- 1The First Affiliated Hospital of Guangxi University of Science and Technology, Guangxi University of Science and Technology, Liuzhou, Guangxi, China
- 2Medicine College, Guangxi University of Science and Technology, Liuzhou, Guangxi, China
Introduction: This meta-analysis aimed to evaluate the efficacy and safety of Lenvatinib plus transarterial chemoembolization with or without programmed death-1 inhibitors (PD-1 inhibitors) in the treatment of intermediate or advanced hepatocellular carcinoma (HCC).
Materials and Methods: Four databases (Pubmed, Embase, Web of Science, and Cochrane Library) were searched for studies comparing lenvatinib plus transarterial chemoembolization with PD-1 inhibitors (TACE-L-P) versus Lenvatinib plus transarterial chemoembolization (TACE-L) for intermediate or advanced HCC. Meta-analyses were conducted for progression-free survival (PFS), overall survival (OS), objective response rate (ORR), disease control rate (DCR), and Grade ≥ 3 treatment-related adverse events (Grade ≥ 3 AEs).
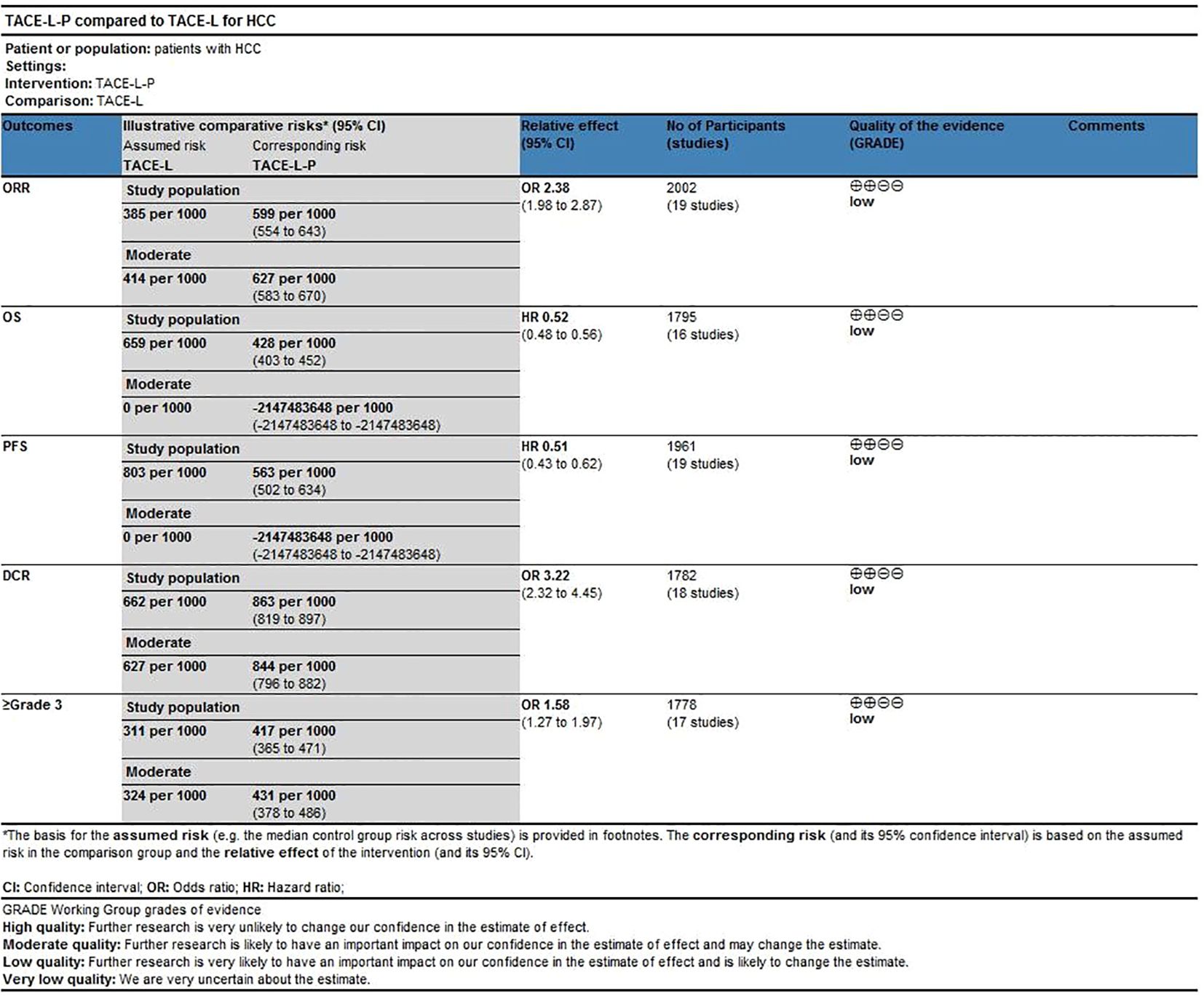
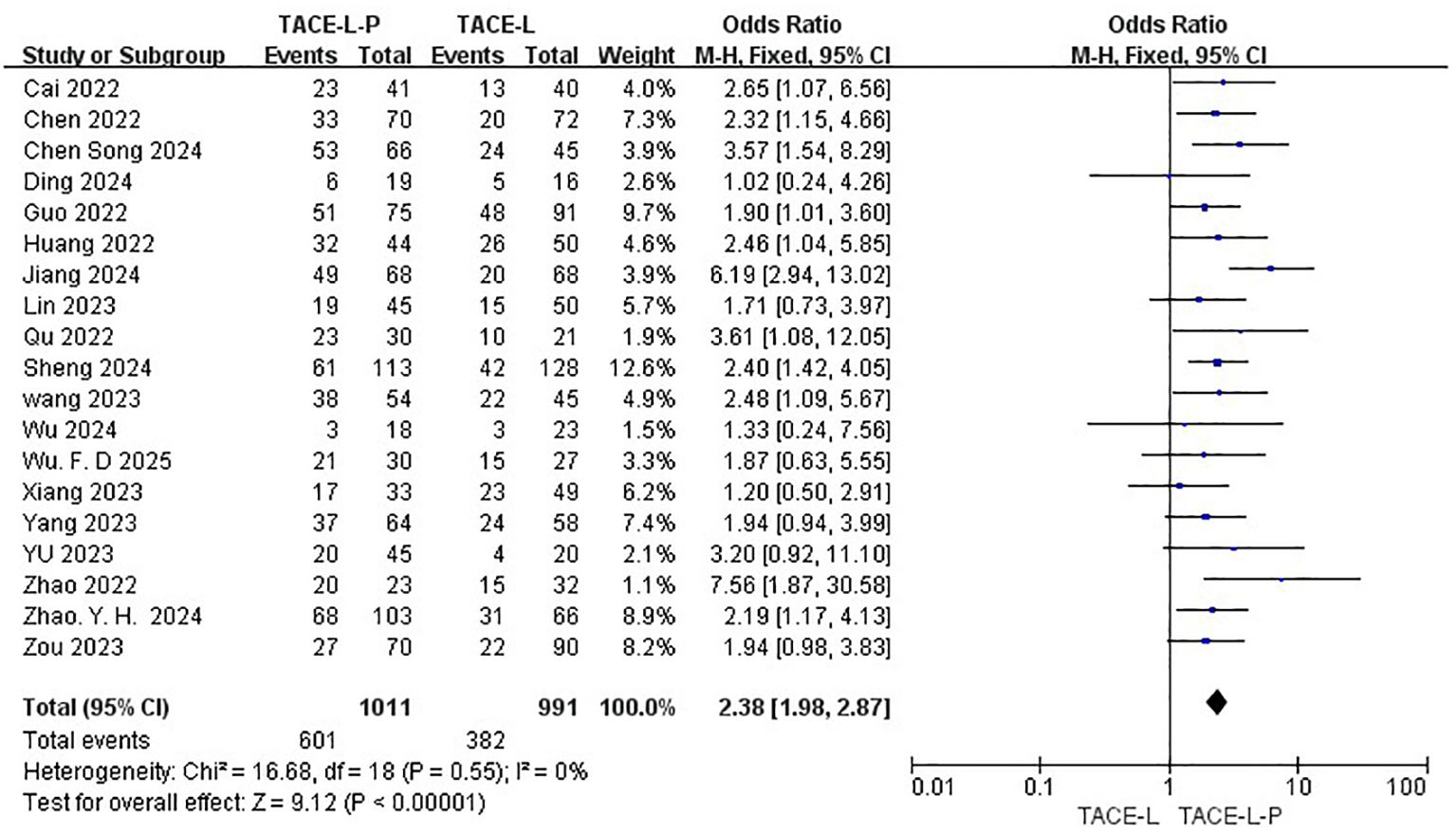
Results: The meta-analysis comprised 19 retrospective cohort studies, including of 2002 patients diagnosed with intermediate or advanced HCC. In this cohort, 1011 individuals were administered TACE-L-P, while 991 patients received TACE-L. In comparison to TACE-L, TACE-L-P demonstrated a superior ORR [odds ratio (OR) = 2.38, 95% confidence interval (CI) 1.98 ~ 2.87, P < 0.00001] and DCR (OR = 3.22, 95% CI, 2.32 ~ 4.45, P < 0.00001). TACE-L-P showed superior efficacy compared to TACE-L regarding PFS (HR: 0.56, 95%CI 0.50 to 0.62, P<0.0001) and OS (HR: 0.70, 95%CI 0.60 to 0.80, P<0.0001). Regarding safety, the incidence of Grade ≥ 3 AEs was more prevalent in the TACE-L-P group compared to the TACE-L group (OR=1.58, 95% CI: 1.27 ~ 1.97, P<0.0001).
Conclusions: The present meta-analysis present a comparison of the efficacy and safety of TACE-L-P against TACE-L for intermediate or advanced HCC. TACE-L-P enhanced ORR, DCR, PFS, and OS relative to TACE-L. Furthermore, the improved efficacy of TACE-L-P was correlated with a rise in the incidence of Grade ≥ 3 AEs.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024590414, identifier CRD42024590414.
1 Introduction
Hepatocellular carcinoma (HCC) is the predominant form of primary liver cancer, representing 80-90% of incidence (1). This malignancy imposes a significant health and economic cost worldwide, particularly in Asia (2). With a 5-year survival rate of around 18%, it ranks as the third most common cause of cancer-related mortality worldwide (3). Possible therapeutic interventions encompass surgical excision, image-guided ablation, liver transplantation, transarterial chemoembolization (TACE), sorafenib, and lenvatinib. Specific therapeutic interventions are recommended during certain clinical phases, and it is common to employ combination therapies (4–6). While surgical resection, ablation, and liver transplantation have the potential to completely cure HCC, most patients are found with advanced disease that is not responsive to these treatments. As a result, their prognosis is dismal, with an anticipated median life of 6–8 months (7–9).
The REFLECT trial validated those patients who received lenvatinib treatment had extended progression-free survival (PFS) and improved objective response rate (ORR). The findings corroborated those of prior investigations on sorafenib, leading to the recognition of lenvatinib as a novel therapy for advanced-stage HCC (10). Lenvatinib is an orally delivered, multi-targeted tyrosine kinase inhibitor that precisely blocks vascular endothelial growth factor receptor, fibroblast growth factor receptor (FGFR) 1–4, platelet-derived growth factor receptor- α (PDGFR α) and receptor tyrosine kinase (KIT) (11, 12). Lenvatinib has been shown in preclinical investigations to effectively inhibit vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) induced angiogenesis and VEGFR3-associated lymphangiogenesis (12–15). Furthermore, TACE has demonstrated the capacity to be used with immunotherapy (16). The anticancer mechanism of TACE entails the lowering of tumor size via the obstruction of blood flow to acquire a therapeutic outcome (17, 18). Considering the high incidence of tumor recurrence following TACE, this operation is often performed multiple times. Nevertheless, the repeated administration of TACE may cause failure of liver function, leading to a negative prognosis for the patient (19). Moreover, TACE induces tumor hypoxia, resulting in the increased expression of hypoxia inducible factor-1α (HIF-1α). Elevated levels of HIF-1α subsequently stimulate the promotion of vascular endothelial growth factor and platelet-derived growth factor (PDGF) production, hence enhancing tumor angiogenesis (20–22). Compared to TACE monotherapy, a previous study indicated that combination therapy with lenvatinib and TACE had a better tendency for extending the survival of patients with unresectable HCC (23).
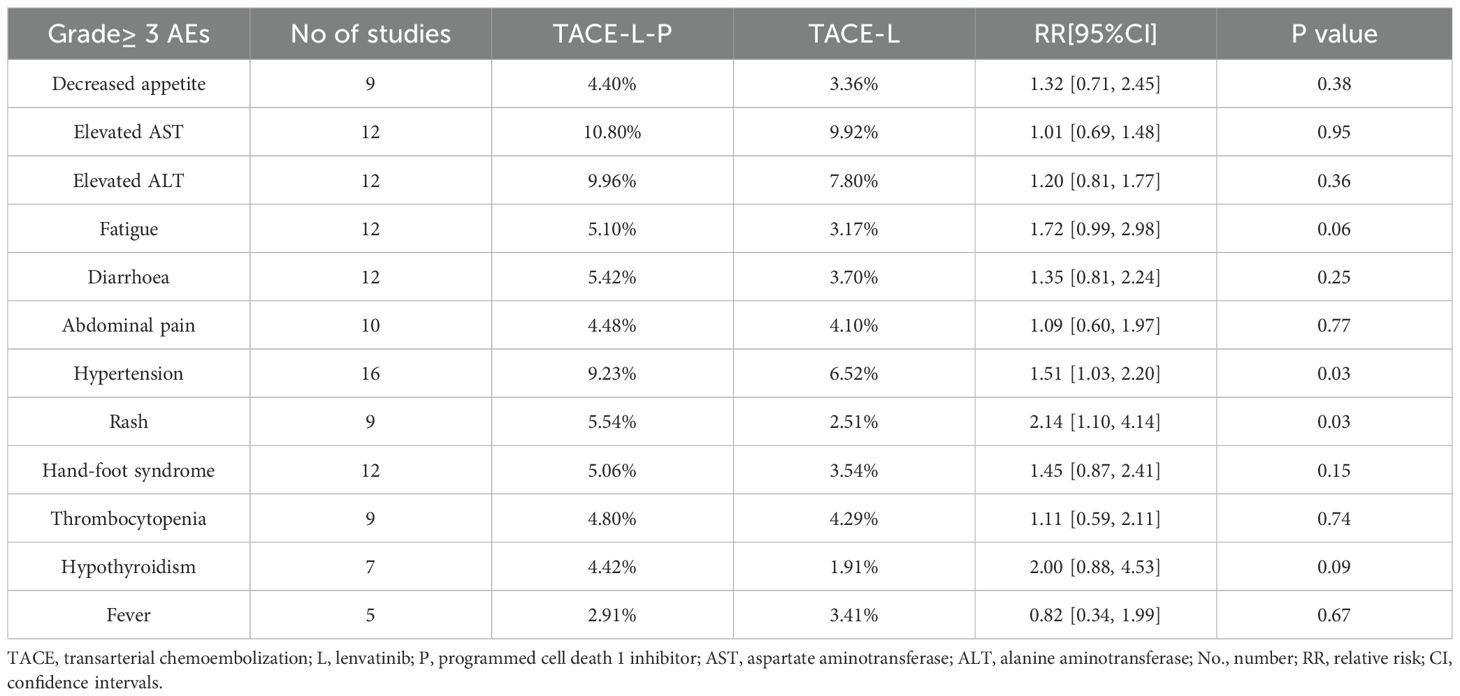
Immune checkpoint inhibitors (ICIs) immunotherapies enhance the immune tresponses against cancer by specifically targeting immunologic receptors located on the surface of T-lymphocytes or cancer cells (24). T cells express programmed death-1 inhibitors (PD-1 inhibitors) and cytotoxic T lymphocyte antigen 4 as co-inhibitory receptors on their surface to suppress T cell-mediated immune responses. However, cancer cells manipulate these inhibitory molecules to promote tumor tolerance and T cell exhaustion (25). More recently, immune checkpoint inhibitors, such as PD-1 and programmed death ligand 1 (PD-L1) inhibitors, have shown a potential therapeutic advantage for patients with advanced HCC (26). Recent studies indicated that utilizing triple therapy of TACE, Lenvatinib, and PD-1/L1 inhibitor might enhance the combined anti-cancer effects in advanced HCC and lead to better efficacy (27, 28). Yan et al. conducted a multicenter retrospective study involving 62 patients with unresectable HCC who received lenvatinib and PD-1 inhibitors in conjunction with TACE; the overall response rate (ORR) was 77.4%, and 53.2% of patients were converted to resectable HCC (29). Other studies also reported that lenvatinib plus transarterial chemoembolization with PD-1 inhibitors (TACE-L-P) provided better ORR and PFS than Lenvatinib plus transarterial chemoembolization (TACE-L) for patients with unresectable HCC (30, 31). The above results have all achieved positive results, indicating that PD-1 inhibitors can improve the prognosis of patients with TACE and lenvatinib. Regarding safety, the treatment-related adverse events (AEs) were controllable and acceptable in both groups. The incidence of grade 3–4 adverse events was greater in the TACE-L-P group compared to the TACE-L group, which remained safe with suitable symptomatic management (32). The primary Grade≥ 3 adverse events reported included abdominal pain, decreased appetite, diarrhea, fever, fatigue, hand-foot syndrome, hypertension, nausea, and rashes, among others. These adverse responses primarily pertain to the therapeutic mechanism of PD-1 immune checkpoint inhibition. The combination therapy for intermediate or advanced hepatocellular carcinoma can result in unavoidable adverse responses, necessitating continuous scrutiny of its long-term safety (32, 33).
Nevertheless, the effectiveness and safety of TACE-L-P in intermediate or advanced HCC remains somewhat contentious. Given the growing use of TACE-L-P, understanding their efficacy and safety profile is critical. Hence, we conducted this meta-analysis with the aim of providing clearer insights into the effectiveness and safety of TACE-L-P versus TACE-L for intermediate or advanced HCC and informing clinical decision-making.
2 Materials and methods
2.1 Search strategy
The current meta-analysis was conducted in compliance with the 2020 guidelines of the Preferred Reporting Project for Systematic Review and Meta-Analysis (PRISMA) (33) and the Assessing the methodological quality of systematic reviews (AMSTAR). A comprehensive literature search was conducted across four electronic databases, including of PubMed, Embase, the Cochrane Library and Web of Science, to identify pertinent articles published in the period from their inception to May 16, 2025. The search keywords were: “hepatocellular carcinoma” AND “Lenvatinib” AND “transarterial chemoembolization” AND “PD-1 inhibitor” AND “study”. Supplementary Tables provides a comprehensive listing of the search results. A comprehensive manual review of the bibliographies of the identified papers, together with relevant reviews and meta-analyses, was undertaken to identify any new research that satisfied the inclusion criteria. Furthermore, we performed a comprehensive search on three clinical trial registries, specifically ClinicalTrials.gov, Controlled-trials.com, and Umin.ac.jp/ctr/index.htm, to ensure the incorporation of unpublished data.
2.2 Inclusion and exclusion criteria
The criteria for inclusion were as follows: (1) patients: diagnosed with intermediate or advanced HCC [Barcelona Clinic Liver Cancer (BCLC) Stage B or C], Eastern Cooperative Oncologic Group (ECOG) score of 0 to 1, Child-Pugh class A/B. Intermediate HCC, or BCLC Stage B HCC, included asymptomatic patients with multinodular tumors without vascular invasion or extrahepatic spread (34, 35). Advanced HCC, or BCLC Stage C HCC, comprised patients with either symptomatic tumors or with an invasive tumoral pattern reflected by the presence of vascular invasion or extrahepatic spread (34, 35). (2) patient in the intervention group received TACE-L-P. (3) patient in the control group received TACE-L. (4) at least one of the following outcomes were reported: ORR, Disease control rate (DCR), Overall survival (OS), PFS, Grade≥ 3AEs. (5) study types: randomized controlled trials, prospective studies, or retrospective studies.
The criteria for exclusion were as follows: (1) other types of articles, including case reports, publications, letters, reviews, meta-analyses, editorials, animal studies, and protocols; (2) other types of cancers; (3) absence of relative outcomes; (4) duplicate group of patients; and (5) inability to compile data for meta-analysis.
2.3 Selection of studies
Selection of studies, including elimination of duplicates, was undertaken using EndNote (Version 20; Clarivate Analytics). An initial search was undertaken by two reviewers who independently deleted duplicate entries, assessed the titles and abstracts for relevance, and classified each study as either included or excluded. The settlement was arrived at through the attainment of consensus. A third author of the review would take on the role of an arbitrator if lacking a consensus.
2.4 Data extraction
Two separate reviewers conducted a thorough examination of the title and abstract, followed by a comprehensive review of the entire text. In order to resolve the discrepancies, expert advice was sought from a third investigator. The collected data comprises the first author’s name, publication year, study area, trial ID, study design, sample size, intervention, participant age, trial phase, study design, sample size, study period, median follow-up duration, ORR, DCR, Kaplan-Meier (KM) curves for PFS, KM curves for OS, and Grade ≥3 AEs. If the same cohort of patients were reported in several publications, only the latest data would be retained to avoid the duplication of information.
2.5 Quality assessment
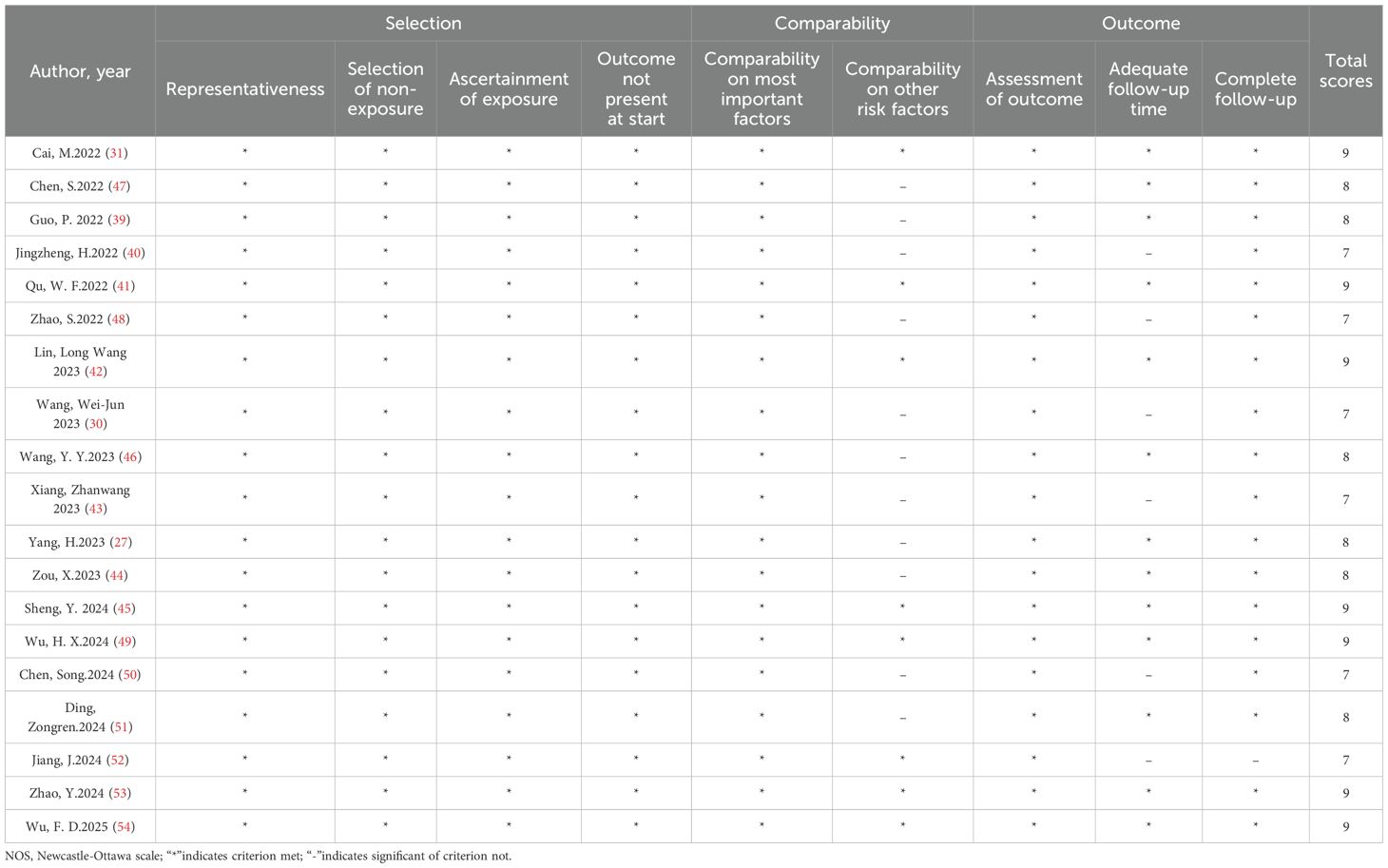
The revised Newcastle-Ottawa Scale (NOS) developed by Lo, Mertz, and Loeb in 2014 was employed to assess the quality of the included studies (36). Two reviewers independently evaluated each study from three domains: (1) Selection of the cohort (4 items), including of representativeness of the case/exposure group (1 point), selection of the non-case/non-exposure group (1 point), definition of exposure (1 point) and no relevant outcome at the start of the study (1 point); (2) Comparability (2 items), including of comparability on most important factors (up to 2 points) and comparability on other risk factors (1 point); (3) Outcome determination (3 items), including of outcome assessment (1 point), dequacy of follow-up time (1 point) and follow-up completeness (1 point). Studies with scores of ≥7 were classified as high quality. In case of any discrepancy, a consensus was formed by mutual discussion with other reviewers.
2.6 Evidence certainty
The certainty of evidence for the systematic review was assessed by two independent reviewers using the GRADEpro GDT (37): GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime, 2021. Available from gradepro.org. In case of any discrepancy, a consensus was formed by mutual discussion with other reviewers.
2.7 Statistical analysis
The data from papers providing Kaplan-Meier curves was extracted using GraphPad Prism software. The individual data were then recreated using the IPDformKM utility. The proven methodology developed by Guyot et al. was employed to recreate data at the level of individual patients (38). The procedure was conducted in a user-friendly Shiny application developed by Guyot et al, which is freely available at https://www.trialdesign.org/one-page-shell.html#IPDfromKM. Quantitative analysis was performed using Review Manager v5.3 software. The choice between fixed - effect and random-effect models was based on the I² value and chi-square test P value. When heterogeneity was high (I² >50%), the random-effect model was used. When heterogeneity was low (I² ≤50%), the fixed-effect model was applicable. Statistical significance was defined as a p-value less than 0.05. Funnel plot was employed to assess the presence of publication bias across different research. Ultimately, a sensitivity analysis was conducted to assess the influence of different research on the combined findings and to evaluate the dependability of the results.
3 Result
3.1 Search results
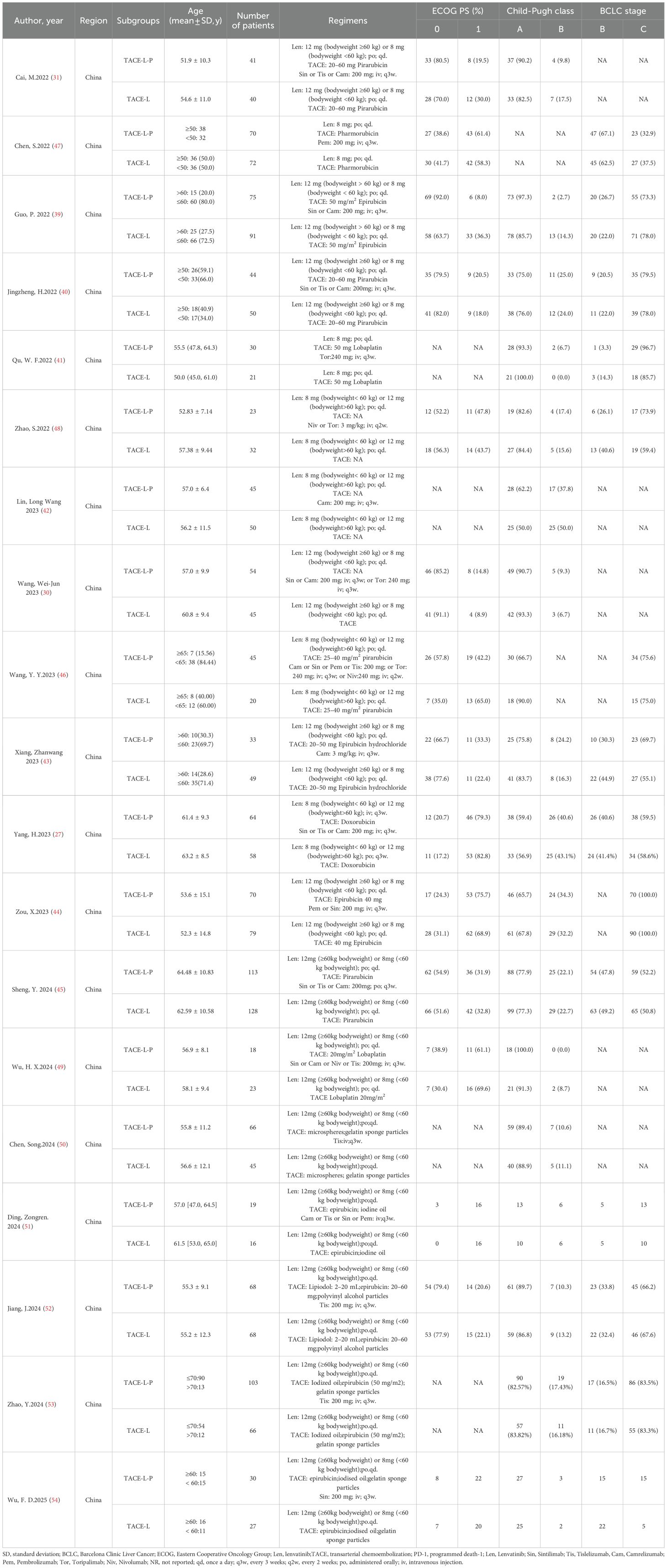
A comprehensive overview of the procedure of selecting and integrating literature is provided in the Figure 1. Our preliminary search yielded a grand total of 683 papers. Once repeat studies were eliminated, the remaining number of studies was only 495. Following an analysis of the titles and abstracts, a grand total of 471 articles were determined to be irrelevant and so eliminated. After conducting a comprehensive study of the entire text, a final selection of 19 articles (27, 30, 31, 39–46) was made for use in this meta-analysis.
3.2 Patient characteristics and quality assessment
A comprehensive summary of the patient characteristics is provided in Table 1. This meta-analysis comprised a total of 2002 individuals diagnosed with intermediate or advanced HCC selected from 19 studies published between 2022 and 2025. All studies included were retrospective cohort studies. Within the patient population, 36.6% of the cancers were classified as BCLC stage B, whereas 67.9% were recognized as stage C. An extensive array of PD-1 inhibitors, including as toripalimab, camrelizumab, pembrolizumab, sintilimab, tislelizumab, and nivolumab, were employed in these studies. The intervention of interest in all the studies considered was the concurrent administration of TACE, lenvatinib, and PD-1 inhibitors. In these studies, the control groups received a combination of TACE-L. All these studies were conducted in China. The authors, year, subgroups, regimens, patients, age, ECOG PS (%), Child-Pugh class and BCLC stage of the included literature are shown in Table 1. Regarding quality assessment, every study included in the analysis obtained a NOS score superior to 7 points, thereby indicating a high level of quality (Table 2).
3.3 Efficacy outcomes
3.3.1 ORR and DCR
All 19 studies included reported data on ORR (27, 30, 31, 40–48), while only 18 studies provided DCR (27, 30, 31, 39–43, 46–49). The ORR of patients in the TACE-L-P group was significantly greater than that of the TACE-L group (59.45% vs 38.55%, OR = 2.38, 95% CI: 1.98 to 2.87, P <0.00001, I2 = 0%) (Figure 2). Sensitive analysis showed that the outcomes were stable (Supplementary Figure S1). The DCR of patients in the TACE-L-P group was also significantly better than that of the TACE-L group (86.41% vs 66.18%, OR = 3.22, 95% CI: 2.32 to 4.45, p <0.00001, I2 = 0%) (Figure 3). Sensitive analysis showed that the outcomes were stable (Supplementary Figure S2).
3.3.2 PFS and OS
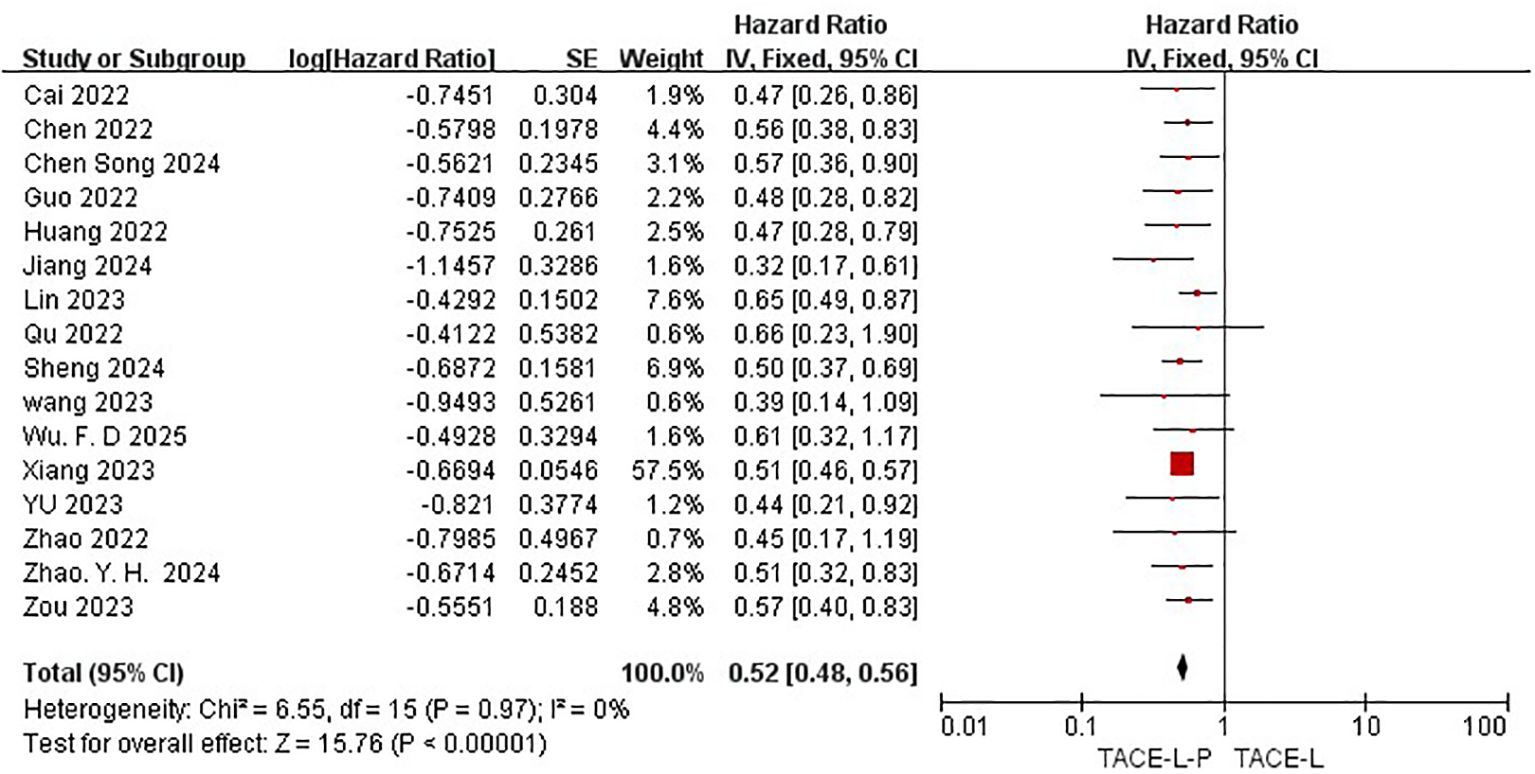
All 19 studies included in the meta-analysis provided KM curves for PFS (27, 30, 31, 39–54), while only 16 studies provided KM curves for OS (30, 31, 39–42, 45–47, 50, 52). TACE-L-P emerged as superior to TACE-L in terms of PFS (HR: 0.54, 95%CI 0.46 to 0.62, P<0.00001, I2 = 46%) (Figure 4) and OS (HR: 0.52, 95%CI 0.48 to 0.56, P<0.0001, I2 = 0%) (Figure 5). Sensitive analysis showed that the outcomes were stable (Supplementary Figures S3, S4).
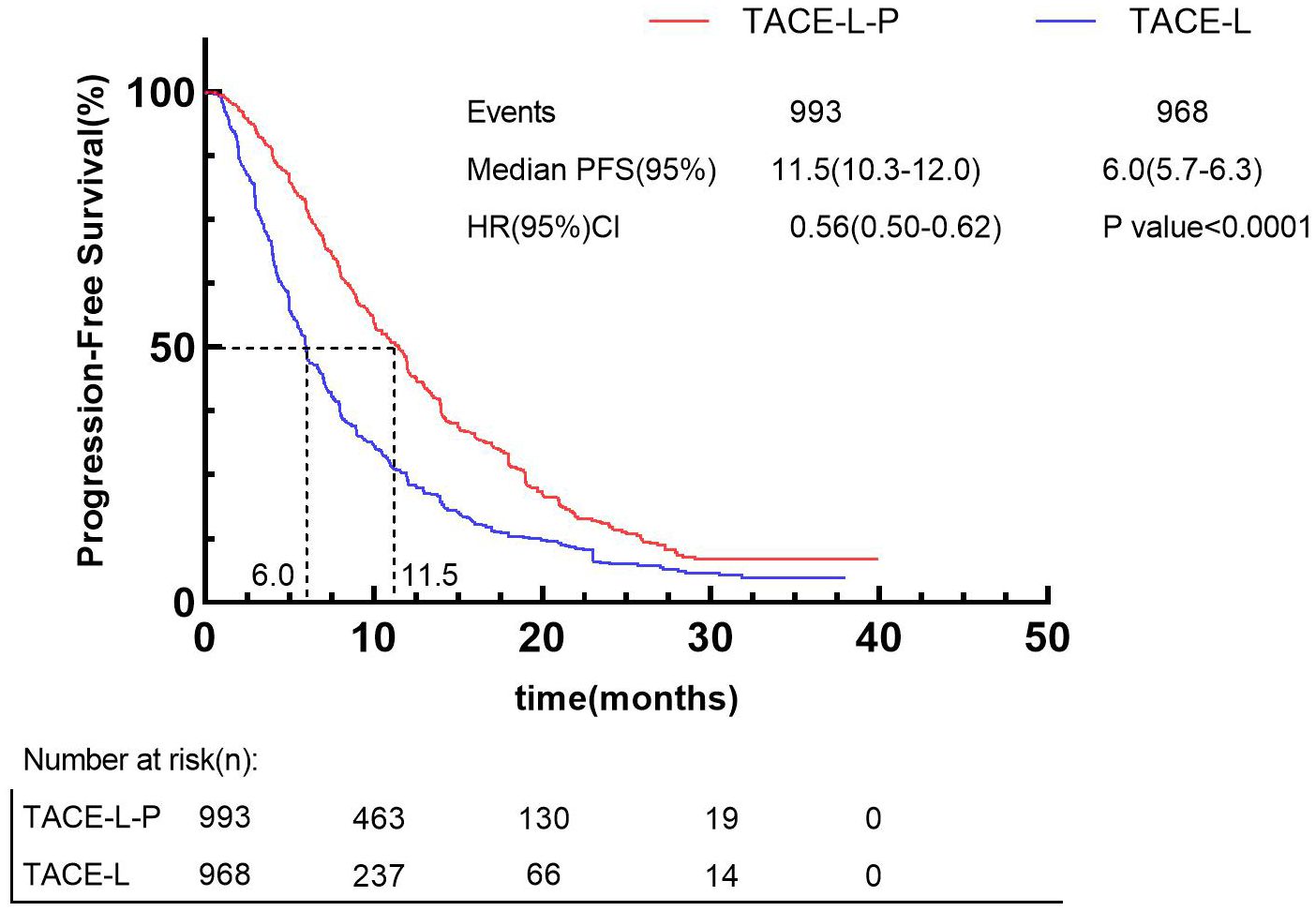
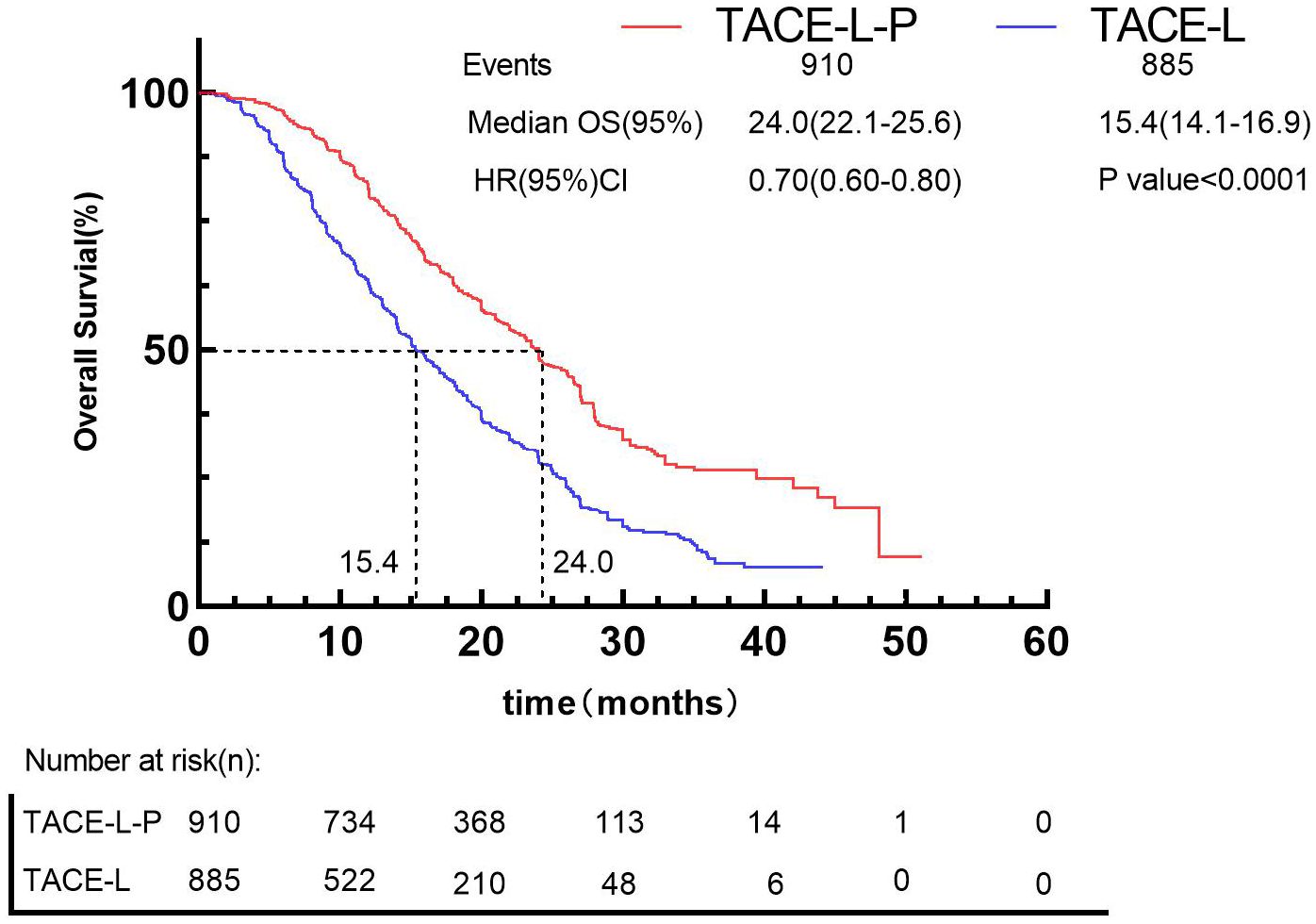
Besides, by using the IPDformKM program, reconstruction of Kaplan-Meier curves provided a clear and comprehensible representation of oncological outcomes for PFS (median survival time: 11.5 months versus 6.0 months, HR: 0.56, 95%CI 0.50 to 0.62, P<0.0001) (Figure 6) and OS (median survival time: 24.0 months versus 15.4 months, HR: 0.70, 95%CI 0.60 to 0.80, P<0.0001) (Figure 7).
3.4 Safety
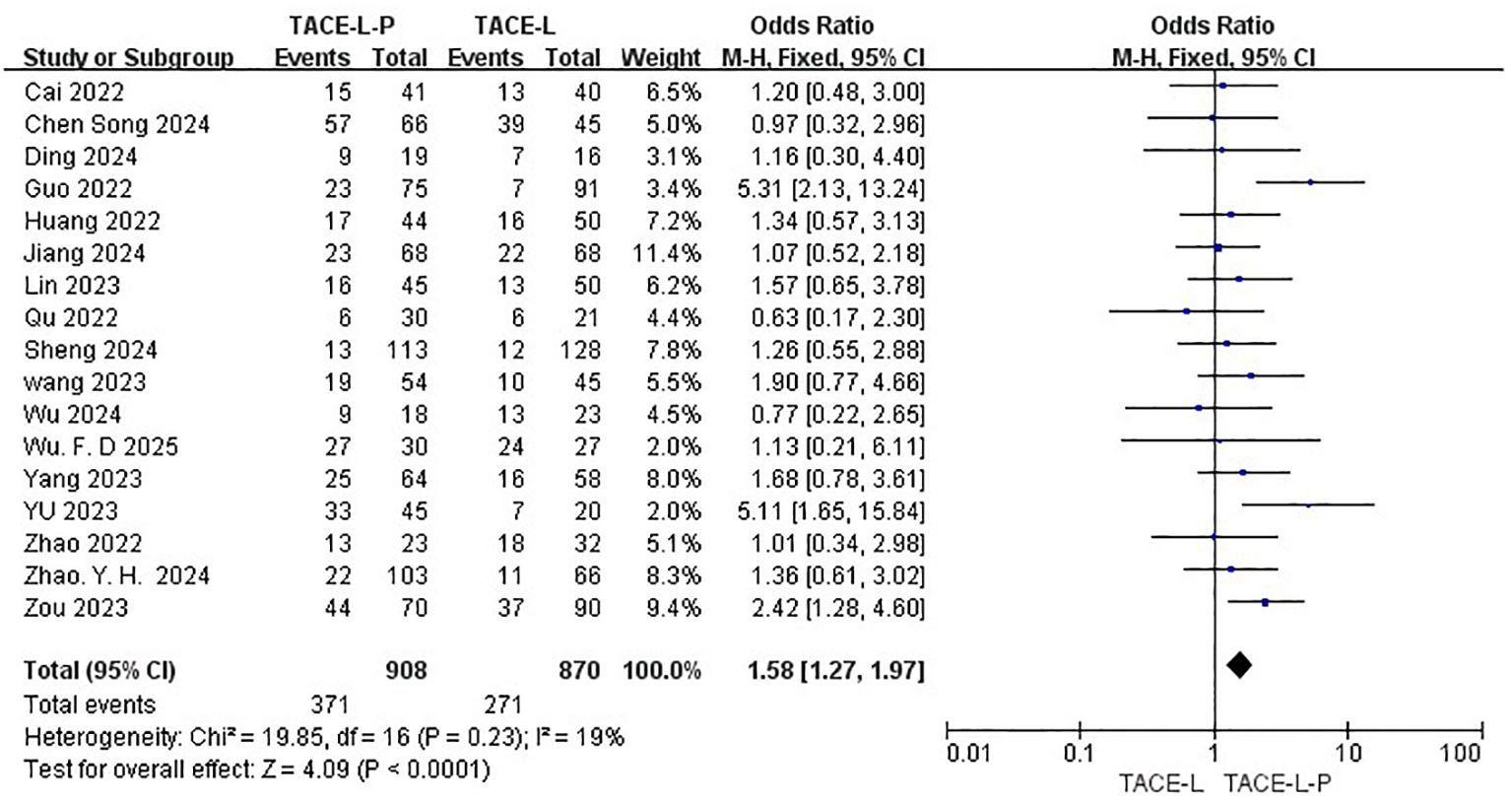
Safety was assessed by evaluating the rate of Grade≥ 3 AEs reported in a total of 17 studies (27, 30, 31, 39–46, 48–54). The TACE-L-P group showed a higher probability of experiencing Grade≥ 3 AEs compared to the TACE-L group(40.86% vs 31.15%, OR=1.58, 95% CI: 1.27 to 1.97, P<0.0001, I2 = 19%) (Figure 8). Sensitive analysis showed that the outcomes were stable (Supplementary Figure S5).
3.5 Publication bias
Publication bias was assessed by analyzing a funnel plot in connection to the ORR (Supplementary Figure S6), DCR (Supplementary Figure S7), PFS (Supplementary Figure S8), OS (Supplementary Figure S9) and Grade≥ 3 AEs (Supplementary Figure S10). The bilateral symmetric funnel plots indicated that there was no substantial evidence of publication bias.
3.6 Subgroup analyses for individual Grade≥3 AEs
Subgroup analyses for individual Grade≥3 AEs were performed (Table 3, Supplementary Figures S11–S22). TACE-L-P significantly increased the incidence of hypertension (OR 1.72, 95% CI 1.03–2.20; P = 0.03) and rash (OR 2.00, 95% CI 0.88–4.53; P = 0.02) compared with TACE-L. There was no statistically significant differences between two groups regarding decreased appetite, elevated AST, elevated ALT, fatigue, diarrhea, abdominal pain, hand-foot syndrome, thrombocytopenia, hypothyroidism or fever.
3.7 Subgroup analysis regarding ORR, DCR, PFS and OS for individual PD-1 Inhibitors
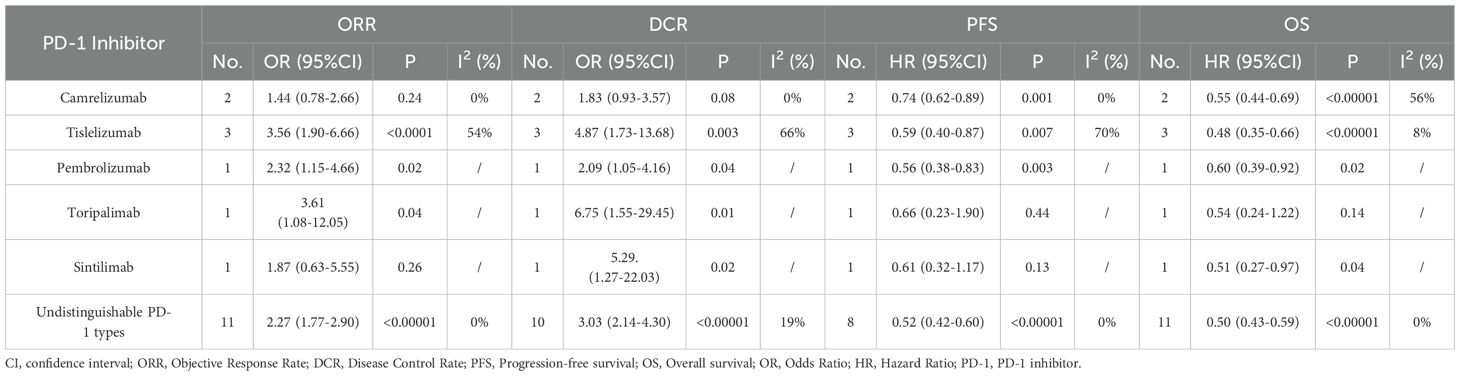
Different PD-1 Inhibitors were employed among the included 19 studies. camrelizumab was employed in two studies, tislelizumab was employed in three studies, pembrolizumab was employed in one study, toripalimab was employed in one study and sintilimab was employed in one study. However, more than one type of PD-1 Inhibitors were employed in another 11 studies and the outcomes data were undistinguishable for individual PD-1 Inhibitors (Table 1, regimens). Subgroup analyses regarding ORR, DCR, PFS and OS for individual PD-1 Inhibitors were performed (Table 4, Supplementary Figures S23–S26). The ORR of patients in the TACE-L-P group was significantly greater than that of the TACE-L group when tislelizumab, pembrolizumab or toripalimab was employed. The DCR of patients in the TACE-L-P group was significantly greater than that of the TACE-L group when tislelizumab, pembrolizumab, sintilimab or toripalimab was employed. The PFS of patients in the TACE-L-P group was significantly better than that of the TACE-L group when tislelizumab, pembrolizumab or camrelizumab was employed. The OS of patients in the TACE-L-P group was significantly better than that of the TACE-L group when tislelizumab, pembrolizumab or camrelizumab was employed.
3.8 Evidence certainty
The certainty of evidence assessed for the various outcomes as per GRADE (Grading of Recommendations, Assessment, Development and Evaluations) criteria were of low certainty category (Figure 9).
4 Discussion
Efficacy and safety of TACE-L-P versus TACE-L for intermediate or advanced HCC were compared in this meta-analysis. Our findings indicate that TACE-L-P greatly enhanced ORR, DCR, PFS and OS, with a rise in the probability of Grade≥ 3 AEs. The co-administration of TACE-L has demonstrated a positive outlook for the management of primary liver cancer.
A previous meta-analysis demonstrated that TACE-L increased ORR, DCR, and 6-month, 12-month, and 18-month PFS in patients with advanced HCC, while lowering blood AFP and VEGF expression levels. Compared with TACE alone, TACE-L did not significantly improve 6-month OS, but it significantly improved 12-month and 18-month OS (55). Nevertheless, the co-administration of TACE-L may not be advantageous for many patients, particularly those with extrahepatic metastases, as this may be linked to the process of T cell escape recognition. The ability of cancer cells to evade the immune system may explain why HCC can elude treatment with TACE-L and other conventional therapeutic approaches (56–58). Over the last ten years, significant advancements have been achieved in the systematic therapy of advanced HCC using specific anticancer drugs and ICIs (41). The phase 1b trial, Study 116–KEYNOTE-524, demonstrated that the combination of lenvatinib and pembrolizumab showed impressive antitumor activity in first-line treatment. These patients had a median overall survival of 22.0 months, a median progression-free survival of 8.6 months, and a manageable safety profile (59). Atezolizumab combined with bevacizumab was granted approval by the US Food and Drug Administration in May 2020 as the primary treatment for advanced HCC (60, 61). All these studies suggested the cooperation between local treatment, immunotherapy, and targeted therapy (41). Furthermore, the effectiveness and safety of immune checkpoint inhibitors, such as pembrolizumab and camrelizumab, which block immune evasion through the PD-1/PD-L1 pathway, have been proven in several prior studies for advanced HCC. TACE enhances adaptive immunity by liberating tumor antigens under hypoxia, creating a “vascular-immune priming” condition (62). TACE-L-P combined with PD-1 inhibitors enhances outcomes via synergistic biological interactions. To reinstate T-cell depletion, PD-1 inhibition interferes with the PD-1/PD-L1 axis, whereas lenvatinib rectifies tumor vasculature to enhance immune cell infiltration into the tumor microenvironment (42, 62). These inhibitors appear to counteract the effects of tumor evasion induced by conventional therapy, so serving a supplementary function (63). Mechanistically, the combination therapy creates a “vascular- immune priming” microenvironment: lenvatinib normalizes tumor vasculature to enhance immune cell infiltration, whereas PD-1 inhibitors augment T-cell activity (63–66). This synergy results in improved survival rates: a phase II trial reported a median progression-free survival (PFS) of 8.0 months and overall survival (OS) of 18.4 months in triple therapy groups, far surpassing existing dual therapy statistics (PFS: 5.1 months; OS: 10.7 months) (62). Possible explanations could be as follows: (1) TACE causes significant local tissue death and can thereafter trigger anticancer immune responses that can be enhanced with immunomodulatory PD-1 drugs (67, 68); (2) A multikinase inhibitor with antiproliferative and antiangiogenic properties (69), lenvatinib may prevent hypoxia-induced angiogenesis following TACE (67, 70) and modulate the tumor immune microenvironment to increase immune response to PD-1 inhibitor in HCC (69, 71); (3)Blockade of PD-1 inhibitor impedes the transmission of immune assault signals to tumors, therefore enhancing the immune response against tumour cells (72). TACE can efficiently decrease the blood flow to urine-derived hepatocellular carcinoma (uHCC) and stimulate the secretion of tumor-specific antigens, so improving the therapeutic effectiveness of PD-1 inhibitor (73, 74). Hence, the concurrent use of TACE, lenvatinib, and PD-1 inhibitor may result in a synergistic anticancer effect, hence enhancing clinical outcomes in patients with advanced HCC (31).
The therapeutic advantages of combination treatments may differ among subpopulations. Patients with BCLC-C stage HCC and extrahepatic metastases provide a unique clinical challenge. A recent multicenter investigation revealed that patients with distant metastases have markedly poorer response rates to TACE-L-P compared to those without metastases (ORR 28% vs 52%, p=0.009), likely attributable to systemic immunosuppressive characteristics and tumor heterogeneity in metastatic lesions (75). The diminished efficacy in this category corresponds with the suggested causes of T cell exhaustion and PD-L1 overexpression in circulating tumor cells from metastatic locations (76, 77). Yan et al. reported 52.4% of patients with BCLC-B stage HCC were converted to resectable HCC after the treatment of TACE-L-P. However, none of the included studies provided individual data of BCLC-B1 stage and BCLC-B2 stage. More detailed exploration about BCLC subclassification should be considered in future studies.
The choice of TACE methods and chemotherapeutic drugs may significantly impact the synergistic results with lenvatinib and PD-1 inhibitors. Recent evidence suggests that drug-eluting bead transarterial chemoembolization (DEB-TACE) utilizing doxorubicin-loaded microspheres exhibits enhanced local tumor control relative to conventional lipiodol-based transarterial chemoembolization (cTACE), especially when integrated with systemic therapy (78). A multicenter study comparing cTACE (doxorubicin 50 mg + lipiodol) with DEB-TACE (100-300 μm doxorubicin-loaded microspheres) in conjunction with lenvatinib revealed that DEB-TACE attained superior objective response rates (63% vs. 48%, p=0.02) and extended median progression-free survival (11.2 vs. 8.4 months, p=0.03), presumably attributable to sustained drug release and diminished systemic exposure (79). Moreover, embolic agents may variably influence the immune microenvironment; experimental models demonstrate that 70-150 μm microspheres induce greater tumor necrosis while maintaining peri-tumoral dendritic cells, hence enhancing antigen presentation and subsequent efficacy of PD-1 inhibitors (80). The data indicate that DEB-TACE utilizing calibrated particle size and a doxorubicin dosage of 150 mg (recommended for HCC >5 cm) may serve as an effective foundation for combination therapies (81).
Regarding safety, our findings indicated that the incidence of Grade≥ 3 AEs was 40.86% following TACE-L-P, whereas the incidence of Grade≥ 3 AEs was 31.15% following TACE-L. Considering the improved efficacy of TACE-L-P, median PFS from 6.0 months to 11.5 months (Figure 7) and median OS from 15.4 months to 24.0 months (Figure 8), the higher incidence of Grade ≥ 3 AEs seems to be acceptable. The occurrence of adverse events is unavoidably heightened by triple or dual therapy, with the most prevalent adverse events being impaired liver function, hypertension, and reduced appetite. The increased occurrence of hypertension in patients may be attributed to the combined impact on angiogenesis. Additionally, the lower appetite is largely caused by the increased toxicities related to the combination therapy (82, 83). The elevated incidence of AEs noted in the TACE-L-P triple therapy group may be ascribed to the overlapping and synergistic toxicities of its constituents. Initially, PD-1 inhibitors can induce immune-related adverse events including hypothyroidism, hepatitis, and pneumonitis as a result of systemic immune activation that affects both neoplastic and normal tissues (84). Second, Lenvatinib, a multi-targeted tyrosine kinase inhibitor (TKI), obstructs VEGF receptors (VEGFR1-3), FGFR, and PDGFRα, leading to hypertension (35% compared to 18% in dual therapy), proteinuria, and hepatic dysfunction via vascular destabilization and metabolic dysregulation (62). Third, TACE-induced hypoxia may aggravate lenvatinib-associated hepatotoxicity by hindering drug clearance in cirrhotic livers, while simultaneously facilitating the release of pro-inflammatory cytokines that enhance immune-mediated toxicity (42). The synergy enhances anticancer activity but also elevates off-target consequences. A phase II trial indicated that grade ≥3 hypertension occurred in 35% of patients treated with TACE-L-P, possibly attributable to endothelial dysfunction generated by VEGF suppression, exacerbated by the vascular toxicity of ICIs (62). The simultaneous administration of TACE and lenvatinib may hinder liver regeneration in cirrhotic patients, resulting in increased transaminases and bilirubin levels (42). Active management techniques, including biomarker-guided patient selection (e.g., excluding Child-Pugh B/C cirrhosis) and sequential administration (commencing TACE prior to PD-1 inhibitors), may alleviate these hazards without diminishing efficacy (84). Future research should investigate pharmacodynamic biomarkers, such as circulating exosomal circCCAR1, to anticipate adverse event susceptibility and refine dosing regimens (85). It is advisable to formulate clinical response strategies for these high-frequency or severe AEs to offer more thorough guidance for clinical applications. Fortunately, most of these patients had relief by lenvatinib dose decrease, cessation of anti-programmed death-1 antibodies, and symptomatic treatment. Hence, both the triple and double combination therapies did not elevate the likelihood of uncontrollable adverse events. The safety outcomes underscore the necessity of educating both healthcare professionals and patients; prompt intervention for adverse events is crucial for effectively treating patients with the combination. Physicians should prioritize monitoring of medication toxicity and liver function following the introduction of the treatment. It is important to analyze the long-term safety profile in more detail.
The choice between triple therapy (TACE-L-P) and dual therapy (TACE-L) for intermediate or advanced HCC should be informed by a thorough evaluation of tumor biology, baseline liver function, and immune-microenvironment attributes. Recent research indicates that triple therapy may provide enhanced survival advantages in particular populations, including patients with portal vein tumor thrombosis (PVTT) or those demonstrating early biomarker responses (e.g., AFP reduction >20% post-treatment) (86, 87) A multicenter retrospective study revealed that patients with BCLC stage B/C, possessing preserved liver function (Child-Pugh A) and satisfactory performance status (ECOG 0-1), experienced a significantly extended median overall survival (26.8 vs. 18.3 months, p=0.003) when treated with TACE-L-P as opposed to TACE-L, despite a higher occurrence of grade ≥3 hypertension (35% vs. 18%) (87) To alleviate negative effects, sequential administration strategies—such as commencing TACE to diminish tumor burden prior to the introduction of PD-1 inhibitors—have demonstrated potential in reducing immune-related hepatotoxicity while preserving efficacy (63). Molecular profiling, such as elevated expression of CDC20, LPCAT1, and SPP1, may identify patients predisposed to TACE resistance, therefore warranting early escalation to triple therapy. Subsequent randomized trials should further corroborate these selection criteria to enhance risk-benefit ratios (88).
The present study had several notable strengths. First, our study was an updated meta-analysis to compare the effectiveness and safety of TACE-L-P versus TACE-L for intermediate or advanced HCC. Considering the growing use of TACE-L-P and the remaining contentious regarding its effectiveness and safety, this meta-analysis provided clearer insights into the effectiveness and safety of TACE-L-P versus TACE-L for intermediate or advanced HCC and informing clinical decision-making. In addition, the heterogeneity of this meta-analysis was low, and the results were stable. Furthermore, the IPDformKM software was used to reconstruct KM curves for OS and PFS, which provide a clear and understandable representation of oncological outcomes. According to the findings of this meta-analysis, TACE-L-P should be recommended for intermediate or advanced HCC, especially for patients with BCLC stage B/C, possessing preserved liver function (Child-Pugh A) and satisfactory performance status (ECOG 0-1). Physicians must emphasize the surveillance of medication toxicity and hepatic function subsequent to the initiation of treatment, along with timely management for adverse events.
However, our study was subject to various limitations. First, as was the case with any meta-analysis, the intrinsic variability across the studies included in terms of patient baseline characteristics, disease stage, and treatment approaches might have influenced the findings. Second, since our study mostly encompassed studies conducted in China, additional research is necessary to assess the efficacy of this treatment combination in other ethnic groups and geographic populations. Third, the heterogeneity in outcomes like PFS or AEs might be caused by BCLC stage, genotype, patient age, the choice of TACE methods and chemotherapeutic drugs, but we failed to conduct further subgroup analysis due to the limitation of data. Besides, all the studies included were of a retrospective nature and resulted in low GRADE evidence grading, leading to potential bias and compromising the validity of the results. Well planned randomized controlled trials are necessary for future prospective validation of these findings. Real-world studies are also recommended to verify the observed advantages of TACE-L-P.
In conclusion, the current meta-analysis confirmed the efficacy and safety of TACE-L-P compared to TACE-L in patients with intermediate or advanced HCC. Enhancements in ORR, DCR, PFS, and OS were observed in patients with intermediate or advanced HCC who received TACE-L-P, as compared to those who received TACE-L. Furthermore, the increased effectiveness of TACE-L-P therapy was accompanied by an increase in adverse effects.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
YL: Conceptualization, Data curation, Formal Analysis, Project administration, Writing – original draft. HZ: Conceptualization, Data curation, Formal Analysis, Writing – original draft. XL: Conceptualization, Data curation, Formal Analysis, Writing – original draft. JW: Investigation, Methodology, Software, Supervision, Writing – original draft. XZ: Funding acquisition, Resources, Validation, Visualization, Writing – review & editing. SD: Funding acquisition, Resources, Validation, Visualization, Writing – review & editing. WL: Funding acquisition, Resources, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Scientific Foundation of China (No. 82460594), Guangxi Natural Science Foundation Program (No. 2024GXNSFAA010167), the Guangxi Medical and Healthcare Appropriate Technology Development and Popularization and Application Project (S2023125), Key Laboratory Construction Project of Guangxi Health Commission (ZPZH2020007), the Scientific Research Foundation of Guangxi University of Science and Technology(19Z26,20Z13), the Scientific Research Foundation of Guangxi Health Commission (Z-B20220927,Z-B20220930).
Acknowledgments
Everyone who contributed significantly to this study has been listed.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1586914/full#supplementary-material
Supplementary Material 1 | Comprehensive listing of the search results.
Supplementary Material 2 | Supplementary Figures.
Supplementary Material 3 | Survival curve reconstruction data.
Abbreviations
VEGF, vascular endothelial growth factor; FGF, Fibroblast Growth Factor; PD-1, inhibitor programmed death-1 inhibitors; HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; TACE-L, lenvatinib plus TACE; TACE-L-P, lenvatinib plus TACE with PD-1 inhibitor; PFS, progression-free survival; OS, overall survival; ORR, objective response rate; DCR, disease control rate; Grade ≥ 3 AE Grade ≥ 3 treatment-related adverse event; FGFR, fibroblast growth factor receptor; PDGFR α, platelet-derived growth factor receptor- α; VEGF, vascular endothelial growth factor; PDGF, platelet-derived growth factor; ICI, immune checkpoint inhibitors; PRISMA, Preferred Reporting Project for Systematic Review and Meta-Analysis; AMSTAR, Assessing the methodological quality of systematic reviews; KM, Kaplan-Meier; IL-6, Interleukin-6; NOS, Newcastle-Ottawa Scale; RR, risk ratios; MD, mean differences; CI, confidence intervals.
References
1. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. (2021) 7:6. doi: 10.1038/s41572-020-00240–3
2. Kulik L and El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. (2019) 156:477–91. doi: 10.1053/j.gastro.2018.08.065
3. Siegel RL, Miller KD, Fuchs HE, and Jemal A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
4. Bruix J, Gores GJ, and Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. (2014) 63:844–55. doi: 10.1136/gutjnl-2013–306627
5. Forner A, Reig M, and Bruix J. Hepatocellular carcinoma. Lancet. (2018) 391:1301–14. doi: 10.1016/s0140-6736(18)30010–2
6. Xiang YJ, Wang K, Yu HM, Li XW, Cheng YQ, Wang WJ, et al. Transarterial chemoembolization plus a PD-1 inhibitor with or without lenvatinib for intermediate-stage hepatocellular carcinoma. Hepatol Res. (2022) 52:721–9. doi: 10.1111/hepr.13773
7. Clinical Practice Guidelines EASL. Management of hepatocellular carcinoma. J Hepatol. (2018) 69:182–236. doi: 10.1016/j.jhep.2018.03.019
8. Benson AB, D’Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:541–65. doi: 10.6004/jnccn.2021.0022
9. Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. (2020) 9:682–720. doi: 10.1159/000509424
10. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. (2018) 391:1163–73. doi: 10.1016/s0140-6736(18)30207–1
11. Tohyama O, Matsui J, Kodama K, Hata-Sugi N, Kimura T, Okamoto K, et al. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. (2014) 2014:638747. doi: 10.1155/2014/638747
12. Yamamoto Y, Matsui J, Matsushima T, Obaishi H, Miyazaki K, Nakamura K, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. (2014) 6:18. doi: 10.1186/2045-824x-6–18
13. Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, and Asada M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res. (2008) 14:5459–65. doi: 10.1158/1078-0432.Ccr-07–5270
14. Matsui J, Yamamoto Y, Funahashi Y, Tsuruoka A, Watanabe T, Wakabayashi T, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. (2008) 122:664–71. doi: 10.1002/ijc.23131
15. Okamoto K, Kodama K, Takase K, Sugi NH, Yamamoto Y, Iwata M, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. (2013) 340:97–103. doi: 10.1016/j.canlet.2013.07.007
16. Sun T, Ren Y, Sun B, Chen L, Zhu L, Zhang L, et al. The feasibility of TACE combined with TKIs plus PD-1 antibody for advanced HCC. J Hepatocell Carcinoma. (2023) 10:447–57. doi: 10.2147/jhc.S400948
17. Llovet JM and Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. (2003) 37:429–42. doi: 10.1053/jhep.2003.50047
18. Lv WF, Liu KC, Lu D, Zhou CZ, Cheng DL, Xiao JK, et al. Transarterial chemoembolization for hepatocellular carcinoma combined with portal vein tumor thrombosis. Cancer Manag Res. (2018) 10:4719–26. doi: 10.2147/cmar.S166527
19. Hiraoka A, Kumada T, Kudo M, Hirooka M, Koizumi Y, Hiasa Y, et al. Hepatic Function during Repeated TACE Procedures and Prognosis after Introducing Sorafenib in Patients with Unresectable Hepatocellular Carcinoma: Multicenter Analysis. Dig Dis. (2017) 35:602–10. doi: 10.1159/000480256
20. Carmeliet P and Jain RK. Angiogenesis in cancer and other diseases. Nature. (2000) 407:249–57. doi: 10.1038/35025220
21. Li X, Feng GS, Zheng CS, Zhuo CK, and Liu X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. (2004) 10:2878–82. doi: 10.3748/wjg.v10.i19.2878
22. Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, and Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. (2008) 49:523–9. doi: 10.1080/02841850801958890
23. Fu Z, Li X, Zhong J, Chen X, Cao K, Ding N, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int. (2021) 15:663–75. doi: 10.1007/s12072-021-10184–9
24. Shiravand Y, Khodadadi F, Kashani SMA, Hosseini-Fard SR, Hosseini S, Sadeghirad H, et al. Immune checkpoint inhibitors in cancer therapy. Curr Oncol. (2022) 29:3044–60. doi: 10.3390/curroncol29050247
25. Sadeghi Rad H, Monkman J, Warkiani ME, Ladwa R, O’Byrne K, Rezaei N, et al. Understanding the tumor microenvironment for effective immunotherapy. Med Res Rev. (2021) 41:1474–98. doi: 10.1002/med.21765
26. Cheng AL, Hsu C, Chan SL, Choo SP, and Kudo M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol. (2020) 72:307–19. doi: 10.1016/j.jhep.2019.09.025
27. Yang H, Yang T, Qiu G, and Liu J. Efficacy and safety of TACE combined with lenvatinib and PD-(L)1 inhibitor in the treatment of unresectable hepatocellular carcinoma: A retrospective study. J Hepatocell Carcinoma. (2023) 10:1435–43. doi: 10.2147/jhc.S423684
28. Sun L, Xu X, Meng F, Liu Q, Wang H, Li X, et al. Lenvatinib plus transarterial chemoembolization with or without immune checkpoint inhibitors for unresectable hepatocellular carcinoma: A review. Front Oncol. (2022) 12:980214. doi: 10.3389/fonc.2022.980214
29. Wu JY, Yin ZY, Bai YN, Chen YF, Zhou SQ, Wang SJ, et al. Lenvatinib combined with anti-PD-1 antibodies plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: A multicenter retrospective study. J Hepatocell Carcinoma. (2021) 8:1233–40. doi: 10.2147/jhc.S332420
30. Wang WJ, Liu ZH, Wang K, Yu HM, Cheng YQ, Xiang YJ, et al. Efficacy and safety of TACE combined with lenvatinib and PD-1 inhibitors for unresectable recurrent HCC: A multicenter, retrospective study. Cancer Med. (2023) 12:11513–24. doi: 10.1002/cam4.5880
31. Cai M, Huang W, Huang J, Shi W, Guo Y, Liang L, et al. Transarterial chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: A retrospective cohort study. Front Immunol. (2022) 13:848387. doi: 10.3389/fimmu.2022.848387
32. Khoja L, Day D, Wei-Wu Chen T, Siu LL, and Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. (2017) 28:2377–85. doi: 10.1093/annonc/mdx286
33. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
34. Llovet JM, Brú C, and Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. (1999) 19:329–38. doi: 10.1055/s-2007–1007122
35. Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. (1999) 29:62–7. doi: 10.1002/hep.510290145
36. Lo CK, Mertz D, and Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. (2014) 14:45. doi: 10.1186/1471-2288-14–45
37. Prasad M. Introduction to the GRADE tool for rating certainty in evidence and recommendations. Clin Epidemiol Global Health. (2024) 25:101484. doi: 10.1016/j.cegh.2023.101484
38. Liu N, Zhou Y, and Lee JJ. IPDfromKM: reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med Res Methodol. (2021) 21:111. doi: 10.1186/s12874-021-01308–8
39. Guo P, Pi X, Gao F, Li Q, Li D, Feng W, et al. Transarterial chemoembolization plus lenvatinib with or without programmed death-1 inhibitors for patients with unresectable hepatocellular carcinoma: A propensity score matching study. Front Oncol. (2022) 12:945915. doi: 10.3389/fonc.2022.945915
40. Jingzheng H, Mingyue C, Wensou H, Yongjian G, Jingjun H, Qunfang Z, et al. Transarterial chemoembolization combined with lenvatinib plus programmed death 1 inhibitor for the treatment of unresectable intermediate−advanced hepatocellular carcinoma. Chin J Radiol (China). (2022) 56:879–85. doi: 10.3760/cma.j.cn112149-20211104–00977
41. Qu WF, Ding ZB, Qu XD, Tang Z, Zhu GQ, Fu XT, et al. Conversion therapy for initially unresectable hepatocellular carcinoma using a combination of toripalimab, lenvatinib plus TACE: Real-world study. BJS Open. (2022) 6:1–8. doi: 10.1093/bjsopen/zrac114
42. Lin LW, Yan L-Y, Ke K, Yang W-Z, Lin J-Q, and Huang N. Efficacy and safety of transarterial chemoembolization combined with lenvatinib, programmed death-1 inhibitor, and iodine-125 seed brachytherapy for hepatocellular carcinoma with portal vein tumor thrombosis. Brachytherapy. (2023) 22:858–71. doi: 10.1016/j.brachy.2023.06.229
43. Xiang Z, Li G, Mu L, Wang H, Zhou C, Yan H, et al. TACE combined with lenvatinib and camrelizumab for unresectable multiple nodular and large hepatocellular carcinoma (<5 cm). Technol Cancer Res Treat. (2023) 22:1–8. doi: 10.1177/15330338231200320
44. Zou X, Xu Q, You R, and Yin G. Correlation and efficacy of TACE combined with lenvatinib plus PD-1 inhibitor in the treatment of hepatocellular carcinoma with portal vein tumor thrombus based on immunological features. Cancer Med. (2023) 12:11315–33. doi: 10.1002/cam4.5841
45. Sheng Y, Wang Q, Liu H, Wang Q, Chen W, and Xing W. Prognostic nomogram model for selecting between transarterial chemoembolization plus lenvatinib, with and without PD-1 inhibitor in unresectable hepatocellular carcinoma. Br J Radiol. (2024) 97:668–79. doi: 10.1093/bjr/tqae018
46. Wang YY, Yang X, Wang YC, Long JY, Sun HS, Li YR, et al. Clinical outcomes of lenvatinib plus transarterial chemoembolization with or without programmed death receptor-1 inhibitors in unresectable hepatocellular carcinoma. World J Gastroenterol. (2023) 29:1614–26. doi: 10.3748/wjg.v29.i10.1614
47. Chen S, Wu Z, Shi F, Mai Q, Wang L, Wang F, et al. Lenvatinib plus TACE with or without pembrolizumab for the treatment of initially unresectable hepatocellular carcinoma harbouring PD-L1 expression: a retrospective study. J Cancer Res Clin Oncol. (2022) 148:2115–25. doi: 10.1007/s00432-021-03767–4
48. Zhao S, Zhou M, Wang P, Yang J, Zhang D, Yin F, et al. Sorafenib, lenvatinib, or lenvatinib combining PD-1 inhibitors plus TACE in unresectable hepatocellular carcinoma: A retrospective analysis. Technol Cancer Res Treat. (2022) 21:15330338221133640. doi: 10.1177/15330338221133640
49. Wu HX, Ding XY, Xu YW, Yu MH, Li XM, Deng N, et al. Transcatheter arterial chemoembolization combined with PD-1 inhibitors and Lenvatinib for hepatocellular carcinoma with portal vein tumor thrombus. World J Gastroenterol. (2024) 30:843–54. doi: 10.3748/wjg.v30.i8.843
50. Chen S, Tang S, Shi F, Cai H, Wu Z, Wang L, et al. TACE plus lenvatinib and tislelizumab for intermediate-stage hepatocellular carcinoma beyond up-to-11 criteria: a multicenter cohort study. Front Immunol. (2024) 15:1430571. doi: 10.3389/fimmu.2024.1430571
51. Ding Z, Fang G, Tang Y, and Zeng Y. The impact of PD-1 inhibitors on prognosis in unresectable hepatocellular carcinoma treated with TACE and lenvatinib: a retrospective study. Sci Rep. (2024) 14:1–24. doi: 10.1038/s41598-024-63571-1
52. Jiang J, Zhang H, Lai J, Zhang S, Ou Y, Fu Y, et al. Efficacy and safety of transarterial chemoembolization plus lenvatinib with or without tislelizumab as the first-line treatment for unresectable hepatocellular carcinoma: A propensity score matching analysis. J Hepatocell Carcinoma. (2024) 11:1607–22. doi: 10.2147/jhc.S472286
53. Zhao Y, Wen S, Xue Y, Dang Z, Nan Z, Wang D, et al. Transarterial chemoembolization combined with lenvatinib plus tislelizumab for unresectable hepatocellular carcinoma: a multicenter cohort study. Front Immunol. (2024) 15:1449663. doi: 10.3389/fimmu.2024.1449663
54. Wu FD, Zhou HF, Yang W, Zhu D, Wu BF, Shi HB, et al. Transarterial chemoembolization combined with lenvatinib and sintilimab vs lenvatinib alone in intermediate-advanced hepatocellular carcinoma. World J Gastrointestinal Oncol. (2025) 17:1–11. doi: 10.4251/wjgo.v17.i1.96267
55. Li D, Liu S, Cheng C, Xu L, and Zhao P. Efficacy and safety of transarterial chemoembolization plus lenvatinib in the treatment of advanced hepatocellular carcinoma: A meta-analysis. Med (Baltimore). (2023) 102:e34811. doi: 10.1097/md.0000000000034811
56. Chakraborty E and Sarkar D. Emerging therapies for hepatocellular carcinoma (HCC). Cancers (Basel). (2022) 14:1–23. doi: 10.3390/cancers14112798
57. Chen C, An L, Cheng Y, Luo X, Li Z, and Liu X. Clinical outcomes and prognosis factors of nivolumab plus chemotherapy or multitarget tyrosine kinase inhibitor in multi-line therapy for recurrent hepatitis B virus-related hepatocellular carcinoma: A retrospective analysis. Front Oncol. (2020) 10:1404. doi: 10.3389/fonc.2020.01404
58. Pinato DJ, Murray SM, Forner A, Kaneko T, Fessas P, Toniutto P, et al. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J Immunother Cancer. (2021) 9:1–9. doi: 10.1136/jitc-2021–003311
59. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. (2020) 38:2960–70. doi: 10.1200/jco.20.00808
60. Casak SJ, Donoghue M, Fashoyin-Aje L, Jiang X, Rodriguez L, Shen YL, et al. FDA approval summary: atezolizumab plus bevacizumab for the treatment of patients with advanced unresectable or metastatic hepatocellular carcinoma. Clin Cancer Res. (2021) 27:1836–41. doi: 10.1158/1078-0432.Ccr-20–3407
61. Qin S, Ren Z, Feng YH, Yau T, Wang B, Zhao H, et al. Atezolizumab plus Bevacizumab versus Sorafenib in the Chinese Subpopulation with Unresectable Hepatocellular Carcinoma: Phase 3 Randomized, Open-Label IMbrave150 Study. Liver Cancer. (2021) 10:296–308. doi: 10.1159/000513486
62. Cai M, Huang W, Liang W, Guo Y, Liang L, Lin L, et al. Lenvatinib, sintilimab plus transarterial chemoembolization for advanced stage hepatocellular carcinoma: A phase II study. Liver Int. (2024) 44:920–30. doi: 10.1111/liv.15831
63. Wang L, Lin L, and Zhou W. Efficacy and safety of transarterial chemoembolization combined with lenvatinib and PD-1 inhibitor in the treatment of advanced hepatocellular carcinoma: A meta-analysis. Pharmacol Ther. (2024) 257:108634. doi: 10.1016/j.pharmthera.2024.108634
64. Kato Y, Tabata K, Kimura T, Yachie-Kinoshita A, Ozawa Y, Yamada K, et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One. (2019) 14:e0212513. doi: 10.1371/journal.pone.0212513
65. Kudo M. Scientific rationale for combined immunotherapy with PD-1/PD-L1 antibodies and VEGF inhibitors in advanced hepatocellular carcinoma. Cancers (Basel). (2020) 12:1–12. doi: 10.3390/cancers12051089
66. Qu S, Zhang X, Wu Y, Meng Y, Pan H, Fang Q, et al. Efficacy and safety of TACE combined with lenvatinib plus PD-1 inhibitors compared with TACE alone for unresectable hepatocellular carcinoma patients: A prospective cohort study. Front Oncol. (2022) 12:874473. doi: 10.3389/fonc.2022.874473
67. Chang Y, Jeong SW, Young Jang J, and Jae Kim Y. Recent updates of transarterial chemoembolilzation in hepatocellular carcinoma. Int J Mol Sci. (2020) 21:1–20. doi: 10.3390/ijms21218165
68. Cheu JW and Wong CC. Mechanistic rationales guiding combination hepatocellular carcinoma therapies involving immune checkpoint inhibitors. Hepatology. (2021) 74:2264–76. doi: 10.1002/hep.31840
69. Kimura T, Kato Y, Ozawa Y, Kodama K, Ito J, Ichikawa K, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1–6 hepatocellular carcinoma model. Cancer Sci. (2018) 109:3993–4002. doi: 10.1111/cas.13806
70. Kishore SA, Bajwa R, and Madoff DC. Embolotherapeutic strategies for hepatocellular carcinoma: 2020 update. Cancers (Basel). (2020) 12:1–21. doi: 10.3390/cancers12040791
71. Yi C, Chen L, Lin Z, Liu L, Shao W, Zhang R, et al. Lenvatinib targets FGF receptor 4 to enhance antitumor immune response of anti-programmed cell death-1 in HCC. Hepatology. (2021) 74:2544–60. doi: 10.1002/hep.31921
72. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. (2017) 389:2492–502. doi: 10.1016/s0140-6736(17)31046–2
73. Hack SP, Zhu AX, and Wang Y. Augmenting anticancer immunity through combined targeting of angiogenic and PD-1/PD-L1 pathways: challenges and opportunities. Front Immunol. (2020) 11:598877. doi: 10.3389/fimmu.2020.598877
74. Kudo M. A new treatment option for intermediate-stage hepatocellular carcinoma with high tumor burden: initial lenvatinib therapy with subsequent selective TACE. Liver Cancer. (2019) 8:299–311. doi: 10.1159/000502905
75. Li Z, Zhou L, Zhao Z, Ma X, Zhang H, Jiang Y, et al. Impact of extrahepatic metastases on clinical outcomes of immune checkpoint inhibitor-based combination therapy in advanced hepatocellular carcinoma. J Hepatol. (2023) 79:682–93. doi: 10.1016/j.jhep.2023.05.015
76. Kurebayashi Y, Morioka K, Ueno A, Kimura T, Komatsu S, Matsui O, et al. Immunogenomic profiling of circulating tumor cells reveals mechanistic insights into immune evasion in metastatic hepatocellular carcinoma. Hepatology. (2022) 76:1082–96. doi: 10.1002/hep.32456
77. Chen S, Qiu C, Wen W, Zhang L, Zhou J, Sun M, et al. Hypoxia-Induced EMT Promotes Metastatic Dissemination in HCC via Exosomal miR-21-5p-Mediated PD-L1 Upregulation. Cancer Res. (2022) 82:3590–604. doi: 10.1158/0008-5472.CAN-22-0991
78. Lencioni R, de Baere T, Burrel M, Caridi JG, Lammer J, Malagari K, et al. Transcatheter treatment of hepatocellular carcinoma with Doxorubicin-loaded DC Bead (DEBDOX): technical recommendations. Cardiovasc Intervent Radiol. (2012) 35:980–5. doi: 10.1007/s00270-011-0287–7
79. Hoffmann K, Czaja F, Hinrichs JB, Rodt T, Ittrich H, Pech M, et al. Conventional versus drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma: emphasis on the impact of survival. J Hepatol. (2022) 76:1095–105. doi: 10.1016/j.jhep.2021.12.033
80. Iezzi R, Lucatelli P, Torre MFL, Flore A, Cotroneo AR, Pacioni M, et al. Drug-eluting beads transarterial chemoembolization prolongs time to progression compared to conventional transarterial chemoembolization in patients with unresectable hepatocellular carcinoma. Eur Radiol. (2020) 30:6816–27. doi: 10.1007/s00330-020-07049-4
81. Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. (2014) 111:255–64. doi: 10.1038/bjc.2014.199
82. Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. (2015) 16:1473–82. doi: 10.1016/s1470-2045(15)00290–9
83. Ruohoniemi DM, Taslakian B, Aaltonen EA, Hickey R, Patel A, Horn JC, et al. Comparative analysis of safety and efficacy of transarterial chemoembolization for the treatment of hepatocellular carcinoma in patients with and without pre-existing transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. (2020) 31:409–15. doi: 10.1016/j.jvir.2019.11.020
84. Guo X, Nie H, Zhang W, Li J, Ge J, Xie B, et al. Contrasting cytotoxic and regulatory T cell responses underlying distinct clinical outcomes to anti-PD-1 plus lenvatinib therapy in cancer. Cancer Cell. (2025) 43:248–268.e249. doi: 10.1016/j.ccell.2025.01.001
85. Shi Y, Cui D, Xia L, Shi D, Jin G, Wang S, et al. Efficacy and safety of lenvatinib plus gefitinib in lenvatinib-resistant hepatocellular carcinomas: a prospective, single-arm exploratory trial. Signal Transduct Target Ther. (2024) 9:359. doi: 10.1038/s41392-024-02085–8
86. Mu C, Shen J, Zhu X, Peng W, Zhang X, and Wen T. The efficacy and safety of lenvatinib plus transarterial chemoembolization in combination with PD-1 antibody in treatment of unresectable recurrent hepatocellular carcinoma: a case series report. Front Oncol. (2023) 13:1096955. doi: 10.3389/fonc.2023.1096955
87. Ma KP, Fu JX, Duan F, and Wang MQ. Efficacy and predictive factors of transarterial chemoembolization combined with lenvatinib plus programmed cell death protein-1 inhibition for unresectable hepatocellular carcinoma. World J Gastrointest Oncol. (2024) 16:1236–47. doi: 10.4251/wjgo.v16.i4.1236
Keywords: hepatocellular carcinoma, lenvatinib, PD-1 inhibitor, transarterial chemoembolization, progression-free survival, overall survival, objective response rate, meta-analysis
Citation: Lei Y, Liang X, Zhu H, Wang J, Zhang X, Duan S and Liang W (2025) Efficacy and safety of lenvatinib plus transarterial chemoembolization with or without programmed death-1 inhibitors in the treatment of intermediate or advanced hepatocellular carcinoma: a systematic review and meta-analysis. Front. Immunol. 16:1586914. doi: 10.3389/fimmu.2025.1586914
Received: 03 March 2025; Accepted: 08 July 2025;
Published: 24 July 2025.
Reviewed by:
Elena Niculet, Dunarea de Jos University, RomaniaKazuto Tajiri, University of Toyama University Hospital, Japan
Feng Zhang, Nanjing University of Chinese Medicine, China
Xiujuan Chang, Fifth Medical Center of the PLA General Hospital, China
Copyright © 2025 Lei, Liang, Zhu, Wang, Zhang, Duan and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaochen Zhang, emhhbmd4aWFvY2hlbkBneHVzdC5lZHUuY24=; Siliang Duan, ZHVhbnNpbGlhbmdAZ3h1c3QuZWR1LmNu; Weiming Liang, d2VpbWluZ2xpYW5nQGd4dXN0LmVkdS5jbg==
†These authors share first authorship
‡ORCID: Siliang Duan, orcid.org/0000-0003-4054-9367
 Yongfa Lei
Yongfa Lei Xiaotian Liang
Xiaotian Liang Hua Zhu1†
Hua Zhu1† Jin Wang
Jin Wang Weiming Liang
Weiming Liang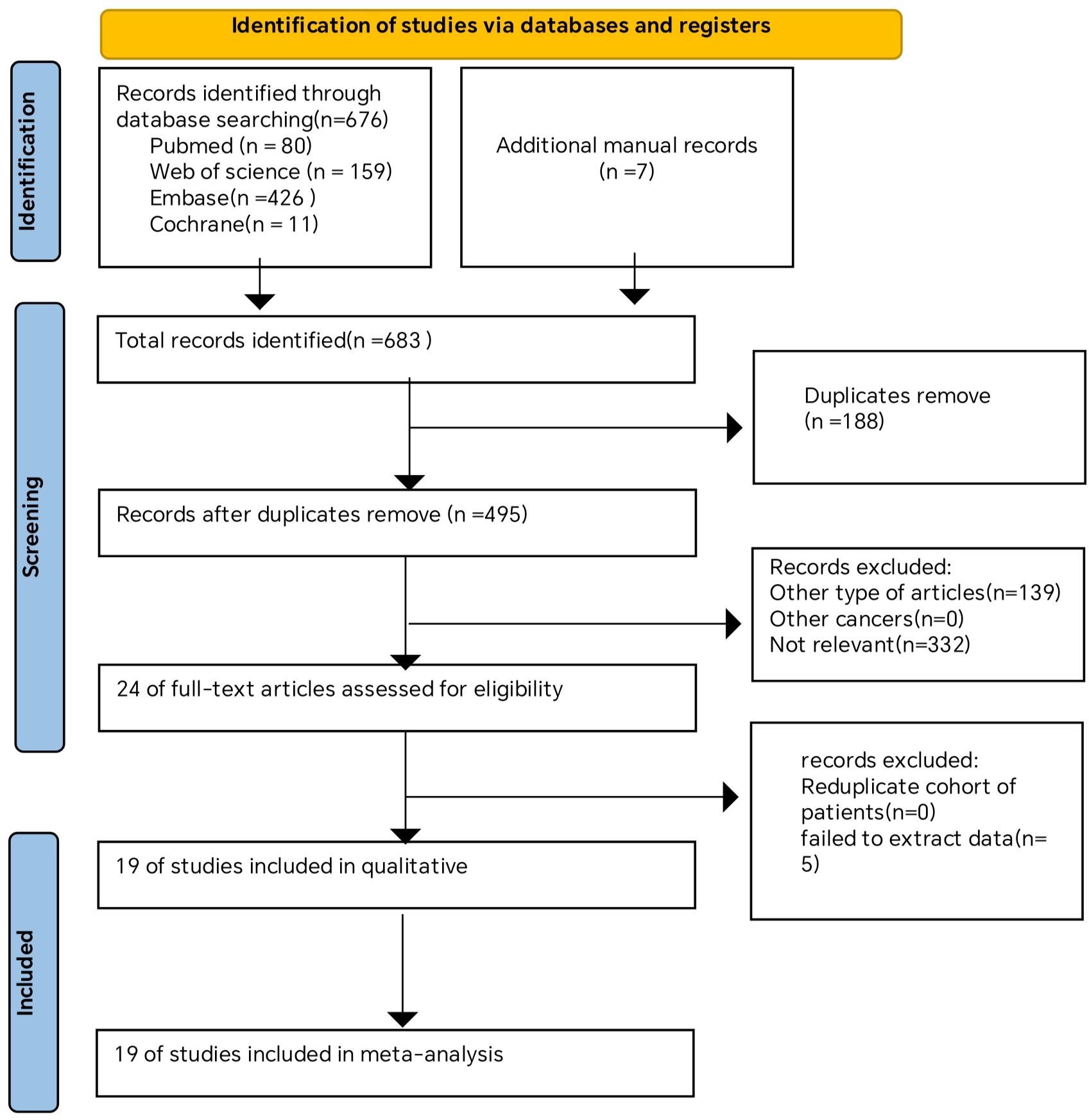
![Forest plot comparing TACE-L-P and TACE-L across various studies, showing odds ratios and confidence intervals. Each study is listed with event counts and weights. The overall effect odds ratio is 3.22 with confidence interval [2.32, 4.45], indicating heterogeneity with Tau²=0.15 and I²=34%.](https://www.frontiersin.org/files/Articles/1586914/fimmu-16-1586914-HTML/image_m/fimmu-16-1586914-g003.jpg)
![Forest plot showing hazard ratios with confidence intervals for various studies or subgroups. Each entry lists the study name, log hazard ratio, standard error, weight, and hazard ratio with confidence interval. A diamond at the bottom represents the total effect size, with a confidence interval of 0.54 [0.46, 0.62]. Heterogeneity statistics include Tau² = 0.05, Chi² = 33.51, degrees of freedom = 18, I² = 46%. The overall effect size is statistically significant with Z = 7.96 and P-value less than 0.00001. Horizontal lines with dots represent individual study data in the plot.](https://www.frontiersin.org/files/Articles/1586914/fimmu-16-1586914-HTML/image_m/fimmu-16-1586914-g004.jpg)