- The Department of Medical Oncology, Cancer Hospital of Shantou University Medical College, Shantou, China
Background: Type 2 papillary renal cell carcinoma (PRCC) is an aggressive subtype of renal cell carcinoma with a poor prognosis. Diagnosis is challenging, particularly when presenting as a metastatic lung mass. This report describes a case of metastatic type 2 PRCC treated with first-line toripalimab (anti-PD-1) and lenvatinib (multikinase inhibitor), highlighting sustained clinical benefit.
Case presentation: A 73-year-old man presented with cough, hemoptysis, and a large left lung mass initially misdiagnosed as primary lung cancer. Multidisciplinary re-evaluation, including immunohistochemistry (PAX8+, P504s+, also known as AMACR), confirmed metastatic type 2 PRCC. After 11 months of toripalimab (flat dose of 240 mg every 3 weeks) and lenvatinib (flat dose of 8 mg daily), serial imaging demonstrated partial response (PR) with regression of pulmonary metastases, lymphadenopathy, and obstructive pneumonia. No serious adverse events occurred.
Conclusions: This case underscores the potential efficacy and tolerability of toripalimab-lenvatinib combination therapy in metastatic type 2 PRCC. Further clinical trials are warranted to validate this approach.
1 Introduction
Type 2 papillary renal cell carcinoma (PRCC) accounts for 10–15% of renal cell carcinomas and is distinguished by aggressive behavior, frequent metastasis, and poor prognosis (1). Unlike type 1 PRCC, type 2 tumors often exhibit rapid progression and resistance to conventional therapies (2). Metastatic type 2 PRCC poses diagnostic challenges, particularly when presenting as a lung mass mimicking primary pulmonary malignancy (3).
Therapeutic advances in renal cell carcinoma include tyrosine kinase inhibitor (TKI) such as lenvatinib, which targets VEGF receptors, and immune checkpoint inhibitor (ICI) like toripalimab (4, 5). Preclinical and clinical studies suggest synergy between TKI and immunotherapy, but data specific to type 2 PRCC remain limited (6). This report details a case of metastatic type 2 PRCC achieving sustained response to toripalimab-lenvatinib therapy, emphasizing the need for further investigation.
2 Case presentation
A 73-year-old man presented in May 2023 with cough and hemoptysis. Chest CT revealed a left lung mass. Initial biopsies suggested sarcomatoid carcinoma, but the patient declined chemotherapy. By December 2023, he developed a productive cough, worsening dyspnea, lower limb edema, and pleural effusions. Re-evaluation at Shantou Shengbao Cerebrovascular Disease Hospital identified mediastinal lymphadenopathy and bilateral lung nodules. The patient presented no significant medical history and no family history of relevant disorders or malignancies.
In early January 2024, the patient presented to our hospital for medical evaluation with a Karnofsky Performance Status (KPS) score of 50. Immunohistochemistry (IHC) of a supraclavicular lymph node biopsy revealed PAX8++ and P504s++, consistent with metastatic renal cell carcinoma (Figure 1A). Further IHC of the lung mass confirmed type 2 PRCC (PAX8++, P504s+++) (Figures 1B, C). PD-L1 expression was additionally assessed, revealing a tumor proportion score (TPS) of 68% (Figure 1D). Laboratory investigations revealed serum creatinine (Cr) levels ranging from 60 to 99 µmol/L (reference range: 57–111 µmol/L), blood urea nitrogen (BUN) levels ranging from 4.05 to 7.19 mmol/L (reference range: 3.6–9.5 mmol/L), alanine aminotransferase (ALT) levels ranging from 34 to 61 U/L (reference range: 9–50 U/L), and aspartate aminotransferase (AST) levels ranging from 25 to 49 U/L (reference range: 15–40 U/L). These parameters indicate preserved hepatic and renal functional reserve in the patient. Furthermore, to establish a prognostic risk model for advanced renal cell carcinoma (RCC), we analyzed laboratory parameters including lactate dehydrogenase (LDH) levels (range: 188–325 U/L; reference: 114–240 U/L), corrected calcium (Ca) levels (range: 2.01-2.23 mmol/L; reference: 2.1-2.6 mmol/L), hemoglobin (Hb) levels (range: 123–154 g/L; reference: 130–175 g/L), neutrophil (NE) counts (range: 4.32-10.97 × 109/L; reference: 1.8-6.3 × 109/L), and platelet (PLT) counts (range: 160-296 × 109/L; reference: 125-350 × 109/L). Using both the Memorial Sloan Kettering Cancer Center (MSKCC) criteria and the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) criteria, patients were stratified into the intermediate-risk group by MSKCC criteria and the poor risk group by IMDC criteria at baseline.
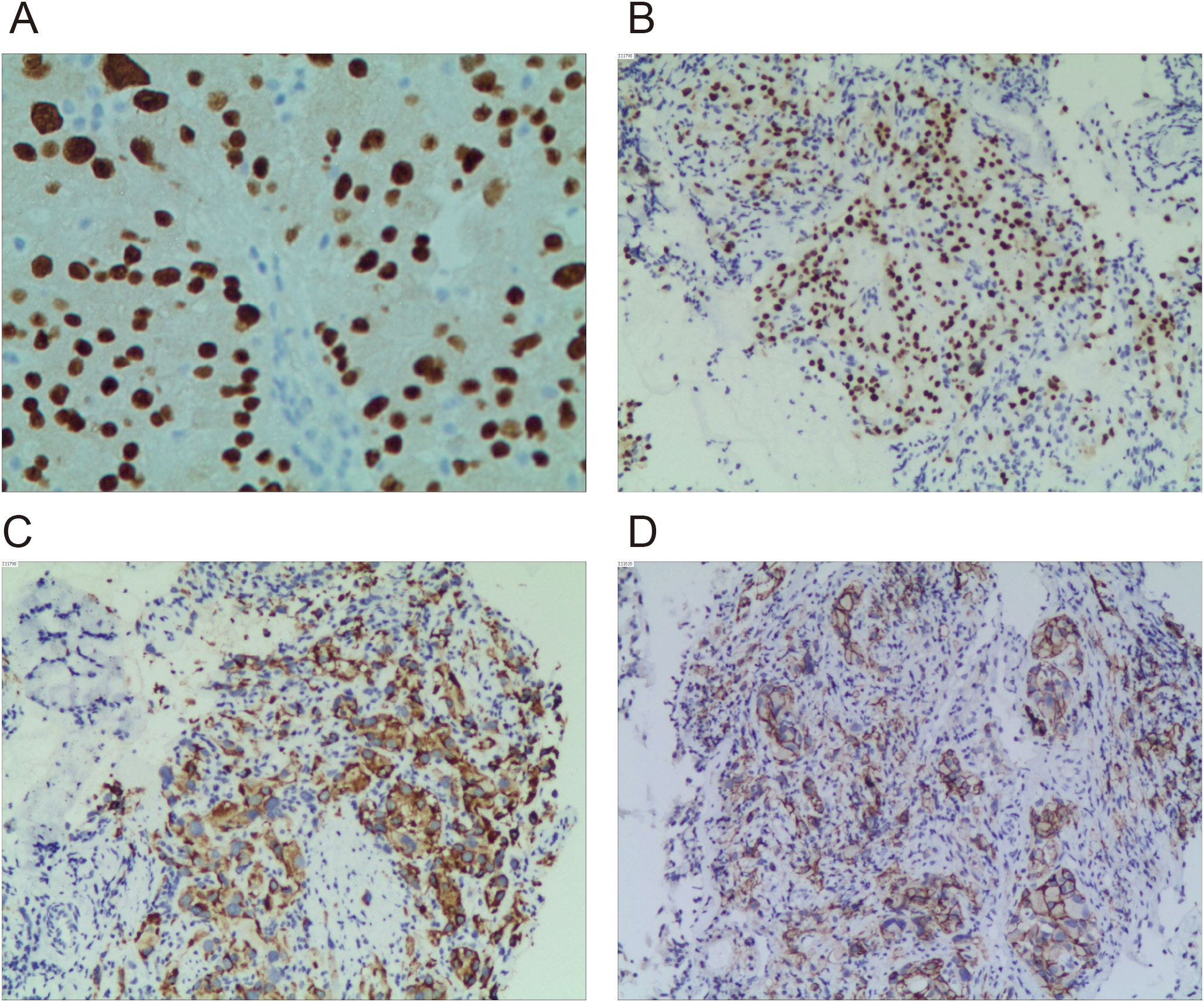
Figure 1. Immunohistochemical staining images of PAX8, P504s, and PD-L1. (A) Immunohistochemical detection of PAX8 in left supraclavicular lymph node specimens (×400). (B) Immunohistochemical detection of PAX8 in left upper lobe specimen (×200). (C) Immunohistochemical detection of P504s in left upper lobe specimen (×200). (D) Immunohistochemical detection of PD-L1 in left upper lobe specimen (×200).
In January 2024, toripalimab (flat dose of 240 mg every 3 weeks) and lenvatinib (flat dose of 8 mg daily) were initiated. Considering the patient’s financial situation, drug accessibility, and the demonstrated superior efficacy of toripalimab in advanced clear cell renal cell carcinoma (ccRCC) from the phase 3 RENOTORCH clinical trial, toripalimab was selected. At initial diagnosis, the patient was in poor clinical condition, exhibiting symptoms including a productive cough, exertional dyspnea, and bilateral lower limb edema. To optimize treatment tolerance, lenvatinib was initiated at a lower dose of 8 mg. According to the criteria defined by RECIST 1.1, follow-up CT at 3 months demonstrated stable disease (SD), with a 25.50% reduction in the target lesion and resolution of obstructive pneumonia. By September 2024 (10 cycles), metastases in both lungs and right renal nodes regressed further. Efficacy remained PR at 11 months, with no grade ≥3 adverse events (Figure 2). Furthermore, the patient’s KPS improved significantly, reaching 90-100. We plan to maintain immunotherapy combined with antiangiogenic therapy until disease progression or the occurrence of intolerable toxicity. A timeline of the major treatment process and CT evaluation of this case is shown in (Figure 3).
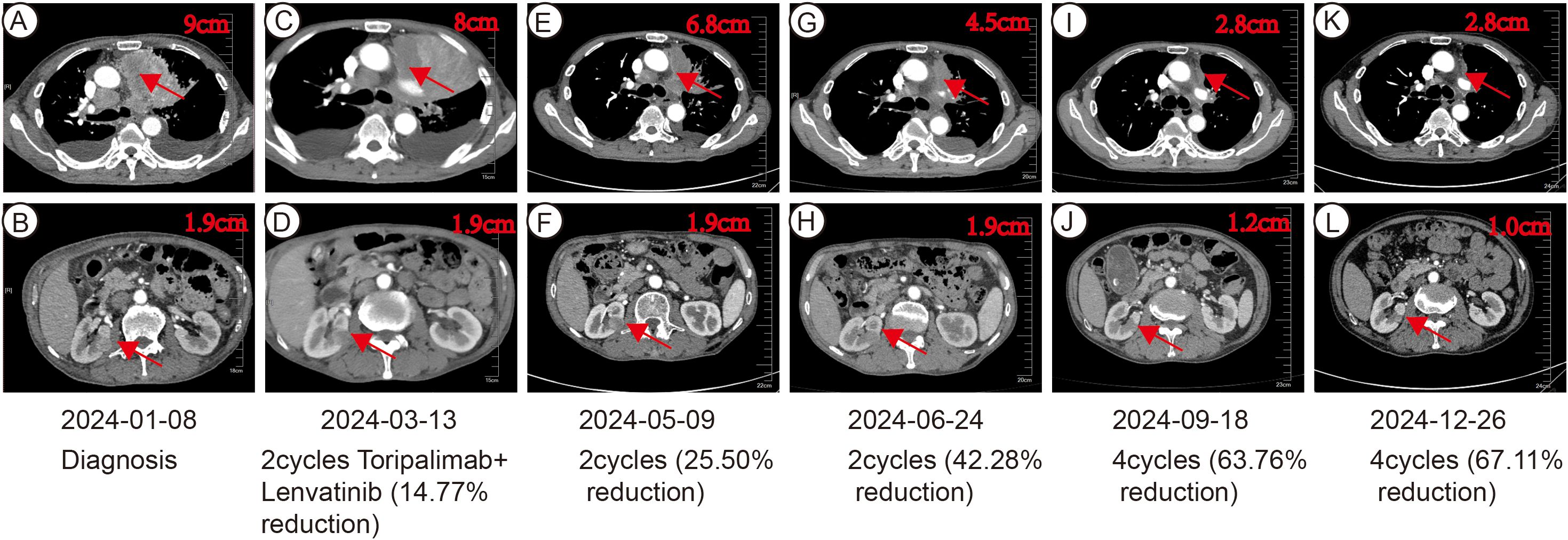
Figure 2. CT imaging assessment of left lung lesion and right kidney mass throughout the course of disease corresponding to their respective time points. (A, B) CT image at initial diagnosis on January 8, 2024. (C, D) CT image on March 13, 2024. (E, F) CT image on May 9, 2024. (G, H) CT image on June 24, 2024. (I, J) CT image on September 18, 2024. (K, L) CT image on December 26, 2024.
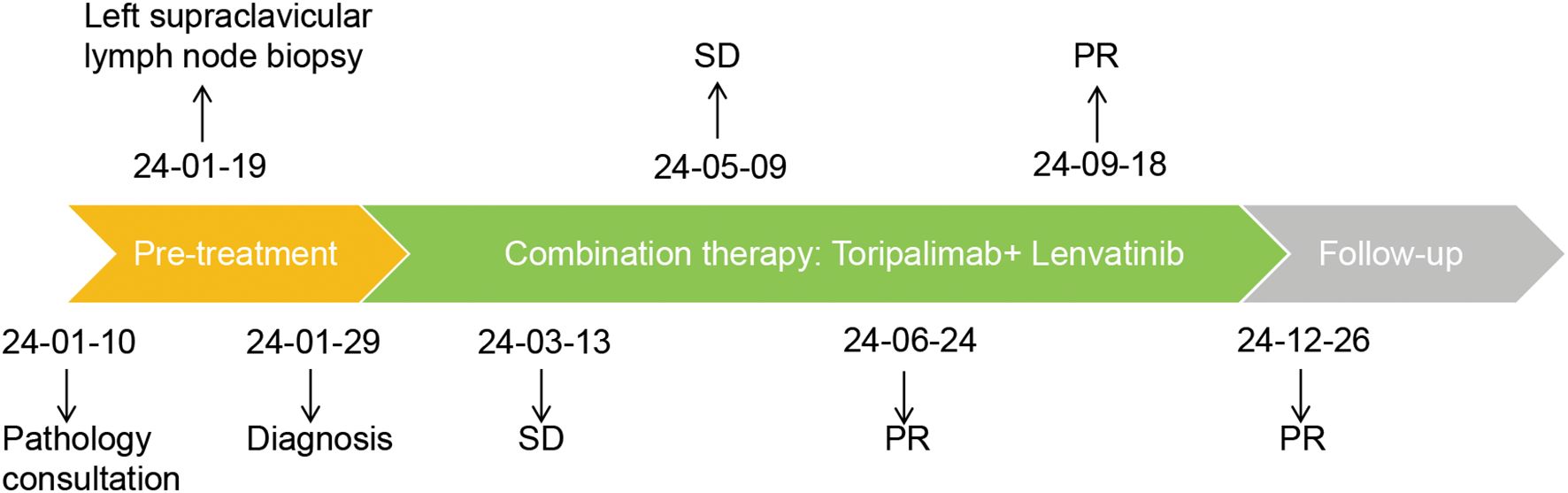
Figure 3. Timeline of the major treatment process and CT evaluation of this case. The tumor response was evaluated according to the criteria defined by RECIST 1.1. PR, Partial Response; SD, Stable Disease.
3 Discussion
Type 2 PRCC is characterized by its aggressive nature and poorer prognosis compared to type 1 PRCC (7). The distinct features of type 2 PRCC, including its tendency for lung metastasis and the diagnostic challenges it presents, highlight the urgent need for effective therapeutic strategies. In this case report, we present a 73-year-old male patient with metastatic type 2 PRCC who exhibited a sustained benefit from a combination of toripalimab and lenvatinib, underscoring the potential of this therapeutic approach in managing this complex malignancy.
The diagnosis of metastatic type 2 PRCC can be particularly challenging, especially when it presents as a large lung mass accompanied by respiratory symptoms. In this case, initial diagnostic uncertainty was resolved through a multidisciplinary approach, which included a comprehensive review of pathology slides and re-cutting of specimens. To date, no molecular or IHC markers specific to PRCC have been identified. PRCC typically shows reactivity for PAX8, AE1/AE3, Cam5.2, CD10, vimentin, P504s, and CK7, while demonstrating negativity for CD117 (KIT). Among these markers, P504s and CK7 are the most valuable IHC markers for distinguishing PRCC from other renal tumor types. P504s serves as a sensitive marker for PRCC, although it is not entirely specific (8). CK7 and MET IHC staining was more prevalent in type 1 PRCC and frequently absent (null phenotype) in type 2 PRCC. MET alterations also demonstrate higher frequency in type 1 PRCC. Additionally, type 2 PRCC exhibit Topoisomerase IIα overexpression. Notably, FH deficiency defines a distinct, clinically aggressive subtype that maintains papillary architecture and may histologically mimic type 2 PRCC. This emphasizes the critical importance of accurate histopathological evaluation in diagnosing renal cell carcinoma, particularly in cases where imaging findings are ambiguous. The integration of advanced imaging techniques and expert pathological consultation is essential for ensuring timely and accurate diagnoses, which can significantly impact treatment decisions and patient outcomes.
Currently, there are no established individualized treatment guidelines specifically for type 2 PRCC, with clinical management strategies generally derived from those for RCC. Several phase 2 studies evaluating targeted therapies have been conducted specifically in metastatic PRCC (mPRCC). Those investigated sunitinib (SUPAP) (9), everolimus (RAPTOR) (10), cabozantinib (PAPMET) (11) and axitinib (AXIPAP) (12) as first-line agents. Cabozantinib treatment led to a significantly longer progression-free survival (PFS) period (median 9.0 months, 95% CI 6% to 12%) compared to the sunitinib group (5.6 months, 3% to 7%; HR for progression or death 0.60, 0.37 to 0.97, one-sided p=0.019). The response rate for cabozantinib was 23% versus 4% for sunitinib (two-sided p=0.010) (11). Additionally, axitinib demonstrated promising efficacy in patients with mPRCC, particularly in type 2 PRCC, with manageable toxicity. The progression-free rate at 24 weeks, the primary endpoint, was 45.2% (95% CI 32.6% to not reached), the objective response rate (ORR) was 28.6% (95% CI 15.7% to 44.6%) with 7.7% in type 1 and 35.7% in type 2. The median overall survival (OS) was 18.9 months (95% CI 12.8 to not reached) (12). Furthermore, combination therapies have demonstrated superior efficacy over sunitinib monotherapy across pivotal trials. In CheckMate 9ER (13), nivolumab plus cabozantinib significantly extended median PFS to 16.6 months versus 8.3 months with sunitinib (HR 0.51; P<0.001) and doubled the ORR (55.7% vs. 27.1%; P<0.001). Similarly, in JAVELIN Renal 101 (14), avelumab plus axitinib provided a longer median PFS (13.8 vs. 7.2 months; HR 0.61; P<0.001) and a higher ORR (55.2% vs. 25.5%) in PD-L1-positive patients. Consistent with these findings, the KEYNOTE-426 trial showed that pembrolizumab plus axitinib improved median PFS (15.1 vs. 11.1 months; HR 0.69; P<0.001) and ORR (59.3% vs. 35.7%; P<0.001) compared to sunitinib (15). The clinical efficacy of immunotherapy, either as monotherapy or in combination, has been established in metastatic RCC with a clear cell histologic component (16, 17). However, pivotal studies of ICI excluded non-clear cell renal cell carcinoma (nccRCC). Small retrospective cohorts have reported conflicting results regarding the response of nccRCC to CPI (18–22).
This case illustrates the diagnostic complexity of metastatic type 2 PRCC and the therapeutic potential of combining PD-1 inhibition with antiangiogenic therapy. Initial misdiagnosis as lung cancer underscores the necessity of multidisciplinary collaboration and advanced IHC (e.g., PAX8, P504s) to confirm renal origin (23).
Lenvatinib inhibits tumor angiogenesis via VEGFR/FGFR blockade, while toripalimab enhances antitumor immunity by disrupting PD-1-mediated immunosuppression (24, 25). Synergy between these agents may explain the sustained PR observed here, aligning with recent trials demonstrating improved progression-free survival with TKI-immunotherapy combinations in renal cell carcinoma (13). Interestingly, this case exhibited a high PD-L1 tumor proportion score of 68%, which raises the possibility of its role as a predictive biomarker for response to PD-1 inhibition in nccRCC. Notably, the absence of severe toxicity contrasts with prior reports of combination-related adverse effects, suggesting careful patient selection and monitoring are critical (14). Limitations of the case report include the single-case design and lack of long-term follow-up. We acknowledge that the treatment duration of 11 months is relatively short for assessing long-term outcomes or disease progression. Larger studies are needed to define optimal dosing, duration, and biomarkers for response.
In summary, Toripalimab-lenvatinib combination therapy achieved durable response in metastatic type 2 PRCC with favorable tolerability. This case supports further exploration of dual-targeted strategies in rare, aggressive renal malignancies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee and the Institutional Review Board of Cancer Hospital of Shantou University Medical College. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MC: Methodology, Supervision, Visualization, Writing – review & editing. WX: Data curation, Software, Supervision, Writing – review & editing. DZ: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1591489/full#supplementary-material
References
1. Delahunt B and Eble JN. Papillary renal cell carcinoma: a clinicopathologic and immunohistochemical study of 105 tumors. Mod Pathol. (1997) 10:537–44.
2. Cancer Genome Atlas Research N, Linehan WM, Spellman PT, Ricketts CJ, Creighton CJ, Fei SS, et al. Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med. (2016) 374:135–45. doi: 10.1056/NEJMoa1505917
3. Kuroda N, Karashima T, Inoue K, Kasajima A, Ohe C, Kawakami F, et al. Review of renal cell carcinoma with rhabdoid features with focus on clinical and pathobiological aspects. Pol J Pathol. (2015) 66:3–8. doi: 10.5114/pjp.2015.51147
4. Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomized, phase 2, open-label, multicenter trial. Lancet Oncol. (2015) 16:1473–82. doi: 10.1016/S1470-2045(15)00290-9
5. Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, et al. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Res. (2019) 25:515–23. doi: 10.1158/1078-0432.CCR-18-2484
6. Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicenter, open-label, phase 3, randomized controlled trial. Lancet. (2019) 393:2404–15. doi: 10.1016/S0140-6736(19)30723-8
7. Ricketts CJ, De Cubas AA, Fan H, Smith CC, Lang M, Reznik E, et al. The cancer genome atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep. (2024) 43:113063. doi: 10.1016/j.celrep.2023.113063
8. Tretiakova MS, Sahoo S, Takahashi M, Turkyilmaz M, Vogelzang NJ, Lin F, et al. Expression of alpha-methylacyl-CoA racemase in papillary renal cell carcinoma. Am J Surg Pathol. (2004) 28:69–76. doi: 10.1097/00000478-200401000-00007
9. Ravaud A, Oudard S, De Fromont M, Chevreau C, Gravis G, Zanetta S, et al. First-line treatment with sunitinib for type 1 and type 2 locally advanced or metastatic papillary renal cell carcinoma: a phase II study (SUPAP) by the French Genitourinary Group (GETUG)dagger. Ann Oncol. (2015) 26:1123–28. doi: 10.1093/annonc/mdv149
10. Escudier B, Molinie V, Bracarda S, Maroto P, Szczylik C, Nathan P, et al. Open-label phase 2 trial of first-line everolimus monotherapy in patients with papillary metastatic renal cell carcinoma: RAPTOR final analysis. Eur J Cancer. (2016) 69:226–35. doi: 10.1016/j.ejca.2016.08.004
11. Pal SK, Tangen C, Thompson IM Jr., Balzer-Haas N, George DJ, Heng DYC, et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomized, open-label, phase 2 trial. Lancet. (2021) 397:695–703. doi: 10.1016/S0140-6736(21)00152-5
12. Negrier S, Rioux-Leclercq N, Ferlay C, Gross-Goupil M, Gravis G, Geoffrois L, et al. Axitinib in first-line for patients with metastatic papillary renal cell carcinoma: Results of the multicenter, open-label, single-arm, phase II AXIPAP trial. Eur J Cancer. (2020) 129:107–16. doi: 10.1016/j.ejca.2020.02.001
13. Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2021) 384:829–41. doi: 10.1056/NEJMoa2026982
14. Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2019) 380:1103–15. doi: 10.1056/NEJMoa1816047
15. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2019) 380:1116–27. doi: 10.1056/NEJMoa1816714
16. Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. (2015) 373:1803–13. doi: 10.1056/NEJMoa1510665
17. Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. (2018) 378:1277–90. doi: 10.1056/NEJMoa1712126
18. Koshkin VS, Barata PC, Zhang T, George DJ, Atkins MB, Kelly WJ, et al. Clinical activity of nivolumab in patients with non-clear cell renal cell carcinoma. J Immunother Cancer. (2018) 6:9. doi: 10.1186/s40425-018-0319-9
19. McKay RR, Bosse D, Xie W, Wankowicz SAM, Flaifel A, Brandao R, et al. The clinical activity of PD-1/PD-L1 inhibitors in metastatic non-clear cell renal cell carcinoma. Cancer Immunol Res. (2018) 6:758–65. doi: 10.1158/2326-6066.CIR-17-0475
20. Yip SM, Wells C, Moreira R, Wong A, Srinivas S, Beuselinck B, et al. Checkpoint inhibitors in patients with metastatic renal cell carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium. Cancer. (2018) 124:3677–83. doi: 10.1002/cncr.31595
21. Stukalin I, Wells JC, Graham J, Yuasa T, Beuselinck B, Kollmansberger C, et al. Real-world outcomes of nivolumab and cabozantinib in metastatic renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Curr Oncol. (2019) 26:e175–e79. doi: 10.3747/co.26.4595
22. Chahoud J, Msaouel P, Campbell MT, Bathala T, Xiao L, Gao J, et al. Nivolumab for the treatment of patients with metastatic non-clear cell renal cell carcinoma (nccRCC): A single-institutional experience and literature meta-analysis. Oncologist. (2020) 25:252–58. doi: 10.1634/theoncologist.2019-0372
23. Tickoo SK, dePeralta-Venturina MN, Harik LR, Worcester HD, Salama ME, Young AN, et al. Spectrum of epithelial neoplasms in end-stage renal disease: an experience from 66 tumor-bearing kidneys with emphasis on histologic patterns distinct from those in sporadic adult renal neoplasia. Am J Surg Pathol. (2006) 30:141–53. doi: 10.1097/01.pas.0000185382.80844.b1
24. Kato Y, Tabata K, Kimura T, Yachie-Kinoshita A, Ozawa Y, Yamada K, et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PloS One. (2019) 14:e0212513. doi: 10.1371/journal.pone.0212513
Keywords: type 2 papillary renal cell carcinoma, immunotherapy, toripalimab, lenvatinib, p504s
Citation: Wang W, Chen M, Xue W and Zeng D (2025) Combination therapy with toripalimab and lenvatinib in metastatic type 2 papillary renal cell carcinoma: a Case Report. Front. Immunol. 16:1591489. doi: 10.3389/fimmu.2025.1591489
Received: 11 March 2025; Accepted: 21 October 2025;
Published: 06 November 2025.
Edited by:
Yanzhu Zhu, Jilin Agricultural Science and Technology College, ChinaCopyright © 2025 Wang, Chen, Xue and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: De Zeng, ZGV6ZW5nQHN0dS5lZHUuY24=
 Wende Wang
Wende Wang Minna Chen
Minna Chen De Zeng
De Zeng