: 10.1016/B978-0-12-800267-4.00003-1
20. Wang X, Lin Z. Immunomodulating effect of Ganoderma (Lingzhi) and possible mechanism. Ganoderma Health: Pharmacol Clin Appl. (2019) 1182:1–37. doi: 10.1007/978-981-32-9421-9_1
21. Yang C, Qian C, Zhang T, Zheng W, Zhang S, Wang S, et al. The killing effect of Tanshinol on breast cancer cells: insight into the reversion of TGF-. Front Bioscience-Landmark. (2021) 26:1106–18. doi: 10.52586/5013
22. Christofides A, Strauss L, Yeo A, Cao C, Charest A, Boussiotis VA. The complex role of tumor-infiltrating macrophages. Nat Immunol. (2022) 23:1148–56. doi: 10.1038/s41590-022-01267-2
23. Zhang X, Zhou W, Zhang Y. Immunoregulation and lycium barbarum. Lycium barbarum Hum Health. (2015) 27–44.
24. Li S, Sun Y, Huang J, Wang B, Gong Y, Fang Y, et al. Anti-tumor effects and mechanisms of Astragalus membranaceus (AM) and its specific immunopotentiation: Status and prospect. J Ethnopharmacol. (2020) 258:112797. doi: 10.1016/j.jep.2020.112797
25. Tang S, Ning Q, Yang L, Mo Z, Tang S. Mechanisms of immune escape in the cancer immune cycle. Int immunopharmacology. (2020) 86:106700. doi: 10.1016/j.intimp.2020.106700
26. Li C, Jiang P, Wei S, Xu X, Wang J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol cancer. (2020) 19:1–23. doi: 10.1186/s12943-019-1085-0
27. Zeng L, Yang T, Yang K, Yu G, Li J, Xiang W, et al. Curcumin and curcuma longa extract in the treatment of 10 types of autoimmune diseases: A systematic review and meta-analysis of 31 randomized controlled trials. Front Immunol. (2022) 13:896476. doi: 10.3389/fimmu.2022.896476
28. Tan BL, Norhaizan ME. Curcumin combination chemotherapy: The implication and efficacy in cancer. Molecules. (2019) 24:2527. doi: 10.3390/molecules24142527
29. Wang Y, Lu J, Jiang B, Guo J. The roles of curcumin in regulating the tumor immunosuppressive microenvironment. Oncol letters. (2020) 19:3059–70. doi: 10.3892/ol.2020.11437
30. Talib WH, Alsayed AR, Farhan F, Al Kury LT. Resveratrol and tumor microenvironment: Mechanistic basis and therapeutic targets. Molecules. (2020) 25:4282. doi: 10.3390/molecules25184282
31. Wang H, Yung MM, Ngan HY, Chan KK, Chan DW. The impact of the tumor microenvironment on macrophage polarization in cancer metastatic progression. Int J Mol Sci. (2021) 22:6560. doi: 10.3390/ijms22126560
32. Liu M-Q, Bao C-J, Liang X-F, Ji X-Y, Zhao L-Q, Yao A-N, et al. Specific molecular weight of Lycium barbarum polysaccharide for robust breast cancer regression by repolarizing tumor-associated macrophages. Int J Biol Macromolecules. (2024) 261:129674. doi: 10.1016/j.ijbiomac.2024.129674
33. Li C, Pan X-Y, Ma M, Zhao J, Zhao F, Lv Y-P. Astragalus polysacharin inhibits hepatocellular carcinoma-like phenotypes in a murine HCC model through repression of M2 polarization of tumour-associated macrophages. Pharmaceutical Biol. (2021) 59:1531–7. doi: 10.1080/13880209.2021.1991384
34. Prendergast G. Immune escape as a fundamental trait of cancer: focus on IDO. Oncogene. (2008) 27:3889–900. doi: 10.1038/onc.2008.35
35. Li S, Hao L, Zhang J, Deng J, Hu X. Focus on T cell exhaustion: new advances in traditional Chinese medicine in infection and cancer. Chin Medicine. (2023) 18:76. doi: 10.1186/s13020-023-00785-x
36. Li J, Xu L, Sang R, Yu Y, Ge B, Zhang X. Immunomodulatory and anti-inflammatory effects of total flavonoids of Astragalus by regulating NF-ΚB and MAPK signalling pathways in RAW 264.7 macrophages. Die Pharmazie-An Int J Pharmaceutical Sci. (2018) 73:589–93. doi: 10.1691/ph.2018.8633
37. Xu X, Lv H, Li X, Su H, Zhang X, Yang J. Danshen attenuates osteoarthritis-related cartilage degeneration through inhibition of NF-κB signaling pathway in vivo and in vitro. Biochem Cell Biol. (2017) 95:644–51. doi: 10.1139/bcb-2017-0025
38. Dong N, Li X, Xue C, Zhang L, Wang C, Xu X, et al. Astragalus polysaccharides alleviates LPS-induced inflammation via the NF-κB/MAPK signaling pathway. J Cell Physiol. (2020) 235:5525–40. doi: 10.1002/jcp.v235.7-8
39. Zhang Y, Wei Y, Jiang S, Dang Y, Yang Y, Zuo W, et al. Traditional Chinese medicine CFF-1 exerts a potent anti-tumor immunity to hinder tumor growth and metastasis in prostate cancer through EGFR/JAK1/STAT3 pathway to inhibit PD-1/PD-L1 checkpoint signaling. Phytomedicine. (2022) 99:153939. doi: 10.1016/j.phymed.2022.153939
40. Petiti J, Rosso V, Lo Iacono M, Panuzzo C, Calabrese C, Signorino E, et al. Curcumin induces apoptosis in JAK2-mutated cells by the inhibition of JAK2/STAT and mTORC1 pathways. J Cell Mol Medicine. (2019) 23:4349–57. doi: 10.1111/jcmm.2019.23.issue-6
41. Ma C, Wang Y, Dong L, Li M, Cai W. Anti-inflammatory effect of resveratrol through the suppression of NF-κB and JAK/STAT signaling pathways. Acta Biochim Biophys Sinica. (2015) 47:207–13. doi: 10.1093/abbs/gmu135
42. Golmohammadi M, Zamanian MY, Al-Ani AM, Jabbar TL, Kareem AK, Aghaei ZH, et al. Targeting STAT3 signaling pathway by curcumin and its analogues for breast cancer: A narrative review. Anim Models Exp Medicine. (2024) 7:853–67. doi: 10.1002/ame2.12491
43. Sundqvist B, Kilpinen S, Böhling T, Koljonen V, Sihto H. Activation of oncogenic and immune-response pathways is linked to disease-specific survival in Merkel cell carcinoma. Cancers. (2022) 14:3591. doi: 10.3390/cancers14153591
44. Qi J, Qi X, Chen H, Rui W. Preparation of lycium barbarum active glycopeptide and investigation of its apoptotic effects on melanoma. Anti-Cancer Agents Medicinal Chemistry-Anti-Cancer Agents). (2024) 24:132–45. doi: 10.2174/0118715206274639231103050807
45. Li G-L, Tang J-F, Tan W-L, Zhang T, Zeng D, Zhao S, et al. The anti-hepatocellular carcinoma effects of polysaccharides from Ganoderma lucidum by regulating macrophage polarization via the MAPK/NF-κB signaling pathway. Food Funct. (2023) 14:3155–68. doi: 10.1039/D2FO02191A
46. Ran L, Chen F, Zhang J, Mi J, Lu L, Yan Y, et al. Antitumor effects of pollen polysaccharides from Chinese wolfberry on DU145 cells via the PI3K/AKT pathway in vitro and in vivo. Int J Biol macromolecules. (2020) 152:1164–73. doi: 10.1016/j.ijbiomac.2019.10.206
47. Tang Q, Chen Y, Li X, Long S, Shi Y, Yu Y, et al. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front Immunol. (2022) 13:964442. doi: 10.3389/fimmu.2022.964442
48. Medler TR, Cotechini T, Coussens LM. Immune response to cancer therapy: mounting an effective antitumor response and mechanisms of resistance. Trends cancer. (2015) 1:66–75. doi: 10.1016/j.trecan.2015.07.008
49. Xie J, Huang H, Li X, Ouyang L, Wang L, Liu D, et al. The role of traditional Chinese Medicine in Cancer Immunotherapy: current status and future directions. Am J Chin medicine. (2023) 51:1627–51. doi: 10.1142/S0192415X2350074X
50. An E-K, Zhang W, Kwak M, Lee PC-W, Jin J-O. Polysaccharides from Astragalus membranaceus elicit T cell immunity by activation of human peripheral blood dendritic cells. Int J Biol Macromolecules. (2022) 223:370–7. doi: 10.1016/j.ijbiomac.2022.11.048
51. Lin Z, Sun L. Antitumor effect of Ganoderma (Lingzhi) mediated by immunological mechanism and its clinical application. Ganoderma Health: Pharmacol Clin Appl. (2019) 1182:39–77. doi: 10.1007/978-981-32-9421-9
52. Chang F-L, Tsai K-C, Lin T-Y, Yang T-W, Lo Y-N, Chen W-C, et al. Astragalus membranaceus–Derived Anti-Programmed Death-1 Monoclonal Antibodies with Immunomodulatory Therapeutic Effects against Tumors. BioMed Res Int. (2020) 2020:3415471. doi: 10.1155/2020/3415471
53. Ji H, Lou X, Jiao J, Li Y, Dai K, Jia X. Preliminary structural characterization of selenium nanoparticle composites modified by astragalus polysaccharide and the cytotoxicity mechanism on liver cancer cells. Molecules. (2023) 28:1561. doi: 10.3390/molecules28041561
54. Gao X, Homayoonfal M. Exploring the anti-cancer potential of Ganoderma lucidum polysaccharides (GLPs) and their versatile role in enhancing drug delivery systems: a multifaceted approach to combat cancer. Cancer Cell Int. (2023) 23:324. doi: 10.1186/s12935-023-03146-8
55. Song M, Qian C, Zhang T, Tang Y, Zhou Y, Wei Z, et al. Salvia mitiorrhiza Bunge aqueous extract attenuates infiltration of tumor-associated macrophages and potentiates anti-PD-L1 immunotherapy in colorectal cancer through modulating Cox2/PGE2 cascade. J Ethnopharmacol. (2023) 316:116735. doi: 10.1016/j.jep.2023.116735
56. Li W, Zhou Q, Lv B, Li N, Bian X, Chen L, et al. Ganoderma lucidum polysaccharide supplementation significantly activates T-cell-mediated antitumor immunity and enhances anti-PD-1 immunotherapy efficacy in colorectal cancer. J Agric Food Chem. (2024) 72:12072–82. doi: 10.1021/acs.jafc.3c08385
57. Xiao Z, Deng Q, Zhou W, Zhang Y. Immune activities of polysaccharides isolated from Lycium barbarum L. What do we know so far? Pharmacol Ther. (2022) 229:107921. doi: 10.1016/j.pharmthera.2021.107921
58. Cheng J, Zhou ZW, Sheng HP, He LJ, Fan XW, He ZX, et al. An evidence-based update on the pharmacological activities and possible molecular targets of Lycium barbarum polysaccharides. Drug Des Devel Ther. (2014) 9:33–78. doi: 10.2147/DDDT.S72892
59. Su Q, Zhu EC, Wu J-B, Li T, Hou Y-L, Wang D-Y, et al. Risk of pneumonitis and pneumonia associated with immune checkpoint inhibitors for solid tumors: a systematic review and meta-analysis. Front Immunol. (2019) 10:108. doi: 10.3389/fimmu.2019.00108
60. Hercun J, Vincent C, Bilodeau M, Lapierre P. Immune-mediated hepatitis during immune checkpoint inhibitor cancer immunotherapy: lessons from autoimmune hepatitis and liver immunology. Front Immunol. (2022) 13:907591. doi: 10.3389/fimmu.2022.907591
61. Chen JH, Pezhouh MK, Lauwers GY, Masia R. Histopathologic features of colitis due to immunotherapy with anti-PD-1 antibodies. Am J Surg pathology. (2017) 41:643–54. doi: 10.1097/PAS.0000000000000829
62. Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. (2018) 19:345–61. doi: 10.1007/s40257-017-0336-3
63. Meng T, Xiao D, Muhammed A, Deng J, Chen L, He J. Anti-inflammatory action and mechanisms of resveratrol. Molecules. (2021) 26:229. doi: 10.3390/molecules26010229
64. Guo Z, Xu H-Y, Xu L, Wang S-S, Zhang X-M. In vivo and in vitro immunomodulatory and anti-inflammatory effects of total flavonoids of astragalus. Afr J Traditional Complementary Altern Medicines. (2016) 13:60–73. doi: 10.21010/ajtcam.v13i4.10
65. Liu S, Xiao G, Wang Q, Tian J, Feng X, Zhang Q, et al. Effects of dietary Astragalus membranaceus and Codonopsis pilosula extracts on growth performance, antioxidant capacity, immune status, and intestinal health in broilers. Front Veterinary Science. (2023) 10:1302801. doi: 10.3389/fvets.2023.1302801
66. Cheng M, Hu J, Zhao Y, Jiang J, Qi R, Chen S, et al. Efficacy and safety of astragalus-containing traditional Chinese medicine combined with platinum-based chemotherapy in advanced gastric cancer: A systematic review and meta-analysis. Front oncology. (2021) 11:632168. doi: 10.3389/fonc.2021.632168
67. Zhang X, Qiu H, Li C, Cai P, Qi F. The positive role of traditional Chinese medicine as an adjunctive therapy for cancer. Bioscience trends. (2021) 15:283–98. doi: 10.5582/bst.2021.01318
68. Lin S, An X, Guo Y, Gu J, Xie T, Wu Q, et al. Meta-analysis of astragalus-containing traditional Chinese medicine combined with chemotherapy for colorectal cancer: efficacy and safety to tumor response. Front oncology. (2019) 9:749. doi: 10.3389/fonc.2019.00749
69. Wang Z-X, Li P-P, Li C-N, Guo Y-N, Shao Y-Z, Yan Q, et al. Study on the mechanism of Codonopsis pilosula polysaccharide inhibiting gastric cancer precancerous lesions by regulating Wnt/β-catenin signaling pathway. Pharmacol Research-Modern Chin Medicine. (2024) 10:100391. doi: 10.1016/j.prmcm.2024.100391
70. Cheng Y-Y, Hsieh C-H, Tsai T-H. Concurrent administration of anticancer chemotherapy drug and herbal medicine on the perspective of pharmacokinetics. J Food Drug analysis. (2018) 26:S88–95. doi: 10.1016/j.jfda.2018.01.003
71. Wang J, Guo Z, Shen M, Xie Q, Xiang H. Potential application mechanism of traditional Chinese medicine in treating immune checkpoint inhibitor-induced colitis. Front Immunol. (2024) 15:1366489. doi: 10.3389/fimmu.2024.1366489
72. Suo H, Hochnadel I, Petriv N, Franke R, Schmidt J, Limanska N, et al. Elucidating the mechanism behind and investigating the efficacy of Traditional Chinese Medicine and Traditional Tibetan Medicine in combination with standard therapeutics in hepatocellular carcinoma and cholangiocarcinoma in vitro. Front Pharmacol. (2022) 13:906468. doi: 10.3389/fphar.2022.906468
73. Singh K, Singh G, Bhushan B, Kumar S, Dhurandhar Y, Dixit P. A comprehensive pharmacological review of Atractylodes Macrocephala: Traditional uses, phytochemistry, pharmacokinetics, and therapeutic potential. Pharmacol Research-Modern Chin Medicine. (2024) 100394:100394. doi: 10.1016/j.prmcm.2024.100394
74. Zhang X, Liu Z, Chen S, Li H, Dong L, Fu X. A new discovery: Total Bupleurum saponin extracts can inhibit the proliferation and induce apoptosis of colon cancer cells by regulating the PI3K/Akt/mTOR pathway. J Ethnopharmacol. (2022) 283:114742. doi: 10.1016/j.jep.2021.114742
75. Jiang H, Li M, Du K, Ma C, Cheng Y, Wang S, et al. Traditional Chinese Medicine for adjuvant treatment of breast cancer: Taohong Siwu Decoction. Chin medicine. (2021) 16:1–20. doi: 10.1186/s13020-021-00539-7
76. Cohen I, Tagliaferri M, Tripathy D. Traditional Chinese medicine in the treatment of breast cancer. Semin Oncol. (2002) 29(6):563–74. doi: 10.1053/sonc.2002.50005
77. Suri C, Swarnkar S, Bhaskar L, Verma HK. Non-coding RNA as a biomarker in lung cancer. Non-coding RNA. (2024) 10:50. doi: 10.3390/ncrna10050050
78. Lam CS, Cheng LP, Zhou LM, Cheung YT, Zuo Z. Herb–drug interactions between the medicinal mushrooms Lingzhi and Yunzhi and cytotoxic anticancer drugs: a systematic review. Chin medicine. (2020) 15:1–18. doi: 10.1186/s13020-020-00356-4
79. Thiagarajan S, Arapoc DJ, Husna Shafie N, Keong YY, Bahari H, Adam Z, et al. Momordica charantia (Indian and Chinese Bitter Melon) Extracts Inducing Apoptosis in Human Lung Cancer Cell Line A549 via ROS-Mediated Mitochodria Injury. Evidence-Based Complementary Altern Medicine. (2019) 2019:2821597. doi: 10.1155/2019/2821597
80. Huang S, Li Q, Song Y. Codonopsis pilosula polysaccharide suppresses the progression of non-small cell lung cancer by triggering NLRP3/GSDMD-dependent pyroptosis. Discover Oncology. (2024) 15:510. doi: 10.1007/s12672-024-01361-x
81. Liu X, Wu J, Zhang D, Wang K, Duan X, Meng Z, et al. Network pharmacology-based approach to investigate the mechanisms of Hedyotis diffusa willd. In the treatment of gastric cancer. Evidence-Based Complementary Altern Medicine. (2018) 2018:7802639. doi: 10.1155/2018/7802639
82. Zhang J, Liu L, Wang J, Ren B, Zhang L, Li W. Formononetin, an isoflavone from Astragalus membranaceus inhibits proliferation and metastasis of ovarian cancer cells. J Ethnopharmacol. (2018) 221:91–9. doi: 10.1016/j.jep.2018.04.014
Keywords: apoptosis, expression, inflammation, extract, activation, antioxidant
Citation: Lei Y and Chen C (2025) Bibliometric analysis of traditional Chinese medicine in cancer treatment via immune system modulation (2015–2025). Front. Immunol. 16:1581885. doi: 10.3389/fimmu.2025.1581885
Received: 23 February 2025; Accepted: 14 April 2025;
Published: 08 May 2025.
Edited by:
Johnson O. Oladele, Royal Scientific Research Institute, NigeriaReviewed by:
Bo Zhang, Guilin Medical University, ChinaKusmardi Kusmardi, University of Indonesia, Indonesia
Yuanyuan Yan, The Third People’s Hospital of Hainan Province, China
Isaac Babatunde, Osun State University, Nigeria
Copyright © 2025 Lei and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunyan Chen, Y2hlbmNodW55YW5Ac2hhcGhjLm9yZw==
 Youfeng Lei
Youfeng Lei Chunyan Chen
Chunyan Chen