- 1Department of Medical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Breast Radiotherapy Ward, Department of Radiotherapy, Shanxi Province Cancer Hospital, Shanxi Hospital Affiliated to Cancer Hospital, Chinese Academy of Medical Sciences, Cancer Hospital Affiliated to Shanxi Medical, Shanxi, China
- 3Department of Respiratory and Critical Care Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
- 4Department of Medical Oncology, Cancer Hospital of HuanXing ChaoYang District, Beijing, China
Drug-induced sarcoidosis-like reaction (DISR) is a rare adverse event associated with immunotherapy. Currently, there is no standardized treatment protocol for DISR linked to immune checkpoint inhibitors (ICIs). This study presents a case of an early-stage triple-negative breast cancer (TNBC) patient who developed hepatic sarcoidosis-like reactions during neoadjuvant pembrolizumab therapy. We provide an overview of ICI-induced sarcoidosis-like reactions in cancer patients, including incidence, mechanisms, clinical manifestations, treatment, and prognosis. Additionally, we discuss the significance of one-year adjuvant immunotherapy for early-stage TNBC patients who achieved pathological complete response after neoadjuvant therapy, offering insights for individualized therapeutic strategies in this population.
1 Case presentation
1.1 Treatment for locally advanced triple-negative breast cancer
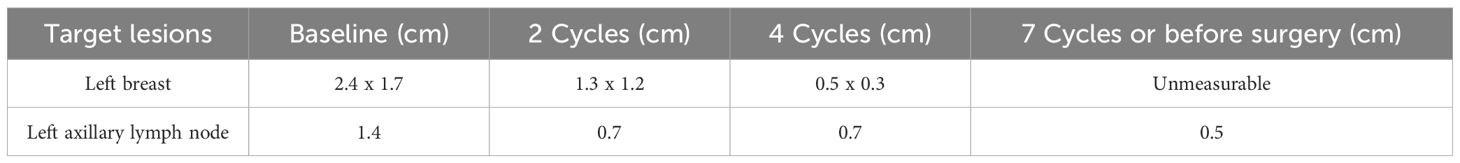
A female patient in her 40s, premenopausal, was diagnosed with locally advanced TNBC. Pathological analysis confirmed invasive ductal carcinoma (IDC), grade III, negative status of estrogen receptor (ER), progesterone receptor (PR) and HER2, a high Ki-67 index of 80%, and a combined positive score of 10% for programmed death ligand 1 (PD-L1) expression. Biopsies of the left supraclavicular and axillary lymph nodes showed cancer cells. MRI and whole-body PET-CT scans indicated a clinical stage of T2 (2.4cm x 1.7cm) N3c (positive left supraclavicular lymph nodes) M0.
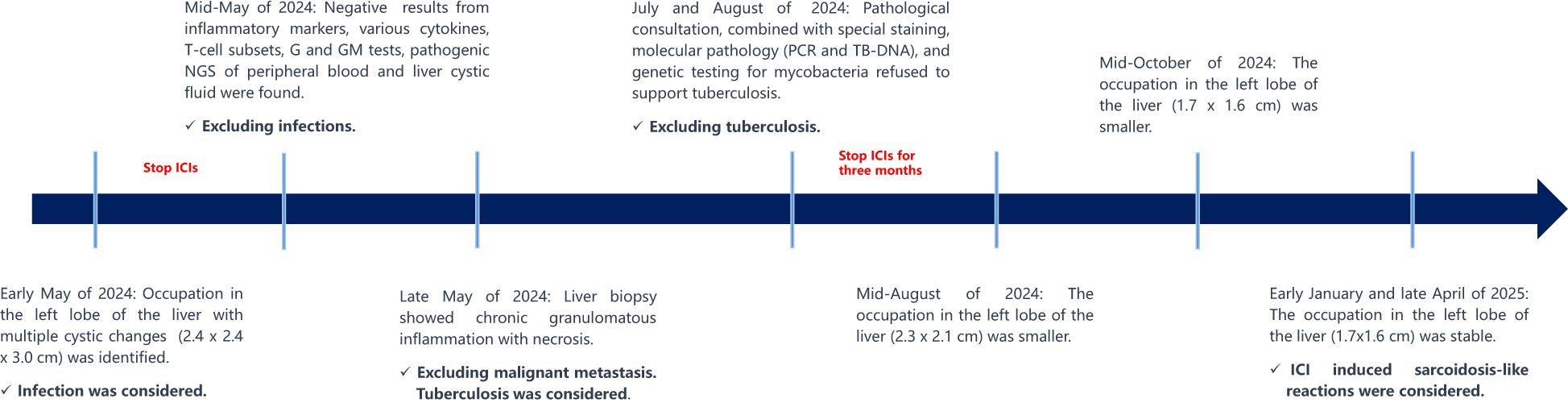
Inspired by the promising outcomes from the KEYNOTE-522 study (1, 2), the patient would receive four cycles of neoadjuvant paclitaxel (175 mg/m²) plus pembrolizumab (200 mg) every three weeks, followed by four cycles of epirubicin (90 mg/m²) and cyclophosphamide (600 mg/m²) (EC regimen) + pembrolizumab. However, a contrast-enhanced MRI after six cycles revealed a suspicious lesion (2.4 x 2.4 x 3.0 cm) with cystic changes in the left lobe of the liver (Supplementary Figure S2A). To mitigate potential immune checkpoint inhibitors (ICIs)-related adverse events (AEs) and enhance the efficacy of neoadjuvant therapy, cycle 7 was treated with EC chemotherapy alone, omitting ICIs. After seven cycles, the tumor lesions were confirmed as clinical CR. The efficacy of neoadjuvant therapy is summarized in Table 1; Supplementary Figure S3.
The patient underwent modified radical surgery after neoadjuvant therapy. Postoperative pathology revealed a pathological complete response (pCR). Due to the uncertain nature of the liver lesion, adjuvant pembrolizumab was postponed. Adjuvant radiotherapy was administered according to the radiation therapists.
1.2 ICI-induced hepatic sarcoidosis-like reaction and follow-up
A liver puncture biopsy unraveled some swelling hepatocytes accompanied by local steatosis and siltation, as well as local epithelioid cell nodules with necrosis, which are infiltrated by high levels of inflammatory cells and hyperplasia fibrotic cells (Figure 1A). Immunohistochemistry: CD34 (vascular +), CD163 (partial +), CD68 (partial +), Ki-67 (10% +), GPC-3 (-). Special staining: PAS (-) (Figure 1B, 40X), weak antacid stain (-), silver hexamine (-), antacid-TB (-) (Figure 1C, 40X). Peripheral blood and hepatic cystic fluid were further tested for multiple nucleic acid high-throughput sequences (covering 44 bacteria, 13 fungi, 7 DNA viruses, 4 parasites, and 7 atypical pathogens), as well as inflammatory markers (including CRP, PCT and ESR), various cytokines, T-cell subsets, G and GM tests, to identify potential pathogens. As a result, no evidence of infection in a spectrum of pathogens was observed. Additionally, the patient denied any infection-related symptoms during the overall course of disease, such as fever, cough, sputum, and diarrhea, etc. Doctors draw out the opinion that liver metastasis and infection are not considered, while tuberculosis needs to be precluded.
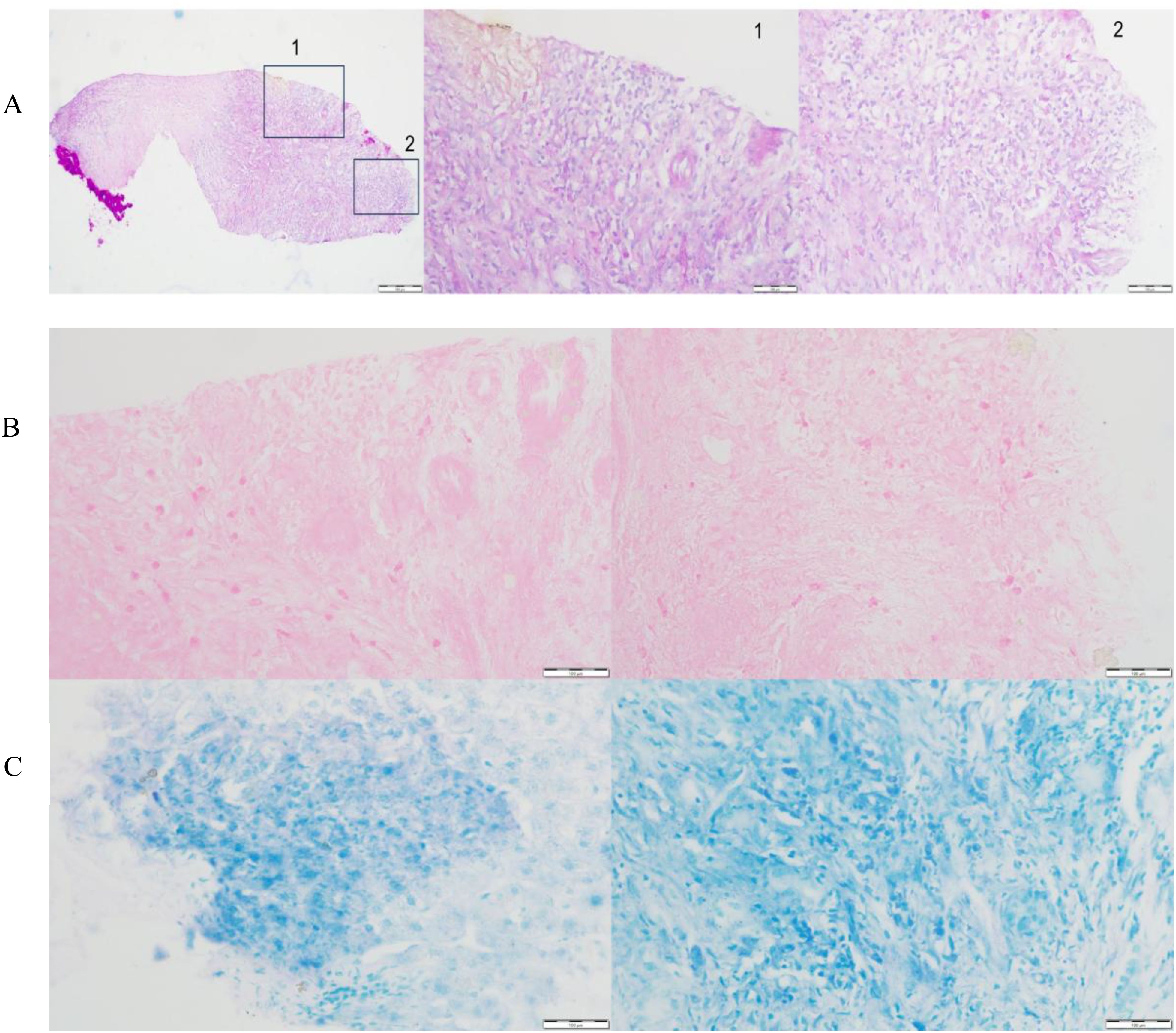
Figure 1. Pathological evaluation associated with tuberculosis of the liver tissue. (A) High levels of inflammatory cells and hyperplasia fibrotic cells were observed in the marked areas, indicating chronic granulomatous inflammation. Special staining of antacid-TB (B) and PAS (C) were negative.
The patient then consulted two specialized hospitals and came up with similar conclusions. Primary T/B-spot screening was negative. Further examination associated with tuberculosis using the biopsy, including pathology consultation, molecular pathology (such as PCR, TB-DNA), special staining, and genetic testing for mycobacteria, unraveled negative results. The pathology indicated chronic granulomatous inflammation with necrosis. Several months after discontinuing immunotherapy, follow-up MRI showed a reduction in the liver lesion’s size (Mid-August of 2024: 2.3 x 2.1 cm, Mid-October of 2024 to late April of 2025: 1.7x1.6 cm), confirming its stable condition (Supplementary Figures S2B-S2D). Figure 2 summarizes the diagnosis and treatment process for the liver lesion.
Since the patient reported no liver discomfort or functional abnormalities, combined with the reduction of the sarcoidosis-like lesion after discontinuing ICIs, hormonal or anti-tuberculosis therapy was deemed unnecessary. The enlarged liver lesion exhibited no anomalies and spontaneously diminished without medical intervention. Therefore, the hepatic cystic lesion that arose during pembrolizumab treatment is likely an ICI-induced AE.
2 Discussion
2.1 ICI induced sarcoidosis-like reaction
ICI-induced sarcoidosis-like reaction (ISR) is relatively rare, which can be attributed to anti-PD-1 therapy, anti-PD-L1 therapy, and anti-CTLA-4 therapy (3). In a retrospective real-world study, the incidence of DISR caused by anti-PD-1 treatment, anti-CTLA-4 treatment, and anti-PD-1 + anti-CTLA-4 treatment was 3.7% (11/297), 3.7% (3/81), and 6.3% (5/79), respectively (4). In another study, the incidence was reported as 2.4% (2/83) (5). The median interval from initiation of ICIs to DISR has been documented as 5.5 months (range: 2.3-13.5 months) (6, 7), which is highly consistent with the present case. The mechanism of ISR remains unclear. Possibly, the use of ICIs triggers a series of enhanced immune responses in a pathologic way, leading to systemic sarcoidosis-like reactions (4, 8).
The clinical manifestations of ISR vary, which mainly present with enlarged lymph nodes. It also exhibits sarcoidosis-like reactions in distinct organs, accompanied by fever, dysfunction of the liver or kidney, or endocrine disorders (Supplementary Table S1) (5, 9–12). Nevertheless, hepatic sarcoidosis-like reactions from neoadjuvant pembrolizumab have not been reported.
Most patients with ISR are asymptomatic, and do not require systemic therapy (8). In general, ISR can automatically and gradually improve once immunotherapy is discontinued (8). For some cases with organ dysfunction or severe clinical symptoms, hormonal therapy, like dexamethasone, is highly effective and can rapidly improve and eliminate systemic symptoms (4, 8, 11). Interestingly, such patients with severe adverse events frequently develop superior drug responses and survival outcomes to ICI treatment. It has been demonstrated that ISR is significantly, and positively correlated with the long-term survival of cancer patients (11, 13). Therefore, oncologists support the continual use of immunotherapy following the onset of ISR, due to favorable controllability. A patient with advanced esophageal squamous cell carcinoma, who developed pulmonary sarcoidosis-like reactions induced by sintilimab, was reported to receive the anti-PD-1 inhibitor of sintilimab for one year in a routine, contributing to a manageable safety profile (11). Regarding drug efficacy, the tumor lesions of the patient were kept in a stable condition, showing satisfactory prognostic outcomes (11).
2.2 Neoadjuvant immunotherapy for patients with early-stage TNBC
As far as we know, it represents the first case of hepatic sarcoidosis-like reactions induced by neoadjuvant pembrolizumab for patients with early-stage TNBC, which represents a rare adverse event for pembrolizumab. Although ISR have been previously reported in the advanced cancer patients (9–12), it is infrequent in the population of patients with TNBC, especially in the early stage. To see, the case is highly innovative from the perspective of the disease population, as well as the specific type and therapeutic stage of immunotherapy.
Emerging studies have elaborated the clinical benefits of ICIs in TNBC patients, such as the famous KEYNOTE-522 study (2, 14, 15). In the clinical trial, the addition of the PD-1 inhibitor pembrolizumab to chemotherapy as neoadjuvant therapy was proved to be effective for patients with early-stage TNBC, significantly improving pCR rates (short-term efficacy), event-free survival (EFS), and overall survival (OS, long-term efficacy) in the intention-to-treat population (1, 2, 14). Up to now, the survival benefit of pembrolizumab after surgery for those who achieve pCR is controversial. Published data provide some insights into the issue. In the subgroup analyses focusing on patients achieving pCR, EFS and OS were not significant for patients who received pembrolizumab in the post-surgery setting versus those who did not, with survival curves almost overlapping (2). It means that the survival benefit of pembrolizumab therapy subsequent to surgery for one year is weak, inferring the limited necessity to continue pembrolizumab in the specific patient population. However, in terms of hazard ratios (HR), pembrolizumab-combined therapy following surgery was suggested to reduce 31% of risks of recurrence or death for patients achieving pCR (HR=0.69, 95% CI = 0.38-1.26)2, reflecting profound clinical benefits of pembrolizumab in early-stage TNBC patients with pCR.
Overall, patients with early-stage TNBC are more likely to derive survival benefits when they receive pembrolizumab combined with neoadjuvant chemotherapy compared to those who receive neoadjuvant chemotherapy alone. For the targeted patients with pCR, a great number of factors need to be weighed regarding the subsequent adjuvant immunotherapy lasting one year. They include patients’ own wishes, tolerability and safety, drug accessibility, and economic conditions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YT: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. YW: Data curation, Investigation, Writing – review & editing. XL: Data curation, Investigation, Writing – review & editing. QM: Data curation, Investigation, Writing – review & editing. CZ: Data curation, Investigation, Writing – review & editing. AZ: Data curation, Investigation, Writing – review & editing. XS: Data curation, Investigation, Writing – review & editing. FM: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. JW: Conceptualization, Data curation, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by National Natural Science Foundation of China (82230058).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1589191/full#supplementary-material
References
1. Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kummel S, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. (2022) 386:556–67. doi: 10.1056/NEJMoa2112651
2. Schmid P, Cortes J, Dent R, McArthur H, Pusztai L, Kummel S, et al. Overall survival with pembrolizumab in early-stage triple-negative breast cancer. N Engl J Med. (2024) 391:21. doi: 10.1056/NEJMoa2409932
3. Jayan A, Sukumar JS, Fangman B, Patel T, Raghavendra AS, Liu D, et al. Real-world immune-related adverse events in patients with early triple-negative breast cancer who received pembrolizumab. JCO Oncol Pract. (2004) 2024:OP2400371. doi: 10.1200/OP.24.00371
4. Li Y, Flavell RR, Juarez R, Chow M, Wu C, Tsai K, et al. Retrospective study of the incidence of sarcoidosis-like reaction in patients treated with immunotherapy. Clin Radiol. (2023) 78:e131–6. doi: 10.1016/j.crad.2022.09.127
5. Rizzo M, Pezzicoli G, Ganini C, Carone L, Calio A, Brunelli M, et al. Sarcoidosis-like reactions in metastatic renal cell carcinoma patients treated with immune-based combinations. Immunotherapy. (2024) 16:603–9. doi: 10.1080/1750743X.2024.2342222
6. Bronstein Y, Ng CS, Hwu P, and Hwu WJ. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. AJR Am J Roentgenol. (2011) 197:W992–W1000. doi: 10.2214/AJR.10.6198
7. Torres-Jimenez J, Esteban-Villarrubia J, Garcia-Abellas P, Cortes-Salgado A, Soria-Rivas A, Gajate-Borau P, et al. Sarcoidosis-like reactions in cancer patients treated with immune checkpoint inhibitors: experience in a Spanish hospital. Clin Transl Oncol. (2021) 23:1474–80. doi: 10.1007/s12094-020-02546-w
8. Gkiozos I, Kopitopoulou A, Kalkanis A, Vamvakaris IN, Judson MA, and Syrigos KN. Sarcoidosis-like reactions induced by checkpoint inhibitors. J Thorac Oncol. (2018) 13:1076–82. doi: 10.1016/j.jtho.2018.04.031
9. Park SD, Kim MS, Han MH, Kim YJ, Jung HY, Choi JY, et al. Renal sarcoidosis-like reaction induced by PD-1 inhibitor treatment in non-small cell lung cancer: A case report and literature review. Medicina (Kaunas). (2023) 59(5):991. doi: 10.3390/medicina59050991
10. Sagawa T, Sato Y, Nagashima H, Takada K, Takahashi M, Hirakawa M, et al. Hilar/mediastinal and cutaneous drug-induced sarcoidosis-like reaction associated with immune checkpoint inhibitors in metastatic colorectal cancer: a case report. Front Immunol. (2023) 14:1203621. doi: 10.3389/fimmu.2023.1203621
11. Li H, Mu F, Zou B, and Wang L. Pulmonary sarcoidosis-like reactions induced by sintilimab in esophageal cancer: A case report. Med (Baltimore). (2023) 102:e34432. doi: 10.1097/MD.0000000000034432
12. Tsunoda A, Mizuno T, Iida S, Uchida K, Yamashita M, Sukeno K, et al. Atezolizumab-induced sarcoidosis-like reaction in a patient with metastatic breast cancer. Case Rep Oncol Med. (2022) 2022:2709062. doi: 10.1155/2022/2709062
13. Shi Y, Li J, Chen M, Liu H, Ma D, Lin Y, et al. Sarcoidosis-like reaction after neoadjuvant pembrolizumab combined with chemotherapy mimicking disease progression of NSCLC induced encouraging discovery of pathological complete response. Thorac Cancer. (2021) 12:3433–6. doi: 10.1111/1759-7714.14228
14. Schmid P, Cortes J, Pusztai L, McArthur H, Kummel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. (2020) 382:810–21. doi: 10.1056/NEJMoa1910549
Keywords: triple-negative breast cancer, pembrolizumab, sarcoidosis-like reaction, neoadjuvant immunotherapy, early breast cancer
Citation: Tan Y, Wang Y, Liu X, Ma Q, Zeng C, Zhu A, Sun X, Ma F and Wang J (2025) Case Report: Hepatic sarcoidosis-like reaction from neoadjuvant pembrolizumab in early-stage triple-negative breast cancer. Front. Immunol. 16:1589191. doi: 10.3389/fimmu.2025.1589191
Received: 07 March 2025; Accepted: 29 May 2025;
Published: 18 June 2025.
Edited by:
Ahmed Fahim, Royal Wolverhampton Hospitals NHS Trust, United KingdomReviewed by:
Yan Guo, The Second Military Medical University, ChinaMohamed Essameldin Abdelgawad, Wake Forest University, United States
Copyright © 2025 Tan, Wang, Liu, Ma, Zeng, Zhu, Sun, Ma and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiani Wang, bmNjX3dhbmdqaWFuaUAxMjYuY29t; Fei Ma, ZHJtYWZlaUAxMjYuY29t
†The authors have contributed equally to the work
 Yujing Tan
Yujing Tan Yu Wang2†
Yu Wang2† Cheng Zeng
Cheng Zeng Fei Ma
Fei Ma Jiani Wang
Jiani Wang