- Department of Thoracic Surgery, Chongqing General Hospital, Chongqing University, Chongqing, China
Brain metastases occur in 40% of advanced NSCLC patients, with poorer prognosis in squamous subtypes. Immune checkpoint inhibitors (ICIs) combined with chemotherapy have revolutionized treatment, yet data on the systematic treatment of stage IV squamous non-small cell lung cancer with surgery remain limited. A 59-year-old male smoker presented with stage cT4N2M1b IVA squamous NSCLC and a solitary brain metastasis. Next-generation sequencing revealed programmed cell death ligand 1 (PD-L1) high expression (TPS=75%) and TMB-High (28.49 Mut/Mb) without driver mutations. After pembrolizumab plus platinum-based chemotherapy induced conversion therapy for 3 cycles, the brain lesion achieved pathological complete response (pCR) following resection, while the primary lung tumor showed major pathological response (MPR) post-surgery. Postoperative adjuvant chemoimmunotherapy and 2-year pembrolizumab maintenance were administered. Serial circulating tumor DNA (ctDNA) monitoring remained negative, with no recurrence observed over 50 months. This is the first reported case of long-term survival (PFS >50 months) in a PD-L1-high/TMB-High squamous NSCLC patient with brain metastasis treated with immunotherapy-based multimodal therapy. Our findings suggest that biomarker-guided strategies integrating systemic therapy, surgery, and MRD monitoring may enable curative potential in select advanced NSCLC patients. Further studies are warranted to validate this “sandwich” approach (drug-surgery-drug) and optimize treatment duration.
Introduction
Advanced non-small cell lung cancer (NSCLC) frequently metastasizes to distant sites, with brain involvement observed in 40% of cases (1). Treatment-naïve patients with driver gene-positive tumors exhibit a higher incidence of brain metastases (30%-40%) compared to driver gene-negative counterparts (15%-35%), during systemic therapy, the cumulative incidence of brain metastases escalates to 60% (2). Squamous cell carcinoma (SqCC) demonstrates a lower propensity for intracranial spread (7%) relative to adenocarcinoma (3). Local interventions, including surgery and stereotactic radiosurgery (SRS), remain cornerstone strategies for NSCLC brain metastases, conferring survival benefits (4). However, the recurrence rate of brain metastases within one year post-surgery is as high as 50–60% (5), and combination therapies may reduce intracranial recurrence.
The prognosis of NSCLC with brain metastases is generally poor, with a median OS of approximately 17 months for adenocarcinoma patients (6) and 8 months for other NSCLC subtypes (7). However, with the rapid development and remarkable efficacy of targeted therapy and immunotherapy (8, 9), some patients with unresectable advanced NSCLC can be converted to an operable state after systematic treatment, providing an opportunity for long-term survival for patients with lung cancer brain metastases. Herein, we present a case of brain metastatic pulmonary SqCC with high programmed cell death ligand 1 (PD-L1, tumor proportion score [TPS]=75%, combined positive score [CPS]=75) expression and high tumor mutational burden (TMB-H, 28.49 Mut/Mb) who underwent sequential resection of brain and lung lesions after immunotherapy combined with chemotherapy. The patient achieved pathological complete response (pCR) in the brain metastasis and major pathological response (MPR) in the primary lung tumor. Postoperative longitudinal molecular residual disease (MRD) monitoring and follow-up have shown no recurrence, with progression-free survival (PFS) exceeding 50 months.
Case description
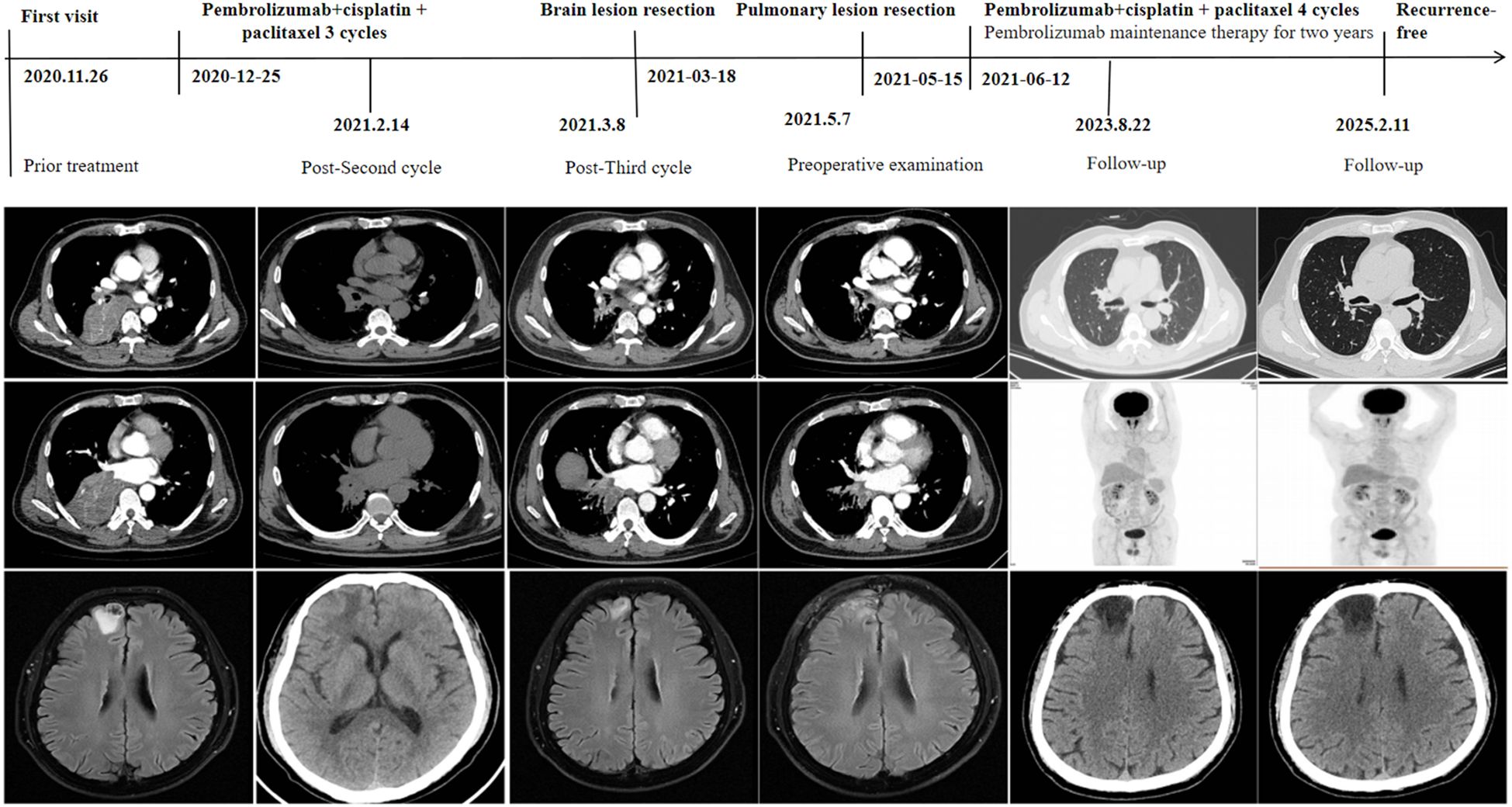
A 59-year-old male presented to our hospital on November 26, 2020, following the detection of a right lower lung mass during a routine health checkup one week prior. The patient had no history of chronic diseases but reported a 40-year smoking history (20 cigarettes/day) and occasional alcohol consumption. Physical examination was unremarkable. Breath sounds were normal without obvious abnormalities, and no palpable enlargement of superficial lymph nodes was noted. Admission chest contrast-enhanced computed tomography (CT) demonstrated occlusion of the right lower lobar bronchus, collapse of the right lower lobe with a mass-like lesion (8.9 cm × 7.8 cm), invasion and occlusion of the right inferior pulmonary vein, narrowing of partial branches of the right inferior pulmonary artery, and mild stenosis of the right middle lobar bronchus (Figure 1). Enlarged mediastinal and right hilar lymph nodes were observed, most notably a subcarinal lymph node measuring 2.9 cm × 2.1 cm (Figure 1). These findings suggested a neoplastic lesion (suspected lung cancer) in the right lower lobe accompanied by lobar collapse, mediastinal/hilar lymph node metastases, and vascular involvement. Fiberoptic bronchoscopy revealed stenosis at the right middle lobar orifice and a neoplasm at the right lower lobar orifice, located 3 cm distal to the carina and 1.5 cm from the right upper lobar orifice (Figures 2A, B). Endoscopic biopsy indicated severe squamous dysplasia with papillary growth (Figure 3A). Percutaneous lung biopsy confirmed squamous cell carcinoma (Figure 3B). Brain contrast-enhanced magnetic resonance imaging (MRI) identified a right frontal lobe lesion (1.6 cm × 1.2 cm) (Figure 1), consistent with lung cancer metastasis. Whole-body bone scintigraphy and abdominal ultrasonography showed no distant metastases. The patient was staged as cT4N2M1b IVA. Next-generation sequencing (NGS) and immunohistochemistry revealed no driver gene mutations, high PD-L1 expression (TPS=75%, CPS=75; DAKO 22C3 antibody) and high TMB (28.49 mutations/megabase [Mut/Mb]) from puncture tissue sample.
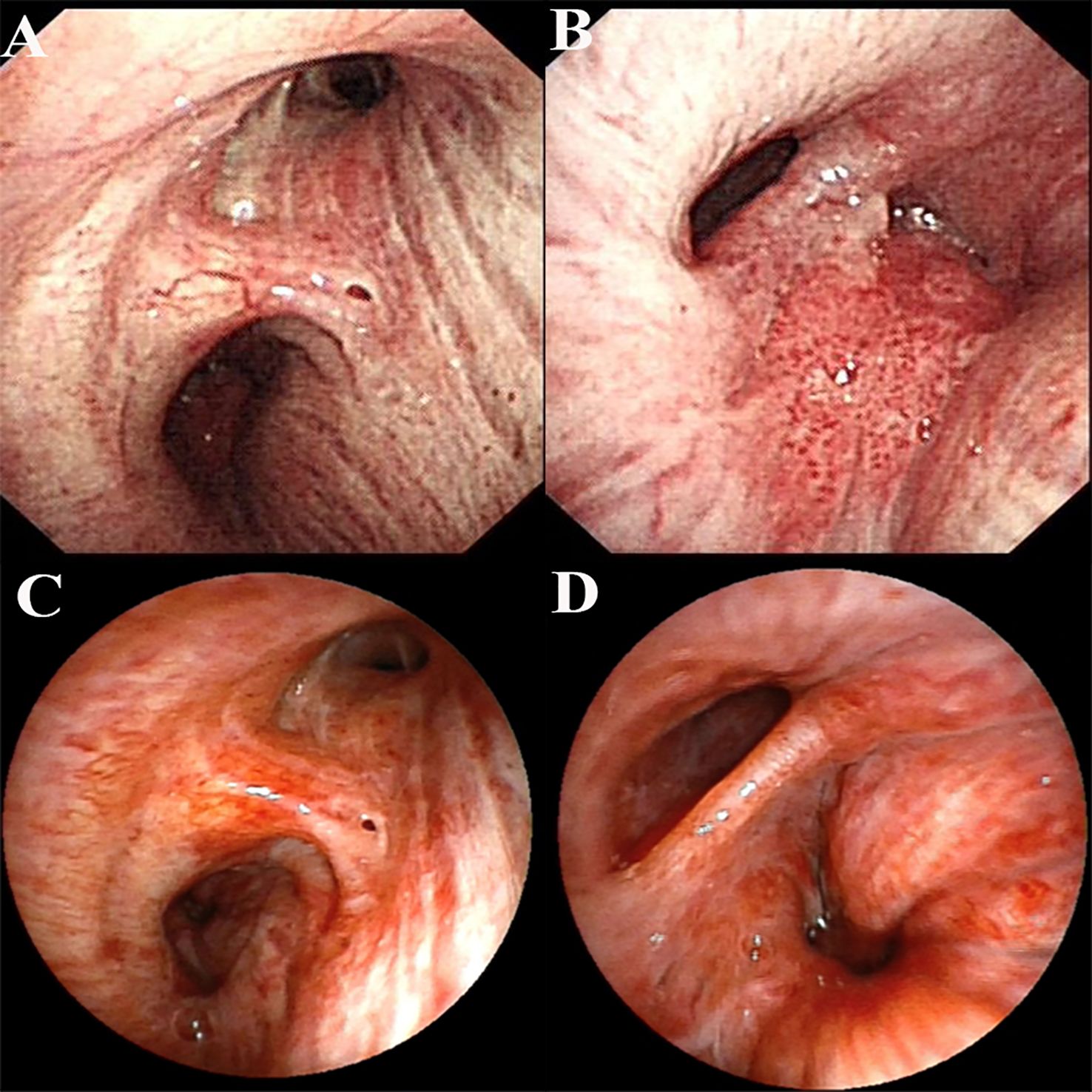
Figure 2. Bronchoscopic images before and after treatment. (A) Bronchoscopic image before treatment (right subcarina); (B) Bronchoscopic image before treatment (right intermediate bronchus); (C) Bronchoscopic image after conversion therapy (right subcarina); (D) Bronchoscopic image after conversion therapy (right intermediate bronchus).
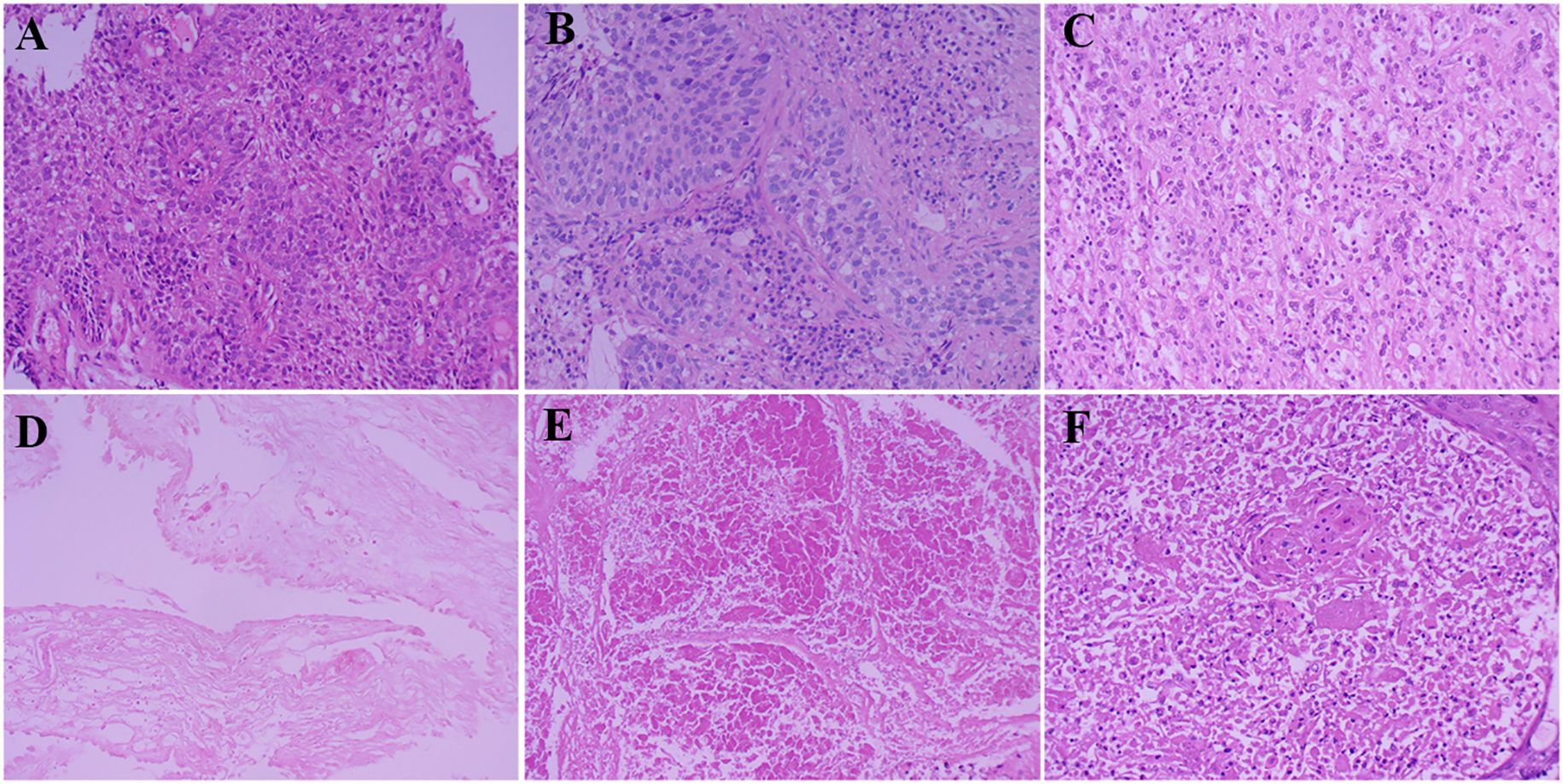
Figure 3. Pathological results of specimens before and after surgery. (A) Bronchoscopic specimen before conversion therapy; (B) Percutaneous biopsy specimen before conversion therapy; (C) Resected specimen of frontal lobe lesion after treatment; (D) Bronchoscopic specimen after conversion therapy; (E) Lung tissue specimen after surgery; (F) Specimen of group 11 lymph nodes after surgery.
Following multidisciplinary team (MDT) discussion (thoracic surgery, oncology, pulmonology, radiotherapy) and informed consent, the patient initiated anti-PD-1 therapy combined with platinum-doublet chemotherapy (pembrolizumab 200 mg IV, cisplatin 75 mg/m² + paclitaxel 135 mg/m² on day 1 every 3 weeks) on December 25, 2020. After one treatment cycle, chest X-ray (digital radiography, DR) showed reduced opacity in the right lower lung field. Post-cycle 2 imaging (February 14, 2021) revealed shrinkage of the right lower lobe lesion (4.9 cm × 3.5 cm) (Figure 1), alleviated bronchial obstruction, and decreased subcarinal lymph node short axis to 1.81 cm (Figure 1). Brain CT demonstrated a nodular isodense lesion in the right frontal lobe (Figure 1). After three cycles (March 8, 2021), chest contrast-enhanced CT showed further reduction of the pulmonary lesion (3.6 cm × 3.3 cm) (Figure 1) and subcarinal lymph node (short axis: 1.7 cm), with persistent vascular involvement. Brain MRI revealed a smaller right frontal lesion (0.8 cm × 0.6 cm) (Figure 1) with reduced density. Positron emission tomography-computed tomography (PET-CT) demonstrated a soft tissue mass (3.58 cm × 3.29 cm, SUVmax 4.09) in the right lower lobe hilum, enlarged subcarinal lymph nodes (short axis 1.42 cm, SUVmax 4.7), and a right frontal hypodense lesion (1.45 cm, SUVmax 7.41) without other metastases. Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 indicated partial response (PR). The patient developed grade 1 myelosuppression during treatment, managed with granulocyte colony-stimulating factor, without grade 3–5 adverse events. Repeat MDT assessment confirmed resectability of the right lower lung lesion. Five weeks after cycle 3 (March 18, 2021), the patient underwent navigated right frontal metastasectomy. Postoperative pathology revealed chronic inflammation, reactive gliosis, and keratin-positive cellular debris, consistent with treated metastatic carcinoma (Figure 3C), according to the Expert Consensus of Chinese Medical Association achieved pCR, which have no residual viable tumor cells following conversion therapy (10). Re-admitted on May 6, 2021, preoperative chest/abdominal CT (May 7, 2021) showed a residual right lower lobe lesion (3.1 cm × 3.9 cm) with stable lymphadenopathy (Figure 1). Brain MRI and bone scintigraphy revealed no metastases (Figure 1). Repeat bronchoscopy demonstrated mucosal protrusion and stenosis at the right lower lobar orifice, with biopsy showing chronic inflammation (Figures 2C, D). Restaged as ycT2aN2aM0 IIIA, the patient underwent thoracoscopic right middle-lower lobectomy with mediastinal lymphadenectomy and partial pericardiectomy on May 15, 2021, due to intraoperative findings of middle lobe invasion.
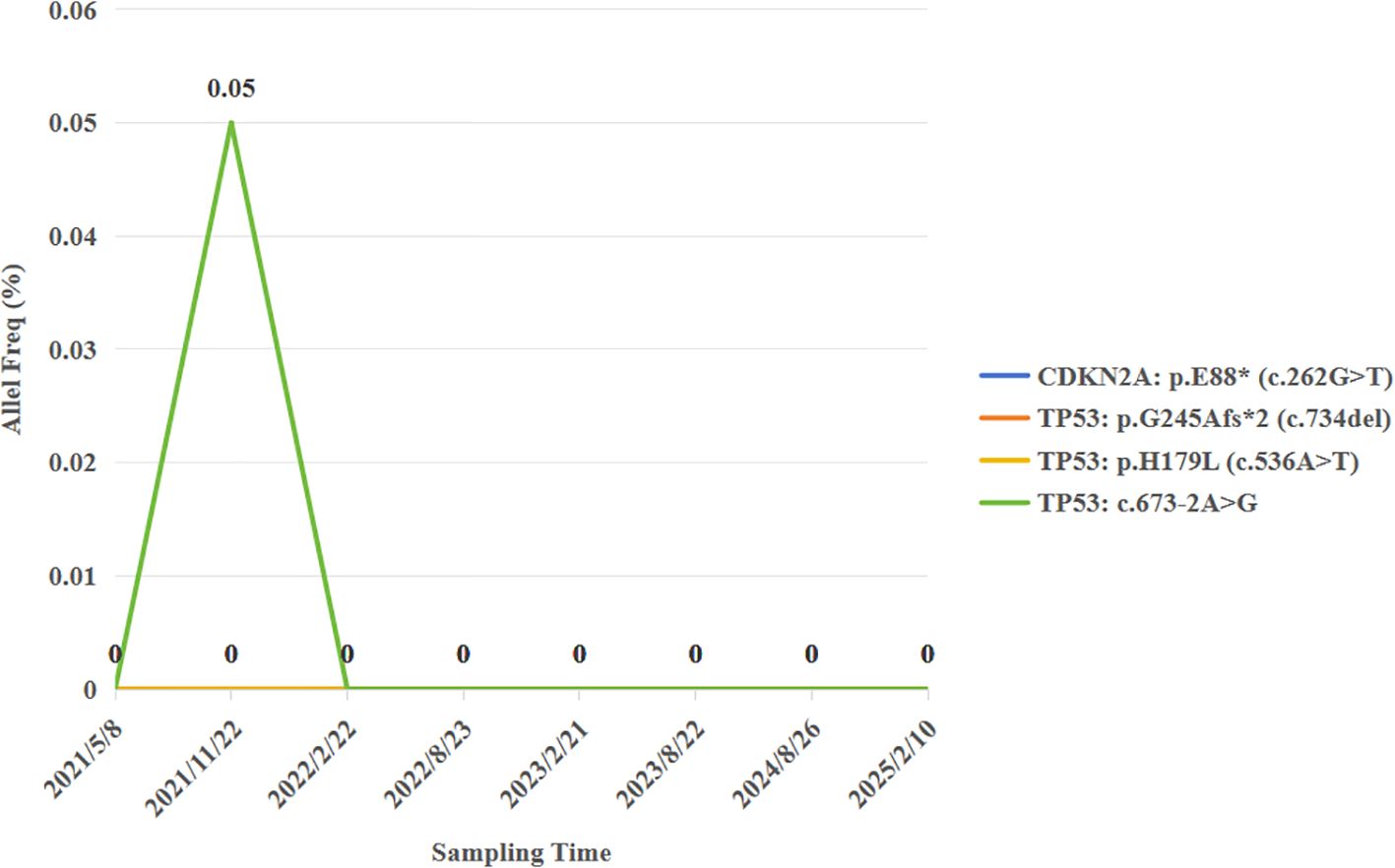
Pathological examination identified a 2.5 cm × 2.2 cm tumor bed with no residual carcinoma (Figures 3D, E), absent lymphovascular or perineural invasion, and bronchial stump without cancer. Lymph nodes submitted for pathological examination included groups 2R (0/2), 4R (0/11), 7 (0/3), 10 (0/1), 11 (1/3), and 12 (0/1). Residual tumor was detected at 1 of group 11, along with evidence of a mild post-chemotherapy response, that suggesting a partial response to treatment (Figure 2F). Notably, groups 7 and 12 exhibited extensive necrosis, stromal fibrosis with cholesterol crystals, and hemosiderin deposition, suggestive of prior tumor involvement. However, no residual tumor cells were detected, indicating effective tumor clearance following conversion therapy (Supplementary Figure 1). Final pathology confirmed ypT0N1M0 IIA and achieved MPR, which met the Expert Consensus of Chinese Medical Association of containing less than 10% viable tumor cells in excised tissue following conversion therapy (10). The patient recovered uneventfully and received four cycles of adjuvant chemoimmunotherapy (pembrolizumab 200 mg, cisplatin 75 mg/m² + nab-paclitaxel 260 mg/m², q3w) starting June 12, 2021, followed by 2-year pembrolizumab maintenance. Serial peripheral blood circulating tumor DNA (ctDNA) monitoring detected no molecular residual disease (Figure 4). Regular imaging surveillance (chest/abdominal CT, brain MRI, PET-CT) showed no recurrence, with ongoing PFS exceeding 50 months. The gene list of NGS tissue and ctDNA is provided in the Supplementary Table 1.
Discussion
For driver-negative stage IV squamous NSCLC, immune checkpoint inhibitor (ICI) plus chemotherapy has become the first-line standard treatment regimen (11). KEYNOTE-407 five-follow-up data demonstrated pembrolizumab + chemotherapy improved median PFS (8.0 vs. 5.1 months) and OS (17.2 vs. 11.6 months) in SqCC, regardless of PD-L1 status (3). However, evidence regarding immunotherapy efficacy in squamous NSCLC with brain metastases remains scarce, as most trials excluded these patients or enrolled only those with stable metastases. The blood-brain barrier limits intracranial ICI efficacy, with historical single-agent ICI objective response rates (ORR) of 16%-33% (12). Pooled analysis of KEYNOTE-021, -189, and -407 revealed superior median OS (18.8 vs. 7.6 months) and PFS (6.9 vs. 4.1 months) for pembrolizumab-chemotherapy versus chemotherapy in NSCLC patients with brain metastases (13). CheckMate 227 reported that dual immunotherapy (nivolumab + ipilimumab) significantly improved 5-year intracranial PFS rates (16% vs. 6%) (14). Chemotherapy may potentiate ICIs by enhancing T-cell responses and disrupting tumor-associated macrophage (TAM) activity (15), potentially overcoming blood-brain barrier limitations (16).
This case achieved intracranial pCR after three cycles of pembrolizumab + chemotherapy, suggesting intracranial penetration of combined therapy. Such outcomes are exceptionally rare in brain metastatic squamous NSCLC, where reported intracranial pCR rates with chemoimmunotherapy remain below 5% (13, 17). The patient’s high PD-L1 expression (TPS=75%) and TMB-H (28.49 Mut/Mb) likely contributed to this response, as both biomarkers correlate positively with immunotherapy efficacy. KEYNOTE-042 established median OS of 20.0 months for PD-L1 TPS ≥50% versus 12.2 months with chemotherapy (18), while TMB-H (≥10 Mut/Mb) associates with ≥40% ICI response rates (19, 20). Synergistic effects of PD-L1 and TMB may amplify antitumor immunity by enhancing T-cell infiltration and activity (21), potentially breaching the blood-brain barrier. Notably, spatial and temporal heterogeneity exists between primary NSCLC and brain metastases in tumor immune microenvironments (22), with intensified immunosuppression in intracranial lesions (23). Bischof et al. demonstrated significantly higher extracranial versus intracranial PD-L1 TPS (p=0.013) in matched samples, with longer intracranial PFS (54.8 vs. 15.4 months) in patients with high intracranial PD-L1 (≥40%) (24). In this case, the brain lesions reached pCR, and the status of PD-L1 and TMB of the brain lesions could not be further confirmed.
The role of surgery in systemically treated stage IV squamous NSCLC remains debated. Dong Tian’s team advocates conversion surgery as a promising strategy to improve outcomes (25). The research of Haibin Wang shows that the median OS of patients with stage IVA who received surgical treatment reached 28.6 months, which was significantly better than that of the non-surgical group (16.2 months) (26). Successful surgical conversion in this case aligns with emerging evidence that PD-L1-high/TMB-H status predicts favorable responses to immunochemotherapy. However, the feasibility of surgery after conversion therapy depends on the biological characteristics of the tumor. Currently, only a few cases have shown that patients with stage IV lung squamous cell carcinoma can be downstaged to an operable condition through the combination of immunotherapy and chemotherapy. Moreover, high PD-L1 expression or high tumor mutational burden (TMB-H) may be the key predictive factors for surgery after successful conversion (27). In this case, the pulmonary lesion significantly regressed after the combination of immunotherapy and chemotherapy (from 8.9 cm to 3.6 cm). The postoperative pathology confirmed MPR, but residual lesions still remained in the interlobar lymph nodes (group 11). Based on this, 4 cycles of chemotherapy combined with immunotherapy were carried out after surgery, followed by maintenance with single-agent immunotherapy for up to 2 years. This phenomenon suggests that surgery can resect radiologically visible drug-resistant clones, while systemic treatment (such as postoperative adjuvant immunotherapy) can further control micrometastases. The success of this case indicates that for specific populations (oligometastasis, high immune activity markers), the “sandwich” model (drug + surgery + drug) may become a strategy to break through the survival bottleneck. Anyway, this still requires verification through prospective studies.
Brain metastases tend to recur relatively easily after surgery. Therefore, the standard treatment for resectable brain metastases is postoperative SRS after surgical resection. But this patient remains recurrence-free for >50 months without SRS, possibly attributable to immunotherapy’s “tail effect”—sustained immune-mediated eradication of residual cells. The ctDNA was undetectable prior to surgery after conversion therapy, and the molecular residual disease (MRD) status remained negative throughout the subsequent long-term follow-up. This phenomenon further validates that the ctDNA status can serve as a predictor of the patient’s prognosis. Numerous studies have indicated that, in contrast to imaging examinations, ctDNA can more sensitively predict the recurrence risk of early-stage lung cancer (28). This implies that ctDNA holds a distinctive and crucial significance in assessing the patient’s condition and prognosticating the outcome.
There are also some issues during the conversion therapy. After the conversion therapy with the combination of immunotherapy and chemotherapy, PET-CT indicates that the tumor is active with increased radioactive uptake. However, after resection, no cancer residue is found. This inconsistency in results interferes with clinical diagnosis and also highlights the limitations of imaging examinations in determining the true state of the tumor. Existing research has confirmed (29) that the clearance of ctDNA is more accurate than imaging examinations in predicting pCR, and patients with persistent negative postoperative MRD often have a longer survival period. Therefore, for patients with brain lesions achieving pCR after immunotherapy and ctDNA clearance, whether SRS treatment can be waived becomes a question worthy of exploration. Due to the existence of the blood-brain barrier, ctDNA in the blood may not comprehensively and accurately reflect the true state of brain lesions (30). Therefore, in the future, attempting to combine the detection of blood ctDNA and cerebrospinal fluid ctDNA may provide a more reliable basis for the decision-making of postoperative SRS treatment. This direction awaits further exploration.
In addition, the withdrawal time of single-agent immunotherapy after surgery for patients with brain metastases is also worthy of further discussion. Long-term immunotherapy may bring certain adverse reactions and economic burdens. How to determine the optimal withdrawal time while ensuring the treatment effect is an urgent problem to be solved in clinical practice. Formulating a more precise and individualized treatment plan based on MRD test results is expected to provide better medical services for patients. However, the realization of this goal still requires a large amount of research and rich clinical data as support.
In conclusion, we report the first case of a lung squamous cell carcinoma patient with brain metastases, high PD-L1 expression and high TMB, who achieved a PFS of over 50 months after surgery following the combination of immunotherapy and chemotherapy. This case suggests that precision medicine guided by biomarkers such as PD-L1, TMB, and MRD, combined with a multidisciplinary comprehensive strategy, may bring the possibility of radical treatment for patients with advanced NSCLC. In the future, it is necessary to further optimize the treatment mode and withdrawal criteria to achieve the best balance between efficacy and safety.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Chongqing People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XX: Writing – original draft, Writing – review & editing. ZM: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fimmu.2025.1660020.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1601125/full#supplementary-material
References
1. Villano JL, Durbin EB, Normandeau C, Thakkar JP, and Moirangthem V. and Davis, FG Incidence of brain metastasis at initial presentation of lung cancer. Neuro-Oncol. (2015) 17:122–8. doi: 10.1093/neuonc/nou099
2. Hockemeyer KG, Rusthoven CG, and Pike LRG. Advances in the management of lung cancer brain metastases. Cancers. (2024) 16:3780. doi: 10.3390/cancers16223780
3. Novello S, Kowalski DM, Luft A, Gümüş M, Vicente D, Mazières J, et al. Pembrolizumab plus chemotherapy in squamous non-small-cell lung cancer: 5-year update of the phase III KEYNOTE-407 study. J Clin Oncol. (2023) 41:1999–2006. doi: 10.1200/JCO.22.01990
4. Shelley D and Gkogkou P. Prognostic factors in non-small cell lung cancer metastatic to the brain. Neuro-Oncology. (2023) 25:iii21–2. doi: 10.1093/neuonc/noad147.093
5. Kocher M, Soffietti R, Abacioglu U, Villà S, Fauchon F, Baumert BG, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol. (2011) 29:134–41. doi: 10.1200/JCO.2010.30.1655
6. Sperduto PW, Yang TJ, Beal K, Pan H, Brown PD, Bangdiwala A, et al. Estimating survival in patients with lung cancer and brain metastases: an update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molgpa). JAMA Oncol. (2017) 3:827–31. doi: 10.1001/jamaoncol.2016.3834
7. Sperduto PW, De B, Li J, Carpenter D, Kirkpatrick J, Milligan M, et al. Graded prognostic assessment (gpa) for patients with lung cancer and brain metastases: initial report of the small cell lung cancer gpa and update of the non-small cell lung cancer gpa including the effect of programmed death ligand 1 and other prognostic factors. Int J Radiat Oncol Biol Phys. (2022) 114:60–74. doi: 10.1016/j.ijrobp.2022.03.020
8. Weller M, Remon J, Rieken S, Vollmuth P, Ahn MJ, Minniti G, et al. Central nervous system metastases in advanced non-small cell lung cancer: A review of the therapeutic landscape. Cancer Treat Rev. (2024) 130:102807. doi: 10.1016/j.ctrv.2024.102807
9. Wang S, Uriel M, and Cheng H. Lung cancer with brain metastasis—treatment strategies and molecular characteristics. J Clin Med. (2024) 13:7371. doi: 10.3390/jcm13237371
10. Expert Committee on Quality Control of Lung Cancer, National Quality Control Center for Cancer. Expert consensus on the pathological evaluation of neoadjuvant therapy efficacy for non-small cell lung cancer. Chin J Pathologi. (2021) 50:1002–7. doi: 10.3760/cma.j.cn112151-20210429-00335
11. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Non-Small Cell Lung Cancer. Version 3.2025. Available online at: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450 (Accessed January 14, 2025).
12. Zhang S, Liu J, Yang C, Li S, and Cheng Y. Research progress of immunotherapy for brain metastases in patients with drive gene negative NSCLC. Zhongguo Fei Ai Za Zhi. (2018) 21(8):610–4. doi: 10.3779/j.issn.1009-3419.2018.08.06
13. Powell SF, Rodríguez-Abreu D, Langer CJ, Tafreshi A, Paz-Ares L, Kopp HG, et al. Outcomes with pembrolizumab plus platinum-based chemotherapy for patients with nsclc and stable brain metastases: pooled analysis of KEYNOTE-021, -189, and -407. J Thorac Onco. (2021) 16:1883–92. doi: 10.1016/j.jtho.2021.06.020
14. Reck M, Ciuleanu TE, Lee JS, Schenker M, Zurawski B, Kim SW, et al. Systemic and intracranial outcomes with first-line nivolumab plus ipilimumab in patients with metastatic nsclc and baseline brain metastases from checkmate 227 part 1. J Thorac Oncol. (2023) 18:1055–69. doi: 10.1016/j.jtho.2023.04.021
15. Emens LA and Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. (2015) 3:436–43. doi: 10.1158/2326-6066.CIR-15-0064
16. Fares J, Ulasov I, Timashev P, and Lesniak MS. Emerging principles of brain immunology and immune checkpoint blockade in brain metastases. Brain. (2021) 144:1046–66. doi: 10.1093/brain/awab012
17. Huang Z, Wu F, Xu Q, Song L, Zhang X, Wang Z, et al. Intracranial activity of first-line immune checkpoint inhibitors combined with chemotherapy in advanced non-small cell lung cancer. Chin Med J. (2023) 136:1422–9. doi: 10.1097/CM9.0000000000002720
18. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomized, open-label, controlled, phase 3 trial. Lancet (London England). (2019) 393:1819–30. doi: 10.1016/S0140-6736(18)32409-7
19. Mok TSK, Lopes G, Cho BC, Kowalski DM, Kasahara K, Wu YL, et al. Associations of tissue tumor mutational burden and mutational status with clinical outcomes in KEYNOTE-042: pembrolizumab versus chemotherapy for advanced PD-L1-positive NSCLC. Ann Oncol. (2023) 34:377–88. doi: 10.1016/j.annonc.2023.01.011
20. Hellmann MD, Ciuleanu T-E, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus Ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. (2018) 378:2093–104. doi: 10.1056/NEJMoa1801946
21. Li SJ, Meng CT, Hao Q, Dai LY, Shi JH, Xu J, et al. A multistage-responsive antibody-delivery strategy to improve immunotherapy for NSCLC brain metastasis by ultrasensitive releasing and tumor-anchoring. Adv Funct Mater. (2024) 34:2312595. doi: 10.1002/adfm.202312595
22. Liu W, Powell CA, and Wang Q. Tumor microenvironment in lung cancer-derived brain metastasis. Chin Med J. (2022) 135:1781–91. doi: 10.1097/CM9.0000000000002127
23. Li M, Hou X, Sai K, Wu L, Chen J, Zhang B, et al. Immune suppressive microenvironment in brain metastatic non-small cell lung cancer: comprehensive immune microenvironment profiling of brain metastases versus paired primary lung tumors (GASTO 1060). Oncoimmunol. (2022) 11:2059874. doi: 10.1080/2162402X.2022.2059874
24. Wasilewski D, Onken J, Höricke P, Bukatz J, Murad S, Früh A, et al. Predictive role of intracranial PD-L1 expression in a real-world cohort of NSCLC patients treated with immune checkpoint inhibition following brain metastasis resection. J Neuro-Oncol. (2024) 167:155–67. doi: 10.1007/s11060-024-04590-w
25. Yan HJ, Zheng XY, Zeng YT, Wan JX, Chen J, Deng ZQ, et al. Salvage surgery and conversion surgery for patients with nonsmall cell lung cancer: a narrative review. Int J Surg. (2025) 111:1032–41. doi: 10.1097/JS9.0000000000001921
26. Wang H, Yang D, Lv Y, Lin J, Wang H, and Zhu Y. Operative versus nonoperative treatment in patients with advanced non-small-cell lung cancer: recommended for surgery. Canad Respir J. (2023) 2023:1–14. doi: 10.1155/2023/4119541
27. Hu C, Ma Q, Li N, Luo N, Hao S, Jiang M, et al. Case Report: pathological complete response in a brain-metastatic lung squamous cell carcinoma patient with long-term benefit from chemo-immunotherapy. Front Oncol. (2021) 11. doi: 10.3389/fonc.2021.693704
28. Peng Y, Mei W, Ma K, and Zeng C. Circulating tumor dna and minimal residual disease (mrd) in solid tumors: current horizons and future perspectives. Front Oncol. (2021) 11. doi: 10.3389/fonc.2021.763790
29. Deutsch JS, Cimino-Mathews A, Thompson E, Provencio M, Forde PM, Spicer J, et al. Association between pathologic response and survival after neoadjuvant therapy in lung cancer. Nat Med. (2023) 30:218–28. doi: 10.1038/s41591-023-02660-6
Keywords: non-small cell lung cancer (NSCLC), brain metastasis, programmed cell death ligand 1, tumor mutational burden (TMB), pathological complete response (PCR), circulating tumor DNA (ctDNA)
Citation: Xu X and Ma Z (2025) A stage IV lung squamous cell cancer patient with brain metastases, high PD-L1 & TMB, achieves pCR and long-term survival after immune-chemotherapy and radical surgery: a case report and literature review. Front. Immunol. 16:1601125. doi: 10.3389/fimmu.2025.1601125
Received: 27 March 2025; Accepted: 23 June 2025;
Published: 04 July 2025; Corrected: 22 July 2025.
Edited by:
Guilherme Finger, The Ohio State University, United StatesReviewed by:
Zhiyuan Xu, University of Virginia, United StatesKyoungmin Lee, Korea University Guro Hospital, Republic of Korea
Copyright © 2025 Xu and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zheng Ma, ZHIubWFyQHFxLmNvbQ==
 Xiangyu Xu
Xiangyu Xu Zheng Ma*
Zheng Ma*