- 1College of Basic Medical Science, Zhejiang Chinese Medical University, Hangzhou, China
- 2the First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, China
- 3Zhejiang Provincial Hospital of Chinese Medicine, Hangzhou, China
Objectives: This meta-analysis evaluated the direction and strength of associations between air pollution, emerging chemical pollutants, and systemic lupus erythematosus (SLE) incidence, clarifying distinct relationships by pollutant type.
Method: By utilizing medical subject headings and keywords from the PubMed and EMBASE databases, a thorough search was conducted for published observational studies linking air pollution and SLE from inception until August 2024. The Newcastle-Ottawa Scale (NOS) was utilized to evaluate the quality of the studies. Statistical analyses were performed using STATA software (version 14.0), with the assessment of publication bias conducted through funnel plots and Egger’s test.
Result: This meta-analysis encompassed 8 studies published between 2018 and 2024, involving a total of 1,390,348 individuals. We assessed exposure to standard air pollutants and emerging chemical pollutants, specifically including perfluoroalkyl and polyfluoroalkyl substances (PFASs, a type of persistent chemical widely used in nonstick cookware and waterproof products) and bisphenol compounds (BPs, a synthetic chemical primarily used in plastic products and resins). These eight studies identified significant positive associations between SLE incidence and exposure to PM2.5 [OR = 1.16, 95% CI (1.02-1.32), I2 = 62.4%, p=0.031], NO2 [OR = 1.24, 95% CI (1.11-1.38), I² = 0.0%, P = 0.603], and PFASs [OR = 2.47, 95% CI (1.54-2.57)], while O3 exhibited a negative association [OR = 0.83, 95% CI (0.70-0.98), I² = 19.3%, P = 0.290]. No significant links were found for PM10 [OR = 1.11, 95% CI (0.90–1.36), I² = 66.3%, P = 0.031], SO2 [OR = 0.99, 95% CI (0.66-1.48), I² = 79.0%, P = 0.001], and BPs [OR = 1.26, 95% CI (0.80-1.99)]. Sensitivity analyses supported robustness without evidence of publication bias.
Conclusion: The results of this meta-analysis suggest that air pollutants PM2.5 and NO2 may be potential environmental risk factors for SLE, while the negative correlation with O3 requires further research to validate its potential mechanisms. It is worth noting that although a study on PFASs showed a strong association with SLE, this finding requires further evidence due to the limited number of relevant studies currently available. These findings imply that improving air quality and strengthening regulation of emerging pollutants may reduce the disease burden of SLE. Based on the current strength of evidence, public health policies should prioritize reducing population exposure levels to PM2.5 and NO2, which may help reduce the potential risk of SLE onset. Concurrently, larger-scale studies should be conducted to confirm the association between other environmental pollutants such as PFASs and SLE, providing more comprehensive scientific evidence for the development of targeted environmental health policies.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, PROSPERO (CRD42024581931).
1 Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease. It features autoantibodies, immune complexes, and activated autoreactive B and T lymphocytes (1). Globally, SLE is estimated to have an incidence of 5.14 cases per 100,000 person-years, with approximately 0.40 million new cases diagnosed annually (2). SLE can lead to multi-organ damage, presenting symptoms ranging from skin lesions and joint issues to severe fatigue, cognitive impairment, renal disease, and thrombosis (3). SLE often causes diverse joint problems, affecting up to 95% of patients (4). These chronic joint issues in SLE patients reduce quality of life, impact functional performance, and are among the most disabling aspects of SLE (5). These issues significantly reduce quality of life and functional capacity, with 12% developing permanent joint damage and 19%-40% experiencing work disability within 5 years of onset (6). The complex etiology of SLE involves genetic, infectious, lifestyle, and environmental factors, with environmental pollution being a significant risk factor impacting disease onset and progression (7, 8).
Global industrialization has made air pollution a major health concern (9). Airborne pollutants such as particulate matter, sulfur dioxide, and nitrogen oxides—primarily affect respiratory health and induce oxidative stress and inflammatory responses (10). Current evidence on air pollution and SLE reveals significant variations by pollutant type. Particulate matter (PM2.5 and PM10) demonstrates more consistent associations with SLE outcomes—ranging from disease activity (11) to serological autoantibody levels (12). In contrast, gaseous pollutants (NO2, SO2, O3) exhibit weaker or null associations in multiple studies (13, 14), and the findings from these studies are less conclusive. In addition to air pollutants, increasing evidence suggests that other environmental chemicals may also be involved in the pathogenesis of SLE. Bisphenol compounds (e.g., BPA, BPS), widely used in plastics and consumer products, may disrupt immune homeostasis through estrogen-like activity and epigenetic regulation (15, 16). Similarly, perfluoroalkyl and polyfluoroalkyl substances (PFASs)—persistent chemicals found in waterproof coatings and drinking water—have been linked to immune dysfunction and autoantibody production (17). Although epidemiological data on these chemicals remain limited, their widespread environmental presence and biological persistence warrant further investigation alongside traditional air pollutants.
While previous studies have mainly concentrated on occupational hazards, smoking, and alcohol consumption as non-shared factors, recent research indicates that environmental factors contribute significantly to the phenotypic variance observed in SLE. Shared environmental factors account for 25.8% of this variance, and non - shared ones for 30.3% (18), highlighting the need to explore non-occupational exposures like air pollution (19).
While previous meta-analyses have explored the association between air pollution and autoimmune diseases, there have been few studies specifically targeting SLE for a comprehensive assessment covering multiple pollutants, including emerging chemical exposures. Existing studies, such as the work by Rezayat et al. (20), have primarily emphasized the association between PM2.5 and SLE disease activity, but have lacked systematic analysis of other key pollutants, such as NO2, PFASs, and BPs. This study conducted the first meta-epidemiological analysis to quantify the direction and strength of associations between multiple air pollutants and SLE incidence, as well as to explore differential effects across population subgroups and study designs. By integrating updated epidemiological data and expanding the scope of the study to include emerging chemicals, our findings help clarify the potential associations between SLE and environmental factors, which may inform public health strategy and promote further research into potential mechanisms.
2 Methods
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The protocol was pre-registered in the International Register of Prospective Systematic Reviews (PROSPERO) under approval number CRD42024581931.
2.1 Data sources
We systematically searched the PubMed and EMBASE databases using Medical Subject Headings (MeSH for PubMed and Emtree for EMBASE) and relevant keywords with the following strategy before August 14, 2024. Search terms included “Systemic lupus erythematosus,” “Air Pollutants,” and “Environmental Exposure.” The complete search strategy is detailed in Supplementary Material Tables 1, 2.
2.2 Eligibility criteria
Studies were included based on the following criteria: (1) prospective or retrospective cohort designs or case-control studies; (2) investigations of the association between SLE and air pollution; (3) primary outcomes relating air pollution or environmental exposure to SLE risk, reported as hazard ratios (HR), odds ratios (OR), or relative risks (RR) during follow-up; (4) publications in English.
Exclusion criteria included: (1) duplicate studies; (2) reviews, conference abstracts, comments, or letters; (3) incomplete data or lack of outcomes of interest; (3)Time series study evaluating air pollution and the risk of hospitalization for SLE.
2.3 Study selection
Study selection was performed by two reviewers (YL Xu and HJ Pan), who independently screened literature according to the eligibility and exclusion criteria. If results were consistent, the final analysis proceeded; discrepancies were resolved by consulting full texts and discussing within the group.
2.4 Data extraction
Data extraction was conducted independently by the two reviewers (YL Xu and HJ Pan) using Microsoft Excel to create a data extraction table. This table captured information such as the first author, publication year, study type, sample size, follow-up duration, age, SLE diagnosis, and adjusted confounders. Extracted data were cross-checked, and discrepancies were resolved through group discussions.
2.5 Quality assessment
The Newcastle-Ottawa Scale (NOS) was employed to assess the quality of included studies. The NOS ranges from 0 to 9 points, with higher scores indicating better quality. It evaluates participant selection (4 points), comparability between groups (2 points), and exposure measurement (3 points). NOS scores of ≥7, 4–6, and 0–3 are classified as high, medium, and low quality, respectively.
2.6 Statistical analysis
The adjusted OR and 95% CI from each study were utilized to assess the association between air pollution, emerging chemical pollutants and SLE. We selected random or fixed effects models based on heterogeneity test results. Heterogeneity was considered high if P < 0.1 or I² > 50%. Due to the inherent heterogeneity in meta-analyses—clinical, methodological, and statistical—we employed a random effects model. To explore the sources of heterogeneity, we conducted a priori subgroup analyses based on predefined variables, including study design (cohort vs. case-control), age (adult vs. non-adult), population characteristics (Asian vs. non-Asian populations), and diagnostic criteria. These variables were selected based on clinical plausibility and prior evidence of their potential impact on the risk of SLE. For each subgroup, pooled risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using the random-effects model. Heterogeneity within each subgroup was evaluated by the I2 statistic, and differences between subgroups were assessed by comparing the magnitudes of effect sizes and the overlap of confidence intervals. Sensitivity analyses were conducted to evaluate the robustness of results, and a case-by-case elimination method was used to explore sources of heterogeneity. Subgroup analyses were performed based on air pollution type, age, and study type. Publication bias was assessed using funnel plots and Egger’s regression tests.
3 Results
3.1 Literature search
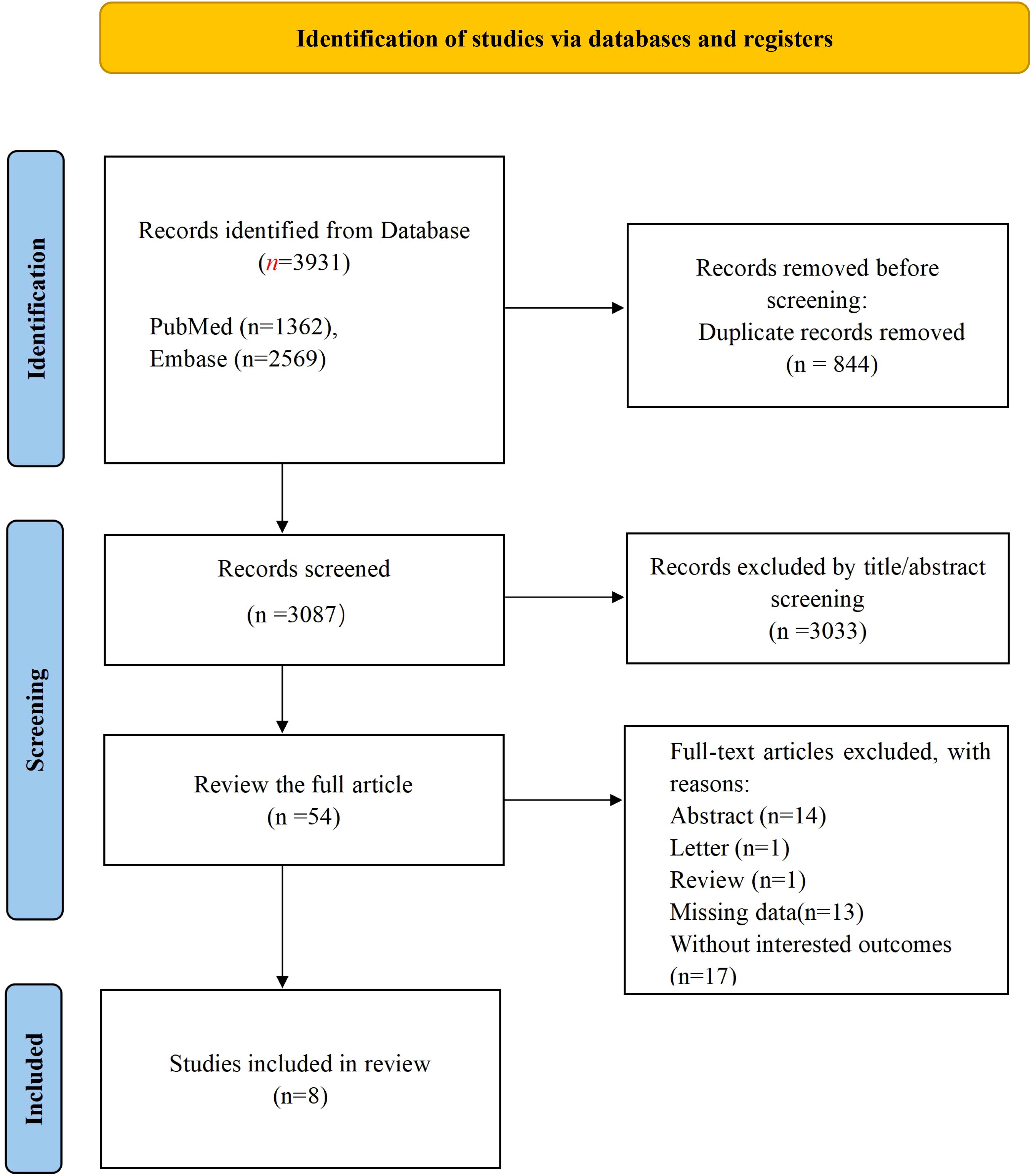
The initial literature search identified 3,931 pertinent documents, comprising 1,362 articles from PubMed and 2,569 from EMBASE. These records were imported into Note Express reference management software. Upon screening titles and abstracts, irrelevant records were excluded, resulting in 54 articles for full-text evaluation. Ultimately, 8 studies met the eligibility criteria (21–28) and were included in the analysis, as depicted in Figure 1.
3.2 Basic characteristics
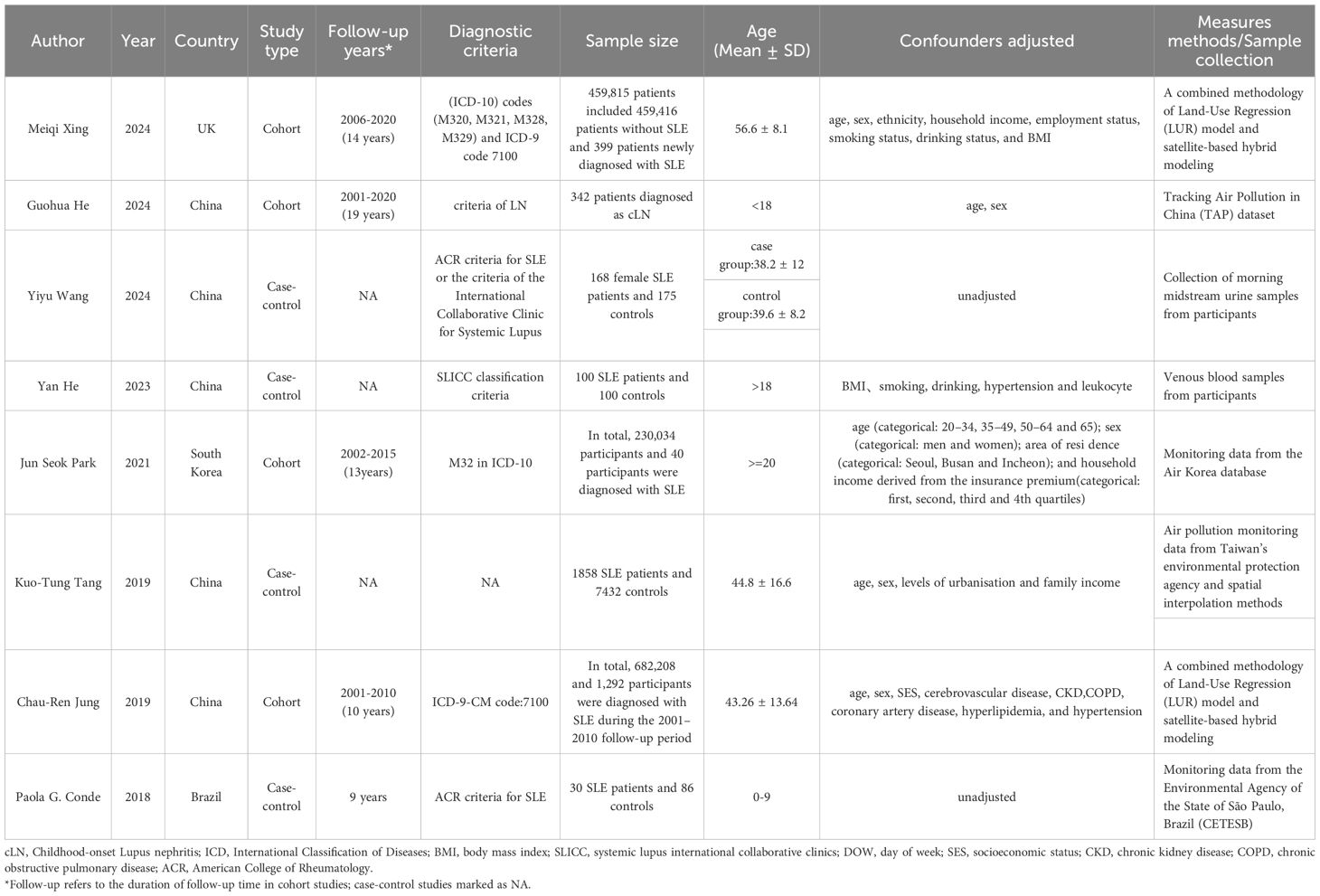
This meta-analysis encompasses 8 studies involving a total of 1,390,348 individuals, published between 2018 and 2024. Among these, four studies were retrospective cohort studies and four were case-control studies. The primary characteristics of the included subjects are outlined in Table 1. All eight included studies (21–28) reported associations between air pollution exposure and SLE. The diagnostic criteria for SLE varied across studies, with most using established classification systems (ACR or ICD criteria), while one study (26) did not explicitly report the diagnostic criteria used.
3.3 Quality assessment
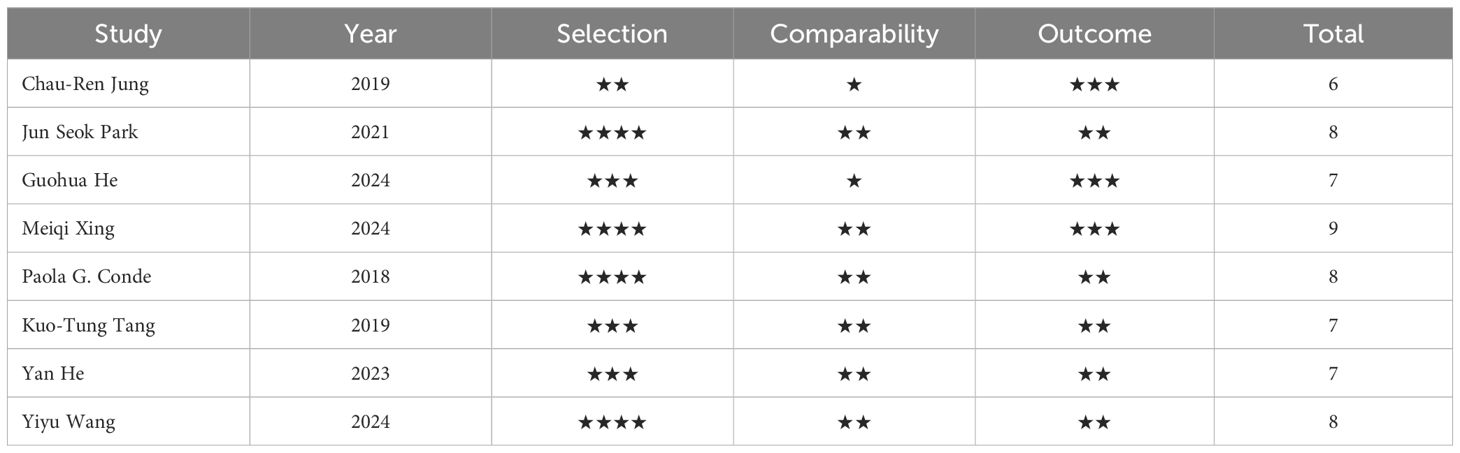
The average quality score across the studies was ≥ 6 points, comprising one studies scoring 6 points, three studies scoring 7 points, three studies scoring 8 points, and one study scoring 9 points. This distribution underscores the high quality of research incorporated in this meta-analysis. The NOS scoring scale is detailed in Table 2.
3.4 Meta-analysis
3.4.1 Air pollution and the association with SLE
This meta-analysis included eight studies (21–28) investigating the association between air pollution exposure and SLE, including case-control studies and cohort studies. Five studies analyzed the association between PM2.5 and SLE [OR = 1.16, 95% CI (1.02-1.32), I2 = 62.4%, p=0.031, Figure 2], indicating a significant positive correlation. Four studies examined the effects of inhalable PM10 [OR = 1.11, 95% CI (0.90-1.36), I2 = 66.3%, p=0.031], showing a positive correlation trend but not reaching statistical significance. Three studies assessed the exposure effects of NO2 [OR = 1.24, 95% CI (1.11-1.38), I2 = 0.0%, p=0.603], indicating a significant positive correlation. Exposure to SO2 was not significantly associated with SLE [OR = 0.77, 95% CI (0.59-1.00), I2 = 19.0%, p=0.291]. Three studies assessed the effects of O3 [OR = 0.83, 95% CI (0.70-0.98), I2 = 19.3%, p=0.290], both showing a negative correlation but only O3 reaching statistical significance. One study examined the exposure effects of PFASs [OR = 2.47, 95% CI (1.54-2.57)]. One study analyzed the effects of BPs [OR = 1.26, 95%CI (0.80-1.99)]. Sensitivity analyses confirmed that none of the studies altered the overall effect, indicating the reliability of these findings regarding air pollution’s impact on SLE (Supplementary Figure A).
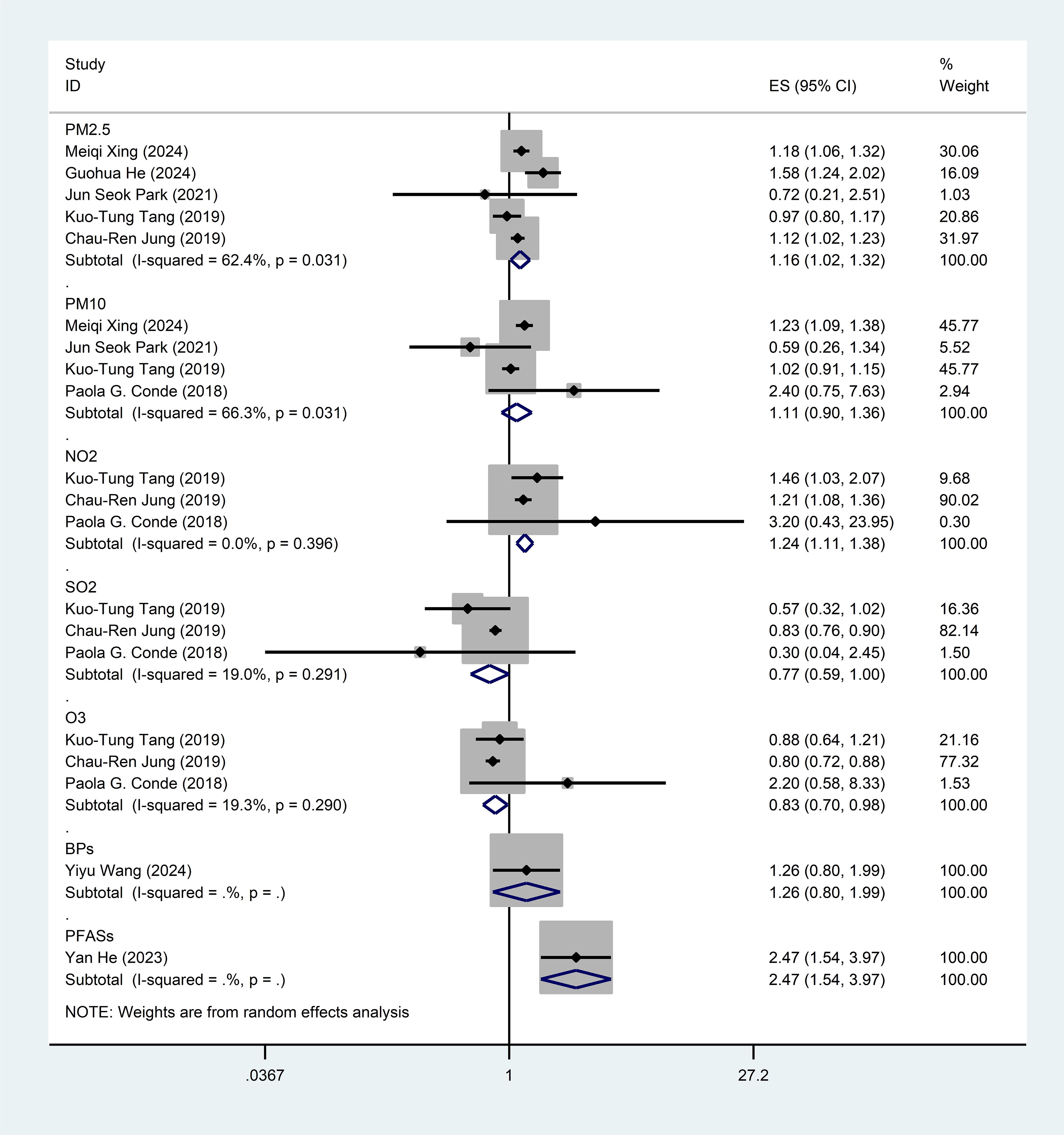
Figure 2. Forest plot for the associations between air pollution, emerging chemical pollutants and SLE. PM2.5, Particulate Matter 2.5; PM10, Particulate Matter 10; NO2, nitrogen dioxide; SO2, sulfur dioxide; O3, ozone; BPs, bisphenol analogue such as bisphenol A(BPA), bisphenol F(BPF), and bisphenol S(BPS); PFASs, Perfluoroalkyl and polyfluoroalkyl substances.
3.4.2 Subgroup analysis
Based on pre-specified clinically relevant variables (age, study design, ethnicity, and diagnostic criteria), we conducted subgroup analyses to systematically assess potential effect modification of the association between air pollution and SLE (Table 3). Six studies (23–28) in adult populations showed a positive but non-statistically significant association [OR = 1.10, 95% CI (0.91–1.33), I2 = 87.4%, P < 0.001]. Meanwhile, two studies (21, 22) targeting non-adult populations showed a stronger and statistically significant association [OR = 1.58, 95% CI (1.25–1.99), I2 = 0.0%, P = 0.938]. However, the number of studies in the non-adult subgroup was limited (n = 2). So, although a larger effect size was observed, caution is warranted when interpreting this difference. Analysis by study design showed that cohort studies (22, 24, 25, 28) demonstrated a moderate positive association [OR = 1.12, 95% CI (0.88–1.42), I2 = 88.3%, P < 0.001], while case - control studies (21, 23, 26, 27) yielded higher but less stable estimates [OR = 1.40, 95% CI (0.85–2.29), I2 = 84.0%, P < 0.001]. In the ethnic subgroup analysis, six Asian studies yielded an OR of 1.17 [95% CI (0.92-1.48)] with high heterogeneity (I2 = 85.8%, P < 0.001), while two non-Asian studies showed an OR of 0.18 [95% CI (0.10-0.26)] with minimal heterogeneity (I2 = 0.0%, P = 0.537). When stratified by diagnostic criteria, two studies using ACR criteria reported an OR of 1.33 [95% CI (0.90-1.96)] with no heterogeneity (I2 = 0.0%, P = 0.672), whereas three studies using ICD criteria demonstrated an OR of 1.00 [95% CI (0.77-1.29)] but significant heterogeneity (I2 = 88.5%, P < 0.001). Significant heterogeneity exists between subgroups (I2 > 80% in most analyses), and there is an imbalance in the number of studies between adult and non - adult populations. This suggests that these stratified results should be interpreted with caution. Although the pattern suggests a possible age-dependent effect, the current evidence remains inconclusive due to limited pediatric population data and methodological differences in study designs.
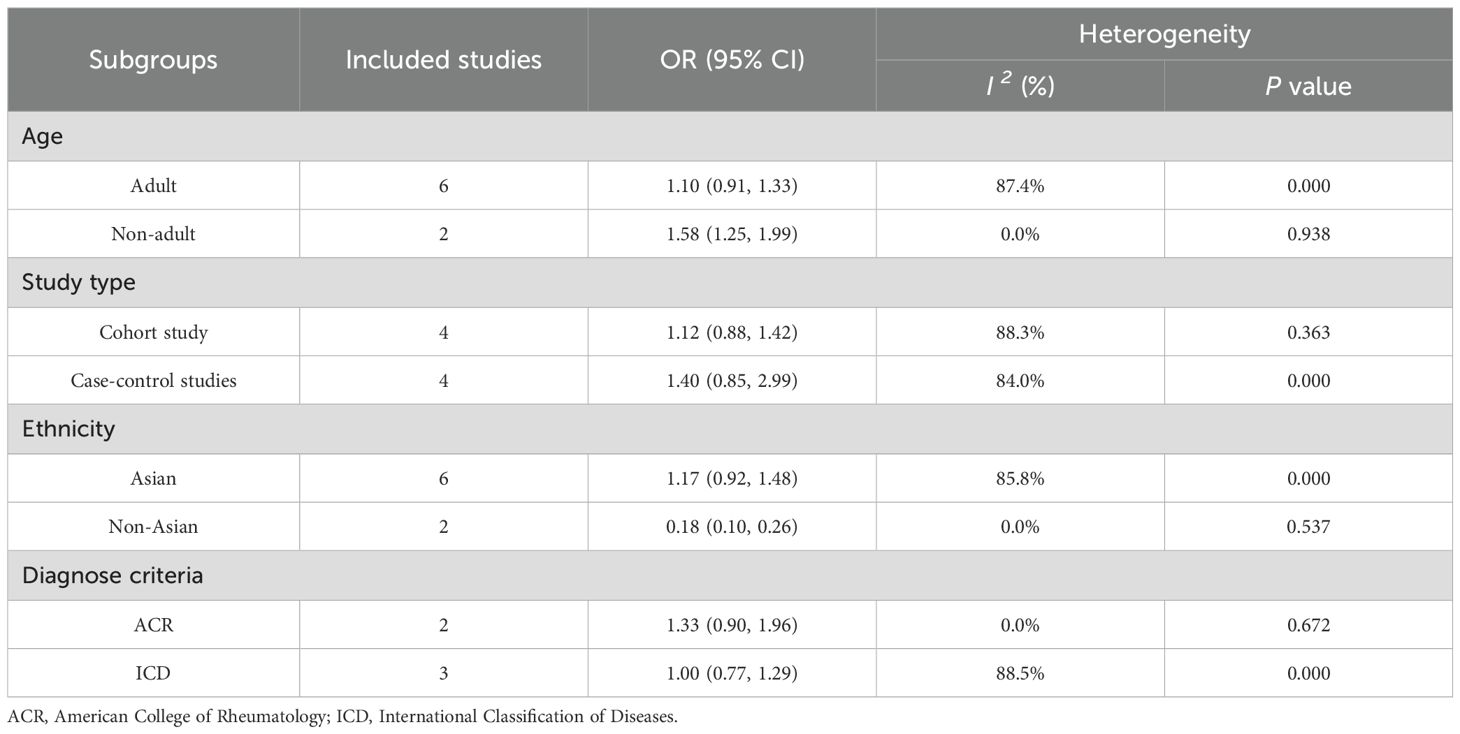
Table 3. Subgroup analysis for the associations between air pollution, emerging chemical pollutants and SLE.
3.5 Publication bias
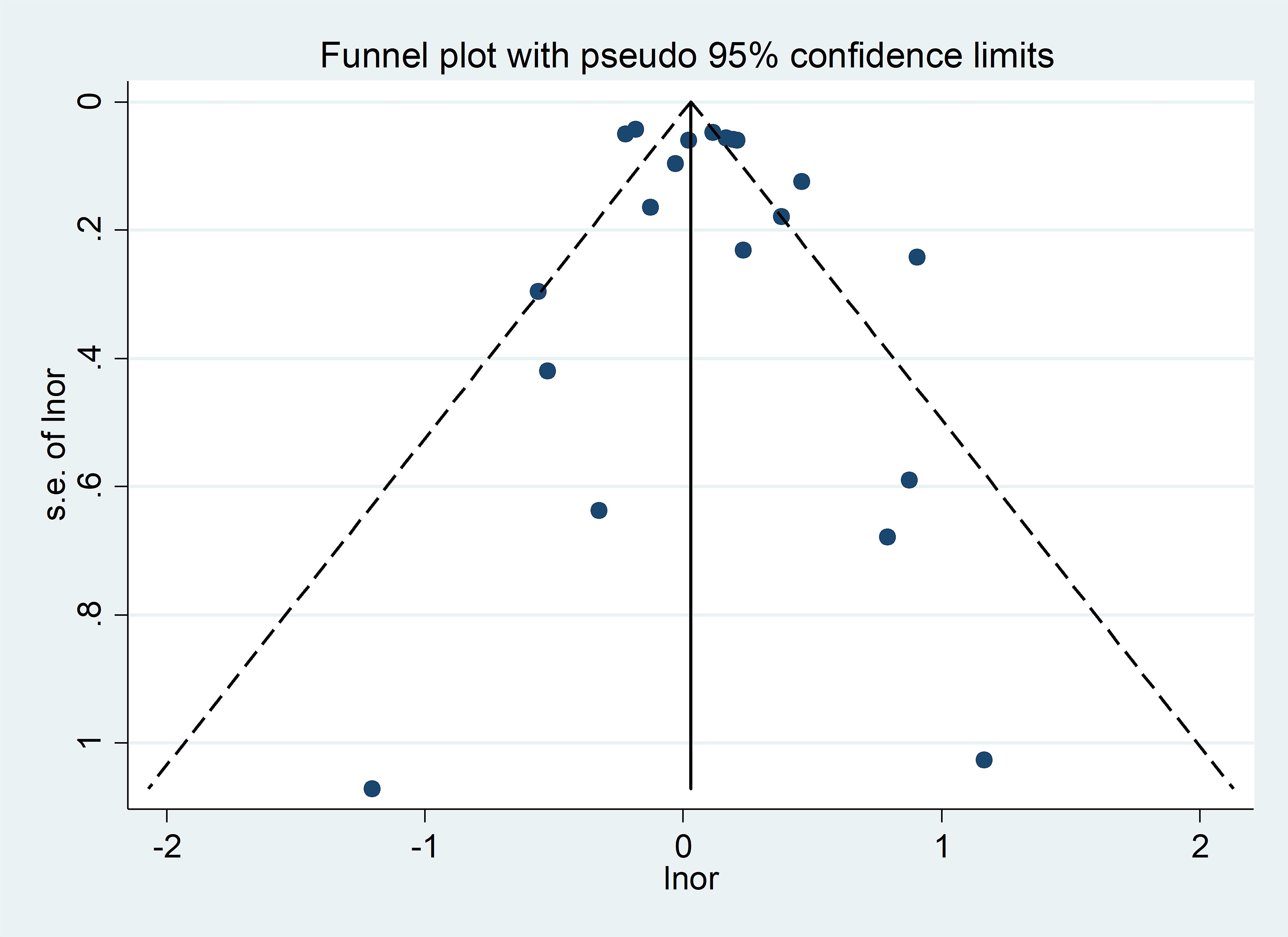
Visual inspection of the funnel plots revealed a rough symmetry, suggesting no significant publication bias in the association between SLE and air pollution (Figure 3). Consequently, we have a high degree of confidence in the validity of our findings. Furthermore, the Egger regression test yielded a p-value of 0.352 (p > 0.05), reinforcing the absence of publication bias in our meta-analysis.
3.6 Results of heterogeneity assessment
In this meta-analysis, the moderate heterogeneity observed for PM2.5 (I2 = 56.2%) and PM10 (I2 = 66.3%) may stem from multiple factors. In terms of population characteristics, the adult subgroup included studies from different regions and only partial studies controlled for socioeconomic status, while the non-adult subgroup showed 0.0% heterogeneity but was limited by small sample size. Regarding study design, case-control studies (I2 = 84.0%) and cohort studies (I2 = 88.3%) differed in exposure assessment methods, with the former susceptible to recall bias and the latter’s model estimation potentially ignoring individual activity differences. In terms of exposure windows, the follow-up duration of cohort studies varied widely, and short-term exposure might fail to capture the long latency effect of SLE. Diverse measurement methods for pollutants, such as satellite data, dataset extraction, and spatial interpolation for PM2.5, also contributed. Additionally, sensitivity analysis using leave-one-out method showed persistent high heterogeneity after excluding individual studies, indicating that heterogeneity was driven by multiple factors including exposure windows, measurement methods, and population differences rather than bias from a single study.
In addition, among the eight included studies, only one study was available for each emerging pollutant exposure, making it impossible to calculate heterogeneity indices for PFASs and BPs.
4 Discussion
4.1 Main findings
This meta-analysis included eight cohort studies comprising 1,390,348 participants to investigate the association between air pollution exposure and SLE. The analysis demonstrated significant positive associations between SLE incidence and exposure to PM2.5 [OR = 1.16, 95%CI (1.02-1.32)] as well as nitrogen dioxide (NO2) [OR = 1.24, 95%CI (1.11-1.38)]. While these findings provide epidemiological evidence supporting an association between specific air pollutants and SLE, the observational nature of the included studies limits causal inference, and residual confounding cannot be excluded.
4.2 Interpretation of findings
A prior meta-analysis (20) focused on the association of PM2.5 with an increased risk of SLE, highlighting its correlation with SLE Disease Activity Index (SLEDAI) scores. Notably, the time-dependence of PM2.5’s impact was observed, with no significant association on the third day of exposure but a positive correlation on the sixth day.
In our meta-analysis, encompassing eight articles and 1,390,348 participants, we investigate the association between PM2.5 and various air pollutants with SLE.Our findings further confirm the significant association between PM2.5 and SLE. Notably, we observed a pronounced association between NO2 exposure and SLE. Moreover, we expanded the analysis to include specific chemical environmental pollutants (such as BPs, PFASs), revealing their substantial contribution to SLE.
By incorporating updated research data, our analysis offers a comprehensive assessment of the association between air pollution exposure and SLE, encompassing a wide array of pollutants.
We conducted subgroup analyses based on age and study design. The results showed that the effect size in the non-adult group [OR = 1.58, 95% CI (1.25-1.99)] was higher than that in the adult group [OR = 1.10, 95% CI (0.91-1.33)], suggesting a possible age-related difference. However, it is important to note that the analysis of the non-adult group was based on only two studies with a total of 372 cases, which is insufficient in terms of sample size and number of studies, limiting the reliability of this result. This finding requires further validation in prospective pediatric cohort studies, particularly in conjunction with recent reports of a declining trend in the incidence of childhood SLE (29) for more in-depth exploration. Second, subgroup analysis showed that the association between air pollution and SLE was consistent in both cohort studies [OR = 1.12, 95% CI (0.88-1.42)] and case-control studies [OR = 1.40, 95% CI (0.85-2.29)], although neither reached statistical significance. The effect size observed in case-control studies was larger, a pattern consistent with previously reported methodological differences between observational study designs, suggesting that methodological characteristics should be considered when interpreting assessments of air pollution’s health effects across different study designs.
In our study, although the Egger regression did not suggest significant publication bias (P = 0.352>0.05), the funnel plot showed that small sample studies clustered at the high end of the effect size. This phenomenon implies that there may be a small study effect. Further observation revealed that some of the small-sample studies had relatively low quality scores. This may be related to study design flaws due to small sample sizes, thus increasing the likelihood of the existence of small-sample study effects. For example, 30 patients with childhood-onset systemic lupus erythematosus (cSLE) and 86 healthy controls were selected for the study by Conde, P.G. et al. (21). The sample size was relatively small. This makes the results of the study potentially not representative enough to accurately reflect the relationship between environmental factors and the onset of cSLE in the overall population, and makes it impossible to completely exclude the influence of possible publication bias and small-scale effects of the study. He, G. et al. (23) analyzed the relationship between PFASs and SLE. Their study, included 100 normal and 100 SLE patients, had a limited sample size and was susceptible to the confounding influence of small-scale study effects.
Furthermore, we analyzed the impact of potential confounders that may have been present in the studies. Nearly half of the studies showed that socioeconomic status was a common potential confounder. In general, areas with poorer economic conditions tend to have poorer environmental quality. Residents are more likely to be exposed to high concentrations of PM and have a relatively higher risk of developing SLE. Also, economic conditions may influence residents’ diet and healthcare, which may indirectly affect the onset of SLE. Of the 8 studies included in this review, 4 controlled for socioeconomic status, making the results relatively robust (24–26, 28). However, some studies (21) suggest that occupational exposures or air pollutants of the mother during pregnancy may affect the development of the foetal immune system. In turn, the development of the foetal immune system has been linked to the development of cSLE. This suggests that our genetic susceptibility and occupational exposures may be potential factors influencing the results of the study. However, very few original studies (21) have moderated the two variables of occupational exposure and genetic susceptibility, a factor that contributes to the uncertainty of the study results.
Among the studies we included, air pollution detection methods varied, including environmental monitoring and self-reported exposure. Differences in measurement methods may affect pooled effect estimates to some extent, thereby interfering with accurate judgements of the air pollution-disease relationship. For example, Jung, C. R. et al. (24) used a 1 - km resolution land use regression (LUR) model and a satellite estimation model to estimate air pollutant concentrations. This approach can better reflect the spatial distribution of air pollutants in a large region and effectively capture the differences in pollution in different areas within a city. However, this method may not accurately reflect the actual exposure of individuals due to their different indoor activity times and frequencies; He, G. et al. (23) extracted the residential addresses of patients in their study and obtained air pollutant data with the help of the China Tracking Air Pollution (TAP) dataset. This dataset integrates multiple factors to a certain extent and enables a more comprehensive assessment of air pollutant exposure. These data have been integrated with various factors to some extent, providing a more comprehensive assessment of air pollution exposure. However, the veracity of the data remains susceptible to the influence of various factors, including but not limited to model assumptions and data fusion methodologies. As a consequence, this may introduce biases into the estimates of the pooled effects; Conde, P. G. et al. (21) used a questionnaire to assess exposure. Participants were asked to provide information including the mother’s occupational exposures during pregnancy and the home surroundings. This method of self-reporting exposure is simple and easy to administer, but suffers from recall bias and inaccurate information. Participants may not be able to accurately recall past exposures or may have biased perceptions of certain exposures, leading to errors in exposure data. Therefore, future studies should incorporate multiple methods to improve exposure measures to more accurately assess the relationship between air pollution and SLE and to improve study reliability.
At present, the biological pathogenesis of air pollutants inducing or influencing the occurrence of SLE is not well understood. Several views on its mechanism are mainly related to oxidative stress and immune disorders, and epigenetic alterations (30–32). Air pollution can disrupt helper T cell (Th) homeostasis. It can also activate nuclear factor-κB, which then regulates Th1. Th1 binds to aryl hydrocarbon receptors to regulate Th17 and regulatory T cells. This process triggers the production of pro-inflammatory cytokines (32, 33). This concept is supported by a recent study by Dellaripa et al., which demonstrated that air pollutant exposure can induce specific immune perturbations that are characteristic of early autoimmunity (34). PM, as a representative substance of air pollution, can cause or amplify oxidative stress due to the presence of heavy metals, organic carbon and other complex elements on its surface (35). PM from air pollution, when inhaled, produces oxidants locally in the alveoli, triggering local chronic inflammation. The toxicity of PM depends on its size, shape, and composition, and the transition metals (e.g., Fe, V, Cr, etc.) present in it can produce oxidative stress through a Fenton-type reaction, which can have a negative effect on cells (36, 37). For example, silica particles are toxic to macrophages, inducing cell death and exposing intracellular self-antigens to immune cells (38), which in turn triggers an immune response (39). Inhalation of nanoparticles stimulates alveolar macrophages, triggering an acute systemic inflammatory response, and airway inflammation leads to an increased secretion of pro-inflammatory mediators such as interleukin-8 and granulocyte-macrophage colony-stimulating factor, as well as an influx of neutrophils (40–42). In addition, PM may act as an adjuvant (43), inducing an immune response against otherwise non-immunogenic antigens (44). All of these processes may interfere with normal immune regulation, leading to immune imbalances and increasing the risk of autoimmune diseases. Furthermore, epigenetic modifications play a crucial role in the pathogenesis of SLE (45). Abnormal methylation patterns can lead to aberrant gene activation. For example, hypomethylation of genomic DNA and immune - related genes in CD4+ T cells can cause overexpression of certain genes, thus triggering an autoimmune response (46, 47). Histone modifications, such as acetylation, phosphorylation, ubiquitination, and methylation, affect chromatin structure and gene expression (48). RNA methylation, particularly N6 - methyladenosine (m6A) modification (49), is also implicated in SLE. Downregulation of the demethylase AlkB homolog 5 (ALKBH5) in PBMCs and T cells of SLE patients inhibits apoptosis and promotes T - cell proliferation (50). Upregulation of methyltransferase 3 (METTL3) in the kidneys of SLE patients promotes IRF4 - mediated plasma cell infiltration, resulting in kidney damage (51). In summary, these epigenetic modifications interact with each other and affect the immune system, ultimately driving the occurrence and progression of SLE.
4.3 Implications and limitations
This study conducted a systematic review and meta-analysis to comprehensively assess the epidemiological association between air pollution exposure and the incidence of SLE. Through subgroup analysis, we further explored the specific association patterns between different types of air pollutants (such as PM2.5 and PM10) and SLE. These findings provide new epidemiological evidence for a deeper understanding of the potential role of environmental factors in the pathogenesis of SLE and offer guidance for future mechanistic studies.
Despite the significant contributions of this study, several limitations warrant consideration. Firstly, the restriction to PubMed/Embase searches might raise concerns about incomplete literature coverage. Although our meta-analysis searched only PubMed and Embase, this approach is methodologically justified. Embase complements PubMed by uniquely covering European and Asian journals in air pollution research (52), ensuring geographical diversity. Methodological analyses further show that including additional databases (e.g., Web of Science, Scopus, or Cochrane Library) identifies fewer than 3% more eligible studies in observational meta-analyses (53, 54). Given the high specificity of our topic—air pollution and SLE—this marginal increase is methodologically insignificant. Our study aligns with the AMSTAR 2 tool’s recommendation (55) to prioritize ‘reasonable’ database selection over exhaustive searches.
Second, Although SLE exhibits well-documented sex disparities in prevalence and clinical manifestations, our meta-analysis could not perform a robust sex-stratified analysis due to insufficient reporting of sex-specific associations between air pollution exposure and SLE in the included studies. Despite our initial intention to explore potential effect modification by sex, the original studies either lacked stratified outcome data or did not explicitly examine interactions between air pollution and sex, precluding meaningful subgroup comparisons. We hope that future research will incorporate more detailed data on sex-specific responses to air pollution, thereby enriching the understanding of how sex may modify the association between air pollution and SLE.
Third, the temporal relationship between pollution exposure and SLE onset exhibited substantial heterogeneity across studies, profoundly influencing the interpretability of exposure-response associations. For instance, studies adopting longitudinal cohort designs (24, 28) evaluated cumulative exposure over 10–15 years prior to SLE diagnosis, aligning with the disease’s postulated latency period (11.77 years median follow-up). Such long-term assessments captured PM2.5/NO2 concentrations during critical preclinical phases, identifying threshold effects (e.g., NO2 28–38 ppb, PM2.5 18–46 μg/m3) that correlated with increased risk. In contrast, cross-sectional or case-control studies (23, 27) measured biomarkers (PFASs/BPs) at the time of diagnosis without clear temporal linkage to disease initiation, precluding inference of exposure-onset causality. Studies focusing on pediatric SLE (22) further highlighted temporal complexity, with perinatal exposures (maternal occupational vapor/secondhand smoke) and childhood PM2.5 demonstrating associations with disease activity and renal failure. However, short-term evaluations (25, 26) using 2-4-year exposure windows (2008-2011) might have underestimated chronic risks, as seen in RA-specific analyses where PM2.5 exposure 3–5 years prior to diagnosis showed stronger associations (aHR=1.74) than concurrent measurements. This methodological variance introduces bias in pooled effect estimates. Shorter-term assessments (e.g., ≤2 years) in cross-sectional designs (26) may reflect disease-related behavioral changes rather than causal exposure, while self-reported data (21) risk recall bias. For SLE—a chronic disease with protracted preclinical phases—standardizing exposure windows to 5–10 years before diagnosis (28) in future meta-analyses will enhance causal inference, particularly when integrating biological plausibility.
It should also be noted that this meta-analysis has limitations in the geographic distribution of included studies: among all 8 included studies, 6 focused on Asian populations. This lack of diversity in study populations partially limits the generalizability of the findings to other ethnicities and regions (such as North America and Europe). It is important to note that the impact of environmental exposures on autoimmune diseases is mediated by multiple factors, including variations in genetic susceptibility, lifestyle characteristics, baseline disease prevalence, and the specific composition and concentration levels of pollutants in the environment. These factors often exhibit significant regional differences. Therefore, the pooled effect estimates derived from this study may not be directly generalizable to Western populations. This limitation reveals a critical gap in the current literature and the urgent need for large-scale, rigorously designed epidemiological studies in non-Asian populations to examine the links between air pollution, emerging chemical exposures, and SLE. Such studies are essential to validate the reliability of the findings from this research and to refine our understanding within a broader population context.
Additionally, while we identified significant associations for BPs and PFASs in individual studies, the lack of comparable data precluded their inclusion in quantitative synthesis. Future systematic reviews with expanded chemical exposure categories are warranted.
5 Conclusion
This meta-analysis revealed a significant association between the incidence of SLE and exposure to specific air pollutants, particularly PM2.5 and NO2. Exposure to emerging chemicals, such as PFASs, showed a strong association, but due to limited research, the association was not sufficiently robust. However, the relationship between BPs and SLE remains unclear, as existing evidence only suggests a non-significant positive trend. These findings emphasize the need for further large-scale epidemiological and mechanistic studies to clarify the role of environmental pollutants in the pathogenesis of SLE. Improving air quality and regulating known risk factors such as PM2.5 and NO2 may help reduce the risk of SLE, but further research is needed to validate the potential impacts of other environmental exposures.
Author contributions
YX: Methodology, Formal analysis, Writing – original draft. HP: Methodology, Writing – original draft. YW: Methodology, Writing – original draft. XL: Conceptualization, Investigation, Writing – review & editing. QD: Conceptualization, Investigation, Writing – review & editing. LH: Software, Supervision, Writing – review & editing. CW: Methodology, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China(grant number: 82374395); Key R&D Program of Zhejiang(grant number: 2024C03191); the National Natural Science Foundation of China (grant number: U21A20402).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1613441/full#supplementary-material
References
1. Hoi A, Igel T, Mok CC, and Arnaud L. Systemic lupus erythematosus. Lancet. (2024) 403:2326–38. doi: 10.1016/S0140-6736(24)00398-2
2. Tian J, Zhang D, Yao X, Huang Y, and Lu Q. Global epidemiology of systemic lupus erythematosus: a comprehensive systematic analysis and modelling study. Ann Rheum Dis. (2023) 82:351–6. doi: 10.1136/ard-2022-223035
4. Zoma A. Musculoskeletal involvement in systemic lupus erythematosus. Lupus. (2004) 13:851–3. doi: 10.1191/0961203303lu2021oa
5. Natalucci F, Ceccarelli F, Cipriano E, Perricone C, Olivieri G, Pirone C, et al. Joint involvement influences quality of life in systemic lupus erythematosus patients. Lupus. (2021) 30:478–83. doi: 10.1177/0961203320979039
6. Baker K and Pope J. Employment and work disability in systemic lupus erythematosus: a systematic review. Rheumatol (Oxford). (2009) 48:281–4. doi: 10.1093/rheumatology/ken477
7. Rosetti F, de la Cruz A, and Crispín JC. Gene-function studies in systemic lupus erythematosus. Curr Opin Rheumatol. (2019) 31:185–92. doi: 10.1097/BOR.0000000000000572
8. Woo J, Parks CG, Jacobsen S, Costenbader KH, and Bernatsky S. The role of environmental exposures and gene-environment interactions in the etiology of systemic lupus erythematous. J Intern Med. (2022) 291:755–78. doi: 10.1111/joim.13448
9. Brugha R and Grigg J. Urban air pollution and respiratory infections. Paediatr Respir Rev. (2014) 15:194–9. doi: 10.1016/j.prrv.2014.03.001
10. Ghio AJ, Carraway MS, and Madden MC. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J Toxicol Environ Health B Crit Rev. (2012) 15:1–21. doi: 10.1080/10937404.2012.632359
11. Alves A, de Azevedo GM, Braga A, Sallum A, Pereira L, Farhat LC, et al. Influence of air pollution on airway inflammation and disease activity in childhood-systemic lupus erythematosus. Clin Rheumatol. (2018) 37:683–90. doi: 10.1007/s10067-017-3893-1
12. Bernatsky S, Fournier M, Pineau CA, Clarke AE, Vinet E, and Smargiassi A. Associations between ambient fine particulate levels and disease activity in patients with systemic lupus erythematosus (sle). Environ Health Perspect. (2011) 119:45–9. doi: 10.1289/ehp.1002123
13. Bernatsky S, Smargiassi A, Johnson M, Kaplan GG, Barnabe C, Svenson L, et al. Fine particulate air pollution, nitrogen dioxide, and systemic autoimmune rheumatic disease in calgary, alberta. Environ Res. (2015) 140:474–8. doi: 10.1016/j.envres.2015.05.007
14. Vidotto JP, Pereira LA, Braga AL, Silva CA, Sallum AM, Campos LM, et al. Atmospheric pollution: influence on hospital admissions in paediatric rheumatic diseases. Lupus. (2012) 21:526–33. doi: 10.1177/0961203312437806
15. Dong Y, Gao L, Sun Q, Jia L, and Liu D. Increased levels of il-17 and autoantibodies following bisphenol a exposure were associated with activation of pi3k/akt/mtor pathway and abnormal autophagy in mrl/lpr mice. Ecotoxicol Environ Saf. (2023) 255:114788. doi: 10.1016/j.ecoenv.2023.114788
16. Hong Y, Wang D, Lin Y, Yang Q, Wang Y, Xie Y, et al. Environmental triggers and future risk of developing autoimmune diseases: molecular mechanism and network toxicology analysis of bisphenol a. Ecotoxicol Environ Saf. (2024) 288:117352. doi: 10.1016/j.ecoenv.2024.117352
17. Wang L, Liu T, Yang S, Sun L, Zhao Z, Li L, et al. Perfluoroalkyl substance pollutants activate the innate immune system through the aim2 inflammasome. Nat Commun. (2021) 12:2915. doi: 10.1038/s41467-021-23201-0
18. Kuo CF, Grainge MJ, Valdes AM, See LC, Luo SF, Yu KH, et al. Familial aggregation of systemic lupus erythematosus and coaggregation of autoimmune diseases in affected families. JAMA Intern Med. (2015) 175:1518–26. doi: 10.1001/jamainternmed.2015.3528
19. Jin H, Zhao C, Chen Y, Zhang Y, Yong Z, Lei Y, et al. Environmental exposure to polycyclic aromatic hydrocarbons: an underestimated risk factor for systemic lupus erythematosus onset and progression. Sci Total Environ. (2024) 926:171841. doi: 10.1016/j.scitotenv.2024.171841
20. Rezayat AA, Jafari N, Mir Nourbakhsh SH, Hasheminezhad Hoseini FS, Hooshmand N, Ghasemi Nour M, et al. The effect of air pollution on systemic lupus erythematosus: a systematic review and meta-analysis. Lupus. (2022) 31:1606–18. doi: 10.1177/09612033221127569
21. Conde PG, Farhat LC, Braga A, Sallum A, Farhat S, and Silva CA. Are prematurity and environmental factors determinants for developing childhood-onset systemic lupus erythematosus? Mod Rheumatol. (2018) 28:156–60. doi: 10.1080/14397595.2017.1332508
22. He G, Wang Y, Cheng C, Guo J, Lin Z, Liang Z, et al. Pm(2.5) constituents associated with mortality and kidney failure in childhood-onset lupus nephritis: a 19-year cohort study. Sci Total Environ. (2024) 949:175333. doi: 10.1016/j.scitotenv.2024.175333
23. He Y, Qu C, Tian J, Miszczyk J, Guan H, and Huang R. Association of perfluoroalkyl and polyfluoroalkyl substances (pfass) exposures and the risk of systemic lupus erythematosus: a case–control study in China. Environ Health. (2023) 22:78. doi: 10.1186/s12940-023-01019-1
24. Jung CR, Chung WT, Chen WT, Lee RY, and Hwang BF. Long-term exposure to traffic-related air pollution and systemic lupus erythematosus in Taiwan: a cohort study. Sci Total Environ. (2019) 668:342–9. doi: 10.1016/j.scitotenv.2019.03.018
25. Park JS, Choi S, Kim K, Chang J, Kim SM, Kim SR, et al. Association of particulate matter with autoimmune rheumatic diseases among adults in South Korea. Rheumatol (Oxford). (2021) 60:5117–26. doi: 10.1093/rheumatology/keab127
26. Tang KT, Tsuang BJ, Ku KC, Chen YH, Lin CH, and Chen DY. Relationship between exposure to air pollutants and development of systemic autoimmune rheumatic diseases: a nationwide population-based case-control study. Ann Rheum Dis. (2019) 78:1288–91. doi: 10.1136/annrheumdis-2019-215230
27. Wang Y, Wu H, Li K, Huang R, Liu J, Lu Z, et al. Environmental triggers of autoimmunity: the association between bisphenol analogues and systemic lupus erythematosus. Ecotoxicol Environ Saf. (2024) 278:116452. doi: 10.1016/j.ecoenv.2024.116452
28. Xing M, Ma Y, Cui F, Li D, Wang J, Tang L, et al. Air pollution, genetic susceptibility, and risk of incident systemic lupus erythematosus: a prospective cohort study. Arthritis Rheumatol. (2024) 76:1530–1537. doi: 10.1002/art.42929
29. Gao S, Yu Z, Ma X, Sun J, Ren A, Gao S, et al. Childhood-onset systemic lupus erythematosus in China 2016–21: a nationwide study. Lancet Child Adolesc Health. (2024) 8:762–72. doi: 10.1016/S2352-4642(24)00172-X
30. Adams DE and Shao WH. Epigenetic alterations in immune cells of systemic lupus erythematosus and therapeutic implications. Cells. (2022) 11:506. doi: 10.3390/cells11030506
31. Gawda A, Majka G, Nowak B, and Marcinkiewicz J. Air pollution, oxidative stress, and exacerbation of autoimmune diseases. Cent Eur J Immunol. (2017) 42:305–12. doi: 10.5114/ceji.2017.70975
32. Glencross DA, Ho TR, Camiña N, Hawrylowicz CM, and Pfeffer PE. Air pollution and its effects on the immune system. Free Radic Biol Med. (2020) 151:56–68. doi: 10.1016/j.freeradbiomed.2020.01.179
33. O'Driscoll CA and Mezrich JD. The aryl hydrocarbon receptor as an immune-modulator of atmospheric particulate matter-mediated autoimmunity. Front Immunol. (2018) 9:2833. doi: 10.3389/fimmu.2018.02833
34. Dellaripa PF, Sung LH, Bain PA, Lanata C, Blazer A, Miller FW, et al. American college of rheumatology white paper: the effects of climate change on rheumatic conditions-an evolving landscape and a path forward. Arthritis Rheumatol. (2024) 76:1459–66. doi: 10.1002/art.42919
35. Mazzoli-Rocha F, Fernandes S, Einicker-Lamas M, and Zin WA. Roles of oxidative stress in signaling and inflammation induced by particulate matter. Cell Biol Toxicol. (2010) 26:481–98. doi: 10.1007/s10565-010-9158-2
36. Negre-Salvayre A, Coatrieux C, Ingueneau C, and Salvayre R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol. (2008) 153:6–20. doi: 10.1038/sj.bjp.0707395
37. Shi T, Schins RP, Knaapen AM, Kuhlbusch T, Pitz M, Heinrich J, et al. Hydroxyl radical generation by electron paramagnetic resonance as a new method to monitor ambient particulate matter composition. J Environ Monit. (2003) 5:550–6. doi: 10.1039/b303928p
38. Davis GS, Pfeiffer LM, and Hemenway DR. Persistent overexpression of interleukin-1beta and tumor necrosis factor-alpha in murine silicosis. J Environ Pathol Toxicol Oncol. (1998) 17:99–114.
39. Brown JM, Archer AJ, Pfau JC, and Holian A. Silica accelerated systemic autoimmune disease in lupus-prone New Zealand mixed mice. Clin Exp Immunol. (2003) 131:415–21. doi: 10.1046/j.1365-2249.2003.02094.x
40. Behndig AF, Mudway IS, Brown JL, Stenfors N, Helleday R, Duggan ST, et al. Airway antioxidant and inflammatory responses to diesel exhaust exposure in healthy humans. Eur Respir J. (2006) 27:359–65. doi: 10.1183/09031936.06.00136904
41. Buzea C, Pacheco II, and Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. (2007) 2:MR17–71. doi: 10.1116/1.2815690
42. Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, et al. Passage of inhaled particles into the blood circulation in humans. Circulation. (2002) 105:411–4. doi: 10.1161/hc0402.104118
43. Takano H, Ichinose T, Miyabara Y, Shibuya T, Lim HB, Yoshikawa T, et al. Inhalation of diesel exhaust enhances allergen-related eosinophil recruitment and airway hyperresponsiveness in mice. Toxicol Appl Pharmacol. (1998) 150:328–37. doi: 10.1006/taap.1998.8437
44. Granum B, Gaarder PI, Groeng E, Leikvold R, Namork E, and Lovik M. Fine particles of widely different composition have an adjuvant effect on the production of allergen-specific antibodies. Toxicol Lett. (2001) 118:171–81. doi: 10.1016/s0378-4274(00)00292-7
45. Zhou X, Zhou S, and Li Y. An updated review on abnormal epigenetic modifications in the pathogenesis of systemic lupus erythematosus. Front Immunol. (2024) 15:1501783. doi: 10.3389/fimmu.2024.1501783
46. Lu Q, Wu A, and Richardson BC. Demethylation of the same promoter sequence increases cd70 expression in lupus t cells and t cells treated with lupus-inducing drugs. J Immunol. (2005) 174:6212–9. doi: 10.4049/jimmunol.174.10.6212
47. Wu H, Zhao M, Tan L, and Lu Q. The key culprit in the pathogenesis of systemic lupus erythematosus: aberrant dna methylation. Autoimmun Rev. (2016) 15:684–9. doi: 10.1016/j.autrev.2016.03.002
48. Rice JC and Allis CD. Histone methylation versus histone acetylation: new insights into epigenetic regulation. Curr Opin Cell Biol. (2001) 13:263–73. doi: 10.1016/s0955-0674(00)00208-8
49. Yang Y, Hsu PJ, Chen YS, and Yang YG. Dynamic transcriptomic m(6)a decoration: writers, erasers, readers and functions in rna metabolism. Cell Res. (2018) 28:616–24. doi: 10.1038/s41422-018-0040-8
50. Deng LJ, Fang XY, Wu J, Li QR, Mao YM, Leng RX, et al. Alkbh5 expression could affect the function of t cells in systemic lupus erythematosus patients: a case-control study. Curr Pharm Des. (2022) 28:2270–8. doi: 10.2174/1381612828666220617154204
51. Liu Y, Wang X, Huang M, Luo A, Liu S, Cai M, et al. Mettl3 facilitates kidney injury through promoting irf4-mediated plasma cell infiltration via an m6a-dependent manner in systemic lupus erythematosus. BMC Med. (2024) 22:511. doi: 10.1186/s12916-024-03735-y
52. Gusenbauer M and Haddaway NR. Which academic search systems are suitable for systematic reviews or meta-analyses? Evaluating retrieval qualities of google scholar, pubmed, and 26 other resources. Res Synth Methods. (2020) 11:181–217. doi: 10.1002/jrsm.1378
53. Frandsen TF, Bruun NM, Lindhardt CL, and Eriksen MB. Using the full pico model as a search tool for systematic reviews resulted in lower recall for some pico elements. J Clin Epidemiol. (2020) 127:69–75. doi: 10.1016/j.jclinepi.2020.07.005
54. Halladay CW, Trikalinos TA, Schmid IT, Schmid CH, and Dahabreh IJ. Using data sources beyond pubmed has a modest impact on the results of systematic reviews of therapeutic interventions. J Clin Epidemiol. (2015) 68:1076–84. doi: 10.1016/j.jclinepi.2014.12.017
Keywords: air pollution, systemic lupus erythematosus, environmental exposure, particulate matter, meta-epidemiology study
Citation: Xu Y, Pan H, Chen W, Wang Y, Li X, Dai Q and Huang L (2025) Air pollution, emerging chemical exposures, and systemic lupus erythematosus: a meta-epidemiology study. Front. Immunol. 16:1613441. doi: 10.3389/fimmu.2025.1613441
Received: 17 April 2025; Accepted: 30 September 2025;
Published: 17 October 2025.
Edited by:
Luca Ferrari, University of Milan, ItalyReviewed by:
Eric Toussirot, INSERM CIC1431 Centre d’Investigation Clinique Besançon, FranceLishi Zhang, Sichuan University, China
Copyright © 2025 Xu, Pan, Chen, Wang, Li, Dai and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Huang, aHVhbmdsaW5AemNtdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Yaling Xu
Yaling Xu Hejing Pan
Hejing Pan Wu Chen1†
Wu Chen1† Xuanlin Li
Xuanlin Li Lin Huang
Lin Huang