- 1IBD Center, Humanitas Research Hospital - IRCCS, Rozzano, Milan, Italy
- 2Department of Biomedical Sciences, Humanitas University, Milan, Italy
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) represent a cornerstone in the treatment of diabetes and obesity and have emerged as a promising option for other metabolic disorders, including hepatic steatosis. Recent evidence highlights the direct and indirect anti-inflammatory properties of GLP-1, suggesting a potential additional therapeutic strategy for patients with inflammatory bowel disease (IBD). However, side effects of GLP-1 RAs, particularly those affecting the gastrointestinal system, may limit their use in patients with IBD. The rising prevalence of IBD worldwide and the ageing of the IBD population will likely increase the number of patients with metabolic comorbidities who may potentially benefit from a combination treatment with GLP-1 RAs. A profound comprehension of the physiological function of intestinal homeostasis and permeability is essential to more accurately evaluate the prospective application of GLP-1 RAs in patients with ongoing inflammation. While preclinical studies support this hypothesis, robust clinical evidence remains limited. This narrative review aims to provide a synthesis of current knowledge regarding the anti-inflammatory properties of GLP-1, with a particular focus on safety concerns and potential future directions for its use in IBD management.
1 Introduction
Inflammatory bowel disease (IBD), comprising Crohn’s disease (CD) and ulcerative colitis (UC), is a multifactorial, chronic, immune-mediated inflammatory disorder that affects the gastrointestinal system. The prevalence and incidence of IBD are rising worldwide, placing a significant burden on healthcare systems and social resources (1). Although significant progress in the development of immunotherapy over the past two decades (2), approximately 50% of IBD patients show response failure after initial advanced therapies, with response rates exhibiting a further decline for second- and third-line treatments (3, 4). As a result, the exploration of novel therapeutic strategies is crucial for improving IBD management.
A promising explanation could be represented by the complex interplay between inflammation and metabolism, which is still largely unknown in the field of IBD management. Recent evidence suggests that Westernized lifestyles, including dietary habits and sedentary behavior, could have contributed to the increasing global prevalence of IBD, particularly in newly industrialized countries (5, 6). Notably, high sugar intake has been identified as a potential risk factor for gut inflammation in preclinical studies, leading to colitis and a significant accumulation of inflammatory cells in mesenteric fat and lymph nodes in genetically susceptible mice (7).
GLP-1 receptor agonists (GLP-1 RAs) have gained attention due to their numerous effects on gut metabolism and immune regulation. By stimulating glucose-dependent insulin secretion and suppressing glucagon production, they are a cornerstone of the treatment of type 2 diabetes (DM2), particularly in patients with a higher risk of cardiovascular disease (8). Additionally, GLP-1 RAs have been approved for obesity treatment given their ability to delay gastric emptying, with enhanced satiety and reduction of energy intake (9). In addition, GLP-1 RAs have shown to present direct anti-inflammatory effects, by modulating immune cell signalling and preventing the release of reactive oxygen species (ROS) (10). Preclinical studies indicate that GLP-1 RAs may have an impact on gut microbiota composition (11) and contribute to the maintenance of intestinal mucosal barrier integrity, thereby reducing gut permeability (12). Furthermore, by addressing metabolic dysfunction in obese patients, whose prevalence is increasing in the IBD population (13), GLP-1 RAs could indirectly mitigate inflammation by decreasing the pro-inflammatory activity of adipose tissue, particularly visceral adipose one. The convergence of metabolic and inflammatory pathways suggests that GLP-1 RAs hold promise as an adjunctive therapy for IBD. Nevertheless, their therapeutic potentials are still subject of debate, particularly given their known gastrointestinal side effects (e.g., nausea, vomiting, and diarrhea), which may limit their application in IBD patients, especially those with clinically active disease (14).
This narrative review describes the complex interplay between GLP-1 signalling and intestinal inflammation, highlighting both the potential benefits and limitations of GLP1 RAs in IBD management, with a particular focus on their promising role in selected obese IBD patients.
2 GLP-1: a key player in metabolic homeostasis
GLP-1 is a 31 aminoacid-long peptide derived from proglucagon (15), which is produced in response to both nutritional and inflammatory stimuli. It is mainly produced by enteroendocrine L-cells, which are distributed throughout the gastrointestinal tract, with increasing density from the proximal jejunum to the colon (16). Additionally, GLP-1 is secreted by brainstem neuronal cells (17). The release of GLP-1 follows a biphasic pathway: an initial peak mediated by neural signalling, followed by a second phase which is triggered by direct mechanical stimulation of L-cells, as nutrients pass through the gut (18).
GLP-1 is best known for its role in glucose homeostasis. It enhances insulin secretion from pancreatic β-cells in a glucose-dependent manner, whilst simultaneously suppressing glucagon release from α-cells (19). It also promotes β-cell survival and proliferation, strengthening its role in metabolic regulation (20). Apart from its metabolic effects, GLP-1 plays a key role in gastrointestinal motility. It is the main mediator of the phenomenon known as the “ileal brake” (21), which delays both gastric emptying and small bowel transit, with resulting slowed nutrient absorption in direct proportion to carbohydrate intake (22, 23). This effect is primarily mediated by GLP-1 receptors (GLP-1Rs) located on myenteric neurons of the digestive system, involving nitrergic and cyclic adenosine monophosphate (cAMP)-dependent mechanisms (22, 24).
Notably, GLP-1 appears to be a key messenger of the gut-brain axis. In response to significant and/or high-nutrient meals, GLP-1 reaches high blood concentrations, exerting its effects on GLP-1Rs located in the area postrema, the nucleus tractus solitarius, and the hypothalamus. As a result, it induces satiety and regulates food intake (25). This effect is significantly more pronounced in patients undergoing GLP-1 RAs compared to the endogenous one (26), thus justifying the use of these drugs in the treatment of obesity. In support of their role in weight management, GLP-1Rs are expressed in adipose tissue, where they regulate the proliferation of pre-adipocyte cells and lipid homeostasis (27).
Apart from its metabolic effects, GLP-1 exerts a wide range of physiological actions. Its receptors are expressed in multiple tissues, including the heart, kidneys, lungs, and smooth muscle. The protective effects of GLP-1 RAs against cardiovascular events have been demonstrated in several studies, thus supporting their application in patients with heart failure with preserved ejection fraction and DM2 (28). These benefits primarily derived from improved glucose control, weight reduction, enhanced cardiac output, and lower blood pressure. Additionally, GLP-1 plays a protective role for the endothelium. It reduces atherosclerotic plaque formation, inhibits the expression of vascular adhesion molecules, and prevents LDL-induced immune cell adhesion (29–31). Notably, GLP-1Rs have also been found in the atrial cavities and in the sinoatrial node, still their precise physiological function remains unclear (32). Additionally, the therapeutic potentials of GLP-1 could extend to renal and hepatic health. Recent studies suggest that GLP-1 RAs could improve renal function in patients with chronic kidney disease (CKD) and DM2 (33, 34). Despite the absence of GLP-1Rs on hepatocytes, GLP-1 is involved in hepatic lipid and glucose metabolism, by contributing to fibrosis reversal and liver cells protection in patients with nonalcoholic steatohepatitis (NASH) undergoing GLP-1 RAs (35).
Taken together, GLP-1 is a major metabolic and regulatory hormone, with many potential therapeutic implications beyond glycemic control, encompassing cardiovascular protection, enhanced renal function, and the potential benefits in hepatic disease.
3 GLP-1’s anti-inflammatory properties
Recent scientific research has increasingly highlighted the potential anti-inflammatory role of GLP-1. This premise is reinforced by the discovery that different immune cells, including B and T lymphocytes, as well as myeloid-lineage cells such as monocytes, eosinophils, and neutrophils, express GLP-1Rs (36). Notably, GLP-1Rs are also expressed by intestinal intraepithelial lymphocytes (IELs), suggesting a role in immune homeostasis of the digestive system (36, 37). Moreover, studies conducted on animal models have shown that enteroendocrine L-cells increase the secretion of GLP-1 in response to inflammatory cytokines (e.g., interleukin-6 (IL-6) and lipopolysaccharide (LPS) (38)) and ischaemic injury (39). Furthermore, Kahlen et al. found that critically ill ICU patients presented significantly higher plasma GLP-1 levels versus healthy controls, which were directly correlated to increased inflammatory biomarkers such as IL-6). These results underscore a potential connection between GLP-1 and inflammatory processes (40). Current evidence suggests that GLP-1 has a regulatory role in both innate and adaptive immunity while also supporting intestinal barrier integrity and gut microbiota health.
3.1 Innate and adaptive immune response
In rat models of systemic inflammation, a GLP-1 RA, exendin, significantly lowered pro-inflammatory cytokine levels, including IL-1β, IL-6, TNF-α, and interferon-gamma (IFN-γ) (41). These effects are primarily mediated by the inhibition of NF-κB and mitogen-activated protein kinase (MAPK) pathways, both of which are associated with stress, inflammation, and apoptosis responses (10, 42). Interestingly, in the context of several in vitro studies, GLP-1 RAs have demonstrated the capacity of promoting an anti-inflammatory state by influencing immune cell differentiation. For instance, exenatide, the first GLP-1-analogue developed, demonstrated to promote human monocyte differentiation into alternatively activated M2 macrophages, leading to an increase in anti-inflammatory cytokines such as IL-10 while significantly reducing pro-inflammatory cytokines, including IL-6, TNF-α and IL-1β (43). Shiraishi et al. demonstrated that GLP-1/GLP-1R signalling plays a crucial role in activating signal transducer and activator of transcription 3 (STAT3), which directly promotes human M2 macrophage polarization while inhibiting classically activated M1 macrophages, known for their pro-inflammatory and tissue-destructive properties (44). In animal models, GLP-1/GLP-1R signalling has demonstrated to be involved in key macrophage functions such as phagocytosis and migration, though further research is needed to confirm these effects in humans (45). Additionally, both eosinophils and neutrophils express GLP-1R on their surface. Notably, eosinophils of asthmatic patients exhibit lower GLP-1Rs’ expression compared to healthy controls (46). The interaction between GLP-1 and its receptor on eosinophils has been shown to reduce the production of pro-inflammatory cytokines, including IL-4, IL-8 and IL-13 (47). Although the limited research on the role of GLP-1 in neutrophils, preliminary findings have shown that it may mitigate their activation, potentially reducing myocardial ischemic injury rodent models (48). In conclusion, the GLP-1/GLP-1R signalling plays a significant role in the balance of innate immunity, particularly in the polarisation of macrophages.
Furthermore, GLP-1 has been hypothesised to act as a mediator between innate and adaptive immune responses. In mice single-cell RNA sequencing identified a subpopulation of GLP-1R-positive memory T-cells that was mainly composed of exhausted CD8+ T cells: functionally, stimulation of the GLP-1R on these cells was found to mediate apoptosis and anergic signals, thereby suppressing effector T-cell function and the inflammatory response (49, 50). In humans, the GLP-1 pathways act as a modulator of a specific subset of T-cells, known as invariant natural killer T (iNKT) cells, and this activity might be responsible for the improvements of some immune-mediated disorders (such as psoriasis and suppurative hidradenitis) observed in obese patients treated with GLP-1 RAs (51–54). In mice, GLP-1 RA treatment also showed to inhibit the differentiation of T helper (Th) cells into Th1 and Th17 subsets and reduce the release of related proinflammatory cytokines, including IFN-y, TNF-α, and IL-17. Instead, GLP-1 promotes the polarization of Th2 and regulatory T (Treg) cells, increasing anti-inflammatory cytokines such as IL-10 and IL-5 (55, 56).
3.2 Intestinal mucosal barrier
IELs are a heterogeneous population of T cells located among intestinal epithelial cells (IECs), where they contribute to maintaining mucosal barrier integrity (57). A particular subset of IELs (Tαβ and Tγδ) has been found to be enriched with GLP-1Rs (37, 58). Their proximity to enteroendocrine L-cells suggests a potential contribution of lymphoid tissue in GLP-1/GLP-1R signalling, as mediator of L-cell proliferation (59, 60). Genetically modified Glp1r−/− mice displayed greater levels of epithelial damage in comparison to wild-type (WT) mice following inflammatory stimulation. Conversely, WT mice exhibited higher expression of antimicrobial and anti-inflammatory genes (37). Similarly, Wong et al. described that IEL-expressing GLP-1Rs play a crucial role in controlling gut inflammation of mice, by reducing IFN-γ production in IELs and promoting IEC survival and intestinal barrier integrity (38). Additionally, GLP-1 showed to directly stimulate murine Brunner’s glands to produce and release mucin, thereby strengthening the mucosal barrier (61). Furthermore, GLP-1Rs on IELs have been found to regulate the metabolic effects of GLP-1 in animal models by entrapping it, thereby reducing its systemic availability and lowering its plasma concentrations (62). Moreover, GLP-1 could be involved in mechanisms of growth and expansion of IECs, as the loss of GLP-1Rs on IELs has been associated with shorter and smaller intestines in mice (59, 63, 64).
The anti-inflammatory role of GLP-1 in the gut is not limited to the maintenance of IELs and L-cells but it is also interconnected with gut microbiota. Thus, several microbiota-derived metabolites have been found to stimulate enteroendocrine L-cells producing GLP-1. Short-chain fatty acids (SCFAs) such as butyrate, a bacterial metabolite produced from dietary fibre, can directly trigger GLP-1 release by binding to the membrane receptor GPR43 (65). Additionally, dietary protein-derived metabolites, including tryptophan-indole, as well as LPS from Gram-negative bacteria, directly promote the production of GLP-1 (66, 67). Interestingly, bile acid metabolites have a dual effect on GLP-1 secretion: they can both stimulate and inhibit its production (66). Furthermore, the ileum of germ-free and antibiotic-treated mice showed lower Glp1r gene expression compared to controls, with higher incretin effect resistance (68).
Both preclinical and clinical studies have demonstrated that GLP-1’s modulate the gut microbiota composition by delaying gastric emptying and altering luminal glucose (12). Zhao et al. observed that the gut microbiota of diet-induced obese (DIO) mice treated with liraglutide for four weeks displayed a similar phylogenetic composition in comparison to the start of the treatment, but was characterized by a significant decrease in microbial phenotypes associated with obesity (e.g., Firmicutes Lachnospiraceae and Clostridiales), alongside an increase in Proteobacteria (e.g., Burkholderiales bacterium YL45) and Akkermansia muciniphila—a species largely associated with high-fibre diets and mucosal barrier health (11, 69–71). Notably, the administration of GLP-1 RA resulted in an elevated Firmicutes/Bacteroidetes ratio and an increase in Prevotella, species that have been linked to lower inflammation (12, 70). Similar results were reported by Wang et al., where GLP-1 RA treatment had a notable impact on the abundance of weight-related microbial phylotypes, though no significant change in the Firmicutes/Bacteroidetes ratio was observed (72).
The findings concerning human gut microbiota remain controversial. Although liraglutide treatment led to a decrease in pro-inflammatory microbial species (e.g., Escherichia–Shigella, Megamonas, and Bacillus) in DM2 patients, no statistically significant differences were observed when compared to metformin treatment. However, a significant increase in Bifidobacterium, Dialister, and Alistipes was reported (73). A recent study conducted on 41 diabetic patients demonstrated that exposure to the GLP-1 RA dulaglutide over a 48-week period was associated with a substantial decrease in non-butyrate-producing Firmicutes (e.g., Ruminococcus and Blautia), accompanied by an increase in Bacteroides, Lactobacillus, and Prevotella (74).
In conclusion, GLP-1 contributes to gut homeostasis and inflammation control through its modulatory effects on immunity, epithelial cell proliferation, and microbial composition (Figure 1). Whilst emerging evidence suggests the extensive therapeutic potential of GLP-1, further studies are needed to fully elucidate its mechanisms and its clinical applications in gastrointestinal disorders.
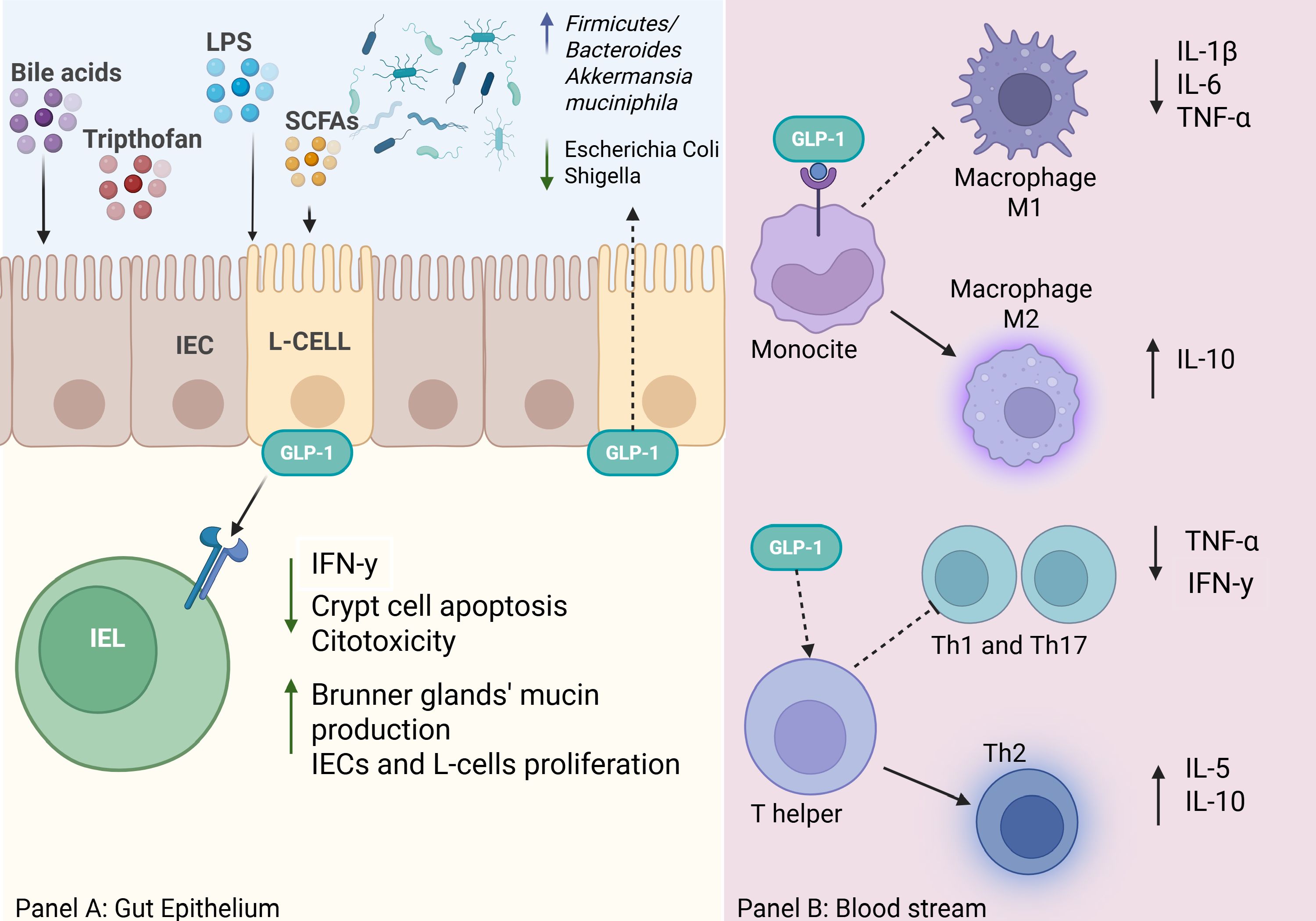
Figure 1. The image in panel (A) illustrates how various metabolites derived from diet and gut microbiota (bile acids, tryptophan, LPS, SCFAs) stimulate enteroendocrine L-cells in the intestinal mucosa to secrete GLP-1. GLP-1 acts both locally at the level of the intestinal epithelium and systemically through blood vessels and immune cells. GLP-1 reduces IFN-γ, crypt cell apoptosis, and cytotoxicity by intraepithelial lymphocytes (IELs) and at the same time, it stimulates the proliferation of intestinal epithelial cells (IECs) and L-cells, as well as mucin production by Brunner’s glands (37, 41, 49, 50, 61). Moreover, GLP-1 RAs are found to be associated with alteration of intestinal microbiota (11, 72). Panel B is a concise representation of the role of GLP-1 in immune responses. GLP-1 modulates monocytes/macrophages, promoting polarization toward M2 macrophages (anti-inflammatory), which release IL-10 and suppress pro-inflammatory cytokines (IL-1β, IL-6, TNF-α). Furthermore, it Influences indirectly T helper lymphocytes polarisation to Th2 cells and release of IL-5 and IL-10, contributing to a more tolerogenic immune response (41, 44). Overall, GLP-1 acts as an immuno-metabolic modulator, supporting intestinal barrier protection and a regulated inflammatory response.
4 Metabolic disorders and IBD
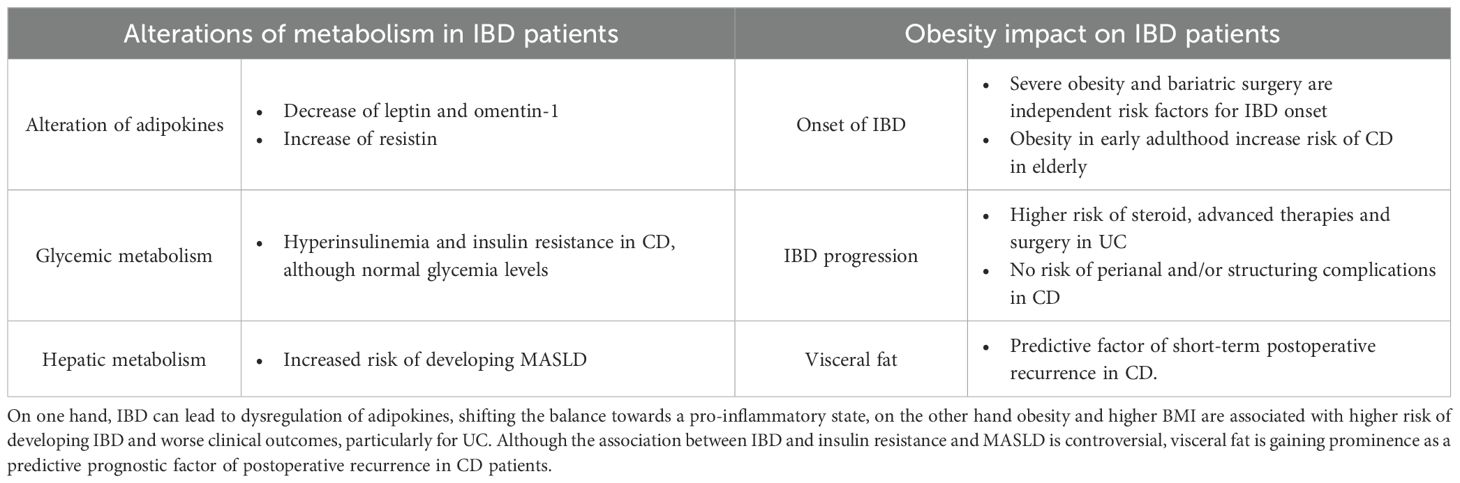
The prevalence of metabolic disorders, including DM2 and obesity, is increasingly rising in the IBD population. Approximately 15-40% of individuals diagnosed with IBD and an additional 20-40% are estimated to be obese and overweight respectively (13). IBD and metabolism are strongly interconnected (Table 1). Adipokine levels are frequently altered in IBD patients (75, 76). Leptin, a key regulator of satiety and appetite, is decreased in active IBD, probably due to exhaustion after transient overproduction related to TNF-α hyperactivity. In contrast, high levels of resistin levels have been found in active IBD, correlating with NF-κB pathway activation and increased secretion of TNF-α, IL-6, and IL-1β (77). Moreover, despite normal glycemic levels, elevated serum resistin has been linked to hyperinsulinemia in active IBD (78). IBD patients, present higher risk of developing insulin resistance, particularly those diagnosed with CD (79, 80). However, a recent study did not support this finding but rather attributed the increased risk of insulin resistance to the concomitant presence of metabolic dysfunction–associated steatotic liver disease (MASLD) (81). Furthermore, omentin-1, an anti-inflammatory adipokine that inhibits TNF-induced vascular inflammation, exhibit low levels in patients with active CD and UC (82), emphasising the profound connection between IBD and adipose tissue.
The impact of obesity on IBD onset and progression is an area of growing research, though evidence remains controversial. Severe obesity and bariatric surgery have been recognized as independent risk factors for the development of IBD (83). Additionally, obesity in early adults has been linked to a substantially increased risk of CD onset in the elderly (84). A recent propensity-matched cohort study also identified obesity as a risk factor for corticosteroid use, therapy escalation, and colectomy in UC patients (85). However, no increased risk of perianal or stricturing complications has been observed in CD patients (86, 87). Interestingly, a retrospective analysis of 202 UC patients showed that higher BMI was inversely related to disease severity and IBD extent (88). Nonetheless, BMI was directly associated with higher risk of severe hospitalization, longer hospital stays and increased surgical intervention rates, mainly due to metabolic comorbidities (89). The discrepancies may be attributed to the limitations of BMI as a lone evaluator of metabolic disorders. A recent cohort study involving 200 IBD patients identified visceral adiposity, rather than BMI, as a predictive risk factor for a shorter time to IBD flare, particularly for CD patients (90). Additionally, recent data recognized visceral fat as a risk factor for postoperative recurrence in CD patients (76).
4.1 The role of GLP-1 RAs in IBD
The previously mentioned intrinsic connection between metabolism and the inflammatory response led researchers to investigate the role of GLP-1 modulation in the management of IBD. Evidence suggests that IBD pathogenesis is closely linked to gut failure in controlling inflammation, with enteroendocrine cells (EECs) playing a pivotal role in the process (91). In this regard, TNF-α has been demonstrated to trigger the NF-κB pathway in EECs, which are a target of the GLP-1/GLP-1R pathway. This, in turn, results in the production of IL-17C, a process that contributes to the propagation of inflammation in individuals suffering from IBD (92). Preclinical models found that GLP-1 RAs reduce intestinal inflammation in dextran sulfate sodium (DSS)-induced colitis, by increasing IL-22 production by colonic IELs and several beneficial bacteria, including Firmicutes, Proteobacteria and Lactobacillus reuteri (93). Furthermore, in human samples, GLP-1R was deregulated in IBD active biopsies (94), with elevated GLP-1 plasmatic levels being associated with severe active disease (95). Anecdotal evidence suggests that GLP-1 RAs may be beneficial in IBD treatment. A case report documented clinical remission in a 42-year-old UC patient following liraglutide administration for obesity treatment (96). This lends further weight to prospective investigation of the benefits and safety of GLP-1 RAs in the real world which are resumed in Table 2.
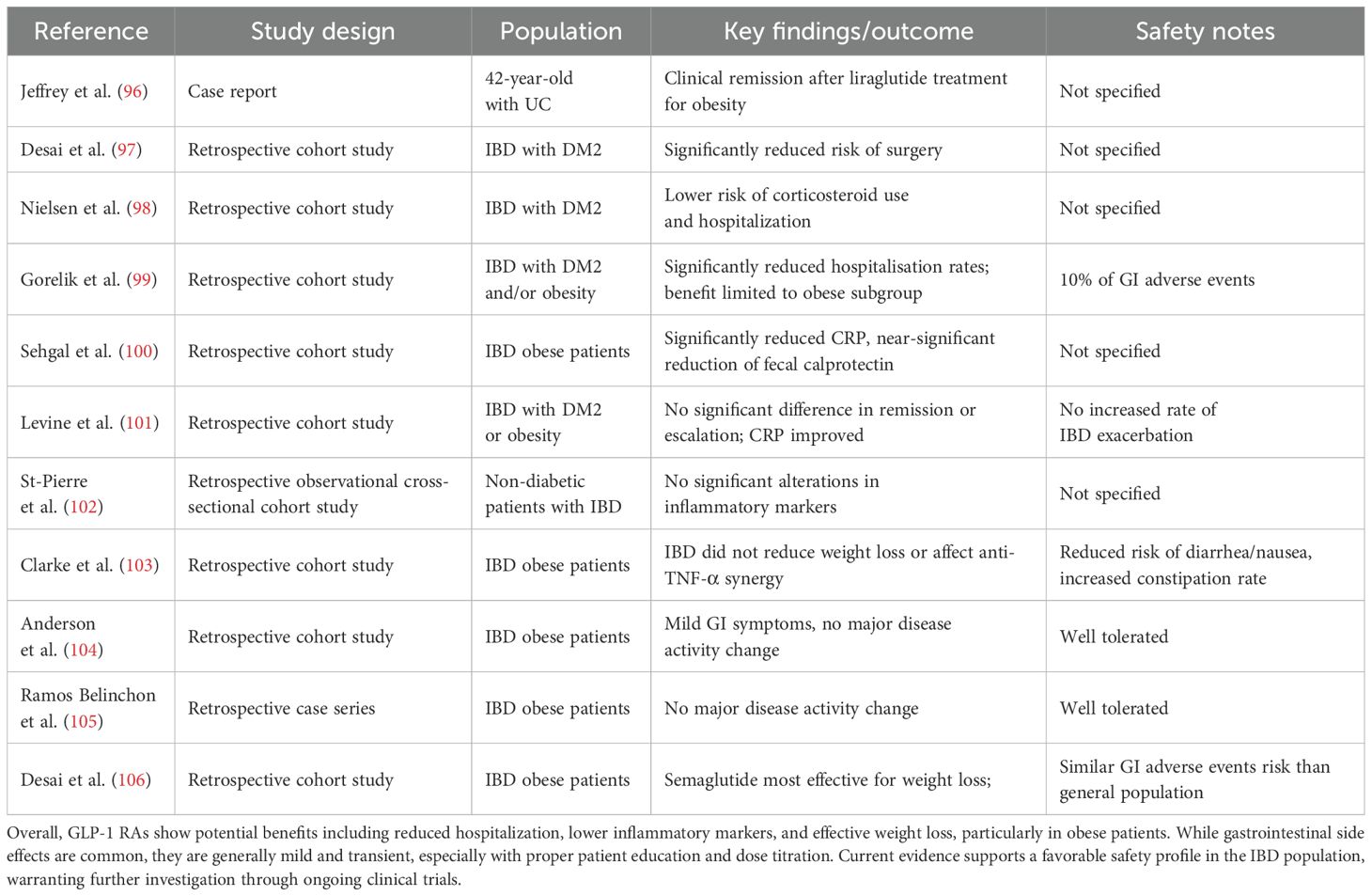
Table 2. This table compiles current knowledge evaluating the impact of GLP-1 RAs on disease outcomes and safety in IBD patients.
Desai et al. found that both UC and CD patients with DM2 presented a reduced risk of surgery when treated with GLP-1 RAs compared to other hypoglycemic agents (97). A Danish nationwide cohort study also reported a lower risk of corticosteroid use and hospitalization in IBD patients with DM2 undergoing GLP-1 RAs rather than other antidiabetic treatments (98). Additionally, a nationwide study conducted in Israel found improved IBD outcomes, with a significant reduction of hospitalization rates; however, these benefits were limited only to obese patients (99). Furthermore, recent finding reported that GLP-1 RAs significantly reduced C-reactive protein (CRP) levels in obese IBD patients, alongside a nearly statistically significant reduction in fecal calprotectin (100). However, not all studies align with these results. Levine et al. observed no statistically significant differences in disease exacerbation, corticosteroid-free remission, or therapy escalation in the same cohort of 224 IBD patients after one year of GLP-1 RAs treatment. Despite this, CRP levels showed improvement (101). A further study conducted on IBD patients and not diagnosed with DM2 also reported no significant alterations in inflammatory markers. However, only a small number of patients were included in the study, and median levels were not elevated even before the commencement of GLP-1 RAs’ treatment (102). These discrepancies may stem from short observation periods and small sample sizes respectively (101, 102).
With regard to safety concerns, the most prevalent adverse effects associated with GLP-1 RAs are gastrointestinal, such as bloating, dyspepsia, nausea, vomiting, diarrhea and constipation (107). It is noteworthy that the majority of these gastrointestinal manifestations are of a mild nature and predominantly occur during the titration phase (108). These symptoms are often the reasons why patients stop their treatment. Registration clinical trials show that 16-37% of patients discontinue within a year (109–111). However, real-life analyses indicate a higher discontinuation rate, of approximately 70% stopping within 2 years, especially in non-diabetic patients (112, 113). Interestingly, GLP-1 RAs showed a favorable safety profile in IBD patients, exhibiting comparable tolerability to non-IBD populations. Clarke et al. found that IBD did not affect weight loss outcomes in obese patients and that anti-TNF-α therapy did not reduce the likelihood of achieving ≥5% total weight loss (TWL) (66% vs. 58%, P = 0.33). This indicates that the combination of these agents can be safely and effectively administered to patients with IBD (103). Moreover, IBD patients exhibited lower prevalence of nausea, vomiting, and diarrhea, but increased rates of constipation (11%) in comparison to the general population (103). Two retrospective studies further corroborated the safety and efficacy of GLP-1 RAs in treatment of obesity in IBD patients. They reported mild gastrointestinal symptoms and no substantial changes in disease activity scores (104, 105). Semaglutide has been observed to induce the most substantial weight loss in IBD patients, with no discrepancies in the attainment of >5% TWL when compared to the general population (104–106). Additionally, semaglutide has been shown to have a comparable risk profile to other GLP-1 RAs with respect to gastrointestinal adverse effects in the IBD population (106). Consequently, some authors consider GLP-1 RAs to be safe in the IBD population, however, they emphasise the necessity of providing educational advice to patients (e.g. small and frequent meals) and employing a gradual dose-up titration strategy (114).
Currently, two ongoing clinical trials are investigating the role of GLP-1 RAs in IBD management, that would lead to further knowledge: a French study (ID NCT05196958) evaluating the safety and efficacy of GLP-1 RAs in treatment of DM2 in overweight IBD patients (115), and an American study (ID NCT06774079) comparing the efficacy of GLP-1 RAs tirzepatide versus diet in CD patients (116).
In summary, the interplay between IBD and metabolism represents a growing area of research and therapeutic interest, with GLP-RAs emerging as a promising therapeutic option. While preclinical and clinical studies have reported anti-metabolic and anti-inflammatory benefits with a favourable safety profile, further evidence from long-term and large-scale trials is necessary to guide clinicians in real-life scenarios.
5 Conclusions
In conclusion, GLP-1 plays a pivotal role in controlling both blood glucose levels and body weight. The interaction between GLP-1, IELs and gut microbiota highlights its vital role in preserving the integrity of the intestinal mucosal barrier and the gut immunity homeostasis. Emerging evidence suggests the potential benefits of GLP-1 RAs in the treatment of IBD through enhanced mucosal healing and reduced inflammation. Furthermore, GLP-1 RAs seem to have similar safety profile in the IBD population to the one observed in the general population, based on real-world observations. However, further research is required to ascertain the long-term outcomes of GLP-1 RAs in IBD patients, with some studies indicating potential benefits and others highlighting concerns regarding altered gut immunity. Further clinical evidence is needed to clarify their role, optimise treatment strategies, and assess their impact on disease progression and patient outcomes.
However, further research is required to ascertain the long-term outcomes of GLP-1 RAs in IBD patients, with some studies indicating potential benefits and others highlighting concerns regarding altered gut immunity. Further clinical research is needed to clarify their role, optimise treatment strategies, and assess their impact on disease progression and patient outcomes.
Author contributions
GM: Writing – review & editing, Writing – original draft. RG: Writing – review & editing. AD: Writing – review & editing. MF: Writing – review & editing. GP: Writing – review & editing. LP: Writing – review & editing. PB: Writing – review & editing. CB: Writing – review & editing. AA: Data curation, Supervision, Methodology, Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Italian Ministry of Health’s “Ricerca Corrente” funding to the IRCCS Humanitas Research Hospital.
Acknowledgments
The publication fee for this work was covered by the Italian Ministry of Health’s “Ricerca Corrente” funding to the IRCCS Humanitas Research Hospital.
Conflict of interest
Author AA has received consulting fees from AbbVie, Allergan, Amgen, Arena, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion, Eli-Lilly, Ferring, Galapagos, Gilead, Janssen, MSD, Mylan, Pfizer, Protagonist Therapeutics, Roche, Samsung Bioepis, Sandoz and Takeda; speaker’s fees from AbbVie, Amgen, Arena, Biogen, Bristol-Myers Squibb, Eli-Lilly, Ferring, Galapagos, Gilead, Janssen, MSD, Novartis, Pfizer, Roche, Samsung Bioepis, Sandoz, Takeda, and Tigenix; and research support from Biogen, MSD, Takeda, and Pfizer. Author CB received lecture fees and served as a consultant for Takeda, MSD, Ferring, Abbvie, Galapagos and Janssen. R. Author RB has received speaker’s fees from Pfizer, MSD, Celltrion and Takeda. Author AB has received speaker’s fees from AbbVie, Galapagos, Celltrion and Pfizer. G. Author GP has received speaker’s fees from Janssen and Alphasigma.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Caron B, Honap S, and Peyrin-Biroulet L. Epidemiology of inflammatory bowel disease across the ages in the era of advanced therapies. J Crohns Colitis. (2024) 18:ii3–15. doi: 10.1093/ecco-jcc/jjae082
2. Bertin L, Crepaldi M, Zanconato M, Lorenzon G, Maniero D, de Barba C, et al. Advancing therapeutic frontiers: a pipeline of novel drugs for luminal and perianal Crohn’s disease management. Therap Adv Gastroenterol. (2024) 17. doi: 10.1177/17562848241303651
3. Moss AC. Approach to treatment failure in inflammatory bowel disease. Gastroenterol Hepatol (N Y). (2022) 18:360–3.
4. Singh S, George J, Boland BS, Vande Casteele N, and Sandborn WJ. Primary non-response to tumor necrosis factor antagonists is associated with inferior response to second-line biologics in patients with inflammatory bowel diseases: A systematic review and meta-analysis. J Crohns Colitis. (2018) 12:635–43. doi: 10.1093/ecco-jcc/jjy004
5. Adolph TE, Meyer M, Schwärzler J, Mayr L, Grabherr F, and Tilg H. The metabolic nature of inflammatory bowel diseases. Nat Rev Gastroenterol Hepatol. (2022) 19:753–67. doi: 10.1038/s41575-022-00658-y
6. Adolph TE, Meyer M, Jukic A, and Tilg H. Heavy arch: from inflammatory bowel diseases to metabolic disorders. Gut. (2024) 73:1376–87. doi: 10.1136/gutjnl-2024-331914
7. Paik J, Fierce Y, Treuting PM, Brabb T, and Maggio-Price L. High-fat diet-induced obesity exacerbates inflammatory bowel disease in genetically susceptible mdr1a male mice. J Nutr. (2013) 143:1240–7. doi: 10.3945/jn.113.174615
8. Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. (2022) 65:1925–66. doi: 10.1007/s00125-022-05787-2
9. Moll H, Frey E, Gerber P, Geidl B, Kaufmann M, Braun J, et al. GLP-1 receptor agonists for weight reduction in people living with obesity but without diabetes: a living benefit–harm modelling study. EClinicalMedicine. (2024) 73:102661. doi: 10.1016/j.eclinm.2024.102661
10. Alharbi SH. Anti-inflammatory role of glucagon-like peptide 1 receptor agonists and its clinical implications. Ther Adv Endocrinol Metab. (2024) 15:20420188231222370. doi: 10.1177/20420188231222367
11. Madsen MSA, Holm JB, Pallejà A, Wismann P, Fabricius K, Rigbolt K, et al. Metabolic and gut microbiome changes following GLP-1 or dual GLP-1/GLP-2 receptor agonist treatment in diet-induced obese mice. Sci Rep. (2019) 9:15582. doi: 10.1038/s41598-019-52103-x
12. Abdalqadir N and Adeli K. GLP-1 and GLP-2 orchestrate intestine integrity, gut microbiota, and immune system crosstalk. Microorganisms. (2022) 10. doi: 10.3390/microorganisms10102061
13. Singh S, Dulai PS, Zarrinpar A, Ramamoorthy S, and Sandborn WJ. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. (2017) 14:110–21. doi: 10.1038/nrgastro.2016.181
14. Sodhi M, Rezaeianzadeh R, Kezouh A, and Etminan M. Risk of gastrointestinal adverse events associated with glucagon-like peptide-1 receptor agonists for weight loss. JAMA. (2023) 330:1795. doi: 10.1001/jama.2023.19574
15. Zheng Z, Zong Y, Ma Y, Tian Y, Pang Y, Zhang C, et al. Glucagon-like peptide-1 receptor: mechanisms and advances in therapy. Signal Transduct Target Ther. (2024) 9:234. doi: 10.1038/s41392-024-01931-z
16. Hickey JW, Becker WR, Nevins SA, Horning A, Perez AE, Zhu C, et al. Organization of the human intestine at single-cell resolution. Nature. (2023) 619:572–84. doi: 10.1038/s41586-023-05915-x
17. Latorre R, Sternini C, De Giorgio R, and Greenwood-Van Meerveld B. Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol motility. (2016) 28:620–30. doi: 10.1111/nmo.12754
18. Spreckley E. The L-cell in nutritional sensing and the regulation of appetite. Front Nutr. (2015) 2. doi: 10.3389/fnut.2015.00023
19. Andersen A, Lund A, Knop FK, and Vilsbøll T. Glucagon-like peptide 1 in health and disease. Nat Rev Endocrinol. (2018) 14:390–403. doi: 10.1038/s41574-018-0016-2
20. Campbell JE and Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. (2013) 17:819–37. doi: 10.1016/j.cmet.2013.04.008
21. Chegeni M, Hayes AMR, Gonzalez TD, Manderfeld MM, Lim J, Menon RS, et al. Activation of gastrointestinal ileal brake response with dietary slowly digestible carbohydrates, with no observed effect on subjective appetite, in an acute randomized, double-blind, crossover trial. Eur J Nutr. (2022) 61:1965–80. doi: 10.1007/s00394-021-02770-2
22. Marathe CS, Rayner CK, Jones KL, and Horowitz M. Effects of GLP-1 and incretin-based therapies on gastrointestinal motor function. Exp Diabetes Res. (2011) 2011:1–10. doi: 10.1155/2011/279530
23. Jalleh RJ, Marathe CS, Rayner CK, Jones KL, Umapathysivam MM, Wu T, et al. Physiology and pharmacology of effects of GLP-1-based therapies on gastric, biliary and intestinal motility. Endocrinology. (2024) 166. doi: 10.1210/endocr/bqae155
24. Halim MA, Degerblad M, Sundbom M, Karlbom U, Holst JJ, Webb DL, et al. Glucagon-like peptide-1 inhibits prandial gastrointestinal motility through myenteric neuronal mechanisms in humans. J Clin Endocrinol Metab. (2018) 103:575–85. doi: 10.1210/jc.2017-02006
25. Jones LA and Brierley DI. GLP-1 and the neurobiology of eating control: recent advances. Endocrinology. (2025) 166. doi: 10.1210/endocr/bqae167
26. Astrup A. Reflections on the discovery GLP-1 as a satiety hormone: Implications for obesity therapy and future directions. Eur J Clin Nutr. (2024) 78:551–6. doi: 10.1038/s41430-024-01460-6
27. Challa TD, Beaton N, Arnold M, Rudofsky G, Langhans W, and Wolfrum C. Regulation of adipocyte formation by GLP-1/GLP-1R signaling. J Biol Chem. (2012) 287:6421–30. doi: 10.1074/jbc.M111.310342
28. Kosiborod MN, Abildstrøm SZ, Borlaug BA, Butler J, Rasmussen S, Davies M, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. New Engl J Med. (2023) 389:1069–84. doi: 10.1056/NEJMoa2306963
29. Skrobucha A, Pindlowski P, Krajewska N, Grabowski M, and Jonik S. Anti-inflammatory effects of glucagon-like peptide-1 (GLP-1) in coronary artery disease: a comprehensive review. Front Cardiovasc Med. (2024) 11. doi: 10.3389/fcvm.2024.1446468
30. Manubolu VS, Lakshmanan S, Kinninger A, Ahmad K, Susarla S, Seok HJ, et al. Effect of semaglutide on epicardial adipose tissue in type 2 diabetes. J Am Coll Cardiol. (2024) 84:865–7. doi: 10.1016/j.jacc.2024.05.065
31. Hullon D, Subeh GK, Volkova Y, Janiec K, Trach A, and Mnevets R. The role of glucagon-like peptide-1 receptor (GLP-1R) agonists in enhancing endothelial function: a potential avenue for improving heart failure with preserved ejection fraction (HFpEF). Cardiovasc Diabetol. (2025) 24:70. doi: 10.1186/s12933-025-02607-w
32. Baggio LL, Yusta B, Mulvihill EE, Cao X, Streutker CJ, Butany J, et al. GLP-1 receptor expression within the human heart. Endocrinology. (2018) 159:1570–84. doi: 10.1210/en.2018-00004
33. Selvarajah V, Robertson D, Hansen L, Jermutus L, Smith K, Coggi A, et al. A randomized phase 2b trial examined the effects of the glucagon-like peptide-1 and glucagon receptor agonist cotadutide on kidney outcomes in patients with diabetic kidney disease. Kidney Int. (2024) 106:1170–80. doi: 10.1016/j.kint.2024.08.023
34. Perkovic V, Tuttle KR, Rossing P, Mahaffey KW, Mann JFE, Bakris G, et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. New Engl J Med. (2024) 391:109–21. doi: 10.1056/NEJMoa2403347
35. Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. (2016) 387:679–90. doi: 10.1016/S0140-6736(15)00803-X
36. Bendotti G, Montefusco L, Lunati ME, Usuelli V, Pastore I, Lazzaroni E, et al. The anti-inflammatory and immunological properties of GLP-1 Receptor Agonists. Pharmacol Res. (2022) 182:106320. doi: 10.1016/j.phrs.2022.106320
37. Yusta B, Baggio LL, Koehler J, Holland D, Cao X, Pinnell LJ, et al. GLP-1R agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte GLP-1R. Diabetes. (2015) 64:2537–49. doi: 10.2337/db14-1577
38. Wong CK, Yusta B, Koehler JA, Baggio LL, McLean BA, Matthews D, et al. Divergent roles for the gut intraepithelial lymphocyte GLP-1R in control of metabolism, microbiota, and T cell-induced inflammation. Cell Metab. (2022) 34:1514–31.e7. doi: 10.1016/j.cmet.2022.08.003
39. Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. (2018) 27:740–56. doi: 10.1016/j.cmet.2018.03.001
40. Kahles F, Meyer C, Möllmann J, Diebold S, Findeisen HM, Lebherz C, et al. GLP-1 secretion is increased by inflammatory stimuli in an IL-6–dependent manner, leading to hyperinsulinemia and blood glucose lowering. Diabetes. (2014) 63:3221–9. doi: 10.2337/db14-0100
41. Yanay O, Bailey AL, Kernan K, Zimmerman JJ, and Osborne WR. Effects of exendin-4, a glucagon like peptide-1 receptor agonist, on neutrophil count and inflammatory cytokines in a rat model of endotoxemia. J Inflammation Res. (2015) 129. doi: 10.2147/JIR.S84993
42. Zhao YY, Chen LH, Huang L, Li YZ, Yang C, Zhu Y, et al. Cardiovascular protective effects of GLP-1: a focus on the MAPK signaling pathway. Biochem Cell Biol. (2022) 100:9–16. doi: 10.1139/bcb-2021-0365
43. Bułdak Ł, Machnik G, Bułdak RJ, Łabuzek K, Bołdys A, Belowski D, et al. Exenatide (a GLP-1 agonist) expresses anti-inflammatory properties in cultured human monocytes/macrophages in a protein kinase A and B/Akt manner. Pharmacol Rep. (2016) 68:329–37. doi: 10.1016/j.pharep.2015.10.008
44. Shiraishi D, Fujiwara Y, Komohara Y, Mizuta H, and Takeya M. Glucagon-like peptide-1 (GLP-1) induces M2 polarization of human macrophages via STAT3 activation. Biochem Biophys Res Commun. (2012) 425:304–8. doi: 10.1016/j.bbrc.2012.07.086
45. Que Q, Guo X, Zhan L, Chen S, Zhang Z, Ni X, et al. The GLP-1 agonist, liraglutide, ameliorates inflammation through the activation of the PKA/CREB pathway in a rat model of knee osteoarthritis. J Inflamm. (2019) 16:13. doi: 10.1186/s12950-019-0218-y
46. Posso-Osorio I, Vargas-Potes CJ, Mejía M, and Cañas CA. Eosinophil-related diseases during treatment with glucagon-like peptide one receptor (GLP-1 RA): a case report and review of the literature. Clin Rheumatol. (2023) 42:2501–6. doi: 10.1007/s10067-023-06612-w
47. Mitchell PD, Salter BM, Oliveria JP, El-Gammal A, Tworek D, Smith SG, et al. Glucagon-like peptide-1 receptor expression on human eosinophils and its regulation of eosinophil activation. Clin Exp Allergy. (2017) 47:331–8. doi: 10.1111/cea.12860
48. Dokken BB, La Bonte LR, Davis-Gorman G, Teachey MK, Seaver N, and McDonagh PF. Glucagon-like peptide-1 (GLP-1), immediately prior to reperfusion, decreases neutrophil activation and reduces myocardial infarct size in rodents. Hormone Metab Res. (2011) 43:300–5. doi: 10.1055/s-0031-1271777
49. Ben Nasr M, Usuelli V, Dellepiane S, Seelam AJ, Fiorentino TV, D’Addio F, et al. Glucagon-like peptide 1 receptor is a T cell-negative costimulatory molecule. Cell Metab. (2024) 36:1302–19.e12. doi: 10.1016/j.cmet.2024.05.001
50. Watada H. One step closer to solving the mystery of the anti-inflammatory effects of glucagon-like peptide-1 receptor agonists. J Diabetes Investig. (2025) 16:180–2. doi: 10.1111/jdi.14346
51. Petković-Dabić J, Binić I, Carić B, Božić L, Umičević-Šipka S, Bednarčuk N, et al. Effects of semaglutide treatment on psoriatic lesions in obese patients with type 2 diabetes mellitus: an open-label, randomized clinical trial. Biomolecules. (2025) 15:46. doi: 10.3390/biom15010046
52. Krajewski PK, Złotowska A, and Szepietowski JC. The therapeutic potential of GLP-1 receptor agonists in the management of hidradenitis suppurativa: A systematic review of anti-inflammatory and metabolic effects. J Clin Med. (2024) 13:6292. doi: 10.3390/jcm13216292
53. Hogan AE, Gaoatswe G, Lynch L, Corrigan MA, Woods C, O’Connell J, et al. Glucagon-like peptide 1 analogue therapy directly modulates innate immune-mediated inflammation in individuals with type 2 diabetes mellitus. Diabetologia. (2014) 57:781–4. doi: 10.1007/s00125-013-3145-0
54. Hogan AE, Tobin AM, Ahern T, Corrigan MA, Gaoatswe G, Jackson R, et al. Glucagon-like peptide-1 (GLP-1) and the regulation of human invariant natural killer T cells: lessons from obesity, diabetes and psoriasis. Diabetologia. (2011) 54:2745–54. doi: 10.1007/s00125-011-2232-3
55. Pang J, Feng JN, Ling W, and Jin T. The anti-inflammatory feature of glucagon-like peptide-1 and its based diabetes drugs—Therapeutic potential exploration in lung injury. Acta Pharm Sin B. (2022) 12:4040–55. doi: 10.1016/j.apsb.2022.06.003
56. Chiou HYC, Lin MW, Hsiao PJ, Chen CL, Chiao S, Lin TY, et al. Dulaglutide modulates the development of tissue-infiltrating Th1/Th17 cells and the pathogenicity of encephalitogenic Th1 cells in the central nervous system. Int J Mol Sci. (2019) 20:1584. doi: 10.3390/ijms20071584
57. Neurath MF, Artis D, and Becker C. The intestinal barrier: a pivotal role in health, inflammation, and cancer. Lancet Gastroenterol Hepatol. (2025). doi: 10.1016/S2468-1253(24)00390-X
58. He S, Kahles F, Rattik S, Nairz M, McAlpine CS, Anzai A, et al. Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease. Nature. (2019) 566:115–9. doi: 10.1038/s41586-018-0849-9
59. Rosario W and D’Alessio D. An innate disposition for a healthier gut: GLP-1R signaling in intestinal epithelial lymphocytes. Diabetes. (2015) 64:2329–31. doi: 10.2337/db15-0436
60. Tsai S, Winer S, and Winer DA. Gut T cells feast on GLP-1 to modulate cardiometabolic disease. Cell Metab. (2019) 29:787–9. doi: 10.1016/j.cmet.2019.03.002
61. Grunddal KV, Jensen EP, Ørskov C, Andersen DB, Windeløv JA, Poulsen SS, et al. Expression profile of the GLP-1 receptor in the gastrointestinal tract and pancreas in adult female mice. Endocrinology. (2021) 163(1). doi: 10.1210/endocr/bqab216
62. Barra NG, Anhê FF, and Schertzer JD. Immunometabolism sentinels: gut surface T-cells regulate GLP-1 availability. Endocrinology. (2019) 160:1177–8. doi: 10.1210/en.2019-00215
63. Simonsen L, Pilgaard S, Orskov C, Rosenkilde MM, Hartmann B, Holst JJ, et al. Exendin-4, but not dipeptidyl peptidase IV inhibition, increases small intestinal mass in GK rats. Am J Physiology-Gastrointestinal Liver Physiol. (2007) 293:G288–95. doi: 10.1152/ajpgi.00453.2006
64. Koehler JA, Baggio LL, Yusta B, Longuet C, Rowland KJ, Cao X, et al. GLP-1R agonists promote normal and neoplastic intestinal growth through mechanisms requiring Fgf7. Cell Metab. (2015) 21:379–91. doi: 10.1016/j.cmet.2015.02.005
65. Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein–coupled receptor FFAR2. Diabetes. (2012) 61:364–71. doi: 10.2337/db11-1019
66. Zeng Y, Wu Y, Zhang Q, and Xiao X. Crosstalk between glucagon-like peptide 1 and gut microbiota in metabolic diseases. mBio. (2024) 15:e0203223. doi: 10.1128/mbio.02032-23
67. Chimerel C, Emery E, Summers DK, Keyser U, Gribble FM, and Reimann F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. (2014) 9:1202–8. doi: 10.1016/j.celrep.2014.10.032
68. Grasset E, Puel A, Charpentier J, Collet X, Christensen JE, Tercé F, et al. A specific gut microbiota dysbiosis of type 2 diabetic mice induces GLP-1 resistance through an enteric NO-dependent and gut-brain axis mechanism. Cell Metab. (2017) 25:1075–90.e5. doi: 10.1016/j.cmet.2017.04.013
69. Moreira G, Azevedo F, Ribeiro L, Santos A, Guadagnini D, Gama P, et al. Liraglutide modulates gut microbiota and reduces NAFLD in obese mice. J Nutr Biochem. (2018) 62:143–54. doi: 10.1016/j.jnutbio.2018.07.009
70. Zhao L, Chen Y, Xia F, Abudukerimu B, Zhang W, Guo Y, et al. A glucagon-like peptide-1 receptor agonist lowers weight by modulating the structure of gut microbiota. Front Endocrinol (Lausanne). (2018) 9. doi: 10.3389/fendo.2018.00233
71. Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci. (2013) 110:9066–71. doi: 10.1073/pnas.1219451110
72. Wang L, Li P, Tang Z, Yan X, and Feng B. Structural modulation of the gut microbiota and the relationship with body weight: compared evaluation of liraglutide and saxagliptin treatment. Sci Rep. (2016) 6:33251. doi: 10.1038/srep33251
73. Ying X, Rongjiong Z, Kahaer M, Chunhui J, and Wulasihan M. Therapeutic efficacy of liraglutide versus metformin in modulating the gut microbiota for treating type 2 diabetes mellitus complicated with nonalcoholic fatty liver disease. Front Microbiol. (2023) 14. doi: 10.3389/fmicb.2023.1088187
74. Liang L, Su X, Guan Y, Wu B, Zhang X, and Nian X. Correlation between intestinal flora and GLP-1 receptor agonist dulaglutide in type 2 diabetes mellitus treatment—A preliminary longitudinal study. iScience. (2024) 27:109784. doi: 10.1016/j.isci.2024.109784
75. Massironi S, Viganò C, Palermo A, Pirola L, Mulinacci G, Allocca M, et al. Inflammation and malnutrition in inflammatory bowel disease. Lancet Gastroenterol Hepatol. (2023) 8:579–90. doi: 10.1016/S2468-1253(23)00011-0
76. Yang L, Liu G, Zhang Y, Yao B, Wu Q, Peng L, et al. Quantitative analysis of adipose tissue for predicting Crohn’s disease postoperative endoscopic recurrence and anastomotic ulcer. Int J Colorectal Dis. (2023) 38:170. doi: 10.1007/s00384-023-04456-z
77. Waluga M. Serum adipokines in inflammatory bowel disease. World J Gastroenterol. (2014) 20:6912. doi: 10.3748/wjg.v20.i22.6912
78. Valentini L, Wirth EK, Schweizer U, Hengstermann S, Schaper L, Koernicke T, et al. Circulating adipokines and the protective effects of hyperinsulinemia in inflammatory bowel disease. Nutrition. (2009) 25:172–81. doi: 10.1016/j.nut.2008.07.020
79. Dagli N, Poyrazoglu OK, Ferda Dagli A, Sahbaz F, Karaca I, Ali Kobat M, et al. Is inflammatory bowel disease a risk factor for early atherosclerosis? Angiology. (2010) 61:198–204. doi: 10.1177/0003319709333869
80. Rodrigues VS, Milanski M, Fagundes JJ, Torsoni AS, Ayrizono MLS, Nunez CEC, et al. Serum levels and mesenteric fat tissue expression of adiponectin and leptin in patients with Crohn’s disease. Clin Exp Immunol. (2012) 170:358–64. doi: 10.1111/j.1365-2249.2012.04660.x
81. Carrillo-Palau M, Hernández-Camba A, Hernández Alvarez-Buylla N, Ramos L, Alonso-Abreu I, Hernández-Pérez A, et al. Insulin resistance is not increased in inflammatory bowel disease patients but is related to non-alcoholic fatty liver disease. J Clin Med. (2021) 10:3062. doi: 10.3390/jcm10143062
82. Yin J, Hou P, Wu Z, and Nie Y. Decreased levels of serum omentin-1 in patients with inflammatory bowel disease. Med Sci Monit. (2015) 21:118–22. doi: 10.12659/MSM.892081
83. Cañete F, Vela E, Calafat M, Piera J, Mañosa M, and Domènech E. Severe obesity, a susceptibility factor for developing inflammatory bowel disease: results of a population-based study. J Crohns Colitis. (2025) 19. doi: 10.1093/ecco-jcc/jjaf010
84. Chan SSM, Chen Y, Casey K, Olen O, Ludvigsson JF, Carbonnel F, et al. Obesity is Associated With Increased Risk of Crohn’s disease, but not Ulcerative Colitis: A Pooled Analysis of Five Prospective Cohort Studies. Clin Gastroenterol Hepatol. (2022) 20:1048–58. doi: 10.1016/j.cgh.2021.06.049
85. Desai A, Sehgal P, Khataniar H, Lewis JD, Farraye FA, Lichtenstein GR, et al. Obesity is associated with worsened outcomes in patients with ulcerative colitis on advanced therapies: A propensity matched cohort study from the U.S. Aliment Pharmacol Ther. (2025) 61:1197–207. doi: 10.1111/apt.18513
86. Youn J, Hsia K, Khadilkar S, Zeina T, Rai P, Rastogi A, et al. The impact of obesity on the prevalence and complications of perianal fistulas of Crohn’s disease. Dig Dis Sci. (2025) 70:323–32. doi: 10.1007/s10620-024-08729-7
87. Flores A, Burstein E, Cipher DJ, and Feagins LA. Obesity in inflammatory bowel disease: A marker of less severe disease. Dig Dis Sci. (2015) 60:2436–45. doi: 10.1007/s10620-015-3629-5
88. Stabroth-Akil D, Leifeld L, Pfützer R, Morgenstern J, and Kruis W. The effect of body weight on the severity and clinical course of ulcerative colitis. Int J Colorectal Dis. (2015) 30:237–42. doi: 10.1007/s00384-014-2051-3
89. Singh S, Khera R, and Sandborn WJ. Obesity is associated with worse outcomes in hospitalized patients with inflammatory bowel diseases: A nationwide analysis. Am J Gastroenterol. (2016) 111:S271. doi: 10.14309/00000434-201610001-00591
90. Sehgal P, Su S, Zech J, Nobel Y, Luk L, Economou I, et al. Visceral adiposity independently predicts time to flare in inflammatory bowel disease but body mass index does not. Inflammation Bowel Dis. (2024) 30:594–601. doi: 10.1093/ibd/izad111
91. Zietek T and Rath E. Inflammation meets metabolic disease: gut feeling mediated by GLP-1. Front Immunol. (2016) 7. doi: 10.3389/fimmu.2016.00154
92. Friedrich M, Diegelmann J, Schauber J, Auernhammer CJ, and Brand S. Intestinal neuroendocrine cells and goblet cells are mediators of IL-17A-amplified epithelial IL-17C production in human inflammatory bowel disease. Mucosal Immunol. (2015) 8:943–58. doi: 10.1038/mi.2014.124
93. Sun H, Shu J, Tang J, Li Y, Qiu J, Ding Z, et al. GLP-1 receptor agonists alleviate colonic inflammation by modulating intestinal microbiota and the function of group 3 innate lymphoid cells. Immunology. (2024) 172:451–68. doi: 10.1111/imm.13784
94. Bang-Berthelsen CH, Holm TL, Pyke C, Simonsen L, Søkilde R, Pociot F, et al. GLP-1 induces barrier protective expression in Brunner's glands and regulates colonic inflammation. Inflammation Bowel Dis. (2016) 22:2078–97. doi: 10.1097/MIB.0000000000000847
95. Keller J, Binnewies U, Rösch M, Juul Holst J, Beglinger C, Andresen V, et al. Gastric emptying and disease activity in inflammatory bowel disease. Eur J Clin Invest. (2015) 45:1234–42. doi: 10.1111/eci.12542
96. Jeffrey L. A novel use of liraglutide: induction of partial remission in ulcerative colitis and ankylosing spondylitis. Clin Med Rev Case Rep. (2019) 6. doi: 10.23937/2378-3656/1410281
97. Desai A, Petrov J, Hashash JG, Patel H, Brahmbhatt B, Kochhar GS, et al. Use of glucagon-like peptide-1 receptor agonists for type 2 diabetes mellitus and outcomes of inflammatory bowel disease. Aliment Pharmacol Ther. (2024) 60:620–32. doi: 10.1111/apt.18138
98. Villumsen M, Schelde AB, Jimenez-Solem E, Jess T, and Allin KH. GLP-1 based therapies and disease course of inflammatory bowel disease. EClinicalMedicine. (2021) 37:100979. doi: 10.1016/j.eclinm.2021.100979
99. Gorelik Y, Ghersin I, Lujan R, Shlon D, Loewenberg Weisband Y, Ben-Tov A, et al. GLP-1 analog use is associated with improved disease course in inflammatory bowel disease: a report from the Epi-IIRN. J Crohns Colitis. (2024) 19(4). doi: 10.1093/ecco-jcc/jjae160
100. Sehgal P, Lichtenstein GR, Khanna T, Profka K, Pickett-Blakely O, Nandi N, et al. Sa1950 impact of GLP1 agonists on inflammatory biomarkers in patients with inflammatory bowel disease. Gastroenterology. (2024) 166:S–591. doi: 10.1016/S0016-5085(24)01827-4
101. Levine I, Sekhri S, Schreiber-Stainthorp W, Locke B, Delau O, Elhawary M, et al. GLP-1 receptor agonists confer no increased rates of IBD exacerbation among patients with IBD. Inflammation Bowel Dis. (2025) 31:467–75. doi: 10.1093/ibd/izae250
102. St-Pierre J, Klein J, Choi NK, Fear E, Pannain S, and Rubin DT. Efficacy and safety of GLP-1 agonists on metabolic parameters in non-diabetic patients with inflammatory bowel disease. Dig Dis Sci. (2024) 69:4437–45. doi: 10.1007/s10620-024-08720-2
103. Clarke L, Passam RT, Falahee B, Jirapinyo P, Allegretti JR, and Kelly CR. Tolerability and effectiveness of glucagon-like peptide-1 receptor agonists in patients with inflammatory bowel disease. Dig Dis Sci. (2025). doi: 10.1007/s10620-025-08964-6
104. Anderson SR, Ayoub M, Coats S, McHenry S, Tan T, and Deepak P. Safety and effectiveness of glucagon-like peptide-1 receptor agonists in inflammatory bowel disease. Am J Gastroenterol. (2024) 120(5):1152–5. doi: 10.14309/ajg.0000000000003208
105. Ramos Belinchón C, Martínez-Lozano H, Serrano Moreno C, Hernández Castillo D, Lois Chicharro P, Ferreira Ocampo P, et al. Effectiveness and safety of GLP-1 agonist in obese patients with inflammatory bowel disease. Rev Española Enfermedades Digestivas. (2024) 116(9):478–83. doi: 10.17235/reed.2024.10305/2024
106. Desai A, Khataniar H, Hashash JG, Farraye FA, Regueiro M, and Kochhar GS. Effectiveness and safety of semaglutide for weight loss in patients with inflammatory bowel disease and obesity. Inflammation Bowel Dis. (2025) 31:696–705. doi: 10.1093/ibd/izae090
107. Liu L, Chen J, Wang L, Chen C, and Chen L. Association between different GLP-1 receptor agonists and gastrointestinal adverse reactions: A real-world disproportionality study based on FDA adverse event reporting system database. Front Endocrinol (Lausanne). (2022) 13. doi: 10.3389/fendo.2022.1043789
108. Wharton S, Davies M, Dicker D, Lingvay I, Mosenzon O, Rubino DM, et al. Managing the gastrointestinal side effects of GLP-1 receptor agonists in obesity: recommendations for clinical practice. Postgrad Med. (2022) 134:14–9. doi: 10.1080/00325481.2021.2002616
109. Lincoff AM, Brown-Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. New Engl J Med. (2023) 389:2221–32. doi: 10.1056/NEJMoa2307563
110. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. New Engl J Med. (2016) 375:311–22. doi: 10.1056/NEJMoa1603827
111. Robinson S, Boye KS, Mody R, Strizek AA, Konig M, Malik RE, et al. Real-world effectiveness of dulaglutide in patients with type 2 diabetes mellitus: A literature review. Diabetes Ther. (2020) 11:1437–66. doi: 10.1007/s13300-020-00839-5
112. Weiss T, Carr RD, Pal S, Yang L, Sawhney B, Boggs R, et al. Real-world adherence and discontinuation of glucagon-like peptide-1 receptor agonists therapy in type 2 diabetes mellitus patients in the United States. Patient Prefer Adherence. (2020) 14:2337–45. doi: 10.2147/PPA.S277676
113. Rodriguez PJ, Zhang V, Gratzl S, Do D, Goodwin Cartwright B, Baker C, et al. Discontinuation and reinitiation of dual-labeled GLP-1 receptor agonists among US adults with overweight or obesity. JAMA Netw Open. (2025) 8:e2457349. doi: 10.1001/jamanetworkopen.2024.57349
114. Arvanitakis K, Koufakis T, Popovic D, Maltese G, Mustafa O, Doumas M, et al. GLP-1 receptor agonists in obese patients with inflammatory bowel disease: from molecular mechanisms to clinical considerations and practical recommendations for safe and effective use. Curr Obes Rep. (2023) 12:61–74. doi: 10.1007/s13679-023-00506-3
115. Study details | Interest of GLP1 analogues in overweight type 2 diabetic patients with chronic inflammatory bowel disease | ClinicalTrials.gov. Available online at: https://clinicaltrials.gov/study/NCT05196958?cond=IBD&intr=GLP1&rank=1 (Accessed April 28, 2025).
116. Study details | Glucagon-like peptide-1 receptor agonist (GLP-1 RA) and diet in inflammatory bowel disease (IBD) patients | ClinicalTrials.gov. Available online at: https://clinicaltrials.gov/study/NCT06774079?cond=IBD&intr=GLP1&rank=4 (Accessed April 28, 2025).
Keywords: IBD, GLP-1, GLP-1 receptor agonists, diabetes, obesity
Citation: Migliorisi G, Gabbiadini R, Dal Buono A, Ferraris M, Privitera G, Petronio L, Bertoli P, Bezzio C and Armuzzi A (2025) GLP-1 receptor agonists in IBD: exploring the crossroads of metabolism and inflammation. Front. Immunol. 16:1610368. doi: 10.3389/fimmu.2025.1610368
Received: 11 April 2025; Accepted: 26 June 2025;
Published: 15 July 2025.
Edited by:
Li-Tung Huang, Kaohsiung Chang Gung Memorial Hospital, TaiwanReviewed by:
Beata Kasztelan-Szczerbinska, Medical University of Lublin, PolandEdit Posta, University of Debrecen, Hungary
Copyright © 2025 Migliorisi, Gabbiadini, Dal Buono, Ferraris, Privitera, Petronio, Bertoli, Bezzio and Armuzzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandro Armuzzi, QWxlc3NhbmRyby5hcm11enppQGh1bmltZWQuZXU=
 Giulia Migliorisi
Giulia Migliorisi Roberto Gabbiadini
Roberto Gabbiadini Arianna Dal Buono
Arianna Dal Buono Matteo Ferraris1,2
Matteo Ferraris1,2 Lorenzo Petronio
Lorenzo Petronio