- 1Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Clinical Research Center for Rare Diseases Aldo e Cele Daccò and Centro Anna Maria Astori, Science and Technology Park Kilometro Rosso, Bergamo, Italy
- 2Department of Medicine, University of Colorado School of Medicine, Aurora, CO, United States
- 3Preclinical Research, Q32 Bio Inc., Waltham, MA, United States
Atypical hemolytic uremic syndrome (aHUS) is a rare and severe thrombotic microangiopathy caused by genetic or acquired abnormalities leading to activation of the complement alternative pathway on cell surfaces. This process leads to endothelial dysfunction and microvascular thrombosis. The introduction of anti-C5 antibodies has dramatically improved aHUS prognosis; however, these treatments require regular intravenous infusions and block systemic complement activity, exposing the patient to risk of infections. Recently complement inhibitors have been developed to selectively bind injury-associated target molecules, thereby concentrating the drug at specific cellular or tissue sites while preserving systemic complement function. This study evaluated the local complement inhibitory activity of new molecules that exploit the natural localization of C3d at complement activation sites on cells: namely the anti-C3d monoclonal antibody 3d8b conjugated with the first 10 or 17 short consensus repeats (SCRs) of complement receptor 1 (CR11–10 and CR11-17, respectively) or the first 5 SCRs of complement factor H (FH1-5). To this purpose we tested their capability to block C3 deposition and C5b-9 formation on microvascular endothelial cells (HMEC-1) exposed to serum from patients with aHUS. We also assessed their ability to prevent loss of anti-thrombogenic properties in HMEC-1 pre-exposed to aHUS serum and then perfused with control blood. We demonstrate that anti-C3d-antibody conjugated with CR11-10, or CR11-17, or FH1–5 effectively prevented aHUS serum-induced complement activation on HMEC-1, outperforming their non-targeted soluble counterparts. The efficacy of C3 convertase inhibition varied depending on the complement inhibitory component (CR11-17 > CR11-10 > FH1-5). However, all the inhibitors successfully blocked C5 convertase activity and eliminated the pro-thrombogenic effects of aHUS patients’ serum. These findings support the potential of tissue-targeted complement inhibition as a novel, non-systemic therapeutic strategy for aHUS and other diseases characterized by localized complement dysregulation.
Introduction
Atypical hemolytic uremic syndrome (aHUS) is a rare and severe form of thrombotic microangiopathy caused by genetic or acquired abnormalities of the alternative pathway (AP) of complement activation, leading to uncontrolled and sustained complement activation on cell surfaces (1, 2). This results in endothelial cell dysfunction and formation of microvascular thrombi, with a particular predilection for renal vasculature (1, 2). About 60% of patients with aHUS carry loss-of-function rare variants (RVs) in genes encoding complement regulatory proteins (complement factor H, CFH; complement factor I, CFI; MCP/CD46) or genomic rearrangements in CFH-CFHRs, or gain-of-function RVs in components of the AP C3 convertase (C3 and complement factor B, CFB), or anti-CFH autoantibodies (1).
The introduction of anti-C5 therapies (eculizumab or ravulizumab) has significantly improved aHUS management, allowing patients to maintain hematological remission and preserve renal function (3, 4). Despite these benefits, these anti-C5 treatments come with limitations. They are associated with high costs and require regular, prolonged hospital visits for drug administration. Moreover, by inhibiting the terminal complement products C5a and C5b-9, these therapies increase the risk of serious infections, particularly with encapsulated bacteria. Consequently, vaccination against Neisseria meningitidis is mandatory prior to the initiation of anti-C5 treatments, and in some cases, prophylactic antibiotic therapy is recommended throughout the duration of treatment (3).
The complement cascade is a key part of the innate immune system, acting as a primary line of defense against invading pathogens and playing a role in the clearance of damaged cells and tissue regeneration (5). Thus, a challenge for complement inhibitory therapeutics is to block complement deleterious effects without impairing its beneficial functions.
To address this, several complement inhibitors have been developed to selectively bind target molecules, thereby concentrating the drug at specific cellular or tissue sites (6). One recent approach involves bispecific antibodies, which inhibit complement activation on particular cells by bringing endogenous complement regulators (such as CFH or C4BP) close to specific cell surface antigens (7). A more focused strategy involves directing complement inhibitors precisely to sites of complement activation. All three complement pathways (classical, lectin or alternative) converge at the proteolytic cleavage of C3, by C3 convertases, resulting in the generation of C3a and C3b (5). C3b serves as a component of the C5 convertase, which subsequently cleaves C5 to produce the anaphylatoxin C5a and the membrane attack complex (C5b-9) (5). To regulate this process, C3b is inactivated by CFI through a cofactor-driven mechanism, being processed first into iC3b and then into C3dg and C3d. These fragments are deposited at high density on tissue surfaces via covalent binding at sites where complement activation has occurred.
By exploiting the natural localization of C3d at complement activation sites, various complement inhibitors have been developed through the conjugation of complement regulators to C3d-recognizing molecules, including the 4N-terminal short consensus repeats (SCRs) domains of complement receptor type 2 (8) and anti-C3d antibodies (9, 10). In this context, recent studies have described fusion proteins composed of a monoclonal antibody (mAb) raised against human C3d, linked to fragments of endogenous complement regulators containing their complement regulatory domains, specifically the first 10 SCRs of complement receptor type 1 (CR1) or the first 5 SCRs of CFH (9, 10). CR1 and CFH negatively regulate the complement AP by accelerating the decay of the C3 convertase and acting as cofactors for the proteolytic activity of CFI (5). The selected mAb clone, 3d8b, binds with high affinity to the C-terminal sequence of C3d, also present in the related fragments iC3b and C3dg (9). The term “anti-C3d” is used when describing the activity of the C3d mAb fusion proteins (9, 10). The mAb is linked to two moieties of either CR11–10 or FH1–5 fragments, conferring a synergistic inhibitory effect. In vivo studies in experimental models of complement-mediated diseases have demonstrated that both anti-C3d-mAb-CR11–10 and anti-C3d-mAb-FH1–5 constructs inhibit complement activity in tissues with localized complement activation at low concentrations that do not affect systemic complement activation (9, 10).
The objective of this study was to evaluate the effectiveness of the anti-C3d mAb 3d8b conjugated with the first 10 or 17 SCRs of CR1 (CR11–10 and CR11–17 including one and two C3b binding sites, respectively) or the first 5 SCRs of CFH (FH1-5), in inhibiting the uncontrolled activity of C3 and C5 convertases, as well as in preventing the loss of anti-thrombogenic properties induced ex-vivo on microvascular endothelial cells by serum from patients with aHUS, taken as prototype of cell-surface restricted complement-related diseases.
Methods
Study participants
Participants were 9 adult patients with aHUS in remission, included in the International Registry of Recurrent and Familial Haemolytic Uremic Syndrome/Thrombotic Thrombocytopenic Purpura (HUS/TTP). The Registry was established in 1996 at the Aldo e Cele Daccò Clinical Research Center for Rare Diseases (Ranica, Bergamo) (villacamozzi.marionegri.it/seu). Samples collected were stored at the Mario Negri Institute Biological Resources Center, in Biobank for Rare Diseases and Kidney Diseases (Ranica, Bergamo).
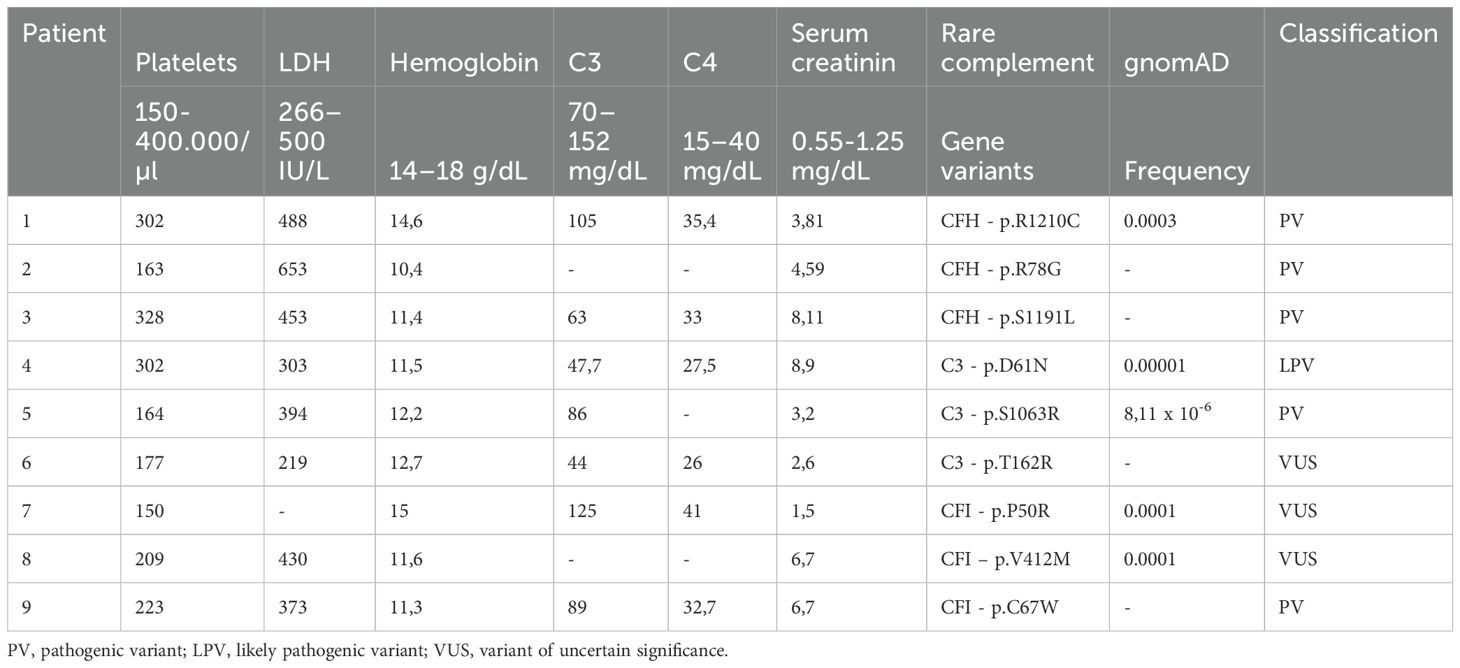
Three patients carry pathogenic RVs in CFH gene (#1, p.R1210C; #2, p.R78G; #3, p.S1191L), three patients in C3 gene (#4, p.D61N; #5, p.S1063R; #6, p.T162R), and three patients in CFI gene (#7, p.P50R; #8, p.V412M; #9, p.C67W). No patients have anti-CFH autoantibodies. Table 1 summarizes clinical and genetic characteristics of the patients. Rare functional variants (missense, nonsense, indel, or splicing variants) with minor allele frequency (MAF) <0.001 in the Genome Aggregation Database (gnomAD, https://gnomad.broadinstitute.org/) were selected. Stop-gain, frameshift and splicing variants, and missense variants with published functional studies, were categorized as pathogenic variants (PV). The other variants were categorized as likely pathogenic variants (LPV) or variants of uncertain significance (VUS), using guidelines from the American College of Medical Genetics and Genomics (ACMG) and from the KDIGO conference on aHUS and C3G (11–13).
aHUS was diagnosed in patients who have had one or more episodes of microangiopathic hemolytic anemia and thrombocytopenia, with hematocrit (Ht) <30%, hemoglobin (Hb) <10 g/dL, serum lactate dehydrogenase (LDH) >500 IU/L, undetectable haptoglobin, fragmented erythrocytes in the peripheral blood smear, and platelet count <150,000/μL, associated with acute renal failure (serum creatinine >1.3 mg/dL, and/or urinary protein/creatinine ratio >200 mg/g, or an increase in serum creatinine or a urinary protein/creatinine ratio >15% compared to baseline levels) (14). TTP was ruled out on the basis of ADAMTS13 activity >10% and no anti-ADAMTS13 antibodies. Stx-E.Coli infection was ruled out based on negative assays for stx and eae genes (by PCR) or Shiga-toxin (Vero cell assay) in the stool and/or anti-Shiga-toxin antibodies (ELISA) and/or LPS O157, O26, O111, or O145 (ELISA) in serum.
Full remission was defined as the normalization of both hematological parameters (Ht >30%; Hb >10 g/dL; LDH <500 IU/L; platelets >150,000/μL) and renal function (serum creatinine <1.3 mg/dL for adults, urinary protein/creatinine ratio <200 mg/g).
Patients had not taken C5 inhibitory drugs for at least 8 weeks prior to blood draws. The protocol was approved by the Ethical Committee of the Azienda Sanitaria Locale Bergamo, Italy.
Evaluation of serum-induced C3 deposition on microvascular endothelial cells
The human microvascular endothelial cell line of dermal origin (HMEC-1, a kind gift of Dr. Edwin Ades and Francisco J. Candal of CDC and Dr. Thomas Lawley of Emory University, Atlanta, GA) was cultured as described (14, 15). For the test, HMEC-1 were plated on glass coverslips and used when confluent. Cells were washed three times with test medium (HBSS: 137 mmol/l NaCl, 5.4 mmol/l KCl, 0.7 mmol/l Na2HPO4, 0.73 mmol/l KH2PO4, 1.9 mmol/l CaCl2, 0.8 mmol/l MgSO4, 28 mmol/l Trizma base pH 7.3, 0.1% dextrose; with 0.5% BSA) and then activated with 10 µM ADP (Sigma-Aldrich) for 10 minutes. At the end, cells were washed and then incubated for 4 hours with aHUS serum or with a pool of 10 control sera (NHS) run in each experiment diluted 1:2 with test medium (50% of serum final concentration) in the presence or absence of: the pan-complement inhibitor sCR1 (150 µg/ml, i.e. 600 nM), anti-C3d mAb conjugated with CR11-10 (1, 10, 100 nM, i.e. 0.290, 2.901, 29.008 µg/ml) or non-targeted CR11-10 (1, 10, 100 nM, i.e. 0.073, 0.726, 7.258 µg/ml), anti-C3d mAb conjugated with CR11-17 (1, 10, 100 nM, i.e. 0.389, 3.887, 38.874 µg/ml) or non-targeted CR11-17 (1, 10, 100 nM, i.e. 0.122, 1.219, 12.191 µg/ml), anti-C3d mAb conjugated with FH1-5 (10, 100, 1000 nM, i.e. 2.171, 21.707, 217.068 µg/ml) or non-targeted FH1-5 (10, 100, 1000 nM, i.e. 0.361, 3.607, 36.072 µg/ml). Table 2 summarizes structural characteristics of the complement inhibitors (anti-C3d mAb conjugated and unconjugated) used. The anti-C3d mAb, 3d8b, recognizes a shared epitope with iC3b, C3dg and C3d. The term “anti-C3d” is used when describing the activity of the C3d mAb fusion proteins.
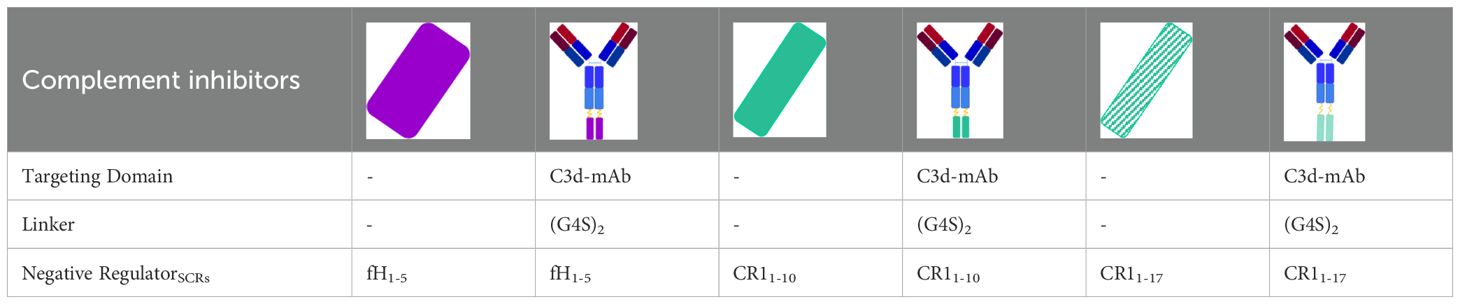
Table 2. structural characteristics of anti-C3d mAb conjugated and unconjugated complement inhibitors.
At the end of the incubation step, HMEC-1 were washed, fixed in 3% paraformaldehyde, washed again and then blocked with PBS with 2% BSA for one hour. The cells were stained with FITC-conjugated rabbit anti-human C3c (Dako, that recognizes C3c, part of C3 and C3b, 1:300 final dilution in Dapi 1 µg/mL). In selected experiments, to confirm target presence on treated HMEC-1, cells have been stained with mouse anti-human C3d (3d8b, the same clone used for the anti-C3d conjugated complement inhibitors, 1.75 µg/ml) or the isotype control (mouse IgG1, k [MOPC-21] from murine myeloma, Sigma) followed by PE-conjugated rat anti-mouse light chain kappa secondary antibody (H139-52.1, Abcam, 1:100 final dilution in 1 µg/mL Dapi).
The fluorescent staining was acquired through the Apotome Axio Imager Z2 microscope (Zeiss) and the stained area was evaluated using Image J (NIH, Bethesda, MD). For each sample, 15 fields were analyzed, the highest and the lowest values were discarded and the mean was calculated on the remaining 13 fields.
Results were expressed in pixel2 or as percentage of stained area relative to area measured after exposure to aHUS serum alone, which was set as 100%. Half maximal inhibitory concentration (IC50) curves for C3d-targeted complement inhibitors were generated using non-linear regression in GraphPad PRISM v10 with normalized response (aHUS serum alone, 100%), values were expressed as mean ± SEM.
To assess whether the anti-C3d mAb (3d8b) alone, which targets complement inhibitors (CR11-10, CR11-17, and FH1-5) to C3d, affects complement activation and deposition on HMEC-1 cells incubated with aHUS serum, preliminary experiments were conducted using aHUS serum added with 3d8b mAb (10, 100, and 1000 nM). C3 deposits on ADP-activated HMEC-1 exposed to aHUS serum added with 3d8b mAb, were comparable to those observed with serum alone, indicating that the 3d8b mAb alone did not affect complement activation and C3 deposition on HMEC-1 exposed to aHUS serum (Supplementary Figure 1), and did not mask C3 detection on cells as well.
Evaluation of serum-induced C5b-9 formation on microvascular endothelial cells
Experiments were performed as described above for C3 deposition evaluation. HMEC-1 cells were incubated with serum from aHUS patients in the presence or absence of anti-C3d mAb conjugated with CR11–10 or non-targeted CR11-10 (10 nM), anti-C3d mAb conjugated with CR11–17 or non-targeted CR11-17 (10 nM), anti-C3d mAb conjugated with FH1–5 or non-targeted FH1-5 (100, 1000 nM). At the end of the incubation step, fixed cells were stained with rabbit anti–human complement C5b-9 complex (Calbiochem, 1:200 final dilution) followed by FITC-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, 1:50 final dilution in 1 µg/mL Dapi). The fluorescent staining was acquired through the Apotome Axio Imager Z2 microscope (Zeiss) and the stained area was evaluated using Image J (NIH, Bethesda, MD). For each sample, 15 fields were analyzed, the highest and the lowest values were discarded and the mean was calculated on the remaining 13 fields.
Results were expressed as stained area in pixel2.
Evaluation of platelet adhesion and aggregation on HMEC-1 exposed to aHUS serum and then perfused with control blood
Platelet adhesion and aggregation on HMEC-1 under flow condition was performed as described previously (16). HMEC-1 were plated on a glass slide and used when they formed a monolayer. Cells were activated with ADP (10 μM) for 10 minutes and then exposed for 4 hours to serum (aHUS or control serum pool) diluted 1:2 with test medium, in the presence or absence of anti-C3d conjugated with CR11-17 (10 nM) or with FH1-5 (100 and 1000 nM), or sCR1 (150 µg/ml), or eculizumab (100 µg/ml, based on recommended Cthrough target level of 50–100 µg/ml). After a quick flush with HBSS, HMEC-1 were perfused with heparinized (10 IU/ml heparin) whole blood from healthy subjects (pre-labeled with the fluorescent dye mepacrine 10 μM) in a thermostatic flow chamber (37°C). A constant flow rate of 1500 sec-1 (60 dynes/cm2) was applied to mimic the shear stress conditions found in the microcirculation (16). Three different healthy subjects were used as blood donors for all the experiments. The same blood donor was used for all the slides/conditions evaluated in each experiment. After 3 minutes, perfusion was stopped and the slide with the endothelial cell monolayer was dehydrated and fixed in acetone for 20 minutes. The integrity of the cell monolayer after the exposure to serum was verified in parallel slides in which cells were stained with May-Grunwald Giemsa.
The fluorescent staining was acquired through the Apotome Axio Imager Z2 microscope (Zeiss) and the stained area was evaluated using Image J (NIH, Bethesda, MD). For each sample, 15 fields were analyzed, the highest and the lowest values were discarded and the mean was calculated on the remaining 13 fields. Results were expressed as area covered by platelet aggregates, in pixel2.
Statistical analysis
Data were reported as mean ± SD. The normality of value distribution was assessed using the D’Agostino-Pearson’s test. Comparisons of groups were performed with one-way analysis of variance followed by post hoc Student-Newman-Keuls test or Kruskal-Wallis test followed by post hoc Conover test, as appropriate. Statistically significant differences were assumed at a 5% level of probability. All calculations were made using MedCalc 10.0.1 statistical software (MedCalc Software, Mariakerke, Belgium).
Results
Deposition of C3 activation and degradation products on the surface of HMEC-1 exposed to serum from aHUS patients
We included in this study 9 aHUS patients in remission out of anti-C5 therapy who carried rare variants (RVs) in the CFH, or CFI, or C3 genes. Table 1 summarizes clinical and genetic characteristics of the patients.
Consistently with previously published reports (14–18), C3 deposition induced on ADP-activated HMEC-1 by serum from aHUS patients was significantly higher than that obtained with control serum pool, regardless of the genetic defect (Figure 1). C3 deposits were evaluated by anti-C3c antibody that recognizes an epitope shared by intact C3 and C3b. The addition of the pan-complement inhibitor sCR1 at the concentration of 150µg/ml (600 nM), which has been shown to completely block complement activation (16), fully prevented the effect of aHUS serum on C3 deposits (Figure 1). These data indicated that aHUS serum-induced C3 deposition entirely reflected complement activation.
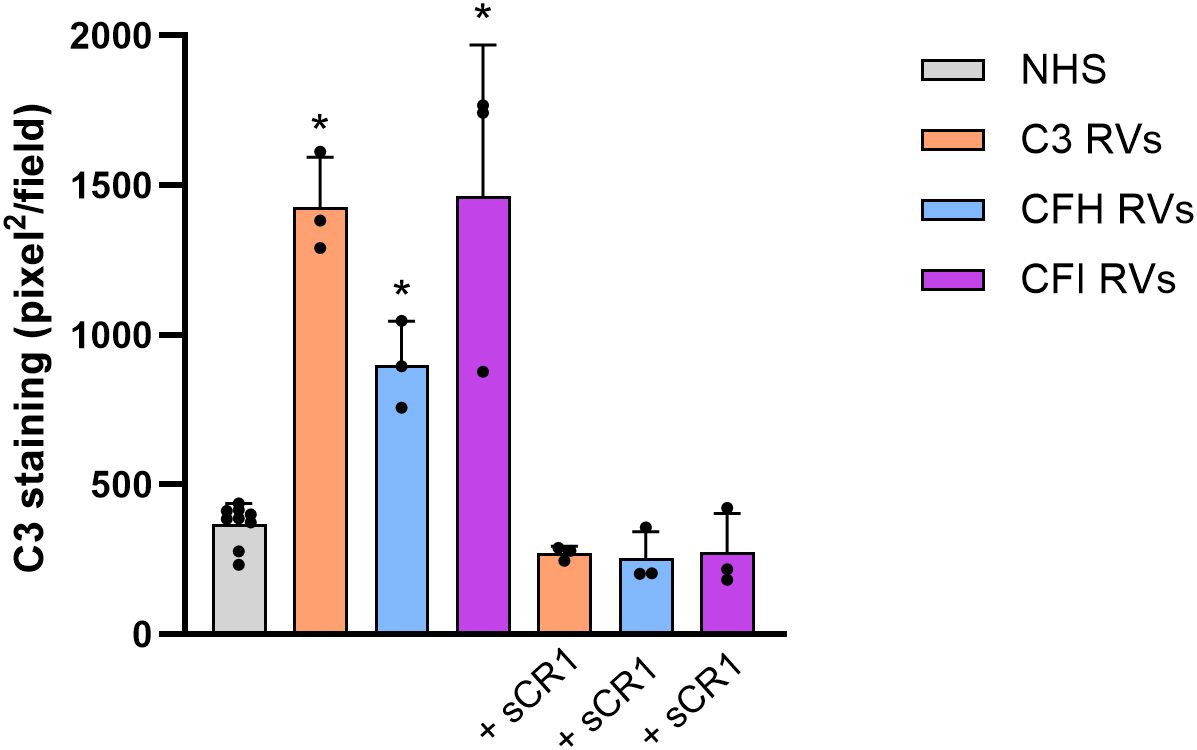
Figure 1. C3 deposition induced on activated HMEC-1 by serum from aHUS patients carrying RVs in C3 (n=3), CFH (n=3) or CFI (n=3) genes. Data are expressed as area covered by C3 staining measured by anti-C3c mAb (mean ± SD). * p<0.05 vs NHS and corresponding +sCR1. RVs, rare variants; NHS, normal human serum.
To further confirm the above findings, we evaluated C3d deposited on ADP-activated HMEC-1 exposed to aHUS serum using the anti-C3d mAb 3d8b. This antibody recognizes a neoepitope shared by the complement fragments iC3b, C3dg, and C3d without binding to C3/C3b/C3c, and it has been documented to detect C3d deposition on biopsy specimens from various complement-related autoimmune kidney and skin conditions (9, 10). We observed a significant increase in C3d staining on ADP-activated cells exposed to aHUS serum compared to those exposed to control serum (Supplementary Figure 2).
Anti-C3d mAb conjugated with CR11-10, CR11–17 or FH1–5 inhibit C3 deposition on HMEC-1 exposed to serum from aHUS patients
We then evaluated whether fusion proteins combining an anti-C3d monoclonal antibody (mAb) with complement inhibitors could efficiently block aHUS serum-induced complement activation on endothelial cell surface.
Both anti-C3d mAb conjugated with CR11–10 and soluble CR11–10 added to serum from aHUS patients, dose dependently reduced C3 deposition on ADP-activated HMEC-1 (Figures 2A, 3). Notably, at the dose of 10nM, C3d-targeted CR11–10 was more effective than equimolar non-targeted CR11-10 (Figure 2A). The anti-C3d mAb alone had no effect on aHUS serum-induced C3 deposition on HMEC-1, indicating that it did not independently influence complement activation on HMEC-1 exposed to aHUS serum and did not interact with anti-C3c Ab staining (Supplementary Figure 1).
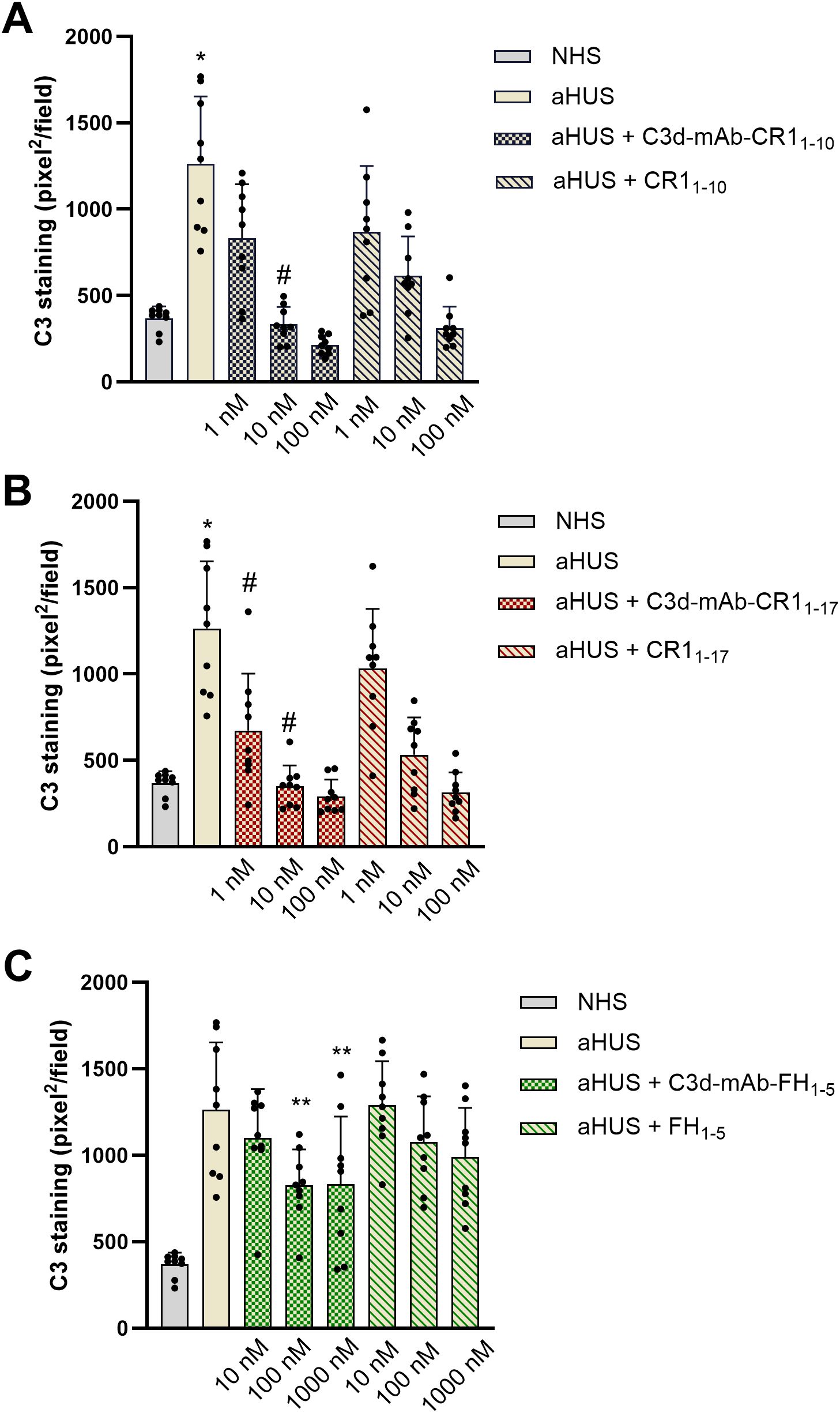
Figure 2. C3 deposition induced on activated HMEC-1 by serum from aHUS patients (three with CFH RVs, three with CFI RVs, and three with C3 RVs) in the presence or absence of anti-C3d mAb conjugated with CR11-10 (1, 10, 100 nM) or the corresponding unconjugated CR11-10 (1, 10, 100 nM) (A); anti-C3d mAb conjugated with CR11-17 (1, 10, 100 nM) or the corresponding unconjugated CR11-17 (1, 10, 100 nM) (B); and anti-C3d mAb conjugated with FH1-5 (10, 100, 1000 nM) or the corresponding unconjugated FH1-5 (10, 100, 1000 nM) (C). Data are expressed as area covered by C3 staining (mean ± SD, nine independent experiments). Each experiment includes cells exposed to NHS, or serum from a specific aHUS patient, with or without complement inhibitors. *p<0.05 vs all groups; #p<0.05 vs equimolar CR11–10 or CR11-17; **p<0.05 vs aHUS. NHS: normal human serum.
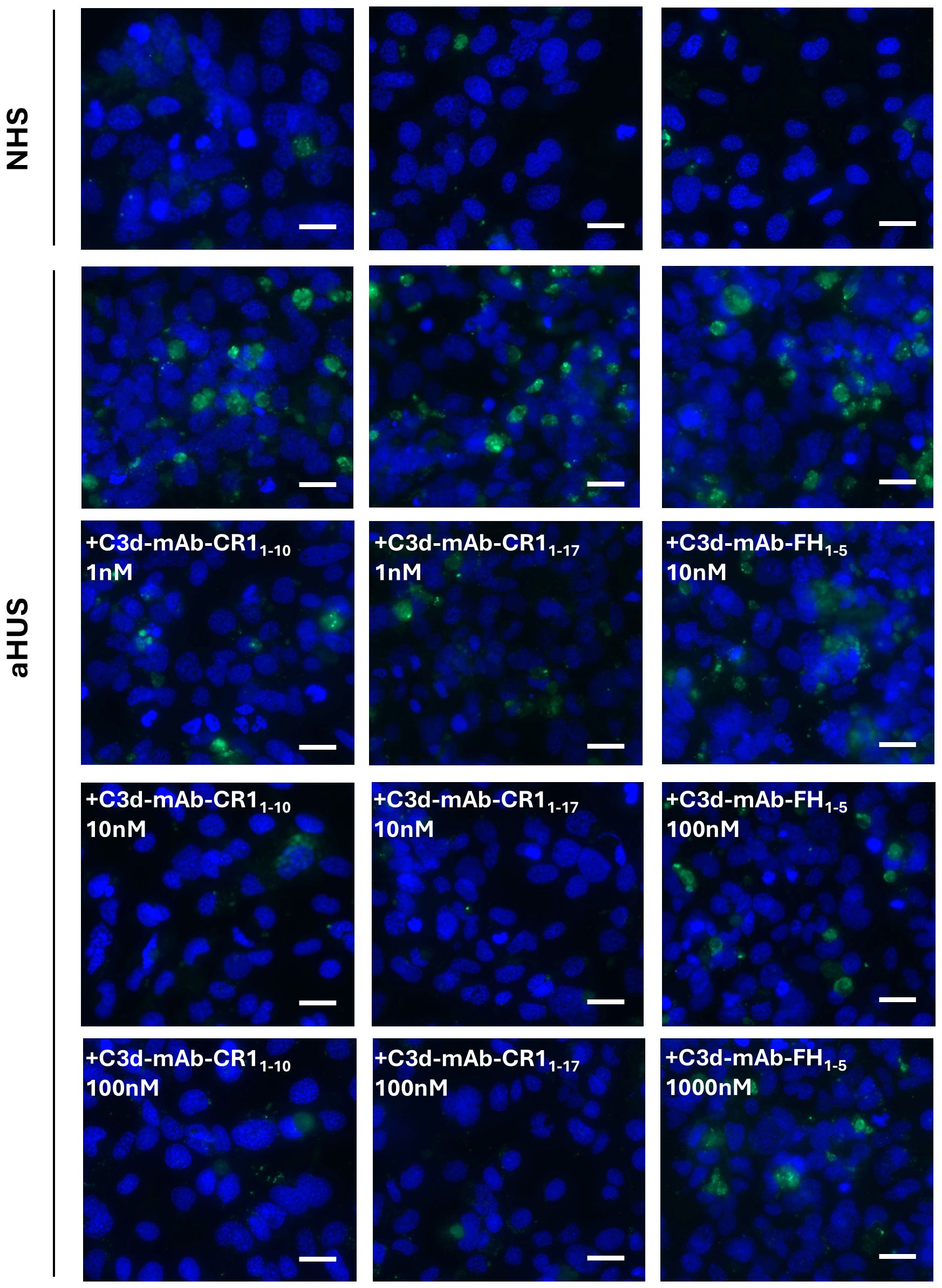
Figure 3. Representative images of C3 staining (green) on activated HMEC-1 exposed to normal human serum (NHS) or serum from aHUS patients in the presence or absence of anti-C3d-CR11-10, anti-C3d-CR11-17, and anti-C3d-FH1-5. Blue: DAPI. Scale bar: 20 µm.
Similar results were obtained with C3d-targeted CR11–17 added to aHUS serum, although it was more effective in reducing C3 deposition than non-targeted CR11-17 both at 1nM and 10 nM doses (Figures 2B, 3).
Half maximal inhibitory concentrations (IC50) on C3 deposition were 2.61 ± 0.48 nM and 1.05 ± 0.60 nM for C3d-targeted CR11–10 and C3d-targeted CR11-17, respectively. Of note the IC50s of the non-targeted inhibitors were about 4-fold higher than the corresponding C3d-targeted ones (CR11-10: 8.16 ± 1.96 nM; CR11-17: 6.61 ± 1.67 nM).
At 10 nM concentration, neither the C3d-targeted FH1-5, which combines the anti-C3d mAb with the complement-regulatory domain of CFH (SCRs 1-5), nor the non-targeted FH1–5 reduced serum-induced C3 deposition on ADP-activated HMEC-1 (Figures 2C, 3). Increasing the concentrations to 100 and 1000 nM, led to a partial but significant reduction of C3 deposits on HMEC-1 exposed to aHUS serum added with C3d-targeted FH1-5, while equimolar non-targeted FH1–5 had no effect (Figures 2C, 3).
To compare the inhibitory potential of C3d-targeted complement inhibitors among patients with RVs in different genes, we expressed the results as percentage of C3 deposition induced by aHUS serum in the presence of complement inhibitors, relative to C3 deposition in their absence, which was set to 100%. The analysis of individual data revealed that, at the 1 nM dose, C3 deposition was minimally or not prevented on HMEC-1 exposed to sera from patients #1 and #3 – carrying the p.R1210C and p.S1191L RVs in CFH gene, respectively – with either C3d-targeted CR11–10 or C3d-targeted CR11-17 (Figures 4A, B). At the 10nM and 100nM doses, C3 deposits were nearly and completely in the normal range, respectively (Figures 4A, B). On the other hand, patients with either C3 or CFI RVs were more sensitive to the inhibitory effects of both C3d-targeted CR11–10 and C3d-targeted CR11-17 (Figures 4A, B). As for fusion proteins combining anti-C3d mAb with FH1-5, the analysis of results in individual patients evidenced that a subgroup of patients was more sensitive to the inhibitory effect of C3d-targeted FH1-5. Indeed, the compound was able to substantially reduce ex-vivo serum-induced C3 deposition even at the dose of 10 nM in patient #2 carrying the p.R78G RV in CFH gene, in patient #5 carrying the p.S1063R RV in C3 gene, and in patients #7 and #9, who carry the p.P50A and p.C67W RVs in CFI gene (Figure 4C).
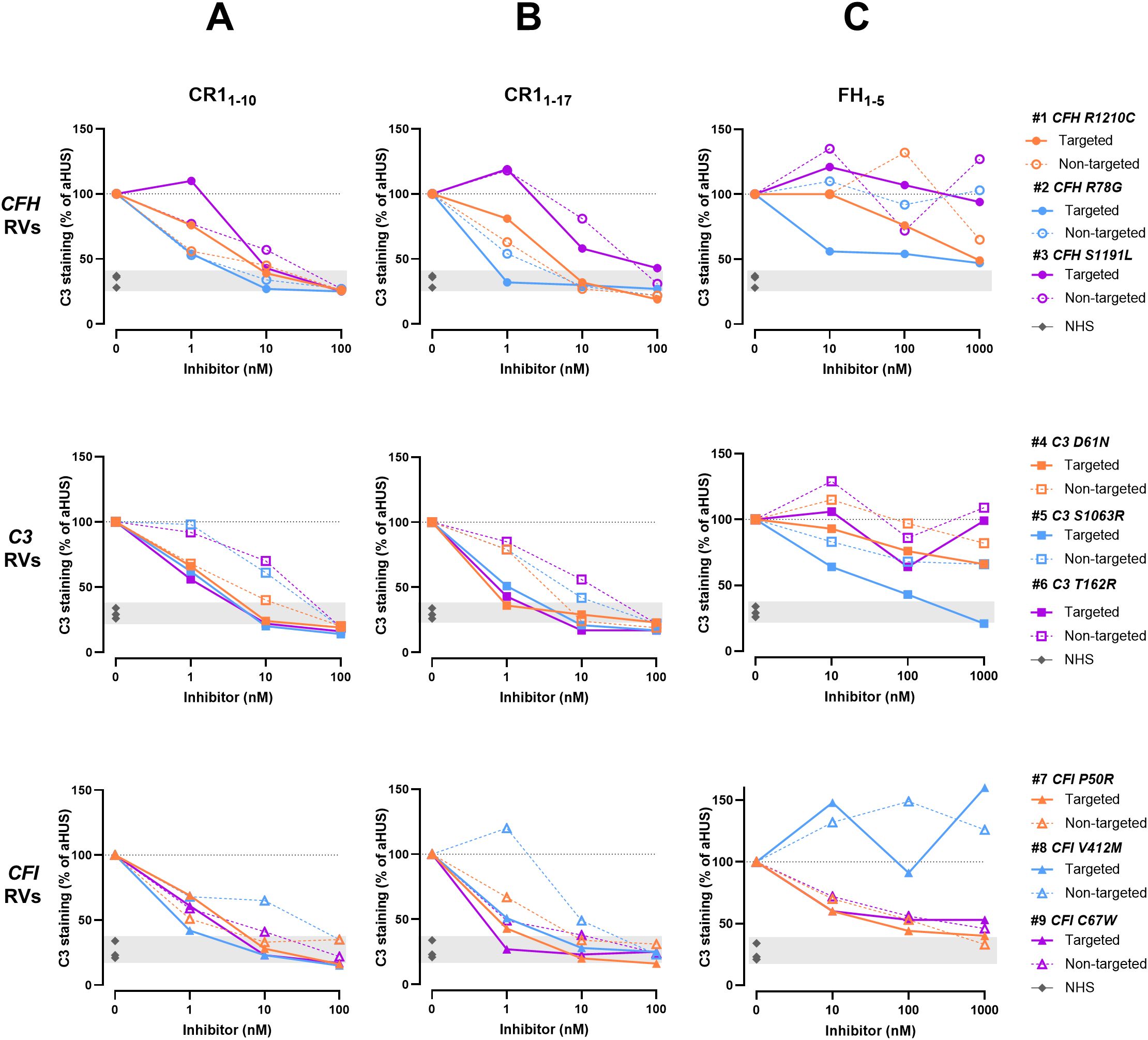
Figure 4. C3 deposition induced on activated HMEC-1 by serum from aHUS patients in the presence or absence of C3d-targeted or non-targeted CR11-10 (A), C3d-targeted or non-targeted CR11-17 (B), and C3d-targeted or non-targeted FH1-5 (C). Data are expressed as percentages of serum-induced C3 deposition in the presence of complement inhibitors, relative to aHUS serum alone, which is set at 100%. Upper panels: Patients carrying CFH RVs (n=3 independent experiments). Each experiment includes cells exposed to NHS, or serum from a specific aHUS patient with or without complement inhibitors. Colored points represent the percentage value for individual patients/experiments. #1: patient carrying p.R1210C RV in CFH gene. #2: patient carrying p.R78G RV in CFH gene. #3: patient carrying p.S1191L RV in CFH gene. Middle panels: Patients carrying C3 RVs (n=3 independent experiments). Each experiment includes cells exposed to NHS, or serum from a specific aHUS patient with or without complement inhibitors. Colored points represent the percentage value for individual patients/experiments. #4: patient carrying p.D61N RV in C3 gene. #5: patient carrying p.S1063R RV in C3 gene. #6: patient carrying p.T162R RV in C3 gene. Lower panels: Patients carrying CFI RVs (n=3 independent experiments). Each experiment includes cells exposed to NHS, or serum from a specific aHUS patient with or without complement inhibitors. Colored points represent the percentage value for individual patients/experiments. #7: patient carrying p.P50R RV in CFI gene. #8: patient carrying p.V412M RV in CFI gene. #9: patient carrying p.C67W RV in CFI gene. RVs, rare variants; NHS, normal human serum. Dashed line: 100% aHUS serum.
Anti-C3d mAb conjugated with CR11-10, CR11–17 or FH1–5 inhibit C5b-9 formation on HMEC-1 exposed to serum from aHUS patients
As in aHUS the terminal product of the complement cascade is strongly activated on endothelium (14, 15), we then tested the efficacy of C3d-targeted complement inhibitors in preventing C5b-9 formation induced by aHUS serum on HMEC-1. For 3 patients, namely patient #1 (p.R1210C RV in CFH), #5 (p.S1063R RV in C3) and #7 (p.P50A RV in CFI), there were sufficient serum samples available.
Data showed a significant reduction of C5b-9 formation on cells exposed to sera added with either C3d-targeted CR11–10 or CR11-17 (Figures 5A, 6), or C3d-targeted FH1-5 (Figures 5B, 6), as well as with non-targeted CR11-10, CR11-17, or FH1-5 (Figures 5A, B). Notably, values of C5b-9 staining were comparable to those observed with control serum pool with C3d-targeted CR11–10 and CR11–17 at 10nM and with C3d-targeted FH1–5 at 1000 nM concentration (Figures 5A, B).
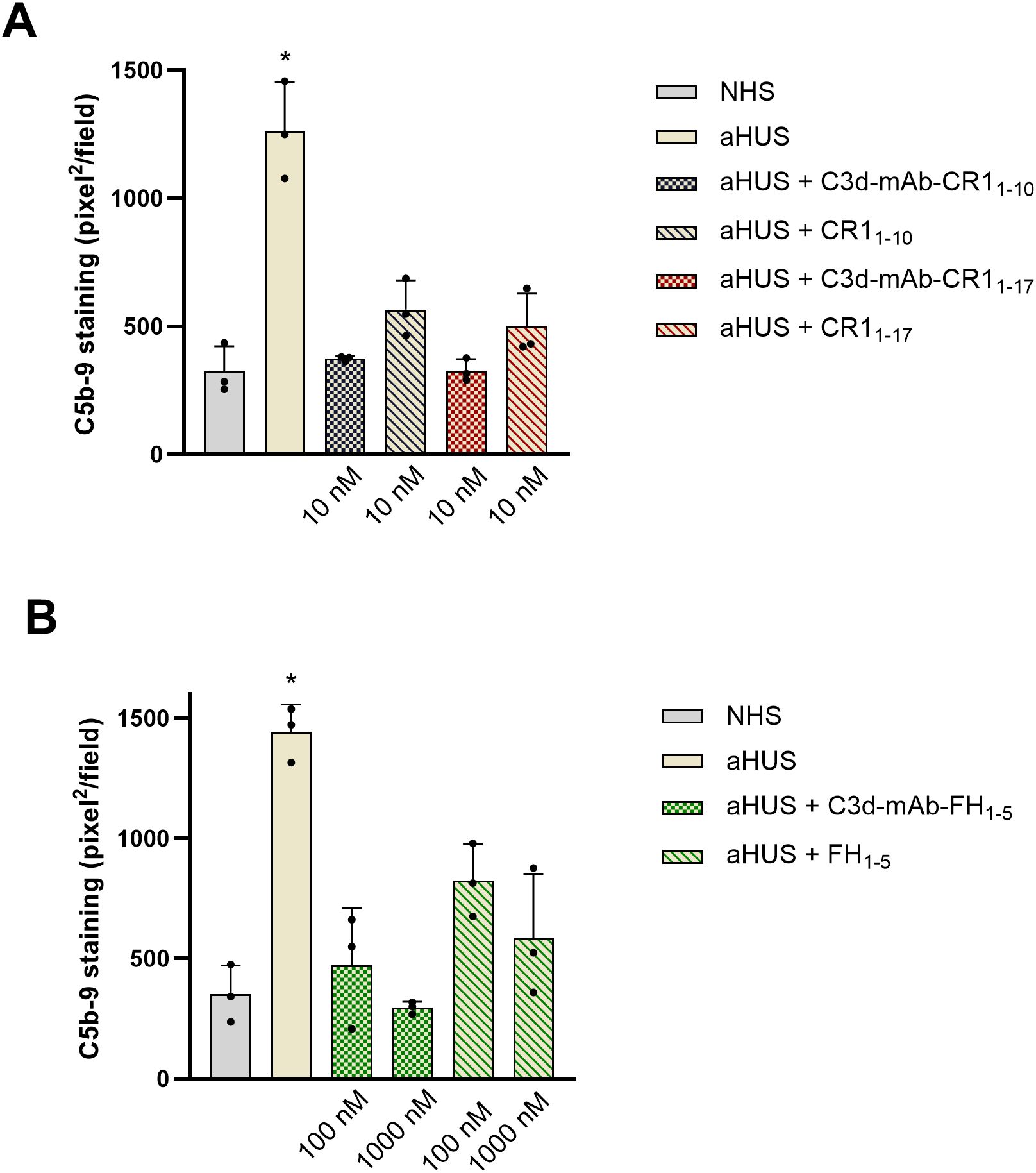
Figure 5. Effect of anti-C3d mAb conjugated with CR11–10 or CR11-17 (10 nM) and their corresponding non-targeted CR11-10, CR11-17 (10 nM) (A); or anti-C3d mAb conjugated with FH1-5 (100 and 1000 nM) and its corresponding non-targeted FH1-5 (100 and 1000 nM) (B), on C5b-9 formation on activated HMEC-1 cells exposed to serum from aHUS patients (one with RV in CFH, one with RV in CFI, and one with RV in C3). Data are expressed as area covered by C5b-9 staining (mean ± SD of three independent experiments). *p<0.05 vs all groups. NHS, normal human serum.
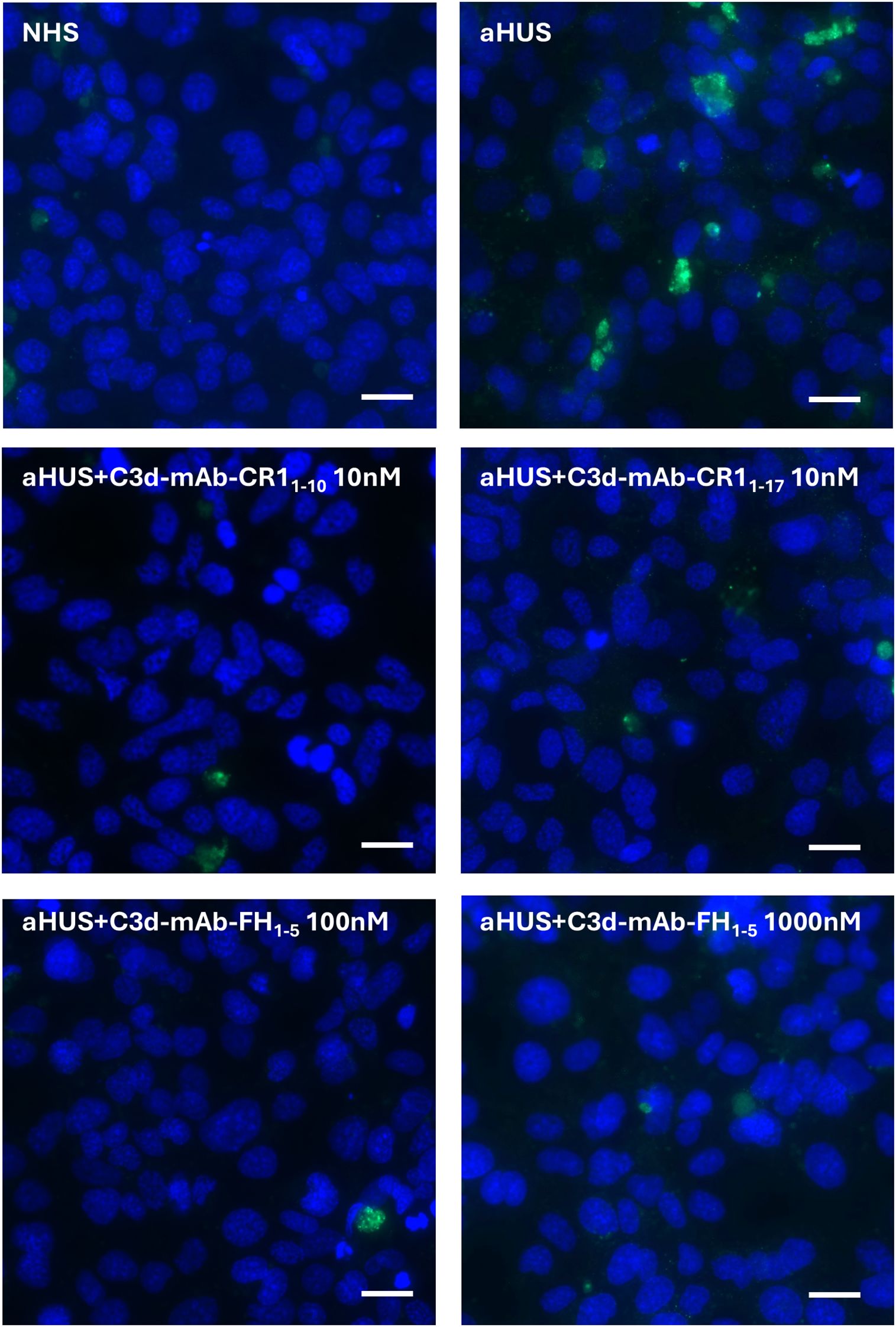
Figure 6. Representative images of C5b-9 staining (green) on activated HMEC-1 exposed to normal human serum (NHS) or serum from aHUS patients in the presence or absence of anti-C3d-CR11-10 (10 nM), anti-C3d-CR11-17 (10 nM), and anti-C3d-FH1-5 (100 and 1000 nM). Blue: DAPI. Scale bar: 20 µm.
Anti-C3d mAb conjugated with CR11–17 or FH1–5 inhibit platelet adhesion and aggregation on HMEC-1 exposed to serum from aHUS patients
In aHUS complement activation on endothelial cell surface is associated with loss of anti-thrombotic properties (16). Thus, we then moved to evaluate the effect of C3d-targeted CR11–17 and C3d-targeted FH1–5 on platelet adhesion and aggregation on HMEC-1 exposed to aHUS serum and then perfused with control whole blood. C3d-targeted CR11–17 and FH1–5 were tested with sera from the same 3 patients selected for the above-described experiments of C5b-9 formation.
As shown in Figure 7, the area covered by platelet aggregates was about 10-fold wider on HMEC-1 pre-exposed to aHUS serum as compared with that observed on endothelium pre-exposed to control serum pool, confirming our previously published results (16). Addition of C3d-targeted CR11–17 at 10 nM to aHUS serum fully prevented platelet adhesion and aggregation induced on HMEC-1 by the exposure to aHUS serum (Figures 7A, B). Similar effects were achieved by C3d-targeted FH1-5, at both 100 and 1000 nM (Figures 7A, B). The inhibitory effect was comparable to that achieved by the pan-complement inhibitor sCR1 as well as by the potent inhibitor of the complement terminal pathway, eculizumab.
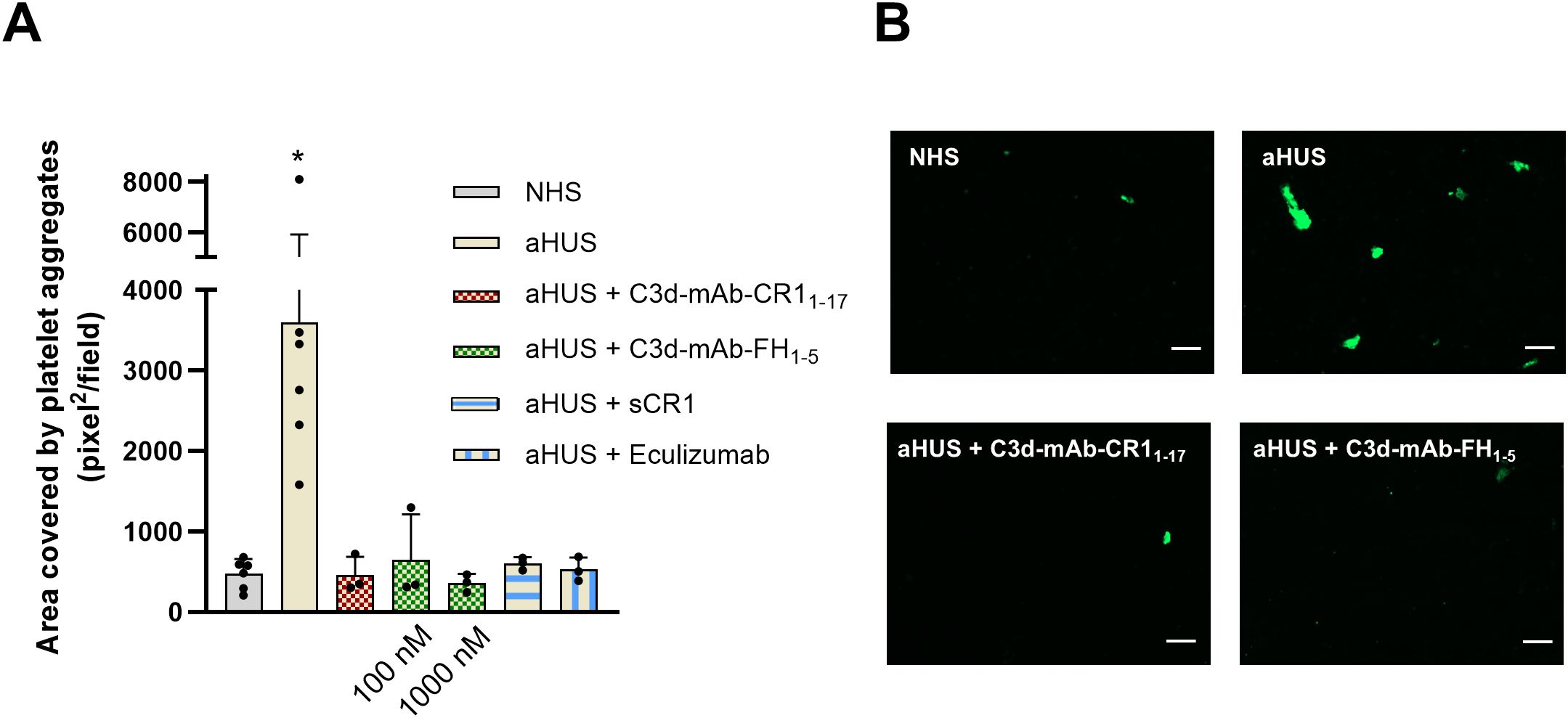
Figure 7. (A) Platelet adhesion and aggregation on activated HMEC-1 exposed to serum from aHUS patients (one with RV in CFH, one with RV in CFI, and one with RV in C3), in the presence or absence of anti-C3d mAb conjugated with CR11-17 (10 nM), or anti-C3d mAb conjugated with FH1-5 (100 or 1000 nM), or sCR1 (150 µg/ml), or eculizumab (100 µg/ml), and then perfused with control whole blood. Data are expressed as area covered by platelet aggregates (mean ± SD). *p<0.05 vs all groups. (B) Representative images of experiments of platelet adhesion and aggregation (green staining) on HMEC-1 cells exposed to NHS, or aHUS serum with or without C3d-mAb-CR11-17 (10 nM) or C3d-mAb-FH1-5 (1000 nM). NHS, normal human serum. Scale bar: 100 µm.
Discussion
Here we evaluated the effectiveness of anti-C3d antibodies -recognizing iC3b/C3dg/C3d- conjugated with fragments of endogenous complement inhibitors (CR11-10, CR11–17 or FH1-5) to correct complement dysregulation at the cell surface level. To assess this, we used ex-vivo assays that measure complement activation – C3 deposition and C5b-9 formation – induced by aHUS serum on microvascular endothelial cells. These assays have proven to be reliable in detecting complement dysregulation in aHUS patients, effectively replicating the endothelial-specific complement activation that characterizes the disease (14–19), and for evaluating the in-vitro efficacy of novel complement inhibitors (20).
Our findings confirm that exposure of microvascular endothelial cells to serum from aHUS patients results in abnormal deposition of active and degradation fragments of C3, and increased formation of C5b-9 (14–19). Importantly, we document that anti-C3d-conjugated CR11-10, CR11-17, and FH1-5, when added to aHUS serum, effectively prevented complement activation on endothelial cells, outperforming their soluble counterparts (CR11-10, CR11-17, and FH1-5). This highlights the potential of localizing complement endogenous negative regulators to C3d deposited on biologic surfaces as a promising strategy for cell/tissue-targeted complement inhibition, avoiding systemic blockade and thereby preserving the homeostatic functions of complement system (5). Furthermore, the observation that the inhibitory effects on complement activation on both C3/C3b and C5b-9 level, achieved with all the three C3d-targeted inhibitors, translated into complete prevention of platelet adhesion and aggregation on endothelial cells pre-exposed to aHUS serum, underscore their potential to counteract the direct consequence of complement activation on endothelium and highlight the potential clinical applicability of C3d-targeted CR1 and FH fragments for aHUS treatment.
Of relevance, we found that the effectiveness of tested compounds, varies according to the specific complement inhibitor conjugated to the anti-C3d antibody, and to the complement activation step being evaluated (C3 vs C5 convertase).
At low doses C3d-targeted CR11–17 exerted more robust inhibition of aHUS serum-induced C3 deposits as compared to C3d-targeted CR11-10. This finding may be interpreted considering that CR1 exerts its regulatory activities by binding C3b through two binding sites located within the regions comprising SCRs 8–11 and SCRs 15–18 (21), so that C3d-targeted CR11–10 and CR11–17 can bind one and two C3b molecules, respectively. In general, fragments of CR1 proved to be more effective than FH1–5 in inhibiting C3 deposition, while both strategies were equally effective in inhibiting C5b-9 formation. The AP C5 convertase is formed by the addition of extra C3b molecules to the C3 convertase, and its activity relies heavily on the density of C3b on cell surfaces (22). Thus, even partial inhibition of C3b deposition may be enough to effectively block C5 convertase. Finding that, even an uncomplete inhibition of C3 convertase is sufficient to penetrate to C5 convertase level and translate to anti-thrombotic activity, is consistent with the above hypothesis.
Another finding of this study is that the complement inhibitory effectiveness of C3d-targeted complement regulators is influenced by the specific genetic defect carried by the patient. All three C3d-targeted complement inhibitors required higher concentration to effectively correct complement dysregulation in the presence of RVs affecting the SCRs 19–20 of CFH. These SCRs contain a C3b-binding site and a polyanion-binding site that are crucial for CFH binding and complement regulatory activity on cell surfaces (23). As a consequence, mutations in these regions have a profound impact on CFH regulatory effect (23).
On the other hand, C3d-targeted FH1–5 corrected C3 convertase dysregulation more effectively than soluble FH1–5 in samples from two patients: one carrying the p.R78G RV in CFH and the other carrying the p.S1063R RV in C3. These findings may be interpreted as to indicate that increasing the local concentration of functional FH1–5 by C3d-anchoring helps compensate for the reduced C3b-CFH binding affinity associated with these RVs (24, 25).
Preclinical studies have recently tested the potential therapeutic effect of C3d-targeted CR11–10 and FH1–5 in C3 glomerulopathies (C3G) by studying their tissue localization and complement inhibition effectiveness in CfH knockout mice (CfH-/-) (9, 10). These mice exhibit uncontrolled systemic complement activation, which results in a 10- to 20-fold reduction in circulating intact C3 protein levels, and increased C3 deposition in the liver and kidneys. This leads to complement-mediated kidney injury, closely mimicking the clinical features of C3G (10, 26). Both C3d-targeted CR11–10 and C3d-targeted FH1-5, administered to CfH-/- mice at low doses, effectively localized to injured tissues and reduced C3 fragments deposition for durations exceeding 10 days (9, 10), with circulating levels of the fusion proteins remaining below 3 µg/ml. Notably at these low doses, unlike soluble FH1-5, treatment of CfH-/- mice with C3d-targeted FH1–5 resulted in marked and sustainable complement inhibition in both the liver and kidneys, while avoiding circulating complement blockade as documented by only a negligible increase in intact C3 plasma levels after drug administration (10). In addition, C3d-targeted FH1–5 treatment achieved similar effects when administered either intravenously or subcutaneously (10), with the subcutaneous route offering the advantage of an easier administration in potential human applications.
Beyond aHUS and C3G, persistent, uncontrolled complement activation plays a major role in several other diseases, including paroxysmal nocturnal hemoglobinuria (PNH) (27). Similar to aHUS patients, those with PNH are treated with anti-C5 therapies. However, some patients on these therapies experience frequent C3-mediated extravascular hemolysis flares (28). Current guidelines recommend switching these patients to therapies that inhibit complement cascade upstream, such as the recently approved C3 inhibitor, pegcetacoplan (29). Nevertheless, like anti-C5 blockade, C3-blocking therapies carry a significant risk of serious infections, which supports the potential of local cell membrane C3d-targeted complement inhibitors as a safer alternative therapeutic approach for PNH patients who do not respond adequately to anti-C5 therapies. The possibility that the fusion proteins described here might recognize certain pathogens opsonized with iC3b, C3dg, or C3d cannot be excluded based on the current data. However, previous studies have shown that both C3d-targeted CR11–10 and C3d-targeted FH1–5 influenced complement-mediated hemolysis of rabbit erythrocytes—used as a model for complement-activating surfaces—only at concentrations far exceeding those effective in rodent models of complement-mediated diseases (9, 10). These findings support the potential of the two fusion proteins to control tissue-localized complement activation effectively, without impairing systemic antibacterial responses.
In summary, despite the limitations posed by the small sample size, our findings indicate that targeting complement inhibitors to C3d effectively corrects complement dysregulation on endothelial cells in aHUS patients. While the efficacy of C3 convertase inhibition varied depending on the complement inhibitor used (CR11-17 > CR11-10 > FH1-5) and the specific genetic abnormalities of the patients, all the inhibitors successfully blocked C5 convertase activity and eliminated the pro-thrombogenic effects of aHUS patients’ serum. These results highlight the potential of tissue-targeted complement inhibition, paving the way to new therapeutic approaches that are effective at low doses without affecting systemic complement activity. This approach may benefit aHUS as well as other diseases characterized by cell/tissue-restricted complement activation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Comitato Etico di Bergamo ASST - Papa Giovanni XXIII. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
VG: Data curation, Formal Analysis, Writing – original draft, Investigation. DS: Data curation, Formal Analysis, Investigation, Writing – original draft. SG: Investigation, Writing – review & editing. JT: Writing – review & editing. VMH: Writing – review & editing. SV: Writing – review & editing. FL: Writing – review & editing. KF: Writing – review & editing. CG: Investigation, Writing – review & editing. AB: Writing – review & editing. GR: Writing – review & editing. MN: Conceptualization, Data curation, Formal Analysis, Writing – original draft. SA: Conceptualization, Data curation, Formal Analysis, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. DS is the recipient of a fellowship from Fondazione Aiuti per la Ricerca sulle Malattie Rare ARMR ONLUS (Bergamo, Italy). This study has been partially supported by a research grant from Q32 Bio. The funding sources had no role in study design, nor in the collection, analysis or interpretation of data, nor in the writing of the report or in the decision to submit the paper for publication. The authors are deeply grateful for the generous support provided in memory of Mr. Rodolfo Luzzana.
Conflict of interest
MN has received honoraria from Alexion Pharmaceuticals, Sobi and Novartis for giving lectures, and for participating in advisory boards. GR has had consultancy agreement with Alexion Pharmaceuticals Inc., Janssen Pharmaceutical, Akebia Therapeutics, Biocryst Pharmaceuticals, Menarini Ricerche SpA, AstraZeneca; speaker honoraria/travel reimbursement from Boehringer Ingelheim, Novartis. VMH and JT hold equity in Q32 Bio and have received consulting income.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. AI was used for English editing.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1620996/full#supplementary-material
References
1. Noris M, Bresin E, Mele C, and Remuzzi G. Genetic atypical hemolytic-uremic syndrome. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, and Amemiya A, editors. GeneReviews®. University of Washington, Seattle, Seattle (WA) (1993). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK1367/.
2. Fakhouri F and Frémeaux-Bacchi V. Thrombotic microangiopathy in aHUS and beyond: clinical clues from complement genetics. Nat Rev Nephrol. (2021) 17:543–53. doi: 10.1038/s41581-021-00424-4
3. Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, et al. Terminal complement inhibitor eculizumab in atypical hemolytic–uremic syndrome. N Engl J Med. (2013) 368:2169–81. doi: 10.1056/NEJMoa1208981
4. Rondeau E, Scully M, Ariceta G, Barbour T, Cataland S, Heyne N, et al. The long-acting C5 inhibitor, Ravulizumab, is effective and safe in adult patients with atypical hemolytic uremic syndrome naïve to complement inhibitor treatment. Kidney Int. (2020) 97:1287–96. doi: 10.1016/j.kint.2020.01.035
5. Mastellos DC, Hajishengallis G, and Lambris JD. A guide to complement biology, pathology and therapeutic opportunity. Nat Rev Immunol. (2024) 24:118–41. doi: 10.1038/s41577-023-00926-1
6. Tomlinson S and Thurman JM. Tissue-targeted complement therapeutics. Mol Immunol. (2018) 102:120–8. doi: 10.1016/j.molimm.2018.06.005
7. Wang H, van de Bovenkamp FS, Dijkstra DJ, Abendstein L, Borggreven NV, Pool J, et al. Targeted complement inhibition using bispecific antibodies that bind local antigens and endogenous complement regulators. Front Immunol. (2024) 15:1288597. doi: 10.3389/fimmu.2024.1288597
8. Fridkis-Hareli M, Storek M, Mazsaroff I, Risitano AM, Lundberg AS, Horvath CJ, et al. Design and development of TT30, a novel C3d-targeted C3/C5 convertase inhibitor for treatment of human complement alternative pathway–mediated diseases. Blood. (2011) 118:4705–13. doi: 10.1182/blood-2011-06-359646
9. Fahnoe KC, Liu F, Morgan JG, Ryan ST, Storek M, Stark EG, et al. Development and optimization of bifunctional fusion proteins to locally modulate complement activation in diseased tissue. Front Immunol. (2022) 13:869725. doi: 10.3389/fimmu.2022.869725
10. Liu F, Ryan ST, Fahnoe KC, Morgan JG, Cheung AE, Storek MJ, et al. C3d-Targeted factor H inhibits tissue complement in disease models and reduces glomerular injury without affecting circulating complement. Mol Ther. (2024) 32:1061–79. doi: 10.1016/j.ymthe.2024.02.001
11. Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, and Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. (2014) 46:310–5. doi: 10.1038/ng.2892
12. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the american college of medical genetics and genomics and the association for molecular pathology. Genet Med. (2015) 17:405–24. doi: 10.1038/gim.2015.30
13. Goodship THJ, Cook HT, Fakhouri F, Fervenza FC, Frémeaux-Bacchi V, Kavanagh D, et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. (2017) 91:539–51. doi: 10.1016/j.kint.2016.10.005
14. Galbusera M, Noris M, Gastoldi S, Bresin E, Mele C, Breno M, et al. An ex vivo test of complement activation on endothelium for individualized eculizumab therapy in hemolytic uremic syndrome. Am J Kidney Dis. (2019) 74:56–72. doi: 10.1053/j.ajkd.2018.11.012
15. Noris M, Galbusera M, Gastoldi S, Macor P, Banterla F, Bresin E, et al. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood> . (2014) 124:1715–26. doi: 10.1182/blood-2014-02-558296
16. Aiello S, Gastoldi S, Galbusera M, Ruggenenti P, Portalupi V, Rota S, et al. C5a and C5aR1 are key drivers of microvascular platelet aggregation in clinical entities spanning from aHUS to COVID-19. Blood Adv. (2022) 6:866–81. doi: 10.1182/bloodadvances.2021005246
17. Gastoldi S, Aiello S, Galbusera M, Breno M, Alberti M, Bresin E, et al. An ex vivo test to investigate genetic factors conferring susceptibility to atypical haemolytic uremic syndrome. Front Immunol. (2023) 14. doi: 10.3389/fimmu.2023.1112257
18. Aiello S, Gastoldi S, Bresin E, Galbusera M, Mele C, Daina E, et al. Exuberant endothelial C5b-9 formation in recurrent and de novo posttransplant thrombotic microangiopathy. Kidney Int Rep. (2024) 9:3318–23. doi: 10.1016/j.ekir.2024.08.014
19. Timmermans SAMEG, Abdul-Hamid MA, Potjewijd J, Theunissen ROMFIH, Damoiseaux JGMC, Reutelingsperger CP, et al. C5b9 formation on endothelial cells reflects complement defects among patients with renal thrombotic microangiopathy and severe hypertension. J Am Soc Nephrol. (2018) 29:2234. doi: 10.1681/ASN.2018020184
20. Tschongov T, Konwar S, Busch A, Sievert C, Hartmann A, Noris M, et al. Moss-produced human complement factor H with modified glycans has an extended half-life and improved biological activity. Front Immunol. (2024) 15:1383123. doi: 10.3389/fimmu.2024.1383123
21. Hardy MP, Mansour M, Rowe T, and Wymann S. The molecular mechanisms of complement receptor 1—It is complicated. Biomolecules. (2023) 13:1522. doi: 10.3390/biom13101522
22. Zwarthoff SA, Berends ETM, Mol S, Ruyken M, Aerts PC, Józsi M, et al. Functional characterization of alternative and classical pathway C3/C5 convertase activity and inhibition using purified models. Front Immunol. (2018) 9:1691. doi: 10.3389/fimmu.2018.01691
23. Morgan HP, Schmidt CQ, Guariento M, Blaum BS, Gillespie D, Herbert AP, et al. Structural basis for engagement by complement factor H of C3b on a self surface. Nat Struct Mol Biol. (2011) 18:463–70. doi: 10.1038/nsmb.2018
24. Pechtl IC, Kavanagh D, Mcintosh N, Harris CL, and Barlow PN. Disease-associated N-terminal complement factor H mutations perturb cofactor and decay-accelerating activities*. J Biol Chem. (2011) 286:11082–90. doi: 10.1074/jbc.M110.211839
25. Schramm EC, Roumenina LT, Rybkine T, Chauvet S, Vieira-Martins P, Hue C, et al. Mapping interactions between complement C3 and regulators using mutations in atypical hemolytic uremic syndrome. Blood. (2015) 125:2359–69. doi: 10.1182/blood-2014-10-609073
26. Pickering MC, Cook HT, Warren J, Bygrave AE, Moss J, Walport MJ, et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet. (2002) 31:424–8. doi: 10.1038/ng912
27. Risitano AM, Frieri C, Urciuoli E, and Marano L. The complement alternative pathway in paroxysmal nocturnal hemoglobinuria: From a pathogenic mechanism to a therapeutic target. Immunol Rev. (2023) 313:262–78. doi: 10.1111/imr.13137
28. McKinley CE, Richards SJ, Munir T, Griffin M, Mitchell LD, Arnold L, et al. Extravascular hemolysis due to C3-loading in patients with PNH treated with eculizumab: defining the clinical syndrome. Bloodgn> . (2017) 130:3471. doi: 10.1182/blood.V130.Suppl_1.3471.3471
Keywords: complement system, complement inhibitors, aHUS, endothelial cell (EC), thrombus formation
Citation: Guaschino V, Santarsiero D, Gastoldi S, Thurman JM, Holers VM, Violette SM, Liu F, Fahnoe KC, Guarinoni C, Benigni A, Remuzzi G, Noris M and Aiello S (2025) C3d-targeted complement inhibitors to correct complement dysregulation in aHUS patients. Front. Immunol. 16:1620996. doi: 10.3389/fimmu.2025.1620996
Received: 30 April 2025; Accepted: 03 June 2025;
Published: 20 June 2025.
Edited by:
Peter F. Zipfel, Leibniz Institute for Natural Product Research and Infection Biology, GermanyReviewed by:
Lubka T. Roumenina, INSERM U1138 Centre de Recherche des Cordeliers (CRC), FranceHéctor Martín Merinero, Spanish National Research Council (CSIC), Spain
Copyright © 2025 Guaschino, Santarsiero, Gastoldi, Thurman, Holers, Violette, Liu, Fahnoe, Guarinoni, Benigni, Remuzzi, Noris and Aiello. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marina Noris, bWFyaW5hLm5vcmlzQG1hcmlvbmVncmkuaXQ=
†These authors have contributed equally to this work and share first authorship
 Valeria Guaschino
Valeria Guaschino Donata Santarsiero
Donata Santarsiero Sara Gastoldi
Sara Gastoldi Joshua M. Thurman
Joshua M. Thurman V. Michael Holers2
V. Michael Holers2 Shelia M. Violette
Shelia M. Violette Kelly C. Fahnoe
Kelly C. Fahnoe Ariela Benigni
Ariela Benigni Giuseppe Remuzzi
Giuseppe Remuzzi Marina Noris
Marina Noris Sistiana Aiello
Sistiana Aiello