- 1Department of Emergency, The First Hospital of China Medical University, Shenyang, China
- 2Trauma Center, The First Hospital of China Medical University, Shenyang, China
- 3Department of Clinical Epidemiology and Evidence-based Medicine, The First Hospital of China Medical University, Shenyang, China
- 4Department of Gastrointestinal Surgery, The First Hospital of China Medical University, Shenyang, China
Background: Sex and age are significant factors influencing the prognosis of various types of cancer. However, the impact of sex and age on the prognosis of gastrointestinal stromal tumors (GISTs) remains unclear. Investigating the interaction between sex and age may facilitate a more precise assessment of the prognosis of GISTs.
Methods: A retrospective analysis was conducted on a cohort of 5318 patients with GISTs, utilizing the Cox regression model to analyze the disparities in disease-specific survival (DSS) across sex. Subsequently, the cohort after propensity score matching was employed to investigate the prognostic differences attributable to age variations, and restricted cubic spline analysis was utilized to assess the prognostic disparities associated with different sexes and ages in GIST.
Results: This investigation demonstrated substantial sex-based disparities in the clinical characteristics of GIST. With respect to prognosis, males exhibited a significantly elevated hazard ratio (HR) for DSS in comparison to females (HR = 1.40, p<0.001), which persisted following multivariate (HR = 1.38, p=0.006) and propensity score matching analyses (HR = 1.36, p=0.014). Moreover, a significant interaction between age and sex was observed in predicting DSS, notably indicating that younger female subjects (≤50 years) demonstrated a more favorable prognosis relative to their male counterparts.
Conclusions: Female patients with GIST exhibit a more favorable prognosis than males, with this advantage decreasing with advancing age.
Introduction
Gastrointestinal stromal tumors (GISTs) predominantly occur in elderly individuals and demonstrate similar prevalence across sexes (1–3). Most GISTs exhibit genetic mutations, predominantly activating mutations in KIT and PDGFRA, which function as crucial targets for tyrosine kinase inhibitors (TKIs) in adjuvant therapy (4–6). While the prognosis and risk classification of GIST remain contentious, tumor size, tumor site, and mitotic index are considered critical factors in risk classification (7, 8). However, with the advancement of precision therapy for GISTs, other prognostic factors such as gastrointestinal bleeding, Ki-67, and age > 50 years have attracted the attention of researchers (9–11).
The etiology and progression of numerous cancers are influenced by sex-specific biological disparities, including variations in chromosomal composition and hormonal profiles (12). These disparities are associated with differences in cancer incidence and survival rates. Sex-based differences are correlated with human behavior, attitudes, and social and cultural expectations of the same. Although controversial, GISTs exhibit sex-specific differences (13). Males are predominant in most cancers (14). Studies have reported the discovery of estrogen receptors in GIST (15, 16); however, research examining the disparities in estrogen levels between females and males and their influence on tumor prognosis remains limited. Similarly, age is a significant prognostic factor (10). Emerging evidence suggests that sex hormones, genetics, and the immune system play specific roles in regulating the risk and progression of other malignant tumors (17, 18); however, this has not been conclusively demonstrated in GIST.
The incidence of GIST is marginally higher in males compared to females (19). Moreover, male patients with GIST exhibit more aggressive characteristics, including larger tumor size, higher mitotic index, and increased likelihood of progression to tumor rupture and metastasis. A study confirmed that the prognosis of gist was significantly correlated with sex (20). Another study revealed that patients with GIST aged ≤60 years have a more favorable prognosis compared to those aged >60 years, suggesting that age may serve as an independent prognostic factor for GIST (21). In a multicenter cohort study, the combination of age and sex demonstrated a more favorable prognosis for GIST in patients under 50 years of age and in females (10). However, further validation using large-scale cohort studies is necessary.
Age and sex, as potential prognostic factors, have yet to be incorporated into the existing prognostic risk classification system. This study aimed to assess the prognostic disparities between male and female patients with GISTs through comprehensive cohort analyses, accounting for potential confounding variables that may influence prognosis. This study not only provides a better assessment of the prognosis for patients with GIST but also serves as a reference for the intensity of adjuvant therapy.
Methods
Data sources
The research cohort data were derived from the SEER Research Data (2000–2018), 18 Registries, and November 2020 Sub database. A total of 9,957 patients’ information and follow-up data were collected. The criteria for patient inclusion were the availability of data based on the International Classification of Diseases for Oncology, third edition (ICD-O-3), and disease-specific survival (DSS) for individuals diagnosed with GISTs. Screening requirements for potentially eligible patients with GIST included a histologic type of ICD-O-38936 from one of the specified sites, including the Appendix, Colon, Esophagus, Peritoneum, Rectum, Small intestine, and stomach. As the SEER is a publicly available database with anonymized data, this study was exempted by the Ethics Committee of China Medical University.
Study population
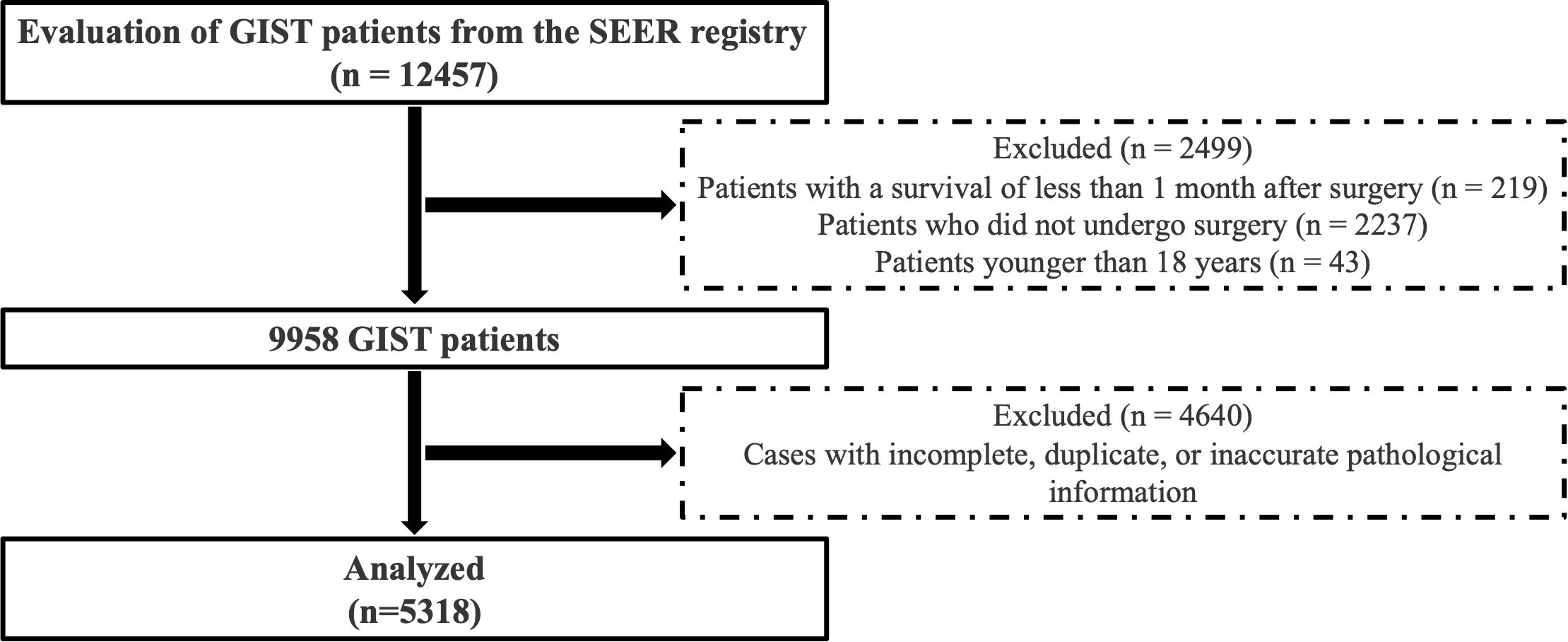
Patients with GIST obtained from the SEER database were excluded based on the following criteria: (a) patients who did not undergo surgical intervention; (b) patients under 18 years of age; (c) patients with a survival duration of less than one month post-surgery; and (d) patients with incomplete, duplicate, or inaccurate pathological data. A total of 5318 cases were included in the statistical analysis. The cohort selection process is illustrated in Figure 1.
We excluded patients with missing, duplicate, or inconsistent pathological data. The variables with missing values included tumor size, mitotic index, AJCC stage and tumor site. Other variables such as age, sex, and marital status were complete. Given the lack of a robust multiple imputation strategy for the SEER database in this context and to maintain consistency in the analysis, we opted for complete-case analysis. We acknowledge that this approach may introduce selection bias, particularly if the missing data are not random. However, sensitivity analyses excluding patients with survival <1 month were conducted to assess the robustness of our findings.
Statistical analysis
Comparisons of baseline characteristics between the sexes were conducted utilizing t-tests and chi-square tests. Survival rates were ascertained using the Kaplan-Meier method, and survival curves were graphically represented. Univariate analysis was performed using the log-rank test, whereas multivariate analysis was performed utilizing the Cox test, with all baseline factors incorporated as adjustment variables. To account for mortality as a competing event, we employed the Fine and Gray subdistribution hazard model in sensitivity analyses to assess the relationship between baseline characteristics and GIST-specific mortality.
Given the potential disparities in variables between the sexes at baseline, propensity score matching (PSM) was employed to address the imbalance. Nearest-neighbor matching with a ratio of 1:1 and a caliper of 0.1 was implemented between different sex groups. The adjusted factors included age, tumor size, marital status, race, origin, tumor site, T stage, N stage, M stage, AJCC stage, radiation, and mitotic index. A standardized mean difference (SMD) of less than 0.1 was considered indicative of balance between variables.
A multiplicative interaction model was employed to analyze the influence of age and sex on the outcomes of patients with GIST. We performed a stratified analysis based on tumor location (gastric vs. non-gastric) to further investigate potential interaction effects between age and sex. To ensure the robustness of the results, a sensitivity analysis was conducted by excluding patients with a survival time of less than one month. To assess the potential non-linear relationship between age and DSS and to visualize how the HR changes continuously with age for sex, we employed restricted cubic spline (RCS) regression. This method is advantageous as it does not impose a pre-specified linear or parametric form on the relationship, allowing the data to reveal the true functional form. The number and positions of the knots, which define the smoothness and flexibility of the spline curve, were chosen based on established statistical recommendations to balance model flexibility and prevent overfitting. The relationships between age, sex, and DSS were investigated utilizing an RCS model. The patients were stratified into three age groups (< 50 years, 50–60 years, and > 60 years), and the association between sex and DSS was examined. To evaluate whether the mediating effect could elucidate the survival differences between females and males, Causal mediation analysis was performed using the ‘mediation’ package in R to quantify the direct and indirect effects of sex on GIST-specific survival. This method is grounded within the counterfactual framework for causal mediation analysis. The mediator variables included AJCC stage, tumor site, T stage, N stage, M stage, radiation, tumor size, and mitotic index. Among these, tumor size was treated as a continuous variable, while all others were included as categorical variables. R software (version 4.3.1) was utilized for all statistical analyses, with the primary packages including survival, tidycmprsk, Matchit, RCS, and mediation. Statistical significance was set at p < 0.05.
Results
Sex differences in clinicopathological characteristics of GIST
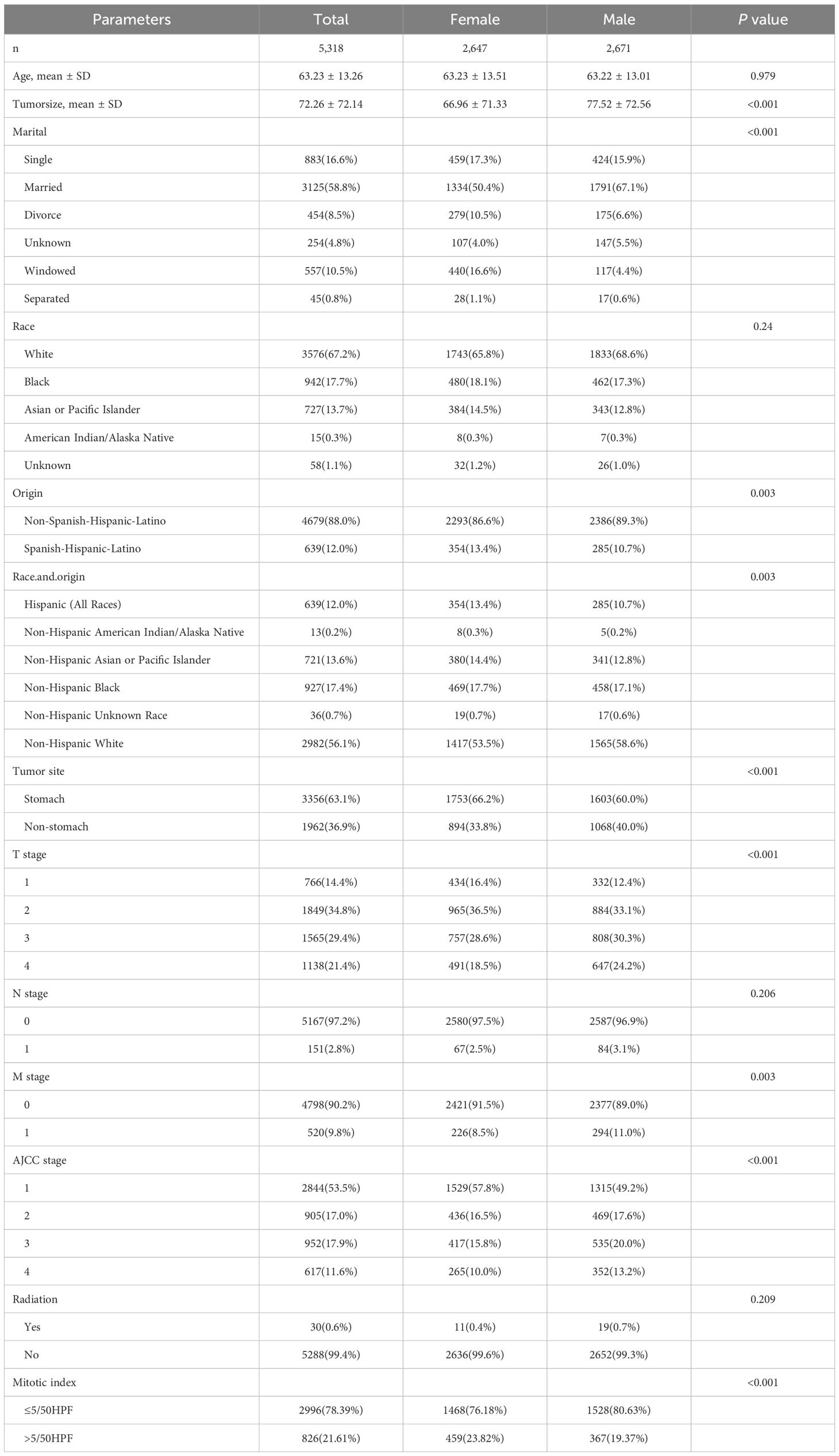
The study encompassed 9,957 patients with GIST from the SEER database, spanning the period from 2000 to 2018. Following the exclusion of non-qualifying cases, 5,318 patients were ultimately included in the analysis, comprising 2,647 females and 2,671 males. Table 1 summarizes the clinical and pathological characteristics of the overall cohort and sex subgroups. The demographic factors of men and women differed with respect to their race and marital status. Regarding clinical and pathological features, females exhibited smaller tumor sizes compared to males (66.96 mm vs. 77.52 mm, p < 0.001) and a higher proportion of gastric site involvement (66.2% vs. 60.0%, p < 0.001). Additionally, females demonstrated lower T stage and lower rates of distant metastasis compared to males (8.5% vs. 11%, p = 0.003), lower american joint committee on cancer (AJCC) stage, and lower nuclear mitotic rates (76.18% vs. 80.63%, p < 0.001).
Male as an independent risk factor for GIST prognosis
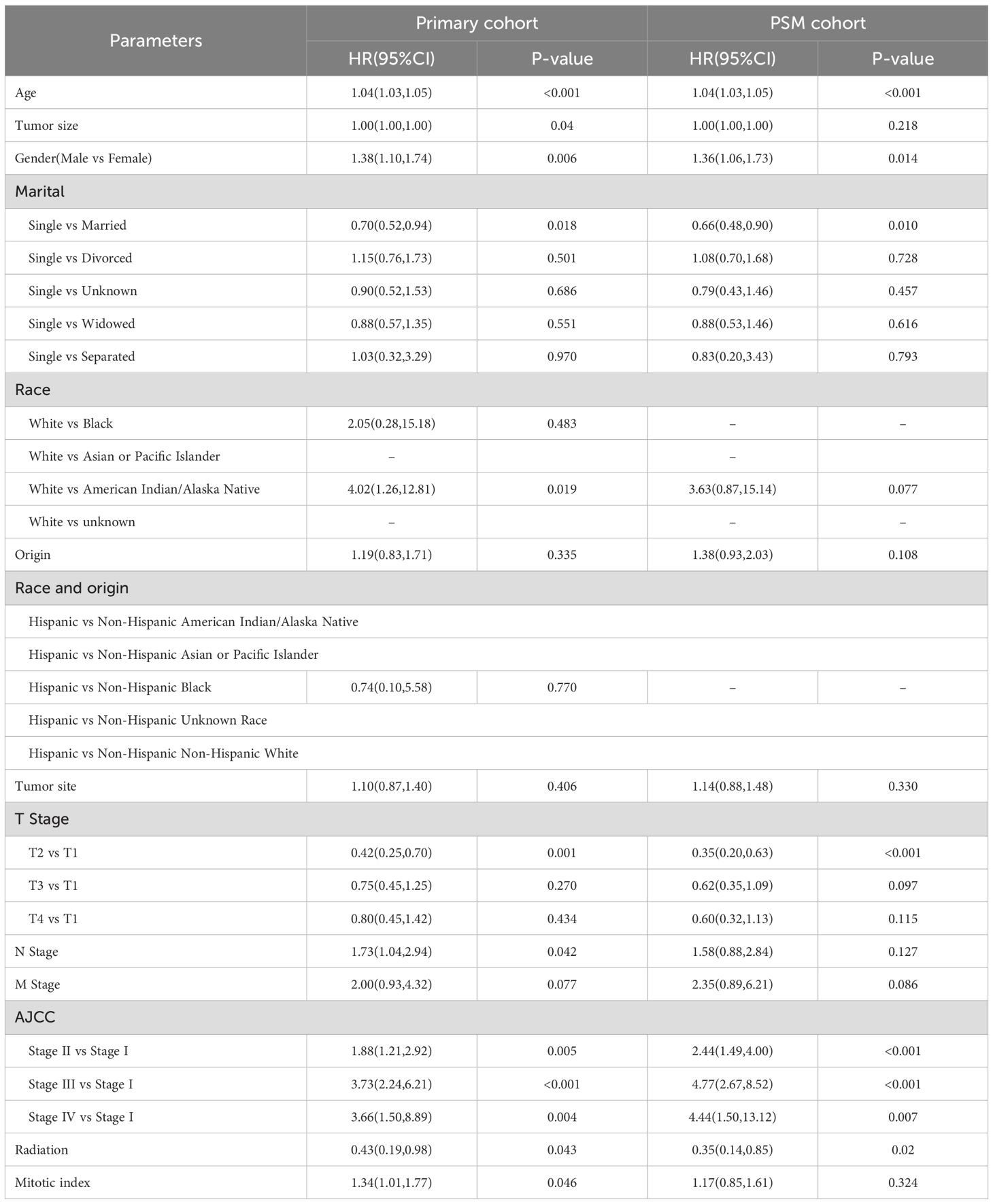
In the entire cohort, females had a median follow-up time of 44 months, whereas males had a median follow-up time of 43 months. In the female GIST cohort, 190 patients experienced disease progression (7.18%), whereas in the male cohort, 262 patients experienced disease progression (9.81%). Univariate analysis revealed that males exhibited a higher hazard ratio (HR) for DSS than females (HR = 1.40, 95%CI: 1.16-1.68, p<0.001) (Figure 2). Multivariate analysis was adjusted for the following variables: race, origin, tumor site, T stage, N stage, M stage, radiation, age, tumor size, marital status, AJCC stage, and mitotic rate. The results demonstrated that the HR for DSS remained significantly higher in males compared to females (HR = 1.38, 95%CI: 1.10-1.74, p=0.006). The associations between sex and DSS remained robust in the competing risk models (Supplementary Table S1).
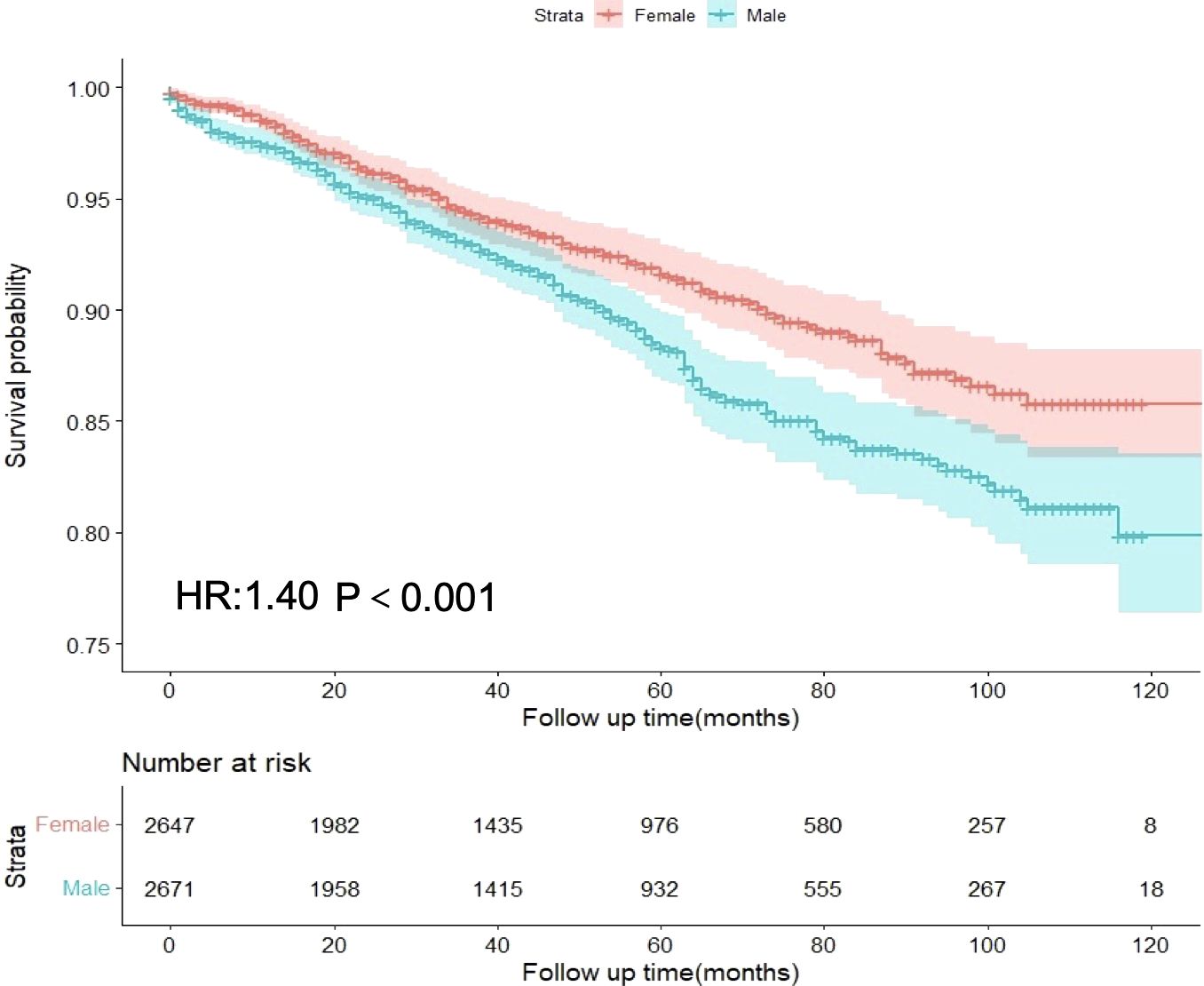
Figure 2. Differences in disease-specific survival between male and female GIST patients in the overall cohort.
Following PSM for sex, all variables between the two subgroups exhibited adequate balance (Supplementary Figure S1, Supplementary Table S2). Prognostic analysis of the PSM-adjusted cohort revealed statistically significant differences in DSS for HR between sexes (HR = 1.36, 95% CI: 1.06-1.73, p=0.014) (Table 2). Consequently, both multivariable analysis and PSM models indicated that male sex remained an independent risk factor for GIST.
Sex-age interaction in GIST prognosis
A statistically significant interaction was observed between age and sex in predicting the prognosis of GIST (p = 0.025). In the gastric tumor cohort, no significant interaction was observed between age and sex (P = 0.23). In contrast, a statistically significant interaction between age and sex was identified in the non-gastric tumor cohort (P = 0.027). Sensitivity analysis after excluding patients with a survival time of less than one month confirmed that the interaction between age and sex remained statistically significant in predicting GIST-specific survival (P = 0.029). RCS analysis revealed that the HR for DSS in males gradually increased with age, demonstrating a significant elevation after 65 years (p < 0.001 for non-linearity). Conversely, the HR for DSS in females displayed a gradual increase between the ages of 20 and 55, maintained relative stability between 55 and 65 years, and subsequently increased rapidly after 65 years (p = 0.010 for non-linearity). Moreover, the HR for DSS in males consistently exceeded that in females (Figure 3).
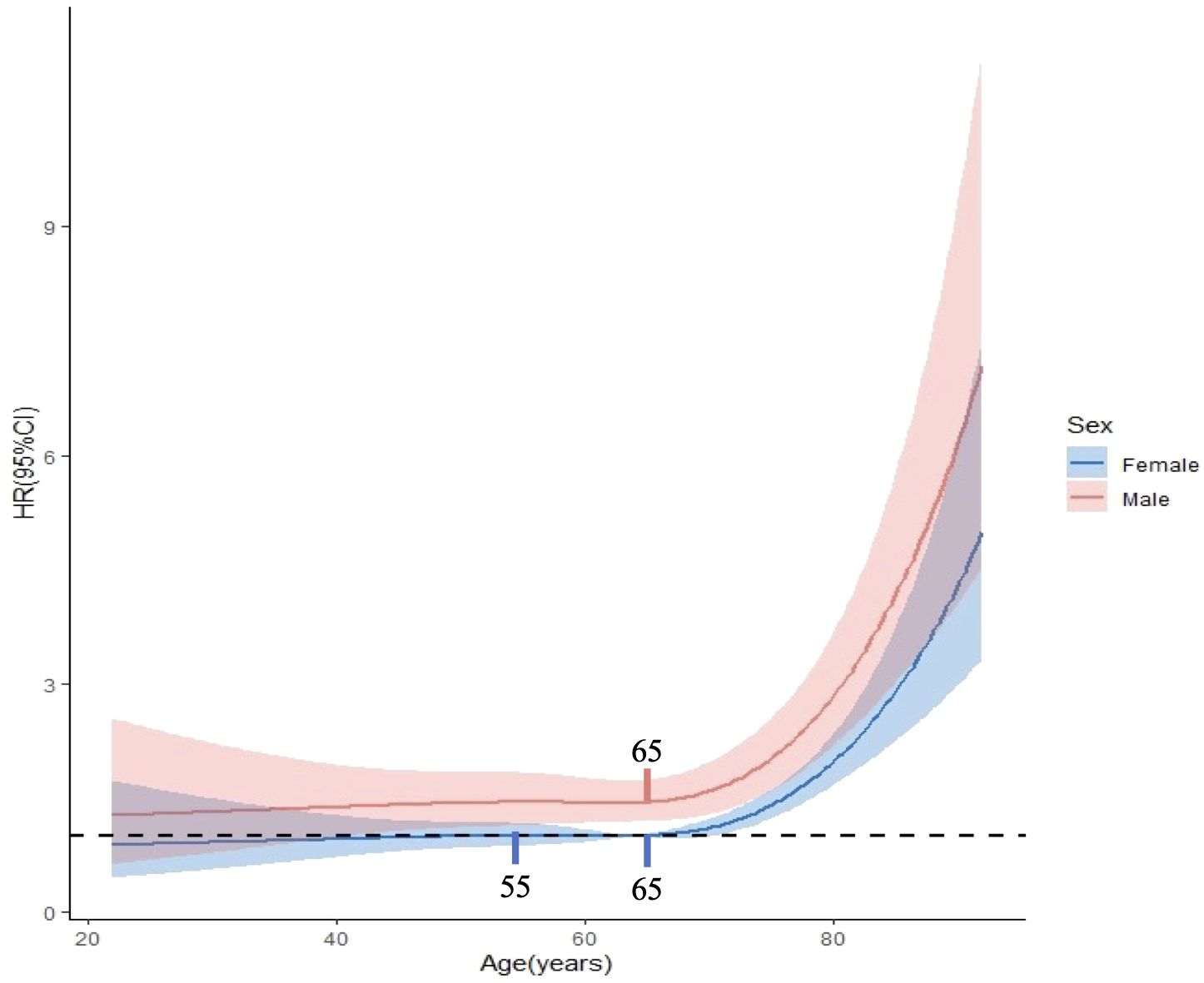
Figure 3. Interaction between age and gender in prognostic prediction of GISTs. The critical age was identified as 65 years for males and at both 55 and 65 years for females.
Age attenuates sex prognosis disparities in GIST
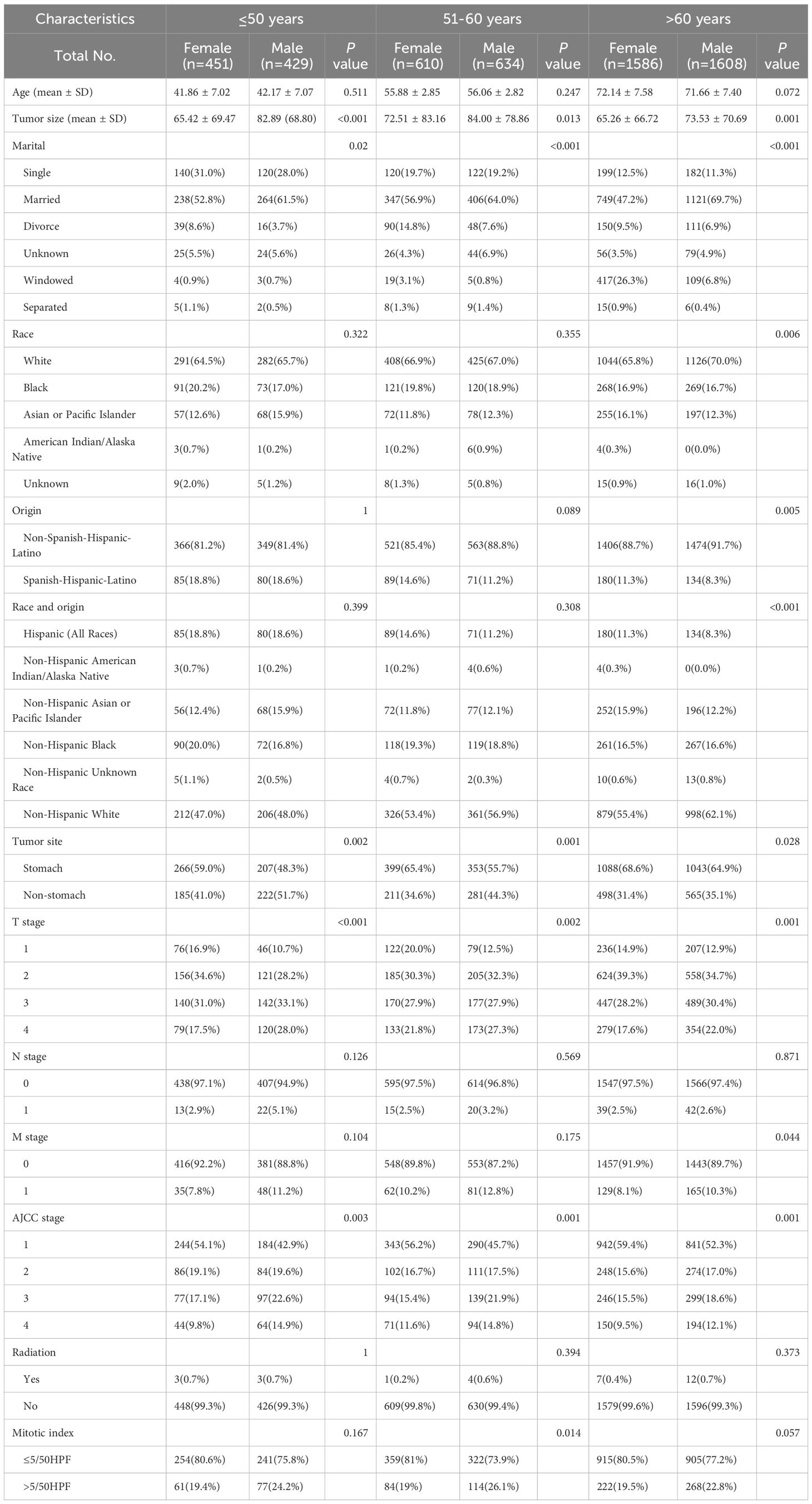
To investigate the differences in prognosis between sexes across different age subgroups, we categorized age into three subgroups (≤ 50 years, 51–60 years, and > 60 years) and conducted an analysis, as described in Table 3. Through subgroup analysis, we identified variations in the pathological parameters between males and females across different age groups. To validate these potential differences, we performed PSM to adjust for baseline characteristics between the different sexes within each age subgroup. After PSM, the intergroup variables within each age subgroup were well-balanced (Supplementary Figure S2). The results of the subgroup analysis indicated that the differences in DSS between sexes diminished with increasing age, demonstrating statistical significance only in the ≤ 50 years age subgroup. No significant differences were observed between the other age groups (Figure 4). In these three subgroups, survival analysis after PSM revealed that the prognostic differences between males and females were present only in the ≤ 50 years subgroup, where the HR for DSS in males was higher than that in females, with no such differences observed in the other two subgroups (Figure 5). This suggests that after adjusting for various factors, younger and middle-aged female patients with GIST exhibit a favorable prognosis; however, this advantage diminishes with increasing age.
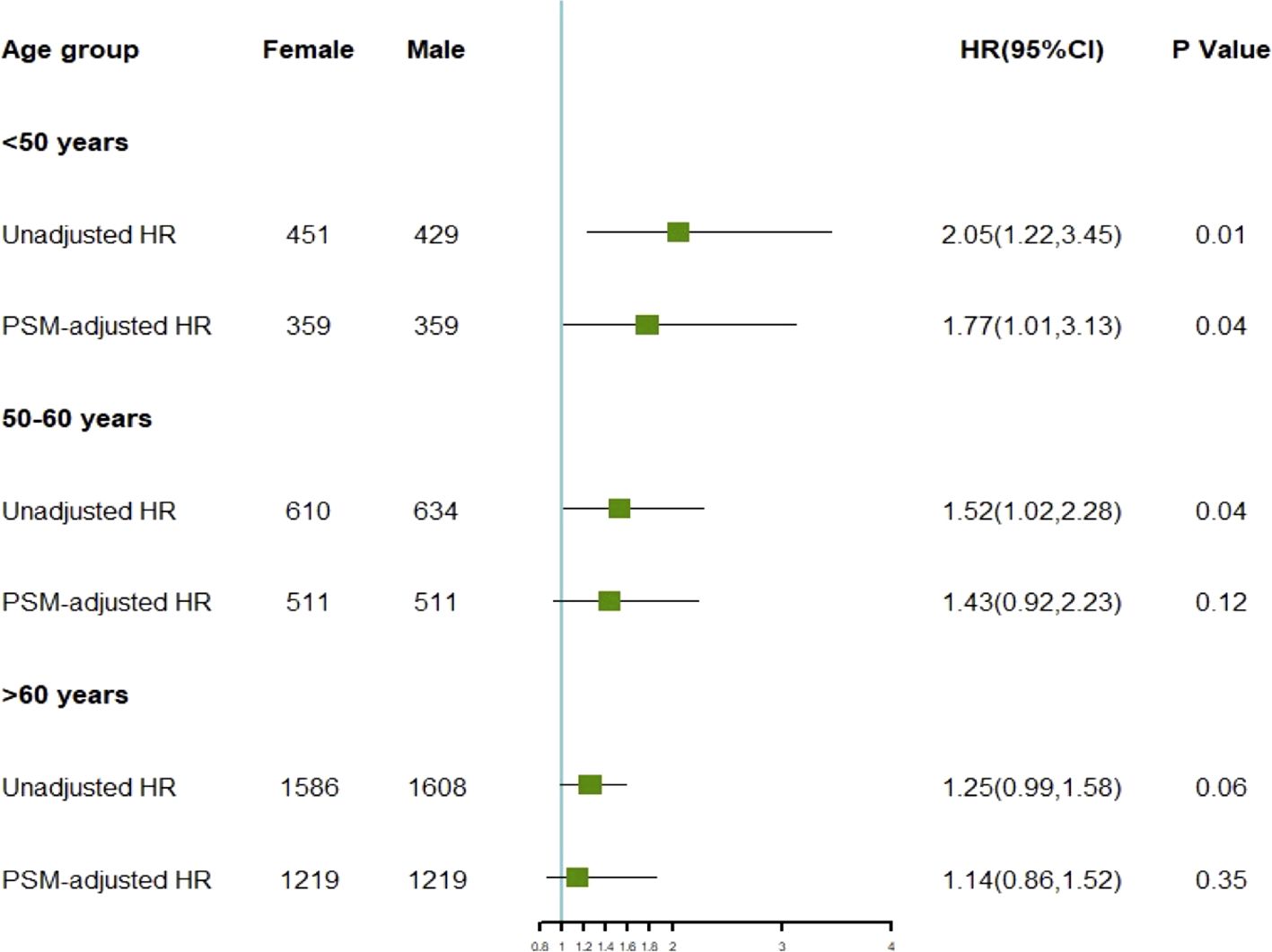
Figure 4. HR of disease-specific survival for different genders before and after adjustment across various age subgroups.
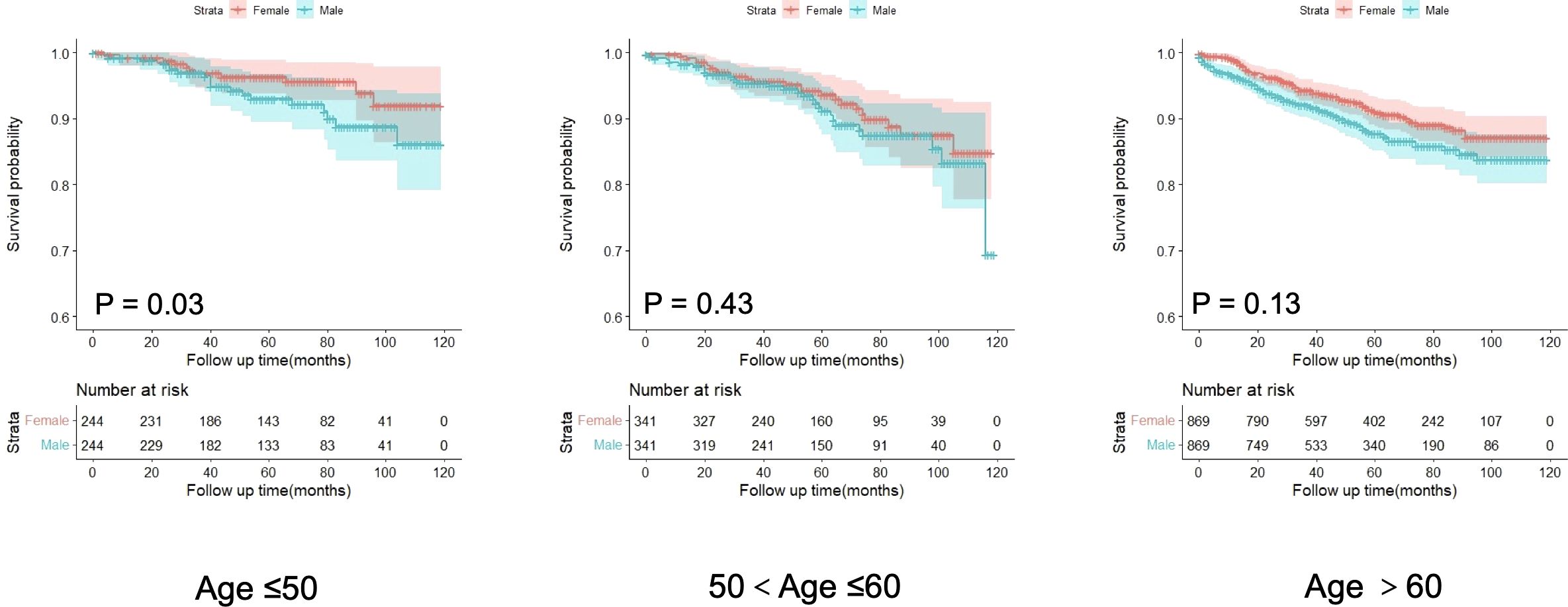
Figure 5. Analysis of the differences in disease-specific survival of GISTs among age subgroups after propensity score matching.
Sex-mediated GIST prognosis via tumor staging and biological behavior
To further investigate the impact of sex on DSS mediated by other factors, we conducted a multiple mediation analysis (Figure 6). Based on the contribution of sex to the mediation of DSS, the five most influential variables, ranked in descending order, were AJCC stage (36.8%), M stage (11.6%), T stage (11.2%), tumor size (6.8%), and N stage (0.9%). These findings suggest that sex may indirectly influence the prognosis of GIST by affecting its biological behavior.
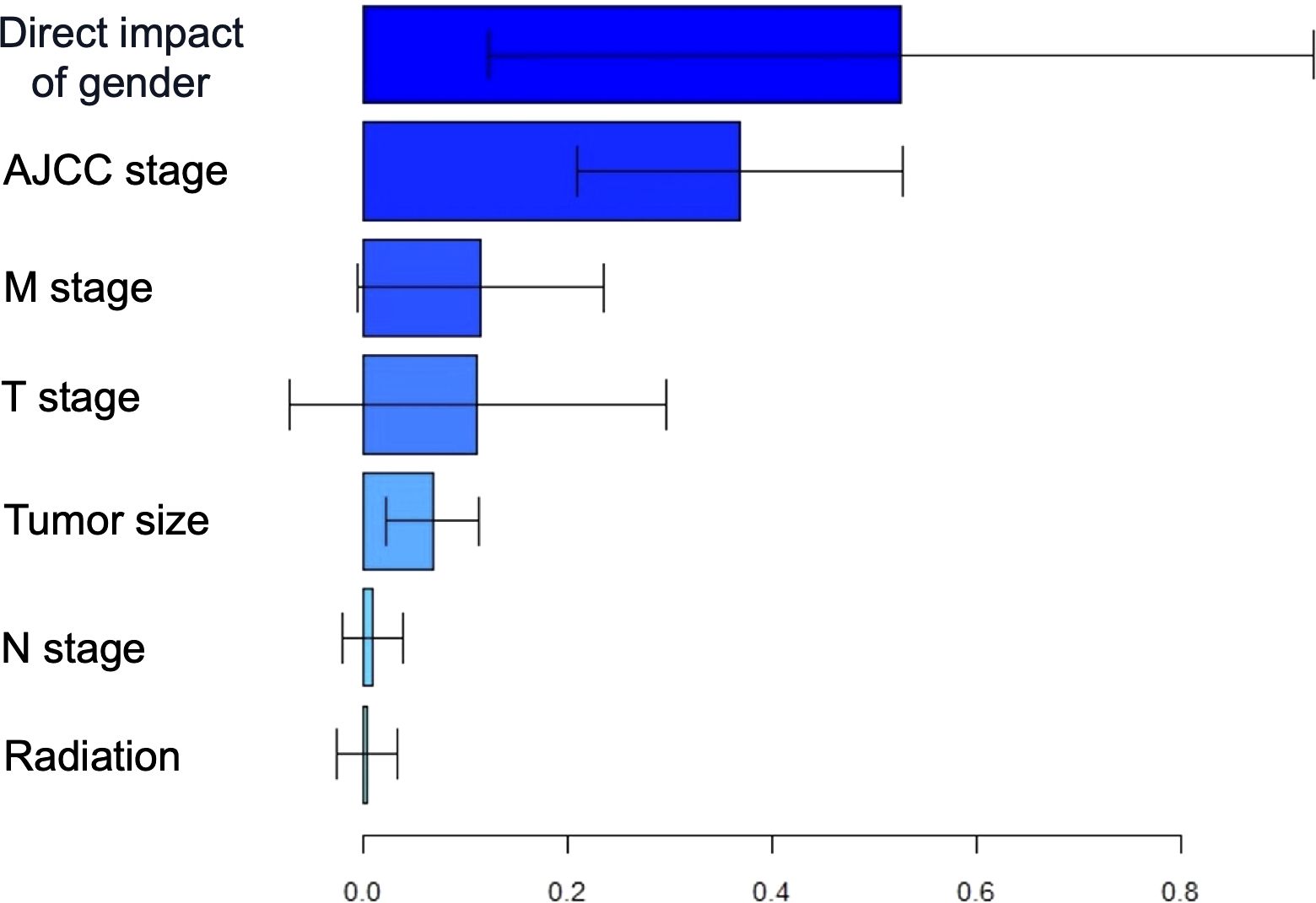
Figure 6. The estimation of direct and indirect effects contributing to the using multiple mediation analyses with gender disparity in DSS.
Discussion
Over the past few decades, the incidence of GIST has increased (22). Currently, the impact of sex on the prognosis of GIST remains a subject of debate. In this extensive retrospective cohort study, our findings indicated that females exhibited an advantage in prognosis. However, this advantage gradually diminished with advancing age. Our results suggest an interaction between sex and age in GIST, potentially conferring a protective effect in middle-aged and younger female patients. These findings may contribute to the development of tailored, personalized treatments for patients with GISTs and potentially improve their prognosis. The predominant methodologies currently employed for the prognostic evaluation of GIST are the NIH or modified NIH risk classification systems. As precision therapy continues to advance, alternative assessment approaches have begun to contribute to the risk stratification of GIST. Numerous studies have substantiated that several factors may contribute to poor prognosis in GIST, including tumor rupture, positive surgical margins, KIT exon 11 mutations, and gastrointestinal hemorrhage (9, 23). Furthermore, studies have indicated that an elevated preoperative Glasgow Prognostic Score may function as a predictive factor for long-term prognosis in patients with GISTs (24). Nevertheless, the combined influence of age and sex on GIST prognosis remains unexplored in the current literature.
Several studies have indicated that elderly patients generally exhibit poorer prognoses than younger patients. Physiological alterations leading to decreased immune function, coupled with relatively poor tolerance and treatment responses, may contribute to this phenomenon. Consequently, when investigating the impact of sex on the prognosis of GIST, age-related factors should be considered. As age increases, the risk of various adverse outcomes significantly increases, resulting in a poorer prognosis for patients with GISTs. The disparities between males and females in GIST, as well as the confounding effects of age and sex on different tumor progressions, have been established in thyroid cancer and nasopharyngeal carcinoma (25, 26).However, these relationships remain unclear in the context of GIST.
Approximately 75% of patients with GIST are diagnosed after the age of 50 (27). This factor significantly influenced the decision to establish an age threshold of 50 years. In the ≤ 50 years age subgroup, females initially demonstrated a more favorable prognosis compared to males. Nevertheless, as age increases, the prognostic disparity between the two sexes progressively diminishes, resulting in a reduction in the initial prognostic advantage observed in females. This phenomenon may be attributed to the decline in immune function and organ dysfunction associated with the aging process. However, younger female patients with GISTs maintain higher levels of estrogen compared to their male counterparts. After the age of 50 years, estrogen levels decrease significantly, consequently leading to older female patients with GISTs losing their prognostic advantage. Furthermore, the decline in estrogen levels following menopause may impair anti-tumor immune responses, which could partly explain why the survival advantage observed in women diminishes with age. Estrogen is known to modulate the function of immune cells, including enhancing the anti-tumor activity of CD8+ T cells (28, 29). Additionally, estrogen receptors are expressed on various immune cells, and estrogen signaling has been shown to suppress M2 tumor-associated macrophages (30). The different effects of male and female sex hormones on cancer immunity may lead to a higher incidence of cancer in men and a poorer prognosis (31, 32). The decline in estrogen levels, coupled with age-related immune dysfunction, may synergistically undermine anti-tumor immunity in older women, thereby diminishing their survival advantage in GIST.
A multicenter study investigated the influence of sex and age on prognosis, demonstrating that female patients with GISTs under 50 years of age exhibit a favorable prognosis (10). This finding is consistent with the results of the present study. However, this investigation solely categorized patients by age and sex without examining the dynamic changes in the HR of DSS with increasing age. A separate study similarly identified advanced age as an independent adverse prognostic factor for patients with GIST, while female sex was considered a protective factor in comparison to male (33). Contemporary research on sex generally indicates that females exhibit a more favorable prognosis than males (34, 35). A favorable prognosis has been observed in female patients aged ≤ 21 years with gastric GIST (36). Collectively, these findings indicate that female patients with GISTs may benefit from the protective effects associated with estrogen levels.
Estrogen plays a crucial role in regulating the female reproductive system and normal physiological functions; however, it may also contribute to certain malignancies. Specifically, estrogen is hypothesized to promote endometrial and breast cancers (37, 38). Conversely, estrogen may exert a protective effect against certain neoplasms. Specifically, estrogen has been observed to reduce the risk of colorectal cancer (39). Consequently, the dual role of estrogen in tumors necessitates further investigation. Estrogen may exert an inhibitory effect on GIST progression, potentially elucidating the improved prognosis observed in female patients. However, additional research is required to substantiate this hypothesis.
Nevertheless, this study has several limitations. Most notably, the SEER database does not include information on KIT and PDGFRA mutation status, nor does it provide records of TKI therapy, which is a critical treatment for GIST and significantly influences patient outcomes. The absence of this information may confound the interpretation of the observed sex-based survival differences. If there are sex-related variations in mutation profiles or responses to TKI treatment, our results may overstate or understate the true effect of sex on survival. Furthermore, the lack of these data complicates mediation analyses aimed at elucidating the indirect pathways through which sex influences survival via tumor stage and biological behavior. Without accounting for mutation status and TKI treatment, the estimated contributions of tumor stage and other factors may be biased, as these factors are themselves influenced by molecular characteristics and the efficacy of TKI therapy. Therefore, although our findings highlight significant prognostic disparities related to sex and age, they require validation in studies incorporating genetic mutation and TKI treatment data.
In conclusion, this study investigated the prognostic disparities among various age groups and sexes within a GIST cohort. Elucidating the interaction between sex and age in GIST prognosis will contribute to a more comprehensive understanding of the biological characteristics of GIST, potentially improving patient treatment outcomes and facilitating the development of more efficacious individualized treatment strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
YZ: Conceptualization, Writing – original draft. QL: Conceptualization, Software, Writing – original draft. BM: Software, Data curation, Writing – original draft. HX: Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1617019/full#supplementary-material
References
1. Patel N and Benipal B. Incidence of gastrointestinal stromal tumors in the United States from 2001-2015: A United States cancer statistics analysis of 50 states. Cureus. (2019) 11:e4120. doi: 10.7759/cureus.4120
2. Soreide K, Sandvik OM, Soreide JA, Giljaca V, Jureckova A, and Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. (2016) 40:39–46. doi: 10.1016/j.canep.2015.10.031
3. Alvarez CS, Piazuelo MB, Fleitas-Kanonnikoff T, Ruhl J, Perez-Fidalgo JA, and Camargo MC. Incidence and survival outcomes of gastrointestinal stromal tumors. JAMA Netw Open. (2024) 7:e2428828. doi: 10.1001/jamanetworkopen.2024.28828
4. Blay JY, Kang YK, Nishida T, and von Mehren M. Gastrointestinal stromal tumours. Nat Rev Dis Primers. (2021) 7:22. doi: 10.1038/s41572-021-00254-5
5. Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. (2003) 299:708–10. doi: 10.1126/science.1079666
6. Rubin BP, Singer S, Tsao C, Duensing A, Lux ML, Ruiz R, et al. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res. (2001) 61:8118–21.
7. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. (2002) 10:81–9. doi: 10.1177/106689690201000201
8. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. (2002) 33:459–65. doi: 10.1053/hupa.2002.123545
9. Zhang H and Liu Q. Prognostic indicators for gastrointestinal stromal tumors: A review. Transl Oncol. (2020) 13:100812. doi: 10.1016/j.tranon.2020.100812
10. Kramer K, Knippschild U, Mayer B, Bogelspacher K, Spatz H, Henne-Bruns D, et al. Impact of age and gender on tumor related prognosis in gastrointestinal stromal tumors (GIST). BMC Cancer. (2015) 15:57. doi: 10.1186/s12885-015-1054-y
11. Zhang QW, Gao YJ, Zhang RY, Zhou XX, Chen SL, Zhang Y, et al. Personalized CT-based radiomics nomogram preoperative predicting Ki-67 expression in gastrointestinal stromal tumors: a multicenter development and validation cohort. Clin Transl Med. (2020) 9:12. doi: 10.1186/s40169-020-0263-4
12. Clocchiatti A, Cora E, Zhang Y, and Dotto GP. Sexual dimorphism in cancer. Nat Rev Cancer. (2016) 16:330–9. doi: 10.1038/nrc.2016.30
13. Joensuu H, Vehtari A, Riihimaki J, Nishida T, Steigen SE, Brabec P, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. (2012) 13:265–74. doi: 10.1016/S1470-2045(11)70299-6
14. Edgren G, Liang L, Adami HO, and Chang ET. Enigmatic sex disparities in cancer incidence. Eur J Epidemiol. (2012) 27:187–96. doi: 10.1007/s10654-011-9647-5
15. Brodsky SV, Gimenez C, Ghosh C, Melamed M, and Ramaswamy G. Estrogen and progesterone receptors expression in gastrointestinal stromal tumors and intramural gastrointestinal leiomyomas. Int J Gastrointest Cancer. (2006) 37:129–32. doi: 10.1007/s12029-007-9003-x
16. Valkov A, Sorbye S, Kilvaer TK, Donnem T, Smeland E, Bremnes RM, et al. Estrogen receptor and progesterone receptor are prognostic factors in soft tissue sarcomas. Int J Oncol. (2011) 38:1031–40. doi: 10.3892/ijo.2011.920
17. Shobab L, Burman KD, and Wartofsky L. Sex differences in differentiated thyroid cancer. Thyroid. (2022) 32:224–35. doi: 10.1089/thy.2021.0361
18. Costa AR, Lanca de Oliveira M, Cruz I, Goncalves I, Cascalheira JF, and Santos CRA. The sex bias of cancer. Trends Endocrinol Metab. (2020) 31:785–99. doi: 10.1016/j.tem.2020.07.002
19. NS IJ, van Werkhoven E, Mohammadi M, Hollander DD, Bleckman RF, Reyners AKL, et al. Sex differences in patients with gastrointestinal stromal tumours: do they exist and does it affect survival? ESMO Open. (2022) 7:100649. doi: 10.1016/j.esmoop.2022.100649
20. Rong J, Chen S, Song C, Wang H, Zhao Q, Zhao R, et al. The prognostic value of gender in gastric gastrointestinal stromal tumors: a propensity score matching analysis. Biol Sex Differ. (2020) 11:43. doi: 10.1186/s13293-020-00321-8
21. Ge XY, Lei LW, Ge F, and Jiang X. Analysis of risk factors of gastrointestinal stromal tumors in different age groups based on SEER database. Scand J Gastroenterol. (2019) 54:480–4. doi: 10.1080/00365521.2019.1604798
22. Amadeo B, Penel N, Coindre JM, Ray-Coquard I, Ligier K, Delafosse P, et al. Incidence and time trends of sarcoma (2000-2013): results from the French network of cancer registries (FRANCIM). BMC Cancer. (2020) 20:190. doi: 10.1186/s12885-020-6683-0
23. Liu Q, Kong F, Zhou J, Dong M, and Dong Q. Management of hemorrhage in gastrointestinal stromal tumors: a review. Cancer Manag Res. (2018) 10:735–43. doi: 10.2147/CMAR.S159689
24. Maruyama T, Shimoda M, Sako A, Ueda K, Hakoda H, Sakata A, et al. Predictive effectiveness of the glasgow prognostic score for gastrointestinal stromal tumors. Nutr Cancer. (2021) 73:1333–9. doi: 10.1080/01635581.2020.1800763
25. Li WZ, Lv SH, Liu GY, Liang H, Xia WX, and Xiang YQ. Age-dependent changes of gender disparities in nasopharyngeal carcinoma survival. Biol Sex Differ. (2021) 12:18. doi: 10.1186/s13293-021-00361-8
26. Liu Q, Ma B, Song M, Sun W, and Zhang H. Age-dependent changes in the prognostic advantage of papillary thyroid cancer in women: A SEER-based study. Clin Endocrinol (Oxf). (2023) 99:342–9. doi: 10.1111/cen.14896
27. DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, and Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. (2000) 231:51–8. doi: 10.1097/00000658-200001000-00008
28. Yuan B, Clark CA, Wu B, Yang J, Drerup JM, Li T, et al. Estrogen receptor beta signaling in CD8(+) T cells boosts T cell receptor activation and antitumor immunity through a phosphotyrosine switch. J Immunother Cancer. (2021) 9:e001932. doi: 10.1136/jitc-2020-001932
29. Milette S, Hashimoto M, Perrino S, Qi S, Chen M, Ham B, et al. Sexual dimorphism and the role of estrogen in the immune microenvironment of liver metastases. Nat Commun. (2019) 10:5745. doi: 10.1038/s41467-019-13571-x
30. Wang DH, He DW, Lv TT, Zhang XK, Li ZJ, and Wang ZY. Estrogen receptor alpha suppresses hepatocellular carcinoma by restricting M2 macrophage infiltration through the YAP-CCL2 axis. BMC Cancer. (2025) 25:550. doi: 10.1186/s12885-025-13676-1
31. Ozdemir BC and Dotto GP. Sex hormones and anticancer immunity. Clin Cancer Res. (2019) 25:4603–10. doi: 10.1158/1078-0432.CCR-19-0137
32. Zhang Z, Zeng P, Gao W, Zhou Q, Feng T, and Tian X. Circadian clock: a regulator of the immunity in cancer. Cell Commun Signal. (2021) 19:37. doi: 10.1186/s12964-021-00721-2
33. Tran T, Davila JA, and El-Serag HB. The epidemiology of Malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol. (2005) 100:162–8. doi: 10.1111/j.1572-0241.2005.40709.x
34. Fujimoto Y, Nakanishi Y, Yoshimura K, and Shimoda T. Clinicopathologic study of primary Malignant gastrointestinal stromal tumor of the stomach, with special reference to prognostic factors: analysis of results in 140 surgically resected patients. Gastric Cancer. (2003) 6:39–48. doi: 10.1007/s101200300005
35. Singer S, Rubin BP, Lux ML, Chen CJ, Demetri GD, Fletcher CD, et al. Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J Clin Oncol. (2002) 20:3898–905. doi: 10.1200/JCO.2002.03.095
36. Miettinen M, Lasota J, and Sobin LH. Gastrointestinal stromal tumors of the stomach in children and young adults: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases with long-term follow-up and review of the literature. Am J Surg Pathol. (2005) 29:1373–81. doi: 10.1097/01.pas.0000172190.79552.8b
37. Yager JD and Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. (2006) 354:270–82. doi: 10.1056/NEJMra050776
38. Rizner TL. Estrogen biosynthesis, phase I and phase II metabolism, and action in endometrial cancer. Mol Cell Endocrinol. (2013) 381:124–39. doi: 10.1016/j.mce.2013.07.026
Keywords: gastrointestinal stromal tumor, SEER, survival, sex-difference, prognosis
Citation: Zhang Y, Liu Q, Ma B and Xu H (2025) Age-dependent diminution of female prognostic advantage in gastrointestinal stromal tumors: a retrospective cohort analysis. Front. Immunol. 16:1617019. doi: 10.3389/fimmu.2025.1617019
Received: 23 April 2025; Accepted: 28 October 2025;
Published: 10 November 2025.
Edited by:
Gambhir Shrestha, Institute of Medicine, NepalReviewed by:
Megha Das, University of Miami, United StatesIldar Fakhradiyev, Kazakh National Medical University, Kazakhstan
Copyright © 2025 Zhang, Liu, Ma and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Xu, aHh1QGNtdS5lZHUuY24=
 Yue Zhang1
Yue Zhang1 Qi Liu
Qi Liu Bing Ma
Bing Ma Hao Xu
Hao Xu