- 1Department of Hematology and Oncology, Children’s Hospital of Soochow University, Suzhou, China
- 2Department of Medical Affairs, Acornmed Biotechnology Co., Ltd., Beijing, China
- 3Department of Pediatric Hematology and Oncology, Key Laboratory of Higher Education Institutions in Jiangsu Province, Suzhou, China
- 4Department of Pediatric Intensive Care Unit, Children’s Hospital of Soochow University, Suzhou, China
Background: Calcineurin inhibitors (CNIs), such as cyclosporine A (CsA), are widely used as immunosuppressants for both prophylactic and therapeutic purposes in patients with graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (allo-HSCT). CsA-related transporters and metabolic enzymes single nucleotide polymorphisms (SNPs) are associated with the efficacy of CsA in individuals. However, few studies have explored how CsA-related SNPs correlate with post-transplant complications and prognosis.
Methods: Here, our study involved 128 pediatric hematological malignancy patients undergoing allo-HSCT with GVHD prophylaxis based on CsA. All patients were detected for CsA-related SNPs. We investigated the associations between the CsA-related SNPs and post-transplant complications and prognosis.
Results: We examined twenty-three CsA-related SNPs. Based on multivariate analysis using Cox regression, we identified umbilical cord blood HSCT and donor-recipient HLA matches of 9/10-10/10 as independent factors for peri-engraftment syndrome (hazard ratio (HR) = 2.82, P = 0.008; HR = 0.30, P = 0.021, respectively); recipient weight ≤ 26 kg, donor-recipient major or minor ABO blood type mismatch, and CYP2C19 (99T>C) variant genotype as independent risk factors for grades II-IV acute GVHD (aGVHD) (HR = 2.08, P = 0.008; HR = 2.56, P = 0.008; HR = 2.22, P = 0.014; HR = 1.80, P = 0.042, respectively); matched unrelated donor HSCT and donor-recipient HLA matches of 9/10-10/10 as independent factors for Epstein-Barr virus infection (HR = 5.22, P = 0.019; HR = 0.13, P = 0.003); CYP3A5 (219-237C>T) variant genotype as an independent protective factor for cytomegalovirus infection (HR = 0.58, P = 0.025); recipient being male, age at transplantation ≤ 104 months, ABCB1 (1236C>T) CT/TT genotype, and SLCO1B1 (1865 + 4846T>C) TC/CC genotype as independent factors for hemorrhagic cystitis (HR = 2.65, P = 0.024; HR = 0.46, P = 0.023; HR = 0.39, P = 0.030; HR = 0.32, P = 0.001, respectively); and donor-recipient HLA matches of 9/10-10/10 as an independent protective factor for capillary leak syndrome (CLS) (HR = 0.19, P = 0.031). Additionally, we found a body weight ≤ 26 kg, CLS after HSCT, SLC29A1 (-162 + 228A>C) AC/CC genotype were independent factors for both disease-free survival (HR = 0.38, P = 0.022; HR = 2.64, P = 0.023; HR = 0.29, P = 0.016, respectively) and overall survival (HR = 0.27, P = 0.007; HR = 3.83, P = 0.003; HR = 0.22, P = 0.005, respectively).
Conclusion: Our study revealed correlations between CsA-related transporters and metabolic enzymes SNPs and post-transplant complications and prognosis, contributing to a better understanding of the interindividual difference in efficacy. Future studies on adjusting the dosage of drugs based on SNPs in clinical practice may be one of the options for improving the HSCT outcomes.
1 Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) has emerged as an effective treatment for refractory or relapsed hematological malignancies, including leukemia, lymphoma and myelodysplastic syndrome (1). Despite its therapeutic benefits, graft-versus-host disease (GVHD) remains one of the most significant complications following HSCT and a leading cause of transplant-related morbidity and mortality. The prognosis for patients with severe (grade III-IV) GVHD remains particularly poor, with reported survival rates varying from 25% in the adult population to 55% in pediatric cohorts (2). While the treatment with immunosuppressants has reduced the incidence and mortality of GVHD, this has been accompanied by an increased risk of other clinically significant complications, including opportunistic infections (particularly cytomegalovirus (CMV) and Epstein-Barr virus (EBV) reactivation), hemorrhagic cystitis (HC), and capillary leak syndrome (CLS) (3).
Calcineurin inhibitors (CNIs), like cyclosporine A (CsA), remain a cornerstone in both the prophylaxis and treat GVHD after HSCT, which significantly enhanced the overall survival (OS) rates after transplantation (4). Cyclosporine A (CsA) is an 11-amino acid cyclic peptide obtained from fungal fermentation. CsA works by selectively inhibiting the activity of calcineurin, which suppresses the lymphocyte activation and proliferation, leading to its immunosuppressive effects (5). The early post-transplant concentration of CsA is closely associated with GVHD risk, highlighting the importance of individualized drug management (6).
CsA is mainly metabolized by enzymes from the CYP450 family, predominantly CYP3A4 and CYP3A5 (7). For example, CYP3A5*3C, a splicing variant in intron 3, leads to aberrant mRNA splicing and a premature stop codon, resulting in a truncated, non-functional protein (8). Several studies have demonstrated that the CYP3A5*3C/*3C genotype correlates with a higher CsA C0/dose in early renal transplant patients (9, 10). Moreover, CYP3A4*1G and CYP3A5*3C are in strong linkage disequilibrium in Asians, and individuals carrying the CYP3A5*1 allele are more likely to co-inherit the CYP3A4*1G allele than the CYP3A4*1 allele. These indicate differences in individual single nucleotide polymorphism (SNP) are the primary factors affecting the metabolic activities of these enzymes.
In addition to metabolic enzymes, CsA acts as a substrate for various influx and efflux transporters, such as ABCB1 and SLCO1B1 (11, 12). SNP variations in transporter coding gene, such as ABCB1, have been associated with altered CsA pharmacokinetics. Notably, ABCB1 3435CC genotype carriers tend to require higher CsA doses than 3435TT carriers, particularly in Asian populations and during the early post-transplant period (13). Other metabolic pathways, including UGT1A8 and BMP-related pathways, may also contribute to CsA disposition, although these associations remain less well defined (14, 15).
However, limited reports have examined the relationship between gene SNPs and clinical complications after transplantation.Our study aimed to identity gene SNPs of CsA-related transporters and metabolic enzymes, including ABC, BMP, CYP, MTHFR, MTRR, SLC, STIM1, UGT1A8 family, and correlate these SNPs with common post-transplant clinical complications in 128 pediatric hematological malignancy patients.
2 Materials and methods
2.1 Study designs
We performed a retrospective cohort study of 128 patients who received allo-HSCT in Children’s Hospital of Soochow University (Suzhou, China) between June 2019 and June 2021 (Table 1). The Institutional Review Board at Children’s Hospital of Soochow University approved the study (No. 2023CS105) and all recipients and donors provided written informed consent according to the Declaration of Helsinki. The study inclusion criteria were as follows: (1) allo-HSCT; (2) first-time transplant; (3) patients aged less than 18 years old; (4) received CsA-based GVHD prophylaxis; (5) presence of informed consent to CsA-related genotyping. Patients were excluded if they: (1) discontinued CsA prematurely for any reason; (2) had a documented allergy or intolerance to CsA.
2.2 Conditioning regimen and GVHD prophylaxis
All patients in the conditioning stage received a myeloablative regimen incorporating the combination of busulfan and cyclophosphamide (BuCy). Drug dosages were tailored according to individual patient parameters including height, weight, body surface area to optimize the therapeutic regimen in terms of both scientific rationale and efficacy. Busulfan was administered intravenously at 0.8 mg/kg every 6 hours for 3 or 4 consecutive days, and cyclophosphamide was given intravenously at a total dose of 120 mg/kg over 2 or 3 days. In certain cases, supplementary regimens like fludarabine and cytarabine (FLAG) or cladribine and cytarabine (CLAG) may be introduced as bridging therapies alongside the BuCy transplantation. In the FLAG group, fludarabine (30 mg/m²/day) and cytarabine (2 g/m²/day) were administered intravenously for 4 days, with G-CSF (5 µg/kg/day) given subcutaneously for 5 days. In the CLAG group, cladribine (5 mg/m²/day) and cytarabine (2 g/m²/day) were administered intravenously for 3 or 5 days, along with subcutaneous granulocyte-colony stimulating factor (G-CSF, 5 µg/kg/day) for 4 or 6 days.
The GVHD prophylaxis included oral CsA and MMF. CsA was administered at 6–10 mg/kg/day every 12 hours. MMF was given at 15 mg/kg/day every 12 hours, with the dose halved on day 30 post-transplant and discontinued by day 45. Umbilical cord blood (UCB) transplantation and fully matched sibling transplantation typically require CsA levels of approximately 150–200 ng/ml, while unrelated fully matched transplantation and haploidentical transplantation require CsA levels of around 200–250 ng/ml. Additionally, some patients concurrently received oral methotrexate (MTX) as a prophylactic measure against GVHD, administered at 15 mg/m² on day +1 and 10 mg/m² on days +3, +6, and +11.
2.3 Post-transplant evaluation
Neutrophil engraftment was defined as achieving an absolute neutrophil count ≥ 0.5×109/L for three consecutive days. Platelet engraftment was defined as reaching a platelet count ≥ 20×109/L without requiring transfusion support for seven consecutive days. A myeloablative conditioning regimen, defined as Bu exceeding 8 mg/kg. Primary graft failure (GF) was defined as the failure of neutrophil engraftment at day +28 post-HSCT, secondary GF was defined as the development of neutrophil count < 0.5×109/L occurring after the initial engraftment. The diagnosis and grading of aGVHD and chronic GVHD (cGVHD) were performed by the transplant center using standard criteria (16, 17). The definition of hemorrhagic cystitis (HC), capillary leak syndrome (CLS) and Peri-engraftment syndrome (Peri-ES) follow as per previous standards (18–20). CMV and EBV viremia was defined by a viral DNA level ≥ 500 copies/mL in plasma.
2.4 Next-generation sequencing experiments
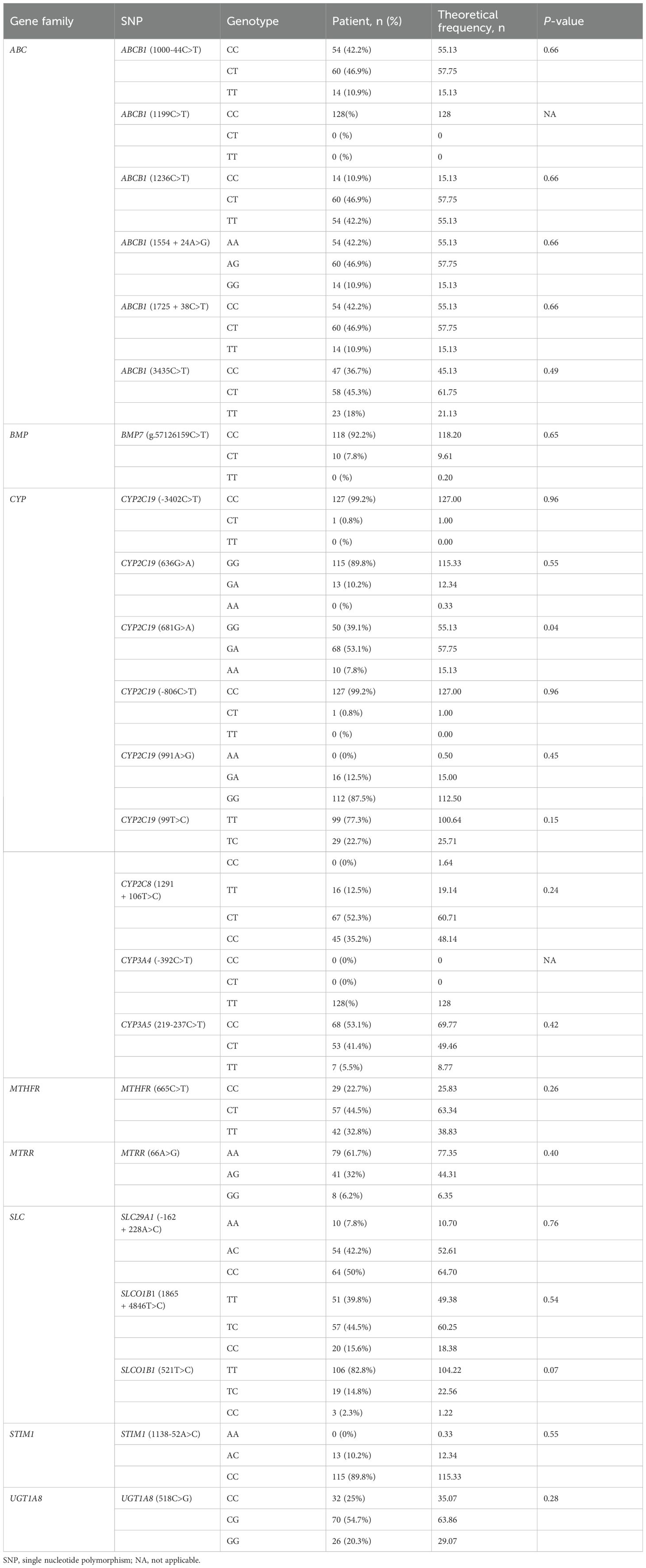
Genomic DNA was isolated from bone marrow diagnostic samples. A total of 27 genes was used for next-generation sequencing (NGS) at Acornmed Biotechnology Co., Ltd (Tianjin, China). The rest standards were same as our previous study (21). Based on literature review (13, 22–27), a total of 23 SNPs of CsA-related transporters and metabolic enzymes were analyzed in our cohort (Table 2).
2.5 Statistical analysis
All data analyses were performed using SPSS software (version 22.0) or R (version 3.5.2). Differences between two groups were compared using independent sample t-tests or Mann-Whitney U non-parametric tests for quantitative variables. For categorical variables, Chi-square tests or Fisher’s exact test were employed. Factors with a P-value < 0.2 via univariate analysis using the Log-rank test were further included in a Cox regression model for multivariate analysis of factors related to clinical outcome events. Genetic conformity to Hardy-Weinberg equilibrium was analyzed using the Chi-square test, with P > 0.05 considered to indicate adherence to Hardy-Weinberg equilibrium. All tests were two-sided, and statistical significance was set at P < 0.05.
3 Results
3.1 Patient characteristics
This study involved 128 pediatric patients with hematologic malignancies who underwent HSCT, with their clinical characteristics detailed in Table 1. The median age at HSCT was 104 months. The most common disease types included acute lymphoblastic leukemia (ALL, 44.5%), acute myeloid leukemia (AML, 48.4%), mixed phenotype acute leukemia (MAPL, 3.9%), and juvenile myelomonocytic leukemia (JMML, 1.6%). All patients received a prophylaxis regimen for GVHD based on CsA. Ultimately, all patients achieved hematopoietic recovery. The median time for neutrophil and platelet engraftment were 12 days (interquartile range (IQR), 11-13) and 11 days (IQR, 10-15), respectively.
3.2 CsA-related transporters and metabolic enzymes SNP
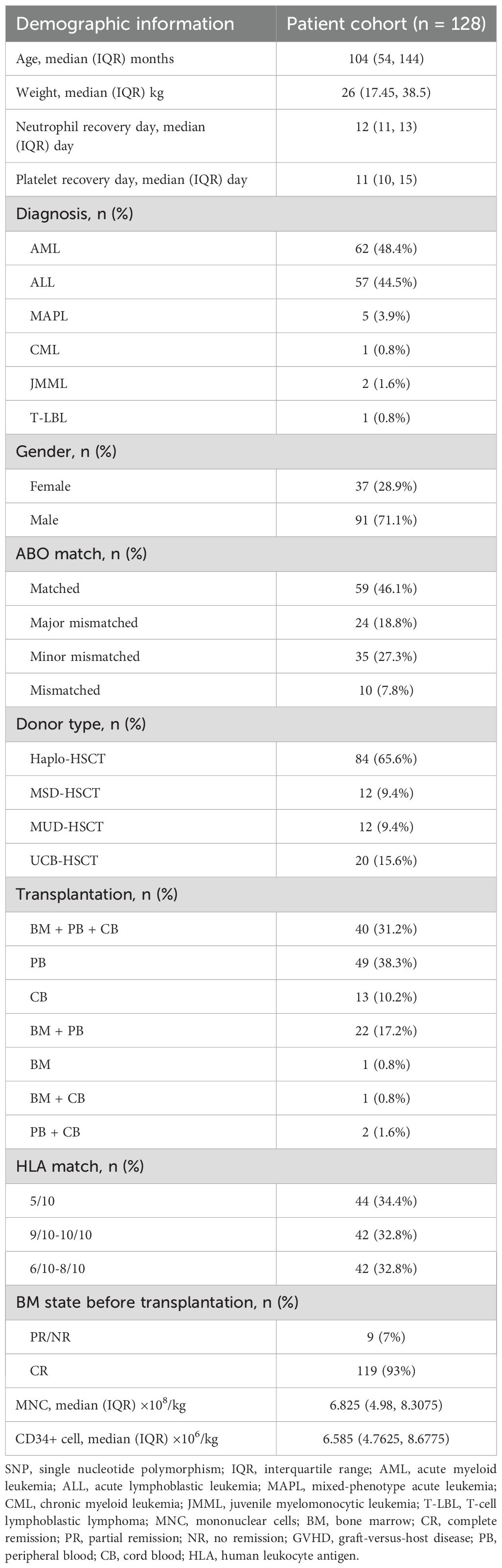
Figure 1 and Table 2 provide descriptions of SNPs related to CsA-related transporters and metabolic enzymes. We examined twenty-three CsA-related SNPs. The most frequently observed genotypes were SLC29A1 (-162 + 228A>C), followed by ABCB1 (1236C>T) and CYP2C8 (1291 + 106T>C) (Figure 1). Among the CsA-related SNPs, CYP2C19 (681G>A), ABCB1 (1199C>T), and CYP3A4 (-392C>T) did not conform to Hardy-Weinberg equilibrium, whereas the remaining SNPs did (P > 0.05, Table 2).
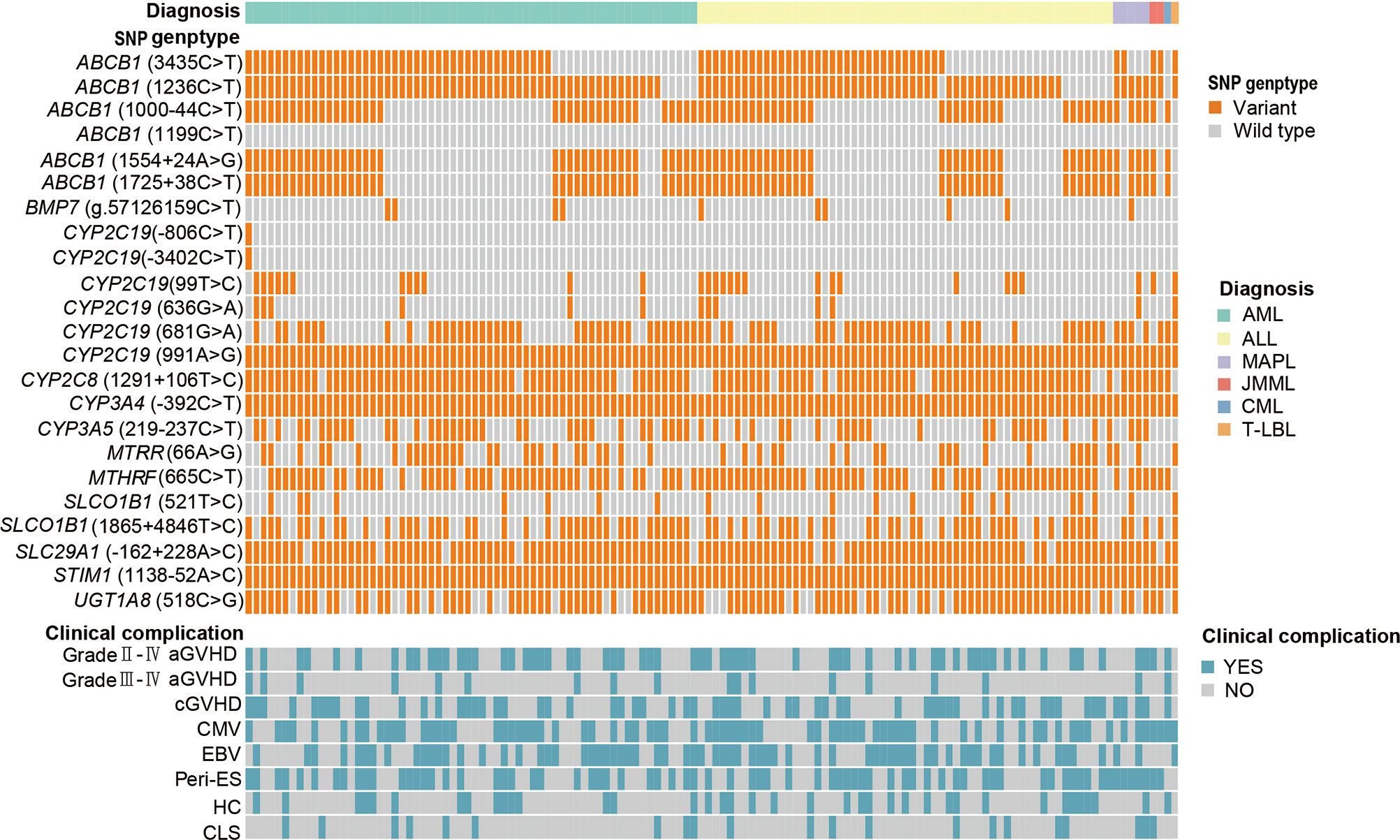
Figure 1. CsA-related transporters and metabolic enzymes gene SNP frequencies in 128 pediatric patients receiving allo-HSCT. Heatmap shows the specific variants in each patient based on different genotypes, according to different subtypes of malignant hematological diseases. CsA, cyclosporine A; SNP, single nucleotide polymorphism; allo-HSCT, allogeneic hematopoietic stem cell transplantation; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MAPL, mixed-phenotype acute leukemia; CML, chronic myeloid leukemia; JMML, juvenile myelomonocytic leukemia; T-LBL, T-cell lymphoblastic lymphoma. aGVHD, acute graft-versus-host disease; cGVHD, chronic GVHD; CMV, cytomegalovirus; EBV, Epstein-Barr virus; Peri-ES, peri-engraftment syndrome; HC, hemorrhagic cystitis; CLS, capillary leak syndrome.
3.3 Correlation between CsA-related SNPs and post-transplant complications
We examined how CsA-related transporters and metabolic enzymes SNPs are associated with post-transplant complications, including GVHD, viral infection, Peri-ES, HC and CLS. This study involved 128 patients, of whom 61 (47.7%) had grades II-IV acute GVHD (aGVHD), and 18 (14.1%) experienced grades III-IV aGVHD. Additionally, 61 patients (47.7%) had cGVHD, 74 (57.8%) had CMV infection, 68 (53.1%) had EBV infection, 71 (55.5%) had Peri-ES, 38 (29.7%) had HC, and 21 (16.4%) had CLS.
We performed univariate analysis with the Log-rank test, and found that the recipients with major ABO blood type mismatches had a significantly higher incidence of Peri-ES than those with fully matched cases (P = 0.043). Moreover, recipients of HLA-matched sibling donor HSCT (MSD-HSCT) and matched unrelated donor HSCT (MUD-HSCT) experienced lower incidences of Peri-ES compared to those undergoing recipients of HLA-matched sibling donor HSCT (MSD-HSCT) and matched unrelated donor HSCT (MUD-HSCT) experienced lower incidences of Peri-ES compared to those undergoing haploidentical HSCT (Haplo-HSCT) (P = 0.014, P = 0.043, respectively). In contrast, recipients of umbilical cord blood HSCT (UCB-HSCT) had a significantly higher incidence of Peri-ES than those receiving Haplo-HSCT (P = 0.049). Additionally, the incidence of Peri-ES was significantly lower in recipients with HLA matches of 9/10-10/10 compared to those with 5/10 match (P < 0.001). Furthermore, patients with BMP7 (g.57126159C>T) CT/TT genotypes showed an increased incidence of Peri-ES (HR = 0.28, P = 0.045) (Supplementary Table S1). Regarding GVHD, we found that recipients with weight ≤ 26 kg had a significantly higher incidence of grades II-IV aGVHD compared to those with weight > 26kg (P = 0.014). UCB-HSCT recipients exhibited a higher incidence of grades II-IV aGVHD compared to those receiving Haplo-HSCT (P = 0.035) (Supplementary Table S2). Recipients with donor-recipient major ABO blood type mismatches had a significantly higher incidence of grades III-IV aGVHD compared to those with fully matched cases (P = 0.040) (Supplementary Table S3). MUD-HSCT recipients exhibited a significantly lower incidence of cGVHD than those receiving Haplo-HSCT (P < 0.001). Recipients with HLA matching of 9/10-10/10 had a significantly lower incidence of cGVHD than those with HLA matching of 5/10 (P = 0.002). Moreover, recipients with lower mononuclear nuclear cell (MNC) infusion dose (≤ 6.83×108/kg) had a significantly lower incidence of cGVHD than those with a higher dose (P = 0.005). Carriers of the SLCO1B1 (521T>C) TT genotype had a higher incidence of cGVHD compared to carriers of the TC/CC genotype (P = 0.005) (Supplementary Table S4). The incidence of EBV infection was significantly lower in UCB-HSCT recipients compared to Haplo-HSCT recipients (P = 0.010). Recipients with HLA matches of 9/10-10/10 showed a lower incidence compared to those with 5/10 HLA matches (P = 0.018). Wild-type alleles of ABCB1 (1000-44C>T), ABCB1 (1554 + 24A>G), ABCB1 (1725 + 38C>T), and CYP3A5 (219-237C>T) are associated with a significantly higher incidence of EBV infection compared to those with variant alleles (all P < 0.050). (Supplementary Table S5). MSD-HSCT recipients experienced significantly fewer CMV infections than those who received Haplo-HSCT (P = 0.046). Recipients with donor HLA matches of 9/10-10/10 had a significantly lower incidence of CMV infection compared to those with HLA matches of 5/10 (P = 0.006). Patients with CYP2C8 (1291 + 106T>C) TT genotypes exhibited a significantly higher incidence of CMV infection than those carrying the variant alleles (P = 0.015) (Supplementary Table S6). Age at transplantation > 104 months and recipient weight ≥ 26 kg both increased incidence of HC (P = 0.040, P = 0.047, respectively). Recipients with donor HLA matches of 9/10-10/10 had a significantly lower incidence of HC than those with HLA matches of 5/10 (P = 0.014). Patients with ABCB1 (1236C>T) CC genotype and SLCO1B1 (1865 + 4846T>C) TT genotype showed a significantly higher incidence of HC compared to carriers with the variant alleles (P = 0.047, P = 0.007, respectively) (Supplementary Table S7). Recipients with donor HLA matches of 9/10-10/10 had a significantly lower incidence of CLS compared to those with HLA matches of 5/10 (P = 0.016) (Supplementary Table S8).
We constructed multivariate Cox regression analysis using variables selected by univariate Log-rank test analysis with P < 0.2, and the results are presented in Supplementary Table S1-S8. We identified UCB-HSCT was an independent risk factor for peri-ES (P = 0.008), while donor-recipient HLA matches of 9/10-10/10 was an independent protective factor for peri-ES (P = 0.021) (Supplementary Table S1). Recipients with weight ≤ 26 kg, major or minor ABO blood type mismatches, and CYP2C19 (99T>C) TC/CC genotype were all independent risk factors for grades II-IV aGVHD (all P < 0.050) (Supplementary Table S2). MUD-HSCT increased the risk of EBV infection (P = 0.019), while donor-recipient HLA matches of 9/10-10/10 served as an independent protective factor for EBV infection (P = 0.003) (Supplementary Table S5). CYP3A5 (219-237C>T) CT/TT acted independently to protect against CMV infection (P = 0.025) (Supplementary Table S6). The risk factors for HC included recipient being male, age at transplantation > 104 months, ABCB1 (1236C>T) CC genotype, and SLCO1B1 (1865 + 4846T>C) TT genotype (Supplementary Table S7). HLA matches between donors and recipients, particularly 9/10-10/10, were an independent protective factor for CLS (P = 0.031) (Supplementary Table S8).
3.4 Correlation between CsA-related SNPs and post-transplant prognosis
In this study, we evaluated the DFS and OS of the patients in our cohort, founding that the 5-year DFS was 78.8% ± 3.6%, and the 5-year OS was 81.9% ± 3.4%. We examined the factors that might influence patient prognosis, including DFS and OS, using both univariate Log-rank test and multivariate Cox regression analyses.
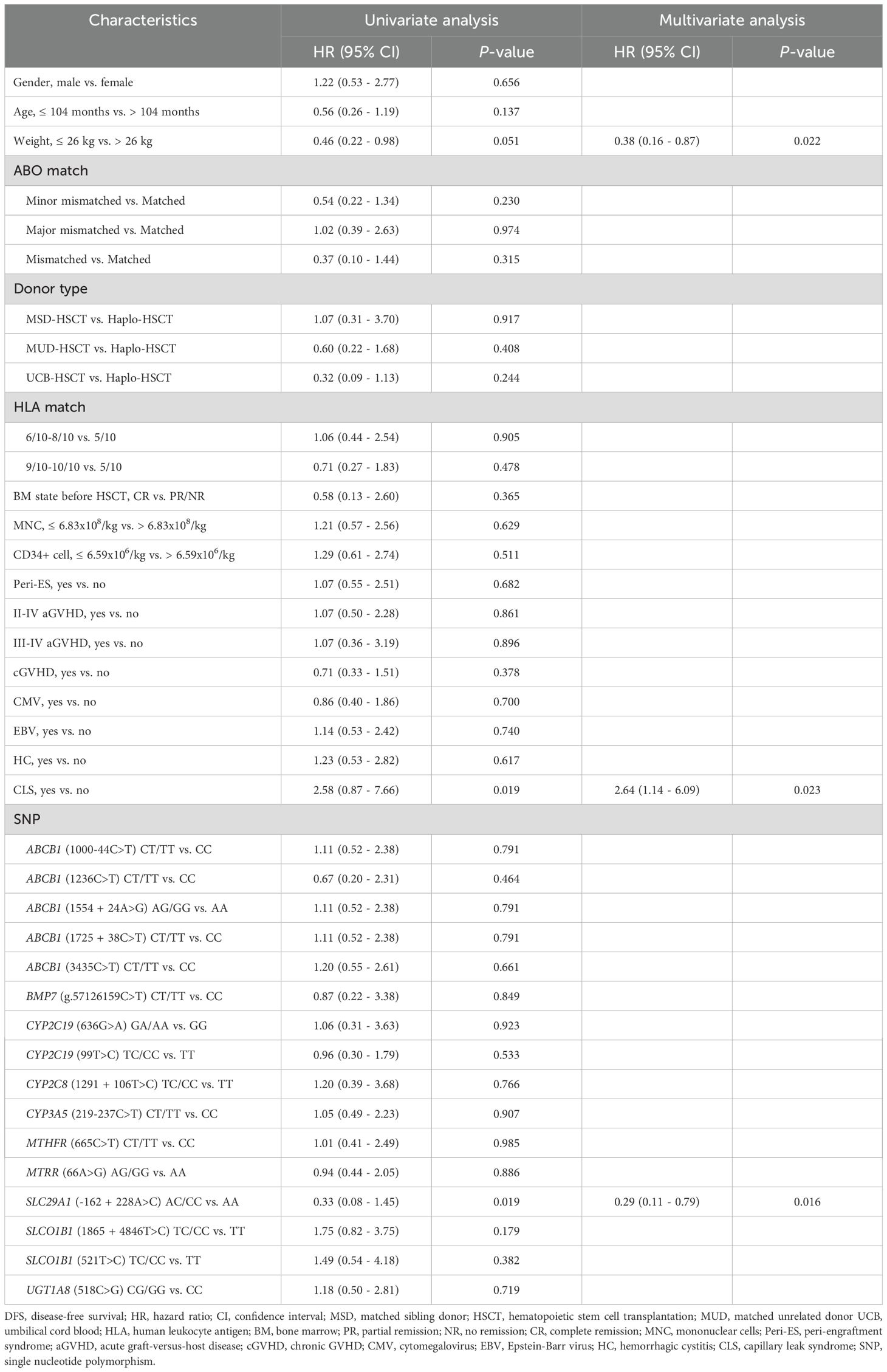
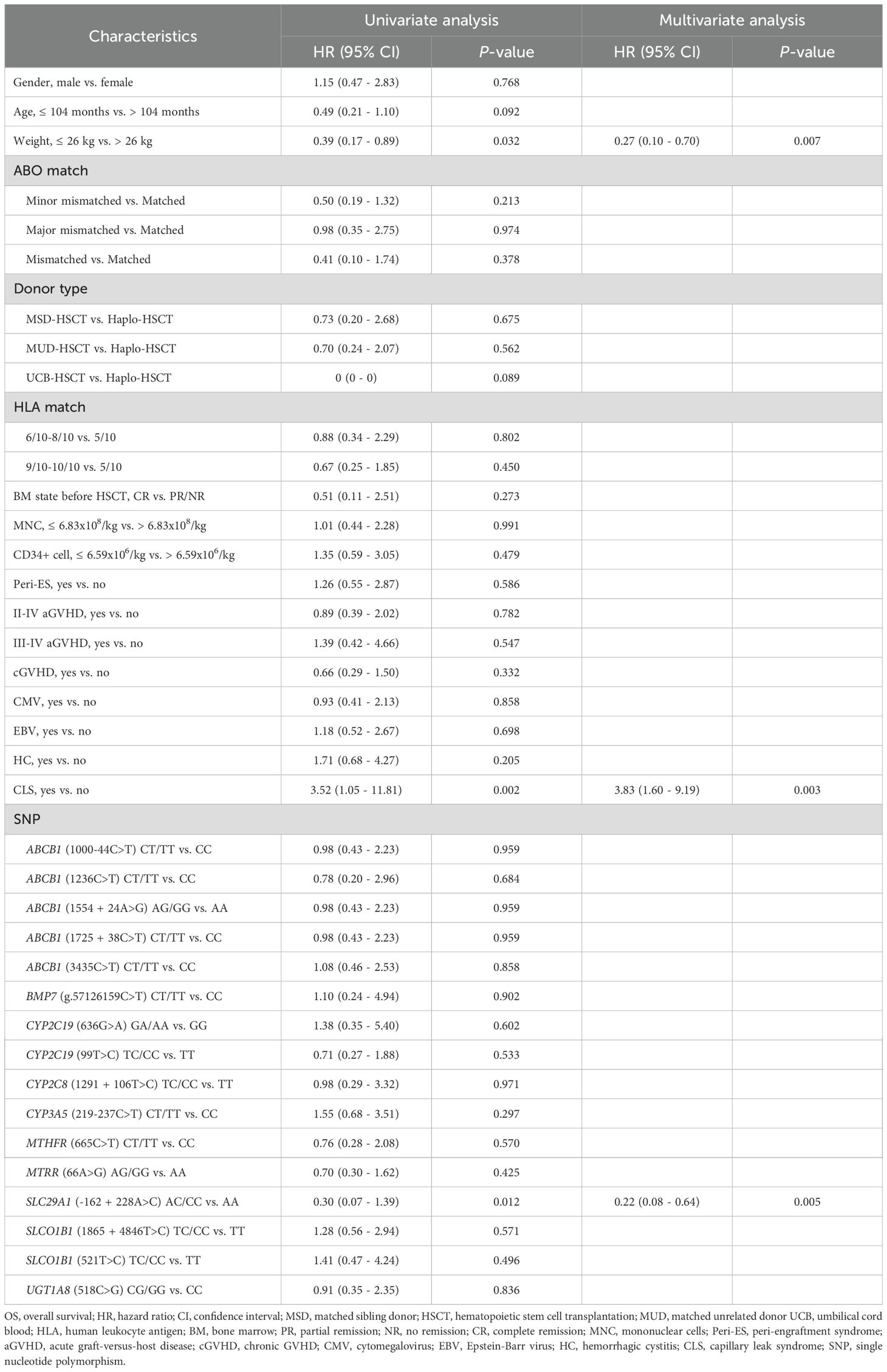
Our univariate analysis revealed that CLS and SLC29A1 (-162 + 228A>C) AA genotype were both associated with poor prognosis in DFS (P = 0.019, P = 0.019, respectively) (Figure 2A, Table 3), and body weight ≥ 26 kg, CLS, and SLC29A1 (-162 + 228A>C) AA genotype were identified as adverse prognostic factors in OS (P = 0.032, P = 0.002, P = 0.012, respectively) (Figure 2B, Table 4).
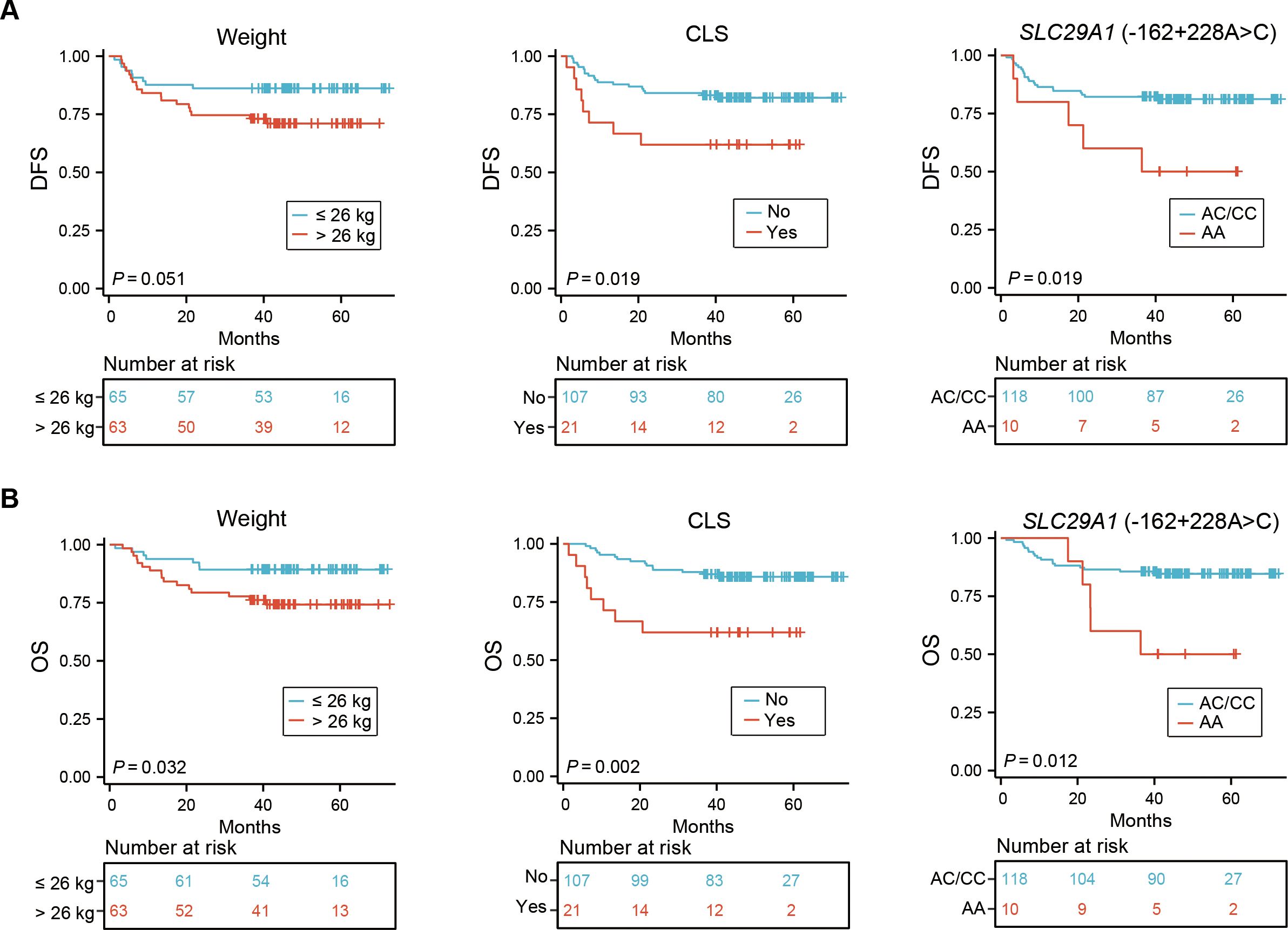
Figure 2. Impact of weight, CLS, and CsA-related SNP on DFS (A) and OS (B) in 128 pediatric patients receiving allo-HSCT. CLS, capillary leak syndrome; CsA, cyclosporine A; SNP, single nucleotide polymorphism; DFS, disease-free survival; OS, overall survival; allo-HSCT, allogeneic hematopoietic stem cell transplantation.
Multivariate analyses showed that patients with a body weight ≥ 26 kg and CLS after HSCT were independent risk factors for both DFS and OS (DFS, P = 0.022, P = 0.023; OS, P = 0.007, P = 0.003, respectively). Additionally, the study revealed that the SLC29A1 (-162 + 228A>C) AA genotype was an independent risk factor for both DFS and OS (P = 0.016, P = 0.005, respectively) (Tables 3, 4).
4 Discussion
Various SNPs are associated with changes in the drug metabolism and effects, explaining differences in drug responses among individuals (28, 29). SNPs affecting the metabolism of drugs like CsA, used in GVHD prophylaxis, have been related to post-HSCT complications (26).
Our study investigates the correlation between CsA-related SNPs and the transplantation outcomes in 128 patients with malignant hematological diseases patients who underwent HSCT. The incidences of various complications were as follows: grades II-IV aGVHD occurred in 47.7% of cases, grades III-IV aGVHD in 14.1%, cGVHD in 47.7%, CMV infection in 57.8%, EBV infection in 53.1%, HC in 29.7%, and CLS in 16.4%. These complication rates are consistent with previously reported data in the literature (21, 30, 31).
The multivariate analysis in this study identifies UCB-HSCT as an independent factor for peri-ES, consistent with previous reports. This observation may due to the critical role played by granulocyte-macrophage colony-stimulating factor, produced by cord blood-derived inflammatory monocytes, in driving the pathology of peri-ES in UCB transplantation (32). Also, the donor-recipient HLA matches of 9/10-10/10 have been identified as an independent protective factor for peri-ES. Previous studies indicate that mismatched donor-recipient HLA matches in allo-HSCT result in poor transplant outcomes, high incidence and severity of GVHD, slow immune reconstitution, and increased risk of severe infections (33). Some studies also suggest that peri-ES represents as an early form of aGVHD, both being immune reactions occurring post-transplant, implying a certain correlation between the occurrence of peri-ES and HLA matching (34, 35). We observed a significant decrease in CLS incidence with donor-recipient HLA matches of 9/10-10/10. Research indicates that calcineurin inhibitors, like cyclosporine and tacrolimus, used during transplantation and donor graft implantation, can activate and damage endothelial cells (36, 37). HLA matches of 9/10-10/10 can effectively reduce immunosuppressant use, decrease the GVHD incidence, endothelial cell damage and CLS incidence. Moreover, Peri-ES, CLS, and GVHD are increasingly recognized as complications driven by the release of inflammatory cytokines (38–40). It is well established that a higher degree of HLA matching results in reduced activation of donor T cells. In the early post-transplant period, donor T cells secrete pro-inflammatory cytokines such as TNF-α, IL-1, and IFN-γ, which contribute to tissue inflammation and endothelial activation (41). HLA mismatch exacerbates this cytokine storm, leading to endothelial injury. Future prospective studies are warranted to investigate the cytokine profiles and immune cell subset dynamics associated with these complications.
Regarding GVHD, the study identifies several independent risk and protective factors. This study is the first to identify independent risk factors for grades II-IV aGVHD at the time of transplantation, including recipient weight ≤ 26 kg, major and minor ABO blood type mismatched between donor and recipient, and CYP2C19 (99T>C) TC/CC genotype. The relationship between recipient weight and GVHD remains controversial. Mehdi Yaseri et al. conducted a meta-analysis examining the relationship between obesity and post-transplant mortality and clinical outcomes in children, finding an increased risk of post-transplant mortality and aGVHD in obese children (42). Additionally, Lam T Khuat et al. suggested that obesity may increase the risk of aGVHD by affecting the microbiota composition (43). However, other studies argue that obesity has no impact on aGVHD (44). Currently, no studies have shown a correlation between low weight and high incidence of aGVHD. However, recipients with lower weight may still face GVHD-related issues, including immune reactions caused by the immunological imbalance between the graft and the host. Lower weights may result in an insufficient immune system to regulate these reactions effectively. Nevertheless, research in this area is relatively scarce, and further in-depth studies are needed to confirm this relationship. This study identified a significant increase in the incidence of grades II-IV aGVHD with major and minor ABO blood type mismatched between donor and recipient, which is identified as an independent risk factor, which aligns with previous research (45). ABO antigens are expressed on the surfaces of tissues like the gastrointestinal tract, liver, and skin, and they retain their original types due to the newly implanted hematopoietic system. Therefore, donor T lymphocytes will attack the incompatible antigens on tissue surfaces, leading to transplant rejection of corresponding organs. Hence, theoretically, HSCT patients with ABO blood type mismatched have a higher risk of GVHD occurrence. CYP2C19 enzyme is highly polymorphic, and its encoding gene is located on human chromosome 10. CYP2C19 SNPs cause variations in enzyme activity, which affects the concentration of related drugs in the body, thereby affecting their efficacy and may lead to serious adverse reactions (46). The CYP2C19 (99T>C) variants, known as CYP2C19*2, reduce the metabolic rate of drugs, including voriconazole and proton pump inhibitors. Following HSCT, cyclosporine is often co-administered with voriconazole or proton pump inhibitors, which can alter its concentration (47). Therefore, we speculate that CYP2C19 (99T>C) variants may influence cyclosporine concentrations through drug interactions, thereby affecting aGVHD, which is an unexpected finding requiring further exploration and validation in subsequent studies.
Recent studies have identified that risk factors for EBV infection in patients after allo-HSCT, including the use of ATG, selective depletion of T cells, HLA mismatched, age ≥ 50 years old at the time of transplantation, and secondary transplantation (48). Since most patients lack fully HLA matched sibling donors, the main obstacles to using such donors for HSCT are graft rejection and GVHD (49–51). Strengthening pre-transplant conditioning regimens and T-cell depletion transplantation have reduced the incidence of these complications but may lead to slow immune reconstitution and increased rates of various infections. Therefore, we hypothesize that HLA mismatching increases the risk of EBV infection. In this study, we observed a significant reduction in EBV infection rates among recipients with HLA matches of 9/10-10/10. Similar to EBV infection, CMV reactivation is closely associated with the use of immunosuppressants. A previous study indicates that, in kidney transplant patients receiving immunosuppressive regimens with CsA, those with CYP3A5 (219-237C>T) CC genotype exhibited higher dose-adjusted trough concentrations and dose-adjusted peak concentrations of CsA than those with variant genotypes. Consequently, patients with CYP3A5 (219-237C>T) CC genotype may experience greater fluctuations in CsA dose adjustment to achieve target concentrations, thereby increasing the risk of CMV infection (25).
HC is a common complication of HSCT, with previous studies identifying factors as associated with its occurrence, including type of transplantation, age at transplantation, presence of GVHD, donor source, and the composition and intensity of the pre-transplant conditioning regimen (52–54). Consistent with prior literature, our study showed that male recipients and older children at the time of transplantation have a significantly higher incidence of HC (55). This could be attributed to estrogen’s protective roles in stabilizing microvasculature and the bladder mucosa, thereby increases the risk of HC in males. Moreover, older children experience higher rates of HC compared to younger children and are more likely to develop severe HC. This may be related to the less mature urinary and central nervous systems in younger children, which lead to more frequent urination, reducing the dwell time of drugs in the bladder and thereby minimizing the irritation and damage to the bladder mucosa by toxic metabolites, ultimately lowering the incidence of HC. Transporters such as the ATP-binding cassette family and the solute carrier family play key roles in drug transport and absorption. Additionally, this study identified wild-type ABCB1 (1236C>T) and SLCO1B1 (1865 + 4846T>C) as independent risk factors for developing HC.
Previous studies have found that CsA inhibits various influx and efflux transporters, including ABCB1 and SLCO1B1, thereby increasing the area under the curve of other substrates such as bosentan, carprofen, and methotrexate. The degree of inhibition varies with different transporter polymorphisms, leading to variable increases in substrate concentrations (56–58). It was demonstrated that the increase in repaglinide concentration was 42% lower in subjects with the SLCO1B1 (521T>C) variant genotype compared to those with the SLCO1B1 (521T>C) TT genotype. This observation is due to the reduced function of OATP1B1 in individuals carrying the variant SLCO1B1 c.521C allele (59). Therefore, we hypothesize that the wild-type ABCB1 (1236C>T) and SLCO1B1 (1865 + 4846T>C) alleles may exhibit higher activity than variant alleles. This could result in CsA significantly increasing the concentrations of other ABCB1 and SLCO1B1 substrates, including immunosuppressants (MTX, tacrolimus), leading to BK virus activation and subsequent HC. On the other hand, HC is influenced by various factors, often associated with high-dose pre-transplant chemotherapy toxicity, genetic polymorphisms in drug-metabolizing enzymes, viral infections, GVHD, patient age, gender, donor type, and transplantation method. Future studies should incorporate additional factors and expand sample sizes for further validation.
Furthermore, the present study identified that the SLC29A1 (-162 + 228A>C) AC/CC genotype is an independent protective factor for both DFS and OS. SLC29A1 encodes human equilibrative nucleoside transporter 1 (hENT1) (60). As an equilibrative nucleoside transporter, hENT1 facilitates the influx of approximately 80% of cytarabine (Ara-C) into leukemia cells. A study involving 103 AML patients aged 16 to 76 years revealed that SLC29A1 variants and haplotypes may influence the Ara-C uptake activity and the complete remission rate (60, 61). Wan et al. analyzed 19 SLC29A1 SNPs in AML patients and found patients with low SLC29A1 gene expression had shorter DFS and OS during Ara-C treatment. Moreover, significant differences were observed in the expression of the rs9394992 and rs324148 genotypes of SLC29A1 gene between remission and relapse phases (62). The SLC29A1 (-162 + 228A>C) variant, also known as rs693955, has been reported to be associated with a shorter duration of neutropenia following chemotherapy (63). However, no studies have yet demonstrated the relationship between this locus and prognosis. We hypothesize that the SLC29A1 (-162 + 228A>C) variants may influence DFS and OS by affecting the hENT1 protein, thereby interfering with the absorption of cytarabine. In the future, we may verify this hypothesis by measuring plasma cytarabine levels using high-performance liquid chromatography.
However, our study has several limitations. First, the sample size is relatively small. Second, potential confounding factors, such as donor source variability, prior treatments, and differences in post-transplant care, could not be fully accounted for. Third, due to the retrospective nature of the study, external validation in independent cohorts and functional experimental confirmation are currently lacking. To address these issues, we will conduct a prospective, multicenter study with expanded sample size to validate our findings, construct clinically applicable predictive models, and establish mouse models of bone marrow transplantation harboring specific SNPs to investigate the pharmacokinetics and pharmacodynamics of CsA in the future.
In conclusion, our study has revealed correlations between CsA-related transporters and metabolic enzymes SNPs and post-transplant complications and prognosis, contributing to a better understanding of the interindividual difference in drug efficacy. Future studies on adjusting the dosage of drugs based on SNPs in clinical practice may be one of the options for improving the HSCT outcomes.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://db.cngb.org, CNP0004415, CNP0005143, CNP0007618.
Ethics statement
The studies involving humans were approved by Children’s Hospital of Soochow University Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
QJ: Data curation, Writing – original draft, Investigation, Formal analysis, Conceptualization, Writing – review & editing, Methodology. SZ: Methodology, Writing – original draft, Data curation, Formal analysis. ML: Writing – original draft, Data curation, Formal analysis, Methodology. WZ: Data curation, Writing – original draft, Formal analysis, Methodology. LL: Formal analysis, Writing – original draft, Data curation, Methodology. YC: Methodology, Formal analysis, Writing – original draft, Data curation. LG: Data curation, Methodology, Funding acquisition, Writing – original draft. BL: Writing – original draft, Funding acquisition, Methodology, Data curation. ZD: Methodology, Writing – original draft, Data curation. YH: Writing – original draft, Funding acquisition, Data curation, Methodology. PX: Methodology, Writing – original draft, Data curation. JLing: Writing – original draft, Methodology, Data curation. LF: Writing – original draft, Data curation, Methodology. XB: Writing – original draft, Methodology, Data curation. HC: Writing – original draft, Methodology, Data curation. JLi: Data curation, Methodology, Writing – original draft. JLu: Data curation, Writing – original draft, Methodology. YZ: Writing – review & editing, Conceptualization, Funding acquisition, Validation. SW: Writing – review & editing, Conceptualization, Validation, Funding acquisition. JQ: Validation, Conceptualization, Writing – original draft, Supervision, Writing – review & editing. SH: Writing – review & editing, Funding acquisition, Validation, Conceptualization, Writing – original draft, Supervision. YL: Supervision, Writing – review & editing, Conceptualization, Funding acquisition, Validation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by following grants: National Key R&D Program of China (2022YFC2502700), the National Natural Science Foundation of China (No. 82470221, 82170218, 82100229, 82200177, 82300244, 82470127, 82300182, 82470160, 82400264), Jiangsu Natural Science Foundation (No. BK20240373), Gusu Innovation and Entrepreneurship Leading Talent Program (ZXL2024387), Suzhou project (No. DZXYJ202305, SZS201615, SKY2022012 and SZS2023014), Soochow Medical School project (ML13101223) and Children’s Hospital of Soochow University grant (2023SYLCYJ01).
Acknowledgments
We also thank all the patients who participated in this study.
Conflict of interest
Authors LL, HC, and JQ are employed by the company Acornmed Biotechnology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1615976/full#supplementary-material
References
1. Muñiz P, Andrés-Zayas C, Carbonell D, Chicano M, Bailén R, Oarbeascoa G, et al. Association between gene polymorphisms in the cyclophosphamide metabolism pathway with complications after haploidentical hematopoietic stem cell transplantation. Front Immunol. (2022) 13:1002959. doi: 10.3389/fimmu.2022.1002959
2. Zaidman I, Even-Or E, Aharoni E, Averbuch D, Dinur-Schejter Y, NaserEddin A, et al. Risk and promise: an 11-year, single-center retrospective study of severe acute gvhd in pediatric patients undergoing allogeneic hsct for nonmalignant diseases. Front Pediatr. (2023) 11:1194891. doi: 10.3389/fped.2023.1194891
3. Annaloro C, Serpenti F, Saporiti G, Galassi G, Cavallaro F, Grifoni F, et al. Viral infections in hsct: detection, monitoring, clinical management, and immunologic implications. Front Immunol. (2020) 11:569381. doi: 10.3389/fimmu.2020.569381
4. Yanagimachi M, Naruto T, Tanoshima R, Kato H, Yokosuka T, Kajiwara R, et al. Influence of cyp3a5 and abcb1 gene polymorphisms on calcineurin inhibitor-related neurotoxicity after hematopoietic stem cell transplantation. Clin Transplant. (2010) 24:855–61. doi: 10.1111/j.1399-0012.2009.01181.x
5. Kapturczak MH, Meier-Kriesche HU, and Kaplan B. Pharmacology of calcineurin antagonists. Transplant Proc. (2004) 36:25s–32s. doi: 10.1016/j.transproceed.2004.01.018
6. Leclerc V, Ducher M, and Bleyzac N. Bayesian networks: A new approach to predict therapeutic range achievement of initial cyclosporine blood concentration after pediatric hematopoietic stem cell transplantation. Drugs R D. (2018) 18:67–75. doi: 10.1007/s40268-017-0223-7
7. Dai Y, Iwanaga K, Lin YS, Hebert MF, Davis CL, Huang W, et al. In vitro metabolism of cyclosporine a by human kidney cyp3a5. Biochem Pharmacol. (2004) 68:1889–902. doi: 10.1016/j.bcp.2004.07.012
8. Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, et al. Sequence diversity in cyp3a promoters and characterization of the genetic basis of polymorphic cyp3a5 expression. Nat Genet. (2001) 27:383–91. doi: 10.1038/86882
9. Hu YF, Qiu W, Liu ZQ, Zhu LJ, Liu ZQ, Tu JH, et al. Effects of genetic polymorphisms of cyp3a4, cyp3a5 and mdr1 on cyclosporine pharmacokinetics after renal transplantation. Clin Exp Pharmacol Physiol. (2006) 33:1093–8. doi: 10.1111/j.1440-1681.2006.04492.x
10. Qiu XY, Jiao Z, Zhang M, Zhong LJ, Liang HQ, Ma CL, et al. Association of mdr1, cyp3a4*18b, and cyp3a5*3 polymorphisms with cyclosporine pharmacokinetics in chinese renal transplant recipients. Eur J Clin Pharmacol. (2008) 64:1069–84. doi: 10.1007/s00228-008-0520-8
11. Kim RB. Mdr1 single nucleotide polymorphisms: multiplicity of haplotypes and functional consequences. Pharmacogenetics. (2002) 12:425–7. doi: 10.1097/00008571-200208000-00002
12. Kalliokoski A and Niemi M. Impact of oatp transporters on pharmacokinetics. Br J Pharmacol. (2009) 158:693–705. doi: 10.1111/j.1476-5381.2009.00430.x
13. Lee J, Wang R, Yang Y, Lu X, Zhang X, Wang L, et al. The effect of abcb1 C3435t polymorphism on cyclosporine dose requirements in kidney transplant recipients: A meta-analysis. Basic Clin Pharmacol Toxicol. (2015) 117:117–25. doi: 10.1111/bcpt.12371
14. Johnson LA, Oetting WS, Basu S, Prausa S, Matas A, and Jacobson PA. Pharmacogenetic effect of the ugt polymorphisms on mycophenolate is modified by calcineurin inhibitors. Eur J Clin Pharmacol. (2008) 64:1047–56. doi: 10.1007/s00228-008-0501-y
15. Tuğlular S, Gogas Yavuz D, Cakalağaoğlu F, Citak L, Arikan H, Koçak H, et al. Cyclosporine-a induced nephrotoxicity is associated with decreased renal bone morphogenetic protein-7 expression in rats. Transplant Proc. (2004) 36:131–3. doi: 10.1016/j.transproceed.2003.11.018
16. Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: A report from the mount sinai acute gvhd international consortium. Biol Blood Marrow Transplant. (2016) 22:4–10. doi: 10.1016/j.bbmt.2015.09.001
17. Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. (2015) 21:389–401.e1. doi: 10.1016/j.bbmt.2014.12.001
18. Cesaro S, Dalianis T, Hanssen Rinaldo C, Koskenvuo M, Pegoraro A, Einsele H, et al. Ecil guidelines for the prevention, diagnosis and treatment of bk polyomavirus-associated haemorrhagic cystitis in haematopoietic stem cell transplant recipients. J Antimicrob Chemother. (2018) 73:12–21. doi: 10.1093/jac/dkx324
19. Lucchini G, Willasch AM, Daniel J, Soerensen J, Jarisch A, Bakhtiar S, et al. Epidemiology, risk factors, and prognosis of capillary leak syndrome in pediatric recipients of stem cell transplants: A retrospective single-center cohort study. Pediatr Transplant. (2016) 20:1132–6. doi: 10.1111/petr.12831
20. Hong KT, Kang HJ, Kim NH, Kim MS, Lee JW, Kim H, et al. Peri-engraftment syndrome in allogeneic hematopoietic sct. Bone Marrow Transplant. (2013) 48:523–8. doi: 10.1038/bmt.2012.171
21. Ji Q, Zhang Y, Hu Y, Liu L, Cao S, Gao L, et al. The influence of methotrexate-related transporter and metabolizing enzyme gene polymorphisms on peri-engraftment syndrome and graft-versus-host disease after haplo-hematopoietic stem cell transplantation in pediatric patients with Malignant hematological diseases. Front Immunol. (2023) 14:1229266. doi: 10.3389/fimmu.2023.1229266
22. Sanchez-Lazaro I, Herrero MJ, Jordan-De Luna C, Boso V, Almenar L, Rojas L, et al. Association of snps with the efficacy and safety of immunosuppressant therapy after heart transplantation. Pharmacogenomics. (2015) 16:971–9. doi: 10.2217/pgs.15.39
23. Chen Z, Zhang L, Yang C, Jiang Z, Shen H, and Gui G. Effect of mdr1 C1236t polymorphism on cyclosporine pharmacokinetics: A systematic review and meta-analysis. Med (Baltimore). (2017) 96:e8700. doi: 10.1097/MD.0000000000008700
24. Crettol S, Venetz JP, Fontana M, Aubert JD, Ansermot N, Fathi M, et al. Influence of abcb1 genetic polymorphisms on cyclosporine intracellular concentration in transplant recipients. Pharmacogenet Genomics. (2008) 18:307–15. doi: 10.1097/FPC.0b013e3282f7046f
25. Zhu HJ, Yuan SH, Fang Y, Sun XZ, Kong H, and Ge WH. The effect of cyp3a5 polymorphism on dose-adjusted cyclosporine concentration in renal transplant recipients: A meta-analysis. Pharmacogenom J. (2011) 11:237–46. doi: 10.1038/tpj.2010.26
26. Rocha V, Porcher R, Fernandes JF, Filion A, Bittencourt H, Silva W Jr., et al. Association of drug metabolism gene polymorphisms with toxicities, graft-versus-host disease and survival after hla-identical sibling hematopoietic stem cell transplantation for patients with leukemia. Leukemia. (2009) 23:545–56. doi: 10.1038/leu.2008.323
27. Woillard JB, Rerolle JP, Picard N, Rousseau A, Drouet M, Munteanu E, et al. Risk of diarrhoea in a long-term cohort of renal transplant patients given mycophenolate mofetil: the significant role of the ugt1a8–2 variant allele. Br J Clin Pharmacol. (2010) 69:675–83. doi: 10.1111/j.1365-2125.2010.03625.x
28. Ahmed S, Zhou Z, Zhou J, and Chen SQ. Pharmacogenomics of drug metabolizing enzymes and transporters: relevance to precision medicine. Genom Proteomics Bioinf. (2016) 14:298–313. doi: 10.1016/j.gpb.2016.03.008
29. Cheng Y, Zhao J, Jia H, Yuan Z, and Li Z. Ligase chain reaction coupled with rolling circle amplification for high sensitivity detection of single nucleotide polymorphisms. Analyst. (2013) 138:2958–63. doi: 10.1039/c3an36920j
30. Lai YR, Chen YH, Hu DM, Jiang M, Liu QF, Liu L, et al. Multicenter phase ii study of a combination of cyclosporine a, methotrexate and mycophenolate mofetil for gvhd prophylaxis: results of the chinese bone marrow transplant cooperative group (Cbmtcg). J Hematol Oncol. (2014) 7:59. doi: 10.1186/s13045-014-0059-3
31. Xu L, Chen H, Chen J, Han M, Huang H, Lai Y, et al. The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China-recommendations from the chinese society of hematology. J Hematol Oncol. (2018) 11:33. doi: 10.1186/s13045-018-0564-x
32. Jin L, Sun Z, Liu H, Zhu X, Zhou Y, Fu B, et al. Inflammatory monocytes promote pre-engraftment syndrome and tocilizumab can therapeutically limit pathology in patients. Nat Commun. (2021) 12:4137. doi: 10.1038/s41467-021-24412-1
33. Bierings M, Nachman JB, and Zwaan CM. Stem cell transplantation in pediatric leukemia and myelodysplasia: state of the art and current challenges. Curr Stem Cell Res Ther. (2007) 2:53–63. doi: 10.2174/157488807779317035
34. Kishi Y, Kami M, Miyakoshi S, Kanda Y, Murashige N, Teshima T, et al. Early immune reaction after reduced-intensity cord-blood transplantation for adult patients. Transplantation. (2005) 80:34–40. doi: 10.1097/01.tp.0000163289.20406.86
35. Cho BK, Rao VP, Ge Q, Eisen HN, and Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med. (2000) 192:549–56. doi: 10.1084/jem.192.4.549
36. Mercanoglu F, Turkmen A, Kocaman O, Pinarbasi B, Dursun M, Selcukbiricik F, et al. Endothelial dysfunction in renal transplant patients is closely related to serum cyclosporine levels. Transplant Proc. (2004) 36:1357–60. doi: 10.1016/j.transproceed.2004.05.073
37. Woywodt A, Haubitz M, Buchholz S, and Hertenstein B. Counting the cost: markers of endothelial damage in hematopoietic stem cell transplantation. Bone Marrow Transplant. (2004) 34:1015–23. doi: 10.1038/sj.bmt.1704733
38. Mehta AK and Koreth J. Toward improving initial therapy of acute graft versus host disease. Am J Hematol. (2025) 100 Suppl 3:40–54. doi: 10.1002/ajh.27593
39. Komara NL, Paragomi P, Greer PJ, Wilson AS, Breze C, Papachristou GI, et al. Severe acute pancreatitis: capillary permeability model linking systemic inflammation to multiorgan failure. Am J Physiol Gastrointest Liver Physiol. (2020) 319:G573–G83. doi: 10.1152/ajpgi.00285.2020
40. Khandelwal P, Mellor-Heineke S, Rehman N, Lane A, Smiley K, Villanueva J, et al. Cytokine profile of engraftment syndrome in pediatric hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant. (2016) 22:690–7. doi: 10.1016/j.bbmt.2015.12.016
41. Tvedt THA, Ersvaer E, Tveita AA, and Bruserud O. Interleukin-6 in allogeneic stem cell transplantation: its possible importance for immunoregulation and as a therapeutic target. Front Immunol. (2017) 8:667. doi: 10.3389/fimmu.2017.00667
42. Yaseri M, Alipoor E, Seifollahi A, Rouhifard M, Salehi S, and Hosseinzadeh-Attar MJ. Association of obesity with mortality and clinical outcomes in children and adolescents with transplantation: A systematic review and meta-analysis. Rev Endocr Metab Disord. (2021) 22:847–58. doi: 10.1007/s11154-021-09641-5
43. Khuat LT, Vick LV, Choi E, Dunai C, Merleev AA, Maverakis E, et al. Mechanisms by which obesity promotes acute graft-versus-host disease in mice. Front Immunol. (2021) 12:752484. doi: 10.3389/fimmu.2021.752484
44. Ladhani M, Lade S, Alexander SI, Baur LA, Clayton PA, McDonald S, et al. Obesity in pediatric kidney transplant recipients and the risks of acute rejection, graft loss and death. Pediatr Nephrol. (2017) 32:1443–50. doi: 10.1007/s00467-017-3636-1
45. Chung HJ, Lee JH, and Kwon SW. Significance of donor-derived isoagglutinins in abo-incompatible hematopoietic stem cell transplantation. J Clin Lab Anal. (2008) 22:383–90. doi: 10.1002/jcla.20269
46. Mirabbasi SA, Khalighi K, Wu Y, Walker S, Khalighi B, Fan W, et al. Cyp2c19 genetic variation and individualized clopidogrel prescription in a cardiology clinic. J Community Hosp Intern Med Perspect. (2017) 7:151–6. doi: 10.1080/20009666.2017.1347475
47. Fu R, Tajima S, Suetsugu K, Watanabe H, Egashira N, and Masuda S. Biomarkers for individualized dosage adjustments in immunosuppressive therapy using calcineurin inhibitors after organ transplantation. Acta Pharmacol Sin. (2019) 40:151–9. doi: 10.1038/s41401-018-0070-2
48. Law N, Logan C, and Taplitz R. Ebv reactivation and disease in allogeneic hematopoietic stem cell transplant (Hsct) recipients and its impact on hsct outcomes. Viruses. (2024) 16:1294. doi: 10.3390/v16081294
49. Landgren O, Gilbert ES, Rizzo JD, Socié G, Banks PM, Sobocinski KA, et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood. (2009) 113:4992–5001. doi: 10.1182/blood-2008-09-178046
50. Uhlin M, Wikell H, Sundin M, Blennow O, Maeurer M, Ringden O, et al. Risk factors for epstein-barr virus-related post-transplant lymphoproliferative disease after allogeneic hematopoietic stem cell transplantation. Haematologica. (2014) 99:346–52. doi: 10.3324/haematol.2013.087338
51. Juvonen E, Aalto S, Tarkkanen J, Volin L, Hedman K, and Ruutu T. Retrospective evaluation of serum epstein barr virus DNA levels in 406 allogeneic stem cell transplant patients. Haematologica. (2007) 92:819–25. doi: 10.3324/haematol.10751
52. Silva Lde P, Patah PA, Saliba RM, Szewczyk NA, Gilman L, Neumann J, et al. Hemorrhagic cystitis after allogeneic hematopoietic stem cell transplants is the complex result of bk virus infection, preparative regimen intensity and donor type. Haematologica. (2010) 95:1183–90. doi: 10.3324/haematol.2009.016758
53. Mori T, Aisa Y, Shimizu T, Ikeda Y, Okamoto S, Okada K, et al. Hemorrhagic cystitis caused by adenovirus type 34 after allogeneic bone marrow transplantation. Transplantation. (2005) 79:624. doi: 10.1097/01.tp.0000147653.16324.ca
54. Lunde LE, Dasaraju S, Cao Q, Cohn CS, Reding M, Bejanyan N, et al. Hemorrhagic cystitis after allogeneic hematopoietic cell transplantation: risk factors, graft source and survival. Bone Marrow Transplant. (2015) 50:1432–7. doi: 10.1038/bmt.2015.162
55. Asano Y, Kanda Y, Ogawa N, Sakata-Yanagimoto M, Nakagawa M, Kawazu M, et al. Male Predominance among Japanese Adult Patients with Late-Onset Hemorrhagic Cystitis after Hematopoietic Stem Cell Transplantation. Bone Marrow Transplant. (2003) 32:1175–9. doi: 10.1038/sj.bmt.1704274
56. Binet I, Wallnöfer A, Weber C, Jones R, and Thiel G. Renal hemodynamics and pharmacokinetics of bosentan with and without cyclosporine A. Kidney Int. (2000) 57:224–31. doi: 10.1046/j.1523-1755.2000.00838.x
57. Fox RI, Morgan SL, Smith HT, Robbins BA, Choc MG, and Baggott JE. Combined oral cyclosporin and methotrexate therapy in patients with rheumatoid arthritis elevates methotrexate levels and reduces 7-hydroxymethotrexate levels when compared with methotrexate alone. Rheumatol (Oxford). (2003) 42:989–94. doi: 10.1093/rheumatology/keg277
58. Niemi M. Role of oatp transporters in the disposition of drugs. Pharmacogenomics. (2007) 8:787–802. doi: 10.2217/14622416.8.7.787
59. Kajosaari LI, Niemi M, Neuvonen M, Laitila J, Neuvonen PJ, and Backman JT. Cyclosporine markedly raises the plasma concentrations of repaglinide. Clin Pharmacol Ther. (2005) 78:388–99. doi: 10.1016/j.clpt.2005.07.005
60. Kim JH, Lee C, Cheong HS, Koh Y, Ahn KS, Kim HL, et al. Slc29a1 (Ent1) polymorphisms and outcome of complete remission in acute myeloid leukemia. Cancer Chemother Pharmacol. (2016) 78:533–40. doi: 10.1007/s00280-016-3103-x
61. Kulsoom B, Shamsi TS, and Afsar NA. Gene expression of hent1, dck, cda, dcmpd and topoisomerase iiα as an indicator of chemotherapy response in aml treated with cytarabine and daunorubicin. Cancer Manag Res. (2018) 10:5573–89. doi: 10.2147/cmar.S181299
62. Wan H, Zhu J, Chen F, Xiao F, Huang H, Han X, et al. Slc29a1 single nucleotide polymorphisms as independent prognostic predictors for survival of patients with acute myeloid leukemia: an in vitro study. J Exp Clin Cancer Res. (2014) 33:90. doi: 10.1186/s13046-014-0090-9
Keywords: HSCT, CSA, SNP, complication, prognosis
Citation: Ji Q, Zhang S, Liu M, Zhang W, Liu L, Chai Y, Gao L, Li B, Du Z, Hu Y, Xiao P, Ling J, Fan L, Bian X, Chen H, Li J, Lu J, Zhang Y, Wu S, Qin J, Hu S and Li Y (2025) Impact of cyclosporine A-related single nucleotide polymorphisms on post-transplant outcomes in pediatric hematologic malignancy patients undergoing allogeneic hematopoietic stem cell transplantation. Front. Immunol. 16:1615976. doi: 10.3389/fimmu.2025.1615976
Received: 22 April 2025; Accepted: 24 June 2025;
Published: 22 July 2025.
Edited by:
Qi Zhang, Yale University, United StatesReviewed by:
Debmalya Sengupta, Bidhannagar College, IndiaZheng Yuan, China Academy of Chinese Medical Sciences, China
Yuanyin Teng, Zhejiang University, China
Copyright © 2025 Ji, Zhang, Liu, Zhang, Liu, Chai, Gao, Li, Du, Hu, Xiao, Ling, Fan, Bian, Chen, Li, Lu, Zhang, Wu, Qin, Hu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongping Zhang, eXB6aGFuZ0BhbHUuc3VkYS5lZHUuY24=; Shuiyan Wu, d3VzaHVpeWFueUAxNjMuY29t; Jiayue Qin, anlxaW5AbGl2ZS5jbg==; Shaoyan Hu, aHVzaGFveWFuQHN1ZGEuZWR1LmNu; Yizhen Li, bGl5ekBzdWRhLmVkdS5jbg==
†These authors have contributed equally to this work
 Qi Ji
Qi Ji Senlin Zhang
Senlin Zhang Minyuan Liu1†
Minyuan Liu1† Weiliang Zhang
Weiliang Zhang Lixia Liu
Lixia Liu Li Gao
Li Gao Bohan Li
Bohan Li Zhizhuo Du
Zhizhuo Du Yixin Hu
Yixin Hu Peifang Xiao
Peifang Xiao Jie Li
Jie Li Jun Lu
Jun Lu Yongping Zhang
Yongping Zhang Shuiyan Wu
Shuiyan Wu Jiayue Qin
Jiayue Qin Shaoyan Hu
Shaoyan Hu