- 1Department of Radiation Oncology and CyberKnife Center, Tianjin Medical University Cancer Institute and Hospital, Key Laboratory of Cancer Prevention and Therapy, Tianjin’s Clinical Research Center for Cancer, National Clinical Research Center for Cancer, Tianjin, China
- 2Department of Pathology, Tianjin Medical University Cancer Institute and Hospital, Key Laboratory of Cancer Prevention and Therapy, Tianjin’s Clinical Research Center for Cancer, National Clinical Research Center for Cancer, Tianjin, China
- 3Department of Colorectal Surgery, Tianjin Union Medical Center, Nankai University, Tianjin, China
- 4Department of Epidemiology and Biostatistics, Tianjin Medical University Cancer Institute and Hospital, Key Laboratory of Molecular Cancer Epidemiology, Key Laboratory of Prevention and Control of Human Major Diseases, Ministry of Education, Tianjin’s Clinical Research Center for Cancer, National Clinical Research Center for Cancer, Tianjin, China
- 5Department of GI Medical Oncology, Tianjin Medical University Cancer Institute and Hospital, Key Laboratory of Cancer Prevention and Therapy, Tianjin’s Clinical Research Center for Cancer, National Clinical Research Center for Cancer, Tianjin, China
- 6Department of Colorectal Oncology, Tianjin Medical University Cancer Institute and Hospital, Key Laboratory of Cancer Prevention and Therapy, Tianjin’s Clinical Research Center for Cancer, National Clinical Research Center for Cancer, Tianjin, China
- 7Department of Radiation Oncology, West China Hospital, West China School of Medicine, Sichuan University, Chengdu, China
- 8Department of Radiation Oncology, University Hospital Seidman Cancer Center, Case Western Reserve School of Medicine, Cleveland, OH, United States
Background: For locally advanced rectal cancer (LARC) with a deficient mismatch repair/microsatellite instability-high (dMMR/MSI-H), particularly in patients not eligible for immunotherapy, the optimal treatment remains undetermined. This study was to evaluate the efficacy and safety of surgery, surgery and chemotherapy, surgery and chemoradiotherapy, in patients with LARC harboring dMMR/MSI-H.
Methods: Patients included from three university centers between August 1, 2012 and March 1, 2023, were categorized into three treatment groups: surgery vs. surgery + chemotherapy vs. surgery + chemoradiotherapy. The primary endpoint was overall survival (OS), with secondary endpoints of progression-free survival (PFS), local recurrence (LR), distant metastasis (DM), and toxicity. The Kaplan-Meier method was utilized to analyze OS and PFS; competing risk methods were employed to evaluate rates of LR and DM. Adjustments were performed utilizing inverse probability of treatment weighting (IPTW) and overlap weighting (OW) based on propensity score, employing logistic regression model. The Cox proportional hazards model was applied for both univariate and multivariate analyses to assess prognostic factors influencing patient OS and PFS.
Results: A total of 119 patients were included, with 45 patients (37.8%) receiving surgery alone, 32 (26.9%) receiving surgery + chemotherapy, and 42 (35.3%) undergoing surgery + chemoradiotherapy. In both the unadjusted cohort and after IPTW and OW adjustments, the surgery alone group (vs. surgery + chemoradiotherapy) had improved OS, PFS, LR, but no significant differences in DM. However, no statistical difference was found between the surgery vs. surgery + chemotherapy groups in OS, PFS, and DM, except for significant differences in LR. Similar results were found in both neoadjuvant and adjuvant treatment cohorts. No adverse events of grade 5 occurred.
Conclusion: This study suggests surgery alone (without chemotherapy and/or radiotherapy) may be an optimal treatment for LARC patients with dMMR/MSI-H, particularly in those who cannot tolerate or access immunotherapy. The results of this study may be used to power a randomized trial for the approaches.
1 Introduction
Rectal cancer is one of most common malignancies and has an annual incidence of approximately 732,000 cases worldwide (1). Locally advanced rectal cancer (LARC), defined as clinical tumor stage 3–4 or clinical lymph node positive disease, accounts for a large proportion of rectal cancers. Approximately 10% of rectal cancers are deficient DNA mismatch repair (dMMR)/microsatellite instability-high (MSI-H) (2), more commonly seen in those < 50 years of age (3, 4).
Over a long period of time, the standard treatment strategy for patients with LARC harboring either dMMR/MSI-H or proficient DNA mismatch repair (pMMR)/microsatellite stability (MSS), as per the ESMO and NCCN guidelines, involves multimodal therapy based on total neoadjuvant therapy (TNT), with chemoradiotherapy and then resection (2, 5–8). There may be several disadvantages to trimodality therapy for these patients: (1) Patients who undergo trimodality therapy may have life-threatening perioperative complications such as anastomotic leakage, poor healing, as well as long-term impairment in urinary, anal, fertility, and sexual functional (9, 10). (2) The distant metastatic rate remains high, reaching about 20% at 3 years (11–13). (3) dMMR/MSI-H cancers may not respond well to chemotherapy or chemoradiotherapy, as they have low rates of complete response (14, 15). Thus, some clinicians argue that LARC with dMMR/MSI-H should be treated with surgery alone, rather than multimodal therapy.
Based on these considerations, and recognizing that surgery with or without chemo(radio)therapy remains an important treatment option for a subset of dMMR/MSI-H LARC patients who are ineligible for immunotherapy, even in the current immunotherapy era, we conducted a multicenter retrospective cohort study to evaluate outcomes with these three approaches. We hypothesize that outcomes are similar among the three approaches. The findings could serve as a valuable reference for further randomized controlled trials, which are essential to investigate and refine optimal treatment strategies beyond traditional chemotherapy or chemoradiotherapy in this context.
2 Patients and methods
2.1 Eligible patients
This retrospective cohort study included patients with LARC harboring dMMR/MSI-H and treated with surgery ± chemo(radio)therapy at Tianjin Medical University Cancer Institute and Hospital, Tianjin Union Medical Center of Nankai University and Tongji Hospital of Huazhong University of Science & Technology from August 1, 2012 to March 1, 2023. The inclusion criteria were as follows: (1) pathologically confirmed rectal adenocarcinoma; (2) dMMR/MSI-H determined using immunohistochemistry (IHC) or polymerase chain reaction (PCR) testing; (3) clinically diagnosed with LARC (T3-4N0 or T1-4N+) according to the AJCC Cancer Staging Eighth Edition; (4) underwent surgery and received either chemo(radio)therapy or not; (5) treated with long course/conventionally fractionated radiotherapy. Patients were excluded if they had recurrent or second primary rectal adenocarcinoma; lacked follow-up or had incomplete data collection; had discordant dMMR/MSI-H testing, or treated with short course radiotherapy. Figure 1 provides a detailed overview of the specific inclusion process for this study. This study adhered to the ethical principles of the Helsinki Declaration and obtained approval from the independent ethics committee of three hospital centers.
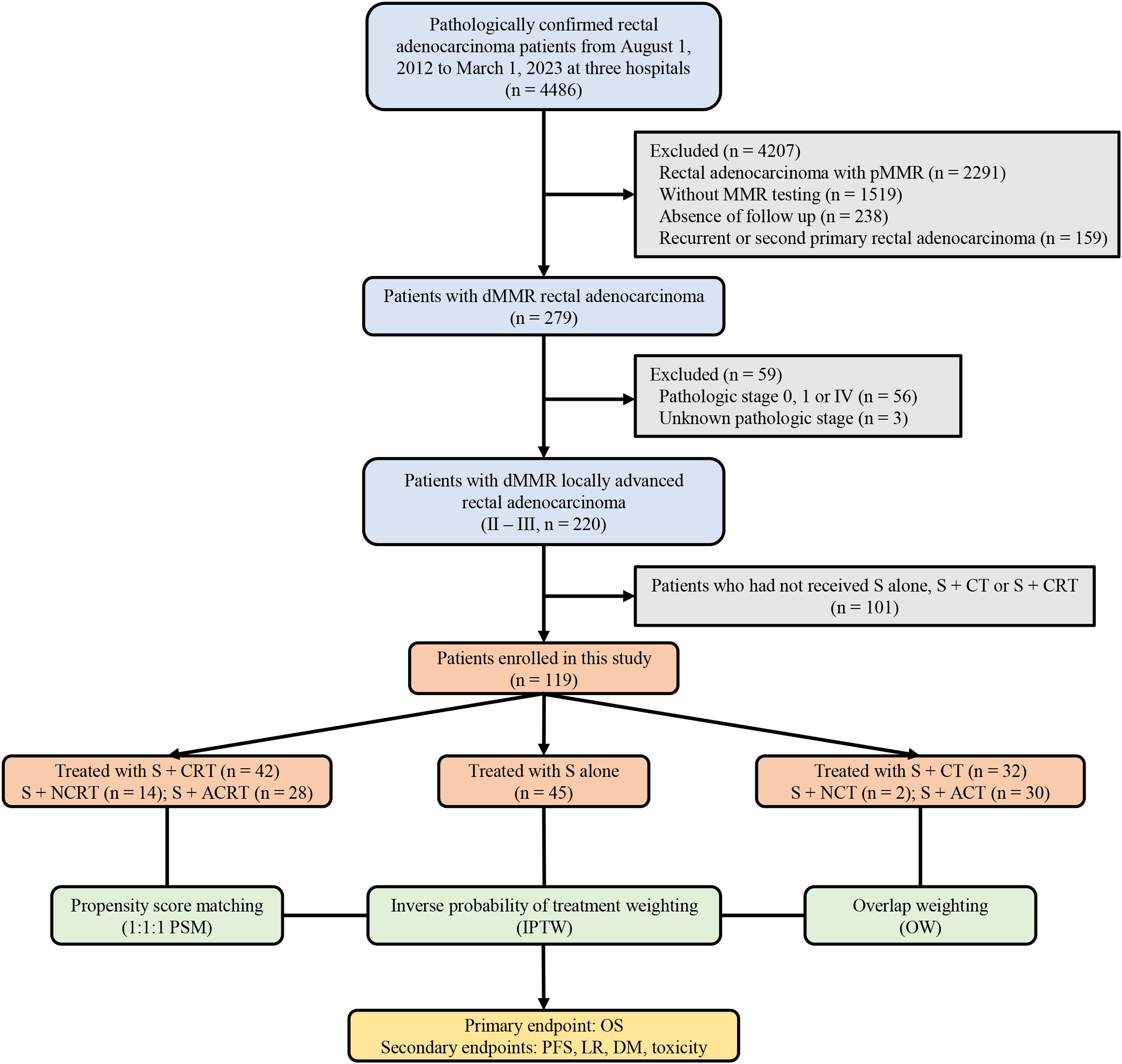
Figure 1. A detailed overview of the specific inclusion process for this study. pMMR, proficient DNA mismatch repair; dMMR, deficient mismatch repair; S+CT, surgery plus chemotherapy; S+CRT, surgery plus chemoradiotherapy; S+NCRT, surgery plus neoadjuvant chemoradiotherapy; S+ACRT, surgery plus adjuvant chemoradiotherapy; S, surgery; S+NCT, surgery plus neoadjuvant chemotherapy; S+ACT, surgery plus adjuvant chemotherapy; PSM, propensity score matching; IPTW, inverse probability of treatment weighting; OW, overlap weighting; OS, overall survival; PFS, progression-free survival; LR, local recurrence; DM, distant metastasis.
2.2 MMR status determination and analyses
MMR status with IHC evaluated the expression of MMR proteins-MLH1, MSH2, MSH6, and PMS2 (16). The absence of any one of these proteins in the tumor classified the patient as dMMR. Additionally, microsatellite instability status was evaluated with PCR, involved analyzing the stability of two mononucleotide (BAT25 and BAT26) and three dinucleotide repeats loci (D5S346, D2S123, and D17S250) in both normal rectal mucosa and tumor tissue. Two or more loci out of the five were found to be unstable (MSI-H), the patient was classified as dMMR. If one locus was unstable (MSI-L) or all five loci were stable (MSS), the patient was categorized as proficient (pMMR) (17, 18).
2.3 Treatments
All patients were treated with definitive-intent surgery, which included Dixon, Miles, Hartmann, Total proctococectomy, or Intersphincteric resection. Long course/conventionally fractionated radiotherapy was administered with three-dimensional conformal radiotherapy or intensity-modulated radiotherapy, with a total dose ranging from 45.0 to 50.4 Gy delivered in 25 to 28 daily fractions. The clinical target volume (CTV) of radiotherapy encompassed primary rectal carcinoma and lymphatic drainage areas such as the mesorectum, internal iliac, and presacral lymph nodes. Chemotherapy cycles were administered every 21 days, and the median number of chemotherapy cycles was approximately 6 (interquartile range [IQR], 4-8). If patients received concurrent chemoradiotherapy, fluorouracil-based monotherapy was administered concurrently with radiotherapy. A subset analysis was performed with neoadjuvant treatment and adjuvant treatment. The interval between neoadjuvant therapy and surgery was about 6–8 weeks, and the interval between adjuvant therapy and surgery was approximately 4–6 weeks.
2.4 Endpoints
The primary endpoint of this study was overall survival (OS), defined as the time from treatment initiation to death or the last follow-up. Secondary endpoints included: (1) progression-free survival (PFS), defined as the duration from treatment initiation to disease progression (based on RECIST 1.1) or last follow up for censored patients; (2) local recurrence, defined as local disease progression (based on RECIST 1.1) after treatment initiation; (3) distant metastasis, defined as distant disease progression (based on RECIST v1.1) after treatment initiation; and (4) Common Terminology Criteria for Adverse Events (CTCAE v4.0) grade toxicity, based on multidisciplinary evaluation.
2.5 Statistical analysis
Categorical variables were compared using the χ2 test and Fisher’s exact test. OS and PFS were generated using the Kaplan-Meier method, and comparisons between groups were conducted via the log-rank test. The cumulative incidence of LR and DM were compared using doubly robust multivariable Fine and Gray regression models, enabling estimation of the subdistribution hazard function that models the hazard function in the presence of competing risks. All-cause mortality was considered a competing risk for LR and DM (19, 20). Univariate analyses (UVA) and multivariate analyses (MVA) were conducted using the Cox proportional hazards model to assess the prognostic factors influencing patient OS and PFS. Variables significant in the univariate analysis were considered for inclusion in the final multivariate model. Variables with a p-value < 0.2 were retained in the final multivariate model. Statistical analyses were conducted using R version 4.3.2 (R Foundation for Statistical Computing). In this study, the Benjamini-Hochberg correction was applied to adjust for multiple testing of pairwise comparisons of survival curves. Both these comparisons and other statistical analyses were considered statistically significant if the p value was < 0.05.
2.6 PSM, IPTW, and OW adjustments
To minimize data biases and confounding factors in terms of baseline characteristics, we utilized the multivariable logistic regression model approach described by Rubin and Rosenbaum to calculate propensity scores for Propensity score matching (PSM), inverse probability of treatment weighting (IPTW), and overlap weighting (OW) (21, 22). Variables involved in the regression model included gender (male vs. female), age (≤ 60 vs. > 60), pathological stage (II vs. III), distance from anal verge (≤ 5 cm vs. > 5 cm), differentiation (poorly vs. moderately vs. well), resection status (R0 vs. R1), and lymphovascular or neural invasion (negative vs. positive). Collinearity was tested using variance inflation factor to ensure the independence of each variable included in the regression model. The collinearities within the overall cohort and the adjuvant cohort were illustrated in Supplementary Figure 1. PSM included a logistic regression model and a 1:1:1 ratio matching with nearest-neighbor matching and a caliper of 0.2 times the standard deviation of the propensity score’s logit. For IPTW and OW, stabilized weights were calculated from the propensity score and used as weights (23). Standardized mean difference (SMD) was used to assess the balance of baseline covariates between treatment groups in the adjusted sample with that in the unadjusted sample. SMD values less than 0.2 indicated high levels of covariate balances. The SMD values within the overall cohort and the adjuvant cohort were illustrated in Supplementary Figure 2. Because PSM yielded inferior SMD results compared to IPTW and OW, IPTW and OW were ultimately selected as the preferred methods.
3 Results
3.1 Patient baseline characteristics
Between August 1, 2012 to March 31, 2023, 4486 patients were screened for MMR testing and eligibility, and 119 patients with dMMR/MSI-H were included. Among included patients, 45 patients (37.8%) underwent surgery alone, 32 patients (26.9%) received surgery + chemotherapy, included 2 and 30 patents who underwent neoadjuvant and adjuvant chemotherapy, as well as 42 patients (35.3%) received surgery + chemoradiotherapy, included 14 and 28 patents who underwent neoadjuvant and adjuvant surgery + chemoradiotherapy, respectively. The median follow-up periods for the three groups were 46.0 months (IQR, 42.5-88.6), 45.5 months (IQR, 28.3-93.0), and 47.9 months (IQR, 29.7-77.0) respectively.
In the unadjusted cohort, significant imbalances were observed in nearly half of patient baseline characteristics. In contrast, this cohort exhibited a good balance in patents baseline characteristics after both IPTW and OW adjustments (Table 1). Similar to the overall cohort, the postoperative adjuvant treatment cohort exhibited balanced patient baseline characteristics after IPTW and OW adjustments. Treatment characteristics of the overall cohort were outlined in Table 2. However, IPTW and OW adjustments could not be conducted for the neoadjuvant treatment cohort due to its small sample size. The MMR protein defect style of the overall cohort, adjuvant treatment cohort, and neoadjuvant treatment cohort were depicted in Supplementary Figure 3.
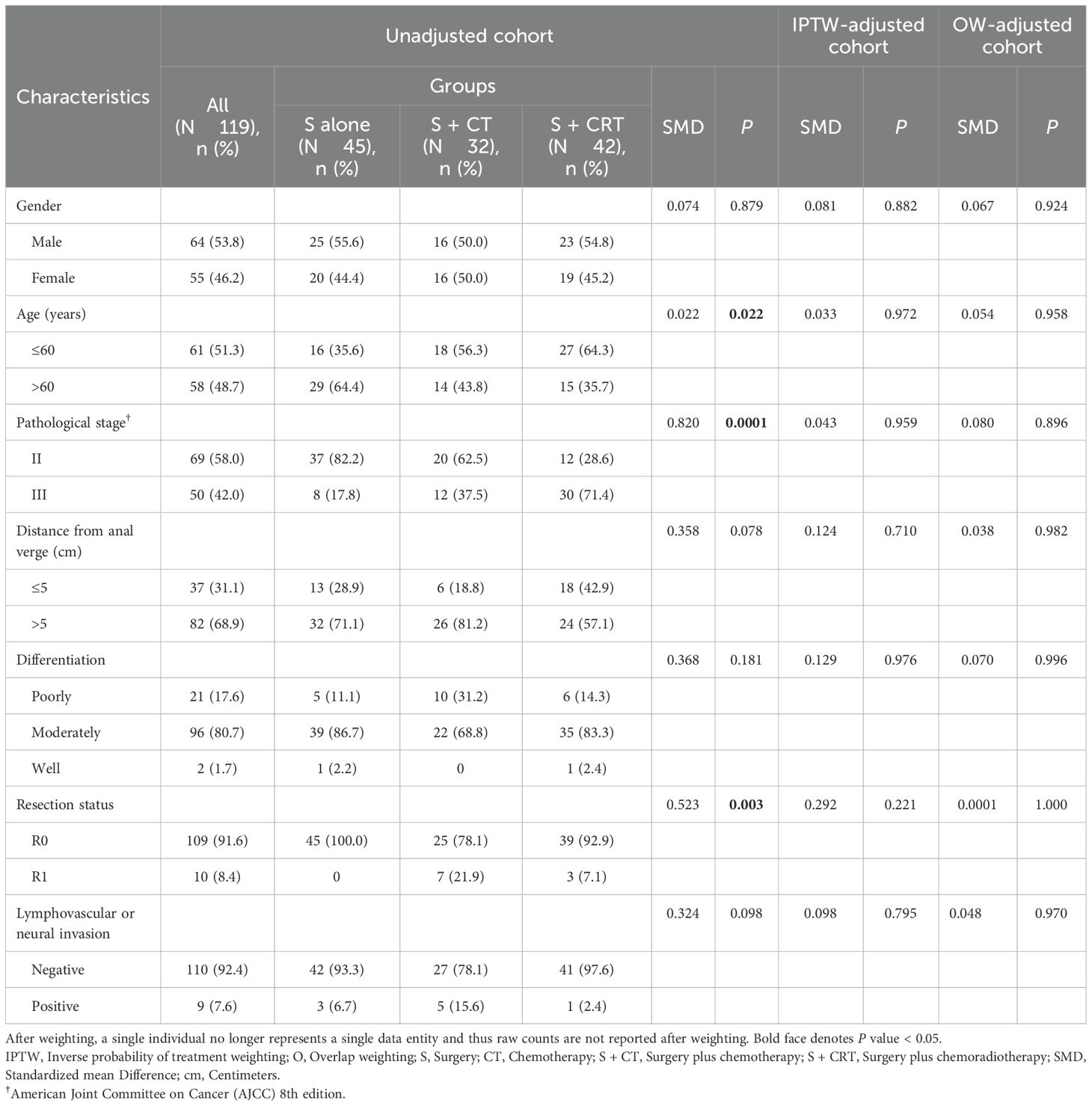
Table 1. Baseline demographic and clinical characteristics for patients received surgery alone, surgery plus CT, and surgery plus CRT.
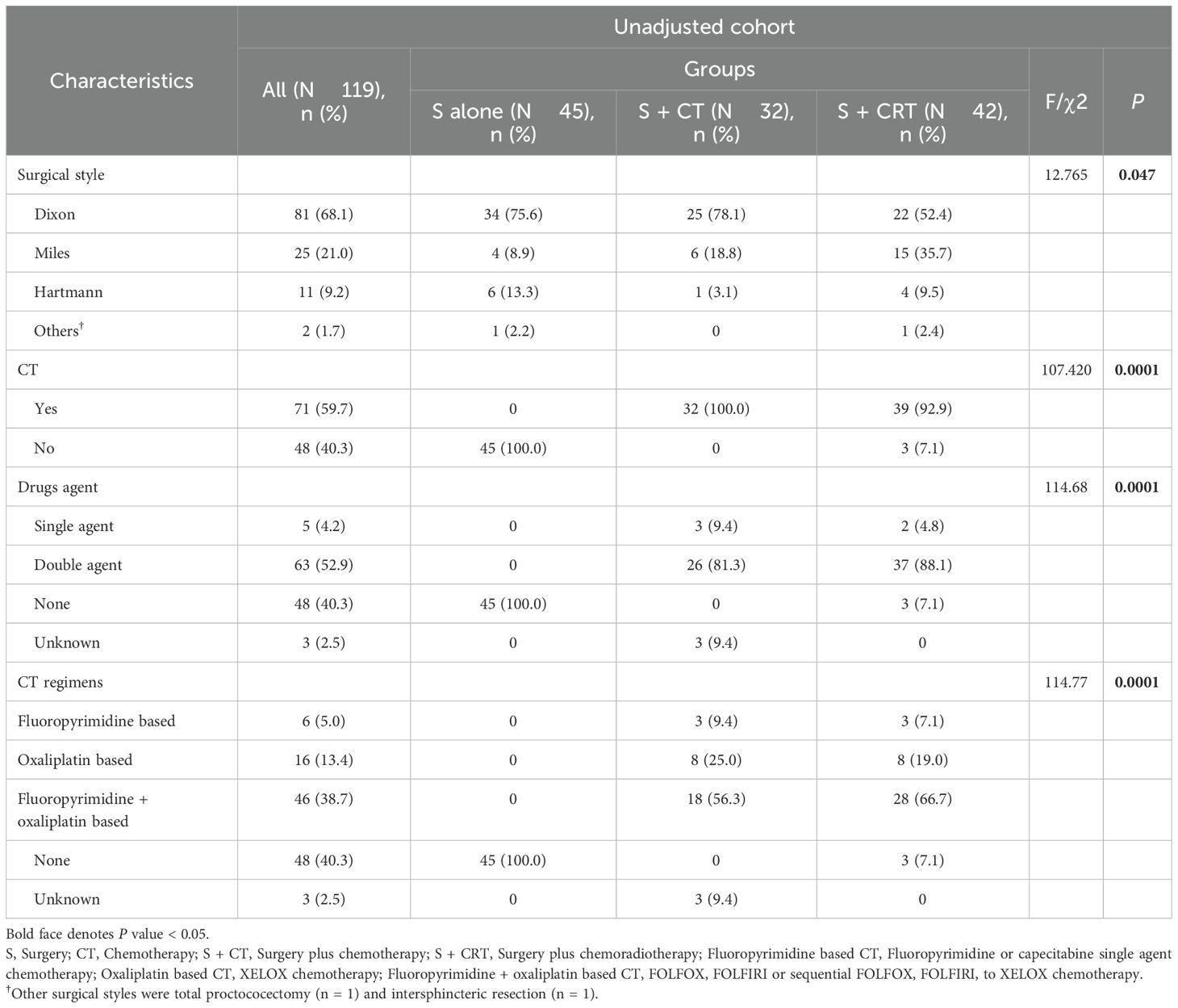
Table 2. Treatment characteristics for patients received surgery alone, surgery plus CT, and surgery plus CRT.
3.2 Outcomes overall cohort
In both the unadjusted cohort and after IPTW and OW adjustments, patients in the surgery alone (vs. surgery + chemoradiotherapy) was associated with improved in OS, PFS, and LR, but no significant differences in DM. However, no statistically significant difference was found between the surgery alone vs. surgery + chemotherapy groups in OS, PFS, and DM, except for significant differences in LR (Figure 2, Supplementary Figure 4). We conducted a subgroup analysis based on treatment modalities, where surgery alone was associated with improved outcomes vs. the surgery + adjuvant chemoradiotherapy group in the unadjusted cohort. However, no statistical difference was found among these three groups in both IPTW (Supplementary Figure 5) and OW adjustments. Although only 13.4% (16/119) included patients received neoadjuvant therapy, similar results were seen in the surgery vs. neoadjuvant chemoradiotherapy group comparison (Supplementary Figure 6). After adjustments, there was no statistically significant difference in outcomes in the surgery vs. the surgery + chemotherapy groups, except for significant differences in LR.
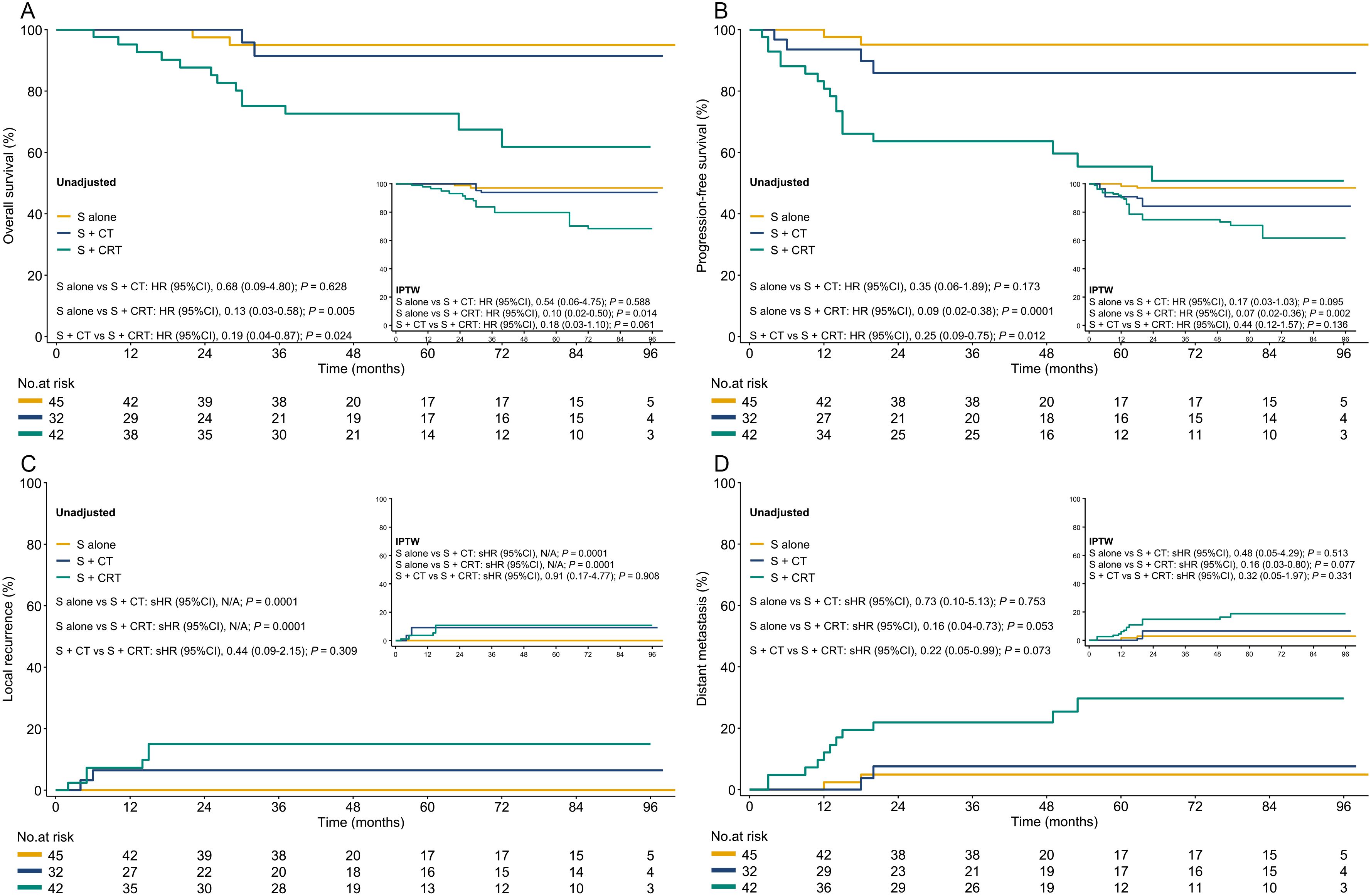
Figure 2. The OS, PFS, LR, and DM in both the unadjusted and IPTW-adjusted cohorts. (A): OS; (B) PFS; (C) LR; (D) DM. S, surgery; S+CT, surgery plus chemotherapy; S+CRT, surgery plus chemoradiotherapy; HR, hazard ratio; IPTW, inverse probability of treatment weighting; sHR, subdistribution hazard ratio.
3.3 Prognostic factors of OS and PFS
Presence of pathological stage III, lymphovascular or neural invasion, non-Dixon surgery style, and surgery + chemo(radio)therapy were significantly associated with worse OS or PFS in the unadjusted cohort. After adjustments using IPTW and OW, only surgery + chemoradiotherapy were found to be correlated with worse OS and PFS after IPTW and OW adjustments. Results of UVA and MVA for clinical factors affecting OS and PFS are presented in Figures 3, 4, and Supplementary Figure 7. Pathological stage III and surgery + chemoradiotherapy were an adverse prognostic factor for OS and PFS in both UVA and MVA in both unadjusted and adjusted matching for adjuvant treatment cohort (Supplementary Figure 8), while only surgery + chemoradiotherapy was adverse prognostic factor for OS and PFS in both UVA and MVA in the unadjusted cohort for neoadjuvant treatment cohorts.
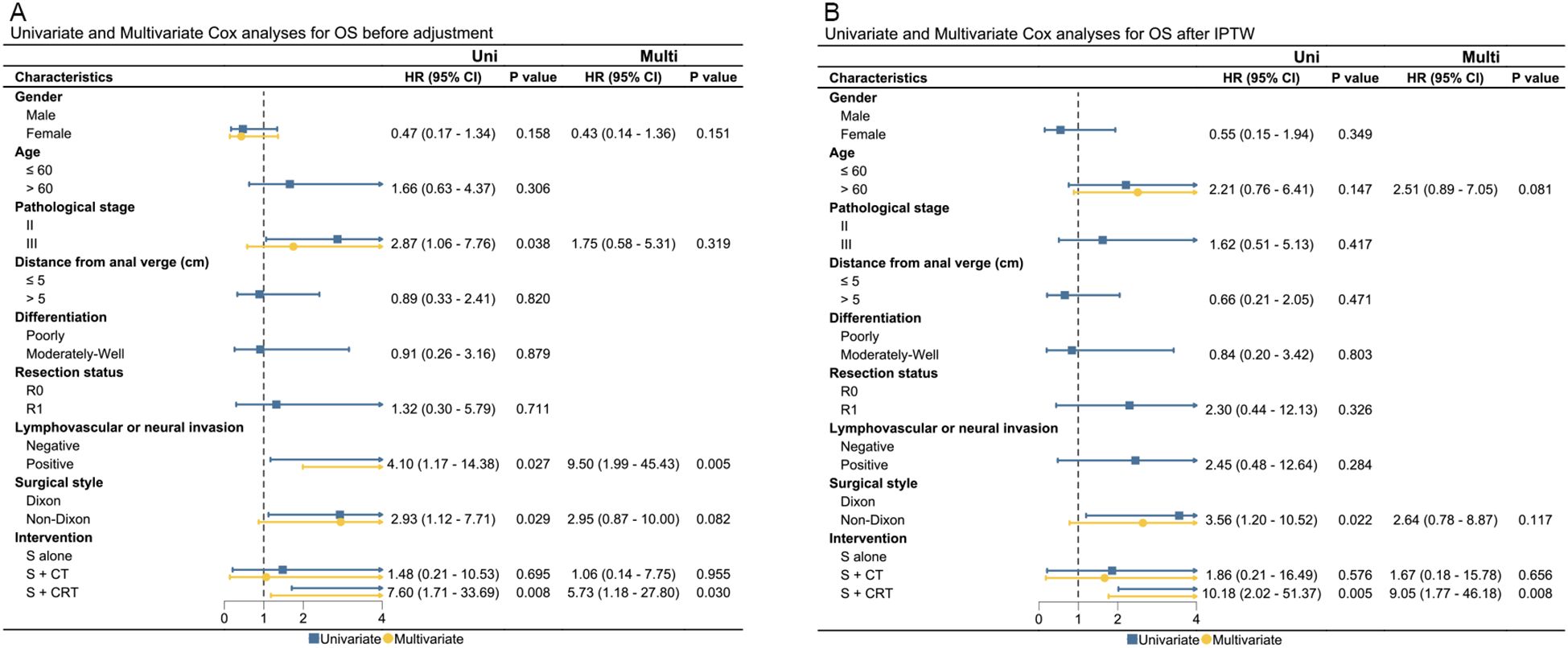
Figure 3. The UVA and MVA analysis for clinical factors affecting OS in both the unadjusted and IPTW-adjusted cohorts. (A) OS before adjustment; (B) OS after IPTW. OS, overall survival; Uni, univariate analysis; Multi, multivariate analysis; HR, hazard ratio; CI, confidence interval; IPTW, inverse probability of treatment weighting; HR, hazard ratio; cm, centimeter.
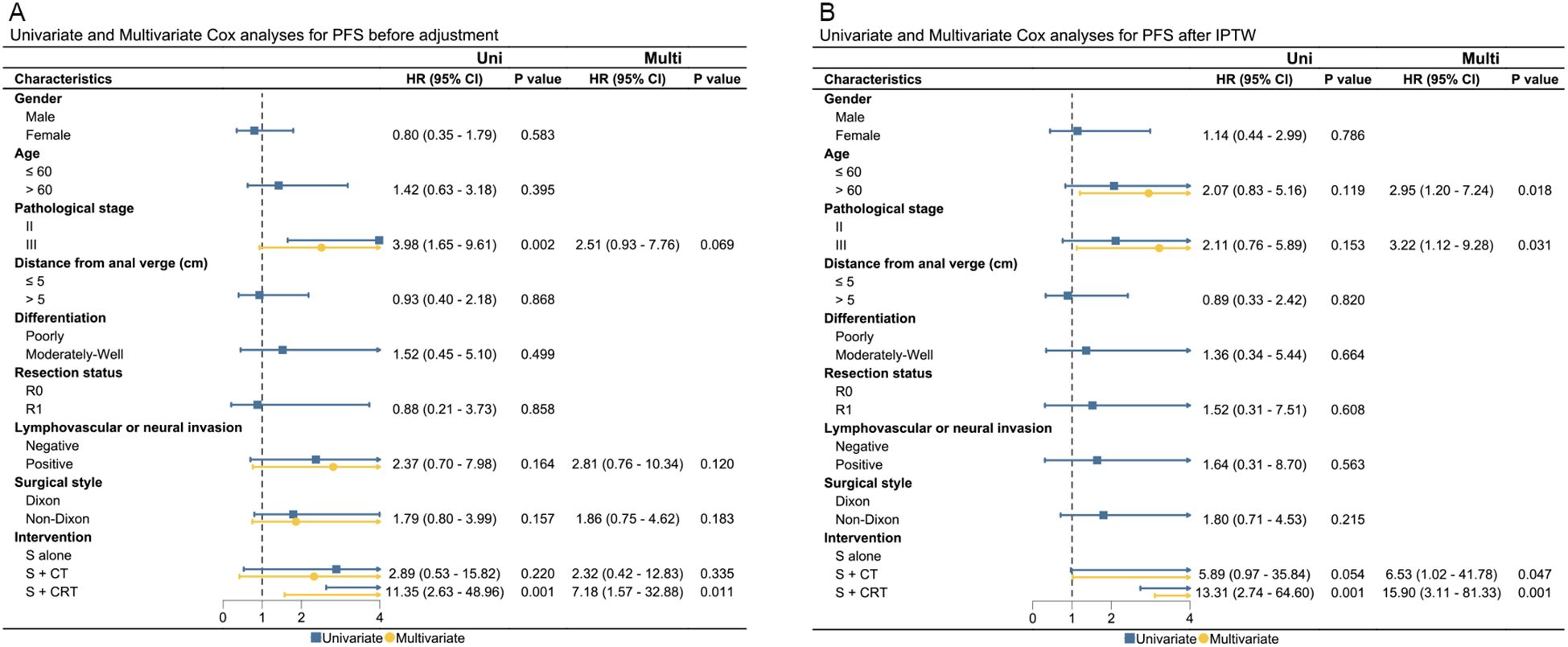
Figure 4. The UVA and MVA analysis for clinical factors affecting PFS in both the unadjusted and IPTW-adjusted cohorts. (A) PFS before adjustment; (B) PFS after IPTW. PFS, progression-free survival; Uni, univariate analysis; Multi, multivariate analysis; HR, hazard ratio; CI, confidence interval; IPTW, inverse probability of treatment weighting; HR, hazard ratio; cm, centimeter.
3.4 Toxicities
The most common treatment-emergent adverse events of any grade ≥ 5% were nausea and vomiting (51/119, 42.9%), diarrhea (48/119, 40.3%), hepatotoxicity (33/119, 27.7%), neutropenia (27/119, 22.7%), neurotoxicity (22/119, 18.5%), and leukopenia (17/119, 14.3%). The grade 3–4 treatment-emergent adverse events were diarrhea (18/119, 15.1%), leukopenia (10/119, 8.4%), neurotoxicity (9/119, 7.6%), nausea and vomiting (4/119, 3.4%), and thrombocytopenia (4/119, 3.4%). All surgery-related adverse events were grade 1–2, including colostomy and subsequent reversal (4/119, 3.3%), anastomotic leakage (3/119, 2.5%), postoperative bleeding (5/119, 4.2%), and urogenital dysfunction (6/119, 5.0%). No adverse events of grade 5 occurred. There was no significant difference among the three groups with respect to any grade and grade 3–4 adverse events.
4 Discussion
To the best of our knowledge, few retrospective studies have evaluated the suitability of chemotherapy or chemoradiotherapy in patients with LARC harboring dMMR/MSI-H, but the contradictory results have arisen from a comparison of LARC patients between those with dMMR/MSI-H and pMMR/MSS (24–27). This difference may partly be attributable to the low incidence of dMMR/MSI-H in rectal cancer and thereby to the small sample size (28). Importantly, our results suggested that there was no improvement in outcomes from the addition of chemotherapy or chemoradiotherapy. A possible biological explanation for these results may lies in the MMR protein biological function. In the absence of a functional MMR system, repair may only occur through the “base excision repair” system, a process that is less affected by the disequilibrium or methylation induced by chemotherapy or chemoradiotherapy (29–31). Therefore, identifying the optimal treatment strategy to improve the response of LARC patients with dMMR/MSI-H, particularly those who are not eligible for immunotherapy, is of utmost importance, necessitating further large-scale studies to refine the optimal treatment strategy.
Theoretically, neoadjuvant therapy results in downstaging, lymph node clearance, and clearance of lymphovascular involvement; however, only 13.4% (16/119) included patients received neoadjuvant therapy, while the majority underwent surgery combined with adjuvant chemoradiotherapy instead of the current neoadjuvant treatment era. We believe this may be due to two main factors. First, the treatment paradigm for LARC has evolved gradually over time—from surgery alone, to the incorporation of adjuvant therapy, and eventually to the widespread adoption of neoadjuvant strategies. The timing of this transition has varied across countries and regions (32–35). Although the utilization of neoadjuvant chemoradiotherapy has been gradually increasing in certain Chinese medical centers, the overall adoption of this treatment strategy occurred later in China than in some Western countries and may still lag behind levels seen in developed countries (33, 34). Second, dMMR/MSI-H tumors exhibit distinct biological behavior, including reduced sensitivity to chemo(radio)therapy, as reported in prior studies (14, 25, 36). As this resistance has become increasingly recognized, clinicians often prioritize surgery and reserve adjuvant therapy for patients with high-risk postoperative features. Importantly, we conducted a subgroup analysis based on treatment modalities, where surgery alone was associated with improved outcomes compared to surgery combined with (neo)adjuvant therapies, including chemotherapy or chemoradiotherapy in the unadjusted cohort. However, no statistically significant differences were observed among these three groups in both IPTW and OW adjustments.
Early studies on immunotherapy in rectal cancer patients without specific molecular characteristics showed disappointing results (37). Nevertheless, there is a growing interest in the use of immunotherapy and immune-based strategies for treating LARC with dMMR/MSI-H. We comprehensively reviewed published research on immunotherapy and immune-based strategies for LARC patients with dMMR/MSI-H by searching PubMed and the clinical trial database. The final search date was June 1, 2024, and resulted in the inclusion of a total of 24 articles (as detailed in Supplementary Table 1) (38–65).
Emerging evidence indicates the extraordinary response of immunotherapy in treating LARC with dMMR/MSI-H. Based on these data, the latest NCCN guideline (https://www.nccn.org/guidelines) provides the preferred treatment strategy for patients with LARC harboring dMMR/MSI-H, recommending the initiation of immunotherapy for patients who have not received prior immune checkpoint inhibitors (ICIs). It suggests adopting a W&W if a clinical complete response (cCR) is achieved. Otherwise, continue with chemoradiotherapy combined with or without surgery. However, neoadjuvant monotherapy with ICIs has shown cCR or pathological complete response (pCR) rates ranging from 37.5% to 100%, comparable to those achieved with neoadjuvant immune-based combination therapy, where cCR or pCR rates range from 60% to 100%. These results suggest that surgery may be essential for some LARC patients with dMMR/MSI-H who do not achieve cCR.
Currently, many questions remain unanswered, such as the optimal timing for initiating ICIs, the appropriate dosage of immunotherapeutic agents, the ideal duration of ICIs, and the optimal combination strategy of ICIs and chemotherapy or chemoradiotherapy before surgery. Additionally, distinguishing masses containing inflammatory cells, necrotic tissue, and/or fibrous tissues from those containing tumor cells poses a challenge. Furthermore, the OS and PFS outcomes for neoadjuvant monotherapy with ICIs have not yet been finalized. Nevertheless, these promising results, albeit primarily derived from small clinical series, provide optimism for the future. Therefore, the optimal treatment strategy of LARC with a dMMR/MSI-H has yet to be clearly defined, necessitating additional studies and efforts to refine the optimal treatment strategy.
For patients with LARC harboring dMMR/MSI-H who achieve cCR after ICIs monotherapy or combination therapy, the option of omitting surgery and proceeding with observation alone may offer the possibility of cure without functional impairment. However, resistance to ICIs is frequently observed in the neoadjuvant setting, with the rate ranging between 10% and 40% (37, 48, 50, 51, 64–69). This results in the forfeiture of the optimal surgical opportunity for these patients. Apart from the biological mechanisms underlying resistance to immunotherapy in LARC with dMMR/MSI-H, consistent with our study (8.4%), nearly 10% of cases can largely be attributed to misdiagnosis of MMR/MSI status (70). Therefore, the combined use of both IHC and PCR is recommended to prevent misdiagnosis of dMMR/MSI-H status. Importantly, there is no well-established biomarker for ICIs resistance in these patients. Next-generation molecular profiling may provide further insight into biologic underpinnings, including potential drivers of oncogenesis or antioncogesis (e.g., BRAF V600E, AKT1, CDH1, PTEN, or PIK3CA) (71–74).
This study has several limitations that need to be acknowledged. To the best of our knowledge, this study comprises a large sample size of LARC harboring dMMR/MSI-H who have undergone surgery with or without chemo(radio)therapy as curative intent treatment and could provide useful indications for future prospective trials. Firstly, we acknowledge your observation that the sample size exceeds the recommended ten events per variable (EPV) guideline in logistic regression analysis. However, previous studies published in prestigious journal, have suggested that even lower ratios, such as five events per variable, have been employed successfully in small patient cohorts with rare diseases (73–77). Indeed, the appropriateness of EPV ratios is context-dependent and influenced by factors such as outcome variability, type of variables, and survival analysis considerations. Additionally, this study possesses the following characteristics: (1) it is an exploration study; (2) independent variables were screened prior to conducting multivariate regression; (3) the HR and 95% CI of the results exhibit normalcy; (4) goodness-of-fit statistics indicate successful modeling; (5) despite some instability, the results consistently reflect the characteristic under consideration. In recent years, numerous high-quality scholarly work have adopted similar analytical method (73–78).
Secondly, the retrospective nature of this study introduces inherent selection bias given its span over one decade. Although we attempted to adjust for confounding factors using PSM, IPTW, and OW, there may still be some uncontrolled potential biases and confounding factors, such as the majority of patients undergoing surgery plus adjuvant chemoradiotherapy instead of the current neoadjuvant treatment era. Thirdly, no patients in the present cohort receive ICIs. Fourth, MMR status in this study was mainly determined through IHC, with only a small number of patients undergoing dual testing with IHC and PCR, potentially leading to false-positive results (57). Among patients who underwent both IHC and PCR testing, we observed concordance rates consistent with previously reported levels ranging from 91.4% to 99.6% (79–82). Given this high level of agreement between the two methods, the likelihood of misclassification within our cohort is expected to be low. Fifth, we collected follow-up data using electronic medical records and telephone follow-ups, resulting in missing data related to treatment-related adverse events, thus precluding analysis of this data. In addition, none of included patients underwent detection of germline genes and confirmed a diagnosis of Lynch syndrome. Given the familial heritability of Lynch syndrome and the potential for multiple primary malignancies, we will continue to encourage subjects who are young or suspected of Lynch syndrome to undergo germline genetic testing for better long-term management and follow-up.
5 Conclusion
Neoadjuvant chemoradiotherapy followed by rectal surgery resection is a standard treatment for LARC, but it is unclear if this approach is ideal for dMMR/MSI-H patients. This multicenter retrospective cohort study suggests no improvement in outcomes from the addition of chemotherapy or chemoradiotherapy vs. surgery alone, and a possible detriment in outcomes with either neoadjuvant or adjuvant chemoradiotherapy. Although caution should be used in the interpretation of retrospective comparative effectiveness research, the results of this study may be used to support the use of surgery alone for select patients (particularly in those who cannot tolerate or access immunotherapy) and to power a randomized controlled trial to evaluate the different treatment approaches.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the corresponding author (Meng M-B), without undue reservation.
Ethics statement
This study adhered to the ethical principles of the Helsinki Declaration and obtained approval from the independent ethics committee of three hospital centers. The ethics statement is already provided in the Methods section of the manuscript. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HW: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. YY: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. HZ: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. YW: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. KN: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – review & editing. XY: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – review & editing. JS: Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – review & editing. HL: Formal Analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – review & editing. JW: Visualization, Writing – review & editing, Formal Analysis, Investigation, Methodology, Resources, Software, Validation. ZY: Investigation, Resources, Software, Validation, Visualization, Writing – review & editing. QW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – review & editing. NZ: Investigation, Resources, Software, Validation, Visualization, Writing – review & editing. CZ: Investigation, Resources, Software, Validation, Visualization, Writing – review & editing. FZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Key Project of Tianjin Education Commission (No. 2020ZD09), the Clinical Talent Training 123 Climbing Plan of Tianjin Medical University (No. 2023-1-20), and the Tianjin Key Medical Discipline (Specialty) Construction Project (No. TJYXZDXK-010A).
Acknowledgments
We also would like to emphasize our appreciation for the concise and thoughtful comments of the reviewers and editors, which have significantly improved this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1626438/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, et al. Rectal cancer, version 2. 2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20:1139–67. doi: 10.6004/jnccn.2022.0051
3. Siegel RL, Wagle NS, Cercek A, Smith RA, and Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. (2023) 73:233–54. doi: 10.3322/caac.21772
4. Papke DJ, Yurgelun MB, Noffsinger AE, Turner KO, Genta RM, and Redston M. Prevalence of mismatch-repair deficiency in rectal adenocarcinomas. N Engl J Med. (2022) 387:1714–6. doi: 10.1056/NEJMc2210175
5. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann Oncol. (2017) 28:iv22–40. doi: 10.1093/annonc/mdx224
6. Fokas E, Allgäuer M, Polat B, Klautke G, Grabenbauer GG, Fietkau R, et al. Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ARO/AIO-12. J Clin Oncol. (2019) 37:3212–22. doi: 10.1200/JCO.19.00308
7. Conroy T, Bosset JF, Etienne PL, Rio E, François É, Mesgouez-Nebout N, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22:702–15. doi: 10.1016/S1470-2045(21)00079-6
8. Lo Greco MC, La Rocca M, Marano G, Finocchiaro I, Liardo RLE, Milazzotto R, et al. Integrated intensified chemoradiation in the setting of total neoadjuvant therapy (TNT) in patients with locally advanced rectal cancer: A retrospective single-arm study on feasibility and efficacy. Cancers. (2023) 15:921. doi: 10.3390/cancers15030921
9. Fazio VW, Zutshi M, Remzi FH, Parc Y, Ruppert R, Fürst A, et al. A randomized multicenter trial to compare long-term functional outcome, quality of life, and complications of surgical procedures for low rectal cancers. Ann Surg. (2007) 246:481–8. doi: 10.1097/SLA.0b013e3181485617
10. Bruheim K, Guren MG, Skovlund E, Hjermstad MJ, Dahl O, Frykholm G, et al. Late side effects and quality of life after radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. (2010) 76:1005–11. doi: 10.1016/j.ijrobp.2009.03.010
11. Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22:29–42. doi: 10.1016/S1470-2045(20)30555-6
12. Braendengen M, Tveit KM, Berglund A, Birkemeyer E, Frykholm G, Påhlman L, et al. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol. (2008) 26:3687–94. doi: 10.1200/JCO.2007.15.3858
13. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. (2012) 30:1926–33. doi: 10.1200/JCO.2011.40.1836
14. Cercek A, Dos Santos Fernandes G, Roxburgh CS, Ganesh K, Ng S, Sanchez-Vega F, et al. Mismatch repair-deficient rectal cancer and resistance to neoadjuvant chemotherapy. Clin Cancer Res. (2020) 26:3271–9. doi: 10.1158/1078-0432.CCR-19-3728
15. Hasan S, Renz P, Wegner RE, Finley G, Raj M, Monga D, et al. Microsatellite instability (MSI) as an independent predictor of pathologic complete response (PCR) in locally advanced rectal cancer: A National Cancer Database (NCDB) Analysis. Ann Surg. (2020) 271:716–23. doi: 10.1097/SLA.0000000000003051
16. Luchini C, Bibeau F, Ligtenberg MJL, Singh N, Nottegar A, Bosse T, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumor mutational burden: a systematic review-based approach. Ann Oncol. (2019) 30:1232–43. doi: 10.1093/annonc/mdz116
17. Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. (1998) 58:5248–57.
18. Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. (2004) 96:261–8. doi: 10.1093/jnci/djh034
19. Dignam JJ, Zhang Q, and Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res. (2012) 18:2301–8. doi: 10.1158/1078-0432.CCR-11-2097
20. Chappell R. Competing risk analyses: how are they different and why should you care? Clin Cancer Res. (2012) 18(8):2127–9. doi: 10.1158/1078-0432.Ccr-12-0455
21. Thomas LE, Li F, and Pencina MJ. Using propensity score methods to create target populations in observational clinical research. JAMA. (2020) 323:466–7. doi: 10.1001/jama.2019.21558
22. Thomas LE, Li F, and Pencina MJ. Overlap weighting: a propensity score method that mimics attributes of a randomized clinical trial. JAMA. (2020) 323:2417–8. doi: 10.1001/jama.2020.7819
23. Zlotta AR, Ballas LK, Niemierko A, Lajkosz K, Kuk C, Miranda G, et al. Radical cystectomy versus trimodality therapy for muscle-invasive bladder cancer: a multi-institutional propensity score matched and weighted analysis. Lancet Oncol. (2023) 24:669–81. doi: 10.1016/S1470-2045(23)00170-5
24. Ni K, Zhan Y, Liu Z, Zhao XZ, Wang W, Wang G, et al. Mismatch repair system deficiency is associated with chemoradiotherapy resistance in locally advanced rectal adenocarcinoma patients. J Surg Oncol. (2022) 125:692–702. doi: 10.1002/jso.26771
25. Ye SB, Cheng YK, Zhang L, Zou YF, Chen P, Deng YH, et al. Association of mismatch repair status with survival and response to neoadjuvant chemo(radio)therapy in rectal cancer. NPJ Precis Oncol. (2020) 4:26. doi: 10.1038/s41698-020-00132-5
26. Meillan N, Vernerey D, Lefèvre JH, Manceau G, Svrcek M, Augustin J, et al. Mismatch repair system deficiency Is associated with response to neoadjuvant chemoradiation in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. (2019) 105:824–33. doi: 10.1016/j.ijrobp.2019.07.057
27. Pretta A, Ziranu P, Giampieri R, Pinna G, Randon G, Donisi C, et al. Mismatch Repair system protein deficiency as a resistance factor for locally advanced rectal adenocarcinoma patients receiving neoadjuvant chemo-radiotherapy. Br J Cancer. (2023) 129:1619–24. doi: 10.1038/s41416-023-02444-2
28. Ni K, Zhan Y, Liu Z, Yuan Z, Wang S, Zhao XZ, et al. Survival outcomes in locally advanced dMMR rectal cancer: surgery plus adjunctive treatment vs. surgery alone. BMC Cancer. (2023) 23:1013. doi: 10.1186/s12885-023-11525-7
29. Fischer F, Baerenfaller K, and Jiricny J. 5-Fluorouracil is efficiently removed from DNA by the base excision and mismatch repair systems. Gastroenterology. (2007) 133:1858–68. doi: 10.1053/j.gastro.2007.09.003
30. Stojic L, Brun R, and Jiricny J. Mismatch repair and DNA damage signaling. DNA Repair (Amst). (2004) 3:1091–101. doi: 10.1016/j.dnarep.2004.06.006
31. Duckett DR, Drummond JT, Murchie AI, Reardon JT, Sancar A, Lilley DM, et al. Human MutSalpha recognizes damaged DNA base pairs containing O6-methylguanine, O4-methylthymine, or the cisplatin-d(GpG) adduct. Proc Natl Acad Sci U S A. (1996) 93:6443–7. doi: 10.1073/pnas.93.13.6443
32. Wong DL, Hendrick LE, Guerrero WM, Monroe JJ, Hinkle NM, Deneve JL, et al. Adherence to neoadjuvant therapy guidelines for locally advanced rectal cancers in a region with sociodemographic disparities. Am J surg. (2021) 222:395–401. doi: 10.1016/j.amjsurg.2020.11.049
33. Kennecke HF, Bahnson HT, Lin B, O'Rourke C, Kaplan J, Pham H, et al. Patterns of practice and improvements in survival among patients with stage 2/3 rectal cancer treated with trimodality therapy. JAMA Oncol. (2022) 8:1466–70. doi: 10.1001/jamaoncol.2022.2831
34. Zhai ML, Zhang FY, Yang JR, Zhang S, Zhao L, Lin ZY, et al. Current status of neoadjuvant therapy for locally advanced rectal cancer in Wuhan Union Hospital Cancer Center. Radiat Oncol (London England). (2022) 17:109. doi: 10.1186/s13014-022-02081-8
35. Liu J, Ladbury C, Glaser S, Fakih M, Kaiser AM, Chen YJ, et al. Patterns of care for patients with locally advanced rectal cancer treated with total neoadjuvant therapy at predominately academic centers between 2016-2020: an NCDB analysis. Clin colorectal cancer. (2023) 22:167–74. doi: 10.1016/j.clcc.2023.01.005
36. Du C, Zhao J, Xue W, Dou F, and Gu J. Prognostic value of microsatellite instability in sporadic locally advanced rectal cancer following neoadjuvant radiotherapy. Histopathology. (2013) 62:723–30. doi: 10.1111/his.12069
37. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. (2017) 357:409–13. doi: 10.1126/science.aan6733
38. Zhang J, Cai J, Deng Y, and Wang H. Complete response in patients with locally advanced rectal cancer after neoadjuvant treatment with nivolumab. Oncoimmunology. (2019) 8:e1663108. doi: 10.1080/2162402X.2019.1663108
39. Deng Y and Zhang J. The efficacy of neoadjuvant treatment in locally advanced rectal cancer with dMMR. J Clin Oncol. (2019) 37:615–5. doi: 10.1200/JCO.2019.37.4_suppl.615
40. Demisse R, Damle N, Kim E, Gong J, Fakih M, Eng C, et al. Neoadjuvant immunotherapy-based systemic treatment in MMR-deficient or MSI-high rectal cancer: case series. J Natl Compr Canc Netw. (2020) 18:798–804. doi: 10.6004/jnccn.2020.7558
41. Liu DX, Li DD, He W, Ke CF, Jiang W, Tang JH, et al. PD-1 blockade in neoadjuvant setting of DNA mismatch repair-deficient/microsatellite instability-high colorectal cancer. Oncoimmunology. (2020) 9:1711650. doi: 10.1080/2162402X.2020.1711650
42. Zhang Z, Cheng S, Gong J, Lu M, Zhou J, Zhang X, et al. Efficacy and safety of neoadjuvant immunotherapy in patients with microsatellite instability-high gastrointestinal Malignancies: A case series. Eur J Surg Oncol. (2020) 46:e33–9. doi: 10.1016/j.ejso.2020.06.034
43. Mans L, Pezzullo M, D’Haene N, Van de Stadt J, and Van Laethem JL. Pathological complete response after neoadjuvant immunotherapy for a patient with microsatellite instability locally advanced rectal cancer: should we adapt our standard management for these patients? Eur J Cancer. (2020) 135:75–7. doi: 10.1016/j.ejca.2020.04.046
44. Wang Q, Xiao B, Jiang W, Steele S, Cai J, Pan Z, et al. P-187: Watch-and-wait strategy for DNA mismatch repair-deficient/microsatellite instability-high rectal cancer with a clinical complete response after neoadjuvant immunotherapy: An observational cohort study. Ann Oncol. (2021) 32:S163–4. doi: 10.1016/j.annonc.2021.05.242
45. Lin Z, Cai M, Zhang P, Li G, Liu T, Li X, et al. Phase II, single-arm trial of preoperative short-course radiotherapy followed by chemotherapy and camrelizumab in locally advanced rectal cancer. J Immunother Cancer. (2021) 9:e003554. doi: 10.1136/jitc-2021-003554
46. Trojan J, Stintzing S, Haase O, Koch C, Ziegler P, Demes M, et al. Complete pathological response after neoadjuvant short-course immunotherapy with ipilimumab and nivolumab in locally advanced MSI-H/dMMR rectal cancer. Oncologist. (2021) 26:e2110–4. doi: 10.1002/onco.13955
47. Wang QX, Xiao BY, Cheng Y, Wu AW, Zhang T, Wang H, et al. Anti-PD-1-based immunotherapy as curative-intent treatment in dMMR/MSI-H rectal cancer: A multicenter cohort study. Eur J Cancer. (2022) 174:176–84. doi: 10.1016/j.ejca.2022.07.016
48. Cercek A, Lumish M, Sinopoli J, et al, Weiss J, Shia J, and Lamendola-Essel M. PD-1 Blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med. (2022) 386:2363–76. doi: 10.1056/NEJMoa2201445
49. Zhang X, Yang R, Wu T, Cai X, Li G, Yu K, et al. Efficacy and safety of neoadjuvant monoimmunotherapy with PD-1 inhibitor for dMMR/MSI⁃H locally advanced colorectal cancer: a single-center real-world study. Front Immunol. (2022) 13:913483. doi: 10.3389/fimmu.2022.913483
50. Bando H, Tsukada Y, Inamori K, Togashi Y, Koyama S, Kotani D, et al. Preoperative Chemoradiotherapy plus Nivolumab before Surgery in Patients with Microsatellite Stable and Microsatellite Instability-High Locally Advanced Rectal Cancer. Clin Cancer Res. (2022) 28:1136–46. doi: 10.1158/1078-0432.CCR-21-3213
51. Hu H, Kang L, Zhang J, Wu Z, Wang H, Huang M, et al. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): a single-center, parallel-group, non-comparative, randomized, phase 2 trial. Lancet Gastroenterol Hepatol. (2022) 7:38–48. doi: 10.1016/S2468-1253(21)00348-4
52. Kothari A, White MG, Peacock O, Kaur H, Palmquist SM, You N, et al. Pathological response following neoadjuvant immunotherapy in mismatch repair-deficient/microsatellite instability-high locally advanced, non-metastatic colorectal cancer. Br J Surg. (2022) 109:489–92. doi: 10.1093/bjs/znac050
53. Zhang M, Yang H, Chen L, Du K, Zhao L, and Wei L. Pathological complete response in MMR-deficient/MSI-high and KRAS-mutant patient with locally advanced rectal cancer after neoadjuvant chemoradiation with immunotherapy: A case report. Front Oncol. (2022) 12:926480. doi: 10.3389/fonc.2022.926480
54. Mori R, Uemura M, Sekido Y, Hata T, Ogino T, Takahashi H, et al. Locally advanced rectal cancer receiving total neoadjuvant therapy combined with nivolumab: a case report and literature review. World J Surg Oncol. (2022) 20:166. doi: 10.1186/s12957-022-02624-z
55. Yang R, Wu T, Yu J, Cai X, Li G, Li X, et al. Locally advanced rectal cancer with dMMR/MSI-H may be excused from surgery after neoadjuvant anti-PD-1 monotherapy: a multiple-center, cohort study. Front Immunol. (2023) 14:1182299. doi: 10.3389/fimmu.2023.1182299
56. Chen G, Jin Y, Guan WL, Zhang RX, Xiao WW, Cai PQ, et al. Neoadjuvant PD-1 blockade with sintilimab in mismatch-repair deficient, locally advanced rectal cancer: an open-label, single-center phase 2 study. Lancet Gastroenterol Hepatol. (2023) 8:422–31. doi: 10.1016/S2468-1253(22)00439-3
57. Li YJ, Liu XZ, Yao YF, Chen N, Li ZW, Zhang XY, et al. Efficacy and safety of preoperative immunotherapy in patients with mismatch repair-deficient or microsatellite instability-high gastrointestinal Malignancies. World J Gastrointest Surg. (2023) 15:222–33. doi: 10.4240/wjgs.v15.i2.222
58. Tissera NS, Esteso F, Luca R, Enrico D, Waisberg F, Rodriguez A, et al. Atypical pattern of response in rectal cancer after neoadjuvant pembrolizumab treatment: a case report, literature review, and proposed management model. J Gastrointest Oncol. (2023) 14:1635–42. doi: 10.21037/jgo-22-1140
59. Pei F, Wu J, Zhao Y, He W, Yao Q, Huang M, et al. Single-agent neoadjuvant immunotherapy with a PD-1 antibody in locally advanced mismatch repair-deficient or microsatellite instability-high colorectal cancer. Clin Colorectal Cancer. (2023) 22:85–91. doi: 10.1016/j.clcc.2022.11.004
60. Eefsen RL, Larsen JS, Klarskov LL, Altaf R, Høgdall E, Ingeholm P, et al. Therapy with pembrolizumab in treatment-naïve patients with nonmetastatic, mismatch repair deficient colorectal cancer. Int J Cancer. (2023) 152:2145–52. doi: 10.1002/ijc.34420
61. Yu JH, Liao LE, Xiao BY, Zhang X, Wu AW, Cheng Y, et al. Long-term outcomes of dMMR/MSI-H rectal cancer treated with anti–PD-1–based immunotherapy as curative-intent treatment. J Natl Compr Canc Netw. (2024) 22:e237096. doi: 10.6004/jnccn.2023.7096
62. Li Y, Liang F, Li Z, Zhang X, and Wu A. Neoadjuvant immunotherapy for patients with microsatellite instability-high or POLE-mutated locally advanced colorectal cancer with bulky tumors: new optimization strategy. Clin Colorectal Cancer. (2024) 24(1):18–31.e2. doi: 10.2139/ssrn.4819108
63. Li Y, Tan L, Chen N, Liu X, Liang F, Yao Y, et al. Neoadjuvant immunotherapy alone for patients with locally advanced and resectable metastatic colorectal cancer of dMMR/MSI-H status. Dis Colon Rectum. (2024) 67:1413–22. doi: 10.1097/DCR.0000000000003290
64. Tosi F, Salvatore L, Tamburini E, Artale S, Lonardi S, Marchetti S, et al. Curative immune checkpoint inhibitors therapy in patients with mismatch repair-deficient locally advanced rectal cancer: a real-world observational study. ESMO Open. (2024) 9:103929. doi: 10.1016/j.esmoop.2024.103929
65. Veselovsky E, Lebedeva A, Kuznetsova O, Kravchuk D, Belova E, Taraskina A, et al. Evaluation of blood MSI burden dynamics to trace immune checkpoint inhibitor therapy efficacy through the course of treatment. Sci Rep. (2024) 14:23454. doi: 10.1038/s41598-024-73952-1
66. Le DT, Kim TW, Van Cutsem E, Geva R, Jäger D, Hara H, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol. (2020) 38:11–9. doi: 10.1200/JCO.19.02107
67. Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicenter, phase 2 study. Lancet Oncol. (2017) 18:1182–91. doi: 10.1016/s1470-2045(17)30422-9
68. Diaz LA Jr, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomized, open-label, phase 3 study. Lancet Oncol. (2022) 23:659–70. doi: 10.1016/S1470-2045(22)00197-8
69. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. (2021) 398:27–40. doi: 10.1016/S0140-6736(21)00797-2
70. Cohen R, Hain E, Buhard O, Guilloux A, Bardier A, Kaci R, et al. Association of primary resistance to immune checkpoint inhibitors in metastatic colorectal cancer with misdiagnosis of microsatellite instability or mismatch repair deficiency status. JAMA Oncol. (2019) 5:551–5. doi: 10.1001/jamaoncol.2018.4942
71. Sahin IH, Goyal S, Pumpalova Y, Sonbol MB, Das S, Haraldsdottir S, et al. Mismatch repair (MMR) gene alteration and BRAF V600E mutation are potential predictive biomarkers of immune checkpoint inhibitors in MMR-deficient colorectal cancer. Oncologist. (2021) 26:668–75. doi: 10.1002/onco.13741
72. Sahin IH, Akce M, Alese O, Shaib W, Lesinski GB, El-Rayes B, et al. Immune checkpoint inhibitors for the treatment of MSI-H/MMR-D colorectal cancer and a perspective on resistance mechanisms. Br J Cancer. (2019) 121:809–18. doi: 10.1038/s41416-019-0599-y
73. Wang Z, Zhang Q, Qi C, Bai Y, Zhao F, Chen H, et al. Combination of AKT1 and CDH1 mutations predicts primary resistance to immunotherapy in dMMR/MSI-H gastrointestinal cancer. J Immunother Cancer. (2022) 10:e004703. doi: 10.1136/jitc-2022-004703
74. Chida K, Kawazoe A, Kawazu M, Suzuki T, Nakamura Y, Nakatsura T, et al. A low tumor mutational burden and PTEN mutations are predictors of a negative response to PD-1 blockade in MSI-H/dMMR gastrointestinal tumors. Clin Cancer Res. (2021) 27:3714–24. doi: 10.1158/1078-0432.CCR-21-0401
75. Song KD, Lim HK, Rhim H, Lee MW, Kim YS, Lee WJ, et al. Repeated Hepatic Resection versus Radiofrequency Ablation for Recurrent Hepatocellular Carcinoma after Hepatic Resection: A Propensity Score Matching Study. Radiology. (2015) 275:599–608. doi: 10.1148/radiol.14141568
76. Biederman DM, Titano JJ, Bishay VL, Durrani RJ, Dayan E, Tabori N, et al. Radiation Segmentectomy versus TACE Combined with Microwave Ablation for Unresectable Solitary Hepatocellular Carcinoma Up to 3 cm: A Propensity Score Matching Study. Radiology. (2017) 283:895–905. doi: 10.1148/radiol.2016160718
77. Chiang CL, Lee FAS, Chan KSK, Lee VWY, Chiu KWH, Ho RLM, et al. Survival outcome analysis of stereotactic body radiotherapy and immunotherapy (SBRT-IO) versus SBRT-alone in unresectable hepatocellular carcinoma. Liver cancer. (2024) 13:265–76. doi: 10.1159/000533425
78. Kulkarni GS, Hermanns T, Wei Y, Bhindi B, Satkunasivam R, Athanasopoulos P, et al. Propensity score analysis of radical cystectomy versus bladder-sparing trimodal therapy in the setting of a multidisciplinary bladder cancer clinic. J Clin Oncol. (2017) 35:2299–305. doi: 10.1200/JCO.2016.69.2327
79. Lindor NM, Burgart LJ, Leontovich O, Goldberg RM, Cunningham JM, Sargent DJ, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol. (2002) 20:1043–8. doi: 10.1200/JCO.2002.20.4.1043
80. Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). New Engl J Med. (2005) 352:1851–60. doi: 10.1056/NEJMoa043146
81. Hissong E, Crowe EP, Yantiss RK, and Chen YT. Assessing colorectal cancer mismatch repair status in the modern era: a survey of current practices and re-evaluation of the role of microsatellite instability testing. Modern Pathol. (2018) 31:1756–66. doi: 10.1038/s41379-018-0094-7
Keywords: locally advanced rectal cancer, deficient mismatch repair, surgery, chemotherapy, chemoradiotherapy, progression-free survival, overall survival
Citation: Wang H-H, Yan Y-Y, Zeng H-Y, Wang Y, Ni K-M, Yu X-R, Shi J-M, Li H-L, Wang J-F, Yuan Z-Y, Wen Q-L, Zaorsky NG, Zhang C-Z, Zang F-L and Meng M-B (2025) The efficacy and safety of neoadjuvant and adjuvant chemo(radio)therapy combined with surgery in patients with locally advanced rectal cancer harboring defective mismatch repair system: a large-scale multicenter propensity score analysis. Front. Immunol. 16:1626438. doi: 10.3389/fimmu.2025.1626438
Received: 10 May 2025; Accepted: 16 June 2025;
Published: 07 July 2025.
Edited by:
Haibo Zhang, Zhejiang Provincial People’s Hospital, ChinaReviewed by:
Corrado Spatola, University of Catania, ItalyCan Chen, Zhejiang University School of Medicine, China
Copyright © 2025 Wang, Yan, Zeng, Wang, Ni, Yu, Shi, Li, Wang, Yuan, Wen, Zaorsky, Zhang, Zang and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng-Lin Zang, emFuZ2ZlbmdsaW5AdGptdWNoLmNvbQ==; Mao-Bin Meng, bW1lbmdAdG11LmVkdS5jbg==
†These authors have contributed equally to this work
 Huan-Huan Wang1†
Huan-Huan Wang1† Ke-Min Ni
Ke-Min Ni Jin-Ming Shi
Jin-Ming Shi Zhi-Yong Yuan
Zhi-Yong Yuan Qing-Lian Wen
Qing-Lian Wen Nicholas G. Zaorsky
Nicholas G. Zaorsky Chun-Ze Zhang
Chun-Ze Zhang Feng-Lin Zang
Feng-Lin Zang Mao-Bin Meng
Mao-Bin Meng