- 1Department of Neurology, Second Affiliated Hospital of Army Medical University, Chong Qing, China
- 2Basic Medical College, Army Medical University, Chong Qing, China
Background: Nivolumab, a human immunoglobulin IgG4 monoclonal antibody targeting PD-1 receptor, received initial FDA approval in 2014 for treating unresectable or metastatic malignant melanoma (MM), followed by approval for metastatic squamous and non-squamous non-small cell lung cancer (NSCLC) in 2015. With expanding clinical applications of nivolumab, comprehensive evaluation of its safety profile in real-world healthcare settings becomes increasingly crucial.
Methods: We compiled a real-world safety dataset of nivolumab from the FDA Adverse Event Reporting System (FAERS) database, encompassing reports from Q4–2014 through Q2 2024. To evaluate the association between nivolumab and adverse events (AEs), we employed four distinct disproportionality analysis methods: Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Multi-item Gamma Poisson Shrinker (MGPS) and Bayesian Confidence Propagation Neural Network (BCPNN). Additionally, we utilized Weibull distribution modeling to characterize the temporal risk patterns of identified adverse events.
Results: Our analysis identified 64,627 AEs reports associated with nivolumab. The most frequently reported AEs included fatigue, dyspnea, musculoskeletal pain, decreased appetite, cough, nausea, and constipation. Notably, we detected several potential safety signals not currently listed in the prescribing information: Malignant neoplasm progression, weight decreased, sepsis myocarditis, encephalitis and hypotension.
Conclusions: Our large-scale pharmacovigilance study identified significant safety signals associated with nivolumab, including previously unrecognized adverse drug reactions. The identification of these safety signals underscores the importance of ongoing post-marketing surveillance for immune checkpoint inhibitors. Future studies should investigate the mechanisms underlying these associations and develop targeted monitoring protocols.
1 Introduction
Nivolumab, a human monoclonal antibody targeting programmed cell death protein 1 (PD-1), represents a breakthrough in cancer immunotherapy with its unique immunomodulatory properties. As an immune checkpoint inhibitor, it has been approved for treating various advanced or metastatic malignancies (1). The PD-1 pathway plays a crucial role in immune regulation. PD-1, expressed on activated T cells, serves as a key checkpoint molecule that downregulates T-cell responses through multiple mechanisms. Many tumors exploit this pathway by upregulating PD-1 ligands (PD-L1/PD-L2), creating an immunosuppressive microenvironment that evades T-cell-mediated immune surveillance. Nivolumab specifically binding to PD-1 receptors, thereby blocking their interaction with both PD-L1 and PD-L2. This inhibition releases the PD-1 pathway-mediated suppression of immune responses and exerts its antitumor therapeutic effects (2).
Nivolumab received FDA approval in 2014 for treating unresectable or metastatic advanced melanoma (MM). MM represents the most lethal form of skin cancer, originating from malignant transformation of melanocytes (3). These neural crest-derived cells are normally distributed in the leptomeninges, uvea, brain parenchyma, mucous membranes, and skin (4). The anatomical distribution of melanocytes correlates with potential melanoma development sites. For primary CNS malignant melanoma(MM), three pathogenic origins have been proposed: (1) mesoderm-derived pigment cells migrating via leptomeningeal vasculature; (2) abnormal embryonic ectodermal cell origins; (3) neural crest-derived melanocytic precursors (5). While gross total resection or subtotal resection significantly improves survival, combination non-surgical therapies remain the optimal approach for unresectable cases. Monotherapies (ipilimumab, nivolumab, pembrolizumab) and combination ipilimumab-nivolumab have demonstrated clinical efficacy in advanced MM, improving 5-year survival rates from <5% to approximately 30% in patients receiving immunotherapy or targeted therapies (6–8). The phase III CheckMate 067 trial established nivolumab plus ipilimumab as achieving the longest median overall survival (72.1 months) among phase III melanoma studies to date (9). Furthermore, this trial demonstrated superior 5-year overall survival rates for combination therapy (52%) and nivolumab monotherapy (44%) versus ipilimumab alone (26%) (10).
Nivolumab received FDA approval in 2015 for the treatment of metastatic squamous or non-squamous non-small cell lung cancer (NSCLC). Lung cancer remains the leading cause of global cancer-related mortality, with NSCLC exhibiting particularly dismal cure rates and survival outcomes, especially when presenting as metastatic disease (11). The high mortality stems from frequent metastatic dissemination at diagnosis, underscoring the critical need for more effective systemic therapies to improve long-term survival (12, 13). Current immune checkpoint blockade (ICB) strategies approved or under development for NSCLC include anti-PD-1 antibodies (nivolumab, pembrolizumab) and anti-PD-L1 antibodies (atezolizumab, durvalumab, avelumab) (14). The CheckMate 816 trial demonstrated that neoadjuvant nivolumab plus chemotherapy significantly improved complete response rates (24% vs. 2.2%, P < 0.001) and event-free survival (HR 0.68, 95% CI 0.49-0.93) compared to chemotherapy alone, with enhanced benefits observed in patients exhibiting ≥1% PD-L1 tumor expression (15). Furthermore, perioperative nivolumab-chemotherapy combination therapy yielded higher rates of pathological complete response and prolonged survival compared to chemotherapy alone in resectable Stage IIIA or selected IIIB NSCLC patients (16).
Nivolumab has been widely used in clinical practice, particularly for the treatment of MM and NSCLC, demonstrating significant survival benefits in these patient populations. Given its broad application, understanding the safety profile of nivolumab in real-world settings is crucial. According to the prescribing information, the most frequently reported adverse reactions include fatigue, dyspnea, musculoskeletal pain, decreased appetite, cough, nausea, and constipation. However, clinical trials typically enroll specific patient populations with strict inclusion and exclusion criteria, meaning that the adverse events (AEs) listed in the prescribing information may only reflect those observed in a selected subset of patients. To further evaluate the real-world safety of nivolumab, this study analyzed data from the FDA Adverse Event Reporting System (FAERS) database. This comprehensive analysis provides additional evidence to guide healthcare professionals in the clinical use of nivolumab, complementing findings from controlled clinical trials.
2 Materials and methods
2.1 Data source and processing
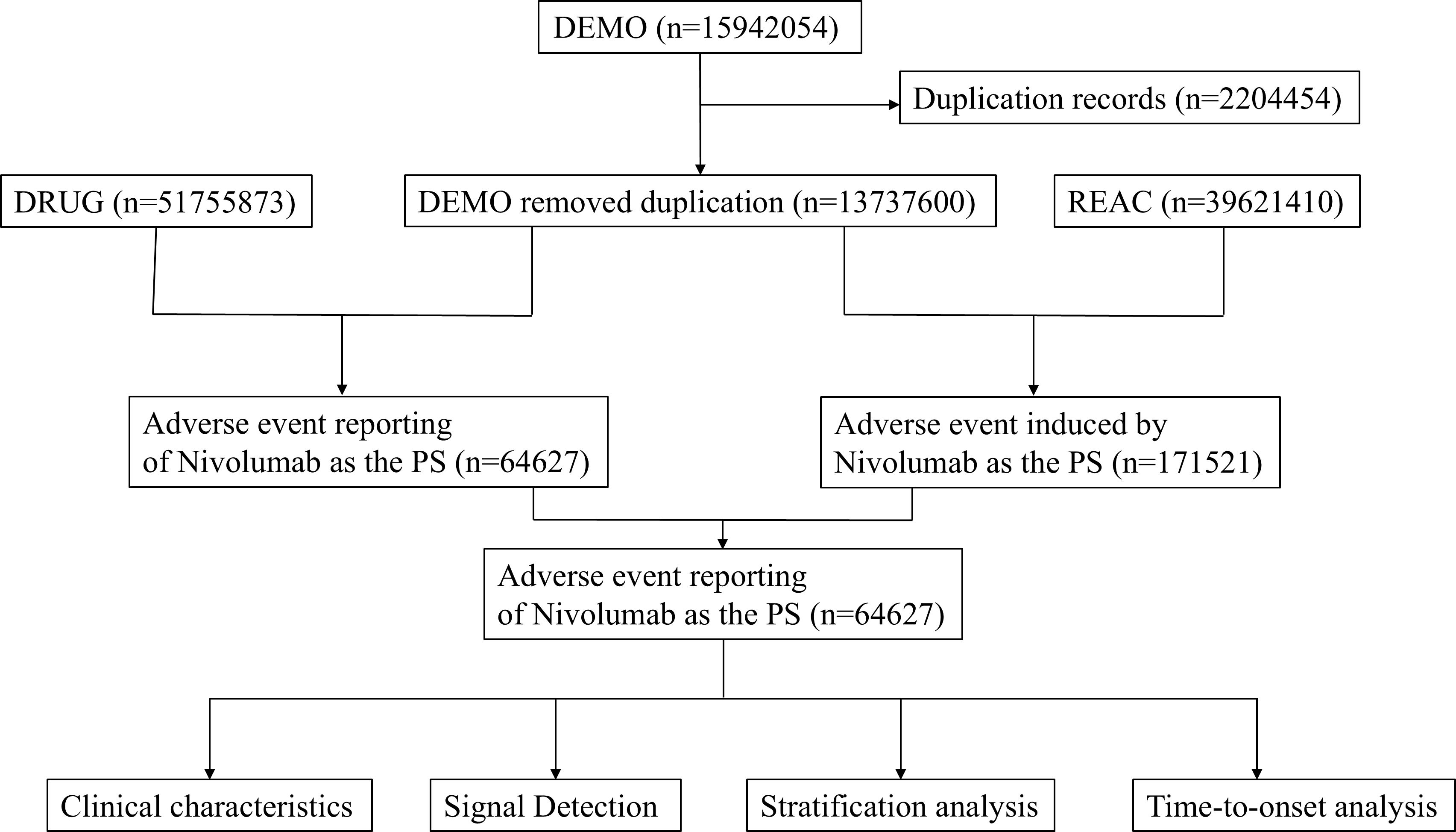
This study compiled AE reports from the FAERS database, spanning from the fourth quarter of 2014 to the second quarter of 2024. Only reports where nivolumab was designated as the primary suspect (PS) drug were included. Data extraction and cleaning were performed using R software (version 4.4.1). The initial database contained 15,942,054 reports, from which 2,204,454 duplicate entries were excluded in accordance with FDA guidance (17). Key fields, including PRIMARYID, CASEID, and FDA_DT, were extracted from the original dataset and sorted. For reports sharing the same CASEID, only the entry with the most recent FDA_DT was retained. In cases where CASEID and FDA_DT were identical, the record with the highest PRIMARYID was selected. AEs were classified based on Preferred Term (PT) and System Organ Class (SOC) using Medical Dictionary for Regulatory Activities (MedDRA, version 27.0) (18). Figure 1 illustrates the flowchart of nivolumab-associated AEs identified in the FAERS database.
2.2 Data analysis
In this study, we employed multiple pharmacovigilance methodologies including the reporting odds ratio(ROR) (6–8), proportional reporting ratio(PRR) (19), multi-item gamma Poisson shrinker (MGPS) (20), and Bayesian confidence propagation neural network (BCPNN) (21) to evaluate significant associations between nivolumab and adverse events (AEs). AEs exceeding the positivity threshold in at least one of these methods were identified as potential signals. Supplementary Table S1 presents the detailed 2×2 contingency matrices, while Supplementary Table S2 provides the specific parameters for the four primary signal detection algorithms. Furthermore, Weibull distribution modeling was applied to analyze the time-to-onset of adverse events.
3 Results
3.1 Basic characteristics of AEs and population
Our analysis encompassed 64,627 adverse event (AE) reports associated with nivolumab. Table 1 presents the baseline characteristics of these reports. Among reporters, males constituted a significantly higher proportion (36,457, 56.4%) compared to females (19,698, 30.5%). The elderly population (65–85 years) represented the largest age group reporting AEs (22,802, 35.3%), followed by adults aged 18–65 years (20,038, 31.0%). Consistent with clinical indications, the primary reported use of nivolumab was for NSCLC (9,809 cases, 15.2%), with MM being the secondary indication (8,020 cases, 12.4%). The FAERS database revealed death as the most frequently documented serious outcome (18,616 cases, 28.8%), followed by hospitalization, life-threatening events, and disability. Geographically, the United States contributed the majority of reports (27,714, 42.9%), with Japan ranking second (10,656, 16.5%). Healthcare professionals submitted the largest proportion of reports (20,176, 31.2%), followed by consumers (15,887, 24.6%). Following FDA approval of nivolumab for MM in 2014, AE reports demonstrated an annual increase, peaking in 2019 (9,533 cases). Subsequently, reports declined yearly, reaching 3,626 cases in 2024. But our analysis only included data from the first two quarters of 2024.
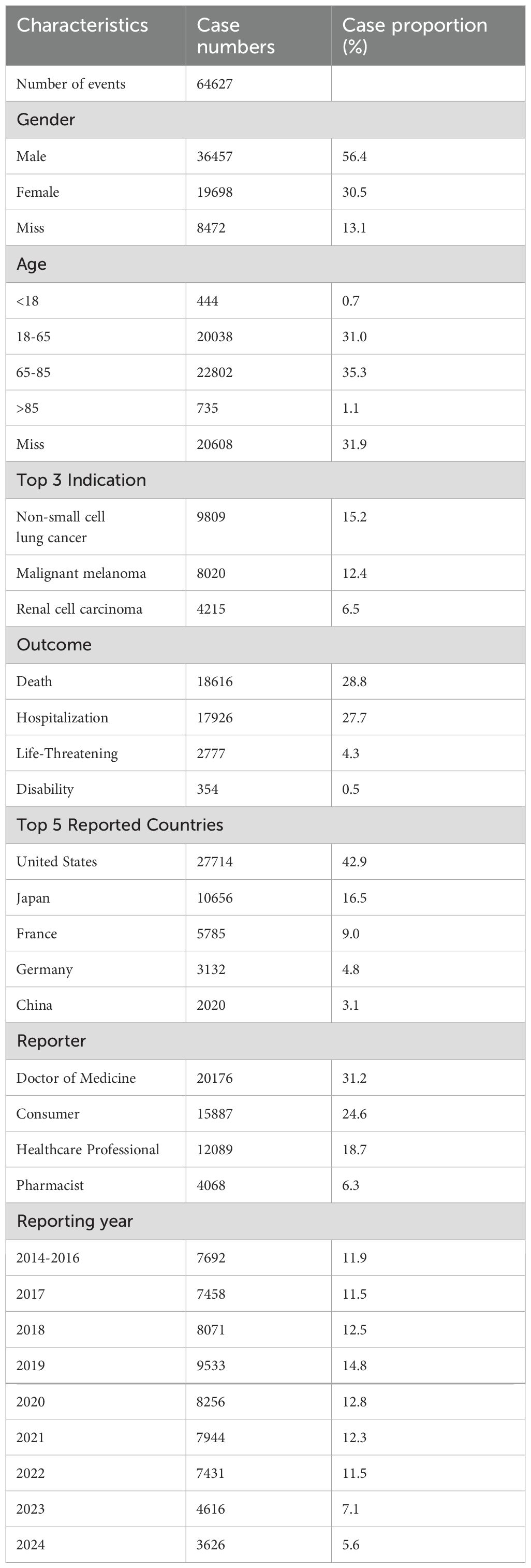
Table 1. Clinical characteristics of Nivolumab adverse event reports from the FAERS database (Q4 2014 – Q3 2024).
3.2 Signal detection related to SOC levels
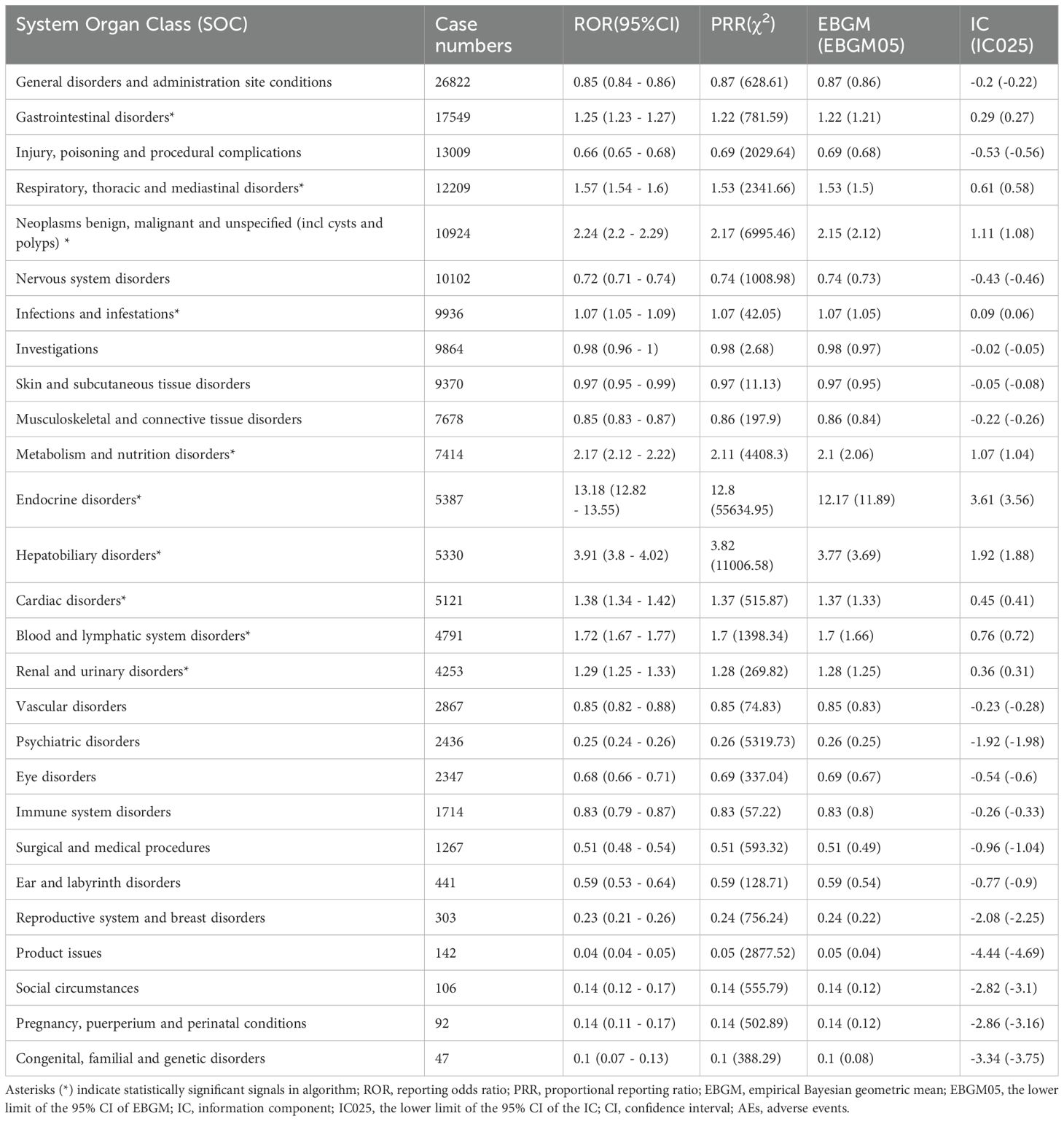
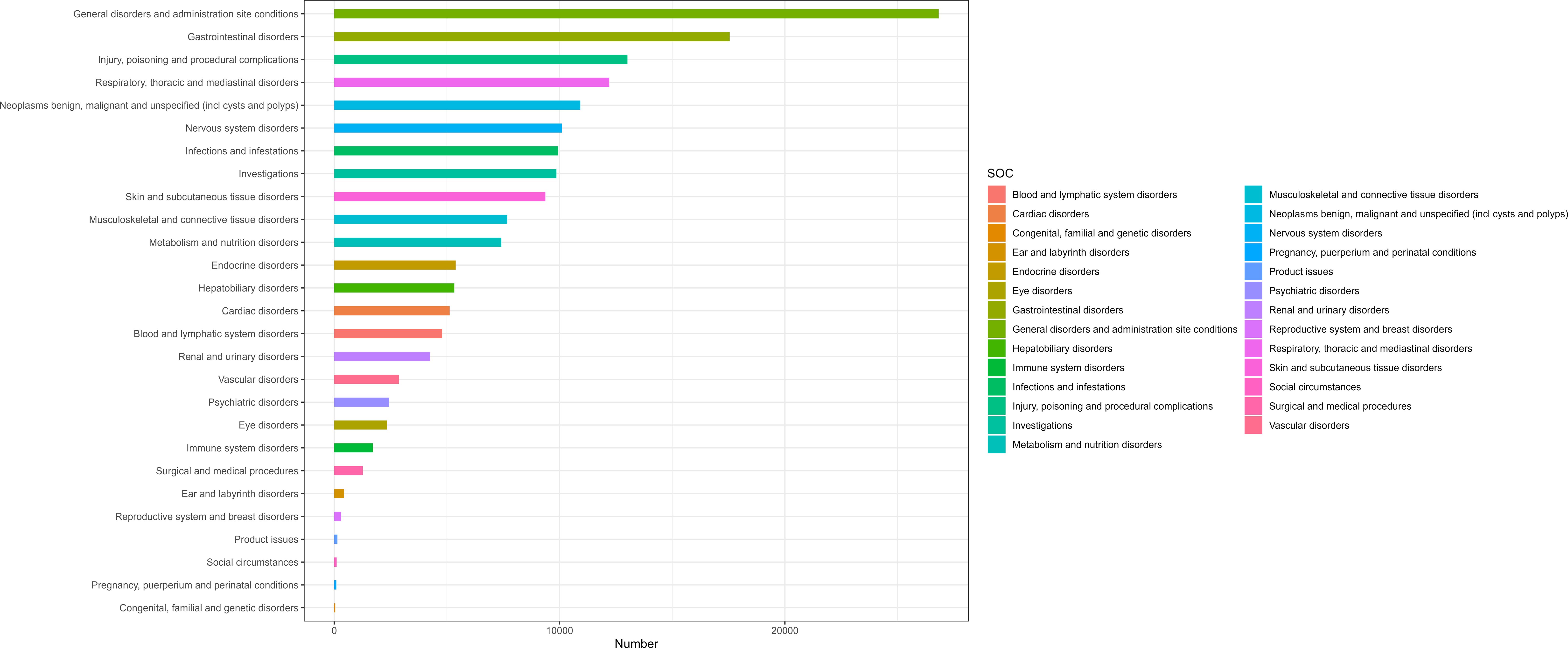
Table 2 presents nivolumab-associated AEs across all 27 SOCs, while Figure 2 illustrate the signal strengths at the SOC level in the FAERS database. The most frequently reported category was general disorders and administration site conditions [n=26,822, ROR (95% CI) = 0.85 (0.84-0.86)], whereas the strongest signal intensity was observed for endocrine disorders [n=5,387, ROR (95% CI) = 13.18 (12.82-13.55)]. Several additional SOCs demonstrated robust signal detection, including gastrointestinal disorders [n=17,549, ROR (95% CI) = 1.25 (1.23-1.27)], respiratory, thoracic and mediastinal disorders [n=12,209, ROR (95% CI) = 1.57 (1.36-1.43)], Neoplasms benign, malignant and unspecified (including cysts and polyps) [n=10,924, ROR (95% CI) = 2.24(2.20-2.29)]. The absence of statistical significance for certain SOCs (e.g., congenital/familial/genetic disorders, surgical/medical procedures, social circumstances) was attributed to the lower bounds of their ROR 95% confidence intervals falling below 1.
3.3 Signal detection related to PT levels
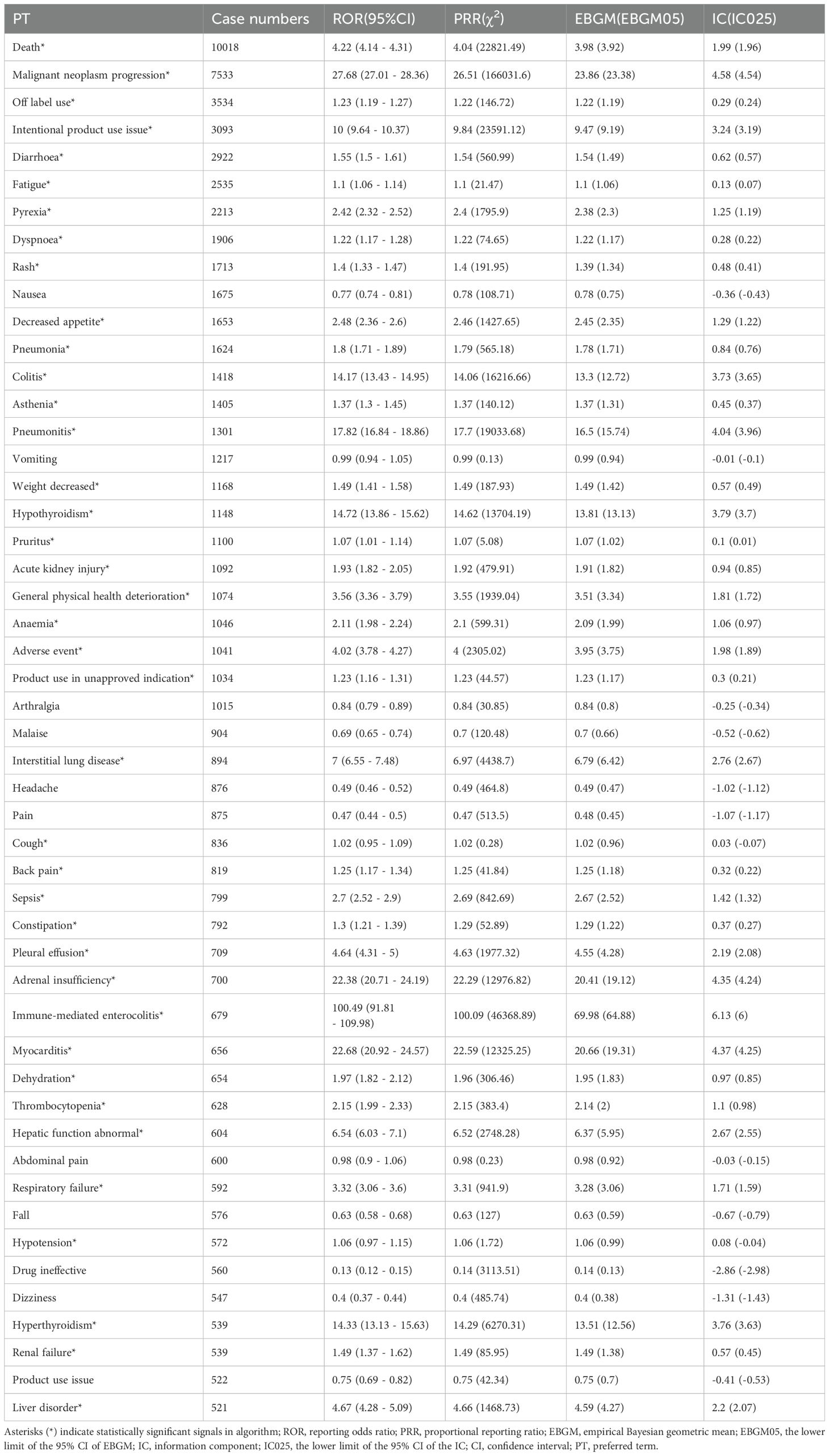
We systematically classified all nivolumab-associated AEs by frequency, with comprehensive results presented in Table 3. All AEs meeting the positive signal criteria were meticulously documented in Supplementary Table S3. The top 10 reported PTs included Death, Malignant neoplasm progression, Off label use, Intentional product use issue, Diarrhoea, Fatigue, Pyrexia, Dyspnoea, Rash and Nausea. Several PTs were consistent with those listed in the drug label, including fatigue, dyspnoea, musculoskeletal pain, decreased appetite, cough, nausea, and constipation. Notably, we identified potential adverse reactions not currently mentioned in the prescribing information, such as: Malignant neoplasm progression, Weight decreased, Sepsis, Myocarditis and Hypotension.
3.4 Information from subgroup
Supplementary Tables S4-S11 present comprehensive subgroup analyses of nivolumab-associated adverse events. In the gender subgroup analysis, death was the most frequently reported adverse event in both males and females, while males exhibited a higher propensity for diarrhea and females demonstrated greater susceptibility to fatigue. Age-stratified analysis revealed that pyrexia was more commonly reported in individuals under 18 years old, whereas mortality rates showed a relative increase in patients aged 18 years and above. Healthcare professionals were more likely to report malignant neoplasm progression, reflecting their clinical awareness in cancer patient monitoring, while consumer reports predominantly involved fatal outcomes. These findings highlight distinct adverse event profiles across different demographic subgroups, with detailed statistical results including reporting odds ratios and confidence intervals provided in the Supplementary Tables.
3.5 Sensitivity analysis
Based on baseline data from the FAERS database, the primary indications for nivolumab were NSCLC and MM. Consequently, we excluded certain concomitant medications potentially used to treat these two malignancies, primarily ipilimumab. After excluding cases requiring additional combination therapies, a total of 48,923 case reports comprising 126,385 AEs were identified. The predominant reported adverse reactions included death, malignant neoplasm progression, off-label use, intentional product use issue, and diarrhea (see Supplementary Table 12 for detailed information).
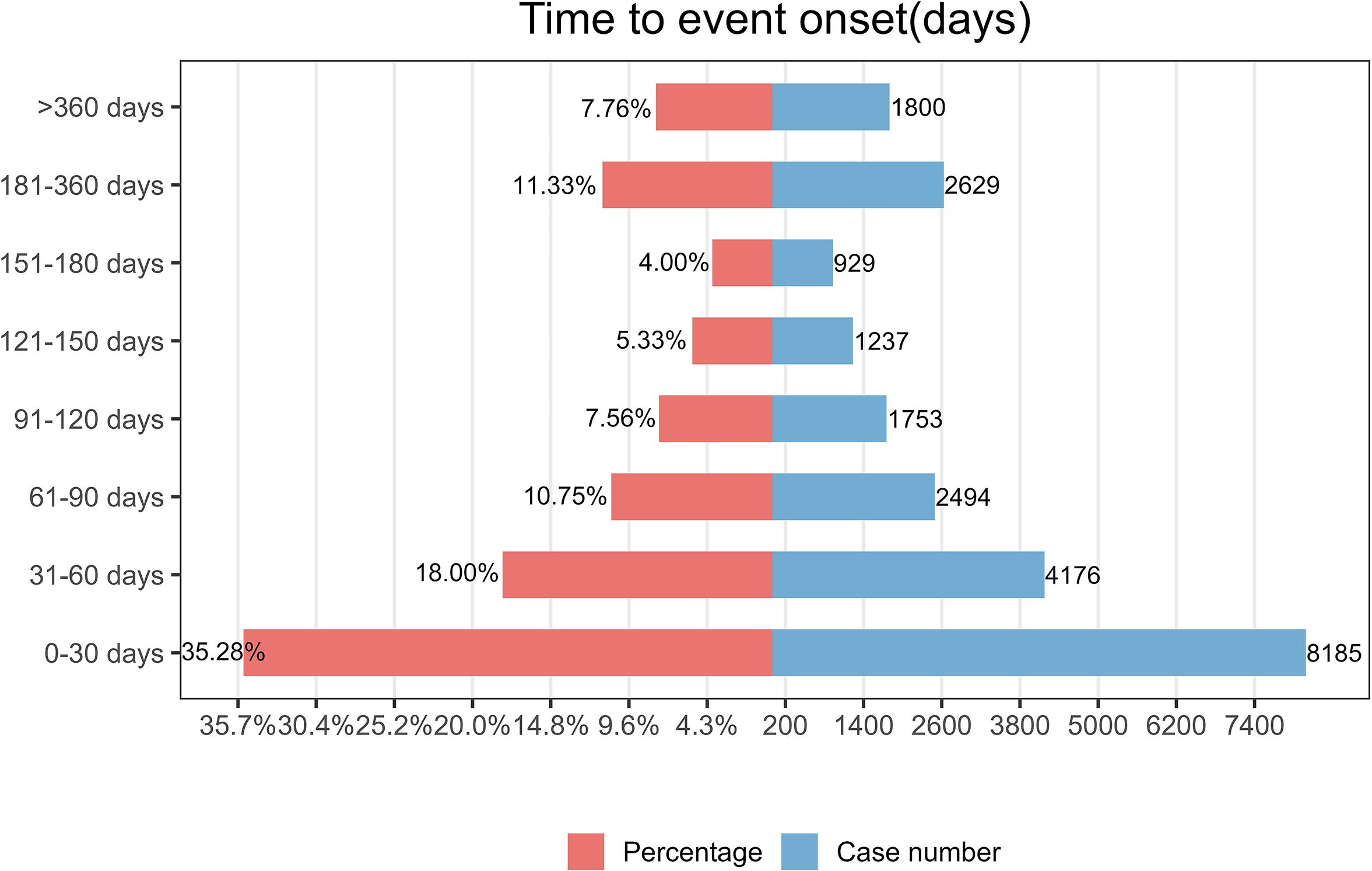
3.6 Time-to-onset and weibull distribution analysis of AEs based on nivolumab
As illustrated in Figure 3, the majority of nivolumab-associated AEs occurred within the initial 30-day period following treatment initiation. Furthermore, Weibull distribution analysis demonstrated an early failure pattern, with specific distribution parameters detailed in Table 4.
4 Discussion
The present study analyzed adverse events associated with nivolumab recorded in the FAERS database following its FDA approval and market introduction in the fourth quarter of 2014. These findings not only confirmed various adverse reactions previously documented in the prescribing information, including fatigue, dyspnea, musculoskeletal pain, decreased appetite, cough, nausea and constipation, but also identified additional adverse events not currently included in the labeling information, such as malignant neoplasm progression, weight loss, sepsis, myocarditis and hypotension.
The present study demonstrates partial concordance between the top 10 most frequently reported adverse drug reactions (ADRs) associated with nivolumab and those listed in the prescribing information, thereby corroborating that fatigue, nausea, and dyspnea represent common adverse effects of nivolumab. A meta-analysis has shown fatigue to be the most prevalent ADR induced by PD-1/PD-L1 inhibitors, consistently ranking first among both all-grade and grade ≥3 adverse events (22). The frequent reporting of these ADRs may be attributed to multiple factors: their characteristic clinical manifestations facilitate accurate recognition and documentation by healthcare providers, while concurrent non-drug-related symptoms might be erroneously ascribed to nivolumab therapy. The prolonged duration of these symptoms could reflect the sustained immunological activation underlying the drug’s antitumor mechanism, potentially leading to persistent or progressive immune-related adverse events over time (23). Notably, the prescribing information also highlights potentially fatal immune-mediated adverse reactions including pneumonitis, colitis, hepatitis, nephritis, and renal dysfunction, which demand heightened clinical vigilance across medical specialties. A comprehensive meta-analysis incorporating data from hundreds of clinical studies on fatal toxicities associated with immune checkpoint inhibitors (ICIs) revealed that immune-mediated colitis accounted for the highest number of reported mortality cases, followed by immune-mediated pneumonitis, hepatitis, and nephritis. Importantly, the diagnostic challenges posed by these rare but serious ADRs warrant emphasis - for instance, immune-mediated hepatitis may be clinically indistinguishable from hepatic dysfunction secondary to metastatic infiltration or perfusion abnormalities (resulting from distributive, hypovolemic, or cardiogenic shock) (24). Enhanced recognition of these ADRs is crucial for implementing timely preventive measures and therapeutic interventions, which are of paramount importance for cancer patients receiving immunotherapy.
Epidemiological studies demonstrate that the overall incidence rates of NSCLC and malignant melanoma follow similar age-related patterns as most malignancies, showing a progressive increase with advancing age (25, 26). Furthermore, male patients exhibit significantly higher susceptibility to both NSCLC and malignant melanoma compared to females. These epidemiological characteristics directly correlate with our baseline data, which revealed a substantially higher number of adverse event (AE) reports among male patients. Additionally, the 65–85 year age group showed the highest proportion of AE reports compared to other age strata. The United States accounted for the majority of reported cases, likely attributable to the domestic nature of the FAERS reporting system. The temporal analysis demonstrated a steady increase in nivolumab-associated AE reports from 2017 to 2019, reflecting its expanding clinical utilization. Notably, 2020 marked the first observed decline in reporting frequency, which may primarily reflect the impact of the COVID-19 pandemic on therapeutic administration patterns.
While nivolumab has demonstrated significant survival benefits in NSCLC and malignant melanoma, therapeutic response varies substantially among patients, resulting in malignant neoplasm progression emerging as the most frequently reported adverse event besides mortality. In the CheckMate 816 trial evaluating nivolumab plus platinum-based chemotherapy for NSCLC, disease progression rates were 23.9% at 1-year follow-up, increasing to 36.2% by 2 years (15). Similarly, the CheckMate 274 trial investigating nivolumab as adjuvant therapy for high-risk, muscle-invasive urothelial carcinoma post-radical surgery reported progression rates of 25.1% at 6 months, with 48.2% of patients experiencing tumor recurrence or death at median follow-up (approximately 20 months) (27).
Weight loss frequently co-occurs with tumor progression as a clinically significant paraneoplastic phenomenon. These findings strongly suggest that such treatment-related outcomes, including both malignant neoplasm progression and associated weight loss, warrant explicit inclusion in the prescribing information to enhance clinical recognition and management.
Myocarditis, encephalitis, and sepsis constitute severe treatment-related complications that may directly endanger patients’ lives, warranting particular clinical attention. In the RELATIVITY-047 clinical study, 0.6% (2 cases) of patients receiving nivolumab monotherapy developed myocarditis, including one fatal case complicated by concurrent myocarditis and septicemia (15). The CheckMate 451 study demonstrated that among 278 patients treated with nivolumab plus ipilimumab, seven experienced fatal adverse drug reactions, including one case each of myocarditis and sepsis with end-organ failure, while one case of fatal encephalitis occurred among 279 patients receiving nivolumab monotherapy (28). A study analyzing two fatal myocarditis cases in malignant melanoma patients treated with nivolumab-ipilimumab combination therapy suggested that compared with nivolumab monotherapy, the combination regimen may lead to more frequent and severe myocarditis (29). Animal studies have confirmed that PD-1 plays a regulatory role in T-cell-mediated myocardial immune responses in experimental myocarditis models, potentially preventing inflammation and myocyte damage (30). PD-1 gene deficiency in mice can also induce cardiomyopathy triggered by anti-cardiac troponin I autoantibodies (31, 32). Furthermore, cases of immunotherapy-associated encephalitis followed by interstitial granulomatous dermatitis have been reported in metastatic melanoma patients treated with nivolumab and ipilimumab (33). Regarding sepsis, one clinical trial reported two fatal cases among 361 patients receiving nivolumab-ipilimumab combination therapy (34), with similar cases documented in detailed reports (35).
Hypotension represents a frequently observed yet currently unlisted adverse effect in nivolumab’s prescribing information. A clinical trial investigating nivolumab for metastatic sarcoma reported four cases of hypotension occurring during combination therapy with ipilimumab (36). Beyond direct causation, nivolumab may induce hypotension through immune-related adrenal insufficiency, as evidenced by a documented case of refractory hypotension secondary to nivolumab-induced adrenal crisis in a 52-year-old male patient (37). Furthermore, nivolumab can trigger autoimmune autonomic ganglionopathy, potentially leading to severe orthostatic hypotension resistant to conventional management. The underlying pathophysiology may involve autoreactive T-cell activation, as PD-1/CTLA-4 blockade disrupts critical self-tolerance mechanisms, precipitating various autoimmune manifestations. Alternative hypotheses suggest B-cell mediated autoantibody production via T-cell dependent activation. Additional proposed mechanisms of immune checkpoint inhibitor neurotoxicity include inflammatory processes affecting neural microstructures, such as endoneurial microvilli inflammation and subperineurial inflammatory edema (38). These findings underscore the necessity for comprehensive monitoring beyond symptomatic management of immune-related adverse events during nivolumab therapy. Elucidation of the multifactorial pathogenic mechanisms will facilitate more effective management of these complex pharmacological complications.
This study has several limitations that warrant consideration. First, the FAERS database relies primarily on voluntary reporting by healthcare professionals and consumers, which may introduce selection bias through underreporting, duplicate entries, and inaccurate documentation. Second, the database lacks standardized classification of adverse event (AE) severity and does not distinguish between drug-induced AEs and those attributable to underlying comorbidities or concomitant therapies. This limitation creates challenges in determining AE causality and grading, as disease-related symptoms may be inadvertently reported as treatment-emergent AEs. Consequently, clinical judgment remains essential for accurate AE assessment in practice. Third, the FAERS data reflect only immediate post-treatment outcomes without longitudinal follow-up information, limiting the ability to evaluate long-term drug safety profiles in real-world settings. Furthermore, the database exhibits significant geographical bias, with predominant representation of U.S. case reports, thereby constraining the generalizability of findings to global populations and diverse disease contexts. These inherent constraints emphasize the need for complementary prospective studies to validate the pharmacovigilance signals identified in this analysis.
5 Conclusion
This pharmacovigilance study utilized the FAERS database to evaluate the real-world safety profile of nivolumab. The analysis confirmed frequently reported AEs consistent with the drug label, including fatigue, dyspnea, musculoskeletal pain, decreased appetite, cough, nausea, and constipation. Importantly, we identified several clinically significant AEs not currently listed in the prescribing information: malignant neoplasm progression, weight loss, sepsis, myocarditis, encephalitis, and hypotension. These findings provide healthcare professionals with enhanced awareness of potential nivolumab-related toxicities, enabling more comprehensive risk-benefit assessments and improved clinical management of treated patients. The detection of these safety signals underscores the value of post-marketing surveillance in complementing data from controlled clinical trials.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.
Author contributions
YW: Conceptualization, Methodology, Writing – review & editing, Software, Investigation, Writing – original draft, Visualization, Data curation. YZ: Conceptualization, Writing – original draft, Writing – review & editing, Investigation. SX: Validation, Software, Writing – original draft. ZM: Writing – review & editing, Writing – original draft, Funding acquisition, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (NSFC, No. 82171342).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1605958/full#supplementary-material
References
1. Paik J. Nivolumab plus relatlimab: first approval. Drugs. (2022) 82:925–31. doi: 10.1007/s40265-022-01723-1
2. Malmberg R, Zietse M, Dumoulin DW, Hendrikx JJMA, Aerts JGJV, van der Veldt AAM, et al. Alternative dosing strategies for immune checkpoint inhibitors to improve cost-effectiveness: a special focus on nivolumab and pembrolizumab. Lancet Oncol. (2022) 23:e552–61. doi: 10.1016/S1470-2045(22)00554-X
3. Mullick N and Nambudiri VE. Relatlimab-nivolumab: A practical overview for dermatologists. J Am Acad Dermatol. (2023) 89:1031–7. doi: 10.1016/j.jaad.2023.06.024
4. Tang K, Kong X, Mao G, Qiu M, Zhu H, and Zhou L. Primary cerebral Malignant melanoma: A case report with literature review. Med (Baltimore). (2017) 96:e5805. doi: 10.1097/MD.0000000000005805
5. Guarrera B, Coati I, and Giarletta M. Unusual case of intraosseous primary intracranial Malignant melanoma. BMJ Case Rep. (2024) 17:e256623. doi: 10.1136/bcr-2023-256623
6. Rothman KJ, Lanes S, and Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. (2004) 13:519–23. doi: 10.1002/pds.1001
7. Sharma A and Kumar A. Identification of novel signal of clobazam-associated drug reaction with eosinophilia and systemic symptoms syndrome: A disproportionality analysis. Acta Neurol Scand. (2022) 146:623–7. doi: 10.1111/ane.13690
8. Javed F and Kumar A. Identification of signal of clindamycin associated renal failure acute: A disproportionality analysis. Curr Drug Saf. (2024) 19:123–8. doi: 10.2174/1574886318666230228142856
9. Wolchok JD, Chiarion-Sileni V, González R, Grob JJ, Rutkowski P, Lao CD, et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol. (2022) 40:127–37. doi: 10.1200/JCO.21.02229
10. Larkin J, Chiarion-Sileni V, González R, Grob JJ, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. (2019) 381:1535–46. doi: 10.1056/NEJMoa1910836
11. Herbst RS, Morgensztern D, and Boshoff C. The biology and management of non-small cell lung cancer. Nature. (2018) 553:446–54. doi: 10.1038/nature25183
12. Herbst RS, Heymach JV, and Lippman SM. Lung cancer. New Engl J Med. (2008) 359:1367–80. doi: 10.1056/NEJMra0802714
13. Morgensztern D, Ng SH, Gao F, and Govindan R. Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J thoracic Oncol. (2010) 5:29–33. doi: 10.1097/JTO.0b013e3181c5920c
14. Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr, Wu YL, et al. Lung cancer: current therapies and new targeted treatments. Lancet (London England). (2017) 389:299–311. doi: 10.1016/S0140-6736(16)30958-8
15. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. (2022) 386:1973–85. doi: 10.1056/NEJMoa2202170
16. Provencio M, Nadal E, González-Larriba JL, Martínez-Martí A, Bernabé R, Bosch-Barrera J, et al. Perioperative nivolumab and chemotherapy in stage III non-small-cell lung cancer. N Engl J Med. (2023) 389:504–13. doi: 10.1056/NEJMoa2215530
17. Kumar A. Signal Analysis in Pharmacovigilance: Principles and Processes. Boca Raton: CRC Press (2024).
18. Brown EG. Using MedDRA: implications for risk management. Drug Saf. (2004) 27:591–602. doi: 10.2165/00002018-200427080-00010
19. Evans SJ, Waller PC, and Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. (2001) 10:483–6. doi: 10.1002/pds.677
20. Rivkees SA and Szarfman A. Dissimilar hepatotoxicity profiles of propylthiouracil and methimazole in children. J Clin Endocrinol Metab. (2010) 95:3260–7. doi: 10.1210/jc.2009-2546
21. Ang PS, Chen Z, Chan CL, and Tai BC. Data mining spontaneous adverse drug event reports for safety signals in Singapore - a comparison of three different disproportionality measures. Expert Opin Drug Saf. (2016) 15:583–90. doi: 10.1517/14740338.2016.1167184
22. Wang Y, Zhou S, Yang F, Qi X, Wang X, and Guan X. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: A systematic review and meta-analysis. JAMA Oncol. (2019) 5:1008–19. doi: 10.1001/jamaoncol.2019.0393
23. Baxi S, Yang A, Gennarelli RL, Khan N, Wang Z, and Boyce L. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. (2018) 360:k793. doi: 10.1136/bmj.k793
24. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis [published correction appears in JAMA Oncol. 2018 Dec 1;4(12):1792. doi: 10.1001/jamaoncol.2018.5346. JAMA Oncol. (2018) 4:1721–8. doi: 10.1001/jamaoncol.2018.3923
25. Radkiewicz C, Dickman PW, Johansson ALV, Wagenius G, Edgren G, and Lambe M. Sex and survival in non-small cell lung cancer: A nationwide cohort study. PloS One. (2019) 14:e0219206. doi: 10.1371/journal.pone.0219206
26. Helvind NM, Brinch-Møller Weitemeyer M, Chakera AH, Hendel HW, Ellebæk E, and Svane IM. Stage-specific risk of recurrence and death from melanoma in Denmark, 2008-2021: A national observational cohort study of 25 720 patients with stage IA to IV melanoma. JAMA Dermatol. (2023) 159:1213–22. doi: 10.1001/jamadermatol.2023.3256
27. Bajorin DF, Witjes JA, Gschwend JE, Schenker M, Valderrama BP, and Tomita Y. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma [published correction appears in N Engl J Med. 2021 Aug 26;385(9):864. doi: 10.1056/NEJMx210012. N Engl J Med. (2021) 384:2102–14. doi: 10.1056/NEJMoa2034442
28. Owonikoko TK, Park K, Govindan R, Ready N, Reck M, and Peters S. Nivolumab and ipilimumab as maintenance therapy in extensive-disease small-cell lung cancer: checkMate 451. J Clin Oncol. (2021) 39:1349–59. doi: 10.1200/JCO.20.02212
29. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. (2016) 375:1749–55. doi: 10.1056/NEJMoa1609214
30. Tarrio ML, Grabie N, Bu DX, Sharpe AH, and Lichtman AH. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol. (2012) 188:4876–84. doi: 10.4049/jimmunol.1200389
31. Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, and Matsumori A. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. (2001) 291:319–22. doi: 10.1126/science.291.5502.319
32. Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. (2003) 9:1477–83. doi: 10.1038/nm955
33. Starling CT, Messer AA, Kleiman A, McQuade JL, Glitza IC, Torres-Cabala CA, et al. Interstitial granulomatous dermatitis and concurrent immunotherapy associated encephalitis with nivolumab and ipilimumab. Dermatol Online J. (2022) 28:10. doi: 10.5070/D328257399
34. Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, and Menezes J. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial [published correction appears in Lancet Oncol. 2021 Mar;22(3):e92. doi: 10.1016/S1470-2045(21)00082-6. Lancet Oncol. (2021) 22:198–211. doi: 10.1016/S1470-2045(20)30641-0
35. Brazel D, Lee S, Mahadevan A, Warnecke B, and Parajuli R. Multiorgan failure from nivolumab and ipilimumab: A case report and literature review. Cureus. (2023) 15:e41781. doi: 10.7759/cureus.41781
36. D'Angelo SP, Mahoney MR, Van Tine BA, Atkins J, Milhem MM, and Jahagirdar BN. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. (2018) 19:416–26. doi: 10.1016/S1470-2045(18)30006-8
37. Tsukizawa Y, Kondo K, Ichiba T, Naito H, Mizuki K, and Masuda K. Refractory hypotension due to Nivolumab-induced adrenal insufficiency. Nagoya J Med Sci. (2018) 80:285–8. doi: 10.18999/nagjms.80.2.285
Keywords: nivolumab, FAERS, mm, NSCLC, pharmacovigilance, adverse events
Citation: Wu Y, Zhou Y, Xia S and Meng Z (2025) The real-world safety of Nivolumab: a pharmacovigilance analysis based on the FDA adverse event reporting system. Front. Immunol. 16:1605958. doi: 10.3389/fimmu.2025.1605958
Received: 04 April 2025; Accepted: 08 May 2025;
Published: 26 May 2025.
Edited by:
Mingzhou Guo, People’s Liberation Army General Hospital, ChinaReviewed by:
Lukasz Chlewicki, Eli Lilly, United StatesIvan Arni Caballero Preclaro, Department of Health- Tondo Medical Center, Philippines
Copyright © 2025 Wu, Zhou, Xia and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaoyou Meng, bWVuZ3poYW95b3VAdG1tdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Yutong Wu
Yutong Wu Yue Zhou
Yue Zhou Shiyue Xia
Shiyue Xia Zhaoyou Meng
Zhaoyou Meng