- 1St Vincent’s Institute of Medical Research, Fitzroy, VIC, Australia
- 2Department of Medicine (St Vincent’s), University of Melbourne, Fitzroy, VIC, Australia
T cell development and function depend on precise remodeling of the actin cytoskeleton, which regulates migration, cell division, immunological synapse formation, and signal transduction. Regulators of actin include nucleators (Arp2/3, Formins) and binding proteins (coronins, cofilin, myosin) that orchestrate cytoskeletal dynamics to ensure efficient antigen recognition and signaling, while Rho GTPases (Rac1, Cdc42, RhoA) link extracellular cues to actin rearrangements, influencing both conventional T cell activation and function. Dysregulated actin dynamics contribute to immunodeficiencies and autoimmunity, and thus understanding how the actin cytoskeleton is regulated in T cells has important implications.
Introduction
The actin cytoskeleton is a dynamic filamentous (F)-actin network that shapes T cell morphology, motility and immunological function (1, 2). Constant F-actin polymerization and depolymerization enable T cells to migrate, exit the thymus, navigate lymphoid organs, and scan antigen-presenting cells (APCs) for the recognition of antigens. Upon recognizing a peptide–Major Histocompatibility complex (MHC), a T cell forms an immunological synapse (3, 4). The mature immunological synapse consists of a central supramolecular activation clusters (cSMAC) enriched in the T cell receptor (TCR) and an integrin-rich peripheral (p)SMAC ring (5, 6). Actin polymerization is essential for assembling and maintaining this structure. TCR engagement triggers a burst of actin filament assembly that drives large-scale reorganization of the T cell lamellipodium region (7, 8). There is continuous flow of actin at the synapse. F-actin polymerizes at the periphery and flows inward – corralling TCR micro-clusters towards the center and facilitating signal amplification and APC-contact stabilization (9–12). In cytotoxic T cells, actin also facilitates directed secretion of lytic granules (1). Beyond motility, actin is important for signal transduction, converting receptor engagement into structural reorganization essential for T cell function and activation (8).
Understanding the function of actin regulators is crucial for elucidating mechano-transduction in T cell biology and exploring the role of cytoskeletal dysfunction in immune tolerance and autoimmunity. T cell development and activation depend on precise actin regulation, controlled by proteins that mediate filament nucleation, stabilization, and turnover. Key regulators, including coronins, actin nucleators, actin-binding proteins and Rho GTPases, coordinate cytoskeletal remodeling for migration, synapse formation, and signaling. This review will address the roles of these regulators in T cells as highlighted in Figure 1.
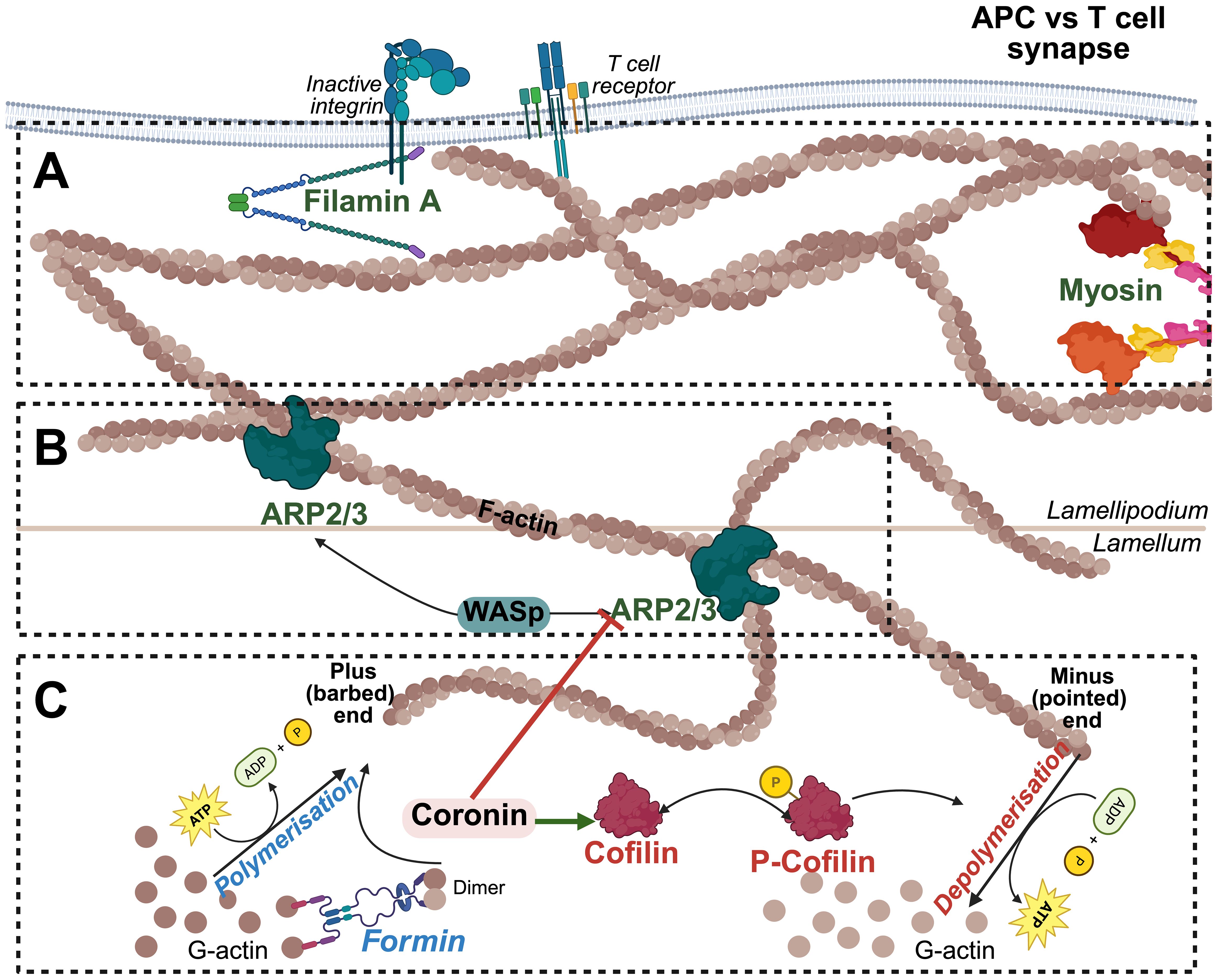
Figure 1. Actin Cytoskeletal Dynamics at the T Cell-APC Interface. (A) Actin remodeling and tension Generation: Myosin II generates contractile forces along the actin cytoskeleton, providing cortical tension necessary for immune synapse stability, T cell migration, and force-dependent signaling. Filamin A crosslinks actin filaments, stabilizing the branched architecture and reinforcing mechanical strength at T cell-APC contact site to facilitate membrane receptor anchoring. (B) Branched actin assembly and network stabilization: The Arp2/3 complex, activated by WASp, promotes the formation of branched actin networks that drive lamellipodia extension and immune synapse formation. (C) Actin polymerization and depolymerization: G-actin monomers polymerize at the plus (barbed) end in an ATP-dependent manner, a process facilitated by Formins. Formin-mediated nucleation and elongation generate linear actin filaments that serve as the foundation for cytoskeletal remodeling during T cell activation. Cofilin and coronins regulate actin filament turnover by promoting filament severing and depolymerization at the pointed ends.
Actin nucleators: Arp2/3 complex and formins
T cells rely on two primary actin nucleation systems: the actin-related protein (Arp)2/3 complex, which drives branched actin polymerization, and formin proteins, which generate linear actin filaments. The Arp2/3 complex is a seven-subunit complex that binds to the side of existing “mother” filaments and initiates the growth of a new filaments at ~70° angle, thereby creating branched actin network (13). Arp2/3 on its own has low activity; it requires nucleation-promoting factors (NPFs) such as the Wiskott-Alrich Syndrome protein (WASP) and the WAVE2 complex (also called SCAR) to trigger branch formation (1, 14). T cells express a hematopoietic-specific as well as the hematopoietic WAVE complex subunit Hem-1 (Nap1) (1). Upon TCR stimulation, these NPFs are activated by upstream signals (e.g. small GTPases) and recruit Arp2/3 to form the branched actin meshwork in the lamellipodium of the immunological synapse (15, 16).
The importance of Arp2/3-mediated actin polymerization in T cell activation is highlighted by WAS, an X-linked immunodeficiency caused by WASP mutation (17, 18). Patients with WAS have defective T cell activation and cytoskeletal organization, and present with autoimmunity, highlighting the importance of actin dynamics in immune function and tolerance (18, 19). At the cellular level, WASP-deficient T cells (but not B cells) to polymerize actin at the synapse, leading to the impaired TCR clustering, PLCγ1 activation, and calcium signaling (1, 19, 20).
Similarly, mutations in ARPC1B, a core Arp2/3 subunit, results in severe immunodeficiency, autoimmunity, and defective cytotoxic function due to aberrant actin polymerization (21–23). T cells from these patients cannot generate normal lamellipodia and instead form aberrant actin spikes and filopodia, leading to unstable T cell-APC conjugates and defective cytotoxic function (23, 24). These findings underscore the importance of Arp2/3-mediated branched actin networks for T cell activation and effector functions.
In parallel, T cells rely on formin proteins (e.g., mDia1, mDia3) to generate linear actin filaments by stabilizing initial actin dimers and promoting barbed-end elongation, often producing long unbranched filaments (25). At the T cell synapse, formins cooperate with Arp2/3 to pattern the actin cytoskeleton. Live-cell imaging studies have shown that arc-like structures in the inner synapse (lamellar region) are composed of linear actin filaments generated by formin activity (26). These actin arcs are aligned concentric rings that undergo a myosin II-dependent contraction, helping to transport TCR clusters inward (26). These actomyosin arcs originate from formin-nucleated filaments behind the leading-edge synapse (25). When formin function is inhibited, these actin arcs are disorganized and TCR micro-cluster movement perturbed, indicating that formin-mediated filament elongation is required to scaffold proper synaptic architecture, Hong and colleagues also showed that the formation of organized actomyosin arcs depends on the strength of TCR signal and the activity of myosin II motor proteins (26). Strong agonist peptides induce robust formin-dependent arc formation, which correlates with higher mechanical forces (evidence by the tension-sensitive molecule phosphorylation) and enhanced central accumulation of signaling complexes (26). By contrast, weak TCR ligands lead to only patchy, irregular arcs. Notably, blocking myosin II contractility abrogates the difference between strong vs weak ligand responses, suggesting that formin-based actin structures and myosin-generated tension together amplify signals for potent antigens (25, 26). This is a form of mechano-transduction that formins facilitate. Consistently with a role in regulating the synapse, mDia1 (Diap1) knockout mice exhibit T cell activation defects, and one study found that T cells lacking both mDia1 and mDia3 had impaired division and migration (13). However, unlike Arp2/3, the role of formin proteins in T cell development has yet to be addressed.
Together, Arp2/3 and formins serve distinct yet complementary roles in T cell cytoskeletal remodeling. Arp2/3 drives broad membrane protrusions and receptors clustering, while formins stabilize actin arcs and filopodial structure. Their coordinated activity is thus essential for T cell activation, migration, and immune regulation (Figure 2).

Figure 2. Rho GTPase-mediated signaling pathways regulating actin polymerization in T cells. Summary of the major signaling cascades initiated by surface receptors (e.g., GPCRs, cytokine receptors, TCRs) that activate Rho family GTPases via Rho GEFs. RhoA activate Formins, such as mDia1 and mDia3, to promote stress fiber formation and cortical contractility, supporting T cell squeezing and immune synapse stability. Rac1 activates WAVE2, and Cdc42 activates WASp, both of which stimulate the Arp2/3 complex, driving actin polymerization. These cascades coordinate T cell migration, activation, trafficking, polarization, and immunological synapse formation via dynamic actin remodeling.
Beyond the nucleation of filaments, numerous actin-binding proteins (ABPs) regulate filament turnover, organization, and linkage to other cellular structures. These are few exemplars with known importance in T cells: myosin motors, the filament-severing protein cofilin, the actin crosslinker filamin A, and the Ezrin-Radixin-Moesin (ERM) family that links actin to the plasma membrane.
Myosins
Myosins are motor proteins that bind F-actin and use ATP to generate force and movement along filaments (10). T cells express non-muscle myosin II, especially Myosin IIA (encoded by MYH9), which assembles into bipolar filaments that can slide actin filaments relative to each other. In the immunological synapse, myosin II localizes to the medial zone, overlapping with the formin-derived arcs, and generates contractile tension that drives the movement of TCR micro-clusters toward the synapse center, thus sustain synapse structure (27). Kumari and colleagues showed that Myosin IIA is required for full immune synapse maturation (28). T cells lacking Myosin IIA (or treated with myosin inhibitors) formed asymmetrical or unstable synapses and had reduced central clustering of signaling molecules (28).
Myosin II also contributes to the retrograde flow of actin, where the motor pulls on filaments, adding to the inward movement initiated by polymerization at the periphery. The forces exerted by myosin II are a key part of T cell mechano-sensing, enabling the cell to probe the stiffness of the APC interface and to discriminate between strong and weak TCR signals (up to differences on the order of a few piconewtons) (26).
Besides Myosin II, T cells also have unconventional myosin, such as Myosin1g (Myo1g), which is a membrane-binding myosin implicated in forming membrane protrusions and regulating cortical tension in lymphocytes (29). Myo1g-defecient T cells exhibit altered spreading and migration, suggesting that this myosin helps T cell maintain proper cell cortex stiffness during squeezing through tissues (29, 30). Overall, myosin in T cells act as force generators and organizers, ensuring that actin filaments are appropriately positioned and tensioned for effective interaction with signaling complexes and adhesion molecules.
Cofilin
Cofilin (also known as cofilin-1 or CFL1 in T cells) is a small protein that binds to actin filaments to promote their disassembly. Cofilin increases the off rate of actin monomers from the pointed end and can sever filaments, creating new barbed ends for polymerization. By depolymerizing actin, cofilin replenishes the pool of G-actin monomers and allows actin turnover, which is critical for dynamic processes like cell migration and synapse recycling. The importance of cofilin in T cell development was strikingly demonstrated by a genetic study in mice where Cfl1 was replaced with a non-functional mutant specifically in T cells. This results in a severe early block in thymocyte development (31). Cofilin-deficient thymocytes were arrested at the double negative (DN) stage before successful TCRβ surface expression, leading to an absence of peripheral αβ T cells (31). These cofilin-mutant thymocytes accumulates F-actin, indicating a failure to dismantle actin structures, which likely interfered with cell division, migration within the thymus, or passage through the β-selection checkpoint. Interestingly, γδ T cells (which develop via a parallel pathway) were unaffected, highlighting that cofilin’s role is especially critical for conventional αβ T cells (31, 32).
In mature T cells, cofilin is required for effective migratory responses and synapse disassembly (33, 34). During immunological synapse formation, cofilin is initially inhibited by phosphorylation, which results in actin accumulation, but later gets activated to break down actin filaments and enable synapse dissolution and cell disengagement (10). If cofilin cannot perform this function, T cells may form overly stable contacts and fail to recycle components to interact with other APCs (35). There is also evidence that cofilin activity influences TCR signaling by facilitating actin turnover and preventing excessive F-actin build-up that could physically constrain signalosomes (36). Indeed, antigen-experienced conventional CD4 T cells have higher cofilin activity and a softer cellular cortex than naïve T cells, correlating with stronger TCR signaling (35, 37).
Filamin A
Filamin A (FLNA) is a large actin-crosslinking protein that organizes actin filaments into orthogonal networks and links them to membrane proteins. FLNA is expressed in T cells and interacts with integrin β-chains, essentially acting as a scaffold connecting integrins like LFA-1 to actin cytoskeleton (38–40). This linkage is crucial when T cells need to apply force or resist shear stress while adhering to other cells or the endothelium. Fagerholm and colleagues found that in T cell-specific FLNA-deficient mice primary T cells, FLNA is required for optimal integrin function, which is contrary to some earlier in vitro findings suggesting filamin is an inhibitor of integrin activation (38, 41). FLNA-/- T cells displayed impaired adhesion under flow conditions and reduced homing to lymph nodes and sites of inflammation (38, 40). In essence, without FLNA, T cells could still activate LFA-1 to some degree but could not transmit forces effectively in vivo (38). FLNA tethers integrins to retrograde flowing actin, functioning as part of the “molecular clutch that allows force coupling (42). In line with this, FLNA-/- T cells have altered distribution of LFA-1 at the synapse and may form less stable immune synapse (43).
There is also evidence that FLNA can impact TCR signaling. One study reported that knocking-down FLNA impaired PKC-θ recruitment to the immunological synapse and subsequent IL-2 production, possibly because cytoskeletal anchoring of signaling complexes was affected. Notably, regulatory T cells (Tregs) express higher LFA-1 than conventional T cells and use it to form aggregates with dendritic cells (44). Tregs with compromised FLNA might not sustain these aggregates under blood flow or in the dynamic lymph node environment, potentially reducing their suppressive capacity (38, 44).
Ezrin-Radixin-Moesin
The Ezrin-Radixin-Moesin (ERM) proteins are membrane-cytoskeletal linkers that tether actin filaments to the plasma membrane. T cells predominantly express Ezrin and Moesin (with little Radixin) (45). In resting T cells, ERM proteins are phosphorylated (active state) and help form microvilli and a rigid cortical actin shell, which maintains cell shape and spatial distribution of receptors (46). Upon T cell activation, ERMs undergo a transient dephosphorylation and re-localization, where they are briefly inactivated at the contact site to allow actin reorganization and receptor clustering and later concentrate at the distal pole complex (the face of the T cell opposite the synapse) (47). ERM proteins organize the distal pole by anchoring transmembrane proteins (like CD43 and PD-1) and preventing them from entering the synapse (47). This segregation of inhibitory or bulky molecules away from the synapse is important for efficient TCR signaling. Perturbing ERM function indeed leads to defects in T cell activation (46). For example, overexpression of dominant-negative Ezrin in Jurkat T cells causes poorly focused synapses and reduce IL-2 production (48). Conversely, T cells from Ezrin Moesin double-knockdown mice show impaired proliferation and motility (48). Upon antigen recognition, ERM proteins are rapidly inactivated via a Vav1-Rac1 signaling pathway leading to the disassociation of the cortical actin cytoskeleton from the plasma membrane (49). This un-anchoring reduces cellular rigidity, facilitating the formation of stable T cell–APC conjugates (49). Thus, ERM proteins act as architects of the T cell actin cortex, ensuring that during synapse formation certain regions of the membrane are scaffolded and others are permissive to movement.
Coronins
Coronins are a family of WD-repeat actin-binding proteins that coordinate actin filament branching and disassembly. In mammals, Coronin-1a (CORO1A) is the best-studied member, especially in T cells where it is abundantly expressed (50–53). CORO1A localizes to F-actin-rich regions and can bind the Arp2/3 complex as well as actin filaments, positioning it to modulate actin dynamics (52, 53). Gene-knockout mouse models and the analysis of human mutations have revealed that CORO1A is essential for T cell homeostasis (54). CORO1A deficient mice have a profound T cell lymphopenia. Thymocyte development proceeds relatively normally, but thymic egress is impaired and mature naïve T cells fail to survive in the periphery (52). This phenotype mirrors a form of severe combined immunodeficiency (SCID) in humans – Immunodeficiency 8 – caused by biallelic CORO1A mutation (54). Shiow and colleagues found that a CORO1A point mutant (K26E), identified in an N-ethyl-N-nitrosourea mutagenesis screen form mice with T cell lymphopenia, disrupts thymic egress, with mutant T cells exhibiting impaired migration, abnormal actin-rich protrusions, and retention in the thymus (54). Mechanistically, this mutation enhanced CORO1A’s ability for inhibition of Arp2/3, leading to excessive actin branching that mis-localized CORO1A away from the leading edge of migrating T cells. The same study identified Coro1A gene mutations in a SCID patient, establishing a crucial role for T cell development in humans (51, 54). In the absence of functional CORO1A, T cells abnormally accumulate F-actin and cannot respond properly to chemokine cues for egress.
Aside from the egress defect, Coro1a-/- T cells have intrinsic signaling anomalies, exhibiting abnormal TCR-induced actin dynamics and impaired calcium and NF-κB signaling (55). CORO1A thus links cytoskeletal dynamics to TCR signaling, helping to tune the strength and duration of signals (55). Notably, CORO1A deficient T cells form overly stable synapses with prolonged contact time, correlating with hyper-accumulation of F-actin and Arp2/3 at the synapse (55). This suggests CORO1A normally promotes actin turnover at the synapse, preventing excessive actin buildup that could dampen signaling or cellular mobility.
Despite these broad peripheral T cell defects, regulatory T cells appear relatively preserved in Coro1a-/- mice. One study reported that Coro1a-/- mice were resistant to experimental autoimmune encephalomyelitis (EAE) due to loss of effector T cells, and this resistance persisted even after Treg depletion, indicating Tregs were not the main cause (56, 57). In fact, Coro1a-/- Tregs appear functionally competent in vitro, suggesting that primary role of CORO1A is in maintaining the naive T cell pool rather than for Treg function (57). Nonetheless, Tregs in CORO1A SCID patients have not been extensively profiled. It is possible that subtle Treg migratory or homeostatic abnormalities exist but are overshadowed by the dramatic loss of conventional T cells.
There are other members of the coronin family that may also have roles in T cells. CORO2A (also known as coronin-2 or IRF-3 binding protein) is a type II coronin and has been shown in fibroblasts to localizes to stress fibers and focal adhesions rather than the leading edge (58). In non-immune cells, CORO2A regulates focal adhesion turnover and cell motility (58, 59). Its function in T cells is less well-characterized. Type I coronins, like CORO1A, can coordinate Arp2/3 and ADF/cofilin to ensure efficient actin turnover at the leading edge (60). It will be interesting to determine if type II coronins also plays a similar regulatory role in T cell development.
Regulatory pathways: Rho family GTPases (Rac1, Cdc42, RhoA)
Upstream of the actin regulators are signaling pathways that respond to extracellular cues to orchestrate cytoskeletal changes (Figure 2). Among these are the Rho family GTPases – molecular switches that cycle between an active GTP-bound state and an inactive GDP-bound state, controlling the organization of actin cytoskeleton necessary for T cell activation, polarization and migration (61). Their activation is tightly regulated by guanine nucleotide exchange factors (GEFs), which promote the exchange of GDP for GTP, and GTPase-activating proteins (GAPs), which enhance GTP hydrolysis, thereby returning them to an inactive state (62).
Rac1 is crucial for actin polymerization and lamellipodia formation, which are essential for T cell motility and immunological synapse formation (63). Upon TCR stimulation, Rac1 is activated by GEFs, such as Vav1, leading to the recruitment of WAVE2, which in turn activates the Arp2/3 complex to promote actin branching and cell spreading (14). This process is necessary for effective T cell activation, as Rac1 deficient T cells exhibit impaired TCR clustering and reduced IL-2 production (64, 65). Additionally, Rac1 facilitates T cell migration by regulating integrin-mediated adhesion and actin remodeling, which are crucial for T cell trafficking within lymphoid organs (66).
Cdc42 is another key regulator of actin dynamics and cell polarity in T cells. It controls filopodia formation, which enhances cell migration and antigen scanning (67). Cdc42 interacts with the Par3/Par6 polarity complex and atypical protein kinase C (aPKC), establishing front-rear polarity during T cell migration (68). It also plays a role in centrosome reorientation and TCR clustering at the immunological synapse, ensuring efficient signaling and sustained T cell activation (2). Loss of Cdc42 impairs the ability of T cells to form stable interactions with antigen-presenting cells (APCs), leading to defective immune responses (69).
RhoA primarily regulates actomyosin contractility through the Rho-associated kinase (ROCK) pathway, facilitating T cell contraction and immune synapse stabilization (70). It promotes stress fiber formation and cortical tension, which are necessary for T cell squeezing through tight endothelial barriers during migration (71). RhoA also controls the phosphorylation of myosin light chain, contributing to T cell shape changes required for trans-endothelial migration (71, 72). Additionally, RhoA signaling is critical for maintaining the stability of the immunological synapse, ensuring prolonged TCR signaling and proper effector function (72, 73). Rac1 and Cdc42 generally promote actin polymerization and membrane protrusions, whereas RhoA modulates actomyosin contractility and synapse stabilization (74, 75). Dysregulation of these pathways has been implicated in immune deficiencies and autoimmunity, underscoring the importance of Rho GTPase-mediated cytoskeletal control in T cell biology (74, 75).
Concluding comments
Actin-binding proteins work in concert to shape the cytoskeleton throughout both T cell development and mature T cell function. These proteins are essential for processes such as migration, thymic selection, immune synapse formation, and signal transduction. In mature T cells, dynamic actin remodeling supports polarization, effector function, and trafficking within tissues. Despite advances in understanding these mechanisms, important questions remain, particularly regarding how specific cytoskeletal regulators coordinate with signaling pathways at distinct stages and how these dynamics influence long-term T cell fate and function. Further research is needed to fully define the roles of actin regulators across the T cell lifespan.
Author contributions
TL: Writing – original draft, Writing – review & editing, Conceptualization. MC: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research in the Chong laboratory is supported by grants from the National Health and Medical Research Council (NHMRC), Breakthrough T1D, mRNA Victoria, Diabetes Australia, and the United States Department of Defense.
Acknowledgments
All figures used in this manuscript were created by the authors using BioRender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kumari S, Depoil D, Martinelli R, Judokusumo E, Carmona G, Gertler FB, et al. Actin foci facilitate activation of the phospholipase C-gamma in primary T lymphocytes via the wasp pathway. Elife. (2015) 4:e04953. doi: 10.7554/eLife.04953
2. Billadeau DD, Nolz JC, and Gomez TS. Regulation of T-cell activation by the cytoskeleton. Nat Rev Immunol. (2007) 7:131–43. doi: 10.1038/nri2021
3. Reinherz EL, Tan K, Tang L, Kern P, Liu J, Xiong Y, et al. The crystal structure of a T cell receptor in complex with peptide and mhc class ii. Science. (1999) 286:1913–21. doi: 10.1126/science.286.5446.1913
4. Victora GD and Nussenzweig MC. Germinal centers. Annu Rev Immunol. (2012) 30:429–57. doi: 10.1146/annurev-immunol-020711-075032
5. Monks CR, Freiberg BA, Kupfer H, Sciaky N, and Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. (1998) 395:82–6. doi: 10.1038/25764
6. Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, et al. The immunological synapse: A molecular machine controlling T cell activation. Science. (1999) 285:221–7. doi: 10.1126/science.285.5425.221
7. Dustin ML, Chakraborty AK, and Shaw AS. Understanding the structure and function of the immunological synapse. Cold Spring Harb Perspect Biol. (2010) 2:a002311. doi: 10.1101/cshperspect.a002311
8. Roy NH and Burkhardt JK. The actin cytoskeleton: A mechanical intermediate for signal integration at the immunological synapse. Front Cell Dev Biol. (2018) 6:116. doi: 10.3389/fcell.2018.00116
9. Bunnell SC, Kapoor V, Trible RP, Zhang W, and Samelson LE. Dynamic actin polymerization drives T cell receptor-induced spreading: A role for the signal transduction adaptor lat. Immunity. (2001) 14:315–29. doi: 10.1016/s1074-7613(01)00112-1
10. Babich A, Li S, O’Connor RS, Milone MC, Freedman BD, and Burkhardt JK. F-actin polymerization and retrograde flow drive sustained plcgamma1 signaling during T cell activation. J Cell Biol. (2012) 197:775–87. doi: 10.1083/jcb.201201018
11. Yi J, Wu XS, Crites T, and Hammer JA 3rd. Actin retrograde flow and actomyosin ii arc contraction drive receptor cluster dynamics at the immunological synapse in jurkat T cells. Mol Biol Cell. (2012) 23:834–52. doi: 10.1091/mbc.E11-08-0731
12. Comrie WA, Li S, Boyle S, and Burkhardt JK. The dendritic cell cytoskeleton promotes T cell adhesion and activation by constraining icam-1 mobility. J Cell Biol. (2015) 208:457–73. doi: 10.1083/jcb.201406120
13. Zhang Y, Shen H, Liu H, Feng H, Liu Y, Zhu X, et al. Arp2/3 complex controls T cell homeostasis by maintaining surface tcr levels via regulating tcr(+) endosome trafficking. Sci Rep. (2017) 7:8952. doi: 10.1038/s41598-017-08357-4
14. Nolz JC, Gomez TS, Zhu P, Li S, Medeiros RB, Shimizu Y, et al. The wave2 complex regulates actin cytoskeletal reorganization and crac-mediated calcium entry during T cell activation. Curr Biol. (2006) 16:24–34. doi: 10.1016/j.cub.2005.11.036
15. Liu Y, Blanchfield L, Ma VP, Andargachew R, Galior K, Liu Z, et al. DNA-based nanoparticle tension sensors reveal that T-cell receptors transmit defined pn forces to their antigens for enhanced fidelity. Proc Natl Acad Sci U.S.A. (2016) 113:5610–5. doi: 10.1073/pnas.1600163113
16. Goode BL, Wong JJ, Butty AC, Peter M, McCormack AL, Yates JR, et al. Coronin promotes the rapid assembly and cross-linking of actin filaments and may link the actin and microtubule cytoskeletons in yeast. J Cell Biol. (1999) 144:83–98. doi: 10.1083/jcb.144.1.83
17. Devriendt K, Kim AS, Mathijs G, Frints SG, Schwartz M, Van Den Oord JJ, et al. Constitutively activating mutation in wasp causes X-linked severe congenital neutropenia. Nat Genet. (2001) 27:313–7. doi: 10.1038/85886
18. Humblet-Baron S, Sather B, Anover S, Becker-Herman S, Kasprowicz DJ, Khim S, et al. Wiskott-aldrich syndrome protein is required for regulatory T cell homeostasis. J Clin Invest. (2007) 117:407–18. doi: 10.1172/JCI29539
19. Snapper SB, Rosen FS, Mizoguchi E, Cohen P, Khan W, Liu CH, et al. Wiskott-aldrich syndrome protein-deficient mice reveal a role for wasp in T but not B cell activation. Immunity. (1998) 9:81–91. doi: 10.1016/s1074-7613(00)80590-7
20. Cai E, Marchuk K, Beemiller P, Beppler C, Rubashkin MG, Weaver VM, et al. Visualizing dynamic microvillar search and stabilization during ligand detection by T cells. Science. (2017) 356:eaal3118. doi: 10.1126/science.aal3118
21. Kahr WH, Pluthero FG, Elkadri A, Warner N, Drobac M, Chen CH, et al. Loss of the arp2/3 complex component arpc1b causes platelet abnormalities and predisposes to inflammatory disease. Nat Commun. (2017) 8:14816. doi: 10.1038/ncomms14816
22. Somech R, Lev A, Lee YN, Simon AJ, Barel O, Schiby G, et al. Disruption of thrombocyte and T lymphocyte development by a mutation in arpc1b. J Immunol. (2017) 199:4036–45. doi: 10.4049/jimmunol.1700460
23. Kuijpers TW, Tool ATJ, van der Bijl I, de Boer M, van Houdt M, de Cuyper IM, et al. Combined immunodeficiency with severe inflammation and allergy caused by arpc1b deficiency. J Allergy Clin Immunol. (2017) 140:273–7.e10. doi: 10.1016/j.jaci.2016.09.061
24. Brigida I, Zoccolillo M, Cicalese MP, Pfajfer L, Barzaghi F, Scala S, et al. T-cell defects in patients with arpc1b germline mutations account for combined immunodeficiency. Blood. (2018) 132:2362–74. doi: 10.1182/blood-2018-07-863431
25. Murugesan S, Hong J, Yi J, Li D, Beach JR, Shao L, et al. Formin-generated actomyosin arcs propel T cell receptor microcluster movement at the immune synapse. J Cell Biol. (2016) 215:383–99. doi: 10.1083/jcb.201603080
26. Hong J, Murugesan S, Betzig E, and Hammer JA. Contractile actomyosin arcs promote the activation of primary mouse T cells in a ligand-dependent manner. PloS One. (2017) 12:e0183174. doi: 10.1371/journal.pone.0183174
27. Cane S, Ponnappan S, and Ponnappan U. Impairment of non-muscle myosin iia in human cd4+ T cells contributes to functional deficits in the elderly. Cell Mol Immunol. (2012) 9:86–96. doi: 10.1038/cmi.2011.41
28. Kumari S, Vardhana S, Cammer M, Curado S, Santos L, Sheetz MP, et al. T lymphocyte myosin iia is required for maturation of the immunological synapse. Front Immunol. (2012) 3:230. doi: 10.3389/fimmu.2012.00230
29. Gerard A, Patino-Lopez G, Beemiller P, Nambiar R, Ben-Aissa K, Liu Y, et al. Detection of rare antigen-presenting cells through T cell-intrinsic meandering motility, mediated by myo1g. Cell. (2014) 158:492–505. doi: 10.1016/j.cell.2014.05.044
30. Fowell DJ and Kim M. The spatio-temporal control of effector T cell migration. Nat Rev Immunol. (2021) 21:582–96. doi: 10.1038/s41577-021-00507-0
31. Seeland I, Xiong Y, Orlik C, Deibel D, Prokosch S, Kublbeck G, et al. The actin remodeling protein cofilin is crucial for thymic alphabeta but not gammadelta T-cell development. PloS Biol. (2018) 16:e2005380. doi: 10.1371/journal.pbio.2005380
32. Adams EJ, Gu S, and Luoma AM. Human gamma delta T cells: evolution and ligand recognition. Cell Immunol. (2015) 296:31–40. doi: 10.1016/j.cellimm.2015.04.008
33. Lee KH, Meuer SC, and Samstag Y. Cofilin: A missing link between T cell co-stimulation and rearrangement of the actin cytoskeleton. Eur J Immunol. (2000) 30:892–9. doi: 10.1002/1521-4141(200003)30:3<892::AID-IMMU892>3.0.CO;2-U
34. Samstag Y, John I, and Wabnitz GH. Cofilin: A redox sensitive mediator of actin dynamics during T-cell activation and migration. Immunol Rev. (2013) 256:30–47. doi: 10.1111/imr.12115
35. Ramirez-Munoz R, Castro-Sanchez P, and Roda-Navarro P. Ultrasensitivity in the cofilin signaling module: A mechanism for tuning T cell responses. Front Immunol. (2016) 7:59. doi: 10.3389/fimmu.2016.00059
36. Beemiller P and Krummel MF. Regulation of T-cell receptor signaling by the actin cytoskeleton and poroelastic cytoplasm. Immunol Rev. (2013) 256:148–59. doi: 10.1111/imr.12120
37. Thauland TJ, Hu KH, Bruce MA, and Butte MJ. Cytoskeletal adaptivity regulates T cell receptor signaling. Sci Signal. (2017) 10:eaah3737. doi: 10.1126/scisignal.aah3737
38. Savinko T, Guenther C, Uotila LM, Llort Asens M, Yao S, Tojkander S, et al. Filamin a is required for optimal T cell integrin-mediated force transmission, flow adhesion, and T cell trafficking. J Immunol. (2018) 200:3109–16. doi: 10.4049/jimmunol.1700913
39. Moser M, Bauer M, Schmid S, Ruppert R, Schmidt S, Sixt M, et al. Kindlin-3 is required for beta2 integrin-mediated leukocyte adhesion to endothelial cells. Nat Med. (2009) 15:300–5. doi: 10.1038/nm.1921
40. Takala H, Nurminen E, Nurmi SM, Aatonen M, Strandin T, Takatalo M, et al. Beta2 integrin phosphorylation on thr758 acts as a molecular switch to regulate 14-3–3 and filamin binding. Blood. (2008) 112:1853–62. doi: 10.1182/blood-2007-12-127795
41. Das M, Ithychanda SS, Qin J, and Plow EF. Migfilin and filamin as regulators of integrin activation in endothelial cells and neutrophils. PloS One. (2011) 6:e26355. doi: 10.1371/journal.pone.0026355
42. Niedergang F, Di Bartolo V, and Alcover A. Comparative anatomy of phagocytic and immunological synapses. Front Immunol. (2016) 7:18. doi: 10.3389/fimmu.2016.00018
43. Waldt N, Seifert A, Demiray YE, Devroe E, Turk BE, Reichardt P, et al. Filamin a phosphorylation at serine 2152 by the serine/threonine kinase ndr2 controls tcr-induced lfa-1 activation in T cells. Front Immunol. (2018) 9:2852. doi: 10.3389/fimmu.2018.02852
44. Wohler J, Bullard D, Schoeb T, and Barnum S. Lfa-1 is critical for regulatory T cell homeostasis and function. Mol Immunol. (2009) 46:2424–8. doi: 10.1016/j.molimm.2009.04.004
45. Shaffer MH, Dupree RS, Zhu P, Saotome I, Schmidt RF, McClatchey AI, et al. Ezrin and moesin function together to promote T cell activation. J Immunol. (2009) 182:1021–32. doi: 10.4049/jimmunol.182.2.1021
46. Cannon JL. 4.1 R: A ferm player at the immunologic synapse. Blood J Am Soc Hematol. (2009) 113:6043–4. doi: 10.1182/blood-2009-03-208504
47. Allenspach EJ, Cullinan P, Tong J, Tang Q, Tesciuba AG, Cannon JL, et al. Erm-dependent movement of cd43 defines a novel protein complex distal to the immunological synapse. Immunity. (2001) 15:739–50. doi: 10.1016/s1074-7613(01)00224-2
48. Rivas MN, Burton OT, Wise P, Charbonnier L-M, Georgiev P, Oettgen HC, et al. Regulatory T cell reprogramming toward a th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity. (2015) 42:512–23. doi: 10.1016/j.immuni.2015.02.004
49. Faure S, Salazar-Fontana LI, Semichon M, Tybulewicz VL, Bismuth G, Trautmann A, et al. Erm proteins regulate cytoskeleton relaxation promoting T cell-apc conjugation. Nat Immunol. (2004) 5:272–9. doi: 10.1038/ni1039
50. Pick R, Begandt D, Stocker TJ, Salvermoser M, Thome S, Bottcher RT, et al. Coronin 1a, a novel player in integrin biology, controls neutrophil trafficking in innate immunity. Blood. (2017) 130:847–58. doi: 10.1182/blood-2016-11-749622
51. Shiow LR, Roadcap DW, Paris K, Watson SR, Grigorova IL, Lebet T, et al. The actin regulator coronin 1a is mutant in a thymic egress-deficient mouse strain and in a patient with severe combined immunodeficiency. Nat Immunol. (2008) 9:1307–15. doi: 10.1038/ni.1662
52. Mueller P, Massner J, Jayachandran R, Combaluzier B, Albrecht I, Gatfield J, et al. Regulation of T Cell Survival through Coronin-1-Mediated Generation of Inositol-1,4,5-Trisphosphate and Calcium Mobilization after T Cell Receptor Triggering. Nat Immunol. (2008) 9:424–31. doi: 10.1038/ni1570
53. Mueller P, Liu X, and Pieters J. Migration and homeostasis of naive T cells depends on coronin 1-mediated prosurvival signals and not on coronin 1-dependent filamentous actin modulation. J Immunol. (2011) 186:4039–50. doi: 10.4049/jimmunol.1003352
54. Shiow LR, Paris K, Akana MC, Cyster JG, Sorensen RU, and Puck JM. Severe combined immunodeficiency (Scid) and attention deficit hyperactivity disorder (Adhd) associated with a coronin-1a mutation and a chromosome 16p11.2 deletion. Clin Immunol. (2009) 131:24–30. doi: 10.1016/j.clim.2008.11.002
55. Mugnier B, Nal B, Verthuy C, Boyer C, Lam D, Chasson L, et al. Coronin-1a links cytoskeleton dynamics to tcrαβ-induced cell signaling. PloS One. (2008) 3:e3467. doi: 10.1371/journal.pone.0003467
56. Foger N, Rangell L, Danilenko DM, and Chan AC. Requirement for coronin 1 in T lymphocyte trafficking and cellular homeostasis. Science. (2006) 313:839–42. doi: 10.1126/science.1130563
57. Siegmund K, Zeis T, Kunz G, Rolink T, Schaeren-Wiemers N, and Pieters J. Coronin 1-mediated naive T cell survival is essential for the development of autoimmune encephalomyelitis. J Immunol. (2011) 186:3452–61. doi: 10.4049/jimmunol.1003491
58. Marshall TW, Aloor HL, and Bear JE. Coronin 2a regulates a subset of focal-adhesion-turnover events through the cofilin pathway. J Cell Sci. (2009) 122:3061–9. doi: 10.1242/jcs.051482
59. Rastetter RH, Blömacher M, Drebber U, Marko M, Behrens J, Solga R, et al. Coronin 2a (Crn5) expression is associated with colorectal adenoma-adenocarcinoma sequence and oncogenic signalling. BMC Cancer. (2015) 15:638. doi: 10.1186/s12885-015-1645-7
60. Holtta-Vuori M, Vainio S, Kauppi M, Van Eck M, Jokitalo E, and Ikonen E. Endosomal actin remodeling by coronin-1a controls lipoprotein uptake and degradation in macrophages. Circ Res. (2012) 110:450–5. doi: 10.1161/CIRCRESAHA.111.256842
61. Heasman SJ and Ridley AJ. Mammalian rho gtpases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. (2008) 9:690–701. doi: 10.1038/nrm2476
62. Rossman KL, Der CJ, and Sondek J. Gef means go: turning on rho gtpases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. (2005) 6:167–80. doi: 10.1038/nrm1587
63. Tybulewicz VL and Henderson RB. Rho family gtpases and their regulators in lymphocytes. Nat Rev Immunol. (2009) 9:630–44. doi: 10.1038/nri2606
64. Saoudi A, Kassem S, Dejean A, and Gaud G. Rho-gtpases as key regulators of T lymphocyte biology. Small GTPases. (2014) 5:e983862. doi: 10.4161/sgtp.28208
65. Guo F, Cancelas JA, Hildeman D, Williams DA, and Zheng Y. Rac gtpase isoforms rac1 and rac2 play a redundant and crucial role in T-cell development. Blood. (2008) 112:1767–75. doi: 10.1182/blood-2008-01-132068
66. Fritz RD and Pertz O. The dynamics of spatio-temporal rho gtpase signaling: formation of signaling patterns. F1000Res. (2016) 5:F1000 Faculty Rev–749. doi: 10.12688/f1000research.7370.1
67. Dustin ML and Cooper JA. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat Immunol. (2000) 1:23–9. doi: 10.1038/76877
68. Etienne-Manneville S. Cdc42–the centre of polarity. J Cell Sci. (2004) 117:1291–300. doi: 10.1242/jcs.01115
69. Beyersdorf N, Kerkau T, and Hunig T. Cd28 co-stimulation in T-cell homeostasis: A recent perspective. Immunotargets Ther. (2015) 4:111–22. doi: 10.2147/ITT.S61647
70. Tzima E, Del Pozo MA, Kiosses WB, Mohamed SA, Li S, Chien S, et al. Activation of rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J. (2002) 21:6791–800. doi: 10.1093/emboj/cdf688
71. Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. (2008) 453:51–5. doi: 10.1038/nature06887
72. Smith A, Bracke M, Leitinger B, Porter JC, and Hogg N. Lfa-1-induced T cell migration on icam-1 involves regulation of mlck-mediated attachment and rock-dependent detachment. J Cell Sci. (2003) 116:3123–33. doi: 10.1242/jcs.00606
73. Rougerie P and Delon J. Rho gtpases: masters of T lymphocyte migration and activation. Immunol Lett. (2012) 142:1–13. doi: 10.1016/j.imlet.2011.12.003
Keywords: T cells, T cell development, coronin, actin cytoskeletal dynamics, actin-binding proteins, immunological synapse
Citation: Lam TT and Chong MMW (2025) Regulation of actin cytoskeletal dynamics in T cell development and function. Front. Immunol. 16:1622928. doi: 10.3389/fimmu.2025.1622928
Received: 05 May 2025; Accepted: 26 May 2025;
Published: 10 June 2025.
Edited by:
Eilon Sherman, Hebrew University of Jerusalem, IsraelReviewed by:
Yasushi Sawanobori, Dokkyo Medical University, JapanCopyright © 2025 Lam and Chong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark M. W. Chong, bWNob25nQHN2aS5lZHUuYXU=
 Trang T. Lam
Trang T. Lam Mark M. W. Chong
Mark M. W. Chong