- 1Zhuzhou Clinical College, Jishou University, Zhuzhou, Hunan, China
- 2Department of Infectious Disease, Zhuzhou Hospital Affiliated to Xiangya School of Medicine, Central South University, Zhuzhou, Hunan, China
- 3Hunan Traditional Chinese Medicine College, Zhuzhou, Hunan, China
- 4Department of Assisted Reproductive Centre, Zhuzhou Hospital Affiliated to Xiangya School of Medicine, Central South University, Zhuzhou, Hunan, China
Lung cancer is a prevalent malignant tumor and the leading cause of cancer-related mortality worldwide. LC is a complex respiratory condition that poses significant challenges for both clinicians and researchers. Crucially, dysregulation of molecular signaling pathways is a key message point in LC. Numerous reviews have highlighted effective treatments for LC by targeting disrupted signaling pathways. Understanding the roles and interconnections of various signaling pathways in LC is crucial. Therefore, this paper reviews the pathogenesis, biological functions and their important interactions in lung cancer. Frist, we reviewed relevant signaling pathways involved in LC, including Wnt, PI3K/Akt, Notch, PD-1/PD-L1, NF-κB, Hippo, MAPK, Hedgehog, AMPK. Immediately thereafter, we further explored the biological functions of LC in this area of pathophysiology, such as apoptosis, metastasis and proliferation. In conclusion, after our deeper understanding of the interactions of these signaling pathways in LC. And we must recognize that the interactions between the above signaling pathways can lead to comprehensive as well as novel therapeutic approaches for LC.
Introduction
Lung cancer (LC) is one of the most prevalent malignant tumors in the world, noted for its high incidence and mortality rates (1, 2). The main reasons for this outcome are the absence of early symptoms of LC and the insufficient emphasis on cancer prevention strategies in developing countries, leading to unexpected global harm (1, 3). Although surgical resection has some clinical efficacy in early-stage LC, treatment options and survival are limited by late symptom onset, restricted screening platforms, and limitations of conventional radiotherapy and chemotherapy for advanced LC (4).
LC is a complex respiratory condition that poses significant challenges for both clinicians and researchers (5). Various studies have shown that Inflammation, dysbiosis of lung and gut microbiota, genetic mutations, metabolic disorders, immune dysfunction, epigenetic modifications, oxidative stress, genetic factors and aberrant hormone expression are major risk factors for LC (6–13). During host defense or therapeutic injury, various molecular mechanisms are modified, such as mitophagy (14), mucin expression anomalies (15), epithelial–mesenchymal transition (EMT) (16), reactive oxygen species (ROS) generation (17), angiogenesis (18), abnormal glycosylation (19), and processes like cell apoptosis, proliferation, survival, metastasis, and invasion. The dysregulation of molecular mechanisms alone appears insufficient to fully explain the origins and progression of LC, and it is reasonable to suspect that genetic and epigenetic events have had an impact on genetic aspects (20, 21).
The functions and interactions of molecular pathways are involved in a wide range of cancers and have significant effects. Research indicates that the dysregulation of various signaling pathways, including Wnt (22), PI3K/Akt (23, 24), Notch (25), PD-1/PD-L1 (4), NF-κB (26), Hippo (27), MAPK (17, 28), Hedgehog (Hh) (29), AMPK (30), can facilitate LC progression and metastasis. Furthermore, the interactions between these pathways are precise and complex. Numerous studies indicate that genetic and epigenetic disturbances both contribute to and result from the development of LC (31, 32). Cristian et al. found that arsenic ingestion can cause cancer by producing epigenetic modifications and disrupting normal microRNAs (miRNAs) expression (33). Gao et al. the study indicated that radicicchioidin (SFN) could potentially prevent LC by restoring miR-9–3 levels through the inhibition of DNMTs, HDACs, and the target gene CDH1 protein levels (34). Yang and colleagues elucidate the role of epigenetic generation in LC progression (35).
In the current review, we provide a deep understanding and summary of the formation of the molecular mechanisms of these studies and the impact they have on LC, which we believe will provide a more effective and comprehensive therapeutic strategy for LC.
Multiple oncogenic and anticancer intracellular pathways in LC
We summarize recent advances in LC in the desire to gain a deep understanding of the molecular pathogenesis of LC (Table 1). Research has shown that intracellular signaling pathways can induce oncogenic effects, and targeting the driver genes within these pathways is highly effective in tumor treatment. Furthermore, expanding research on molecular diseases in LC offers crucial insights into its carcinogenesis, elucidated by various molecular mechanisms that function diversely across cancer development stages or contexts.
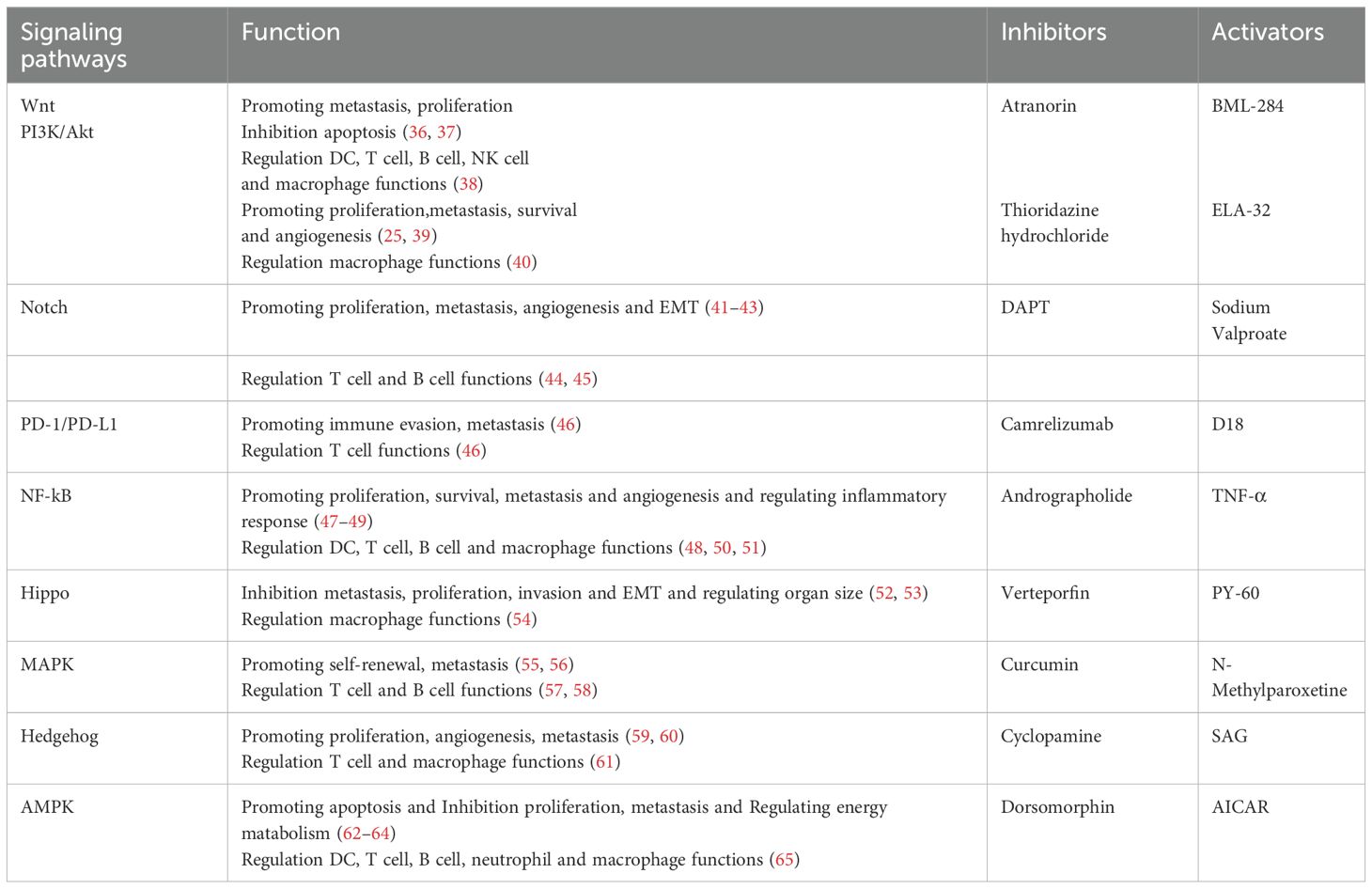
Table 1. The roles of the following signaling pathways in LC and associated inhibitors and activators.
Wnt pathway
The Wnt pathway consists of two canonical and noncanonicall types, one of which is the classical pathway in which Wnt binds to the LRP-5/6 receptor (LDL receptor) and Frizzled receptor, resulting in Disheveled (DVL) being phosphorylated, allowing the complex (Axin, GSK-3β, CK1, APC) to inhibit the activity of GSK-3β and reduce β-catenin ubiquitination and proteasomal degradation, allowing enrichment of unphosphorylated β-catenin (66–68). Unphosphorylated β-catenin translocated to the nucleus binds to TCF/LEF and induces the expression of multiple target genes (66–68).
When Wnt binds to Frizzled receptors and interacts with Daam2, it initiates a Wnt non-canonical pathway called planar cell polarity (PCP). This interaction activates small GTPases like RhoA and Rac, subsequently triggering downstream stress kinases JNK and ROCK, which play roles in cytoskeletal remodeling and actin alignment (69). Wnt/Ca2+ pathway is another Wnt noncanonicall pathway that is activated by Wnt5a and Fzd2 receptors, leading to G protein-mediated activation of PLC, which allows for a large amount of Ca2+ inward flow. The increased intracellular Ca2+ then stimulates calmodulin phosphatase and CAMKII, which in turn promotes TCF phosphorylation, which inhibits the Wnt classical pathway (70, 71).
The Wnt signaling pathway is a key regulator of LC development, metastasis, and drug resistance, as identified in previous studies (36, 37, 72). The study identified that isoform 1 of the neurogenesis-associated protein ASPM (ASPM-I1) plays a crucial role in SCLC development by stabilizing the Hh transcription factor GLI3 via a unique coding region in exon 3 and activating the Wnt-DVL3-β-catenin signaling pathway, which sustains the transcription of the Hh pathway regulator Smoothened (SMO) (73). Cisplatin (CP) is a widely used chemotherapeutic agent, and studies have shown that miRNAs can trigger the Wnt/β-catenin signaling pathway, leading to varying degrees of resistance to this chemotherapeutic agent in LC cells (74). In addition, it has been found that a variety of non-coding RNAs (ncRNAs) can regulate the expression of the Wnt/β-catenin signaling pathway, which affects the progression of LC to varying degrees, and thus targeting ncRNAs appears to be a good gene therapy (75). Studies have shown that Wnt can be mutually promoted with PD-L1, suggesting that Wnt can be controlled by PD-L1 (76), PD-L1 is important for lung tumorigenesis and progression in mice by regulating Yes-associated protein (YAP) (77). And it is the potential mechanism of the Wnt pathway on the PD-1/PD-L1 pathway that makes non-small cell lung cancer (NSCLC) patients somewhat resistant to immune checkpoint inhibitors (ICIs), so it seems that the combination of Wnt inhibitors and ICIs could be a new option for the treatment of LC patients (78). The study revealed that fibronectin overexpression in LC activates FAK and MAPK/ERK signaling pathways, which in turn overactivate the Wnt pathway, facilitating tumor progression (79).
Tumor metastasis is a leading factor in poor prognosis for LC patients (80), with the Wnt signaling pathway significantly contributing to its development (36). In addition, metastasis of cancer cells is not a one-sided problem, but requires the acquisition of a series of conditions to realize this process (81). Among other things, we found that tumor cells are mainly powered by uptake of stromal cells and immune cells from the tumor environment during this process (82, 83). Research indicates that the tumor microenvironment (TME) significantly influences tumor progression, with various specialized microenvironments within the TME interacting with cancer (84). Notably, hypoxia is a critical factor in tumor development (85). Research indicates a link between cancer incidence and chronic inflammation, with microenvironmental dysregulation causing persistent inflammatory lesions, reinforcing the validation that TME after tumor metastasis contributes to LC development (80, 86, 87). While Wnt signaling seems to interact with TME by regulating different components of TME (88). In addition, the Wnt signaling pathway can be involved in the functioning of a variety of immune cells such as dendritic cells (DCs), T cells, B cells, natural killer (NK) cells, macrophages, granulocytes, etc, which is the main cause of cancer, but it also provides us with a good strategy for immunotherapy (38). Cancer stem cells (CSCs) can promote lung tumor metastasis, and studies have found that by targeting the Wnt pathway associated with CSCs, it seems to have good efficacy in the treatment of LC (29, 89). Latency competent cancer (LCC) cells are cells that can enter the quiescent state very easily, and we found that the main mechanism is that the Wnt signaling pathway leads to the ability of LCC cells to escape from NK cell immunosurveillance through autocrine DKK1 (90). In addition, overexpression of Metadherin (MTDH) upregulates the Wnt pathway and depletes cytotoxic T cells, which promotes LC metastasis and progression (91, 92).
PI3K/Akt pathway
Inhibiting the PI3K/AKT signaling pathway, crucial for LC progression, could offer a significant therapeutic strategy (93). PI3K is a lipid kinase that converts phosphatidylinositol-4,5-bisphosphate (PIP2) into the second messenger phosphatidylinositol-3,4,5-trisphosphate (PIP3), which facilitates Akt translocation to the endosomal membrane for phosphorylation and thus facilitates signal expression (94). AKT functions downstream of PI3K, with PIP3 signaling being terminated by the lipid phosphatase PTEN. The PI3K/AKT pathway, often dysregulated in human cancers, contributes to cancer cell growth and metastasis (95).
This pathway phosphorylates NF-κB to enhance cell survival by having IKK phosphorylate inhibitory IκBα, facilitating NF-κB’s nuclear translocation to promote cell survival and vascular production, thereby inducing oncogenesis (39, 96). This pathway enhances angiogenesis in LC by modulating vascular endothelial factor (VEGF), leading to hypoxia-inducible factors (HIFs)-1α binding to the HRE in the VEGF promoter region (25, 97). Arsenic and benzo[α]pyrene (BaP), key contributors to LC, enhance integrin α4 (ITGA4) expression, thereby activating the PI3K/AKT pathway (98). This activation reduces suppressor of fused (SUFU) protein stability and concentration, allowing the Hh ligand to bind Patched (Ptch) and release SMO. Consequently, the GLI transcription factor is expressed, activating the Hh pathway and significantly increasing CSCs properties and tumorigenesis (98). This pathway regulates the EMT process, one of the major molecular mechanisms regulating lung tumor metastasis, which acts by inhibiting E-calmodulin expression and upregulating mesenchymal markers and EMT-specific transcription factors (99, 100). Long non-coding RNA (lncRNA) significantly regulates the PI3K/AKT signaling pathway in LC (101, 102). Specifically, lncRNA FOXD3-AS1 is highly recruited by exosomes in LC cells, where it interacts with ELAV-like RNA-binding protein 1 (ELAVL1) to activate this pathway, promoting LC progression (103). The study identified that Go-Ichi-Ni-San complex subuint 2 (GINS2) enhances phosphorylated proteins via PI3K/AKT and MEK/ERK pathways, thereby facilitating NSCLC growth, metastasis and EMT in mice (104). Wang et al. identified that CXCL5, a chemokine associated with NSCLC prognosis, is overexpressed in LC cells and to enhance LC progression through the activation of PI3K/AKT and MAPK/ERK1/2 signaling pathways (105). Radiation therapy is one of the main options for the treatment of various types of cancer, and it destroys cancer cells by means of ionizing radiation (IR) (106). However, we found that while destroying cancer cells, IR induces ROS generation and EMT, and to a certain extent leads to changes in TME and thus promotes metastasis, and it has been found that targeting the PI3K/Akt pathway seems to enhance the efficacy of the treatment by inhibiting EMT (107). KRAS G12D mutation in LC was found to activate the PI3K/Akt pathway and thus promote LC progression (108). In contrast, Hou et al. found that Salvianolic acid F (SalF) inhibits PI3K/Akt pathway expression by targeting KRAS G12D mutants (109). In addition, in NSCLC patients with PI3K/Akt pathway activation combined with epidermal growth factor receptor (EGFR) mutations, it was found that the combination of inhibitors of this pathway with EGFR Tyrosine Kinase Inhibitors (TKIs) improved the resistance of such patients to EGFR-TKIs (110). It was found that the Akt pathway can influence metabolic signaling and convergent inflammation to regulate macrophage function and can promote its M1/M2 polarization (40). Research on the PI3K/AKT pathway’s impact on immune cells and TME regulation revealed that combining immunotherapy with targeted therapy enhances efficacy in LC patients (111).
The PI3K/Akt pathway activates mTOR by inhibiting tuberous sclerosis complex 1 (TSC1), enhancing protein synthesis and promoting cellular metabolism, growth, and proliferation, thereby regulating cancer cell growth and metastasis (39). Collectively, the findings indicate that this pathway facilitates LC growth and metastasis.
Notch pathway
The Notch signaling pathway, comprising four receptors (Notch1-4) and five ligands (Delta-like 1, 3, 4, and Jagged 1, 2), plays a crucial role in regulating the biological functions of LC cells (112).
Various studies have shown that this pathway promotes the progression of LC through multifaceted effects (41, 113). Initially, ADAM10 hydrolyzes Notch protein near the membrane, followed by γ-secretase cleavage of the Notch intracellular domain (NICD) (113). This cleavage allows NICD to translocate into the nucleus, where it binds to CBF-1/suppressor of hairless/Lag1 (CSL), thereby promoting the transcription of downstream targets (41). RFC4 enhances the Notch signaling pathway by binding to NICD1, thereby preventing CDK8/FBXW7-mediated phosphorylation and polyubiquitination (114). Delta-like ligand 3 (DLL3), a Notch inhibitory ligand, is markedly up-regulated on SCLC cell surfaces, enhancing cell growth, metastasis and proliferation, which contributes to resistance against platinum-based chemotherapy (115). Therefore, against the high expression of DLL3, it was found that Tarlatamab (AMG 757) could cause tumors to regress to different degrees by combining DLL3 and CD3 on T cells, which provides a new direction for targeted immunotherapy for SCLC (116). In addition, we found that the chimeric antigen receptor (CAR) for DLL3 has an antitumor effect in SCLC in mice, and thus therapies targeting CAR T cells for DLL3 may provide a new strategy for the treatment of SCLC (117). Notch and Wnt/β-catenin signaling pathways are closely interconnected, as β-catenin enhances Notch signaling by binding to the Dll4 promoter, which subsequently influences the Wnt/β-catenin pathway through Nrarp regulation (118).
Numerous studies indicate that the Notch pathway is upregulated in LC patients, leading to cancer cell proliferation, metastasis, EMT and angiogenesis (41–43). For the Notch pathway to promote metastasis in cancer cells, a key point is that it promotes angiogenesis and appears to be related to the ability of the pathway to induce EMT (119). In cancer cells undergoing distant metastasis, aberrant angiogenesis in TME mainly plays a role in providing energy to cancer cells (120). The promotion of tumor angiogenesis by the Notch pathway is primarily attributed to the roles of ligands DLL4 and JAG1 (121, 122). Furthermore, EMT is a key factor contributing to chemotherapy resistance in NSCLC (123). Lu et al. found that the ADAM17 inhibitor ZLDI-8 could inhibit the Notch pathway and EMT thereby significantly promoting apoptosis in chemotherapy-resistant NSCLC and inhibiting NSCLC invasion and metastasis (124). SCLC has a poor prognosis due to its resistance to chemotherapeutic agents (125). Research on targeted therapies revealed that LSD1 inhibitors can impede SCLC progression by targeting Notch signaling and reducing ASCL1 transcription factor expression (126). The Notch pathway is crucial for T cell differentiation and B cell development (44, 45). It is well known that the current main strategy of immunotherapy is to modulate the immune system in order to enhance its power to destroy cancer cells (127). Instead, the study reports a novel T-cell therapy that uses synthetic Notch (synNotch) receptors to alter tailored behavior in T cells and control their differentiation, and also specifically targets tumors through such T cells (128).
PD-1/PD-L1 pathway
The PD-1/PD-L1 pathway is crucial in LC development (46, 129), in which PD-1 is expressed in a variety of immune cells such as T cells and dendritic cells (130), while PD-L1 is present in macrophages, epithelial cells, etc (131). PD-L1 binds to and acts on PD-1 to inhibit T cell expression and facilitating immune evasion (46).
PD-L1 expression in LC patients is regulated by various pathways (132–134). NF-kB directly binds to the PD-L1 promoter to enhance its expression and also promotes HIF-1α transcription (132), which subsequently stimulates glycolysis in lung tumors and up-regulates PD-L1 by modulating glycolytic enzymes (135). YAP proteins act as effectors in the Hippo pathway signaling cascade, which is linked to tumor cell metastasis and proliferation (136). EGFR, a transmembrane tyrosine kinase receptor (137) that phosphorylates Hippo kinase, which enhance YAP protein expression and regulate PD-L1 (133). The study identified an association between chemoresistance in lung squamous cell carcinoma (LUSC) and the interaction of the NRF2 and PD-1/PD-L1 pathways (134). In addition, PD-L1 promotes the expression of hexokinase 2 (HK2) and glycolysis in LC cells thereby inhibiting the function of effector T cells in LC (135). Interleukin-1β (IL-1β) promotes tumor growth and metastasis and appears to synergize with the PD-1/PD-L1 pathway to enhance lung tumor development (138).
PD-1 is currently a well-studied immune checkpoint (ICP) and its ligand, PD-L1, is overexpressed in LC, whereas metastasis of cancer cells occurs by promoting evasion of immune surveillance (46, 139). PD-L1, also referred to as B7-H1, has been identified in prior research as a key mechanism for promoting immune evasion in lung tumor cells by inducing apoptosis in activated tumor-reactive T cells (140). It has been previously stated that the poor prognosis of LC patients is due to the relative limitations of radiotherapy in the treatment of advanced LC (4). With immunotherapy research, targeting the PD-1/PD-L1 pathway and combining it with other therapeutic options has resulted in a significant increase in the survival of patients with advanced LC, making immune checkpoint inhibitors (ICIs) the preferred treatment for advanced LC at this time (4, 141, 142). In addition, for patients with advanced NSCLC, it has been shown that the bispecific antibody AK112 works by targeting both PD-1 and VEGF and can be combined with chemotherapeutic agents to achieve a good anti-tumor effect (143, 144). In addition, gene editing therapy mediated by transcription activator-like effector nuclease (TALEN) can inhibit PD-1 expression in CAR-T cells thereby reducing T-cell depletion and thus prolonging anti-tumor activity (145). Hypoxia significantly contributes to metastasis in advanced cancers, with HIFs serving as central mediators in this process (146). HIFs are overexpressed in hypoxic conditions, enhancing PD-L1 levels, which suggests that inhibiting the HIF/PD-L1 pathway could enhance cancer treatment efficacy (147). It was found that targeting High-mobility group box 1 (HMGB1) could remodel TME and enhance its efficacy in combination with anti-PD-1/PD-L1 immunotherapy for anti-cancer purposes (148). Immunotherapy targeting the PD-1/PD-L1 signaling pathway is a therapeutic option for advanced LC due to its sustained anti-tumor immune response (149).
NF-κB pathway
NF-κB, a stress-regulated transcription factor from the Rel family, is crucial in connecting inflammation with tumor cell survival (47). The NF-κB signaling pathway is pivotal in both innate and adaptive immune responses and is divided into classical and non-classical pathways (48).
The canonical NF-κB pathway can be activated by various agents, including interferon-based stimulators (STING), interleukin 1 (IL-1), tumor necrosis factor (TNF-α) and numerous drugs (150, 151). IKK activation is usually mediated by IKKβ-promoted IκB phosphorylation, and the activated IKK complex consists of two kinase subunits (IKKα and IKKβ) and a regulatory subunit (IKKγ/NF-κB essential modulator (NEMO)). The IKK complex facilitates IκBα phosphorylation and degradation, enabling NF-κB translocation to the nucleus and induce gene expression (151, 152). The noncanonical NF-κB pathway is primarily mediated by CD40, LTβR and NF-κB receptor activators, which stabilize NF-κB-induced kinase (NIK). This stabilization promotes IκB kinase-α (IKKα) activation, leading to the phosphorylation, ubiquitination, and processing of p100. Consequently, the p52/ReIB NF-κB complex translocate to the nucleus and thus induces gene expression (153).
Furthermore, it was found that STING and NF-κB can promote each other, where the classical NF-κB pathway increases STING expression by regulating microtubule depolymerization (150). NF-κB signaling enhances EMT by upregulating ZEB1/2, transforming growth factor-β and Slug gene transcription, which suppresses the epithelial marker E-calreticulin and promotes N-calreticulin and poikilodulin expression (154). Interleukin-6 (IL-6) is a key cytokine in immune regulation, but its abnormal expression is linked to inflammation and cancer progression (155). Aberrant expression of T-cell immunoglobulin domain and mucin domain 4 (TIM-4) correlates with poor LC prognosis, and IL-6 can upregulate TIM-4 via NF-κB activation, promoting EMT expression and LC development (156). In addition, Xiang et al. found that semaphorin 4A (Sema4A) could promote phosphorylated NF-κB pathway-related proteins as well as IL-6 expression to promote LC cell migration and proliferation (157). Mesoderm-specific transcripts (MEST) were found to induce STAT3 expression to enhance IκBα and P65 phosphorylation activity, and it also promotes IκBα degradation and thus modulates NF-κB signaling by interacting with valine-containing protein (VCP) (158). STAT3 signaling enhances the NF-κB pathway by upregulating miRNAs through interaction with IL-6. These miRNAs regulate various pathways and promote angiogenesis in the NF-κB pathway by inhibiting the deubiquitinating enzyme CYLD (159). In patients with NSCLC, studies have shown that the NF-κB pathway induces PD-L1 expression leading to immune evasion of cancer cells, and thus the combination of NF-κB inhibitors with ICIs appears to be useful for the treatment of NSCLC (160). S-adenosylmethionine (SAM) is a natural metabolite, and recent studies have found that SAM can target P62 and thus inhibit the NF-κB pathway in NSCLC, thus SAM may become a relatively safe adjuvant therapeutic agent (161).
In LC, NF-κB is also one of the important pathways that promote metastasis in cancer cells, in which the induction of EMT expression by this pathway is an important mechanism to achieve this process (154). Among them, the classical NF-κB pathway regulates multiple pro-inflammatory and pro-angiogenic factors, leading to chronic inflammation and promoting angiogenesis (48, 49). Because of this, anti-inflammatory drugs can be used as clinical therapeutic options to inhibit the NF-κB pathway (48). DCs facilitating T cell activation by presenting antigens, thus bridging innate and adaptive immunity (162). Whereas the non-classical NF-κB pathway regulates DC, T and B cell development and plays a role in mediating lymphoid organ development and osteoclast differentiation (48, 50). The infiltration of immunosuppressive macrophages, crucial for primary tumor metastasis, is driven by tumor-derived exosomes (TDE) that polarize macrophages into an immunosuppressive phenotype via NF-κB activation and glycolytic metabolic reprogramming (51). Platelets create a protective barrier for tumor cells against immune cell attacks and boost their metastatic potential by inducing EMT through transforming growth factor-β (TGFβ) (26). Activation of the NF-κB pathway during interactions between platelets and cancer cells can increase pro-metastatic potential (26). In conclusion, the NF-κB signaling pathway’s regulation of immune function facilitates cancer cell metastasis, yet it also offers a crucial target for clinical treatment of LC.
Hippo pathway
Hippo signaling is one of the important signaling during tumorigenesis and cancer cell development (163), which was first discovered in Drosophila melanogaster and plays an important role in regulating stem cell proliferation, differentiation, migration, apoptosis, organ size and self-renewal (52). In addition, Hippo signaling mainly consists of MST1/2, SAV1, MOB1A/B, LATS1/2, YAP, transcriptional coactivator with PDZ-binding motif (TAZ) and transcription enhancement-associated structural domain (TEAD) family of multiple key proteins (164), and importantly the pathway can inhibit tumor development through these components (163). Activation of the Hippo kinase cascade enhances MST phosphorylation of LATS, which subsequently binds to MOB1 to phosphorylate YAP (165–167). This process sequesters YAP in the cytoplasm, inhibiting the YAP/TAZ complex’s interaction with TEAD (165–167), ultimately contributing to lung tumorigenesis (53).
In LC, dysregulation of the Hippo signaling pathway induces migration, invasion, proliferation, drug resistance and EMT in LC cells (53). It was found that by targeting PFKFB3 (6-phosphofructose-2-kinase) it could down-regulate YAP/TAZ expression to inhibit the glycolytic process of CSCs in SCLC and enhance the chemosensitivity of SCLS (168). In addition, the herbal medicine cryptotanshinone (CT) can regulate TAZ expression to activate Hippo, thereby inhibiting the progression of NSCLC and reducing its chemoresistance (169). YAP upregulates EGFR ligands like Amphiregulin (ARGE) and Neuregulin 1 (NRG-1), creating a positive feedback loop in the MAPK signaling pathway (133). This process also inactivates the oncogene k-ras, which subsequently interacts with FOS to activate MAPK signaling, thereby promoting EMT expression (133, 170). In addition, YAP/TAZ can enhance PD-L1 protein expression to promote NSCLS immune evasion (170).
YAP/TAZ is a major effector downstream of the Hippo signaling pathway that promotes metastasis by reprogramming cancer cells, but the Hippo cascade reaction can ultimately phosphorylate and inhibit YAP/TAZ (136). In addition, we found that YAP/TAZ promoted glycolysis and glutamine catabolism, which provided energy support to metastatic tumor cells (171). YAP has been reported to interact with the transcription factors TEAD and PRDM4 to induce leukocyte-specific integrin β2 (ITGB2) expression thereby mimicking the behavior of leukocyte endothelial invasion and ultimately promoting cancer cell invasion and distant metastasis (172). Mitotic Spindle Positioning (MISP) suppresses MST1/2 activity, resulting in YAP hyperactivation and increased SCL7A11 expression. This process enhances lung cancer cell resistance to ferroptosis, promoting tumor metastasis, yet also offers a potential therapeutic target for LC (173). It is well known that the level of tumor immunogenicity affects the survival of tumor cells, and it has been found that inhibition of LATS1/2 kinase in the Hippo pathway seems to enhance tumor immunogenicity and thus can achieve the purpose of LC treatment (174). The Hippo pathway influences specific immune cells, impacting immunotherapy effectiveness (164). Hippo pathway dysregulation leads to increased YAP activity, which elevates CCL2 and CXCL5 cytokine levels, facilitating the recruitment of M2 macrophages and polymorphonuclear myeloid-derived suppressor cells (MDSCs), thereby contributing to immunotherapy resistance (54, 175).
Ras/Raf/MEK/MAPK/ERK pathway
The MAPK pathway is crucial in human tumors, influencing cell proliferation, differentiation, apoptosis and angiogenesis. The RAS gene is tumorigenically mutated in approximately 30% of tumors. Activated Ras mediates the activation and phosphorylation of Raf membrane translocation, which in turn promotes MEK activation, which then phosphorylates and activates MAPK/extracellular signaling-associated kinase (ERK) by phosphorylating Tyr and Thr residues (176).
In LC, gene mutations in the MAPK pathway are activated. In EGFR-mutated NSCLC cells, the MAPK pathway enhances PD-L1 expression by modulating drug resistance mechanisms like c-MET amplification and EGFR-T790M mutation, resulting in resistance to EGFR-TKIs (177, 178). Mutations in T790M and C797 within the ATP receptor cause NSCLC to be resistant to EGFR-TKIs, and it has been found that the EAI045 inhibitor works by targeting such mutants and has good efficacy in combination with cetuximab for the treatment of EGFR-TKIs resistant mutants in LC (179). In addition, studies have shown that the inhibitor Sotorasib directly targets the KRAS G12C mutant protein, thereby improving survival in patients with KRAS G12C mutations in NSCLC (180). Fentanyl has been found to reduce the sensitivity of cisplatin (DDP) chemotherapy by inducing a MAPK signaling cascade response through the promotion of ROS expression (181). In addition, the oncoprotein hepatitis B X-interacting protein (HBXIP) was found to be upregulated in NSCLC, which reduces MEK1 protein degradation to promote MAPK/ERK pathway expression (182). In addition, the MAPK/ERK and PI3K/AKT signaling pathways seem to play a synergistic role in promoting self-renewal of lung CSCs during LC development (55). This suggests that the MAPK pathway can interact with the PI3K/AKT pathway in LC.
The MAPK signaling pathway is a central pathway in the regulation of LC metastasis (56). Among the major molecular mechanisms driving LC metastasis is the Ras gene mutation that ultimately triggers the MEK/ERK cascade reaction, leading to extracellular matrix remodeling (ECM) and EMT (183). In addition, the MAPK pathway can enable the formation of a metastasis-promoting immunosuppressive environment by regulating the TME (58, 184, 185). First, this pathway promotes VEGF expression, leading to increased angiogenesis (185). Secondly, mutant KRAS activates the MEK/ERK/AP-1 pathway and thus promotes the expression of TGF-β1 and IL-10. This process recruits regulatory T cells (Tregs), further diminishing the antitumor immune response (58). Moreover, KRAS mutations can also upregulate PD-L1 via the ERK pathway, leading to depletion of T cells, resulting in immune evasion (184). Currently, because KRAS-mutant lung adenocarcinoma (LUAD) is resistant to the MEK inhibitor trametinib, it has been found that trametinib in combination with ICIs for the treatment of LUAD exerts a synergistic anti-tumor effect (186). In addition, early B cell development as well as late B cell maturation are also regulated by the MAPK pathway (57). Despite the current efficacy of ICI in advanced LC (4), it is limited by the fact that patients with KRAS mutations result in immunosuppressive TME formation (187). Therefore, combination therapy is particularly important, and it has been found that KRAS G12C inhibitors combined with ICI can not only inhibit the proliferation of lung tumors but also lift TME immunosuppression (188). In KRAS-mutated lung cancer, combining MEK with CDK4/6 inhibitors suppressed cell proliferation and enhanced NK cell-mediated immunosurveillance (189).
Hedgehog pathway
Initially discovered in Drosophila, the Hh signaling pathway is crucial for embryonic development regulation (190). The HH protein family is important in the regulation of cell proliferation, apoptosis, differentiation, metastasis and invasion (59). HH proteins (SHH, IHH, or DHH) bind to the PTCH1 receptor, prompting its lysosomal degradation and reducing its repression of Smo (191). This process activates GLI protein expression, causing GLI1 and GLI2 transcription factors to the nucleus and promotes gene expression (192).
Research indicates that the Hh signaling pathway is important in regulating lung tumor cell proliferation, drug resistance, stemness and the tumor microenvironment (193, 194). ASPM-I1 is a stemness gene that is significantly upregulated in SCLC cells, which regulates the activity of the transcription factor GLI1 and promotes SMO transcription through signaling with Wnt-DVL3-β-catenin (73). In addition, it was found that tretinoin could mediate HNF1A/SHH expression to inhibit Hh signaling thereby enhancing the sensitivity of LC cells to paclitaxel (195). In addition, Wang et al. found that SFN inhibited the expression of SHH, SMO and GLI1 in LC cells and thus inhibited LC cell proliferation (196). Research indicates that Sonic Hedgehog (Shh) is upregulated in A549 and H520 cells, enhancing NSCLC angiogenesis by modulating collagen production in fibroblasts (60).
The HH signaling pathway is crucial for promoting metastasis in LC by inducing EMT expression via the metastasis factor Gli, which is the primary mechanism driving cancer cell metastasis (197). Bone is frequently affected by LC metastasis. The HH pathway enhances receptor activator of NFkB ligand (RANKL) expression in osteoblasts, which stimulates osteoclast activation and accelerates osteolytic destruction, perpetuating a vicious cycle (198). In addition, this pathway induces activation of cancer-associated fibroblasts (CAFs) leading to ECM remodeling and thus formation of pro-metastatic TME, but this also provides an approach for anti-fibrotic therapy (199). The pathway also regulates the self-renewal capacity of CSCs, causing them to be resistant to chemotherapy and thus promoting metastasis (200). Tumor-associated macrophages (TAMs) predominantly exhibit an M2-like phenotype in TME, which has been shown to promote tumor metastasis and progression (201). In contrast, the ligand SHH in the Hh signaling pathway induces TAM M2 polarization, resulting in a decrease in the expression of CXCL9 and CXCL10 leading to a significant down-regulation of CD8+ T cells infiltrating into the TME, which ultimately leads to an attenuation of the immunosuppressive function (61). Interleukin-4 (IL-4) is crucial in inhibiting anti-tumor immune responses and facilitating tumor cell proliferation (202), while the Hh pathway enhances IL-4 expression by stimulating T-helper 2 (Th2) cells transcription (203). Inhibition of the Hh pathway was observed to decrease PD-L1 expression and increase CD8+ lymphocyte expression, thereby enhancing anti-tumor activity (204). For targeted therapy against the Hh pathway, SMO and GLI inhibitors are currently important therapeutic options and have achieved good efficacy in the clinical treatment of LC (205, 206).
AMPK pathway
AMPK, a serine/threonine kinase composed of regulatory β and γ subunits and a catalytic α subunit, acts as an energy sensor sensitive to the AMP/ATP ratio (62–64). It regulates energy homeostasis and metabolic stress responses, influencing cell proliferation, growth, stress response, autophagy and cell polarity to inhibit tumorigenesis (62–64).
AMPK acts mainly through oxidative phosphorylation and regulation of malignant tumor metabolism (63). In particular, AMPK activates p53 thereby delaying the cell cycle and can induce apoptosis (207). Resveratrol (RSV) enhances nerve growth factor receptor (NGFR) expression by modulating mRNA levels and the stability of mRNAs and proteins, thereby promoting AMPK phosphorylation and inhibiting mTOR phosphorylation, offering a potential targeted therapy for NSCLC (208). Metformin can activate AMPK to inhibit mTOR phosphorylation and suppress cell proliferation, but its antiproliferative effects appear to be independent of Liver kinase B1 (LKB1) (30). Metformin-activated AMPK at the S655 site phosphorylates downstream PHF2 to promote epigenetic H3K9me2 demethylation during EMT, thereby inhibiting LC metastasis (209). Metformin-activated AMPK recruits anti-inflammatory factors, including IL-1β, IL-6, TNF-α and NF-kB, resulting in reduced vascular endothelial growth factor levels (210, 211). LKB1 mutation is prevalent in NSCLC and crucial for activating AMPK family kinases (212). SIK1 and SIK3 are two tumor suppressor kinases of the AMPK family, and it has been found that in Kras-driven LC, the results of LKB1 deletion and SIK1/SIK3 deletion in regulating gene expression are highly overlapping and LKB1 deletion seems to activate the IL6/JAK/STAT pathway, which gives us a new direction for the treatment of LKB1 mutant LC (213). In addition, phosphorylation of AMPK promotes phosphorylation of downstream acetyl-coenzyme A carboxylase (ACC), thereby inhibiting lipid synthesis, a crucial process for nutrient acquisition in cancer cell growth and proliferation (63). The Warburg effect is profound in cancer metabolism by affecting glucose, amino acid, and lipid metabolism (214). Previous studies have found that β-elemene can activate AMPK pathways to counteract the Warburg effect in LC (215). This finding indicates that various signaling pathways may activate the AMPK pathway, either directly or indirectly, to influence the Warburg effect. The findings indicate that the AMPK pathway significantly impacts LC development.
The AMPK pathway influences LC metastasis by modulating energy metabolism, the immune microenvironment, and various other factors (63, 216). This pathway reduces VEGF expression by inhibiting HIF-1α, which inhibits tumor angiogenesis (216). Sustained activation of AMPK can inhibit protein synthesis by inhibiting the mTORC1 pathway or phosphorylating eEF2k, which can indirectly inhibit tumor cell proliferation (217, 218). TAMs is important for cancer cell proliferation, invasion, metastasis and angiogenesis (219). Studies have shown that astragaloside (AS-IV) can inhibit M2 polarization of TAM by targeting the AMPK signaling pathway, thereby inhibiting the progression and metastasis of LC (220). Most activated immune cells derive part of their energy from glycolysis, such as macrophages, neutrophils, B cells, dendritic cells, etc, and AMPK can promote cellular catabolism to inhibit immune cell activation (65). The LKB1-AMPK pathway is crucial for T cell differentiation and function through its regulation of metabolic reprogramming (221). In patients with KRAS and LKB1 co-mutant lung cancer, autophagy in cancer cells increases acetyl-coenzyme A (acetyl-CoA) levels, inducing EMT and promoting metastasis through the acetylation of the transcription factor Snail. CAMKK2 and ACLY inhibitors have been shown to effectively reduce cancer cell metastasis by targeting the autophagy/acetyl-CoA axis (222). Tregs contribute to cancer immunosuppression by expressing various immunomodulatory cytokines and inhibitory receptors. Conversely, the AMPK pathway can suppress PD-1 expression in Tregs via the HMGCR/P38 MAPK/GSK3β axis, thereby boosting anti-tumor immunity and offering a potential combination therapy for LC treatment (223).
Importance of the interconnectedness of these pathways
The linear progression of the Wnt, PI3K/Akt, Notch, PD-1/PD-L1, NF-KB, Hippo, MAPK, Hedgehog and AMPK pathways has been summarized in Figure 1. However, there has been a great deal of research on the linkage of these pathways showing that these signaling pathways are intimately connected by directly or indirectly interfering with each other (224, 225).
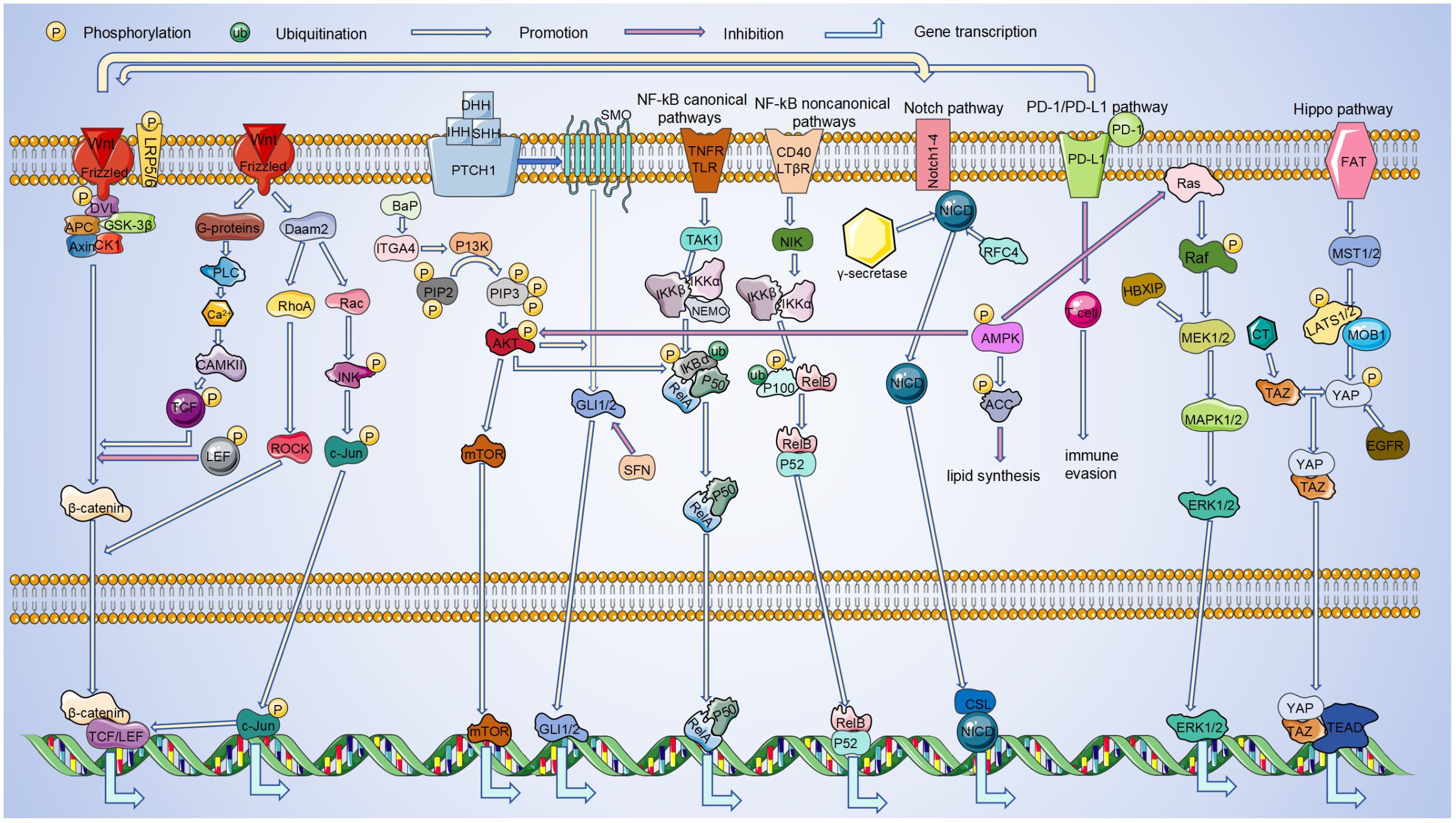
Figure 1. Linkages between pathways in LC. During the occurrence and development of LC, it is regulated by signaling pathways such as Wnt, PI3K/Akt, Notch, PD-1/PD-L1, NF-κB, Hippo, MAPK, Hedgehog, AMPK. And these pathways can be linked to each other. Among these signaling pathways, Hippo and AMPK pathways inhibit LC progression. The remaining pathways play an oncogenic role in LC progression. By interacting in the cytoplasm and nucleus, these pathways can ultimately regulate the transcription of target genes.
Wnt signaling has been shown to influence YAP expression and modulate Gli3 via the Hh pathway (27, 73). In addition, it can interact with Notch (118), and PD-L1 (76) signaling. Most RAS proteins predominantly exist in a GTP-bound state primarily due to mutations that enhance their stability, conferring structural activity and resistance to exogenous growth factors like EGFR. Thus, such K-RAS proteins appear to cause tumor cells to be less sensitive to ErbB-targeting drugs, including cetuximab or panitumumab (226, 227). The PI3K/AKT pathway activation enhances the Hh pathway’s involvement in LC progression (98). Wnt/β-catenin enhances Notch signaling, which reciprocally influences the Wnt/β-catenin pathway through Nrarp regulation (118). Similarly, Wnt can be activated not only by MAPK (79), but also regulated by PD-L1 (76). Activation of AMPK inhibits activation of PI3K/Akt (228) and Ras (229), in addition to it also promotes the biological function of NF-KB (210, 211). PI3K/AKT plays a synergistic role with MAPK in the self-renewal of lung CSCs (55). Hippo not only enhances MAPK function (133), but also inactivates Ras (170). PD1/PD-L1 activation involves the NF-KB pathway (132), and is regulated by the Hippo pathway (133).
The interplay of these pathways is crucial in cancer formation and progression. Although feedback loops are a fundamental part of carcinogenesis, their impact is indeed significant, suggesting that fine-tuning of one link may also affect the whole.
Conclusion
This article examines the pathophysiological roles and interactions of various signaling pathways in LC. Pathophysiological studies have demonstrated that dysregulated signaling pathways significantly contribute to LC by enhancing cell proliferation and metastasis, while their interactions and feedback loops may suppress cell differentiation and apoptosis (224, 225).
In past studies, pathways that play a role in tumors continue to be unearthed, bringing our understanding of targeted therapy as a tool to new heights. Studies have shown that these pathways act in connection with each other rather than in isolation, with changes in one pathway acting as a chain reaction, leading to changes in another pathway (230). The growing research on signaling pathways highlights the critical need to understand their interactions, offering diverse strategies for cancer treatment. Dysregulation of several pathways is involved in the process of LC occurrence and development, mainly including Wnt (22), P13/Akt (23), Notch (25), PD-1/PD-L1 (4), NF-KB (26), Hippo (27), MAPK (17), Hedgehog (29) and AMPK (30).
A thorough investigation into the etiology, causative factors, and clinical treatments for LC is essential to develop a comprehensive and effective treatment strategy (4). However, if we only target a single gene in the treatment of LC, we often fail to achieve the expected efficacy, in which the complex etiology of LC has a significant impact. Furthermore, “cocktail therapy,” which combines multiple drugs, has proven more effective in treating the disease. In conclusion, exploring epigenetic mechanisms in LC development and progression offers unexpected insights, potentially enhancing therapeutic options and improving early diagnosis and treatment (37, 231).
Author contributions
HL: Writing – review & editing, Writing – original draft, Data curation. DZ: Writing – original draft, Writing – review & editing. YO: Writing – review & editing, Writing – original draft. SC: Writing – review & editing, Writing – original draft. YZL: Writing – original draft, Writing – review & editing. TY: Writing – review & editing, Writing – original draft. YKL: Writing – review & editing, Writing – original draft. YT: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Tan N, Li Y, Ying J, and Chen W. Histological transformation in lung adenocarcinoma: Insights of mechanisms and therapeutic windows. J Transl Int Med. (2024) 12:452–65. doi: 10.1515/jtim-2024-0019
3. Li C, Lei S, Ding L, Xu Y, Wu X, Wang H, et al. Global burden and trends of lung cancer incidence and mortality. Chin Med J. (2023) 136:1583–90. doi: 10.1097/CM9.0000000000002529
4. Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. (2023) 22:40. doi: 10.1186/s12943-023-01740-y
5. Nooreldeen R and Bach H. Current and future development in lung cancer diagnosis. Int J Mol Sci. (2021) 22(16):8661. doi: 10.3390/ijms22168661
6. Forder A, Zhuang R, Souza VGP, Brockley LJ, Pewarchuk ME, Telkar N, et al. Mechanisms contributing to the comorbidity of COPD and lung cancer. Int J Mol Sci. (2023) 24(3):2859. doi: 10.3390/ijms24032859
7. Heng WS, Kruyt FAE, and Cheah SC. Understanding lung carcinogenesis from a morphostatic perspective: prevention and therapeutic potential of phytochemicals for targeting cancer stem cells. Int J Mol Sci. (2021) 22(17):5697. doi: 10.3390/ijms22115697
8. Malhotra J, Malvezzi M, Negri E, La Vecchia C, and Boffetta P. Risk factors for lung cancer worldwide. Eur Respir J. (2016) 48:889–902. doi: 10.1183/13993003.00359-2016
9. Musial C, Zaucha R, Kuban-Jankowska A, Konieczna L, Belka M, Marino Gammazza A, et al. Plausible role of estrogens in pathogenesis, progression and therapy of lung cancer. Int J Environ Res Public Health. (2021) 18(12):648. doi: 10.3390/ijerph18020648
10. Schabath MB and Cote ML. Cancer progress and priorities: lung cancer. Cancer Epidemiol Biomarkers Prev. (2019) 28:1563–79. doi: 10.1158/1055-9965.EPI-19-0221
11. Smok-Kalwat J, Mertowska P, Mertowski S, Smolak K, Kozińska A, Koszałka F, et al. The importance of the immune system and molecular cell signaling pathways in the pathogenesis and progression of lung cancer. Int J Mol Sci. (2023) 24(2):1506. doi: 10.3390/ijms24021506
12. Zhao Y, Liu Y, Li S, Peng Z, Liu X, Chen J, et al. Role of lung and gut microbiota on lung cancer pathogenesis. J Cancer Res Clin Oncol. (2021) 147:2177–86. doi: 10.1007/s00432-021-03644-0
13. Zou Y, Zhang H, Liu F, Chen ZS, and Tang H. Intratumoral microbiota in orchestrating cancer immunotherapy response. J Transl Int Med. (2024) 12:540–2. doi: 10.1515/jtim-2024-0038
14. Zhou Z, Qu C, Zhou P, Zhou Q, Li D, Wu X, et al. Extracellular vesicles activated cancer-associated fibroblasts promote lung cancer metastasis through mitophagy and mtDNA transfer. J Exp Clin Cancer Res. (2024) 43:158. doi: 10.1186/s13046-024-03077-w
15. Ning Y, Zheng H, Zhan Y, Liu S, Yang Y, Zang H, et al. Comprehensive analysis of the mechanism and treatment significance of Mucins in lung cancer. J Exp Clin Cancer Res. (2020) 39:162. doi: 10.1186/s13046-020-01662-3
16. Odarenko KV, Zenkova MA, and Markov AV. The nexus of inflammation-induced epithelial-mesenchymal transition and lung cancer progression: A roadmap to pentacyclic triterpenoid-based therapies. Int J Mol Sci. (2023) 24(24):17325. doi: 10.3390/ijms242417325
17. Fan J, Ren D, Wang J, Liu X, Zhang H, Wu M, et al. Bruceine D induces lung cancer cell apoptosis and autophagy via the ROS/MAPK signaling pathway in vitro and in vivo. Cell Death Dis. (2020) 11:126. doi: 10.1038/s41419-020-2317-3
18. Wheatley-Price P and Shepherd FA. Targeting angiogenesis in the treatment of lung cancer. J Thorac Oncol. (2008) 3:1173–84. doi: 10.1097/JTO.0b013e318187220f
19. Reticker-Flynn NE and Bhatia SN. Aberrant glycosylation promotes lung cancer metastasis through adhesion to galectins in the metastatic niche. Cancer Discov. (2015) 5:168–81. doi: 10.1158/2159-8290.CD-13-0760
20. Willers H, Azzoli CG, Santivasi WL, and Xia F Basic mechanisms of therapeutic resistance to radiation and chemotherapy in lung cancer. Cancer J. (2013) 19:200–7. doi: 10.1097/PPO.0b013e318292e4e3
21. Zhang J, Tang M, and Shang J. PPARγ Modulators in lung cancer: molecular mechanisms, clinical prospects, and challenges. Biomolecules. (2024) 14(2):190. doi: 10.3390/biom14020190
22. Krishnamurthy N and Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat Rev. (2018) 62:50–60. doi: 10.1016/j.ctrv.2017.11.002
23. Gao Y, Yang J, Cai Y, Fu S, Zhang N, Fu X, et al. IFN-γ-mediated inhibition of lung cancer correlates with PD-L1 expression and is regulated by PI3K-AKT signaling. Int J Cancer. (2018) 143:931–43. doi: 10.1002/ijc.v143.4
24. Ke M, Zhu H, Lin Y, Zhang Y, Tang T, Xie Y, et al. Actin-related protein 2/3 complex subunit 1B promotes ovarian cancer progression by regulating the AKT/PI3K/mTOR signaling pathway. J Transl Int Med. (2024) 12:406–23. doi: 10.2478/jtim-2024-0025
25. Tirpe AA, Gulei D, Ciortea SM, Crivii C, and Berindan-Neagoe I. Hypoxia: overview on hypoxia-mediated mechanisms with a focus on the role of HIF genes. Int J Mol Sci. (2019) 20(24):6140. doi: 10.3390/ijms20246140
26. Labelle M, Begum S, and Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. (2011) 20:576–90. doi: 10.1016/j.ccr.2011.09.009
27. Harvey KF, Zhang X, and Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. (2013) 13:246–57. doi: 10.1038/nrc3458
28. Shen X, Niu N, and Xue J. Oncogenic KRAS triggers metabolic reprogramming in pancreatic ductal adenocarcinoma. J Transl Int Med. (2023) 11:322–9. doi: 10.2478/jtim-2022-0022
29. Clara JA, Monge C, Yang Y, and Takebe N. Targeting signalling pathways and the immune microenvironment of cancer stem cells - a clinical update. Nat Rev Clin Oncol. (2020) 17:204–32. doi: 10.1038/s41571-019-0293-2
30. Fatehi Hassanabad A and MacQueen KT. Molecular mechanisms underlining the role of metformin as a therapeutic agent in lung cancer. Cell Oncol (Dordr). (2021) 44:1–18. doi: 10.1007/s13402-020-00570-0
31. Duruisseaux M and Esteller M. Lung cancer epigenetics: From knowledge to applications. Semin Cancer Biol. (2018) 51:116–28. doi: 10.1016/j.semcancer.2017.09.005
32. Teng PC, Liang Y, Yarmishyn AA, Hsiao YJ, Lin TY, Lin TW, et al. RNA modifications and epigenetics in modulation of lung cancer and pulmonary diseases. Int J Mol Sci. (2021) 22(19):10592. doi: 10.3390/ijms221910592
33. Soza-Ried C, Bustamante E, Caglevic C, Rolfo C, Sirera R, and Marsiglia H. Oncogenic role of arsenic exposure in lung cancer: A forgotten risk factor. Crit Rev Oncol Hematol. (2019) 139:128–33. doi: 10.1016/j.critrevonc.2019.01.012
34. Gao L, Cheng D, Yang J, Wu R, Li W, and Kong AN. Sulforaphane epigenetically demethylates the CpG sites of the miR-9–3 promoter and reactivates miR-9–3 expression in human lung cancer A549 cells. J Nutr Biochem. (2018) 56:109–15. doi: 10.1016/j.jnutbio.2018.01.015
35. Yang S, Huang Y, and Zhao Q. Epigenetic alterations and inflammation as emerging use for the advancement of treatment in non-small cell lung cancer. Front Immunol. (2022) 13:878740. doi: 10.3389/fimmu.2022.878740
36. Yang S, Liu Y, Li MY, Ng CSH, Yang SL, Wang S, et al. FOXP3 promotes tumor growth and metastasis by activating Wnt/β-catenin signaling pathway and EMT in non-small cell lung cancer. Mol Cancer. (2017) 16:124. doi: 10.1186/s12943-017-0700-1
37. Zhang Z, Westover D, Tang Z, Liu Y, Sun J, Sun Y, et al. Wnt/β-catenin signaling in the development and therapeutic resistance of non-small cell lung cancer. J Transl Med. (2024) 22:565. doi: 10.1186/s12967-024-05380-8
38. Haseeb M, Pirzada RH, Ain QU, and Choi S. Wnt signaling in the regulation of immune cell and cancer therapeutics. Cells. (2019) 8(11):1380. doi: 10.3390/cells8111380
39. Ahmad A, Biersack B, Li Y, Kong D, Bao B, Schobert R, et al. Targeted regulation of PI3K/Akt/mTOR/NF-κB signaling by indole compounds and their derivatives: mechanistic details and biological implications for cancer therapy. Anticancer Agents Med Chem. (2013) 13:1002–13. doi: 10.2174/18715206113139990078
40. Vergadi E, Ieronymaki E, Lyroni K, Vaporidi K, and Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J Immunol. (2017) 198:1006–14. doi: 10.4049/jimmunol.1601515
41. Yuan X, Wu H, Han N, Xu H, Chu Q, Yu S, et al. Notch signaling and EMT in non-small cell lung cancer: biological significance and therapeutic application. J Hematol Oncol. (2014) 7:87. doi: 10.1186/s13045-014-0087-z
42. He C. Activating invasion and metastasis in small cell lung cancer: role of the tumour immune microenvironment and mechanisms of vasculogenesis, epithelial-mesenchymal transition, cell migration, and organ tropism. Cancer Rep (Hoboken). (2024) 7:e70018. doi: 10.1002/cnr2.70018
43. Pancewicz-Wojtkiewicz J. Epidermal growth factor receptor and notch signaling in non-small-cell lung cancer. Cancer Med. (2016) 5:3572–8. doi: 10.1002/cam4.944
44. Garis M and Garrett-Sinha LA. Notch signaling in B cell immune responses. Front Immunol. (2020) 11:609324. doi: 10.3389/fimmu.2020.609324
45. Radtke F, MacDonald HR, and Tacchini-Cottier F. Regulation of innate and adaptive immunity by Notch. Nat Rev Immunol. (2013) 13:427–37. doi: 10.1038/nri3445
46. Cha JH, Chan LC, Li CW, Hsu JL, and Hung MC. Mechanisms controlling PD-L1 expression in cancer. Mol Cell. (2019) 76:359–70. doi: 10.1016/j.molcel.2019.09.030
47. Piva R, Belardo G, and Santoro MG. NF-kappaB: a stress-regulated switch for cell survival. Antioxid Redox Signal. (2006) 8:478–86. doi: 10.1089/ars.2006.8.478
48. Yu H, Lin L, Zhang Z, Zhang H, and Hu H. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther. (2020) 5:209. doi: 10.1038/s41392-020-00312-6
49. Mantovani A, Allavena P, Sica A, and Balkwill F. Cancer-related inflammation. Nature. (2008) 454:436–44. doi: 10.1038/nature07205
50. Sun SC. The non-canonical NF-κB pathway in immunity and inflammation. Nat Rev Immunol. (2017) 17:545–58. doi: 10.1038/nri.2017.52
51. Morrissey SM, Zhang F, Ding C, Montoya-Durango DE, Hu X, Yang C, et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. (2021) 33:2040–2058.e10. doi: 10.1016/j.cmet.2021.09.002
52. Clark KL, George JW, Przygrodzka E, Plewes MR, Hua G, Wang C, et al. Hippo signaling in the ovary: emerging roles in development, fertility, and disease. Endocr Rev. (2022) 43:1074–96. doi: 10.1210/endrev/bnac013
53. Liang H, Xu Y, Zhao J, Chen M, and Wang M. Hippo pathway in non-small cell lung cancer: mechanisms, potential targets, and biomarkers. Cancer Gene Ther. (2024) 31:652–66. doi: 10.1038/s41417-024-00761-z
54. Guo X, Zhao Y, Yan H, Yang Y, Shen S, Dai X, et al. Single tumor-initiating cells evade immune clearance by recruiting type II macrophages. Genes Dev. (2017) 31:247–59. doi: 10.1101/gad.294348.116
55. Li J, Wang J, Xie D, Pei Q, Wan X, Xing HR, et al. Characteristics of the PI3K/AKT and MAPK/ERK pathways involved in the maintenance of self-renewal in lung cancer stem-like cells. Int J Biol Sci. (2021) 17:1191–202. doi: 10.7150/ijbs.57871
56. Bahar ME, Kim HJ, and Kim DR. Targeting the RAS/RAF/MAPK pathway for cancer therapy: from mechanism to clinical studies. Signal Transduct Target Ther. (2023) 8:455. doi: 10.1038/s41392-023-01705-z
57. Chen Y, Zheng Y, You X, Yu M, Fu G, Su X, et al. Kras is critical for B cell lymphopoiesis. J Immunol. (2016) 196:1678–85. doi: 10.4049/jimmunol.1502112
58. Zdanov S, Mandapathil M, Abu Eid R, Adamson-Fadeyi S, Wilson W, Qian J, et al. Mutant KRAS conversion of conventional T cells into regulatory T cells. Cancer Immunol Res. (2016) 4:354–65. doi: 10.1158/2326-6066.CIR-15-0241
59. McMahon AP, Ingham PW, and Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. (2003) 53:1–114. doi: 10.1016/S0070-2153(03)53002-2
60. Bermudez O, Hennen E, Koch I, Lindner M, and Eickelberg O. Gli1 mediates lung cancer cell proliferation and Sonic Hedgehog-dependent mesenchymal cell activation. PLoS One. (2013) 8:e63226. doi: 10.1371/journal.pone.0063226
61. Petty AJ, Li A, Wang X, Dai R, Heyman B, Hsu D, et al. Hedgehog signaling promotes tumor-associated macrophage polarization to suppress intratumoral CD8+ T cell recruitment. J Clin Invest. (2019) 129:5151–62. doi: 10.1172/JCI128644
62. Faubert B, Vincent EE, Poffenberger MC, and Jones RG. The AMP-activated protein kinase (AMPK) and cancer: many faces of a metabolic regulator. Cancer Lett. (2015) 356:165–70. doi: 10.1016/j.canlet.2014.01.018
63. Hsu CC, Peng D, Cai Z, and Lin HK. AMPK signaling and its targeting in cancer progression and treatment. Semin Cancer Biol. (2022) 85:52–68. doi: 10.1016/j.semcancer.2021.04.006
64. Rehman G, Shehzad A, Khan AL, and Hamayun M. Role of AMP-activated protein kinase in cancer therapy. Arch Pharm (Weinheim). (2014) 347:457–68. doi: 10.1002/ardp.201300402
65. Chou WC, Rampanelli E, Li X, and Ting JP. Impact of intracellular innate immune receptors on immunometabolism. Cell Mol Immunol. (2022) 19:337–51. doi: 10.1038/s41423-021-00780-y
66. Albrecht LV, Tejeda-Muñoz N, and De Robertis EM. Cell biology of canonical wnt signaling. Annu Rev Cell Dev Biol. (2021) 37:369–89. doi: 10.1146/annurev-cellbio-120319-023657
67. Parsons MJ, Tammela T, and Dow LE. WNT as a driver and dependency in cancer. Cancer Discov. (2021) 11:2413–29. doi: 10.1158/2159-8290.CD-21-0190
68. Zhan T, Rindtorff N, and Boutros M. Wnt signaling in cancer. Oncogene. (2017) 36:1461–73. doi: 10.1038/onc.2016.304
69. Maeda K, Kobayashi Y, Koide M, Uehara S, Okamoto M, Ishihara A, et al. The regulation of bone metabolism and disorders by wnt signaling. Int J Mol Sci. (2019) 20(22):5525. doi: 10.3390/ijms20225525
70. Okamoto M, Udagawa N, Uehara S, Maeda K, Yamashita T, Nakamichi Y, et al. Noncanonical Wnt5a enhances Wnt/β-catenin signaling during osteoblastogenesis. Sci Rep. (2014) 4:4493. doi: 10.1038/srep04493
71. Qin K, Yu M, Fan J, Wang H, Zhao P, Zhao G, et al. Canonical and noncanonical Wnt signaling: Multilayered mediators, signaling mechanisms and major signaling crosstalk. Genes Dis. (2024) 11:103–34. doi: 10.1016/j.gendis.2023.01.030
72. Rapp J, Jaromi L, Kvell K, Miskei G, and Pongracz JE. WNT signaling - lung cancer is no exception. Respir Res. (2017) 18:167. doi: 10.1186/s12931-017-0650-6
73. Cheng LH, Hsu CC, Tsai HW, Liao WY, Yang PM, Liao TY, et al. ASPM activates hedgehog and wnt signaling to promote small cell lung cancer stemness and progression. Cancer Res. (2023) 83:830–44. doi: 10.1158/0008-5472.CAN-22-2496
74. Ashrafizadeh M, Zarrabi A, Hushmandi K, Hashemi F, Moghadam ER, Owrang M, et al. Lung cancer cells and their sensitivity/resistance to cisplatin chemotherapy: Role of microRNAs and upstream mediators. Cell Signal. (2021) 78:109871. doi: 10.1016/j.cellsig.2020.109871
75. Zhong Y, He JW, Huang CX, Lai HZ, Li XK, Zheng C, et al. The NcRNA/Wnt axis in lung cancer: oncogenic mechanisms, remarkable indicators and therapeutic targets. J Transl Med. (2025) 23:326. doi: 10.1186/s12967-025-06326-4
76. Ma Y, Marinkova R, Nenkov M, Jin L, Huber O, Sonnemann J, et al. Tumor-intrinsic PD-L1 exerts an oncogenic function through the activation of the wnt/β-catenin pathway in human non-small cell lung cancer. Int J Mol Sci. (2022) 23(19):11031. doi: 10.3390/ijms231911031
77. Yu W, Hua Y, Qiu H, Hao J, Zou K, Li Z, et al. PD-L1 promotes tumor growth and progression by activating WIP and β-catenin signaling pathways and predicts poor prognosis in lung cancer. Cell Death Dis. (2020) 11:506. doi: 10.1038/s41419-020-2701-z
78. Muto S, Enta A, Maruya Y, Inomata S, Yamaguchi H, Mine H, et al. Wnt/β-catenin signaling and resistance to immune checkpoint inhibitors: from non-small-cell lung cancer to other cancers. Biomedicines. (2023) 11(1):190. doi: 10.3390/biomedicines11010190
79. Zhou F, Sun J, Ye L, Jiang T, Li W, Su C, et al. Fibronectin promotes tumor angiogenesis and progression of non-small-cell lung cancer by elevating WISP3 expression via FAK/MAPK/HIF-1α axis and activating wnt signaling pathway. Exp Hematol Oncol. (2023) 12:61. doi: 10.1186/s40164-023-00419-w
80. Altorki NK, Markowitz GJ, Gao D, Port JL, Saxena A, Stiles B, et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat Rev Cancer. (2019) 19:9–31. doi: 10.1038/s41568-018-0081-9
81. Wan L, Pantel K, and Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nat Med. (2013) 19:1450–64. doi: 10.1038/nm.3391
82. Gerstberger S, Jiang Q, and Ganesh K. Metastasis. Cell. (2023) 186:1564–79. doi: 10.1016/j.cell.2023.03.003
83. Wang B, Hao X, Yan J, Li X, Zhao M, and Han T. A bibliometric analysis of immune-related adverse events in cancer patients and a meta-analysis of immune-related adverse events in patients with hepatocellular carcinoma. J Transl Int Med. (2024) 12:225–43. doi: 10.2478/jtim-2024-0003
84. Peng J, Liu D, Zhang H, Hu Q, Chen W, Zou J, et al. Identification of a novel prognostic lymphangiogenesis-related signature associated with tumor immunity for guiding therapy in lung adenocarcinoma. Analytical Cellular Pathology. (2024) 29:2090450. doi: 10.1155/2024/2090450
85. Jin MZ and Jin WL. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther. (2020) 5:166. doi: 10.1038/s41392-020-00280-x
86. Grivennikov SI, Greten FR, and Karin M. Immunity, inflammation, and cancer. Cell. (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
87. Quail DF and Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. (2013) 19:1423–37. doi: 10.1038/nm.3394
88. Peri SS, Narayanaa YK, Hubert TD, Rajaraman R, Arfuso F, Sundaram S, et al. Navigating tumour microenvironment and wnt signalling crosstalk: implications for advanced cancer therapeutics. Cancers (Basel). (2023) 15(24):5847. doi: 10.3390/cancers15245847
89. Chu X, Tian W, Ning J, Xiao G, Zhou Y, Wang Z, et al. Cancer stem cells: advances in knowledge and implications for cancer therapy. Signal Transduct Target Ther. (2024) 9:170. doi: 10.1038/s41392-024-01851-y
90. Malladi S, Macalinao DG, Jin X, He L, Basnet H, Zou Y, et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell. (2016) 165:45–60. doi: 10.1016/j.cell.2016.02.025
91. Hu G, Wei Y, and Kang Y. The multifaceted role of MTDH/AEG-1 in cancer progression. Clin Cancer Res. (2009) 15:5615–20. doi: 10.1158/1078-0432.CCR-09-0049
92. Shen M, Xie S, Rowicki M, Michel S, Wei Y, Hang X, et al. Therapeutic targeting of metadherin suppresses colorectal and lung cancer progression and metastasis. Cancer Res. (2021) 81:1014–25. doi: 10.1158/0008-5472.CAN-20-1876
93. Iksen, Pothongsrisit S, and Pongrakhananon V. Targeting the PI3K/AKT/mTOR signaling pathway in lung cancer: an update regarding potential drugs and natural products. Molecules. (2021) 26(13):4100. doi: 10.3390/molecules26134100
94. Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, and González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. (2004) 30:193–204. doi: 10.1016/j.ctrv.2003.07.007
95. Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, and Abraham RT. The PI3K pathway in human disease. Cell. (2017) 170:605–35. doi: 10.1016/j.cell.2017.07.029
96. Thapa R, Moglad E, Goyal A, Bhat AA, Almalki WH, Kazmi I, et al. Deciphering NF-kappaB pathways in smoking-related lung carcinogenesis. Excli J. (2024) 23:991–1017. doi: 10.17179/excli2024-7475
97. Feng W, Ting Y, Tang X, Liu D, Zhou WC, Li Y, et al. The role of ESM1 in the lipids metabolic reprogramming and angiogenesis of lung adenocarcinoma cells. Heliyon. (2024) 10:e36897. doi: 10.1016/j.heliyon.2024.e36897
98. Xie J, Yang P, Lin HP, Li Y, Clementino M, Fenske W, et al. Integrin α4 up-regulation activates the hedgehog pathway to promote arsenic and benzo[α]pyrene co-exposure-induced cancer stem cell-like property and tumorigenesis. Cancer Lett. (2020) 493:143–55. doi: 10.1016/j.canlet.2020.08.015
99. Moghbeli M. PI3K/AKT pathway as a pivotal regulator of epithelial-mesenchymal transition in lung tumor cells. Cancer Cell Int. (2024) 24:165. doi: 10.1186/s12935-024-03357-7
100. Tian B, Pang Y, Gao Y, Meng Q, Xin L, Sun C, et al. A pan-cancer analysis of the oncogenic role of Golgi transport 1B in human tumors. J Transl Int Med. (2023) 11:433–48. doi: 10.2478/jtim-2023-0002
101. Almalki WH. Beyond the genome: lncRNAs as regulators of the PI3K/AKT pathway in lung cancer. Pathol Res Pract. (2023) 251:154852. doi: 10.1016/j.prp.2023.154852
102. Xiong Z, Han Z, Pan W, Zhu X, and Liu C. Correlation between chromatin epigenetic-related lncRNA signature (CELncSig) and prognosis, immune microenvironment, and immunotherapy in non-small cell lung cancer. PLoS One. (2023) 18:e0286122. doi: 10.1371/journal.pone.0286122
103. Mao G, Mu Z, and Wu DA. Exosomal lncRNA FOXD3-AS1 upregulates ELAVL1 expression and activates PI3K/Akt pathway to enhance lung cancer cell proliferation, invasion, and 5-fluorouracil resistance. Acta Biochim Biophys Sin (Shanghai). (2021) 53:1484–94. doi: 10.1093/abbs/gmab129
104. Liu X, Sun L, Zhang S, Zhang S, and Li W. GINS2 facilitates epithelial-to-mesenchymal transition in non-small-cell lung cancer through modulating PI3K/Akt and MEK/ERK signaling. J Cell Physiol. (2020) 235:7747–56. doi: 10.1002/jcp.v235.11
105. Wang L, Shi L, Gu J, Zhan C, Xi J, Ding J, et al. CXCL5 regulation of proliferation and migration in human non-small cell lung cancer cells. J Physiol Biochem. (2018) 74:313–24. doi: 10.1007/s13105-018-0619-z
106. Huber SM, Butz L, Stegen B, Klumpp D, Braun N, Ruth P, et al. Ionizing radiation, ion transports, and radioresistance of cancer cells. Front Physiol. (2013) 4:212. doi: 10.3389/fphys.2013.00212
107. Lee SY, Jeong EK, Ju MK, Jeon HM, Kim MY, Kim CH, et al. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol Cancer. (2017) 16:10. doi: 10.1186/s12943-016-0577-4
108. McDaid WJ, Wilson L, Adderley H, Martinez-Lopez A, Baker MJ, Searle J, et al. The PI3K-AKT-mTOR axis persists as a therapeutic dependency in KRAS(G12D)-driven non-small cell lung cancer. Mol Cancer. (2024) 23:253. doi: 10.1186/s12943-024-02157-x
109. Hou X, Zhou C, Liang Z, Qiu H, Zhou Z, Zheng H, et al. Salvianolic acid F suppresses KRAS-dependent lung cancer cell growth through the PI3K/AKT signaling pathway. Phytomedicine. (2023) 121:155093. doi: 10.1016/j.phymed.2023.155093
110. Liu X, Mei W, Zhang P, and Zeng C. PIK3CA mutation as an acquired resistance driver to EGFR-TKIs in non-small cell lung cancer: Clinical challenges and opportunities. Pharmacol Res. (2024) 202:107123. doi: 10.1016/j.phrs.2024.107123
111. O'Donnell JS, Massi D, Teng MWL, and Mandala M. PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux. Semin Cancer Biol. (2018) 48:91–103. doi: 10.1016/j.semcancer.2017.04.015
112. Zhang H, Yang Y, Li X, Yuan X, and Chu Q. Targeting the Notch signaling pathway and the Notch ligand, DLL3, in small cell lung cancer. BioMed Pharmacother. (2023) 159:114248. doi: 10.1016/j.biopha.2023.114248
113. Galluzzo P and Bocchetta M. Notch signaling in lung cancer. Expert Rev Anticancer Ther. (2011) 11:533–40. doi: 10.1586/era.10.158
114. Liu L, Tao T, Liu S, Yang X, Chen X, Liang J, et al. An RFC4/Notch1 signaling feedback loop promotes NSCLC metastasis and stemness. Nat Commun. (2021) 12:2693. doi: 10.1038/s41467-021-22971-x
115. Rudin CM, Reck M, Johnson ML, Blackhall F, Hann CL, Yang JC, et al. Emerging therapies targeting the delta-like ligand 3 (DLL3) in small cell lung cancer. J Hematol Oncol. (2023) 16:66. doi: 10.1186/s13045-023-01464-y
116. Paz-Ares L, Champiat S, Lai WV, Izumi H, Govindan R, Boyer M, et al. Tarlatamab, a first-in-class DLL3-targeted bispecific T-cell engager, in recurrent small-cell lung cancer: an open-label, phase I study. J Clin Oncol. (2023) 41:2893–903. doi: 10.1200/JCO.22.02823
117. Jaspers JE, Khan JF, Godfrey WD, Lopez AV, Ciampricotti M, Rudin CM, et al. IL-18-secreting CAR T cells targeting DLL3 are highly effective in small cell lung cancer models. J Clin Invest. (2023) 133(9):1–11. doi: 10.1172/JCI166028
118. Corada M, Nyqvist D, Orsenigo F, Caprini A, Giampietro C, Taketo MM, et al. The Wnt/beta-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Dev Cell. (2010) 18:938–49. doi: 10.1016/j.devcel.2010.05.006
119. Misiorek JO, Przybyszewska-Podstawka A, Kałafut J, Paziewska B, Rolle K, Rivero-Müller A, et al. Context matters: NOTCH signatures and pathway in cancer progression and metastasis. Cells. (2021) 10(1):94. doi: 10.3390/cells10010094
120. Dudley AC and Griffioen AW. Pathological angiogenesis: mechanisms and therapeutic strategies. Angiogenesis. (2023) 26:313–47. doi: 10.1007/s10456-023-09876-7
121. Hultgren NW, Fang JS, Ziegler ME, Ramirez RN, Phan DTT, Hatch MMS, et al. Slug regulates the Dll4-Notch-VEGFR2 axis to control endothelial cell activation and angiogenesis. Nat Commun. (2020) 11:5400. doi: 10.1038/s41467-020-18633-z
122. Pedrosa AR, Trindade A, Fernandes AC, Carvalho C, Gigante J, Tavares AT, et al. Endothelial Jagged1 antagonizes Dll4 regulation of endothelial branching and promotes vascular maturation downstream of Dll4/Notch1. Arterioscler Thromb Vasc Biol. (2015) 35:1134–46. doi: 10.1161/ATVBAHA.114.304741
123. Raoof S, Mulford IJ, Frisco-Cabanos H, Nangia V, Timonina D, Labrot E, et al. Targeting FGFR overcomes EMT-mediated resistance in EGFR mutant non-small cell lung cancer. Oncogene. (2019) 38:6399–413. doi: 10.1038/s41388-019-0887-2
124. Lu HY, Zu YX, Jiang XW, Sun XT, Liu TY, Li RL, et al. Novel ADAM-17 inhibitor ZLDI-8 inhibits the proliferation and metastasis of chemo-resistant non-small-cell lung cancer by reversing Notch and epithelial mesenchymal transition in vitro and in vivo. Pharmacol Res. (2019) 148:104406. doi: 10.1016/j.phrs.2019.104406
125. Peng J, Sun BF, Chen CY, Zhou JY, Chen YS, Chen H, et al. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer. (2017) 17:725–37. doi: 10.1038/nrc.2017.87
126. Bergenfelz C and Leandersson K. Targeting NOTCH activation in small cell lung cancer through LSD1 inhibition. Sci Signal. (2019) 12(567):1–31. doi: 10.1126/scisignal.aau2922
127. Szeto GL and Finley SD. Integrative approaches to cancer immunotherapy. Trends Cancer. (2019) 5:400–10. doi: 10.1016/j.trecan.2019.05.010
128. Gibbons DL, Creighton CJ, Zhu L, Richardson AL, and Kurie JM. Engineering T cells with customized therapeutic response programs using synthetic notch receptors. Cell. (2016) 167:419–432.e16. doi: 10.1016/j.cell.2016.09.011
129. Han Y, Liu D, and Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. (2020) 10:727–42.
130. Engblom C, Pfirschke C, Rickelt S, Rohde M, Rau K, von Boehmer L, et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol. (2020) 21:1346–58. doi: 10.1038/s41590-020-0769-3
131. Sari MI and Ilyas S. The expression levels and concentrations of PD-1 and PD-L1 proteins in septic patients: A systematic review. Diagnostics (Basel). (2022) 12(8):2004. doi: 10.3390/diagnostics12082004
132. Hagerling C, Karpinich NO, Whitaker EE, Moore DL, Ford HJ, Berwin B, et al. Hypoxia-inducible factor-1α and nuclear factor-κB play important roles in regulating programmed cell death ligand 1 expression by epidermal growth factor receptor mutants in non-small-cell lung cancer cells. Cancer Sci. (2019) 110:1665–75. doi: 10.1111/cas.2019.110.issue-5
133. Kratochvill F, Neale G, Huse JT, Rodriguez FJ, Cheng L, Iwai N, et al. Epidermal growth factor receptor (EGFR) pathway, yes-associated protein (YAP) and the regulation of programmed death-ligand 1 (PD-L1) in non-small cell lung cancer (NSCLC). Int J Mol Sci. (2019) 20(15):3821. doi: 10.3390/ijms20153821
134. Duan J, Zhang Y, Chen R, Liang L, Huo Y, Lu S, et al. Tumor-immune microenvironment and NRF2 associate with clinical efficacy of PD-1 blockade combined with chemotherapy in lung squamous cell carcinoma. Cell Rep Med. (2023) 4:101302. doi: 10.1016/j.xcrm.2023.101302
135. Kim S, Jang JY, Koh J, Kwon D, Kim YA, Paeng JC. Programmed cell death ligand-1-mediated enhancement of hexokinase 2 expression is inversely related to T-cell effector gene expression in non-small-cell lung cancer. J Exp Clin Cancer Res. (2019) 38:462. doi: 10.1186/s13046-019-1407-5
136. Piccolo S, Dupont S, and Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. (2014) 94:1287–312. doi: 10.1152/physrev.00005.2014
137. He J, Huang Z, Han L, Gong Y, Xie C. Mechanisms and management of 3rd−generation EGFR−TKI resistance in advanced non−small cell lung cancer (Review). Int J Oncol. (2021) 59(5):90. doi: 10.3892/ijo.2021.5270
138. Castillo DR, Jeon WJ, Park D, Pham B, Yang C, Joung B, et al. Comprehensive review: unveiling the pro-oncogenic roles of IL-1ß and PD-1/PD-L1 in NSCLC development and targeting their pathways for clinical management. Int J Mol Sci. (2023) 24(14):11547. doi: 10.3390/ijms241411547
139. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. (2012) 12:252–64. doi: 10.1038/nrc3239
140. Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. (2002) 8:793–800. doi: 10.1038/nm730
141. Xia L, Liu Y, and Wang Y. PD-1/PD-L1 blockade therapy in advanced non-small-cell lung cancer: current status and future directions. Oncologist. (2019) 24:S31–s41. doi: 10.1634/theoncologist.2019-IO-S1-s05
142. Tang M, Li Y, Luo X, Xiao J, Wang J, Zeng X, et al. Identification of biomarkers related to CD8(+) T cell infiltration with gene co-expression network in lung squamous cell carcinoma. Front Cell Dev Biol. (2021) 9:606106. doi: 10.3389/fcell.2021.606106
143. Frentzas S, Austria Mislang AR, Lemech C, Nagrial A, Underhill C, Wang W, et al. Phase 1a dose escalation study of ivonescimab (AK112/SMT112), an anti-PD-1/VEGF-A bispecific antibody, in patients with advanced solid tumors. J Immunother Cancer. (2024) 12(4):1–11. doi: 10.1136/jitc-2023-008037
144. Zhao Y, Chen G, Chen J, Zhuang L, Du Y, Yu Q, et al. AK112, a novel PD-1/VEGF bispecific antibody, in combination with chemotherapy in patients with advanced non-small cell lung cancer (NSCLC): an open-label, multicenter, phase II trial. EClinicalMedicine. (2023) 62:102106. doi: 10.1016/j.eclinm.2023.102106
145. Gautron AS, Juillerat A, Guyot V, Filhol JM, Dessez E, Duclert A, et al. Fine and predictable tuning of TALEN gene editing targeting for improved T cell adoptive immunotherapy. Mol Ther Nucleic Acids. (2017) 9:312–21. doi: 10.1016/j.omtn.2017.10.005
146. Wicks EE and Semenza GL. Hypoxia-inducible factors: cancer progression and clinical translation. J Clin Invest. (2022) 132(11):1–10. doi: 10.1172/JCI159839
147. Shurin MR and Umansky V. Cross-talk between HIF and PD-1/PD-L1 pathways in carcinogenesis and therapy. J Clin Invest. (2022) 132(9):1–4. doi: 10.1172/JCI159473
148. Chan CY, Chiu DK, Yuen VW, Law CT, Wong BP, Thu KL, et al. Extracellular HMGB1 blockade inhibits tumor growth through profoundly remodeling immune microenvironment and enhances checkpoint inhibitor-based immunotherapy. J Immunother Cancer. (2021) 9(32):1–11. doi: 10.1136/jitc-2020-001966
149. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. (2016) 387:1837–46. doi: 10.1016/S0140-6736(16)00587-0
150. Zhang L, Wei X, Wang Z, Liu P, Hou Y, Xu Y, et al. NF-κB activation enhances STING signaling by altering microtubule-mediated STING trafficking. Cell Rep. (2023) 42:112185. doi: 10.1016/j.celrep.2023.112185
151. Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. (2009) 1:a001651. doi: 10.1101/cshperspect.a001651
152. Morgan MJ and Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. (2011) 21:103–15. doi: 10.1038/cr.2010.178
153. Sun SC. Non-canonical NF-κB signaling pathway. Cell Res. (2011) 21:71–85. doi: 10.1038/cr.2010.177
154. Mirzaei S, Saghari S, Bassiri F, Raesi R, Zarrabi A, Hushmandi K, et al. NF-κB as a regulator of cancer metastasis and therapy response: A focus on epithelial-mesenchymal transition. J Cell Physiol. (2022) 237:2770–95. doi: 10.1002/jcp.v237.7
155. Yao X, Huang J, Zhong H, Shen N, Faggioni R, Fung M, et al. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. (2014) 141:125–39. doi: 10.1016/j.pharmthera.2013.09.004
156. Liu W, Wang H, Bai F, Ding L, Huang Y, Lu C, et al. IL-6 promotes metastasis of non-small-cell lung cancer by up-regulating TIM-4 via NF-κB. Cell Prolif. (2020) 53:e12776. doi: 10.1111/cpr.12776
157. Wei X, Liu Z, Shen Y, Dong H, Chen K, Shi X, et al. Semaphorin4A promotes lung cancer by activation of NF-κB pathway mediated by PlexinB1. PeerJ. (2023) 11:e16292. doi: 10.7717/peerj.16292
158. Wang Y, Zhang J, Li YJ, Yu NN, Liu WT, Liang JZ, et al. MEST promotes lung cancer invasion and metastasis by interacting with VCP to activate NF-κB signaling. J Exp Clin Cancer Res. (2021) 40:301. doi: 10.1186/s13046-021-02107-1
159. Zhao J, Wang X, Mi Z, Jiang X, Sun L, Zheng B, et al. STAT3/miR-135b/NF-κB axis confers aggressiveness and unfavorable prognosis in non-small-cell lung cancer. Cell Death Dis. (2021) 12:493. doi: 10.1038/s41419-021-03773-x
160. Antonangeli F, Natalini A, Garassino MC, Sica A, Santoni A, Di Rosa F, et al. Regulation of PD-L1 expression by NF-κB in cancer. Front Immunol. (2020) 11:584626. doi: 10.3389/fimmu.2020.584626
161. Jin X, Zhang L, Ying C, Yu L, Guo X, Pan K, et al. S-adenosylmethionine inhibits non-small cell lung cancer and enhances chemosensitivity by targeting the P62/NF-κB axis and regulating autophagy and oxidative stress. Bioorg Chem. (2025) 160:108509. doi: 10.1016/j.bioorg.2025.108509
162. Eisenbarth SC. Dendritic cell subsets in T cell programming: location dictates function. Nat Rev Immunol. (2019) 19:89–103. doi: 10.1038/s41577-018-0088-1
163. Lv L and Zhou X. Targeting Hippo signaling in cancer: novel perspectives and therapeutic potential. MedComm (2020). (2023) 4:e375. doi: 10.1002/mco2.v4.5
164. Fu M, Hu Y, Lan T, Guan KL, Luo T, Luo M, et al. The Hippo signalling pathway and its implications in human health and diseases. Signal Transduct Target Ther. (2022) 7:376. doi: 10.1038/s41392-022-01191-9
165. Cho YS and Jiang J. Hippo-independent regulation of yki/yap/taz: A non-canonical view. Front Cell Dev Biol. (2021) 9:658481. doi: 10.3389/fcell.2021.658481
166. Snigdha K, Gangwani KS, Lapalikar GV, Singh A, and Kango-Singh M. Hippo signaling in cancer: lessons from drosophila models. Front Cell Dev Biol. (2019) 7:85. doi: 10.3389/fcell.2019.00085
167. Wu Z and Guan KL. Hippo signaling in embryogenesis and development. Trends Biochem Sci. (2021) 46:51–63. doi: 10.1016/j.tibs.2020.08.008
168. Thirusangu P, Ray U, Sarkar Bhattacharya S, Oien DB, Jin L, Staub J, et al. PFKFB3 regulates cancer stemness through the hippo pathway in small cell lung carcinoma. Oncogene. (2022) 41:4003–17. doi: 10.1038/s41388-022-02391-x
169. Jin L, Wu Z, Wang Y, and Zhao X. Cryptotanshinone attenuates the stemness of non-small cell lung cancer cells via promoting TAZ translocation from nuclear to cytoplasm. Chin Med. (2020) 15:66. doi: 10.1186/s13020-020-00348-4
170. Hsu PC, Yang CT, Jablons DM, and You L. The role of yes-associated protein (YAP) in regulating programmed death-ligand 1 (PD-L1) in thoracic cancer. Biomedicines. (2018) 6(4):114. doi: 10.3390/biomedicines6040114
171. Koo JH and Guan KL. Interplay between YAP/TAZ and metabolism. Cell Metab. (2018) 28:196–206. doi: 10.1016/j.cmet.2018.07.010
172. Liu H, Dai X, Cao X, Yan H, Ji X, Zhang H, et al. PRDM4 mediates YAP-induced cell invasion by activating leukocyte-specific integrin β2 expression. EMBO Rep. (2018) 19(6):1–14. doi: 10.15252/embr.201745180
173. Zhang F, Huang B, Xu Y, Cao G, Shen M, Liu C, et al. MISP suppresses ferroptosis via MST1/2 kinases to facilitate YAP activation in non-small cell lung cancer. Adv Sci (Weinh). (2025) p:e2415814. doi: 10.1002/advs.202415814
174. Moroishi T, Hayashi T, Pan WW, Fujita Y, Holt MV, Qin J, et al. The hippo pathway kinases LATS1/2 suppress cancer immunity. Cell. (2016) 167:1525–1539.e17. doi: 10.1016/j.cell.2016.11.005
175. Wang G, Lu X, Dey P, Deng P, Wu CC, Jiang S, et al. Targeting YAP-dependent MDSC infiltration impairs tumor progression. Cancer Discov. (2016) 6:80–95. doi: 10.1158/2159-8290.CD-15-0224
176. Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, and Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. (2020) 19:1997–2007. doi: 10.3892/etm.2020.8454
177. Peng S, Wang R, Zhang X, Ma Y, Zhong L, Li K, et al. EGFR-TKI resistance promotes immune escape in lung cancer via increased PD-L1 expression. Mol Cancer. (2019) 18:165. doi: 10.1186/s12943-019-1073-4
178. Li N, Zuo R, He Y, Gong W, Wang Y, Chen L, et al. PD-L1 induces autophagy and primary resistance to EGFR-TKIs in EGFR-mutant lung adenocarcinoma via the MAPK signaling pathway. Cell Death Dis. (2024) 15:555. doi: 10.1038/s41419-024-06945-7
179. Jia Y, Yun CH, Park E, Ercan D, Manuia M, Juarez J, et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature. (2016) 534:129–32. doi: 10.1038/nature17960
180. de Langen AJ, Johnson ML, Mazieres J, Dingemans AC, Mountzios G, Pless M, et al. Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRAS(G12C) mutation: a randomised, open-label, phase 3 trial. Lancet. (2023) 401:733–46. doi: 10.1016/S0140-6736(23)00221-0
181. Yao J, Ma C, Gao W, Liang J, Liu C, Yang H, et al. Fentanyl induces autophagy via activation of the ROS/MAPK pathway and reduces the sensitivity of cisplatin in lung cancer cells. Oncol Rep. (2016) 36:3363–70. doi: 10.3892/or.2016.5183
182. Zhang J, Sun B, Ruan X, Hou X, Zhi J, Meng X, et al. Oncoprotein HBXIP promotes tumorigenesis through MAPK/ERK pathway activation in non-small cell lung cancer. Cancer Biol Med. (2021) 18:105–19. doi: 10.20892/j.issn.2095-3941.2020.0098
183. Lee JH, Sánchez-Rivera FJ, He L, Basnet H, Chen FX, Spina E, et al. TGF-β and RAS jointly unmask primed enhancers to drive metastasis. Cell. (2024) 187:6182–6199.e29. doi: 10.1016/j.cell.2024.08.014
184. Chen N, Fang W, Lin Z, Peng P, Wang J, Zhan J, et al. KRAS mutation-induced upregulation of PD-L1 mediates immune escape in human lung adenocarcinoma. Cancer Immunol Immunother. (2017) 66:1175–87. doi: 10.1007/s00262-017-2005-z
185. Simons M, Gordon E, and Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. (2016) 17:611–25. doi: 10.1038/nrm.2016.87
186. Puyalto A, Rodríguez-Remírez M, López I, Macaya I, Guruceaga E, Olmedo M, et al. Trametinib sensitizes KRAS-mutant lung adenocarcinoma tumors to PD-1/PD-L1 axis blockade via Id1 downregulation. Mol Cancer. (2024) 23:78. doi: 10.1186/s12943-024-01991-3
187. Molina-Arcas M and Downward J. Exploiting the therapeutic implications of KRAS inhibition on tumor immunity. Cancer Cell. (2024) 42:338–57. doi: 10.1016/j.ccell.2024.02.012
188. Xu M, Zhao X, Wen T, and Qu X. Unveiling the role of KRAS in tumor immune microenvironment. BioMed Pharmacother. (2024) 171:116058. doi: 10.1016/j.biopha.2023.116058
189. Ruscetti M, Leibold J, Bott MJ, Fennell M, Kulick A, Salgado NR, et al. NK cell-mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science. (2018) 362:1416–22. doi: 10.1126/science.aas9090
190. Yang L, Xie G, Fan Q, and Xie J. Activation of the hedgehog-signaling pathway in human cancer and the clinical implications. Oncogene. (2010) 29:469–81. doi: 10.1038/onc.2009.392
191. Skoda AM, Simovic D, Karin V, Kardum V, Vranic S, and Serman L. The role of the Hedgehog signaling pathway in cancer: A comprehensive review. Bosn J Basic Med Sci. (2018) 18:8–20. doi: 10.17305/bjbms.2018.2756
192. Hanna A and Shevde LA. Hedgehog signaling: modulation of cancer properies and tumor mircroenvironment. Mol Cancer. (2016) 15:24. doi: 10.1186/s12943-016-0509-3
193. Ma C, Hu K, Ullah I, Zheng QK, Zhang N, Sun ZG, et al. Molecular mechanisms involving the sonic hedgehog pathway in lung cancer therapy: recent advances. Front Oncol. (2022) 12:729088. doi: 10.3389/fonc.2022.729088
194. Cao L, Xie Y, Chen Z, and Ashby CR. A cycloruthenated complex, ruthenium (II) Z (RuZ) overcomes in vitro and in vivo multidrug resistance in cancer cells: A pivotal breakthrough. J Transl Int Med. (2023) 11:95–7. doi: 10.2478/jtim-2023-0081
195. Li LB, Yang LX, Liu L, Liu FR, Li AH, Zhu YL, et al. Targeted inhibition of the HNF1A/SHH axis by triptolide overcomes paclitaxel resistance in non-small cell lung cancer. Acta Pharmacol Sin. (2024) 45:1060–76. doi: 10.1038/s41401-023-01219-y
196. Wang F, Wang W, Li J, Zhang J, Wang X, Wang M, et al. Sulforaphane reverses gefitinib tolerance in human lung cancer cells via modulation of sonic hedgehog signaling. Oncol Lett. (2018) 15:109–14. doi: 10.3892/ol.2017.7293
197. Yue D, Li H, Che J, Zhang Y, Tseng HH, Jin JQ, et al. Hedgehog/Gli promotes epithelial-mesenchymal transition in lung squamous cell carcinomas. J Exp Clin Cancer Res. (2014) 33:34. doi: 10.1186/1756-9966-33-34
198. Cannonier SA and Sterling JA. The role of hedgehog signaling in tumor induced bone disease. Cancers (Basel). (2015) 7:1658–83. doi: 10.3390/cancers7030856
199. Hauge A and Rofstad EK. Antifibrotic therapy to normalize the tumor microenvironment. J Transl Med. (2020) 18:207. doi: 10.1186/s12967-020-02376-y
200. Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. (2020) 5:8. doi: 10.1038/s41392-020-0110-5
201. Qian BZ and Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. (2010) 141:39–51. doi: 10.1016/j.cell.2010.03.014
202. Li Z, Jiang J, Wang Z, Zhang J, Xiao M, Wang C, et al. Endogenous interleukin-4 promotes tumor development by increasing tumor cell resistance to apoptosis. Cancer Res. (2008) 68:8687–94. doi: 10.1158/0008-5472.CAN-08-0449
203. Furmanski AL, Saldana JI, Ono M, Sahni H, Paschalidis N, D'Acquisto F, et al. Tissue-derived hedgehog proteins modulate Th differentiation and disease. J Immunol. (2013) 190:2641–9. doi: 10.4049/jimmunol.1202541
204. Onishi H, Fujimura A, Oyama Y, Yamasaki A, Imaizumi A, Kawamoto M, et al. Hedgehog signaling regulates PDL-1 expression in cancer cells to induce anti-tumor activity by activated lymphocytes. Cell Immunol. (2016) 310:199–204. doi: 10.1016/j.cellimm.2016.08.003
205. Didiasova M, Schaefer L, and Wygrecka M. Targeting GLI transcription factors in cancer. Molecules. (2018) 23(5):1003. doi: 10.3390/molecules23051003
206. Rimkus TK, Carpenter RL, Qasem S, Chan M, and Lo HW. Targeting the sonic hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancers (Basel). (2016) 8(2):22. doi: 10.3390/cancers8020022
207. Li H, Liu L, Chen HY, Yan X, Li RL, Lan J, et al. Mogrol suppresses lung cancer cell growth by activating AMPK-dependent autophagic death and inducing p53-dependent cell cycle arrest and apoptosis. Toxicol Appl Pharmacol. (2022) 444:116037. doi: 10.1016/j.taap.2022.116037
208. Li J, Fan Y, Zhang Y, Liu Y, Yu Y, Ma M, et al. Resveratrol induces autophagy and apoptosis in non-small-cell lung cancer cells by activating the NGFR-AMPK-mTOR pathway. Nutrients. (2022) 14(12):2413. doi: 10.3390/nu14122413
209. Dong Y, Hu H, Zhang X, Zhang Y, Sun X, Wang H, et al. Phosphorylation of PHF2 by AMPK releases the repressive H3K9me2 and inhibits cancer metastasis. Signal Transduct Target Ther. (2023) 8:95. doi: 10.1038/s41392-022-01302-6
210. Gadducci A, Biglia N, Tana R, Cosio S, and Gallo M. Metformin use and gynecological cancers: A novel treatment option emerging from drug repositioning. Crit Rev Oncology/Hematology. (2016) 105:73–83. doi: 10.1016/j.critrevonc.2016.06.006
211. Morales DR and Morris AD. Metformin in cancer treatment and prevention. Annu Rev Med. (2015) 66:17–29. doi: 10.1146/annurev-med-062613-093128
212. Shackelford DB and Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. (2009) 9:563–75. doi: 10.1038/nrc2676
213. Hollstein PE, Eichner LJ, Brun SN, Kamireddy A, Svensson RU, Vera LI, et al. The AMPK-related kinases SIK1 and SIK3 mediate key tumor-suppressive effects of LKB1 in NSCLC. Cancer Discov. (2019) 9:1606–27. doi: 10.1158/2159-8290.CD-18-1261
214. Liberti MV and Locasale JW. The warburg effect: how does it benefit cancer cells? Trends Biochem Sci. (2016) 41:211–8. doi: 10.1016/j.tibs.2015.12.001
215. Li L, Zhao D, Cheng G, Li Q, Chu Y, Chu H, et al. β-elemene suppresses Warburg effect in NCI-H1650 non-small-cell lung cancer cells by regulating the miR-301a-3p/AMPKα axis. Biosci Rep. (2020) 40(6):1–10. doi: 10.1042/BSR20194389
216. Li Y, Sun R, Zou J, Ying Y, and Luo Z. Dual roles of the AMP-activated protein kinase pathway in angiogenesis. Cells. (2019) 8(7):752. doi: 10.3390/cells8070752
217. Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. (2008) 30:214–26. doi: 10.1016/j.molcel.2008.03.003
218. Leprivier G, Remke M, Rotblat B, Dubuc A, Mateo AR, Kool M, et al. The eEF2 kinase confers resistance to nutrient deprivation by blocking translation elongation. Cell. (2013) 153:1064–79. doi: 10.1016/j.cell.2013.04.055
219. Li W, Yuan Q, Li M, He X, Shen C, Luo Y, et al. Research advances on signaling pathways regulating the polarization of tumor-associated macrophages in lung cancer microenvironment. Front Immunol. (2024) 15:1452078. doi: 10.3389/fimmu.2024.1452078
220. Xu F, Cui WQ, Wei Y, Cui J, Qiu J, Hu LL, et al. Astragaloside IV inhibits lung cancer progression and metastasis by modulating macrophage polarization through AMPK signaling. J Exp Clin Cancer Res. (2018) 37:207. doi: 10.1186/s13046-018-0878-0
221. Saravia J, Raynor JL, Chapman NM, Lim SA, and Chi H. Signaling networks in immunometabolism. Cell Res. (2020) 30:328–42. doi: 10.1038/s41422-020-0301-1
222. Han JH, Kim YK, Kim H, Lee J, Oh MJ, Kim SB, et al. Snail acetylation by autophagy-derived acetyl-coenzyme A promotes invasion and metastasis of KRAS-LKB1 co-mutated lung cancer cells. Cancer Commun (Lond). (2022) 42:716–49. doi: 10.1002/cac2.12332
223. Pokhrel RH, Acharya S, Ahn JH, Gu Y, Pandit M, Kim JO, et al. AMPK promotes antitumor immunity by downregulating PD-1 in regulatory T cells via the HMGCR/p38 signaling pathway. Mol Cancer. (2021) 20:133. doi: 10.1186/s12943-021-01420-9
224. Geissler K and Zach O. Pathways involved in Drosophila and human cancer development: the Notch, Hedgehog, Wingless, Runt, and Trithorax pathway. Ann Hematol. (2012) 91:645–69. doi: 10.1007/s00277-012-1435-0
225. Lin GL and Hankenson KD. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J Cell Biochem (2011) 112:3491–501. doi: 10.1002/jcb.v112.12
226. Konstantinopoulos PA, Karamouzis MV, and Papavassiliou AG. Post-translational modifications and regulation of the RAS superfamily of GTPases as anticancer targets. Nat Rev Drug Discov. (2007) 6:541–55. doi: 10.1038/nrd2221
227. Shao X, Xie N, Chen Z, Wang X, Cao W, Zheng Y, et al. Inetetamab for injection in combination with vinorelbine weekly or every three weeks in HER2-positive metastatic breast cancer: A multicenter, randomized, phase II clinical trial. J Transl Int Med. (2024) 12:466–77. doi: 10.1515/jtim-2024-0022
228. Pernicova I and Korbonits M. Metformin—mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. (2014) 10:143–56. doi: 10.1038/nrendo.2013.256
229. Zhang H-H and Guo X-L. Combinational strategies of metformin and chemotherapy in cancers. Cancer Chemotherapy Pharmacol. (2016) 78:13–26. doi: 10.1007/s00280-016-3037-3
230. Takebe N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. (2015) 12:445–64. doi: 10.1038/nrclinonc.2015.61
Keywords: signal transduction, lung cancer, therapeutic targets, oncogene, metastasis
Citation: Liu H, Zhou D, Ou Y, Chen S, Long Y, Yuan T, Li Y and Tan Y (2025) Multiple signaling pathways in the frontiers of lung cancer progression. Front. Immunol. 16:1593793. doi: 10.3389/fimmu.2025.1593793
Received: 14 March 2025; Accepted: 26 May 2025;
Published: 10 June 2025.
Edited by:
Paulo Rodrigues-Santos, University of Coimbra, PortugalReviewed by:
Ting Ye, Southwest Medical University, ChinaYi-Ju Chen, National Yang Ming Chiao Tung University, Taiwan
Copyright © 2025 Liu, Zhou, Ou, Chen, Long, Yuan, Li and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingzheng Tan, eWluZ3poZW5nVEAxMjYuY29t; Yukun Li, eXVrdW5fbGlAZm94bWFpbC5jb20=; eXVrdW5fbGlAY3N1LmVkdS5jbg==
†These authors have contributed equally to this work
 Hao Liu
Hao Liu Dujuan Zhou2†
Dujuan Zhou2† Ting Yuan
Ting Yuan Yukun Li
Yukun Li