- 1Department of Pediatrics, Hokkaido University Hospital, Sapporo, Japan
- 2Department of Pediatrics, Obihiro-Kosei General Hospital, Obihiro, Japan
- 3Department of Food and Human Wellness, Rakuno Gakuen University, Ebetsu, Japan
STAT3-hyper IgE syndrome (STAT3-HIES) is a primary immunodeficiency disorder caused by dominant-negative mutations in STAT3, leading to defects in Th17 cell differentiation, immune regulation, and tissue repair. Patients are susceptible to recurrent infections and vascular abnormalities, such as vasculopathy and pseudoaneurysms. While involvement of cerebral, bronchial, and coronary arteries has been reported, hepatic artery involvement is rare. We describe a 25-year-old woman with genetically confirmed STAT3-HIES who presented with biliary hemorrhage secondary to a ruptured hepatic pseudoaneurysm. Emergency transcatheter arterial embolization successfully controlled the hemorrhage, and the patient was discharged without complications. Systemic vascular screening revealed an asymptomatic right coronary artery dilation, necessitating medical management with statin therapy. This case highlights hepatic pseudoaneurysm as a rare but life-threatening vascular complication in STAT3-HIES. Given the potential for multi-organ vasculopathy, systemic vascular screening by contrast-enhanced CT or MRI is crucial for early detection and management. Further research is needed to elucidate the mechanisms underlying vasculopathy in STAT3-HIES and establish optimal screening strategies to improve patient outcomes.
Introduction
Inborn errors of immunity (IEIs) are diseases caused by pathogenic variants in genes that affect immune cell function (1). Primary immunodeficiency (PID) is one of the IEIs susceptible to infections, which can sometimes lead to severe or disseminated infections. STAT3-hyper IgE syndrome (STAT3-HIES) is a type of PID caused by dominant-negative pathogenic variants in STAT3. Patients with STAT3-HIES typically present with the classic triad of cold abscesses, elevated serum IgE levels, and refractory eczema beginning in the neonatal period (2). Impaired activation of STAT3 affects the JAK-STAT signaling pathway, such as Interleukin (IL)-6, IL-10, and IL-11 signaling. Consequently, this affects immunological activation including Th17 cell differentiation, vascular endothelial cell function, and tissue repair. Some patients with STAT3-HIES develop vasculopathy or pseudoaneurysms in the bronchial, coronary, or cerebral arteries due to endothelial fragility and impaired tissue repair (3, 4). However, reports of hepatic artery involvement are rare.
In this report, we describe a case of biliary hemorrhage due to the rupture of an intrahepatic pseudoaneurysm in a patient with STAT3-HIES and provide a review of the literature on vasculopathy and pseudoaneurysms in STAT3-HIES patients. Patients with STAT3-HIES have been reported to develop vasculopathy involving the aortic, intracranial, coronary, and pulmonary arteries. In addition, as demonstrated in our patient, vasculopathy may also affect abdominal arteries, including the hepatic artery.
Case description
A 25-year-old woman with STAT3-HIES presented with a three-week history of worsening epigastric pain and vomiting. She was transported to the emergency hospital by ambulance and underwent endoscopy because of possible biliary tract obstruction by elevated levels of hepatobiliary enzymes, hyperbilirubinemia, and elevated pancreatic enzymes. Endoscopy showed biliary hemorrhage. Enhanced-contrast CT revealed an extravasation of hepatic artery suggesting ruptured hepatic pseudoaneurysm. She was subsequently transferred to our hospital for intravenous catheter treatment.
The patient had a history of intractable eczema since birth and recurrent viral and bacterial infections during infancy. At 7 months of age, she was hospitalized for pneumonia. At 1 year of age, she was clinically diagnosed with HIES based on recurrent infections, characteristic facial features, and elevated serum IgE levels, and she was started on sulfamethoxazole-trimethoprim prophylaxis. At age 9, genetic testing identified a de novo reported heterozygous dominant-negative variant, p.R382W, in STAT3, as previously described (5). At age 12, she experienced recurrent lung abscesses due to co-infection with MSSA and MRSA (Supplementary Figures 1A, B). Vancomycin treatment was not tolerated due to Redman syndrome, and a left lower lobe pneumonectomy was ultimately performed without complications. Pathology showed infection with both Staphylococcus and Aspergillus species. At 13, she developed MRSA pneumonia (Supplementary Figures 1C, D), which did not respond to arbekacin or panipenem-betamipron but improved with rifampicin based on susceptibility testing. With sulfamethoxazole-trimethoprim and fluconazole prophylaxis, she remained free of further infections. She had no history of smoking, alcohol use, significant trauma, or vasculitis-related diseases including Kawasaki disease.
At 25 years old, she presented with epigastric pain and vomiting due to biliary hemorrhage. Laboratory findings showed normocytic anemia (Hb 8.7 g/dL), elevated hepatobiliary enzymes, hyperbilirubinemia, and elevated pancreatic enzymes, but no evidence of inflammation or coagulation abnormalities (Supplementary Table 1). Endoscopy showed bleeding from the ampulla of Vater (Figure 1A). Contrast-enhanced CT revealed a hematoma in the gallbladder, active bleeding from the hepatic artery (Figures 1B, C). The patient was diagnosed with biliary hemorrhage due to the rupture of a hepatic pseudoaneurysm and underwent emergency transcatheter arterial embolization (Figures 1D-F). She was discharged on the sixth day of hospitalization without further bleeding.

Figure 1. Images at the rupture of hepatic pseudoaneurysm and biliary hemorrhage in the patient with STAT3-HIES. Endoscopy revealed bleeding from the ampulla of Vater (A). Contrast-enhanced CT showed an active hepatic bleeding (B) and highly resorbed area in the gallbladder likely representing a hematoma (C). Angiography identified an intrahepatic pseudoaneurysm and active bleeding (D). Catheter embolism was successfully performed, effectively controlling the bleeding (E, F).
A few days later, coronary CT angiography and brain MRI with MR angiography (MRA) were performed for possible complication with asymptomatic vasculopathy. Coronary CT angiography showed spindle-shaped dilation in the right coronary artery, while no abnormalities were detected in the left coronary artery (Figures 2A, B). Brain MRI and MRA revealed no abnormalities (Figure 2C). She received rosuvastatin calcium therapy to prevent the atherosclerosis and has been recovering without any complications for 2 years.
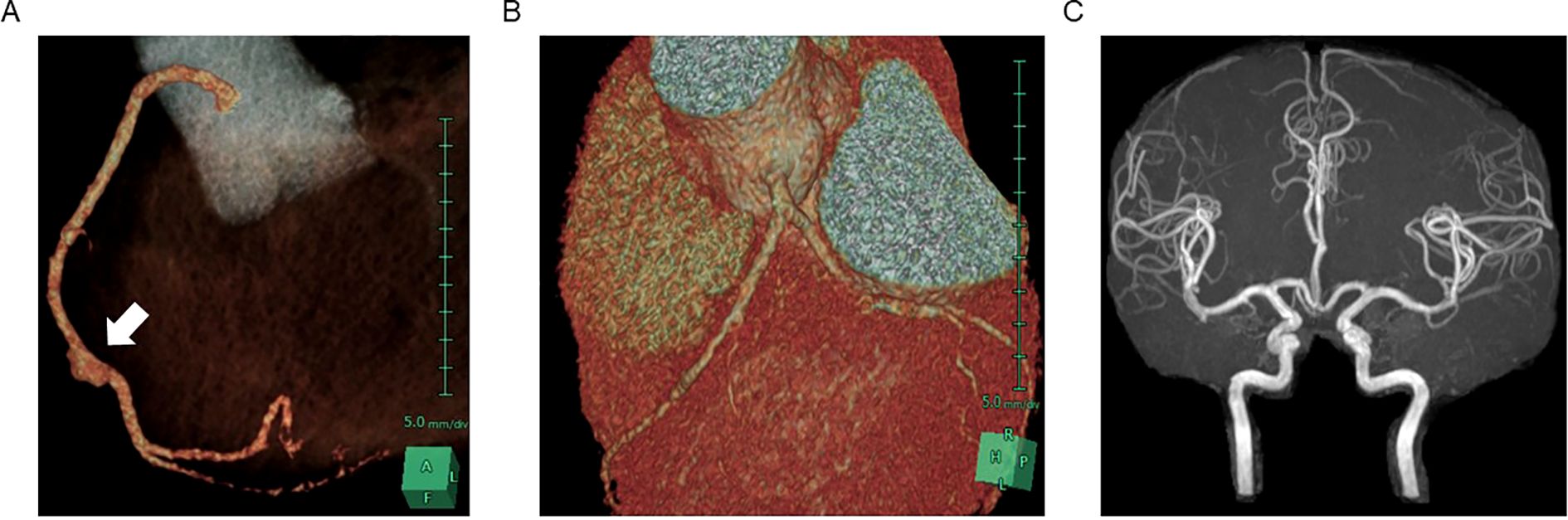
Figure 2. CT image of asymptomatic pseudoaneurysm in the right coronary artery. 3D reconstruction of contrast-enhanced CT image in coronary arteries demonstrated pseudoaneurysm in right coronary artery (A) and no dilation observed in left coronary arteries (B). MR angiography showed no dilation in cerebral arteries (C).
We reviewed previous reports of vasculopathy in patients with STAT3-HIES including autosomal dominant or sporadic HIES without consanguinity (3, 4, 6–14) (Table 1). Reported vascular abnormalities include aneurysms, arterial dilation, cerebral infarction, and imaging findings indicative of tissue ischemia and vascular injury. A total of 33 patients (20 males, 13 females) across 11 reports were included. The most frequently affected vessels were the cerebral arteries (22 patients), followed by the coronary arteries (15 patients). Among patients with cerebrovascular abnormalities, 9 (40.9%) had concomitant coronary artery dilation. Five patients (14.3%) were diagnosed in childhood, with the youngest being 2 years old. The median age at diagnosis of vasculopathy was 27.9 years. The median age at diagnosis for cerebral arterial dilation was 28 years, and for coronary arterial dilation, 29 years.
Discussion
In this report, we present a case of biliary hemorrhage due to hepatic pseudoaneurysm rupture in a STAT3-HIES patient, along with an asymptomatic pseudoaneurysm in the right coronary artery.
In STAT3-HIES, immunodeficiency is primarily due to defective IL-6 signaling, which plays a crucial role in Th17 cell differentiation. Th17 cell deficiency leads to reduced IL-17 production, impairing neutrophil migration and activation. Consequently, neutrophils fail to migrate effectively to infection sites, leading to inadequate immune responses against Staphylococcus aureus and fungal infections (2). Managing drug resistant Staphylococcus aureus infections, such as MRSA, is critical in patients with STAT3-HIES. Although vancomycin is the first-line therapy, its use can be intolerable by Redman syndrome, as observed in this case. Combination therapy with rifampicin and vancomycin or clindamycin has been reported effective for MRSA pneumonia (15). However, as demonstrated in this case, rifampicin monotherapy may also be effective, although careful monitoring for the emergence of drug-resistant strains is necessary.
Impaired tissue repair and angiogenesis, involving FGF1, FGF2, TGF-β, VEGF, and HIF-1α, has been implicated in vasculopathy and pseudoaneurysm formation in STAT3-HIES (16). Additionally, eosinophilic inflammation and Th17 deficiency may contribute to vascular pathology (3, 17). As observed in eosinophilic granulomatosis with polyangiitis, persistent eosinophilia may be associated with vasculitis. Furthermore, in a mouse model, IL-17 neutralization led to increased aneurysm severity and fatal rupture, although the underlying mechanism remains unclear. T cell–mediated inflammation may contribute to severe aneurysm formation, as increased T cell infiltration in the aneurysm was observed in these mice (4). Previous report showed the thickness of coronary artery wall and increased vessel area in patients with STAT3-HIES (18). However, the risk factors of vasculopathy remain unclear. Although vasculopathy is relatively common in adult patients, the factors such as hypertension, atherosclerosis, and infections remain uncertain (3, 17). Furthermore, our review of previous cases identified 5 pediatric patients, including 2 adolescents, suggesting that aging, hypertension, atherosclerosis, and recurrent infections are not the sole risk factor (Table 1). Identification of risk factors, especially for children, is essential, to improve the prognosis, although we could not determine them through our review. The most commonly affected arteries are the cerebral and coronary arteries, with severe complications such as aortic aneurysm rupture, hemoptysis, and myocardial infarction reported. Notably, vasculopathy could appear more frequent in males, which has not been previously highlighted (4, 17).
STAT3-HIES vasculopathy can involve any large or medium-sized artery, since the hepatic artery was also affected in this case. Therefore, systemic vascular screening is crucial. Although there are no standardized vascular screening guidelines for STAT3-HIES, early detection and intervention (e.g., medical therapy, catheter-based procedures, or surgery) are essential. In our review, screening for vascular involvement was performed using MRI or contrast-enhanced CT. A total of ten patients, including the present case, exhibited vasculopathy affecting two or more arteries. These findings suggest that patients who develop vasculopathy should undergo comprehensive whole-body vascular screening. Regarding treatment, anticoagulants and lipid-lowering agents, such as aspirin or statins, may be considered, although standardized treatment guidelines have not yet been established. In this case, rosuvastatin calcium was administered to prevent atherosclerosis and to reduce the risk of cardiovascular and cerebrovascular complications (19). Further patient-based research is needed to elucidate pathophysiological mechanisms, risk factors, optimal screening strategies and treatments to improve patient outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the committee on ethics of Hokkaido University Faculty of Medicine and Graduate School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
DF: Investigation, Writing – review & editing, Data curation, Writing – original draft. MU: Writing – review & editing, Funding acquisition, Methodology, Conceptualization, Writing – original draft. HY: Data curation, Investigation, Writing – review & editing. KW: Data curation, Investigation, Writing – review & editing. SY: Writing – review & editing, Conceptualization, Supervision. AM: Supervision, Project administration, Writing – review & editing. MY: Writing – review & editing, Supervision, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was partially supported by a grant from the Research on Intractable Diseases from the Japanese Ministry of Health, Labor and Welfare (to MU and MY) and JSPS KAKENHI grants (23K15335 to MU).
Acknowledgments
We appreciate the gastroenterologists, cardiologists, and radiologists who treated the patients.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. We used the generative AI for proofreading of the article.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1601776/full#supplementary-material
Supplementary Figure 1 | Images of the recurrent pulmonary infections. At age 9, the patient developed lung abscess, which was ultimately treated with surgical resection of left lung lobe, in the left lung by chest X ray (A) and CT (B) images. At age 12, the patient developed right pneumonia by Methicillin-resistant Staphylococcus aureus, successfully treated with rifampicin. Infiltration in the right lung was confirmed by chest X ray (C) and CT (D).
References
1. Tangye SG, Al-Herz W, Bousfiha A, Cunningham-Rundles C, Franco JL, Holland SM, et al. Human inborn errors of immunity: 2022 update on the classification from the international union of immunological societies expert committee. J Clin Immunol. (2022) 42:1473–507. doi: 10.1007/s10875-022-01289-3
2. Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, et al. STAT3 mutations in the hyper-IgE syndrome. New Engl J Med. (2007) 357:1608–19. doi: 10.1056/NEJMoa073687
3. Yavuz H and Chee R. A review on the vascular features of the hyperimmunoglobulin E syndrome. Clin Exp Immunol. (2010) 159:238–44. doi: 10.1111/j.1365-2249.2009.04044.x
4. Chandesris MO, Azarine A, Ong KT, Taleb S, Boutouyrie P, Mousseaux E, et al. Frequent and widespread vascular abnormalities in human signal transducer and activator of transcription 3 deficiency. Circ Cardiovasc Genet. (2012) 5:25–34. doi: 10.1161/CIRCGENETICS.111.961235
5. Saito M, Nagasawa M, Takada H, Hara T, Tsuchiya S, Agematsu K, et al. Defective IL-10 signaling in hyper-IgE syndrome results in impaired generation of tolerogenic dendritic cells and induced regulatory T cells. J Exp Med. (2011) 208:235–49. doi: 10.1084/jem.20100799
6. Connolly B, Manson D, Khattak S, and Burrows P. Bronchial artery aneurysm in hyperimmunoglobulinemia E syndrome. Pediatr Radiol. (1994) 24:592–3. doi: 10.1007/BF02012742
7. Ling JC, Freeman AF, Gharib AM, Arai AE, Lederman RJ, Rosing DR, et al. Coronary artery aneurysms in patients with hyper IgE recurrent infection syndrome. Clin Immunol (Orlando Fla). (2007) 122:255–8. doi: 10.1016/j.clim.2006.10.005
8. Ma BH, Ng CS, Lam R, Wan S, Wan IY, Lee TW, et al. Recurrent hemoptysis with Penicillium marneffei and Stenotrophomonas maltophilia in Job’s syndrome. Can Respir J. (2009) 16:e50–2. doi: 10.1155/2009/586919
9. Lee WI, Huang JL, Lin SJ, Yeh KW, Chen LC, Hsieh MY, et al. Clinical aspects and genetic analysis of Taiwanese patients with the phenotype of hyper-immunoglobulin E recurrent infection syndromes (HIES). J Clin Immunol. (2011) 31:272–80. doi: 10.1007/s10875-010-9479-1
10. Falah O, Thwaites SE, and Chalmers RT. Ruptured thoracoabdominal aneurysm in a 27-year-old with hyper IgE syndrome. J Vasc Surg. (2012) 55:830–2. doi: 10.1016/j.jvs.2011.08.011
11. Kim Y, Nard JA, Saad A, Casselman J, Wessell KR, Toller-Artis E, et al. Cerebral aneurysm in a 12-year-old boy with a STAT3 mutation (hyper-IgE syndrome). Ann Allergy Asthma Immunol. (2015) 114:430–1. doi: 10.1016/j.anai.2015.02.016
12. Hakim A, Bazan IS, Sanogo ML, Manning EP, Pollak JS, and Chupp GL. Pulmonary artery pseudoaneurysm causing massive hemoptysis in hyperimmunoglobulin E syndrome: a case report. BMC Pulm Med. (2019) 19:34. doi: 10.1186/s12890-019-0797-7
13. Nussbaum ES, Torok CM, Carroll J, and Gunderman AM. Delayed development of a de novo contralateral middle cerebral artery aneurysm in a patient with hyperimmunoglobulin E syndrome: A case report. Interv Neuroradiol. (2019) 25:442–6. doi: 10.1177/1591019919828657
14. Ponsford MJ, Clark J, Mock J, Abinun M, Carne E, El-Shanawany T, et al. Hematopoietic stem cell transplantation and vasculopathy associated with STAT3-dominant-negative hyper-igE syndrome. Front Pediatr. (2020) 8:575. doi: 10.3389/fped.2020.00575
15. Liu Q, He D, Wang L, Wu Y, Liu X, Yang Y, et al. Efficacy and safety of antibiotics in the treatment of methicillin-resistant staphylococcus aureus (MRSA) infections: A systematic review and network meta-analysis. Antibiot (Basel). (2024) 13. doi: 10.3390/antibiotics13090866
16. Dmitrieva NI, Walts AD, Nguyen DP, Grubb A, Zhang X, Wang X, et al. Impaired angiogenesis and extracellular matrix metabolism in autosomal-dominant hyper-IgE syndrome. J Clin Invest. (2020) 130:4167–81. doi: 10.1172/JCI135490
17. Tsilifis C, Freeman AF, and Gennery AR. STAT3 hyper-igE syndrome-an update and unanswered questions. J Clin Immunol. (2021) 41:864–80. doi: 10.1007/s10875-021-01051-1
18. Abd-Elmoniem KZ, Ramos N, Yazdani SK, Ghanem AM, Holland SM, Freeman AF, et al. Coronary atherosclerosis and dilation in hyper IgE syndrome patients: Depiction by magnetic resonance vessel wall imaging and pathological correlation. Atherosclerosis. (2017) 258:20–5. doi: 10.1016/j.atherosclerosis.2017.01.022
Keywords: hyper IgE syndrome, STAT3, biliary hemorrhage, intrahepatic pseudoaneurysm, coronary pseudoaneurysm
Citation: Fujita D, Ueki M, Yamanaka H, Watanabe K, Yakuwa S, Manabe A and Yamada M (2025) Case Report: Biliary hemorrhage by intrahepatic pseudoaneurysm and asymptomatic right coronary artery pseudoaneurysm in a patient with STAT3 hyper IgE syndrome. Front. Immunol. 16:1601776. doi: 10.3389/fimmu.2025.1601776
Received: 28 March 2025; Accepted: 07 May 2025;
Published: 26 May 2025.
Edited by:
Yoji Sasahara, Tohoku University, JapanReviewed by:
Julian Joseph Bosco, The Alfred Hospital, AustraliaYusuke Matsuda, Kanazawa University, Japan
Copyright © 2025 Fujita, Ueki, Yamanaka, Watanabe, Yakuwa, Manabe and Yamada. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masahiro Ueki, bS51ZWtpQG1lZC5ob2t1ZGFpLmFjLmpw
 Daiki Fujita
Daiki Fujita Masahiro Ueki
Masahiro Ueki Hiroshi Yamanaka1,2
Hiroshi Yamanaka1,2 Masafumi Yamada
Masafumi Yamada