- 1State Key Laboratory of Experimental Hematology, National Clinical Research Center for Blood Diseases, Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Tianjin, China
- 2Department of Hematology, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, Yantai, Shandong, China
Introduction: The incidence of platelet transfusion refractoriness (PTR) and its impact on survival outcomes in patients with severe aplastic anemia (SAA) undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT) remains unclear.
Methods: We investigated the incidence of early PTR (within one month post-allo-HSCT) and its clinical implications in 215 aplastic anemia (AA) patients in a retrospective study.
Results: Among the enrolled patients, 24 (11.7%) developed PTR within the first month post-transplantation. Propensity score matching (PSM) was performed, resulting in 24 PTR cases and 96 matched non-PTR controls, with balanced baseline characteristics. No significant differences were observed between the two groups in bloodstream infections, grade II–IV or III–IV acute graft-versus-host disease (aGVHD), viral infections, or engraftment rates. However, PTR patients required significantly more red blood cell (median: 13.5 units vs. 8 units, P = 0.003) and platelet transfusions (median: 10.5 units vs. 5 units, P < 0.001) compared to non-PTR patients. The 3-year overall survival (OS) rate was numerically lower in the PTR group (66.7%; 95% CI, 44.3–81.7) than in the non-PTR group (81.2%; 95% CI, 71.1–88.0), although this difference was not statistically significant (P = 0.106). Multivariate analysis identified haploidentical donor and patient age as independent risk factors for OS.
Conclusion: Our findings suggest that early PTR occurs at a relatively low frequency (11.7%) in AA patients post-allo-HSCT and may not significantly compromise survival outcomes following successful engraftment.
1 Introduction
Platelet transfusion refractoriness (PTR), characterized by inadequate post-transfusion platelet count recovery (1), results from both immunologic and non-immunologic mechanisms. Unlike chemotherapy patients, PTR following allogeneic hematopoietic stem cell transplantation (allo-HSCT) predominantly stems from non-immunologic factors such as infection, fever, and bleeding (2). The clinical impact of post-allo-HSCT PTR remains controversial, with studies reporting conflicting outcomes (3, 4); this discrepancy may reflect variations in PTR onset timing, underlying diseases, and conditioning regimens (4). Of note, recent work by Gao et al. have demonstrated that rabbit anti-thymocyte globulin (rATG) can effectively reverse PTR in severe aplastic anemia (SAA) patients receiving intensive immunosuppressive therapy (5). However, the incidence and survival implications of early PTR, within one month post-allo-HSCT, in aplastic anemia (AA) patients have not been established. To address this knowledge gap, we conducted a retrospective study examining the incidence and clinical consequences of early PTR in AA patients undergoing allo-HSCT.
2 Methods
2.1 Patients
From 2010 to July 2023, 257 patients with AA who consecutively underwent allo-HSCT were screened. Inclusion criteria included: AA diagnosis according to established criteria (6); and completion of allo-HSCT during the study period. The exclusion criteria included patients who died before engraftment (n=6), those with primary engraftment failure (n=1), or patients not evaluated for PTR within one month post-HSCT (n=35). Ultimately, 215 patients were enrolled. This study was approved by the Institutional Review Board of our hospital (IIT2021011-EC-1) and complied with the Declaration of Helsinki. Written informed consent was obtained from all participants or their legal guardians.
2.2 Conditioning regimen and transplantation procedure
Our institutional transplantation protocol followed established procedures as previously reported (7) and incorporated insights from haploidentical donor HSCT (HID-HSCT) protocols in China (8, 9). The regimen included: fludarabine (FLU) 150mg/m2 IV in divided doses on days -6 to -2, cyclophosphamide (CY) 80 or 150 mg/kg IV in a divided dose on days -5 to -2, and rATG (Thymoglobulin®, Genzyme, Cambridge, MA) 12.5mg/kg or porcine antilymphocyte globulin (pALG) (Anti-lymphocyte Immunoglobulin®, Wuhan Institute of Biological Products Co., Ltd., China) 100 or 125mg/kg IV in divided doses on days -5 to -2. For patients undergoing HID-HSCT or with transfusion-dependent AA, busulfan (BU) 6.4 mg/kg IV, divided over days –7 to –6, was added. Prophylaxis for acute GVHD (aGVHD), infection prevention, and surveillance followed prior protocols (7).
2.3 Definitions
Days of neutrophil and platelet engraftment (10), aGVHD (11), chronic GVHD (12), graft failure (GF) (13), and primary cause of death (COD) (14) were defined based on previously reported criteria. All patients received single-donor apheresis platelet products. The 12-hour corrected count increment (CCI) was calculated between 8 and16 hours post-transfusion (typically measured at 8 AM following transfusions administered between 4:00–5:00 PM or 11:00 PM–12:00 AM) using the standard formula:
where the denominator (2.5 × 10¹¹) represents the average platelet dose administered at our center. PTR was defined as a CCI<5 × 109/L on two sequential occasions (5). Transplant-related mortality (TRM) was defined as death without GF. Transplant failure after HSCT was defined as death or GF, whichever occurred first. Failure-free survival (FFS) was defined as the time from the Day +30 post-HSCT to treatment failure or last follow-up. Overall survival (OS) was defined as the time from the Day +30 post-HSCT to death or last follow-up.
2.4 Statistical analysis
This study aimed to compare OS between AA patients with and without PTR within one month after allo-HSCT.
All patients attended outpatient follow-up visits or were contacted by telephone. The final follow-up date was July 2023. Continuous and categorical variables were compared using the Mann–Whitney U test and chi-square test, respectively. When cell counts were ≤ 5, Fisher’s exact test was used. The median follow-up of surviving patients was calculated using the reverse Kaplan–Meier method. The cumulative incidences (CIs) of GVHD and TRM were calculated using the competing risk model and compared using the Gray’s test. Death or graft failure was considered a competing event for GVHD. The probabilities of OS and FFS were calculated using the Kaplan–Meier method, and differences were assessed using the log–rank test. Variables with P-values ≤0.1 in univariate analysis were entered into multivariate models to identify factors affecting survival. Variables including donor type, patient age, interval from diagnosis to transplantation, and ferritin levels before transplantation were used as covariates in propensity score matching (PSM). Patients in the PTR group were matched to those in the non-PTR group using 1:4 nearest neighbor matching with a caliper width of 0.2. R (version 4.0.5), GraphPad Prism (version 5), and SPSS (version 25.0) were used for statistical analyses. Figures were generated using GraphPad Prism 5. All P-values were two-sided, and results were considered statistically significant at P < 0.05.
3 Results
3.1 Characteristics of patients
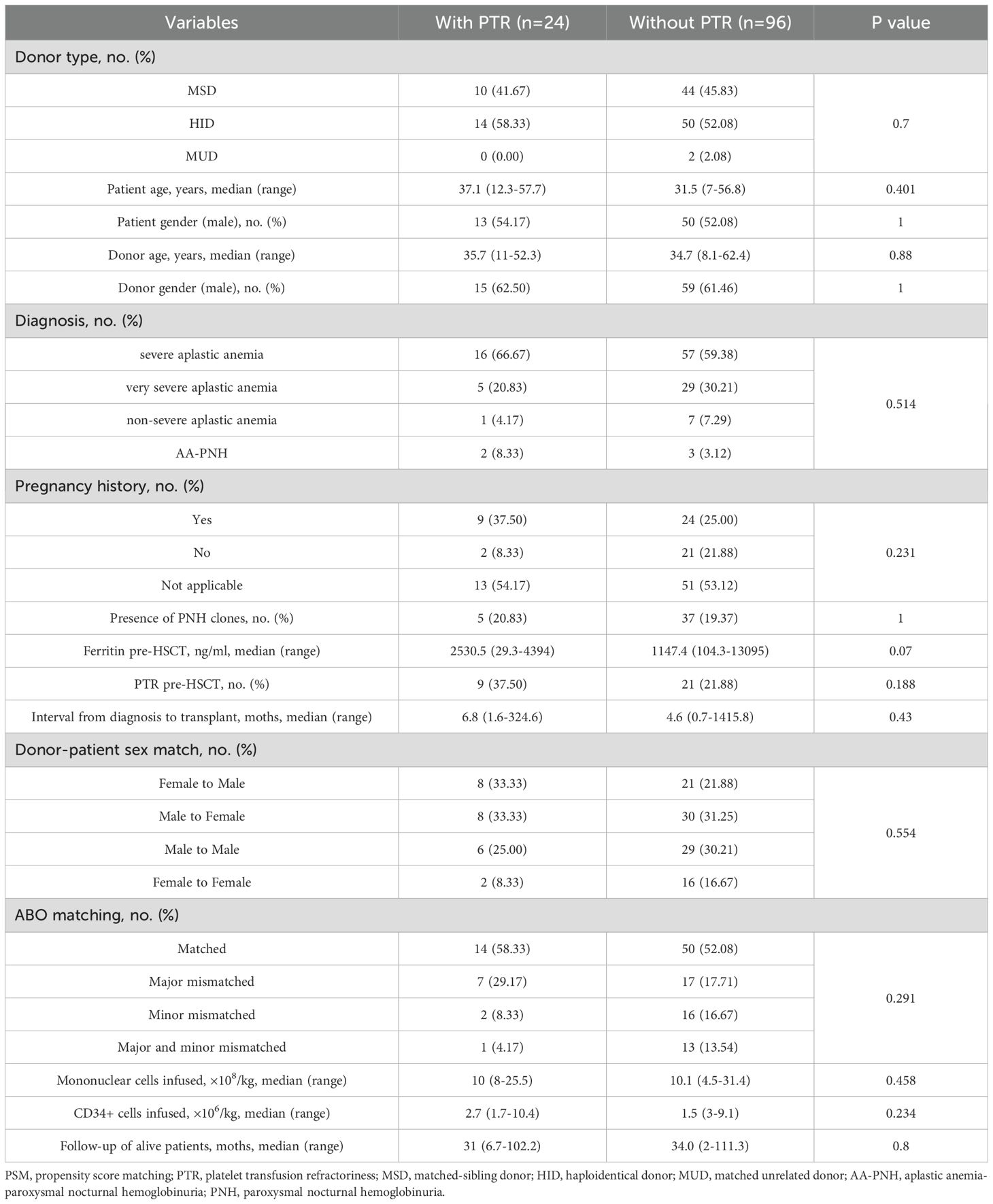
The study cohort included 215 AA patients undergoing allogeneic HSCT, among whom 24 (11.7%) developed platelet transfusion refractoriness (PTR) within the first post-transplant month (Supplementary Table 1). Propensity score matching (PSM) yielded well-balanced cohorts of 24 PTR and 96 non-PTR patients (Table 1). Key clinical characteristics, including donor age and sex, patient age, interval from diagnosis to transplantation, and ferritin levels before allo-HSCT were balanced after PSM. Notably, HID accounted for more than half of the patients in each cohort (14 [PTR] vs. 50 [non-PTR]). The median age of patients in the PTR group was 37.1 years (range: 12.3–57.7) versus 31.5 years (range: 7–56.8) in the non-PTR group (P = 0.401), while the median age of donors was 35.7 years (range: 11–52.3) versus 34.7 years (8.1–62.4), respectively (P = 0.88). No significant differences were observed between groups in pre-HSCT PTR, diagnosis, donor–patient sex match, blood types of donors to recipients, mononuclear cells, and CD34+ cells infused. Follow-up duration was comparable between surviving patients (PTR: 31 months vs. non-PTR: 34 months; P = 0.8).
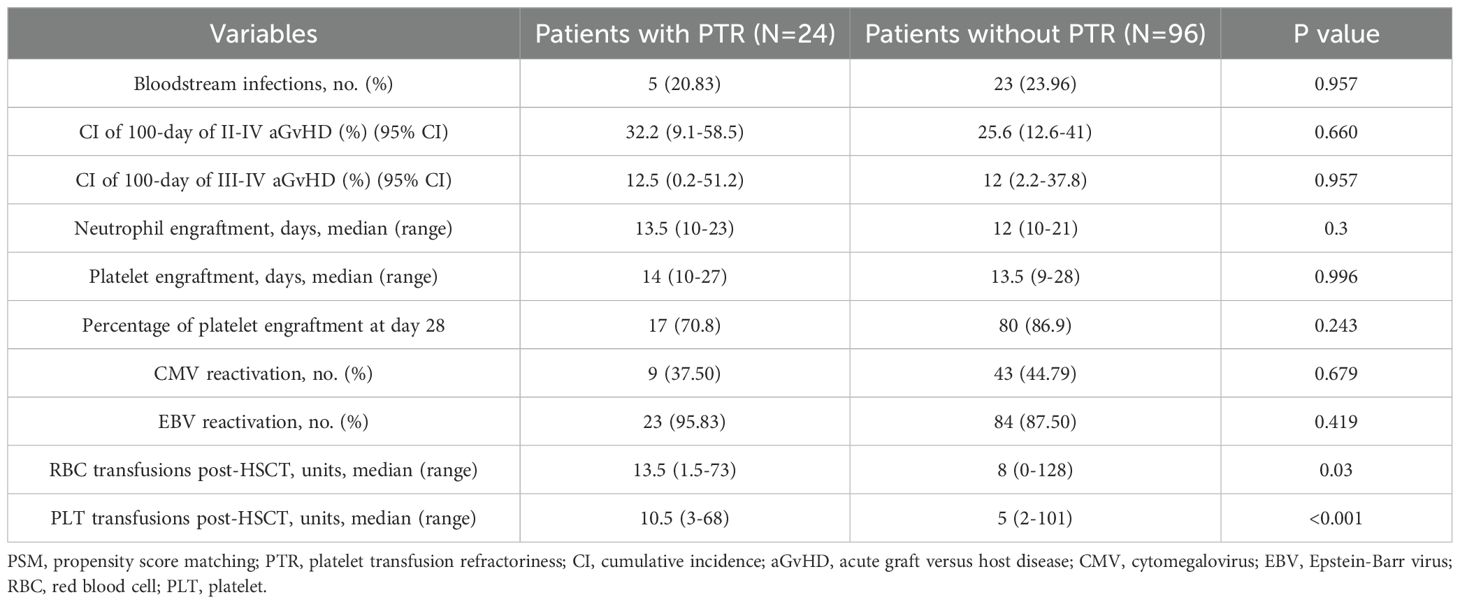
3.2 Clinical outcomes
Table 2 summarizes the major clinical outcomes of patients after PSM. The CI of 100-day grade II-IV aGVHD was 32.2% (95% confidence interval [CI], 9.1–58.5) in the PTR group and 25.6% (95% CI, 12.6–41.0) (P = 0.66) in the non-PTR group; the CI of 100-day grade III–IV aGVHD was 12.5% (95% CI, 0.2–51.2) versus 12% (95% CI, 2.2–37.8) (P = 0.957), respectively (Figure 1). No differences were observed in terms of bloodstream infection, virus infection, and engraftment between the two groups. Notably, transfusion requirements were significantly higher in the PTR group: red blood cells: median 13.5 units (PTR) vs. 8 units (non-PTR; P = 0.003); platelets: median 10.5 units vs. 5 units (P < 0.001). Regarding TRM, the CI of 1-year TRM in the PTR group was 33.3% (95% CI, 11.0–57.9) compared to 16.9% (95% CI, 5.5–33.7) in the non-PTR group (P = 0.106).
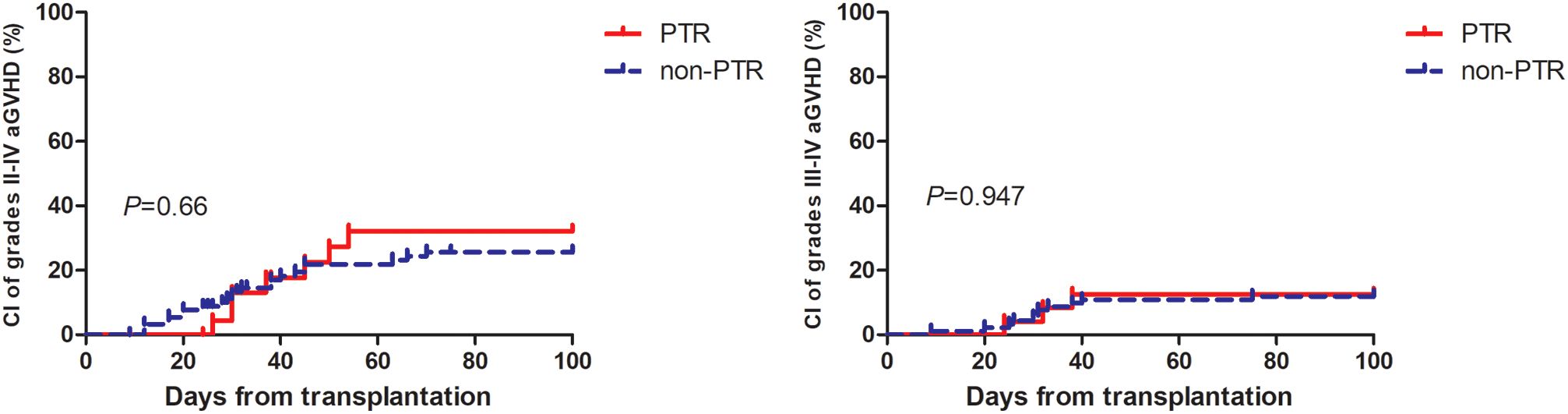
Figure 1. Cumulative incidence of grade II to IV aGvHD and III to IV aGvHD among AA patients with PTR were similar to patient without PTR.
3.3 Survival
After PSM, the probability of 3-year OS in the PTR group was numerically lower, 66.7% (95% CI, 44.3–81.7) versus 81.2% (95% CI, 71.1–88) in the non-PTR group. However, the difference was not statistically significant (P = 0.106) while the probability of 3-year FFS was 66.7% (95% CI, 44.3–81.7) versus 67.1% (95% CI, 56.1–73.9) (P = 0.083) (Figure 2).
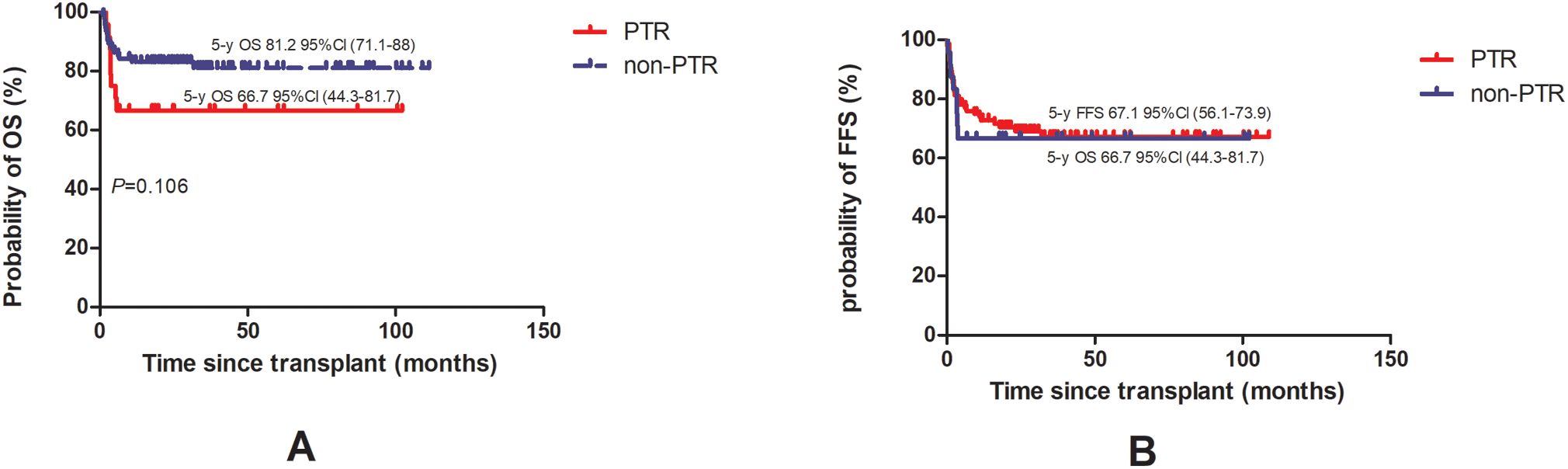
Figure 2. Overall survival [OS, (A)] and failure-free survival [FFS, (B)] of AA patients with PTR were compared to patient without PTR.
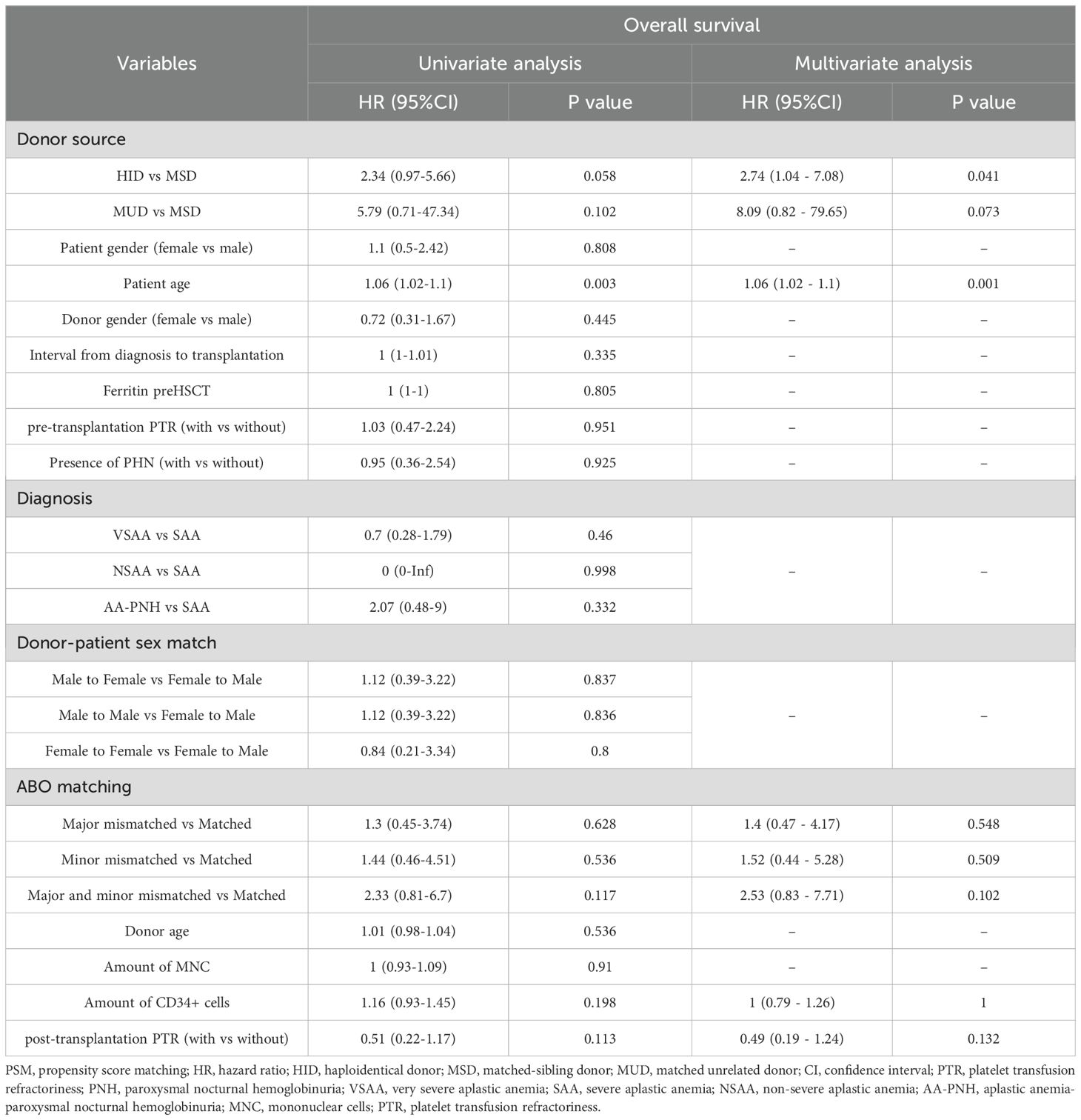
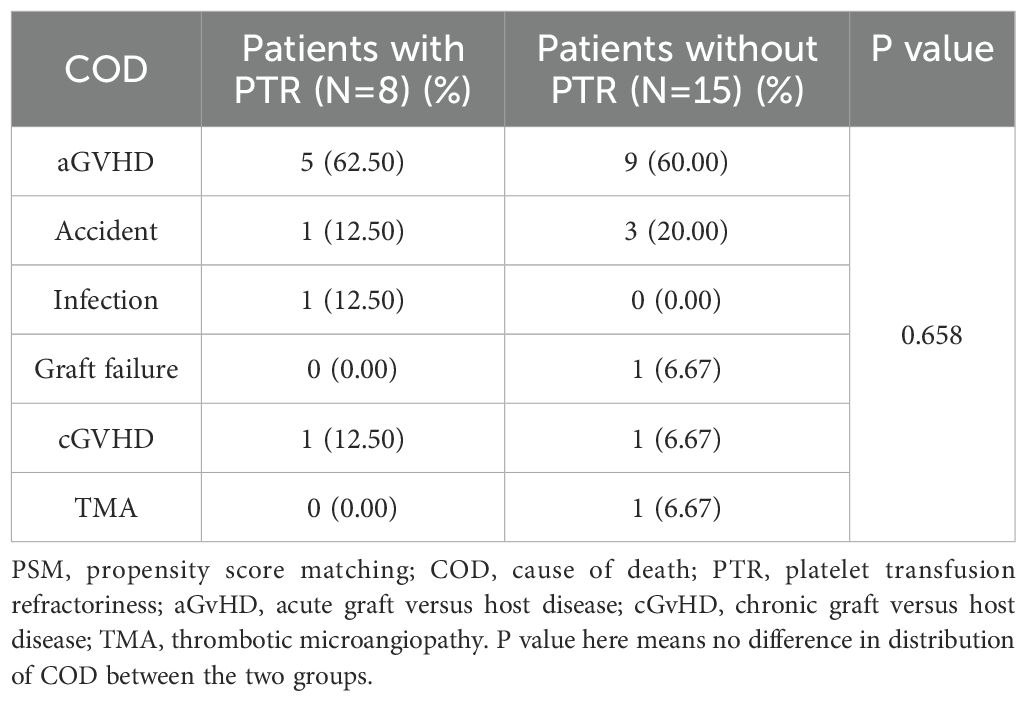
In multivariate analysis, as shown in Table 3, HID and patient age were independent risk factors for OS. More patients died in the PTR group (n=8) compared to those in the non-PTR group (n=15) (P = 0.049) while distribution of COD were similar between the two groups (Table 4, P = 0.658). The PTR group demonstrated significantly higher overall mortality (n=8 vs. n=15, P = 0.049). A GVHD was the predominant cause of death in both cohorts and no fatal bleeding events occurred in either group.
4 Discussion
To our knowledge, this represents the first study to report the incidence of PTR within the critical one-month period following HSCT in AA patients. Our data demonstrate a PTR incidence of 11.7%, which contrasts markedly with the 59.6% incidence reported by Solves et al. (15) in a mixed cohort (peripheral blood and cord blood transplants) and our own findings of 34.9% PTR incidence in myelodysplastic syndrome/myeloproliferative neoplasms (16). This lower incidence rate may be an effect of high-dose ATG universally used in conditioning regimens for AA, regardless of donor type. Supporting this, Gao et al. reported, 21 (72.40%) of the 29 PTR patients with SAA treated with ATG showed a response, while 13 (44.8%) patients had a rapid response after the first dose of ATG administration (5).
While multiple studies have associated post-HSCT platelet transfusion refractoriness (PTR) with inferior survival outcomes (3, 15, 17), our analysis of AA patients revealed no significant survival difference. This discrepancy may be explained by several key factors. First, different disease types were enrolled. We analyzed only AA patients undergoing allo-HSCT, whereas other studies included various hematological diseases. Second, different time points were studied. In this study, we focused on the period from stem cell infusion to one-month post-HSCT to reduce the effect of viral reactivation and aGVHD occurring after one month on PTR. Similarly, Tanoue et al. demonstrated that only PTR occurring 31–45 days after cord blood transplantation, rather than PTR before or after HSCT within one month, was significantly associated with inferior survival (4). Third, combined therapeutic approaches may reduce the hazards of PTR. Currently, thrombopoietin receptor ( (18–21), HLA-matched platelets, and double platelet transfusions can overcome the risk of PTR, and once engraftment is achieved within one-month post-allo-HSCT, the adverse effect of PTR may not persist.
Consistent with established literature, advanced patient age remains a significant prognostic factor for allo-HSCT outcomes in SAA patients across all donor types. Gupta et al. reported markedly increased mortality risks in older recipients of HLA-matched sibling transplants, with relative risks of 2.70 (P < 0.0001) for patients >40 years and 1.69 (P < 0.001) for those aged 20–40 years compared to younger patients (<20 years) (22). This age-dependent survival pattern has been consistently observed in matched unrelated donor (MUD) transplant settings as well (23, 24). Notably, our analysis identified HID-HSCT as an additional independent risk factor for overall survival. This finding likely reflects the selective use of HID-HSCT as salvage therapy at our institution, primarily for patients with both prolonged diagnosis-to-transplant intervals and extensive pre-transfusion histories that may collectively contribute to the poorer outcomes observed in this subgroup.
This study has several important limitations that should be acknowledged. First, our sample size was small which may undermine statistical power and selection bias was unavoidable. For example, patients who died before engraftment may have been more likely to experience PTR; however, we did not analyze this population in our study to avoid blowing up the effect of PTR instead of infections or conditioning toxicities. Second, the reasons for and risk factors of PTR were not explored in detail in our study. In recent years at our center, for patients proceeding to or undergoing allo-HSCT, we have applied tests including anti-class I human leukocyte antigens, anti-human platelet antigens, anti-membrane glycoproteins, and anti-CD36 antibodies to screen for the causes of PTR. Of note, HLA antibodies derived from donor cells post-HSCT have been reported (25). However, non-immunologic factors such as infection, drugs, and increased consumption caused by fever post-allo-HSCT remain the major causes in clinical practice (26, 27). Third, our results should be confirmed in prospective, multi-centers studies.
Through PSM analysis, we demonstrate that early-onset PTR, within one month post-HSCT, may not adversely affect survival outcomes in AA patients. Our findings suggest that incorporation of rATG or pALG in conditioning regimens may mitigate PTR incidence while patient age and HID status remain critical prognostic factors for overall survival. The retrospective design and moderate sample size constrain definitive conclusions. These observations warrant validation through prospective multicenter studies with larger patient cohorts and standardized PTR assessment protocols. This study provides important preliminary evidence that early post-transplant PTR may represent a manageable complication in AA patients receiving modern transplant protocols.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Institute of Hematology and Blood Diseases Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
YZ: Writing – original draft. YW: Writing – original draft, Data curation, Investigation. LL: Writing – review & editing. TZ: Writing – review & editing. JS: Writing – review & editing. XC: Writing – review & editing. DY: Writing – review & editing. AP: Writing – review & editing. RZ: Writing – review & editing. QM: Writing – review & editing. WZ: Writing – review & editing. YH: Writing – review & editing. JW: Writing – review & editing. YC: Writing – review & editing. CL: Writing – review & editing. EJ: Writing – review & editing. MH: Writing – review & editing. SF: Writing – review & editing, Funding acquisition, Formal analysis, Methodology, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2023-I2M-2-007; 2021-I2M-1-017), National Natural Sciences Foundation of China (82470208), National Key R&D Program of China (2024YFC2510500), Noncommunicable Chronic Diseases-National Science and Technology Major Project (2023ZD0510400), Beijing Xisike Clinical Oncology Research Foundation (Y-SYBLD2022RWR-0017), and the Fundamental Research Funds for the Central Universities, Peking Union Medical College (3332024077).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1623004/full#supplementary-material
References
1. Hod E and Schwartz J. Platelet transfusion refractoriness. Br J Haematol. (2008) 142:348–60. doi: 10.1111/j.1365-2141.2008.07189.x
2. Li G, Liu F, Mao X, and Hu L. The investigation of platelet transfusion refractory in 69 Malignant patients undergoing hematopoietic stem cell transplantation. Transfus Apher Sci. (2011) 45:21–4. doi: 10.1016/j.transci.2011.06.017
3. Fu Q, Xu L, Zhang X, Wang Y, Chang Y, Liu K, et al. Platelet transfusion refractoriness after T-cell-replete haploidentical transplantation is associated with inferior clinical outcomes. Sci China Life Sci. (2018) 61:569–77. doi: 10.1007/s11427-017-9110-0
4. Tanoue S, Konuma T, Kato S, Oiwa-Monna M, Isobe M, Jimbo K, et al. Platelet transfusion refractoriness in single-unit cord blood transplantation for adults: risk factors and clinical outcomes. Biol Blood Marrow Transplant. (2018) 24:1873–80. doi: 10.1016/j.bbmt.2018.05.006
5. Gao M, Huang J, Shao Y, Ge M, Li X, Zhang J, et al. Efficacy of anti-thymocyte globulin for platelet transfusion refractoriness in serious aplastic anemia patients. Transfus Apher Sci. (2022) 61:103376. doi: 10.1016/j.transci.2022.103376
6. Kulasekararaj A, Cavenagh J, Dokal I, Foukaneli T, Gandhi S, Garg M, et al. Guidelines for the diagnosis and management of adult aplastic anaemia: A British Society for Haematology Guideline. Br J Haematol. (2024) 204:784–804. doi: 10.1111/bjh.19236
7. Zhang Y, Huo J, Liu L, Shen Y, Chen J, Zhang T, et al. Comparison of hematopoietic stem cell transplantation outcomes using matched sibling donors, haploidentical donors, and immunosuppressive therapy for patients with acquired aplastic anemia. Front Immunol. (2022) 13:837335. doi: 10.3389/fimmu.2022.837335
8. Xu Z-L, Zhou M, Jia J-S, Mo W-J, Zhang X-H, Zhang Y-P, et al. Immunosuppressive therapy versus haploidentical transplantation in adults with acquired severe aplastic anemia. Bone Marrow Transplant. (2019) 54:1319–26. doi: 10.1038/s41409-018-0410-3
9. Liu L, Zhang Y, Jiao W, Zhou H, Wang Q, Jin S, et al. Comparison of efficacy and health-related quality of life of first-line haploidentical hematopoietic stem cell transplantation with unrelated cord blood infusion and first-line immunosuppressive therapy for acquired severe aplastic anemia. Leukemia. (2020) 34:3359–69. doi: 10.1038/s41375-020-0933-7
10. Xu LP, Liu KY, Liu DH, Han W, Chen H, Chen YH, et al. A novel protocol for haploidentical hematopoietic SCT without in vitro T-cell depletion in the treatm ent of severe acquired aplastic anemia. Bone Marrow Transplant. (2012) 47:1507–12. doi: 10.1038/bmt.2012.79
11. Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: A report from the Mount Sinai acute GVHD international consortium. Biol Blood Marrow Transplant. (2016) 22:4–10. doi: 10.1016/j.bbmt.2015.09.001
12. Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. (2015) 21:389–401.e381. doi: 10.1016/j.bbmt.2014.12.001
13. Champlin RE, Horowitz MM, van Bekkum DW, Camitta BM, Elfenbein GE, Gale RP, et al. Graft failure following bone marrow transplantation for severe aplastic anemia: risk factors and treatment results. Blood. (1989) 73:606–13. doi: 10.1182/blood.V73.2.606.bloodjournal732606
14. Copelan E, Casper JT, Carter SL, van Burik J-AH, Hurd D, Mendizabal AM, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transplant. (2007) 13:1469–76. doi: 10.1016/j.bbmt.2007.08.047
15. Solves P, Sanz J, Freiria C, Santiago M, Villalba A, Gomez I, et al. Factors influencing platelet transfusion refractoriness in patients undergoing allogeneic hematopoietic stem cell transplantation. Ann Hematol. (2018) 97:161–7. doi: 10.1007/s00277-017-3168-6
16. Zhang Y, Wang Y, Ma R, Liu L, Sun J, Chen X, et al. Impact of platelet transfusion refractoriness in the first 30 days post-hematopoietic stem cell transplantation on outcomes of patients with myelodysplastic syndrome. Front Immunol. (2024) 15:1437176. doi: 10.3389/fimmu.2024.1437176
17. Toor AA, Choo SY, and Little JA. Bleeding risk and platelet transfusion refractoriness in patients with acute myelogenous leukemia who undergo autologous stem cell transplantation. Bone Marrow Transplant. (2000) 26:315–20. doi: 10.1038/sj.bmt.1702490
18. Reid R, Bennett JM, Becker M, Chen Y, Milner L, Phillips GL, et al. Use of eltrombopag, a thrombopoietin receptor agonist, in post-transplantation thrombocytopenia. Am J Hematol. (2012) 87:743–5. doi: 10.1002/ajh.23225
19. Tanaka T, Inamoto Y, Yamashita T, Fuji S, Okinaka K, Kurosawa S, et al. Eltrombopag for treatment of thrombocytopenia after allogeneic hematopoietic cell transplantation. Biol Blood Marrow tr. (2016) 22:919–24. doi: 10.1016/j.bbmt.2016.01.018
20. Zhou M, Qi J, Gu C, Wang H, Zhang Z, Wu D, et al. Avatrombopag for the treatment of thrombocytopenia post hematopoietic stem-cell transplantation. Ther Adv Hematol. (2022) 13:20406207221127532. doi: 10.1177/20406207221127532
21. Zheng X, Zhang H, Guo W, Cao Y, Liu X, Zhai W, et al. Herombopag promotes platelet engraftment and decreases platelet transfusion after allogeneic hematopoietic stem cell transplantation. Eur J Haematol. (2023) 110:527–33. doi: 10.1111/ejh.13925
22. Gupta V, Eapen M, Brazauskas R, Carreras J, Aljurf M, Gale RP, et al. Impact of age on outcomes after bone marrow transplantation for acquired aplastic anemia using HLA-matched sibling donors. Haematologica. (2010) 95:2119–25. doi: 10.3324/haematol.2010.026682
23. Bacigalupo A, Socie G, Hamladji RM, Aljurf M, Maschan A, Kyrcz-Krzemien S, et al. Current outcome of HLA identical sibling versus unrelated donor transplants in severe aplastic anemia: an EBMT analysis. Haematologica. (2015) 100:696–702. doi: 10.3324/haematol.2014.115345
24. Gadalla SM, Wang T, Dagnall C, Haagenson M, Spellman SR, Hicks B, et al. Effect of recipient age and stem cell source on the association between donor telomere length and survival after allogeneic unrelated hematopoietic cell transplantation for severe aplastic anemia. Biol Blood Marrow Transplant. (2016) 22:2276–82. doi: 10.1016/j.bbmt.2016.09.012
25. Hatakeyama N, Hori T, Yamamoto M, Inazawa N, Iesato K, Miyazaki T, et al. Platelet transfusion refractoriness attributable to HLA antibodies produced by donor-derived cells after allogeneic bone marrow transplantation from one HLA-antigen-mismatched mother. Pediatr Transplant. (2011) 15:E177–182. doi: 10.1111/j.1399-3046.2010.01365.x
26. Ishida A, Handa M, Wakui M, Okamoto S, Kamakura M, and Ikeda Y. Clinical factors influencing posttransfusion platelet increment in patients undergoing hematopoietic progenitor cell transplantation–a prospective analysis. Transfusion. (1998) 38:839–47. doi: 10.1046/j.1537-2995.1998.38998409004.x
Keywords: aplastic anemia, transplantation, platelet transfusion refractoriness, propensity score, survival
Citation: Wang Y, Zhang Y, Liu L, Zhang T, Sun J, Chen X, Yang D, Pang A, Zhang R, Ma Q, Zhai W, He Y, Wei J, Cao Y, Liang C, Jiang E, Han M and Feng S (2025) Platelet transfusion refractoriness within one month post-hematopoietic stem cell transplantation does not impair survival in aplastic anemia patients after engraftment: a propensity score-matched analysis. Front. Immunol. 16:1623004. doi: 10.3389/fimmu.2025.1623004
Received: 05 May 2025; Accepted: 07 July 2025;
Published: 24 July 2025.
Edited by:
Luciana Tucunduva, Hospital Sirio Libanes, BrazilReviewed by:
Mohsen Saleh Elalfy, Ain Sham University, EgyptLouise Helander, University of Colorado Anschutz Medical Campus, United States
Shuhei Kurosawa, Tokyo Metropolitan Komagome Hospital, Japan
Copyright © 2025 Wang, Zhang, Liu, Zhang, Sun, Chen, Yang, Pang, Zhang, Ma, Zhai, He, Wei, Cao, Liang, Jiang, Han and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiali Sun, c3VuamlhbGlAaWhjYW1zLmFjLmNu; Sizhou Feng, c3pmZW5nQGloY2Ftcy5hYy5jbg==; ZG9jdG9yX3N6aGZlbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yan Wang1,2†
Yan Wang1,2† Yuanfeng Zhang
Yuanfeng Zhang Li Liu
Li Liu Tingting Zhang
Tingting Zhang Jiali Sun
Jiali Sun Donglin Yang
Donglin Yang Aiming Pang
Aiming Pang Weihua Zhai
Weihua Zhai Yi He
Yi He Yigeng Cao
Yigeng Cao Erlie Jiang
Erlie Jiang MingZhe Han
MingZhe Han Sizhou Feng
Sizhou Feng